Abstract
Respiratory syncytial virus (RSV) is one of the most important causes of respiratory disease in infants, immunocompromised individuals, and the elderly. Natural infection does not result in long-term immunity, and there is no licensed vaccine. Vesicular stomatitis virus (VSV) is a commonly used vaccine vector platform against infectious diseases, and has been used as a vector for a licensed Ebola vaccine. In this study, we expressed the RSV fusion (F) protein, the RSV F protein stabilized in either a pre-fusion or a post-fusion configuration, the attachment glycoprotein (G), or the G and F proteins of RSV in combination in a VSV vector. Cotton rats were immunized with these recombinants intranasally or subcutaneously to test immunogenicity. RSV F stabilized in either a pre-fusion or a post-fusion configuration proved to be poorly immunogenic and protective when compared to unmodified F. RSV G provided partial protection and moderate levels of neutralizing antibody production, both of which improved with intranasal administration compared to subcutaneous inoculation. The most successful vaccine vector was VSV expressing both the G and F proteins after intranasal inoculation. Immunization with this recombinant induced neutralizing antibodies and provided protection from RSV challenge in the upper and lower respiratory tract for at least 80 days. Our results demonstrate that co-expression of F and G proteins in a VSV vector provides synergistic effects in inducing RSV-specific neutralizing antibodies and protection against RSV infection.
Keywords: Respiratory syncytial virus, cotton rat, vesicular stomatitis virus, vaccine vector, glycoproteins
Introduction
Respiratory syncytial virus (RSV) is one of the most important causes of respiratory disease in infants, the elderly, and immunocompromised individuals [1,2]. Currently, vaccine development for RSV is an active area of research, using a wide array of platforms and approaches [3–5]. Among the various viral vector vaccine platforms, vesicular stomatitis virus (VSV) has several advantages. It has inherent adjuvant function as a strong inducer of type I interferon (IFN) [6–8]. It can be grown at a higher titer than many other viral vector systems, express up to 4 kb of foreign genetic material, and is highly stable, as it is not prone to genetic reassortment or recombination [9]. Additionally, attenuated VSV does not cause disease in humans, and the exposure of the general human population to VSV is low, thus reducing the likelihood that vaccination of an individual would be inhibited by an immune response to the vaccine vector itself [6]. Last but not least, it has already been approved by FDA as a platform for an Ebola virus vaccine [10].
Of RSV’s three surface glycoproteins, small hydrophobic (SH), fusion (F), and attachment (G), only F and G induce neutralizing antibodies. Neutralizing antibodies are an important parameter of immune protection from viral infection with RSV, as indicated by work demonstrating that passive immunization with polyclonal or monoclonal antibodies prevents severe disease in infants and infection in adults, and that higher antibody titers are protective against RSV infection in adults [11–13]. The F protein has two forms, a pre-fusion metastable configuration wherein the F protein exists as a spring-loaded trimer, and a post-fusion form following the triggering of a conformational change, at which point the F protein exists in a highly stable conformation. The G protein has a central conserved domain with a CX3C sequence mimicking the chemokine fractalkine, flanked by two mucin-like domains [14]. For vaccine development, both the F and G proteins of RSV have been targeted, with equivocal success [15]. The F protein is more avidly pursued as a vaccine candidate, as monoclonal antibodies specific for the G protein are less neutralizing than those produced against the F protein [16]. In natural infection in infants, the titers of G protein-specific antibodies are typically lower than F protein-specific antibodies [17]. However, individuals that generate G protein antibodies have less severe clinical disease [17].
In this study, we used a VSV vector system to create recombinant viruses expressing the RSV G protein and different versions of the F protein, which was either stabilized in its pre-fusion or post-fusion configuration, or expressed in its naïve form. We also created a recombinant virus expressing both G and F, linked by a self-cleaving peptide. These recombinant viruses were used to immunize and protect cotton rats against RSV infection.
Materials and Methods
Animal experiments:
Inbred female cotton rats (Sigmodon hispidus) were purchased from Envigo (Indianapolis, Indiana) and housed in polysulfone microisolator cages (NextGen Rat 900, Allentown Inc., Allentown, NJ, USA) in a barrier facility with a 12:12 hour light cycle at 20 ± 2 °C and 30% to 70% relative humidity. Cotton rats were 4–6 weeks of age, and were specific pathogen-free. Four cotton rats (n=4) were allocated for each experimental group. Cotton rats were anesthetized via isoflurane inhalation prior to all procedures. Cotton rats were immunized once or twice with a four week interval either subcutaneously (500μl volume) or intranasally (100μl volume). Blood was collected from the retro-orbital plexus into serum separator tubes every 2 weeks after initial immunization. After immunization, cotton rats were challenged intranasally with 105 TCID50 RSV A2 (100μl volume). Four days post-challenge, all rats were euthanized via carbon dioxide inhalation followed by exsanguination. The nasal turbinate mucosa and left lung lobe was removed and homogenized in either 3 ml or 2 ml of DMEM. The lung was homogenized using a Precellys 24 tissue homogenizer (Bertin, MD)The lung and nasal homogenates were aliquoted and stored at −80 °C. The presence of infectious virus was determined by TCID50 assay in HEp-2 cells. All studies were approved by the Institutional Animal Care and Use Committee of The Ohio State University (OSU).
Respiratory Syncytial Virus
Stocks of RSV-A/2 (GenBank accession #M74568) were grown in HEp-2 cells in MEM/2% fetal bovine serum. When infection reached a cytopathic effect of ~80%, 1M MgSO4/0.25M HEPES was added and cells were scraped from the flask in the growth media. The cells were briefly frozen at −80° C, thawed, and centrifuged at 3000 rpm for 15 minutes at 4° C. The supernatant was collected and placed on 15 ml of a 35% sucrose cushion and centrifuged at 15000 rpm in a Sorval SS34 rotor for 5 hours at 4° C to pellet the virus. Virus pellets were re-suspended in MEM/10% trehalose, and TCID50 was determined by titration on HEp-2 cells [18]. Each virus stock was aliquoted and frozen in liquid nitrogen
Tissue culture infectious dose 50 (TCID50) assay
Ten-fold serial dilutions of the viral stock, or lung or nasal homogenates, were added to a 48-well plate with 80% to 90% confluent HEp-2 cells. After one hour, the monolayer was washed three times with PBS, and 500 μL of MEM/2% FCS was added per well. After five days, plates were scored microscopically for cytopathic effect. TCID50 was calculated according Reed and Muench [19].
RSV-specific neutralizing antibody assay:
Two-fold dilutions of the serum samples in Advanced MEM were prepared in 96-well tissue culture flat-bottom plate with the starting dilution of 1:10 (50 μl/well). 50 TCID50 of RSV A2 in Advanced MEM media was added in a 50 μl volume to each well and mixed. After incubation of 1 hour, HEp-2 cells (5000 cells/well in 100μL of MEM/5% FBS) were added and the plate was incubated at 37 °C and 5% CO2 for 4 days. Endpoint neutralization titer was determined as the reciprocal of the highest dilution at which 100% inhibition of RSV induced CPE was observed.
Generation of recombinant vesicular stomatitis viruses (VSV)
Recombinant VSV based on the Indiana strain were generated as described previously [20–23]. Recombinant VSVs were generated by inserting the transgene (RSV-G, RSV-F mutants, or RSV-G-2A-F) into restriction sites XmaI and XhoI between the G and L gene junction of a plasmid of a VSV (Indiana strain) molecular clone [23].
The sequences for RSV-G, RSV-pre-F, RSV-HEK-pre-F, RSV-post-F, and RSV-G-2A-F (based on the RSV A2 strain) were codon-optimized for expression in eukaryotic (human) cells using the software on the website of Integrated DNA Technologies (Coralville, IA). The codon-optimized sequence for RSV-F could not be rescued, and a non-codon-optimized sequence was used instead. Oligonucleotides comprising the RSV-G, RSV-F, RSV-pre-F, RSV-HEK-pre-F, RSV-post-F, and RSV-G-2A-F DNA gene sequences were also obtained from Integrated DNA Technologies (Coralville, IA). The RSV-pre-F construct was codon-optimized for expression in eukaryotic (human) cells as described above, with the following mutations: mutations in the furin cleavage sites (RRAR → KAKK, KKRKRR → KKKKKK), an extra disulfide bond establishment by mutation in S155C and S290C, and cavity-filling hydrophobic substitutions S190F and V207L. The RSV-HEK-pre-F incorporated the same mutations, with the additions K66E and Q101P to make the sequence identical to a clinical isolate demonstrated to increase protein expression [24]. The RSV-post-F was also codon-optimized, with a 10 amino acid deletion (137–146) of the fusion peptide which did not affect the furin cleavage sites [24]. The RSV-G-2A-F gene construct consisted of the RSV-G and RSV-F genes from RSV A2 strain, linked by a self-cleaving peptide 2A sequence [25]. The RSV-F construct is based on the F protein of the RSV-A2 strain (GenBank accession FJ614814.1). All other sequences have been submitted to GenBank, and are available under accession numbers MZ611511 (pre-F), MZ611512 (post-F), MZ611513 (HEK-pre-F), MZ611514 (G-2A-F), and MZ611515 (G). Oligonucleotides of the recombinants, as well as the pVSV1(+)-GxxL plasmid, were incubated overnight with restriction enzymes XhoI and XmaI, CutSmart Buffer, and water, at 37°C. The digestion products were then purified using the GeneJET PCR Purification Kit (Thermo Fisher Scientific, Waltham, MA). After purification, the vector DNA, insert, T4 ligase buffer, T4 ligase, and water were combined and incubated at room temperature for 2 hours. The insertion of these genes into pVSV1(+)-GxxL was confirmed by amplifying the genes with the forward primer 5′-CGAGTTGGTATTTATCTTTGC-3′ and the reverse primer 5′-GTACGTCATGCGCTCATCG-3′ which were designed to amplify inserted genes between the VSV G gene at position 4524 to 4545 and the L gene at position 4831 to 4850 (numbering refers to the nucleotide position on the complete VSV Indiana genome sequence), which are respectively 214 nts upstream and 127 nts downstream of the transgene. Once the presence of the target gene was confirmed, the remaining PCR product was submitted for Sanger sequencing through the OSU Genomics Shared Resource, to confirm that no mutations had been introduced during the rescue or plaque purification.
The rescue of the recombinant VSVs from the respective VSV molecular clones was performed as previously described [21]. Briefly, vaccinia virus expressing T7-polymerase (vTF7–3) was grown and titrated on BSC-40 cells as described [21]. BSRT7 cells were infected for 1 hr at 10 MOI with a recombinant vaccinia virus (vTF7–3) expressing T7 RNA polymerase. The cells were washed with Opti-MEM (Gibco) after removing the vTF7–3 and co-transfected with pVSV expressing one of the transgenes [pVSV1(+)-G, pVSV1(+)-F, or pVSV1(+)-G-2A-F] and support plasmids pN, pP, and pL mixed with Lipofectamine 2000 transfection reagent. After 96 to 108 hr post-transfection, the recovered viruses, designated as rVSV-G, rVSV-F, rVSV-pre-F, rVSV-HEK-pre-F, rVSV-post-F, or rVSV-G-2A-F, were isolated by plaque purification. The expressed transgenes were confirmed in the seed stock virus by RT-PCR and sequence analysis. Finally, viral titers were determined by plaque assay on Vero cells as described [22].
Flow cytometry
A monolayer of L929 cells was infected with recombinant VSV at an MOI of 5. At 12 hours post-infection, the virus and media were aspirated and infected cells were removed from the flasks using trypsin. Cells were washed with PBS/0.1% FCS/0.02% NaN3 and then incubated for one hour with a monoclonal antibody specific for either the F protein (Motavizumab), the prefusion configuration of the F protein (D25), the postfusion configuration of the F protein (F2), or the G protein (m131–2G). Both D25 and F2 were labeled with FITC. Cells stained with Motavizumab or m131–2G were incubated for an hour with donkey anti-mouse IgG FITC. Flow cytometry was performed on the Attune NxT Flow Cytometer (Thermo Fisher Scientific), which was also used to analyze the data.
Western blotting:
BHK21 cells were infected with VSV constructs at a multiplicity of infection (MOI) of 4 for 1 hour. After 12 hours incubation, cells were lysed in 150 ul of radioimmunoprecipitation assay (RIPA) cell lysis buffer (Sigma) to extract proteins. Cell lysate was then boiled with beta-mercaptoethanol for 5 minutes. Cell lysate was separated by 10% SDS-PAGE in a Mini-Protean 3 electrophoresis cell module (Bio-Rad) and transferred to a nitrocellulose membrane (Bio-Rad) in an XCell IITM blot module (Invitrogen). The blot was probed with specific primary antibodies, followed by species-specific secondary antibodies linked to horseradish peroxidase (HRP) as follows: for all RSV-G proteins, goat anti-RSV polyclonal IgG (1:3,000, Biotin) and chicken anti-goat IgG-HRP (1:20,000); for the RSV-F proteins, Motavizumab, a humanized anti-F monoclonal IgG (1:100,000) and donkey anti-human IgG-HRP (1:20,000, Jackson). The blot was developed with SuperSignal West Dura chemiluminescent substrate (Thermo Scientific) and exposed to Biomax MR film (Kodak).
Statistical analysis:
The data from groups of four cotton rats were expressed as the mean ± standard deviation. Statistical analysis was performed by one-way analysis of variance followed by Tukey’s multiple comparison post-hoc test, and a P-value below 0.05 (P <0.05) was considered to indicate statistically significant difference between indicated groups and represented by asterisks (*). Prism 8 (GraphPad Software, San Diego, CA) was used for all data analysis.
Results
Characterization of VSV recombinants (rVSV) expressing the RSV G attachment and F fusion protein.
Natural infection with RSV does not lead to long term immunity, and recent studies indicate that declining immunity is at least partially related to declining neutralizing antibody levels [26,27]. To strengthen the antibody response against the G and F proteins, we used the vesicular stomatitis virus (VSV, Indiana strain) platform to generate candidate vaccine vectors. VSV induces a strong immune response by inducing type I interferon, with high protein expression but only limited replication in humans. The genes for RSV-G, modified versions of RSV-F, or both the RSV-G and RSV-F linked by a self-cleaving 2A peptide (RSV-G-2A-F) were inserted at the VSV G-L junction. RSV-F genes encoded either the native unmodified F, or a mutated form that encoded the F protein stabilized in either the pre-fusion or post-fusion configuration (Figure 1) [16]. These pre-fusion and post-fusion configurations were chosen based on previous work in the literature, which suggests that stabilized pre-fusion F protein is more immunogenic than F in its native configuration (Figure 1) [28]. We tested two forms of the pre-fusion form, one of which (HEK-pre-F) was derived from a clinical isolate of RSV, with mutations K66E and Q101P, and had been shown in the literature to produce approximately 6-fold more protein than the pre-F form (Figure 1) [24]. We also wished to determine if combining G and F genes in a single vaccine would improve immune responses to RSV, and therefore integrated both genes in a single recombinant (Figure 1). Recombinant VSVs were generated using support plasmids according to published procedures [22]. Insertion of the target genes was confirmed by PCR and sequencing.
Figure 1. Diagram of RSV F and G protein constructs.
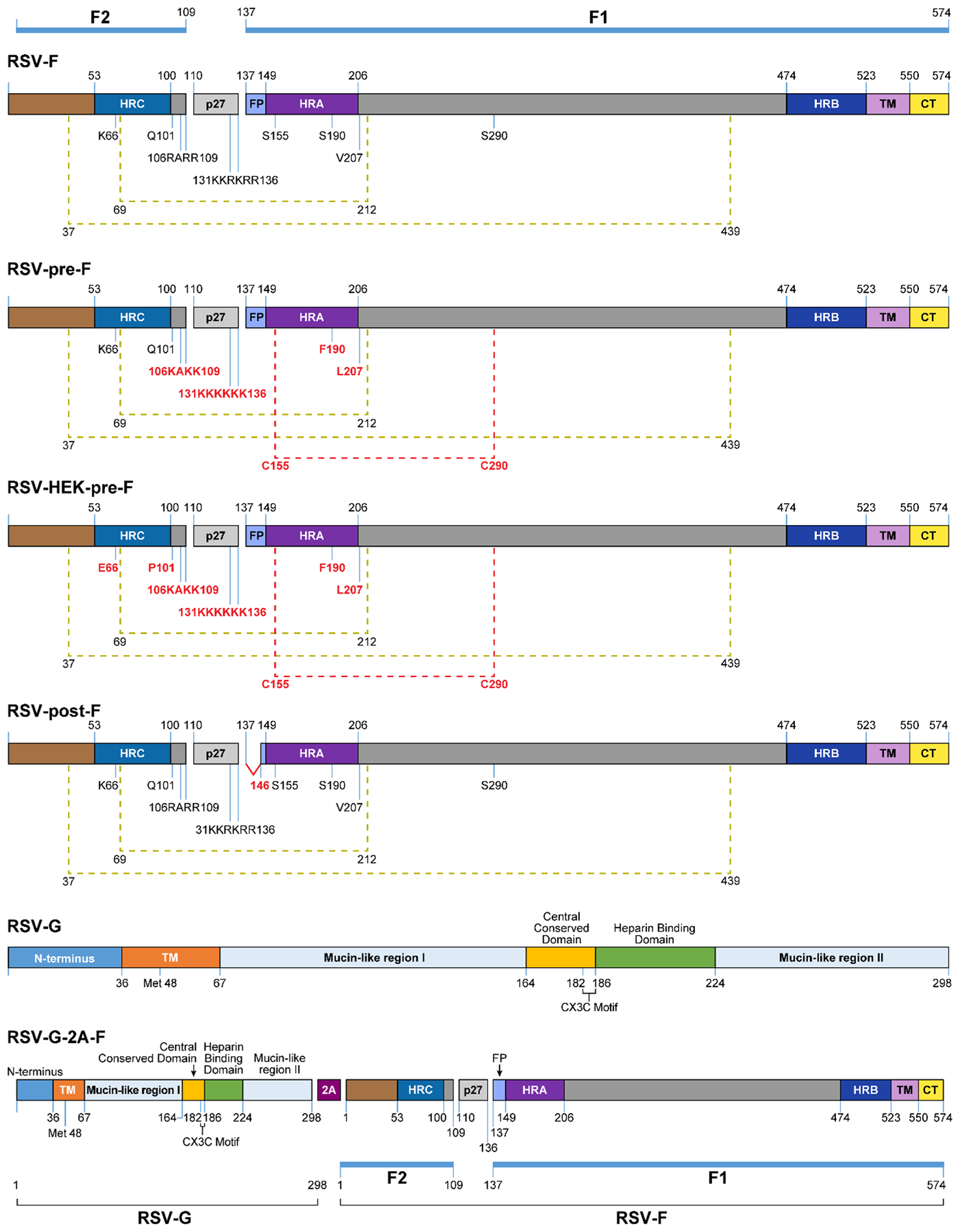
Linear diagram of RSV-F and RSV-G constructs used in this study, showing functional domains, motifs, and alterations to the original sequences. All changes to the RSV-F sequences are represented in red font in the other constructs. These include: mutations in the furin cleavage sites at amino acids 106–109 and 131–136 (RRAR → KAKK, KKRKRR → KKKKKK) to be protease resistant, an extra disulfide bond establishment at amino acids 155 and 290, and cavity-filling substitutions at amino acids 190 and 207 (RSV-pre-F, RSV-HEK-pre-F); mutations of amino acids 66 and 101 to reflect a clinically-isolated strain of RSV (HEK-pre-F); deletion of the first 10 amino acids of the fusion peptide (post-F); and insertion of a self-cleaving 2A peptide between the G and F sequences (G-2A-F). Other features of RSV-F include: RSV-F subunits F1 and F2 (blue bars); heptad repeats A, B, and C (HRA, purple; HRB, dark blue; HRC, blue); p27 fragment (gray); disulfide bonds (olive green brackets); transmembrane domain (TM, lavender); cytoplasmic tail (CT, yellow). Features of RSV-G include: transmembrane domain (TM, orange); mucin-like regions I and II; central conserved domain (yellow), heparin binding domain (green), and CX3C motif (brackets).
The expression of the RSV glycoproteins at the cell surface of rVSV-infected cells was evaluated via Western blotting. Both the F and G proteins were detected in cell lysates previously infected with rVSV constructs, as determined by labeling with an RSV specific antibody or an F-specific antibody (Motavizumab) (Figure 2). Depending on the cell type in which it is expressed, the G protein varies in size from 55–100 kDa, due to its heavy and variable glycosylation [16,29–33]. These bands were visible following labeling with an RSV-specific antibody, with more protein being produced by the rVSV-G construct than the rVSV-G-2A-F construct (Figure 2). Under reducing conditions, the F protein typically dissociates into two bands, one approximately 67 kDa (representing F0), and another at approximately 50 kDa (representing F1) [34,35]. Interestingly, rVSV-F and rVSV-G-2A-F yielded two bands representing F0 and F1, but rVSV-HEK-pre-F only produced a band consistent in size with F0, supporting the fact that this protein is being expressed in the pre-fusion form only (Figure 2). The faint band seen at a higher molecular weight in the G-2A-F construct when labeled with Motavizumab likely represents oligomers that incompletely dissociated [34]. Post-F also produced a band consistent with F0, while pre-F produced a band consistent with F1 (Figure 2). When 100 ug of total protein was loaded, as opposed to 25 ug, faint bands could be seen representing F1 and F0 in pre-F and post-F, respectively, indicating that these proteins were formed, although in low quantities (data not shown). Cell surface protein expression was further evaluated via flow cytometry. rVSV-G- and rVSV-G-2A-F-infected L929 cells expressed the G protein on the cell surface, based on labeling with the G-specific antibody 131–2G (Figure 3). Motavizumab, an F-specific human monoclonal IgG, indicated protein expression on the surface of rVSV-pre-F and rVSV-post-F infected cells, confirming that the F protein was being produced by these recombinants. As expected, L929 cells infected with rVSV-F and rVSV-G-2A-F expressed the F protein in both the pre-fusion and post-fusion configurations (Figure 3), which were detected by pre-fusion and post-fusion-specific antibodies (D25 and 2F, respectively). Cells infected with rVSV-pre-F and rVSV-post-F had limited pre-F-specific or post-F-specific antibody binding respectively (Figure 3), indicating a reduced level of protein expression on the cell surface. Cells infected with rVSV-HEK-pre-F expressed the F protein in the pre-fusion configuration only (Figure 3).
Figure 2. Protein expression of RSV-G or RSV-F proteins in BHK21 cells infected with rVSV-F, rVSV-post-F, rVSV-HEK-pre-F, rVSV-G-2A-F, or rVSV-G.
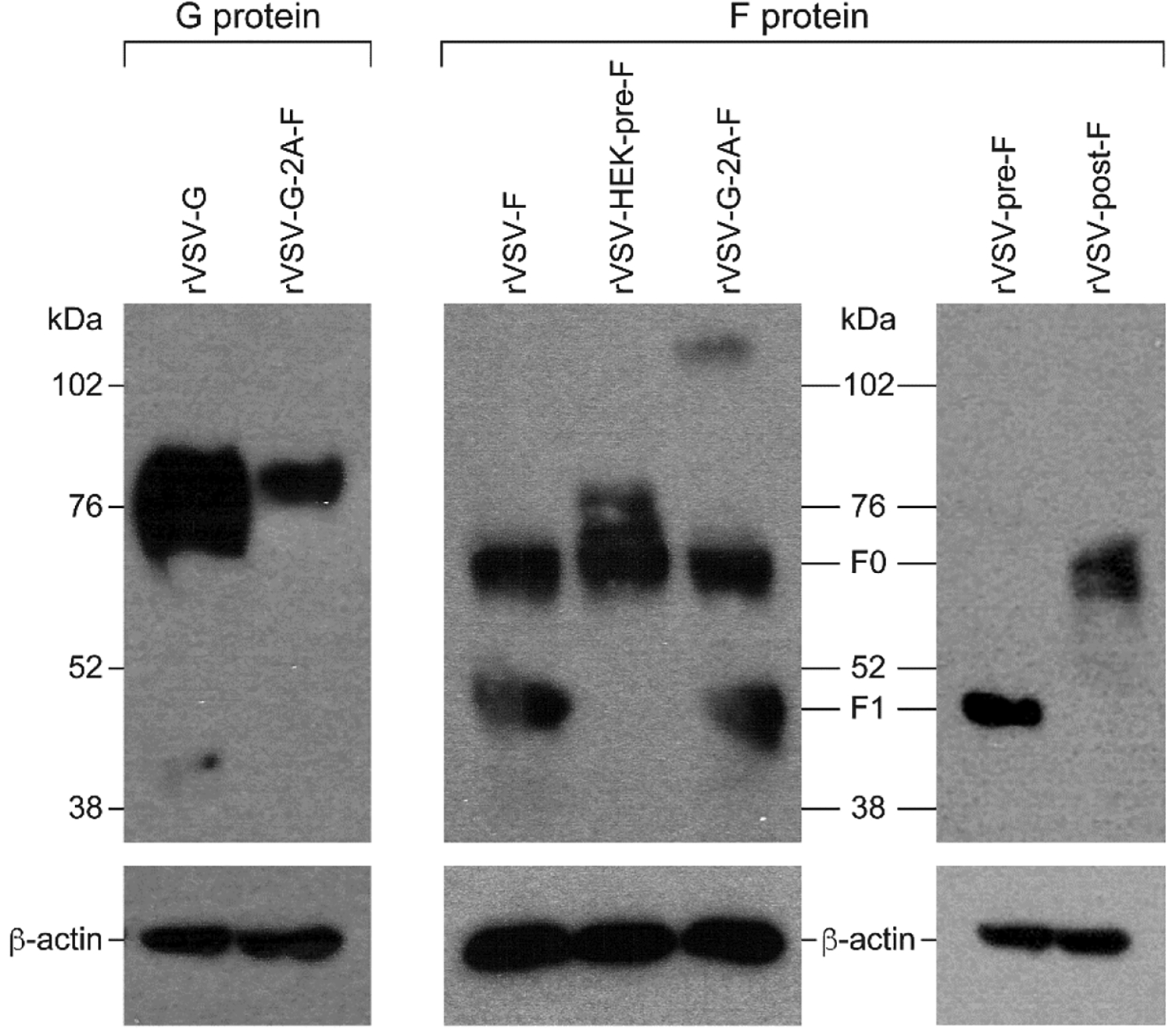
BHK21 cells were infected for 12 hours with an MOI of 4, and protein expression level of G (left panel) or F (middle and right panels) was assessed via Western blotting under dissociating (reducing) conditions. A representative blot of the control protein beta-actin is also shown. Left panel: 25 ug total protein cell lysates of rVSV-G or rVSV-G-2A-F infected cells was loaded and analyzed using a goat anti-RSV primary antibody with a chicken anti-goat + HRP secondary antibody. Bands representing the G protein can be seen at approximately 72–85 kDa.
Middle panel: 25 ug total protein of cell lysates of rVSV-F, rVSV-HEK-pre-F or rVSV-G-2A-F infected cells was loaded and analyzed using an F specific antibody (Motavizumab) and a secondary donkey anti-human + HRP antibody. All three constructs have a band at approximately 67 kDa representing F0, and the unstabilized F proteins (F and G-2A-F) also have bands at approximately 50 kDa, representing the F1 subunit. The high molecular weight band in G-2A-F likely represents an oligomer of the F protein that did not dissociate.
Right panel: 25 ug total protein of cell lysates of rVSV-pre-F or rVSV-post-F was loaded and analyzed using a primary F specific antibody (Motavizumab) and a donkey anti-human + HRP secondary antibody. There is a band representing the F1 subunit in pre-F, and a band representing F0 in post-F.
Figure 3. Protein expression on the surface of L929 cells infected with rVSV-F, rVSV-post-F, rVSV-HEK-pre-F, rVSV-G-2A-F, or rVSV-G.
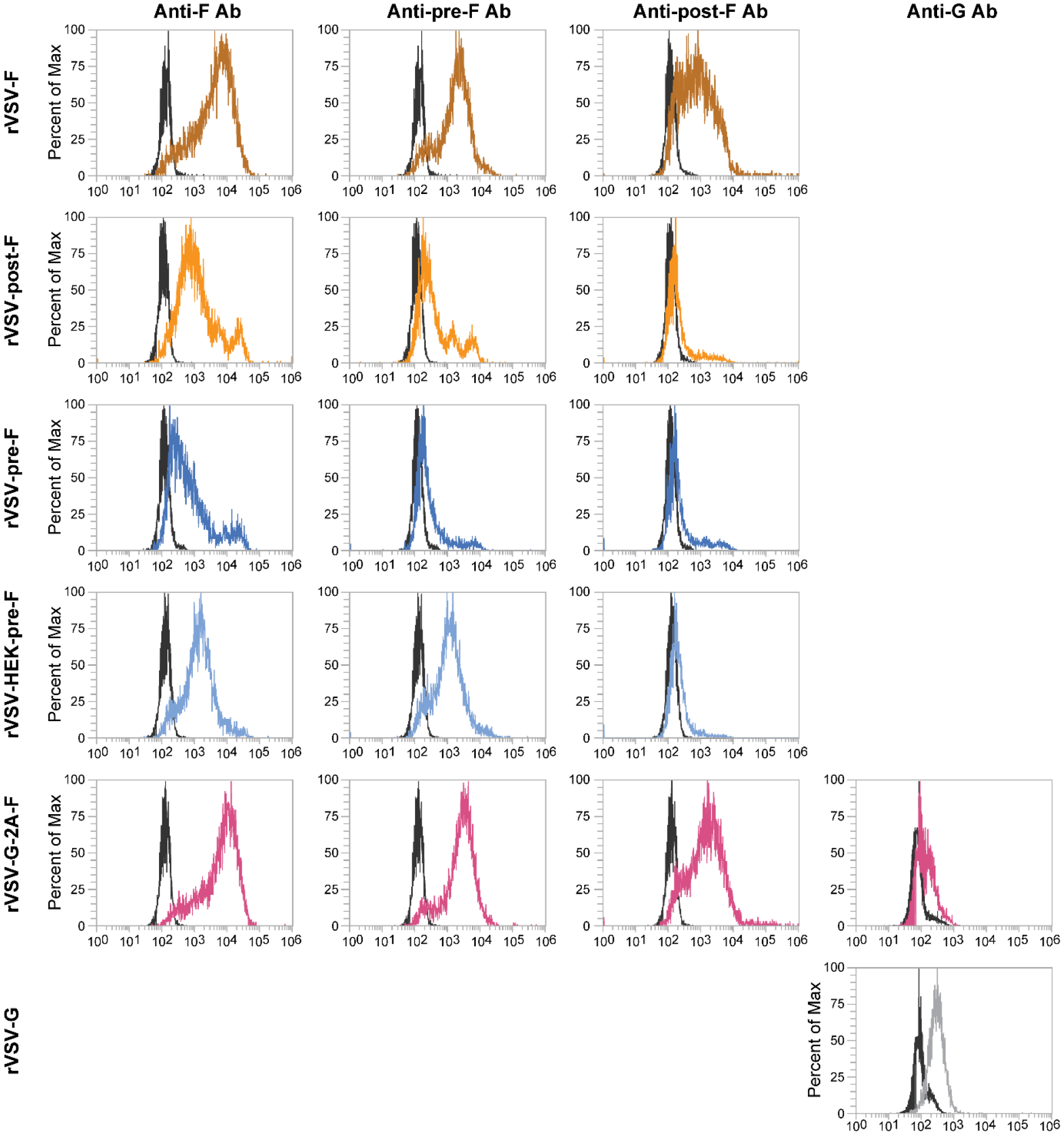
L929 cells were infected for 12 hours with an moi of 5 and protein expression level of F or G protein was assessed via flow cytometry, using an anti-F antibody (Motavizumab), an anti-pre-fusion-F antibody (D25), an anti-post-fusion-F antibody (2F), or an anti-G antibody (m131–2G). Black overlays on the histograms represent unstained, infected L929 cells, while the colored overlays represent stained and infected L929 cells.
Stabilizing the F protein in the pre- or post-fusion form does not improve immunogenicity over native F protein.
To test the immunogenicity and protective capacity of the different forms of the F protein expressed by rVSVs, cotton rats were immunized subcutaneously with a single dose of 107 pfu of rVSV-pre-F, rVSV-HEK-pre-F, rVSV-post-F, or rVSV-F, or rWT-VSV as a control. None of the animals developed neutralizing antibodies after 4 weeks (Figure 4). After challenge with RSV, animals immunized with rVSV-pre-F, rVSV-HEK-pre-F, and rVSV-post-F demonstrated a reduction of viral titers in lung tissue of 1–2 log10 TCID50/g tissue compared to rWT-VSV, while in the upper respiratory tract only immunization with rVSV-pre-F led to a reduction in viral titers of about 1 log10 TCID50/g tissue (Figure 4). This partial protection correlated with the absence of neutralizing antibodies (Figure 4).
Figure 4. Protection against RSV in cotton rats after single subcutaneous immunization with 107 pfu rVSV-pre-F, rVSV-HEK-pre-F, rVSV-post-F, or rVSV-F.
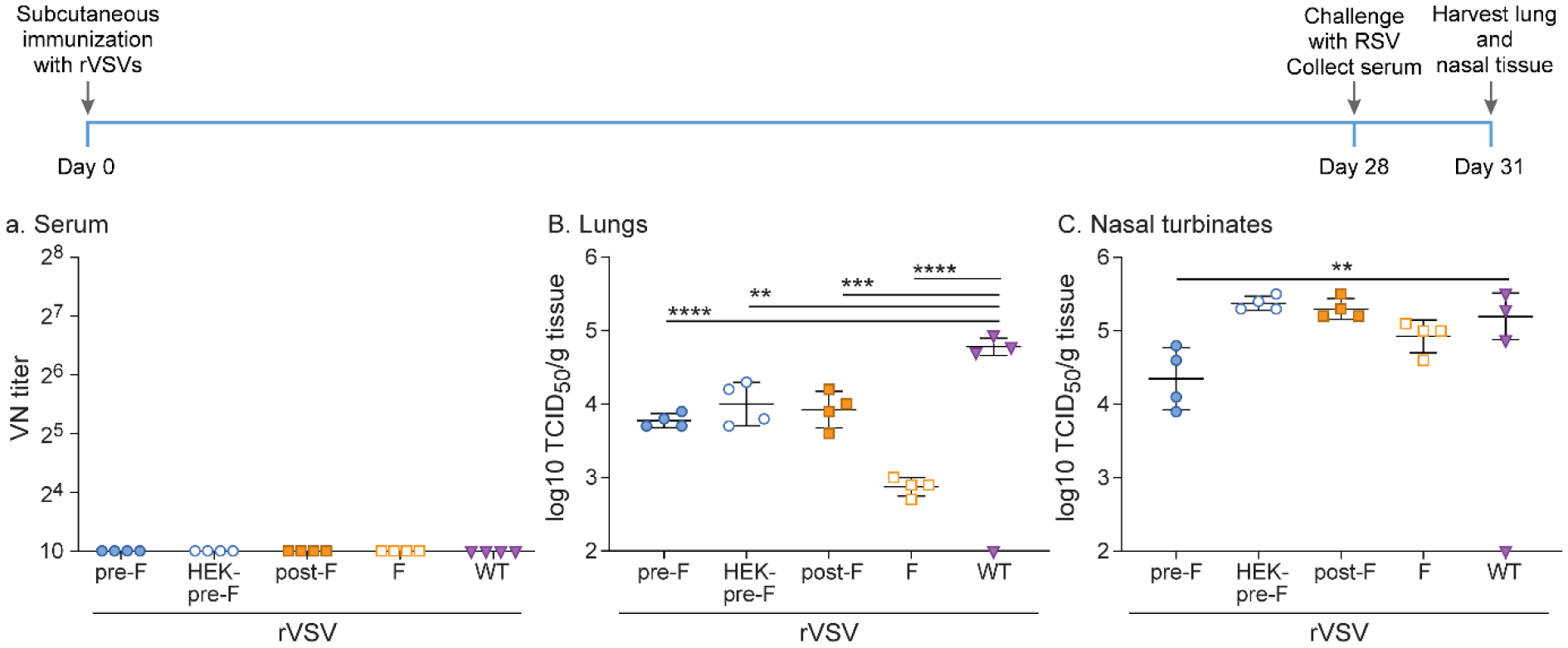
Cotton rats were immunized with 107 pfu rVSV-pre-F, rVSV-HEK-pre-F, rVSV-post-F, or rVSV-F via subcutaneous route. After RSV-A2 challenge 4 weeks later, viral titers were assessed via TCID50, with the limit of detection at 2log10. Serum antibody levels were assessed via neutralizing antibody assay, with the limit of detection at 10. A. Neutralizing titers against RSV in serum. No recombinant generated neutralizing antibody titers. B. Lung homogenates. Immunization with all recombinants provided partial protection against RSV A2 challenge. C. Nasal turbinate homogenates. rVSV-pre-F immunization provided partial protection against RSV A2 challenge. * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001
In order to assess whether booster immunization would improve immunization outcomes, cotton rats were immunized subcutaneously with a dose of 107 pfu of rVSV-pre-F, rVSV-HEK-pre-F, rVSV-post-F, or rVSV-F, or rWT-VSV, and then boosted with the same viruses subcutaneously at the same dose 4 weeks later. After an additional 4 weeks, all animals were challenged intranasally with RSV A2. This immunization schedule led to the induction of neutralizing antibodies in animals immunized with either rVSV-HEK-pre-F or rVSV-F after 8 weeks (Figure 5). However, the second immunization did not improve viral clearance in the upper or lower respiratory tract. Immunization with rVSV-F and rVSV-post-F provided partial protection in the lower respiratory tract, with a reduction of viral titers of about 1 log10 TCID50/g tissue compared to rWT-VSV, as seen after single immunization, while immunization with rVSV-F and rVSV-HEK-pre-F again provided partial protection in the upper respiratory tract, with a reduction of viral titers of 1–2 log10 TCID50/g tissue (Figure 5).
Figure 5. Protection against RSV in cotton rats immunized twice subcutaneously at 107 pfu rVSV-pre-F, rVSV-HEK-pre-F, rVSV-post-F, or rVSV-F.
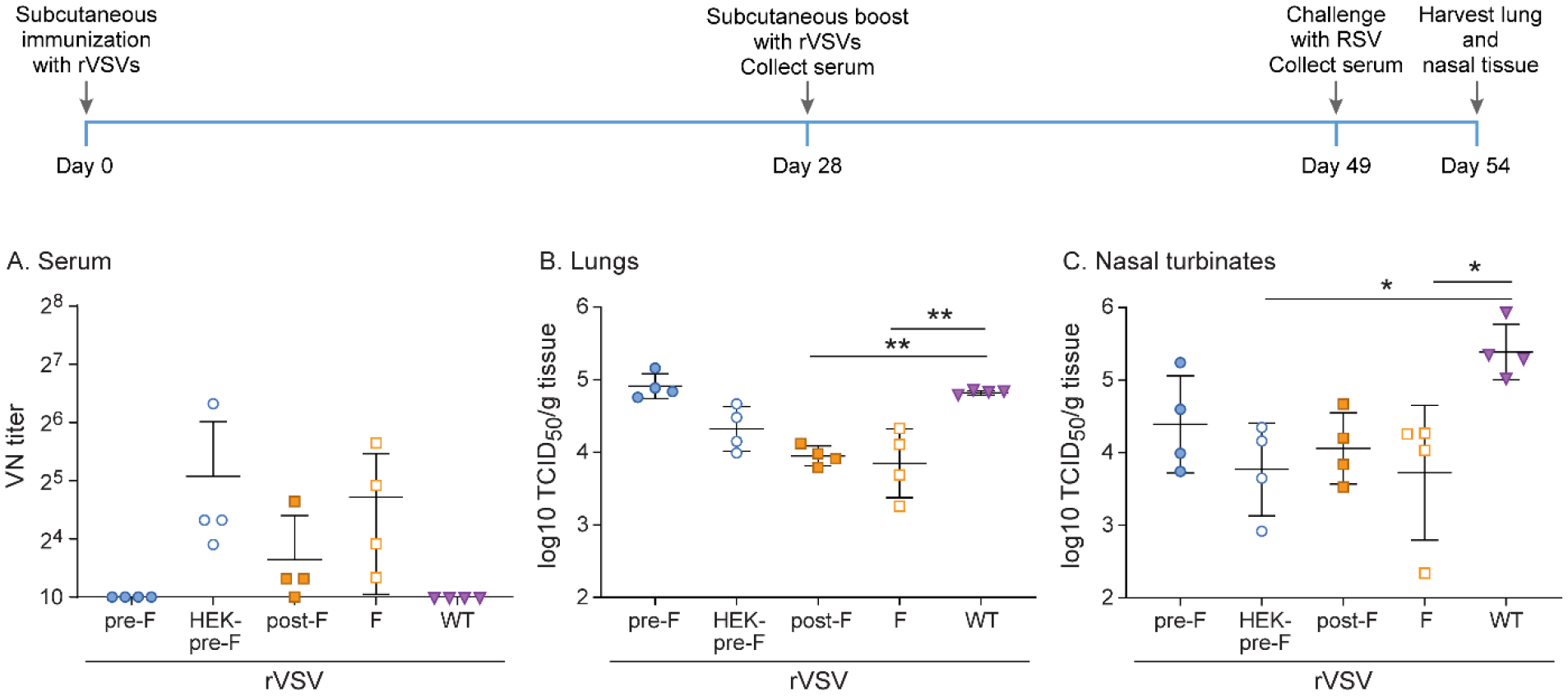
Cotton rats were immunized with 107 pfu rVSV-pre-F, rVSV-HEK-pre-F, rVSV-post-F, or rVSV-F via subcutaneous route, and then boosted with the same dose via the same route 4 weeks later. Cotton rats were challenged with RSV-A2 after a total of 7 weeks, and viral titers were assessed via TCID50, with the limit of detection at 2log10. Serum antibody levels were assessed via neutralizing antibody assay, with the limit of detection set at 10. A. Neutralizing titers against RSV in serum. Groups rVSV-HEK-pre-F and rVSV-F generated higher titers of neutralizing antibodies than all other groups. B. RSV titer in lung homogenate. rVSV-post-F and rVSV-F immunization provided partial protection against RSV challenge. C. RSV titer in nasal turbinate homogenate. rVSV-HEK-pre-F and rVSV-F immunization provided partial protection against RSV A2 challenge. * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001
Since booster immunization led only to the generation of low levels of neutralizing antibodies but did not provide complete protection with any of the F mutants or the native F, we tested whether changing the route of immunization from subcutaneous to intranasal inoculation would improve protection. All other aspects of the experimental design remained the same as in the previous experiment. Intranasal immunization with rVSV-F resulted in higher levels of neutralizing antibodies, complete protection from RSV A2 challenge in the lower respiratory tract, and a reduction of viral titers in the upper respiratory tract by about 2 log10 TCID50/g tissue compared to rWT-VSV (Figure 6). Immunization with rVSV-HEK-pre-F led to low levels of neutralizing antibodies and provided partial protection in the upper and lower respiratory tract, with a reduction of 1.5–2.5 log10 TCID50/g tissue (Figure 6). None of the other recombinants generated neutralizing antibodies or provided protection, with the exception of rVSV-pre-F, which provided partial protection in the lower respiratory tract, with a reduction of 1 log10 TCID50/g tissue (Figure 6).
Figure 6. Protection against RSV in cotton rats immunized twice intranasally with 107 pfu rVSV-pre-F, rVSV-HEK-pre-F, rVSV-post-F, or rVSV-F.
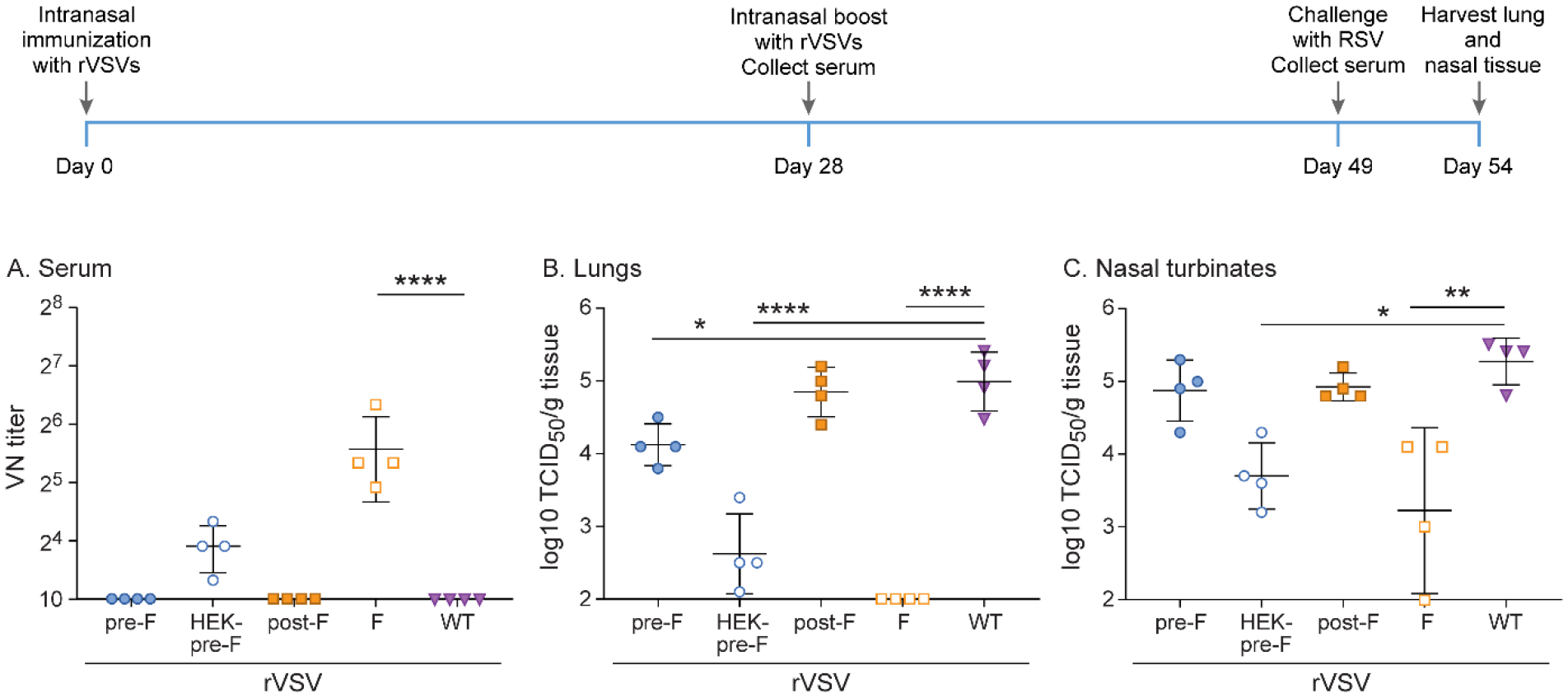
Cotton rats were immunized with 107 pfu rVSV-pre-F, rVSV-HEK-pre-F, rVSV-post-F, or rVSV-F via intranasal route, and then boosted with the same dose via the same route 4 weeks later. Cotton rats were challenged with RSV-A2 after a total of 7 weeks, and viral titers were assessed via TCID50, with the limit of detection at 2log10. Serum antibody levels were assessed via neutralizing antibody assay, with the limit of detection set at 10. A. Neutralizing titers against RSV in serum. Group rVSV-F generated statistically significant higher titers of neutralizing antibodies. B. RSV titer in lung homogenate. rVSV-pre-F and rVSV-HEK-pre-F immunization provided partial protection against RSV A2 challenge, while rVSV-F immunization provided complete protection. C. RSV titer in nasal turbinate homogenate. rVSV-HEK-pre-F and rVSV-F immunization provided partial protection against RSV A2 challenge. * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001
A VSV recombinant expressing both G and F improves the induction of neutralizing antibody levels compared to single expression of G or F alone.
During natural RSV infection, G and F proteins are expressed on the surface of the same cell. They potentially interact with each other, thus providing presumably additional target sites for neutralizing antibodies. To test whether co-expression of both proteins in one cell would improve immunization, we generated a recombinant VSV which expressed the G and the F proteins, separated by a 2A self-cleaving peptide linker sequence. Given that in the previous experiments we demonstrated that arresting the F protein in its pre-fusion or post-fusion configuration did not improve its immunogenicity, we chose to use an unmodified F protein for this construct. Cotton rats were immunized subcutaneously with a dose of 107 pfu of rVSV-G, rVSV-G-2A-F, rVSV-F, or rWT-VSV as a control. After 4 weeks, all animals were boosted with the same VSV recombinant subcutaneously at the same dose. Four weeks following boosting, all animals were challenged intranasally with 105 TCID50 of RSV A2. Immunization with rVSV-G-2A-F led to significantly higher neutralizing antibody titers than those immunized with rVSV-G or rVSV-F alone (Figure 7). As in the previous experiments, animals who received the F protein via rVSV-F or rVSV-G-2A-F were partially protected from RSV A2 infection in the upper and lower respiratory tract, with a reduction of 2–2.5 log10 TCID50/g tissue compared to rWT-VSV (Figure 7). Animals who received rVSV-G showed partial viral clearance in the lower respiratory tract, with a reduction of 2 log10 TCID50/g tissue, but no viral clearance in the upper respiratory tract (Figure 7).
Figure 7. Protection against RSV in cotton rats immunized twice subcutaneously with 107 pfu rVSV-F, rVSV-G, or rVSV-G-2A-F.
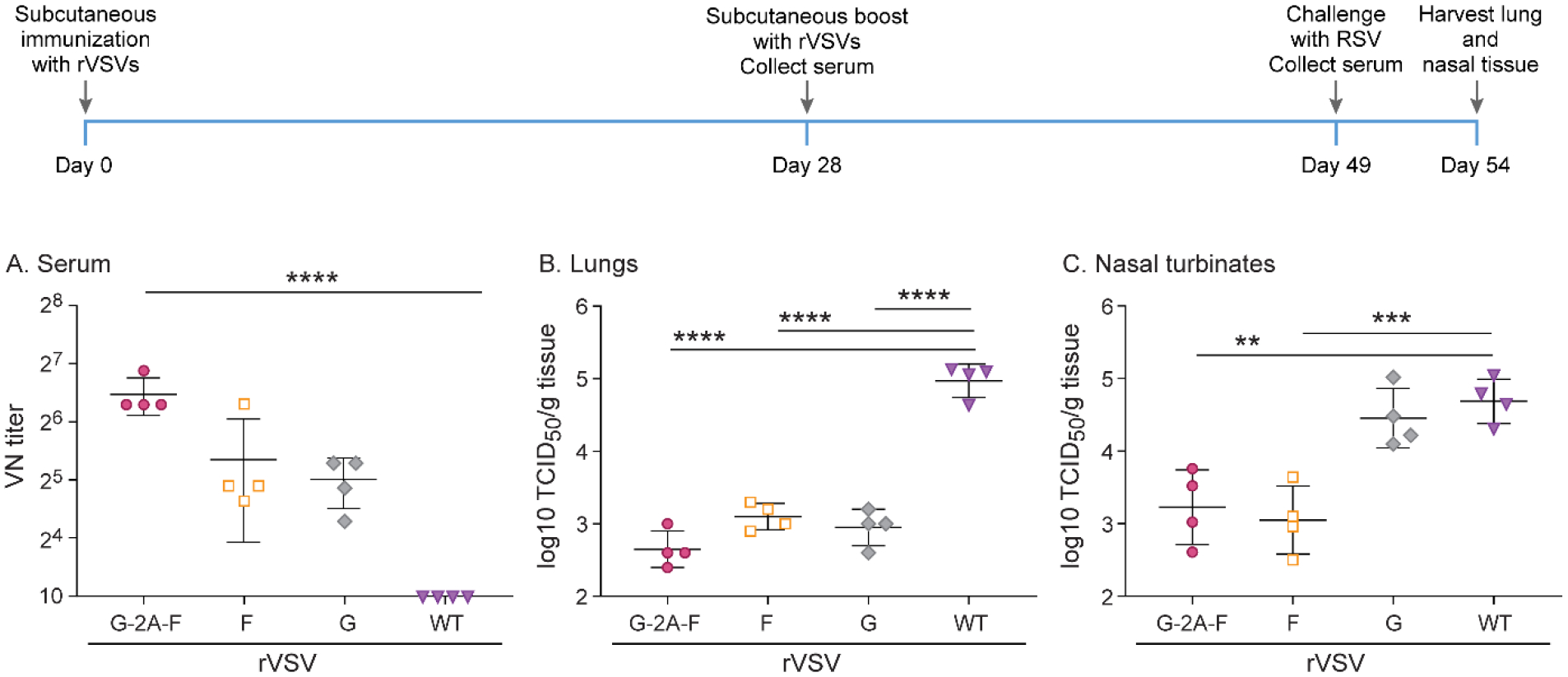
Cotton rats were immunized with 107 pfu rVSV-F, rVSV-G, or rVSV-G-2A-F via subcutaneous route, and then boosted with the same dose via the same route 4 weeks later. Cotton rats were challenged with RSV-A2 after a total of 7 weeks, and viral titers were assessed via TCID50, with the limit of detection at 2log10. Serum antibody levels were assessed via neutralizing antibody assay, with the limit of detection set at 10. A. Neutralizing titers against RSV in serum. Group rVSV-G-2A-F generated statistically significant higher titers of neutralizing antibodies. B. RSV titer in lung homogenate. rVSV-F, rVSV-G, and rVSV-G-2A-F immunization provided partial protection against RSV A2 challenge. C. RSV titer in nasal turbinate homogenate. rVSV-F and rVSV-G-2A-F immunization provided partial protection against RSV A2 challenge. * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001
To determine if the higher levels of neutralizing antibodies generated by rVSV-G-2A-F could be further enhanced, and if complete protection against RSV A2 infection could be obtained, we immunized cotton rats intranasally rather than subcutaneously, using the same experimental design as in the previous experiment in all other aspects. Neutralizing antibody levels remained the same in cotton rats immunized intranasally with rVSV-G-2A-F as with subcutaneous immunization (Figure 8). However, intranasal immunization with rVSV-G resulted in increased neutralizing antibody levels as compared to subcutaneous immunization (Figure 8). Additionally, intranasal immunization improved protection in the lower and upper respiratory tract with rVSV-G, which resulted in complete protection (Figure 5). rVSV-G-2A-F provided nearly complete protection in the lower respiratory tract, and complete protection in the upper respiratory tract (Figure 8).
Figure 8. Protection against RSV in cotton rats immunized twice intranasally with 107 pfu rVSV-F, rVSV-G or rVSV-G-2A-F.
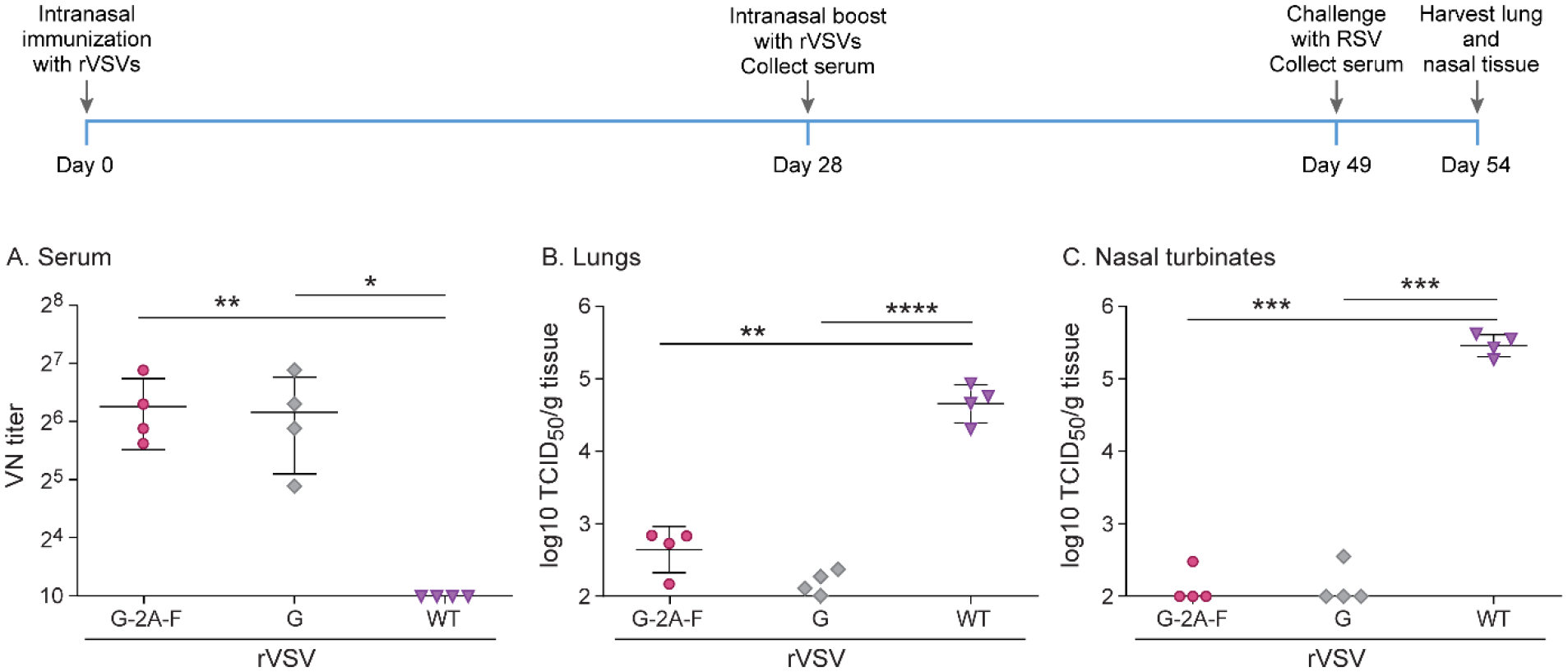
Cotton rats were immunized with 107 pfu rVSV-F, rVSV-G, or rVSV-G-2A-F via intranasal route, and then boosted with the same dose via the same route 4 weeks later. Cotton rats were challenged with RSV-A2 after a total of 7 weeks, and viral titers were assessed via TCID50, with the limit of detection at 2log10. Serum antibody levels were assessed via neutralizing antibody assay, with the limit of detection set at 10. A. Neutralizing titers against RSV in serum. Groups rVSV-G and rVSV-G-2A-F generated statistically significant higher titers of neutralizing antibodies. B. RSV titer in lung homogenate. rVSV-G-2A-F immunization provided partial protection against RSV A2 challenge, while rVSV-G and rVSV-F immunization provided complete protection. C. RSV titer in nasal turbinate homogenate. rVSV-G and rVSV-G-2A-F immunization provided complete protection against RSV A2 challenge, while rVSV-F immunization provided partial protection. * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001
Combining G and F results in the induction of higher long-term neutralizing antibody levels than after immunization with G or F alone.
RSV infection leads to a short-lived immune response and lack of long-lasting neutralizing antibody levels after natural infection. To test the longevity of the antibody response after immunization with rVSVs, cotton rats were immunized subcutaneously with a dose of 107 pfu of rVSV-G, rVSV-G-2A-F, rVSV-F, or rWT-VSV as a control. After 4 weeks, cotton rats were boosted with the same recombinant virus subcutaneously at the same dose. Eight weeks following boosting, all animals were challenged intranasally with 105 TCID50 of RSV A2. Four days post-challenge, virus titers were established on lung and nasal homogenates of infected animals, and neutralizing antibodies were measured on serum harvested immediately before intranasal RSV A2 infection. Neutralizing antibodies were initially high for animals immunized with rVSV-F and rVSV-G-2A-F, with subsequently declining antibody titers, while rVSV-G always had low levels for all 12 weeks, and did not show an increase following booster immunization (Figure 9). All groups receiving VSV recombinants showed complete protection in the lower respiratory tract, while there was no significant difference between immunized groups and the rWT-VSV control in the upper respiratory tract (Figure 9).
Figure 9. Long-term immunity after subcutaneous immunization with 107 pfu rVSV-F, rVSV-G, or rVSV-G-2A-F.
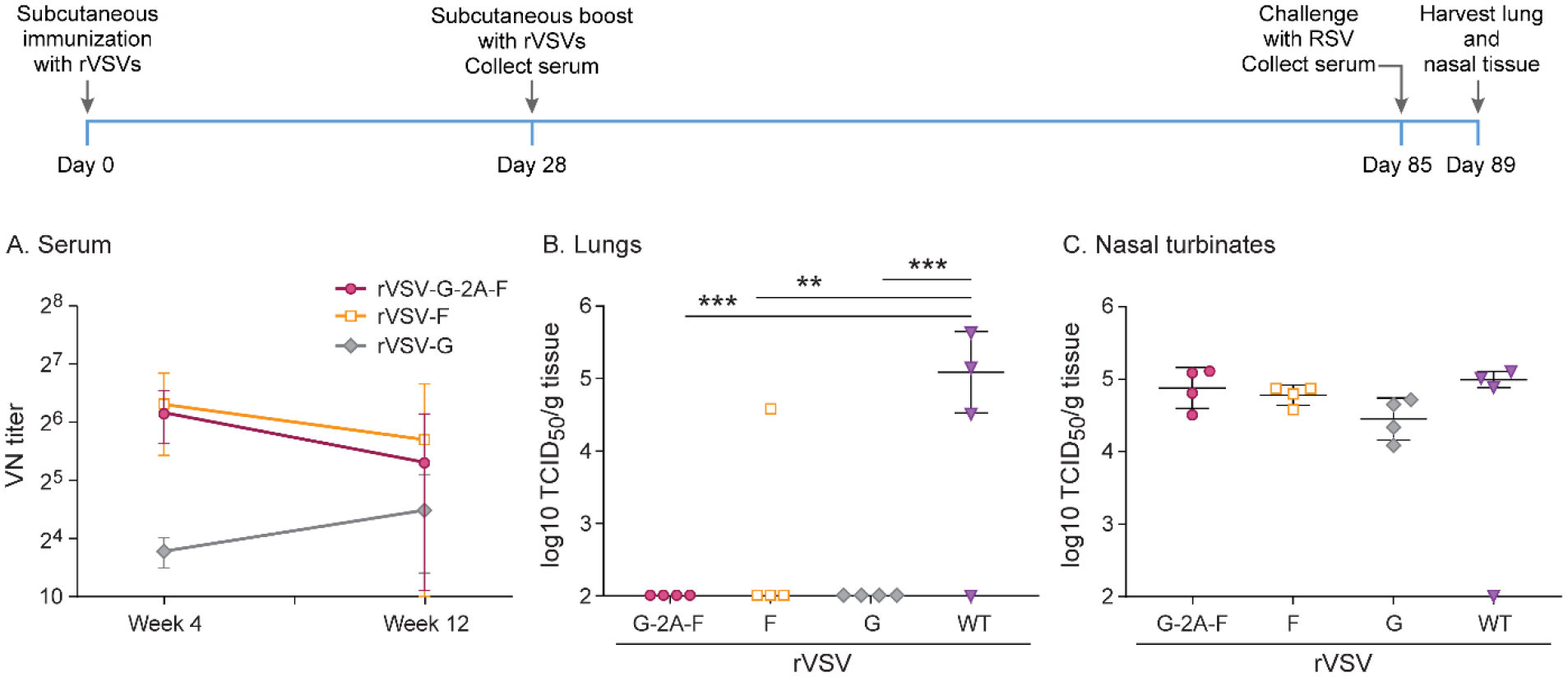
Cotton rats were immunized with 107 pfu rVSV-F, rVSV-G, or rVSV-G-2A-F via subcutaneous route, and then boosted with the same dose via the same route 4 weeks later. Cotton rats were challenged with RSV-A2 after a total of 12 weeks, and viral titers were assessed via TCID50, with the limit of detection at 2log10. Serum antibody levels were assessed via neutralizing antibody assay, with the limit of detection set at 10. A. Neutralizing titers against RSV in serum. Immunization with rVSV-G generated low levels of neutralizing antibodies that did not change over 12 weeks, while immunization with rVSV-F and rVSV-G-2A-F generated 4–5-fold higher levels of antibodies that slowly waned over the course of 12 weeks. B. RSV titer in lung homogenate. rVSV-F, rVSV-G, and rVSV-G-2A-F all provided complete protection against RSV A2 challenge. C. RSV titer in nasal homogenate. No treatment group was protected against RSV A2 challenge. * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001
Based on short-term experiments, we hypothesized that immunogenicity could be improved with intranasal immunization, and repeated the previous experiment with the following modifications: the immunization route was intranasal rather than subcutaneous, and the cotton rats were not boosted after 4 weeks. This resulted in complete protection by immunization with rVSV-F of the lungs but only partial protection in the nasal tissue. Immunization with rVSV-G and rVSV-G-2A-F led to near-complete protection in the lungs and nasal tissue (Figure 10). Initially, neutralizing antibody levels generated by immunization with rVSV-F were highest, but steadily declined over the course of 12 weeks (Figure 10). Immunization with rVSV-G generated low levels of RSV neutralizing antibodies, which rose slightly over the course of 12 weeks but remained lower than antibody levels after immunization with rVSV-G-2A-F (Figure 10). rVSV-G-2A-F induced neutralizing antibody levels peaked at week 8, but remained significantly higher than either rVSV-F or rVSV-G alone by week 12 (Figure 10). These data suggest that although immunization with rVSV-F initially generates high levels of neutralizing antibodies, combining F with G ultimately leads to generation of longer-lasting antibodies and superior protection in the upper and lower respiratory tract than either F or G alone.
Figure 10. Long-term immunity after intranasal immunization with 107 pfu rVSV-F, rVSV-G, or rVSV-G-2A-F.
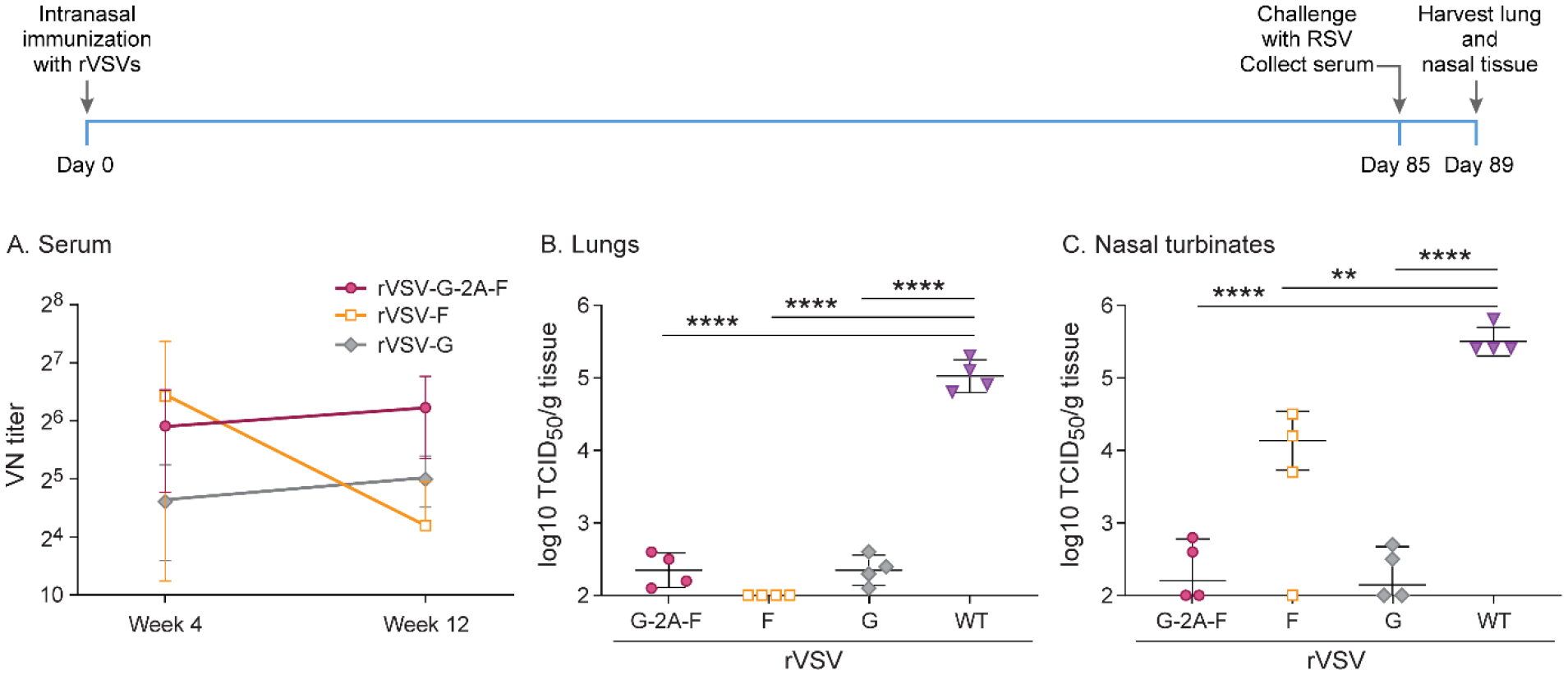
Cotton rats were immunized with 107 pfu rVSV-F, rVSV-G, or rVSV-G-2A-F via intranasal route, challenged with RSV-A2 after a total of 12 weeks, and viral titers were assessed via TCID50, with the limit of detection at 2log10. Serum antibody levels were assessed via neutralizing antibody assay, with the limit of detection set at 10. A. Neutralizing titers against RSV in serum. rVSV-G-2A-F provided the highest levels of neutralizing antibodies at 8 weeks and 12 weeks post-immunization. B. RSV titer in lung homogenate. rVSV-G-2A-F and rVSV-G immunization provided partial protection against RSV A2 challenge, while rVSV-F provided complete protection. C. RSV titer in nasal turbinate homogenate. rVSV-G-2A-F and rVSV-G immunization provided near-complete protection against RSV A2 challenge, while rVSV-F immunization provided partial protection. * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001
Discussion
Recombinant vesicular stomatitis virus (VSV) have been used as a vector-based delivery platform for foreign proteins, and are the basis for a successful Ebola virus vaccine [10]. In addition, this virus vector is used in vaccine development against infectious diseases and cancer, and is being explored as a vector platform for RSV vaccination [3–5,22]. In this study, we developed recombinant vesicular stomatitis viruses expressing RSV-G, RSV-F, RSV-HEK-pre-F, RSV-pre-F, RSV-post-F, or both RSV-G and RSV-F, and tested their immunogenicity in a cotton rat model. We chose to investigate subcutaneous and intranasal routes of inoculation, since current vaccine work on RSV is pursuing both of these routes [22,36–39]. We found that intranasal immunization resulted in the highest level of protection from viral replication in the lung and the nose, with lower viral titers than with subcutaneous immunization. It also increased the neutralizing antibody levels with all recombinants tested and, in the case of the rVSV-G-2A-F recombinant, resulted in high levels of neutralizing antibodies for at least 80 days. This is consistent with observations that mucosal immunization stimulates IgA production and the mucosal associated lymphoid tissue (MALT), providing an immune defense against RSV infection at the site of entry [40]. However, mucosal immunization can sometimes require a higher dose of vaccine and additional adjuvant compared to subcutaneous vaccination [41]. Theoretically, mucosal immunization may be easier to administer [42] and there are some indications that systemic vaccine application is not always effective in inducing mucosal immunity [43]. Subcutaneous immunization seems to be less likely to induce a strong mucosal response, but does typically induce strong T cell responses, which is important for viral clearance [44]. We found that the immunogenicity of our vaccine candidates could be improved with a boost after 4 weeks, with the most marked improvement after subcutaneous immunization. These experiments only explored the results of a homologous prime-boost vaccine regimen. We have found that neutralizing antibody titers to the rVSVs used in these experiments do not significantly change relative to each other over the course of an 8-week prime-boost experiment (unpublished data). As a result, we do not anticipate that any neutralizing antibodies to VSV contributed to variations in the relative protection between constructs. However, future experiments using a heterologous prime-boost regimen may yield different results.
Both the G and F proteins are important targets for antibodies in natural RSV infection, but antibody levels are typically low and the immune response is short-lived [45–48]. Previous studies have investigated the effect of mutating the F protein to stabilize it in its pre-fusion or post-fusion configuration, with conflicting results [7,24,36,49–55]. Our results demonstrate that when expressed in a VSV vector, neither the stabilized pre-fusion or post-fusion form of the F protein was superior to the native F protein in terms of protection in the upper and lower respiratory tract or in terms of neutralizing antibody levels generated. We also demonstrated that intranasal immunization, with a boost after 4 weeks, was superior to either subcutaneous immunization with or without a boost, or intranasal immunization without a boost. The improved protection provided by the native F over the stabilized recombinants may be due to the reduced level of protein expression on the cell surface by the stabilized recombinants, which likely results in lower antigen levels available for B cell recognition. This is supported by the flow cytometry and Western blot results for rVSV-pre-F and rVSV-post-F, which had reduced antibody binding as compared to rVSV-F and overall reduced protein expression levels. However, rVSV-HEK-pre-F had antibody binding levels on flow cytometry comparable to rVSV-F, as well as comparable protein expression on Western blotting, and was still not as protective as rVSV-F. A study in which wild-type F, HEK-pre-F, and post-fusion RSV-F were expressed in a PIV3 vector system demonstrated that post-F was less immunogenic than either wild-type F or HEK-pre-F when used to immunize hamsters, but there was no difference in immunogenicity between HEK-pre-F and wild-type F [28]. Similarly, a study evaluating pre-F and wild-type F in a PIV5 vector system also found that pre-F did not confer superior protection in mice or cotton rats [36]. This study also performed flow cytometry on their constructs, and determined that there was reduced surface expression of the F protein in constructs expressing pre-F, as compared to constructs expressing wild-type F. Taken together with our findings, these results suggest that RSV-F expressed in live-virus vectors may have reduced cell surface expression when stabilized in the pre-fusion or post-fusion form. It is possible that changing the transmembrane domain to a VSV-specific domain would improve cell surface expression in the VSV vector. Studies using PIV3 to vector either RSV-F or RSV-G have variably found that using a PIV3 transmembrane domain either improves or has no effect on protein packaging [56,57].
Although the predominant antibodies produced in natural infection target the F protein, individuals who generate antibodies against the G protein have reduced clinical signs, indicating the desirability of a G protein specific antibody response [17,58]. In our study, antibodies were significantly elevated when F and G were combined together in a recombinant vector and then administered to cotton rats subcutaneously. However, subcutaneous immunization with the F protein, regardless of single expression or co-expression with the G protein, had the most significant impact on protection in the upper and lower respiratory tract. This difference became less striking with intranasal immunization, which improved protection against infection in animals immunized with rVSV-G and brought neutralizing antibodies to levels comparable to those in animals immunized with rVSV-G-2A-F. Increased neutralizing antibody levels and improved protection from infection following immunization with rVSV-G-2A-F indicate that immunizing with both RSV glycoproteins is superior to immunizing with either individually.
Given that G and F in combination resulted in increased neutralizing antibody levels, we hypothesized that these initially high neutralizing antibody levels would help counteract the loss in protection typically seen following RSV infection in humans [48]. In cotton rats, antibodies levels after infection with RSV decrease after 60 days [27]. To test decrease of antibody levels with our vaccine vectors, we immunized cotton rats and measured their immune responses 80 days following initial immunization, and found that G and F expressed in a VSV vector administered intranasally resulted in higher neutralizing antibody levels and improved protection than either G or F alone. So far, there have been no other studies reporting viral titers and neutralizing antibody levels after 80 days in cotton rats immunized with RSV-G and RSV-F.
In conclusion, we demonstrated that in the VSV vector system, the unmodified RSV-F is superior to the F protein stabilized in either the pre-fusion or post-fusion form in terms of protection and induction of neutralizing antibody levels. We also demonstrated that combining the F and G proteins into a single recombinant vector results in improved protection as compared to either the F or G protein alone, and that this protection, along with increased neutralizing antibody levels, has longer longevity than immunizing with either protein singly. Therefore, a recombinant VSV vector expressing both RSV-G and RSV-F could be used as a potential vaccine against RSV to provide longer-lasting immunity and superior protection.
Acknowledgements
Funding:
This work was supported by the NIH National Institute of Allergy and Infectious Diseases [P01AI112524].
The authors would like to thank Tim Vojt for artistic and technical assistance with the figures, especially Figure 1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med 2005;352:1749–59. 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- [2].Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 2010;375:1545–55. 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Meyer G, Deplanche M, Schelcher F. Human and bovine respiratory syncytial virus vaccine research and development. Comp Immunol Microbiol Infect Dis 2008;31:191–225. 10.1016/j.cimid.2007.07.008. [DOI] [PubMed] [Google Scholar]
- [4].Wilmschen S, Schneider S, Peters F, Bayer L, Issmail L, Bánki Z, et al. RSV vaccine based on rhabdoviral vector protects after single immunization. Vaccines 2019;7. 10.3390/vaccines7030059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Johnson JE, McNeil LK, Megati S, Witko SE, Roopchand VS, Obregon JH, et al. Non-propagating, recombinant vesicular stomatitis virus vectors encoding respiratory syncytial virus proteins generate potent humoral and cellular immunity against RSV and are protective in mice. Immunol Lett 2013;150:134–44. 10.1016/j.imlet.2012.12.005. [DOI] [PubMed] [Google Scholar]
- [6].Roberts A, Buonocore L, Price R, Forman J, Rose JK, Roberts A, et al. Attenuated Vesicular Stomatitis Viruses as Vaccine Vectors. J Virol 1999;73:3723–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Siler CA, McGettigan JP, Dietzschold B, Herrine SK, Dubuisson J, Pomerantz RJ, et al. Live and killed rhabdovirus-based vectors as potential hepatitis C vaccines. Virology 2002;292:24–34. 10.1006/viro.2001.1212. [DOI] [PubMed] [Google Scholar]
- [8].Lichty BD, Power AT, Stojdl DF, Bell JC. Vesicular stomatitis virus: Re-inventing the bullet. Trends Mol Med 2004;10:210–6. 10.1016/j.molmed.2004.03.003. [DOI] [PubMed] [Google Scholar]
- [9].Geisbert TW, Feldmann H. Recombinant vesicular stomatitis virus-based vaccines against Ebola and marburg virus infections. J Infect Dis 2011;204. 10.1093/infdis/jir349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Heppner DG, Kemp TL, Martin BK, Ramsey WJ, Nichols R, Dasen EJ, et al. Safety and immunogenicity of the rVSVΔG-ZEBOV-GP Ebola virus vaccine candidate in healthy adults: a phase 1b randomised, multicentre, double-blind, placebo-controlled, dose-response study. Lancet Infect Dis 2017;17:854–66. 10.1016/S1473-3099(17)30313-4. [DOI] [PubMed] [Google Scholar]
- [11].Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. the IMpact-RSV study group. Pediatrics 1998;102:531–7. [PubMed] [Google Scholar]
- [12].Hamdy RF. Efficacy of motavizumab for the prevention of respiratory syncytial virus disease in healthy native American infants: A phase 3 randomised double-blind placebo-controlled trial. J Pediatric Infect Dis Soc 2015;5:93–5. 10.1093/jpids/piv087. [DOI] [PubMed] [Google Scholar]
- [13].Bagga B, Cehelsky JE, Vaishnaw A, Tomwilkinson T, Meyers R, Harrison LM, et al. Effect of Preexisting Serum and Mucosal Antibody on Experimental Respiratory Syncytial Virus (RSV) Challenge and Infection of Adults. J Infect Dis 2015;212:1719–25. 10.1093/infdis/jiv281. [DOI] [PubMed] [Google Scholar]
- [14].Zhivaki D, Lemoine S, Lim A, Morva A, Vidalain PO, Schandene L, et al. Respiratory Syncytial Virus Infects Regulatory B Cells in Human Neonates via Chemokine Receptor CX3CR1 and Promotes Lung Disease Severity. Immunity 2017;46:301–14. 10.1016/j.immuni.2017.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Melero JA, Mas V, McLellan JS. Structural, antigenic and immunogenic features of respiratory syncytial virus glycoproteins relevant for vaccine development. Vaccine 2017;35:461–8. 10.1016/j.vaccine.2016.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].McLellan JS, Ray WC, Peeples ME. Structure and function of respiratory syncytial virus surface glycoproteins. Curr Top Microbiol Immunol 2013;372:83–104. 10.1007/978-3-642-38919-1_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Capella C, Chaiwatpongsakorn S, Gorrell E, Risch ZA, Ye F, Mertz SE, et al. Prefusion F, postfusion F, G antibodies, and disease severity in infants and young children with acute respiratory syncytial virus infection. J Infect Dis 2017;216:1398–406. 10.1093/infdis/jix489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gia Green M, Petroff N, La Perle KMD, Niewiesk S. Characterization of cotton rat (Sigmodon hispidus) eosinophils, including their response to respiratory syncytial virus infection. Comp Med 2018;68:31–40. [PMC free article] [PubMed] [Google Scholar]
- [19].Reed LJ, Muench H. A simple method of estimating fifty per cent endpoints. Am J Epidemiol 1938;27:493–7. 10.1093/oxfordjournals.aje.a118408. [DOI] [Google Scholar]
- [20].Ma Y, Duan Y, Wei Y, Liang X, Niewiesk S, Oglesbee M, et al. Heat Shock Protein 70 Enhances Mucosal Immunity against Human Norovirus When Coexpressed from a Vesicular Stomatitis Virus Vector. J Virol 2014;88:5122–37. 10.1128/jvi.00019-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ma Y, Li J. Vesicular Stomatitis Virus as a Vector To Deliver Virus-Like Particles of Human Norovirus: a New Vaccine Candidate against an Important Noncultivable Virus. J Virol 2011;85:2942–52. 10.1128/jvi.02332-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Binjawadagi B, Ma Y, Binjawadagi R, Brakel K, Harder O, Peeples M, et al. Mucosal delivery of recombinant VSV vectors expressing envelope proteins of RSV induces protective immunity in cotton rats. J Virol 2021;95. 10.1128/jvi.02345-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Whelan SPJ, Ball LA, Barr JN, Wertz GTW. Efficient recovery of infectious vesicular stomatitis virus entirely from cDNA clones. Proc Natl Acad Sci U S A 1995;92:8388–92. 10.1073/pnas.92.18.8388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Liang B, Surman S, Amaro-Carambot E, Kabatova B, Mackow N, Lingemann M, et al. Enhanced Neutralizing Antibody Response Induced by Respiratory Syncytial Virus Prefusion F Protein Expressed by a Vaccine Candidate. J Virol 2015;89:9499–510. 10.1128/jvi.01373-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Liu Z, Chen O, Wall JBJ, Zheng M, Zhou Y, Wang L, et al. Systematic comparison of 2A peptides for cloning multi-genes in a polycistronic vector. Sci Rep 2017;7:1–9. 10.1038/s41598-017-02460-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Prince GA, Horswood RL, Camargo E, Koenig D, Chanock RM. Mechanisms of immunity to respiratory syncytial virus in cotton rats. Infect Immun 1983;42:81–7. 10.1128/iai.42.1.81-87.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Widjojoatmodjo MN, Boes J, van Bers M, van Remmerden Y, Roholl PJ, Luytjes W. A highly attenuated recombinant human respiratory syncytial virus lacking the G protein induces long-lasting protection in cotton rats. Virol J 2010;7:114. 10.1186/1743-422X-7-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Liang B, Surman S, Amaro-Carambot E, Kabatova B, Mackow N, Lingemann M, et al. Enhanced Neutralizing Antibody Response Induced by Respiratory Syncytial Virus Prefusion F Protein Expressed by a Vaccine Candidate. J Virol 2015;89:9499–510. 10.1128/jvi.01373-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kwilas S, Liesman RM, Zhang L, Walsh E, Pickles RJ, Peeples ME. Respiratory Syncytial Virus Grown in Vero Cells Contains a Truncated Attachment Protein That Alters Its Infectivity and Dependence on Glycosaminoglycans. J Virol 2009;83:10710. 10.1128/JVI.00986-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Johnson PR, Spriggs MK, Olmsted RA, Collins PL. The G glycoprotein of human respiratory syncytial viruses of subgroups A and B: extensive sequence divergence between antigenically related proteins. Proc Natl Acad Sci U S A 1987;84:5625–9. 10.1073/pnas.84.16.5625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].F S, C EM, G H, K S, Fuentes S, Coyle EM, et al. Nonglycosylated G-Protein Vaccine Protects against Homologous and Heterologous Respiratory Syncytial Virus (RSV) Challenge, while Glycosylated G Enhances RSV Lung Pathology and Cytokine Levels. J Virol 2015;89:8193–205. 10.1128/jvi.00133-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].King T, Mejias A, Ramilo O, Peeples ME. The larger attachment glycoprotein of respiratory syncytial virus produced in primary human bronchial epithelial cultures reduces infectivity for cell lines. PLoS Pathog 2021;17:e1009469. 10.1371/journal.ppat.1009469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Collins PL. O glycosylation of glycoprotein G of human respiratory syncytial virus is specified within the divergent ectodomain. J Virol 1990;64:4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Smith G, Raghunandan R, Wu Y, Liu Y, Massare M, Nathan M, et al. Respiratory Syncytial Virus Fusion Glycoprotein Expressed in Insect Cells Form Protein Nanoparticles That Induce Protective Immunity in Cotton Rats. PLoS One 2012;7:50852. 10.1371/journal.pone.0050852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Rixon HWML, Brown C, Brown G, Sugrue RJ. Multiple glycosylated forms of the respiratory syncytial virus fusion protein are expressed in virus-infected cells. J Gen Virol 2002;83:61–6. 10.1099/0022-1317-83-1-61. [DOI] [PubMed] [Google Scholar]
- [36].Phan SI, Zengel JR, Wei H, Li Z, Wang D, He B. Parainfluenza Virus 5 Expressing Wild-Type or Prefusion Respiratory Syncytial Virus (RSV) Fusion Protein Protects Mice and Cotton Rats from RSV Challenge. J Virol 2017;91:2021. 10.1128/jvi.00560-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Hu KF, Elvander M, Merza M, Åkerblom L, Brandenburg A, Morein B. The immunostimulating complex (ISCOM) is an efficient mucosal delivery system for respiratory syncytial virus (RSV) envelope antigens inducing high local and systemic antibody responses. Clin Exp Immunol 1998;113:235–43. 10.1046/j.1365-2249.1998.00650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Diaz FE, Guerra-Maupome M, Soto JA, Gálvez NMS, Ríos M, McGill JL, et al. Safety, immunogenicity, and protective efficacy of a recombinant vaccine against RSV: Evidence from murine and bovine models. J Immunol 2020;204. [Google Scholar]
- [39].Mapletoft JW, Latimer L, Babiuk LA, Van Drunen Littel-Van Den Hurk S. Intranasal immunization of mice with a bovine respiratory syncytial virus vaccine induces superior immunity and protection compared to those by subcutaneous delivery or combinations of intranasal and subcutaneous prime-boost strategies. Clin Vaccine Immunol 2010;17:23–35. 10.1128/CVI.00250-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Holmgren J, Czerkinsky C. Mucosal immunity and vaccines. Nat Med 2005;11:S45. 10.1038/nm1213. [DOI] [PubMed] [Google Scholar]
- [41].Neutra MR, Kozlowski PA. Mucosal vaccines: The promise and the challenge. Nat Rev Immunol 2006;6:148–58. 10.1038/nri1777. [DOI] [PubMed] [Google Scholar]
- [42].Citron MP, Patel M, Purcell M, Lin SA, Rubins DJ, McQuade P, et al. A novel method for strict intranasal delivery of non-replicating RSV vaccines in cotton rats and non-human primates. Vaccine 2018;36:2876–85. 10.1016/j.vaccine.2018.02.110. [DOI] [PubMed] [Google Scholar]
- [43].Su F, Patel GB, Hu S, Chen W. Induction of mucosal immunity through systemic immunization: Phantom or reality? Hum Vaccines Immunother 2016;12:1070–9. 10.1080/21645515.2015.1114195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Clements JD, Freytag LC. Parenteral vaccination can be an effective means of inducing protective mucosal responses. Clin Vaccine Immunol 2016;23:438–41. 10.1128/CVI.00214-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of Primary Infection and Reinfection With Respiratory Syncytial Virus. Am J Dis Child 1986;140:543–6. 10.1001/archpedi.1986.02140200053026. [DOI] [PubMed] [Google Scholar]
- [46].Henderson FW, Collier AM, Clyde WA, Denny FW. Respiratory-Syncytial-Virus Infections, Reinfections and Immunity. N Engl J Med 1979;300:530–4. 10.1056/nejm197903083001004. [DOI] [PubMed] [Google Scholar]
- [47].Power UF. Respiratory syncytial virus (RSV) vaccines-Two steps back for one leap forward. J Clin Virol 2008;41:38–44. 10.1016/j.jcv.2007.10.024. [DOI] [PubMed] [Google Scholar]
- [48].Hall CB, Walsh EE, Long CE, Schnabel KC. Immunity to and frequency of reinfection with respiratory syncytial virus. J Infect Dis 1991;163:693–8. 10.1093/infdis/163.4.693. [DOI] [PubMed] [Google Scholar]
- [49].McLellan JS, Chen M, Leung S, Graepel KW, Du X, Yang Y, et al. Structure of RSV fusion glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Science (80- ) 2013;340:1113–7. 10.1126/science.1234914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Magro M, Mas V, Chappell K, Vázquez M, Cano O, Luque D, et al. Neutralizing antibodies against the preactive form of respiratory syncytial virus fusion protein offer unique possibilities for clinical intervention. Proc Natl Acad Sci U S A 2012;109:3089–94. 10.1073/pnas.1115941109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Ngwuta JO, Chen M, Modjarrad K, Joyce MG, Kanekiyo M, Kumar A, et al. Prefusion F-specific antibodies determine the magnitude of RSV neutralizing activity in human sera. Sci Transl Med 2015;7:309ra162–309ra162. 10.1126/scitranslmed.aac4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].McLellan JS, Chen M, Joyce MG, Sastry M, Stewart-Jones GBE, Yang Y, et al. Structure-based design of a fusion glycoprotein vaccine for respiratory syncytial virus. Science (80-) 2013;342:592–8. 10.1126/science.1243283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Falloon J, Yu J, Esser MT, Villafana T, Yu L, Dubovsky F, et al. An adjuvanted, postfusion F protein-based vaccine did not prevent respiratory syncytial virus illness in older adults. J Infect Dis 2017;216:1362–70. 10.1093/infdis/jix503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Swanson KA, Settembre EC, Shaw CA, Dey AK, Rappuoli R, Mandl CW, et al. Structural basis for immunization with postfusion respiratory syncytial virus fusion F glycoprotein (RSV F) to elicit high neutralizing antibody titers. Proc Natl Acad Sci U S A 2011;108:9619–24. 10.1073/pnas.1106536108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Cimica V, Boigard H, Bhatia B, Fallon JT, Alimova A, Gottlieb P, et al. Novel respiratory syncytial virus-like particle vaccine composed of the postfusion and prefusion conformations of the F glycoprotein. Clin Vaccine Immunol 2016;23:451–9. 10.1128/CVI.00720-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Liang B, Kabatova B, Kabat J, Dorward DW, Liu X, Surman S, et al. Effects of Alterations to the CX3C Motif and Secreted Form of Human Respiratory Syncytial Virus (RSV) G Protein on Immune Responses to a Parainfluenza Virus Vector Expressing the RSV G Protein. J Virol 2019;93:2043–61. 10.1128/jvi.02043-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Liang B, Ngwuta JO, Herbert R, Swerczek J, Dorward DW, Amaro-Carambot E, et al. Packaging and Prefusion Stabilization Separately and Additively Increase the Quantity and Quality of Respiratory Syncytial Virus (RSV)-Neutralizing Antibodies Induced by an RSV Fusion Protein Expressed by a Parainfluenza Virus Vector. J Virol 2016;90:10022–38. 10.1128/jvi.01196-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Wright PF, Karron RA, Belshe RB, Thompson J, Crowe JE, Boyce TG, et al. Evaluation of a live, cold-passaged, temperature-sensitive, respiratory syncytial virus vaccine candidate in infancy. J Infect Dis 2000;182:1331–42. 10.1086/315859. [DOI] [PubMed] [Google Scholar]


