Abstract
Early life adversities (ELA), include experiences such as child maltreatment, household dysfunction, bullying, exposure to crime, discrimination, bias, and victimization, and are recognized as social determinants of cardiovascular disease (CVD). Strong evidence shows exposure to ELA directly impacts cardiometabolic risk in adulthood and emerging evidence suggests there may be continuity in ELA’s prediction of cardiometabolic risk over the life course.
Extant research has primarily relied on a cumulative risk framework to evaluate the relationship between ELA and CVD. In this framework, risk is considered a function of the number of risk factors or adversities that an individual was exposed to across developmental periods. The cumulative risk exposure approach treats developmental periods and types of risk as equivalent and interchangeable. Moreover, cumulative risk models do not lend themselves to investigating the chronicity of adverse exposures or consider individual variation in susceptibility, differential contexts, or adaptive resilience processes, which may modify the impact of ELA on CVD risk.
To date, however, alternative models have received comparatively little consideration. Overall, this paper will highlight existing gaps and offer recommendations to address these gaps that would extend our knowledge of the relationship between ELA and CVD development. We focus specifically on the roles of: 1) susceptibility and resilience, 2) timing and developmental context; and 3) variation in risk exposure. We propose to expand current conceptual models to incorporate these factors to better guide research that examines ELA and CVD risk across the life course.
Overview
Early life adversities (ELA), which include experiences such as child maltreatment, household dysfunction (Felitti et al., 1998), bullying, exposure to crime, discrimination, bias, and victimization (Slopen et al., 2014; Suglia et al., 2015) are recognized as social determinants of cardiovascular disease (CVD). Strong evidence shows exposure to ELA leads to elevated cardiometabolic risk in adulthood; for example, ELA is prospectively associated with blood pressure trajectories in young adults, (Su et al., 2015) and higher blood pressure (Alastalo et al., 2013; Janicki-Deverts et al., 2012; Riley et al., 2010) and obesity in adulthood (Midei and Matthews, 2011). In addition, ELA is associated with a range of outcomes in adulthood, including elevated Hemoglobin A1c (Widom et al., 2012), incident metabolic syndrome (Midei et al., 2013), Type 2 diabetes (Rich-Edwards et al., 2010), and inflammation (Danese et al., 2007). Emerging evidence suggests similar relationships between ELA and cardiometabolic risk factors in childhood and adolescence. A recent study reported that verbally abusive behavior among mothers predicted higher systolic blood pressure, independent of body mass index, in children ages 5–6 (Smarius et al., 2018). The accumulation of ELA is also associated with obesity in early childhood (Suglia et al., 2012) with evidence suggesting adult obesity may be shaped by patterns of weight gain in early childhood (Rundle et al., 2020; Rundle et al., 2019; Stettler et al., 2003). Associations between ELA and inflammation have also been observed in adolescents in parallel to adult study findings (Miller and Chen, 2010). In sum, these research findings suggest there may be continuity in ELA’s impact on cardiometabolic risk over the life course. However, additional studies of children and adolescents are needed to precisely delineate the unique trajectories of individual cardiometabolic risk factors and the emergence of cardiometabolic disease over time. Addressing key gaps in our understanding of the role of timing, duration, and differential susceptibility to the effects of ELA, can inform important prevention and intervention efforts.
Experiences of ELA tend to cluster, particularly among racial/ethnic minorities and children in low-income households. As a result, extant research has primarily relied on a cumulative risk framework to evaluate the relationship between ELA and CVD. In this framework, the risk is considered a function of the total number of adversities that an individual is exposed to across developmental periods. The cumulative risk exposure approach treats developmental periods and numbers and types of adversities as equivalent and interchangeable, yet, the evidence indicates that adversity exposures may be more formative during specific developmental periods, and certain types of adversity may influence health differentially. Moreover, the research on the health consequences of ELA has primarily relied on a ‘risk factor-disease model’ (i.e., purely examining predictors of disease), without considering individual variation in susceptibility, differential contexts, or most importantly adaptive resilience processes, which may modify the impact of ELA on CVD risk.
An alternative to the cumulative risk model, a model that considers the timing, duration, type and context of exposure may be better suited to informing interventions (Figure 1). To date, however, alternative models have received comparatively little consideration. This conceptual review will highlight gaps in existing research on the relation between ELA and CVD and offer recommendations to address these gaps, specifically examining: 1) variability in susceptibility and resilience, 2) timing and developmental context; and 3) variability in risk exposure.
Fig. 1.
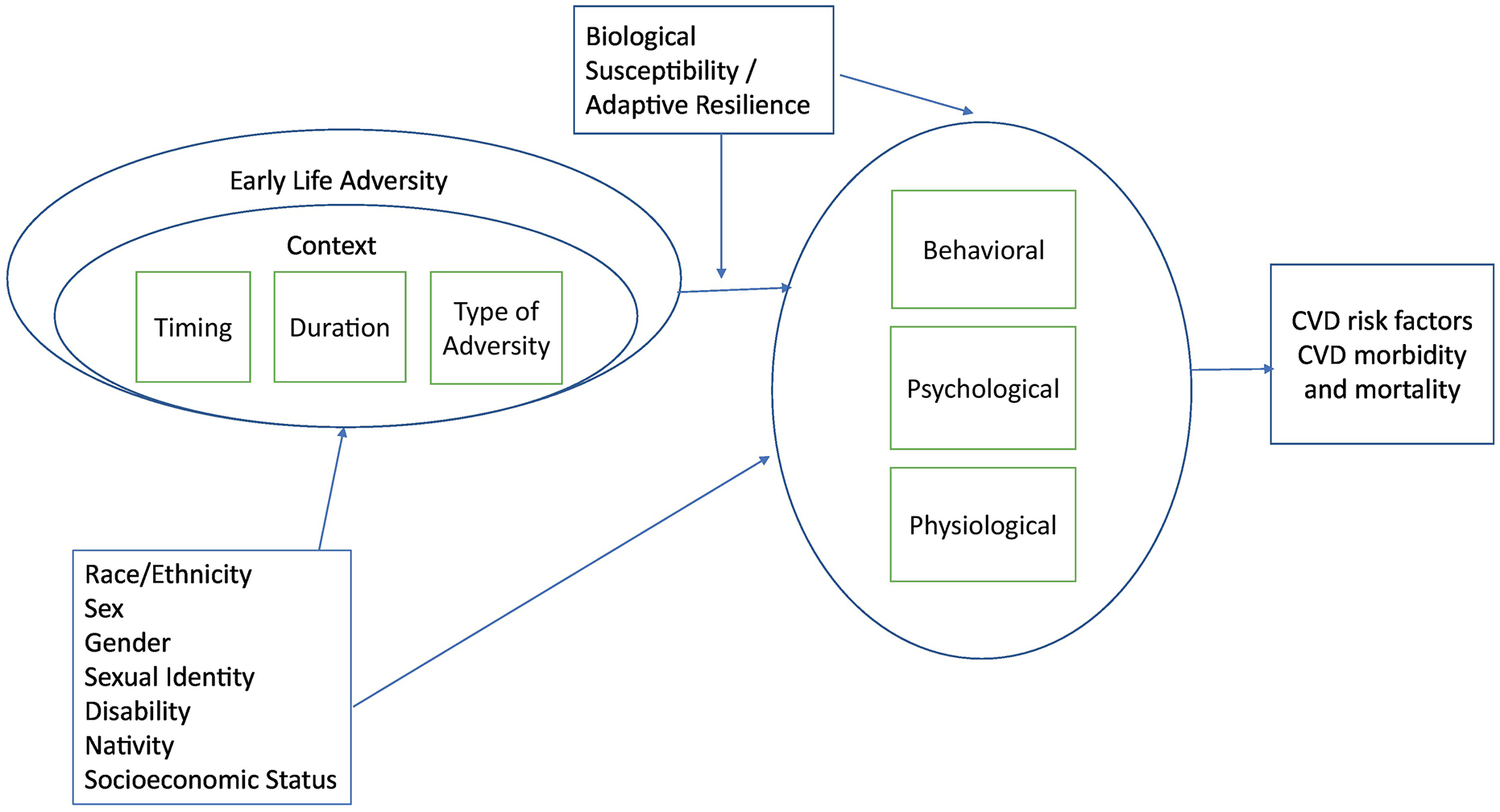
Expanded conceptual model of the relation between childhood adversities and cardiometabolic health.
VARIATION IN SUSCEPTIBILITY AND RESILIENCE
Individual Differences in Biological Reactivity to Early Adversity
Studies examining the relationship between ELA and health outcomes demonstrate considerable heterogeneity in outcomes among those who experienced ELA. For example, although evidence for the link between ELA and increased inflammation exists, this pathway is not evident for all individuals. Numerous studies assessing childhood stress and inflammation have failed to find associations between these two variables, or have found this link only among select subsets of youths, such as those with high adiposity, individual cognitive appraisal styles, socioeconomic backgrounds, or among particular racial or ethnic groups (Blevins et al., 2017; Chiang et al., 2017; Ehrlich et al., 2016; Giletta et al., 2018; Heard-Garris et al., 2020; Hostinar et al., 2017; Low et al., 2013). Differential response to ELA may be due to differences in biological sensitivity to a range of environmental influences or differential social context.
Biological Sensitivity to Context (BSC) theory (Boyce et al., 2005) proposes that children differ in their susceptibility to environmental influence “for better and for worse”, depending on their psychobiologic reactivity to the environment. Aligned with this theory, studies show that more reactive children (as indexed by heightened autonomic or adrenocortical responses to laboratory challenges, for example) display an increased sensitivity to both positive and negative environmental conditions (Bush and Boyce, 2016; Ellis et al., 2011). Research indicates that ELA exposures can result in either hyper-reactivity (high BSC) or hypo-reactivity (low BSC) of the autonomic nervous system (ANS) and hypothalamic-pituitary-adrenal (HPA) axis (Del Giudice et al., 2011). Several studies have noted that hyper- stress reactivity is associated with conditions that promote the development of CVD (Chida and Steptoe, 2010; Ginty et al., 2017). Hypo- stress reactivity has emerged as a marker of chronic dysregulation of the neuroendocrine stress response which is associated with increased risk for CVD (Ginty et al., 2017; Wiggert et al., 2016) including increases in blood pressure, coronary artery calcification and higher carotid intima media thickness (Turner et al., 2020). Studies to evaluate interventions designed to reduce CVD or CVD risk should consider heterogeneity in stress-reactivity and how it may impact the effects of the intervention. This is particularly important given that relatively strong intervention effects may occur among more reactive children and adults, representing a subset of those exposed to ELA.
Resilience
Risk models typically focus on measuring inherent risk characteristics and estimating the odds of disease given a specific risk factor or combination of risk factors. For example, a commonly cited statistic is that among individuals with no ELA, the lifetime prevalence of depression is 20%. In contrast, among individuals with five or more ELA, the lifetime prevalence of depression increases to 60% (Chapman et al., 2004). This is a large difference and an important finding as it points to the role of resiliency among the 40% of ELA-exposed individuals who did not become depressed. Given the “disease risk” orientation of research, however, the vast majority of studies linking ELA and health have focused on the population that develops the disease, with limited research on the population that remains healthy even in the face of ELA exposures (i.e., those who are resilient). Resilience is domain specific, and this orientation to disease risk extends to health outcomes as well. For example, in a recent study of 8609 young women (Loxton et al., 2021), the prevalence of current smoking increased in a graded fashion across categories of ELA exposures, ranging from 10.5%, to 32.1% among those who reported 0 and 4+ ELA, respectively. Similarly, the prevalence of severe obesity (BMI≥35) was 4.0% among those not reporting any adversities to 13.2% among those reporting 4+ ELA. While these patterns show an increasing level of risk associated with greater ELA exposure, from a resiliency perspective, more emphasis should be placed on better understanding the characteristics and circumstances of the majority of individuals who are exposed to a high number of ELA but do not smoke (67.9%) or become severely obese (86.8%). Further examination of the characteristics and circumstances of individuals who have ELA exposures but do not experience negative outcomes could identify targets for intervention.
One common definition of resilience is the capacity to realize better than expected outcomes, given an assessment of risk (Masten and Barnes, 2018; Wortman, 2004). This explanation highlights the development of resilience, namely that resilience does not occur in the absence of risk. However, it neglects consideration or quantification of positive outcomes that exceed the ‘absence of disease’. This limitation both contributes to and is driven by limitations in our conventional measures. For example, many chronic diseases have clinically-defined healthy, pre-clinical, and clinical disease states (e.g., healthy weight, overweight, obese), without a clear understanding of what outcomes are especially beneficial for long-term wellbeing in the context of risk exposure. In some cases, it may be possible to identify a resilient biological state – that is, a biological adaptation to adversity that protects against later disease. Likewise, several early life positive psychosocial and behavioral assets can promote the development of favorable cardiovascular health in adulthood (Slopen et al., 2017). Identifying these positive factors and mechanisms would open direct links to intervention that would offer some advantages over current approaches that aim to achieve biological states in adversity-exposed individuals that mirror those of non-adversity exposed individuals. It may be that what works well in low-risk conditions is, in fact, not adaptive in adverse conditions and that a more nuanced consideration would better direct intervention dollars once the risk has occurred (vs. in prevention models).
TIMING, TYPE AND DEVELOPMENTAL CONTEXT
Timing/Duration
There is increasing interest in identifying factors that underlie individual variation in responses to ELA, such as the developmental period of exposure. The term “sensitive period” refers to specific time points in development when individuals are most likely to be influenced by (or have the maximal response to) social or environmental exposures (Ben-Shlomo and Kuh, 2002; Hertzman and Boyce, 2010). Studies have investigated sensitive periods at multiple points in development, including the prenatal (Bale et al., 2010; Brown et al., 2000), childhood (Zeanah et al., 2011), and adolescent periods (Andersen and Teicher, 2008; Blakemore and Mills, 2014; Gunnar et al., 2019). However, it is challenging to rigorously test for sensitive periods in observational cohort designs given that negative (or positive) social exposures naturally cluster and also can influence the likelihood of subsequent exposures (Evans et al., 2013; McLaughlin, 2016). Studies that have examined the timing of ELA concerning outcomes in youth that are associated with later cardiovascular risk, such as adiposity (Jun et al., 2011; Ziol-Guest et al., 2009) suggest that timing of exposure to ELA matters – however, across existing research, studies are not consistent about which period of exposure presents the greatest risk.
ELA is hypothesized to have a negative impact on physical health because it occurs during a sensitive developmental window when a child first develops close relationship bonds (i.e., attachment relationships). Individual differences in attachment insecurity have a significant impact on both mental and physical health across the lifespan (Anderson et al., 2012; Anderson and Whitaker, 2011; Goossens et al., 2012). Adolescence may be an especially important period for investigating these individual differences because of the heightened sensitivity of adolescents to social-environmental cues (Blakemore & Mills, 2014). Evidence from psychology and neuroscience underscores adolescence as a sensitive period for the development and consolidation of emotion regulation skills as well as the maturation of neural regions involved in affect regulation (Steinberg, 2005).
Developmentally oriented models have largely focused on sensitive periods when risk exposures are hypothesized to be particularly salient for cardiovascular health (Barker, 1992; Bleil et al., 2015; Jasik and Lustig, 2008). For example, the Fetal Origins Hypothesis (Barker, 1992)—or Developmental Origins of Health and Disease Hypothesis—focuses on a range of detrimental exposures, including ELA, that occur during the period of gestation, ultimately shaping trajectories of cardiovascular health into adulthood (Barker et al., 1990; Hales et al., 1991; Valdez et al., 1994). Mechanisms of such effects, although not fully elucidated, may include epigenetic pathways through which early environments alter gene expression (without changing DNA sequences) (Dunn et al., 2019; Hao et al., 2018; Klengel et al., 2013).
Relative to prenatal exposures, ELA during other developmental periods such as puberty are less well studied. Yet, like the gestational period, puberty is a discrete developmental period during which time a dynamic set of biological events occur, shaping sexual maturation and the potential for human reproduction. It is plausible that the critical organizational processes that occur during puberty, if disrupted, could impact reproductive development and associated hormonal and metabolic factors relevant to adulthood cardiometabolic diseases (Bleil et al., 2015). Interestingly, related evidence shows ELA exposures influence the timing of puberty itself. That is, ELA exposures predict earlier pubertal timing (Belsky et al., 1991; Ellis, 2004; Moffitt et al., 1992), which, in turn, is a risk factor for post-pubertal weight gain, worsening CVD risk factor profiles, incident cardio-metabolic disease, and early mortality (Cooper et al., 1999; Feng et al., 2008; Frontini et al., 2003; Jacobsen et al., 2009; Lakshman et al., 2009). Recent work has shown that exposure to ELA during pubertal development is associated with higher adiposity in adulthood compared to experiencing ELA at other developmental periods (Riem and Karreman, 2019). In addition, adolescence is a life stage characterized in part by the uptake of health behaviors and social relationships, which can influence mental and physical health over the life course (Sawyer et al., 2012). Moreover, health risk behaviors, including tobacco use, alcohol, and substance abuse, tend to be initiated earlier among those who experience ELA and may be a pathway linking ELA to later health outcomes (Doom et al., 2017; Duke, 2018). Finally, it is notable that the prenatal and pubertal periods are not only periods of increased sensitivity biologically but are also associated with distinct changes in family and social functioning, possibly further compounding effects of prior ELA exposures on health.
Type of adversities
Individual ELA have been associated with cardiovascular health outcomes. Experiences of child maltreatment for example have been shown to impact several mental health and physical health outcomes including CVD. A systematic review noted that 22 out of 24 studies reviewed noted child maltreatment to be associated with cardiovascular disease (Basu et al., 2017). Specific types of maltreatment, specifically sexual and physical abuse, have also been associated with CVD (Fuller-Thomson et al., 2012; Rich-Edwards et al., 2012; Thurston et al., 2017). Other individual ELA, however have not been as extensively studied for their individual association with CVD. As noted, ELA tend to cluster, thus making it difficult to examine individual forms of adversity for their impact on health outcomes. Partly due to this clustering, the predominant research relies on a cumulative risk framework where adversities are aggregated without consideration of whether different adversities may impart a differential impact on health outcomes. Recent work has proposed a dimensional approach to childhood adversity proposing two dimensions, threat and deprivation (McLaughlin and Sheridan, 2016). Work examining whether different dimensions of child adversity differentially impact cardiometabolic health outcomes is lacking, but recent work has demonstrated this differential impact in relation to biological aging markers (Colich et al., 2020; Sumner et al., 2019; Sun et al., 2020). An important consideration when examining different types of adversity is the interrelatedness to timing, duration and developmental context which may serve to modify the impact of specific types of adversities on cardiometabolic health.
The Moderating Role of Developmental Context
Given substantial heterogeneity in outcomes among individuals exposed to similar profiles of adversity, there is growing focus on moderators of the effects of ELA on later health and developmental outcomes, both positive and negative (Bethell et al., 2019; Traub and Boynton-Jarrett, 2017). Protective factors are conceptualized as health-promoting resources, relationships, or contexts that help children cope with or recover from ELA (i.e., not mere absence of a negative exposure or experience). Potentially protective processes and factors occur across levels of a child’s social ecology (Cicchetti and Toth, 2016). These include individual (e.g., genetic factors, self-regulation) (Bakker et al., 2011; Niitsu et al., 2019) familial (e.g., stable and nurturing relationships) (Farrell et al., 2017), community (e.g., availability of supportive programs and social supports in times of need) (Luthar et al., 2015) and social and policy factors (e.g., policies that provide access to high-quality accessible childcare) (Gartland et al., 2019; Watamura et al., 2011).
Caregiving relationships play a central role in human development, parent and family systems are a key protective factor. High-quality parent-child relationships may prevent or mitigate lasting changes resulting from ELA, as shown in animal studies (Gunnar et al., 2015; Sanchez, 2006; Winslow et al., 2003), observational studies of children (Asok et al., 2013; Bernard et al., 2019; Kertes et al., 2009; Suglia et al., 2009), parenting intervention studies (Chen et al., 2018; Miller et al., 2014), and retrospective studies of adults (Carroll et al., 2013; Chen et al., 2011). For example, numerous studies assessing childhood stress and dysregulation of the stress-response system have shown that maternal warmth buffers the impact of early life stress on physical health outcomes (Farrell et al., 2017; Howell et al., 2017). Studies suggest that warm and/or responsive caregivers can buffer children exposed to ELA from poor outcomes relevant to cardiometabolic health measured in childhood (e.g., body mass index (BMI) (Bernard et al., 2019), telomere shortening (Asok et al., 2013)) and in adulthood (e.g., pro-inflammatory signaling (Chen et al., 2011), metabolic syndrome (Miller et al., 2011), and other measures of multisystem biological risk (Carroll et al., 2013)). Accordingly, adversities that compromise the capacity of parents to provide nurturing care may exacerbate the impact of ELA, thus presenting a greater risk for negative outcomes among children. Further research on the impact of ELA on specific developmental periods may inform whether interventions may work better if timed to occur in particular periods. In addition, an examination of the relative impact of particular exposures on health outcomes may identify “key” exposures that if targeted with interventions might be sufficient or generalize to protecting against other correlated exposures.
VARIATION IN RISK OF EXPOSURE TO ELA
Individuals belonging to marginalized groups are at greater risk of experiencing ELA. Common experiences of bias and discrimination among these individuals result in individual- and societal-level experiences of victimization (e.g., bullying, harassment) and stigmatization, possibly contributing to disparities in health (Hatzenbuehler et al., 2013). Moreover, individuals’ identification with multiple marginalized groups (e.g., transgender identity and African American) termed ‘intersectionality’ may combine to make one uniquely vulnerable to the experience and health impacts of ELA (Crenshaw, 1990).
Racial and ethnic health disparities seen in cardiometabolic health conditions, such as diabetes, hypertension, and obesity, have roots in structural inequities (Havranek et al., 2015). Disparities can be stratified by race and ethnicity, in addition to SES and other demographic factors. Specifically, differences in SES, neighborhood resources, and exposure to adversity, including race/ethnicity-based adversities, such as discrimination may explain racial/ethnic disparities in cardiometabolic disease prevalence and outcomes (Liu et al., 2018; Suglia et al., 2020; Vasquez et al., 2019).
The risk of exposure to ELA may vary based on demographic characteristics, including race/ethnicity, sex/gender, sexual orientation, family SES, urbanicity, and disability status. ELA disproportionately impact underserved groups (Austin et al., 2016a; Llabre et al., 2017). For example, Non-Hispanic Blacks and Hispanics are exposed to a high number of ELA compared to Non-Hispanic whites (Merrick et al., 2018). Racial/ethnic minority children and those from financially disadvantaged households experience higher levels of the most common adversities, including high rates of economic hardship, parental separation, and incarcerated parents (N. Heard-Garris et al., 2018; Merrick et al., 2018). Racial/ethnic minorities may experience higher levels of adversity as well as additional adversities that Non-Hispanic white groups do not experience and may experience racism during “sensitive periods”, such as early childhood and adolescence (N. J. Heard-Garris et al., 2018; Priest et al., 2014).
Some research suggests the prevalence of ELA may be higher among sexual and gender minorities (SGM) than their non-SGM peers (Andersen and Blosnich, 2013; Andersen et al., 2015). Sexual and gender minorities are more likely to experience physical and verbal bullying in childhood as compared to their heterosexual peers (Andersen and Blosnich, 2013; Austin et al., 2016a). Transgender individuals may experience more emotional abuse and both physical and emotional neglect as compared to similar cisgender individuals (Schnarrs et al., 2019). SGM are more likely to have psychological distress and engage in risky health behaviors, like smoking and alcohol use, potentially widening health disparities (Hart et al., 2018; Schnarrs et al., 2019). For SGM, bullying and ELA exposure may help to explain disparities that disadvantage this population (Andersen and Blosnich, 2013).
Individuals with disabilities are more likely to report ELA than their counterparts without disabilities, including sexual abuse (Austin et al., 2016b; Schussler-Fiorenza Rose et al., 2014). Berg et al. found children with autism are more likely to experience ELA (Berg et al., 2016), which, in turn, is associated with unmet healthcare needs (Berg et al., 2018). However, more studies that examine the role of ELA in the development of cardiometabolic health disparities based on ability status and developmental conditions are needed.
Increased risk of exposure to ELA among specific subgroups, as described above, has relevance for the timing, duration, and developmental context within which ELA are experienced. Living in underserved neighborhoods or low SES households may expose children to adversities across multiple developmental periods, which may include periods of increased vulnerability for experiences of adversity. Families experiencing social adversities are the most vulnerable, often lacking interpersonal support, economic resources, or access to governmental programmatic support to navigate these adversities. The additional burden of experiencing multiple adversities, and for longer durations, may impact children’s biological susceptibility to adversities as well as their families’ resilience.
The increased likelihood of exposure, differential duration, and exposure to multiple adversities may translate into a higher risk of adverse cardiometabolic outcomes. For example, Black individuals with ELA histories of child abuse or household difficulties are 3 times more likely to drink alcohol heavily as compared to non-Hispanic whites with similar backgrounds (Lee and Chen, 2017). Hispanic/Latinx populations also have an increased prevalence of ELA as compared to whites, second only to non-Hispanic Blacks. In a nationally representative study published in 2010, Hispanics with ELA histories of child abuse were 11 times more likely to drink alcohol heavily than non-Hispanic whites with similar histories (Lee and Chen, 2017). In contrast, a South Dakota-based study demonstrated American Indians had more ELA as compared to non- American Indians; however, there was no evidence for differential associations between negative health outcomes and health risk behaviors (i.e., mental health conditions, alcohol abuse, or smoking) among American Indians as compared to non-American Indians (Warne et al., 2017). These findings suggest that racial or ethnic minority status does not always signify worse outcomes. Some studies have suggested that women in comparison to men are more vulnerable to the impact of adversity as it relates to cardiometabolic outcomes, either because women are exposed to more adversity or because they are more vulnerable to its effects (Pedersen et al., 2016). For example, among young children, sex differences have been noted in the relation between adversity and obesity, with girls being at increased risk of obesity in relation to adversity in early childhood (Liu et al., 2019).
Despite the existing evidence linking ELA to CVD, few studies have examined how the duration, timing and context of exposure help to explain the increased burden of cardiometabolic outcomes among underserved populations including racial/ethnic minorities, immigrants, individuals with disabilities, and SGM. Furthermore, few studies have examined how structural factors that drive these inequities increase exposure to ELA and limit resources that would help vulnerable populations in limiting the impact of these adversities.
Conclusions and Future Research Directions
There are several gaps in the evidence linking ELA and cardiometabolic risk that limit our ability to explain patterns of disease risk and resilience over time. Most notably, studies often use a blunt instrument to define multiple co-occurring ELA exposures across broad swaths of development (sometimes from birth to age 18) (Felitti et al., 1998). There are, however, sensitive periods in development that can affect, in the short- and long-term, underlying risk for cardiometabolic disease. In these periods, the impact of adversity exposures is likely to be amplified (Geelhoed and Jaddoe, 2010; Johnson W, 2015). Similarly, there is expected developmental variability in the association between ELA and biological processes such as DNA methylation (Dunn et al., 2019; Klengel et al., 2013). Therefore, future studies should aim to characterize this developmental variability by 1) comparing the impact of similar exposures at different points in development; 2) examining level of severity of similar exposures at different points in development. For example, number of times the exposure occurred, along with duration of the particular ELA exposure under investigation.
Alongside attention to developmental processes, additional research is needed to describe individual and contextual risk and resilience factors that modulate the relationship between ELA and cardiometabolic risk. Individual factors such as sex/gender, racial or ethnic identity (Slopen et al., 2010), SGM, ability status, coping styles (Bergh et al., 2015), and genetic and behavioral risks (Gooding et al., 2014) have received relatively little study. Contextual and structural factors as wells as social conditions, such as poverty are connected with abuse, neglect and many of other adversities children face. Because of their contribution as critical ELA factors, they need sustained attention in research studies especially when examining the ELA and cardiometabolic health association. Studies should explicitly measure the quality of caregiver/child relationships, and family systems given their critical role in buffering or potentiating the effects of ELA on health and developmental outcomes (Shonkoff and Garner, 2012). Finally, future research should also include systematic assessments of characteristics beyond the individual and family-level. These studies would incorporate the role of social policies, place-based characteristics, and other contextual factors to provide a more holistic understanding of the influence of ELA on health (Maguire-Jack et al., 2021; Slopen and Williams, 2021).
Additionally, significant gaps exist in the literature that examines ELA as a driver of disparities in cardiometabolic outcomes. Current research indicates multiple minority groups may be exposed to adversity differentially in early life. Thus, these groups experience not only higher levels of adversity as compared to non-minority groups but also additional adversities increasing the likelihood they are experienced during sensitive developmental periods. Thus, it is plausible that both the timing and accumulation of ELA among minority children matter for later life CVD risk. However, this literature is still immature, and many minority groups have been understudied. Additionally, ELA as a contributor to health disparities are rarely studied considering multiple minority statuses simultaneously (e.g., intersectionality). When this and other potential factors that give rise to differential ELA exposure are more thoroughly studied, we may better explain disparities in cardiometabolic health outcomes and, therefore, better understand how to intervene effectively.
The next wave of research should inform the development of interventions that address both ELA and population variation in ELA that drives health inequality. For example, in addition to traditional “lifestyle” behavioral intervention targets, psychosocial factors such as emotional counseling, stress reduction, and social support may be potential intervention targets for those at risk of poor cardiometabolic health outcomes due to ELA exposure. Specifically, such interventions may help to mitigate how ELA become biologically embedded and internalized (e.g., depression) amongst at-risk individuals (Cheong et al., 2017). Evidence exists that nurturing home environments, characterized by warmth, safety, support, and empathetic parenting relationships, are associated with positive cardiovascular health outcomes (Appleton et al., 2013; Slopen et al., 2017). Building on these studies, interventions that foster positive family functioning/environments, affect interventions, or the use of stress coping skills which may not have been designed to address physical health outcomes should be evaluated for their potential impact on cardiometabolic health outcomes. For example the Strong African-American Families program designed to enhance supportive parenting has been shown to ameliorate the impact of ELA on prediabetes in young adulthood (Brody et al., 2017).
Moreover, as ELA exposure disproportionately affects marginalized groups, prevention and intervention efforts must also be tailored to meet the needs of a range of populations made vulnerable. Research that addresses the type, timing, duration and context of ELA exposures within marginalized groups would aid in the development of specialized interventions for these groups uniquely. Doing so may not only improve and promote good cardiovascular health but may also work to reduce cardiovascular health disparities as well.
Acknowledgement:
This work was supported by grant R01HL125761 (Suglia), K01HL147995 (Heard-Garris).
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Disclaimer: The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alastalo H, Raikkonen K, Pesonen AK, Osmond C, Barker DJP, Heinonen K, Kajantie E, Eriksson JG, 2013. Early life stress and blood pressure levels in late adulthood. J Hum Hypertens 27:90–94. [DOI] [PubMed] [Google Scholar]
- Andersen JP, Blosnich J, 2013. Disparities in adverse childhood experiences among sexual minority and heterosexual adults: results from a multi-state probability-based sample. PLoS One 8:e54691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen JP, Zou C, Blosnich J, 2015. Multiple early victimization experiences as a pathway to explain physical health disparities among sexual minority and heterosexual individuals. Soc Sci Med 133:111–9. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Teicher MH, 2008. Stress, sensitive periods and maturational events in adolescent depression. Trends in Neurosciences 31:183–91. [DOI] [PubMed] [Google Scholar]
- Anderson SE, Gooze RA, Lemeshow S, Whitaker RC, 2012. Quality of early maternal–child relationship and risk of adolescent obesity. Pediatrics 129:132–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SE, Whitaker RC, 2011. Attachment security and obesity in US preschool-aged children. Archives of pediatrics & adolescent medicine 165:235–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleton AA, Buka SL, Loucks EB, Rimm EB, Martin LT, Kubzansky LD, 2013. A prospective study of positive early-life psychosocial factors and favorable cardiovascular risk in adulthood. Circulation 127:905–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asok A, Bernard K, Roth TL, Rosen JB, Dozier M, 2013. Parental responsiveness moderates the association between early-life stress and reduced telomere length. Development and Psychopathology 25:577–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin A, Herrick H, Proescholdbell S, 2016a. Adverse Childhood Experiences Related to Poor Adult Health Among Lesbian, Gay, and Bisexual Individuals. Am J Public Health 106:314–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin A, Herrick H, Proescholdbell S, Simmons J, 2016b. Disability and Exposure to High Levels of Adverse Childhood Experiences: Effect on Health and Risk Behavior. N C Med J 77:30–6. [DOI] [PubMed] [Google Scholar]
- Bakker MP, Ormel J, Verhulst FC, Oldehinkel AJ, 2011. Adolescent family adversity and mental health problems: the role of adaptive self-regulation capacities. The TRAILS study. J Abnorm Child Psychol 39:341–50. [DOI] [PubMed] [Google Scholar]
- Bale TL, Baram TZ, Brown AS, Goldstein JM, Insel TR, McCarthy MM, Nemeroff CB, Reyes TM, Simerly RB, et al. , 2010. Early Life Programming and Neurodevelopmental Disorders. Biological Psychiatry 68:314–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJP, 1992. The fetal and infant origins of adult disease, 1st ed. British Medical Journal Books, London. [Google Scholar]
- Barker DJP, Bull AR, Osmond C, Simmonds SJ, 1990. Fetal and placental size and risk of hypertension in adult life. British Medical Journal 301:259–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu A, McLaughlin KA, Misra S, Koenen KC, 2017. Childhood Maltreatment and Health Impact: The Examples of Cardiovascular Disease and Type 2 Diabetes Mellitus in Adults. Clin Psychol (New York) 24:125–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, Steinberg L, Draper P, 1991. Childhood experience, interpersonal development, and reproductive strategy - an evolutionary theory of socialization. Child Development 62:647–70. [DOI] [PubMed] [Google Scholar]
- Ben-Shlomo Y, Kuh D, 2002. A life course approach to chronic disease epidemiology: conceptual models, empirical challenges and interdisciplinary perspectives. International Journal of Epidemiology 31:285–93. [PubMed] [Google Scholar]
- Berg KL, Shiu CS, Acharya K, Stolbach BC, Msall ME, 2016. Disparities in adversity among children with autism spectrum disorder: a population-based study. Dev Med Child Neurol 58:1124–31. [DOI] [PubMed] [Google Scholar]
- Berg KL, Shiu CS, Feinstein RT, Msall ME, Acharya K, 2018. Adverse Childhood Experiences Are Associated with Unmet Healthcare Needs among Children with Autism Spectrum Disorder. J Pediatr 202:258–64 e1. [DOI] [PubMed] [Google Scholar]
- Bergh C, Udumyan R, Fall K, Almroth H, Montgomery S, 2015. Stress resilience and physical fitness in adolescence and risk of coronary heart disease in middle age. Heart 101:623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard K, Frost A, Jelinek C, Dozier M, 2019. Secure attachment predicts lower body mass index in young children with histories of child protective services involvement. Pediatric Obesity:e12510. [DOI] [PubMed] [Google Scholar]
- Bethell C, Jones J, Gombojav N, Linkenbach J, Sege R, 2019. Positive childhood experiences and adult mental and relational health in a statewide sample: Associations across adverse childhood experiences levels. JAMA pediatrics 173:e193007–e07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore S-J, Mills KL, 2014. Is Adolescence a Sensitive Period for Sociocultural Processing? Annual Review of Psychology 65:187–207. [DOI] [PubMed] [Google Scholar]
- Bleil ME, Appelhans BM, Latham MD, Irving MA, Gregorich SE, Adler NE, Cedars MI, 2015. Neighborhood socioeconomic status during childhood versus puberty in relation to endogenous sex hormone levels in adult women. Nurs. Res. 64:211–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blevins CL, Sagui SJ, Bennett JMJB, behavior,, immunity, 2017. Inflammation and positive affect: examining the stress-buffering hypothesis with data from the National Longitudinal Study of Adolescent to Adult Health. 61:21–26. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Ellis BJJD, psychopathology, 2005. Biological sensitivity to context: I. An evolutionary–developmental theory of the origins and functions of stress reactivity. 17:271–301. [DOI] [PubMed] [Google Scholar]
- Brody GH, Yu T, Chen E, Miller GE, 2017. Family-centered prevention ameliorates the association between adverse childhood experiences and prediabetes status in young black adults. Preventive Medicine 100:117–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS, Os J.v., Driessens C, Hoek HW, Susser ES, 2000. Further Evidence of Relation Between Prenatal Famine and Major Affective Disorder. American Journal of Psychiatry 157:190–95. [DOI] [PubMed] [Google Scholar]
- Bush NR, Boyce W.T.J.D.p., 2016. Differential sensitivity to context: Implications for developmental psychopathology. 2:107–37. [Google Scholar]
- Carroll JE, Gruenewald TL, Taylor SE, Janicki-Deverts D, Matthews KA, Seeman TE, 2013. Childhood abuse, parental warmth, and adult multisystem biological risk in the Coronary Artery Risk Development in Young Adults study. Proceedings of the National Academy of Sciences 110:17149–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman DP, Whitfield CL, Felitti VJ, Dube SR, Edwards VJ, Anda RF, 2004. Adverse childhood experiences and the risk of depressive disorders in adulthood. J Affect Disord 82:217–25. [DOI] [PubMed] [Google Scholar]
- Chen E, Miller GE, Kobor MS, Cole SW, 2011. Maternal warmth buffers the effects of low early-life socioeconomic status on pro-inflammatory signaling in adulthood. Mol Psychiatry 16:729–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E, Miller GE, Yu T, Brody GH, 2018. Unsupportive parenting moderates the effects of family psychosocial intervention on metabolic syndrome in African American youth. Int J Obes (Lond) 42:634–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong EV, Sinnott C, Dahly D, Kearney PM, 2017. Adverse childhood experiences (ACEs) and later-life depression: perceived social support as a potential protective factor. BMJ Open 7:e013228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang JJ, Bower JE, Irwin MR, Taylor SE, Fuligni AJJB, behavior,, immunity, 2017. Adiposity moderates links from early adversity and depressive symptoms to inflammatory reactivity to acute stress during late adolescence. 66:146–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chida Y, Steptoe A, 2010. Greater cardiovascular responses to laboratory mental stress are associated with poor subsequent cardiovascular risk status: a meta-analysis of prospective evidence. Hypertension 55:1026–32. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Toth S, 2016. Cicchetti D, Toth S. Child maltreatment and developmental psychopathology: A multilevel perspective. Cichetti D (Ed.), in: Cicchetti D (Ed.), Developmental Psychopathology. Wiley & Sons. [Google Scholar]
- Colich NL, Rosen ML, Williams ES, McLaughlin KA, 2020. Biological aging in childhood and adolescence following experiences of threat and deprivation: A systematic review and meta-analysis. Psychol Bull 146:721–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper GS, Ephross SA, Weinberg CR, Baird DD, Whelan EA, Sandler DP, 1999. Menstrual and reproductive risk factors for ischemic heart disease. Epidemiology 10:255–59. [PubMed] [Google Scholar]
- Crenshaw K, 1990. A Black Feminist Critique of Antidiscrimination Law and Politics, in: Kairys D (Ed.), The politics of law : a progressive critique Pantheon Books, New York, pp. 195–217. [Google Scholar]
- Danese A, Pariante CM, Caspi A, Taylor A, Poulton R, 2007. Childhood maltreatment predicts adult inflammation in a life-course study. Proceedings of the National Academy of Sciences of the United States of America 104:1319–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Giudice M, Ellis BJ, Shirtcliff EAJN, Reviews B, 2011. The adaptive calibration model of stress responsivity. 35:1562–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doom JR, Mason SM, Suglia SF, Clark CJ, 2017. Pathways between childhood/adolescent adversity, adolescent socioeconomic status, and long-term cardiovascular disease risk in young adulthood. Soc Sci Med 188:166–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke NN, 2018. Adolescent Adversity and Concurrent Tobacco, Alcohol, and Marijuana Use. Am J Health Behav 42:85–99. [DOI] [PubMed] [Google Scholar]
- Dunn E, Soare T, Zhu Y, 2019. Sensitive periods for the effect of childhood adversity on DNA methylation: Results from a prospective, longitudinal study. Biological Psychiatry 85:838–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich KB, Miller GE, Rohleder N, Adam EKJD, Psychopathology, 2016. Trajectories of relationship stress and inflammatory processes in adolescence. 28:127–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis BJ, 2004. Timing of pubertal maturation in girls: An integrated life history approach. Psychological Bulletin 130:920–58. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Boyce WT, Belsky J, Bakermans-Kranenburg MJ, Van IJzendoorn MHJD, psychopathology, 2011. Differential susceptibility to the environment: An evolutionary–neurodevelopmental theory. 23:7–28. [DOI] [PubMed] [Google Scholar]
- Evans GW, Li D, Sepanski Whipple S, 2013. Cumulative Risk and Child Development. Psychological Bulletin. [DOI] [PubMed] [Google Scholar]
- Farrell AK, Simpson JA, Carlson EA, Englund MM, Sung S, 2017. The impact of stress at different life stages on physical health and the buffering effects of maternal sensitivity. Health Psychol 36:35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, Marks JS, 1998. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. American Journal of Preventive Medicine 14:245–58. [DOI] [PubMed] [Google Scholar]
- Feng Y, Hong XM, Wilker E, Li ZP, Zhang WB, Jin DL, Liu X, Zang TH, Xu XP, et al. , 2008. Effects of age at menarche, reproductive years, and menopause on metabolic risk factors for cardiovascular diseases. Atherosclerosis 196:590–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frontini MG, Srinivasan SR, Berenson GS, 2003. Longitudinal changes in risk variables underlying metabolic Syndrome X from childhood to young adulthood in female subjects with a history of early menarche: The Bogalusa Heart Study. International Journal of Obesity 27:1398–404. [DOI] [PubMed] [Google Scholar]
- Fuller-Thomson E, Bejan R, Hunter JT, Grundland T, Brennenstuhl S, 2012. The link between childhood sexual abuse and myocardial infarction in a population-based study. Child Abuse and Neglect 36:656–65. [DOI] [PubMed] [Google Scholar]
- Gartland D, Riggs E, Muyeen S, Giallo R, Afifi TO, MacMillan H, Herrman H, Bulford E, Brown SJ, 2019. What factors are associated with resilient outcomes in children exposed to social adversity? A systematic review. BMJ Open 9:e024870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geelhoed JJM, Jaddoe VWV, 2010. Early influences on cardiovascular and renal development. European journal of epidemiology 25:677–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giletta M, Slavich GM, Rudolph KD, Hastings PD, Nock MK, Prinstein M.J.J.J.o.C.P., Psychiatry, 2018. Peer victimization predicts heightened inflammatory reactivity to social stress in cognitively vulnerable adolescents. 59:129–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginty AT, Kraynak TE, Fisher JP, Gianaros PJ, 2017. Cardiovascular and autonomic reactivity to psychological stress: Neurophysiological substrates and links to cardiovascular disease. Auton Neurosci 207:2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooding HC, Milliren C, McLaughlin KA, Richmond TK, Katz-Wise SL, Rich-Edwards J, Austin SB, 2014. Child maltreatment and blood pressure in young adulthood. Child Abuse & Neglect 38:1747–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens L, Braet C, Van Durme K, Decaluwe V, Bosmans G, 2012. The parent-child relationship as predictor of eating pathology and weight gain in preadolescents. J Clin Child Adolesc Psychol 41:445–57. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, DePasquale CE, Reid BM, Donzella B, 2019. Pubertal stress recalibration reverses the effects of early life stress in postinstitutionalized children. Proc Natl Acad Sci U S A 116:23984–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Hostinar CE, Sanchez MM, Tottenham N, Sullivan RM, 2015. Parental buffering of fear and stress neurobiology: Reviewing parallels across rodent, monkey, and human models. Social neuroscience 10:474–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales CN, Barker DJP, Clark PMS, Cox LJ, Fall C, Osmond C, Winter PD, 1991. Fetal and infant growth and impaired glucose tolerance at age 64. BMJ-British Medical Journal 303:1019–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao G, Youssef N, Davis C, Su S, 2018. The role of DNA methylation in the association between childhood adversity and cardiometabolic disease. International journal of cardiology. International Journal of Cardiology 255:168–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart TA, Noor SW, Vernon JRG, Kidwai A, Roberts K, Myers T, Calzavara L, 2018. Childhood Maltreatment, Bullying Victimization, and Psychological Distress Among Gay and Bisexual Men. J Sex Res 55:604–16. [DOI] [PubMed] [Google Scholar]
- Hatzenbuehler ML, Phelan JC, Link BG, 2013. Stigma as a fundamental cause of population health inequalities. Am J Public Health 103:813–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havranek EP, Mujahid MS, Barr DA, Blair IV, Cohen MS, Cruz-Flores S, Davey-Smith G, Dennison-Himmelfarb CR, Lauer MS, et al. , 2015. Social Determinants of Risk and Outcomes for Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 132:873–98. [DOI] [PubMed] [Google Scholar]
- Heard-Garris N, Davis MM, Estabrook R, Burns J, Briggs-Gowan M, Allen N, Carnethon M, Aguayo L, Wakschlag L, et al. , 2020. Adverse childhood experiences and biomarkers of inflammation in a diverse cohort of early school-aged children. Brain, Behavior, & Immunity - Health 1:100006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heard-Garris N, Winkelman TNA, Choi H, Miller AK, Kan K, Shlafer R, Davis MM, 2018. Health Care Use and Health Behaviors Among Young Adults With History of Parental Incarceration. Pediatrics 142. [DOI] [PubMed] [Google Scholar]
- Heard-Garris NJ, Cale M, Camaj L, Hamati MC, Dominguez TP, 2018. Transmitting Trauma: A systematic review of vicarious racism and child health. Soc Sci Med 199:230–40. [DOI] [PubMed] [Google Scholar]
- Hertzman C, Boyce T, 2010. How Experience Gets Under the Skin to Create Gradients in Developmental Health, Annual Review of Public Health, Vol 31. Annual Reviews, Palo Alto, pp. 329–47. [DOI] [PubMed] [Google Scholar]
- Hostinar CE, Ross KM, Chen E, Miller G.E.J.P.m., 2017. Early-life socioeconomic disadvantage and metabolic health disparities. 79:514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell BR, McMurray MS, Guzman DB, Nair G, Shi Y, McCormack KM, Hu X, Styner MA, Sanchez MM, 2017. Maternal buffering beyond glucocorticoids: impact of early life stress on corticolimbic circuits that control infant responses to novelty. Soc Neurosci 12:50–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen BK, Oda K, Knutsen SF, Fraser GE, 2009. Age at menarche, total mortality and mortality from ischaemic heart disease and stroke: the Adventist Health Study, 1976–88. International Journal of Epidemiology 38:245–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janicki-Deverts D, Cohen S, Matthews KA, Jacobs DR, 2012. Sex differences in the association of childhood socioeconomic status with adult blood pressure change: The CARDIA Study. Psychosomatic Medicine 74:728–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasik CB, Lustig RH, 2008. Adolescent obesity and puberty: The “Perfect Storm”, in: Gordon CM, Welt C, Rebar RW, Hillard PJA, Matzuk MM, Nelson LM (Eds.), Menstrual Cycle and Adolescent Health, pp. 265–79. [DOI] [PubMed] [Google Scholar]
- Johnson W KD, Hardy R. In: Burton-Jeangros C, Cullati S, Sacker A, et al. , editors. [Internet]. Cham (CH): Springer; 2015. Chapter 4. Available from: doi: 10.1007/978-3-319-20484-0_4, 2015. A Life Course Perspective on Body Size and Cardio-metabolic Health, in: Burton-Jeangros C, Cullati S, Sacker A, Blane D (Eds.), A Life Course Perspective on Health Trajectories and Transitions. Springer, Internet. [DOI] [PubMed] [Google Scholar]
- Jun H-J, Corliss HL, Boynton-Jarrett R, Spiegelman D, Austin SB, Wright RJ, 2011. Growing up in a domestic violence environment: relationship with developmental trajectories of body mass index during adolescence into young adulthood. Journal of Epidemiology and Community Health. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertes DA, Donzella B, Talge NM, Garvin MC, Van Ryzin MJ, Gunnar MR, 2009. Inhibited Temperament and Parent Emotional Availability Differentially Predict Young Children’s Cortisol Responses to Novel Social and Nonsocial Events. Developmental Psychobiology 51:521–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klengel T, Mehta D, Anacker C, 2013. Allele-specific fkbp5 DNA demethylation mediates gene–childhood trauma interactions. Nature Neuroscience 16:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshman R, Forouhi NG, Sharp SJ, Luben R, Bingham SA, Khaw KT, Wareham NJ, Ong KK, 2009. Early age at menarche is associated with cardiovascular disease and mortality. Journal of Clinical Endocrinology & Metabolism 94:4953–60. [DOI] [PubMed] [Google Scholar]
- Lee RD, Chen J, 2017. Adverse childhood experiences, mental health, and excessive alcohol use: Examination of race/ethnicity and sex differences. Child Abuse Negl 69:40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Shelton RC, Eldred-Skemp N, Goldsmith J, Suglia SF, 2019. Early Exposure to Cumulative Social Risk and Trajectories of Body Mass Index in Childhood. Child Obes 15:48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SR, Kia-Keating M, Nylund-Gibson K, 2018. Patterns of adversity and pathways to health among White, Black, and Latinx youth. Child Abuse Negl 86:89–99. [DOI] [PubMed] [Google Scholar]
- Llabre MM, Schneiderman N, Gallo LC, Arguelles W, Daviglus ML, Gonzalez F 2nd, Isasi CR, Perreira KM, Penedo FJ, 2017. Childhood Trauma and Adult Risk Factors and Disease in Hispanics/Latinos in the US: Results From the Hispanic Community Health Study/Study of Latinos (HCHS/SOL) Sociocultural Ancillary Study. Psychosom Med 79:172–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low CA, Matthews KA, Hall M.J.P.m., 2013. Elevated CRP in adolescents: roles of stress and coping. 75:449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loxton D, Forder PM, Cavenagh D, Townsend N, Holliday E, Chojenta C, Melka AS, 2021. The impact of adverse childhood experiences on the health and health behaviors of young Australian women. Child Abuse Negl 111:104771. [DOI] [PubMed] [Google Scholar]
- Luthar S, Crossman E, Small P, 2015. Resilience and adversity, in: Lerner R (Ed.), Handbook of child psychology and developmental science, 7th ed. Wiley, pp. 247–86. [Google Scholar]
- Maguire-Jack K, Font S, Dillard R, Dvalishvili D, Barnhart S, 2021. Neighborhood Poverty and Adverse Childhood Experiences over the First 15 Years of Life. International Journal on Child Maltreatment: Research, Policy and Practice 4:93–114. [Google Scholar]
- Masten AS, Barnes AJ, 2018. Resilience in Children: Developmental Perspectives. Children (Basel) 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, 2016. Future Directions in Childhood Adversity and Youth Psychopathology. J Clin Child Adolesc Psychol 45:361–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, 2016. Beyond Cumulative Risk: A Dimensional Approach to Childhood Adversity. Curr Dir Psychol Sci 25:239–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrick MT, Ford DC, Ports KA, Guinn AS, 2018. Prevalence of Adverse Childhood Experiences From the 2011–2014 Behavioral Risk Factor Surveillance System in 23 States. JAMA Pediatr 172:1038–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midei AJ, Matthews KA, 2011. Interpersonal violence in childhood as a risk factor for obesity: a systematic review of the literature and proposed pathways. Obesity Reviews 12:e159–e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midei AJ, Matthews KA, Chang YF, Bromberger JT, 2013. Childhood physical abuse is associated with incident metabolic syndrome in mid-life women. Health Psychology 32:121–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Brody GH, Yu T, Chen E, 2014. A family-oriented psychosocial intervention reduces inflammation in low-SES African American youth. Proc Natl Acad Sci U S A 111:11287–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, 2010. Harsh family climate in early life presages the emergence of a proinflammatory phenotype in adolescence. Psychological Science 21:848–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Lachman ME, Chen E, Gruenewald TL, Karlamangla AS, Seeman TE, 2011. Pathways to Resilience: Maternal Nurturance as a Buffer Against the Effects of Childhood Poverty on Metabolic Syndrome at Midlife. Psychological Science 22:1591–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt TE, Caspi A, Belsky J, Silva PA, 1992. Childhood experience and the onset of menarche - a test of a sociobiological model. Child Development 63:47–58. [DOI] [PubMed] [Google Scholar]
- Niitsu K, Rice MJ, Houfek JF, Stoltenberg SF, Kupzyk KA, Barron CR, 2019. A Systematic Review of Genetic Influence on Psychological Resilience. Biol Res Nurs 21:61–71. [DOI] [PubMed] [Google Scholar]
- Pedersen LR, Frestad D, Michelsen MM, Mygind ND, Rasmusen H, Suhrs HE, Prescott E, 2016. Risk Factors for Myocardial Infarction in Women and Men: A Review of the Current Literature. Curr Pharm Des 22:3835–52. [DOI] [PubMed] [Google Scholar]
- Priest N, Perry R, Ferdinand A, Paradies Y, Kelaher M, 2014. Experiences of racism, racial/ethnic attitudes, motivated fairness and mental health outcomes among primary and secondary school students. J Youth Adolesc 43:1672–87. [DOI] [PubMed] [Google Scholar]
- Rich-Edwards JW, Mason S, Rexrode K, Spiegelman D, Hibert E, Kawachi I, Jun HJ, Wright RJ, 2012. Physical and sexual abuse in childhood as predictors of early-onset cardiovascular events in women. Circulation 126:920–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich-Edwards JW, Spiegelman D, Hibert ENL, Jun HJ, Todd TJ, Kawachi I, Wright RJ, 2010. Abuse in Childhood and Adolescence As a Predictor of Type 2 Diabetes in Adult Women. American Journal of Preventive Medicine 39:529–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riem MME, Karreman A, 2019. Childhood Adversity and Adult Health: The Role of Developmental Timing and Associations With Accelerated Aging. Child Maltreat 24:17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley EH, Wright RJ, Jun HJ, Hibert EN, Rich-Edwards JW, 2010. Hypertension in adult survivors of child abuse: observations from the Nurses’ Health Study II. Journal of Epidemiology and Community Health 64:413–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rundle AG, Factor-Litvak P, Suglia SF, Susser ES, Kezios KL, Lovasi GS, Cirillo PM, Cohn BA, Link BG, 2020. Tracking of Obesity in Childhood into Adulthood: Effects on Body Mass Index and Fat Mass Index at Age 50. Child Obes 16:226–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rundle AG, Suglia SF, Susser ES, Factor-Litvak P, March D, Kezios KL, Lovasi GS, Fader KM, Andrews H, et al. , 2019. Body mass index across the life course: emergence of race-by-sex disparities in early childhood. Ann Epidemiol 33:44–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez MM, 2006. The impact of early adverse care on HPA axis development: Nonhuman primate models. Hormones and Behavior 50:623–31. [DOI] [PubMed] [Google Scholar]
- Sawyer SM, Afifi RA, Bearinger LH, Blakemore SJ, Dick B, Ezeh AC, Patton GC, 2012. Adolescence: a foundation for future health. Lancet 379:1630–40. [DOI] [PubMed] [Google Scholar]
- Schnarrs PW, Stone AL, Salcido R Jr., Baldwin A, Georgiou C, Nemeroff CB, 2019. Differences in adverse childhood experiences (ACEs) and quality of physical and mental health between transgender and cisgender sexual minorities. J Psychiatr Res 119:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schussler-Fiorenza Rose SM, Xie D, Stineman M, 2014. Adverse childhood experiences and disability in U.S. adults. PM R 6:670–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonkoff JP, Garner AS, 2012. The lifelong effects of early childhood adversity and toxic stress. Pediatrics 129:e232–46. [DOI] [PubMed] [Google Scholar]
- Slopen N, Chen Y, Guida JL, Albert MA, Williams DR, 2017. Positive childhood experiences and ideal cardiovascular health in midlife: Associations and mediators. Prev Med 97:72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slopen N, Koenen KC, Kubzansky LD, 2014. Cumulative adversity in childhood and emergent risk factors for long-term health. The Journal of Pediatrics 164:631–38. e2. [DOI] [PubMed] [Google Scholar]
- Slopen N, Lewis TT, Gruenewald TL, Mujahid MS, Ryff CD, Albert MA, Williams DR, 2010. Early life adversity and inflammation in African Americans and whites in the midlife in the United States survey. Psychosom Med 72:694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slopen N, Williams DR, 2021. Resilience-promoting policies and contexts for children of color in the United States: Existing research and future priorities. Dev Psychopathol 33:614–24. [DOI] [PubMed] [Google Scholar]
- Smarius L, Strieder TGA, Doreleijers TAH, Vrijkotte TGM, de Rooij SR, 2018. Maternal verbally aggressive behavior in early infancy is associated with blood pressure at age 5–6. Journal of Developmental Origins of Health and Disease 9:344–50. [DOI] [PubMed] [Google Scholar]
- Stettler N, Kumanyika SK, Katz SH, Zemel BS, Stallings VA, 2003. Rapid weight gain during infancy and obesity in young adulthood in a cohort of African Americans. Am J Clin Nutr 77:1374–8. [DOI] [PubMed] [Google Scholar]
- Su S, Wang X, Pollock JS, Treiber FA, Xu X, Snieder H, McCall WV, Stefanek M, Harshfield GA, 2015. Adverse childhood experiences and blood pressure trajectories from childhood to young adulthood: the Georgia stress and Heart study. Circulation 131:1674–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suglia SF, Campo RA, Brown AGM, Stoney C, Boyce CA, Appleton AA, Bleil ME, Boynton-Jarrett R, Dube SR, et al. , 2020. Social Determinants of Cardiovascular Health: Early Life Adversity as a Contributor to Disparities in Cardiovascular Diseases. J Pediatr 219:267–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suglia SF, Duarte CS, Chambers EC, Boynton-Jarrett R, 2012. Cumulative social risk and obesity in early childhood. Pediatrics 129:e1173–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suglia SF, Enlow MB, Kullowatz A, Wright RJ, 2009. Maternal Intimate Partner Violence and Increased Asthma Incidence in Children Buffering Effects of Supportive Caregiving. Archives of Pediatrics & Adolescent Medicine 163:244–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suglia SF, Sapra KJ, Koenen KC, 2015. Violence and cardiovascular health: a systematic review. Am J Prev Med 48:205–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner JA, Colich NL, Uddin M, Armstrong D, McLaughlin KA, 2019. Early Experiences of Threat, but Not Deprivation, Are Associated With Accelerated Biological Aging in Children and Adolescents. Biol Psychiatry 85:268–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Fang J, Wan Y, Su P, Tao F, 2020. Association of Early-Life Adversity With Measures of Accelerated Biological Aging Among Children in China. JAMA Netw Open 3:e2013588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurston RC, Chang Y, Barinas-Mitchell E, von Kanel R, Jennings JR, Santoro N, Landsittel DP, Matthews KA, 2017. Child Abuse and Neglect and Subclinical Cardiovascular Disease Among Midlife Women. Psychosom Med 79:441–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub F, Boynton-Jarrett R, 2017. Modifiable Resilience Factors to Childhood Adversity for Clinical Pediatric Practice. Pediatrics. [DOI] [PubMed] [Google Scholar]
- Turner AI, Smyth N, Hall SJ, Torres SJ, Hussein M, Jayasinghe SU, Ball K, Clow AJ, 2020. Psychological stress reactivity and future health and disease outcomes: A systematic review of prospective evidence. Psychoneuroendocrinology 114:104599. [DOI] [PubMed] [Google Scholar]
- Valdez R, Athens MA, Thompson GH, Bradshaw BS, Stern MP, 1994. Birthweight and adult health outcomes in a biethnic population in the USA. Diabetologia 37:624–31. [DOI] [PubMed] [Google Scholar]
- Vasquez E, Udo T, Corsino L, Shaw BA, 2019. Racial and Ethnic Disparities in the Association Between Adverse Childhood Experience, Perceived Discrimination and Body Mass Index in a National Sample of U.S. Older Adults. J Nutr Gerontol Geriatr 38:6–17. [DOI] [PubMed] [Google Scholar]
- Warne D, Dulacki K, Spurlock M, Meath T, Davis MM, Wright B, McConnell KJ, 2017. Adverse Childhood Experiences (ACE) among American Indians in South Dakota and Associations with Mental Health Conditions, Alcohol Use, and Smoking. J Health Care Poor Underserved 28:1559–77. [DOI] [PubMed] [Google Scholar]
- Watamura SE, Phillips DA, Morrissey TW, McCartney K, Bub K, 2011. Double jeopardy: poorer social-emotional outcomes for children in the NICHD SECCYD experiencing home and child-care environments that confer risk. Child Dev 82:48–65. [DOI] [PubMed] [Google Scholar]
- Widom CS, Czaja SJ, Bentley T, Johnson MS, 2012. A Prospective Investigation of Physical Health Outcomes in Abused and Neglected Children: New Findings From a 30-Year Follow-Up. American Journal of Public Health 102:1135–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggert N, Wilhelm FH, Nakajima M, al’Absi M, 2016. Chronic Smoking, Trait Anxiety, and the Physiological Response to Stress. Subst Use Misuse 51:1619–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow JT, Noble PL, Lyons CK, Sterk SM, Insel TR, 2003. Rearing effects on cerebrospinal fluid oxytocin concentration and social buffering in rhesus monkeys. Neuropsychopharmacology 28:910. [DOI] [PubMed] [Google Scholar]
- Wortman C, 2004. Posttraumatic Growth: Progress and Problems. Psychological Inquiry 15:81–90. [Google Scholar]
- Zeanah CH, Gunnar MR, McCall RB, Kreppner JM, Fox NA, 2011. VI. Sensitive Periods. Monographs of the Society for Research in Child Development 76:147–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziol-Guest KM, Duncan GJ, Kalil A, 2009. Early Childhood Poverty and Adult Body Mass Index. American Journal of Public Health 99:527–32. [DOI] [PMC free article] [PubMed] [Google Scholar]


