Abstract
Tetanus neurotoxin (TeNT) is an A-B toxin with three functional domains: endopeptidase, translocation (HCT), and receptor binding. Endosomal acidification triggers HCT to interact with and insert into the membrane, translocating the endopeptidase across the bilayer. Although the function of HCT is well defined, the mechanism by which it accomplishes this task is unknown. To gain insight into the HCT membrane interaction on both local and global scales, we utilized an isolated, beltless HCT variant (bHCT), which retained the ability to release potassium ions from vesicles. To examine which bHCT residues interact with the membrane, we widely sampled the surface of bHCT using 47 single-cysteine variants labeled with the environmentally sensitive fluorophore NBD. At neutral pH, no interaction was observed for any variant. In contrast, all NBD-labeled positions reported environmental change in the presence of acidic pH and membranes containing anionic lipids. We then examined the conformation of inserted bHCT using circular dichroism and intrinsic fluorescence. Upon entering the membrane, bHCT retained predominantly α-helical secondary structure, whereas the tertiary structure exhibited substantial refolding. The use of lipid-attached quenchers revealed that at least one of the three tryptophan residues penetrated deep into the hydrocarbon core of the membrane, suggesting formation of a bHCT transmembrane conformation. The possible conformational topology was further explored with the hydropathy analysis webtool MPEx, which identified a large, potential α-helical transmembrane region. Altogether, the spectroscopic evidence supports a model in which, upon acidification, the majority of TeNT bHCT entered the membrane with a concurrent change in tertiary structure.
Significance
The tetanus neurotoxin protein (TeNT) disrupts mammalian neuronal function. To accomplish this, the TeNT toxic component must be transported across the cellular endosomal membrane by the action of the TeNT heavy chain translocation domain (HCT). Although the function of HCT is known, the mechanism and structure by which HCT accomplishes its task are not. We used various spectroscopic techniques to demonstrate that HCT inserted into membranes only under conditions that mimic the endosomal lumen and that insertion was accompanied by membrane-induced refolding. Experimental results were complemented with sequence-based predictions of membrane interactions. This is the first study, to our knowledge, to directly demonstrate membrane-induced refolding of HCT and provides a framework for subsequent structural studies.
Introduction
Tetanus neurotoxin (TeNT) is a clostridial neurotoxin (1). This family of proteinaceous toxins are the deadliest known, with an estimated median lethal dose (LD50) of less than 1 ng/kg of toxin per body weight (2,3). The other clostridial neurotoxins are the eight botulinum neurotoxin serotypes A–G and X (4, 5, 6, 7). The clostridial neurotoxins belong to a larger family of A-B toxins, which are composed of two functional subunits (8). The active (A) subunit is responsible for the toxicity of the toxin; the binding (B) subunit is responsible for mediating entry of the toxin into the target cell.
In the clostridial neurotoxins, the A and B subunits are produced as a single polypeptide of ∼150 kDa. This polypeptide then undergoes proteolytic cleavage into an ∼50 kDa light chain (LC, A subunit, zinc-dependent endopeptidase; Fig. 1 a, red) and an ∼100 kDa heavy chain (B subunit) that are connected by a single disulfide bond (9,10). The heavy chain possesses two functional domains. The ∼50 kDa N-terminal heavy chain translocation domain (HCT; Fig. 1 a, yellow) is responsible for translocating the LC endopeptidase across the endosomal membrane. The ∼50 kDa C-terminal heavy chain receptor binding domain binds to the host cell receptors to facilitate cellular entry (Fig. 1 a, green) (9,11,12). The HCT domain contains a long N-terminal loop (∼80 residues) referred to as the “belt,” which wraps around the LC endopeptidase (13). Once the HCT translocates the LC endopeptidase across the endosomal membrane, endogenous thioredoxin reductase reduces the linking disulfide bond to release the LC endopeptidase into the cytosol, where it can cleave a member of the soluble N-ethylmaleimide–sensitive factor attachment protein receptors (SNARE) complex to cause toxicity (14,15).
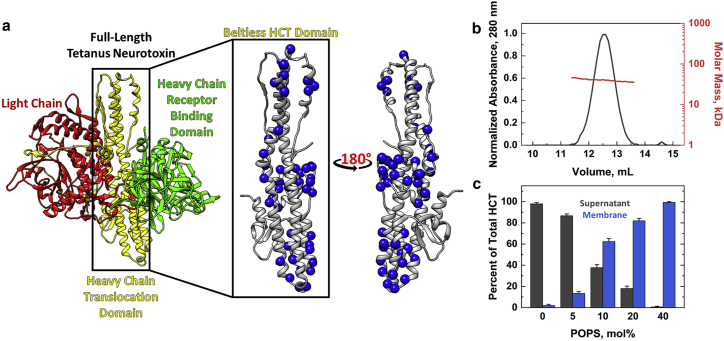
Figure 1.
Crystal structure of tetanus neurotoxin and characterization of bHCT. (a) The crystal structure of the soluble TeNT (left, PDB: 5N0B (13)) was represented as a backbone ribbon and colored by functional domain: endopeptidase (red), translocation domain (yellow), and receptor binding domain (green). The structure of the bHCT fragment (right, residues 555–868) and rotated 180° around the y axis is shown. Blue spheres represent the positions of single cysteine replacements that were labeled with the environmentally sensitive fluorescent NBD probe. (b) Size-exclusion chromatography coupled with multiangle light scattering for bHCT. The experimentally determined molecular mass (40 ± 1.3 kDa, red line) matches closely with the theoretical molecular mass (39.92 kDa) for bHCT. (c) Cosedimentation of bHCT with liposomes. bHCT (0.5 nmol) was incubated with liposomes at pH 4.4 containing a mixture of POPC/POPS at the following mol%: 100:0, 95:5, 90:10, 80:20, and 60:40. After liposomes were pelleted, the proteins in the supernatant and pellet fractions were analyzed by SDS-PAGE. The data indicate the purified protein was a monomer with similar partitioning assay results to that published for full-length toxin (16). Experiments were performed in triplicate, and quantification of protein band intensities is shown. To see this figure in color, go online.
In recent years, much progress has been made in understanding the molecular basis for clostridial neurotoxin binding to neuronal receptors and LC endopeptidase cleavage of SNARE proteins. In contrast, the mechanism by which clostridial neurotoxins deliver the LC endopeptidase across the membrane bilayer remains poorly understood. One model proposes that, at acidic pH, HCT inserts into the endosomal membrane and experiences a dramatic refolding, resulting in the formation of an ion-conducting pore through which the partially unfolded LC is subsequently delivered (17, 18, 19, 20). However, this model has not been conclusively demonstrated for any clostridial neurotoxin. Indeed, accumulating evidence suggests that LC endopeptidase translocation may occur through a different mechanism (16,21, 22, 23, 24, 25, 26).
To illuminate the mechanism, we need to better understand aspects of HCT membrane insertion. Here, we gain insight into the HCT membrane-inserted structure using a variant of the TeNT HCT domain as a model. This isolated, “beltless” HCT domain (bHCT) retained the TeNT functions of membrane interaction and pore formation. Therefore, we studied the membrane interaction of bHCT using a variety of circular dichrosim (CD) and fluorescence spectroscopy techniques on purified bHCT variants incubated with anionic large unilamellar vesicles (LUVs) at neutral or acidic pH. These techniques provided evidence that all regions of bHCT interacted with the membrane and that bHCT underwent membrane-induced refolding.
Materials and methods
Materials
The fluorescent dye IANBD-amide (N,N′-dimethyl-N-(iodoacetyl)-N′-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)ethylenediamine) was obtained from Setareh Biotech (Eugene, OR). The phospholipids, 1-palmitoyl-2-oleoyl-phosphatidylcholine (POPC), 1-palmitoyl-2-oleoyl-phosphatidylserine (POPS), 1-palmitoyl-2-(4,5-dibromo)stearoyl-sn-glycero-3-phosphocholine (4-5BrPC), 1-palmitoyl-2-(6,7-dibromo)stearoyl-sn-glycero-3-phosphocholine (6-7BrPC), 1-palmitoyl-2-(9,10-dibromo)stearoyl-sn-glycero-3-phosphocholine (9-10BrPC), 1-palmitoyl-2-(11,12-dibromo)stearoyl-sn-glycero-3-phosphocholine (11-12BrPC), asolectin (soybean polar lipid extract), and mini extruder unit and associated filters were purchased from Avanti Polar Lipids (Alabaster, AL). All other chemicals and salts were purchased from Millipore Sigma (Burlington, MA) or Thermo Fisher Scientific (Waltham, MA).
Construction of pET28-bHCT
DNA encoding bHCT (residues 555–868) with a C-terminal extension encoding a Strep Tag II epitope was synthesized (Genscript, Piscataway, NJ) with optimal codon usage for expression in Escherichia coli. DNA encoding bHCT was subcloned into a modified pET28a expression vector that contained unique KpnI and PstI sites. Correct insertion of the DNA fragment verified by automated DNA sequencing (MU DNA Core facility).
bHCT mutagenesis
Cysteine point mutations were introduced into the pET28 plasmid encoding bHCT using the QuikChange Lightning site-directed mutagenesis kit according to the manufacturer’s instructions (Agilent Technologies, Santa Clara, CA). Mutagenesis was confirmed by automated DNA sequencing of the entire gene (MU DNA Core facility). Mutagenesis primers used are listed in Table S1.
bHCT expression
E. coli BL21 AI cells were transformed with pET28-bHCT plasmids and grown overnight on Luria broth (LB) agar with 50 μg/mL kanamycin at 37°C. Transformants were grown overnight in 3 mL LB broth with 50 μg/mL kanamycin and then stored at −80°C in 12% (v/v) glycerol. For protein expression, E. coli cells harboring pET28-bHCT were cultured overnight on LB agar plates with 50 μg/mL kanamycin at 37°C. An isolated colony was inoculated into 3 mL LB broth with 50 μg/mL kanamycin; subsequently, 1 mL of the overnight culture was inoculated into 50 mL of Turbo Prime Oleate (Athena Enzyme Systems, Baltimore, MD) with 50 μg/mL kanamycin and 1/1000 Antifoam 204. Cultures were grown for 3 h at 37°C until an optical density at 600 nm (OD600) of ∼0.6. Isopropyl-β-D-1-thiogalactosidase and arabinose were then added to final concentrations of 1 mM and 2% w/v, respectively, and culture incubation continued overnight at 16°C.
bHCT purification and labeling
Cells were harvested by centrifugation (6000 × g, 15 min) and suspended in Ni2+-NTA binding buffer (BB, 20 mM Tris (pH 8), 500 mM NaCl, 5 mM imidazole) supplemented with protease inhibitor cocktail (1:100; Millipore Sigma) and universal nuclease (1:5000; Thermo Fisher Scientific). Cells were lysed with two passes in a French press and then clarified by centrifugation (20,000 × g, 30 min) and 0.22 μm filtration. The clarified lysate was applied to 0.5 mL pre-equilibrated Ni2+-NTA (nitrilotriacetic acid) resin (Qiagen, LLC., Germantown, MD) and then washed with 5 mL BB with 20 mM imidazole. bHCT was eluted with 2.5 mL of a modified buffer (20 mM MOPS (3-(N-morpholino)propanesulfonic acid) (pH 8), 500 mM NaCl, 250 mM imidazole) and collected in 10 fractions. The concentration of protein in each fraction was estimated by measuring the absorbance at 280 nm, and the three peak fractions were pooled.
Next, TCEP (tris(2-carboxyethyl)phosphine) and IANBD-amide (dissolved in DMSO (dimethyl sulfoxide)) were added in 20-fold molar excess of the protein and incubated overnight in the dark at 4°C. After clarification of the sample by centrifugation, the supernatant was diluted with three volumes of streptavidin binding buffer (SBB, 100 mM Tris pH 8, 150 mM NaCl) supplemented with 1 mM reduced glutathione. Samples were applied to 0.5 mL pre-equilibrated StrepTactin Sepharose (IBA Lifesciences, Göttingen, Germany), and the flowthrough was collected and reapplied to the same column. The column was then washed with SBB and eluted with 2 mL SBB with 10 mM desthiobiotin as 10 fractions. The concentration of protein in each fraction was estimated by measuring the absorbance at 280 nm. Protein samples (2 μg) were separated by sodium dedecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and visualized by both ultraviolet (UV) excitation (NBD fluorescence) and staining with Coomassie blue (total protein).
NBD labeling efficiency was calculated using Eq. 1:
| (1) |
where A478 is the sample absorbance at 478 nm, A280 is sample absorbance at 280 nm, ϵ478, NBD = 22,000 cm−1 M−1 is the molar extinction coefficient for NBD at 478 nm, and ϵ280, bHCT = 48,820 cm−1 M−1 is the molar extinction coefficient for bHCT at 280 nm. Labeling efficiencies of the mutants used in this study were in the range of 70–100%.
LUV preparation
LUVs were prepared by mixing lipids dissolved in chloroform in the indicated ratios. To transfer the lipids to buffer, the chloroform mixtures was dried under a gentle stream of nitrogen and subjected to vacuum centrifugation overnight to remove all trace chloroform. The dried lipids were hydrated in phosphate buffer (50 mM HPO42− (pH 8.0)) or Tris buffer (50 mM Tris, 150 mM NaCl (pH 7.4)) to bring lipids to 20 mM final concentration. These suspensions were vortexed every 20 min for 3 h to dissolve the lipid film. The rehydrated lipid was then subjected to extrusion through a 0.1 μm filter until the syringe writing was readable through the extruded lipids using at least 25 passes (27). The vesicle stocks were stored at 4°C until use.
Association of bHCT with LUV
LUV were combined with bHCT in low-pH buffer (10 mM sodium acetate-acetic acid, 150 mM NaCl, 1 mM EDTA (pH 4.4)) and incubated for 20 min at 4°C. Subsequently, LUVs were isolated by centrifugation at 100,000 × g for 30 min at 4°C. Supernatants containing unbound proteins were recovered and held on ice for further analysis. LUVs were suspended in fresh low-pH buffer and recovered by centrifugation as above. Supernatants (wash fractions) were collected and, along with those from the first centrifugation step, concentrated to ∼50 μL using Microcon centrifugal filter devices (Millipore Sigma). Liposomes were suspended in 50 μL of buffer, and all fractions were mixed with an equal volume of SDS-PAGE buffer. Volume equivalents of each fraction were resolved on SDS-polyacrylamide gels and visualized by staining with Coomassie blue dye. Gels were imaged using a LiCor Odyssey system (Lincoln, NE), and bands were quantified by densitometry.
Potassium release assay
LUVs were diluted into low-pH buffer (10 mM sodium acetate-acetic acid, 150 mM NaCl, 1 mM EDTA (pH 4.0)) with constant stirring and allowed to equilibrate for 5 min. bHCT (0.25 nmol) was then added to the solution, and potassium ion release was monitored using a potassium-selective electrode (Orion; Thermo Fisher Scientific). After 10 min, 0.01 M KCl was added to the solution to ensure that the electrode was functioning as expected. Specific K+ release was determined by subtraction of basal release values obtained from liposomes incubated in buffer alone.
Steady-state fluorescence measurements
Ensemble steady-state fluorescence emission measurements were performed in a Fluorolog FL3-22 steady-state fluorescence spectrometer (Jobin Yvon, Edison, NJ) equipped with double-grating excitation and emission monochromators. The experiments were performed using a 2 × 10 mm cuvette oriented parallel to the excitation beam. The sample temperature was maintained constant at 25°C using a Peltier device from Quantum Northwest (Spokane, WA) and a refrigerated, circulating water bath. All measurements were collected after at least a 15 min equilibration period, after which all spectral measurements were collected with 1 nm steps using a 4 nm slit on the excitation monochromator and 5 nm on the emission monochromator, averaged over three scans. NBD fluorescence measurements were collected from 500 to 650 nm using an excitation wavelength of 475 nm. For intrinsic fluorescence spectra measurements, the excitation slit was adjusted to 2 nm. Intrinsic fluorescence spectra were collected 300–500 nm at both 280 and 295 nm excitation.
Lifetime fluorescence measurements
Fluorescence decay was measured with a time-resolved fluorescence spectrometer (FluoTime 200; PicoQuant, Berlin, Germany) using a standard time-correlated, single-photon counting scheme. Samples were excited at 439 nm by a subnanosecond pulsed diode laser (LDH 440; PicoQuant) with a repetition rate of 10 MHz. Fluorescence emission was detected at 520 nm, selected by a Sciencetech model 9030 monochromator (1200 lines/mm, spectral range 350–800 nm, linear dispersion 8 nm/mm; Sciencetech, London, ON, Canada), using a PMA-182 photomultiplier (PicoQuant). The fluorescence intensity was recorded within 4096 channels (34 ps/channel). Data normally were collected to a constant peak value of 10,000 counts. The instrument response function was recorded under the same conditions at the excitation wavelength by replacing the sample with a scattering solution of colloidal silica (Ludox; Grace, Columbia, MD). The total width on the half-height of the instrument response function was ∼0.8 ns.
Spectral analysis of fluorescence
Data analysis and spectral curve fitting were performed using GraphPad Prism 7 (GraphPad Software, San Diego, CA). Spectra were baseline corrected using either a buffer blank spectrum or LUV-only sample and then fitted using Eq. 2 (28):
| (2) |
where I0 is the intensity maximum, λmax is the wavelength of maximal intensity, Γ is the spectral width at half-maximal intensity I0/2, and ρ is the asymmetry of the distribution as determined by nonlinear least-squares fitting. Error fitting was manually performed for representative spectra using support plane analysis in Origin (OriginLab, Northampton, MA).
Circular dichroism
CD measurements were performed using an upgraded Jasco-720 spectropolarimeter (Japan Spectroscopic Company, Tokyo, Japan). For most far-UV CD experiments, 30–50 scans were recorded using a 1 mm optical path cuvette. For most near-UV CD experiments, 200–250 scans were recorded using a 10 mm optical path cuvette. All CD spectra were corrected for background.
Structure visualization and analysis
All ribbon structures of TeNT and bHCT were generated using UCSF Chimera (29) on Protein Data Bank (PDB): 5N0B (13). The distances between each tyrosine and tryptophan pair were calculated for the α-carbon using the built-in distance function of Chimera. The solvent-exposed surface area for each tryptophan residue was calculated using the areaSES (solvent-excluded surface area) generated from the surface command and normalized to the areaSES for Gly-X-Gly.
Sequence-based hydropathy analysis
Hydropathy analysis was carried out using the Membrane Protein Explorer (MPEx) webtool (https://blanco.biomol.uci.edu/mpex/ (30)), updated to account for interactions with anionic membranes (31). Residues 555–868 of tetanus neurotoxin from UniProt P04958 were used as sequence input. The sequence was analyzed using the water-octanol scale, the water-interface scale, and the β-barrel formation propensity. To mimic acidification, all glutamate and aspartate side chains were protonated. To reflect the membrane composition, the interfacial scale was analyzed with the latest 3.3.0 version of MPEx that accounts for interactions with anionic lipids (31). Specifically, a surface potential of Ψ0 = −60 mV was used to approximate 75:25 mol% POPC/POPS or Ψ0 = −110 mV for 25:75 mol% POPC/POPS, as described in (32).
Results
The goal of this study was to obtain molecular insights into how TeNT interacts with and inserts into the endosomal membrane. Ideally, experiments would be performed on full-length TeNT, but several practical limitations prevented this approach. First, a powerful approach for studying the insertion domains of many proteins, including other bacterial toxins, is to chemically link the environmentally sensitive fluorophore NBD to a cysteine (33). To utilize site-selective labeling, the protein should have only one reactive cysteine (34). Creating a unique NBD labeling site in the full-length TeNT would have required removing the 10 endogenous cysteines in the LC endopeptidase domain, the belt region of HCT, and the heavy chain receptor binding domain. Fortunately, bHCT does not contain endogenous cysteines. Second, results from spectroscopic studies, such as the measurements of ellipticity and intrinsic fluorescence in this study, would have been impossible to interpret in the context of the full-length protein. Thus, we first demonstrated that the isolated bHCT recapitulated several key functions of full-length TeNT and could be produced in the milligram quantities required for biophysical studies. Using this construct, we aimed to identify which region(s) of bHCT interacted with LUVs at acidic pH and whether any structural rearrangements accompanied membrane interaction.
Biochemical studies of the bHCT domain
Initial attempts to purify the full-length HCT domain resulted in the formation of inclusion bodies during recombinant expression. We reasoned this was due to the large unstructured belt region, as was observed for the related botulinum neurotoxin A (35). Fortunately, a truncated form of HCT, lacking the belt region, could be expressed in soluble form and readily purified by conventional affinity chromatography. The purified bHCT protein (residues 555–868; Fig. 1 a, middle), was monomeric in solution, as shown by size exclusion chromatography in combination with multiangle light scattering (Fig. 1 b). Purified protein eluted as a single peak with an estimated molar mass of 40 ± 1.3 kDa, which is consistent with bHCT’s theoretical molecular mass of 39.9 kDa.
Next, we determined whether bHCT partitioned to LUVs and resulted in potassium leakage at acidic pH, as previously reported for full-length TeNT (16). Wild-type bHCT partitioned to membranes at pH 4.4, and partitioning increased when the LUV contained a greater mol% of the anionic lipid phosphatidylserine, POPS (Fig. 1 c). In addition, bHCT facilitated potassium ion release from LUVs at low pH, but not at neutral pH (Fig. S1). Furthermore, at pH 4.4, the release of potassium ions was dependent on LUV composition; increasing the negative membrane surface potential by increasing the anionic lipid content with POPS led to an increase in ion release (Fig. S1, blue). When asolectin (a natural mix of polar lipids containing ∼25% mixed anionic lipids) was used, bHCT also demonstrated ion release (Fig. S1). This indicated that anionic lipids promoted bHCT interaction with LUV anionic lipids; notably, as the main polar lipid in asolectin is phosphatidylinositol, the interaction was not due to a specific headgroup. Results for bHCT in both assays (Figs. 1 c and S1) were consistent with the previously published results for full-length TeNT (16). Therefore, we elected to use bHCT as a model for full-length TeNT insertion.
Study of membrane interactions using fluorescence of NBD-labeled bHCT
Although potassium release required membrane insertion of bHCT, the exact region(s) of bHCT that entered the membrane bilayer were unknown. To monitor which region(s) of bHCT were involved, 47 residues were selected to sample the bHCT surface (Fig. 1 a, spheres). Particular attention was paid to residues or regions that were previously identified in botulinum neurotoxin A and TeNT as potentially membrane interacting (35, 36, 37). To assess the environment of each selected residue, we substituted the wild-type residue with cysteine to serve as an attachment site for the environmentally sensitive fluorophore NBD. To ensure the substitution and NBD label did not affect the function of bHCT, the potassium release assay was performed for a subset of the variants with and without the NBD label. Neither the cysteine substitutions nor addition of the NBD label altered potassium release activity relative to wild-type bHCT (Table S2).
When TeNT (or bHCT) inserts into a membrane, it moves from a hydrophilic to a hydrophobic environment. This insertion can be monitored using the NBD-labeled bHCT because when NBD enters a hydrophobic environment, the emission spectrum increases in fluorescent intensity and shifts toward shorter wavelengths. Thus, the steady-state fluorescence emission spectra were collected for the series of NBD-labeled bHCT variants in the absence and presence of anionic LUVs (75:25 mol% POPC/POPS) at neutral and acidic pH. Results for the entire series of variants showed similar changes in their emission spectral responses to the addition of LUV and subsequent acidification.
As an example of the NBD response to the change in environment, the spectra for bHCT Q608C-NBD are shown in Fig. 2 a. Soluble, NBD-labeled protein at pH 8.0 (Fig. 2 a, black) showed a low intensity and a position of the emission maximum near 545 nm, consistent with a hydrophilic environment. At pH 8.0 with anionic LUVs, the emission spectrum remained similar (Fig. 2 a, red), which demonstrated no partitioning, as expected. At pH 4.5 with anionic LUVs, the enhanced intensity and blue-shifted emission spectrum indicated that region of the protein entered a hydrophobic environment (Fig. 2 a, blue). As a control, spectra were obtained for protein acidified without LUVs (Fig. 2 a, green). This spectrum displayed some change in the emission profile, but not to the same extent as when LUVs were present, which suggested that the extreme shift seen with acid + LUVs was not due to aggregation. From these observations, we concluded that Q608C-NBD entered the membrane only at acidic pH. The remaining 46 positions assayed displayed similar responses to LUVs and acidic pH (Fig. S2).
Figure 2.
Representative steady-state and time-resolved fluorescence emission spectra of NBD-labeled bHCT. (a) bHCT Q608C-NBD emission spectra are shown either in solution at pH 8.0 (black) and pH 4.5 (green) or in the presence of LUVs at pH 8.0 (red) and pH 4.5 (blue). (b) Fluorescence decay kinetics of bHCT Q608C-NBD in solution at pH 8.0 (black), with LUVs at pH 8.0 (red), and with LUVs at pH 4.5 (blue). The dashed line corresponds to the instrument response function. Both steady-state and time-resolved fluorescence data indicate that the NBD probe selectively attached to bHCT at position Q608C enters a hydrophobic environment upon the interaction of the protein with the lipid bilayer at acidic pH. Labeled bHCT was 300 nM for all measurements. 75:25 mol% POPC/POPS LUVs were added to a final concentration of 0.8 mM. The data shown are representative of two independent experiments. a.u., arbitrary unit. To see this figure in color, go online.
To quantify these changes in fluorescence, we assessed the position of the emission maximum and the relative change in fluorescence intensity at 520 nm (Fig. 3). For all 47 variants, the soluble protein’s position of the maximum indicated that the NBD label was in a hydrophilic environment, as expected (Fig. 3 a, black). At pH 8.0 with anionic LUVs, both the position of the maximum and the fluorescent intensity remained similar for all positions (Fig. 3, red), which indicated no partitioning to the membrane. In contrast, at pH 4.5 with anionic LUVs, all 47 variants displayed a blue-shifted position of the maximum, and most also displayed an increase in fluorescent intensity (Fig. 3, blue), which indicated all positions entered a hydrophobic environment.
Figure 3.
NBD Position of the emission maximum and fluorescence intensity increases for all bHCT positions. (a) Position of the emission maximum for all NBD-labeled cysteine substitutions in solution at pH 8.0 (black), with LUVs at pH 8.0 (red), and with LUVs at pH 4.5 (blue). (b) Intensity fold increase at 520 nm for all single-cysteine point mutants labeled with NBD for LUVs at pH 8.0 (red) and LUVs at pH 4.5 (blue). The horizontal dashed line at 1 indicates no change in intensity. An increase in the fluorescence occurred only with both LUVs and acidic pH. The data together suggest that all NBD probes entered a hydrophobic environment in the presence of LUVs and low pH. Experiments were carried out at 300 nM NBD-labeled bHCT and 0.8 mM 75:25 mol% POPC/POPS LUVs. The data are representative of two independent experiments with the standard error of fitting under 1 nm. To see this figure in color, go online.
The magnitudes of the NBD blue shift and intensity increase were unexpectedly large for many positions. Thus, to confirm the large response, lifetime fluorescence was measured for a representative variant (Q608C-NBD). At pH 8.0 with and without anionic LUVs, soluble protein displayed an extremely short lifetime (Fig. 2 b, black and red), which indicated the NBD label was in a very hydrophilic environment, as expected and consistent with steady-state measurements. At pH 4.5 with anionic LUVs, the sample lifetime increased by over an order of magnitude, consistent with the NBD label entering a very hydrophobic environment. Thus, the lifetime fluorescence and steady-state fluorescence results were consistent.
Effect of pH and lipid composition on membrane binding
In addition to altered pH, another change as TeNT is endocytosed is the enrichment of anionic lipids in the endosomal membrane bilayer (38). This can be simulated using anionic lipids such as POPS in the LUVs. Indeed, increased anionic lipid content increased LUV partitioning of TeNT (16) and unlabeled bHCT (Fig. 1 c) at pH 4.4. The use of NBD-labeled bHCT provided the opportunity to obtain a highly sensitive pH titration as the domain inserted into LUVs of various compositions. For these experiments, Q608C-NBD was selected because it demonstrated the largest signal change. Spectra were collected as the pH was titrated, and the position of the maximum and change in fluorescence intensity were assessed. For Q608C-NBD in solution, the spectral profile remained similar at all pH values, which indicated the protein did not undergo aggregation. For zwitterionic LUVs (100:0 mol% POPC/POPS, Fig. 4 a, green) at either pH 7.4 or 4.5, the spectral profiles also remained similar, consistent with a lack of partitioning to the membrane. In contrast, acidification in the presence of the two anionic LUV compositions (75:25 and 25:75 mol% POPC/POPS, Fig. 4, red and blue) showed large decreases in spectral position of the maximum (Fig. 4 a) and increases in NBD fluorescence intensity (Fig. 4 b), indicating bHCT partitioning. LUVs with larger mol% of POPS displayed greater NBD signal changes, which could arise from a greater fraction partitioning to the membrane and/or from a deeper penetration into the membrane. Consistent with the data in Fig. S1, the addition of anionic lipids increased the interaction at all pH values. Therefore, bHCT insertion was a function of both pH and anionic lipid content.
Figure 4.
Effect of pH on position of the emission maximum and fluorescence intensity increase. (a) Change in position of the emission maximum for bHCT Q608-NBD in solution (black) or with various mol% POPC/POPS LUVs: 100:0 (green), 75:25 (red), and 75:25 (blue). (b) Intensity change (FpH/FpH7.5) at 520 nm for the NBD-labeled Q608C bHCT in solution (black) or with various mol% POPC/POPS mixture LUVs: 100:0 (green), 75:25 (red), and 75:25 (blue). Neither the position of the emission maximum nor intensity changed in the absence of membranes or when 100% zwitterionic POPC membranes were used. Labeled bHCT (300 nM) was incubated with the corresponding LUV (0.8 mM) before being titrated with acetic acid. The data are representative of two independent experiments. To see this figure in color, go online.
Circular dichroism measurements
To determine whether bHCT underwent structural rearrangement upon membrane insertion, circular dichroism measurements were obtained. Far-UV CD (200–260 nm) was used to assess changes to secondary structural content, and near-UV CD (260–300 nm) was used to assess changes to the environment of aromatic side chains. Experiments used unlabeled wild-type bHCT.
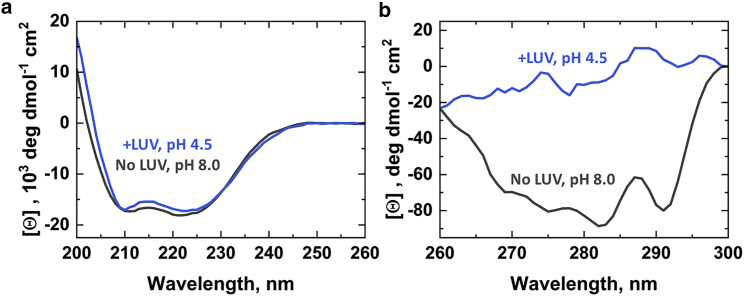
At pH 7.4, the far-UV CD spectrum displayed a strong α-helical profile (Fig. 5 a, black), consistent with the crystal structure of the soluble structure (Fig. 1 a, yellow), which is ∼70% α-helices. The spectrum at pH 4.5 in the presence of LUVs indicated similar α-helical content when bHCT inserted in the membrane (Fig. 5 a, blue). In contrast, the near-UV CD showed a loss of peaks at 275 and 290 nm upon the acidification in the presence of LUV (Fig. 5 b). Because near-UV CD reports on the environment of aromatic side chains, this indicated a change in the local environment of the bHCT aromatic residues. This change in the local aromatic environment, taken together with the far-UV CD, indicated a change in tertiary structure without changing the overall secondary structural content, consistent with α-helical refolding.
Figure 5.
Far- and near-UV circular dichroism of bHCT in the absence and presence of anionic LUVs. (a) Far-UV CD spectra for wild-type bHCT in solution at pH 7.4 (black) and with 75:25 mol% POPC/POPS LUVs at pH 4.5 (blue). Minimal changes in the far-UV spectra indicated that the bHCT retained α-helical secondary structure upon insertion. (b) Near-UV spectra for wild-type bHCT in solution at pH 7.4 (black) and with 75:25 mol% POPC/POPS LUVs at pH 4.5 (blue). The pronounced change in the near-UV spectra indicated the loss of asymmetry in the environment of aromatic residues, consistent with tertiary structure refolding upon insertion. bHCT was at 1.4 μM for far UV and 2.8 μM for near UV, with 0.8 mM LUVs for both. Spectra are representative of three experiments with 20–50 scans for near UV and 200–500 for far UV. To see this figure in color, go online.
Measurements of intrinsic fluorescence from tyrosine and tryptophan residues
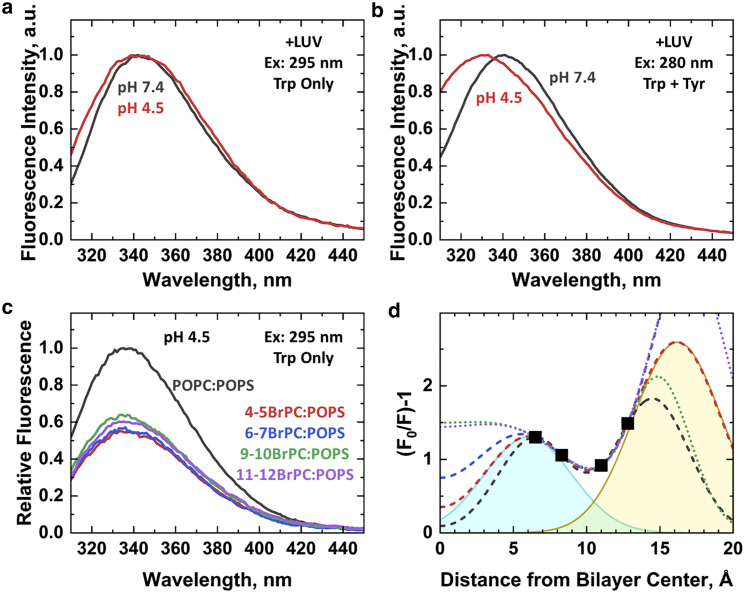
To further investigate the changes in aromatic residue environment observed by the near-UV CD, the fluorescence emission spectra for tyrosine and tryptophan residues were collected for unlabeled wild-type bHCT. These fluorescent residues are widely dispersed over bHCT (Fig. S3 a), and therefore the spectra represent an integrated contribution for the entire protein. The contributions from tyrosine and tryptophan can be differentiated by utilizing two different excitation wavelengths: 280 nm excites both fluorophores, whereas 295 nm selectively excites tryptophan residues (28).
Selective tryptophan excitation at 295 nm in the presence of LUV at pH 7.4 resulted in a fluorescence emission position of the maximum at 342 nm (Fig. 6 a, black). When both tyrosine and tryptophan residues were excited at 280 nm (Fig. 6 b, black), the position of the maximum is the same, suggesting little direct contribution of tyrosine residues to the emission spectrum. This is often observed in well-folded soluble proteins and is commonly attributed to the energy transfer from tyrosine to tryptophan residues (39). Acidification to pH 4.5 in the presence of LUVs showed marked spectral changes that were induced by protein insertion (Fig. 6, a and b, red). First, the fluorescence spectrum of tryptophan residues exhibited some broadening (Fig. 6 a, red) relative to the spectrum at neutral pH (Fig. 6 a, black). This indicated that the three tryptophan residues experienced a more heterogenic environment (28) in the membrane-inserted bHCT, which can arise from different depths of penetration into the bilayer (e.g., as in the membrane-proximal external region (MPER) domain of HIV gp41 (40)). Second, the spectrum for the combined tyrosine and tryptophan fluorescence (excitation at 280 nm) in the presence of LUVs underwent more drastic changes upon acidification and was blue shifted to 330 nm (Fig. 6 b). Because this spectral shift was only observed when tyrosine residues were also excited, it was most likely related to the disruption of the efficient tyrosine to tryptophan energy transfer, which allowed the direct contribution of tyrosine fluorescence to be observed in the emission spectrum. Such disruption of the transfer indicated substantial structural rearrangements of the bHCT upon acid-induced insertion into membranes.
Figure 6.
Spectra of intrinsic fluorescence of bHCT in the presence and absence of LUVs. (a) Intrinsic fluorescence in the presence of LUV using excitation at 295 nm to selectively excite tryptophan (28). (b) Intrinsic fluorescence in the presence of LUVs using excitation at 280 nm to excite both tryptophan and tyrosine. For the ease of comparison, all spectra were normalized to a maximal peak intensity of 1.0. In both (a) and (b), spectra of bHCT (1.4 μM) were acquired with 25:75 mol% POPC/POPS LUVs (0.8 mM). The spectra shown are representatives of three independent experiments. (c) Depth-dependent tryptophan quenching at acidic pH using bromolipid LUVs composed of 50:50 mol% BrPC/POPS or POPC/POPS; bHCT was 1.4 μM, and LUV was 0.8 mM. Data are representative of two independent experiments. (d) To estimate the depth of the tryptophan residues in the membrane, the depth-dependent quenching profile (black squares) was generated from the spectra shown in Fig. 6c and plotted on the y axis versus the known depth of each bromine quencher (41). Because the quenching profile was concave up, fitting required two distributions. A family of equivalent solutions can be obtained for this quenching profile; the details and values of the fittings are given in Table S3. Individual distributions were graphed for when one variable was fixed (orange and teal shaded areas) to illustrate how two distributions fit the data. The sum of the two distributions was plotted with a red dashed line. Other equivalent solutions given in Table S3 are also plotted. This family of quenching profiles illustrates that the data can be explained by the simultaneous presence of shallow (hm > 14 Å) and deep (hm < 5 Å) populations of tryptophan residues in the inserted bHCT. To see this figure in color, go online.
Excitation with either wavelength in the absence of LUVs at pH 7.4 produced fluorescence emission spectra identical to when LUVs were present (Fig. S4, black). The spectra did not change with the addition of LUVs (Fig. 6, a and b, black versus S4, black), consistent with the lack of membrane interaction at neutral pH as demonstrated in Figs. 1 c, S1, S2, and S3. Furthermore, the position of the emission maximum at 342 nm was consistent with the partial solvent exposure of all three tryptophan residues in the structure (Fig. S3, b and c). In contrast to when LUVs were present, acidification of bHCT in the absence of membranes had no appreciable change in the emission spectrum for either wavelength (Fig. S4, red), indicating little change in the chromophores’ environment. The appearance of a small, short-wavelength shoulder in the spectrum excited at 280 nm at pH 4.5 (Fig. S4 b, red) can be attributed to some loosening of the structure, reducing the energy transfer from tyrosine residues and slightly increasing their spectral contribution. However, the large reduction in energy transfer was only observed when LUVs were present and when insertion was triggered by acidic pH (Fig. 6 b, red).
Depth-dependent fluorescence quenching with brominated lipids
To gain further insight into the topology of membrane-inserted bHCT, we conducted a series of depth-dependent fluorescence quenching measurements. This methodology relied on the comparison of fluorescence intensity in the presence of quenching groups, which were specifically attached at different positions on lipid molecules (42). Specifically, we examined the quenching of tryptophan fluorescence by a set of brominated lipids, to which bromine atoms were attached at various positions along the acyl chains with a known depth in the bilayer (41). Quenching efficiency was proportional to tryptophan proximity to a particular bromine quencher. Experiments used a full set of commercially available bromolipids, with bromine quenchers at positions 6.5, 8.3, 11.0, and 12.8 Å from the bilayer center (41).
At pH 7.4, the tryptophan fluorescence spectra of wild-type bHCT showed no significant differences among brominated LUV or with vesicles lacking bromine quenchers (data not shown). The absence of bromine-induced changes indicated that bHCT did not interact with membranes at this pH, consistent with NBD fluorescence measurements (Fig. 3). Acidification to pH 4.5 in the presence of brominated lipids led to an approximately twofold decrease in fluorescence intensity, regardless of bromine depth (Fig. 6 c). These decreases in fluorescence intensity were attributed to the pH-dependent interaction of bHCT with the lipid bilayer, which placed the tryptophan(s) in the vicinity of bromine quenchers. Strong tryptophan quenching indicated that at least one of the three tryptophan residues penetrated beyond the interfacial region of the bilayer into the hydrocarbon core.
The small differences in quenching efficiencies among the four quenchers were further modeled as a system that contained both deeply and shallowly penetrating tryptophan residues (43). The modeled systems were generated using the quenching profile (Fig. 6 d), which were quantitatively examined using distribution analysis methodology (42). This quenching profile was calculated from intensity in the presence (F) and absence of quenching (F0) as (F0/F − 1) and plotted against the corresponding quencher depth (defined as the distance from the center of the bilayer). In distribution analysis, the quenching profile (which usually has a concave down shape) is fitted to a Gaussian distribution with the three independent parameters position of the maximum corresponding to the most-probable depth of the fluorophore (hm), the dispersion of the transverse position (σ), and the area under the curve (S), related to the overall quenching efficiency.
However, the resulting profile for the inserted bHCT had a concave up shape (Fig. 6 d) that suggested the presence of two Gaussian distributions arising from two tryptophan populations. To explicitly describe such a system, a total of six fitting parameters was required, which exceeded the number of available quencher depths. Mathematically, this implied a series of nonunique solutions to the problem. Nevertheless, an underlying trend could be identified by systematically fixing individual parameters during the fits. This trend was illustrated with a series of simulated fits by fixing either the most-probable depth (hm) for each tryptophan population or the width of the distributions (σ). Parameters from representative fits are tabulated in Table S3. Regardless of the limitations imposed on parameters and the variability of the overall profiles, all profiles in the series possessed both a deep tryptophan population located ∼5 Å from the bilayer center and a shallow population located at least 14 Å from the bilayer center.
The presence of three tryptophan residues, as well as the lack of additional, commercially available bromolipids, prevented the precise depth determination of individual tryptophan populations. Nonetheless, these results demonstrated that at low pH, some tryptophan-containing regions of bHCT penetrated deeply into the hydrocarbon core of the bilayer, whereas others did not.
Hydropathy analysis of bHCT using MPEx
To aid interpretation of the biophysical data, we used a sequence-based prediction webtool MPEx (https://blanco.biomol.uci.edu/mpex) (30) in its latest modification (31). MPEx utilizes a sliding window hydropathy analysis to find sequences with the energetic potential to enter either the membrane interface or the hydrocarbon core, either or both of which may occur as proteins convert from a soluble to a membrane-interacting conformation (44,45). Unlike many other hydropathy calculators, MPEx accounts for difference in membrane interactions with protonated and unprotonated side chains, which is particularly useful for pH-dependent insertion of bacterial toxins (31,45).
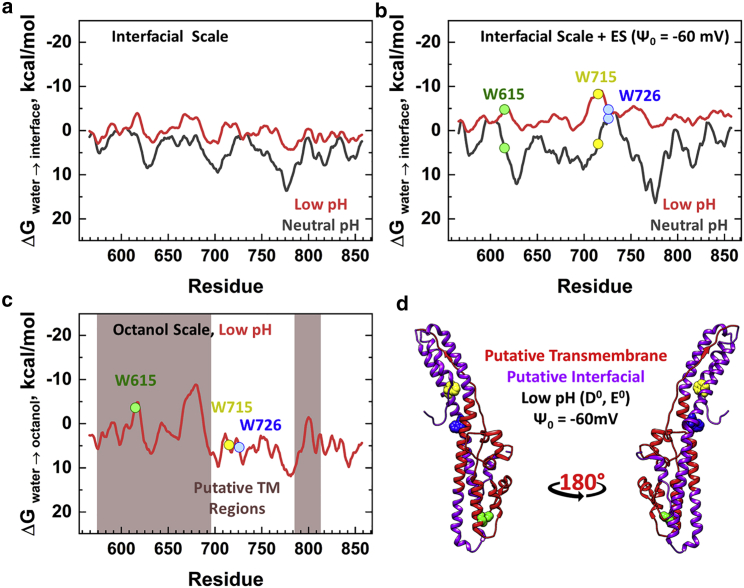
MPEx analyses of the interfacial partitioning for bHCT into a pure zwitterionic lipid bilayer and a bilayer containing 25% of anionic lipid (modeled by a membrane surface potential of −60 mV (46)) are shown in Fig. 7, a and b. In both predictions, protonation resulted in more favorable free energies, providing clues to the thermodynamics of pH-dependent conformational switching. For zwitterionic lipid membranes (e.g., POPC), the resulting low-pH profile ranged from a mildly unfavorable value of +4 kcal/mol to a mildly favorable value of −4 kcal/mol across the sequence. For membranes containing anionic lipids (e.g., POPC/POPS), the entire sequence showed favorable free energy predictions, with some regions reaching up to −9 kcal/mol. This difference was consistent with the experimental observation that membrane-induced refolding required the presence of anionic lipids (Figs. 1 c, S1, and S4).
Figure 7.
Hydropathy analysis of the bHCT sequence and visualization on the crystal structure. Potential bHCT membrane interacting regions were estimated using the online application MPEx (30). (a) bHCT regions with sufficient hydrophobicity to interact with lipid bilayers were determined using the interfacial Wimley-White scale (47). Predictions were calculated for “neutral pH” (black: E−, D−) and “acidic pH” (red: E0, D0); as the domain contains no histidine residues, their protonation state was ignored. MPEx-determined, favorable interactions are denoted by the negative value of free energies (traditionally plotted above zero). (b) Predictions of interfacial partitioning that includes contributions arising from Coulombic interactions with anionic membranes were estimated as described (31). The latter analysis used a membrane surface potential Ψ0 = −60 mV, which approximated 75:25 mol% POPC/POPS under the salt conditions used in this study (46). The more favorable partitioning free energies predicted at acidic pH in anionic membranes were consistent with the biochemical and spectroscopic measurements (Figs. 1c, S1, and S4). (c) Putative α-helical transmembrane regions at “acidic pH” (E0, D0) were estimated using the Wimley-White octanol scale (47) using a 19-residue sliding window, as described in (48). Regions of the sequence with possible transmembrane α-helices are indicated by the brown shading. (d) The crystal structure of the bHCT domain in the soluble TeNT (PDB: 5N0B (13)) was represented as a backbone ribbon and colored to illustrate the MPEx predictions for low pH insertion into anionic lipid bilayer: transmembrane α-helix (red) or interfacial (purple). On the right, the same structure was rotated 180° about the y axis. The three tryptophan residues were represented as space-filling spheres and colored individually. Note that W615 is predicted to be in a transmembrane segment, whereas W715 and W726 are expected to have interfacial locations. This predicted difference in membrane penetration is consistent with the depth-dependent quenching data in Fig. 6d. To see this figure in color, go online.
We further analyzed the propensity of the protonated bHCT sequence to form inserted transmembrane α-helices (Fig. 7 c) and β-structures (Fig. S5). The latter were identified in MPEx using scores for β-strand and β-hairpin that exceed their corresponding thresholds (49). As seen in Fig. S5, only small regions of the sequence crossed the corresponding thresholds (dashed lines), which ruled out the possibility of an inserted β-barrel. In contrast, the octanol scale, which predicts transmembrane α-helical segments (47,48), revealed several sequences with potential for transmembrane insertion (Fig. 7 c). These sequences overlapped and formed a continuous, large region that spanned residues 574–696 (Fig. 7 d) and could potentially accommodate several transmembrane α-helices. In the known membrane protein structures, single transmembrane α-helices range from 17 to 43 residues, with an average of 28 residues (50). Thus, the large region predicted in bHCT could reasonably form four α-helices. This region also covers the sequence of the bHCT-derived peptide (residues 669–691) that formed cation-selective ion channels in planar bilayers (51). Note that out of the three tryptophan residues, only W615 resided in the potential transmembrane region.
Discussion
Cellular entry of A-B toxins usually occurs by the translocation of the catalytic moiety (A subunit; LC) across a cellular membrane by its corresponding translocation domain (B subunit; part of the heavy chain). The interaction between a translocation domains and membranes often led to the formation of ion-conducting structures that share similar properties, as has been demonstrated for tetanus, botulinum, and diphtheria toxins (11,52, 53, 54). In some cases, such as anthrax toxin, the ion channel provided a passageway for the translocation of the catalytic moiety through the membrane (55,56). In other cases, such as diphtheria toxin, the formation of the ion-conducting open channel state (57) likely occurred after translocation (58). Models for the translocation of tetanus and botulinum toxins often depict a through-channel translocation (59), but the experimental evidence for or against such a mechanism is extremely difficult to obtain, especially in the absence of the high-resolution structures of the protein in the membrane. Because such structures are extremely difficult to obtain, various spectroscopic and computational techniques are often used to gain structural insights into the interactions between the translocation domain and the membrane; such studies can also provide thermodynamic insights into this critical interaction (33,60,61). Here, we present an application of several spectroscopic techniques, complemented by hydropathy analysis, to the study of membrane interactions of the bHCT domain from tetanus neurotoxin.
As with full-length TeNT, the isolated bHCT domain remained monomeric and well folded in solution at neutral pH (Fig. 1 b) and inserted into the membrane at low pH to promote potassium release (Fig. S1). The ability to promote potassium release was retained by numerous bHCT mutants with single-cysteine variants (Table S2), allowing for site-selective labeling with the small, environmentally sensitive fluorophore NBD. A wide sampling of NBD-labeled bHCT mutants showed that all regions of bHCT entered a hydrophobic environment upon membrane insertion, which argued against the existence of a particular membrane “docking” site on the domain. On the other hand, this result did not indicate that the protein entered into the middle of the bilayer as a rigid body, as such penetration would be thermodynamically unfavorable. Instead, we suggest that bHCT refolded at the membrane interface and inserted into the bilayer in a new conformation, similar to what was observed for other bacterial toxins and colicins (33,60, 61, 62).
This model for membrane-induced refolding of bHCT was supported by the observed changes in ellipticity measured by CD spectroscopy. The conformation of the inserted bHCT maintained high α-helical content, as evidenced by the far-UV CD spectrum in Fig. 5 a. In contrast, the near-UV CD spectrum displayed a loss of ellipticity (Fig. 5 b), consistent with the disruption of packing around aromatic residues (three tryptophan and 18 tyrosine residues). These patterns of change were very similar to those observed upon the formation of “molten globule” states known for some soluble proteins (63). Molten globule-like states have also been identified for membrane-inserting proteins and are known as extended α-helical arrays (62) or molten disks (64).
The reduction in Förster resonance energy transfer (FRET) was manifested in the pronounced shift of the overall emission upon excitation at 280 nm (Fig. 6 b). This shift was not associated with tryptophan fluorescence, as it was not observed upon 295 nm excitation (Fig. 6 a). Because tyrosine fluorescence is not environmentally sensitive, this change in the tyrosine band must arise from the less efficient energy transfer in the inserted form (65); the latter must be due to increased distances between the two classes of fluorophores relative to the soluble state (Fig. S3 a; Table S4).
Refolding of bHCT was also predicted by an MPEx-based analysis that identified large regions of sequence with putative α-helical transmembrane segments (Fig. 7, c and d). In contrast, the prediction of transmembrane β-structures predicted virtually no propensity (Fig. S5). One of the putative transmembrane α-helical regions included residues 669–691, which was previously synthesized as a short peptide and demonstrated to form cation-selective ion channels in planar bilayers (51). Notably, the remaining bHCT sequence was predicted to interact with the anionic lipid bilayer interfacially, under conditions mimicking low pH (E0, D0) (Fig. 7 b, red). The predicted interfacial region contained residue K768, which had been experimentally demonstrated to play an important role in translocation (37). Coarse-grained molecular dynamics simulations, which restricted the conformation of bHCT, suggested that K768 did not contribute to the original interactions with anionic lipids (37,66). It is not clear, however, whether K768 contributed to membrane interaction in addition to the special role that it played in translocation.
Finally, we used fluorescence quenching with lipid-attached bromine atoms to investigate the possibility of deep membrane penetration of bHCT. These studies were complicated by two factors. First, the NBD probe was insensitive to quenching with bromine atoms, leaving us little choice but to use intrinsic tryptophan fluorescence. Second, measurements had to be performed in the context of wild-type bHCT, which contained three tryptophan residues; replacement of any of the endogenous tryptophan with tyrosine, phenylalanine, alanine, or methionine resulted in misfolding within E. coli. Nevertheless, the quenching data in Fig. 6, c and d demonstrated the existence of two classes of tryptophan residues, with deep and shallow penetration. The deep population was most likely to be located ∼5 Å from the bilayer center and thus belonged to a transmembrane segment. This was consistent with the position of W615 in the putative transmembrane region (Fig. 7 c). The shallow population, located ∼15 Å from the bilayer center, likely comprised W715 and W726, which were both predicted to be in the interfacial region (Fig. 7 b). Although further experiments will be required to establish the overall topology of bHCT in the membrane, it was clear that insertion resulted in substantial refolding into a conformation containing both transmembrane and interfacial α-helical segments.
How does the described membrane-induced refolding of bHCT compare to membrane insertion of other proteins?
Comparing the translocation action of TeNT HCT to that of other A-B toxins revealed some similarities and important differences. Like many toxins that exploit the endosomal pathway of cellular entry, insertion of bHCT required low pH and anionic lipids. The cellular entry of TeNT HCT had a multistep insertion and translocation mechanism, a feature also shared with diphtheria toxin (16,61,67, 68, 69). In both cases, the translocation domains underwent global refolding upon insertion into the target membrane. This was different, for example, from the pathway of the anthrax toxin, for which only a small fragment underwent a refolding transition to a single long strand that contributed to a heptameric transmembrane β-barrel (55,56). Moreover, one of the models suggested for TeNT translocation (66) was similar to the data that demonstrated that the channel activity of diphtheria toxin was formed after translocation and likely did not facilitate the translocation pathway for the cellular entry of toxin (58).
In contrast, the overall topology of the inserted bHCT likely differed from that of the diphtheria toxin translocation domain, which was half the length and had only three transmembrane α-helices (33). Notably, the experimentally confirmed transmembrane α-helices of the diphtheria toxin translocation domain were also identified using the MPEx prediction tool (45). However, the hydrophobicity pattern of diphtheria toxin was very different from that of bHCT. Indeed, a curious feature of the bHCT hydropathy plot in Fig. 7 c was the presence of a very large region in which transmembrane α-helices are predicted to reside, without a clear indication for α-helical boundaries. This was instead reminiscent of the membrane-spanning domain of HIV-1 gp41 (40) that was involved in viral entry to the host cell. This large region in bHCT implies that different segments might experience conformational plasticity during the insertion step(s) of the translocation event. The absence of the well-defined boundaries for transmembrane helices is also expected to increase lipid perturbation during insertion.
Finally, because bHCT has no histidine residues, the triggering mechanism for acid-induced insertion must differ from that of diphtheria toxin. Diphtheria toxin experienced pronounced histidine protonation (70,71), whereas acidic residues played an auxiliary role (72). In stark contrast, bHCT lacks histidine residues but possesses 16 aspartate and 21 glutamate residues. For TeNT bHCT, the increased efficiency of pH-dependent insertion that was coincident with the increased fraction of anionic lipids (Fig. 4, red versus blue) was consistent with both the insertion being triggered by protonation of acidic residues and with their pKas being dependent on the membrane surface potential. This was similar to observations for the apoptotic inhibitor Bcl-xL (73) and tumor-targeting peptide pHLIP (74). Our future mutagenesis studies will be aimed at identifying acidic residues responsible for triggering conformational switching in bHCT.
Conclusions
In this study, we found the bHCT domain of TeNT refolded and inserted deeply into the membrane bilayer to permit ion conductance. Insertion required the presence of anionic lipids and was triggered by acidic pH. Spectroscopic evidence indicated that the inserted conformation had a predominantly α-helical secondary structure; however, the tertiary structure was likely to be very different from that of the known soluble structure. An MPEx-based hydropathy analysis, corroborated by depth-dependent fluorescence quenching, indicated that the inserted conformation is expected to have both transmembrane and interfacial α-helical segments. The regions in which putative transmembrane segments were predicted spanned residues 574–696 and 785–813. Mapping these putative segments to the crystallographic structure of the toxin (Fig. 7 d) indicates that long α-helices present in the solution structure did not slide into the membrane but were likely to refold into separate transmembrane and interfacial segments. Further study is necessary to demonstrate whether TeNT acts as a monomer or oligomer; however, previous work has shown that the related full-length botulinum toxin interacts with membranes as a monomer (75). Similarly, it is conceivable that the ability of bHCT to interact with the membrane may be modulated by the presence of the LC and receptor binding domain. Another challenge in determining the structure organization of membrane-inserted bHCT is experimental verification of the topology of the putative transmembrane segments. Because the insertion occurs at acidic conditions, simple topological methods like dithionite quenching of NBD are not applicable (76). Our subsequent studies will be directed toward measuring topology by depth-dependent fluorescence quenching, which were instrumental for studies of other membrane-inserting proteins (33,43).
Author contributions
M.R.B. and A.S.L. conceived and directed the research. P.T.O. and M.R.B. selected sites for mutagenesis. P.T.O. and M.R.B. expressed the protein. M.R.B. performed the biochemical controls. P.T.O. and V.V.-M. performed and analyzed the spectroscopy. P.T.O. generated protein models. All authors interpreted and reviewed results, contributed to manuscript writing, and approved the final version of the manuscript.
Acknowledgments
We acknowledge Dr. Mark T. Fisher for being a great mentor, colleague, and friend and for starting this work.
We also acknowledge our funding 5R21AI121492 (M.R.B. and Mark T. Fisher), AI145960 (M.R.B.), and GM126778 (A.S.L.). V.V.-M. was supported in part by KUMC Biomedical Research Training Program fellowship from the University of Kansas Medical Center (KUMC).
Editor: Charles M Deber.
Footnotes
Supporting material can be found online at https://doi.org/10.1016/j.bpj.2021.09.030.
Supporting material
References
- 1.Dong M., Masuyer G., Stenmark P. Botulinum and tetanus neurotoxins. Annu. Rev. Biochem. 2019;88:811–837. doi: 10.1146/annurev-biochem-013118-111654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rossetto O., Montecucco C. Tables of toxicity of botulinum and tetanus neurotoxins. Toxins (Basel) 2019;11:E686. doi: 10.3390/toxins11120686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gill D.M. Bacterial toxins: a table of lethal amounts. Microbiol. Rev. 1982;46:86–94. doi: 10.1128/mr.46.1.86-94.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lacy D.B., Stevens R.C. Sequence homology and structural analysis of the clostridial neurotoxins. J. Mol. Biol. 1999;291:1091–1104. doi: 10.1006/jmbi.1999.2945. [DOI] [PubMed] [Google Scholar]
- 5.Masuyer G., Zhang S., et al. Stenmark P. Structural characterisation of the catalytic domain of botulinum neurotoxin X - high activity and unique substrate specificity. Sci. Rep. 2018;8:4518. doi: 10.1038/s41598-018-22842-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang S., Masuyer G., et al. Stenmark P. Identification and characterization of a novel botulinum neurotoxin. Nat. Commun. 2017;8:14130. doi: 10.1038/ncomms14130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schiavo G., Matteoli M., Montecucco C. Neurotoxins affecting neuroexocytosis. Physiol. Rev. 2000;80:717–766. doi: 10.1152/physrev.2000.80.2.717. [DOI] [PubMed] [Google Scholar]
- 8.Gill D. In: Bacterial Toxins and Cell Membranes. Jeljaszewicz J., Wadstrom T., editors. Academic Press; 1978. Seven toxic peptides that cross cell membranes; pp. 291–332. [Google Scholar]
- 9.Lacy D.B., Tepp W., et al. Stevens R.C. Crystal structure of botulinum neurotoxin type A and implications for toxicity. Nat. Struct. Biol. 1998;5:898–902. doi: 10.1038/2338. [DOI] [PubMed] [Google Scholar]
- 10.De Filippis V., Vangelista L., et al. Montecucco C. Structural studies on the zinc-endopeptidase light chain of tetanus neurotoxin. Eur. J. Biochem. 1995;229:61–69. doi: 10.1111/j.1432-1033.1995.tb20437.x. [DOI] [PubMed] [Google Scholar]
- 11.Hoch D.H., Romero-Mira M., et al. Simpson L.L. Channels formed by botulinum, tetanus, and diphtheria toxins in planar lipid bilayers: relevance to translocation of proteins across membranes. Proc. Natl. Acad. Sci. USA. 1985;82:1692–1696. doi: 10.1073/pnas.82.6.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen C., Fu Z., et al. Baldwin M.R. Gangliosides as high affinity receptors for tetanus neurotoxin. J. Biol. Chem. 2009;284:26569–26577. doi: 10.1074/jbc.M109.027391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Masuyer G., Conrad J., Stenmark P. The structure of the tetanus toxin reveals pH-mediated domain dynamics. EMBO Rep. 2017;18:1306–1317. doi: 10.15252/embr.201744198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pirazzini M., Bordin F., et al. Montecucco C. The thioredoxin reductase-thioredoxin system is involved in the entry of tetanus and botulinum neurotoxins in the cytosol of nerve terminals. FEBS Lett. 2013;587:150–155. doi: 10.1016/j.febslet.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 15.Schiavo G., Benfenati F., et al. Montecucco C. Tetanus and botulinum-B neurotoxins block neurotransmitter release by proteolytic cleavage of synaptobrevin. Nature. 1992;359:832–835. doi: 10.1038/359832a0. [DOI] [PubMed] [Google Scholar]
- 16.Burns J.R., Baldwin M.R. Tetanus neurotoxin utilizes two sequential membrane interactions for channel formation. J. Biol. Chem. 2014;289:22450–22458. doi: 10.1074/jbc.M114.559302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Montecucco C., Schiavo G., Dasgupta B.R. Effect of pH on the interaction of botulinum neurotoxins A, B and E with liposomes. Biochem. J. 1989;259:47–53. doi: 10.1042/bj2590047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Montecucco C., Schiavo G., et al. DasGupta B.R. Interaction of botulinum and tetanus toxins with the lipid bilayer surface. Biochem. J. 1988;251:379–383. doi: 10.1042/bj2510379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Puhar A., Johnson E.A., et al. Montecucco C. Comparison of the pH-induced conformational change of different clostridial neurotoxins. Biochem. Biophys. Res. Commun. 2004;319:66–71. doi: 10.1016/j.bbrc.2004.04.140. [DOI] [PubMed] [Google Scholar]
- 20.Finkelstein A. Channels formed in phospholipid bilayer membranes by diphtheria, tetanus, botulinum and anthrax toxin. J. Physiol. (Paris) 1990;84:188–190. [PubMed] [Google Scholar]
- 21.Sun S., Tepp W.H., et al. Chapman E.R. Botulinum neurotoxins B and E translocate at different rates and exhibit divergent responses to GT1b and low pH. Biochemistry. 2012;51:5655–5662. doi: 10.1021/bi3004928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun S., Suresh S., et al. Chapman E.R. Receptor binding enables botulinum neurotoxin B to sense low pH for translocation channel assembly. Cell Host Microbe. 2011;10:237–247. doi: 10.1016/j.chom.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pirazzini M., Rossetto O., et al. Montecucco C. Double anchorage to the membrane and intact inter-chain disulfide bond are required for the low pH induced entry of tetanus and botulinum neurotoxins into neurons. Cell. Microbiol. 2011;13:1731–1743. doi: 10.1111/j.1462-5822.2011.01654.x. [DOI] [PubMed] [Google Scholar]
- 24.Montecucco C., Schiavo G., et al. Roa M. Tetanus toxin is labeled with photoactivatable phospholipids at low pH. Biochemistry. 1986;25:919–924. doi: 10.1021/bi00352a027. [DOI] [PubMed] [Google Scholar]
- 25.Pirazzini M., Rossetto O., et al. Montecucco C. Time course and temperature dependence of the membrane translocation of tetanus and botulinum neurotoxins C and D in neurons. Biochem. Biophys. Res. Commun. 2013;430:38–42. doi: 10.1016/j.bbrc.2012.11.048. [DOI] [PubMed] [Google Scholar]
- 26.Eswaramoorthy S., Kumaran D., et al. Swaminathan S. Role of metals in the biological activity of Clostridium botulinum neurotoxins. Biochemistry. 2004;43:2209–2216. doi: 10.1021/bi035844k. [DOI] [PubMed] [Google Scholar]
- 27.Mayer L.D., Hope M.J., Cullis P.R. Vesicles of variable sizes produced by a rapid extrusion procedure. Biochim. Biophys. Acta. 1986;858:161–168. doi: 10.1016/0005-2736(86)90302-0. [DOI] [PubMed] [Google Scholar]
- 28.Ladokhin A.S., Jayasinghe S., White S.H. How to measure and analyze tryptophan fluorescence in membranes properly, and why bother? Anal. Biochem. 2000;285:235–245. doi: 10.1006/abio.2000.4773. [DOI] [PubMed] [Google Scholar]
- 29.Pettersen E.F., Goddard T.D., et al. Ferrin T.E. UCSF Chimera--a visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 30.Snider C., Jayasinghe S., et al. White S.H. MPEx: a tool for exploring membrane proteins. Protein Sci. 2009;18:2624–2628. doi: 10.1002/pro.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vasquez-Montes V., Ladokhin A.S. Expanding MPEx hydropathy analysis to account for electrostatic contributions to protein interactions with anionic membranes. J. Membr. Biol. 2021;254:109–117. doi: 10.1007/s00232-021-00170-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McLaughlin S. The electrostatic properties of membranes. Annu. Rev. Biophys. Biophys. Chem. 1989;18:113–136. doi: 10.1146/annurev.bb.18.060189.000553. [DOI] [PubMed] [Google Scholar]
- 33.Ladokhin A.S., Kyrychenko A., et al. Vasquez-Montes V. Conformational switching, refolding and membrane insertion of the diphtheria toxin translocation domain. Methods Enzymol. 2021;649:341–370. doi: 10.1016/bs.mie.2020.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaback H.R., Sahin-Tóth M., Weinglass A.B. The kamikaze approach to membrane transport. Nat. Rev. Mol. Cell Biol. 2001;2:610–620. doi: 10.1038/35085077. [DOI] [PubMed] [Google Scholar]
- 35.Lam K.H., Guo Z., et al. Jin R. A viral-fusion-peptide-like molecular switch drives membrane insertion of botulinum neurotoxin A1. Nat. Commun. 2018;9:5367. doi: 10.1038/s41467-018-07789-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mushrush D.J., Koteiche H.A., et al. Lacy D.B. Studies of the mechanistic details of the pH-dependent association of botulinum neurotoxin with membranes. J. Biol. Chem. 2011;286:27011–27018. doi: 10.1074/jbc.M111.256982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zuverink M., Bluma M., Barbieri J.T. Tetanus toxin cis-loop contributes to light-chain translocation. MSphere. 2020;5:e00244-20. doi: 10.1128/mSphere.00244-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hullin-Matsuda F., Taguchi T., et al. Kobayashi T. Lipid compartmentalization in the endosome system. Semin. Cell Dev. Biol. 2014;31:48–56. doi: 10.1016/j.semcdb.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 39.Stryer L. Energy transfer in proteins and polypeptides. Radiat. Res. 1960;2:432–451. [PubMed] [Google Scholar]
- 40.Kyrychenko A., Freites J.A., et al. Ladokhin A.S. Structural plasticity in the topology of the membrane-interacting domain of HIV-1 gp41. Biophys. J. 2014;106:610–620. doi: 10.1016/j.bpj.2013.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McIntosh T.J., Holloway P.W. Determination of the depth of bromine atoms in bilayers formed from bromolipid probes. Biochemistry. 1987;26:1783–1788. doi: 10.1021/bi00380a042. [DOI] [PubMed] [Google Scholar]
- 42.Ladokhin A.S. Measuring membrane penetration with depth-dependent fluorescence quenching: distribution analysis is coming of age. Biochim. Biophys. Acta. 2014;1838:2289–2295. doi: 10.1016/j.bbamem.2014.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ladokhin A.S. Analysis of protein and peptide penetration into membranes by depth-dependent fluorescence quenching: theoretical considerations. Biophys. J. 1999;76:946–955. doi: 10.1016/S0006-3495(99)77258-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Posokhov Y.O., Rodnin M.V., et al. Ladokhin A.S. Membrane insertion pathway of annexin B12: thermodynamic and kinetic characterization by fluorescence correlation spectroscopy and fluorescence quenching. Biochemistry. 2008;47:5078–5087. doi: 10.1021/bi702223c. [DOI] [PubMed] [Google Scholar]
- 45.Ladokhin A.S., Legmann R., et al. White S.H. Reversible refolding of the diphtheria toxin T-domain on lipid membranes. Biochemistry. 2004;43:7451–7458. doi: 10.1021/bi036157w. [DOI] [PubMed] [Google Scholar]
- 46.Vasquez-Montes V., Vargas-Uribe M., et al. Ladokhin A.S. Lipid-modulation of membrane insertion and refolding of the apoptotic inhibitor Bcl-xL. Biochim. Biophys. Acta. Proteins Proteomics. 2019;1867:691–700. doi: 10.1016/j.bbapap.2019.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wimley W.C., White S.H. Experimentally determined hydrophobicity scale for proteins at membrane interfaces. Nat. Struct. Biol. 1996;3:842–848. doi: 10.1038/nsb1096-842. [DOI] [PubMed] [Google Scholar]
- 48.Jayasinghe S., Hristova K., White S.H. Energetics, stability, and prediction of transmembrane helices. J. Mol. Biol. 2001;312:927–934. doi: 10.1006/jmbi.2001.5008. [DOI] [PubMed] [Google Scholar]
- 49.Wimley W.C. Toward genomic identification of beta-barrel membrane proteins: composition and architecture of known structures. Protein Sci. 2002;11:301–312. doi: 10.1110/ps.29402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jayasinghe S., Hristova K., White S.H. MPtopo: a database of membrane protein topology. Protein Sci. 2001;10:455–458. doi: 10.1110/ps.43501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Montal M.S., Blewitt R., et al. Montal M. Identification of an ion channel-forming motif in the primary structure of tetanus and botulinum neurotoxins. FEBS Lett. 1992;313:12–18. doi: 10.1016/0014-5793(92)81173-j. [DOI] [PubMed] [Google Scholar]
- 52.Koriazova L.K., Montal M. Translocation of botulinum neurotoxin light chain protease through the heavy chain channel. Nat. Struct. Biol. 2003;10:13–18. doi: 10.1038/nsb879. [DOI] [PubMed] [Google Scholar]
- 53.Neale E.A. Moving across membranes. Nat. Struct. Biol. 2003;10:2–3. doi: 10.1038/nsb0103-2. [DOI] [PubMed] [Google Scholar]
- 54.Borochov-Neori H., Yavin E., Montal M. Tetanus toxin forms channels in planar lipid bilayers containing gangliosides. Biophys. J. 1984;45:83–85. doi: 10.1016/S0006-3495(84)84117-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Katayama H., Wang J., et al. Fisher M.T. Three-dimensional structure of the anthrax toxin pore inserted into lipid nanodiscs and lipid vesicles. Proc. Natl. Acad. Sci. USA. 2010;107:3453–3457. doi: 10.1073/pnas.1000100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jiang J., Pentelute B.L., et al. Zhou Z.H. Atomic structure of anthrax protective antigen pore elucidates toxin translocation. Nature. 2015;521:545–549. doi: 10.1038/nature14247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Senzel L., Gordon M., et al. Finkelstein A. Topography of diphtheria Toxin’s T domain in the open channel state. J. Gen. Physiol. 2000;115:421–434. doi: 10.1085/jgp.115.4.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ladokhin A.S., Vargas-Uribe M., et al. Sharma O. Cellular entry of the diphtheria toxin does not require the formation of the open-channel state by its translocation domain. Toxins (Basel) 2017;9:E299. doi: 10.3390/toxins9100299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Montal M. Botulinum neurotoxin: a marvel of protein design. Annu. Rev. Biochem. 2010;79:591–617. doi: 10.1146/annurev.biochem.051908.125345. [DOI] [PubMed] [Google Scholar]
- 60.Hayashibara M., London E. Topography of diphtheria toxin A chain inserted into lipid vesicles. Biochemistry. 2005;44:2183–2196. doi: 10.1021/bi0482093. [DOI] [PubMed] [Google Scholar]
- 61.Ladokhin A.S. pH-triggered conformational switching along the membrane insertion pathway of the diphtheria toxin T-domain. Toxins (Basel) 2013;5:1362–1380. doi: 10.3390/toxins5081362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zakharov S.D., Lindeberg M., et al. Cramer W.A. Membrane-bound state of the colicin E1 channel domain as an extended two-dimensional helical array. Proc. Natl. Acad. Sci. USA. 1998;95:4282–4287. doi: 10.1073/pnas.95.8.4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ptitsyn O.B. How the molten globule became. Trends Biochem. Sci. 1995;20:376–379. doi: 10.1016/s0968-0004(00)89081-7. [DOI] [PubMed] [Google Scholar]
- 64.Tamm L.K., Arora A., Kleinschmidt J.H. Structure and assembly of beta-barrel membrane proteins. J. Biol. Chem. 2001;276:32399–32402. doi: 10.1074/jbc.R100021200. [DOI] [PubMed] [Google Scholar]
- 65.Davis K.B., Zhang Z., et al. Zhang J. Application of tyrosine-tryptophan fluorescence resonance energy transfer in monitoring protein size changes. Anal. Biochem. 2018;557:142–150. doi: 10.1016/j.ab.2018.07.022. [DOI] [PubMed] [Google Scholar]
- 66.Pirazzini M., Azarnia Tehran D., et al. Montecucco C. On the translocation of botulinum and tetanus neurotoxins across the membrane of acidic intracellular compartments. Biochim. Biophys. Acta. 2016;1858:467–474. doi: 10.1016/j.bbamem.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 67.Hudson T.H., Scharff J., et al. Neville D.M., Jr. Energy requirements for diphtheria toxin translocation are coupled to the maintenance of a plasma membrane potential and a proton gradient. J. Biol. Chem. 1988;263:4773–4781. [PubMed] [Google Scholar]
- 68.Gambale F., Montal M. Characterization of the channel properties of tetanus toxin in planar lipid bilayers. Biophys. J. 1988;53:771–783. doi: 10.1016/S0006-3495(88)83157-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rodnin M.V., Li J., et al. Ladokhin A.S. The pH-dependent trigger in diphtheria toxin T domain comes with a safety latch. Biophys. J. 2016;111:1946–1953. doi: 10.1016/j.bpj.2016.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kurnikov I.V., Kyrychenko A., et al. Ladokhin A.S. pH-triggered conformational switching of the diphtheria toxin T-domain: the roles of N-terminal histidines. J. Mol. Biol. 2013;425:2752–2764. doi: 10.1016/j.jmb.2013.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vargas-Uribe M., Rodnin M.V., et al. Ladokhin A.S. Crucial role of H322 in folding of the diphtheria toxin T-domain into the open-channel state. Biochemistry. 2013;52:3457–3463. doi: 10.1021/bi400249f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ghatak C., Rodnin M.V., et al. Ladokhin A.S. Role of acidic residues in helices TH8-TH9 in membrane interactions of the diphtheria toxin T domain. Toxins (Basel) 2015;7:1303–1323. doi: 10.3390/toxins7041303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vargas-Uribe M., Rodnin M.V., Ladokhin A.S. Comparison of membrane insertion pathways of the apoptotic regulator Bcl-xL and the diphtheria toxin translocation domain. Biochemistry. 2013;52:7901–7909. doi: 10.1021/bi400926k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kyrychenko A., Vasquez-Montes V., et al. Ladokhin A.S. Lipid headgroups modulate membrane insertion of pHLIP peptide. Biophys. J. 2015;108:791–794. doi: 10.1016/j.bpj.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fischer A., Montal M. Single molecule detection of intermediates during botulinum neurotoxin translocation across membranes. Proc. Natl. Acad. Sci. USA. 2007;104:10447–10452. doi: 10.1073/pnas.0700046104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.O’Neil P. A limitation of using dithionite quenching to determine the topology of membrane-inserted proteins. J. Membr. Biol. 2021 doi: 10.1007/s00232–021–00199–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.