Abstract
We have employed gene targeting coupled with conditional expression to construct a chicken DT40 cell line in which a tetracycline (Tet)-repressible promoter is exclusively responsible for expression of cTAFII31, a histone-like TAFII residing in both the transcription factor TFIID and the histone acetylase complex PCAF/SAGA. Tet addition resulted in rapid loss of cTAFII31 mRNA and protein, eventually leading to apoptotic cell death. Significantly, five of six other TAFIIs tested were also rapidly depleted, but levels of the TATA binding protein and subunits of PCAF/SAGA were at most modestly compromised. Strikingly, pulse-labeling experiments indicate that total poly(A)+ mRNA transcription was not significantly reduced after cTAFII31 depletion, and steady-state levels of several specific transcripts remained the same or decreased only mildly. Moreover, activation of c-fos transcription following serum starvation occurred efficiently in the absence of cTAFII31. These data, which contrast with comparable studies in yeast, strongly suggest that cTAFII31 and perhaps other TAFIIs are not essential for general mRNA transcription in DT40 cells. We propose that this is due to extensive functional degeneracy in the highly complex metazoan transcriptional machinery.
In eukaryotes, RNA polymerase II (Pol II)-mediated mRNA transcription requires several general transcription factors (GTFs). One important factor is TFIID, which recognizes core promoter elements to initiate preinitiation complex formation (45). TFIID is a multiprotein complex, consisting of the TATA-binding protein (TBP), which is primarily responsible for recognition of the TATA box, and approximately 10 to 12 phylogenetically conserved polypeptides called TBP-associated factors (TAFIIs) (6, 55, 59, 62).
Early biochemical data suggested an important function of TAFIIs in transcriptional activation (6, 62). In reconstituted in vitro systems, TFIID, but not TBP alone, can stimulate transcription in response to activators in the presence of other GTFs and Pol II. Furthermore, interactions between different types of activators and distinct TAFIIs correlated well with the ability of the activators to enhance transcription. In light of these data, TAFIIs were considered to be essential coactivators, presumably functioning as the direct and specific targets of various activators. More recently, however, TAFII-independent activated transcription was found, under appropriate conditions, to occur in both yeast and mammalian reconstituted systems (12, 27, 43). Moreover, additional coactivators, including PC2 and PC4, were found to be required for TFIID-dependent transcriptional activation in cell-free systems (7), and in a highly purified system, PC4 can suffice for activation in the absence of TAFIIs (68). While the coactivator function of TAFIIs has thus not been fully established in vitro, another line of biochemical analysis provided strong evidence for core promoter functions of TAFIIs. Drosophila TAFII150 was first shown to be involved in recognition of the initiator element present in some promoters (62). A novel core promoter element, termed downstream promoter element (DPE), was identified in some TATA-less promoters, and this element might be recognized by dTAFII60 and dTAFII40 (5). These additional TAFII-promoter interactions might compensate for the absence of a TATA box to allow efficient initiation at a variety of promoters.
The function of TAFIIs has also been extensively studied in vivo, mostly in yeast. While almost all TAFIIs studied are essential for cell viability, genetic depletion or inactivation of several TAFIIs appeared not to cause a significant decrease in general mRNA transcription (39, 63). Transcription of a subset of genes was, however, affected. Notably, depletion of yTAFII145 effected a reduction in transcription of several cell cycle genes, in agreement with the phenotypes of cell cycle arrest at the G1 phase observed in both yTAFII145 and homologous TAFII250 mutant cells (52, 57, 64, 66). In both yeast and mammals, the dependence on yTAFII145/TAFII250 was mapped, at least in part, to the core promoter region, suggesting a functional conservation of this TAFII. In Drosophila, a sensitized genetic assay was utilized to provide evidence suggesting roles for dTAFII110 and dTAFII60 in Dorsal-mediated activation of snail gene transcription, perhaps through direct interactions between TAFIIs and the Dorsal activator protein (70). Most recently, murine cells deprived of TAFII30 were shown to arrest at the G1/G0 phase, which correlated well with the impaired expression of cyclin E in these (37). The above in vivo data support a role for TAFIIs in transcription of specific genes, but they do not necessarily suggest a general requirement of TAFIIs for Pol II transcription.
The possibility of a general role of TAFIIs in Pol II transcription in yeast was further explored in a recent set of studies focusing on a group of TAFIIs known as the histone-like TAFIIs (1, 38, 40, 42). Several lines of evidence strongly suggest the existence of canonical histone-fold motifs in three TAFIIs, yTAFII17/dTAFII40/42/hTAFII32/31, yTAFII60/dTAFII62/hTAFII80, and yTAFII61/68/dTAFII30α/hTAFII20, which have highest sequence similarity with histones H3, H4, and H2B, respectively (6). Inactivation of each of the histone-like TAFIIs was shown to cause an apparent decrease in general Pol II transcription as measured by reduced accumulation of total and specific mRNAs. Through similar assays, yTAFII40, a TFIID-specific TAFII, was also demonstrated to be essential for bulk Pol II transcription (28). Additionally, a genome-wide expression study showed that while a yTAFII145 mutation reduced expression of only 16% of genes tested, 67% required yTAFII17 for optimal expression (22). It is noteworthy that two earlier studies had suggested a limited role of yTAFII60, yTAFII68, and yTAFII40 in Pol II transcription (39, 63). Thus, while the essential role of yTAFII17 in transcription seems firmly established, a consensus regarding these other three yTAFIIs awaits additional studies.
The histone-like TAFIIs are also components of a histone acetyl transferase (HAT)-containing complex, known as SAGA in yeast (14) and PCAF in humans (44). The discovery of TAFIIs in complexes other than TFIID (2) has added another level of complexity to the in vivo functions of TAFIIs: phenotypes observed after TAFII depletion could be due to TAFII function in the context of TFIID, SAGA, or both. Supporting an important role for SAGA in transcription, it has been found that SAGA can interact with TBP and some acidic activators, and mutational studies of SAGA revealed transcriptional defects (54). By contrast, only a small fraction of genes appear to be transcriptionally dependent on GCN5, the HAT activity of SAGA (22), and the non-TAFII components of SAGA, which are not required for viability. The exact role of PCAF/SAGA in transcriptional activation remains to be determined.
Studies on the possible physiological functions of TAFIIs have been carried out mostly in yeast, and it remains unclear whether higher eukaryotic TAFIIs possess similar transcriptional and cellular functions as their yeast counterparts. To address this question, and to resolve the controversies regarding the general requirement for TAFIIs in activated transcription, we have employed gene targeting coupled with conditional expression (58, 65) to construct a chicken DT40 cell line in which the only source of cTAFII31, the homologue of yTAFII17 and dTAFII40, was from a tetracycline (Tet)-repressible promoter. Following the addition of Tet to the culture medium, cTAFII31 mRNA and protein were efficiently depleted, cell growth slowed, and the cells eventually died by an apoptotic pathway. Genetic depletion of cTAFII31 also significantly reduced the intracellular levels of several other TAFIIs, but there were only minimal or undetectable decreases of TBP and of several subunits of the PCAF complex. Surprisingly, 3H pulse-labeling experiments indicate that transcription of total poly(A)+ mRNA was not significantly compromised by cTAFII31 depletion and the abundance of several specific transcripts was either not affected or only minimally reduced. Taken together, these data suggest that cTAFII31 and perhaps other TAFIIs are not universally required for transcription in DT40 cells. We discuss these results in terms of the existence of extensive functional degeneracy in vertebrate cells.
MATERIALS AND METHODS
Cloning of chicken TAFII31 cDNA and genomic sequences.
Based on the dTAFII40 and hTAFII31 sequence alignment (20), a 36-mer oligonucleotide with 64-fold degeneracy (5′-AACCAGMTGCTGGAGTTCRCCTTCCGITATGTIACC-3′; M, A or C; R, A or G; I, inosine) was designed to correspond to a peptide sequence (NQLLEFTFRYVT) from dTAFII40. This oligonucleotide was used to screen a chicken fibroblast cDNA library (Stratagene). Out of 400,000 plaques screened, seven positives were identified and purified. Sequence analysis of these clones revealed that they were all nearly full length, with a poly(A) tail and a small truncation at the start of the coding region. One cDNA clone with a truncation of only the first 3 bp (ATG) was sequenced on both strands and also used to screen a chicken genomic DNA library (Stratagene). Fourteen positives were purified. Southern blotting and partial sequencing of a 4.5-kb genomic DNA fragment showed that the chicken TAFII31 gene carries seven exons comprising its coding region and 3′ untranslated region (UTR).
Cell culture and transfections.
Chicken DT40 cells were maintained in RPMI 1640 medium (Gibco) supplemented with 10% fetal bovine serum (HyClone) and 1% chicken serum (Sigma) at 37°C and 5% CO2. Transfections were carried out based on the protocol previously described (65). Thirty to forty micrograms of each linearized plasmid was used in transfections.
Plasmid constructs.
To construct targeting vectors, the chicken β-actin promoter-driven neomycin and hygromycin resistance genes (65) were used to replace a BstXI fragment spanning exon 3 to intron 5 of the chicken TAFII31 genomic sequence. The resulting vectors were named neo-TAF and hygro-TAF, respectively. Both constructs were linearized by BamHI before transfection. The vector tTA, which encodes the transactivator Tet-VP16, has been described previously (65). A NotI site was used for linearization.
A flu-cTAFII31 cDNA that carries a Flu tag at its 5′ end and a truncated 3′ UTR was inserted after the TetO-minimal promoter (13). A β-globin splicing-poly(A) sequence was inserted downstream of flu-cTAFII31 to enhance expression efficiency. Finally, a histidinol resistance gene (65) was included to make the expression vector pKH(+)TFG. The expression vector pRSET-TAF31 encoding His-tagged cTAFII31 was made by subcloning an EcoRI-PvuII fragment that contains the full-length cTAFII31 coding region except for the start codon into the NheI-PvuII-digested pRSET.C vector (Invitrogen) by blunt-end ligation. A BamHI-PflMI cTAFII31 cDNA fragment covering the Flu tag sequence and exons 1 and 2 was ligated with BamHI-XhoI-digested pKS(+) vector (Stratagene) to yield the template plasmid for the 5′-end probe used in RNase protection. The template plasmid for the deletion probe was constructed by inserting a BstXI-ApaI cTAFII31 cDNA fragment (corresponding to most of exon 3 and part of exon 4) into the EcoRV site of pKS(+).
Southern blotting and RNase protection assays.
Genomic DNA purification and Southern blotting were carried out essentially as previously described (48). Total RNA samples used in RNase protection experiments were prepared as previously described (9). To generate [α-32P]CTP-labeled probes, 1 μg of linearized template DNAs was transcribed in vitro by either T3 or T7 RNA polymerase. The labeled probes were purified from 5% polyacrylamide–8 M urea gels. For each reaction, 1 to 10 μg of total RNA (depending on the abundance of the transcript analyzed) and 2 × 105 to 106 cpm of probe were used, following standard protocols (48) with only minor changes. All RNase protection assays were quantitated using a STORM Phosphorimager (Molecular Dynamics).
Western blot analysis.
His-tagged cTAFII31 was expressed in Escherichia coli BL21 cells transformed with the expression vector pRSET-TAF31 following induction by isopropyl-β-d-thiogalactopyranoside (IPTG) for 3 h at 37°C. His-cTAFII31 was purified using Ni2+ beads under denaturing conditions according to the manufacturer's instructions (Qiagen). The protein band was excised from a preparative sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel (17) and directly used to raise a rabbit polyclonal antibody (Cocalico). Affinity purification was performed essentially as previously described (17). The anti-Flu monoclonal antibody (HA.11) was from Babco. The polyclonal anti-CPSF-100 antibody was supplied by K. G. K. Murthy. Other antibodies were kindly provided by several labs (see Acknowledgments). These were all raised against mammalian proteins, but most reacted strongly and specifically with their chicken counterparts, and all yielded readily detectable signals.
Whole-cell lysates of DT40 cells were prepared as previously described (48). Protein concentrations were quantitated by the Bradford method (Bio-Rad) with bovine serum albumin as the standard. Each sample was measured in duplicate to minimize quantification error. Equal amounts of samples, ranging from 30 to 100 μg, were used for each experiment. After transfer of proteins from SDS-PAGE gels to nitrocellulose membranes, membranes were blocked in phosphate-buffered saline (PBS) plus 5% milk, incubated with primary antibodies in PBS plus 1% milk, and washed in PBS plus 0.05 to 0.5% Tween 20. Following incubation with secondary antibodies, target proteins were detected using an ECL kit (Amersham). Membranes were routinely stained with Ponceau S to confirm equal loading.
Quantitation of TAFII31 protein levels following Tet addition utilized both the purified anti-cTAF31 and anti-Flu antibodies. Specifically, Western blottings using the anti-cTAF31 antibody revealed ∼50% overexpression of cTAFII31 in DT40-TAF31 cells compared with DT40 cells (see Fig. 3C), and that the TAFII31 levels in DT40-TAF31 cells at 36 h after Tet addition were ≤10% the amount in DT40 cells. Extending these findings, Western blottings using the more sensitive anti-Flu antibody demonstrated <5% of the zero time concentration at 48 h following Tet addition. After adjusting for the different cell types (DT40-TAF31 versus DT40), the amount of TAFII31 remaining after 48 h was estimated to be ∼5% the level in DT40 cells. In light of the further significant decrease observed at 60 h (see Fig. 3D), the amount of TAFII31 was estimated to be ≤2% the amount in wild-type cells after 60 h of Tet treatment.
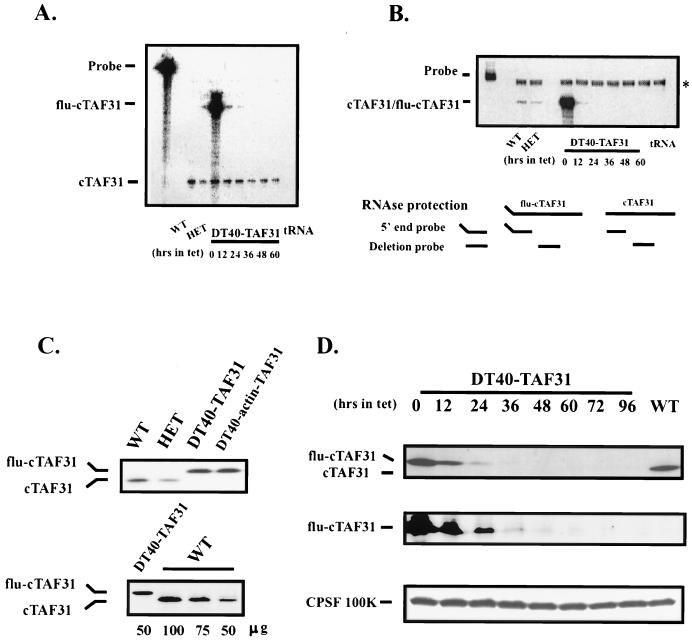
FIG. 3.
Expression of cTAFII31 in DT40-TAF31 cells. (A) RNase protection assay using a 5′-end probe. Ten micrograms of total RNAs from DT40 cells (WT), heterozygous cells (HET), and DT40-TAF31 cells incubated in the presence of Tet (1 μg/ml) for the indicated times was analyzed by RNase protection. The Flu tag sequence accounts for the size difference between endogenous and exogenous expression. tRNA was used as a negative control. (B) RNase protection assay using a deletion probe. The probe corresponds to part of the region deleted during gene targeting events (diagrammed below). Assay conditions are as above. The position of a background band, which is also present in the tRNA control lane, is indicated by an asterisk. (C) Western blot analysis using an affinity-purified polyclonal anti-cTAFII31 antibody. (Top) Thirty micrograms of total cellular proteins from wild-type, heterozygous, DT40-TAF31, and DT40-actin-TAF31 cells that was fractionated on an SDS–10% PAGE gel, transferred to a nitrocellulose membrane, and probed with an anti-chicken TAFII31 polyclonal antibody. Positions of flu-cTAFII31 and cTAFII31 are shown. (Bottom) The indicated amounts of total cell proteins from wild-type or DT40-TAF31 cells were analyzed as shown in the top panel. (D) Western blot of time course of Tet-induced depletion. DT40-TAF31 cells were incubated in the presence of Tet (1 μg/ml) for the indicated times before harvest. Lysates prepared from these cells were analyzed for Tet-repressible expression of flu-cTAFII31 using the above anti-cTAFII31 antibody (upper panel), a monoclonal antibody against the Flu tag (middle panel), and a polyclonal antibody against CPSF-100 (lower panel). Wild-type cell lysates (WT) were analyzed for comparison.
Fluorescence-activated cell sorter (FACS) analysis and DNA fragmentation assays.
One to 2 million cells were harvested. After the medium was removed by aspiration, 300 μl of ice-cold PBS was used to resuspend the cell pellet and the mixture was set on ice for 15 min. Five milliliters of cold methanol was then added dropwise with gentle vortexing, followed by incubation at −20°C for at least 40 min. Cells were spun down, resuspended in 2 ml of cold PBS, and incubated for at least 20 min at 4°C before centrifugation. The pellet was resuspended in 900 μg of a PBS solution containing 60 μg of propidium iodide (Sigma) per ml and 50 μl of RNase A per ml and incubated for 30 min at room temperature. DNA contents were measured by a FACS Calibur (Becton Dickinson), and cell cycle profiles were analyzed by the ModFit program (Verity Software). DNA fragmentation assays were performed as previously described (21).
Pulse labeling and poly(A)+ RNA selection.
Three to five independent pulse-labeling experiments were conducted at each of the indicated times, and for each experiment, two cell cultures were grown in parallel, treated or untreated with Tet (1 μg/ml; Sigma). After incubation for the indicated time, cell densities of both populations were adjusted to the same level, between 1 million and 1.3 million cells per ml. Appropriate splitting ratios were used so that both cell cultures were roughly in the same growth phase when harvested. Thirty to 35 μCi of [5,6-3H]uridine per ml was added to each culture, and the cells were incubated for 10 to 15 min. Cells were then harvested and washed with PBS. Total cellular RNA from both cultures were prepared (9) and checked by DE81 paper (48) to verify no contamination of unincorporated isotope. Concentrations of RNA samples were determined by UV adsorption. Equal amounts of total RNAs from both control and test samples were then subjected to the following poly(A)+ RNA selection. Typically, 20 to 40 mg of oligo(dT) cellulose (Sigma; NEB) was packed into a 1-ml syringe to make a 100- to 150-μl column. Isolation of poly(A)+ mRNA was conducted according to standard protocols (48), except that samples were loaded onto the column for four or five times over 1.5 to 2 h. Ratios of selected RNA to total RNA for both samples were calculated after scintillation counting of equal aliquots of each fraction. The ratio of the control sample (without Tet), usually between 15 and 25%, was designated 100%, while the ratio from the test sample (with Tet) was normalized against this standard to facilitate comparisons. Selection efficiencies of poly(A)+ RNA were measured by RNase protection assays using a TBP cRNA as probe and typically ranged from 65 to 80%. For unusual cases in which the selection efficiency between a test and control sample differed by 10% or more, the ratios of selected RNA to total RNA were normalized against one another. To measure the specificity of selection, human U6 snRNA and chicken 5S rRNA probes were used in RNase protection assays to monitor the distribution of poly(A)− RNAs. In most cases, the amount of poly(A)− RNA in the poly(A)+ samples was 1% or less of the total. In accord with this, an independent assay, ethidium bromide staining of formaldehyde RNA gels, demonstrated that less than 1% of the 28S and 18S rRNAs was present in the poly(A)+ samples.
Induction of c-fos transcription by serum stimulation.
After growing in the presence of Tet (1 μg/ml) for 0, 60, or 84 h, DT40-TAF31 cells were washed once with PBS and resuspended in serum-free RPMI media, with the addition of Tet for the latter two time points. Following starvation for 10 h, cells were washed and stimulated in media with 20% fetal bovine serum and 2% chicken serum. The inclusion of Tet was as described above. Serum stimulation lasted 75 min before harvest. At this point, cells had been incubated in Tet-containing media for a total of 0, 72, or 96 h. Cell cultures incubated in the presence of Tet for the same times but not subjected to serum starvation served as controls. Total cellular RNAs were then purified from these samples and used in RNase protection analysis. Genomic PCR was performed according to QIAGEN protocols to amplify a chicken c-fos fragment encompassing intron 3 and part of exons 3 and 4. This c-fos probe, which is 383 nucleotides long, produced a protected fragment of 229 nucleotides.
RESULTS
Cloning and sequence analysis of cTAFII31.
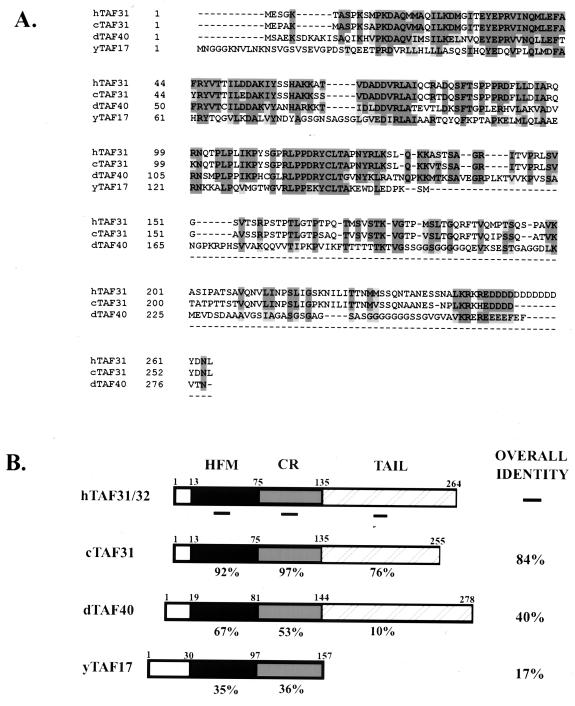
To begin a genetic analysis of cTAFII31, we first isolated the cTAFII31 cDNA (see Materials and Methods). Sequence analysis of purified positive clones revealed that they were all nearly full length at around 1.3 kb with a poly(A) tail at the 3′ end. The cDNA sequence of cTAFII31 has 51 and 42% nucleotide identity with those of hTAFII31 and dTAFII40, respectively. The putative open reading frame encodes a protein of 255 amino acids which, because the apparent molecular mass of both in vitro-translated and E. coli-expressed proteins was about 31 kDa, we named cTAFII31. Protein sequence alignments (Fig. 1A) and homology comparisons (Fig. 1B) indicated an even greater conservation at the amino acid level. In the N-terminal half, the histone-fold motif and the flanking region are highly conserved from yeast to human. However, while the C-terminal half is completely lacking in yTAFII17 and not conserved at all (10% identity) in dTAFII40, it is almost as highly conserved as the N-terminal half between the human and chicken proteins. This is similar to the pattern of conservation observed with the N-terminal “species-specific” domain of TBP (18).
FIG. 1.
Protein sequence alignment and homology comparison of cTAFII31. (A) Protein sequence alignment. The deduced amino acid sequence of cTAFII31 and homologous sequences from a human (hTAF31), Drosophila (dTAF40), and Saccharomyces cerevisiae (yTAF17) were aligned using Clustal W software. At those positions where at least three proteins have conserved residues, amino acids are outlined black (identical) or gray (similar). (B) Evolutionary conservation between homologous sequences. A schematic representation of the homologues is shown, with percent identities compared to the human protein indicated. The positions of the histone-fold motif (HFM), the flanking conserved region (CR), and the C-terminal nonconserved region (TAIL) are shown.
Construction of a cTAFII31 conditional knockout DT40 cell line.
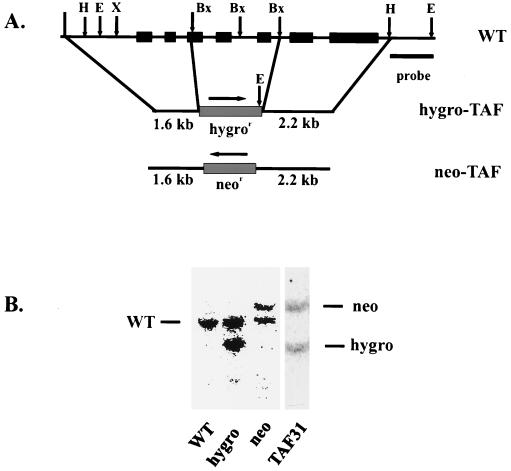
Chicken DT40 cells can undergo homologous recombination at a high frequency, making them ideal for gene-targeting studies (58, 65). Genomic Southern analysis indicated that cTAFII31 is a single-copy gene in DT40 cells (data not shown). A cDNA probe was used to isolate a 4.5-kb genomic clone. Partial sequencing revealed that this fragment contained at least seven exons from the coding region and 3′ UTR (Fig. 2A). BstXI digestion was used to delete exons 3 to 5, corresponding to most of the histone-fold motif and flanking conserved region, and either of two drug selection markers, neomycin or hygromycin resistance, was inserted into the digested plasmid to produce the targeting vectors neo-TAF31 and hygro-TAF31, respectively. DT40 cells were first transfected with neo-TAF31. Southern blotting of DNA from drug-resistant colonies (Fig. 2B) revealed that ∼20% of the clones had an 8.4-kb band, as expected from homologous recombination. Transfection of hygro-TAF31 into DT40 cells also gave rise to an ∼20% homologous recombination efficiency. However, transfection of either type of heterozygous cell line with the reciprocal targeting vector failed to produce any double-allele knockout cells (data not shown), suggesting that cTAFII31 is essential for DT40 cell viability.
FIG. 2.
Disruption of the cTAFII31 gene in DT40 cells. (A) Genomic organization and targeting vectors. The structure of the cTAFII31 gene is shown, along with the two targeting vectors used to disrupt both wild-type alleles. The black boxes represent exons. In both targeting vectors, selection marker genes (neo or hygro, gray boxes) replace exons 3 through 5. Positions of relevant restriction sites (Bx, BstXI; X, XhoI; H, HindIII; E, EcoRI) and the direction of transcription (arrows) are indicated. The flanking region probe (HindIII-EcoRI fragment) and the sizes of the relevant fragments are also denoted. (B) Southern blot analysis. Genomic DNA was purified from wild-type DT40 cells (WT), heterozygous cells transfected with hygro-TAF (hygro) or neo-TAF (neo) targeting vectors, or homozygous cells after the second round of transfections (TAF31). After digestion with EcoRI, DNAs were subjected to Southern blot analysis using the probe depicted above. Positions of wild-type (WT) or targeted fragments (neo and hygro) are indicated.
To disrupt the second allele and introduce instead a conditional version of the cTAFII31 gene, we adopted the Tet-repressible expression system as described by Wang et al. (65). As a preliminary experiment, we first constitutively expressed Flu epitope-tagged cTAFII31 driven by the β-actin promoter in a hygromycin-resistant heterozygous cell line. Subsequent transfection of these cells with neo-TAF31 indeed gave rise to double-knockout cells (data not shown), establishing the ability of flu-cTAFII31 expressed from a strong constitutive promoter to substitute for the endogenous protein. To establish a Tet-repressible allele, we cotransfected hygromycin-resistant heterozygous cells with a construct encoding the chimeric transactivator tetR-VP16 (tTA) and an expression vector encoding Flu-tagged cTAFII31, driven by a Tet-responsive promoter. Drug-resistant colonies were screened by RNase protection, using the 5′ probe depicted in Fig. 3A. Several clones expressing high levels of flu-cTAFII31 transcripts were selected and transfected with the neo-TAF31 construct. Almost 100 drug-resistant clones were screened by Southern blotting, but only one, called DT40-TAF31, was found to have targeted the second allele (Fig. 2B). The reason for this low targeting efficiency is unknown.
We next determined how effectively cTAFII31 mRNA synthesis could be repressed by Tet. The response of cTAFII31 mRNA expression to Tet was measured by RNase protection, first using the same probe as above (Fig. 3A). Heterozygous cells expressed slightly less cTAFII31 mRNA than did wild-type cells, possibly reflecting the loss of one allele (Fig. 3A and B, compare lanes WT and HET). DT40-TAF31 cells expressed flu-cTAFII31 mRNA at a much higher level in the absence of Tet (time point 0). In the presence of Tet, however, the amount of this mRNA was dramatically reduced, becoming undetectable after 24 h. Note that the protected band from the endogenous allele was still present in DT40-TAF31 cells, likely reflecting expression of a 5′ fragment of TAFII31 mRNA. This was confirmed by using a probe from the center of the gene, which revealed only Tet-repressible TAFII31 mRNA (Fig. 3B).
We next examined cTAFII31 protein expression by Western blotting, first using an affinity-purified anti-cTAFII31 polyclonal antibody (Fig. 3C). Levels of cTAFII31 were reduced ∼2-fold in the heterozygous cells, consistent with the loss of one cTAFII31 allele and the absence of a positive autoregulatory mechanism to compensate. In contrast, flu-cTAFII31 accumulated to levels slightly higher than did cTAFII31 in DT40 cells (∼1.5-fold; Fig. 3C, bottom) in both DT40-TAF31 cells and a cTAFII31 knockout cell line with constitutive expression of flu-cTAFII31 (DT40-actin-TAF31). This contrasts with the much greater overexpression of the mRNA and suggests a posttranscriptional regulatory mechanism to limit cTAFII31 accumulation. We next followed the fate of flu-cTAFII31 after addition of Tet, using both the affinity-purified antibody and a sensitive anti-Flu monoclonal antibody (Fig. 3D and results not shown). Only low levels of cTAFII31 (≤10% the amount in DT40 cells) were detectable after a 36-h incubation in Tet, and the protein was undetectable by 60 h, even after extended exposures (≤2% the amount in wild-type cells, determined by comparisons with diluted extracts using both the purified anti-cTAFII31 and anti-Flu antibodies; see Materials and Methods for details). Incubation in a 10-fold-higher concentration of Tet did not significantly affect cTAFII31 depletion efficiency (data not shown). The level of the 100-kDa subunit of polyadenylation factor CPSF (Fig. 3D) and Coomassie blue-stained protein profiles (data not shown) were unchanged by Tet. Taken together, these results demonstrate that exogenous flu-cTAFII31 can functionally replace the endogenous protein and that this expression displayed a sensitive and rapid response to Tet.
Depletion of cTAFII31 induces apoptosis, but not specific cell cycle arrest.
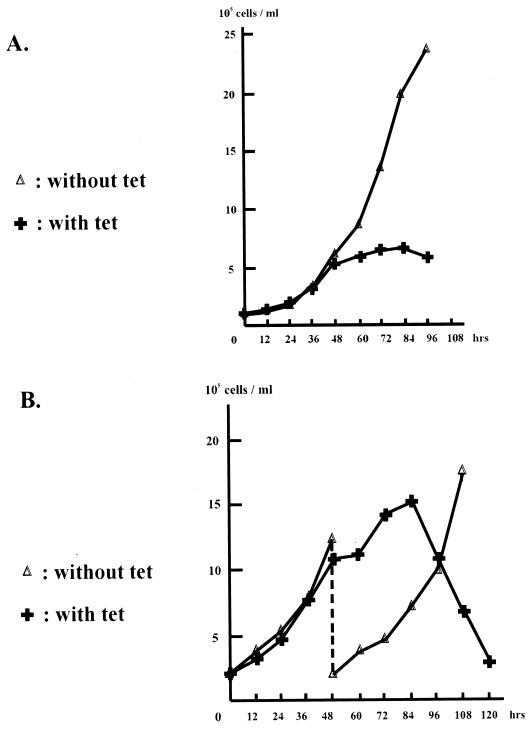
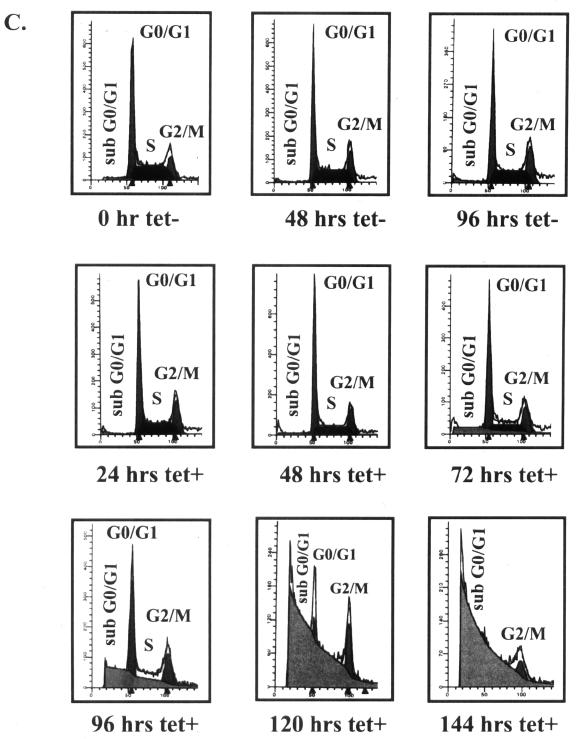
Almost all TAFIIs tested so far are essential for cell survival (30). To provide additional evidence for the essential role of cTAFII31 in DT40 cells, we examined cell growth in the presence of Tet (Fig. 4A and B). Cells grew normally until 48 h after Tet addition but began to grow more slowly thereafter. After 84 h, cell growth ceased and the number of viable cells began to decline. A 10-fold-lower concentration of Tet was also sufficient to elicit cell death, although somewhat more slowly (not shown). These results strongly suggest that expression of at least one gene required for viability is dependent on cTAFII31. We next examined additional morphological properties of the Tet-treated cells. First, membrane blebbing was observed in the cell population treated with Tet (data not shown), suggesting that cell death may involve an apoptotic pathway. To investigate this, as well as any possible effects on cell cycle progression, we performed FACS analysis to measure the DNA content of DT40-TAF31 cells incubated with Tet for different periods of time (Fig. 4C). To eliminate potential effects of cell growth phase on this analysis, cell populations were split at different ratios so that cells grown in the absence or presence of Tet were at similar densities when harvested (see Fig. 4B). In the absence of Tet, cells had essentially the same cell cycle profile throughout the time course, shown here at 0, 48, and 96 h. Cells incubated with Tet displayed this pattern until ∼60 h, after which increases in the sub-G0/G1 fraction, indicative of DNA fragmentation, could be detected. The sub-G0/G1 fraction constituted nearly 85% of the population by 120 h. Consistent with the FACS results, direct visualization of DNA by agarose gel electrophoresis revealed accumulation of nucleosome ladder-sized fragments at 72- and 96-h time points (results not shown).
FIG. 4.
Depletion of cTAFII31 causes apoptic cell death. Shown are growth curves of DT40-TAF31 cells split at low (A) or high (B) densities. DT40-TAF31 cells were passaged into medium with or without Tet (1 μg/ml), and the concentration of cells at the indicated times was determined by hemacytometer counting. (C) FACS analysis. DNA content of DT40-TAF31 cells, cultured in the absence of Tet for 0, 48, and 96 h or in the presence of Tet (1 μg/ml) for 24, 48, 72, 96, 120, and 144 h exactly as shown in panel B, was analyzed by FACS Calibur (Becton Dickinson). (Cells grown in the absence of Tet for 48 h were analyzed prior to passage.) Different phases of the cell cycle are indicated in each diagram. x and y axes represent DNA content and cell number, respectively.
Previous studies have identified specific cell cycle arrest phenotypes after inactivation of yTAFII145/TAFII250, yTAFII150 (TSM), yTAFII90, and murine TAFII30 (reference 37 and references therein). In contrast, similar to the behavior of yTAFII17 (1), depletion of cTAFII31 did not lead to a specific cell cycle arrest (Fig. 4C), as the profile of cell cycle stages remained similar as late as 72 h after Tet addition. However, at later time points, a relative increase in the percentage of G2/M cells was observed. By 120 and 144 h of Tet incubation, when most cells were dead, the great majority of living cells possessed a characteristic G2/M-phase DNA content (Fig. 4C). Since this accumulation of G2/M cells was only seen at later stages when most cells were undergoing apoptosis, and also because the absolute number of G2/M cells at these time points remained roughly the same, it is unlikely that this reflected a specific cell cycle arrest. An alternative explanation is that G2/M cells were resistant to apoptosis. An inhibitor of apoptosis, survivin, was recently found to be expressed specifically in the G2/M phase and may help to negate an M phase-specific apoptotic pathway (31). Whether such an antiapoptotic pathway is operative in DT40 cells is unclear, but in any event, our data show that depletion of cTAFII31 results in apoptotic cell death, but not cell cycle arrest.
Differential effects of cTAFII31 depletion on accumulation of TFIID and PCAF subunits.
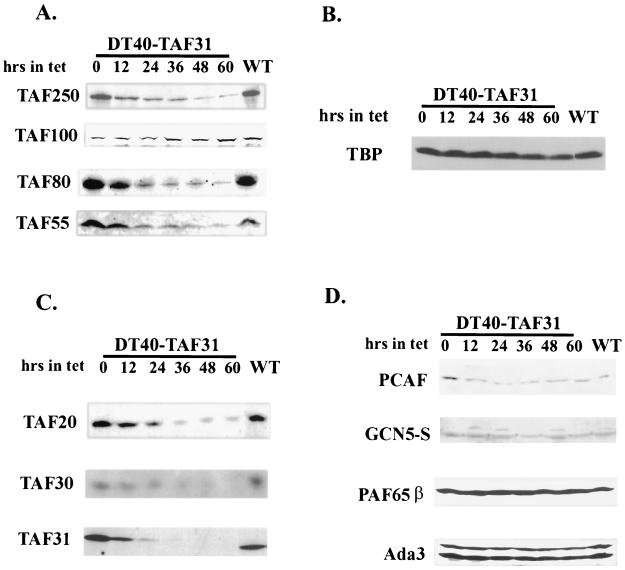
TAFII31 is a component of at least two multisubunit complexes, TFIID and PCAF/SAGA. We therefore next determined the influence of cTAFII31 depletion on accumulation of other subunits of these complexes. It has been suggested that TAFII31, together with two other histone-like TAFIIs, TAFII80 and TAFII20, forms a nucleosomal octomer-like subcomplex that may be important for TFIID structural integrity (6). Upon depletion of one of the histone-like TAFIIs, the subcomplex would disassociate, thereby destabilizing TFIID. The amount of the resulting free subunits, including histone-like TAFIIs and other TAFIIs, would be downregulated, possibly by protein degradation. Evidence consistent with this has been reported for yeast (1, 38, 40, 42). We therefore examined levels of a number of TAFIIs by Western blotting following addition of Tet to DT40-TAF31 cells (Fig. 5A and C). Strikingly, nearly all showed rapid and significant decreases. These include not only TAFII80 and TAFII20 but also TAFII250, TAFII55, and TAFII30. All showed decreased accumulation as early as 12 h after Tet addition and were depleted on the order of 80% after 60 h. Among the TAFIIs tested, only TAFII100 was unaffected throughout the time course. Why this one TAFII was stable is not clear, although it is of interest that this WD-40 repeat-containing protein is thought to help anchor the histone-like TAFIIs into the TFIID complex (60). Importantly, the levels of TBP decreased only slightly (∼2-fold) throughout the time course (Fig. 5B).
FIG. 5.
Immunoblot analysis of subunits of TFIID and PCAF complexes. DT40-TAF31 cells were cultured in the presence of Tet for the indicated time before harvest. Total lysates were prepared and quantitated by the Bradford method. Equal amounts of lysates were fractionated on SDS-PAGE gels and probed with antibodies directed against the indicated proteins. Lysates from wild-type DT40 cells (WT) were analyzed for comparison. (A) TFIID-specific subunits. (B) TBP. (C) Subunits common to TFIID and PCAF. (D) PCAF-specific subunits.
Previous studies have also suggested a structural role for several TAFIIs in the SAGA complex (14, 38, 42), which prompted us to investigate the effect of cTAFII31 depletion on subunits of the SAGA/PCAF complex. As noted above, in addition to cTAFII31, another TAFII common to both complexes, TAFII20, was significantly reduced in concentration (Fig. 5C). In sharp contrast, all other PCAF subunits tested showed, at most, modest reductions (Fig. 5D). Levels of PCAF itself and the related GCN5-S protein, both of which contain HAT activity, were reduced at most very slightly, less than twofold. The TAFII100-like factor, PAF65β, was not affected at all, consistent with the behavior of TAFII100. Another subunit, Ada3, was also only minimally depleted. Taken together, these results suggest that depletion of cTAFII31 exerted a severe effect on the integrity of TFIID, but only slightly affected components of the PCAF complex.
cTAFII31 is not universally required for Pol II-mediated transcription.
The apparent disruption of TFIID caused by cTAFII31 depletion, as well as the reported deleterious effects of yTAFII17 inactivation on total Pol II transcription, suggested that addition of Tet to DT40-TAF31 cells would result in a rapid inhibition of Pol II transcription. To test this, we first performed pulse-labeling experiments to measure total transcription activity at different times after Tet addition to DT40-TAF31 cells. Pulse labeling allows a measure of ongoing transcription at the time of the pulse and is thus preferable to methods that measure total accumulated mRNA, in which mRNA turnover may complicate interpretations.
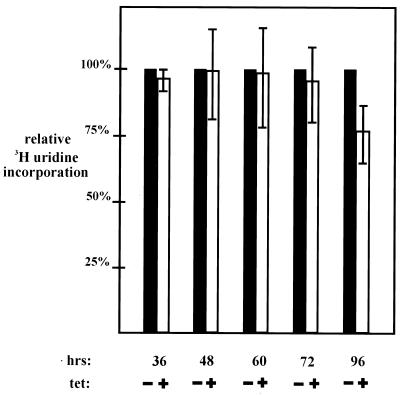
Cells were split as shown in Fig. 4B to ensure that transcription was not influenced by any differences in cell growth state between the control (without Tet) and test samples (with Tet). Equivalent numbers of cells were labeled with [3H]uridine by short pulses (10 to 15 min) at the times indicated in Fig. 6. Equal amounts of total RNA purified from labeled cells were then subjected to oligo(dT) selection to isolate poly(A)+ mRNA. The efficiency and selectivity of the protocol were verified as described in Materials and Methods. The poly(A)+ RNA fraction routinely contained 3 to 5% of total RNA (measured by optical density at 260 nm) and 15 to 25% of the total pulse-labeled RNA. Importantly, 1% or less of the poly(A)− RNA (e.g., rRNA) was detected in the poly(A)+ samples, and the amounts of total RNA and mRNA purified from equal numbers of cells grown in Tet+ and Tet− media were equivalent at all time points up to 60 h. The yield of total RNA from the cells grown in Tet decreased slightly at 72 and 96 h, although the relative amounts of poly(A)+ and poly(A)− RNA were unchanged. This likely reflected the inclusion of dead or dying cells at these times. The ratio of 3H incorporation of the selected poly(A)+ population against that of total RNA was calculated for both control and test samples, and values from the control samples were all set at 100% to facilitate comparisons. Results from the indicated time points, each representing three to five independent experiments, are shown in Fig. 6. Strikingly, poly(A)+ RNA synthesis was unaffected as late as 60 h after Tet addition, a time at which cTAFII31 was undetectable and other TFIID components were significantly depleted (Fig. 5). Even at 72 h, just a minimal (8%) decrease was detected. Only at 96 h, when a large fraction of the cell population was undergoing cell death, was a significant decrease (25%) in poly(A)+ RNA synthesis observed. These data suggest that total Pol II transcription was not influenced to a significant degree by depletion of cTAFII31 and apparent disruption of TFIID.
FIG. 6.
Pulse-labeling analysis of general Pol II-mediated transcription in DT40-TAF31 cells. DT40-TAF31 cells were cultured in medium with or without Tet for the indicated times. Each culture was pulse labeled with [3H]uridine for 10 to 15 min. Total cellular RNAs were purified, and poly(A)+ RNA was selected on an oligo(dT) column. The ratios of selected poly(A)+ RNA to total RNA were determined after scintillation counting of equal aliquots of each sample. The ratios for control samples (cells grown in the absence of Tet [−]) were designated 100%, and the ratios for test samples (cells grown in the presence of Tet [+]) are presented relative to this. Results from three to five independent experiments for each of the indicated time points were averaged and plotted. Error bars represent standard deviation.
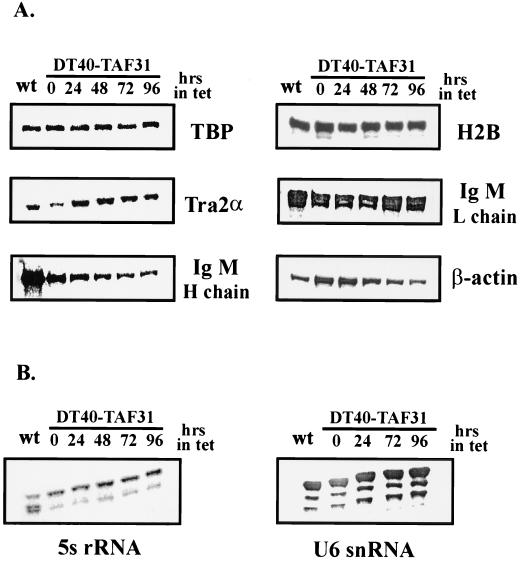
To explore further the consequences of cTAFII31 depletion on mRNA synthesis, we examined the abundance of a number of specific transcripts by RNase protection (Fig. 7). These mRNAs differ significantly in their structures, functions, abundance, half-lives, and expression profiles during B-cell development (Fig. 7A). Consistent with the pulse-labeling results, levels of four different mRNAs, encoding TBP, Tra2α, histone 2B and immunoglobulin M (IgM) L chain (the most abundant transcript in DT40 cells; e.g., 58), remained essentially unchanged, even at 96 h after Tet addition. Two other transcripts, encoding β-actin and IgM H chain, were slightly decreased (two- to threefold) at later time points. Interestingly, these two mRNAs, but not the others tested, showed differences in their abundance in DT40 cells compared to that in DT40-TAF31 cells in the absence of Tet, with actin levels two- to threefold higher in the mutant cell line and H chain decreased by three- to fivefold (Fig. 7A). Although the reason for this is unknown, it may reflect the altered level of cTAFII31 in the knockout cells (Fig. 3C). As expected, accumulation of 5S rRNA and U6 snRNA (RNA Pol III dependent) was not affected by cTAFII31 depletion (Fig. 7B).
FIG. 7.
RNase protection analysis of steady-state levels of specific transcripts. Total cellular RNA was isolated from wild-type DT40 and DT40-TAF31 cells incubated in the presence of Tet for the indicated times. Equal amounts of total RNAs for each sample were hybridized with [32P]CTP-labeled probes. After RNase digestion, the protected bands were separated on polyacrylamide gels. (A) Expression levels of six Pol II transcripts. (B) Expression of 5S rRNA and U6 snRNA.
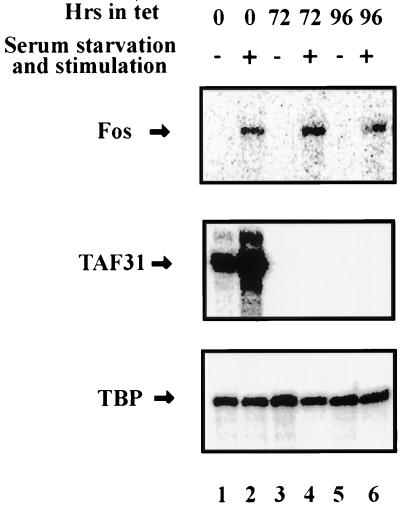
The above assays measure primarily ongoing transcription of genes constitutively expressed in DT40 cells. We next wished to determine whether an inducible gene could be activated in the absence of cTAFII31. To this end, we examined induction of the c-fos gene in response to serum stimulation following serum starvation. The rapid induction of c-fos transcription as a result of various extracellular signals has been well characterized and involves the formation of a ternary protein-DNA complex (61). As shown in Fig. 8, during a time course of up to 96 h of incubation in Tet, c-fos transcript synthesis in cells serum starved for 10 h was efficiently induced by a short period (75 min) of serum stimulation regardless of the time of Tet incubation (Fig. 8, compare lanes 4 and 6, in Tet for 72 and 96 h, respectively, to lane 2, without Tet). By contrast, cells not subjected to serum starvation expressed essentially undetectable levels of c-fos mRNA during the same Tet incubation periods (see lanes 1, 3, and 5). As controls, cTAFII31 expression was indeed abrogated during the time course and TBP mRNA expression remained constant and was unaffected by serum treatment (Fig. 8). Therefore, after cTAFII31 had been totally depleted from the cells, inducible transcription of c-fos in response to serum stimulation was not compromised. Taken together, our experiments strongly suggest that cTAFII31 is not essential for high levels of Pol II-mediated transcription of many genes in chicken DT40 cells.
FIG. 8.
RNase protection analysis of serum-induced c-fos expression. Total cellular RNA was isolated from DT40-TAF31 cells incubated in the presence of Tet for the indicated times, with or without 10 h of serum starvation and 75 min of serum stimulation. Equal amounts of total RNAs for each sample were analyzed by RNase protection using c-fos, cTAFII31, or TBP probes, and the protected bands were separated on polyacrylamide gels.
DISCUSSION
In this study, we established a conditional TAFII31 knockout cell line in chicken DT40 cells. Unexpectedly, results from [3H]uridine pulse-labeling experiments illustrated that a high level of Pol II-mediated transcription occurred in the apparent absence of cTAFII31 and reduced levels of several other TAFIIs. In agreement with this, the transcript levels of several specific genes were unchanged or decreased only modestly, even as the cells began to undergo extensive cell death. Importantly, while in yeast the expression of TBP, histone 2B, and actin mRNAs was reduced in a yTAFII17 mutant strain by 3.1-, 3.7-, and 6.2-fold, respectively (22), chicken TBP and histone 2B maintained the same expression level well after complete cTAFII31 depletion, and β-actin expression exhibited only a minimal reduction at later time points. On a global scale, ongoing differential display experiments also reveal comparable expression of a large majority of genes with or without cTAFII31 (unpublished data). Furthermore, cTAFII31 is also dispensable for the efficient induction of c-fos transcription, indicating that a transcriptionally silent gene can be activated in the absence of cTAFII31. These results strongly suggest that cTAFII31 is not an essential general coactivator for class II gene transcription and that transcription of many genes can continue unabated in the presence of significantly reduced levels of several TAFIIs.
The observed modest effect on transcription by cTAFII31 depletion is, on the one hand, surprising in light of previous studies on yTAFII17, but in retrospect is not inconsistent with several studies analyzing other metozoan TAFIIs. For example, the hamster cell line harboring a weak temperature-sensitive mutant of TAFII250 was shown to be defective in expression of some, but not all, genes tested at the nonpermissive temperature (57, 66), consistent with subsequent experiments on yeast TAFII145 (52, 64). In Drosophila, a weak, nonlethal mutation in dTAFII40, identified in a genetic screen for regulatory genes involved in pigmentation, as well as dominant modifier mutants of dTAFII60 and dTAFII110, conferred selective transcriptional defects (51, 53, 70). Recently, an approach similar to that used here was employed to target TAFII30 in murine F9 cells (37). Human TAFII30 is found in only about 50% of TFIID complexes (24) and thus may be considered a specific TAFII, but it is also present in PCAF/SAGA and TFTC (see below) complexes (14, 44, 67). Depletion of TAFII30 resulted in cell death, but did not appear to have a general effect on transcription, which could have been indicative of either its specific role and/or the fact that the levels of other TAFIIs examined were not affected. Surprisingly, a temperature-sensitive mutant of yTAFII25, the TAFII30 homologue, has been reported to display a strong general transcription defect at the nonpermissive temperature, perhaps reflecting the fact that multiple other TFIID and SAGA components were codepleted (49). Unlike yTAFII17, genome-wide analysis is currently not available to substantiate the broad requirement of yTAFII25 in transcription. Taken together, these studies are consistent with the view that metazoan TAFIIs are necessary for transcription of specific genes, but for different reasons they did not resolve the question of whether they are generally required for transcription in vivo.
Another line of in vivo evidence was provided by studies of TBP recruitment in yeast using chromatin immunoprecipitation procedures (29, 32). It was shown that activator-dependent transcription correlated well with the loading of TBP and that this TBP binding required general factors on a majority of promoters tested, but yTAFII145 enhanced TBP binding only on specific promoters. Further insight into the role of TAFIIs in TBP recruitment was offered by quantitation of transcriptional factors in cells (26, 30). In both yeast and HeLa cells, the reservoir of GTFs, including TBP, is estimated to be almost 10-fold greater than that of TAFIIs. Additionally, only a fraction (∼15%) of the TBP bound to chromatin appears to be associated with the TFIID-specific hTAFII100 (26). Taken together, these findings invoke the possibility that TAFIIs might assist TBP recruitment to only a subset of promoters. A number of more recent in vitro studies have also provided corroborative biochemical evidence for the above in vivo studies (33), consistent with a possible gene-specific, rather than global, role of TAFIIs in transcription.
Our results extend these previous studies in several important ways. First, our experiments are the first in a metazoan system to inactivate completely one of the histone-like TAFIIs, which are believed to play important structural roles in TFIID (6) and, especially yTAFII17, to be generally required for transcription in yeast (1, 38, 40). Second, ours is the only study performed in higher eukaryotes in which the levels of TAFIIs in addition to the targeted TAFII were significantly reduced. In yeast, this is thought to be characteristic of tight conditional mutants, and it has been suggested that only such tight mutants display global transcriptional defects (28, 38). By the criteria of the rapid shut-off of cTAFII31 expression following Tet addition, in addition to the significant codepletion of other TAFIIs, DT40-TAF31 cells have the characteristics of a tight mutant. Finally, to our knowledge, ours is the first analysis of TAFII function in any organism to measure transcription directly, i.e., by pulse labeling, as opposed to measurements of steady-state mRNA. Although such measurements likely do reflect transcription, it is not impossible that apparent reductions in mRNA may in some cases reflect enhanced mRNA turnover, perhaps as a direct effect of TAFII depletion, given recent experiments linking mRNA capping (8, 35, 69) and polyadenylation (11, 19, 36) to transcription. In any case, our experiments provide the most compelling evidence to date that TAFIIs are not essential for activated transcription in higher eukaryotes and, as discussed below, suggest the existence of important functional redundancies in metazoans, as well as significant differences between yeast and vertebrate transcriptional mechanisms.
Depletion of cTAFII31 resulted in specific decreases in the concentrations of multiple TAFIIs, but did not significantly affect accumulation of other proteins. Importantly, the levels of TBP and several subunits of PCAF were much less sensitive to the absence of cTAFII31. In the case of PCAF/SAGA, this might stem from the fact that this complex is much bigger than TFIID, and its more than 20 subunits appear to be organized into several subcomplexes that might possess a certain degree of functional and structural autonomy (15). Previously, a temperature-sensitive mutant of yTAFII60 has been reported to lead to only a mild structural change upon temperature shift and the smaller SAGA complex still retained partial HAT activity (14). Another study has implied that three non-TAFII subunits constitute the structural core of the SAGA complex (54). Our results are consistent with this and further suggest that the integrities of the TFIID and PCAF/SAGA complexes are differentially affected by removal of TAFII31.
How could it be that transcription is not generally affected by complete removal of TAFII31 and significant depletion of several other TAFIIs? The answer, we suggest, involves extensive functional degeneracy. Recent years have witnessed the discovery of much greater complexity within the transcription machinery than previously conceived. For example, a number of variant forms of TFIID and RNA Pol II holoenzyme have been identified in yeast and human (2). A group of TAFIIs, most notably the histone-like TAFIIs, were also found to be components of various TBP-free HAT complexes, including SAGA/PCAF and another similar complex called TFTC (2, 16, 55, 67). Another TAFII31-containing complex contains a novel long form of GCN5 and Spt3, but is devoid of other histone-like TAFIIs (3, 34). Other than TAFIIs, many protein factors or complexes can function as coactivators, including a rapidly growing family of multifunctional chromatin-remodeling activities, such as HATs and ATP-dependent activities (25, 30). Recently, a number of cofactor protein complexes were identified biochemically, such as CRSP as a coactivator to Sp1 (47) and four essentially identical complexes (TRAP, ARC, SMCC, and DRIP) with diverse functional activities (23, 41, 46). All five complexes have with two other complexes (4, 56) common subunits corresponding to yeast SRB/mediator proteins. These burgeoning multifunctional coactivator families, together with previously proposed USA fractions, TFIIA, and TAFIIs, may form a balanced yet flexible cofactor network. In DT40-TAF31 cells depleted of TAFIIs, the remaining large TBP pool can still perform TAFII-independent functions, and other cofactors, such as the partial PCAF complex and/or other coactivators, can, we suggest, substitute for the missing TAFIIs on many promoters that naturally utilize TFIID. It is also possible that in the TAFII31-depleted cells, the low levels of the remaining TAFIIs reflect partial TFIID complexes that are selectively associated with active genes.
Our results suggest that cTAFII31 is required for only a subset of class II gene transcription in chicken. This contrasts sharply with the situation in yeast, in which yTAFII17 is the only yTAFII whose essential role in Pol II transcription is universally supported by various experimental approaches. One explanation for this apparent inconsistency is that the functional degeneracy we suggest to be in vertebrates is absent in yeast. In support of such an evolutionary divergence, numerous examples of transcription factors present in metazoans but lacking in yeast have been uncovered in recent years, including TBP-related factors in higher eukaryotes (11), three mammalian proteins homologous to yGCN5, and TAFII80- and TAFII100-like factors in the PCAF complex (44). Half of the subunits of TRAP identified thus far are missing from the yeast genome (23). Following the same combinatorial principle as in mediator-containing complexes, the number of cofactor complexes would grow exponentially in order to cope with the multitude of metazoan-specific activators and much more complicated developmental and tissue-specific gene regulatory programs (50). As a consequence of such finely tuned gene regulation, however, a large number of factors would function in the same pathway, perhaps in the same step, raising the possibility of much greater functional degeneracy in metazoans than in yeast, with its simpler genetic circuitry. Thus, while yTAFII17 appears to be broadly required for transcription of many yeast genes, in higher eukaryotes the absence of TAFII31 might be compensated for on most, but not all, genes by other factors. It is interesting to speculate that such metazoan-specific functional degeneracy might exist in other complex regulatory pathways.
ACKNOWLEDGMENTS
We thank Y. Nakatani, R. Tjian, R. Roeder, L. Tora, C.-M. Chiang, and K. G. K. Murthy for kindly providing antibodies. We are grateful to Y. Takagaki, T. Kashima, M. Um, and S. Valadkhan for gifts of template DNAs for RNase protection assays. We thank members of Manley's and Prives' labs for helpful discussions. We also thank Inna Boluk for helping prepare this paper.
This work is supported by NIH grant GM 37971.
REFERENCES
- 1.Apone L M, Virbasius C A, Holstege F C, Wang J, Young R A, Green M R. Broad, but not universal, transcriptional requirement for yTAFII17, a histone H3-like TAFII present in TFIID and SAGA. Mol Cell. 1998;2:653–661. doi: 10.1016/s1097-2765(00)80163-x. [DOI] [PubMed] [Google Scholar]
- 2.Bell B, Tora L. Regulation of gene expression by multiple forms of TFIID and other novel TAFII-containing complexes. Exp Cell Res. 1999;246:11–19. doi: 10.1006/excr.1998.4294. [DOI] [PubMed] [Google Scholar]
- 3.Birck C, Poch O, Romier C, Ruff M, Mengus G, Lavigne A C, Davidson I, Moras D. Human TAF(II)28 and TAF(II)18 interact through a histone fold encoded by atypical evolutionary conserved motifs also found in the SPT3 family. Cell. 1998;94:239–249. doi: 10.1016/s0092-8674(00)81423-3. [DOI] [PubMed] [Google Scholar]
- 4.Boyer T G, Martin M E, Lees E, Ricciardi R P, Berk A J. Mammalian Srb/Mediator complex is targeted by adenovirus E1A protein. Nature. 1999;399:276–279. doi: 10.1038/20466. [DOI] [PubMed] [Google Scholar]
- 5.Burke T W, Kadonaga J T. The downstream core promoter element, DPE, is conserved from Drosophila to humans and is recognized by TAFII60 of Drosophila. Genes Dev. 1997;11:3020–3031. doi: 10.1101/gad.11.22.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burley S K, Roeder R G. Biochemistry and structural biology of transcription factor IID (TFIID) Annu Rev Biochem. 1996;65:769–799. doi: 10.1146/annurev.bi.65.070196.004005. [DOI] [PubMed] [Google Scholar]
- 7.Chiang C M, Ge H, Wang Z, Hoffmann A, Roeder R G. Unique TATA-binding protein-containing complexes and cofactors involved in transcription by RNA polymerases II and III. EMBO J. 1993;12:2749–2762. doi: 10.1002/j.1460-2075.1993.tb05936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho E J, Takagi T, Moore C R, Buratowski S. mRNA capping enzyme is recruited to the transcription complex by phosphorylation of the RNA polymerase II carboxy-terminal domain. Genes Dev. 1997;11:3319–3326. doi: 10.1101/gad.11.24.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 10.Dantonel J C, Murthy K G, Manley J L, Tora L. Transcription factor TFIID recruits factor CPSF for formation of 3′ end of mRNA. Nature. 1997;389:399–402. doi: 10.1038/38763. [DOI] [PubMed] [Google Scholar]
- 11.Dantonel J C, Wurtz J M, Poch O, Moras D, Tora L. The TBP-like factor: an alternative transcription factor in metazoa? Trends Biochem Sci. 1999;24:335–339. doi: 10.1016/s0968-0004(99)01436-x. [DOI] [PubMed] [Google Scholar]
- 12.Fondell J D, Guermah M, Malik S, Roeder R G. Thyroid hormone receptor-associated proteins and general positive cofactors mediate thyroid hormone receptor function in the absence of the TATA box-binding protein-associated factors of TFIID. Proc Natl Acad Sci USA. 1999;96:1959–1964. doi: 10.1073/pnas.96.5.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grant P A, Schieltz D, Pray-Grant M G, Steger D J, Reese J C, Yates J R, 3rd, Workman J L. A subset of TAF(II)s are integral components of the SAGA complex required for nucleosome acetylation and transcriptional stimulation. Cell. 1998;94:45–53. doi: 10.1016/s0092-8674(00)81220-9. [DOI] [PubMed] [Google Scholar]
- 15.Grant P A, Sterner D E, Duggan L J, Workman J L, Berger S L. The SAGA unfolds: convergence of transcription regulators in chromatin-modifying complexes. Trends Cell Biol. 1998;8:193–197. doi: 10.1016/s0962-8924(98)01263-x. [DOI] [PubMed] [Google Scholar]
- 16.Hahn S. The role of TAFs in RNA polymerase II transcription. Cell. 1998;95:579–582. doi: 10.1016/s0092-8674(00)81625-6. [DOI] [PubMed] [Google Scholar]
- 17.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1988. [Google Scholar]
- 18.Hernandez N. TBP, a universal eukaryotic transcription factor? Genes Dev. 1993;7:1291–1308. doi: 10.1101/gad.7.7b.1291. [DOI] [PubMed] [Google Scholar]
- 19.Hirose Y, Manley J L. RNA polymerase II is an essential mRNA polyadenylation factor. Nature. 1998;395:93–96. doi: 10.1038/25786. [DOI] [PubMed] [Google Scholar]
- 20.Hisatake K, Ohta T, Takada R, Guermah M, Horikoshi M, Nakatani Y, Roeder R G. Evolutionary conservation of human TATA-binding-polypeptide-associated factors TAFII31 and TAFII80 and interactions of TAFII80 with other TAFs and with general transcription factors. Proc Natl Acad Sci USA. 1995;92:8195–8199. doi: 10.1073/pnas.92.18.8195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hockenbery D, Nunez G, Milliman C, Schreiber R D, Korsmeyer S J. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990;348:334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- 22.Holstege F C, Jennings E G, Wyrick J J, Lee T I, Hengartner C J, Green M R, Golub T R, Lander E S, Young R A. Dissecting the regulatory circuitry of a eukaryotic genome. Cell. 1998;95:717–728. doi: 10.1016/s0092-8674(00)81641-4. [DOI] [PubMed] [Google Scholar]
- 23.Ito M, Yuan C X, Malik S, Gu W, Fondell J D, Yamamura S, Fu Z Y, Zhang X, Qin J, Roeder R G. Identity between TRAP and SMCC complexes indicates novel pathways for the function of nuclear receptors and diverse mammalian activators. Mol Cell. 1999;3:361–370. doi: 10.1016/s1097-2765(00)80463-3. [DOI] [PubMed] [Google Scholar]
- 24.Jacq X, Brou C, Lutz Y, Davidson I, Chambon P, Tora L. Human TAFII30 is present in a distinct TFIID complex and is required for transcriptional activation by the estrogen receptor. Cell. 1994;79:107–117. doi: 10.1016/0092-8674(94)90404-9. [DOI] [PubMed] [Google Scholar]
- 25.Kadonaga J T. Eukaryotic transcription: an interlaced network of transcription factors and chromatin-modifying machines. Cell. 1998;92:307–313. doi: 10.1016/s0092-8674(00)80924-1. [DOI] [PubMed] [Google Scholar]
- 26.Kimura H, Tao Y, Roeder R G, Cook P R. Quantitation of RNA polymerase II and its transcription factors in an HeLa cell: little soluble holoenzyme but significant amounts of polymerases attached to the nuclear substructure. Mol Cell Biol. 1999;19:5383–5392. doi: 10.1128/mcb.19.8.5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koleske A J, Young R A. An RNA polymerase II holoenzyme responsive to activators. Nature. 1994;368:466–469. doi: 10.1038/368466a0. [DOI] [PubMed] [Google Scholar]
- 28.Komarnitsky P B, Michel B, Buratowski S. TFIID-specific yeast TAF40 is essential for the majority of RNA polymerase II-mediated transcription in vivo. Genes Dev. 1999;13:2484–2489. doi: 10.1101/gad.13.19.2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuras L, Struhl K. Binding of TBP to promoters in vivo is stimulated by activators and requires Pol II holoenzyme. Nature. 1999;399:609–613. doi: 10.1038/21239. [DOI] [PubMed] [Google Scholar]
- 30.Lee T I, Young R A. Regulation of gene expression by TBP-associated proteins. Genes Dev. 1998;12:1398–1408. doi: 10.1101/gad.12.10.1398. [DOI] [PubMed] [Google Scholar]
- 31.Li F, Ambrosini G, Chu E Y, Plescia J, Tognin S, Marchisio P C, Altieri D C. Control of apoptosis and mitotic spindle checkpoint by survivin. Nature. 1998;396:580–584. doi: 10.1038/25141. [DOI] [PubMed] [Google Scholar]
- 32.Li X Y, Virbasius A, Zhu X, Green M R. Enhancement of TBP binding by activators and general transcription factors. Nature. 1999;399:605–609. doi: 10.1038/21232. [DOI] [PubMed] [Google Scholar]
- 33.Martinez E, Ge H, Tao Y, Yuan C X, Palhan V, Roeder R G. Novel cofactors and TFIIA mediate functional core promoter selectivity by the human TAFII150-containing TFIID complex. Mol Cell Biol. 1998;18:6571–6583. doi: 10.1128/mcb.18.11.6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martinez E, Kundu T K, Fu J, Roeder R G. A human SPT3-TAFII31-GCN5-L acetylase complex distinct from transcription factor IID. J Biol Chem. 1998;273:23781–23785. doi: 10.1074/jbc.273.37.23781. [DOI] [PubMed] [Google Scholar]
- 35.McCracken S, Fong N, Rosonina E, Yankulov K, Brothers G, Siderovski D, Hessel A, Foster S, Shuman S, Bentley D L. 5′-Capping enzymes are targeted to pre-mRNA by binding to the phosphorylated carboxy-terminal domain of RNA polymerase II. Genes Dev. 1997;11:3306–3318. doi: 10.1101/gad.11.24.3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCracken S, Fong N, Yankulov K, Ballantyne S, Pan G, Greenblatt J, Patterson M, Wickens S D, Bentley D L. The C-terminal domain of RNA polymerase II couples mRNA processing to transcription. Nature. 1997;385:357–361. doi: 10.1038/385357a0. [DOI] [PubMed] [Google Scholar]
- 37.Metzger D, Scheer E, Soldatov A, Tora L. Mammalian TAF(II)30 is required for cell cycle progression and specific cellular differentiation programmes. EMBO J. 1999;18:4823–4834. doi: 10.1093/emboj/18.17.4823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Michel B, Komarnitsky P, Buratowski S. Histone-like TAFs are essential for transcription in vivo. Mol Cell. 1998;2:663–673. doi: 10.1016/s1097-2765(00)80164-1. [DOI] [PubMed] [Google Scholar]
- 39.Moqtaderi Z, Bai Y, Poon D, Weil P A, Struhl K. TBP-associated factors are not generally required for transcriptional activation in yeast. Nature. 1996;383:188–191. doi: 10.1038/383188a0. [DOI] [PubMed] [Google Scholar]
- 40.Moqtaderi Z, Keaveney M, Struhl K. The histone H3-like TAF is broadly required for transcription in yeast. Mol Cell. 1998;2:675–682. doi: 10.1016/s1097-2765(00)80165-3. [DOI] [PubMed] [Google Scholar]
- 41.Naar A M, Beaurang P A, Zhou S, Abraham S, Solomon W, Tjian R. Composite co-activator ARC mediates chromatin-directed transcriptional activation. Nature. 1999;398:828–832. doi: 10.1038/19789. [DOI] [PubMed] [Google Scholar]
- 42.Natarajan K, Jackson B M, Rhee E, Hinnebusch A G. yTAFII61 has a general role in RNA polymerase II transcription and is required by Gcn4p to recruit the SAGA coactivator complex. Mol Cell. 1998;2:683–692. doi: 10.1016/s1097-2765(00)80166-5. [DOI] [PubMed] [Google Scholar]
- 43.Oelgeschlager T, Tao Y, Kang Y K, Roeder R G. Transcription activation via enhanced preinitiation complex assembly in a human cell-free system lacking TAFIIs. Mol Cell. 1998;1:925–931. doi: 10.1016/s1097-2765(00)80092-1. [DOI] [PubMed] [Google Scholar]
- 44.Ogryzko V V, Kotani T, Zhang X, Schlitz R L, Howard T, Yang X J, Howard B H, Qin J, Nakatani Y. Histone-like TAFs within the PCAF histone acetylase complex. Cell. 1998;94:35–44. doi: 10.1016/s0092-8674(00)81219-2. [DOI] [PubMed] [Google Scholar]
- 45.Orphanides G, Lagrange T, Reinberg D. The general transcription factors of RNA polymerase II. Genes Dev. 1996;10:2657–2683. doi: 10.1101/gad.10.21.2657. [DOI] [PubMed] [Google Scholar]
- 46.Rachez C, Lemon B D, Suldan Z, Bromleigh V, Gamble M, Naar A M, Erdjument-Bromage H, Tempst P, Freedman L P. Ligand-dependent transcription activation by nuclear receptors requires the DRIP complex. Nature. 1999;398:824–828. doi: 10.1038/19783. [DOI] [PubMed] [Google Scholar]
- 47.Ryu S, Zhou S, Ladurner A G, Tjian R. The transcriptional cofactor complex CRSP is required for activity of the enhancer-binding protein Sp1. Nature. 1999;397:446–450. doi: 10.1038/17141. [DOI] [PubMed] [Google Scholar]
- 48.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 49.Sanders S L, Klebanow E R, Weil P A. TAF25p, a non-histone-like subunit of TFIID and SAGA complexes, is essential for total mRNA gene transcription in vivo. J Biol Chem. 1999;274:18847–18850. doi: 10.1074/jbc.274.27.18847. [DOI] [PubMed] [Google Scholar]
- 50.Sauer F, Tjian R. Mechanisms of transcriptional activation: differences and similarities between yeast, Drosophila, and man. Curr Opin Genet Dev. 1997;7:176–181. doi: 10.1016/s0959-437x(97)80126-8. [DOI] [PubMed] [Google Scholar]
- 51.Schaeffer V, Janody F, Loss C, Desplan C, Wimmer E A. Bicoid functions without its TATA-binding protein-associated factor interaction domains. Proc Natl Acad Sci USA. 1999;96:4461–4466. doi: 10.1073/pnas.96.8.4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shen W C, Green M R. Yeast TAF(II)145 functions as a core promoter selectivity factor, not a general coactivator. Cell. 1997;90:615–624. doi: 10.1016/s0092-8674(00)80523-1. [DOI] [PubMed] [Google Scholar]
- 53.Soldatov A, Nabirochkina E, Georgieva S, Belenkaja T, Georgiev P. TAFII40 protein is encoded by the e(y)1 gene: biological consequences of mutations. Mol Cell Biol. 1999;19:3769–3778. doi: 10.1128/mcb.19.5.3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sterner D E, Grant P A, Roberts S M, Duggan L J, Belotserkovskaya R, Pacella L A, Winston F, Workman J L, Berger S L. Functional organization of the yeast SAGA complex: distinct components involved in structural integrity, nucleosome acetylation, and TATA-binding protein interaction. Mol Cell Biol. 1999;19:86–98. doi: 10.1128/mcb.19.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Struhl K, Moqtaderi Z. The TAFs in the HAT. Cell. 1998;94:1–4. doi: 10.1016/s0092-8674(00)81213-1. [DOI] [PubMed] [Google Scholar]
- 56.Sun X, Zhang Y, Cho H, Rickert P, Lees E, Lane W, Reinberg D. NAT, a human complex containing Srb polypeptides that functions as a negative regulator of activated transcription. Mol Cell. 1998;2:213–222. doi: 10.1016/s1097-2765(00)80131-8. [DOI] [PubMed] [Google Scholar]
- 57.Suzuki-Yagawa Y, Guermah M, Roeder R G. The ts13 mutation in the TAF(II)250 subunit (CCG1) of TFIID directly affects transcription of D-type cyclin genes in cells arrested in G1 at the nonpermissive temperature. Mol Cell Biol. 1997;17:3284–3294. doi: 10.1128/mcb.17.6.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Takagaki Y, Manley J L. Levels of polyadenylation factor CstF-64 control IgM heavy chain mRNA accumulation and other events associated with B cell differentiation. Mol Cell. 1998;2:761–771. doi: 10.1016/s1097-2765(00)80291-9. [DOI] [PubMed] [Google Scholar]
- 59.Tansey W P, Herr W. TAFs: guilt by association? Cell. 1997;88:729–732. doi: 10.1016/s0092-8674(00)81916-9. [DOI] [PubMed] [Google Scholar]
- 60.Tao Y, Guermah M, Martinez E, Oelgeschlager T, Hasegawa S, Takada R, Yamamoto T, Horikoshi M, Roeder R G. Specific interactions and potential functions of human TAFII100. J Biol Chem. 1997;272:6714–6721. doi: 10.1074/jbc.272.10.6714. [DOI] [PubMed] [Google Scholar]
- 61.Treisman R. Ternary complex factors: growth factor regulated transcriptional activators. Curr Opin Genet Dev. 1994;4:96–101. doi: 10.1016/0959-437x(94)90097-3. [DOI] [PubMed] [Google Scholar]
- 62.Verrijzer C P, Tjian R. TAFs mediate transcriptional activation and promoter selectivity. Trends Biochem Sci. 1996;21:338–342. [PubMed] [Google Scholar]
- 63.Walker S S, Reese J C, Apone L M, Green M R. Transcription activation in cells lacking TAFIIS. Nature. 1996;383:185–188. doi: 10.1038/383185a0. [DOI] [PubMed] [Google Scholar]
- 64.Walker S S, Shen W C, Reese J C, Apone L M, Green M R. Yeast TAF(II)145 required for transcription of G1/S cyclin genes and regulated by the cellular growth state. Cell. 1997;90:607–614. doi: 10.1016/s0092-8674(00)80522-x. [DOI] [PubMed] [Google Scholar]
- 65.Wang J, Takagaki Y, Manley J L. Targeted disruption of an essential vertebrate gene: ASF/SF2 is required for cell viability. Genes Dev. 1996;10:2588–2599. doi: 10.1101/gad.10.20.2588. [DOI] [PubMed] [Google Scholar]
- 66.Wang E H, Tjian R. Promoter-selective transcriptional defect in cell cycle mutant ts13 rescued by hTAFII250. Science. 1994;263:811–814. doi: 10.1126/science.8303298. [DOI] [PubMed] [Google Scholar]
- 67.Wieczorek E, Brand M, Jacq X, Tora L. Function of TAF(II)-containing complex without TBP in transcription by RNA polymerase II. Nature. 1998;393:187–191. doi: 10.1038/30283. [DOI] [PubMed] [Google Scholar]
- 68.Wu S Y, Kershnar E, Chiang C M. TAFII-independent activation mediated by human TBP in the presence of the positive cofactor PC4. EMBO J. 1998;17:4478–4490. doi: 10.1093/emboj/17.15.4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yue Z, Maldonado E, Pillutla R, Cho H, Reinberg D, Shatkin A J. Mammalian capping enzyme complements mutant Saccharomyces cerevisiae lacking mRNA guanylyltransferase and selectively binds the elongating form of RNA polymerase II. Proc Natl Acad Sci USA. 1997;94:12898–12903. doi: 10.1073/pnas.94.24.12898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhou J, Zwicker J, Szymanski P, Levine M, Tjian R. TAFII mutations disrupt Dorsal activation in the Drosophila embryo. Proc Natl Acad Sci USA. 1998;95:13483–13488. doi: 10.1073/pnas.95.23.13483. [DOI] [PMC free article] [PubMed] [Google Scholar]