Abstract
Sleep benefits motor memory consolidation in young adults, but this benefit is reduced in older adults. Here we sought to understand whether differences in the neural bases of encoding between young and older adults contribute to aging-related differences in sleep-dependent consolidation of an explicit variant of the serial reaction time task (SRTT). Seventeen young and 18 older adults completed two sessions (nap, wake) one week apart. In the MRI, participants learned the SRTT. Following an afternoon interval either awake or with a nap (recorded with high-density polysomnography), performance on the SRTT was reassessed in the MRI. Imaging and behavioral results from SRTT performance showed clear sleep-dependent consolidation of motor sequence learning in older adults after a daytime nap, compared to an equal interval awake. Young adults, however, showed brain activity and behavior during encoding consistent with high SRTT performance prior to the sleep interval, and did not show further sleep-dependent performance improvements. Young adults did show reduced cortical activity following sleep, suggesting potential systems-level consolidation related to automatization. Sleep physiology data showed that sigma activity topography was affected by hippocampal and cortical activation prior to the nap in both age groups, and suggested a role of theta activity in sleep-dependent automatization in young adults. These results suggest that previously observed aging-related sleep-dependent consolidation deficits may be driven by aging-related deficiencies in fast learning processes. Here we demonstrate that when sufficient encoding strength is reached with additional training, older adults demonstrate intact sleep-dependent consolidation of motor sequence learning.
Keywords: aging, sleep, motor learning, encoding, consolidation, fMRI
1. Introduction
Motor skills – such as walking, driving, and writing – are vital to the quality of life of older adults. The ability to acquire new skills or patterns is also important for healthy aging, as new skills may be learned for enjoyment (e.g., tennis, piano) or rehabilitation after injury. Several lines of evidence suggest older adults consolidate motor skills poorly during sleep compared to young adults (Fogel et al., 2014; King et al., 2013; King, Saucier, et al., 2017; Nemeth & Janacsek, 2011; Roig et al., 2014; Spencer et al., 2007; Wilson et al., 2012). Understanding the neural correlates of poor consolidation and sleep-related motor skill learning deficits during aging will provide general insight into the process of sleep-dependent consolidation, and may provide avenues to optimize sleep-centered interventions intended to minimize the impact of aging-related motor impairments and stroke (Siengsukon & Boyd, 2009a, 2009b).
Motor skill learning is typically assessed with motor sequence learning paradigms, which entail the learning of a sequence of finger movements through repeated practice (Doyon et al., 2018; Karni et al., 1995; Nissen & Bullemer, 1987; Robertson, 2007). Notably, motor sequence learning paradigms differ widely according to task demands, the underlying neural substrates, and the expression of consolidation-related performance benefits (Doyon et al., 2018; Janacsek et al., 2020; King, Hoedlmoser, et al., 2017). In studies of sleep and motor learning, two paradigms have prevailed – the motor sequence learning (MSL) task and the serial reaction time task (SRTT). In the MSL task (alternately referred to as the explicit sequential finger tapping task [FTT], or the manual sequence task [MST]), participants are explicitly provided with a short sequence in advance (e.g., 4-3-1-2-4 presented on a screen), and then repeatedly execute finger movements in the given sequence as quickly and accurately as possible (e.g., Karni et al., 1995). Learning is measured by increases in speed and accuracy. In the SRTT, movements are instead cued online one at a time, and cues are either random, probabilistic (certain sequences or cues occur at higher frequencies, e.g., Song et al., 2007), or deterministic (following a predefined but unspecified repeating sequence; Hardwick et al., 2013; Janacsek et al., 2020; Nissen & Bullemer, 1987; Robertson, 2007; Robertson et al., 2004). Further, the presence of a sequence in the SRTT can be explicit or implicit to the participant (Hardwick et al., 2013; Janacsek et al., 2020).
Sequence learning in the SRTT is quantified as reaction time improvements during periods of sequentially presented cues relative to periods of randomly presented cues. The SRTT design minimizes the contaminating influences of fatigue and motivation (Nissen & Bullemer, 1987; Robertson, 2007; Willingham et al., 1989; Willingham & Goedert-Eschmann, 1999), controls for individual differences in visuomotor skill (Bennett et al., 2007; Curran, 1997; Feeney et al., 2002; Howard et al., 2004), and avoids the increased cognitive demand of maintaining prior explicit knowledge of the sequence (Howard & Howard, 2001; Willingham et al., 2002; Willingham & Goedert-Eschmann, 1999). These features make the SRTT particularly advantageous for examining aging-related differences in sequence learning, as typical aging can involve decline across these cognitive faculties (Bennett et al., 2007; Curran, 1997; Feeney et al., 2002; Howard et al., 2004). Controlling for them, as occurs in the SRTT, is therefore critical to isolate aging-related differences specific to sequence learning.
1.1. Neural correlates of motor sequence learning and consolidation in young adults
Decades of research using motor sequence learning paradigms have produced insight into the structural and functional neural networks that support motor skill learning and consolidation in young adults (Albouy, Sterpenich, et al., 2013; Curran, 1997; Doyon et al., 2018; Doyon et al., 2009; Hikosaka et al., 2002; Janacsek et al., 2020; Karni et al., 1995; King, Hoedlmoser, et al., 2017; Penhune & Steele, 2012). Such studies have demonstrated that motor skill improvements rely on consolidation-related changes to activation within, and functional interactions between, the hippocampo-cortical, striato-cortical, and cerebello-cortical systems (Doyon et al., 2018; Hardwick et al., 2013; Janacsek et al., 2020; Lohse et al., 2014) (See Figure 1).
Fig. 1. Motor sequence encoding and consolidation timeline.
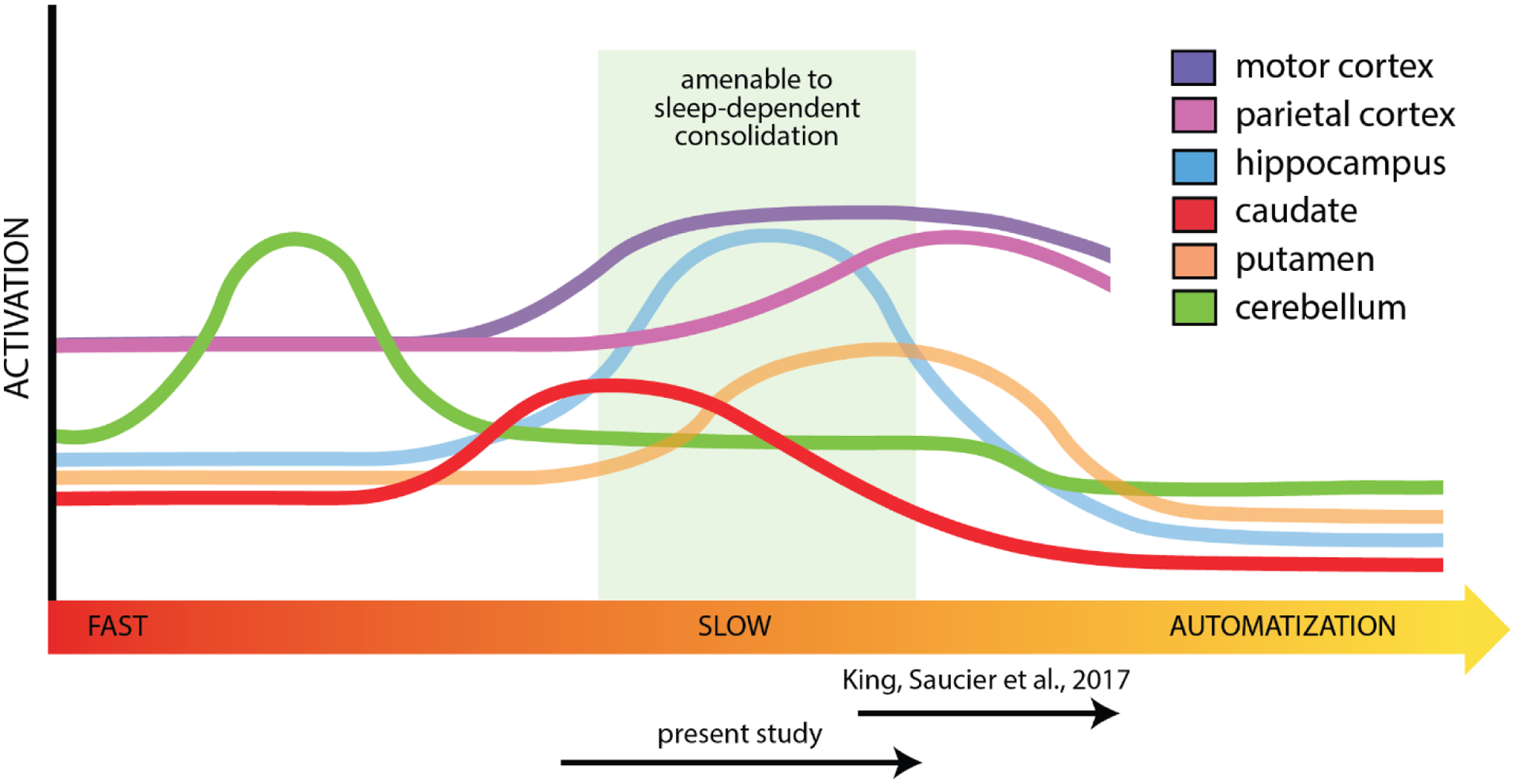
Fast learning is marked by engagement of the cerebellum and caudate. As slow learning emerges, the role of hippocampal and motor cortical regions increases. Engagement of the hippocampus and putamen are thought to be a minimum requirement for sleep-dependent consolidation (green region). Prior work using the MSL task is apt to tap later explicit learning processes as the participants are explicitly told the sequence (King, Saucier, et al., 2017). In the explicit SRTT, participants are aware there is a sequence but it is acquired through practice (present study), which is expected to probe earlier in the consolidation timeline.
Initial experience with a motor sequencing task is characterized by fast learning: adjusting movements according to sensory input to produce accurate motor output. Fast learning involves improved error correction, development of internal models, and more efficient selection, execution, and binding of responses into unified chunks (Boutin et al., 2013; Graybiel & Grafton, 2015; Lungu et al., 2014; Rosenbaum et al., 1987; Wymbs et al., 2012). During the subsequent slow learning phase, incremental performance gains occur with continued practice (Albouy et al., 2008; Doyon et al., 2018). Performance gains across both fast learning and slow learning result from consolidation-related changes in activity within, and functional connectivity between, the hippocampus, striatum (i.e., caudate, putamen), and motor cortical regions (i.e., primary motor cortex, premotor cortex, supplementary motor area [SMA], pre-SMA, anterior cingulate cortex, posterior parietal cortex) (Albouy et al., 2008; Albouy, Sterpenich, et al., 2013; Debas et al., 2014; Doyon et al., 2018; Penhune & Doyon, 2002; Penhune & Steele, 2012) Across fast learning and into slow learning, there is a competitive balance between hippocampo-cortical allocentric (i.e., in an external frame of reference) motor strategies, and striato-cortical egocentric (i.e., in an internal frame of reference) motor strategies, managed by the prefrontal cortex (Albouy et al., 2013).
Allocentric motor strategies involve effortful focus on explicit and spatial aspects of task performance. As a result, they recruit attentional, cognitive control, and pattern searching networks to form spatial maps and abstract representations of the sequence (Ashe et al., 2006). While allocentric representations are forming, hippocampo-prefrontal engagement suppresses activity in striato-cortical networks (Albouy et al., 2013; Destrebecqz et al., 2005; Narayanan & Laubach, 2006) until an appropriate map of the sequence is developed. Once a spatial map is developed, striato-cortical activity gradually increases, supporting a more internal, automatized, and rigid representation of the motoric features of the sequence (Doyon et al., 2009; Hikosaka et al., 2002; Penhune & Steele, 2012) executed egocentrically, that requires less cognitive control and less attentional resources.
With continued practice across slow learning, activity in the putamen and motor cortical regions increases, and activity in the caudate and cerebellum decreases, continuing to shift motor performance from an allocentric strategy to an egocentric strategy (G. Albouy et al., 2015; Grafton et al., 1998; Hikosaka et al., 1999; Penhune & Steele, 2012; Willingham & Goedert-Eschmann, 1999). As asymptotic performance is achieved during the subsequent automatization phase, long-term representations of the motor sequence are increasingly distributed throughout primary motor and parietal cortical memory networks for use during retrieval or further practice, while activity in the putamen and cerebellum decreases (Doyon et al., 2018). Further automatization increases execution efficiency and decreases the need for effortful control over task performance, and is accompanied by gradual performance related decreases in neural activity in motor cortical and parietal brain networks (Picard & Strick, 1996; Poldrack et al., 2005; Toni et al., 1998; Wu et al., 2004).
During sleep, allocentric aspects of motor skills are enhanced but egocentric aspects are not (Albouy et al., 2015; Albouy, Fogel, et al., 2013; Cohen et al., 2005; Pace-Schott & Spencer, 2013). Following sleep compared to wakefulness, there is a sleep-dependent increase in allocentric encoding brain regions in the associative striatum (i.e., anterior caudate and anterior putamen) and motor cortical regions, but not egocentric regions in the sensorimotor striatum (i.e., posterior lateral caudate and posterior lateral putamen) (Albouy et al., 2015; King, Hoedlmoser, et al., 2017; Penhune & Steele, 2012). Further, consolidation during sleep compared to consolidation during awake periods is supported by different changes in the activity and connectivity within hippocampo-cortical and striato-cortical brain networks (Albouy et al., 2008; Albouy, Sterpenich, et al., 2013; Barakat et al., 2013; Debas et al., 2010, 2014; Fogel et al., 2014; Walker et al., 2005). As a result, task-related hippocampal and associative striatal (i.e., anterior caudate and anterior putamen) engagement has been suggested as a prerequisite for sleep-dependent motor sequence consolidation (Binder et al., 2012; Inostroza et al., 2013; Kelemen et al., 2014; Nemeth et al., 2010; Spencer et al., 2006), such that insufficient engagement in these regions during encoding may preclude the emergence of sleep-dependent motor sequence consolidation and contribute to heterogeneity in behavioral findings across motor tasks (King, Hoedlmoser, et al., 2017).
In young adults, sleep-related enhancement of motor skills is supported by elements of sleep physiology (Albouy, Fogel, et al., 2013; Barakat et al., 2011; Barakat et al., 2013; Bottary et al., 2016; Fogel et al., 2014; Korman et al., 2007; Nishida & Walker, 2007; Tamaki et al., 2013; Tucker & Fishbein, 2009; Vahdat et al., 2017; Walker et al., 2002; Wilhelm et al., 2011; Witt et al., 2010). For example, at the macrostructure level greater time spent in NREM2 sleep predicts increased consolidation of allocentric aspects of an acquired motor sequence (Witt et al., 2010). Furthermore, at the microstructure level neural oscillations in the delta (0.5–4Hz), theta (4–8Hz), and sigma (12–15Hz) frequency ranges correlate with post-sleep performance improvements on motor sequence learning tasks (Menicucci et al., 2020; Nishida & Walker, 2007; Tamaki et al., 2013; Tucker & Fishbein, 2009). Interestingly, sleep spindle activity in the sigma range has been specifically linked to functional changes in striato-cortical brain activity following motor sequence learning, suggesting an active role of sleep physiology in motor memory consolidation (Barakat et al., 2013; Fogel et al., 2014; Vahdat et al., 2017).
1.2. Aging-related changes in motor sequence learning and consolation
Like young adults, older adults are able to encode novel motor sequences (Brown et al., 2009; Daselaar et al., 2003; Fraser et al., 2009; Howard & Howard, 1989, 1992; King, Saucier, et al., 2017; Nemeth et al., 2010; Nemeth & Janacsek, 2011; Rieckmann & Bäckman, 2009; Romano et al., 2010; Shea et al., 2006; Wilson et al., 2012). However, older adults demonstrate deficits in both the rate and magnitude of fast learning, deficits that worsen with increased task complexity, such as intervening random elements within sequences (Bennett et al., 2007; Curran, 1997; Feeney et al., 2002; Howard et al., 2004), and when holding the sequence in working memory during task performance (i.e., MSL task; Howard & Howard, 2001; Willingham et al., 2002; Willingham & Goedert-Eschmann, 1999). Further, both middle-aged and older adults exhibit a fast-learning related performance deficit during the first few retest trials following an offline interval (Tucker et al., 2011), which is similar to a deficit observed in young adults performing a difficult bi-manual finger tapping task (Kuriyama et al., 2004; Manoach et al., 2004).
Aging-related deficits in motor sequence learning are associated with changes in the structural and functional neural networks supporting motor skill learning (Aizenstein et al., 2006; Daselaar et al., 2003; Fogel et al., 2014; Hardwick et al., 2013; King et al., 2013). In particular, deficits in executive function (Howard & Howard, 2001; Rieckmann & Bäckman, 2009; Salthouse, 1996), degradations in striato-cortical networks (Rieckmann et al., 2010; Rieckmann & Bäckman, 2009), and decreased working memory capacity (Bo et al., 2009; Bo & Seidler, 2009) negatively affect the initial acquisition of motor sequences in older adults. To overcome these deficits, it is thought that the hippocampus plays a compensatory role during sequence learning in older adults (King et al., 2013; Rieckmann et al., 2010; Rieckmann & Bäckman, 2009). In line with this theory, young adults show increased striatal and decreased hippocampal activity during sequence compared to random blocks, while older adults show increased activation in both regions (Albouy et al., 2008; Rieckmann et al., 2010).
Additionally, age-related deficits in motor sequence consolidation are associated with aging-related changes in sleep physiology (Aizenstein et al., 2006; Brown et al., 2009; Fogel et al., 2014; Hardwick et al., 2013; King et al., 2013; Nemeth & Janacsek, 2011; Pace-Schott & Spencer, 2013; Rieckmann et al., 2010; Roig et al., 2014; Spencer et al., 2007; Wilson et al., 2012). Oscillatory neural activity during sleep changes with typical aging in a frequency- and region-dependent manner; high-density polysomnography demonstrates declines in absolute delta, theta, and sigma activity that are progressively more frontocentrally focused with increasing frequency, as well as increased relative sigma over lateral central regions and relative delta over far frontal regions (Sprecher et al., 2016; Fitzroy et al., under review). These declines contribute to aging-related changes in motor learning, as reduced consolidation of memories over sleep in older adults has been associated with diminished sleep spindles (Fogel et al., 2014) and sigma power (Bottary et al., 2016). Moreover, in older adults decreased activation in the hippocampus, cerebellum, and motor cortical regions has been observed following post-learning sleep, whereas in young adults increased activity is observed in these regions (Fogel et al., 2014). These changes in during-task brain activation relate to sleep physiology in a manner moderated by age; in older adults, sleep spindles positively correlate with greater changes in cerebellum activity, whereas in younger adults, sleep spindles positively correlate with greater changes in putamen and related motor cortical areas (Fogel et al., 2014).
Interestingly, aging-related declines in sleep-dependent motor skill consolidation may be remediated by increased encoding strength. Although older adults with low encoding performance on a motor learning task do not show sleep-dependent performance benefits, older adults with high encoding performance do exhibit consolidation benefits from sleep (Hauptmann et al., 2005; King, Hoedlmoser, et al., 2017; King, Saucier, et al., 2017; Kuriyama et al., 2004; Muehlroth et al., 2020; Sonni & Spencer, 2015; Stickgold, 2009; Tucker et al., 2011; Wilhelm et al., 2012; Wilson et al., 2012). Older adults with low encoding performance may prolong the engagement of early fast-learning brain regions, like cerebellum and caudate, preventing the engagement of the later slow learning brain regions necessary to support consolidation during subsequent sleep like the hippocampus and the putamen. Indeed, additional visuomotor training prior to a motor sequencing paradigm remediated fast learning deficits and prompted greater activation in the cerebellum, putamen, and parietal cortex, producing sleep-related maintenance of motor skills, in older adults (King, Saucier, et al., 2017). However, the neural correlates necessary to support motor sequence consolidation during sleep remain speculative, and the work by King, Saucier, et al. (2017) may be limited by lacking a young adult control group, and by between-group rather than within-group comparisons of consolidation over sleep versus wake (Rickard & Pan, 2017).
1.3. The present study
The purpose of this study was to examine how aging-related changes in neural activity during encoding and subsequent sleep physiology affect sleep-dependent motor sequence consolidation. We hypothesized that aging-related deficits in fast learning extend the engagement of the cerebellum and caudate during encoding, preventing activation of allocentric slow learning structures like the hippocampus and putamen which are necessary for sleep-dependent consolidation to occur. Further, we hypothesized that overcoming aging-related fast-learning deficits with additional visuomotor training prior to a motor sequence learning task would facilitate sleep-dependent motor memory consolidation in older adults. To this end, young and older adults were trained to an equal criterion on the visuomotor elements of the SRTT before they performed an explicit variant of the SRTT in the MRI. Neuroimaging data were collected during SRTT performance prior to and following within-subject nap and wake intervals in both young and older adults, along with high-density polysomnography during the nap.
We predicted that lower encoding activity in cerebellum and caudate, and higher encoding activity in hippocampus, putamen, and motor cortical regions would be associated with increased performance at the end of encoding, and with higher levels of sleep-dependent consolidation. Following previous associations of sleep neuroscillatory activity in the delta (Menicucci et al., 2020; Tamaki et al., 2013), theta (Tucker & Fishbein, 2009), and sigma (Barakat et al., 2013; Fogel et al., 2014; Nishida & Walker, 2007; Tamaki et al., 2013) frequency bands with over-sleep performance improvements on motor sequence learning tasks, we predicted increased activity in these bands would lead to higher levels of sleep-dependent consolidation in both young and older adults. Further, we predicted the relationship between sleep neuroscillatory activity and sleep-dependent consolidation in older adults would be smaller over scalp regions where aging-related declines in sleep EEG amplitude are largest.
2. Material and methods
2.1. Participants
Participants were 17 young adults (8 female) ranging from 18–31 years (M = 22.71, SD = 3.51) and 18 older adults (8 female) ranging from 58–75 years (M = 65.39, SD = 5.80). Data from one additional young adult was collected but excluded from analysis due to the participant performing the task incorrectly. The present data are a subset of those reported in Fitzroy, Kainec, and Spencer (under review), using only the participants who were given different sequences for the SRTT in the nap and wake conditions. Participants were right-handed, excluded for presence of self-reported neurological, psychiatric, cardiac, or sleep disorders; medications or supplements affecting sleep; excessive napping, caffeine, or alcohol consumption; and implanted metal or other contraindications for the MRI environment. Older adult participants were free of self-reported cognitive decline.
2.2. Questionnaires
The Pittsburg Sleep Quality Index (PSQI; Buysse et al., 1989) was used to assess habitual sleep over the previous 30 days, the Epworth Sleepiness Scale (ESS; Johns, 1992) measured participants’ typical daytime sleepiness, and the Stanford Sleepiness Scale (SSS; Hoddes et al., 1973) was used to assess acute subjective sleepiness at multiple times during the experimental protocol. The Morning-Eveningness Questionnaire (MEQ; Horne & Ostberg, 1976) assessed participants’ chronotype. Depressive symptoms were assessed using the Beck Depression Inventory (BDI; Beck et al., 1996; Steer & Clark, 1997). Lastly, in-house questionnaires assessed daytime activities affecting sleep (e.g., caffeine intake, exercise, and prior night sleep), dexterity (e.g., musicianship, hand skills), and self-reported sequence awareness.
2.3. Procedure
All procedures were approved by the Institutional Review Board at the University of Massachusetts, Amherst. Participants completed two experimental sessions (Figure 2A) scheduled one week apart (except for one young adult, scheduled two weeks apart). Participants were instructed to get quality sleep the night before experimental sessions and to abstain from caffeine and alcohol consumption on experimental days and the prior night.
Fig. 2. Study procedures and serial reaction time task.
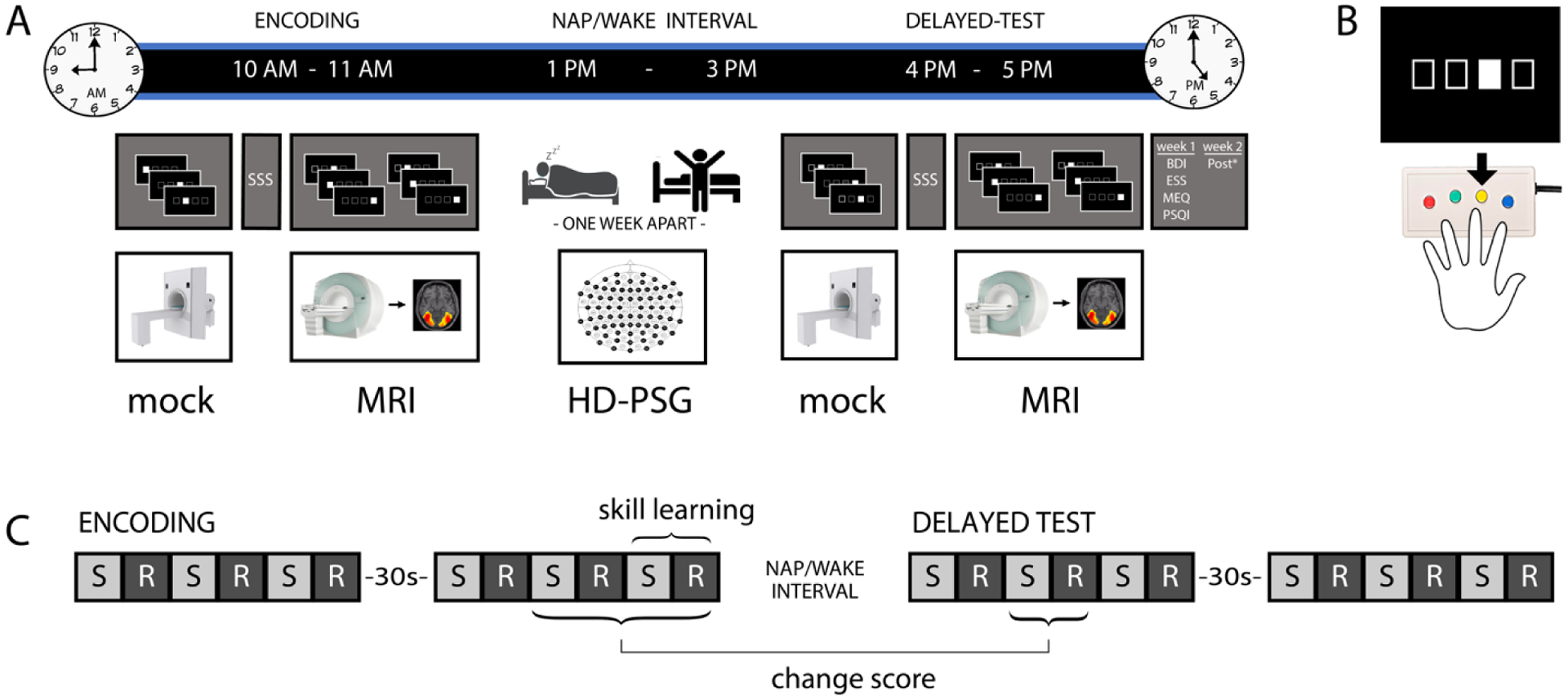
A) On each experimental day, participants first completed the encoding phase of the SRTT, followed by a nap or wake interval (within-subject, separated by 1 week, order counterbalanced), then completed the delayed test phase of the SRTT. During each phase of the SRTT, participants practiced the task in the mock MRI to criterion and completed the SSS prior to beginning the experimental MRI session. Additional questionnaires and sequence awareness assessments were completed after the delayed test phase of the SRTT on each day (*Post: in-house questionnaires regarding dexterity). B) The SRTT utilized a four-button MRI-compatible response box and images were presented on a screen visible to the participant, who was supine in the scanner. Participants were instructed to respond quickly and accurately to stimuli (the location of the white box in a row of four boxes). C) In the MRI scanner, experimental blocks alternated between sequential (S) and random (R) stimulus order. Twelve blocks with 40 stimuli each were performed in each task phase. Skill learning was assessed as the performance in a given sequence block relative to the following random block, and across-interval performance was assessed as a change score comparing the second block-pair of the delayed test phase to the average of the final two block-pairs of the encoding phase.
For each session, participants arrived at the lab at approximately 9 AM. After providing informed consent and undergoing in-person MR safety screening, participants were given SRTT instructions and positioned in a mock MRI scanner to familiarize themselves with the physical and auditory aspects of the MRI environment, and to practice the visuomotor aspects of the SRTT. During this practice, all stimuli were presented in random order. Participants were required to reach criterion performance of 100% on two consecutive random blocks in the mock scanner while simulated scanner audio was playing for practice to complete. Participants then completed the SSS, were positioned in the MRI scanner, and underwent a high-resolution structural (T1) brain scan. After the structural scan was complete, participants performed the encoding phase of the SRTT while functional MRI (fMRI) images were collected.
After completing the encoding phase, participants were given a short break to stretch and use the restroom, followed by 30 minutes for lunch (in the lab). Next, high-density polysomnography (HD-PSG) was applied. At 1 PM, participants were brought to a private bedroom for the nap or wake opportunity (order counterbalanced). In the nap condition, the room was darkened and participants were instructed to nap. In the wake condition, participants watched a (non-arousing) film of their choice on a wall-mounted television while lying in the bed. At 3 PM, participants were awakened (nap condition), had the HD-PSG setup removed, and were given the chance to wash their hair. Participants returned to the mock MRI scanner to complete random blocks of the SRTT, continuing until the performance criterion (100% on two consecutive blocks with simulated scanner audio) was reached. Participants then completed another SSS before being positioned in the MRI scanner for another high-resolution structural brain scan (DTI). Finally, the delayed test phase of the SRTT was completed while fMRI images were collected.
After completing the delayed test phase, participants exited the scanner and completed the remaining questionnaires. The PSQI, ESS, MEQ, BDI were completed on the first experimental day, and the dexterity (e.g., musicianship, hand-skills) questionnaire was completed on the second experimental day. Information regarding daytime activities affecting sleep (e.g., caffeine intake, exercise, and prior night sleep) and self-assessed sequence awareness was collected on both experimental days.
2.4. Serial reaction time task
Participants performed an explicit variant of the SRTT, in which they were made aware there was an underlying pattern in the stimulus sequence, but not directly informed what that pattern was. Specifically, participants were informed that cues would be sequential during the indicated blocks and instructed to notice and learn any patterns they could. Participants were instructed to respond quickly and accurately using a four-button MRI-compatible response pad (Current Designs 932; Figure 2B). Stimuli were presented on a computer screen positioned behind the scanner bore, made visible to the participant via an angled mirror mounted to the head coil directly in front of their eyes.
Movements were cued when one of four horizontally arranged boxes filled white (Figure 2B). Participants were instructed to press the button that corresponded to the location of the stimulus. All responses were made using the non-dominant (left) hand. Cues appeared at a regular interval (1000 ms) either according to a regular repeating eight-item sequence (sequence blocks) that did not contain repeats, trills, or runs of three or more (3-1-4-2-1-3-2-4 or 2-3-1-2-4-1-3-4; session order counterbalanced across participants), or randomly with constraint (random blocks), with 40 cues (trials) in each block. Cue order in random blocks was constrained to have no repeats, and such that trial transitional probabilities were either 0.33 (50%), 0.50 (25%), or 1.00 (25%). Participants completed twelve blocks in each phase of the SRTT, organized in six pairs of alternating block types (sequence, random), with a 30 second rest period between the third and fourth block-pairs (Figure 2C). Prior to the start of each block, the type of block was indicated to the participant by displaying the word “PATTERNED” or “RANDOM” for 1 second prior to the first trial.
2.5. High-density polysomnography
HD-PSG data were acquired with reference to FCz during nap and wake intervals using a custom 129-channel cap (Easycap, Herrsching, Germany) and BrainAmp MR plus amplifiers (Brain Products GmbH, Gilching, Germany). The HD-PSG montage consisted of 123 scalp EEG electrodes placed at 10–10 and intermediary locations, 4 electrooculogram (EOG) electrodes placed beside and below the eyes, and 2 electromyogram (EMG) electrodes placed over the zygomatic major and mylohyoid muscles. Data were recorded using a hardware bandpass of 0.1–1000 Hz and digitized at 500 Hz using BrainVision Recorder (Brain Products GmbH, Gilching, Germany). Scalp impedances were reduced below 20 kΩ using high-chloride abrasive gel before the nap and wake intervals.
2.6. MRI data acquisition
Whole-brain images were collected with a Siemens 3T MAGNETOM Skyra scanner (Siemens Healthcare, Erlangen, Germany) and a 20-channel head coil. During functional scans, after acquiring automated scout images and performing shimming procedures to optimize field homogeneity, 372 blood oxygenation level dependent (BOLD) fMRI image volumes were acquired with an interleaved T2*-weighted echo-planar imaging (EPI) sequence. A total of 60 slices per volume were obtained with the following parameters: TR = 1500 ms, TE = 30.0 ms, FA = 73°, FoV = 68 × 68 mm2, voxel size 3.0 × 3.0 × 2.4 mm3, acceleration factor slice = 3. High-resolution structural scans of 3-dimensional T1-weighted multiecho magnetization prepared rapid acquisition gradient-echo (MPRAGE) sequences were collected in the sagittal plane (TR = 1810 ms, TE = 2.26 ms, FA = 9°, 224 slices, FoV = 224 × 256 × 256 mm2, voxel size 0.8 × 0.797 × 0.797 mm3). Analyses of structural scans in relation to behavioral performance and sleep physiology are reported in a parallel manuscript (Fitzroy et al., under review).
2.7. Behavioral data analysis
For stimulus cues to which multiple responses were recorded, reaction time for only the final response was used (e.g., Cousins et al., 2014). To minimize any potential effects of predictable elements within random blocks (Bennett et al., 2007; Howard et al., 2004; Nemeth & Janacsek, 2011), reaction times were excluded for trials in random blocks that formed trills (e.g., 1-3-1), runs of three or more (e.g., 1-2-3 or 3-2-1), or had transitional probabilities greater than 0.33. Moreover, because the stimuli were presented at a fixed isochronous interval, correct responses could become temporally predictive (i.e., occur prior to stimulus presentation; Repp, 2005; Repp & Su, 2013); to account for this possibility, correct responses were accepted if they occurred between 300 ms prior to trial onset and 1000 ms after trial onset. Due to software limitations, responses that occurred in the 40 ms immediately prior to trial onset were not recorded or evaluated. The acceptable response window was expanded to 800 ms before to 1000 ms after trial onset in two young adults for whom visual inspection of response data suggested high propensity to respond predictively. In these participants, responses earlier than 300 ms prior to trial onset comprised only 0.016% and 0.003% of all correct responses.
All reaction time analyses were performed using reaction times from correct trials only, and reaction times were median-collapsed within each block prior to performing inferential statistics. Following Robertson (2007), skill learning was defined as the median reaction time for correct trials in each sequence block subtracted from the following random block. Behavioral performance at the end of encoding was quantified as the average performance over the final two block-pairs of the encoding phase (Nishida & Walker, 2007; Walker et al., 2002). Behavioral performance during the delayed test was quantified as performance during the second block-pair of the phase, to account for both the performance lag previously reported in older adults following an offline interval, and potential re-learning (Kuriyama et al., 2004; Manoach et al., 2004; Tucker et al., 2011). Change scores were calculated for random reaction time, sequence reaction time, and skill learning, as the difference over the nap or wake interval (i.e., delayed test – end of encoding; Figure 2C).
Changes in behavioral performance across the encoding phase were assessed using mixed-design ANOVAs with within-subjects factors condition (nap, wake), block type (BT: sequence, random), and block-pair (BP: 1 – 6), and between-subjects factor age (young, older). Changes in behavioral performance over the nap/wake interval were assessed using mixed-design ANOVAs with within-subjects factors condition (nap, wake), block type (BT: sequence, random), and phase (end of encoding, beginning of delayed test), and between-subjects factor age (young, older). Interactions among factors in these omnibus ANOVAs were followed with separate ANOVAs at each level of the interacting factors, in the order block type, age, condition, phase. Two participants (one young adult, one older adult) had one sequence block each in which no correct answers, and therefore no valid response times, were recorded (sequence blocks 1 and 2 during wake encoding, respectively). These missing reaction time data were imputed from the median reaction times of the surrounding blocks prior to conducting the during-encoding reaction time ANOVAs. Whenever Mauchly’s Test indicated that the assumption of sphericity had been violated for ANOVA factor comparisons with more than two levels, Greenhouse–Geisser corrected degrees of freedom were used to assess statistical significance.
The relationships between behavioral performance at the end of encoding and subsequent sleep-dependent consolidation were assessed using multiple linear regression predicting change in behavioral performance over the nap or wake interval as a function of end-of-encoding behavioral performance. The regression models predicting change in random or sequence block reaction time included predictors for both random and sequence reaction times from the end of encoding, along with their interactions with condition and age. The regression models predicting change in skill learning included skill learning from the end of encoding and its interactions with condition and age. Interactions among factors in the regressions were followed with separate regressions at each level of the interacting factors, in the order age, condition. Behavioral performance analyses were completed in R (www.r-project.org; R Core Team, 2020) using addon packages rstudio (RStudio Team, 2020), ggplot2 (Wickham, 2016) and gridExtra (Baptiste, 2017).
2.8. High-density polysomnography analysis
To facilitate sleep scoring, HD-PSG data were first filtered offline (0.3–35 Hz EEG, 10–70 Hz EMG) and re-referenced to the contralateral mastoid. Sleep stages were visually scored in 30 sec epochs according to American Academy of Sleep Medicine criteria (Iber et al., 2007) using the Hume toolbox (Saletin & Greer, 2015). Next, to facilitate quantification of oscillatory neural activity in typical sleep frequency bands, the original unfiltered HD-PSG data were re-referenced to the averaged mastoid and bad channels were interpolated. Amplitude envelopes were then extracted from EEG recordings in the delta (0.5–4 Hz), theta (4–8 Hz), and sigma (12–16 Hz) frequency bands using the filter-Hilbert method (Freeman, 2004). Bandpass filter designs were frequency band specific: delta used a 2nd order Butterworth IIR filter, theta used a Chebyshev Type II filter requiring ≥ 20 dB attenuation in the stopband (outside 4–9 Hz) and ≤ 2 dB attenuation in the passband (4.5–8 Hz), and sigma used a 164th order FIR filter after demeaning the data.
Artifactual sections of bandpass filtered EEG data were identified automatically for each electrode using band-specific voltage thresholds (delta: ±250 μV; theta/sigma: ±75 μV). Artifact-free regions of the band-specific amplitude envelopes recorded during the first 60 minutes of NREM2 or NREM3 sleep were averaged into non-overlapping 20 second epochs, then averaged again across epochs to give a single amplitude measure per combination of frequency band, electrode, and participant. Absolute and topographically relative (i.e., divided by the across-channel mean) amplitudes were averaged over frontal and occipital ROIs for delta and theta activity, and frontal and lateral ROIs for sigma activity (Figure 3A, B). These ROIs were selected based on previous reports of aging-related amplitude differences using nonparametric cluster-based permutation testing (Sprecher et al., 2016; Fitzroy et al., under review). Aging-related changes in EEG amplitude were assessed separately within each frequency band and scalp ROI using independent samples t-tests assuming unequal variance. EEG amplitude analyses were completed using a combination of EEGLAB (Delorme & Makeig, 2004), ERPLAB (Lopez-Calderon & Luck, 2014), Fieldtrip (Oostenveld et al., 2011), and in-house MATLAB software (PSGpower; https://osf.io/qsryf/).
Fig. 3. Aging-related differences in sleep microstructure.
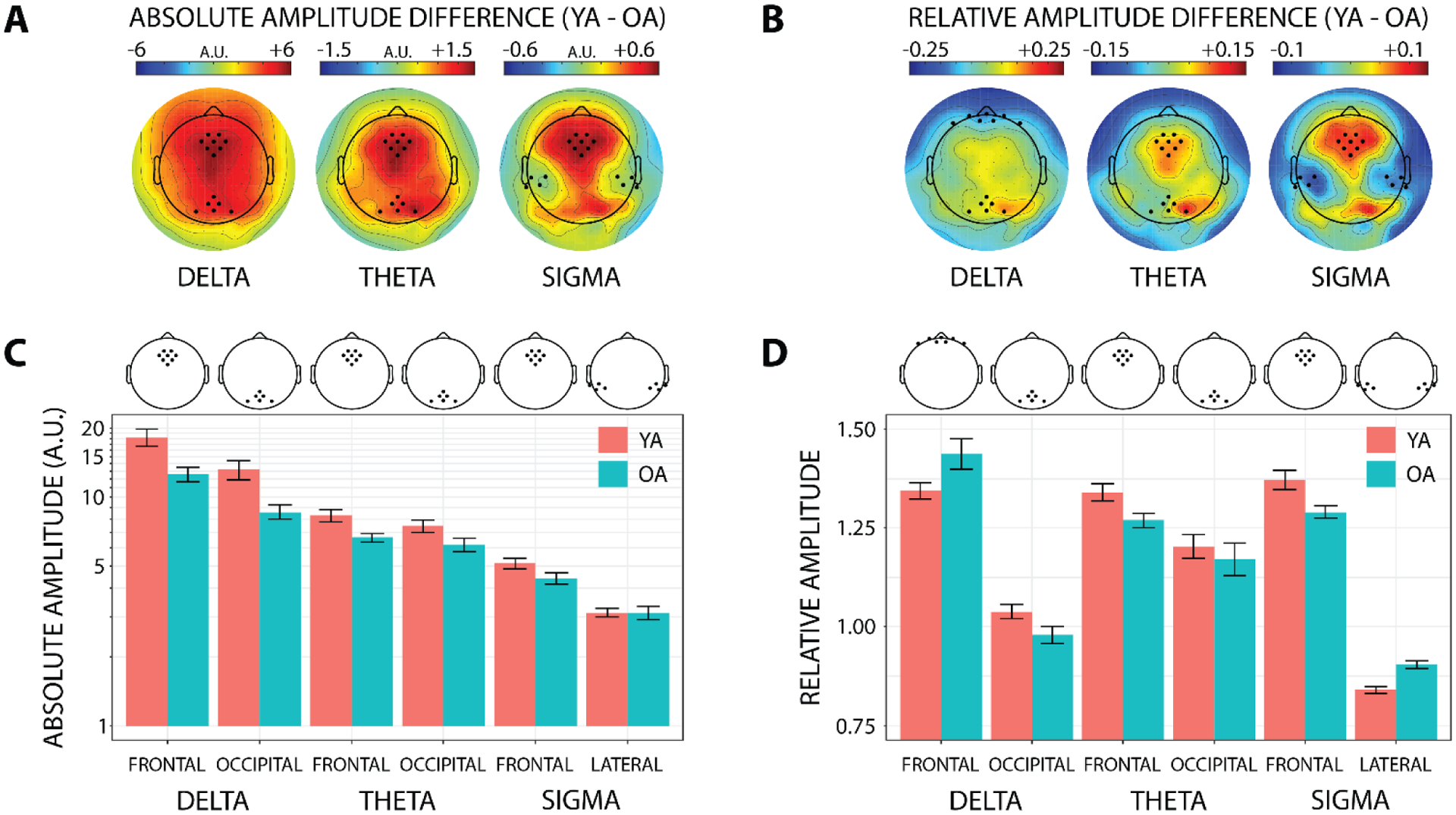
A) Aging-related differences in absolute EEG amplitude are shown across the entire scalp, separately for the delta (0.5–4 Hz), theta (4–8 Hz), and sigma (12–16 Hz) frequency bands. The frontal and occipital delta/theta ROIs and frontal and lateral sigma scalp ROIs used for statistical analyses are illustrated in bold. Absolute EEG amplitude is plotted in arbitrary units (AU) because the analytic amplitude is unitless, but in the context of scalp EEG these units are akin to μV. YA – young adults; OA – older adults. B) Aging-related differences in topographically relative (i.e., divided by the all-electrode mean) EEG amplitude is shown across the entire scalp separately for the same frequency bands as in (A), with scalp ROIs illustrated in bold. Relative amplitude is unitless because it is a ratio. C) Average absolute EEG amplitude within each scalp ROI is plotted on a logarithmic scale separately for each frequency band and age group. D) Average topographically relative EEG amplitude within each scalp ROI is plotted separately for each frequency band and age group.
2.9. fMRI data analysis
Functional images underwent the following preprocessing stages: fieldmap-based distortion correction, realignment, co-registration with the T1 structural image, normalization into Montreal Neurological Institute (MNI) space, and smoothing with an 8 mm full-width half-maximum Gaussian kernel. A temporal highpass filter with 128-second cutoff was applied to remove low-frequency signal drift, and serial correlations from aliased biorhythms in the time series were adjusted for with an autoregressive AR 1 model. The Artifact Detection Toolbox (ART, http://gablab.mit.edu) detected motion spikes in the functional time series data. These motion artifact data, along with movement parameters from the realignment procedure (x, y, z, roll, pitch, and yaw), were used as regressors of non-interest in the first-level analysis. Participants had minimal movement within the functional scan of each SRTT phase (below 3 mm and 3° for all participants). BOLD images were processed and analyzed using Statistical Parametric Mapping (SPM12; Wellcome Trust Centre for Neuroimaging, http://www.fil.ion.ucl.ac.uk) in MATLAB v2018b (Mathworks Inc, Natick, Massachusetts, USA).
Although our task employed a block design, we modeled our conditions using individual event-related task regressors for each participant and SRTT phase to maximize the contrast between sequence and random blocks by excluding ambiguous trials. Specifically, our random task regressors omitted any trials that formed trills, runs of three or more, or had transitional probabilities greater than 0.33 to account for predictability (Bennett et al., 2007; Howard et al., 2004), and our sequence task regressors omitted the first sequence presentation (first 8 trials) in each block to account for within-block task adaptation effects and across-block carryover effects (Kuriyama et al., 2004; Manoach et al., 2004; Tucker et al., 2011). To equate signal-to-noise ratio across sequence and random blocks within each participant and SRTT phase, trials were then randomly removed from the task regressor containing more trials until the pair of sequence and random regressors contained the same number of trials. To obtain sequence-specific brain activation while controlling for visual, motor, and other confounding aspects of task performance (Janacsek et al., 2020), the sequence condition was contrasted with the random condition after balancing the task regressors.
In the first level analysis, we analyzed whole-brain activity during sequence blocks relative to random blocks with regard to within-subjects factors condition (nap, wake) and phase (encoding, delayed test). For each participant, we generated contrast images of the sequence block > random block comparison for each of the 4 levels of condition-by-phase (nap encoding, nap delayed, wake encoding, wake delayed).
Each sequence block vs. random block contrast image from the first level was entered into a second level analysis across subjects, which employed a general linear model with random effects to identify the brain regions that differed with between-subjects factor age (young, older). Four two-sample t-test designs were employed to detect aging-related brain activation differences in the sequence vs. random contrast associated with the encoding and delayed test phases of the nap and wake conditions. We performed the second level analyses on both increased and decreased activation for the sequence relative to random blocks to investigate whether sequence-specific learning was associated with patterns of brain excitation or suppression. The second level analyses used the order of the nap and wake conditions as a covariate.
To test whether the group differences in brain activation were more different than would be expected given chance, a permutation-based voxel-wise nonparametric test (5000 permutations) was performed for each of the four comparisons in the second level analyses using the threshold-free cluster enhancement (TFCE) toolbox (http://dbm.neuro.uni-jena.de/tfce/; r95). The TFCE method can estimate voxel-wise values representing the amount of cluster-like local spatial support without an arbitrary cluster-forming threshold as in the traditional approach (Smith & Nichols, 2009). The resulting TFCE maps were thresholded at a family-wise error (FWE) corrected p < 0.05.
To reduce dimensionality and facilitate individual-differences analyses comparing fMRI activation to behavioral and EEG parameters, we also conducted a region of interest (ROI) analysis. Five ROIs (inferior parietal cortex [IPC], hippocampus, parahippocampal gyrus, caudate, and putamen) were anatomically defined using the Wake Forest University Pick Atlas (http://fmri.wfubmc.edu/software/PickAtlas). Two additional ROIs in premotor cortex (BA6) and cerebellum were defined as 10 mm radius spheres, centered on the peak activations of the within-group whole brain conjunction analysis of brain activation for sequence vs. random. Contrast beta weights were averaged separately within these seven ROIs and extracted for further statistical analyses using the region of interest extraction (REX) toolbox (http://web.mit.edu/swg/software.htm).
2.10. Comparisons among brain activation during encoding, sleep physiology, and motor sequence learning and consolidation
To assess the role of brain activation during encoding in motor sequence learning and subsequent sleep-dependent consolidation, we performed a series of regression-based analyses using the beta weights of the sequence > random contrast during the pre-nap encoding phase averaged within the seven ROIs to predict: 1) behavioral performance at the end of the pre-nap encoding phase, 2) across-nap changes in behavioral performance, and 3) EEG amplitude in the delta, theta, and sigma bands during the nap. Additionally, to assess the role of sleep physiology in motor sequence consolidation, we performed a series of regression-based analyses using EEG amplitude in the delta, theta, and sigma bands during the nap to predict: 1) across-nap changes in behavior, and 2) across-nap changes in the beta weights of the sequence > random contrast averaged within the seven ROIs.
For each of these analyses, we first tested whether age moderated the predictive relationship using interaction models of the general form YOutcome = β0 + β1(Age) + β2(predictor) + β3(Age*predictor). When no interaction with age was present, the predictive relationship was assessed across age groups using a simple linear model of the general form YOutcome = β0 + β1(Age) + β2(predictor); when an interaction with age was present, the relationship was assessed separately in young and older adults using the same simple linear model structure. Coefficient p-values in the interaction and simple models were adjusted within each outcome measure using the Benjamini-Hochberg procedure (Benjamini & Hochberg, 1995) to hold false discovery rate at 0.05 while separately testing multiple predictors.
Second, we tested whether aging-related differences in the outcome measure were mediated by aging-related changes in the predictor using model-based causal mediation analysis (Imai et al., 2010; Tingley et al., 2014). For each mediation analysis, the magnitude and reliability of the total effect (TE), average causal mediation effect (ACME), and average direct effect (ADE) were empirically estimated using a bootstrap procedure with 1000 iterations. It is actively debated whether a significant TE should be considered a criterion for assessing mediation (Agler & De Boeck, 2017; Baron & Kenny, 1986; MacKinnon et al., 2000; Rucker et al., 2011); in the present study, we opted for the more conservative approach of requiring significance of both TE and ACME as criteria for mediation. For significant mediation results (pTE < 0.05 and pACME < 0.05), the proportion of the age TE on the outcome measure mediated by the predictor (PropMediated) was calculated as (ACME + ADE) / ACME.
3. Results
3.1. Questionnaire data
Depressive symptoms (BDI), typical daytime sleepiness (ESS), and levels of habitual sleep quality (PSQI) did not differ between young and older adults. Differences in chronotype (see Table 1) indicated that older adults were typically morning types (M = 59.08, SD = 10.30) while young adults were intermediate types (M = 47.53, SD = 11.21). For self-reported acute sleepiness (SSS), there were no main effects of group or condition, nor interactions of group and condition, across the four samples (ps > 0.3; Table 1).
Table 1. Questionnaire data.
Group means and standard deviations of questionnaire data.
| Young Adults | Older Adults | Age Differences | |||
|---|---|---|---|---|---|
| n | M (SD) | n | M (SD) | (p-value) | |
| BDI | 15 | 7.33 (8.55) | 17 | 4.76 (5.25) | 0.42 |
| ESS | 17 | 8.18 (3.09) | 18 | 7.72 (3.08) | 0.36 |
| MEQ | 17 | 47.53 (11.21) | 13 | 59.08 (10.30) | 0.02 |
| PSQI | 17 | 4.70 (2.47) | 16 | 4.56 (1.41) | 0.95 |
| SSS-1 | 17 | 2.29 (0.69) | 18 | 2.11 (0.76) | 0.46 |
| SSS-2 | 17 | 2.35 (1.17) | 18 | 1.94 (0.80) | 0.38 |
| SSS-3 | 17 | 2.41 (0.87) | 16 | 2.00 (0.73) | 0.19 |
| SSS-4 | 16 | 2.94 (1.24) | 16 | 2.13 (0.89) | 0.08 |
| PTA-1 | 16 | 4.06 (3.32) | 15 | 3.67 (2.47) | 0.71 |
| PTA-2 | 14 | 4.29 (3.32) | 15 | 2.47 (2.75) | 0.12 |
BDI – Beck Depression Inventory; ESS – Epworth Sleepiness Scale; MEQ – Morning-Eveningness Questionnaire; PSQI – Pittsburgh Sleep Quality Index; SSS – Stanford Sleepiness Scale (SSS-1: nap encoding; SSS-2: nap delayed test; SSS-3: wake encoding; SSS-4: wake delayed test); PTA – Post Task Awareness (PTA-1: nap condition; PTA-2: wake condition); n – number of participants with available data; M – mean; SD – standard deviation.
3.2. Sleep characteristics
3.2.1. Sleep macrostructure
All participants were able to nap. Overall nap length and minutes spent in individual sleep stages did not differ between young and older adults (ps > 0.2; Table 2), though there was some evidence that NREM2 made up a larger percentage of the nap in older adults, t(32.38) = −1.884, p = 0.069. Additionally, although the groups did not differ significantly on minutes or percent of the nap spent in REM, a higher proportion of young adults reached REM sleep than did older adults (young = 0.88, older = 0.50; χ2 = 4.29, p = 0.038).
Table 2.
Group means and standard deviations of sleep statistics.
| Young adults | Older adults | Age differences | |
|---|---|---|---|
| M (SD) | M (SD) | (p-value) | |
| TST (min) | 112.5 (12.2) | 105.5 (21.7) | 0.25 |
| NREM1 (min) | 16.4 (13.5) | 11.8 (8.0) | 0.23 |
| NREM2 (min) | 53.8 (16.9) | 58.2 (17.1) | 0.45 |
| NREM3 (min) | 17.1 (14.8) | 15.1 (16.5) | 0.71 |
| REM (min) | 10.9 (8.0) | 7.7 (11.4) | 0.34 |
| NREM1 (% TST) | 15.1 (12.4) | 12.0 (8.8) | 0.40 |
| NREM2 (% TST) | 47.4 (12.9) | 55.3 (11.9) | 0.07 |
| NREM3 (% TST) | 14.9 (12.7) | 13.2 (14.0) | 0.70 |
| REM (% TST) | 9.6 (6.8) | 6.9 (10.0) | 0.34 |
TST – Total sleep time; M – mean; SD – standard deviation.
3.2.2. Sleep microstructure
Older adults had lower absolute EEG amplitude than young adults in the delta, theta, and sigma frequency bands during the first 60 minutes of NREM2/NREM3 in the nap (Figure 3A, C). This aging-related reduction in absolute EEG amplitude was evident over frontal and occipital scalp for delta (frontal: t(26.8) = 3.08, p = 0.005; occipital: t(23.1) = 3.27, p = 0.003), and theta (frontal: t(25.1) = 2.95, p = 0.007; occipital: t(32.0) = 2.06, p = 0.047), and over frontal scalp alone for sigma at trend level (t(32.3) = 1.98, p = 0.056). Absolute sigma amplitude over lateral scalp did not change with age (p > 0.9).
The relative distribution of EEG amplitude across the scalp also changed with age, in a frequency-specific manner (Figure 3B, D). In the delta band, older adults had increased relative frontal amplitude, t(25.4) = −2.09, p = 0.046, and reduced relative occipital amplitude, t(32.6) = 2.07, p = 0.046, compared to young adults. In the theta band, older adults had reduced relative frontal amplitude, t(31.9) = 2.50, p = 0.018, compared to young adults. In the sigma band, older adults had reduced relative frontal amplitude, t(27.5) = 2.80, p = 0.009, and increased relative lateral amplitude, t(32.96) = −5.21, p < 0.001, compared to young adults.
3.3. Behavioral results
3.3.1. Accuracy
Accuracy on the SRTT was high overall: across participants, mean within-block accuracy exceeded 85% for every individual block of the encoding and delayed test phases, and averaged 94.6% across all blocks (Figure S1). The high accuracy on the SRTT was also reflected in high post-task awareness of the sequence in both age groups (Table 1), which was unaffected by age or condition (ps > 0.1). Accuracy during the encoding phase did not change across block-pairs, and was unaffected by age, condition, or block type (ps > 0.05). When considering accuracy from the end of encoding and beginning of delayed test, accuracy was higher overall at the beginning of delayed test relative to the end of encoding, F(1,33) = 8.73, p = 0.006, but was again unaffected by age, condition, or block type (ps > 0.06). Because of the overall high accuracy on the SRTT and limited accuracy differences among experimental conditions, the remainder of our behavioral performance analyses focus on reaction time.
3.3.2. Reaction time
During encoding, correct reaction times were faster overall for young adults compared to older adults, F(1,33) = 28.50, p < 0.001, and for sequence blocks compared to random blocks, F(1,33) = 37.37, p < 0.001, especially for in the nap condition (BT × Condition: F(1,33) = 6.62, p = 0.015; Figure 4A). Reaction times became faster across encoding block-pairs, F(5,165) = 11.56, p < 0.001, especially for sequence blocks, (BT × BP: F(5,165) = 24.71, p < 0.001). Similarly, when considering reaction times from the end of encoding and beginning of delayed test, reaction times were faster for young adults, F(1,33) = 23.58, p < 0.001, for sequence blocks, F(1,33) = 42.05, p < 0.001, and in the nap condition, F(1,33) = 10.90, p = 0.002. Additionally, reaction times were faster overall at the beginning of the delayed test phase compared to the end of the encoding phase, F(1,33) = 33.83, p < 0.001. In the across-interval reaction time comparison, block type interacted with condition, F(1,33) = 5.38, p = 0.027, and showed some evidence of a four-way interaction with age, phase, and condition, F(1,33) = 3.77, p = 0.061. The interactive effects of block type on reaction time across the encoding phase and across the nap/wake interval motivated examining reaction time separately for random and sequence blocks.
Fig. 4. Reaction time results.
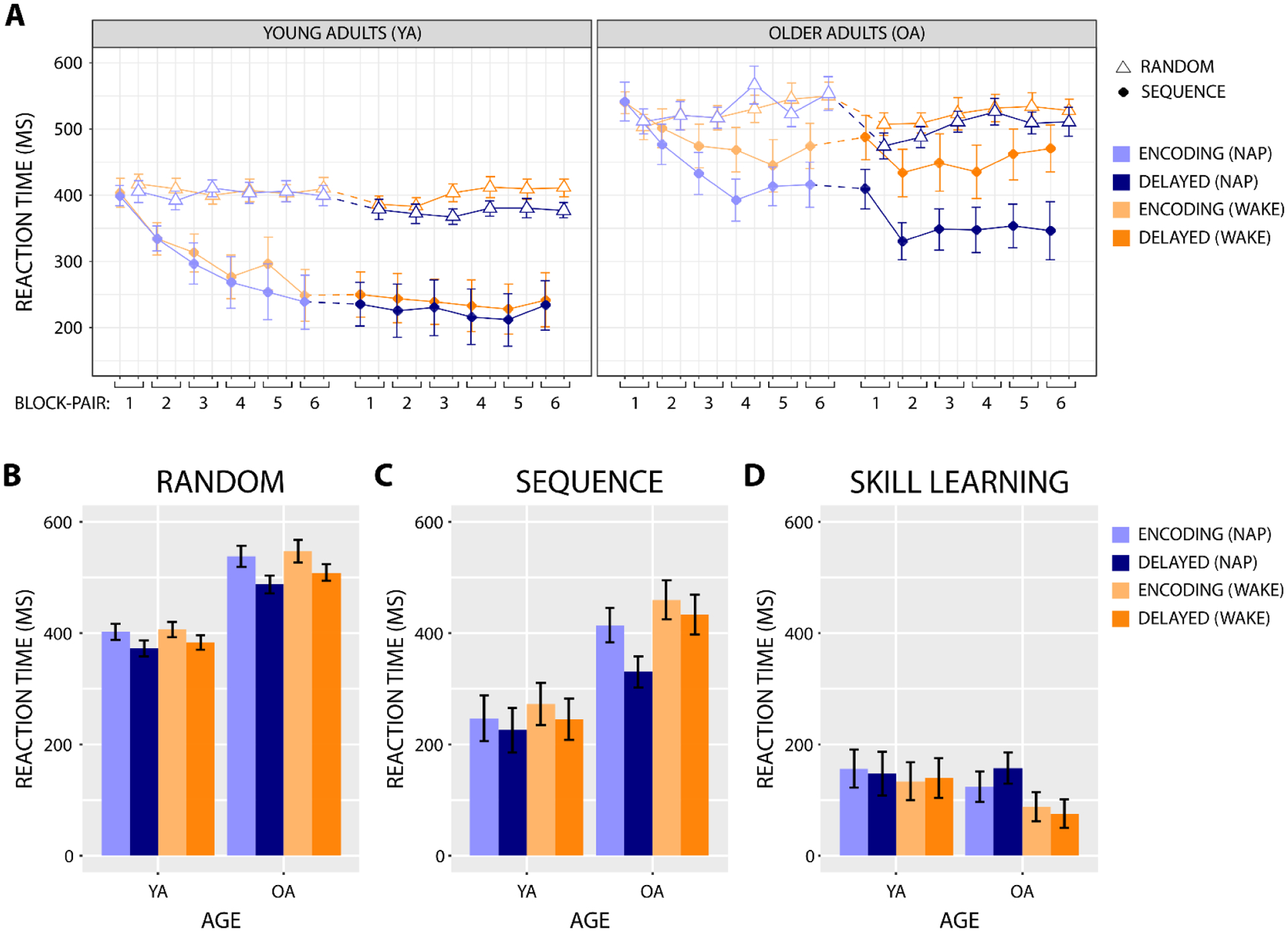
A) Median correct SRTT reaction times are plotted by block across the encoding and delayed test phases. The dotted line segment represents the nap/wake interval. B, C, D) Median correct SRTT reaction times from the end of the encoding phase (average of block-pairs 5 and 6) and the beginning of the delayed test phase (block-pair 2) for random blocks (B), sequence blocks (C), and skill learning (D).
For random blocks, correct reaction times during encoding were faster overall in young adults, F(1,33) = 36.61, p < 0.001, and slowed across encoding block-pairs in older adults (Age × BP: F(5,165) = 2.67, p = 0.043; older adults BP: F(5,85) = 3.49, p = 0.026; young adults BP: ps > 0.5; Figure 4A). When considering reaction times from the end of encoding and beginning of delayed test, correct random block reaction times were again faster overall for young adults, F(1,33) = 43.67, p < 0.001, and also at the beginning of delayed test, F(1,33) = 32.92, p < 0.001 (Figure 4B). Additionally, there was some evidence that slower random reaction times at the end of the encoding phase predicted larger across-interval improvements in random reaction time in older adults, but not young adults (RTRandom × Age: β = −0.492, t = −1.821, p = 0.074; older adults RTRandom: β = −0.700, t = −3.816, p = 0.001; young adults RTRandom: ps > 0.2).
For sequence blocks, correct reaction times during encoding were faster overall in young adults, F(1,33) = 18.82, p < 0.001, and in the nap condition, F(1,33) = 5.14, p = 0.030, and became faster across encoding block-pairs, F(5,165) = 22.62, p < 0.001 (Figure 4A). Notably, the speeding of sequence block reaction time across encoding block-pairs was not affected by age or condition (ps > 0.3), demonstrating that motor sequence learning was comparable across age groups and prior to the nap and wake intervals. When considering reaction times from the end of encoding and beginning of delayed test, correct sequence block reaction times were again faster overall for young adults, F(1,33) = 12.02, p = 0.001, and in the nap condition, F(1,33) = 10.10, p = 0.003, and also at the beginning of delayed test, F(1,33) = 17.53, p < 0.001 (Figure 4C). A three-way interaction indicated that the across-interval sequence block reaction time improvement was largest for the nap condition in older adults (Age × Condition × Phase: F(1,33) = 4.90, p = 0.034). Follow-up ANOVAs for each age group showed that the across-interval reaction time improvement was significant for the nap condition in older adults, but not for the wake condition in older adults or at all in young adults (older adults Condition × Phase: F(1,17) = 6.20, p = 0.023; older adults PhaseNap: F(1,17) = 23.56, p < 0.001; older adults PhaseWake: p > 0.1; young adults: ps > 0.09). The across-interval improvement in sequence reaction time was not predicted by reaction time at the end of the encoding phase (ps > 0.2).
3.3.3. Skill learning
Skill learning, i.e., the reaction time advantage on sequence relative to random blocks, increased across encoding block-pairs, F(5,165) = 24.71, p < 0.001, and was larger overall during encoding in the nap condition, F(1,33) = 6.62, p = 0.015 (Figure 4A). When considering the end of encoding and beginning of delayed test, skill learning was again larger overall in the nap condition, F(1,33) = 5.38, p = 0.027, in a manner that showed some evidence of interacting with age and phase, F(1,33) = 3.77, p = 0.061 (Figure 4D). Follow-up ANOVAs for each age group showed that skill learning in older adults was greater for the nap condition and showed some evidence of increasing more over the nap compared to the wake interval (Condition: F(1,17) = 4.47, p = 0.050; Condition × Phase: F(1,17) = 4.036, p = 0.061; PhaseNap: F(1,17) = 3.81, p = 0.068; PhaseWake: F(1,17) = 0.616, p = 0.443), but in young adults was unaffected by condition or phase (ps > 0.5),. Across-interval changes in skill learning were not predicted by skill learning at the end of the encoding phase (ps > 0.4).
3.4. fMRI results
In the pre-nap encoding phase, young adults showed increased brain activation on sequence relative to random trials in a primarily striato-cortical network of regions consistent with late fast and early slow motor sequence learning: dorsolateral prefrontal cortex (DLPFC), premotor cortex (PMC), SMA, IPC, insula, putamen, pallidum, thalamus, and cerebellum (Figure 5A [Red], Table S1A). Further, young adults showed decreased brain activation on sequence relative to random trials in a hippocampo-cortical network characteristic of fast motor sequence learning: ventromedial prefrontal cortex (vmPFC), left middle temporal gyrus (MTG), and left hippocampus (Figure 5A [Green], Table S1A). Comparatively, in the pre-wake encoding phase, young adults showed increased activation in IPC and decreased activation in vmPFC and left MTG on sequence relative to random trials (Figure S2A, Table S1B).
Fig. 5. Brain activation on sequence relative to random trials for the nap condition.
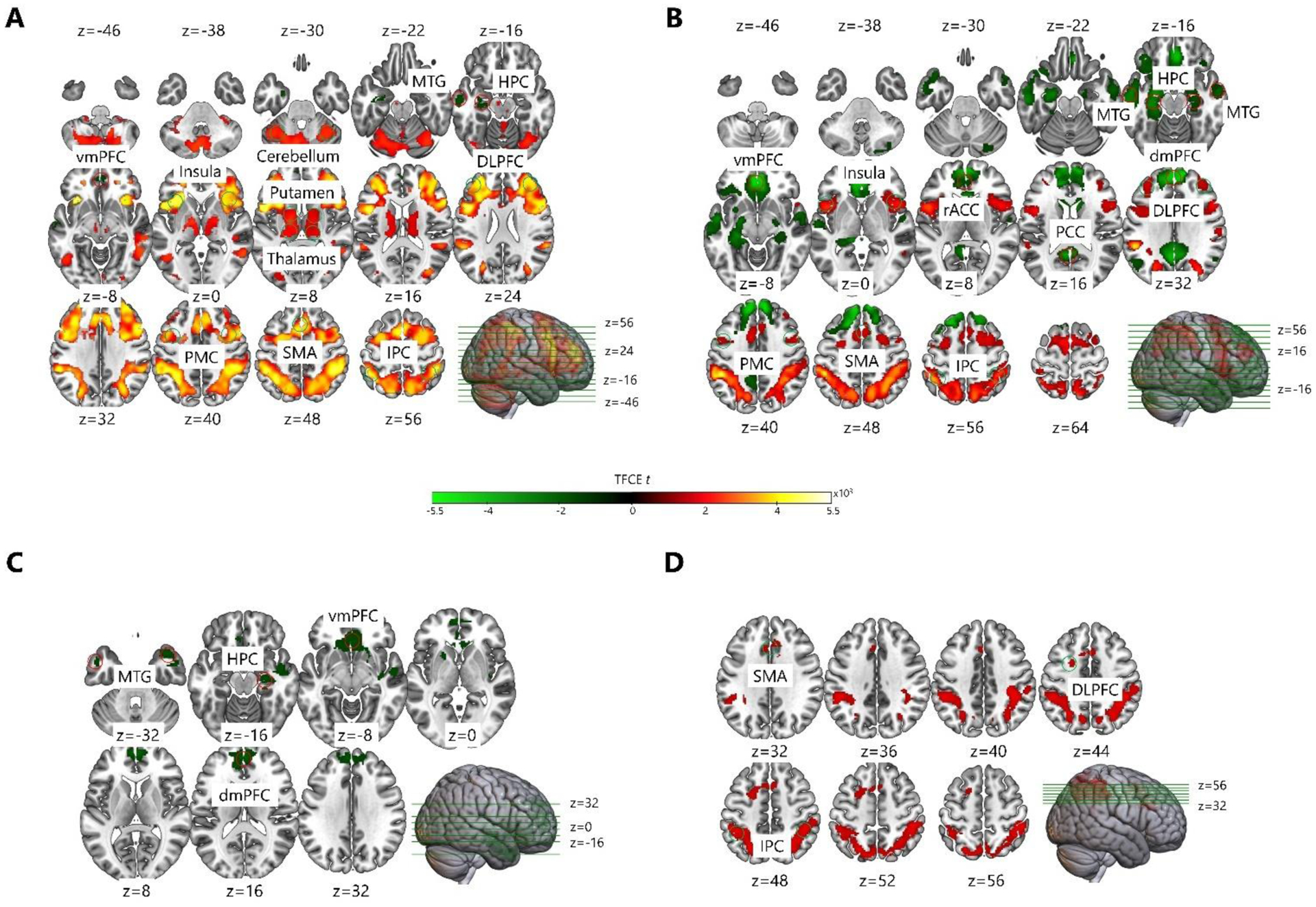
A) During the pre-nap encoding phase, young adults showed increased brain activation in DLPFC, PMC, SMA, IPC, insula, putamen, pallidum, thalamus, and cerebellum, and decreased brain activation in vmPFC, left MTG, and left HPC. B) During the post-nap delayed test phase, young adults showed increased brain activation in DLPFC, PMC, SMA, IPC, and insula, and decreased brain activation in dmPFC, vmPFC, rACC, PCC, MTG, and HPC. C) During the pre-nap encoding phase, older adults showed decreased brain activation in dmPFC, vmPFC, MTG, and right HPC. D) During the post-nap delayed test phase, older adults showed increased brain activation in left DLPFC, SMA, and IPC. DLPFC – dorsolateral prefrontal cortex; dmPFC – dorsomedial prefrontal cortex; vmPFC – ventromedial prefrontal cortex; rACC – rostral anterior cingulate cortex; PCC – posterior cingulate cortex; PMC – premotor cortex; SMA – supplementary motor area; MTG – middle temporal gyrus; IPC – inferior parietal cortex; HPC – hippocampus.
In the post-nap delayed test phase, young adults continued to show increased brain activation on sequence relative to random trials in regions consistent with slow motor sequence learning, but in a smaller (relative to encoding) network of only cortical regions: DLPFC, PMC, SMA, IPC and insula (Figure 5B [Red], Table S1A). Further, young adults continued to show decreased brain activation on sequence relative to random trials in a hippocampo-cortical network, which was larger than that observed during encoding: dorsomedial prefrontal cortex (dmPFC), vmPFC, rostral anterior cingulate cortex (rACC), posterior cingulate cortex (PCC), MTG, and hippocampus (Figure 5B [Green], Table S1A). The increases in activation on sequence relative to random trials were smaller, and the decreases in activation were larger, during the post-nap delayed test phase than they were during pre-nap encoding in young adults (Figure 5A, B, Table S1A). Comparatively, in the post-wake delayed test phase, young adults showed increased activation during sequence relative to random trials in DLPFC, SMA, and IPC, and decreased activation in dmPFC (Figure S2B, Table S1B).
In the pre-nap encoding phase, older adults showed decreased brain activation on sequence relative to random trials in a hippocampo-cortical network similar to that observed in young adults: dmPFC, vmPFC, MTG, and right hippocampus (Figure 5C [Green], Table S1A). Older adults did not show any increases in brain activation on sequence relative to random trials during pre-nap encoding (Figure 5C [Red], Table S1A). Further, the second-level analysis demonstrated that during pre-nap encoding, older adults had smaller activation differences between sequence and random trials than young adults in the striato-cortical network, indicating that older adults had progressed less into slow learning: DLPFC, PMC, SMA, insula, and putamen (Figure 6, Table S1). Older adults also showed smaller activation differences between sequence and random trials than young adults in middle frontal cortex (MFC) and inferior frontal cortex (IFC) during pre-nap encoding (Figure 6, Table S1). Comparatively, in the pre-wake encoding phase, older adults showed increased activation in the right IPC on sequence relative to random trials (Figure S2C, Table S1B). There were no significant effects of aging on the activation difference between sequence and random trials during pre-wake encoding in the second-level analysis.
Fig. 6. Aging-related differences in brain activation on sequence relative to random trials during the pre-nap encoding phase.
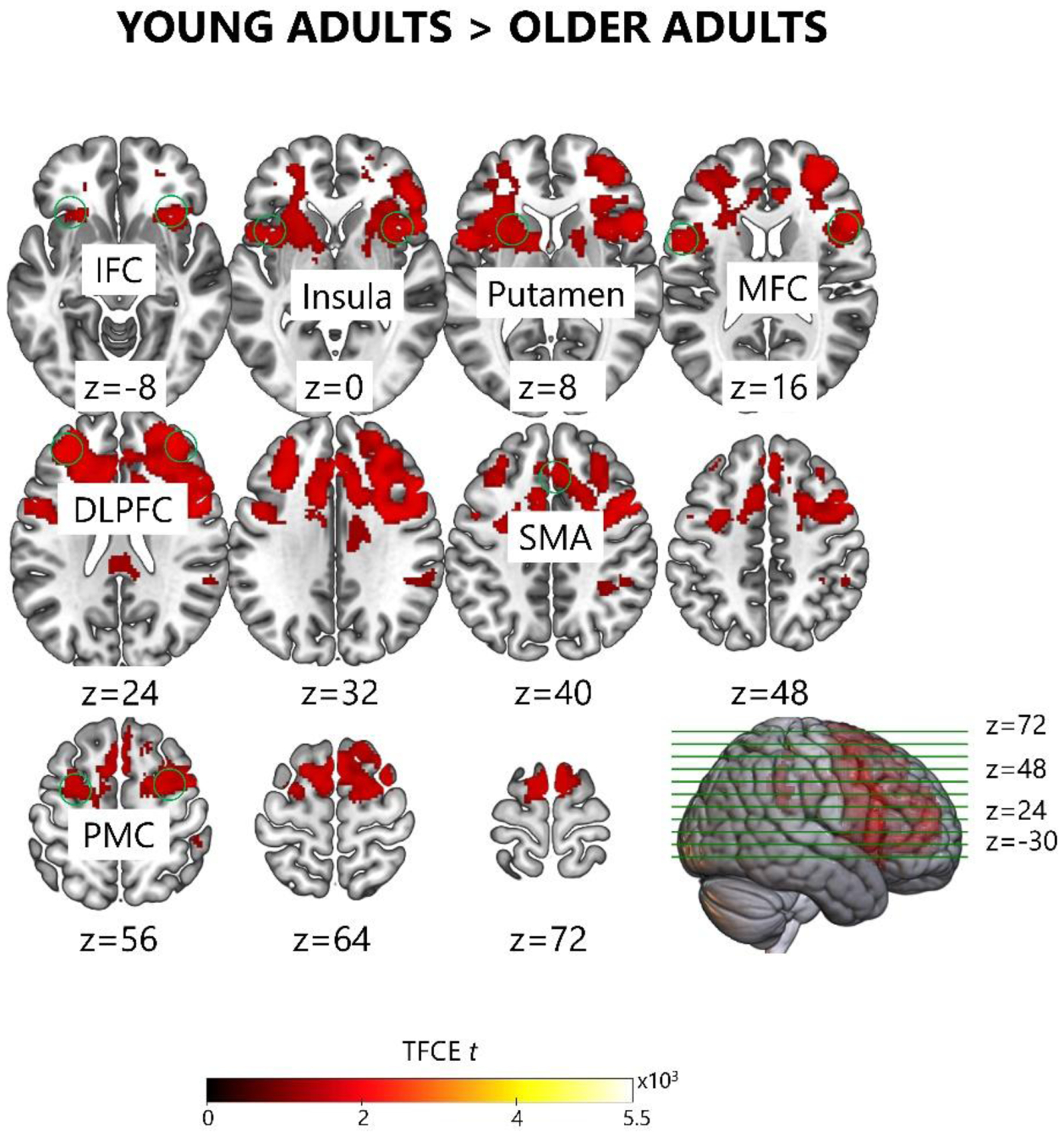
Young adults showed a larger increase in brain activation on sequence relative to random trials than older adults in regions DLPFC, MFC, IFC, PMC, SMA, insula, and putamen during the pre-nap encoding phase. The differences in brain activation on sequence relative to random trials did not significantly change with aging during the post-nap delayed test phase, the pre-wake encoding phase, or the post-wake delayed test phase. DLPFC – dorsolateral prefrontal cortex; MFC – middle frontal cortex; IFC – inferior frontal cortex; PMC – premotor cortex; SMA – supplementary motor area.
In the post-nap delayed test phase, older adults showed increased brain activation on sequence relative to random trials in cortical regions consistent with slow learning: left DLPFC, SMA, and IPC (Figure 5D, Table S1A). Older adults did not show any decreases in brain activation on sequence relative to random trials during the post-nap delayed test phase. Comparatively, older adults did not show any increases or decreases in brain activation on sequence relative to random trials during the post-wake delayed test phase (Figure S2D, Table S1B). There were no significant effects of aging on the activation difference between sequence and random trials during the post-nap or post-wake delayed test phases in the second-level analysis.
3.5. Relationships among brain activation during encoding, sleep physiology, and motor sequence learning and consolidation
3.5.1. Predictive relationships
Increased activation in bilateral premotor cortex on sequence relative to random trials during pre-nap encoding predicted larger skill learning changes across the nap (Left PMC: β = 41.49, t = 3.385, p = 0.013; Right PMC: β = 42.50, t = 3.529, p = 0.013; Figure 7A), in a manner unaffected by age (ps > 0.8). Additionally, increased activation in right hippocampus on sequence relative to random trials during pre-nap encoding predicted higher relative frontal sigma amplitude, β = 0.105, t = 3.152, p = 0.049, and lower relative lateral sigma amplitude, β = −0.051, t = −3.733, p = 0.010, during the nap (Figure 7B), in a manner unaffected by age (ps > 0.8). There were no other significant predictions of behavioral performance at the end of encoding (ps > 0.5), change in behavioral performance over the nap (ps > 0.07), or sleep physiology during the nap (ps > 0.1) by differences in activation on sequence relative to random trials during pre-nap encoding after correcting for false discovery rate.
Fig. 7. Relationships between brain activation during pre-nap encoding and sleep-dependent consolidation.
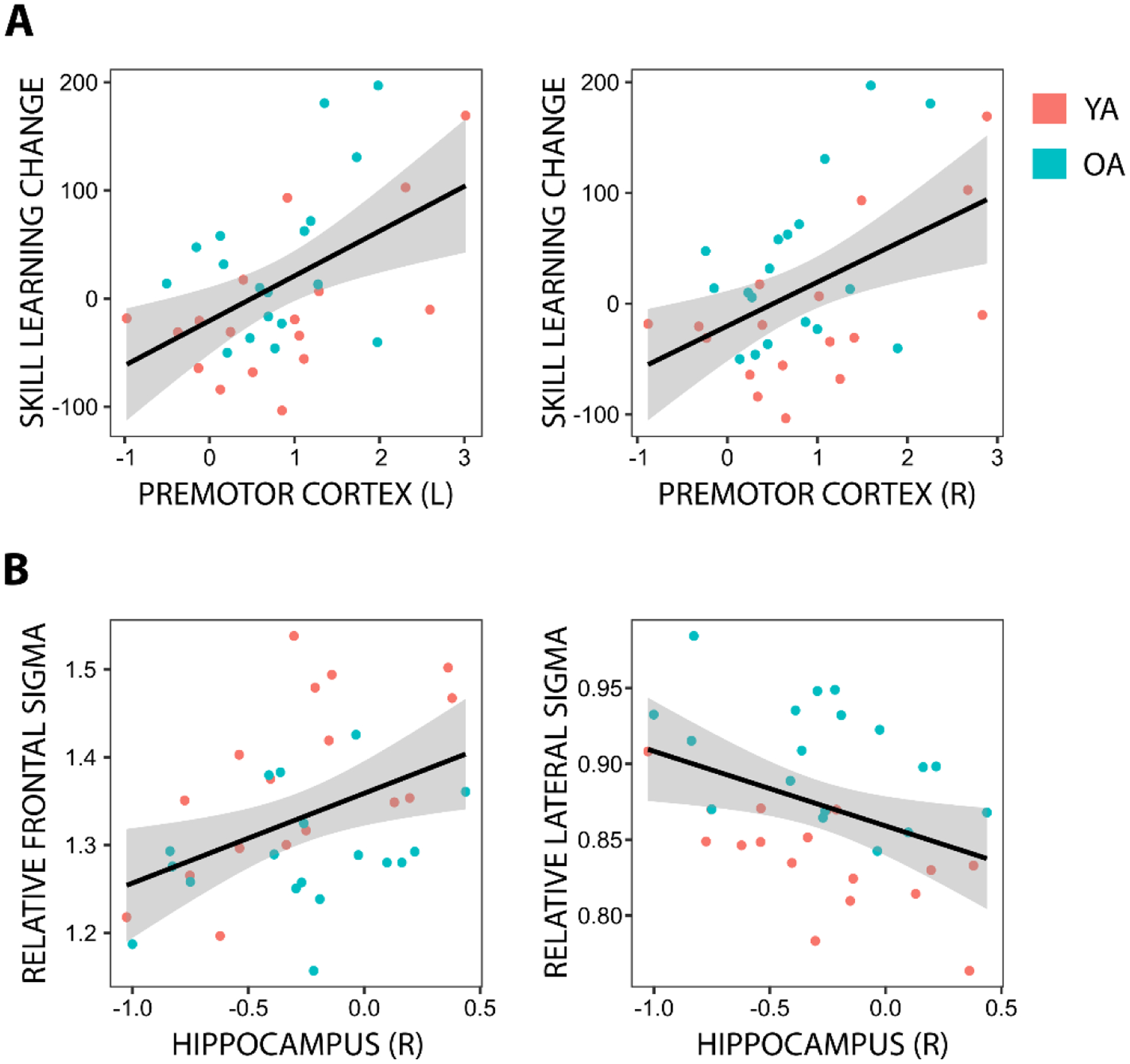
Individual data are shown for significant predictions of A) across-nap changes in behavioral performance, and B) nap sleep physiology, by brain activation differences on sequence relative to random blocks during the pre-nap encoding phase within the seven targeted ROIs in young (YA) and older adults (OA). The simple regression line is plotted for each relationship, with the 95% confidence interval shown as a shaded band. Y-axis units are milliseconds in (A) and unitless in (B), X-axis units are β units (i.e., standard deviations). L – left; R – right.
There was some evidence that relative occipital theta amplitude during the nap predicted across-nap changes in sequence reaction time and skill learning in a manner dependent on age, although the interactions did not meet the typical significance threshold after correcting for false discovery rate (Sequence RT change: βTheta*Age = −405.55, t = −2.607, puncorrected = 0.014, p = 0.164; Skill learning change: βTheta*Age = 480.78, t = 2.974, puncorrected = 0.006, p = 0.068). These marginal interactions motivated separate follow-up regressions for each age group, which demonstrated that in young adults, higher relative occipital theta amplitude during the nap predicted larger across-nap decreases in sequence reaction time, β = −373.24, t = −4.047, p = 0.013, and larger across-nap increases in skill learning, β = 411.17, t = 3.797, p = 0.021 (Figure 8). These relationships were not evident in older adults (ps > 0.6). There were no other significant predictions of across-nap change in performance by sleep physiology after correcting for false discovery rate (ps > 0.2). Further, sleep physiology did not significantly predict change in task-related brain activation over the nap in any ROIs after correcting for false discovery rate (ps > 0.09).
Fig. 8. Relationships between sleep physiology and across-nap changes in behavior.
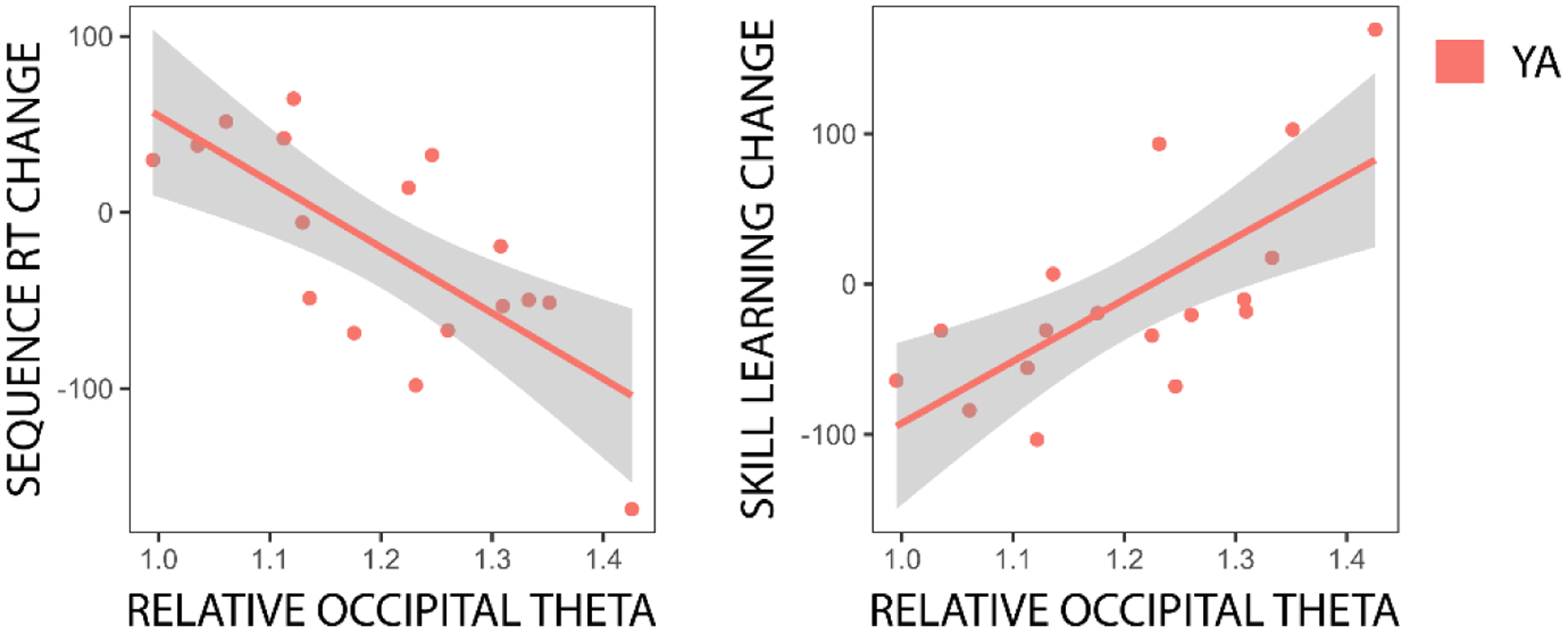
Individual data are shown for significant predictions of across-nap changes in behavioral performance by nap sleep physiology in young adults (YA). The simple regression line is plotted for each relationship, with the 95% confidence interval shown as a shaded band. Y-axis units are milliseconds, X-axis variables are unitless.
3.5.2. Mediation of aging-related differences
Aging-related slowness of sequence block reaction time at the end of pre-nap encoding was partially mediated by aging-related reductions in sequence-trial-specific activation in right IPC during pre-nap encoding (TE = −167.31, pTE < 0.001, ADE = −115.28, pADE = 0.032, ACME = −52.03, pACME = 0.032, PropMediated = 0.292). Additionally, there was some evidence that aging-related increases in relative lateral sigma amplitude during the nap were partially mediated by aging-related reductions in sequence-trial-specific activation in left putamen during pre-nap encoding (TE = −0.07, pTE < 0.001, ADE = −0.05, pADE < 0.001, ACME = −0.01, pACME = 0.054, PropMediated = 0.155). There were no other measures of behavioral performance at the end of encoding (ps > 0.09), change in behavioral performance over the nap (ps > 0.1), or sleep physiology during the nap (ps > 0.07) for which the age effect met the dual criteria (i.e., significant TE and ACME) for mediation by aging-related differences in increased brain activation on sequence relative to random trials in any ROI. Further, there were no measures of change in behavioral performance (ps > 0.09) or change in during-task brain activation (ps > 0.1) over the nap for which the age effect met the dual criteria for mediation by aging-related differences in sleep physiology during the nap.
4. Discussion
In the present study, we examined aging-related changes in brain activation during motor sequence encoding, and in subsequent consolidation over midday intervals spent awake and asleep. We hypothesized that previously reported deficits in sleep-dependent motor sequence consolidation in older adults resulted from aging-related deficits in fast learning, and that increased training would therefore facilitate sleep-dependent motor sequence consolidation in older adults. Consistent with this hypothesis, following extended visuomotor training, older adults showed clear behavioral and neuroimaging evidence of greater serial reaction time task (SRTT) consolidation following midday intervals spent asleep compared to awake. In young adults, the extended training led to an advanced memory representation during encoding, resulting in high behavioral performance and motor cortical engagement during encoding, and no behavioral evidence of consolidation over subsequent sleep or wake. Changes in during-task neural activity following sleep in young adults did however suggest continued systems-level consolidation of the motor sequence memory trace over sleep in young adults, despite static behavioral performance. Additionally, our sleep physiology measures corroborate prior reports of sigma activity contributions to sleep-dependent consolidation of motor sequence learning in both young and older adults, while also demonstrating a contribution of theta activity to motor sequence learning consolidation in young adults.
Broadly speaking, our results are consistent with the motor sequence learning timeline depicted in Figure 1, in which cerebellum and caudate dominate fast learning, followed by engagement in hippocampo-cortical and striato-cortical networks as the motor memory progresses through allocentric and egocentric representations across later fast and slow learning. The explicit SRTT variant and pre-training paradigm facilitated older adults advancing past cerebello-caudate representation during encoding, allowing sleep-dependent consolidation to occur. The extra training in young adults advanced memories to a primarily egocentric striato-cortical representation during encoding, largely precluding subsequent sleep-dependent consolidation. Together, our results indicate that sleep-dependent consolidation of motor sequence learning, and the neural mechanisms thereof, is largely intact in older adults, and provide further support for the argument that aging-related deficits sometimes observed in this process are driven primarily by aging-related differences in fast learning due to differential handling of task complexity (Gudberg et al., 2015).
4.1. Sleep-dependent motor sequence consolidation
We interpret our observation of sleep-dependent performance improvement in older adults to reflect benefits of additional training and lower task complexity relative to other studies using the SRTT (Spencer et al., 2007). While the majority of prior studies has suggested an absence of sleep-dependent memory benefits in older adults (Fogel et al., 2014; Pace-Schott & Spencer, 2013; Spencer et al., 2007; Wilson et al., 2012), our results add to a growing number of studies demonstrating sleep-dependent motor sequence memory benefits in older adults when encoding is sufficiently strong (Gudberg et al., 2015; King, Saucier, et al., 2017; Korman et al., 2015; Tucker et al., 2011; Wilhelm et al., 2012). Sleep-dependent consolidation is thought to predominantly benefit intermediate strength memories (Stickgold, 2009); weak memories lack the encoding depth and related hippocampal representation to engage memory replay processes. We propose the heterogeneity of prior findings regarding sleep-dependent motor sequence memory consolidation in older adults primarily reflects a heterogeneity of encoding depth across studies.
Aging-related decreases in cognitive functioning (e.g., processing speed, working memory) disproportionately impact fast learning aspects of motor sequence learning in older adults (Bennett et al., 2007; Curran, 1997; Feeney et al., 2002; Howard et al., 2004). Degraded fast learning mechanisms in older adults may then extend time spent in fast learning during motor sequence learning, delaying older adults’ engaging in slow learning processes. In the current study, remediating fast learning deficits in older adults with visuomotor training prior to the SRTT decreased the need for effortful focus on fast learning aspects during SRTT performance, facilitating the engagement of slow learning processes. Additionally, predictability afforded by explicit knowledge of sequence presence and temporal regularity of cue presentation likely reduced cognitive demands of the SRTT, and improved attention to sequential cues (Janacsek & Nemeth, 2013; Nobre & Ede, 2017; Shin & Ivry, 2002). As a result, older adults were able to utilize more advanced sequence learning strategies during encoding (e.g., pattern searching, egocentric representations), engaging brain regions further along the progression of the motor sequence learning timeline (Figure 1) and reaching the intermediate representation necessary to support consolidation during subsequent sleep.
Conversely, memories that are very strong may be already established in cortical representations, precluding further sleep-dependent consolidation (Stickgold, 2009). In support of this theory, Wilhelm and colleagues (2012) found that over-sleep performance benefits for motor sequence learning were abolished when young adults were over-trained on a motor sequence learning task. Consistent with these findings, in the current study young adults showed no behavioral evidence of sleep-dependent motor sequence consolidation, likely reflecting a highly established memory representation created during encoding. A high depth of motor sequence encoding was indicated in young adults both behaviorally (faster and more predictive reaction times during encoding), and neurally (striato-cortical activation during encoding indicating a progression to egocentric memory representation prior to sleep). This progression to an egocentric memory representation prior to sleep in young adults precluded subsequent sleep-dependent motor sequence memory consolidation, which preferentially enhances allocentric aspects of motor memory (e.g., Albouy et al., 2015; Albouy, Fogel et al., 2013; Cohen et al., 2005; Pace-Schott & Spencer, 2013).
4.2. Neural mechanisms of sleep-dependent motor sequence consolidation
4.2.1. Cerebellum and caudate
We predicted that lower activity in cerebellum and caudate during encoding, indicative of less time spent in fast learning, would be associated with both increased performance at the end of encoding due to higher encoding strength, and higher levels of sleep-dependent consolidation due to more reliable progression into the sleep-dependent encoding window. Contrary to our prediction, lower activity in cerebellum and caudate did not predict higher encoding strength or sleep-dependent consolidation. This suggests that fast learning progressed sufficiently rapidly in both age groups for individual differences in sleep-dependent consolidation to be driven primarily by progression through hippocampo-cortical and striato-cortical representations, across later fast learning and slow learning, rather than through cerebello-caudate representations in early fast learning. Though contrary to our initial prediction, this finding is consistent with our broader hypothesis that overcoming aging-related fast learning deficits with additional training facilitates sleep-dependent motor memory consolidation in older adults.
4.2.2. Hippocampus
We predicted that higher activity in the hippocampus, putamen, and motor cortical regions, indicative of further progression of the memory through the consolidation timeline, would be associated with increased performance at the end of encoding, but lower levels of sleep-dependent consolidation. Regarding the hippocampus, contrary to our prediction hippocampal activation during encoding did not predict performance at the end of encoding, or the across-nap change in performance. Instead, young adults showed reduced activation on sequence relative to random trials in a hippocampo-cortical network prior to the nap, and an even larger reduction in this network after the nap. Additionally, older adults showed reduced activation in a similar hippocampo-cortical network prior to, but not following, the nap. These results suggest that activity in the hippocampo-cortical network is suppressed during motor sequence task performance once the memory has progressed past hippocampal dependence, into a striato-cortical representation. This is consistent with a competitive balance between hippocampo-cortical allocentric and striato-cortical egocentric motor strategies as motor consolidation progresses through later fast learning into slow learning (e.g., Albouy et al., 2013).
It is notable that hippocampo-cortical network suppression increased over the sleep and wake intervals in young adults, but decreased over sleep in older adults. This result is consistent with prior reports of hippocampal suppression during sequence learning in young adults, but hippocampal activation during sequence learning in older adults (Albouy et al., 2008; Rieckmann et al., 2010). The hippocampus is suggested to play a compensatory role during sequence learning in older adults (King et al., 2013; Rickmann et al., 2010; Rieckmann & Backman, 2009), increasing activation to offset deficits in executive function and working memory, and degraded striato-cortical networks. In keeping with this interpretation, hippocampal activation is decreased relative to baseline when fewer items are maintained in working memory (Axmacher et al., 2007). Through this lens, our hippocampal suppression findings could suggest decreased working memory load during sequence blocks of the SRTT after sufficient motor sequence consolidation in young adults, but increased working memory effort in older adults relative to young adults during sequence blocks of the SRTT following sleep-dependent consolidation. Sequence learning during an implicit SRTT is predicted by both visuospatial working memory and verbal working memory in young adults, but only by verbal working memory in older adults (Bo et al., 2012); it may be then that following sleep-dependent consolidation, older adults are able to make use of visuospatial working memory as well during the SRTT, in a more young adult-like fashion, leading to increased overall working memory load. Alternatively, sleep-dependent consolidation may lead to greater explicit knowledge of the motor sequence memory in older adults, leading to cognitive strategies that involve holding the sequence, in whole or in part, in working memory after the nap and increasing working memory load.
Additionally, increased right hippocampus activation on sequence relative to random blocks during encoding predicted higher relative frontality and lower relative laterality of sigma amplitude during the nap in both young and older adults. Frontal spindles, which are apparent in the sigma band, have previously been associated with sleep-dependent motor memory consolidation (Fogel et al., 2014). We therefore interpret this finding to represent an inverse relationship between hippocampal suppression during the SRTT and sleep-dependent memory consolidation. Specifically, individuals who progress farther past hippocampo-cortical allocentric memory representation (and into striato-cortical egocentric memory representation) during encoding show both greater suppression of the hippocampo-cortical network during the task, and reduced sleep-dependent memory consolidation over the subsequent nap, as indexed by lower relative frontal sigma amplitude. This finding is consistent with prior evidence that sleep preferentially enhances hippocampally-mediated allocentric aspects of motor sequence memories (e.g., Albouy et al., 2015).
4.2.3. Putamen
Consistent with our prediction, young adults had both increased sequence-trial-specific putamen activation and improved behavioral performance during encoding relative to older adults, though individual differences in putamen activation did not directly predict behavioral performance during encoding. Additionally, we found some evidence that aging-related reductions in sequence-trial-specific putamen activation partially mediated aging-related increases in relative sigma laterality, though individual differences in putamen activation did not directly predict the change in behavioral performance over the nap. This finding may indirectly reflect a relationship between decreased putamen activation during encoding and decreased frontal sigma activity during the nap, as decreases in relative sigma frontality (where sigma is predominantly concentrated during sleep) will result in increases in relative sigma laterality. This interpretation would be consistent with previous reports linking aging-related declines in increased putamen activation following post-motor sequence learning sleep with aging-related declines in frontal spindle activity (Fogel et al., 2014). It is unclear why this relationship would not show up directly for relative frontal sigma, but given the statistically marginal nature of the relationship with relative lateral sigma it is possible that a relationship with relative frontal sigma was also present that we were underpowered to detect.
Alternatively, our finding may represent a direct relationship between decreased putamen activation during encoding and increased lateral sigma activity. Such a relationship would be difficult to functionally interpret, as little is known regarding laterally distributed sigma activity during sleep; the majority of sleep microstructure research has focused on oscillatory neural activity over the scalp midline, most typically over frontocentral regions. However, increased relative sigma amplitude over lateral scalp in older adults relative to young adults has been reported in both high-density polysomnography investigations of aging-related changes in sleep microstructure (Sprecher et al., 2016; Fitzroy et al., under review). It may then be that lateral sigma activity plays a functionally distinct role from frontal sigma activity in motor sequence memory consolidation, in a manner that changes with age. Further research into the neural mechanisms and functional associations of laterally distributed sigma activity is necessary to explore this possibility.
4.2.4. Motor cortical regions
Consistent with our predictions, young adults showed greater sequence-trial-specific activation in a motor cortical network (PMC, SMA, DLPFC, insula, IPC) during encoding than older adults, along with better performance at the end of encoding. Further, aging-related deficits in performance at the end of encoding were partially mediated by aging related declines in sequence-trial-specific right IPC activation during encoding. Contrary to our predictions, greater motor cortical activation during encoding in young adults was followed by an absence of behavioral evidence for sleep-dependent consolidation. These results indicate that young adults encoded the motor sequence memory to a high level prior to the nap, leading to a primarily striato-cortical egocentric memory representation (e.g., Doyon et al., 2018), and in turn diminished or absent sleep-dependent consolidation. Older adults, on the other hand, did not reach motor cortical involvement during encoding, but did then show activation in a motor cortical network (DLPFC, SMA, IPC) during the SRTT after sleep-dependent memory consolidation. This supports our behavioral evidence suggesting that older adults encoded the motor sequence memory to an intermediate level prior to the nap, leading to a post-cerebellar but pre-cortical memory representation, and subsequently, clear sleep-dependent consolidation as proposed by Stickgold (2009). The resulting sleep-dependent motor sequence consolidation in older adults then led to a, at least partially, cortically-driven memory representation after the nap, but not after wake.
However, though young adults as a group did not show performance improvements over the nap, changes in cortical activation over the nap interval suggested sleep-dependent consolidation nonetheless occurred in the young adults at the neural systems level. The reduction in activation of the dominant striato-cortical network (and increased, more widespread suppression of the hippocampo-cortical network) in young adults at delayed test relative to encoding is consistent with prior reports of reductions in putamen and motor cortical activity as motor memories become automatized with expertise (Poldrack et al., 2005; Toni et al., 1998; Wu et al., 2004). That these reductions in cortical activation were evident following the nap but not following an equivalent period of wake suggests that sleep may have played an active role in the systems-level consolidation. Moreover, the relative occipitality of theta amplitude robustly predicted over-nap improvements in behavioral performance in young adults at the individual level, indicating a specific role of sleep physiology in automatization-related performance improvements. Given that performance improvements are asymptotic over slow learning and automatization, the positive relationship of relative occipital theta and skill learning improvement suggests that occipital theta plays a diminishing role as automatization continues. Theta-band activity has been previously shown to be involved in cortico-cortical and hippocampo-cortical communication during non-automatized motor activity in awake rats (Young & McNaughton, 2009), and in sleep-dependent procedural learning consolidation in humans (Tucker & Fishbein, 2009). Though speculative, it may be then that our results illustrate a role for theta in the sleep-dependent automatization of motor activity to primarily cortico-cortical representations in humans, that diminishes once cortico-cortical representations are established.
Additionally, we observed a clear relationship between increased bilateral premotor cortex activation during encoding and larger improvements in skill learning over the nap, in both young and older adults. In older adults this relationship is straightforward to interpret, in that as a group older adults showed evidence of both sleep-dependent performance improvement and increases in motor cortical activity over the nap. The positive relationship with premotor cortex suggests that high-encoding older adults progressed farther into a motor cortical memory representation before sleep, and achieved the greatest gains over sleep due to optimal (i.e., intermediate strength) memory encoding for consolidation. Young adults however, did not as a group show evidence of sleep-dependent performance improvements, and showed a decrease in motor cortical activity over the nap. As such, we interpret the positive relationship of premotor cortex activity to skill learning change in young adults to reflect increased efficiency of motor cortical memory representation, as a product of automatization (e.g., Poldrack et al., 2005). Specifically, low-encoding young adults have less efficient cortical memory representations and higher cortical activity during encoding, while also having greater room for behavioral improvement over the sleep interval. Conversely, high-encoding young adults have more efficient cortical memory representations and lower cortical activity during encoding, and are closer to ceiling performance, allowing less improvement over subsequent sleep.
Across age groups, our pattern of group-level results and individual differences results together suggest an inverted U-shaped curve of motor cortical involvement as motor memory representation progresses across slow learning and automatization. Specifically, older adults as a group achieved a post-cerebellar, pre-cortical memory representation prior to the nap (i.e., on the upslope of the inverted U-shaped curve of motor cortical involvement), facilitating sleep-dependent memory consolidation, but with high-encoding older adults showing both greater motor cortical involvement and greater sleep-dependent memory consolidation. Young adults as a group achieved a fully cortical memory representation prior to the nap (i.e., on the downslope of the inverted U-shaped curve of motor cortical involvement), precluding sleep-dependent memory consolidation, but with low-encoding young adults showing greater motor cortical involvement and greater sleep-dependent memory consolidation. This inverted U-shaped pattern of motor cortical involvement tracks with the inverted U-shaped curve of sleep-dependent consolidation as a function of encoding strength proposed by Stickgold, 2009. Moreover, this interpretation is consistent with prior evidence that motor cortical involvement increases across slow learning (e.g, Penhune & Steele, 2012), but then decreases during automatization and with expertise (Picard & Strick, 1996; Poldrack et al., 2005; Toni et al., 1998; Wu et al., 2004).
4.3. Time-dependent motor sequence consolidation
Though unpredicted, in addition to the clear sleep-dependent consolidation of motor sequence learning observed in older adults and potential sleep-dependent automatization observed in young adults, both young and older adults exhibited a performance improvement during random blocks over time that was not dependent on sleep. Further, in older adults, slower random reaction times at the end of encoding predicted larger improvements in random reaction time over the nap or wake interval. This may suggest a ceiling effect on practice-related improvements in random reaction time, such that older adults who were farther away from that ceiling had more room to improve over the interval, whereas high-performing older (and all young) adults who reached the performance ceiling during encoding had a time-dependent improvement of only a set amount. Alternatively, given that random block reaction times slowed across the encoding phase in older adults, it may be that slower random reaction times at the end of encoding in older adults indicated greater fatigue, and that subsequent larger improvements over the nap or wake interval represent larger recovery from fatigue.
4.4. Sleep physiology and motor sequence consolidation
We predicted that increased activity in the delta, theta, and sigma frequency bands would lead to higher levels of sleep-dependent consolidation in both young and older adults. While we did observe reduced absolute delta, theta, and sigma activity with aging, these reductions did not predict lower sleep-dependent consolidation in older adults. To the contrary, older adults showed clear sleep-dependent performance improvements while young adults did not. This suggests that the majority of the observed aging-related declines in absolute delta, theta, and sigma activity during sleep represent reductions in trait-like aspects of neural activity as a result of structural brain changes with aging, rather than reductions in state-like aspects of neural activity as a result of functional cognitive or consolidation mechanism changes (Fitzroy, Kainec, & Spencer, under review). Further, absolute EEG activity during the nap did not directly predict sleep-dependent consolidation across age groups or in either age group separately. Though surprising given prior evidence of delta, theta, and sigma activity predicting sleep-dependent behavioral improvement on procedural tasks (e.g., Tamaki et al., 2013; Tucker & Fishbein, 2009; Nishida & Walker, 2007; Fogel et al., 2014), we do not interpret our null finding as contradictory evidence to this past work. Rather, we posit our conservative significance thresholds correcting for multiple comparisons led to a lack of power to detect marginal effects; indeed, we observed but do not report multiple additional potential predictive relationships of sleep physiology and sleep-dependent performance improvement that met or approached uncorrected significance criteria, but did not remain after adjusting for false discovery rate.
Additionally, though we did not observe relationships between sleep-dependent consolidation and absolute EEG activity during sleep, we did observe relationships with the relative distribution of EEG activity during sleep. Specifically, relative theta occipitality predicted performance improvement over the nap in young adults, right hippocampal activation prior to the nap predicted sigma frontality during the nap in young and older adults, and aging-related increases in relative sigma laterality during the nap were partially mediated by aging-related declines in left IPC activation prior to the nap. These results support and extend prior findings of theta- and sigma-band activity involvement in sleep-dependent consolidation of motor sequence memories. Moreover, the limiting of the mediation of aging-related change in sigma by aging-related decreases in striato-cortical network (i.e., left IPC) activation to lateral scalp regions is in keeping with our prediction that relationships between sleep microstructure and consolidation in older adults would be reduced over scalp regions where aging-related declines in sleep neural activity are largest. Absolute sigma over lateral scalp did not decline with age; it is likely then that lateral sigma differences between young and older adults represent larger contributions of state-like functional mechanism differences with aging, rather than trait-like structural brain differences with aging, leading to more clear relationships with task-related functional activation.
4.5. Limitations
This study examined brain activation before (encoding) and after (delayed test) nap and wake intervals, within-subjects, in young and older adults. While this is an ideal design, with few studies reporting all of these measures in a single data set, it also creates limitations. Most importantly, given the time and energy demands of this paradigm (two eight-hour visits to the lab), the older adults who volunteered to participate are not likely representative of others in their age group. Thus, the older adults in this study may have performed better at encoding than those in previous studies, which provides an alternative account for the present findings. An additional compromise of this design is that parameters which enhanced learning in the older adults caused young adult performance to be at ceiling. This tempers the contrasts of learning and consolidation between the two age groups. Finally, while the wealth of recorded measures is valuable, it yields an extensive number of comparisons. Though we have adjusted for the number of comparisons via bootstrapping methods and false discovery rate controls, we recognize the continued possibility of false positives when examining a large dataset, and the importance of replicating our individual findings in larger datasets to increase confidence in them.
4.6. Conclusions
In conclusion, we demonstrate that when encoding is sufficiently strong, older adults show sleep-dependent consolidation of motor sequence learning as assessed by the explicit variant of the SRTT. The neural mechanisms supporting this consolidation are similar to those in young adults, indicating that these mechanisms are preserved across aging. Specifically, in keeping with the proposal of Stickgold (2009), sleep-dependent motor sequence consolidation does not occur until encoding strength is sufficiently high to engage the hippocampus, and ceases to occur when encoding strength is sufficiently high to establish a motor cortical representation. Together, these results suggest that deficits in sleep-dependent motor sequence consolidation previously reported in older adults stem from insufficient encoding strength, likely due to aging-related increases in difficulty managing task demands, rather than from aging-related changes to the neural mechanisms of memory consolidation.
Supplementary Material
Acknowledgements
The authors would like to acknowledge Phuong Bui, Aazam Najeebi, and Sarah Spagnolo for assistance with aspects of the work contained in this manuscript, and Dr. Bethany Jones for comments on versions of this manuscript. This work was supported by National Institutes of Health award R01 AG040133 (PI: Spencer).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Competing Interest
Declarations of interest: none.
References
- Agler R, & De Boeck P (2017). On the interpretation and use of mediation: Multiple perspectives on mediation analysis. Frontiers in Psychology, 8, 1984. 10.3389/fpsyg.2017.01984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizenstein HJ, Butters MA, Clark KA, Figurski JL, Andrew Stenger V, Nebes RD, Reynolds CF, & Carter CS (2006). Prefrontal and striatal activation in elderly subjects during concurrent implicit and explicit sequence learning. Neurobiology of Aging, 27(5), 741–751. 10.1016/j.neurobiolaging.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Albouy G, Fogel S, King BR, Laventure S, Benali H, Kami A, Carrier J, Robertson EM, & Doyon J (2015). Maintaining vs. enhancing motor sequence memories: Respective roles of striatal and hippocampal systems. NeuroImage, 108, 423–434. 10.1016/j.neuroimage.2014.12.049. [DOI] [PubMed] [Google Scholar]
- Albouy G, Fogel S, Pottiez H, Nguyen VA, Ray L, Lungu O, … van Swinderen B (2013). Daytime Sleep Enhances Consolidation of the Spatial but Not Motoric Representation of Motor Sequence Memory. PLoS ONE, 8(1), e52805. 10.1371/journal.pone.0052805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albouy G, Sterpenich V, Balteau E, Vandewalle G, Desseilles M, Dang-Vu T, Darsaud A, Ruby P, Luppi P-H, Degueldre C, Peigneux P, Luxen A, & Maquet P (2008). Both the hippocampus and striatum are involved in consolidation of motor sequence memory. Neuron, 58(2), 261–272. 10.10l6/j.neuron.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Albouy G, Sterpenich V, Vandewalle G, Darsaud A, Gais S, Rauchs G, … Robertson E (2013). Interaction between Hippocampal and Striatal Systems Predicts Subsequent Consolidation of Motor Sequence Memory. PLoS ONE, 8(3), e59490. 10.1371/journal.pone.0059490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashe J, Lungu OV, Basford AT, & Lu X (2006). Cortical control of motor sequences. Current Opinion in Neurobiology, 16(2), 213–221. 10.1016/j.conb.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Axmacher N, Mormann F, Fernández G, Cohen MX, Elger CE, & Fell J (2007). Sustained neural activity patterns during working memory in the human medial temporal lobe. Journal of Neuroscience, 27(29), 7807–7816. 10.1523/JNEUROSCI.0962-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baptiste A (2017). gridExtra: Miscellaneous Functions for “Grid” Graphics (2.3).
- Barakat M, Carrier J, Debas K, Lungu O, Fogel S, Vandewalle G, Hoge RD, Bellec P, Kami A, Ungerleider LG, Benali H, & Doyon J (2013). Sleep spindles predict neural and behavioral changes in motor sequence consolidation. Human Brain Mapping, 34(11), 2918–2928. 10.1002/hbm.22116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barakat M, Doyon J, Debas K, Vandewalle G, Morin A, Poirier G, Martin N, Lafortune M, Kami A, Ungerleider LG, Benali H, & Carrier J (2011). Fast and slow spindle involvement in the consolidation of a new motor sequence. Behavioural Brain Research, 217(1), 117–121. 10.1016/j.bbr.2010.10.019. [DOI] [PubMed] [Google Scholar]
- Baron RM, & Kenny DA (1986). The Moderator-Mediator Variable Distinction in Social Psychological Research: Conceptual, Strategic, and Statistical Considerations. Journal of Personality and Social Psychology, 51(6), 1173–1182. 10.1037/0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Ball R, & Ranieri WF (1996). Comparison of Beck depression inventories -IA and -II in psychiatric outpatients. Journal of Personality Assessment, 67 (3), 588–597. 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, & Hochberg Y (1995). Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society: Series B (Methodological), 57(1), 289–300. 10.l11l/j.2517-6161.1995.tb02031.x. [DOI] [Google Scholar]
- Bennett IJ, Howard JH, & Howard DV (2007). Age-related differences in implicit learning of subtle third-order sequential structure. Journals of Gerontology - Series B Psychological Sciences and Social Sciences, 62(2), P98–P103. 10.1093/geronb/62.2.P98. [DOI] [PubMed] [Google Scholar]
- Binder S, Baier PC, Mölle M, Inostroza M, Born J, & Marshall L (2012). Sleep enhances memory consolidation in the hippocampus-dependent object-place recognition task in rats. Neurobiology of Learning and Memory, 97(2), 213–219. 10.1016/j.nlm.2011.12.004. [DOI] [PubMed] [Google Scholar]
- Bo J, Borza V, & Seidler RD (2009). Age-related declines in visuospatial working memory correlate with deficits in explicit motor sequence learning. Journal of Neurophysiology, 102(5), 2744–2754. 10.1152/jn.00393.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bo J, & Seidler RD (2009). Visuospatial working memory capacity predicts the organization of acquired explicit motor sequences. Journal of Neurophysiology, 101 (6), 3116–3125. 10.1152/jn.00006.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bo J, Jennett S, & Seidler RD (2012). Differential working memory correlates for implicit sequence performance in young and older adults. Experimental Brain Research, 221(4), 467–477. 10.1007/s00221-012-3189-2. [DOI] [PubMed] [Google Scholar]
- Bottary R, Sonni A, Wright D, & Spencer RMC (2016). Insufficient chunk concatenation may underlie changes in sleep-dependent consolidation of motor sequence learning in older adults. Learning and Memory, 23(9), 455–459. 10.1101/lm.043042.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutin A, Massen C, & Heuer H (2013). Modality-specific organization in the representation of sensorimotor sequences. Frontiers in Psychology, 4, 937. 10.3389/fpsyg.2013.00937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RM, Robertson EM, Press DZ, & Zak P (2009). Sequence skill acquisition and offline learning in normal aging. PLoS ONE, 4(8), e6683. 10.1371/journal.pone.0006683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, & Kupfer DJ (1989). The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Research, 28(2), 193–213. 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Cohen DA, Pascual-Leone A, Press DZ, & Robertson EM (2005). off-line learning of motor skill memory: A double dissociation of goal and movement. Proceedings of the National Academy of Sciences of the United States of America, 102(50), 18237–18241. 10.1073/pnas.0506072102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins JN, El-Deredy W, Parkes LM, Hennies N, & Lewis PA (2014). Cued memory reactivation during slow-wave sleep promotes explicit knowledge of a motor sequence. Journal of Neuroscience, 34(48), 15870–15876. 10.1523/JNEUROSCI.1011-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran T (1997). Effects of aging on implicit sequence learning: Accounting for sequence structure and explicit knowledge. Psychological Research, 60(1–2), 24–41. 10.1007/BF00419678. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Rombouts SARB, Veltman DJ, Raaijmakers JGW, & Jonker C (2003). Similar network activated by young and old adults during the acquisition of a motor sequence. Neurobiology of Aging. 24(7), 1013–1019. 10.1016/S0197-4580(03)00030-7. [DOI] [PubMed] [Google Scholar]
- Debas K, Carrier J, Barakat M, Marrelec G, Bellec P, Tahar AH, Karni A, Ungerleider LG, Benali H, & Doyon J (2014). Off-line consolidation of motor sequence learning results in greater integration within a cortico-striatal functional network. NeuroImage, 99, 50–58. 10.1016/j.neuroimage.2014.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debas K, Carrier J, Orban P, Barakat M, Lungu O, Vandewalle G, Tahar AH, Bellee P, Karni A, Ungerleider LG, Benali H, & Doyon J (2010). Brain plasticity related to the consolidation of motor sequence learning and motor adaptation. Proceedings of the National Academy of Sciences of the United States of America, 107(41), 17839–17844. 10.1073/pnas.1013176107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A, & Makeig S (2004). EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods, 134(1), 9–21. 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Destrebecqz A, Peigneux P, Laureys S, Degueldre C, Fiore GD, Aerts J, Luxen A, Van Der Linden M, Cleeremans A, & Maquet P (2005). The neural correlates of implicit and explicit sequence learning: Interacting networks revealed by the process dissociation procedure. Learning and Memory, 12(5), 480–490. 10.1101/lm.95605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyon J, Gabitov E, Vahdat S, Lungu O, & Boutin A (2018). Current issues related to motor sequence learning in humans. In Current Opinion in Behavioral Sciences (Vol. 20, pp. 89–97). Elsevier Ltd.. 10.1016/j.cobeha.2017.l1.012 [DOI] [Google Scholar]
- Doyon J, Bellec P, Amsel R, Penhune V, Monchi O, Carrier J, … Benali H (2009). Contributions of the basal ganglia and functionally related brain structures to motor learning. Behavioural Brain Research, 199(1), 61–75. 10.1016/j.bbr.2008.11.012. [DOI] [PubMed] [Google Scholar]
- Feeney JJ, Howard JH, & Howard DV (2002). Implicit learning of higher order sequences in middle age. Psychology and Aging, 17(2), 351–355. 10.1037/0882-7974.17.2.351. [DOI] [PubMed] [Google Scholar]
- Fitzroy AB, Kainec KA, & Spencer RMC (under review). Aging-related changes in nap neuroscillatory activity are mediated and moderated by grey matter volume. [DOI] [PMC free article] [PubMed]
- Fogel SM, Albouy G, Vien C, Popovicci R, King BR, Hoge R, … Doyon J (2014). fMRl and sleep correlates of the age-related impairment in motor memory consolidation. Human Brain Mapping. 35(8), 3625–3645. 10.1002/hbm.22426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser SA, Li KZH, & Penhune VB (2009). A comparison of motor skill learning and retention in younger and older adults. Experimental Brain Research, 195(3), 419–427. 10.1007/s00221-009-1806-5. [DOI] [PubMed] [Google Scholar]
- Freeman WJ (2004). Origin, structure, and role of background EEG activity. Part 1. Analytic amplitude. Clinical Neurophysiology, 115(9), 2077–2088. 10.1016/j.clinph.2004.02.029. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Hazeltine E, & Ivry RB (1998). Abstract and effector-specific representations of motor sequences identified with pet. Journal of Neuroscience, 18 (22), 9420–9428. https://doi.oig/10.1523/jneurosci.18-22-09420.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel AM, & Grafton ST (2015). The striatum: Where skills and habits meet. Cold Spring Harbor Perspectives in Biology, 7(8), a021691. 10.1101/cshperspect.a021691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudberg C, Wulff K, & Johansen-Berg H (2015). Sleep-dependent motor memory consolidation in older adults depends on task demands. Neurobiology of Aging, 36(3), 1409–1416. 10.1016/j.neurobiolaging.2014.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick RM, Rottschy C, Miall RC, & Eickhoff SB (2013). A quantitative meta-analysis and review of motor learning in the human brain. NeuroImage. 67, 283–297. 10.1016/j.neuroimage.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauptmann B, Reinhart E, Brandt SA, & Karni A (2005). The predictive value of the leveling off of within-session performance for procedural memory consolidation. Cognitive Brain Research, 24(2), 181–189. 10.10l6/j.cogbrainres.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Sakai K, Lu X, Nakahara H, Rand MK, Nakamura K, … Doya K (1999). Parallel neural networks for learning sequential procedures. Trends in Neurosciences, 22(10), 464–471. 10.1016/S0166-2236(99)01439-3. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Nakamura K, Sakai K, & Nakahara H (2002). Central mechanisms of motor skill learning. Current Opinion in Neurobiology, 12(2), 217–222. Elsevier Ltd. [DOI] [PubMed] [Google Scholar]
- Hoddes E, Zarcone V, Smythe H, Phillips R, & Dement WC (1973). Quantification of Sleepiness: A New Approach. Psychophysiology, 10(4), 431–436. 10.1111/j.l469-8986.1973.tb00801.x. [DOI] [PubMed] [Google Scholar]
- Home JA, & Ostberg O (1976). A self assessment questionnaire to determine Morningness Eveningness in human circadian rhythms. International Journal of Chronobiology, 4(2), 97–110. [PubMed] [Google Scholar]
- Howard DV, & Howard JH (1989). Age differences in learning serial patterns: Direct versus indirect measures. Psychology and Aging, 4(3), 357–364. 10.1037/0882-7974.4.3.357. [DOI] [PubMed] [Google Scholar]
- Howard DV, & Howard JH (1992). Adult age differences in the rate of learning serial patterns: Evidence from direct and indirect tests. Psychology and Aging, 7(2), 232–241. 10.1037/0882-7974.7.2.232. [DOI] [PubMed] [Google Scholar]
- Howard DV, & Howard JH (2001). When it does hurt to try: Adult age differences in the effects of instructions on implicit pattern learning. Psychonomic Bulletin and Review. 8(4), 798–805. 10.3758/BF03196220. [DOI] [PubMed] [Google Scholar]
- Howard DV, Howard JH, Japikse K, DiYanni C, Thompson A, & Somberg R (2004). Implicit Sequence Learning: Effects of Level of Structure, Adult Age, and Extended Practice. Psychology and Aging. 19(1), 79–92. 10.1037/0882-7974.19.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iber C, Ancoli-Israel S, Chesson AL, & Quan SF (2007). The AASM manual for die scoring of sleep and associated events: Rules, terminology, and technical specifications. American Academy of Sleep Medicine. [Google Scholar]
- Imai K, Keele L, & Tingley D (2010). A General Approach to Causal Mediation Analysis. Psychological Methods, 15(4), 309–334. 10.1037/a0020761. [DOI] [PubMed] [Google Scholar]
- Inostroza M, Binder S, & Born J (2013). Sleep-dependency of episodic-like memory consolidation in rats. Behavioural Brain Research, 237, 15–22. 10.1016/j.bbr.2012.09.011. [DOI] [PubMed] [Google Scholar]
- Janacsek K, & Nemeth D (2013). Implicit sequence learning and working memory: Correlated or complicated? Cortex. 49(8), 2001–2006. 10.1016/J.CORTEX.2013.02.012. [DOI] [PubMed] [Google Scholar]
- Janacsek K, Shattuck KF, Tagarelli KM, Lum JAG, Turkeltaub PE, & Ullman MT (2020). Sequence learning in the human brain: A functional neuroanatomical meta-analysis of serial reaction time studies. NeuroImage, 207, 116387. 10.1016/j.neuroimage.2019.l16387. [DOI] [PubMed] [Google Scholar]
- Johns MW (1991). A new method for measuring daytime sleepiness: The Epworth Sleepiness Scale. Sleep, 14(6), 540–545. 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- Karni A, Meyer G, Jezzard P, Adams MM, Turner R, & Ungerleider LG (1995). Functional MRI evidence for adult motor cortex plasticity during motor skill learning. Nature, 377(6545), 155–158. 10.1038/377155a0. [DOI] [PubMed] [Google Scholar]
- Kelemen E, Bahrendt M, Born J, & Inostroza M (2014). Hippocampal corticosterone impairs memory consolidation during sleep but improves consolidation in the wake state. Hippocampus, 24(5), 510–515. 10.1002/hipo.22266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King BR, Fogel SM, Albouy G, & Doyon J (2013). Neural correlates of the age-related changes in motor sequence learning and motor adaptation in older adults. Frontiers in Human Neuroscience, 7, 142. 10.3389/fnhum.2013.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King BR, Hoedlmoser K, Hirschauer F, Dolfen N, & Albouy G (2017). Sleeping on the motor engram: The multifaceted nature of sleep-related motor memory consolidation. Neuroscience & Biobehavioral Reviews, 90, 1–22. 10.1016/j.neubiorev.2017.04.026. [DOI] [PubMed] [Google Scholar]
- King BR, Saucier P, Albouy G, Fogel SM, Rumpf JJ, Klann J, … Doyon J (2017). Cerebral activation during initial motor learning forecasts subsequent sleep-facilitated memory consolidation in older adults. Cerebral Cortex, 27(2), 1588–1601. 10.1093/cercor/bhv347. [DOI] [PubMed] [Google Scholar]
- Korman M, Dagan Y, & Karni A (2015). Nap it or leave it in the elderly: A nap after practice relaxes age-related limitations in procedural memory consolidation. Neuroscience Letters. 606, 173–176. 10.1016/j.neulet.2015.08.051. [DOI] [PubMed] [Google Scholar]
- Korman M, Doyon J, Doljansky J, Carrier J, Dagan Y, & Karni A (2007). Daytime sleep condenses the time course of motor memory consolidation. Nature Neuroscience, 10(9), 1206–1213. 10.1038/nn1959. [DOI] [PubMed] [Google Scholar]
- Kuriyama K, Stickgold R, & Walker MP (2004). Sleep-dependent learning and motor-skill complexity. Learning and Memory, 11(6), 705–713. 10.1101/lm.76304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohse KR, Wadden K, Boyd LA, & Hodges NJ (2014). Motor skill acquisition across short and long time scales: A meta-analysis of neuroimaging data. Neuropsychologia, 59(1), 130–141. 10.1016/j-neuropeychologia.2014.05.001. [DOI] [PubMed] [Google Scholar]
- Lopez-Calderon J, & Luck SJ (2014). ERPLAB: An open-source toolbox for the analysis of event-related potentials. Frontiers in Human Neuroscience, 8, 213. 10.3389/fnhum.2014.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lungu O, Monchi O, Albouy G, Jubault T, Ballarin E, Burnod Y, … Robertson E (2014). Striatal and hippocampal involvement in motor sequence chunking depends on the learning strategy. PLoS ONE, 9(8), e103885. 10.1371/journal.pone.0103885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon DP, Krull JL, & Lockwood CM (2000). Equivalence of the mediation, confounding and suppression effect. Prevention Science, 7(4), 173–181. 10.1023/A:1026595011371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoach DS, Cain MS, Vangel MG, Khurana A, Goff DC, & Stickgold R (2004). A failure of sleep-dependent procedural learning in chronic, medicated schizophrenia. Biological Psychiatry, 56(12), 951–956. 10.1016/j.biopeych.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Menicucci D, Piarulli A, Laurino M, Zaccaro A, Agrimi J, & Gemignani A (2020). Sleep slow oscillations favour local cortical plasticity underlying the consolidation of reinforced procedural learning in human sleep. Journal of Sleep Research, 29(5). 10.1111/jsr.13117. [DOI] [PubMed] [Google Scholar]
- Muehlroth BE, Sander MC, Fandakova Y, Grandy TH, Rasch B, Lee Shing Y, & Werkle-Bergner M (2020). Memory quality modulates the effect of aging on memory consolidation during sleep: Reduced maintenance but intact gain. NecuroImage. 209, 116490. 10.1016/j.neuroimage.2019.116490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan NS, & Laubach M (2006). Top-Down Control of Motor Cortex Ensembles by Dorsomedial Prefrontal Cortex. Neuron, 52(5), 921–931. 10.1016/j.neuron2006.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth D, & Janacsek K (2011). The dynamics of implicit skill consolidation in young and elderly adults. Journals of Gerontology - Series B Psychological Sciences and Social Sciences, 66B(1), 15–22. 10.1093/geronb/gbq063. [DOI] [PubMed] [Google Scholar]
- Nemeth D, Janacsek K, Londe Z, Ullman MT, Howard DV, & Howard JH (2010). Sleep has no critical role in implicit motor sequence learning in young and old adults. Experimental Brain Research, 201(2), 351–358. 10.1007/s00221-009-2024-x. [DOI] [PubMed] [Google Scholar]
- Nishida M, Walker MP, & Miall C (2007). Daytime naps, motor memory consolidation and regionally specific sleep spindles. PLoS ONE, 2(4), e341. 10.1371/journal.pone.0000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissen MJ, & Bullemer P (1987). Attentional requirements of learning: Evidence from performance measures. Cognitive Psychology. 19(1), 1–32. 10.1016/0010-0285(87)90002-8. [DOI] [Google Scholar]
- Nobre AC, & van Ede F (2017). Anticipated moments: temporal structure in attention. Nature Reviews Neuroscience, 19(1), 34–48. 10.1038/nm.2017.141. [DOI] [PubMed] [Google Scholar]
- Oostenveld R, Fries P, Maris E, & Schoffelen J-M (2011). FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Computational Intelligence and Neuroscience, 2011, 1–9. 10.1155/2011/156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace-Schott EF, & Spencer RMC (2013). Age-related changes in consolidation of perceptual and muscle-based learning of motor skills. Frontiers in Aging. Neuroscience, 5. 10.3389/fnagi.2013.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penhune VB, & Doyon J (2002). Dynamic cortical and subcortical networks in learning and delayed recall of timed motor sequences. Journal of Neuroscience, 22(4), 1397–1406. 10.1523/jneurosci.22-04-01397.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penhune VB, & Steele CJ (2012). Parallel contributions of cerebellar, striatal and M1 mechanisms to motor sequence learning. Behavioural Brain Research, 226(2), 579–591. 10.1016/j.bbr.2011.09.044. [DOI] [PubMed] [Google Scholar]
- Picard N, & Strick PL (1996). Motor Areas of the Medial Wall: A Review of Their Location and Functional Activation. Cerebral Cortex, 6(3), 342–353. 10.1093/CERCOR/6.3.342. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Sabb FW, Foerde K, Tom SM, Asarnow RF, Bookheimer SY, & Knowlton BJ (2005). The Neural Correlates of Motor Skill Automaticity. Journal of Neuroscience, 25(22), 5356–5364. 10.1523/JNEUROSCI.3880-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. (2020). R: A language and environment for statistical computing. R Foundation for Statistical Computing. [Google Scholar]
- Repp BH (2005). Sensorimotor synchronization: A review of the tapping literature. Psychonomic Bulletin and Review, 12(6), 969–992. 10.3758/BF03206433. [DOI] [PubMed] [Google Scholar]
- Repp BH, & Su YH (2013). Sensorimotor synchronization: A review of recent research (2006–2012). Psychonomic Bulletin and Review. 20(3), 403–452. 10.3758/s13423-012-0371-2. [DOI] [PubMed] [Google Scholar]
- Rickard TC, & Pan SC (2017). Time for considering the possibility that sleep plays no unique role in motor memory consolidation: Reply to Adi-Japha and Karni (2016). Psychological Bulletin, 143(4), 454–458. 10.1037/bul0000094. [DOI] [PubMed] [Google Scholar]
- Rieckmann A, & Bäckman L (2009). Implicit learning in aging: Extant patterns and new directions. Neuropsychology Review, 19(4), 490–503. 10.1007/s11065-009-9117-y. [DOI] [PubMed] [Google Scholar]
- Rieckmann A, Fischer H, & Bäckman L (2010). Activation in striatum and medial temporal lobe during sequence learning in younger and older adults: Relations to performance. NeuroImage, 50(3), 1303–1312. 10.1016/j.neuroimage.2010.01.015. [DOI] [PubMed] [Google Scholar]
- Robertson EM (2007). The serial reaction time task: Implicit motor skill learning? Journal of Neuroscience, 27(38), 10073–10075. 10.1523/JNEUROSCI.2747-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson EM, Pascual-Leone A, & Press DZ (2004). Awareness Modifies the Skill-Learning Benefits of Sleep. Current Biology, 14(3), 208–212. 10.1016/j.cub.2004.01.027. [DOI] [PubMed] [Google Scholar]
- Roig M, Ritterband-Rosenbaum A, Lundbye-Jensen J, & Nielsen JB (2014). Aging increases the susceptibility to motor memory interference and reduces off-line gains in motor skill learning. Neurobiology of Aging 35(8), 1892–1900. 10.1016/j.neurobiolaging.2014.02.022. [DOI] [PubMed] [Google Scholar]
- Romano JC, Howard JH, & Howard DV (2010). One-year retention of general and sequence-specific skills in a probabilistic, serial reaction time task. Memory, 18(4), 427–441. 10.1080/09658211003742680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum DA, Hindorff V, fit Munro EM (1987). Scheduling and Programming of Rapid Finger Sequences: Tests and Elaborations of the Hierarchical Editor Model. Journal of Experimental Psychology: Human Perception and Performance, 13(2), 193–203. 10.1037/0096-1523.13.2.193. [DOI] [PubMed] [Google Scholar]
- Rucker DD, Preacher KJ, Tormala ZL, & Petty RE (2011). Mediation Analysis in Social Psychology: Current Practices and New Recommendations. Social and Personality Psychology Compass, 5(6), 359–371. 10.1111/j.1751-9004.2011.00355.x. [DOI] [Google Scholar]
- Saletin JM, & Greer S (2015). Hume Software Package (previously sleepSMG): Open-Source MATLAB User Interface for Scoring Sleep [MATLAB]. https://github.com/jsaletin/hume.
- Salthouse TA (1996). The Processing-Speed Theory of Adult Age Differences in Cognition. Psychological Review. 103(3), 403–428. 10.1037/0033-295X.103.3.403. [DOI] [PubMed] [Google Scholar]
- Shea CH, Park JH, & Braden HW (2006). Age-related effects in sequential motor learning. Physical Therapy, 86(4), 478–488. 10.1093/ptj/86.4.478. [DOI] [PubMed] [Google Scholar]
- Shin JC, & Ivry RB (2002). Concurrent Learning of Temporal and Spatial Sequences. Journal of Experimental Psychology: Learning Memory and Cognition, 23(3), 445–457. 10.1037/0278-7393.28.3.445. [DOI] [PubMed] [Google Scholar]
- Siengsukon C, & Boyd LA (2009a). Sleep to learn after stroke: Implicit and explicit off-line motor learning. Neuroscience Letters, 451(1), 1–5. 10.1016/j.neulet.2008.12.040. [DOI] [PubMed] [Google Scholar]
- Siengsukon C, & Boyd LA (2009b). Sleep enhances off-line spatial and temporal motor learning after stroke. Neurorehabilitation and Neural Repair, 23(4), 327–335. 10.1177/1545968308326631. [DOI] [PubMed] [Google Scholar]
- Smith SM, & Nichols TE (2009). Threshold-free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. Ncurolmagc, 44(1), 83–98. 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Song S, Howard JH, & Howard DV (2007). Sleep does not benefit probabilistic motor sequence learning. Journal of Neuroscience, 27(46), 12475–12483. 10.1523/JNEUROSCI.2062-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonni A, & Spencer RMC (2015). Sleep protects memories from interference in older adults. Neurobiology of Aging, 36(7), 2272–2281. 10.1016/j.neurobiolaging.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer RMC, Gouw AM, & Ivry RB (2007). Age-related decline of sleep-dependent consolidation. Learning and Memory, 14(7), 480–484. 10.1101/lm.569407. [DOI] [PubMed] [Google Scholar]
- Spencer RMC, Sunm M, & Ivry RB (2006). Sleep-Dependent Consolidation of Contextual Learning. Current Biology. 16(10), 1001–1005. 10.1016/j.cub.2006.03.094. [DOI] [PubMed] [Google Scholar]
- Sprecher KE, Riedner BA, Smith RF, Tononi G, Davidson RJ, Benca RM, … Mongrain V (2016). High resolution topography of age-related changes in non-rapid eye movement sleep electroencephalography. PLOS ONE, 11(2), e0149770. 10.1371/joumal.pone.0149770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steer RA, & Clark DA (1997). Psychometric characteristics of the beck depression inventory-II with college students. Measurement and Evaluation in Counseling and Development. 30(3), 128–136. 10.1080/07481756.1997.12068933. [DOI] [Google Scholar]
- Stickgold R (2009). How do I remember? Let me count the ways. Sleep Medicine Reviews, 13(5), 305–308. 10.1016/j.smrv.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaki M, Huang TR, Yotsumoto Y, Hämäläinen M, Lin FH, Náñez JE, Watanabe T, & Sasaki Y (2013). Enhanced spontaneous oscillations in the supplementary motor area are associated with sleep-dependent offline learning of finger-tapping motor-sequence task. Journal of Neuroscience, 33(34), 13894–13902. 10.1523/JNEUROSCI.1198-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tingley D, Yamamoto T, Hirose K, Keele L, & Imai K (2014). mediation: R Package for Causal Mediation Analysis. Journal of Statistical Software, 59(5). 10.18637/jss.v059.i05. [DOI] [Google Scholar]
- Toni I, Krams M, Turner R, & Passingham RE (1998). The Time Course of Changes during Motor Sequence Learning: A Whole-Brain fMRI Study. NeuroImage, 8(l), 50–61. 10.1006/NIMG.1998.0349. [DOI] [PubMed] [Google Scholar]
- Tucker MA, & Fishbein W (2009). The impact of sleep duration and subject intelligence on declarative and motor memory performance: How much is enough? Journal of Sleep Research, 13(3), 304–312. 10.l111/j.1365-2869.2009.00740.x. [DOI] [PubMed] [Google Scholar]
- Tucker M, McKinley S, & Stickgold R (2011). Sleep optimizes motor skill in older adults. Journal of the American Geriatrics Society, 59(4), 603–609. 10.1111/j.1532-5415.2011.03324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MP, Stickgold R, Alsop D, Gaab N, & Schlaug G (2005). Sleep-dependent motor memory plasticity in the human brain. Neuroscience, 133(4), 911–917. 10.1016/j.neuroscience.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Vahdat S, FogeL S, Benali H, & Doyon J (2017). Network-wide reorganization of procedural memory during NREM sleep revealed by fMRI. ELife, 6. 10.7554/eLife.24987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker Matthew P., Brakefield T, Morgan A, Hobson JA, & Stickgold R (2002). Practice with sleep makes perfect: Sleep-dependent motor skill learning. Neuron, 35 (1), 205–211. 10.1016/80896-6273(02)00746-8. [DOI] [PubMed] [Google Scholar]
- Wickham H (2016). ggplot2: Elegant Graphics for Data Analysis (Second). Springer International Publishing. [Google Scholar]
- Wilhelm I, Diekelmann S, Molzow I, Ayoub A, Mölle M, & Born J (2011). Sleep selectively enhances memory expected to be of future relevance. Journal of Neuroscience, 31(5), 1563–1569. 10.1523/JNEUROSCI.3575-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm I, Metzkow-Mészàros M, Knapp S, & Born J (2012). Sleep-dependent consolidation of procedural motor memories in children and adults: The pre-sleep level of performance matters. Developmental Science, 15(4), 506–515. 10.1111/j.1467-7687.2012.01146.x. [DOI] [PubMed] [Google Scholar]
- Willingham DB, & Goedert-Eschmann K (1999). The Relation between Implicit and Explicit Learning: Evidence for Parallel Development. Psychological Science, 10(6), 531–534. 10.1111/1467-9280.00201. [DOI] [Google Scholar]
- Willingham DB, Nissen MJ, & Bullemer P (1989). On the Development of Procedural Knowledge. Journal of Experimental Psychology: Learning Memory, and Cognition, 15(6), 1047–1060. 10.1037/0278-7393.15.6.1047. [DOI] [PubMed] [Google Scholar]
- Willingham DB, Salidis J, & Gabrieli JDE (2002). Direct comparison of neural systems mediating conscious and unconscious skill learning. Journal of Neurophysiology, 88(3), 1451–1460. 10.1152/jn.2002.88.3.1451. [DOI] [PubMed] [Google Scholar]
- Wilson JK, Baran B, Pace-Schott EF, Ivry RB, & Spencer RMC (2012). Sleep modulates word-pair learning but not motor sequence learning in healthy older adults. Neurobiology of Aging, 33(5), 991–1000. 10.1016/j.neurobiolaging.2011.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt K, Margraf N, Bieber C, Born J, & Deuschl G (2010). Sleep consolidates the effector-independent representation of a motor skill. Neuroscience, 171(1), 227–234. 10.1016/j.neuroscience.2010.07.062. [DOI] [PubMed] [Google Scholar]
- Wu T, Kansaku K, & Hallett M (2004). How Self-Initiated Memorized Movements Become Automatic: A Functional MRI Study. Journal of Neurophysiology, 91(4), 1690–1698. 10.1152/jn.01052.2003. [DOI] [PubMed] [Google Scholar]
- Wymbs NF, Bassett DS, Mucha PJ, Porter MA, & Grafton ST (2012). Differential Recruitment of the Sensorimotor Putamen and Frontoparietal Cortex during Motor Chunking in Humans. Neuron, 74(5), 936–946. 10.1016/j.neuron.2012.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young CK, & McNaughton N (2009). Coupling of Theta Oscillations between Anterior and Posterior Midline Cortex and with the Hippocampus in Freely Behaving Rats. Cerebral Cortex, 19(1), 24–40. 10.1093/CERCOR/BHN055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


