Abstract
Background:
The Ending the HIV Epidemic (EHE) initiative aims to reduce incident HIV infections by 90% over a span of 10 years. The intensity of interventions needed to achieve this for local epidemics is unclear.
Objective:
To estimate the effect of HIV interventions at the city level.
Design:
A compartmental model of city-level HIV transmission stratified by age, race, sex, and HIV risk factor was developed and calibrated.
Setting:
32 priority metropolitan statistical areas (MSAs).
Patients:
Simulated populations in each MSA.
Intervention:
Combinations of HIV testing and preexposure prophylaxis (PrEP) coverage among those at risk for HIV, plus viral suppression in persons with diagnosed HIV infection.
Measurements:
The primary outcome was the projected reduction in incident cases from 2020 to 2030.
Results:
Absent intervention, HIV incidence was projected to decrease by 19% across all 32 MSAs. Modest increases in testing (1.25-fold per year), PrEP coverage (5 percentage points), and viral suppression (10 percentage points) across the population could achieve reductions of 34% to 67% by 2030. Twenty-five percent PrEP coverage, testing twice a year on average, and 90% viral suppression among young Black and Hispanic men who have sex with men (MSM) achieved similar reductions (13% to 68%). Including all MSM and persons who inject drugs could reduce incidence by 48% to 90%. Thirteen of 32 MSAs could achieve greater than 90% reductions in HIV incidence with large-scale interventions that include heterosexuals. A web application with location-specific results is publicly available (www.jheem.org).
Limitation:
The COVID-19 pandemic was not represented.
Conclusion:
Large reductions in HIV incidence are achievable with substantial investment, but the EHE goals will be difficult to achieve in most locations. An interactive model that can help policymakers maximize the effect in their local environments is presented.
Primary Funding Source:
National Institutes of Health.
Keywords: Health disparities, HIV infections, HIV prevention, Metropolitan statistical areas, Preexposure prophylaxis, Structural racism
TOC blurb
Forty years after its initial recognition, and despite the development of highly effective approaches to prevention and treatment, HIV infection continues to cause major morbidity and mortality in the United States. The Ending the HIV Epidemic initiative has as its ambitious goal the reduction of incident HIV infections by 90% over the next 10 years. An interactive model was developed to assist policymakers in prioritizing interventions at the local level to achieve this goal.
BACKGROUND
With more than 37 000 new infections and 1 million prevalent cases in 2018, HIV imposes substantial health burdens in the United States (1). The Ending the HIV Epidemic (EHE) initiative aims to reduce incident HIV infections in the United States by 90% over a span of 10 years (2, 3). The initiative highlights high-risk populations (young Black and Hispanic men who have sex with men [MSM]) and focuses on 4 strategies: rapid diagnosis of HIV, effective treatment to achieve viral suppression in persons with HIV infection (PWH), prevention of new infections through interventions such as preexposure prophylaxis (PrEP) or needle-exchange programs, and rapid response to HIV outbreaks.
The optimal combination of these strategies is unclear, as is the effect of targeting interventions toward specific subgroups. Furthermore, HIV epidemics are localized, and the most effective interventions likely differ between locations (4). Mathematical models of infectious disease can facilitate evidence-based evaluation of potential strategies (5, 6).
We developed a dynamic model of HIV transmission to help policymakers identify the combinations and intensity of strategies most likely to reduce HIV incidence in their local settings. Specifically, we projected the effect of interventions by implementing the first 3 EHE pillars—HIV screening, viral suppression among PWH, and preventive measures—in 32 priority urban areas (2, 3).
METHODS
Model Structure
The Johns Hopkins HIV Economic-Epidemiologic Mathematical Model is a dynamic, compartmental model of HIV transmission (7, 8). Expanding on that model, we developed a novel HIV transmission model stratified by age (13 to 24, 25 to 34, 35 to 44, 45 to 54, and ≥55 years), race/ethnicity (Black, Hispanic, and other), sex and sexual behavior (female, heterosexual male, and MSM), and intravenous drug use (never use, active use, and prior use) at the local level. We represented the adult population (aged ≥13 years) according to HIV infection status (uninfected, acute HIV—the first 2.9 months after infection [9]—or chronic HIV), awareness of infection, and PrEP status (Figure 1).
Figure 1.
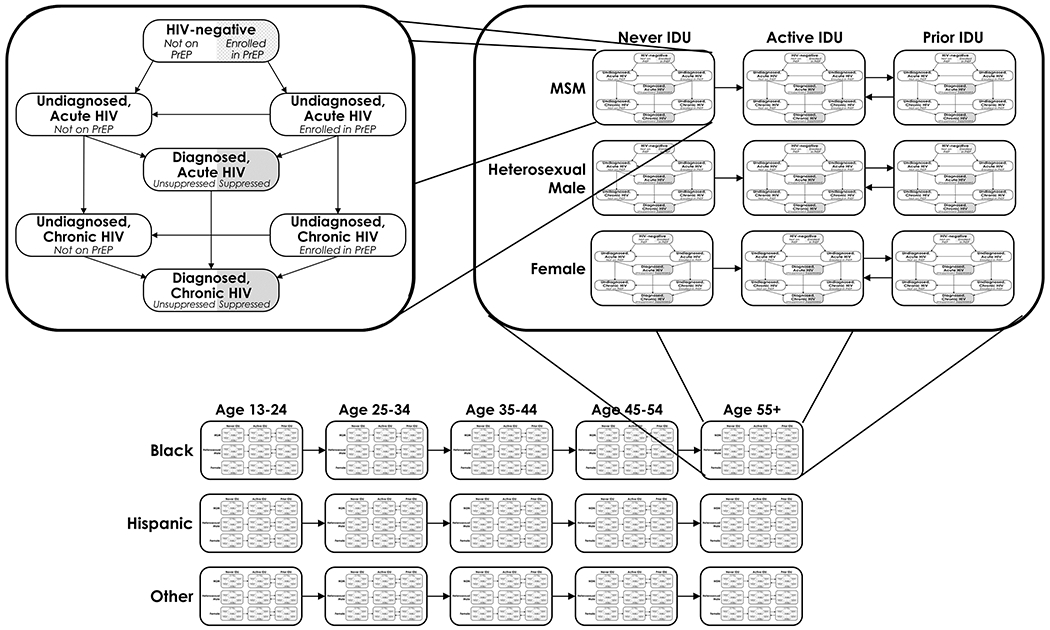
Model structure. The upper left panel shows model populations (compartments) representing HIV disease and continuum of care. Each uninfected population has a proportion who are enrolled in a PrEP program. As persons in the model become infected, they first enter the acute HIV phase, where transmissibility is high, before progressing to chronic HIV. Persons who become infected with HIV while enrolled in a PrEP program are diagnosed at an average rate of once every 3 mo. Persons with HIV infection who are unaware of their diagnosis and not in a PrEP program are diagnosed according to testing rates that depend on their age, race/ethnicity, sex or sexual behavior, intravenous drug use status, location, and calendar year. All populations of persons with HIV infection who are aware of their diagnosis have a proportion who are virally suppressed and do not transmit HIV. Each population is further stratified by sex or sexual behavior and intravenous drug use status (top right) and by age and race/ethnicity (bottom). IDU = injection drug use; MSM = men who have sex with men; PrEP = pre-exposure prophylaxis.
Acquisition of HIV in each population subgroup reflected the frequency of sexual or needle-sharing pairings with other subgroups; prevalence of unsuppressed HIV in partnering subgroups; subgroup-specific transmission rates; and subgroup PrEP coverage and use of needle-exchange programs. We represented these relationships as a set of differential equations (Supplement, available at Annals.org), solved using the odeintr package in R, version 4.0.2 (R Foundation for Statistical Computing) (10, 11).
Study Setting
The EHE initiative identified 48 high-burden counties plus Washington, DC (2), which span 32 metropolitan statistical areas (MSAs). Because mixing between adjacent counties in the same MSA is likely common, we represented epidemics at the MSA level.
Model Calibration
Within each MSA, we estimated 131 parameters that governed subgroups’ risks for HIV infection, frequency of HIV testing, PrEP uptake, viral suppression, use of injection drugs, and propensities for mixing with other subgroups, in time-dependent fashion (Supplement Table 1). Each set of parameter values yielded a different simulation; we used a Bayesian process to generate simulations that closely matched the following 10 calibration targets at the MSA level: reported diagnoses from 2009 to 2017, stratified by sex, age, race, and risk factor (and 2-way combinations thereof) (12–19); estimated number of PWH who are aware of their diagnosis from 2008 to 2016, stratified by sex, age, race, and risk factor (plus 2-way combinations) (12–19); death in PWH from 2009 to 2016, stratified by sex (13–19); the proportion of PWH who are aware of their diagnosis from 2010 to 2018 (at the state level) (12); the proportion of PWH who were virally suppressed, as reported by local health departments from 2010 to 2018 and stratified by age, race, sex, and risk factor when data were available; the number of persons receiving a prescription for emtricitabine–tenofovir for PrEP (20); the probability of receiving an HIV test between 2013 and 2017 (21); the prevalence of injection drug use from 2014 to 2016 (substate estimates) (22); cumulative AIDS mortality up to 2002 (23); and reported AIDS diagnoses from 1998 to 2002 (23).
We formulated a likelihood function that quantified how well each simulation matched calibration targets (detailed in the Supplement). We ran an adaptive Metropolis sampler, a Bayesian method in which thousands of simulations are run to approximate the probability distributions of parameters (24, 25). For each MSA, we ran 4 chains with 50 000 “burn-in” iterations and 50 000 sampling iterations. Thinning to every 200th iteration yielded a posterior set of 1000 simulations per MSA. We projected these 1000 simulations forward to 2030 under different intervention scenarios to estimate the effect on each MSA’s HIV epidemic. We calculated mean absolute percentage errors for reported diagnoses and prevalence to summarize overall model fit (26).
Data Sources
We derived population sizes from U.S. Census Bureau 1990 to 2018 intercensal estimates (27), birth and death rates from the Centers for Disease Control and Prevention’s WONDER database (23), and numbers of MSM from Grey and colleagues (28). We based prior distributions for HIV testing, viral suppression, and PrEP use on national data from Centers for Disease Control and Prevention HIV Surveillance Reports (29–37) and multicity data from the National HIV Behavioral Surveillance program (38–45). Prior distributions for sexual and needle-sharing frequency and assortativity were based on published literature (22, 38, 46–57).
Modeled Interventions
We modeled 3 values that could be affected by interventions for each demographic and risk factor subgroup in each MSA. First, we modeled HIV testing – the average number of tests done per year on undiagnosed PWH – which determines the rate of diagnosis. Second, we modeled the proportion of serostatus-aware PWH who are virally suppressed (and thus do not transmit HIV [58]) representing the combined efficacy of interventions to enhance engagement and retention in care and improve adherence to antiretrovirals in terms of the resulting. Third, we modeled PrEP coverage: the proportion of persons who are HIV negative and at risk for HIV (that is, engaging in needle sharing or nonmonogamous sex [59]) who are enrolled in a PrEP program, including both medication and HIV testing every 3 to 6 months. We based efficacy on clinical trials, so PrEP reduced the rate of HIV acquisition by 86% for male-to-male sexual transmission (60), 75% for heterosexual transmission (61), and 49% for intravenous drug use–related transmission (62).
We assumed that, before 2021, HIV testing, PrEP coverage, and viral suppression changed over time in each subgroup, as seen in national and multicity reporting from 2010 to 2018 (29–45). Absent any additional intervention, we assumed that these levels would continue their trajectories (with randomly sampled variation) into the future, such that, in MSAs where HIV trends are improving, those improvements are carried forward.
Among persons who inject drugs (PWID) who do not have HIV, we additionally represented the proportion in needle-exchange programs, as well as the medications for opioid use disorder among PWID in remission. We let needle-exchange programs reduce the rate of HIV acquisition via needle sharing by 31% (63–65) and increased the rate of remission from injection drug use by 1.36-fold (66–68). Medications for opioid use disorder reduced the rate of relapse to injection use by 0.45-fold (69). We assumed that, absent intervention, needle exchange and medication for opioid use disorder coverage were zero.
We developed a “broad moderate improvement” scenario in which all population subgroups increased PrEP coverage 5 percentage points above what it would have been, testing 1.25 times, and viral suppression 10 percentage points. To explore what it will take to achieve the EHE goals, we also tested intervention scenarios with “intensive” levels of testing (once every 2 years to twice a year), PrEP coverage (10% or 25%), and viral suppression among diagnosed PWH (80% or 90%), alone or in combination. We applied interventions to 3 risk groups identified by the EHE initiative, in decreasing order of HIV risk: Black and Hispanic MSM younger than 35 years, all other MSM plus all PWID, and heterosexuals (2). Finally, we tested the effect of needle-exchange programs and medications for opioid use disorder among PWID.
We presumed that interventions would scale up linearly from the baseline to the target level over a span of 5 years (1 January 2023 through 31 December 2027, with a secondary analysis for 3 years from 1 January 2023 to 31 December 2025), after which they would continue throughout the simulated time period at the target intensity. If the baseline (no additional intervention) level ever exceeded the target, we applied the higher (baseline) value.
Outcomes
Our primary outcome was the reduction in the projected number of incident HIV infections from 2020 to 2030 ([incident cases in 2020 – incidence cases in 2030]/incident cases in 2020) compared with the EHE target of 90% reduction. Our secondary outcome was the reduction in the projected number of incident HIV infections from 2020 to 2025 compared with the EHE target of 75%. For each intervention scenario and MSA, we calculated the mean reduction and 95% credible interval (2.5th and 97.5th quantiles) from 1000 simulations.
Sensitivity Analyses
We did probabilistic sensitivity analyses to identify parameters strongly associated with the primary outcome using the intervention scenario in which MSM and PWID were tested on average twice a year; had 25% PrEP coverage; and 90% of diagnosed MSM, PWID, and PWH were virally suppressed. First, for each MSA, we calculated partial rank correlation coefficients, a multivariate measure of the correlation between the outcome and parameters (70). Second, for each parameter, we ranked the simulations in each MSA by the parameter value and compared the reductions in total incidence—aggregated across all MSAs—from the 20% of simulations with the highest values of that parameter to the reductions in the 20% of simulations with the lowest (6).
Web Tool
We developed an interactive, publicly available web tool at www.jheem.org to visualize projected HIV incidence, prevalence, reported diagnoses, and death under each intervention scenario and under user-customizable scenarios in each of the 32 MSAs.
Role of the Funding Source
The funder had no role in the study’s design, conduct, or reporting.
RESULTS
Simulations in each MSA were consistent with epidemiologic targets (see Figure 2 for New York–Newark–Jersey City). Detailed visualizations for each MSA are available at www.jheem.org. Across all 32 MSAs, the projected new diagnoses from 2010 to 2018 differed from reported diagnoses by 1265 cases on average (mean absolute percentage error, 5% [range, 4% to 11% across cities]) (Supplement Figure 1). Projected prevalence differed by 11 736 cases on average (2% [range, 3% to 12% across cities]) (Supplement Figure 2).
Figure 2.

Model fit for 1000 simulations in New York–Newark–Jersey City, New York–New Jersey–Pennsylvania metropolitan statistical area. The shaded ribbons represent the 95% credible interval. The solid, dotted, and dashed lines in the middle of the ribbons represent the mean across 1000 model simulations. The circles, squares, triangles and diamonds represent data from the Centers for Disease Control and Prevention surveillance (12–19). Data stratified by race/ethnicity, HIV risk factor, and age on reported diagnoses at the metropolitan statistical area-level were available for 2010, 2011, and 2013 to 2017, and on estimated prevalence for 2009, 2010, and 2012 to 2016. IDU = injection drug use; MSM = men who have sex with men.
With current trends continuing, absent additional interventions, HIV incidence was projected to decrease by 19% in total by 2030 across all 32 MSAs (see Figure 3 for New York–Newark–Jersey City, New York–New Jersey–Pennsylvania), ranging from a 2% increase in Sacramento–Roseville–Folsom, California to a 38% decrease in Washington–Arlington–Alexandria, District of Columbia–Virginia–Maryland–West Virginia (Figure 4, column 1). These projections reflected baseline PrEP coverage, testing rates, and viral suppression among diagnosed PWH in each MSA, which differed by demographic subgroup and over time. Averaged across all races and ages, MSM in 2020 were projected to have PrEP coverage ranging from 3% to 12% across MSAs, 0.3 to 0.8 tests per year on average, and 46% to 88% viral suppression. In the subsequent 5 years, PrEP coverage was projected to increase by 0.7 to 2.4 percentage points, whereas testing and viral suppression increased only marginally. Baseline levels for other subgroups and times are presented in Supplement Figures 3 to 5 and at www.jheem.org.
Figure 3.

The effect of 2 potential interventions on incidence and reported diagnoses in the New York–Newark–Jersey City, New York–New Jersey–Pennsylvania metropolitan statistical area. The shaded ribbons represent the 95% CrI. The solid and dashed lines in the middle of the ribbons represent the mean across 1000 model simulations. The black circles represent Centers for Disease Control and Prevention surveillance data for total reported diagnoses (12–19). Interventions begin implementation on 1 January 2023, scale up by 31 December 2027, and continue implementation through 2030. The “no intervention” scenario (solid orange lines) assumes continuation of current rates of improvement in testing, suppression, and PrEP usage over the next 5 years. Intervention 1 (dashed blue lines) targets Black and Hispanic MSM aged younger than 35 years with twice-yearly testing and 25% PrEP uptake among those at risk, plus viral suppression in 80% of those with diagnosed HIV. Intervention 2 (dashed red lines) targets all MSM and PWID with twice-yearly testing and 25% PrEP uptake among those at risk, plus viral suppression in 90% of those with diagnosed HIV. CrI = credible interval; MSM = men who have sex with men; PrEP = preexposure prophylaxis; PWID = persons who inject drugs.
Figure 4.
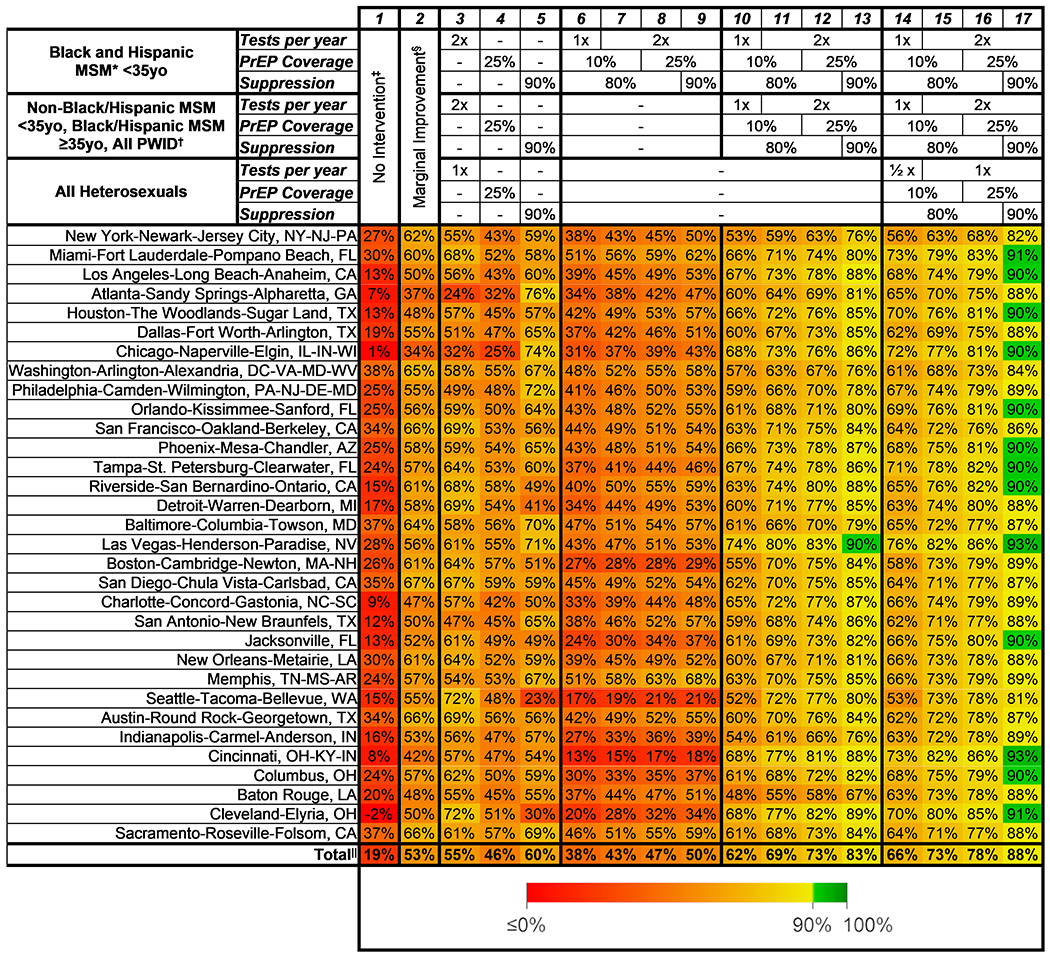
Reduction in HIV incidence from 2020 to 2030 for intervention scenarios across 32 MSAs. Each cell shows the mean percentage reduction across 1000 simulations in model-projected incidence from 2020 to 2030. The interventions begin on 1 January 2023, scale up linearly until 31 December 2027, and continue implementation through 2030. Tests per year denotes the average number of tests per year for those in the group (½ implies an average of 1 test every 2 years), PrEP coverage denotes the proportion of at-risk persons who are enrolled in a PrEP program, and suppression denotes the proportion of persons with HIV who have a suppressed viral load. MSA = metropolitan statistical area; MSM = men who have sex with men; PrEP = preexposure prophylaxis; PWID = persons who inject drugs. * The rates of testing, PrEP coverage, and suppression absent additional intervention differ by age, race, sex, risk factor, and MSA, and change over time. Averaged across all races and ages, MSM in 2020 were projected to have PrEP coverage ranging from 3% to 12% across MSAs, 0.3 to 0.8 tests per year on average, and 46% to 88% viral suppression. By 2025, PrEP coverage was projected to increase by 0.7 to 2.4 percentage points, whereas testing and viral suppression increased only marginally. Baseline levels for other subgroups and times are presented in Supplement Figures 3 to 5 and at www.jheem.org. † In the “broad moderate improvement” scenario, all subgroups increased PrEP coverage by 5 percentage points, testing 1.25 times per year, and viral suppression 10 percentage points. ‡ Total = the reduction in the sum of projected incidence across all 32 MSAs.
Interventions that modestly improved PrEP coverage (5 percentage points), testing (1.25-fold), and suppression (10 percentage points) across the entire population achieved reductions of 34% to 67% (Figure 4, column 2). Single-modality interventions that increased PrEP coverage (up to 25%), testing (up to twice a year), or viral suppression among diagnosed PWH (up to 90%) across all subgroups reduced incidence by 23% to 76% (Figure 4, columns 3 to 5; Supplement Figure 6).
Interventions that focused on young Black and Hispanic MSM could achieve 10-year incidence reductions from 13% to 68% (Figure 4, columns 5 to 8 and Figure 3, A and B). Interventions targeting all MSM and PWID reduced incidence from 48% to 90% (Figure 4, columns 10 to 13 and Figure 3, C and D). Among all 32 MSAs, only Las Vegas was projected to reach a 90% reduction without also expanding to heterosexuals; when including heterosexuals in intensive interventions, 13 of 32 MSAs could reach the EHE targets, with reductions of 53% to 93% (Figure 4, columns 14 to 17).
None of the 17 intervention scenarios in any of the 32 cities achieved the 2025 target of a 75% reduction (Supplement Figure 7). Implementing interventions over a faster time frame (2023 to 2025) allowed 23 MSAs to meet the 2025 goals with broad interventions (Supplement Figure 8) but did not change the ability of any scenario to achieve the 2030 target (Supplement Figure 9).
In specific MSAs, increased coverage of needle-exchange programs reduced HIV incidence by up to 16% (for example, 42% in Boston–Cambridge–Newton, Massachusetts–New Hampshire vs. 26% without such intervention), although in most MSAs, the effect of needle-exchange programs or medications for opioid use disorder was substantially less (<1% additional reduction) (Supplement Figure 10).
In sensitivity analyses, the model parameters most strongly associated with estimated reductions in HIV incidence by 2030 were the HIV transmission rate among Black heterosexuals (mean partial rank correlation coefficient = −0.60) and the degree to which viral suppression increased in the future absent intervention (mean partial rank correlation coefficient = −0.38). Model results were robust to variation (Figure 5). For example, in Baton Rouge, Louisiana—the MSA with the greatest sensitivity to HIV transmission among Black heterosexuals—the 200 simulations with the highest Black heterosexual transmission rate had a mean projected reduction in HIV incidence by 2030 of 75% (95% credible interval, 62% to 83%), whereas the 200 simulations with the lowest transmission rate had a mean projected reduction of 54% (95% credible interval, 9% to 71%). Other parameters were less strongly correlated on average, although there was substantial variation at the MSA level.
Figure 5.
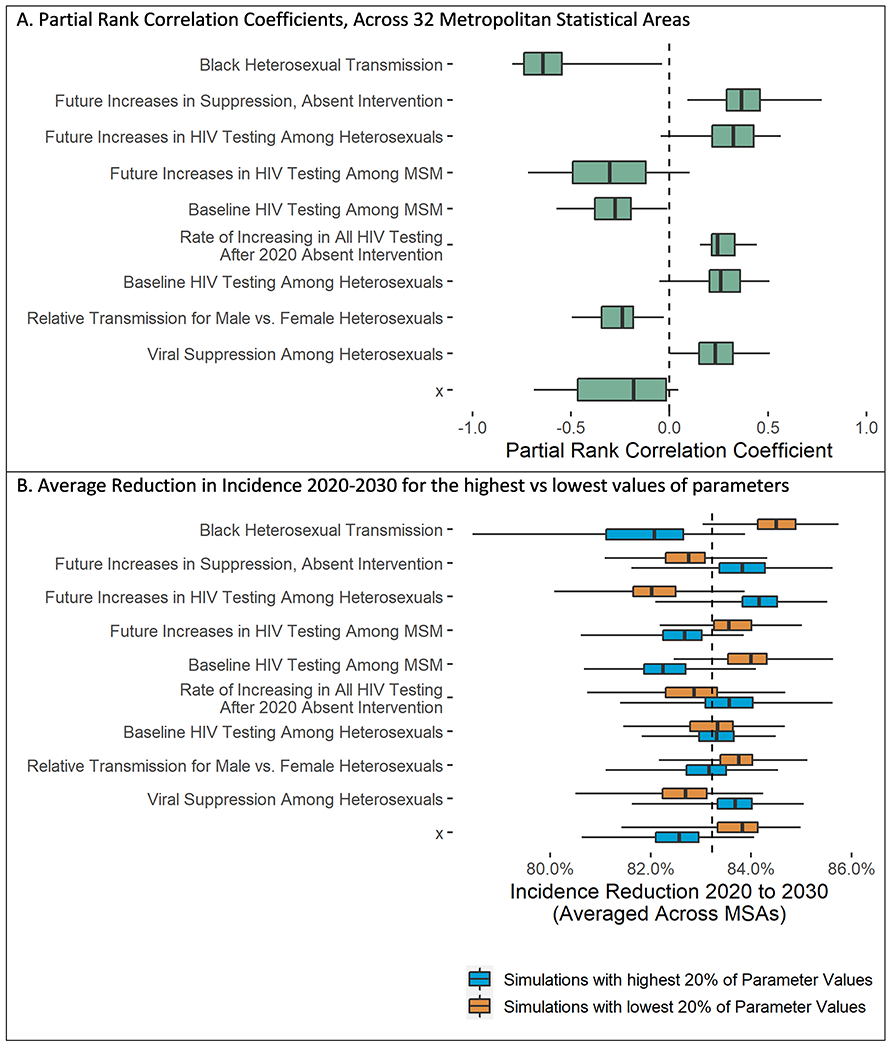
Sensitivity analyses for the 10 parameters most strongly associated with projected 10-year reduction in HIV incidence. MSA = metropolitan statistical area; MSM = men who have sex with men. Top. Box-and-whisker plot showing partial rank correlation coefficients—a measure of the correlation between (ranked) parameter values and the (ranked) outcome: the reduction in incidence from 2020 to 2030 assuming an intervention in which all at-risk MSM and all persons who inject drugs are tested yearly, 50% are receiving preexposure prophylaxis, and 90% of those with HIV are virally suppressed. Values close to 1 or −1 indicate perfect correlation. We show the 10 parameters with the strongest correlation with the outcome. The vertical line represents the median partial rank correlation coefficient across the 32 MSAs, the shaded boxes denote the interquartile range, and the whiskers span the minimum and maximum reduction across all 32 MSAs.
Bottom. The reduction in total incidence (aggregated across the 32 MSAs) from 2020 to 2030 for the 200 simulations with the highest values of each parameter (blue) versus the 200 simulations with the lowest values (orange). This gives a sense for the magnitude of the change in the outcome when each parameter is varied from low to high values across the selected ranges. The vertical line represents the median reduction across the 200 simulations, the shaded boxes denote the interquartile range, and the whiskers span the minimum and maximum reduction for the 200 simulations.
DISCUSSION
This detailed HIV transmission model, calibrated to specific MSAs, shows that EHE targets will be difficult to achieve. Absent additional interventions, we project a total incidence reduction of 19% across 32 cities over a span of 10 years if current trends continue. Modest increases in testing, PrEP coverage, and viral suppression across the population can achieve 10-year incidence reductions up to 67% in some cities. Interventions focused on the highest-risk subgroups (young Black and Hispanic MSM) that concurrently deliver high levels of testing, PrEP coverage, and viral suppression among diagnosed PWH can achieve similar reductions up to 68%. Including all MSM and PWID in interventions could reduce incidence by 48% to 90%. Thirteen of 32 MSAs could achieve greater than 90% reductions in incidence with large-scale interventions targeted across the entire population. Our local-level projections—available at www.jheem.org—can help decision makers identify those interventions most likely to have a population-level effect. They also illustrate the heterogeneity among cities and the need to avoid “one-size-fits-all” thinking.
The levels of HIV testing (once to twice a year), PrEP coverage (10% to 25%), and viral suppression among diagnosed PWH (80% to 90%) needed to sharply reduce HIV transmission will require substantial investments at the local level. Although Centers for Disease Control and Prevention guidelines recommend yearly HIV testing for those at risk (71), the median time since the last HIV test among those at risk was 1.4 years from 2006 to 2016 (72). Boosting testing rates higher than yearly would likely require some form of free, at-home HIV testing, which has been shown to increase the number of tests in MSM by 1.5- to 2.1-fold (73, 74). Preexposure prophylaxis coverage of 10% among MSM has been reported in several urban clinics (75–77). Approaching 25% coverage would demand an intensive effort. In a study in New York City from 2017 to 2018, offering PrEP navigators to all MSM presenting at public sexual health clinics increased PrEP coverage from 14% to 23% (75). Eighty percent viral suppression among serostatus-aware PWH has been found in several international settings (78); nationally, 65% of diagnosed PWH in the United States were suppressed in 2018 (29), but King County, Washington (in the Seattle–Tacoma–Bellevue MSA), has reported more than 80% viral suppression since 2015 (12). To our knowledge, 90% suppression among diagnosed PWH, which roughly corresponds to the World Health Organization’s target of 95% of diagnosed PWH on antiretroviral therapy with 95% of those virally suppressed (79), has not been shown in large-scale settings. Reaching this ambitious target would require improvements all along the continuum of care—from initial linkage, to retention, to suppressing those in care. A breakdown at any step would render the 90% level unattainable.
Other HIV transmission models have explored EHE targets, although not across all MSAs targeted by the EHE initiative. In a national-level model, Bradley and colleagues (80) projected a 67% reduction in reported diagnoses from 2018 to 2030 if 95–95-95 goals were met and approximately 28% of persons at risk were prescribed and adherent to PrEP. We projected a decrease of 74% across 32 MSAs in aggregate under a roughly analogous scenario (95% aware, 25% receiving PrEP, and 90% suppressed by 2025). However, our analysis highlights substantial variations between MSAs, with projected reductions ranging from 49% (in Seattle–Tacoma–Bellevue, Washington) to 83% (in Baltimore–Columbia–Towson, Maryland). At the local level, Nosyk and colleagues (81–83) developed a compartmental model of HIV epidemics in 6 U.S. cities, stratified by race and HIV risk factor. Our model predicted greater declines in incidence under our baseline scenario (that is, absent additional interventions) (Supplement Table 3); for example, in Seattle, we projected a 28% decline, whereas Nosyk and colleagues projected a 15% reduction. This may result from structural differences (we explicitly represent age strata but simplify the HIV continuum of care) or differences in calibration (Nosyk and colleagues calibrated to reported diagnoses, diagnosed PWH, and death from 2012 to 2015, whereas we calibrated to 10 stratified targets through 2018). Jenness and colleagues (84) developed a network-based transmission model focused on MSM in Atlanta, Georgia. Our 2030 incidence projections absent interventions aligned closely with this model, but we projected greater reductions from improved testing (Supplement Tables 4 to 6). The individual-based framework of Jenness and colleagues can explore network effects that our compartmental model cannot, whereas by including other risk groups, we can evaluate interventions for a broader population. Our results expand on these existing models by providing projections across 32 MSAs for a broad spectrum of intervention scenarios.
Our approach has important strengths. First, by representing individual MSAs, our projections reflect the unique conditions of local epidemics. Second, our model is calibrated to epidemiologic data at the intersection of age, race, sex, and HIV risk factor, allowing us to consider targeted interventions that focus on specific demographic and risk subgroups. Third, our Bayesian calibration process replicates a broad array of epidemiologic calibration targets and explores possible scenarios that could lead to the observed epidemic in each city. This enables the model to be fitted to additional locations as long as sufficient epidemiologic data are available. Finally, we provide a publicly available web tool that allows users to visualize projections for all 32 MSAs and also design custom interventions.
Like with any model, our results should be interpreted in light of several limitations. First, we assume all HIV transmission occurs within an MSA. These high-burden cities have more transmission than surrounding areas, so imported cases are likely relatively infrequent, but exclusion of imported and exported cases could result in over-or underestimating the total effect of local interventions. Second, we framed PrEP coverage as the proportion of “persons at risk for HIV infection” within a subgroup who are enrolled in a PrEP program. However, not all persons in a subgroup are equally at risk. Thus, our projections of PrEP effect could be underestimates (for example, if actual PrEP enrollees are higher risk than average) or overestimates (for example, if the highest-risk persons are the most difficult to enroll). We also modeled PrEP effectiveness on available short-acting regimens (60–62), which would underestimate the effect of emerging options, such as long-acting regimens (85). Third, we simplified the HIV continuum of care to include only persons who were diagnosed and virally suppressed versus unsuppressed. This framework enables us to test the effect of meeting specified targets for the overall proportion of persons who are virally suppressed (which could be achieved by a combination of linkage, care retention, reengagement, or adherence interventions). However, it does not allow us to evaluate the particular contributions of individual steps on the continuum. Finally, our projections presume that recent trends in HIV transmission, care, and prevention continue into the near future. This assumption is notably violated, for example, by the COVID-19 pandemic. Although early surveys suggest there have been alterations in transmission behaviors, testing, viral suppression, and PrEP uptake (86–89), the precise effects are unlikely to be clear for some time; 2 other models project a range of possible pandemic effects, from moderate decreases to moderate increases in HIV incidence (90, 91). If pandemic-related changes in HIV care are temporary, the relative effect of interventions may not change substantially; however, the projected ability to meet an absolute 90% incidence reduction may be over- or underestimated.
In conclusion, large reductions in HIV incidence are feasible but only with substantial investments that consider the local dynamics of HIV epidemics. However, reaching the goals set forth by the EHE initiative would require a level of intervention that is likely beyond the reach of most local jurisdictions. We present an interactive, locally tailored model that can help policymakers maximize the epidemiologic effect of HIV-focused interventions in their local environments.
Supplementary Material
Acknowledgment:
The authors thank Joseph Flack and Carolina Fojo for their help in developing the web tool.
Grant Support:
By grants K08MH118094, K01AI138853, P30AI094189, and T32HL007024 from the National Institutes of Health.
Footnotes
Supplement. Supplementary Material
Contributor Information
Anthony Todd Fojo, Johns Hopkins University School of Medicine, Baltimore, Maryland.
Melissa Schnure, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland.
Parastu Kasaie, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland.
David W. Dowdy, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland.
Maunank Shah, Johns Hopkins University School of Medicine, Baltimore, Maryland.
REFERENCES
- 1.Centers for Disease Control and Prevention. Diagnoses of HIV infection in the United States and dependent areas, 2018 (updated). HIV Surveillance Report. 2020;31:1–119. Accessed at www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-report-2018-updated-vol-31.pdf on 5/1/2021. [Google Scholar]
- 2.Fauci AS, Redfield RR, Sigounas G, et al. Ending the HIV epidemic: a plan for the United States. JAMA. 2019;321:844–845. doi: 10.1001/jama.2019.1343 [DOI] [PubMed] [Google Scholar]
- 3.Giroir BP. The time is now to end the HIV epidemic. Am J Public Health. 2020;110:22–24. doi: 10.2105/AJPH.2019.305380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Panagiotoglou D, Olding M, Enns B, et al. ; Localized HIV Modeling Study Group. Building the case for localized approaches to HIV: structural conditions and health system capacity to address the HIV/AIDS epidemic in six US cities. AIDS Behav. 2018;22:3071–3082. doi: 10.1007/s10461-018-2166-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knight GM, Dharan NJ, Fox GJ, et al. Bridging the gap between evidence and policy for infectious diseases: how models can aid public health decision-making. Int J Infect Dis. 2016;42:17–23. doi: 10.1016/j.ijid.2015.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fojo AT, Kendall EA, Kasaie P, et al. Mathematical modeling of “chronic” infectious diseases: unpacking the black box. Open Forum Infect Dis. 2017;4:ofx172. doi: 10.1093/ofid/ofx172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah M, Perry A, Risher K, et al. Effect of the US National HIV/AIDS Strategy targets for improved HIV care engagement: a modelling study. Lancet HIV. 2016;3:e140–6. doi: 10.1016/S2352-3018(16)00007-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shah M, Risher K, Berry SA, et al. The epidemiologic and economic impact of improving HIV testing, linkage, and retention in care in the United States. Clin Infect Dis. 2016;62:220–229. doi: 10.1093/cid/civ801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hollingsworth TD, Anderson RM, Fraser C. HIV-1 transmission, by stage of infection. J Infect Dis. 2008;198:687–93. doi: 10.1086/590501 [DOI] [PubMed] [Google Scholar]
- 10.Keitt TH. odeintr: C++ ODE Solvers Compiled on-Demand. R Foundation for Statistical Computing; 2017. Accessed at https://cran.r-project.org/web/packages/odeintr/index.html on 10/1/2020. [Google Scholar]
- 11.R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing; 2020. Accessed at www.r-project.org/ on X.
- 12.Centers for Disease Control and Prevention. NCHHSTP AtlasPlus. Accessed at www.cdc.gov/nchhstp/atlas/index.htm on 10/1/2020.
- 13.Centers for Disease Control and Prevention . Diagnoses of HIV infection among adults and adolescents in metropolitan statistical areas—United States and Puerto Rico, 2017. HIV Surveillance Supplemental Report. 2019;24:1–87. Accessed at www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-supplemental-report-vol-24-2.pdf on 10/1/2020. [Google Scholar]
- 14.Centers for Disease Control and Prevention. Diagnoses of HIV infection among adults and adolescents in metropolitan statistical areas—United States and Puerto Rico, 2016. HIV Surveillance Supplemental Report. 2018;23:1–87. Accessed at www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-supplemental-report-vol-23-2.pdf on 10/1/2020. [Google Scholar]
- 15.Centers for Disease Control and Prevention. Diagnosed HIV infection among adults and adolescents in metropolitan statistical areas—United States and Puerto Rico, 2015. HIV Surveillance Supplemental Report. 2017;22:1–88. Accessed at www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-supplemental-report-vol-22-1.pdf on 10/1/2020. [Google Scholar]
- 16.Centers for Disease Control and Prevention. Diagnosed HIV infection among adults and adolescents in metropolitan statistical areas—United States and Puerto Rico, 2014. HIV Surveillance Supplemental Report. 2016;21:1–87. Accessed at www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-supplemental-report-vol-21-1.pdf on 10/1/2020. [Google Scholar]
- 17.Centers for Disease Control and Prevention. Diagnosed HIV infection among adults and adolescents in metropolitan statistical areas—United States and Puerto Rico, 2013. HIV Surveillance Supplemental Report. 2015;20:1–88. Accessed at www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-supplemental-report-vol-20-4.pdf on 10/1/2020. [Google Scholar]
- 18.Centers for Disease Control and Prevention. Diagnosed HIV infection among adults and adolescents in metropolitan statistical areas—United States and Puerto Rico, 2011. HIV Surveillance Supplemental Report. 2013;18:1–87. Accessed at www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-supplemental-report-vol-18-8.pdf on 10/1/2020. [Google Scholar]
- 19.Centers for Disease Control and Prevention. Diagnosed HIV infection among adults and adolescents in metropolitan statistical areas—United States and Puerto Rico, 2010. HIV Surveillance Supplemental Report. 2013;18:1–87. Accessed at www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-supplemental-report-vol-18-1.pdf on 10/1/2020. [Google Scholar]
- 20.AIDSVu. Mapping PrEP: first ever data on PrEP users across the U.S. Published 6 March 2018. Accessed at https://aidsvu.org/prep/ on 10/1/2020.
- 21.Centers for Disease Control and Prevention. Behavioral Risk Factor Surveillance System (BRFSS) Data. United States Department of Health and Human Services, Centers for Disease Control and Prevention; 2013-2017. Accessed at https://www.cdc.gov/brfss/smart/Smart_data.htm on 10/1/202.. [Google Scholar]
- 22.U.S. Department of Health and Human Services, Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. National Survey on Drug Use and Health (NSDUH) 2014–2018.
- 23.AIDS Public Information Data Set (APIDS): U.S. Surveillance Data for 1981–2002. CDC WONDER online database. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for HIV, STD and TB Prevention; 2005. Accessed at https://wonder.cdc.gov/wonder/help/aids.html on 10/1/2021. [Google Scholar]
- 24.Haario H, Saksman E, Tamminen J. An adaptive Metropolis algorithm. Bernoulli. 2001;7:223–42. doi: 10.2307/3318737 [DOI] [Google Scholar]
- 25.Andrieu C, Thoms J. A tutorial on adaptive MCMC. Stat Comput. 2008;18:343–73. doi: 10.1007/s11222-008-9110-y [DOI] [Google Scholar]
- 26.Mean Absolute Percentage Error (MAPE). 2000:462. Accessed at https://link.springer.com/referenceworkentry/10.1007%2F1-4020-0612-8_580 on 6/30/2021. [Google Scholar]
- 27.U.S. Census Bureau. Intercensal Estimates 1990–2018. 2018. Accessed at https://data.census.gov/cedsci/ on 10/1/2020.
- 28.Grey JA, Bernstein KT, Sullivan PS, et al. Estimating the population sizes of men who have sex with men in US states and counties using data from the American Community Survey. JMIR Public Health Surveill. 2016;2:e14. doi: 10.2196/publichealth.5365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data—United States and 6 dependent areas, 2018. HIV Surveillance Supplemental Report. 2020;25:1–104. Accessed at www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-supplemental-report-vol-25-2.pdf on 10/1/2020. [Google Scholar]
- 30.Centers for Disease Control and Prevention. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data—United States and 6 dependent areas, 2017. HIV Surveillance Supplemental Report. 2019;24:1–74. Accessed at www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-supplemental-report-vol-24-3.pdf on 10/1/2020. [Google Scholar]
- 31.Centers for Disease Control and Prevention. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data—United States and 6 dependent areas, 2016. HIV Surveillance Supplemental Report. 2018;23:1–51. Accessed at www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-supplemental-report-vol-23-4.pdf on 10/1/2020. [Google Scholar]
- 32.Centers for Disease Control and Prevention. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data—United States and 6 dependent areas, 2015. HIV Surveillance Supplemental Report. 2017;22:1–63. Accessed at www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-supplemental-report-vol-22-2.pdf on 10/1/2020. [Google Scholar]
- 33.Centers for Disease Control and Prevention. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data—United States and 6 dependent areas, 2014. HIV Surveillance Supplemental Report. 2016;21:1–87. Accessed at www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-supplemental-report-vol-21-4.pdf on 10/1/2020. [Google Scholar]
- 34.Centers for Disease Control and Prevention. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data—United States and 6 dependent areas—2013. HIV Surveillance Supplemental Report. 2015;20:1–70. Accessed at www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-supplemental-report-vol-20-2.pdf on 10/1/2020. [Google Scholar]
- 35.Centers for Disease Control and Prevention. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data—United States and 6 dependent areas—2012. HIV Surveillance Supplemental Report. 2014;19:1–61. Accessed at www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-supplemental-report-vol-19-3.pdf on 10/1/2020. [Google Scholar]
- 36.Centers for Disease Control and Prevention. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data—United States and 6 dependent areas—2011. HIV Surveillance Supplemental Report. 2013;18:1–47. Accessed at www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-supplemental-report-vol-18-5.pdf on 10/1/2020. [Google Scholar]
- 37.Centers for Disease Control and Prevention. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data—United States and 6 U.S. dependent areas—2010. Part B. HIV Surveillance Supplemental Report. 2013;18:1–25. Accessed at www.cdc.gov/hiv/pdf/statistics_2010_HIV_Surveillance_Report_vol_18_no_2.pdf on 10/1/2020. [Google Scholar]
- 38.Centers for Disease Control and Prevention. HIV infection risk, prevention, and testing behaviors among persons who inject drugs—national HIV behavioral surveillance: injection drug use, 23 U.S. cities, 2018. HIV Surveillance Special Report 24. Accessed at www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-special-report-number-24.pdf on 10/1/2020.
- 39.Centers for Disease Control and Prevention. HIV infection risk, prevention, and testing behaviors among men who have sex with men—national HIV behavioral surveillance, 23 U.S. cities, 2017. HIV Surveillance Special Report 22. Accessed at www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-special-report-number-22.pdf on 10/1/2020.
- 40.Centers for Disease Control and Prevention. HIV infection, risk, prevention, and testing behaviors among heterosexuals at increased risk for HIV infection—national HIV behavioral surveillance, 17 U.S. cities, 2016. HIV Surveillance Special Report 19. Accessed at www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-report-number-19.pdf on 10/1/2020.
- 41.Centers for Disease Control and Prevention. HIV infection, risk, prevention, and testing behaviors among persons who inject drugs—national HIV behavioral surveillance: injection drug use, 20 U.S. cities, 2015. HIV Surveillance Special Report 18. Accessed at www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-special-report-number-18.pdf on 10/1/2020.
- 42.Centers for Disease Control and Prevention. HIV infection risk, prevention, and testing behaviors among men who have sex with men—national HIV behavioral surveillance, 20 U.S. cities, 2014. HIV Surveillance Special Report 15. Accessed at www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-special-report-number-15.pdf on 10/1/2020.
- 43.Centers for Disease Control and Prevention. HIV infection, risk, prevention, and testing behaviors among heterosexuals at increased risk of HIV infection—national HIV behavioral surveillance, 20 U.S. cities, 2013. HIV Surveillance Special Report 13. Accessed at www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-hssr_nhbs_het_2013.pdf on 10/1/2020.
- 44.Centers for Disease Control and Prevention. HIV infection, risk, prevention, and testing behaviors among persons who inject drugs—national HIV behavioral surveillance: injection drug use, 20 U.S. cities, 2012. HIV Surveillance Special Report 11. Revised August 2015. Accessed at www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-HSSR_NHBS_PWID_2012.pdf on 10/1/2020.
- 45.Centers for Disease Control and Prevention. HIV risk, prevention, and testing behaviors—national HIV behavioral surveillance system: men who have sex with men, 20 U.S. cities, 2011. HIV Surveillance Special Report 8. Accessed at www.cdc.gov/hiv/pdf/HSSR_8_NHBS_MSM_PDF-03.pdf on 10/1/2020.
- 46.Pathela P, Hajat A, Schillinger J, et al. Discordance between sexual behavior and self-reported sexual identity: a population-based survey of New York City men. Ann Intern Med. 2006;145:416–25. [DOI] [PubMed] [Google Scholar]
- 47.Jenness SM, Neaigus A, Hagan H, et al. Heterosexual HIV and sexual partnerships between injection drug users and noninjection drug users. AIDS Patient Care STDS. 2010;24:175–81. doi: 10.1089/apc.2009.0227 [DOI] [PubMed] [Google Scholar]
- 48.Bohl DD, McFarland W, Raymond HF. Improved measures of racial mixing among men who have sex with men using Newman’s assortativity coefficient. Sex Transm Infect. 2011;87:616–20. doi: 10.1136/sextrans-2011-050103 [DOI] [PubMed] [Google Scholar]
- 49.Mustanski B, Birkett M, Kuhns LM, et al. The role of geographic and network factors in racial disparities in HIV among young men who have sex with men: an egocentric network study. AIDS Behav. 2015;19:1037–47. doi: 10.1007/s10461-014-0955-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fujimoto K, Williams ML. Racial/ethnic differences in sexual network mixing: a log-linear analysis of HIV status by partnership and sexual behavior among most at-risk MSM. AIDS Behav. 2015;19:996–1004. doi: 10.1007/s10461-014-0842-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hamilton DT, Morris M. The racial disparities in STI in the U.S.: concurrency, STI prevalence, and heterogeneity in partner selection. Epidemics. 2015;11:56–61. doi: 10.1016/j.epidem.2015.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chow EPF, Read TRH, Law MG, et al. Assortative sexual mixing patterns in male-female and male-male partnerships in Melbourne, Australia: implications for HIV and sexually transmissible infection transmission. Sex Health. 2016;13:451–456. doi: 10.1071/SH16055 [DOI] [PubMed] [Google Scholar]
- 53.Smith MK, Graham M, Latkin CA, et al. Quantifying potentially infectious sharing patterns among people who inject drugs in Baltimore, USA. Epidemiol Infect. 2018;146:1845–1853. doi: 10.1017/S0950268818002042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Glick SN, Burt R, Kummer K, et al. Increasing methamphetamine injection among non-MSM who inject drugs in King County, Washington. Drug Alcohol Depend. 2018;182:86–92. doi: 10.1016/j.drugalcdep.2017.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Twenge JM, Sherman RA, Wells BE. Declines in sexual frequency among American adults, 1989–2014. Arch Sex Behav. 2017;46:2389–2401. doi: 10.1007/s10508-017-0953-1 [DOI] [PubMed] [Google Scholar]
- 56.Abma JC, Martinez GM. Sexual activity and contraceptive use among teenagers in the United States, 2011–2015. Natl Health Stat Report. 2017:1–23. [PubMed] [Google Scholar]
- 57.Lindau ST, Schumm LP, Laumann EO, et al. A study of sexuality and health among older adults in the United States. N Engl J Med. 2007;357:762–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Eisinger RW, Dieffenbach CW, Fauci AS. HIV viral load and transmissibility of HIV infection: undetectable equals untransmittable. JAMA. 2019;321:451–452. doi: 10.1001/jama.2018.21167 [DOI] [PubMed] [Google Scholar]
- 59.Centers for Disease Control and Prevention. Preexposure prophylaxis for the prevention of HIV infection in the United States—2017 update: a clinical practice guideline. Published March 2018. Accessed at www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2017.pdf on 5/1/2021.
- 60.McCormack S, Dunn DT, Desai M, et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet. 2016;387:53–60. doi: 10.1016/S0140-6736(15)00056-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baeten JM, Donnell D, Ndase P, et al. ; Partners PrEP Study Team. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399–410. doi: 10.1056/NEJMoa1108524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Choopanya K, Martin M, Suntharasamai P, et al. ; Bangkok Tenofovir Study Group. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2013;381:2083–90. doi: 10.1016/S0140-6736(13)61127-7 [DOI] [PubMed] [Google Scholar]
- 63.Des Jarlais DC, Marmor M, Paone D, et al. HIV incidence among injecting drug users in New York City syringe-exchange programmes. Lancet. 1996;348:987–91. [DOI] [PubMed] [Google Scholar]
- 64.Monterroso ER, Hamburger ME, Vlahov D, et al. Prevention of HIV infection in street-recruited injection drug users. the collaborative injection drug user study (CIDUS). J Acquir Immune Defic Syndr. 2000;25:63–70. [DOI] [PubMed] [Google Scholar]
- 65.Bruneau J, Lamothe F, Franco E, et al. High rates of HIV infection among injection drug users participating in needle exchange programs in Montreal: results of a cohort study. Am J Epidemiol. 1997;146:994–1002. [DOI] [PubMed] [Google Scholar]
- 66.Hagan H, McGough JP, Thiede H, et al. Reduced injection frequency and increased entry and retention in drug treatment associated with needle-exchange participation in Seattle drug injectors. J Subst Abuse Treat. 2000;19:247–52. [DOI] [PubMed] [Google Scholar]
- 67.Strathdee SA, Celentano DD, Shah N, et al. Needle-exchange attendance and health care utilization promote entry into detoxification. J Urban Health. 1999;76:448–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huo D, Bailey SL, Ouellet LJ. Cessation of injection drug use and change in injection frequency: the Chicago Needle Exchange Evaluation Study. Addiction. 2006;101:1606–13. [DOI] [PubMed] [Google Scholar]
- 69.Biondi BE, Zheng X, Frank CA, et al. A literature review examining primary outcomes of medication treatment studies for opioid use disorder: what outcome should be used to measure opioid treatment success? Am J Addict. 2020;29:249–267. doi: 10.1111/ajad.13051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Marino S, Hogue IB, Ray CJ, et al. A methodology for performing global uncertainty and sensitivity analysis in systems biology. J Theor Biol. 2008;254:178–96. doi: 10.1016/j.jtbi.2008.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Branson BM, Handsfield HH, Lampe MA, et al. ; Centers for Disease Control and Prevention (CDC). Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55:1–17; quiz CE1–4. [PubMed] [Google Scholar]
- 72.Pitasi MA, Delaney KP, Oraka E, et al. Interval since last HIV test for men and women with recent risk for HIV infection-United States, 2006–2016. MMWR Morb Mortal Wkly Rep. 2018;67:677–681. doi: 10.15585/mmwr.mm6724a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Katz DA, Golden MR, Hughes JP, et al. HIV self-testing increases HIV testing frequency in high-risk men who have sex with men: a randomized controlled trial. J Acquir Immune Defic Syndr. 2018;78:505–512. doi: 10.1097/QAI.0000000000001709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jamil MS, Prestage G, Fairley CK, et al. Effect of availability of HIV self-testing on HIV testing frequency in gay and bisexual men at high risk of infection (FORTH): a waiting-list randomised controlled trial. Lancet HIV. 2017;4:e241–e250. doi: 10.1016/S2352-3018(17)30023-1 [DOI] [PubMed] [Google Scholar]
- 75.Pathela P, Jamison K, Blank S, et al. The HIV pre-exposure prophylaxis (PrEP) cascade at NYC sexual health clinics: navigation is the key to uptake. J Acquir Immune Defic Syndr. 2020;83:357–364. doi: 10.1097/QAI.0000000000002274 [DOI] [PubMed] [Google Scholar]
- 76.Shover CL, Javanbakht M, Shoptaw S, et al. HIV preexposure prophylaxis initiation at a large community clinic: differences between eligibility, awareness, and uptake. Am J Public Health. 2018;108:1408–1417. doi: 10.2105/AJPH.2018.304623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rolle CP, Onwubiko U, Jo J, et al. PrEP implementation and persistence in a county health department setting in Atlanta, GA. AIDS Behav. 2019;23:296–303. doi: 10.1007/s10461-019-02654-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Granich R, Gupta S, Hall I, et al. Status and methodology of publicly available national HIV care continua and 90–90-90 targets: a systematic review. PLoS Med. 2017;14:e1002253. doi: 10.1371/journal.pmed.1002253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.UNAIDS. Fast-track: ending the AIDS epidemic by 2030. Published 18 November 2014. Accessed at www.unaids.org/sites/default/files/media_asset/JC2686_WAD2014report_en.pdf on 6/30/2021.
- 80.Bradley H, Rosenberg ES, Holtgrave DR. Data-driven goals for curbing the U.S. HIV epidemic by 2030. AIDS Behav. 2019;23:557–563. doi: 10.1007/s10461-019-02442-7 [DOI] [PubMed] [Google Scholar]
- 81.Zang X, Krebs E, Min JE, et al. ; Localized HIV Modeling Study Group. Development and calibration of a dynamic HIV transmission model for 6 US cities. Med Decis Making. 2020;40:3–16. doi: 10.1177/0272989X19889356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nosyk B, Zang X, Krebs E, et al. ; Localized HIV Modeling Study Group. Ending the HIV epidemic in the USA: an economic modelling study in six cities. Lancet HIV. 2020;7:e491–e503. doi: 10.1016/S2352-3018(20)30033-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nosyk B, Zang X, Krebs E, et al. Ending the epidemic in America will not happen if the status quo continues: modeled projections for human immunodeficiency virus incidence in 6 US cities. Clin Infect Dis. 2019;69:2195–2198. doi: 10.1093/cid/ciz1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jenness SM, Johnson JA, Hoover KW, et al. Modeling an integrated HIV prevention and care continuum to achieve the Ending the HIV Epidemic goals. AIDS. 2020;34:2103–2113. doi: 10.1097/QAD.0000000000002681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Marshall BDL, Goedel WC, King MRF, et al. Potential effectiveness of long-acting injectable pre-exposure prophylaxis for HIV prevention in men who have sex with men: a modelling study. Lancet HIV. 2018;5:e498–e505. doi: 10.1016/S2352-3018(18)30097-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stephenson R, Chavanduka TMD, Rosso MT, et al. Sex in the time of COVID-19: results of an online survey of gay, bisexual and other men who have sex with men’s experience of sex and HIV prevention during the US COVID-19 epidemic. AIDS Behav. 2020:1–9. doi: 10.1007/s10461-020-03024-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shilo G, Mor Z. COVID-19 and the changes in the sexual behavior of men who have sex with men: results of an online survey. J Sex Med. 2020;17:1827–1834. doi: 10.1016/j.jsxm.2020.07.085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hochstatter KR, Akhtar WZ, Dietz S, et al. Potential influences of the COVID-19 pandemic on drug use and HIV care among people living with HIV and substance use disorders: experience from a pilot mHealth intervention. AIDS Behav. 2021;25:354–359. doi: 10.1007/s10461-020-02976-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pinto RM, Park S. COVID-19 pandemic disrupts HIV continuum of care and prevention: implications for research and practice concerning community-based organizations and frontline providers [Editorial]. AIDS Behav. 2020;24:2486–2489. doi: 10.1007/s10461-020-02893-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zang X, Krebs E, Chen S, et al. ; Localized HIV Modeling Study. The potential epidemiological impact of coronavirus disease 2019 (COVID-19) on the human immunodeficiency virus (HIV) epidemic and the cost-effectiveness of linked, opt-out HIV testing: a modeling study in 6 US cities. Clin Infect Dis. 2021;72:e828–e834. doi: 10.1093/cid/ciaa1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jenness SM, Guillou AL, Chandra C, et al. Projected HIV and bacterial STI incidence following COVID-related sexual distancing and clinical service interruption. medRxiv. Preprint posted online 21 October 2020. doi: 10.1101/2020.09.30.20204529 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


