Abstract
The intracellular long-range transport of membrane vesicles and organelles is mediated by microtubule motors (kinesins, dynein), which move cargo with spatiotemporal accuracy and efficiency. How motors navigate the microtubule network and coordinate their activity on membrane cargo are fundamental but poorly understood questions. New studies show that microtubule-dependent membrane traffic is spatially controlled by septins, a unique family of multimerizing GTPases that associate with microtubules and membrane organelles. Here, we review how septins selectively regulate motor interactions with microtubules and membrane cargo. We posit that septins provide a novel traffic code that specifies the movement and directionality of select motor-cargo complexes on distinct microtubule tracks.
Keywords: septins, microtubules, dynein, kinesins, microtubule-associated proteins, scaffold and adaptor proteins
Microtubule-dependent Transport - A New Era
Cellular physiology and homeostasis rely on the proper localization and transport of proteins and organelles. Despite extensive knowledge of the mechanisms that control membrane traffic at sites of origin and destination, it is less understood how movement and directionality are specified on the cytoskeleton en route to destination. Questions such as how membrane cargos select the appropriate motors and cytoskeletal tracks, and how cargo-bound motors of opposite directionality coordinate their activity pose a critical gap in our knowledge of membrane traffic. New insights into these questions have emerged with the discovery of microtubule post-translational modifications (see Glossary) and microtubule-associated proteins that select for the transport of specific motor-cargo complexes [1–3], and cargo adaptor and scaffold proteins that regulate motor binding and activity [4, 5]. These advances have ushered a new era of research into the molecules and regulatory mechanisms that function at the interface of motors with microtubules and membranes. Unexpectedly, the multimeric GTPases of the septin family have emerged as new regulators of motor interactions with microtubules and cargo, posing a novel alternative to regulation by small monomeric GTPases (e.g., Rab GTPases).
Septins are a family of GTP-binding proteins (Box 1), which assemble into non-polar oligomers and polymers that associate with subpopulations of microtubules and actin filaments, and membranes of distinct curvature and phospholipid content [6, 7]. Septins were originally discovered in the budding yeast Saccharomyces cerevisiae as filaments that localize on the plasma membrane of the mother-bud neck cortex and are essential for cell morphology and division [8]. Seminal studies established that septins function as membrane scaffolds and diffusion barriers that control protein localization [9–12]. Although the scaffolding and barrier functions have been conserved and adapted throughout evolution, it is less understood how septins function on the cytoskeleton and endomembrane organelles of mammalian cells. Recent evidence indicates that septins regulate the association of kinesin and dynein motors with microtubules and membrane cargo [13–15]. Here, we review these findings and discuss how septins may provide a code for the spatial regulation of microtubule-dependent transport.
Box 1. The Septin GTPases.
Septin genes express a multitude of paralogs and isoforms. Septins consist of a highly conserved GTP-binding domain (G-domain) and a septin unique element (SUE), and vary mainly in the sequences of their N-terminal extension (NTE) and C-terminal extension (CTE) (Figure IA). Based on sequence similarity, mammalian septins are classified into four groups: SEPT2 (SEPT1, SEPT2, SEPT4, SEPT5), SEPT3 (SEPT3, SEPT9, SEPT12), SEPT6 (SEPT6, SEPT8, SEPT10, SEPT11, SEPT14), and SEPT7 [16]. Septins dimerize in tandem through two alternate interfaces of their G-domain [17]. GTP hydrolysis, which is absent from septins of the SEPT6 group and differs in rate among other septin paralogs (Figure IB), promotes dimerization and in part determines the identity of dimeric partners by favoring certain pairings over others [18, 19]. A palindromic hetero-octamer, two head-to-head tetramers with subunits from each one of the four groups, is considered to be the dominate unit of septin multimers [17] (Figure IC). Septins of the same group can exchange at their respective positions generating oligomers and polymers of diverse combinations, many of which are not fully identified [17, 20]. Oligomeric units of smaller sizes and atypical combinations exist depending on the cell type-specific expression of septin paralogs and isoforms [21]. Septin oligomers assemble into higher-order filamentous multimers, whose localization, binding partners and functional properties are determined by their individual subunits; largely, due to the variable N- and C-terminal extensions that project orthogonally from the linear axis of multimerization [17] (Figure IB). Septins appear to be more stable than typical cytoskeletal polymers with a half-time of subunit turnover at least two-to-four-fold slower than actin and microtubules [22, 23]. The nucleotide exchange rates of septins are much slower [18], and in the absence of any known septin guanine nucleotide exchange factors (GEFs), septin oligomers may turn over in their respective polymers stochastically and/or due to post-translational modifications [24].
Box 1, Figure I. Septin Domains and Assembly.
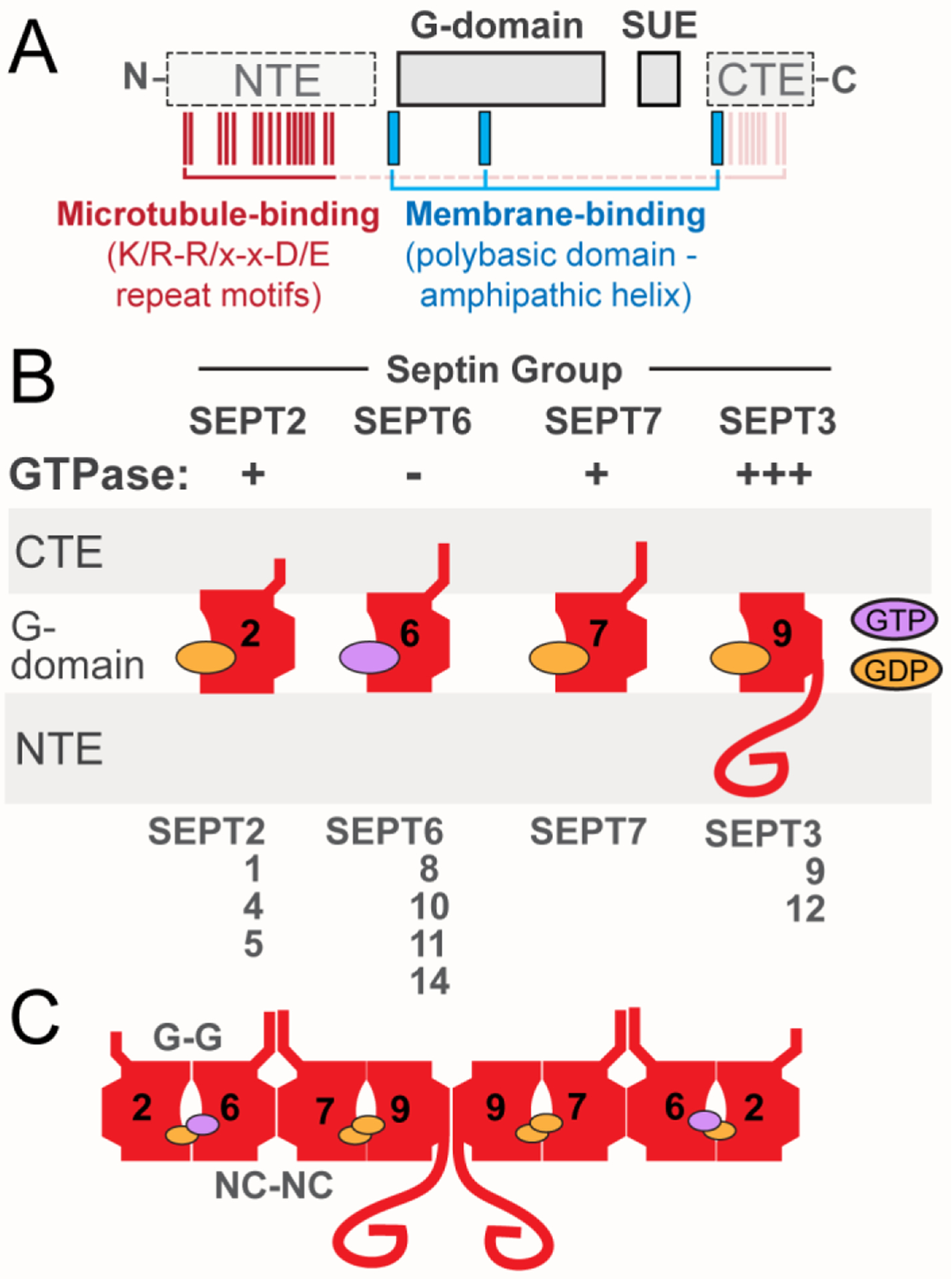
(A) Schematic shows the main domains of septins, which include the highly conserved G-domain and SUE, and the variable NTE and CTE. The positions of the membrane-binding polybasic domains and amphipathic helices (blue), and the microtubule-binding repeat motifs (K/R-R/x-x-D/E; red) are also depicted. Microtubule-binding motifs are present in the NTE of SEPT9, but similar motifs may mediate the interaction of the CTEs of SEPT6 and SEPT7 with microtubules. (B) Schematic of the position and orientation of the major domains of septins and the GTP/GDP nucleotide. GTPase activity is denoted as minus (−) for septins of the SEPT6 group, which do not hydrolyze GTP, and plus (+) for septins with slower GTPase such as SEPT2 and SEPT7. SEPT9, which is depicted as the ubiquitously expressed septin paralog of the SEPT3 group, has faster rates (+++) of GTP hydrolysis than SEPT2 and SEPT7. (C) Schematic representation of the prototypical SEPT2/6/7/9 hetero-octamer, which consists of septin paralogs interacting with one another through two different bindings interfaces, which involve the GTP-binding pockets (G-G interface) and the N- and C-termini of the GTP-binding domains (NC-NC interface).
Septins: Multimeric GTPases with Microtubule- and Membrane-binding Specificities
Septins associate with subpopulations of microtubules, which are often bundled and localize in specific intracellular regions and compartments such as the perinuclear cytoplasm and primary cilia [25–28]. Septins are likely to establish these localizations due to subunit-specific affinities for tubulin isotypes and post-translational modifications [27, 29, 30]. Amplification of septin-microtubule binding in response to taxol suggests that septins prefer the longitudinal α/β-tubulin conformations of stabilized protofilaments [28, 31] (Figure 1A). In support of this possibility, septins (SEPT2/6/7, SEPT9) associate in vitro preferentially with stable GMPCPP-bound microtubule lattices which are characterized by a non-compacted longitudinal inter-dimer interface that is similar to the taxol-stabilized protofilaments [32–34] (Figure 1A). Microtubule bundling may further enhance septin binding, but it is unknown whether the parallel or antiparallel orientation of bundled microtubules plays a role. The regional specificities of microtubule binding might be also indirectly determined by the membrane localizations of septins, which may associate with proximal microtubules upon dissociation from membranes. Indicative of a spatial coupling between membrane- and cytoskeleton-bound septins, septin localization to a subset of actin stress fibers coincides topologically with septin enrichment on phosphatidylinositol 3,5-bisphosphate-rich macropinosomes [21, 35]. Similarly, septin association with microtubule subsets might be linked to endomembrane pools of septins.
Figure 1. Microtubule-associated Septins.

(A) Septins interact directly with and crosslink microtubules into bundles. Septin interaction with microtubules involves the C-terminal tyrosine of α-tubulin, polyglutamylation of the C-terminal tails of tubulin and the C-terminal tail of β-tubulin. Septins associate preferentially with GTP- and taxol-bound microtubules, which are characterized by a non-compacted longitudinal inter-dimer interface. Yellow highlights the structural elements of α-/β-tubulin that play a role in septin-microtubule binding. (B) Septins function in the elongation and trimming of the polyglutamyl sidechains of the tubulin C-terminal tails by interacting with the polyglutamylase enzymes tubulin tyrosine ligase like 1 and 11 (TTLL1, TTLL11) and the cytosolic carboxypeptidase 1 (CCP1), which removes glutamyl residues. (C) Septins have been shown to bundle microtubules, suppress microtubule catastrophe and thereby, promote persistent microtubule growth and elongation. In addition, SEPT9 recruits unpolymerized tubulin dimers to the microtubule lattice, which might promote the incorporation of tubulin subunits at sites of microtubule damage.
Current understanding of how septins interact with microtubules has come from studies of SEPT9, a paralog with a long and structurally disordered N-terminal extension (NTE) which is unique among septins. The NTE of isoform 1 of SEPT9 (SEPT9_i1) contains a repeat motif (K/R-R/x-x-D/E), which resembles the microtubule-binding repeat motifs (KKE) of MAP1, and interacts electrostatically with the microtubule C-terminal tails (CTTs) [29]. Competition experiments with CTT peptides indicate that the SEPT9 NTE interacts preferentially with the βII-tubulin (TUBB2) isotype [29]. Polyglutamylation, the addition of polyglutamylated side chains to tubulin CTTs, and the presence of the C-terminal tyrosine of α-tubulin promote septin-microtubule binding, suggesting a preference for tyrosinated and polyglutamylated microtubules [27, 30] (Figure 1B). It is unknown whether septins of different subunit composition have preferences for microtubule subsets with other tubulin isotypes and post-translational modifications. It is also unclear how septins of the SEPT6 and SEPT7 groups interact with microtubules, but the C-terminal extensions may directly bind microtubules through repeat motifs similar to K/R-R/x-x-D/E. Of note, SEPT2 and possibly more septins of the SEPT2 group have very low affinity for microtubules [29]. SEPT9 associates with unpolymerized tubulin and microtubule-associated SEPT9 recruits tubulin dimers to the microtubule lattice and thereby, may rescue microtubule lattices from depolymerization and repair sites of damage [33]. In vitro assays show that SEPT9 and SEPT2/6/7 complexes promote microtubule growth and elongation by suppressing catastrophe [32, 33] (Figure 1C). In agreement with the in vitro findings, septins associate with microtubule bundles and promote as well as guide microtubule growth in living cells [28].
In addition to their microtubule-binding properties, septins associate with distinct membrane domains and organelles (Figure 2). Septins bind and assemble preferentially on membrane domains of micron-scale curvature, accumulating on the cytoplasmic leaflet of the saddle-shaped domains of the plasma membrane, which often outline the base of protrusive structures (e.g., filipodia, dendritic spines) [36]. Consistent with peak affinities for membrane bilayers on beads with diameters of 1–5 μm [36], septins associate with larger organelles including lipid droplets, macropinosomes, mitochondria and lysosomes [15, 35, 37, 38]. Septin association with these organelles involves recognition of specific phospholipids including phosphoinositides such as phosphatidylinositol 5-phoshate, phosphatidylinositol 3,5-bisphosphate and phosphatidylinositol 4,5-bisphosphate [35, 37, 39, 40]. Growing evidence suggests that septins have differential affinities for phospholipids and membrane curvature due to paralog-specific domains [41]. For example, recognition of membrane curvature involves an amphipathic helix in the C-terminal extension of SEPT6, which lacks a polybasic phosphoinositide-binding domain that is conserved in other septin paralogs [41–43]. Membrane-binding specificity is also influenced by the interactions of septin paralogs with the cytoplasmic tails of integral membrane proteins (e.g., glutamate transporter GLAST) [44], components of the vesicle tethering and fusion machinery [45], Rab GTPases (Rab8) [46], ESCRT complex subunits and nexins (TSG101, SNX21) [47, 48], adaptor (AP3, huntingtin) [47, 49] and membrane scaffold proteins such as anillin [50] (Figure 2).
Figure 2. Membrane-associated Septins.

Septins associate preferentially with membrane domains of micron-scale curvature (radius of 0.5–1.5 um) and phosphoinositide content that includes phosphatidylinositol 4-phosphate, PI4P, phosphatidylinositol 4,5-bisphosphate, PI(4,5)P2, phosphatidylinositol 3,5-bisphosphate, PI(3,5)P2, as well as cone-shaped lipids such as cardiolipin, phosphatidic acid (PA) and diacylglycerol (DAG), which favorably partition in membrane areas of curvature. Septins have been reported to localize on phagosomes, macropinosomes, lipid droplets, multivesicular bodies, lysosomes, mitochondria, bacteria, and Golgi membranes. SEPT2 interacts with the exocyst vesicle-tethering complex and the cytoplasmic tail of the glutamate aspartate receptor (GLAST). SEPT7 interacts with the Rab8 GTPase and the clathrin adaptor protein 3 (AP3). SEPT9 associates with the tumor suppressor gene 101, a component of the endosomal soring complex required for transport (ESCRT), and septins (SEPT2, SEPT7, SEPT9) are in a complex with the nexin SNX-21 and the adaptor protein huntingtin.
The higher-order organization of septins on microtubules and membrane organelles is poorly understood. In cells, microtubule-associated septins often appear as cables that overlap laterally with microtubule bundles, but it is unclear if these are septin filaments [14, 28]. Septins associate with microtubules in vitro and increasing septin concentrations exert biphasic effects on microtubule dynamics, which correlate with a shift from an oligomeric to higher-order polymeric state [32]. On membrane bilayers, septins form networks of parallel paired filaments, whose repeatability and density enable them to function as protein scaffolds [51, 52]. Filamentous networks of septins assemble on domains of micro-scale curvature, but septins are also seen in nanometer-scale domains and patches of membrane organelles, which may consist of septin oligomers [15, 35]. Overall, septins associate with microtubules and membrane domains as both oligomers or higher-order filaments, which can control protein localizations and interactions.
Microtubule-associated Septins Regulate Motor-cargo Motility
The microtubule network was long viewed as a passive scaffold for the transport of membrane cargo by their respective motors. Recent advances, however, indicate that the α/β-tubulin isotype content of microtubules along with their PTMs and MAPs may determine which motors and cargos move on which microtubules [1, 2, 53]. Conceptually, this has altered our view of membrane traffic and septins have emerged among the first few MAPs that differentially regulate the movement of select motor-cargo complexes on subsets of microtubules.
To test whether microtubule-associated septins directly affect the motility of kinesin and dynein motors, translocation of constitutively active cargo-less motors was quantitatively assayed in vitro upon reconstitution of septin (SEPT9_i1)-coated microtubules [14]. SEPT9_i1 impeded the motility of kinesin-1/KIF5, reducing landing rates and velocity, and increasing the frequency of pausing events [14]. The opposite effect, however, was observed for kinesin-3/KIF1A, which lands and moves on SEPT9_i1 coated microtubules with enhanced rates, and pauses less [14]. In contrast, there is no effect on kinesin-2/KIF17, while the velocity and run lengths of the dynein-dynactin-Bicaudal D motor complex decrease [13, 14].
Consistent with the in vitro findings, SEPT9 differentially regulates KIF5 and KIF1A on the microtubules of rat hippocampal neuron dendrites, where SEPT9 is enriched [14]. SEPT9 depletion increases the movement of both kinesin-1/KIF5 and its amyloid precursor protein (APP)-carrying vesicles, and reduces the motility of kinesin-3/KIF1A and vesicles with lipoprotein density receptor (LDLR). In contrast, the opposite effects are observed when SEPT9_i1 is over-expressed [14]. In SEPT9-depleted neurons, loss of the axonal preference of KIF5, APP and presynaptic proteins (Bassoon, synapsin) indicates that SEPT9 inhibits the transport of axonally-destined cargo into dendrites [14]. Conversely, dendritically-destined cargos of KIF1A stall at the dendritic entry sites and proximal dendrites of SEPT9-depleted neurons, suggesting that SEPT9 promotes the dendritic transport of KIF1A motor-cargo [14]. Steady state imaging of kinesin-specific cargos and peroxisomes linked to the motor domains of KIF5B and KIF1A, showed that KIF5-driven transport is indeed impeded during entry into dendrites relative to KIF1A. KIF5 cargos stall and reverse toward the cell body, while KIF1A cargos are more motile and move with anterograde bias [14]. Predictably, SEPT9 depletion reverses these biases, which is also consistent with a shift in the axon-dendrite polarity of KIF5 and KIF1A cargos [14]. Thus, microtubule-associated septins can differentially regulate the motility of specific kinesin-cargo complexes on a subset of neuronal microtubules, and thereby contribute to and maintain the axon-dendrite polarity of neuronal traffic.
These findings raise a number of mechanistic questions (Box 2) and point to the possibility of septin paralog- and complex-specific effects on select motors and cargo. However, given that septins assemble into heteromeric complexes, an important question is whether SEPT9 functions as a homomer or heteromer, and if it retains its properties in complex with SEPT2/6/7. On the dendritic microtubules of hippocampal neurons, SEPT9 exhibits little to no colocalization with its cognate partner SEPT7 or the SEPT2 and SEPT6 subunits of the canonical hetero-octamer [14, 54]. Moreover, SEPT7 depletion does not impact the axon-dendrite localization of kinesin motors [14]. Interestingly, SEPT7 depletion does not phenocopy SEPT9 knock-down in migrating melanomas [55], and divergent localizations and functions between SEPT7 and other septin paralogs have been observed in centrosomes and migrating neurons [56, 57]. Thus, it is possible that SEPT9 functions in heteromeric complexes of alternative composition or as a homomer. This might be due to the expression of the neurospecific paralog SEPT3, which may outcompete SEPT9 in the SEPT3 group slot of the canonical hetero-octameric unit. More work is required to determine whether SEPT9 functions in complex with other paralogs, and if microtubule motor motility is differentially regulated by heteromeric septin complexes in a manner that depends on the identity and combination of their subunits.
Box 2. Mechanisms of Differential Motor Regulation by Microtubule-associated SEPT9_i1.
Microtubule-associated SEPT9_i1 inhibits kinesin-1/KIF5 and dynein, enhances kinesin-3/KIF1A and does not affect kinesin-2/KIF17 (Figure IA). Differential regulation of KIF5 andKIF1A is likely to involve distinct interactions between kinesin-specific residues and the microtubule-associated SEPT9_i1. The motor domain of KIF5 has a longer neck-linker and a shorter loop 12 (L12) than KIF1A, which affect the microtubule-binding and motile properties of these motors. SEPT9_i1 may impede KIF5 motility by one or more of the following possibilities: i) occluding the microtubule-binding sites of the KIF5 motor domain, ii) interacting or stabilizing the necklinker, which would slowdown KIF5 movement by restricting the ATP-triggered swiveling of the neck-linker – the latter shifts forward the unbound motor domain, iii) hindering KIF5 motility by associating with the kinesin neck and stalk domains (Figure IB). Enhancement of KIF1A motility by SEPT9 involves the lysine-rich L12 of the motor domain, which is more positively charged than KIF5 [14]. SEPT9_i1 improves the microtubule association and motility of a hybrid KIF5C motor domain, which contains the L12 of KIF1A, and promotes its entry into dendrites. We hypothesize that the acidic domain of the SEPT9 NTE may interact directly with the L12 of KIF1A, providing additional docking sites at the microtubule-motor interface. This might be similar to the transient interactions of KIF1A with acidic residues of MAP9, which enhances the on-rates and processivity of kinesin-3/KIF1A in a L12-dependent manner [58]. Structural differences in the kinesin neck-linker sequences and domains such as helix-α6, loop11-helix-α4 and loop8-strand-β5 could also account for the differential effects of SEPT9 on the motilities of KIF5 and KIF1A (Figure IC) [59, 60]. It is plausible that SEPT9 may interact with KIF1A-specific residues of these domains or allosterically trigger conformational changes at the microtubule-motor interface, which promote KIF1A motility. By contrast, the NTE of SEPT9 may inhibit dynein-dynactin motility by masking residues of the CTTs of α-/β-tubulin including the C-terminal EEY/F sequence of α-tubulin, which interacts with the CAP-Gly domain of p150GLUED (Figure ID). Of note, α-tubulin detyrosination diminishes septin colocalization with microtubules suggesting that SEPT9 associates with the α-tubulin CTTs. In addition, SEPT9 may occlude the E443 residue of β-tubulin, which is critical for the docking of the dynein microtubule-binding domain [61]. Alternatively, SEPT9 may impede dynein from side-stepping to neighboring protofilaments, which enables dynein-dynactin to maneuver around inhibitory MAPs [62].
Box 2, Figure I. Regulation of Kinesin and Dynein motility by the Microtubule-binding Domain of SEPT9.

(A) Differential regulation of motor motility by microtubule-associated SEPT9. (B) The NTE of SEPT9 may inhibit KIF5 motor motility by interfering with the microtubule docking of the KIF5 motor domain, restricting the ATP-triggered swiveling of the neck linker (NL) domain, which underlies ATP hydrolysis and stepping, and/or tethering the stalk domain. (C) Side (left panel) and top-down (right panel) view schematics of structural elements of kinesin-3/KIF1A, which might be in contact with both microtubules and SEPT9. The NTE of SEPT9 may provide additional binding sites for the lysine-rich L12 (left panel) of KIF1A, and interact with loops L11 and L8, helices α4 and α5 and/or the β-sheet β5 of KIF1A, which vary in sequence from KIF5. (D) Side (left panel) and top-down (right) view schematics of dynein-dynactin inhibition. The SEPT9 NTE may occlude the microtubule-binding sites of the CAP-Gly domain of p150GLUED, and DHC (left panel). Additionally (right panel), it may impede dynein-dynactin from switching protofilaments.
Microtubule-associated Septins Regulate Motor-cargo Transport in Coordination with MAPs and PTMs
Similar to microtubule-associated septins, bone fide MAPs and microtubule PTMs can directly modulate the motility of kinesin and dynein motors. Because MAPs compete with one another for microtubule binding, which is also influenced by PTMs [1, 2], septins are prone to regulatory interplays with MAPs and microtubule PTMs. Early evidence of such coordinate regulation came from studies of Golgi-to-plasma membrane traffic in polarizing epithelia [27]. At trans-Golgi sites of tubulovesicular exit, septins (SEPT2) localize to polyglutamylated microtubules and compete with MAP4 for binding to these microtubule tracks [27, 63, 64]. Over-expression of MAP4, a MAP that inhibits membrane traffic, displaces septins (SEPT2) from microtubules and abrogates vesicle egress from the Golgi to plasma membrane [27]. In addition, MAP4 over-expression diminishes microtubule polyglutamylation suggesting that septins play a role in microtubule polyglutamylation [27]. Indeed, septins were independently found to interact with enzymes that elongate and trim the polyglutamyl sidechains of microtubule CTTs (Figure 1B) [30, 65]. Consistent with a MAP4-septin competition, septins prevent MAP4 from associating with microtubules by directly sequestering MAP4 in the cytoplasm and/or occluding its microtubule binding sites [27, 64], and indirectly by influencing microtubule polyglutamylation, which potentiates MAP-binding [27, 30, 66]. Septins and MAP4 also compete on the axonemal microtubules of primary cilia, where septins counteract the inhibitory effects of MAP4 on ciliary elongation [26]. Taken together, septins promote vesicle transport on the microtubules of the trans-Golgi network by antagonizing MAP4, which inhibits membrane traffic, and by enhancing polyglutamylation, which has been shown to enhance the motility of kinesin-1 and -2 motors [65].
A septin interplay with microtubule PTMs may also exist in the regulation of neuronal membrane traffic by SEPT9. In the dendrites of hippocampal neurons, acetylated and tyrosinated microtubules are oriented with their plus ends pointing toward and away from the cell body, respectively [67]. Kinesin-1/KIF5 preferentially associates with acetylated microtubules, while kinesin-3/KIF1A selectively binds and moves on tyrosinated microtubules [67]. It is plausible that SEPT9 associates with tyrosinated microtubules, inhibiting kinesin-1/KIF5 binding while permitting and enhancing kinesin-3/KIF1A motility. However, it is less likely that SEPT9 localizes to and/or maintains the acetylated microtubules. SEPT9 knock-down does not affect the relative distribution of acetylated microtubules in axon-dendrites [14], and SEPT9 associates with segments of microtubules that are weakly acetylated in non-neuronal cells [68]. Moreover, SEPT7 knock-down increases microtubule acetylation, which would enhance exclusion of KIF5 from dendrites rather than increasing its dendritic localization as observed in SEPT9-depeleted neurons [64, 69]. Further work is required to determine the PTMs and MAPs of the septin-bound microtubules. Knowledge of this landscape is key for understanding how septins regulate motor-driven transport in coordination with MAPs and microtubule PTMs.
Membrane-associated Septins Function as Motor Adaptors and Scaffolds
Regulation of motor-cargo motility on microtubules occurs concomitantly with the mechanisms that recruit, activate and coordinate motors on membrane cargo. Association of kinesin and dynein with cargo is mainly mediated by adaptor and scaffold proteins, many of which induce conformational changes that activate motors by relieving autoinhibitory interactions [4, 5]. Adaptors/scaffold proteins can selectively interact with more than one motor, assembling and coordinating teams of motors of the same or opposing directionality [4, 5]. New findings suggest that septins function as adaptors or scaffolds at the interface of membrane cargo with microtubules motors (Figure 3A–B).
Figure 3. Septin Interactions with Microtubule Motors and Functions in Mitosis.

(A) Summary of direct (solid lines) and potentially indirect (dotted lines) interactions of septin paralogs with dynactin and dynein components, and kinesins KIF17, KIF20A and CENP-E. Dotted rectangles outline the C-terminal kinesin domains that interact directly with septins. (B) Cargo-bound septins recruit and scaffold motors of the same or opposite directionalities (KIF17 and dynein), providing a mechanism for the selective assembly of motor teams and possibly the regulation of bidirectional movement. (C) Septins (SEPT7) interact directly with CENP-E and function in proper chromosome alignment and bi-orientation at the metaphase plate by maintaining proper CENP-E localization. Septins may enhance processive motility of CENP-E, promoting CENP-E-mediated translocation of mono-oriented chromosomes along spindle microtubules toward the metaphase plate. At the metaphase plate, microtubule-associated septin complexes may scaffold CENP-E interactions with microtubule ends and kinetochores, and/or blocking the diffusion and movement of CENP-E away from the kinetochore-microtubule interface. In the latter scenario, microtubule-associated septins are posited to restrict CENP-E diffusion along the microtubule lattice. (D) A hypothetical model of septin roles in the localization and functions of KIF20A in the central spindle of mitotic cells. Septins localize to central spindle microtubules and may trigger the dissociation of CPC from KIF20A as SEPT7 interacts with the same domain of KIF20A, which associates with the INCENP component of the CPC. Additionally, microtubule-associated septins may immobilize KIF20A and promote its localization at the spindle midzone.
In the human interactome of dynein, which was obtained from pulling down proximal partners of components of the dynein-dynactin motor complex using the biotin ligase (BioID), septins co-precipitated with the dynein intermediate and light intermediate chains (DIC, DILC) and the dynein activating adaptor BiCD2 [70]. Although these interactions may not be direct, new studies show that SEPT9 binds directly DIC, and SEPT7 associates with the p150GLUED subunit of dynactin [15, 57]. A yeast two hybrid screen also identified SEPT2 as a binding partner of the dynactin component p50 dynamitin [71]. Septin (SEPT9) coupling to the membranes of mitochondria and peroxisomes enhances dynein recruitment and triggers retrograde transport to the perinuclear cytoplasm [15]. This dynein adaptor-like function of SEPT9 is impaired by a mutation that abrogates its GTPase activity and GDP-dependent multimerization, and disrupts its interaction with DIC [15]. Consistent with these findings, endogenous SEPT9 localizes to microdomains of lysosomal membranes and promotes retrograde traffic at steady state and in response to oxidative stress [15]. Collectively, these findings indicate that SEPT9 functions as a GDP-activated scaffold of dynein motors on lysosomes and possibly other organelles such as lipid droplets, whose retrograde transport in response to hepatitis C infection is dependent on SEPT9 [37].
In contrast to the established mechanisms of dynein recruitment to membrane organelles, which involve adaptor proteins under the control of the Rab7 GTPase, septins recruit dynein in a GDP-activated manner and independently of Rab7 [15]. This might be advantageous under stress conditions of low GTP-to-GDP ratio, but it is unclear whether SEPT9 activates dynein. SEPT9 lacks the DLIC-interacting coiled-coil domains of known dynein adaptors and activators, and does not interact with DLIC [72]. However, SEPT9 interacts with both dynein (DIC) and dynactin (p150GLUED) via its GTP-binding and NTE domains, respectively [15]. Moreover, SEPT9 binds the same DIC domain that associates with the dynein adaptors snapin and huntingtin [15]. Given that the SEPT9 NTE binds actin filaments [73], it may also interact with the Arp1 minifilament of dynactin resembling the coiled-coil domains of bona fide dynein adaptors and spectrin [73, 74]. Multimerization of SEPT9 with itself and/or other septins could enable additional contacts between dynein and dynactin, and the recruitment of multiple copies of dynein-dynactin.
Resembling dynein adaptors (e.g., JIP-1, HOOK3, HAP1), which scaffold motors of opposing directionality, SEPT9 has been found to interact with the kinesin-2 motor KIF17 [4, 5, 13]. Kinesin-2/KIF17 is a homo-dimerizing motor that undergoes autoinhibition through backfolding of its C-terminal cargo-binding tail onto the microtubule-binding motor domain [75]. SEPT9 binds directly and specifically to the cargo-binding tail of KIF17, interacting preferentially with the fully extended cargo-binding conformation of KIF17 [13]. Moreover, SEPT9 competes with mLin-10 of the mLin-2/mLin-7/mLin-10 adaptor complex for binding the tail of KIF17 [13]. Thereby, SEPT9 regulates the vesicular traffic of the N-methyl-d-aspartate (NMDA) receptor, a post-synaptic protein which is transported by KIF17 into dendrites; KIF17 associates with the cytoplasmic tail of the NMDA receptor via the mLin-10 complex [13]. SEPT9 co-traffics with KIF17 in neuronal vesicles, but it is unknown whether it links KIF17 to specific cargo [13]. It is also unclear whether SEPT9 can concomitantly interact with both dynein and KIF17, coordinating a switch between these two motors (Figure 1B). Interestingly, SEPT9 interacts with the c-Jun N-terminal kinase, which triggers a kinesin-to-dynein switch by phosphorylating the cargo adaptor/scaffold protein JIP1 [76, 77]. Thus, SEPT9 may function as a cargo scaffold of motor teams of the same or opposing directionalities, coordinating the bidirectional movement of membrane organelles and vesicles.
Septins Regulate the Localization and Functions of Mitotic Microtubule Motors
During cell division, microtubule motors adapt their functions in the mitotic spindle for the capture, movement and positioning of chromosomes. Microtubule motors enable nuclear envelope breakdown, spindle orientation and elongation, and are linked to the signaling pathways that trigger anaphase and cytokinesis [78]. Consistent with their roles in microtubule-dependent transport in interphase cells, septins interact with mitotic kinesins and dynactin, and function in spindle assembly, chromosome alignment and cytokinesis.
SEPT7 localizes to centrosomes and is critical for the assembly of bipolar spindles [57]. SEPT7 associates with p150GLUED (dynactin), which functionally complements SEPT7 in microtubule nucleation from the centrosome [57]. Because centrosomal levels of SEPT7 decrease during mitosis and SEPT7 depletion impacts entry into S phase [57], SEPT7 appears to function in centrosome duplication prior to mitosis. Although the precise role of SEPT7 is unknown, p150GLUED localizes to the subdistal appendages of the mother centriole, which anchor microtubule minus ends, and impacts the recruitment of the subdistal appendage proteins ninein and CEP170, which interact with SEPT9 and SEPT1, respectively [79–81]. Of note, SEPT1 is also enriched in spindle poles [82]. Thus, septins could scaffold the interactions of p150GLUED with components of the subdistal appendages and thereby, affecting centriole cohesion and microtubule anchoring.
In metaphase, a network of fibrillar septins (SEPT2, SEPT7) partially colocalizes microtubules in close apposition to the kinetochores of aligned chromosomes [83]. Septin (SEPT2, SEPT7) depletion impairs chromosome congression and alignment at the metphase, resulting in a number of chromosomes accumulating at spindle poles [83, 84]. This phenotype correlates with mislocalization of kinesin-7/CENP-E, which mediates microtubule end-on attachments with kinetochores and translocates mono-oriented chromosomes along spindle microtubules to the metaphase plate [84]. Loss of CENP-E from the kinetochores of chromosomes indicates that septins are critical for the maintenance of CENP-E at the interface of microtubule plus ends with kinetochores (Figure 3C). Consistent with lack of proper microtubule-kinetochore attachment, the inter-kinetochore distance between chromatid pairs is reduced in SEPT7-depleted cells [84]. Although SEPT7 does not colocalize with CENP-E at kinetochores, SEPT7 interacts directly with the flexible C-terminal tail of CENP-E [84]. Thus, septins are likely to function at the microtubule-CENP-E interface. SEPT7 may stabilize the microtubule-binding of the C-terminal tail of CENP-E and/or impeding its diffusion along the microtubule lattice upon dissociation from the kinetochore – in the latter possibility, septins would enable CENP-E to re-attach to kinetochores without diffusing or translocating to the spindle poles (Figure 3C). Alternatively, septins may promote the directional transport of mono-oriented chromosomes by CENP-E from the poles to the metaphase plate (Figure 3C), which occurs along a subset of detyrosinated microtubules [85]. Consistent with these possibilities, CENP-E is abnormally concentrated at the spindle poles of SEPT7-depleted cells [83]. Thus, septins may control the localization of CENP-E by regulating its motility and/or diffusion on mitotic microtubules.
On the microtubules of the cytokinetic midbody of neural crest cells, SEPT7 interacts and colocalizes with kinesin-6/KIF20A (mitotic kinesin-like protein 2, MKLP2), a mitotic motor with key roles in central spindle and midbody formation [86]. SEPT7 binds the C-terminal coiled-coil domain of KIF20A and is required for the localization of KIF20A to the midbody of neural crest cells, which undergo precocious differentiation in the absence of SEPT7 [86]. However, the precise function of SEPT7 in the regulation of KIF20A localization and motility is unknown. SEPT7 binds to the same domain of KIF20A that interacts with INCENP, one of the major components of the chromosomal passenger complex (CPC) [86, 87]. Because KIF20A functions as both a microtubule crosslinker and processive motor, which transports CPC to the spindle midzone in late anaphase [87], SEPT7 may promote INCENP release from KIF20A and association of the latter with the anti-parallel microtubules of the central spindle (Figure 3D). In this manner, SEPT7 not only would enable proper localization of KIF20 and INCENP, but also facilitate the functional transition of KIF20A from a processive to a microtubule-crosslinking motor (Figure 3D). In support of this possibility, SEPT2-depleted epithelia are devoid of a central spindle and the spindle does not elongate [83]. Moreover, SEPT7 could synergize with SEPT1, which also localizes to the central spindle and intercellular bridge, and is phosphorylated by the CPC component Aurora B [82]. Taken together, it is evident that septins regulate the localization and function of the motors of the mitotic spindle, and therefore maintain and adapt their functions as regulators of microtubule motors from membrane traffic to mitosis.
Concluding Remarks
The discovery that septins regulate kinesin and dynein motor interactions with microtubules and membrane cargo is an exciting development with implications for the spatial regulation of intracellular transport. With thirteen septin genes expressing as many septin paralogs and isoforms as kinesin motors – over 40 - and a combinatorial type of assembly, septins may provide a code that determines which motors interact with which cargo and microtubules (Figure 4, Key Figure). As septins were recently reported to interact with processive myosins [88, 89], a septin code may encompass myosin-driven transport on the actin cytoskeleton.
Figure 4. A Septin GTPase Code for Motor Selection by Cargo and Microtubules.

Graphical abstract of the septin code hypothesis for the selective attachment of motors to cargo (left panel) and motor-cargo binding and transport on microtubules (right panel). In the left panel, different microtubule motors (kinesins X, Y and dynein) are recruited to different cargos (cargos A, B and C) by cargo-specific septins or septin complexes, which are denoted by roman numerals (septins I, II, III). In the right panel, microtubule tracks with distinct septin complexes select for the attachment and motility of specific motor-cargo (cargo A-motor X, cargo B-motor Y, cargo C-dynein). Thereby, microtubule-associated septins specify the microtubule tracks and intracellular routes of specific motors and their cargo.
A septin code entails a functional specialization among septins for the selective regulation of motors and their cargos. We envisage that the localization and regulatory specificity of septin complexes is determined by the combination of their septin paralogs and/or isoforms. Septin complexes such as SEPT2/6/7 and SEPT5/11/7, for example, might be on separate microtubule tracks, and exert distinct effects on certain motor-cargo. Septins would be functionally integrated with the PTMs and MAPs of microtubules – the tubulin and MAP codes, which have been proposed to regulate membrane traffic [1–3, 53]. Microtubule PTMs not only mark microtubules of distinct orientation and organization, but also differentially regulate kinesin and dynein motility [65, 90]. Similarly, MAPs occupy spatially distinct microtubule subsets and exhibit a functional specialization in the regulation of microtubule motor motility – e.g., MAP7 enhances KIF5 and inhibits KIF1A, while MAP9 is reversely a KIF5 inhibitor and KIF1A enhancer [58, 61, 91]. Hence, septins are likely to function in concert with MAPs and PTMs on the same or different microtubule tracks, selecting the type of motors and cargo that move on a particular microtubule and their directionality. A similar type of selection could also be taking place on membranes with septins and cytoplasmic adaptor or scaffold proteins determining the type of motors that engage with vesicles or organelles.
Central to the hypothesis of a septin GTPase code are two tenets. First, different septin complexes associate with different cytoskeletal polymers and membrane cargos. Second, septins selectively modulate the binding and activity of motors on cargo and cytoskeletal tracks. Evidence for cargo- and microtubule-specific septin complexes is lacking, but septins associate with phosphoinositides, membrane curvature and proteins through unique domains and sequences, some of which are septin paralog-specific [39, 41–43]. It is also evident that septins are present on distinct subpopulation of microtubules and actin filaments, and that these localizations are influenced by septin paralog/isoform-specific domains and properties [29, 68]. However, it is unknown if there are septin paralogs, isoforms or complexes that are specific to distinct microtubule subsets, distinguishing between microtubules of different tubulin isotype, PTM and MAP content. Rigorous mapping of septins to membrane organelles and microtubule subsets is necessary to resolve cargo- and cytoskeleton-binding specificities. Similarly, a more systematic investigation of septin interactions with motors of the kinesin and myosin families will provide more insight into the selectivity of septin-motor interactions, the second tenet of the septin code hypothesis. A handful of septin-motor interactions, which are known to date, might be the proverbial tip of the iceberg - a larger interactome of specific and functionally specialized septin-motor pairings.
In sum, septin emerge as a combinatorial and adaptable GTPase module for the selective regulation of motor interactions with cargo and the microtubules. Alongside cargo adaptors, microtubule-associated and PTMs, septins may constitute a distinct code for the spatial control and coordination of membrane traffic.
Outstanding Questions.
What is the three-dimensional structure of septins in complex with a microtubule? How do septins interact with α-/β-tubulin at the atomic-level, and how are they positioned with respect to microtubule protofilaments and α-/β-tubulin dimers?
Can different septin paralogs or complexes distinguish between microtubules of distinct post-translational modifications, α/β-tubulin isotypes, GTP/GDP content, and bundles of parallel or anti-parallel orientation?
How do MAPs impact septin association with microtubules and vice versa? Do septins determine the MAP signature of individual microtubules?
What is the mechanism by which SEPT9 enhances the motility of kinesin-1/KIF1A, and inhibits kinesin-1/KIF5 and dynein-dynactin? Does SEPT9 function similarly in a complex with SEPT2/6/7?
Do microtubule-associated septins modulate kinesin and dynein motility in a paralog and isoform-specific manner?
Are there membrane cargo-specific septin complexes or paralogs, and do they selectively recruit kinesin or dynein motors? Do septins associate differentially with the cargo-binding tails of kinesins?
Can membrane-bound septins activate kinesin and dynein motors by relieving autoinhibitory interactions or promoting interactions with cofactors?
Can cargo-bound septins interact with multiple motors of the same or opposing directionality? Do they coordinate bidirectional movement by activating or inactivating motors of opposing directionality in response to signaling cues?
Are septin functions at the microtubule- and cargo-motor interfaces mutually exclusive or do they crossover? For example, can microtubule-associated septins impact cargo-motor attachment?
Do septins shift from microtubules to membrane cargo? Can septins both inhibit and activate the same motor depending on whether they are microtubule- or membrane-bound? Can this enable an on-demand mobilization or stalling of certain cargo?
Highlights.
Septins are multimeric GTPases, which associate with subsets of microtubules in distinct intracellular regions. Septins also assemble on membrane domains of micron-scale curvature and distinct phospholipid content.
Microtubule-associated septins differentially regulate the motility of kinesin and dynein motors, spatially guiding motor-cargo transport.
Membrane-associated septins can function as motor adaptors, interacting with the cargo-binding tails of kinesins and scaffolding dynein-dynactin.
Septins regulate the localization and function of microtubule motors in mitosis, promoting spindle assembly, chromosome alignment and cytokinesis.
Septins may provide a code for the selective recruitment of microtubule motors to their respective cargo, and the spatial control of motor-cargo transport on microtubules.
Acknowledgments
We apologize for inadvertent omission of any relevant references under the journal’s requirements and limitations. E.T.S is supported with grant 5R35 GM136337-02 from the National Institute of General Medical Sciences (National Institutes of Health).
Glossary
- Adaptor proteins
Cytoplasmic proteins that link membrane organelle and vesicle cargo to microtubule motors. Adaptor proteins can promote assembly, oligomerization, and/or the activation of microtubule motors.
- Dynactin
A multi-subunit complex and obligate cofactor of dynein, consisting of a central mini-filament with multiple Arp1 subunits and capping proteins (CAPZ, ARP11, p62, p27, p25), and a shoulder complex of p50 dynamitin, p24 and p150GLUED. The C-terminal CAP-Gly domain of p150GLUED interacts with microtubules.
- Dynein
A microtubule minus-end directed motor that comprises a dynein heavy chain (DHC), an intermediate chain (DIC), a light intermediate chain (DLIC) and three light chains. The DHC contains a AAA+ ATPase domain, which harnesses ATP hydrolysis to power dynein motility.
- Kinesin
A motor protein that binds and hydrolyzes ATP to power movement along microtubules or slide microtubules against one another. Motors of the kinesin-1, -2, -3, -6 and -7 subfamilies are microtubule plus end-directed.
- Microtubule
A hollow cylindrical polymer of 10–15 protofilaments consisting of GTP-binding α-/β-tubulin dimers that assemble longitudinally in a head-to-tail fashion. Opposite microtubule ends contain α- and β-tubulin subunits, and are termed minus and plus ends, respectively. Microtubule ends undergo growth and shrinkage with plus ends growing faster than minus ends. Microtubule dynamics is driven by the binding, hydrolysis and exchange of the GTP of β-tubulin, which triggers conformational changes that impact the longitudinal and lateral interactions of tubulin dimers; α-tubulin subunits are constitutively bound to GTP.
- Microtubule-associated proteins (MAPs)
Proteins that bind microtubules and regulate microtubule organization and dynamics, and modulate kinesin and dynein motility.
- Microtubule C-terminal tails (CTTs)
The C-terminal amino sequences of α-/β-tubulin subunits, which project away from the microtubule lattice, are post-translationally modified, and interact electrostatically with motors and MAPs.
- Microtubule post-translational modifications (PTMs; tyrosination, polyglutamylation, acetylation)
Modifications of the α-/β-tubulin subunits of microtubules through the addition and removal of residues such as the C-terminal tyrosine of α-tubulin (tyrosination/detyrosination), polyglutamate side chains on glutamate residues of the C-terminal tails (polyglutamylation/deglutamylation), and an acetyl group on the L40 residue of α-tubulin, which is positioned in microtubule lumen.
- Rab GTPases
GTP binding and hydrolyzing proteins that function as molecular switches that cycle between activated GTP-bound and inactive GDP-bound states. Rab GTPases regulate the location, timing and specificity of protein interactions that underlie a diversity of trafficking events (e.g., vesicle formation, transport and fusion).
- Scaffold proteins
Cytoplasmic or peripheral membrane proteins with multiple protein-binding domains or oligomerizing properties, which form a molecular platform for the attachment and coordination of multiple copies of the same or different proteins.
- Septins
GTP-binding proteins that assemble into higher order oligomers and polymers that control the localization and interactions of cytoskeletal and membrane proteins.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no competing interests.
Refences
- 1.Janke C, and Magiera MM (2020). The tubulin code and its role in controlling microtubule properties and functions. Nat Rev Mol Cell Biol 21, 307–326. [DOI] [PubMed] [Google Scholar]
- 2.Bodakuntla S, Jijumon AS, Villablanca C, Gonzalez-Billault C, and Janke C (2019). Microtubule-Associated Proteins: Structuring the Cytoskeleton. Trends Cell Biol 29, 804–819. [DOI] [PubMed] [Google Scholar]
- 3.Atherton J, Houdusse A, and Moores C (2013). MAPping out distribution routes for kinesin couriers. Biol Cell 105, 465–487. [DOI] [PubMed] [Google Scholar]
- 4.Cross JA, and Dodding MP (2019). Motor-cargo adaptors at the organelle-cytoskeleton interface. Curr Opin Cell Biol 59, 16–23. [DOI] [PubMed] [Google Scholar]
- 5.Fu MM, and Holzbaur EL (2014). Integrated regulation of motor-driven organelle transport by scaffolding proteins. Trends Cell Biol 24, 564–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spiliotis ET, and Nakos K (2021). Cellular functions of actin- and microtubule-associated septins. Curr Biol 31, R651–R666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woods BL, and Gladfelter AS (2020). The state of the septin cytoskeleton from assembly to function. Curr Opin Cell Biol 68, 105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spiliotis ET, and McMurray MA (2020). Masters of asymmetry - lessons and perspectives from 50 years of septins. Mol Biol Cell 31, 2289–2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeMarini DJ, Adams AE, Fares H, De Virgilio C, Valle G, Chuang JS, and Pringle JR (1997). A septin-based hierarchy of proteins required for localized deposition of chitin in the Saccharomyces cerevisiae cell wall. J Cell Biol 139, 75–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shulewitz MJ, Inouye CJ, and Thorner J (1999). Hsl7 localizes to a septin ring and serves as an adapter in a regulatory pathway that relieves tyrosine phosphorylation of Cdc28 protein kinase in Saccharomyces cerevisiae. Mol Cell Biol 19, 7123–7137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barral Y, Mermall V, Mooseker MS, and Snyder M (2000). Compartmentalization of the cell cortex by septins is required for maintenance of cell polarity in yeast. Mol Cell 5, 841–851. [DOI] [PubMed] [Google Scholar]
- 12.Takizawa PA, DeRisi JL, Wilhelm JE, and Vale RD (2000). Plasma membrane compartmentalization in yeast by messenger RNA transport and a septin diffusion barrier. Science 290, 341–344. [DOI] [PubMed] [Google Scholar]
- 13.Bai X, Karasmanis EP, and Spiliotis ET (2016). Septin 9 interacts with kinesin KIF17 and interferes with the mechanism of NMDA receptor cargo binding and transport. Mol Biol Cell 27, 897–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karasmanis EP, Phan CT, Angelis D, Kesisova IA, Hoogenraad CC, McKenney RJ, and Spiliotis ET (2018). Polarity of Neuronal Membrane Traffic Requires Sorting of Kinesin Motor Cargo during Entry into Dendrites by a Microtubule-Associated Septin. Dev Cell 46, 204–218 e207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kesisova IA, Robinson BP, and Spiliotis ET (2021). A septin GTPase scaffold of dynein-dynactin motors triggers retrograde lysosome transport. J Cell Biol 220(2), e202005219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pan F, Malmberg RL, and Momany M (2007). Analysis of septins across kingdoms reveals orthology and new motifs. BMC Evol Biol 7, 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valadares NF, d’ Muniz Pereira H, Ulian Araujo AP, and Garratt RC (2017). Septin structure and filament assembly. Biophys Rev 9, 481–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abbey M, Gaestel M, and Menon MB (2019). Septins: Active GTPases or just GTP-binding proteins? Cytoskeleton (Hoboken) 76, 55–62. [DOI] [PubMed] [Google Scholar]
- 19.Weems A, and McMurray M (2017). The step-wise pathway of septin hetero-octamer assembly in budding yeast. Elife 6, e23689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kinoshita M (2003). Assembly of mammalian septins. J Biochem 134, 491–496. [DOI] [PubMed] [Google Scholar]
- 21.Dolat L, Hu Q, and Spiliotis ET (2014). Septin functions in organ system physiology and pathology. Biol Chem 395, 123–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu Q, Nelson WJ, and Spiliotis ET (2008). Forchlorfenuron alters mammalian septin assembly, organization, and dynamics. J Biol Chem 283, 29563–29571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hagiwara A, Tanaka Y, Hikawa R, Morone N, Kusumi A, Kimura H, and Kinoshita M (2011). Submembranous septins as relatively stable components of actin-based membrane skeleton. Cytoskeleton (Hoboken) 68, 512–525. [DOI] [PubMed] [Google Scholar]
- 24.Hernandez-Rodriguez Y, and Momany M (2012). Posttranslational modifications and assembly of septin heteropolymers and higher-order structures. Curr Opin Microbiol 15, 660–668. [DOI] [PubMed] [Google Scholar]
- 25.Nagata K, Kawajiri A, Matsui S, Takagishi M, Shiromizu T, Saitoh N, Izawa I, Kiyono T, Itoh TJ, Hotani H, et al. (2003). Filament formation of MSF-A, a mammalian septin, in human mammary epithelial cells depends on interactions with microtubules. J Biol Chem 278, 18538–18543. [DOI] [PubMed] [Google Scholar]
- 26.Ghossoub R, Hu Q, Failler M, Rouyez MC, Spitzbarth B, Mostowy S, Wolfrum U, Saunier S, Cossart P, Jamesnelson W, et al. (2013). Septins 2, 7 and 9 and MAP4 colocalize along the axoneme in the primary cilium and control ciliary length. J Cell Sci 126, 2583–2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spiliotis ET, Hunt SJ, Hu Q, Kinoshita M, and Nelson WJ (2008). Epithelial polarity requires septin coupling of vesicle transport to polyglutamylated microtubules. The Journal of cell biology 180, 295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bowen JR, Hwang D, Bai X, Roy D, and Spiliotis ET (2011). Septin GTPases spatially guide microtubule organization and plus end dynamics in polarizing epithelia. The Journal of cell biology 194, 187–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bai X, Bowen JR, Knox TK, Zhou K, Pendziwiat M, Kuhlenbaumer G, Sindelar CV, and Spiliotis ET (2013). Novel septin 9 repeat motifs altered in neuralgic amyotrophy bind and bundle microtubules. J Cell Biol 203, 895–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Froidevaux-Klipfel L, Targa B, Cantaloube I, Ahmed-Zaid H, Pous C, and Baillet A (2015). Septin cooperation with tubulin polyglutamylation contributes to cancer cell adaptation to taxanes. Oncotarget 6, 36063–36080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Froidevaux-Klipfel L, Poirier F, Boursier C, Crepin R, Pous C, Baudin B, and Baillet A (2011). Modulation of septin and molecular motor recruitment in the microtubule environment of the Taxol-resistant human breast cancer cell line MDA-MB-231. Proteomics 11, 3877–3886. [DOI] [PubMed] [Google Scholar]
- 32.Nakos K, Radler MR, and Spiliotis ET (2019). Septin 2/6/7 complexes tune microtubule plus-end growth and EB1 binding in a concentration- and filament-dependent manner. Mol Biol Cell 30, 2913–2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakos K, Rosenberg M, and Spiliotis ET (2019). Regulation of microtubule plus end dynamics by septin 9. Cytoskeleton (Hoboken) 76, 83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alushin GM, Lander GC, Kellogg EH, Zhang R, Baker D, and Nogales E (2014). High-resolution microtubule structures reveal the structural transitions in alphabeta-tubulin upon GTP hydrolysis. Cell 157, 1117–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dolat L, and Spiliotis ET (2016). Septins promote macropinosome maturation and traffic to the lysosome by facilitating membrane fusion. J Cell Biol 214, 517–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bridges AA, Jentzsch MS, Oakes PW, Occhipinti P, and Gladfelter AS (2016). Micron-scale plasma membrane curvature is recognized by the septin cytoskeleton. J Cell Biol 213, 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akil A, Peng J, Omrane M, Gondeau C, Desterke C, Marin M, Tronchere H, Taveneau C, Sar S, Briolotti P, et al. (2016). Septin 9 induces lipid droplets growth by a phosphatidylinositol-5-phosphate and microtubule-dependent mechanism hijacked by HCV. Nat Commun 7, 12203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sirianni A, Krokowski S, Lobato-Marquez D, Buranyi S, Pfanzelter J, Galea D, Willis A, Culley S, Henriques R, Larrouy-Maumus G, et al. (2016). Mitochondria mediate septin cage assembly to promote autophagy of Shigella. EMBO Rep 17, 1029–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang J, Kong C, Xie H, McPherson PS, Grinstein S, and Trimble WS (1999). Phosphatidylinositol polyphosphate binding to the mammalian septin H5 is modulated by GTP. Curr Biol 9, 1458–1467. [DOI] [PubMed] [Google Scholar]
- 40.Tanaka-Takiguchi Y, Kinoshita M, and Takiguchi K (2009). Septin-mediated uniform bracing of phospholipid membranes. Curr Biol 19, 140–145. [DOI] [PubMed] [Google Scholar]
- 41.Omrane M, Camara AS, Taveneau C, Benzoubir N, Tubiana T, Yu J, Guerois R, Samuel D, Goud B, Pous C, et al. (2019). Septin 9 has Two Polybasic Domains Critical to Septin Filament Assembly and Golgi Integrity. iScience 13, 138–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cannon KS, Woods BL, Crutchley JM, and Gladfelter AS (2019). An amphipathic helix enables septins to sense micrometer-scale membrane curvature. J Cell Biol 218, 1128–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lobato-Márquez D, Xu J, Özbaykal Güler G, Ojiakor A, Pilhofer M, and Mostowy S (2021). Mechanistic insight into bacterial entrapment by septin cage reconstitution. Nat Commun (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kinoshita N, Kimura K, Matsumoto N, Watanabe M, Fukaya M, and Ide C (2004). Mammalian septin Sept2 modulates the activity of GLAST, a glutamate transporter in astrocytes. Genes Cells 9, 1–14. [DOI] [PubMed] [Google Scholar]
- 45.Neubauer K, and Zieger B (2017). The Mammalian Septin Interactome. Front Cell Dev Biol 5, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dash SN, Lehtonen E, Wasik AA, Schepis A, Paavola J, Panula P, Nelson WJ, and Lehtonen S (2014). Sept7b is essential for pronephric function and development of left-right asymmetry in zebrafish embryogenesis. J Cell Sci 127, 1476–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Danson CM, Pearson N, Heesom KJ, and Cullen PJ (2018). Sorting nexin-21 is a scaffold for the endosomal recruitment of huntingtin. J Cell Sci 131, jcs211672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Karasmanis EP, Hwang D, Nakos K, Bowen JR, Angelis D, and Spiliotis ET (2019). A Septin Double Ring Controls the Spatiotemporal Organization of the ESCRT Machinery in Cytokinetic Abscission. Curr Biol 29, 2174–2182 e2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baust T, Anitei M, Czupalla C, Parshyna I, Bourel L, Thiele C, Krause E, and Hoflack B (2008). Protein networks supporting AP-3 function in targeting lysosomal membrane proteins. Mol Biol Cell 19, 1942–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu J, Fairn GD, Ceccarelli DF, Sicheri F, and Wilde A (2012). Cleavage furrow organization requires PIP(2)-mediated recruitment of anillin. Curr Biol 22, 64–69. [DOI] [PubMed] [Google Scholar]
- 51.Bridges AA, Zhang H, Mehta SB, Occhipinti P, Tani T, and Gladfelter AS (2014). Septin assemblies form by diffusion-driven annealing on membranes. Proc Natl Acad Sci U S A 111, 2146–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Szuba A, Bano F, Castro-Linares G, Iv F, Mavrakis M, Richter RP, Bertin A, and Koenderink GH (2021). Membrane binding controls ordered self-assembly of animal septins. Elife 10, e63349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roll-Mecak A (2019). How cells exploit tubulin diversity to build functional cellular microtubule mosaics. Curr Opin Cell Biol 56, 102–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xie Y, Vessey JP, Konecna A, Dahm R, Macchi P, and Kiebler MA (2007). The GTP-binding protein Septin 7 is critical for dendrite branching and dendritic-spine morphology. Curr Biol 17, 1746–1751. [DOI] [PubMed] [Google Scholar]
- 55.Farrugia AJ, Rodriguez J, Orgaz JL, Lucas M, Sanz-Moreno V, and Calvo F (2020). CDC42EP5/BORG3 modulates SEPT9 to promote actomyosin function, migration, and invasion. J Cell Biol 219(9), e201912159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shinoda T, Ito H, Sudo K, Iwamoto I, Morishita R, and Nagata K (2010). Septin 14 is involved in cortical neuronal migration via interaction with Septin 4. Mol Biol Cell 21, 1324–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen TY, Lin TC, Kuo PL, Chen ZR, Cheng HL, Chao YY, Syu JS, Lu FI, and Wang CY (2021). Septin 7 is a centrosomal protein that ensures S phase entry and microtubule nucleation by maintaining the abundance of p150(glued). J Cell Physiol 236, 2706–2724. [DOI] [PubMed] [Google Scholar]
- 58.Monroy BY, Tan TC, Oclaman JM, Han JS, Simo S, Niwa S, Nowakowski DW, McKenney RJ, and Ori-McKenney KM (2020). A Combinatorial MAP Code Dictates Polarized Microtubule Transport. Dev Cell 53, 60–72 e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Scarabelli G, Soppina V, Yao XQ, Atherton J, Moores CA, Verhey KJ, and Grant BJ (2015). Mapping the Processivity Determinants of the Kinesin-3 Motor Domain. Biophys J 109, 1537–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Atherton J, Farabella I, Yu IM, Rosenfeld SS, Houdusse A, Topf M, and Moores CA (2014). Conserved mechanisms of microtubule-stimulated ADP release, ATP binding, and force generation in transport kinesins. Elife 3, e03680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ferro LS, Eshun-WIlson L, Golcuk M, Fernandes J, Huijben T, Gerber E, Jack A, Costa M, Gur M, Fang Q, et al. (2020). The mechanism of motor inhibition by microtubule-associated proteins. bioRxiv 2020.10.22.351346. 10.1101/2020.10.22.351346 [DOI] [Google Scholar]
- 62.Ferro LS, Can S, Turner MA, ElShenawy MM, and Yildiz A (2019). Kinesin and dynein use distinct mechanisms to bypass obstacles. Elife 8, e48629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bulinski JC, McGraw TE, Gruber D, Nguyen HL, and Sheetz MP (1997). Overexpression of MAP4 inhibits organelle motility and trafficking in vivo. J Cell Sci 110 ( Pt 24), 3055–3064. [DOI] [PubMed] [Google Scholar]
- 64.Kremer BE, Haystead T, and Macara IG (2005). Mammalian septins regulate microtubule stability through interaction with the microtubule-binding protein MAP4. Mol Biol Cell 16, 4648–4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sirajuddin M, Rice LM, and Vale RD (2014). Regulation of microtubule motors by tubulin isotypes and post-translational modifications. Nat Cell Biol 16, 335–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bonnet C, Boucher D, Lazereg S, Pedrotti B, Islam K, Denoulet P, and Larcher JC (2001). Differential binding regulation of microtubule-associated proteins MAP1A, MAP1B, and MAP2 by tubulin polyglutamylation. J Biol Chem 276, 12839–12848. [DOI] [PubMed] [Google Scholar]
- 67.Tas RP, Chazeau A, Cloin BMC, Lambers MLA, Hoogenraad CC, and Kapitein LC (2017). Differentiation between Oppositely Oriented Microtubules Controls Polarized Neuronal Transport. Neuron 96, 1264–1271 e1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Verdier-Pinard P, Salaun D, Bouguenina H, Shimada S, Pophillat M, Audebert S, Agavnian E, Coslet S, Charafe-Jauffret E, Tachibana T, et al. (2017). Septin 9_i2 is downregulated in tumors, impairs cancer cell migration and alters subnuclear actin filaments. Sci Rep 7, 44976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ageta-Ishihara N, Miyata T, Ohshima C, Watanabe M, Sato Y, Hamamura Y, Higashiyama T, Mazitschek R, Bito H, and Kinoshita M (2013). Septins promote dendrite and axon development by negatively regulating microtubule stability via HDAC6-mediated deacetylation. Nat Commun 4, 2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Redwine WB, DeSantis ME, Hollyer I, Htet ZM, Tran PT, Swanson SK, Florens L, Washburn MP, and Reck-Peterson SL (2017). The human cytoplasmic dynein interactome reveals novel activators of motility. Elife 6, e28257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nakahira M, Macedo JN, Seraphim TV, Cavalcante N, Souza TA, Damalio JC, Reyes LF, Assmann EM, Alborghetti MR, Garratt RC, et al. (2010). A draft of the human septin interactome. PLoS One 5, e13799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee IG, Olenick MA, Boczkowska M, Franzini-Armstrong C, Holzbaur ELF, and Dominguez R (2018). A conserved interaction of the dynein light intermediate chain with dynein-dynactin effectors necessary for processivity. Nat Commun 9, 986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Smith C, Dolat L, Angelis D, Forgacs E, Spiliotis ET, and Galkin VE (2015). Septin 9 Exhibits Polymorphic Binding to F-Actin and Inhibits Myosin and Cofilin Activity. J Mol Biol 427, 3273–3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Holleran EA, Ligon LA, Tokito M, Stankewich MC, Morrow JS, and Holzbaur EL (2001). beta III spectrin binds to the Arp1 subunit of dynactin. J Biol Chem 276, 36598–36605. [DOI] [PubMed] [Google Scholar]
- 75.Hammond JW, Blasius TL, Soppina V, Cai D, and Verhey KJ (2010). Autoinhibition of the kinesin-2 motor KIF17 via dual intramolecular mechanisms. J Cell Biol 189, 1013–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gonzalez ME, Makarova O, Peterson EA, Privette LM, and Petty EM (2009). Up-regulation of SEPT9_v1 stabilizes c-Jun-N-terminal kinase and contributes to its pro-proliferative activity in mammary epithelial cells. Cellular signalling 21, 477–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fu MM, and Holzbaur EL (2013). JIP1 regulates the directionality of APP axonal transport by coordinating kinesin and dynein motors. J Cell Biol 202, 495–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pavin N, and Tolic IM (2021). Mechanobiology of the Mitotic Spindle. Dev Cell 56, 192–201. [DOI] [PubMed] [Google Scholar]
- 79.Kodani A, Salome Sirerol-Piquer M, Seol A, Garcia-Verdugo JM, and Reiter JF (2013). Kif3a interacts with Dynactin subunit p150 Glued to organize centriole subdistal appendages. EMBO J 32, 597–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Song K, Gras C, Capin G, Gimber N, Lehmann M, Mohd S, Puchkov D, Rodiger M, Wilhelmi I, Daumke O, et al. (2019). A SEPT1-based scaffold is required for Golgi integrity and function. J Cell Sci 132(3), jcs225557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Devlin L, Perkins G, Bowen JR, Montgana C, and Spiliotis ET (2019). Proteomic profiling of the oncogenic septin 9 reveals isoform-specific interactions in breast cancer cells. bioRxiv 566513, doi: 10.1101/566513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Qi M, Yu W, Liu S, Jia H, Tang L, Shen M, Yan X, Saiyin H, Lang Q, Wan B, et al. (2005). Septin1, a new interaction partner for human serine/threonine kinase aurora-B. Biochem Biophys Res Commun 336, 994–1000. [DOI] [PubMed] [Google Scholar]
- 83.Spiliotis ET, Kinoshita M, and Nelson WJ (2005). A mitotic septin scaffold required for Mammalian chromosome congression and segregation. Science 307, 1781–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhu M, Wang F, Yan F, Yao PY, Du J, Gao X, Wang X, Wu Q, Ward T, Li J, et al. (2008). Septin 7 interacts with centromere-associated protein E and is required for its kinetochore localization. J Biol Chem 283, 18916–18925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Barisic M, Silva e Sousa R, Tripathy SK, Magiera MM, Zaytsev AV, Pereira AL, Janke C, Grishchuk EL, and Maiato H (2015). Mitosis. Microtubule detyrosination guides chromosomes during mitosis. Science 348, 799–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Qiu R, Runxiang Q, Geng A, Liu J, Xu CW, Menon MB, Gaestel M, and Lu Q (2020). SEPT7 Interacts with KIF20A and Regulates the Proliferative State of Neural Progenitor Cells During Cortical Development. Cereb Cortex 30, 3030–3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Adriaans IE, Hooikaas PJ, Aher A, Vromans MJM, van Es RM, Grigoriev I, Akhmanova A, and Lens SMA (2020). MKLP2 Is a Motile Kinesin that Transports the Chromosomal Passenger Complex during Anaphase. Curr Biol 30, 2628–2637 e2629. [DOI] [PubMed] [Google Scholar]
- 88.O’Loughlin T, Masters TA, and Buss F (2018). The MYO6 interactome reveals adaptor complexes coordinating early endosome and cytoskeletal dynamics. EMBO Rep 19(4), e44884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hecht M, Rosler R, Wiese S, Johnsson N, and Gronemeyer T (2019). An Interaction Network of the Human SEPT9 Established by Quantitative Mass Spectrometry. G3 (Bethesda) 9, 1869–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McKenney RJ, Huynh W, Vale RD, and Sirajuddin M (2016). Tyrosination of alpha-tubulin controls the initiation of processive dynein-dynactin motility. EMBO J 35, 1175–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Monroy BY, Sawyer DL, Ackermann BE, Borden MM, Tan TC, and Ori-McKenney KM (2018). Competition between microtubule-associated proteins directs motor transport. Nat Commun 9, 1487. [DOI] [PMC free article] [PubMed] [Google Scholar]


