Abstract
Polar auxin transport through plant tissue strictly requires polarly localized PIN proteins and uniformly distributed ABCB proteins. A functional synergy between the two types of membrane protein where their localizations overlap may create the degree of asymmetric auxin efflux required to produce polar auxin transport. We investigated this possibility by expressing ABCB4 and PIN2 in human embryonic kidney cells and measuring whole‐cell ionic currents with the patch‐clamp technique and CsCl‐based electrolytes. ABCB4 activity was 1.81‐fold more selective for Cl− over Cs+ and for PIN2 the value was 2.95. We imposed auxin gradients and determined that ABCB4 and PIN2 were 12‐fold more permeable to the auxin anion (IAA−) than Cl−. This measure of the intrinsic selectivity of the transport pathway was 21‐fold when ABCB4 and PIN2 were co‐expressed. If this increase occurs in plants, it could explain why asymmetric PIN localization is not sufficient to create polar auxin flow. Some form of co‐action or synergy between ABCB4 and PIN2 that increases IAA− selectivity at the cell face where both occur may be important. We also found that auxin stimulated ABCB4 activity, which may contribute to a self‐reinforcement of auxin transport known as canalization.
1. INTRODUCTION
A special mechanism for directing auxin through tissues to its sites of action was recognized even before the chemical structure of this important plant hormone was deduced (Went & Thimann, 1937). So‐called polar auxin transport was hypothesized to result from channels releasing the auxin anion (IAA−) across the plasma membrane, down a large thermodynamic gradient, more so at one end of the cell than the other (Goldsmith, 1977; Raven, 1975; Rubery & Sheldrake, 1974). Mathematical models indicated that a twofold higher permeability of the membrane to auxin at one end of each cell in a file would produce the auxin transport rates and high degree of directional bias through tissues (Goldsmith et al., 1981; Mitchison, 1981). The breakthrough discovery of PIN‐FORMED (PIN) proteins accorded well with the theory because their sequences are consistent with a solute transport function, they are localized at the plasma membrane predominantly at the downstream ends of auxin‐transporting cells, and mutant analysis showed them to be required for polar auxin transport (Chen et al., 1998; Gälweiler et al., 1998; Křeček et al., 2009; Okada et al., 1991; Wisniewska et al., 2006). When PIN proteins were shown to reduce the amount of radioactive auxin retained in non‐plant cells engineered to express them, indicating they were capable of auxin transport (Chen et al., 1998; Petrášek et al., 2006), the mechanism of polar auxin transport through tissues appeared to be well understood. However, a PIN‐only explanation became insufficient when members of the B subfamily of ATP‐Binding Cassette (ABCB) transporters were also shown to be essential for polar auxin transport (Noh et al., 2001; Terasaka et al., 2005), and also capable of promoting auxin efflux as indicated by auxin retention assays (Blakeslee et al., 2007; Cho et al., 2007; Geisler et al., 2005; Petrášek et al., 2006; Yang & Murphy, 2009). Of the two types of proteins required for polar auxin transport, only PIN proteins are localized predominantly at one end of cells. Yet the uniformly distributed ABCB proteins are also required for directionally biased release of auxin from cells. It is important to understand how the ABCB transporters play an essential role in the polar auxin transport mechanism. The twd1 mutation prevents multiple auxin‐related ABCB transporters from reaching the plasma membrane (Wu, Otegui, et al., 2010). The twd1 mutation does not affect the polar localization of PIN transporters in the same cells, yet it severely impairs polar auxin transport (Bouchard et al., 2006). Thus, asymmetrically placed PIN proteins appear insufficient to produce polar auxin transport through tissues. ABCB proteins at the plasma membrane are also required. An explanation of this requirement is needed in order to understand the polar auxin transport mechanism.
One published explanation of the requirement for ABCB proteins in polar auxin transport (Noh et al., 2003) required substantial modification after further investigation. Mutations in ABCB genes apparently do not grossly affect the localization of PIN proteins, but they appear to make PIN protein localization more sensitive to disruption by the Triton X‐100 detergent, indicating that ABCB proteins may stabilize PIN proteins in some particular membrane contexts (Titapiwatanakun et al., 2008).
A different potential explanation for why ABCB and PIN proteins are required for polar auxin transport is that they function differently when together than either does separately. This synergistic effect would occur where the two protein types co‐reside at the downstream face of each cell. Consistent with this synergistic function idea, ABCB and PIN proteins have been shown to interact physically (Blakeslee et al., 2007). For a critical review of the topic of ABCB and PIN functional cooperativity and its implications for polar auxin transport, see Geisler et al. (2017).
A functional synergy, or a new transport property that manifests when ABCB and PIN proteins are co‐expressed, may be difficult to detect with an auxin retention assay. A method that directly measures auxin movement across the membrane while controlling the variables that affect transport may be more effective. The electrophysiological patch‐clamp technique can be such a method if the transported substance bears an electric charge. At the pH of cytoplasm, essentially all of the auxin is IAA−, and release of the IAA− from cells is thermodynamically passive (Goldsmith, 1977; Raven, 1975; Spalding, 2013). Therefore, ABCB and PIN proteins are expected to be electrogenic, meaning their transport activities should produce an electric current that the patch‐clamp method may be able to measure. This approach is considered to be a rigorous method for characterizing the functions of plant electrogenic transporters (Dreyer et al., 1999).
A previous report established the feasibility of using heterologous expression and patch clamping to study plant ABCB protein activity (Cho et al., 2014). The ABCB19 protein from Arabidopsis was expressed in human embryonic kidney (HEK) cells. Whole‐cell patch clamp analyses using NaCl‐based electrolytes demonstrated that ABCB19 produced electrogenic transport activity. The ABCB19 currents were carried more by Cl− than Na+. The Cho et al. (2014) study was not designed to measure transport of IAA−. The present paper extends the approach of Cho et al. (2014) to determine if the patch‐clamp technique can directly measure IAA− transport by ABCB and PIN proteins and if some synergy resulting from their co‐expression can be detected. The experiments used ABCB4 and PIN2 because these members of the two protein families are expressed in the same outer cell layers of the Arabidopsis root; both are required for shootward polar auxin transport through the root epidermis (Band et al., 2014; Kubeš et al., 2012), and both participate in the root gravitropism response (Abas et al., 2006; Lewis et al., 2007). They are a logical pair of proteins to study separately and together with electrophysiological methods in order to advance our understanding of the polar auxin transport mechanism.
2. RESULTS
2.1. ABCB4 and PIN2 display weakly anion‐selective electrogenic activity
To test the hypothesis that ABCB4 and PIN2 transport activities are electrogenic and therefore may be studied with the patch clamp technique, cDNA encoding one or the other of these proteins was expressed in cultured HEK cells, which are commonly used for electrophysiological characterization of cloned membrane proteins (Lemtiri‐Chlieh & Ali, 2013). The bi‐cistronic transfection vector also encoded a fluorescent protein marker in order to determine which cells in a field were appropriate subjects. The principal cation in the pipette and bath electrolytes was Cs+, present at a concentration of 140 mM. Cs+ was chosen to preclude currents from the small amount of endogenous sodium and potassium channel activity in HEK cells. Cl− (140 mM) was the principal anion. In response to a voltage‐step protocol, cells expressing ABCB4 or PIN2 displayed time‐independent inward and outward currents that were at least threefold greater than currents recorded from controls cells transfected with a vector containing only the fluorescent marker (Figure 1a,b). Current–voltage (I–V) relationships in these symmetric CsCl conditions were approximately linear (Figure 1c). They reversed (I = 0) between −8 and −11 mV. A reversal potential (E rev ) of 0 mV was expected in this case of equal CsCl concentrations on either side of the membrane. An uncorrected junction potential (Neher, 1992) or some type of active transport process in the HEK cell may have caused this offset. We did not subtract or otherwise remove this offset in any of the analyses because it was similar in all cells.
FIGURE 1.
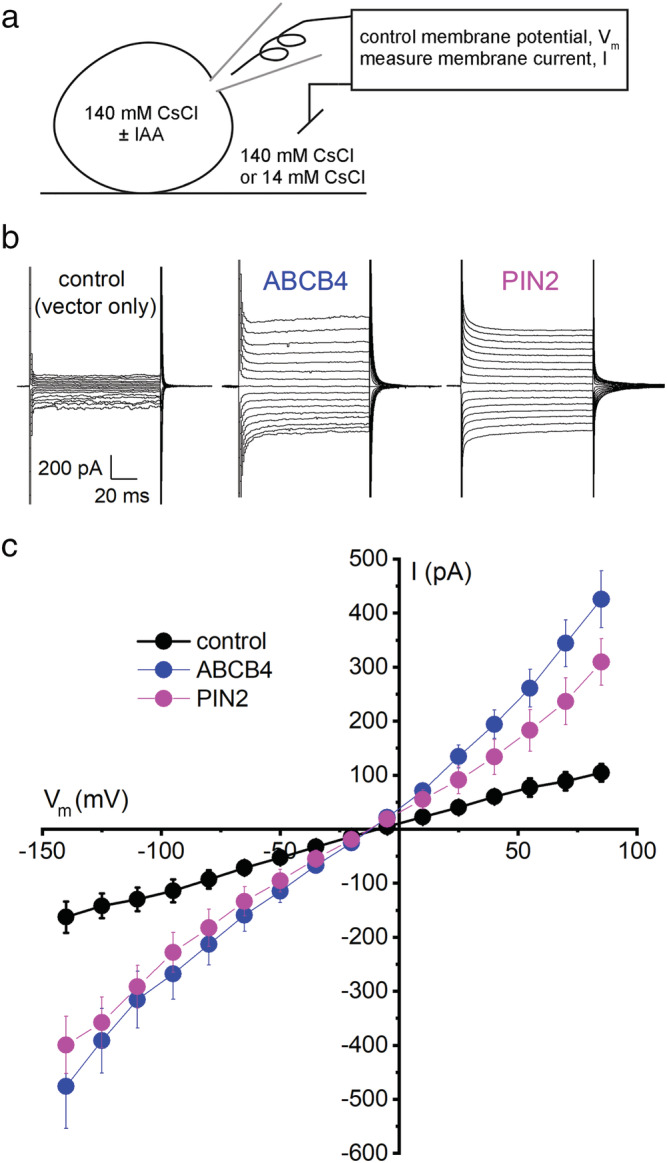
Electrogenic activities of ABCB4 and PIN2 proteins expressed in HEK cells. (a) Diagram of the whole‐cell patch clamp technique employing Cs+ and Cl− as principal charge carriers. The amplifier controlled (clamped) the membrane potential (V m ) while the transmembrane electric currents (I) were measured. The cells were transfected with a vector carrying only EGFP or DsRED cDNA (control), ABCB4 and EGFP cDNA in separate reading frames, or PIN2 and DsRED cDNA in separate reading frames. (b) Example recordings of transmembrane currents elicited by step‐wise changes in V m recorded from control cells and cells transfected with ABCB4 or PIN2. (c) I–V curves represent mean current ± SE at each membrane potential measured in control cells (n = 5), ABCB4‐expressing cells (n = 6), and PIN2‐expressing cells (n = 6). The pipette and bath solutions contained 140 mM CsCl
To determine whether Cs+ or Cl− carried the currents in cells expressing ABCB4 and PIN2, we imposed a concentration difference by shifting the bath solution, measured the resulting shift in reversal voltage of the I‐V curve (ΔErev), and then used the Goldman‐Hodgkin‐Katz (GHK) equation (Equation 1) to measure the membrane's permeability to Cl− (P Cl ) relative to Cs+ (P Cs ) as explained by (Hille, 2001).
| (1) |
R, T, and F have their usual thermodynamic meaning.
Reducing the CsCl concentration in the bath solution from 140 to 14 mM shifted the average reversal voltage (E rev ) of control cells by −11 mV (Figure 2a,d), but this difference was not statistically significant (p = 0.052). The same shift of the bath solution shifted the average E rev of ABCB4‐expressing cells significantly in the opposite direction by 12.2 mV (Figure 2a,d). Using Equation 1, a ΔE rev of 12.2 mV indicates the plasma membrane of cells expressing ABCB4 was 1.81 times as permeable to Cl− as it was to Cs+, that is, PCl:PCs = 1.81. PIN2‐expressing cells displayed a ΔE rev of 21.6 mV, corresponding to a PCl:PCs = 2.95 (Figure 2b,d). When ABCB4 and PIN2 were co‐expressed, the PCl:PCs of the membrane was also 2.95 (Figure 2c,d).
FIGURE 2.
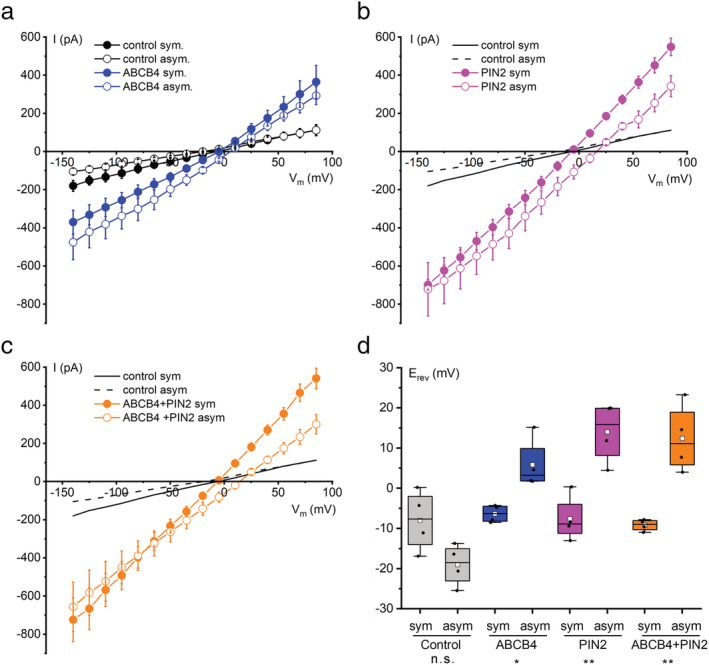
Current–voltage analysis of ABCB4, PIN2, and co‐expressed ABCB4 + PIN2 in symmetrical and asymmetrical conditions demonstrates anion preference. (a) ABCB4 and control cell I–V relationships recorded with 140 mM CsCl in the bath and pipette (symmetrical) and after switching the bath to 14 mM CsCl (asymmetrical). Plotted are the mean currents ± SE at each voltage obtained from four separate cells for each condition. (b) Same as (a) but for cells expressing PIN2. The control cell curves are re‐plotted from (a). (c) Same as (b) but for cells co‐expressing ABCB4 and PIN2. (d) The reversal potential (E rev ) values determined by linear regression performed on a limited portion of the I–V curve and displayed in a box plot. The positive shift in mean E rev (white square symbol within box) between symmetric and asymmetric conditions was used to calculate Cl− to Cs+ permeability ratios (PCl:PCs) with Equation 1 for ABCB4 (1.91), PIN2 (2.95), and ABCB4 + PIN2 (2.95). Notations on the bottom of the graph indicate whether the differences in E rev between the symmetrical and asymmetrical CsCl were not statistically significant (n.s.) or significant with *p < 0.05 or **p < 0.01
2.2. Assumptions
The above analyses and those that follow depend on a framework of assumptions.
The activity of the expressed protein is much greater than any similar activity inherent in the expression system. The currents in cells transfected with ABCB4 or PIN2 were at least threefold above the control cell (background) and endowed the membrane with a significant degree of an anion selectivity not observed in the control cells. Therefore, this assumption is probably valid. If the heterologous proteins did not completely dominate the membrane conductance, the selectivity values will be underestimated. The biological conclusions would probably not be affected.
The measured activity is not a secondary effect of the plant protein on a HEK cell transporter. HEK cells were chosen as an expression system because they have been called “a host of choice for transient heterologous expression of membrane proteins” in part because of low endogenous transporter expression (Ooi et al., 2016). The activities measured here are probably not a HEK cell transporter that responds to a foreign protein because ABCB4 and PIN2 share no structural features, yet both produced anion‐selective activities, which may be expected of proteins that transport IAA−. It seems unlikely that two structurally dissimilar proteins would create a similar artifact. Also, this assumption was tested in a previous study of ABCB19‐dependent currents in HEK cells. Mutating a single conserved amino acid in ABCB19 produced a null phenotype in a plant and prevented the protein from producing any currents in the HEK cell (Cho et al., 2014). A spurious secondary effect would probably not be eliminated by changing one amino acid in this very large protein. Pharmacological evidence also indicates this assumption is valid. The anion channel blocker 5‐nitro‐2‐(3‐phenylpropylamine)‐benzoic acid (NPPB) completely blocked the ABCB19 activity in HEK cells. Briefly pretreating seedlings with the same low concentration of NPPB also blocked polar auxin transport in Arabidopsis roots to the same extent as the abcb19 mutation and impaired gravitropism without affecting root growth rate (Cho et al., 2014). Thus, genetic and pharmacological results link the activity generated in HEK cells with in planta functions of the ABCB protein. We successfully used this platform to study the transport and gating functions of plant GLR proteins (Vincill et al., 2012, 2013). The results matched their in planta functions. No evidence of an artifactual activity was found.
Permeant ions act independently with respect to the conduction pathway. The GHK analysis assumes that the Cs+ ions do not affect the permeation of Cl−, and vice versa. If this assumption is not fully valid, the accuracy of the permeability ratios would be affected, but probably not the biological interpretations of the results to be presented.
Accepting these assumptions leads to the conclusion that ABCB4 and PIN2 perform thermodynamically passive, electrogenic transport. ABCB4 and PIN2 were found to be approximately twofold and threefold selective for Cl− over Cs+, respectively. Whether these ions are natural transport substrates depends on their in vivo cytoplasmic concentrations and the concentrations of other potentially permeant ions. Cs+ is not present in cells at a concentration that could be physiologically relevant, but Cl− would be. Whether the proteins preferentially transport another natural substrate, namely, IAA−, was addressed by the next set of experiments.
2.3. ABCB4 and PIN2 conduct IAA−
Imposing an auxin gradient will positively shift E rev if the expressed protein transports IAA−. Our ability to detect a ΔE rev due to IAA− transport will depend on the magnitude of the IAA− gradient across the membrane, the concentrations of other permeant ions (Cs+ and Cl− in this case), and the selectivity of the transporter for IAA− relative to other permeant ions (PIAA:P x ). We set the first and second of these at levels that should improve the ability to measure the third. We lowered the CsCl concentration to 50 mM and increased the concentration of auxin in the pipette solution from 0.1 μM to 1 mM IAA. Then, we measured I–V relations (Figure 3a,b). Failure of the glass/membrane seal was frequent in these low ionic strength conditions, but we successfully patch‐clamped 57 cells to complete a set of experiments that directly tested for electrogenic transport of IAA− by ABCB4 and PIN2. The results in Figure 3c show that significant positive shifts in E rev were observed in cells expressing ABCB4, PIN2, and ABCB4 + PIN2 but not in control cells. These IAA‐dependent shifts in E rev establish that ABCB4 and PIN2 transport IAA− across the membrane. No such activity could be detected in control cells. An artifactual activity resulting from a secondary effect of ABCB4 or PIN2 on an endogenous HEK cell protein (see above assumptions) would not be expected to respond to an auxin gradient. This is not the first example of a plant membrane protein that transports organic and inorganic anions. ALMT1 transports malate and Cl− as shown by I–V analysis of the protein in Xenopus oocytes. ALMT1 is activated by Al3+ and negatively regulated by gamma‐aminobutyric acid (Piñeros et al., 2008; Ramesh et al., 2015).
FIGURE 3.
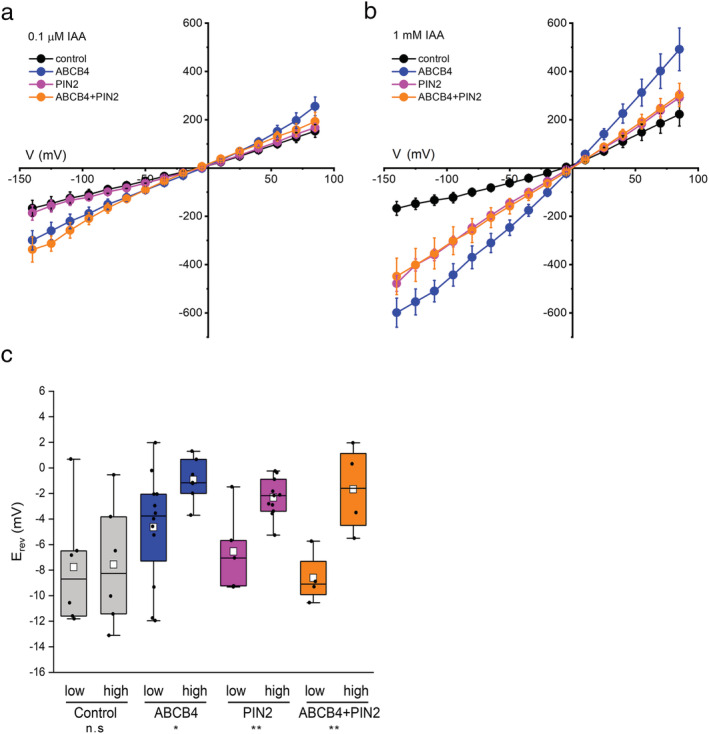
IAA− permeability demonstrated by current–voltage analysis. (a) I–V curves obtained in symmetrical 50 mM CsCl conditions with 0.1 μM IAA in the pipette, that is, on the cytoplasmic side. (b) I–V curves obtained as in (a) but with 1 mM IAA in the pipette. The number of independent cells measured per condition was between 4 and 14. (c) Reversal potentials (E rev ) of I–V curves obtained with either 0.1 μM IAA (low) or 1 mM IAA (high) in the pipette displayed in box plots with the means denoted by white square symbols. The E rev value for each trial (independent cell) was determined by linear regression performed on a limited portion of the I–V curve. Notations on the bottom of the graph indicate whether the differences in E rev between the low and high auxin concentrations were not statistically significant (n.s.), or significant with *p < 0.05 or **p < 0.01
2.4. Synergistic effect of co‐expression on IAA− selectivity
Evidence of an interaction between ABCB4 and PIN2 was found by analyzing the IAA‐dependent shifts in E rev with a GHK‐based model of the experiment (Equation 2). The result appears to help explain the mechanism of polar auxin transport.
| (2) |
The values used to parameterize the GHK model (Equation 2) were derived from the data in Figure 2d. PCl:PCs was 1.81 for ABCB4, 2.95 for PIN2, and 2.95 for ABCB4 + PIN2. The curves for PIN2 and ABCB4 + PIN2 overlap because their PCl:PCs ratios were identical. The resulting curves plotted in Figure 4 show that a high degree of IAA− selectivity equates with a shift in E rev of only a few millivolts. The model shows that large shifts in E rev should not be expected in these ionic conditions, consistent with the measured values of 3.7 mV for ABCB4, 4.2 mV for PIN2, and 6.9 mV for ABCB4 + PIN2. The model shows these shifts reflect a PIAA:PCl of 12 for ABCB4, 12 for PIN2, and 21 for ABCB4 + PIN2. The results in Figures 3 and 4 demonstrate that ABCB4 and PIN2 proteins transport IAA− with a 12‐fold preference over Cl−. The co‐expression results indicate that the permeation pathway becomes even more selective for IAA− over Cl− when both proteins are present. Co‐expression did not increase the amount of activity; the magnitude of current did not increase (Figure 3b). Instead, the apparent increase in PIAA:PCl from 12 to 21 indicates that co‐expressing ABCB4 and PIN2 approximately doubled the contribution of IAA− ions to the transported current (Figure 4). The results are evidence of a synergism between the two types of proteins that increase the selectivity of the transport pathway for IAA− at least with respect to Cl−.
FIGURE 4.
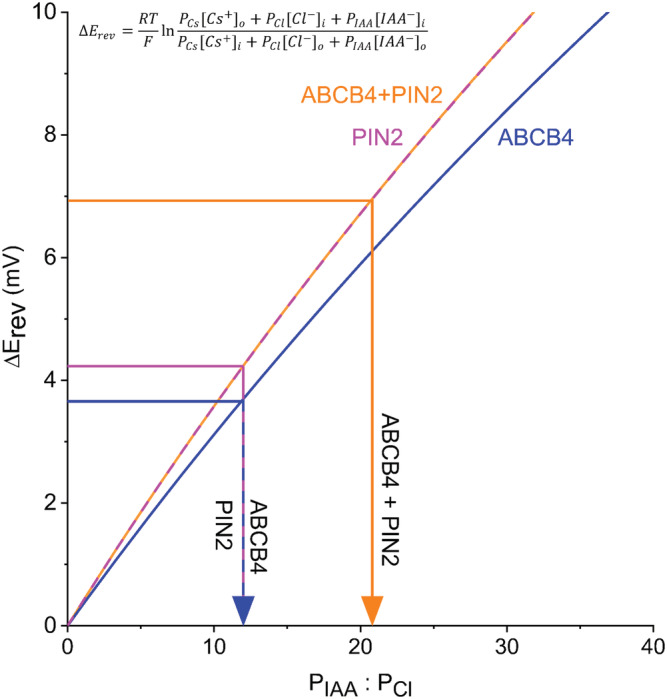
ABCB4 and PIN2 are highly selective for IAA− over Cl−, and the selectivity approximately doubles when the two are co‐expressed. The Goldman‐Hodgkin‐Katz model of membrane transport was parameterized with values of Cl− permeability (PCl) relative to Cs+ permeability (PCs) calculated from the results in Figure 2D to determine the permeability of IAA− relative to Cl− (PIAA: PCl) based on the ΔErev values presented in Figure 3D
2.5. Auxin stimulates the activity of ABCB4
Increasing the auxin concentration on the cytoplasmic side increased the magnitude of the currents in cells expressing ABCB4, which can be seen by comparing the I–V curves in Figure 3a,b. The increase in negative currents could be explained by there being a higher concentration of a selectively transported substrate, IAA−, on the cytoplasmic side. However, the increased positive currents cannot be explained by more IAA− substrate on the cytoplasmic side. Instead, the results indicate that auxin stimulated the activity of ABCB4, in addition to being an electrogenically transported substrate. Additional experiments were performed to investigate the potential for auxin regulation of an auxin transporter's activity, which is a fundamentally different topic than the effects of the auxin gradient on E rev . Figure 5a shows that increasing IAA concentration in the pipette from 0.1to 1 μM increased the negative and positive currents in ABCB4‐expressing cells by approximately 30% but did not affect control cells. Increasing it further to 1 mM further increased the negative currents again by 30%, but the positive currents did not increase. One possible explanation for the lack of increase in positive currents when auxin was increased from 1 μM to 1 mM is that ABCB4 becomes more selective for IAA− when auxin concentrations increase. Positive currents due to Cs+ efflux or Cl− influx were possibly limited by a more stringently selective ABCB4 at the highest auxin concentration. The effect of increasing auxin to 1 mM on the currents in PIN2‐expressing cells could be explained by an increase in substrate (permeant ion) concentration. There is less evidence in I–V curves of an activity‐stimulating effect of auxin. The negative currents did not increase when auxin concentration increased from 0.1 to 1 μM, and none would not be expected if auxin was only a transport substrate and not also an activator. Only at the highest concentration did auxin increase the negative and possibly also the positive currents. This effect would be expected if the transporter were presented with a significant concentration (relative to Cl−) of a permeant, transportable, substrate. In contrast to ABCB4, there appears to be no evidence of auxin stimulating PIN2 transport activity within the presumed physiologically relevant range of 0.1–1 μM (Figure 5b).
FIGURE 5.
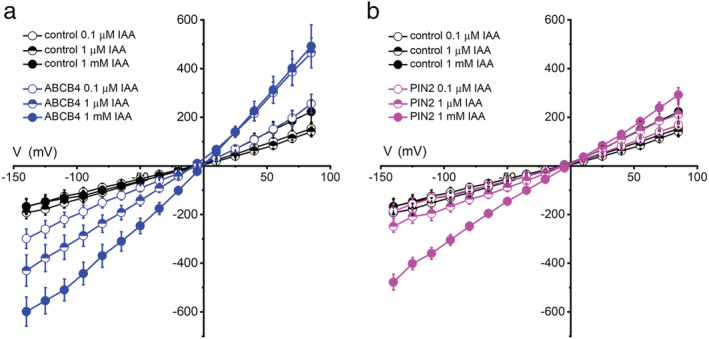
Auxin stimulates ABCB4 activity. (a) I–V relationships for ABCB4 obtained with different concentrations of IAA− in the pipette. (b) I–V curves for PIN2 obtained with different concentrations of IAA− in the pipette. The pipette and bath solutions contained 50 mM CsCl. The same control data are plotted in (a) and (b); the 0.1 μM IAA and 1 mM IAA data are replotted from Figure 3. The 1 μM IAA data are mean currents ± SE obtained from six control cells, four ABCB4 cells, and five PIN2 cells. The increase in negative currents in ABCB4‐expressing cells is evidence of auxin‐stimulated IAA− transport. PIN2 did not display this regulatory behavior
Benzoic acid (BA) is often used as a control compound in polar auxin transport assays. It did not stimulate ABCB4 or PIN2 channel activity (Figure 6a,b). Auxin is structurally related to the amino acid tryptophan (Trp), which did not stimulate activity like auxin (Figure 6a,b). These results demonstrate that auxin activates ABCB4 and PIN2 transport, but another weak acid with an aromatic ring (BA) and a biosynthetic precursor (Trp) did not. Activation specifically by auxin would not be expected of an artifactual activity, further evidence in favor of one of the underlying assumptions discussed above.
FIGURE 6.
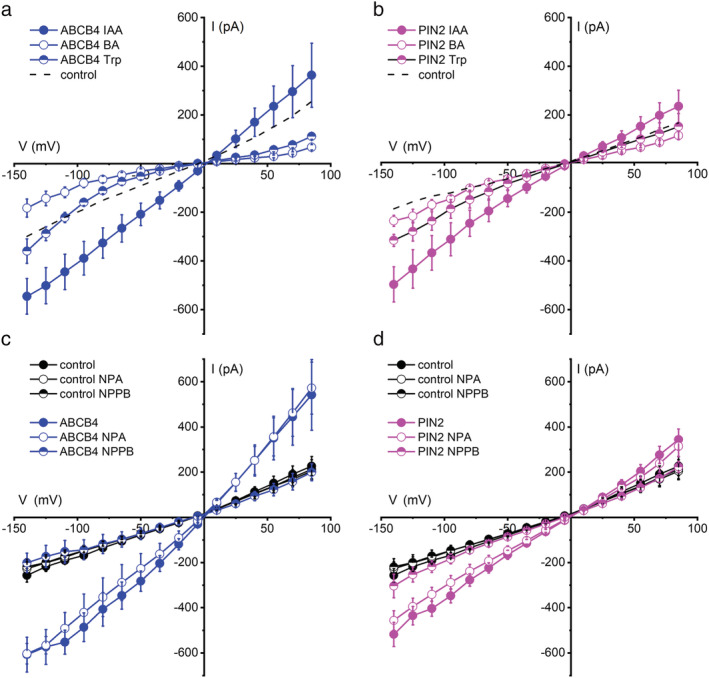
Activation specificity and pharmacology of ABCB4 and PIN2 activity. (a) Neither the indole‐based amino acid Trp nor the aromatic benzoic acid (BA) activates ABCB4 similarly to IAA. The dashed line shows the 0.1 μM IAA ABCB4 baseline copied from Figure 5a. (b) Same as (a) but for PIN2. The dashed line shows the 0.1 μM IAA PIN2 baseline copied from Figure 5B. (c) ABCB4 activity in auxin‐stimulated conditions is blocked by 20 μM NPPB but not 10 μM NPA applied by switching the bath solution. Neither treatment significantly affected the control cell currents. (d) Same as (c) but for PIN2. Plotted is mean current ± SE at each voltage for n = 4 or 6 independent cells for each treatment. The pipette and bath contained 50 mM CsCl. Untreated control cell data are replotted from Figure 5a
These experiments also showed that BA and Trp were not transported by ABCB4 because E rev did not shift significantly when 0.1 μM IAA in the pipette was replaced with 1 mM BA or Trp (Figure S1). In this set of experiments, 1 mM IAA served as a positive control, and it served as a second test of IAA− transport. Again, 1 mM IAA shifted E rev significantly to a more positive voltage in cells expressing ABCB4 (Figure S1). We conclude that ABCB4 transported IAA− but not Trp or BA. Very similar results were obtained for PIN2. The only exception is that PIN2 transported BA, consistent with a previous report (Geisler et al., 2005).
2.6. NPPB but not NPA inhibits ABCB4 and PIN2 activity
NPPB is used to block anion channels including those encoded by mammalian ABC transporters (Csanády & Töröcsik, 2014; Wang et al., 2005), and it reversibly blocks Cl−‐permeable channels in the Arabidopsis plasma membrane (Cho & Spalding, 1996). It displays a half‐inhibition concentration of approximately 5 μM (Noh & Spalding, 1998). The Arabidopsis ABCB19 gene was originally isolated by screening for NPPB‐induced genes (Noh et al., 2001). ABCB19 was shown to possess NPPB‐inhibited activity when expressed in HEK cells (Cho et al., 2014). NPPB also blocks polar auxin transport in roots as effectively as null abcb19 mutations (Cho et al., 2014). Consistent with these results, 20 μM NPPB completely blocked ABCB4 channel activity (Figure 6c). The chemical N‐1‐naphthylphthalamic acid (NPA) is widely used in the low micromolar range to inhibit polar auxin transport and has been reported to bind to ABCB19 (Kim et al., 2010), but it did not inhibit ABCB19 currents in HEK cells (Cho et al., 2014). Figure 6c shows that 10 μM NPA did not inhibit ABCB4 activity either. PIN2 currents displayed the same pharmacological profile. NPPB but not NPA was an effective inhibitor (Figure 6c,d). The lack of an inhibitory effect of NPA on PIN2‐mediated currents carrying IAA− is consistent with a recent report (Abas et al., 2021) that NPA binds to PIN proteins at a dimerization interface, considered to be “distinct from IAA substrate‐binding sites” and therefore probably not part of the intrinsic transport pathway.
HEK cells possess an anion channel that could potentially confound the heterologous expression approach used in the present study (see Section 2.2). However, the HEK cell must be swelled in hypotonic conditions to observe this endogenous activity and 100 μM NPPB is required to suppress it (Hélix et al., 2003). Therefore, this endogenous volume‐regulated anion channel (VRAC) probably did not contribute to the present results.
The Dataset S1 contains the 191 individual I‐V data sets used to generate the results in Figures 1, 2, 3, 4, 5, 6. Each entry is an independent trial obtained from a separate transfected HEK cell.
3. DISCUSSION
Previous studies demonstrated that ABCB19 and PIN1 physically interact strongly enough to enable coimmunoprecipitation of the pair (Blakeslee et al., 2007). Mravec et al. (2008) concluded that ABCBs and PINs “interact intermolecularly at the PIN‐containing polar domain.” The PIAA:PCl measurements in Figure 4 indicate an important functional effect can be expected as a result of the interaction. Where ABCB4 and PIN2 co‐reside, they form an electrogenic transport activity that is 21‐fold more selective for IAA− over Cl−, compared to 12‐fold for either protein by itself. The large increase in auxin selectivity is a fundamentally different effect than the increase in transport capacity that Blakeslee et al. (2007) measured when ABCB and PIN proteins were co‐expressed in yeast and HeLa cells. In that case, co‐transfection with ABCB and PIN genes may have produced the observed greater transport activity by producing more transporters compared to cells transfected with only one gene. In the present study, co‐expression of ABCB4 and PIN2 enhanced an intrinsic property of the conduction pathway in a way that cannot be explained by a difference in the number of proteins present. The results in Figure 4 show that ABCB4 and PIN2 co‐acted, or synergized, to make a more selective transport mechanism. Blakeslee et al. (2007) found PIN2 could transport BA and that co‐expression of ABCB1 reduced that activity. This could be interpreted as evidence of an ABCB protein increasing the selectivity of a PIN protein, consistent with the biophysical evidence shown here.
The co‐action of ABCB4 and PIN2 would be expected where both proteins reside, which is at the shootward ends of epidermal and lateral root cap cells. There the currents they facilitate would be enriched approximately twofold for IAA− relative to currents across the membrane at other cell faces, according to the analysis shown in Figure 4. The magnitude of this enrichment matches the twofold permeability difference between opposite cell ends that mathematical models have shown will produce the measured rate and directional bias of auxin transport through cell files (Goldsmith et al., 1981; Mitchison, 1981). The special accumulation of PIN2 at the shootward end of these root cells is required for polar auxin transport. It should increase the capacity for IAA efflux at this cell face. The new permeability analyses presented here indicate that the presence of ABCB4 in this membrane domain would increase selectivity for IAA−. The enhanced selectivity effect of ABCB4‐PIN2 interaction at one end of the cell may be a key aspect of the cellular asymmetry underpinning the polar auxin transport phenomenon. This could explain why properly positioned PIN proteins in the absence of ABCB proteins is not sufficient to produce polar auxin transport. A diagram summarizing this explanation is shown in Figure 7.
FIGURE 7.
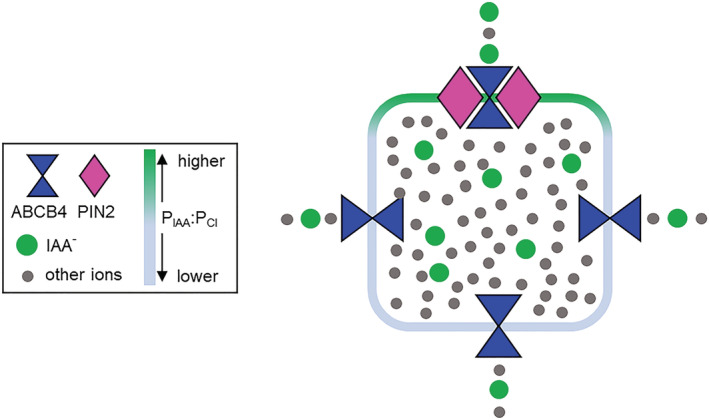
Diagram summarizing how the intrinsic and synergistic properties of ABCB4 and PIN2 measured by patch clamping may produce the twofold asymmetry in P IAA across a cell that results in polar auxin transport through tissues. The diagram shows that ion flux across the end of a cell containing ABCB4 and PIN2 is twofold enriched for IAA− compared to faces of the cell that do not have both components. A synergy or co‐action between ABCB4 and PIN2 that enhances selectivity for IAA− could explain why the phenomenon of polar auxin transport requires ABCB4 in addition to polarly localized PIN proteins
If one accepts that ABCB4 and PIN2 did not enhance endogenous HEK cell conductances to create artifactual currents and that the genuine background HEK cell currents were small enough to ignore, then analyzing reversal potentials with an equation (GHK) derived from electrodiffusion theory is the standard way to quantify a heterologous protein's intrinsic permeability ratios (Hille, 1991). The GHK model showed ABCB4 and PIN2 each selected IAA− over Cl− by a factor of 12. Inferring physiological function from these values requires knowledge of the natural concentrations of potential substrates. An upper limit for the concentration of IAA− in the cytoplasm of Arabidopsis seedling cells is approximately 100 nM. This estimate is based on 19.4 picograms of free IAA per milligram (fresh weight) of Arabidopsis seedling (Wu, Cameron, et al., 2010), and IAA binding constants for the TIR1‐AUX/IAA receptors in the range of 10 to 100 nM (Calderón Villalobos et al., 2012). Cytoplasmic Cl− concentration, on the other hand, is at least 104‐fold greater (Lorenzen et al., 2004). Despite an intrinsic high selectivity for the hormone, a relatively high concentration of Cl− means it will also be transported. Thus, in a natural cellular context, ABCB4 and PIN2 will conduct a current carried by IAA−, Cl−, and other potentially permeant ions.
It may be a mistake to consider the transport of inorganic ions by ABCB and PIN proteins as a purposeless and unavoidable consequence of relatively low auxin concentration. The transport of Cl− and potentially other permeant ions may be physiologically important, as may be the case in the best‐studied human ABCB transporter, the multidrug‐resistance P‐glycoprotein (Fletcher et al., 2010; Higgins, 1995; Hoffman et al., 1996; Roepe, 2000; Valverde et al., 1992). One possible function for the currents in addition to carrying IAA− is to balance other electrogenic activity at the membrane. By convention in the field of cell physiology, negative membrane currents are those carried by anions moving out of the cytoplasm or cations moving in to the cytoplasm (Bertl et al., 1992). The opposite is true for positive currents. For example, a H+‐ATPase pumping proton outward across the plasma membrane creates a positive current. All currents in a circuit must sum to zero according to Kirchoff's laws, and ionic currents across a membrane are no exception. Thus, the positive current created by a plasma membrane H+‐ATPase must be offset by an equal amount of negative current. Auxin increases the activity of plasma membrane H+‐ATPases to promote cell expansion (Du et al., 2020). Auxin also stimulates the activity of ABCB4 (Figure 5). Auxin‐stimulated ABCB4 activity may facilitate the negative currents that balance the positive currents resulting from auxin‐stimulated H+‐ATPase activity. NPPB is a potent inhibitor of ABCB19 (Cho et al., 2014) as well as an inducer of its expression (Noh et al., 2001). NPPB also inhibits ABCB4 and PIN2 (Figure 6c,d), and anion channels of unknown molecular composition that can depolarize the membrane in Arabidopsis plasma membranes (Cho & Spalding, 1996). NPPB could be used to test the role of balancing currents mediated by ABCB and PIN proteins in auxin‐stimulated proton pumping. The results may show that auxin transport and auxin response are not completely independent processes. They may both depend on the electrogenic transport activity of ABCB and PIN proteins.
Auxin stimulation of ABCB4 activity (Figure 5a), especially if it occurs in other members of the ABCB family, would also contribute to the positive reinforcement of auxin transport by auxin transport itself. Self‐strengthening auxin transport is a key feature of canalization, the phenomenon thought to create auxin distribution patterns that guide many aspects of development (Bennett et al., 2014; Sauer et al., 2006; Stoma et al., 2008; van Berkel et al., 2013). The present findings of auxin‐stimulated ABCB4 transport activity and increased selectivity due to ABCB4 and PIN2 interactions would combine with auxin promotion of PIN expression to produce canalization (Bayer et al., 2009; Kramer, 2008; Smith & Bayer, 2009; van Berkel et al., 2013).
The results reported here establish a new category of evidence indicating that ABCB4 and PIN2 directly transport IAA− across a cell membrane. Similar approaches based on voltage‐clamp measurements of charge movement and membrane transport theory could determine if other ABCB proteins associated with auxin transport through tissues, such as ABCB1, ABCB6, ABCB19, and ABCB20 (Noh et al., 2001; Zhang et al., 2018), directly transport IAA− and synergistically affect IAA− selectivity when co‐expressed with appropriate PIN proteins. The experimental methods established here could be used to study ABCB protein structure–function relationships. One structure–function question to address is whether or not the proposed IAA binding sites in ABCB4 (Yang & Murphy, 2009) are responsible for auxin activation of its transport function (Figure 5a). Another is the role of predicted NPA binding sites (Kim et al., 2010) in transporter function, and if a D/E‐P motif (Hao et al., 2020) is important to the IAA− selectivity measurable by electrophysiological methods. The measurement platform could also be used to investigate the regulatory effects of co‐expressed kinases (Weller et al., 2017; Zourelidou et al., 2014). Especially important will be biophysical investigations of how enhanced selectivity apparently results from the interaction of ABCB4 and PIN2. The phenomenon may be analogous to the interaction between mammalian ABCC8 and the Kir6 (KATP) potassium channel which results in regulation not evident in either individual protein (Bryan & Aguilar‐Bryan, 1999; Burke et al., 2008). All of the above would benefit from improvements to the platform such as increasing expression of the plant proteins in HEK cells and finding ways to achieve larger auxin gradients across the membrane. The former would increase the activity to be measured and the latter would create larger shifts in E rev and therefore increase the precision with which IAA− selectivity could be measured. The resulting improved understanding of how auxin transport proteins create directionally biased, self‐reinforcing auxin flow would lead to more accurate mechanistic models of how auxin influences plant growth and development.
3.1. Materials and methods
3.1.1. HEK cell culture and transfection
HEK293T cells from American Type Culture Collection were cultured in Dulbecco's modified Eagle's medium‐GlutaMAX (Invitrogen) with 10% fetal bovine serum, 100 IU ml−1 penicillin, and 100 μg ml−1 streptomycin in an incubator set at 37°C with 95% air and 5% CO2. Prior to transfection, trypsin‐treated HEK293T cells (5 × 105 cells per well) were plated into six‐well tissue culture plates containing collagen‐coated glass cover slips 12 to 24 h before being transfected with 1 μg of the indicated plasmid DNA using FuGENE 6 transfection reagent (Promega) following the manufacturer's protocol. In the case of cotransfections, a plasmid ratio of 1:1 (0.5 μg + 0.5 μg) was used. All experiments were performed 36 to 48 h after transfection and imaged live at room temperature.
3.1.2. DNA cloning
For HEK293T cell expression and electrophysiology experiments, full length ABCB4 and PIN2 cDNA was amplified from total RNA by RT‐PCR as described in Vincill et al. (2012) using the following primers: 5′‐ATCTGTCGACATGGCTTCAGAGA GCGGCTTA‐3′ and 5′‐AATTCCCGGGTCAAGAAGCCGCGGTT‐3′. PCR products were then digested and inserted into the SalI and XmaI sites of the pIRES‐Enhanced Green Fluorescent Protein (EGFP) bicistronic vector used by Vincill et al. (2012) such that a single mRNA would separately code ABCB4 and EGFP. PIN2 was amplified from cDNA template using the following primers: 5′‐TATTTGTCGACATGATCACCGGCAAA GAC‐3′ and 5′‐ATCACCCGGGTTAAAGCCCCAAAAGAAC‐3′. PCR products were digested and inserted into the SalI and XmaI sites of the pIRES2‐DsRed bicistronic vector.
3.1.3. Electrophysiology
The methods and procedures used in this study were similar to those used in previous studies of ABCB19 and glutamate receptor channels expressed in HEK cells (Vincill et al., 2013; Cho et al., 2014. For whole‐cell recording, a coverslip with cells was placed in a recording chamber mounted on the fixed stage of an upright fluorescence microscope (Olympus BX51WI) mounted on an antivibration table equipped with a micromanipulator that controlled the head stage of the patch‐clamp amplifier (Axopatch 200A; Molecular Devices; www.moleculardevices.com). A 40X dipping objective lens was used to view the cells in bright‐field or fluorescence mode in the chamber, which was being continuously perfused with a bath solution containing 140 mM CsCl, 2 mM CaCl2, 2 mM MgCl2, 5 mM KCl, and 10 mM HEPES, adjusted to pH 6 with CsOH. The pipette was filled with 140 mM CsCl, 1 mM CaCl2, 2 mM MgCl2, 5 mM EGTA, 10 mM D‐Glc, 10 mM HEPES, and 3 mM Mg‐ATP, adjusted to pH 7.2 with CsOH. For experiments using 50 mM CsCl, 190 mM sorbitol was added to maintain osmolarity. Bath solutions containing only 14 mM CsCl solutions were supplemented with 252 mM sorbitol. When auxin gradients were imposed, the bath solution always included 0.1 μM IAA and the pipette contained the indicated auxin concentration. Cells displaying strong EGFP or DsRed fluorescence were selected for whole‐cell patch‐clamp analysis using micropipettes pulled from borosilicate glass. Micropipette resistance was between 5 and 8 megaohms when filled. After achieving a gigaohm seal, the patch was ruptured to obtain the whole‐cell configuration. After the baseline current stabilized, a voltage clamp protocol was administered by pCLAMP 10.2 software (Molecular Devices). The measured membrane currents were low‐pass filtered at 5 kHz and digitized at 10 kHz using a Digidata 1440A A/D converter (Molecular Devices). Steady‐state currents at each clamp voltage were measured with Clampfit 10.2 (Molecular Devices) software.
3.1.4. I‐V curve analysis
To obtain accurate and objective measurements of E rev (reversal potential), linear regression was used to fit a straight line to the I‐V data between −20 and 25 mV for each separate trial (cell). This typically linear region of the I–V curve always included the I = 0 point. The x intercept of the fitted line was taken as the value of E rev . Student's t tests were used to determine the statistical significance of differences where indicated. The GHK model of the IAA− permeability experiment was parameterized by setting PCl to 1, using the PCl:PCs values derived from the data in Figure 2c to obtain a relative value for PCs, and then plotting the result obtained when PIAA was varied (swept) across the range shown using the Origin 2020 (Origin Lab Corp) software package. This was done separately for ABCB4, PIN2, and ABCB4 + PIN2. The ΔE rev due to the imposed auxin gradient, determined from Figure 3c, was used to obtain the corresponding PIAA:PCl value from the plotted model curves by using the Origin 2020 data reader tool.
CONFLICT OF INTEREST
The Authors did not report any conflict of interest.
AUTHOR CONTRIBUTIONS
Edgar Spalding and Stephen Deslauriers designed the research; Stephen Deslauriers performed the experiments; Stephen Deslauriers and Edgar Spalding analyzed the data; Edgar Spalding and Stephen Deslauriers wrote the paper.
Supporting information
Supplemental Figure S1. Tests of benzoic acid (BA) and tryptophan (Trp) transport. A, ABCB4 transports auxin but not BA or Trp according to reversal potential analysis. B, PIN2 transports auxin and BA but not Trp. Plotted are individual Erev values obtained from separate patch‐clamped cells with the indicated substance present at the indicated concentration at the cytoplasmic side of the membrane. At the bottom of each graph, the following symbols indicate the statistical significance of a difference from the 0.1 μM IAA condition: n.s. = not significant, * = p < 0.05, ** = p < 0.01.
Supplemental Dataset S1. All of the current–voltage (I‐V) curves are contained in one comma separated value (csv) file. The results are grouped by the figure in which the mean values appear. All of the electrophysiology results in the paper can be reconstructed from these patch clamp trials.
ACKNOWLEDGMENT
This work was funded by National Science Foundation (NSF) grant IOS‐1360751 to E.P.S.
Deslauriers, S. D. , & Spalding, E. P. (2021). Electrophysiological study of Arabidopsis ABCB4 and PIN2 auxin transporters: Evidence of auxin activation and interaction enhancing auxin selectivity. Plant Direct, 5(11), e361. 10.1002/pld3.361
The author responsible for distribution of materials integral to the findings presented in this article is Edgar P. Spalding (spalding@wisc.edu).
REFERENCES
- Abas, L. , Benjamins, R. , Malenica, N. , Paciorek, T. , Wiśniewska, J. , Moulinier‐Anzola, J. C. , Sieberer, T. , Friml, J. , & Luschnig, C. (2006). Intracellular trafficking and proteolysis of the Arabidopsis auxin‐efflux facilitator PIN2 are involved in root gravitropism. Nature Cell Biology, 8, 249–256. 10.1038/ncb1369 [DOI] [PubMed] [Google Scholar]
- Abas, L. , Kolb, M. , Stadlmann, J. , Janacek, D. P. , Lukic, K. , Schwechheimer, C. , Sazanov, L. A. , Mach, L. , Friml, J. , & Hammes, U. Z. (2021). Naphthylphthalamic acid associates with and inhibits PIN auxin transporters. Proceedings of the National Academy of Sciences of the United States of America, 118(1), e2020857118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Band, L. R. , Wells, D. M. , Fozard, J. A. , Ghetiu, T. , French, A. P. , Pound, M. P. , Wilson, M. H. , Yu, L. , Li, W. , Hijazi, H. I. , Oh, J. , Pearce, S. P. , Perez‐Amador, M. A. , Yun, J. , Kramer, E. , Alonso, J. M. , Godin, C. , Vernoux, T. , Hodgman, T. C. , … Bennett, M. J. (2014). Systems analysis of auxin transport in the Arabidopsis root apex. Plant Cell, 26, 862–875. 10.1105/tpc.113.119495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer, E. M. , Smith, R. S. , Mandel, T. , Nakayama, N. , Sauer, M. , Prusinkiewicz, P. , & Kuhlemeier, C. (2009). Integration of transport‐based models for phyllotaxis and midvein formation. Genes & Development, 23, 373–384. 10.1101/gad.497009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett, T. , Hines, G. , & Leyser, O. (2014). Canalization: What the flux? Trends in Genetics, 30, 41–48. 10.1016/j.tig.2013.11.001 [DOI] [PubMed] [Google Scholar]
- Bertl, A. , Blumwald, E. , Coronado, R. , Eisenberg, R. , Findlay, G. , Gradmann, D. , Hille, B. , Köhler, K. , Kolb, H. A. , MacRobbie, E. , Meissner, G. , Miller, C. , Neher, E. , Palade, P. , Pantoja, O. , Sanders, D. , Schroeder, J. , Slayman, C. , Spanswick, R. , … Williams, A. (1992). Electrical measurements on endomembranes. Science, 258, 873–874. 10.1126/science.1439795 [DOI] [PubMed] [Google Scholar]
- Blakeslee, J. J. , Bandyopadhyay, A. , Lee, O. R. , Mravec, J. , Titapiwatanakun, B. , Sauer, M. , Makam, S. N. , Cheng, Y. , Bouchard, R. , Adamec, J. , Geisler, M. , Nagashima, A. , Sakai, T. , Martinoia, E. , Friml, J. , Peer, W. A. , & Murphy, A. S. (2007). Interactions among PIN‐FORMED and P‐glycoprotein auxin transporters in Arabidopsis. Plant Cell, 19, 131–147. 10.1105/tpc.106.040782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard, R. , Bailly, A. , Blakeslee, J. J. , Oehring, S. C. , Vincenzetti, V. , Lee, O. R. , Paponov, I. , Palme, K. , Mancuso, S. , Murphy, A. S. , Schulz, B. , & Geisler, M. (2006). Immunophilin‐like TWISTED DWARF1 modulates auxin efflux activities of Arabidopsis P‐glycoproteins. The Journal of Biological Chemistry, 281, 30603–30612. 10.1074/jbc.M604604200 [DOI] [PubMed] [Google Scholar]
- Bryan, J. , & Aguilar‐Bryan, L. (1999). Sulfonylurea receptors: ABC transporters that regulate ATP‐sensitive K+ channels. Biochimica et Biophysica Acta (BBA) – Biomembranes, 1858, 2959–3204. 10.1016/S0005-2736(99)00164-9 [DOI] [PubMed] [Google Scholar]
- Burke, M. A. , Mutharasan, R. K. , & Ardehali, H. (2008). The sulfonylurea receptor, an atypical ATP‐binding bassette protein, and its regulation of the KATP channel. Circulation Research, 102, 164–176. 10.1161/CIRCRESAHA.107.165324 [DOI] [PubMed] [Google Scholar]
- Calderón Villalobos, L. , Lee, S. , de Oliveira, C. , Ivetac, A. , Brandt, W. , Armitage, L. , Sheard, L. B. , Tan, X. , Parry, G. , Mao, H. , Zheng, N. , Napier, R. , Kepinski, S. , & Estelle, M. (2012). A combinatorial TIR1/AFB–Aux/IAA co‐receptor system for differential sensing of auxin. Nature Chemical Biology, 8, 477–485. 10.1038/nchembio.926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, R. , Hilson, P. , Sedbrook, J. , Rosen, E. , Caspar, T. , & Masson, P. H. (1998). The Arabidopsis thaliana AGRAVITROPIC 1 gene encodes a component of the polar‐auxin‐transport efflux carrier. Proceedings of the National Academy of Sciences of the United States of America, 95, 15112–15117. 10.1073/pnas.95.25.15112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho, M. , Henry, E. M. , Lewis, D. R. , Wu, G. , Muday, G. K. , & Spalding, E. P. (2014). Block of ATP‐binding cassette B19 ion channel activity by 5‐nitro‐2‐(3 ‐phenylpropylamino)‐benzoic acid impairs polar auxin transport and root gravitropism. Plant Physiology, 166, 2091–2099. 10.1104/pp.114.250860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho, M. , Lee, S. H. , & Cho, H. T. (2007). P‐glycoprotein4 displays auxin efflux transporter‐like action in Arabidopsis root hair cells and tobacco cells. Plant Cell, 19, 3930–3943. 10.1105/tpc.107.054288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho, M. H. , & Spalding, E. P. (1996). An anion channel in Arabidopsis hypocotyls activated by blue light. Proceedings of the National Academy of Sciences of the United States of America, 93, 8134–8138. 10.1073/pnas.93.15.8134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csanády, L. , & Töröcsik, B. (2014). Structure–activity analysis of a CFTR channel potentiator: Distinct molecular parts underlie dual gating effects. The Journal of General Physiology, 144, 321–336. 10.1085/jgp.201411246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer, I. , Horeau, C. , Lemaillet, G. , Zimmerman, S. , Bush, D. R. , Rodríguez‐Navarro, A. , Schachtman, D. P. , Spalding, E. P. , Sentenac, H. , & Gaber, R. F. (1999). Identification and characterization of plant transporters using heterologous expression systems. Journal of Experimental Botany, 50, 1073–1087. [Google Scholar]
- Du, M. , Spalding, E. P. , & Gray, W. M. (2020). Rapid auxin‐mediated cell expansion. Annual Review of Plant Biology, 71, 379–402. 10.1146/annurev-arplant-073019-025907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher, J. I. , Haber, M. , Henderson, M. J. , & Norris, M. D. (2010). ABC transporters in cancer: More than just drug efflux pumps. Nature Reviews Cancer, 10, 147–156. 10.1038/nrc2789 [DOI] [PubMed] [Google Scholar]
- Gälweiler, L. , Guan, C. , Müller, A. , Wisman, E. , Mendgen, K. , Yephremov, A. , & Palme, K. (1998). Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science, 282, 2226–2230. 10.1126/science.282.5397.2226 [DOI] [PubMed] [Google Scholar]
- Geisler, M. , Aryal, B. , di Donato, M. , & Hao, P. (2017). A critical view on ABC transporters and their interacting partners in auxin transport. Plant & Cell Physiology, 58, 1601–1614. 10.1093/pcp/pcx104 [DOI] [PubMed] [Google Scholar]
- Geisler, M. , Blakeslee, J. J. , Bouchard, R. , Lee, O. R. , Vincenzetti, V. , Bandyopadhyay, A. , Titapiwatanakun, B. , Peer, W. A. , Bailly, A. , Richards, E. L. , Ejendal, K. F. , Smith, A. P. , Baroux, C. , Grossniklaus, U. , Müller, A. , Hrycyna, C. A. , Dudler, R. , Murphy, A. S. , & Martinoia, E. (2005). Cellular efflux of auxin catalyzed by the Arabidopsis MDR/PGP transporter AtPGP1. The Plant Journal, 44, 179–194. 10.1111/j.1365-313X.2005.02519.x [DOI] [PubMed] [Google Scholar]
- Goldsmith, M. H. M. (1977). The polar transport of auxin. Annual Review of Plant Physiology, 28, 439–478. 10.1146/annurev.pp.28.060177.002255 [DOI] [Google Scholar]
- Goldsmith, M. H. M. , Goldsmith, T. H. , & Martin, M. H. (1981). Mathematical analysis of the chemosmotic polar diffusion of auxin through plant tissues. Proceedings of the National Academy of Sciences of the United States of America, 78, 976–980. 10.1073/pnas.78.2.976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao, P. , Xia, J. , Liu, J. , di Donato, M. , Pakula, K. , Bailly, A. , Jasinski, M. , & Geisler, M. (2020). Auxin‐transporting ABC transporters are defined by a conserved D/E‐P motif regulated by a prolylisomerase. The Journal of Biological Chemistry, 295, 13094–13105. 10.1074/jbc.RA120.014104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hélix, N. , Strøbaek, D. , Dahl, B. , & Christophersen, P. (2003). Inhibition of the endogenous volume‐regulated anion channel (VRAC) in HEK293 cells by acidic di‐aryl‐ureas. The Journal of Membrane Biology, 196, 83–94. 10.1007/s00232-003-0627-x [DOI] [PubMed] [Google Scholar]
- Higgins, C. F. (1995). The ABC of channel regulation. Cell, 82, 693–696. 10.1016/0092-8674(95)90465-4 [DOI] [PubMed] [Google Scholar]
- Hille, B. (1991). Ionic channels of excitable membranes (p. 607). Oxford University Press. (ISBN‐10 0878933239) [Google Scholar]
- Hille, B. (2001). Ion channels of excitable membranes (3rd ed.). Sinauer Associates. [Google Scholar]
- Hoffman, M. M. , Wei, L. Y. , & Roepe, P. D. (1996). Are altered pHi and membrane potential in hu MDR 1 transfectants sufficient to cause MDR protein‐mediated multidrug resistance? The Journal of General Physiology, 108, 295–313. 10.1085/jgp.108.4.295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J. Y. , Henrichs, S. , Bailly, A. , Vincenzetti, V. , Sovero, V. , Mancuso, S. , Pollmann, S. , Kim, D. , Geisler, M. , & Nam, H. G. (2010). Identification of an ABCB/P‐glycoprotein‐specific inhibitor of auxin transport by chemical genomics. The Journal of Biological Chemistry, 285, 23309–23317. 10.1074/jbc.M110.105981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer, E. M. (2008). Computer models of auxin transport: A review and commentary. Journal of Experimental Botany, 59, 45–53. 10.1093/jxb/erm060 [DOI] [PubMed] [Google Scholar]
- Křeček, P. , Skupa, P. , Libus, J. , Naramoto, S. , Tejos, R. , Friml, J. , & Zazímalová, E. (2009). The PIN‐FORMED (PIN) protein family of auxin transporters. Genome Biology, 10, 249. 10.1186/gb-2009-10-12-249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubeš, M. , Yang, H. , Richter, G. L. , Cheng, Y. , Młodzińska, E. , Wang, X. , Blakeslee, J. J. , Carraro, N. , Petrášek, J. , Zažímalová, E. , Hoyerová, K. , Peer, W. A. , & Murphy, A. S. (2012). The Arabidopsis concentration‐dependent influx/efflux transporter ABCB4 regulates cellular auxin levels in the root epidermis. The Plant Journal, 69, 640–654. 10.1111/j.1365-313X.2011.04818.x [DOI] [PubMed] [Google Scholar]
- Lemtiri‐Chlieh, F. , & Ali, R. (2013). Characterization of heterologously expressed transporter genes by patch‐ and voltage‐clamp methods: Application to cyclic nucleotide‐dependent responses. Methods in Molecular Biology, 1016, 67–93. 10.1007/978-1-62703-441-8_6 [DOI] [PubMed] [Google Scholar]
- Lewis, D. R. , Miller, N. D. , Splitt, B. L. , Wu, G. , & Spalding, E. P. (2007). Separating the roles of acropetal and basipetal auxin transport on gravitropism with mutations in two Arabidopsis multidrug resistance‐like ABC transporter genes. Plant Cell, 19, 1838–1850. 10.1105/tpc.107.051599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzen, I. , Aberle, T. , & Plieth, C. (2004). Salt stress‐induced chloride flux: A study using transgenic Arabidopsis expressing a fluorescent anion probe. The Plant Journal, 38, 539–544. 10.1111/j.0960-7412.2004.02053.x [DOI] [PubMed] [Google Scholar]
- Mitchison, G. (1981). The polar transport of auxin and vein patterns in plants. Philosophical Transactions of the Royal Society B, 295, 461–471. 10.1098/rstb.1981.0154 [DOI] [Google Scholar]
- Mravec, J. , Kubes, M. , Bielach, A. , Gaykova, V. , Petrásek, J. , Skůpa, P. , Chand, S. , Benková, E. , Zazímalová, E. , & Friml, J. (2008). Interaction of PIN and PGP transport mechanisms in auxin distribution‐dependent development. Development, 135, 3345–3354. 10.1242/dev.021071 [DOI] [PubMed] [Google Scholar]
- Neher, E. (1992). Correction for liquid junction potentials in patch clamp experiments. Methods in Enzymology., 207, 123–131. 10.1016/0076-6879(92)07008-C [DOI] [PubMed] [Google Scholar]
- Noh, B. , Bandyopadhyay, A. , Peer, W. A. , Spalding, E. P. , & Murphy, A. S. (2003). Enhanced gravi‐ and phototropism in plant mdr mutants mislocalizing the auxin efflux protein PIN1. Nature, 423, 999–1002. 10.1038/nature01716 [DOI] [PubMed] [Google Scholar]
- Noh, B. , Murphy, A. S. , & Spalding, E. P. (2001). Multidrug resistance‐like genes of Arabidopsis required for auxin transport and auxin‐mediated development. Plant Cell, 13, 2441–2454. 10.1105/tpc.010350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh, B. , & Spalding, E. P. (1998). Anion channels and the stimulation of anthocyanin accumulation by blue light in Arabidopsis seedlings. Plant Physiology, 116, 503–509. 10.1104/pp.116.2.503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada, K. , Ueda, J. , Komaki, M. K. , Bell, C. J. , & Shimura, Y. (1991). Requirement of the auxin polar transport system in early stages of Arabidopsis floral bud formation. Plant Cell, 3, 677–684. 10.2307/3869249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooi, A. , Wong, A. , Esau, L. , Lemtiri‐Chlieh, F. , & Gehring, C. (2016). A guide to transient expression of membrane proteins in HEK‐293 cells for functional characterization. Frontiers in Physiology, 7, 300. 10.3389/fphys.2016.00300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrášek, J. , Mravec, J. , Bouchard, R. , Blakeslee, J. J. , Abas, M. , Seifertová, D. , Wisniewska, J. , Tadele, Z. , Kubes, M. , Covanová, M. , Dhonukshe, P. , Skupa, P. , Benková, E. , Perry, L. , Krecek, P. , Lee, O. R. , Fink, G. R. , Geisler, M. , Murphy, A. S. , … Friml, J. (2006). PIN proteins perform a rate‐limiting function in cellular auxin efflux. Science, 312, 914–918. 10.1126/science.1123542 [DOI] [PubMed] [Google Scholar]
- Piñeros, M. A. , Cançado, G. M. A. , & Kochian, L. V. (2008). Novel properties of the wheat aluminum tolerance organic acid transporter (TaALMT1) revealed by electrophysiological characterization in Xenopus oocytes: Functional and structural implications. Plant Physiology, 147, 2131–2146. 10.1104/pp.108.119636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh, S. A. , Tyerman, S. D. , Xu, B. , Bose, J. , Kaur, S. , Conn, V. , Domingos, P. , Ullah, S. , Wege, S. , Shabala, S. , Feijó, J. A. , Ryan, P. R. , & Gilliham, M. (2015). GABA signalling modulates plant growth by directly regulating the activity of plant‐specific anion transporters. Nature Communications, 6, 7879. 10.1038/ncomms8879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven, J. A. (1975). Transport of indoleacetic acid in plant cells in relation to pH and electrical potential gradients, and its significance for polar IAA transport. The New Phytologist, 74, 163–172. 10.1111/j.1469-8137.1975.tb02602.x [DOI] [Google Scholar]
- Roepe, P. D. (2000). What is the precise role of human MDR 1 protein in chemotherapeutic drug resistance. Current Pharmaceutical Design, 6, 241–260. 10.2174/1381612003401163 [DOI] [PubMed] [Google Scholar]
- Rubery, P. H. , & Sheldrake, A. R. (1974). Carrier‐mediated auxin transport. Planta, 118, 101–121. 10.1007/BF00388387 [DOI] [PubMed] [Google Scholar]
- Sauer, M. , Balla, J. , Luschnig, C. , Wisniewska, J. , Reinöhl, V. , Friml, J. , & Benková, E. (2006). Canalization of auxin flow by Aux/IAA‐ARF‐dependent feedback regulation of PIN polarity. Genes & Development, 20, 2902–2911. 10.1101/gad.390806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, R. S. , & Bayer, E. M. (2009). Auxin transport‐feedback models of patterning in plants. Plant, Cell & Environment, 32, 1258–1271. 10.1111/j.1365-3040.2009.01997.x [DOI] [PubMed] [Google Scholar]
- Spalding, E. P. (2013). Diverting the downhill flow of auxin to steer growth during tropisms. American Journal of Botany, 100, 203–214. 10.3732/ajb.1200420 [DOI] [PubMed] [Google Scholar]
- Stoma, S. , Lucas, M. , Chopard, J. , Schaedel, M. , Traas, J. , & Godin, C. (2008). Flux‐based transport enhancement as a plausible unifying mechanism for auxin transport in meristem development. PLoS Computational Biology, 4, e1000207. 10.1371/journal.pcbi.1000207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasaka, K. , Blakeslee, J. J. , Titapiwatanakun, B. , Peer, W. A. , Bandyopadhyay, A. , Makam, S. N. , Lee, O. R. , Richards, E. L. , Murphy, A. S. , Sato, F. , & Yazaki, K. (2005). PGP4, an ATP binding cassette P‐glycoprotein, catalyzes auxin transport in Arabidopsis thaliana roots. Plant Cell, 17, 2922–2939. 10.1105/tpc.105.035816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titapiwatanakun, B. , Blakeslee, J. J. , Bandyopadhyay, A. , Yang, H. , Mravec, J. , Sauer, M. , Cheng, Y. , Adamec, J. , Nagashima, A. , Geisler, M. , Sakai, T. , Friml, J. , Peer, W. A. , & Murphy, A. S. (2008). ABCB19/PGP19 stabilises PIN1 in membrane microdomains in Arabidopsis. The Plant Journal, 57, 27–44. 10.1111/j.1365-313X.2008.03668.x [DOI] [PubMed] [Google Scholar]
- Valverde, M. A. , Diaz, M. , Sepulveda, F. V. , Gill, D. R. , Hyde, S. C. , & Higgins, C. F. (1992). Volume‐regulated chloride channels associated with the human multidrug‐resistance P‐glycoprotein. Nature, 355, 830–833. 10.1038/355830a0 [DOI] [PubMed] [Google Scholar]
- van Berkel, K. , de Boer, R. J. , Scheres, B. , & ten Tusscher, K. (2013). Polar auxin transport: Models and mechanisms. Development, 140, 2253–2268. 10.1242/dev.079111 [DOI] [PubMed] [Google Scholar]
- Vincill, E. D. , Bieck, A. M. , & Spalding, E. P. (2012). Ca2+ conduction by an amino acid‐gated ion channel related to glutamate receptors. Plant Physiology, 159, 40–46. 10.1104/pp.112.197509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincill, E. D. , Clarin, A. E. , Molenda, J. N. , & Spalding, E. P. (2013). Interacting glutamate receptor‐like proteins in phloem regulate lateral root initiation. Plant Cell, 25, 1304–1313. 10.1105/tpc.113.110668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, W. , Li, G. , Clancy, J. P. , & Kirk, K. L. (2005). Activating cystic fibrosis transmembrane conductance regulator channels with pore blocker analogs. The Journal of Biological Chemistry, 280, 23622–23630. 10.1074/jbc.M503118200 [DOI] [PubMed] [Google Scholar]
- Weller, B. , Zourelidou, M. , Frank, L. , Barbosa, I. C. , Fastner, A. , Richter, S. , Jürgens, G. , Hammes, U. Z. , & Schwechheimer, C. (2017). Dynamic PIN‐FORMED auxin efflux carrier phosphorylation at the plasma membrane controls auxin efflux‐dependent growth. Proceedings of the National Academy of Sciences of the United States of America, 114(5), E887–E896. 10.1073/pnas.1614380114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Went, F. W. , & Thimann, K. V. (1937). Phytohormones. MacMillan. [Google Scholar]
- Wisniewska, J. , Xu, J. , Seifertová, D. , Brewer, P. B. , Ruzicka, K. , Blilou, I. , Rouquié, D. , Benková, E. , Scheres, B. , & Friml, J. (2006). Polar PIN localization directs auxin flow in plants. Science, 312, 883. 10.1126/science.1121356 [DOI] [PubMed] [Google Scholar]
- Wu, G. , Cameron, J. N. , Ljung, K. , & Spalding, E. P. (2010). A role for ABCB19‐mediated polar auxin transport in seedling photomorphogenesis mediated by cryptochrome 1 and phytochrome B. The Plant Journal, 62, 179–191. 10.1111/j.1365-313X.2010.04137.x [DOI] [PubMed] [Google Scholar]
- Wu, G. , Otegui, M. S. , & Spalding, E. P. (2010). The ER‐localized TWD1 immunophilin is necessary for localization of multidrug resistance‐like proteins required for polar auxin transport in Arabidopsis roots. Plant Cell, 22, 3295–3304. 10.1105/tpc.110.078360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, H. , & Murphy, A. S. (2009). Functional expression and characterization of Arabidopsis ABCB, AUX 1 and PIN auxin transporters in Schizosaccharomyces pombe. The Plant Journal, 59, 179–191. 10.1111/j.1365-313X.2009.03856.x [DOI] [PubMed] [Google Scholar]
- Zhang, Y. , Nasser, V. , Pisanty, O. , Omary, M. , Wulff, N. , di Donato, M. , Tal, I. , Hauser, F. , Hao, P. , Roth, O. , Fromm, H. , Schroeder, J. I. , Geisler, M. , Nour‐Eldin, H. H. , & Shani, E. (2018). A transportome‐scale amiRNA‐based screen identifies redundant roles of Arabidopsis ABCB6 and ABCB20 in auxin transport. Nature Communications, 9, 4204. 10.1038/s41467-018-06410-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zourelidou, M. , Absmanner, B. , Weller, B. , Barbosa, I. C. , Willige, B. C. , Fastner, A. , Streit, V. , Port, S. A. , Colcombet, J. , de la Fuente van Bentem, S. , Hirt, H. , Kuster, B. , Schulze, W. X. , Hammes, U. Z. , & Schwechheimer, C. (2014). Auxin efflux by PIN‐FORMED proteins is activated by two different PROTEIN kinases, D6 PROTEIN KINASE and PINOID. eLife, 3, e02860. 10.7554/eLife.02860 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure S1. Tests of benzoic acid (BA) and tryptophan (Trp) transport. A, ABCB4 transports auxin but not BA or Trp according to reversal potential analysis. B, PIN2 transports auxin and BA but not Trp. Plotted are individual Erev values obtained from separate patch‐clamped cells with the indicated substance present at the indicated concentration at the cytoplasmic side of the membrane. At the bottom of each graph, the following symbols indicate the statistical significance of a difference from the 0.1 μM IAA condition: n.s. = not significant, * = p < 0.05, ** = p < 0.01.
Supplemental Dataset S1. All of the current–voltage (I‐V) curves are contained in one comma separated value (csv) file. The results are grouped by the figure in which the mean values appear. All of the electrophysiology results in the paper can be reconstructed from these patch clamp trials.


