Abstract
The prevalence of non-alcoholic fatty liver disease (NAFLD) is increasing worldwide. Perinatal development is a critical window for altered, lifelong health trajectory, and evidence supports the role of perinatal programming in chronic metabolic diseases. To examine the impact of diet and bisphenol A (BPA) on the developmental trajectory of NAFLD in offspring, we exposed dams from pre-gestation through lactation to a human-relevant dose of oral BPA coupled with intake of high fat Western or Mediterranean-style diets. We assessed hepatic steatosis by quantifying hepatic triglycerides (TGs) and metabolic health by measuring body weight, relative organ weights, and serum hormone levels in dams and offspring at postnatal day 10 (PND10) and 10-months of age. In dams, consumption of the Western or Mediterranean diet increased hepatic TGs 1.7-2.4-fold, independent of BPA intake. Among offspring, both perinatal diet and BPA exposure had a greater impact on metabolic outcomes than on hepatic steatosis. At PND10, serum leptin levels were elevated 2.6-4.8-fold in pups exposed to the Mediterranean diet, with a trend for sex-specific effects on body and organ weights. At 10-months, sex-specific increases in organ weight and hormone levels were observed in mice perinatally exposed to Western+BPA or Mediterranean+BPA. These findings suggest lifestage-specific interaction of perinatal exposures to experimental diets and BPA on offspring metabolic health without effects on NAFLD later in life. Importantly, alterations in dam phenotype by diet and BPA exposure appear to impact offspring health trajectory, emphasizing the need to define dam diet in assessing effects of environmental exposures on offspring health.
Keywords: Endocrine disrupting chemicals, developmental programming, NAFLD, sexual dimorphism, high-fat diet, bisphenol-A
Introduction
Non-alcoholic fatty liver disease (NAFLD) describes a range of pathologic states in the liver, beginning with simple hepatic steatosis and steatohepatitis, advancing to fibrosis, cirrhosis, and in some cases ending in hepatocellular carcinoma. NAFLD is now the leading cause of liver disease among U.S. children and adults (Giorgio et al., 2013; Loomba et al., 2009). NAFLD prevalence has increased with the rise in global obesity and insulin resistance (IR) and is likely a metabolic precursor to type 2 diabetes (T2DM) and metabolic syndrome (MetSyn) (Lonardo et al., 2015). An estimated 9% of U.S. children have NAFLD, but prevalence estimates rise to 38% among obese children aged 2-19 years (Angulo, 2007; Jeffrey B. Schwimmer et al., 2006). If detected early at the simple steatosis stage, the condition is reversible, making steatosis a prime target for public health intervention. Critical for slowing the incidence of NAFLD in children is improved understanding of early-life factors that contribute to hepatic steatosis.
Many perinatal conditions alter fetal developmental trajectory and culminate in adult metabolic pathologies. Altered maternal status during pregnancy (e.g., disease states, nutrition, environmental exposure to endocrine disrupting chemicals, EDCs) is associated with increased risk of offspring metabolic disease (Hajj et al., 2014; Padmanabhan et al., 2016; Sullivan et al., 2011). For instance, maternal pre-gestational weight and gestational weight gain are associated with increased risk of metabolic syndrome, insulin resistance, and obesity in offspring in both animal models and human studies (Aviram et al., 2011; Catalano et al., 2009; Heerwagen et al., 2010). Furthermore, perinatal overnutrition has been associated with increased hepatic steatosis in offspring (Brumbaugh and Friedman, 2014; Li et al., 2015; Wesolowski et al., 2017).
Despite the evidence suggestive of a fetal programming component to NAFLD, the role played by different hypercaloric diets in producing steatosis in offspring remains to be clarified. For instance, perinatal exposure to a Western-style high fat diet (HFD) is associated with a well-documented increase in offspring hepatic steatosis and metabolic alterations in animal models (Dahlhoff et al., 2014; Kruse et al., 2013; Pruis et al., 2014; Simino De Paula et al., 2017). In human adolescents, consumption of a Western HFD is associated with increased risk of steatosis (Mollard et al., 2014; Oddy et al., 2013). Alternatively, adherence to a Mediterranean-style HFD in human adults is associated with reversal of biopsy-confirmed NAFLD (Kontogianni et al., 2014). The potential ability of maternal consumption of a Mediterranean HFD to protect against hepatic steatosis in offspring is appealing as a low-cost, high-gain public health intervention and remains to be adequately tested in animal models.
In addition to diet, perinatal exposure to the EDC bisphenol A (BPA) has been associated with fetal programming of metabolic diseases like NAFLD (Foulds et al., 2017; Heindel et al., 2017). BPA is a high production volume, synthetic chemical found predominantly in polycarbonate plastics and epoxy resins (Environmental, n.d.); it has been shown to cross the placenta in both humans and murine models (Nahar et al., 2015, 2013; Schönfelder et al., 2002). We previously reported reduced expression of BPA-specific biotransformation enzymes (e.g. UGT2B15, SULT1A1, and STS) in human fetal tissue (Nahar et al., 2013), suggestive of impaired fetal ability to metabolize BPA, making even low-dose exposures concerning. Additionally, perinatal exposure of dams to low-dose BPA via either drinking water or subcutaneous pump is associated with greater body weight and hepatic lipid accumulation in offspring (Ke et al., 2016; Shimpi et al., 2017).
Of even greater importance, it is increasingly apparent that the impact of EDCs is subject to modulation by diet and dietary supplements (D’Angelo et al., 2019; Di Ciaula and Portincasa, 2017). For instance, simultaneous maternal BPA and high fat diet exposure are associated with exacerbated hypertension in adult male offspring (Hsu et al., 2019). Recognizing this, recent studies have begun to investigate the potential interaction between BPA and diet to delineate underlying mechanisms as well as identify beneficial dietary interventions (Kochmanski et al., 2018; Mou et al., 2018). Building upon this scientific premise, we developed a mouse model to compare the impact of co-exposure to Western and Mediterranean experimental diets on developmental effects of perinatal exposure to BPA on metabolic outcomes. Specifically, we tested the hypothesis that the metabolic impact of perinatal BPA treatment is more severe when fed to dams in combination with a Western high fat diet as opposed to when fed in combination with a Mediterranean high fat diet.
Methods
Experimental Design
Mice used for this study originated in the Dolinoy Lab from a viable yellow agouti (Avy) mouse colony. The colony had been maintained by sibling mating for over 250 generations with forced heterozygosity for the Avy allele, producing a genetically invariant background with 93% homology to C57BL/6J and 7% homology to C3H/HeJ (Waterland and Jirtle, 2003; Weinhouse et al., 2014). Mice were housed in polycarbonate-free cages with enrichment, in a climate-controlled room with a 12hr light-dark cycle, in accordance with the Institute for Laboratory Animal Research (ILAR) guidelines (Research, 2011). Mice were treated humanely and provided ad libitum access to food and water 24-hours a day, in accordance with the University of Michigan’s University Committee on Use and Care of Animals (UCUCA) policies on cage enrichment, cleaning, maintenance, and daily mouse health checks (Nowland and Lebowsky, 2016). This study protocol was approved by UCUCA.
To reduce the effect of parity and age, virgin, 8-10-week-old, wild-type (a/a) dams were randomly assigned to one of six exposure groups: Control, Control + 50 μg BPA/kg diet, Western, or Western + 50 μg BPA/kg diet, Mediterranean, or Mediterranean + 50 μg BPA/kg diet (Figure 1).
Figure 1.
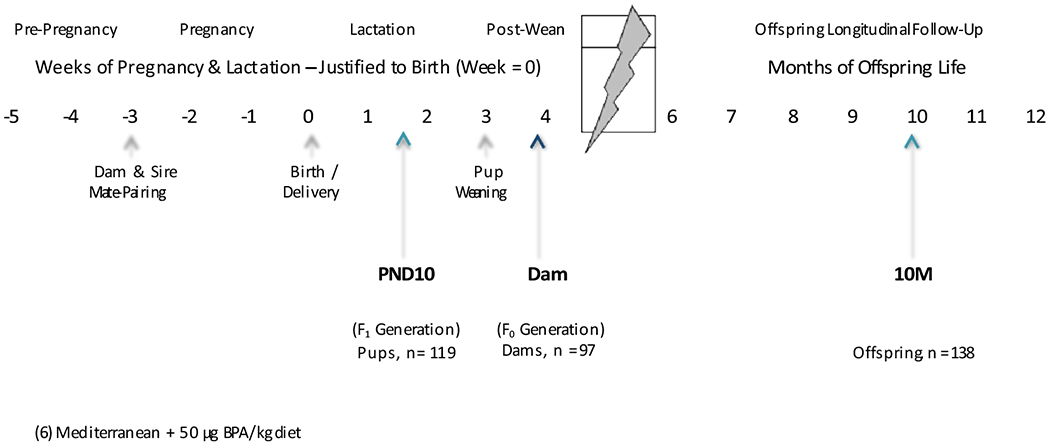
Experimental Design of Longitudinal Mouse Exposure Study
Wild type (a/a), virgin dams (8-10 weeks of age) were randomly assigned to one of six experimental diets. They were mate-paired two weeks later with young (7.5 week old), virile, Avy/a males. Dams remained on their assigned experimental diet throughout pregnancy and lactation. Offspring were exposed to the diet in utero and via mother’s milk. All offspring were weaned onto the Control diet at postnatal day 21 (PND21). Only a/a pups were followed in this study. Thus, 10M offspring were consuming the control diet for nine months at the time of sacrifice and tissue collection. Sacrifices were performed at three time points: (1) dams: 4 days post-weaning (PND25), (2) offspring: at PND10, and (3) offspring: at 10M.
After two weeks on their respective diets, N=121 virgin a/a dams (10-12 weeks of age) were mated with young, virile Avy/a males (7.5 weeks old on average). Dams remained on their assigned diets from pre-gestation through lactation such that dams were directly exposed to their assigned diet for an average of 8-9 weeks beginning two weeks before conception. Of the 121 dams who were mate-paired, only 98 dams successfully mated, as evidenced from copulatory plugs. One of the 98 dams was lost during the 4-day weaning period leaving N=97 dams. All offspring were weaned onto the Control diet at postnatal day 21 (PND21).
Mating a/a females with Avy/a males generates litters of approximately 50% a/a and 50% Avy/a pups. Mice with the Avy allele have a range of coat colors from yellow to pseudoagouti (brown), that phenotypically display differences in epigenetic marks in the Agouti gene promoter (Dolinoy, 2008; Dolinoy et al., 2010; Morgan et al., 1999); wild type a/a mice have black coats and are thus easily distinguishable by eight days of age. To avoid confounding from the obesity and metabolic abnormalities observed in the Avy/a mice (Dolinoy et al., 2007; Duhl et al., 1994; Miltenberger et al., 1997), only a/a pups were followed in this study. Offspring coat color was recorded at postnatal day 8 to determine which pups would be followed longitudinally. One male and one female a/a pup per litter were maintained for longitudinal testing to 10 months of age (10-month). All other a/a pups were sacrificed at postnatal day 10 (PND10). Offspring sacrifices were conducted in the afternoon (2-5pm) to normalize diurnal hormone fluctuations. To further standardize measurements, 10-month females were only sacrificed when in estrus, confirmed by vaginal cytology (Caligioni, 2009). Sacrificing offspring at 10 months approximates human middle age, prior to aging-related health decline (Flurkey et al., 2007).
Composition of Experimental Diets
Experimental diets were modifications of the standard AIN93G mouse diet (Table 1 and Table S1) (Reeves, 1997; Reeves et al., 1993). Like AIN93G, the Control diet contains casein as the sole protein source, but the soybean oil was replaced with corn oil to remove the potential epigenetic programming effect of phytoestrogens in the soybean oil (Dolinoy et al., 2006; Guerrero-Bosagna et al., 2008). The Mediterranean and Western diets differed from the Control diet in their lipid ratio, carbohydrate profile, vitamin and mineral content; protein content was kept constant between all three diets (Table 1, S1).
Table 1.
Composition of Experimental Diets: Mice Perinatal Exposures
| Diet Ingredients | 3 Experimental Mouse Diets |
||
|---|---|---|---|
| Control | Western | Mediterranean | |
|
| |||
| MACRONUTRIENTS | |||
|
| |||
| Calories (Kcal/g chow) | 3.98 | 4.72 | 4.53 |
| % Calories from Fat | 16 | 40 | 42 |
| PUFA : SFA : MUFA | 1 : 0.2 : 0.5 | 1 : 1.9 : 1.6 | 1 : 1.3 : 5.6 |
| Protein (casein) (g/100g chow) | 20 | 19 | 19 |
| Carbohydrate Content (g/100g chow) | |||
| Cornstarch | 40 | 14 | 23 |
| Sucrose | 10 | 25.5 | 9.2 |
| Cellulose | 5 | 2 | 8 |
|
| |||
| VITAMINS & MINERALS | |||
|
| |||
| Vitamin A (IU/kg chow) | 4000 | 4000 | 8000 |
| Vitamin C (mg/kg chow) | 0 | 0 | 500 |
| Vitamin D (IU/kg chow) | 1000 | 400 | 1000 |
| Vitamin E (IU/kg chow) | 75 | 25 | 75 |
| Folic Acid (mg/kg chow) | 2 | 1 | 4 |
| Sodium (mg/kg chow) | 1039 | 7000 | 1039 |
| Potassium (mg/kg chow) | 3600 | 3600 | 8000 |
| Magnesium (mg/kg chow) | 513 | 513 | 850 |
The Control diet is equivalent to AIN-93G, except that corn oil replaced soybean oil as the source of fat. The experimental Mediterranean and Western diets were designed to reflect the nutrient content of human dietary patterns in Crete and the U.S., respectively. Nutrient content of the mouse diets were achieved on a per weight basis.
Mouse diets were formulated at the University of Michigan and manufactured by Harlan Teklad (Madison, WI). The Mediterranean diet was based on a traditional Cretan diet which included high nut, fruit and vegetable content (Kafatos et al., 2000; Trichopoulou et al., 1993b, 1993a). The nutrient content of this diet, as given in (Kafatos et al., 2000) was achieved on a per weight basis. The Western diet was based on U.S. dietary intake as recorded in NHANES II (Block et al., 1985a, 1985b). This resulted in a Western diet composed of more saturated fats (butter and palm oil), lower fiber / higher sugar carbohydrates, higher salt, and lower antioxidant content (Table S1, Figure S1) (Block et al., 1985a, 1985b).
Bisphenol A (BPA) Exposure
The choice of 50 ug BPA/kg dose was based on a previous BPA dose range finding study, which found an intake of 50 ug BPA/kg diet produced on average 2.02 ng BPA/g liver (Anderson et al., 2012). This is within the human exposure levels measured in human fetal liver samples (range: below limit of quantification to 96.8 ng BPA/g liver) (Anderson et al., 2012). BPA was supplied by the National Toxicology Program (NTP, Durham, NC). To create a 0.1% BPA/sucrose mixture, BPA (0.01 g) was mixed into sucrose (9.99 g) in glass containers. Harlan Teklad incorporated this mixture at 0.05 g/kg into three of the six experimental diets: Control+BPA, Western+BPA, and Mediterranean+BPA.
Body and Tissue Weights
A strict protocol was followed for all sacrifices, necropsies, and both animal and tissue weighing (Nowland and Lebowsky, 2016). The liver was dissected and separated by lobe; all analyses were conducted on the left lobe. Dam body weight was recorded three times throughout the study: (1) pre-gestation, at initial exposure to the study diets, (2) at mate-pairing, two weeks later, and (3) at sacrifice, four days post-weaning. Two weight change periods were calculated: dam pre-pregnancy weight change (pre-gestation to mate-pairing), and gestational weight gain (mate-pairing to sacrifice). Organ weights were measured for dam liver and mesenteric white adipose tissue (mWAT). mWAT, a component of visceral WAT that does not include gonadal fat, was separated from abdominal organs (stomach, pancreas, spleen, intestines) prior to weighing.
Total body and liver weights were also recorded for PND10 offspring. Mice at PND10 have negligible mWAT, so no adipose weights were recorded for the pups. 10-month offspring body, liver, and mWAT weights were measured during necropsy. Relative liver and mWAT weights were calculated as a ratio of absolute organ weight / total body weight. All weights were measured on a SLF103 balance (Fisher Scientific) to the hundredths digit.
Hepatic Triglycerides (TG)
TG levels were quantified via the previously published TG extraction protocol, using the Sigma Triglyceride Determination Kit (TRO100) (Crosson et al., 2003). Samples were analyzed in triplicate (sensitivity 0.0625 mg/mL, 10.5% CV). Samples were selected for TG experiments from among the 119 PND10 and 138 10M offspring that had sufficient volume of extract remaining to complete the assay, resulting in N=103 PND 10 and N=132 10 M offspring.
Liver Histology
Two histologic staining methods were used to validate hepatic TG quantification and to independently analyze hepatic lipid accumulation and lesions in 10-month offspring liver tissue: (1) hematoxylin and eosin (H&E), and (2) OilRedO.
H&E Staining:
Liver tissue was fixed in 10% neutral-buffered formalin and transferred to the University of Michigan In-Vivo Animal Core for histologic staining and analysis. Fixed tissues were transferred to 70% ethanol, routinely processed and stained with H&E for histopathology. In livers with visible gross masses, the boundary between mass and normal-appearing tissue was included in the fixed sections, to analyze the potential invasive properties of the mass. Lipid vacuolation was the primary outcome of interest, but additional morphologic changes were observed by the veterinary pathologist including hepatocyte hypertrophy, multinucleated hepatocytes, proliferative alterations (hepatocellular adenoma, nodular hyperplasia, mixed cell, clear cell, and eosinophilic foci), and non-proliferative alterations (cell infiltrates, oval cell and Kupffer cell hyperplasia, and inflammation). Slides were read by a board-certified veterinary pathologist who was blinded to experimental groups.
OilRedO Staining:
Liver tissue was embedded in optimal cutting temperature (OCT) compound, flash frozen in liquid nitrogen, and stored at −80°C until processing. OCT-embedded tissue was sectioned at 4 microns by microtome, each liver was sectioned one section per block, per animal. The standard AbCam OilRedO kit (ab150678) protocol was followed to stain lipids on each slide.
Serum Hormone Analyses
Hormone levels were measured in blood samples collected via closed chest cardiac exsanguination, on semi-fasted mice (Hoff, 2000). At PND21, mice were fasted for four hours prior to euthanasia. At 10 months, mice were fasted for six hours prior to euthanasia. Whole blood was allowed to clot, serum separated, and stored at −80°C. On average, more than 650 μL of whole blood was collected from dams, 100 μL from PND10 pups, and 1565 μL from 10-month offspring. For dams and PND10 offspring, Millipore Mouse ELISA kits were used to quantify serum leptin (EZML-82K, sensitivity 0.05 ng/mL, 3.0% CV) and insulin (EZRMI-13K, sensitivity 0.2 ng/mL, 6.0% CV) levels. Considering the PND10 time point falls within the neonatal leptin surge - a critical developmental time point relative to programming (Bouret and Simerly, 2006) and the limited sample volume from PND10 offspring, we opted to measure leptin instead of insulin.
Serum from 10-month offspring was analyzed on a Luminex xMAP (ThermoFischer), at the MDRC Chemistry Lab. Six serum hormones were simultaneously measured via the Multiplex Mouse Adipokine Magnetic Bead Panel (MADKMAG-71K), with intra-assay CV <10% and inter-assay CV <20%. The assay limits of detection were as follows: leptin (4.2 pg/mL), insulin (13.0 pg/mL), resistin (1.1 pg/mL), IL-6 (2.3 pg/mL), PAI-1 (4.0 pg/mL), and TNFα (5.3 pg/mL). All hormone concentrations reported in this paper are the average of duplicate measures for each mouse.
Statistical Analyses
Measurements of all variables were undertaken for QC inspection and outliers were identified and not used in data analysis. A quantile plot of residuals was used to check the normality of each continuous variable, and variables with right-skewed distribution were log-transformed. The effects of two factors, 3-level diet and 2-level BPA, on the outcome of liver TG levels, serum hormones, body and organ weights were separately analyzed by two-way ANOVA, followed by the Tukey’s post-hoc analyses with the Bonferroni correction for the multiple comparisons when the effects were found to be statistically significant; this allowed comparison across all six perinatal exposure groups defined by the two factors. Statistical significance was set at p < 0.05.
Previous studies have reported sexually dimorphic metabolic responses to prenatal exposures (Anderson et al., 2013; Oben et al., 2010), so sex-stratification was invoked in the cross-sectional PND10 and 10-month offspring comparisons. Two-way ANOVAs were performed to compare all six perinatal exposure groups among dams, PND10 and 10-month offspring. To account for within litter dependence, we constructed linear mixed models (LMM) with random intercepts to examine predictors on clustered repeated measurements of hepatic TGs at PND10 and month 10, with perinatal diet and BPA exposure included as confounding variables. This allowed us to examine assess significance of main effects and interaction effect between experimental diets and BPA on repeatedly measured metabolic outcomes.
A dummy variable of ‘Cohort’ was found significantly correlated to dam and 10-month offspring variables, so it was considered in the LMMs via random intercepts. However, ‘Cohort’ did not improve effect sizes or model fit (by AIC) in PND10 models, and since no PND10 variables differed by cohort, it was removed from those models. Some PND10 mice had littermates for clustered data, so the within ‘Litter’ dependence was accounted via random effects in the LMM in the analysis of correlated PND10 data. The litter size was varying from 3-11pups in this study, and ‘Litter size’ was included in all PND10 and 10-month models. Other covariates potentially related to offspring metabolic changes were also tested for their significance in the LMMs. Final models were selected according to the largest effect size on perinatal exposure variables: experimental diets and BPA. All analyses were conducted in SAS 9.4 (Cary, NC, USA).
Results
Dam’s Metabolic Response to BPA and Experimental Diets
Dam (N=97) body weight did not differ at initial BPA exposure (Table 2A), but pre-pregnancy weight change, during the first two weeks on the experimental diets, was greater in the Western and Mediterranean diet groups versus control (Figure S2). Dams on the Control and Control+BPA diets lost weight, on average, (−0.22 to −0.55g), while the other four groups gained weight (0.15 to 0.79g), especially dams on Western, Western+BPA, and Mediterranean diets (Table 2A). This weight gain was associated with greater mean pre-gestation body weights in Western (19.63g), Western+BPA (19.42g), and Mediterranean (19.66g) dams compared to Controls (18.55g), but the differences among these 4 groups were not significant. Gestational weight gain did not differ between the six groups (Figure S3).
Table 2.
Impact of Experimental Diet Exposure on Dam Hepatic Liver Triglyceride Levels 25 Days Post-Partum (n=97).
| Experimental Diet | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| Difference in TGs | p-value | Difference in TGs | p-value | Difference in TGs | p-value | |
| Western | 15.49 | 0.0058 | 11.85 | 0.0343 | 11.24 | 0.0009 |
| Mediterranean | 19.98 | 0.0002 | 18.65 | 0.0003 | 18.02 | 0.0518 |
| BPA | 5.29 | 0.2918 | 6.37 | 0.1978 | 6.05 | 0.2279 |
| Western*BPA | −9.71 | 0.1891 | −8.76 | 0.2317 | −8.63 | 0.2410 |
| Mediterranean*BPA | −13.58 | 0.0589 | −13.73 | 0.0503 | −13.24 | 0.0634 |
Linear mixed effect models with ‘Cohort’ as a random effect were performed to assess the impact of experimental diet components and their interaction on dam hepatic TG levels, 25 days post-partum, as compared to Control dams. Effect size p-values were bolded if significant (p < 0.05) or borderline significant (p < 0.06) to highlight the experimental diet components that contribute to model prediction of hepatic TG levels.
• Model 1: includes only the experimental diet variables, no additional covariates.
• Model 2: adjusted for dam body weight, 25 days post-partum.
• Model 3: additionally adjusted for dam pre-gestational body weight change.
Relative liver weight in dams did not differ by experimental diet nor BPA exposure; on average, dam livers composed 5.0-5.5% of their overall body weight. However, greater relative mWAT (3.2-3.3%) was observed in the Western and Western+BPA dam as compared to Control dams (Table 2A).
Post-partum, semi-fasting serum insulin levels in dams on Mediterranean and Western diet trended lower (~1.5ng/mL) than Control and Control+BPA (~2.5ng/mL) serum insulin levels, but the differences were not significant. Serum leptin levels were constant in dams across all dietary exposure groups, ranging from about 4.5-5.9 ng/mL.
Use of linear mixed models (LMM) to explore the contribution of perinatal experimental diets, perinatal BPA, and the interaction of these two concurrent exposures revealed both experimental diets were associated with increased mean dam hepatic TG concentrations (Table 3).
Table 3.
Impact of Perinatal Experimental Diet Exposure on Hepatic Liver Triglyceride Levels in PND10 Offspring, Sex-Stratified
| Experimental Diet | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| % Difference TGs | p-value | % Difference TGs | p-value | % Difference TGs | p-value | |
| Female PND10 Offspring | ||||||
| Western | −1.7 | 0.6680 | −3.60 | 0.3820 | −3.53 | 0.3926 |
| Mediterranean | 6.7 | 0.1194 | 5.48 | 0.2034 | 6.14 | 0.1620 |
| BPA | 2.2 | 0.6113 | 2.31 | 0.5836 | 3.38 | 0.4377 |
| Western*BPA | 1.3 | 0.8214 | 2.71 | 0.6479 | 2.70 | 0.6489 |
| Mediterranean*BPA | −7.3 | 0.2367 | −6.47 | 0.2883 | −8.02 | 0.2048 |
| Male PND10 Offspring | ||||||
| Western | 6.80 | 0.0265 | 5.47 | 0.0586 | 5.92 | 0.0543 |
| Mediterranean | 17.75 | <0.0001 | 17.43 | <0.0001 | 18.16 | < 0.0001 |
| BPA | 4.53 | 0.1679 | 4.84 | 0.1165 | 5.29 | 0.1061 |
| Western*BPA | −6.73 | 0.1396 | −5.78 | 0.1678 | −6.19 | 0.1539 |
| Mediterranean*BPA | −12.29 | 0.0145 | −11.77 | 0.0132 | −12.73 | 0.0150 |
Linear mixed effect models were performed to assess the impact of perinatal experimental diet components and their interaction on Ln-transformed hepatic TG levels in PND10 offspring. Models were sex-stratified (female: n=48, male: n=55), and all included ‘Litter ID’ as a random effect. Effect size p-values were bolded if significant (p < 0.05) or borderline significant (p < 0.06) to highlight the experimental diet type that contribute to model prediction of hepatic TG levels.
• Model 1 adjusted for litter size.
• Model 2 additionally adjusted for dam body weight, 25 days post-partum.
• Model 3 additionally adjusted for dam pre-gestational body weight change.
On average Western and Mediterranean dams had higher TGs than Control dams (Figure 2, Table 2A). BPA exposure did not affect dam hepatic TG levels, but the Mediterranean*BPA interaction term indicated marginally lower TG levels in Mediterranean+BPA than Mediterranean diet exposed dams.
Figure 2.
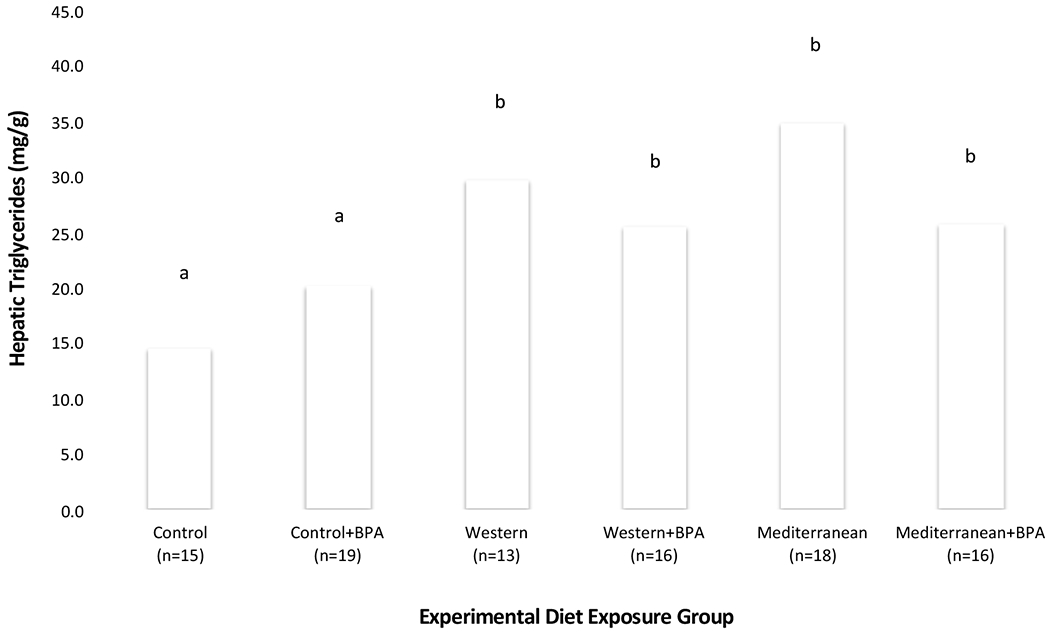
Dam Hepatic Triglyceride Levels, Four Days After Weaning Offspring (25 Days Post-Partum)
Average hepatic triglycerides (TGs) in dams (n=97), four days after weaning offspring, by experimental exposure group. a Denotes the average TGs of Control dams; groups that do not differ significantly from Control are also marked with ‘a’. b Denotes exposure groups with average TGs that differ significantly (p < 0.05) from Control.
Every one-gram increase in dam body weight at sacrifice was associated with 1.43 mg/g (p = 0.0116) greater hepatic TGs. Other potential covariates did not contribute significantly to the model including dam gestational weight gain, dam serum leptin, or insulin levels.
Offspring Response to BPA and Experimental Diets
PND10 Offspring
Metabolic Response to Experimental Diets and BPA:
PND10 offspring (n = 118) were exposed to experimental diets and experimental diets + BPA via maternal in utero transfer for at least three weeks and via mother’s milk for 10 days. The experimental diets did not impact body weight or relative liver weight in PND10 offspring. Body weight only differed by expose group in males with lower average weight in Control+BPA males compared to Control, while the addition of BPA to the Mediterranean and Western diets did not result in decreased body weight (Table 2B). Body weight did not differ among PND10 females based on perinatal exposure group.
Serum leptin levels were consistently highest among both male and female PND10 offspring exposed to the Mediterranean diet. Both males and females had higher serum leptin levels versus Controls (Table 2B). Serum leptin levels were not higher versus control with a Western diet or BPA exposure in male or female PND10 offspring.
Hepatic TG concentrations were highest among Mediterranean PND10 offspring of both sexes. Mediterranean males had 7.3-fold greater hepatic TG concentrations compared to Control males (p < 0.0001) (Table 2B). Although not significant, hepatic TGs were 1.6-fold higher among Mediterranean females compared to Controls. As observed for serum leptin levels, variability in hepatic TG levels was largest among Mediterranean pups of both sexes at PND10 (Figure 3A & B). Neither perinatal Western diet nor BPA exposure altered hepatic TG levels in either sex.
Figure 3.
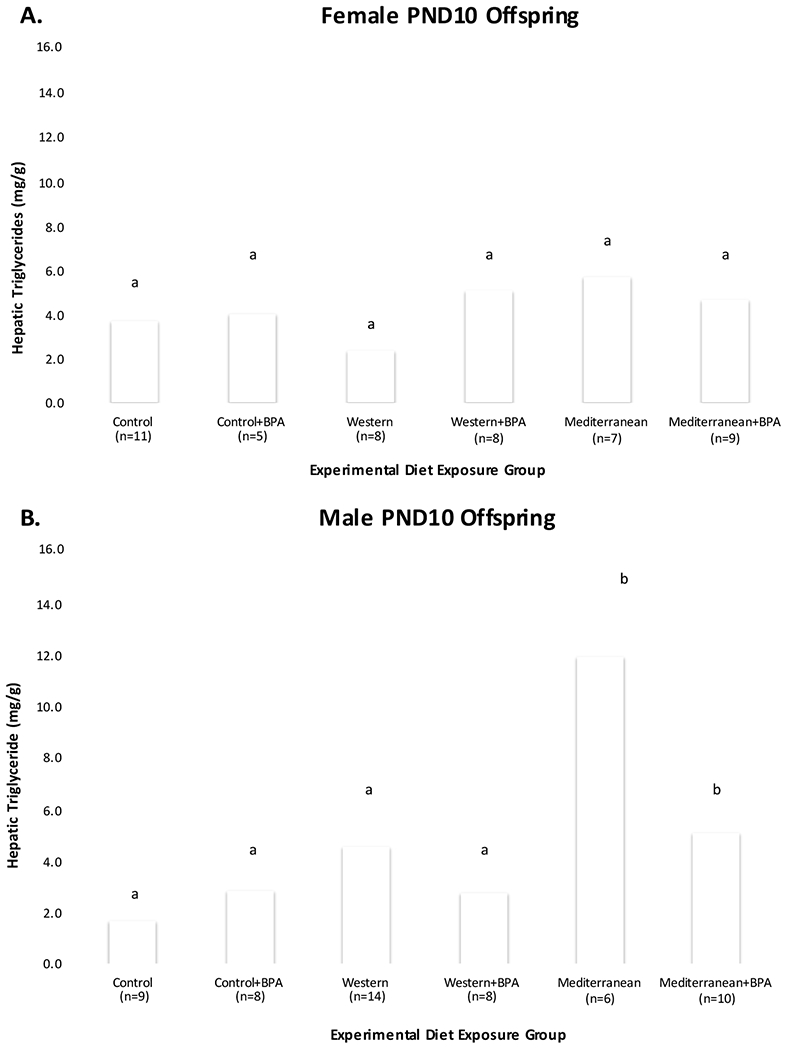
PND10 Offspring Hepatic Triglyceride Levels by Exposure Group
Average hepatic triglycerides (TGs) in PND10 offspring (n = 103), by experimental exposure group: Panel A = female PND10 offspring (n = 48), Panel B = male PND10 offspring (n = 55). aDenotes the average TGs of Control PND10 offspring; groups that do not differ significantly from Control are also marked with ‘a’. b Denotes exposure groups with average TGs that differ significantly (p < 0.05) from Control.
Sex-stratified LMMs predicting Ln-transformed hepatic TGs in PND10 offspring included litter size as a covariate (Table 4). Western PND10 males had 5.9% (p=0.0543) and Mediterranean males had 18.2% (p < 0.0001) greater TGs than Control males. Perinatal BPA exposure did not affect male PND10 hepatic TG levels, but the Mediterranean*BPA interaction term decreased TG levels by 12.7% (p = 0.0150) compared to Mediterranean males. PND10 male hepatic TGs increased by 4.8% (p=0.0761) for every one-gram increase in dam body weight at sacrifice but decreased by 4.6% (n.s.) for every one-gram change in pre-gestational weight. Neither perinatal experimental diets, BPA, nor their interaction terms significantly contributed to hepatic TG levels in female PND10 offspring. Other potential covariates did not contribute significantly to the models.
Table 4.
Correlation of Three Hepatic Steatosis Measures in 10-Month Offspring
| Pearson’s Correlation | Hepatic TGs (mg/g) | OilRedO (score) | Vacuolation (score) | |
|---|---|---|---|---|
| Female 10-month Offspring | ||||
| Hepatic TGs (mg/g) | Coefficient | 1.000 | 0.4130 | 0.6189 |
| OilRedO (score) | Coefficient | 0.4130 | 1.000 | 0.6453 |
| Vacuolation (score) | Coefficient | 0.6189 | 0.6453 | 1.000 |
| Male 10-month Offspring | ||||
| Hepatic TGs (mg/g) | Coefficient | 1.000 | 0.2758 | 0.4163 |
| OilRedO (score) | Coefficient | 0.2758 | 1.000 | 0.5826 |
| Vacuolation (score) | Coefficient | 0.4163 | 0.5826 | 1.000 |
10-Month Offspring
Metabolic Response to Experimental Diets and BPA:
By 10-months of age, offspring (n=138) had not been exposed to the experimental diets nor BPA for more than nine months since weaning. Body weight at 10-months did not differ by perinatal diet or BPA exposure. As expected, male offspring (44.87g) weighed more than females (34.63g) (Table 2C). Relative liver weight did not differ by perinatal HFD or BPA exposure in 10-month males. However, males perinatally exposed to the Western+BPA diet had greater relative mWAT compared to Control males (Figure S3). Females perinatally exposed to Western, Western+BPA, and Mediterranean diets had greater relative liver weights compared to Control females (Figure S4). Relative mWAT weight, however, did not differ by perinatal exposure in 10-month females.
Perinatal diet impacted serum leptin concentrations among 10-month males. Western+BPA serum leptin was 1.6-fold higher and Mediterranean was 1.5-fold higher than Control concentrations (Table 2C). Western+BPA males also had 1.6-fold higher serum resistin concentrations compared to Control males. Serum insulin and PAI-1 levels did not differ by perinatal exposure group among 10-month males. However, in 10-month females, serum PAI-1 levels were 1.6-fold greater in Mediterranean+BPA than Controls. No other serum hormones differed by perinatal exposure among female offspring at 10-months.
Hepatic TGs did not differ by perinatal exposure group among male offspring at 10-months. Hepatic TGs were 2.4-fold higher in females perinatally exposed to the Mediterranean+BPA diet compared to Control females (p = 0.0118) (Table 2C).
Comparison of Hepatic Steatosis Measures in 10-month Offspring
H&E vacuolation and OilRedO staining were highly correlated to hepatic TG levels, in 10-month offspring of both sexes (Table 4).
Hepatic steatosis ranged from negligible to severe (>50% of tissue affected) in liver samples from both male and female 10-month offspring (Figure 4).
Figure 4.
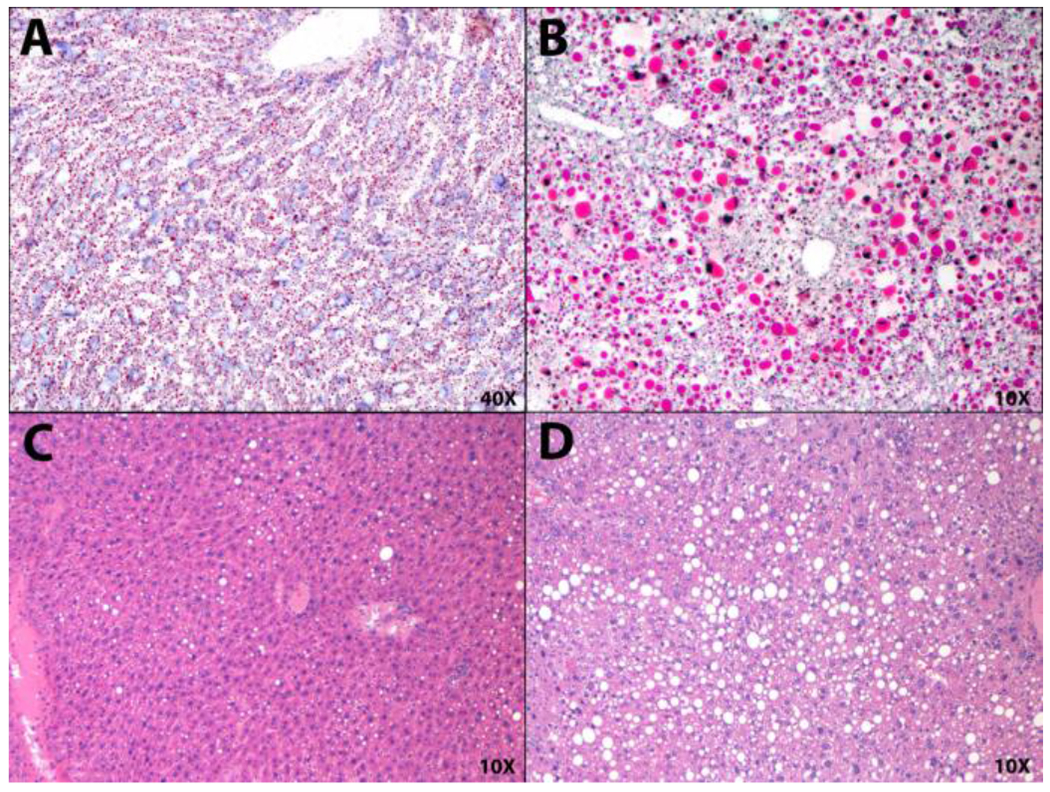
Histopathologic Measures of Hepatic Steatosis at 10-Months
Hepatic steatosis ranged from negligible to severe (>50% of tissue affected) in liver samples from the 10-month offspring. (A) OilRed0 staining, score 0: no visible lipid accumulation, (B) OilRedO staining, score 4: severe accumulation, (C) H&E staining, score 1: minimal (<9% tissue affected), (D) H&E staining, score 4: severe.
Hepatocellular response to perinatal exposures was sexually dimorphic, with multiple morphologic lesions differing among experimental diets (Table S2). Hepatic adenomas were observed in 11 male mice, spread across five of the experimental exposure groups, including Control (Table S2). No adenomas were found in any female mice, but proliferative lesions differed by perinatal exposure groups. Proliferative lesions were only observed in Control+BPA and Mediterranean+BPA females. Non-proliferative lesions also differed by perinatal exposure group in 10-month females; Control females had more non-proliferative responses than Western, Western+BPA, and Mediterranean (p < 0.02).
Liver Triglycerides
Similar to PND10 models, sex-stratified linear mixed models predicting Ln-transformed hepatic TGs in 10-month offspring included litter size as a covariate (Table 6). Perinatal exposure to neither experimental diet nor BPA alone significantly contributed to female 10-month offspring hepatic TG levels.
However, the Mediterranean*BPA interaction term had 15.2% (p = 0.0309) higher TG levels than Mediterranean females. In females, there was a 24.6% (p = 0.0547) increase in hepatic TGs for every 1% increase in 10-month female relative mWAT weight. Although it did not contribute significantly to the model (p = 0.4143), presence of hepatic nodular hyperplasia improved model fit for 10-month female hepatic TGs. In 10-month males, neither perinatal diet nor BPA were significant predictors of Ln-transformed hepatic TGs. However, the best-fit model included the same covariates as the 10-month female model: 10-month relative mWAT (25.4% increase, p = 0.1418) and nodular hyperplasia (6.3% increase, p = 0.0265). No other factors measured in 10-month offspring, or their mothers (dams) were predictive of hepatic TGs in 10-month offspring of either sex.
Discussion
The incidence of NAFLD is increasing as Western diets are being increasingly adopted across the population; this is especially worrisome in children. This study reports on the effects of two different kinds of prenatal high fat diet exposures, alone and in combination with BPA exposure, on metabolic variables and measures of lipid accumulation in the liver of dams and offspring. The findings from this study show that, in dams, consumption of the Western or Mediterranean high fat diets increased hepatic TGs, independent of BPA intake while in offspring, perinatal high fat diet and BPA exposures had a greater impact on weight-related outcomes than on hepatic steatosis. Specifically, at PND10, serum leptin levels were elevated in pups exposed to the Mediterranean diet, with a trend for sex-specific effects on body and organ weights. Similarly, at 10-months sex-specific increases in organ weight and hormone levels and a trend for increase in body weight were observed mainly in mice perinatally exposed to Western+BPA or Mediterranean+BPA groups. Exposure to BPA alone displayed few significant effects on metabolic or hepatic outcomes in either dams or offspring as compared with either prenatal HFD and HFD+BPA exposures. These findings suggest that maternal diet has a greater impact on offspring health trajectory than BPA with both exposures having sexually dimorphic effects on offspring metabolic outcomes more so than on hepatic steatosis.
Dam Metabolic & Hepatic Outcomes
While many studies use perinatal HFD exposure to investigate effects on offspring health (Williams et al., 2014), their impact on maternal health and physiology have largely been ignored. Findings from this study provide evidence that dams that consumed the Western or Mediterranean experimental diets had increased hepatic TGs compared to Control dams with exposure to BPA having no additional impact. The timing of observed hepatic steatosis, 8-9 weeks after dietary exposure, aligns with previously reported time-to-develop-steatosis in non-pregnant adult mice (Fengler et al., 2016; Lohr et al., 2016; Waller-Evans et al., 2013).
In another dimension, dams exposed to the Western, Western+BPA, or Mediterranean diets had greater pre-gestational weight gain and relative mWAT weight. This increase in mWAT weight, without increases in relative liver weight, suggests that increased adiposity contributed to the greater pre-gestational weight gain (Lohr et al., 2016). Gestational weight gain is associated with an increased risk of overweight in children 2-18 years in many human birth cohorts (Lau et al., 2014). However, a large prospective cohort study in the Netherlands reported that maternal pre-gestational obesity increased the odds of childhood obesity, but excessive gestational weight gain had a more limited impact on children’s outcomes (Gaillard et al., 2013). This distinction may explain the lack of effect the differential pre-gestational weight gain had on mouse offspring as none of the dams were obese prior to mate-pairing. The idea that a threshold of dam weight measures may need to be reached to differentially impact offspring health trajectory supports the need to better characterize the maternal environment and metabolism when studying the potential perinatal programming of offspring metabolic disease.
PND10 Hepatic and Metabolic Outcomes
Diet continued to be a persistent factor in offspring hepatic and metabolic outcomes. The impact of diet at PND10 was coupled with a variety of sexually dimorphic responses. For example, perinatal exposure to experimental diets impacted hepatic TGs only in male PND10 offspring. This sexually dimorphic response has been previously observed in mice and rats (Burgueño et al., 2013; Strakovsky et al., 2014), with male offspring consistently more likely to develop hepatic steatosis following perinatal HFD exposure than females. This sex-specific difference in rates of hepatic steatosis may also translate to human populations. Among youth (2-19 years) accidental deaths in San Diego County, males were also more likely to have hepatic steatosis (15%) than females (9%) (Jeffrey B Schwimmer et al., 2006).
Metabolic outcomes at PND10 were most pronounced in offspring perinatally exposed to the Mediterranean diet. The Western diet surprisingly did not elevate leptin in the offspring, despite research demonstrating that maternal HFD during pregnancy was associated with increased serum leptin in 12-week old offspring (Griffiths et al., 2016). The difference in offspring age, however, may account for the difference in effects observed between the two studies. Specifically, the serum leptin levels in our PND10 offspring likely reflected variations in leptin from their mother’s milk. Breast milk contains leptin and is thought to contribute to regulation of food intake in offspring prior to weaning (Casabiell et al., 1997; Nozhenko et al., 2015; Sánchez et al., 2005), whereas 12-week old offspring endogenously produce leptin. Among adults, elevated leptin may be indicative of leptin resistance, which is often observed in metabolic conditions like obesity, MetSyn, or T2DM (Myers et al., 2008). However, the postnatal leptin surge that occurs in the first two weeks of life in rodents is necessary for establishing hypothalamic pathways that will control food intake (Bouret, 2004) and for the maturation of other organs involved in energy homeostasis, including the kidney, pancreas, ovary, and thymus (Attig et al., 2011). Thus, elevated leptin levels in PND10 offspring exposed to the Mediterranean diet likely have a greater impact on organ development than on lifelong metabolic health.
10-month Hepatic and Metabolic Outcomes
Surprisingly, the effect of diet on hepatic outcomes in 10-month-old offspring was fairly modest. While we observed hepatic steatosis among these offspring, it did not differ strongly by perinatal exposure group. C57BL/6J mice are known to develop hepatic cysts and adenomas as they age (Pettan-Brewer and M. Treuting, 2011; Snyder et al., 2016). This age-related pathology has been previously reported in our viable yellow agouti colony, with 10.5% of non-agouti, a/a 10-month male Controls displaying neoplastic lesions and over 50% of 10-month females and males exhibiting simple steatosis (Weinhouse et al., 2014). This evidence combined with our data suggest our observations may have been simply due to normal, age-related steatosis.
Strikingly, histopathologic evaluation of hepatic tissue found Control offspring had more non-proliferative changes than offspring perinatally exposed to the experimental diets. This may suggest Control mice retained the ability to regenerate and repair injured tissue at 10-months compared to other groups. Although age-related damage may be occurring, the liver may be able to better respond to environmental stressors and return to a healthier state when fed a low-fat diet, whereas livers perinatally exposed to high fat diets may not be able to repair as easily, and thus amass more tissue damage.
Perinatal exposure to diet+BPA combinations had the greatest effect on 10-month offspring metabolic outcomes, differing from the effects observed on both dams and PND10 offspring. However, we did observe sexually dimorphic responses. In 10-month female offspring we observed increased liver weights; a finding that is consistent with previous studies demonstrating that HFD consumption is associated with increased relative liver weight in adult animals (Milagro et al., 2006). Given that the 10-month offspring were not exposed to the experimental diets since weaning, these results suggest that perinatal HFD exposure alters the developmental trajectory of liver weight across the life course. Interestingly, the increased liver weight did not translate to greater hepatic TGs. The borderline increase in body weight and significant increase in relative mWAT weight among Western+BPA males at 10-months suggests that these males accumulated more visceral adipose across their life, due to the combined exposure in early life. These Western+BPA males also had the highest serum leptin and resistin levels and the lowest serum PAI-1, suggesting that the greater mWAT mass was metabolically active. These results point to a greater theme: that the impact of various perinatal exposures on offspring health trajectory differ as a function of age.
Multifaceted Effects of the Experimental Diets & BPA
Effects of the six perinatal exposures were much more complex than anticipated in our original hypotheses. The Western diet had the expected, adipogenic effect (Dahlhoff et al., 2014; Kruse et al., 2013; Pruis et al., 2014) in dams but barely impacted offspring. Conversely, we expected our Mediterranean diet would protect against metabolic and hepatic alterations, but in dams it was generally indistinguishable from the adipogenic effect of the Western diet and was the only diet to increase PND10 hepatic TGs. Individual components of the Mediterranean diet (fish oil, olive oil, and polyphenols) have been investigated, but a complete Mediterranean diet has never been created for mice. A previous comparison of two purified perinatal diets found that maternal diets high in saturated fat promoted offspring hyperphagia, but equivalent consumption of a fish oil diet did not (Nakashima, 2008). Further, a recent study in rats reported that adding fish oil to a HFD for dams during pregnancy prevented insulin resistance in adult male offspring, independent of body weight (Albert et al., 2017). In adult mice predisposed to hepatic steatosis, daily 2% DHA/EPA supplementation reduced hepatic TGs by almost 40% (Xu et al., 2015), further supporting the health benefits of a fish-oil enriched diet.
On the other hand, consumption of olive oil, the primary fat component in the Mediterranean diet, is associated with increased steatosis in mice (Arbones-Mainar et al., 2007; Ferramosca, 2014). In mice, olive oil decreases activity of carnitine palmitoyl transferase I (CPT1), the rate limiting step for mitochondrial fatty acid β-oxidation. This depression of CPT1 activity does not occur in humans consuming olive oil, thus may partially explain the differential outcome of the diet between the two species. In human studies, Mediterranean diet is associated with reversal of biopsy-confirmed NAFLD in adults, significantly reducing hepatic steatosis and improving insulin sensitivity, even without weight loss (Baratta et al., 2017; Ryan et al., 2013). A proteomics analysis reported that tissue oxidation and atherosclerotic plaque formation was delayed in mice consuming olive oil, despite increased hepatic steatosis and insulin resistance; the authors attributed this paradoxical response to the differential regulation of 80 hepatic antioxidant enzymes (Arbones-Mainar et al., 2007). Thus, the hepatic steatosis observed in mice consuming olive oil may be protective, as it does not appear to induce the detrimental metabolic alterations. This protective antioxidant effect may also explain why mice perinatally exposed to Mediterranean diet in this study did not exhibit altered hepatic or metabolic responses at 10-months.
Our findings support the previously reported detrimental effect of perinatal BPA exposure on reproductive outcomes (Cabaton et al., 2011; Ferguson et al., 2011; Peretz et al., 2014), but suggest that maternal diet may have a greater impact on offspring health trajectory than BPA exposure. This is encouraging from a public health standpoint, because diet is a more readily modified as compared to the exposures stemming from food and beverage packaging. The evidence suggests, however, that perinatal BPA exposure may not be sufficient to alter offspring health trajectory, and an additional postnatal stressor or dietary challenge may be required. The theory of developmental “priming” by perinatal EDC exposure proposes that exposure-induced changes in gene methylation may prime the loci for increased transcription in response to a later challenge (Walker, 2016). Two mouse studies that examined perinatal BPA exposure followed by a postnatal HFD challenge reported greater hepatic TGs in offspring exposed to both the BPA and HFD (Strakovsky et al., 2015; Wei et al., 2014), supportive of BPA’s potential priming effect. Thus, perinatal low-dose BPA exposure may increase offspring susceptibility to hepatic steatosis, but postnatal diet may be required to trigger the pathologic response.
In contrast to this, perinatal BPA exposure was protective when combined with maternal over-nutrition in a sheep model (Koneva et al., 2017a; Mohankumar et al., 2017). We recently reported that prenatal exposure to BPA protected overfed sheep offspring from the increased blood pressure and greater left ventricle area observed in overfed offspring not exposed to BPA (Mohankumar et al., 2017). Gene network analysis identified a reversal of overfeeding effects on gene expression, by prenatal BPA exposure, at FABP4, A2M, APOD, HLA-C (Koneva et al., 2017b), suggestive of greater fatty acid uptake in adipocytes, more cytokine transport, and greater circulating high-density lipoproteins. Multiple animal models now suggest a diet*BPA interaction, so future perinatal BPA exposure studies should include multiple background diets to elucidate this interaction. Human populations are simultaneously exposed to BPA and a variety of diets; improved understanding this interaction may be critical for interpretation of epidemiologic data on BPA exposure.
Challenges & Limitations
Assessing the tissue-level impact of perinatal exposures across a lifetime in the same animal can be challenging. In order to assess developmental progression of hepatic steatosis in the offspring at two time points (PND10 and 10-months), an invasive biopsy procedure would have been required at PND10. Although liver tissue regenerates (Michalopoulos, 2010), to avoid metabolic and mental stress that surgery induces, we analyzed sex-matched littermates at PND10 and 10-months. Considering our investigation was limited to two time points, subtle changes that could have occurred in between or any compensatory effort to overcome the deficit at PND10 would have been missed. Furthermore, limitations in sample volume prevented our evaluation of more expansive measures of NAFLD like alanine transaminase and aspartate aminotransferase in plasma. While our studies have addressed the functional consequences of perinatal diet and BPA exposure of relevance to NAFLD and metabolic disorders, they do not address underlying causal mechanisms. Nonetheless, these studies establish a unique lifespan model system beginning with preconception exposure to identify molecular and epigenetic targets for developing intervention strategies.
Conclusion
This is the first study examining the interaction of BPA and diet in perinatal programming of NAFLD beginning from the preconceptual period. The differential response of dams, PND10, and 10-month offspring to the six experimental emphasizes the need to study the impact of developmental exposures at different ages and life stages of offspring. The hepatic and metabolic effects of these perinatal diets differed not only by mouse age, but also by offspring sex and diet composition. The substantial impact of experimental diets on maternal phenotype and the subsequent effect of maternal phenotype on PND10 offspring hepatic TGs, suggests that detailed characterization of maternal factors would improve our understanding of developmental programming. To build on these insights, future studies should consider beginning perinatal exposures prior to gestation, continuing to explore diet*EDC interactions, including more detailed maternal phenotyping measures, increasing the number of offspring evaluations, and incorporating molecular analyses with phenotypic outcomes.
Supplementary Material
Table 5.
Impact of Perinatal Experimental Diet Exposure on Hepatic Liver Triglyceride Levels in 10-month Offspring, Sex-Stratified
| Experimental Diet | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| % Difference TGs | p-value | % Difference TGs | p-value | % Difference TGs | p-value | |
| Female 10-month Offspring | ||||||
| Western | 3.21 | 0.5067 | 4.08 | 0.3914 | 4.22 | 0.3765 |
| Mediterranean | 0.69 | 0.8881 | 0.64 | 0.8944 | 0.59 | 0.9022 |
| BPA | −4.13 | 0.4263 | −3.66 | 0.4716 | −2.55 | 0.6281 |
| Western*BPA | 2.36 | 0.7378 | 1.74 | 0.8021 | 5.85 | 0.9341 |
| Mediterranean*BPA | 15.28 | 0.0318 | 14.71 | 0.0355 | 15.21 | 0.0309 |
| Male 10-month Offspring | ||||||
| Western | −6.70 | 0.1430 | −6.86 | 0.1344 | −5.00 | 0.2639 |
| Mediterranean | −1.99 | 0.6466 | −2.09 | 0.6318 | −1.27 | 0.7635 |
| BPA | 2.39 | 0.6000 | 2.13 | 0.6415 | 2.32 | 0.5993 |
| Western*BPA | 1.95 | 0.7649 | 1.07 | 0.8703 | 1.08 | 0.8644 |
| Mediterranean*BPA | −5.00 | 0.4396 | −4.75 | 0.4632 | −3.40 | 0.5878 |
Linear mixed effect models were run to assess the impact of perinatal experimental diet components and their interaction on Ln-transformed hepatic TG levels in 10-month offspring. Models were sex-stratified (female n=65, male: n=67) and all included ‘Cohort’ as a random effect. Effect size p-values were bolded if significant (p < 0.05) or borderline significant (p < 0.06) to highlight the experimental diet components that contribute to model prediction of hepatic TG levels.
• Model 1 adjusted for litter size.
• Model 2 additionally adjusted for relative mWAT in 10-month offspring.
• Model 3 additionally adjusted for the presence of nodular hyperplasia in 10-month offspring.
Highlights:
Maternal high fat diet (HFD) influenced offspring health trajectory.
HFD & BPA exposure: greater impact on metabolic outcomes than on hepatic steatosis.
Prenatal exposure to HFD and BPA displayed sexually dimorphic effects in offspring.
ACKNOWLEDGEMENTS
We are grateful to the following individuals for providing their expert opinion and talents to make this study possible. Ms. Jessica Flowers at Harlan Teklad (now Envigo, Indianapolis, IN) for assistance designing the humanized experimental diets. Mr. Jason Whalen at the Michigan Diabetes Research Center (MDRC) Chemistry Lab for multiplexed hormone analysis on 10-month offspring serum samples.
FUNDING INFORMATION
This work was supported by the University of Michigan (UM) NIEHS/EPA Children’s Environmental Health and Disease Prevention Center P01 ES022844/RD83543601, NIEHS R35 ES031686, the Michigan Lifestage Environmental Exposures and Disease (M-LEEaD) NIEHS Core Center (P30 ES017885), the Rogel Cancer Center (P30 CA046592), and the MDRC Chemistry Lab (P30 DK020572), as well as UM Institutional and Individual Training Grants T32 ES007062 (EHM), T32 HD079342 (EHM), and F31 ES025101 (EHM).
Abbreviations:
- NAFLD
non-alcoholic fatty liver disease
- BPA
bisphenol-A
- HFD
high-fat diet
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Credit Author Statement
Elizabeth Marchlewicz*: Conceptualization, methodology, investigation, writing—original draft; Carolyn McCabe*: conceptualization, writing—original draft, writing—review & editing; Zora Djuric: methodology, writing—review & editing; Mark Hoenerhoff: methodology, resources; John Barks J: conceptualization, writing—review & editing; Lu Tang: data curation, formal analysis, Peter Song: methodology, data curation, formal analysis, writing—review & editing; Karen Peterson: methodology, writing—review & editing; Vasantha Padmanabhan: conceptualization, methodology, writing—original draft; writing—review & editing, Dana Dolinoy: conceptualization, supervision, methodology, resources, project administration, funding acquisition
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
REFERENCES
- Albert BB, Vickers MH, Gray C, Reynolds CM, Segovia SA, Derraik JGB, Garg ML, Cameron-Smith D, Hofman PL, Cutfield WS, 2017. Fish oil supplementation to rats fed high-fat diet during pregnancy prevents development of impaired insulin sensitivity in male adult offspring. Sci. Rep 7, 1–11. 10.1038/s41598-017-05793-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson OS, Nahar MS, Faulk C, Jones TR, Liao C, Kannan K, Weinhouse C, Rozek LS, Dolinoy DC, 2012. Epigenetic Responses Following Maternal Dietary Exposure to Physiologically Relevant Levels of Bisphenol A. Environ. Mol. Mutagen 53, 334–342. 10.1002/em.21692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson OS, Peterson KE, Sanchez BN, Zhang Z, Mancuso P, Dolinoy DC, 2013. Perinatal bisphenol a exposure promotes hyperactivity, lean body composition, and hormonal responses across the murine life course. FASEB J. 27, 1784–1792. 10.1096/fj.12-223545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angulo P, 2007. Obesity and Nonalcoholic Fatty Liver Disease. Nutr. Rev 65, 57–63. 10.1301/nr.2007.jun.S57 [DOI] [PubMed] [Google Scholar]
- Arbones-Mainar JM, Ross K, Rucklidge GJ, Reid M, Duncan G, Arthur JR, Horgan GW, Navarro MA, Carnicer R, Arnal C, Osada J, Roos B. De, 2007. Extra Virgin Olive Oils Increase Hepatic Fat Accumulation and Hepatic Antioxidant Protein Levels in APOE − / − Mice research articles. J. Proteome Res 6, 4041–4054. [DOI] [PubMed] [Google Scholar]
- Attig L, Larcher T, Gertler A, Abdennebi-Najar L, Djiane J, 2011. Postnatal leptin is necessary for maturation of numerous organs in newborn rats. Organogenesis 7, 88–94. 10.4161/org.7.2.14871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviram A, Hod M, Yogev Y, 2011. Maternal obesity: Implications for pregnancy outcome and long-term risks -- a link to maternal nutrition. Int J Gynecol Obs. 115, S6–S10. 10.1016/S0020-7292(11)60004-0 [DOI] [PubMed] [Google Scholar]
- Baratta F, Pastori D, Polimeni L, Bucci T, Ceci F, Calabrese C, Ernesti I, Pannitteri G, Violi F, Angelico F, Del Ben M, 2017. Adherence to Mediterranean Diet and Non-Alcoholic Fatty Liver Disease: Effect on Insulin Resistance. Am. J. Gastroenterol 112, 1832–1839. 10.1038/ajg.2017.371 [DOI] [PubMed] [Google Scholar]
- Block G, Dresser CM, Hartman AM, Carroll MD, 1985a. Nutrient sources in the American diet: quantitative data from the NHANES II survey. Vitamins and minerals. Am. J. Epidemiol 122, 13–26. [DOI] [PubMed] [Google Scholar]
- Block G, Dresser CM, Hartman AM, Carroll MD, 1985b. Nutrient Sources in the American Diet: Quantitative Data From the Nhanes Ii Survey: Macronutrients and Fats. Am. J. Epidemiol 122, 27–40. [DOI] [PubMed] [Google Scholar]
- Boney CM, Verma A, Tucker R, Vohr BR, 2005. Metabolic Syndrome in Childhood: Association With Birth Weight, Maternal Obesity, and Gestational Diabetes Mellitus. Pediatrics 115, e290–e296. 10.1542/peds.2004-1808 [DOI] [PubMed] [Google Scholar]
- Bouret S, 2004. Minireview: Leptin and development of hypothalamic feeding circuits. Endocrinology 145, 2621–2626. [DOI] [PubMed] [Google Scholar]
- Bouret SG, Simerly RB, 2006. Developmental programming of hypothalamic feeding circuits. Clin. Genet 70, 295–301. 10.1111/j.1399-0004.2006.00684.x [DOI] [PubMed] [Google Scholar]
- Brumbaugh DE, Friedman JE, 2014. Developmental origins of nonalcoholic fatty liver disease. Pediatr. Res 75, 140–147. 10.1038/pr.2013.193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgueño AL, Cabrerizo R, Gonzales Mansilla N, Sookoian S, Pirola CJ, 2013. Maternal high-fat intake during pregnancy programs metabolic-syndrome-related phenotypes through liver mitochondrial DNA copy number and transcriptional activity of liver PPARGC1A. J. Nutr. Biochem 24, 6–13. 10.1016/jjnutbio.2011.12.008 [DOI] [PubMed] [Google Scholar]
- Cabaton NJ, Wadia PR, Rubin BS, Zalko D, Schaeberle CM, Askenase MH, Gadbois JL, Tharp AP, Whitt GS, Sonnenschein C, Soto AM, 2011. Perinatal exposure to environmentally relevant levels of bisphenol A decreases fertility and fecundity in CD-1 mice. Environ. Health Perspect 119, 547–552. 10.1289/ehp.1002559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caligioni C, 2009. Assessing Reproductive Status/Stages in Mice. Curr Protoc Neurosci Appendix 4, 1–11. 10.1002/0471142301.nsa04is48.Assessing [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casabiell X, Pineiro V, Tome MA, Peino R, Dieguez C, Casanueva FF, 1997. Presence of leptin in colostrum and/or breast milk from lactating mothers: a potential role in the regulation of neonatal food intake. J. Clin. Endocrinol. Metab 82, 4270–4273. [DOI] [PubMed] [Google Scholar]
- Catalano PM, Farrell K, Thomas A, Huston-Presley L, Mencin P, Mouzon S.H. De, Amini SB, 2009. Perinatal risk factors for childhood obesity and metabolic dysregulation. Am. J. Clin. Nutr 90, 1303–12. 10.3945/ajcn.2008.27416.l [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosson SM, Khan A, Printen J, Pessin JE, Saltiel AR, 2003. PTG gene deletion causes impaired glycogen synthesis and developmental insulin resistance. J. Clin. Invest 111, 1423–1432. 10.1172/JCI200317975.Introduction [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Angelo S, Scafuro M, Meccariello R, 2019. BPA and Nutraceuticals, Simultaneous Effects on Endocrine Functions. Endocrine, Metab. Immune Disord. - Drug Targets 19, 594–604. 10.2174/1871530319666190101120119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlhoff M, P S, Blutke A, Rozman J, Klingenspor M, Deutsch MJ, Rathkolb B, Fink B, Gimp M, Hrab M, Angelis D, Roscher AA, Wolf E, Ensenauer R, 2014. Peri-conceptional obesogenic exposure induces sex-specific programming of disease susceptibilities in adult mouse offspring. Biochim. Biophys. Acta 1842, 304–317. 10.1016/j.bbadis.2013.ll.021 [DOI] [PubMed] [Google Scholar]
- Di Ciaula A, Portincasa P, 2017. Diet and Contaminants: Driving the Rise to Obesity Epidemics? Curr. Med. Chem 26, 3471–3482. 10.2174/0929867324666170518095736 [DOI] [PubMed] [Google Scholar]
- Dolinoy DC, 2008. The agouti mouse model: an epigenetic biosensor for nutritional and environmental alterations on the fetal epigenome. Nutr. Rev 66, S7–S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolinoy DC, Huang D, Jirtle RL, 2007. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc. Natl. Acad. Sci 104, 13056–13061. 10.1073/pnas.0703739104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolinoy DC, Weidman JR, Waterland RA, Jirtle RL, 2006. Maternal genistein alters coat color and protects Avy mouse offspring from obesity by modifying the fetal epigenome. Environ. Health Perspect 114, 567–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolinoy DC, Weinhouse C, Jones TR, Rozek LS, Jirtle RL, 2010. Variable histone modifications at A(vy) metastable epiallele. Epigenetics 5, 637–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duhl D, Vrieling H, Miller K, Wolff G, Barsh G, 1994. Neomorphic agouti mutations in obese yellow mice. Nat. Genet 8, 59–65. [DOI] [PubMed] [Google Scholar]
- Environmental PA, n.d. Risk Management for Bisphenol A (BPA) [WWW Document]. Assess. Manag. Chem. under TSCA. [Google Scholar]
- Fengler VHI, Macheiner T, Kessler SM, Czepukojc B, Gemperlein K, Muller R, Keimer AK, Magnes C, Haybaeck J, Lackner C, Sargsyan K, 2016. Susceptibility of Different Mouse Wild Type Strains to Develop Diet-Induced NAFLD / AFLD-Associated Liver Disease. PLoS One 11, 1–21. 10.1371/journal.pone.0155163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson S, Law C, Abshire J, 2011. Developmental treatment with bisphenol A or ethinyl estradiol causes few alterations on early preweaning measures. Toxicol. Sci 124, 149–160. [DOI] [PubMed] [Google Scholar]
- Ferramosca A, 2014. Modulation of hepatic steatosis by dietary fatty acids. World J. Gastroenterol 20, 1746. 10.3748/wjg.v20.i7.1746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flurkey K, Currer J, Harrison D, 2007. Mouse models in aging research, in: The Mouse in Biomedical Research, 2nd Edition. New York; Elsevier, Volume 3: pp. 637–672. [Google Scholar]
- Foulds CE, Treviño LS, York B, Walker CL, 2017. Endocrine-disrupting chemicals and fatty liver disease. Nat. Rev. Endocrinol 13, 445–457. 10.1038/nrendo.2017.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard R, Durmuş B, Hofman A, MacKenbach JP, Steegers EAP, Jaddoe VWV, 2013. Risk factors and outcomes of maternal obesity and excessive weight gain during pregnancy. Obesity 21, 1046–1055. 10.1002/oby.20088 [DOI] [PubMed] [Google Scholar]
- Giorgio V, Prono F, Graziano F, Nobili V, 2013. Pediatric non alcoholic fatty liver disease: old and new concepts on development, progression, metabolic insight and potential treatment targets. BMC Pediatr. 13, 40. 10.1186/1471-2431-13-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths PS, Walton C, Samsell L, Perez MK, Piedimonte G, 2016. Maternal high-fat hypercaloric diet during pregnancy results in persistent metabolic and respiratory abnormalities in offspring. Pediatr. Res 79, 278–286. 10.1038/pr.2015.226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Bosagna CM, Sabat P, Valdovinos FS, Valladares LE, Clark SJ, 2008. Epigenetic and phenotypic changes result from a continuous pre and post natal dietary exposure to phytoestrogens in an experimental population of mice. BMC Physiol. 8, 17. 10.1186/1472-6793-8-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajj N. El, Schneider E, Lehnen H, Haaf T, 2014. Epigenetics and life-long consequences of an adverse nutritional and diabetic intrauterine environment. Reproduction 148, R111–R120. 10.1530/REP-14-0334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heerwagen MJR, Miller MR, Barbour LA, Friedman JE, 2010. Maternal obesity and fetal metabolic programming: A fertile epigenetic soil. Am J Physiol Regul Integr Comp Physiol 299, R711–R722. 10.1152/ajpregu.00310.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heindel JJ, Blumberg B, Cave M, Machtinger R, Mantovani A, Mendez MA, Nadal A, Palanza P, Panzica G, Sargis R, Vandenberg LN, vom Saal FS, 2017. Metabolism disrupting chemicals and metabolic disorders. Reprod. Toxicol 68, 3–33. 10.1016/j.reprotox.2016.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoff J, 2000. Methods of Blood Collection in the Mouse. Lab Anim. (NY). 29, 47–53. [Google Scholar]
- Hsu C-N, Lin Y-J, Tain Y-L, 2019. Maternal Exposure to Bisphenol A Combined with High-Fat Diet-Induced Programmed Hypertension in Adult Male Rat Offspring: Effects of Resveratrol. Int. J. Mol. Sci 20, 4382. 10.3390/ijms20184382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafatos AG, Verhagen H, Moschandreas J, Apostolaki I, van Westerop JJM, 2000. Mediterranean diet of Crete: foods and nutrient content. J. Am. Diet. Assoc 1000, 1487–1493. [DOI] [PubMed] [Google Scholar]
- Ke Z-H, Pan J-X, Jin L-Y, Xu H-Y, Yu T-T, Ullah K, Rahman TU, Ren J, Cheng Y, Dong X-Y, Sheng J-Z, Huang H-F, 2016. Bisphenol A Exposure May Induce Hepatic Lipid Accumulation via Reprogramming the DNA Methylation Patterns of Genes Involved in Lipid Metabolism. Sci. Rep 6, e31331. 10.1038/srep31331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochmanski J, Marchlewicz EH, Dolinoy DC, 2018. Longitudinal effects of developmental bisphenol A, variable diet, and physical activity on age-related methylation in blood. Environ. Epigenetics 4. 10.1093/eep/dvy017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koneva LA, Vyas AK, Mceachin RC, Puttabyatappa M, Wang H-S, Sartor M, Padmanabhan V, 2017a. Developmental Programming : Interaction Between Prenatal BPA and Postnatal Overfeeding on Cardiac Tissue Gene Expression in Female Sheep. Environ. Mol. Mutagen 58, 4–18. 10.1002/em [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koneva LA, Vyas AK, Mceachin RC, Puttabyatappa M, Wang H-S, Sartor MA, Padmanabhan V, 2017b. Developmental Programming: Interaction Between Prenatal BPA and Postnatal Overfeeding on Cardiac Tissue Gene Expression in Female Sheep. Environ. Mol. Mutagen 58, 4–18. 10.1002/em [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontogianni MD, Tileli N, Margariti A, Georgoulis M, Deutsch M, Tiniakos D, Fragopoulou E, Zafiropoulou R, Manios Y, Papatheodoridis G, 2014. Adherence to the Mediterranean diet is associated with the severity of non-alcoholic fatty liver disease. Clin. Nutr 33, 678–683. 10.1016/j.clnu.2013.08.014 [DOI] [PubMed] [Google Scholar]
- Kruse M, Seki Y, Vuguin PM, Du XQ, Fiallo A, Glenn AS, Singer S, Breuhahn K, Katz EB, Charron MJ, 2013. High-Fat Intake During Pregnancy and Lactation Exacerbates High-Fat Diet-Induced Complications in Male Offspring in Mice. Endocrinology 154, 3565–3576. 10.1210/en.2012-1877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau EY, Liu Junxiu, Archer E, McDonald SM, Liu Jihong, 2014. Maternal weight gain in pregnancy and risk of obesity among offspring: A systematic review. J. Obes 2014, 1–16. 10.1155/2014/524939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Reynolds CM, Segovia SA, Gray C, Vickers MH, 2015. Developmental Programming of Nonalcoholic Fatty Liver Disease : The Effect of Early Life Nutrition on Susceptibility and Disease Severity in Later Life. Biomed Res. Int 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindbäck SM, Gabbert C, Johnson BL, Smorodinsky E, Sirlin CB, Garcia N, Pardee PE, Kistler KD, Schwimmer JB, 2010. Pediatric nonalcoholic fatty liver disease: a comprehensive review. Adv. Pediatr 57, 85–140. 10.1016/j.yapd.2010.08.006 [DOI] [PubMed] [Google Scholar]
- Lohr K, Pachl F, Moghaddas Gholami A, Geillinger KE, Daniel H, Kuster B, Klingenspor M, 2016. Reduced mitochondrial mass and function add to age-related susceptibility toward diet-induced fatty liver in C57BL/6J mice. Physiol. Rep 4, 1–17. 10.14814/phy2.12988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonardo A, Ballestri S, Marchesini G, Angulo P, Loria P, 2015. Nonalcoholic fatty liver disease: A precursor of the metabolic syndrome. Dig. Liver Dis 47, 181–190. 10.1016/j.dld.2014.09.020 [DOI] [PubMed] [Google Scholar]
- Loomba R, Sirlin CB, Schwimmer JB, Lavine JE, 2009. Advances in pediatric nonalcoholic fatty liver disease. Hepatology 50, 1282–93. 10.1002/hep.23119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalopoulos GK, 2010. Liver regeneration after partial hepatectomy: Critical analysis of mechanistic dilemmas. Am. J. Pathol 176, 2–13. 10.2353/ajpath.2010.090675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milagro FI, Campión J, Martíez JA, 2006. Weight gain induced by high-fat feeding involves increased liver oxidative stress. Obesity 14, 1118–1123. 10.1038/oby.2006.128 [DOI] [PubMed] [Google Scholar]
- Miltenberger R, Mynatt R, Wilkinson J, Woychick R, 1997. The role of the agouti gene in the yellow obese syndrome. J Nutr 127, S1902–S1907. [DOI] [PubMed] [Google Scholar]
- Mohankumar SMJ, Rajendran TD, Vyas AK, Hoang V, Asirvatham-Jeyaraj N, Veiga-Lopez A, Olivier NB, Padmanabhan V, Mohankumar PS, 2017. Effects of prenatal bisphenol-A exposure and postnatal overfeeding on cardiovascular function in female sheep. J. Dev. Orig. Health Dis 8, 65–74. 10.1017/S204017441600057X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollard RC, Senechal M, Macintosh AC, Hay J, Wicklow BA, Wittmeier KDM, Sellers EAC, Dean HJ, Ryner L, Berard L, Mcgavock JM, 2014. Dietary determinants of hepatic steatosis and visceral adiposity in overweight and obese youth at risk of type 2 diabetes. Am. J. Clin. Nutr 99, 804–812. 10.3945/ajcn.113.079277.INTRODUCTION [DOI] [PubMed] [Google Scholar]
- Morgan H, Suterhland H, Martin D, Whitelaw E, 1999. Epigenetic inheritance at the agouti locus in the mouse. Nat Genet 23, 314–318. [DOI] [PubMed] [Google Scholar]
- Mou D, Wang J, Liu H, Chen Y, Che L, Fang Z, Xu S, Lin Y, Feng B, Li J, Wu D, 2018. Maternal methyl donor supplementation during gestation counteracts bisphenol A-induced oxidative stress in sows and offspring. Nutrition 45, 76–84. 10.1016/j.nut.2017.03.012 [DOI] [PubMed] [Google Scholar]
- Myers MG, Cowley MA, Münzberg H, 2008. Mechanisms of Leptin Action and Leptin Resistance. Annu. Rev. Physiol 70, 537–556. 10.l146/annurev.physiol.70.113006.100707 [DOI] [PubMed] [Google Scholar]
- Nahar MS, Liao C, Kannan K, Dolinoy DC, 2013. Fetal Liver Bisphenol A Concentrations and Biotransformation Gene Expression Reveal Variable Exposure and Altered Capacity for Metabolism in Humans 27, 116–123. 10.1002/jbt [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahar MS, Liao C, Kannan K, Harris C, Dolinoy DC, 2015. In utero bisphenol A concentration, metabolism, and global DNA methylation across matched placenta, kidney, and liver in the human fetus. Chemosphere 124, 54–60. 10.1016/j.chemosphere.2014.10.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima Y, 2008. Fish-oil high-fat diet intake of dams after day 5 of pregnancy and during lactation guards against excessive fat consumption of their weaning pups. J. Nutr. Sci. Vitaminol. (Tokyo) 54, 46–53. 10.3177/jnsv.54.46 [DOI] [PubMed] [Google Scholar]
- Nowland MH, Lebowsky R, 2016. ULAM Guidelines and SOPs for Mice [WWW Document]. Univ. Michigan, Institutional Anim. Care Use Comm. [Google Scholar]
- Nozhenko Y, Asnani-Kishnani M, Rodríguez AM, Palou A, 2015. Milk leptin surge and biological rhythms of leptin and other regulatory proteins in breastmilk. PLoS One 10, 1–17. 10.1371/journal.pone.0145376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oben JA, Mouralidarane A, Samuelsson AM, Matthews PJ, Morgan ML, Mckee C, Soeda J, Fernandez-Twinn DS, Martin-Gronert MS, Ozanne SE, Sigala B, Novelli M, Poston L, Taylor PD, 2010. Maternal obesity during pregnancy and lactation programs the development of offspring non-alcoholic fatty liver disease in mice. J. Hepatol 52, 913–920. 10.1016/jjhep.2009.12.042 [DOI] [PubMed] [Google Scholar]
- Oddy WH, Herbison CE, Jacoby P, Ambrosini GL, O’Sullivan T. a, Ayonrinde OT, Olynyk JK, Black LJ, Beilin LJ, Mori T. a, Hands BP, Adams L. a, 2013. The Western Dietary Pattern Is Prospectively Associated With Nonalcoholic Fatty Liver Disease in Adolescence. Am. J. Gastroenterol 1–8. 10.1038/ajg.2013.95 [DOI] [PubMed] [Google Scholar]
- Padmanabhan V, Cardoso RC, Puttabyatappa M, 2016. Developmental programming, a pathway to disease. Endocrinology. 10.1210/en.2016-1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peretz J, Vrooman L, Ricke WA, Hunt PA, Ehrlich S, Hauser R, Padmanabhan V, Taylor HS, Swan SH, Vandevoort CA, Flaws JA, 2014. Bisphenol A and Reproductive Health: Update of Experimental and Human Evidence, 2007-2013. Env. Heal. Perpsect 122, 775–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettan-Brewer C, M. Treuting P, 2011. Practical pathology of aging mice. Pathobiol. Aging Age-related Dis 1, 1–16. 10.3402/pba.vli0.7202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruis MGM, Lendvai A, Bloks VW, Zwier MV, Baller JFW, Bruin A. De, Groen AK, Plosch T, 2014. Maternal western diet primes non-alcoholic fatty liver disease in adult mouse offspring. Acta Physiol 210, 215–227. 10.1111/apha.12197 [DOI] [PubMed] [Google Scholar]
- Reeves PG, 1997. Components of the AIN-93 Diets as Improvements in the AIN-76A Diet. J. Nutr 123, S838–S841. [DOI] [PubMed] [Google Scholar]
- Reeves PG, Nielsen FH, Fahey GC, 1993. Committee Report AIN-93 Purified Diets for Laboratory Rodents : Final Report of the American Institute of Nutrition Ad Hoc Writing Committee on the Reformulation of the AIN-76A Rodent Diet. J. Nutr 123, 1939–1951. [DOI] [PubMed] [Google Scholar]
- Research, I. for L.A., 2011. Guide for the Care and Use of Laboratory Animals, National Academy of Sciences. 10.1163/1573-3912_isiam_DUM_3825 [DOI] [Google Scholar]
- Ryan M, Itsiopouls C, Thodis T, Ward G, Trost N, Hofferberth S, 2013. The Mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. J Hepatol 59, 138–143. [DOI] [PubMed] [Google Scholar]
- Sánchez J, Oliver P, Miralles O, Ceresi E, Picó C, Palou A, 2005. Leptin orally supplied to neonate rats is directly uptaken by the immature stomach and may regulate short-term feeding. Endocrinology 146, 2575–2582. 10.1210/en.2005-0112 [DOI] [PubMed] [Google Scholar]
- Schönfelder G, Wittfoht W, Hopp H, Talsness CE, Paul M, Chahoud I, 2002. Parent bisphenol a accumulation in the human maternal-fetal-placental unit. Environ. Health Perspect 110, A703–A707. 10.1289/ehp.110-1241091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwimmer Jeffrey B., Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C, 2006. Prevalence of Fatty Liver in Children and Adolescents. Pediatrics 118, 1388–1393. 10.1542/peds.2006-1212 [DOI] [PubMed] [Google Scholar]
- Schwimmer Jeffrey B, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C, 2006. Prevalence of fatty liver in children and adolescents. Pediatrics 118, 1388–93. 10.1542/peds.2006-1212 [DOI] [PubMed] [Google Scholar]
- Shimpi PC, More VR, Paranjpe M, Donepudi AC, Goodrich JM, Dolinoy DC, Rubin B, Slitt AL, 2017. Hepatic lipid accumulation and Nrf2 expression following perinatal and peripubertal exposure to bisphenol a in a mouse model of nonalcoholic liver disease. Environ. Health Perspect 125, 1–10. 10.1289/EHP664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simino De Paula LA, de Fante T, Fontana MF, Borges FO, Torsoni MA, Milanski M, Velloso LA, Torsoni AS, 2017. Lipid overload during gestation and lactation can independently alter lipid homeostasis in offspring and promote metabolic impairment after new challenge to high-fat diet. Nutr. Metab. (Lond) 14, 1–15. 10.1186/sl2986-017-0168-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JM, Ward JM, Treuting PM, 2016. Cause-of-Death Analysis in Rodent Aging Studies. Vet. Pathol 53, 233–243. 10.1177/0300985815610391 [DOI] [PubMed] [Google Scholar]
- Strakovsky R, Zhang X, Zhou D, Pan Y, 2014. The regulation of hepatic Ponl by a maternal high-fat diet is gender specific and may occur through promoter histone modifications in neonatal rats. J. Nutr. Biochem 25, 170–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strakovsky RS, Wang H, Engeseth NJ, Flaws JA, Helferich WG, Pan Y, Lezmi S, 2015. Developmental bisphenol A (BPA) exposure leads to sex-speci fi c modi fi cation of hepatic gene expression and epigenome at birth that may exacerbate high-fat diet-induced hepatic steatosis. Toxicol. Appl. Pharmacol 284, 101–112. 10.1016/j.taap.2015.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EL, Smith MS, Grove KL, 2011. Perinatal Exposure to High-Fat Diet Programs Energy Balance , Metabolism and Behavior in Adulthood. Neuroendocrinology 93, 1–8. 10.1159/000322038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trichopoulou A, Katsouyanni K, Gnardellis C, 1993a. The traditional Greek diet. Eur. J. Clin. Nutr 47, S76–S81. [PubMed] [Google Scholar]
- Trichopoulou A, Toupadaki N, Tzonou A, Katsouyanni K, Manousos O, Kada E, Trichopoulos D, 1993b. The macronutrient composition of the Greek diet: estimates derived from six case-control studies. Eur J Clin Nutr 47, 549–558. [PubMed] [Google Scholar]
- Walker CL, 2016. Minireview: Epigenomic Plasticity and Vulnerability to EDC Exposures. Mol. Endocrinol 30, 848–855. 10.1210/me.2016-1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller-Evans H, Hue C, Fearnside J, Rothwell AR, Lockstone HE, Caldérari S, Wilder SP, Cazier JB, Scott J, Gauguier D, 2013. Nutrigenomics of high fat diet induced obesity in mice suggests relationships between susceptibility to fatty liver disease and the proteasome. PLoS One 8, 1–12. 10.1371/journal.pone.0082825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterland RA, Jirtle RL, 2003. Transposable Elements : Targets for Early Nutritional Effects on Epigenetic Gene Regulation Transposable Elements : Targets for Early Nutritional Effects on Epigenetic Gene Regulation. Mol Cell Biol 23, 5293–5300. 10.1128/MCB.23.15.5293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Sun X, Chen Y, Li Y, Song L, Zhou Z, Xu B, Lin Y, Xu S, 2014. Perinatal exposure to bisphenol A exacerbates nonalcoholic steatohepatitis-like phenotype in male rat offspring fed on a high-fat diet. J. Endocrinol 222, 313–325. 10.1530/JOE-14-0356 [DOI] [PubMed] [Google Scholar]
- Weinhouse C, Anderson OS, Bergin IL, Vandenbergh DJ, Gyekis JP, Dingman MA, Yang J, Dolinoy DC, 2014. Dose-dependent incidence of hepatic tumors in adult mice following perinatal exposure to bisphenol A. Env. Heal. Perspect 122, 485–491. 10.1289/ehp.1307449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesolowski SR, Kasmi KCE, Jonscher KR, Friedman JE, 2017. Developmental origins of NAFLD: A womb with a clue. Nat. Rev. Gastroenterol. Hepatol 14, 81–96. 10.1038/nrgastro.2016.160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L, Seki Y, Vuguin PM, Charron MJ, 2014. Animal models of in utero exposure to a high fat diet: A review. Biochim. Biophys. Acta 1842, 507–519. 10.1016/j.bbadis.2013.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P, Wang H, Kayoumu A, Wang M, Huang W, Liu G, 2015. Diet rich in Docosahexaenoic Acid / Eicosapentaenoic Acid robustly ameliorates hepatic steatosis and insulin resistance in seipin deficient lipodystrophy mice. Nutr. Metab. (Lond) 12, 1–10. 10.1186/sl2986-015-0054-x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


