Abstract
Although the basic process of intestinal lipid absorption and transport is understood, many critical aspects remain unclear. One question, in particular, is whether intestinal lipid absorption and transport differ between the sexes. Using a well-established lymph fistula model, we found that intact female mice exhibited lower lymphatic output of triacylglycerol (TAG) than male mice. Further analysis revealed that the female mice segregated into two groups: the high group having similar lymphatic TAG transport to the males, and the low group having significantly less lymphatic output, implying the impact of cyclical variation of ovarian hormonal levels. These led us to examine whether estradiol (E2) and progesterone (P) affect intestinal absorption and lymphatic transport of dietary lipids. In ovariectomized (OVX) rats, E2 treatment significantly reduced [3H]-TAG lymphatic output through reducing TAG transport; and P-treatment decreased [14C]-cholesterol (Chol) lymphatic output by inhibiting Chol absorption, compared to vehicle treatment. Gene expression data suggested that E2 enhances vascular endothelial growth factor-A (VEGF-A) signaling to reduce the permeability of lacteals, leading to reduced CM transport through the lymphatic system. Interestingly, E2 treatment also increased lymphatic output of apolipoprotein A-I (apoA-I), but not apoB-48 and apoA-IV, in the OVX rats. Collectively, these data suggested that ovarian hormone-induced reductions of intestinal lipid absorption and lymphatic transport, as well as increased lymphatic output of apoA-I, may contribute to a beneficial protection from atherosclerosis in females.
Keywords: lymphatic lipid transport, estrogen, progesterone, triacylglycerol, cholesterol, apolipoproteins
Graphical Abstract
Introduction
Lipids are an essential part of our diet. The absorption of dietary lipids is important for the acquisition of energy and lipid-soluble vitamins. The main dietary lipids consist of triacylglycerols (TAG), cholesterols (Chol), and phospholipids (PL). While the small intestine absorbs large loads of TAG very efficiently (Jandacek et al., 2004), it only absorbs about half (~50%) of Chol in humans (Pouteau et al., 2003). Dietary lipid absorption is a complex physiological process (Wang, 2007; Wang et al., 2013), and most of our understanding of lipid absorption and transport has been derived from studies using male animal models. In other words, there is a notable lack of information related to possible sex differences in intestinal lymph physiology.
There are fundamental differences between males and females that likely have significant implications for metabolic physiology. Most aspects of female physiology are impacted by the ovarian hormone variations that occur with the estrous cycle. In rodents, a 4 to 5 day estrous cycle is comprised of 4 distinct stages: diestrus 1, diestrus 2, proestrus, and estrus (Ajayi & Akhigbe, 2020). Notably, this hormonal cycle profoundly influences many aspects of energy balance and metabolism. Our group and many others have provided instances of these phenomena (Clegg et al., 2006; Shen et al., 2010, 2017, 2018a; Leeners et al., 2017). However, the potential influence of estrous cycle on intestinal lipid metabolism and physiology have not been sufficiently explored.
The objectives of this study are two-fold. First, intact male and female mice were used to evaluate the extent that lipid absorption varies between the sexes. Second, ovariectomized (OVX) rats were used to determine the role of female sex hormone, including estradiol (E2) and progesterone (P), in the regulation of intestinal absorption and lymphatic transport of radiolabeled TAG and Chol. We took advantage of our well-established conscious lymph fistula animal model to explore the effects of these hormonal treatments on the various steps involved in the absorptive process that may be affected, including lipid uptake, mucosal assimilation, packaging and secretion into the lymph in the OVX rats.
Methods
Ethical approval
All animal experiments were carried out according to the Animal Welfare Act in the United States and approved by the the University of Cincinnati Institutional Animal Care and Use Committee (references #02-01-08-01). The study was conducted in accordance to the National Institutes of Health (NIH) policy on the humane care and use of laboratory animals and the ethical principles of the Journal of Physiology (Grundy, 2015).
Animals
Adult male and female C57BL/6 mice aged 8–10 weeks were obtained from the Jackson Laboratory (Bar Harbor, ME), and adult female Sprague-Dawley rats (10–12 weeks old) were obtained from Envigo (Indianapolis, IN). The rodents were allowed at least two weeks to acclimate to the animal facilities before they were used for the experiments, and were housed in a room under a 12 h:12 h light-dark cycle (lights on from 0600 to 1800) and temperature-controlled (22 ± 1°C) conditions for the duration of the study. The animals were allowed ad libitum access to food (Harlan-Teklad LM485 chow, Madison, WI) and water prior to experiment onset.
Materials
17β-Estradiol-3-benzoate (estradiol, E2), progesterone (P), triolein, cholesterol (Chol), egg phosphatidylcholine (PC), sesame oil, and sodium taurocholate (NaTC) were purchased from Sigma (St. Louis, MO). The radioactive [9,10-3H(N)] triolein (labels were on the 3 fatty acid molecules, not on the glycerol moiety) and [1,2-14C] cholesterol were purchased from Perkin-Elmer (Waltham, MA). Reagents for measuring lymph TAG and Chol were from Randox Laboratories Ltd. (London, UK) and Pointe Scientific Inc. (Canton, MI), respectively. Primary antibodies against apolipoprotein B (apoB), apoA-IV and apoA-I were raised from goats and validated previously (Hayashi et al., 1990b; Shen et al., 2007; Lo et al., 2008). TaqMan Gene Expression Assays for vascular endothelial growth factor-A (vegfa), vascular endothelial growth factor receptor 1 (vegfr1 also known as Flt1), and Neuropilin1 (Nrp1) were purchased from ThermoFisher Scientific (Waltham, MA).
Lymph and duodenal cannulation in mice
The mice were fasted overnight and then anesthetized with isoflurane. Briefly, the animals were placed in the induction chamber and isoflurane in oxygen was administered at a rate of 4 %. Once the mice were unconscious, the vaporizer setting is adjusted to a maintenance rate of 2%. The analgesic buprenorphine was administered subcutaneously at a dose of 0.05 mg/kg. The first dose was given pre-emptively and a second dose was given at 10 h post-surgery. Mouse intestinal lymph ducts were cannulated with polyvinyl chloride (PVC) tubing (inner diameter [ID], 0.2 mm; outside diameter [OD], 0.5 mm) as described previously (BOLLMAN et al., 1948; Nauli et al., 2006). In addition, a PVC tube (ID, 0.5 mm; OD, 0.8 mm) was inserted into the duodenum through a fundal incision in the stomach and secured by a purse-string. Following the surgery, mice were infused with 5% glucose in saline (145 mmol/L NaCl, 4 mmol/L KCl, and 0.28 mol/L glucose) at a rate of 0.3 mL/h to replenish the loss of fluid and electrolytes due to lymphatic drainage. The animals were allowed to recover overnight in Bollman restraining cages maintained at a temperature of 28°C to prevent hypothermia caused by surgical stress and single-animal housing. Despite being restrained, the animals still had considerable freedom to move.
Lipid infusate Preparation
Non-radioactive triolein, [3H] labeled triolein, cholesterol, [14C] labeled cholesterol, and egg phosphatidylcholine were dissolved in chloroform. After evaporation under a steady stream of nitrogen, the chloroform free lipid mixture was then emulsified with 19 mM sodium taurocholate (NaTC) in 30 ml phosphate-buffered saline (PBS, 6.74 mM Na2HPO4, 16.5 mM NaH2PO4, 115 mM NaCl and 5 mM KCl, pH 6.4). The mixture was sonicated with 1 second on/1 second off pulse until the solution appeared homogenous. The homogeneity of the emulsion was verified by determining the radioactivity sampled from the top, middle, and bottom of the emulsion. Emulsions were considered homogeneous if the counts did not vary >1 %.
Lipid infusion and lymph collection in mice
The fasting lymph was collected for one hour (h) prior to the lipid infusion. The lipid emulsion was then infused continuously into the duodenum at a constant rate of 0.3 mL/h for 6 h. The hourly infusate contained 4 μmol triolein with a trace mass of [3H] triolein (1 μCi), 0.78 μmol Chol, 0.78 μmol egg PC, and 5.7 μmol NaTC in 0.3 ml PBS. Lymph samples were collected every h for 6 h into pre-cooled eppendorf tubes. An aliquot of each lymph sample (200 μl) was added to Opti-Fluor (Packard Bioscience, Meriden, CT) and counted in a liquid scintillation spectrometer (LS 6500 multi-purpose scintillation counter, Beckman Coulter). With the information on the volume of the lymph collected hourly (lymph flow rate), we were able to calculate the output of labeled lipids into lymph each hour. With the information on what we infused per hour, we were able to calculate the lymphatic output as a percentage of what we infused.
Ovariectomy (OVX) and hormone treatments
Female rats were food-deprived overnight and anesthetized by ip injection of an anesthetic mix: ketamine (80 mg/kg)/xylazine (8 mg/kg). They then received bilateral OVX through a midline abdominal incision using sterile surgical technique as we described previously (Shen et al., 2010). Six days after OVX, rats were subcutaneously (sc) injected with vehicle (100 μl sesame oil as control), E2 (10 μg/100 μl oil), and/or P (500 μg/100 μl oil), depending on the experimental groups (Oil, E2, P, or E2 + P). As described in Fig. 2, the OVX rats in the Oil group were injected with oil on Days 1, 2, and 3. Animals in the E2 group received injections of E2 on Days 1 and 2, and a single injection of oil on Day 3. Animals in the P group received injections of oil on Days 1 and 2, followed by a single injection of P on Day 3. Finally, animals in the E2 + P group received injections of E2 on Days 1 and 2 and a single injection of P on Day 3. This cyclic dosing regimen was chosen for two reasons. First, it produces plasma levels of E2 and P on Day 3, which approximately occur during the period of proestrus to estrus transition in regularly cycling female rats (Nequin et al., 1979; Figueiredo et al., 2007). Second, it allows us to test the mechanistic contribution of E2 and P alone, as well as in combination, on intestinal lymph biology.
Fig. 2.
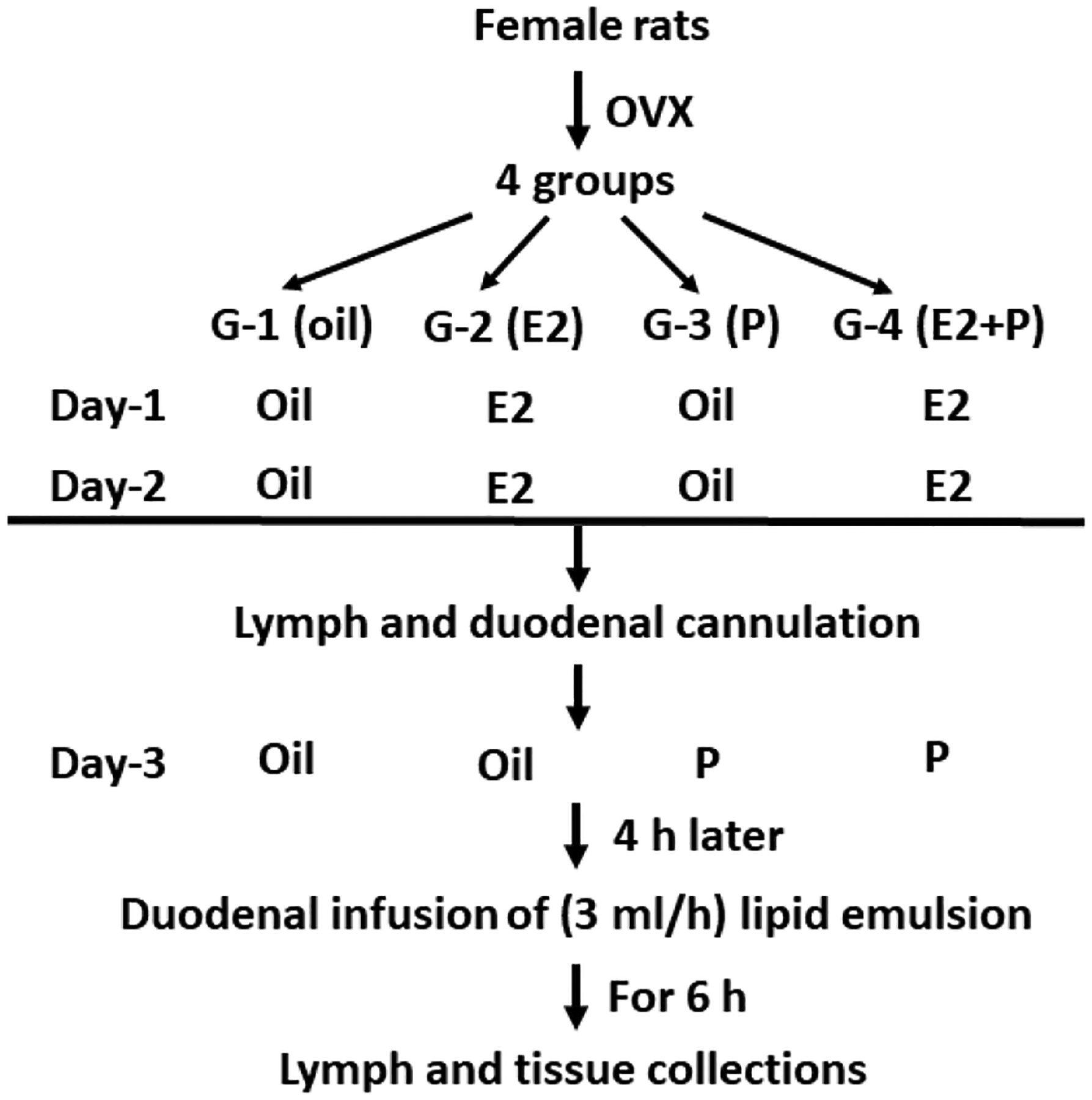
Schematic diagram of the experimental design. After 6-day recovery from OVX surgery, rats were divided into 4 groups and then received sc injections with vehicle (sesame oil, 100 μl), E2 (10 μg), and/or P (500 μg). Four h after the final injections, lymph was collected for 1 h (fasting), and then the radiolabeled lipid emulsion was continuously infused into the duodenum for 6 h. Lymph samples were collected every hour for 6 h. At the end of the 6 h, the rats were deeply anesthetized, and the stomach, small intestine, colon, and their luminal contents were collected for lipid analysis.
Experimental procedure in OVX rats
After treatments on Day 1, the OVX rats were deprived of food overnight with free access to water. On Day 2 after treatments, under isoflurane anesthesia (the vaporizer setting was adjusted to maintain isoflurane in oxygen at a rate of 2%), the intestinal lymph duct was cannulated with a PVC tube (OD 0.8 mm), and the duodenum was cannulated with a soft silicone infusion tube (1.6 mm OD) through a fundal incision of the stomach, as we described previously (Tso et al., 1980; Ji et al., 2012). The analgesic buprenorphine was administered subcutaneously at a dose of 0.02 mg/kg, first dose being given pre-emptively and a second dose being given at 10 h post-surgery. Postoperatively, the rats were intraduodenally infused with a 5% glucose-saline solution at a rate of 3 mL/h to replenish the loss of fluid and electrolytes due to lymphatic drainage.
On the experimental day (Day 3), lipid emulsions were prepared. Triolein (360 mg), [3H] triolein (10 μCi), Chol (30 mg), [14C] Chol (10 μCi), and egg PC (67.4 mg) were dissolved in chloroform. After evaporation, the lipid mixture was then emulsified with 570 μmol NaTC in 30 ml PBS. The mixture was sonicated for the homogeneity of the lipid emulsion. Lymph was collected for 1 h (fasting) before the lipid infusion. The emulsion was then continuously infused into the duodenum for 6 h at a constant rate of 3 mL/h. Lymph samples were collected every h for 6 h into pre-cooled conical centrifuge tubes.
At the end of the 6 h, the rats were anesthetized with an overdose of pentobarbital (60 mg/kg, ip) prior to tissue and organ removal. The stomach, small intestine and colon were collected. The luminal contents of the stomach, small intestine, and colon were collected after washing the luminal contents 3 times with 5 ml of 10 mM NaTC. The small intestine was then divided into 4 equal length segments, and labeled M1–M4 (M1: duodenum, M2 and M3: two equal segments of the jejunum, and M4: ileum). The lipids were extracted from small intestinal segments according to the method described by Folch et al. (FOLCH et al., 1957). An aliquot of each sample was added to Opti-Fluor and counted by liquid scintillation spectrometer. The total count for each sample was calculated from the total volume of the sample and the aliquot count obtained from the scintillation counter.
Calculations of absorptive and transport index
Absorptive and lymph transport indexes were calculated as previously described (Bergstedt et al., 1990). The absorptive index represents the percentage of infused lipid taken up by the enterocyte, which was calculated from the following equation: absorptive index = 100 % − % total dose recovered in the lumen of gastrointestinal tract. The lymphatic transport index represents the percentage of absorbed lipid secreted into the lymph and takes into consideration the uptake of lipid by the small intestine, which was calculated from the equation: lymphatic transport index = % infused dose recovered in lymph/absorptive index × 100. This index is useful in characterizing the ability of the small intestine to transport lipid into lymph as chylomicrons (CM).
Measurements of lymph components
Total TAG and Chol in lymph were measured using total TAG assay kits (Randox Laboratories, Kearneysville, WV) and Infinity cholesterol assay kits (Thermo-Fisher Scientific, Middletown), respectively.
Lymph apolipoprotein determination by Western blot
The lymph sample (2 μl) was separated by 4–15% polyacrylamide/SDS gel, transferred onto nitrocellulose sheets, and blotted with antibodies, including apoB48 (1:8000 dilution), apoA-IV (1:3000) and apoA-I (1:5000) (Hayashi et al., 1990b; Shen et al., 2007; Lo et al., 2008), respectively. The amount of immune complexes was quantitated using an enhanced chemiluminescence detection system (GE Healthcare Bio-Sciences). The images from reacted membranes were acquired and the band density was analyzed by ChemiDoc Imaging Systems (Bio-Rad Laboratories, Inc.). Fold changes in apolipoprotein secretion, including apoB48, apoA-IV and apoA-I, during each subsequent h of infusion were quantified by dividing the relative lymph apolipoprotein level at each hourly time point by the 0 h (fasting) level.
Quantitative real-time PCR (qPCR) for target gene expression measurement
Total RNA was extracted from the M1 segment of intestine in the OVX rats using a PureLink RNA Mini Kit (ThermoFisher Scientific, Waltham, MA). RNA was quantified using a NanoDrop 2000 (Thermo Scientific). Then, 250 ng of total RNA from each sample was used for reverse transcription to cDNA with a Transcriptor First Strand cDNA Synthesis Kit (ThermoFisher Scientific). The mRNA levels of target genes, including vascular endothelial growth factor-A (vegfa), vascular endothelial growth factor receptor 1 (Flt1), and Neuropilin1 (Nrp1), were quantified by qPCR using TaqMan Fast Advanced Master Mix with TaqMan Gene Expression Assays with a StepOneTM Plus device (ThermoFisher Scientific). 45 S mRNA levels from each sample were used as internal controls to normalize the mRNA levels, as we described previously (Shen et al., 2018b).
Statistical analysis
The data shown are mean values ± standard deviation (SD). For comparison of data that have 2 independent variables, 2-way analysis of variance (ANOVA) was used. To compare groups throughout the 6 h infusion period, two-way repeated measures ANOVA with Bonferroni or Tukey test were used. Statistical analyses were performed using GraphPad Prism V8. P values less than 0.05 were considered statistically significant.
Results
Lymphatic TAG output in male and female mice
To address the existence of sex differences in intestinal lipid absorption and lymphatic transport, mice equipped with lymph and duodenal cannulae were intraduodenally infused with a lipid emulsion containing [3H]-TAG for a period of 6 h. The histograms of the lipid absorption of the mice were derived from the distribution of their total lymphatic [3H]-TAG outputs, which were obtained by summing the total [3H] counts recovered in the lymph during the 6 h of infusion. Fig. 1A showed that the male mice had a normal distribution of total lymphatic [3H]-TAG output of 21–70% with the majority of the animals transporting between 30–60% of the total dose of [3H] TAG transported in the 6 h of the experiment. However, the female mice had a distinct bimodal distribution with 20 mice falling in the range of 0–30%, and 12 mice falling in the range of 31–70% (more resembling the distribution we observed in the male mice). Based on this distribution, the female mice were grouped into either high output group (High, 31–70% of the total lymphatic TAG recovery) or low output group (Low, 0–30% of the total lymphatic TAG recovery) (Fig. 1B)
Fig. 1.
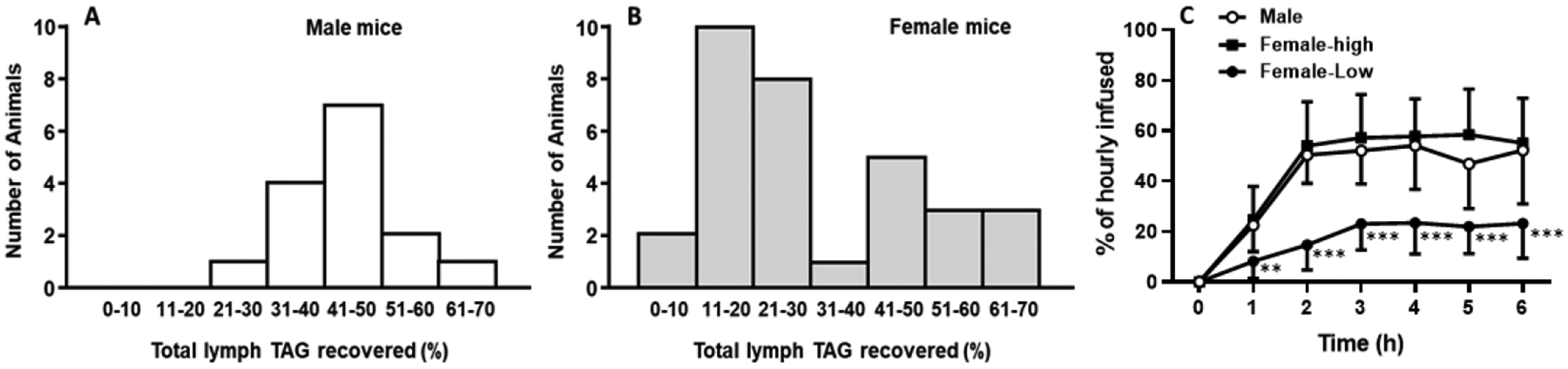
The histograms of total lymphatic TAG recovery in the male (A) and the female mice (B). Mice were equipped with lymph and duodenal cannulae, and were intraduodenally infused with a lipid emulsion containing [3H]-TAG for a period of 6 h. Lymph was collected hourly and analyzed. The total lymphatic [3H]-TAG recoveries were plotted in a histogram. Based on the histogram, the female mice were divided into two groups, the high-output group (female-high) and the low-output group (female-low) (C). Values are means ± SD, male mice: n = 15, female-high: n = 12, and female-low: n = 20; **P = 0.0041 at 1 h, and ***P < 0.0001 at 2 to 6 h, vs. both male and female-high mice.
Fig. 1C displayed hourly lymphatic [3H]-TAG recovery in the male, the low-, and the high-output female groups. The hourly lymphatic TAG output of the high-output group superimposed that of the male group, reaching a maximum of about 55% recovery of hourly infused [3H]-TAG at the 3rd h of the infusion. As expected, the low-output female group had a lower hourly lymphatic TAG recovery, reaching a maximum of only 23% recovery at the 3rd h. The differences in hourly lymphatic TAG output were significant when comparing the low-output female group with both the high-output female and the male groups (from the first h onwards). Although the observation in the female mice was intriguing, it is something that we have not experienced previously because we used mostly male mice or rats for our lymph fistula experiments. Since we have considerable experience working with the female rodents (Shen et al., 2010, 2014, 2018a), we decided in the next series of experiments to determine how the female hormones affect lipid absorption and transport in female rats.
Lymphatic studies in OVX rats
As the mice utilized in the prior experiment were gonadally-intact and freely cycling, we speculate that the above phenomenon in the mice was caused by the natural variation in the hormonal status of the female mice on the day of testing. Since there are 4 distinct stages of estrous cycle, the intact females with high lymphatic 3H-TAG output could be in diestrus (i.e., the most male-like among the stages), whereas those with low lymphatic 3H-TAG output could be in proestrus/estrus. To further determine the roles of ovarian hormones in regulating intestinal lymph biology, female rats were ovariectomized. As described in Fig. 2, the OVX rats were then treated with/without E2 and/or P.
Lymph flow rate in OVX rats
As shown in Fig. 3, the mean fasting lymph flow for all four groups of rats varied between 1.9 and 2.8 ml/h. In all groups, lymph flow increased and reached a maximum output between 3.0 and 4.1 ml/h at the 4th h after the infusion of lipid infusate. The lymph flow then declined slightly and maintained a steady-state output of ~3 ml/h during hours 5 and 6 after lipid infusion. There were no significant differences in the lymph flow rates (P = 0.9139) among the four groups at any time point of lymph collection, indicating that the treatments of E2 and/or P did not significantly affect the lymph flow rate of the lymph fistula OVX rats.
Fig. 3.
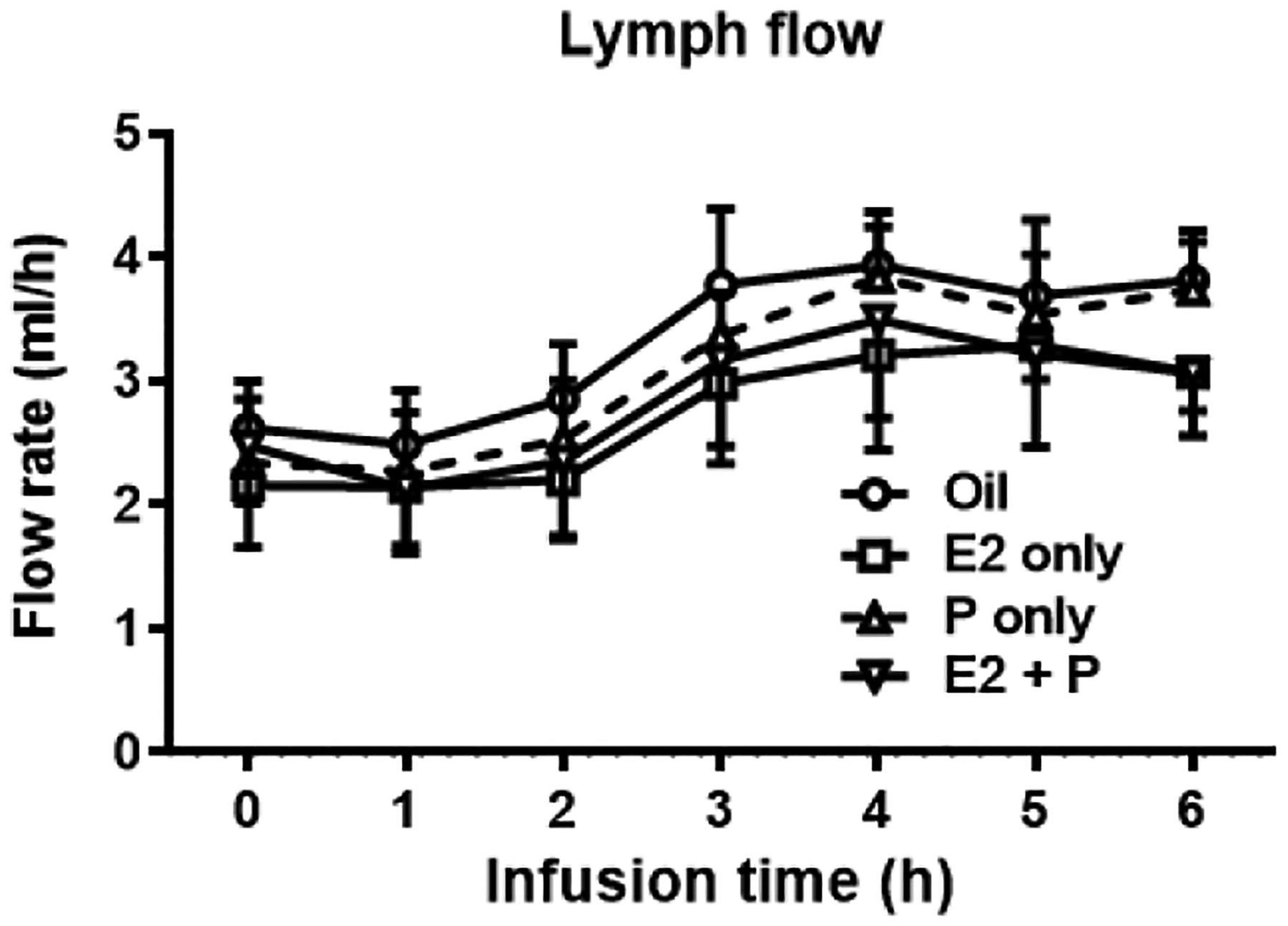
Lymph flow rate throughout the 6 h infusion period in the 4 groups of OVX rats. Mesenteric lymph flow was measured at hourly intervals before and immediately after the lipid infusion for 6 h. No significant differences in the lymph flow rates was found among the 4 groups at any time point of lymph collection (P = 0.9139). Values are means ± SD, n = 6 per group, except P-treated group (n = 7).
Lymphatic TAG output in OVX rats
In Oil-control OVX rats, the lymphatic [3H]-TAG transport rate reached a steady state by 4 h (Fig. 4A), which is consistent with our previous findings in male rats (Tso et al., 1980). Interestingly, the female sex hormones showed different patterns of impact on intestinal lipid absorption and transport. P-treatment significantly reduced lymphatic [3H]-TAG output at both 2 and 3 h (P = 0.0357 and P = 0.0409, respectively), while E2-treatment led to significantly decreased [3H]-TAG output at 4 h and 6 h (P = 0.0157 and P = 0.0003, respectively). With the combination treatment of E2 and P, the OVX rats showed a more potent reduction in the lymphatic [3H]-TAG transport rate that was evident from 2 h until the end of the infusion period (P = 0.0296 at 2 h; P = 0.0022 at 3 h, P = 0.0011 at 4 h, P = 0.0056 at 5 h, and P = 0.0045 at 6 h) (Fig. 4A). Output of total TAG mass in the lymph, as measured by the chemical mass assay, was also reduced in the OVX rats at at 2 h after E2 alone (P = 0.0189) and at 4 h after E2 + P treatment (P = 0.0183). These reductions in TAG mass output (endogenous plus exogenous TAG contributions) were in parallel with their reduced output of radiolabeled tracer (Fig. 4B).
Fig. 4.
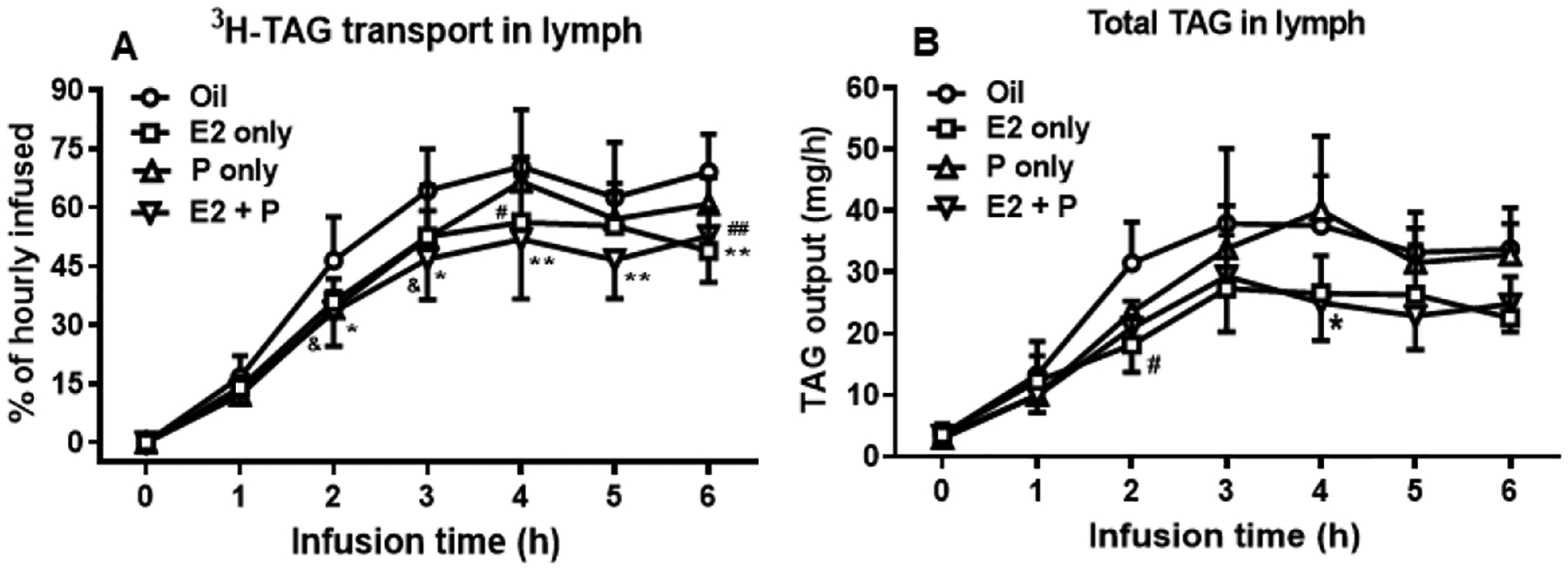
The treatments of E2 and P significantly reduced TAG transport into the lymph of OVX rats. Lymphatic output of [3H]-TAG (A) was expressed as a percentage of the infused hourly dose. Output of total TAG mass in the lymph of OVX rats was measured by TAG assay kit (B).
Compared to oil-treated rats, P-treated rats had significantly reduced lymphatic [3H]-TAG output at 2 and 3 h (&P = 0.0357 and &P = 0.0409, respectively), while in E2-treated rats, [3H]-TAG output was significantly decreased at 4 h and 6 h (#P = 0.0157 and ##P = 0.0003, respectively). The OVX rats receiving the combination of E2 and P treatments showed a more potent reduction in the lymphatic [3H]-TAG transport rate that was evident from 2 h until the end of the infusion period (*P = 0.0296 at 2 h; *P = 0.022 at 3 h, **P = 0.0011 at 4 h, **P = 0.0056 at 5 h, and **P = 0.0045 at 6 h) (A). The output of total TAG mass in the lymph was also reduced in the OVX rats at 2 h after E2 alone (#P = 0.0189) and at 4 h after E2 + P treatment (*P = 0.0183) (B). Values are means ± SD, n = 6 per group, except P-treated group (n = 7).
Lymphatic Chol output in OVX rats
Oil-treated OVX rats achieved a lymphatic [14C]-Chol transport rate of 41% of the hourly dose at 4 h. While there was a trend in the reduction of lymphatic [14C]-Chol transport rate in E2 and/or P-treated rats relative to Oil-treated control rats, the difference did not reach the level of statistical significance (P = 0.7136) (Fig. 5A). Interestingly, the outputs of total lymph Chol, as measured by Chol assay kit, in E2− and E2 + P-treated OVX rats was significantly less than those of Oil-treated controls (E2 vs. Oil, at 4 h, P = 0.0129, and at 6 h, P = 0.0097; E2 + P vs. Oil, at 4 h, P = 0.0112, at 5 h, P = 0.0023, and at 6 h, P = 0.0016) (Fig. 5B). These data suggest that E2 and E2 + P treatment reduced total lymphatic Chol output, which includes both the exogenous (radiolabeled) and endogenous Chol output (derived mostly from the bile) in OVX rats.
Fig. 5.
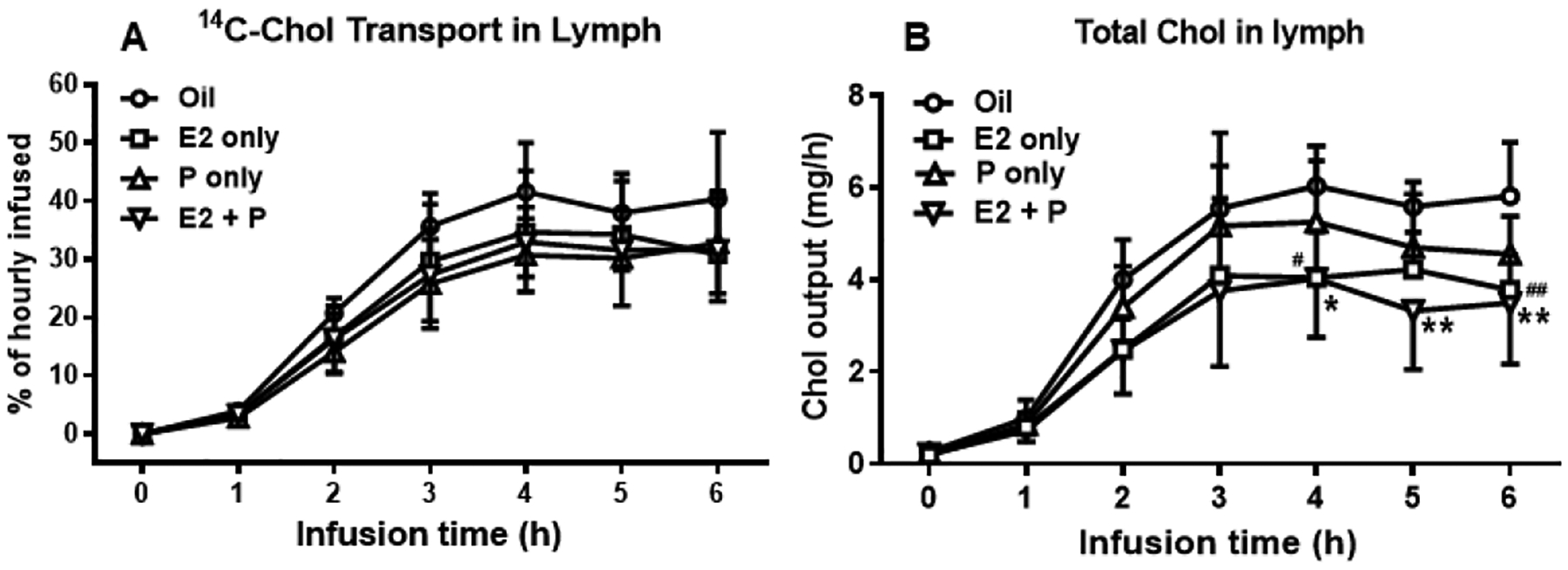
Chol transport into the lymph. The output of [14C]-Chol (A) is expressed as a percentage of the infused hourly dose. The output of total lymph Chol was measured by Chol assay kit (B).
While no significant difference in the lymphatic [14C]-Chol output was found in E2 and/or P-treated rats relative to Oil-treated control rats (P = 0.7136) (A), the outputs of total lymph Chol in E2− and E2 + P-treated OVX rats was significantly less than those of Oil-treated controls (E2 vs. Oil, at 4 h, #P = 0.0129, and at 6 h, ##P = 0.0097; E2 + P vs. Oil, at 4 h, *P = 0.0112, at 5 h, **P = 0.0023, and at 6 h, **P = 0.0016) (B). Values are means ± SD, n = 6 per group, except P-treated group (n = 7).
Distribution of labeled TAG and Chol along various segments of the small intestine
Fig. 6 shows the distribution of the amount of [3H]-TAG (A) and [14C]-Chol (B) in the segments of small intestine, M1–M4 (M1 representing the duodenum, M2 and M3 the jejunum, M4 the ileum). As shown in Fig. 6A, most of 3H TAG accumulated in the first segment (M1) of the small intestine in all four groups of animals. The amount of [3H]-TAG accumulated in M1 was significantly more in both the E2− and E2 + P-treated OVX rats, compared to that in Oil-control rats (P = 0.0167 and P = 0.0132, respectively). Consistent with that, the accumulated [3H]-TAG in the intestine was also significantly more in both the E2− and E2 + P-treated OVX rats (P = 0.0055 and P = 0.0494, respectively). While P-treated rats also had increased levels of [3H]-TAG in the M1 segment, the differences did not reach statistical significance (P = 0.644). There was no significant difference in the amount of [14C]-Chol accumulated in any of the intestinal segments among the four groups of animals (P = 0.5598) (Fig. 6B). The data indicate that the [3H]-TAG, but not [14C]-Chol, accumulated in proximal small intestine.
Fig. 6.
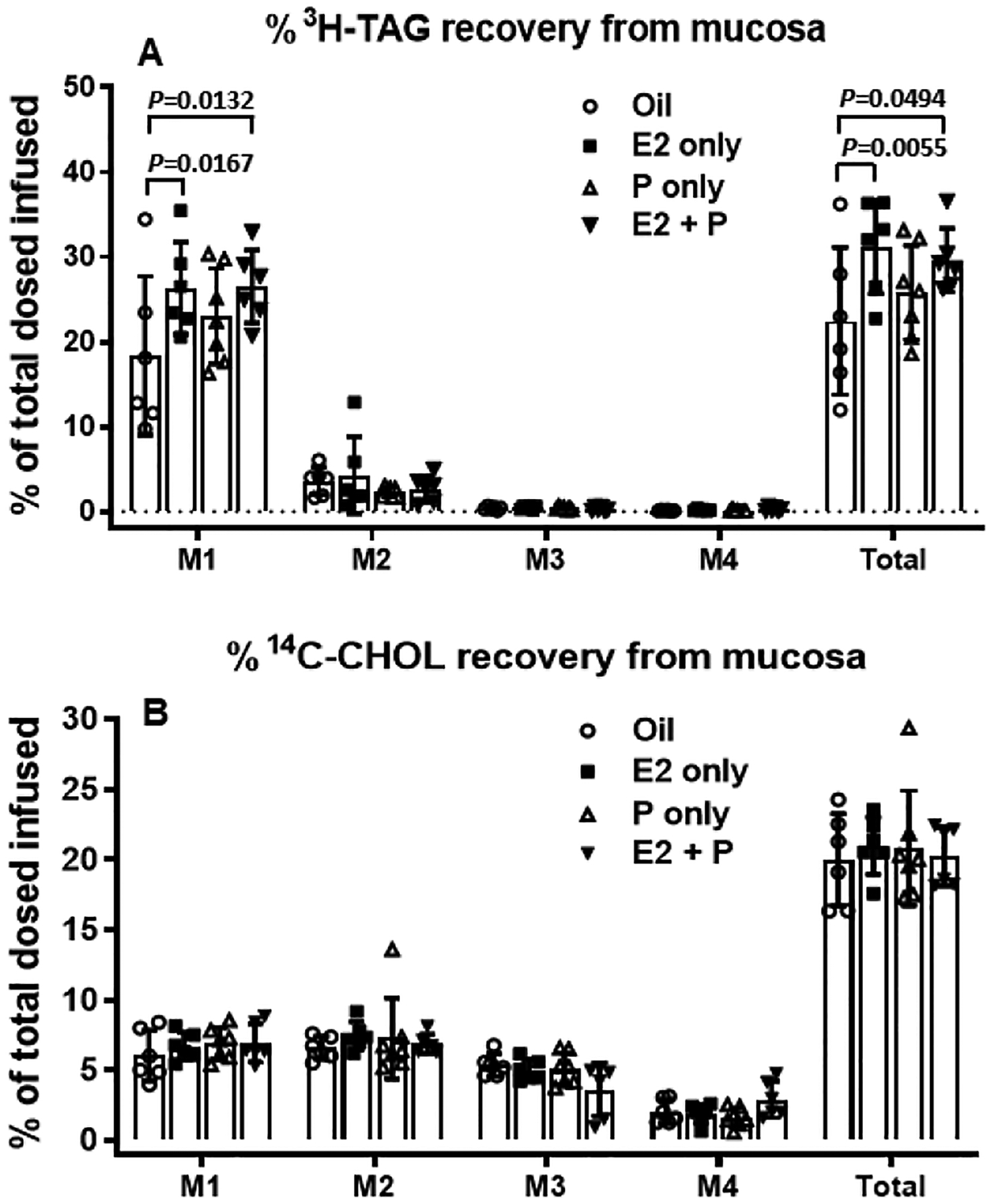
Distribution of [3H]-TAG and [14C]-Chol along the small intestine divided into 4 segments of equal length, from proximal to distal: M1, M2, M3, and M4 (M1: duodenum, M2 and M3: jejunum, M4: ileum). Radioactivity was determined by scintillation counter.
The [3H]-TAG in M1 was accumulated significantly more in both the E2− and E2 + P-treated OVX rats, compared to that in Oil-control rats. Consistent with that, the total [3H]-TAG level accumulated in the intestine was also significantly higher in both the E2− and E2 + P-treated OVX rats than that in Oil-control rats. While P-treated rats also had increased levels of [3H]-TAG in the M1 segment, the differences did not reach statistical significance (P = 0.644). No significant difference in the amount of [14C]-Chol accumulated was found in any of the intestinal segments among the 4 groups of animals (P = 0.5598) (B). Values are means ± SD, n = 6 per group, except P-treated group (n = 7).
Total recovery of labeled TAG and Chol
Fig. 7A shows the percentage of [3H]-TAG recovery in the lymph, as well as the percent recovery of [3H]-TAG in small intestinal lumen, stomach lumen, colonic lumen and the mucosa from the four intestinal segments at the end of experiment. Any [3H]-TAG that could not be recovered at the end of the experiment has been named “Others” in the figure. The “Others” could represent the portal transport of the fatty acids or simply loss during the experiment. The E2− and E2 + P-treated OVX rats had significantly reduced average hourly recoveries of [3H]-TAG in the lymph (P = 0.0002 and P < 0.0001, respectively), but increased levels in the mucosal tissues of the small intestine, compared to Oil-treated OVX rats (P = 0.0042 and P < 0.0257, respectively). A small amount of [3H]-TAG was found in the intestinal lumen and did not differ among the four groups. Little [3H]-TAG refluxed into the stomach and very little was recovered from the colon, indicating that the infused TAG did not reflux back to the stomach from the duodenum and very little passed into the feces during the experiment (Fig. 7A). The percent of the [3H]-TAG that could not be recovered (the Others) was also significantly higher in E2 + P treated rats, compared to that in Oil-control rats (P = 0.0236).
Fig. 7.
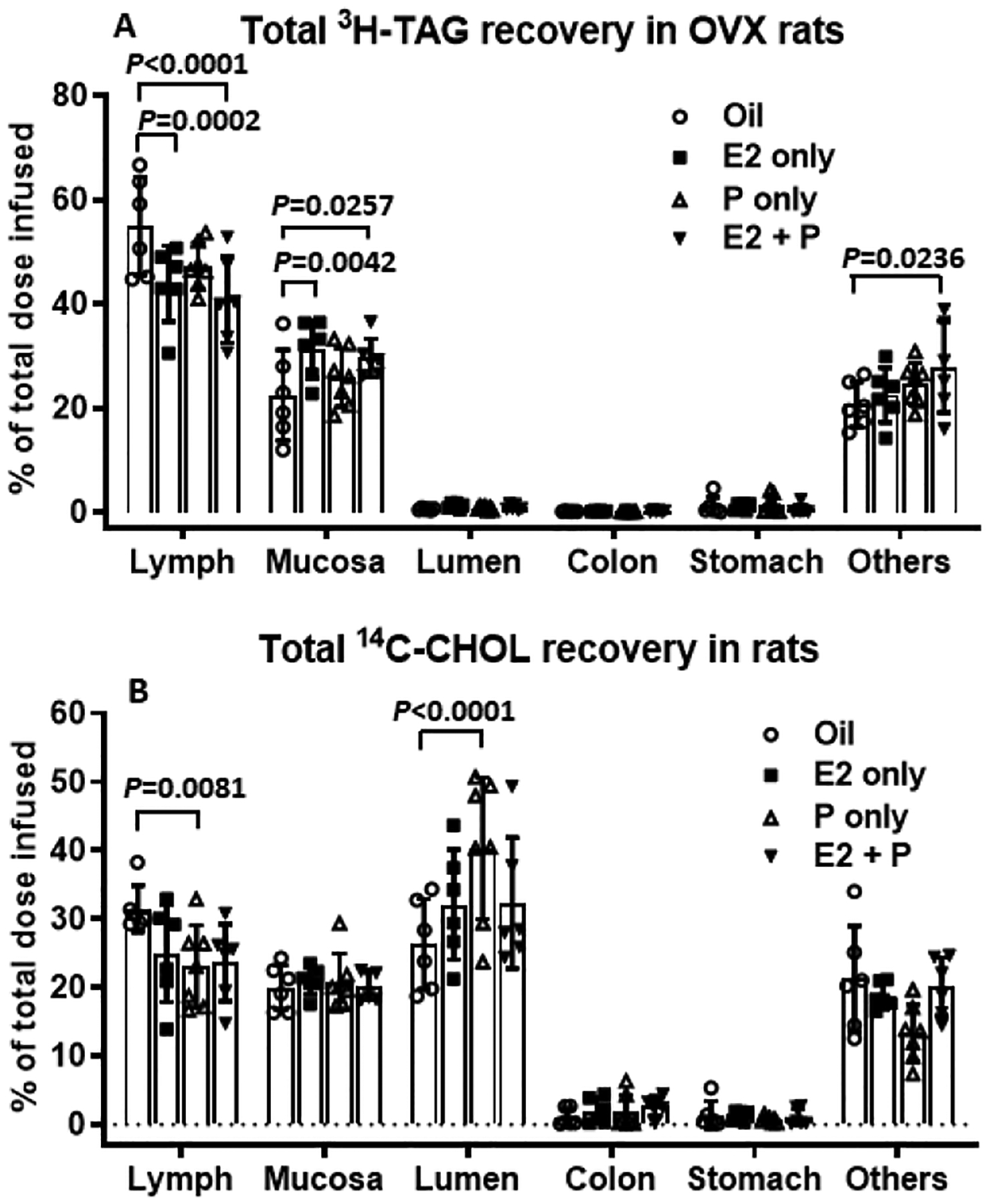
Comparison of the total recovery of a [3H]-TAG (A) and [14C]-Chol (B) among the 4 groups of OVX rats at the end of the 6 h lipid infusion. ‘Lymph’ refers to the average radiolabeled lipid collected from the lymph over the 6-h infusion period. ‘Small Intestine’ refers to radioactivity recovered after homogenization of the small intestine. ‘Lumen’ refers to small intestinal luminal contents that were washed and collected at the end of infusion. ‘Colon’ and ‘Stomach’ refer to luminal contents that were washed and collected from colon and stomach, respectively, at the end of infusion. ‘Others’ refers to the amount of infused radiolabel that was not recovered in the previous tissues. The E2− and E2 + P-treated OVX rats had significantly reduced average hourly recoveries of [3H]-TAG in the lymph, but increased levels in the mucosal tissues of the small intestine, compared to Oil-treated OVX rats. The percent of the [3H]-TAG that could not be recovered (the Others) was also significantly higher in E2 + P treated rats, compared to that in Oil-control rats (A). In addition, P-treated OVX rats showed a significant reduction in total recovery of [14C]-Chol in the lymph during 6-h infusion period, and this was accompanied by a significant increase in total recovery of [14C]-Chol in the intestinal lumen (B). Values are means ± SD, n = 6 per group, except P-treated group (n = 7).
Although there is a reduction in the lymphatic [14C]-Chol transport in E2, P, and the E2 + P-treated rats relative to that of Oil-treated Controls, the difference was not statistically significant at each time point (Fig. 5B). Notably, P-treated OVX rats showed a significant reduction in total recovery of [14C]-Chol in the lymph during 6-h infusion period (P = 0.0081), and this was accompanied by a significant increase in total recovery of [14C]-Chol in the intestinal lumen (P < 0.0001) (Fig. 7B). Very low amounts of [14C]-Chol were found in the lumen of the stomach (Fig. 7B), indicating there was little reflux of cholesterol from the duodenum back to the stomach. Also, there was very little [14C]-Chol recovered in the colon, suggesting that intestinal motility has not furthered the luminal Chol to the colonic lumen yet.
Absorptive and transport index
By quantifying the efficiency of lipid uptake and secretion from the intestines of the OVX rats, we found that E2− and E2 + P-treated rats had a significantly lower lymphatic transport index for [3H]-TAG than Oil-treated rats (P = 0.0477 and P = 0.0098, respectively) (Fig. 8B), while their absorptive index for [3H]-TAG were comparable (P = 0.0739 and P = 0.5075, respectively) (Fig. 8A). These findings suggest that TAG was taken up normally in E2− and E2 + P-treated OVX rats, but their incorporation into CM and the subsequent transport into lymph was compromised. Interestingly, P-treated rats had a significantly reduced absorptive index for [14C]-Chol relative to Oil-treated rats (P = 0.0415), indicating that P treatment decreased the output of [14C]-Chol in lymph by inhibiting Chol absorption in the OVX rats (Fig. 8C). However, the lymphatic transport indexes for [14C]-Chol were comparable among the 4 groups (P = 0.8727) (Fig. 8D).
Fig. 8.
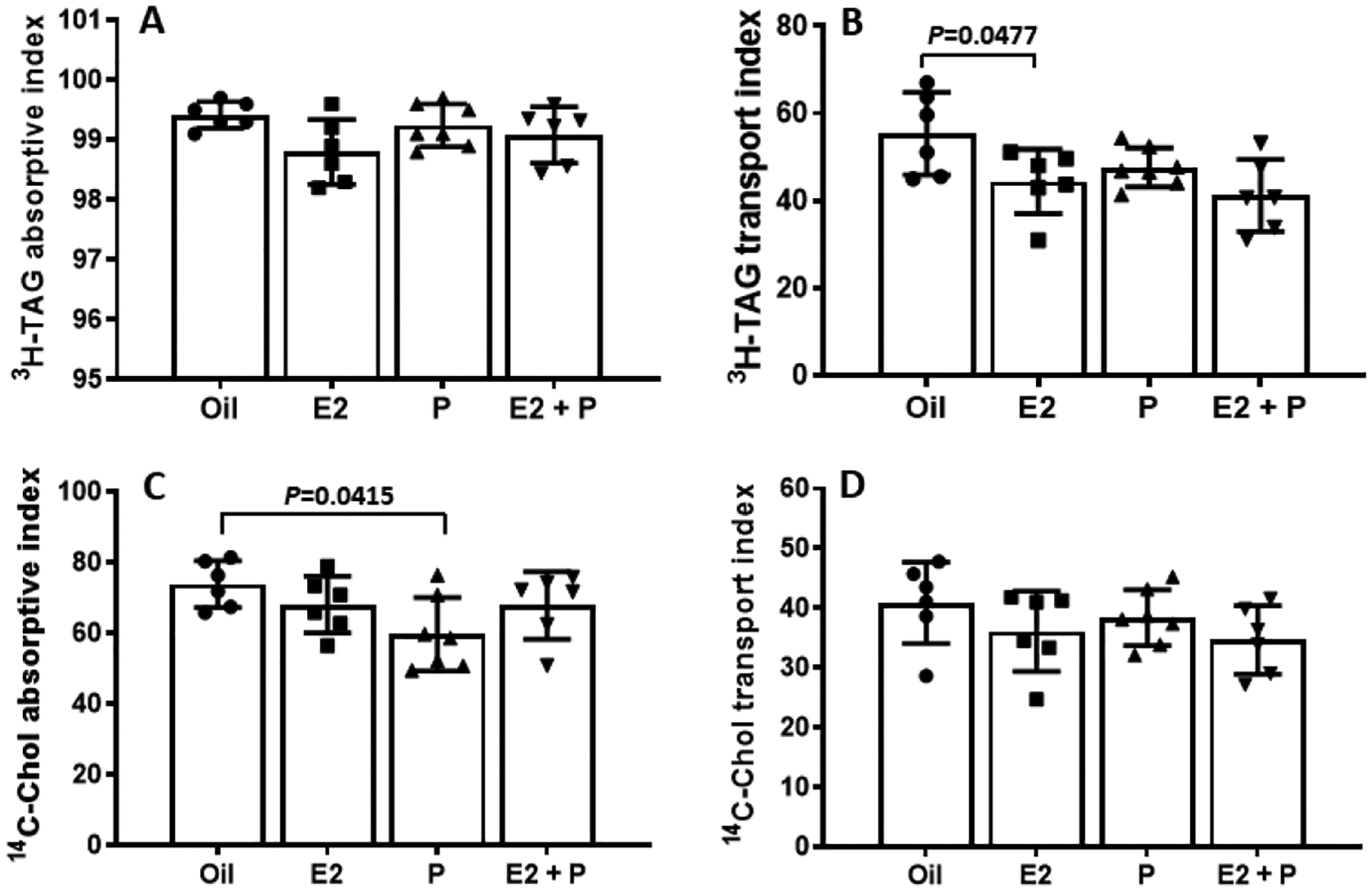
Comparison of lipid absorptive and lymph transport indexes for [3H]-TAG and [14C]-cholesterol in OVX rats with different treatments. While their absorptive index for [3H]-TAG were comparable (A), E2− and E2 + P-treated rats had a significantly lower lymphatic transport index for [3H]-TAG than Oil-treated rats (B). Interestingly, P-treated rats had a significantly reduced absorptive index for [14C]-Chol relative to Oil-treated rats (C). However, the lymphatic transport indexes for [14C]-Chol were comparable among the 4 groups (P = 0.8727) (D). Means ± SD, n = 6 per group, except P-treated group (n = 7).
Expression of genes regulating intestinal lacteal structure and function.
To determine whehter E2 and/or P affect the expression of genes that regulate lacteal structure and function in the M1 segment of intestine, we measured the mRNA levels of 3 important target genes, including vascular endothelial growth factor-A (vegfa), vascular endothelial growth factor receptor 1 (Flt1), and Neuropilin1 (Nrp1), in the M1 segment of the intestine. We found that the treatment with E2 (P = 0.0366) or E2 + P (P = 0.0031), but not P (P = 0.901), significantly increased vegfa gene expression (Fig. 9A). Although there were trends of reductions in Flt1 and Nrp1 mRNA levels in both E and E2 + P groups, compared to oil-treated control rats (P = 0.7005 and P = 0.3920, respectively), those differences were not significant statistically (Fig. 9B and 9C).
Fig. 9.
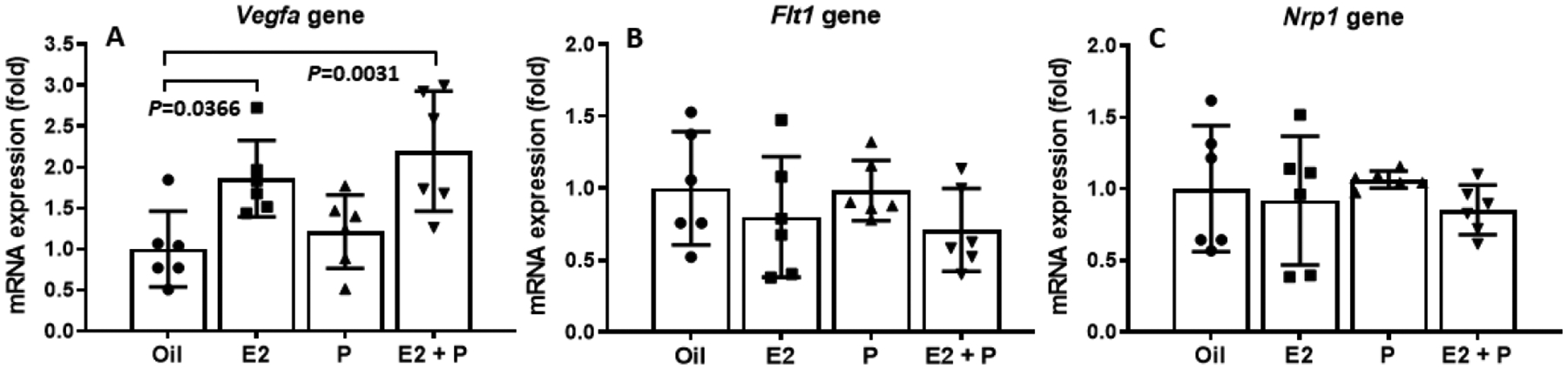
Changes of gene expression in the small intestine of OVX rats treated with E2 and/or P. treatment with E2 or E2 + P, but not P (P = 0.901), significantly increased vegfa gene expression (A). No significant differences in both Flt1 and Nrp1 mRNA levels were found among the 4 groups (B and C). Means ± SD, n = 6 per group.
Lymphatic apolipoprotein output in OVX rats.
We next examined the secretion of apolipoproteins (apo), including apoB48, apoA-IV and apoA-I in the lymph of the four groups of OVX rats. As shown in Fig. 10, the hourly lymphatic output of apoB48 and apoA-IV are comparable among the 4 groups of OVX rats (P = 0.1347 and P = 2709). Interestingly, E2− and E2 + P-treatments significantly increased apoA-I levels in the lymph at 4 h and 5 h, relative to Oil-treatment [E2 vs. Oil, P = 0.0139 (4 h) and P = 0.0124 (5 h); E2 + P vs. Oil, P = 0.0338 (4 h) and P = 0.0177 (5 h)] (Fig. 10).
Fig. 10.
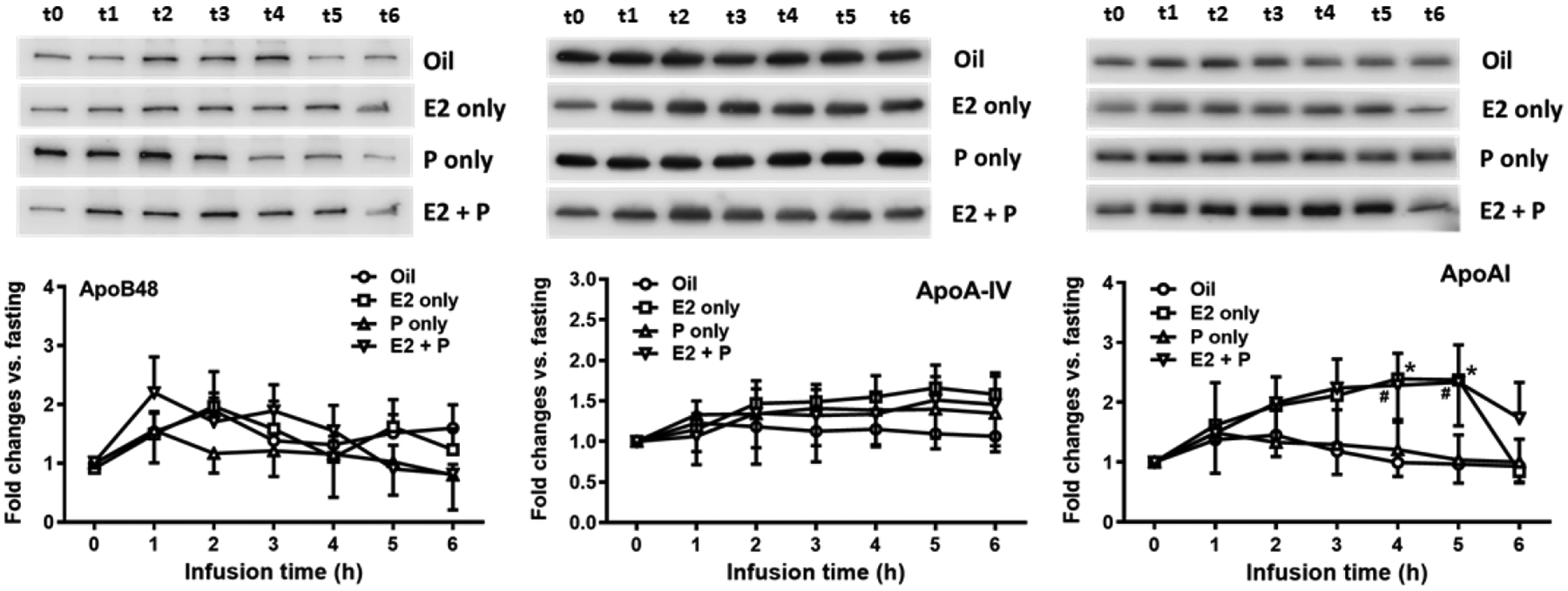
Comparison of lymphatic outputs of apoB-48, apoA-IV and apoA-I among the 4 groups of OVX rats. Top, images of representative bands from the Western blot; and bottom, densitometric quantification of the immunoblots. Although the hourly lymphatic output of apoB48 and apoA-IV are comparable among the 4 groups of OVX rats (P = 0.1347 and P = 2709, respectively), E2− and E2 + P-treatments significantly increased apoA-I levels in the lymph at 4 h and 5 h, relative to Oil-treatment [E2 vs. Oil, #P = 0.0139 (4 h) and #P = 0.0124 (5 h); E2 + P vs. Oil, *P = 0.0338 (4 h) and *P = 0.0177 (5 h)]. Means ± SD, n = 6 per group, except P-treated group (n = 7).
Discussion
Intestinal lipid absorption is a dynamic process that involves many complex steps at the level of enterocyte. Currently, most of our understanding of lipid absorption and transport was derived from studies using male animal models. In the present study, using the lymph fistula model, we were able to identify important functional differences in lipid uptake and transport between male and female rodents.
After lipid infusion, the lymphatic [3H]-TAG output in male mice showed a normal unimodal distribution. In contrast, the lymphatic [3H]-TAG output in female mice segregated into high and low output groups; the high group had lipid absorption comparable to that of the males, and the low group had significantly less lymphatic output (Fig. 1C). One possible explanation for this difference is the ovarian hormone variations that occur with the estrous cycle in the females. In rodents, there are 4 distinct stages of estrous cycle (4 to 5 days per cycle): diestrus 1, diestrus 2, proestrus, and estrus. Both diestrus 1 and diestrus 2 are characterized by low levels of gonadal hormones. During proestrus, E2 increases and reaches its peaks; and P also increases. During the estrus phase, both E2 and P levels drop (Becker et al., 2005; Goldman et al., 2007). We speculate that the intact female mice with high lymphatic TAG output are in diestrus (i.e., the most male-like stage of the estrous cycle), whereas those with low lymphatic TAG output are in proestrus/estrus. Therefore, we hypothesized that E2 and/or P decrease lymphatic TAG output.
To test our hypothesis, female rats were ovariectomized and then treated with E2, P, E2 + P, or vehicle control (Fig. 2). Consistent with our hypothesis, in the OVX rats, E2 and E2 + P treatments led to a decreased lymphatic output of TAG, relative to vehicle (oil) control (Fig. 4). Lipid absorption occurs in the small intestine, with most lipids being absorbed in the duodenum and jejunum and minimal absorption occurring in the ileum. Consistent with this idea, we observed that the majority of the radiolabeled-TAG were found in the upper small intestinal mucosa of the OVX rats (Fig. 6A). The small intestine, specifically the duodenum and the jejunum, was the predominant site of lipid uptake in OVX animals. Our data indicated that TAG absorption was not shifted to utilizing the more distal small intestine in E2 and/or P-treated rats. Thus these hormonal changes did not seem to impart any significant gastrointestinal motility changes in our experiment as the luminal recoveries from the stomach, the different intestinal segments, and the colon were not statistically different among the various groups of experimental animals and the controls.
After coming into contact with the pancreatic enzyme and the bile juice, TAG molecules are hydrolyzed to form 2-monoglycerol (2-MG) and free fatty acids (FA). These digestion products are then solubilized by the bile salts and partial digestion products in the intestinal lumen to form micelles (Hofmann & Borgstrom, 1962; Hofmann & Borgström, 1963). The formation of micelles is important to facilitate the uptake of the digestion produced by the enterocytes (Dietschy et al., 1971; Wilson & Dietschy, 1972). The uptake of the fatty acids is mediated mostly by passive uptake in the intestinal enterocytes, although carrier-mediated process has also been described (Stremmel, 1988; Thomson et al., 1989). After uptake by the enterocytes, the 2-MG and FA are resynthesized back to form TAG (Small, 1991). Note that most dietary Chol that is taken up by enterocytes also undergoes esterification. The TAG and Chol molecules are then packaged with phosphatidylcholine and apolipoproteins, including apoB48, apoA-IV, and apoA-I, to form CM (M.J. van Greevenbroek & W.A. de Bruin, 1998). The CMs are then transported into the lymphatic circulation.
To identify the mechanisms underlying the reduced lymphatic lipid output in E2− and/or P-treated OVX rats, we examined the lipids absorbed into the intestinal enterocytes. We found that the levels of [3H]-TAG were significantly higher in the proximal intestine of both E2− and E2 + P-treated OVX rats, compared to those in Oil-treated rats (Fig. 6A). These results indicated that the E2− and E2 + P-treated rats were less efficient in incorporating the absorbed lipids into CMs, leading to greater accumulation of lipids within the proximal intestine. It is important to note that the regurgitation of the infused emulsion back to the stomach is minimal as reflected by the low recovery of both [3H]-TAG and [14C]-Chol from the stomach in all four groups of animals. Also, the possibility that the infused lipids were excreted cannot explain the difference as shown by the very low radioactivity recovery in the colon.
Although the reduction of lymphatic [14C]-Chol transport rate in E2 and/or P-treated rats did not reach the significant level statistically relative to that in Oil-treated rats (Fig. 5A), P-treated OVX rats showed a significantly reduced total recovery of [14C]-Chol in the lymph during 6-h infusion period, which was accompanied by a significantly increase in the total recovery of [14C]-Chol in the intestinal lumen (Fig. 7B). These results are consistent with the calculation showing that P-treated rats had a significantly lower absorptive index for [14C]-Chol relative to Oil-treated rats (Fig. 8C). These findings suggested that P treatment inhibited the uptake of dietary Chol from the small intestine.
Enterocyte-absorbed lipids are packaged into CMs, which enter the bloodstream through intestinal lymphatic capillaries called lacteals located in the center of the intestinal villi. From there, the CMs are transported through mesenteric lymphatic vessels into the thoracic duct, which drains into the venous circulation (Randolph & Miller, 2014). In the last two decades, the development of genetic approaches (generating lymphatic-reporter mouse models) and intravital imageing techniques (visualizing lymphatic lacteal structure) have improved our understanding of gut lymphatic physiology in health and disease.
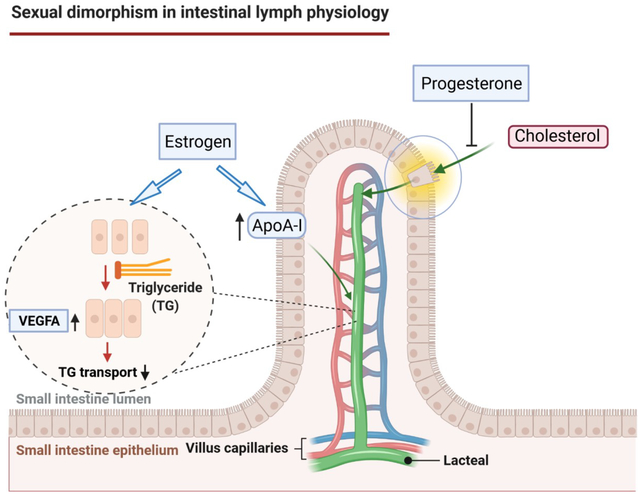
Regulation of CM uptake by lacteals is now emerging. A recent study showed that VEGF-A signaling regulates CM uptake through modulation of lacteal cell-cell junctions (Zhang et al., 2018). As a member of the VEGFs family, VEGF-A plays important roles in vasculogenesis, angiogenesis, and vascular permeability. These effects are mainly mediated through binding to its receptors, VEGFR-1 (also known as Flt1) (Hiratsuka et al., 2005) and semaphorin receptor NRP1 (Soker et al., 1998). Using transgenic mouse model, Zhang et al found that inducible endothelial deletion of Nrp1 and Flt1 genes renders mice resistant to diet-induced obesity because of less lacteal CM uptake. Furhter studies demonstrated that absence of NRP1 and FLT1 receptors increased VEGF-A bioavailability and signaling through VEGFR2, inducing lacteal junction zippering and chylomicron malabsorption (Zhang et al., 2018).
To determine the molecular mechanisms of E2 and P in regulating lipid absorption and transport, we measured the expression of these 3 important genes, including vegfa, flt1, Nrp1 in the M1 segment of intestine of the OVX rats. We found that E2-treatment significantly increased vegfa gene expression in the M1 segment, compared to vehicle control (Fig. 9A). These data suggest that E2 might enhance VEGF-A signaling at the level of the lymphatics of intestine to reduce the permeability of lacteals by closing junctions via transformation of buttons into zippers (Cifarelli & Eichmann, 2019), leading to reduced CM absorption from intestine. This finding is important because it provides valuable knowledge on potential direct effect of E2 treatment on intestinal lymphatic endothelial cells.
Absorbed lipids can be transported via both the lymphatic and the portal routes. The labeled lipids that cannot be recovered from the intestinal lumen and the intestinal mucosa, and were not excreted in the colon was probably transported by the portal circulation, which we named as the “Others” in Fig. 7. A study using both thoracic duct and portal cannulation in rats suggested that up to 50% of the infused lipid can enter the portal circulation (McDonald et al., 1980). The E2-treated OVX rats could have higher portal TAG transport than that of the vehicle-treated rats. The differences between the male and female mice in lymphatic lipid transport could be partially due to the regulation of lipid transport through the lymphatic vs. portal routes. Lipids entering portal route drain into liver, the key metabolic organ. Therefore, our studies raise an interesting question of whether E2 affects lipid transport into the portal circulation in females, which should be addressed in the future.
ApoB-48 is associated with CM particles and plays a critical role in the formation and secretion of CM particles (Lo et al., 2008). It has been demonstrated that one molecule of apoB-48 is associated with one CM particle (Phillips et al., 1997). Accordingly, the mass of lymphatic apoB-48 secretion was used as a surrogate measure to evaluate the relative number of CM particles produced by the gut (Hayashi et al., 1990a). To address the question of whether the reduced lipid transport observed in E2 and P-treated OVX rats was a result of a reduced number of secreted TAG-rich CM following administration of lipid infusate, we measured the levels of apoB-48, as well as apoA-IV, by Western blot. Our results show that E2 and/or P-treated rats had a comparable amount of apoB-48 and apoA-IV mass in the lymph after lipid infusion (Fig. 10), suggesting that the secretion of these apolipoproteins was not influenced by the female sex hormone treatment.
Previous research has demonstrated that female gonadal hormones play important roles in sex differences of a variety of diseases, especially cardiovascular diseases (Arnold et al., 2017; Palmisano et al., 2018). E2 has beneficial effects on circulating lipid profiles, acting to protect against atherosclerotic cardiovascular disease. Specifically, before menopause, the age of onset for the first myocardial infarction is about 10 years later for women compared to men. At any given age, women have one-third to one-half of the risk of cardiovascular disease relative to men (Wilmot et al., 2015; Benjamin et al., 2017). The protective effect of E2 against atherosclerotic cardiovascular disease is further supported by the increased risk that occurs when levels of naturally cycling E2 and P decline with menopause in women. (Bourassa et al., 1996; Middelberg et al., 2002; Guthrie et al., 2004).
The mechanisms conferring protection from atherosclerotic cardiovascular disease in women have been of considerable research interest. The intestine has been demonstrated to synthesize and secrete apo AI and it is an important source of the circulating apo AI (Alpers et al., 1982; Bisgaier & Glickman, 1983). Interestingly, we found that lymphatic apoA-I level was significantly increased in the OVX rats after treatment with E2, compared to the control rats with oil-treatment (Fig. 10). It has been reported that CMs secreted from the intestinal enterocyte also contain apoA-I, and the latter is quickly transferred to high-density lipoprotein (HDL) in the bloodstream (Wasan et al., 2008). While it is unknown whether the increased apoA-I levels affect the intestinal lipid transport in E2-treated OVX rats, the protective action of apoA-I against atherosclerotic cardiovascular disease is very well established (Smith, 2010; Rosenson et al., 2016). As a major protein component of HDL particles in plasma, apoA-I enables efflux of Chol from peripheral tissues for transport elsewhere, including back to LDL particles or through the reverse Chol transport to the liver for biliary excretion. ApoA-I has been often used as a biomarker for prediction of cardiovascular disease (McQueen et al., 2008). The mechanisms mediating E2’s effect on apoA-I production, secretion or degradation also warrant further studies.
Conclusions
Our current studies clearly demonstrated that female sex hormones play important roles in the regulation of intestinal absorption and lymphatic transport of dietary lipids. Specifically, E2 treatment suppressed lymphatic TAG transport in the small intestine, and P treatment reduced Chol uptake from the intestinal lumen. The hormone-associated decrease in lymphatic lipid concentrations and the increased level of lymphatic apoA-I may contribute to the reduced risk of atherosclerosis in premenopausal women.
Key points.
Significant differences in intestinal lipid absorption and lymphatic transport were found between female and male animals.
Estrogen treatment significantly reduced [3H]-triacylglycerol (TAG) lymphatic output through suppressing TAG transport in ovariectomized (OVX) rats, and this effect is associated with enhanced vegfa gene expression in the intestine.
Progesterone treatment significantly decreased the output of [14C]-cholesterol (Chol) in lymph by inhibiting Chol absorption in the OVX rats.
Estrogen treatment also increased lymphatic output of apolipoprotein A-I (apoA-I) in the OVX rats, which may contribute to the reduced risk of atherosclerosis in females.
Funding and additional information
This study was financially supported by National Institutes of Health (United States) grants DK119135 (to P.T., M.L. and Y.U L.), DK59630 (to P.T.), and DK95440 (to M.L.).
Abbreviations:
- E2
estradiol
- P
progesterone
- OVX
ovariectomized
- TAG
triacylglycerol
- Chol
cholesterol
- CM
chylomicron
- PL
phospholipid
- HDL
high-density lipoprotein
- LDL
low-density lipoprotein
- NaTC
sodium taurocholate
- apoB48
apolipoprotein B48
- apoA-I
apolipoprotein A-I
- apoA-IV
apolipoprotein A-IV
Biography

Dr. Min Liu is a professor in the Department of Pathology and Laboratory Medicine, College of Medicine, University of Cincinnati. He has been working extensively with the gastrointestinal absorption and transport of lipids. One of his research interests in physiology is to understand the regulation and mechanisms of sex hormones on the assembly and secretion of triglyceride-rich lipoproteins in lymphatic system.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Data availability statement
The datasets generated and analysed during the present study are available from the corresponding author upon reasonable request. No novel applicable resources were generated or analysed during the study.
References
- Ajayi AF & Akhigbe RE (2020). Staging of the estrous cycle and induction of estrus in experimental rodents: an update. Fertil Res Pract; DOI: 10.1186/s40738-020-00074-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpers DH, Lancaster N & Schonfeld G (1982). The effects of fat feeding on apolipoprotein AI secretion from rat small intestinal epithelium. Metabolism 31, 784–790. [DOI] [PubMed] [Google Scholar]
- Arnold AP, Cassis LA, Eghbali M, Reue K & Sandberg K (2017). Sex Hormones and Sex Chromosomes Cause Sex Differences in the Development of Cardiovascular Diseases. Arterioscler Thromb Vasc Biol 37, 746–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, Herman JP, Marts S, Sadee W, Steiner M, Taylor J & Young E (2005). Strategies and methods for research on sex differences in brain and behavior. Endocrinology 146, 1650–1673. [DOI] [PubMed] [Google Scholar]
- Benjamin EJ et al. (2017). Heart Disease and Stroke Statistics—2017 Update: A Report From the American Heart Association. Circulation 135, e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergstedt SE, Hayashi H, Kritchevsky D & Tso P (1990). A comparison of absorption of glycerol tristearate and glycerol trioleate by rat small intestine. AmJPhysiol 259, G386–G393. [DOI] [PubMed] [Google Scholar]
- Bisgaier CL & Glickman RM (1983). Intestinal synthesis, secretion, and transport of lipoproteins. Annu Rev Physiol 45, 625–636. [DOI] [PubMed] [Google Scholar]
- BOLLMAN JL, CAIN JC & GRINDLAY JH (1948). Techniques for the collection of lymph from the liver, small intestine, or thoracic duct of the rat. J Lab Clin Med 33, 1349–1352. [PubMed] [Google Scholar]
- Bourassa PA, Milos PM, Gaynor BJ, Breslow JL & Aiello RJ (1996). Estrogen reduces atherosclerotic lesion development in apolipoprotein E-deficient mice. Proc Natl Acad Sci U S A 93, 10022–10027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cifarelli V & Eichmann A (2019). The Intestinal Lymphatic System: Functions and Metabolic Implications. Cmgh 7, 503–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg DJ, Brown LM, Woods SC & Benoit SC (2006). Gonadal hormones determine sensitivity to central leptin and insulin. Diabetes 55, 978–987. [DOI] [PubMed] [Google Scholar]
- Dietschy JM, Sallee VL & Wilson FA (1971). Unstirred water layers and absorption across the intestinal mucosa. Gastroenterology 61, 932–934. [PubMed] [Google Scholar]
- Figueiredo HF, Ulrich-Lai YM, Choi DC & Herman JP (2007). Estrogen potentiates adrenocortical responses to stress in female rats. Am J Physiol Endocrinol Metab 292, E1173–82. [DOI] [PubMed] [Google Scholar]
- FOLCH J, LEES M & SLOANE STANLEY GH (1957). A simple method for the isolation and purification of total lipides from animal tissues. JBiolChem 226, 497–509. [PubMed] [Google Scholar]
- Goldman JM, Murr AS & Cooper RL (2007). The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res B Dev Reprod Toxicol 80, 84–97. [DOI] [PubMed] [Google Scholar]
- Grundy D (2015). Principles and standards for reporting animal experiments in The Journal of Physiology and Experimental Physiology. J Physiol 593, 2547–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie JR, Taffe JR, Lehert P, Burger HG & Dennerstein L (2004). Association between hormonal changes at menopause and the risk of a coronary event: a longitudinal study. Menopause 11, 315–322. [DOI] [PubMed] [Google Scholar]
- Hayashi H, Fujimoto K, Cardelli JA, Nutting DF, Bergstedt S & Tso P (1990a). Fat feeding increases size, but not number, of chylomicrons produced by small intestine. AmJ Physiol 259, G709–G719. [DOI] [PubMed] [Google Scholar]
- Hayashi H, Nutting DF, Fujimoto K, Cardelli JA, Black D & Tso P (1990b). Transport of lipid and apolipoproteins A-I and A-IV in intestinal lymph of the rat. J Lipid Res 31, 1613–1625. [PubMed] [Google Scholar]
- Hiratsuka S, Nakao K, Nakamura K, Katsuki M, Maru Y & Shibuya M (2005). Membrane Fixation of Vascular Endothelial Growth Factor Receptor 1 Ligand-Binding Domain Is Important for Vasculogenesis and Angiogenesis in Mice. Mol Cell Biol 25, 346–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann AF & Borgstrom B (1962). Physico-chemical state of lipids in intestinal content during their digestion and absorption. Fed Proc 21, 43–50. [PubMed] [Google Scholar]
- Hofmann AF & Borgström B (1963). Hydrolysis of long-chain monoglycerides in micellar solution by pancreatic lipase. BBA - Biochim Biophys Acta 70, 317–331. [DOI] [PubMed] [Google Scholar]
- Jandacek RJ, Heubi JE & Tso P (2004). A novel, noninvasive method for the measurement of intestinal fat absorption. Gastroenterology 127, 139–144. [DOI] [PubMed] [Google Scholar]
- Ji Y, Sakata Y, Yang Q, Li X, Xu M, Yoder S, Langhans W & Tso P (2012). Activation of rat intestinal mucosal mast cells by fat absorption. Am J Physiol Liver Physiol 302, G1292–G1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeners B, Geary N, Tobler PN & Asarian L (2017). Ovarian hormones and obesity. Hum Reprod Update 23, 300–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo CM, Nordskog BK, Nauli AM, Zheng S, Vonlehmden SB, Yang Q, Lee D, Swift LL, Davidson NO & Tso P (2008). Why does the gut choose apolipoprotein B48 but not B100 for chylomicron formation? AmJ Physiol GastrointestLiver Physiol 294, G344–G352. [DOI] [PubMed] [Google Scholar]
- van Greevenbroek M MJ & de Bruin T WA (1998). Chylomicron synthesis by intestinal cells in vitro and in vivo. Atherosclerosis 141, S9–S16. [DOI] [PubMed] [Google Scholar]
- McDonald GB, Saunders DR, Weidman M & Fisher L (1980). Portal venous transport of long-chain fatty acids absorbed from rat intestine. Am J Physiol 239, G141–50. [DOI] [PubMed] [Google Scholar]
- McQueen MJ, Hawken S, Wang X, Ounpuu S, Sniderman A, Probstfield J, Steyn K, Sanderson JE, Hasani M, Volkova E, Kazmi K & Yusuf S (2008). Lipids, lipoproteins, and apolipoproteins as risk markers of myocardial infarction in 52 countries (the INTERHEART study): a case-control study. Lancet 372, 224–233. [DOI] [PubMed] [Google Scholar]
- Middelberg RPS, Spector TD, Swaminathan R & Snieder H (2002). Genetic and Environmental Influences on Lipids, Lipoproteins, and Apolipoproteins. Arterioscler Thromb Vasc Biol 22, 1142–1147. [DOI] [PubMed] [Google Scholar]
- Nauli AM, Nassir F, Zheng S, Yang Q, Lo C-M, Vonlehmden SB, Lee D, Jandacek RJ, Abumrad NA & Tso P (2006). CD36 is important for chylomicron formation and secretion and may mediate cholesterol uptake in the proximal intestine. Gastroenterology 131, 1197–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nequin LG, Alvarez J & Schwartz NB (1979). Measurement of serum steroid and gonadotropin levels and uterine and ovarian variables throughout 4 day and 5 day estrous cycles in the rat. Biol Reprod 20, 659–670. [DOI] [PubMed] [Google Scholar]
- Palmisano BT, Zhu L, Eckel RH & Stafford JM (2018). Sex differences in lipid and lipoprotein metabolism. Mol Metab 15, 45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Pullinger C, Kroes I, Kroes J, Hardman DA, Chen G, Curtiss LK, Gutierrez MM, Kane JP & Schumaker VN (1997). A single copy of apolipoprotein B-48 is present on the human chylomicron remnant. J Lipid Res 38, 1170–1177. [PubMed] [Google Scholar]
- Pouteau E, Piguet-Welsch C, Berger A & Fay LB (2003). Determination of cholesterol absorption in humans: From radiolabel to stable isotope studies. Isotopes Environ Health Stud 39, 247–258. [DOI] [PubMed] [Google Scholar]
- Randolph GJ & Miller NE (2014). Lymphatic transport of high-density lipoproteins and chylomicrons. J Clin Invest 124, 929–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenson RS, Brewer HB, Ansell BJ, Barter P, Chapman MJ, Heinecke JW, Kontush A, Tall AR & Webb NR (2016). Dysfunctional HDL and atherosclerotic cardiovascular disease. Nat Rev Cardiol 13, 48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Liu Y, Tso P, Wang DQ-H, Davidson WS, Woods SC & Liu M (2018a). Silencing steroid receptor coactivator-1 in the nucleus of the solitary tract reduces estrogenic effects on feeding and apolipoprotein A-IV expression. J Biol Chem 293, 2091–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Liu Y, Tso P, Wang DQ-H, Davidson WS, Woods SC & Liu M (2018b). Silencing steroid receptor coactivator-1 in the nucleus of the solitary tract reduces estrogenic effects on feeding and apolipoprotein A-IV expression. J Biol Chem 293, 2091–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Liu Y, Wang DQH, Tso P, Woods SC & Liu M (2014). Estradiol stimulates apolipoprotein A-IV gene expression in the nucleus of the solitary tract through estrogen receptor-α. Endocrinology 155, 3882–3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Tso P, Woods SC, Sakai RR, Davidson WS & Liu M (2007). Hypothalamic Apolipoprotein A-IV Is Regulated by Leptin. Endocrinology 148, 2681–2689. [DOI] [PubMed] [Google Scholar]
- Shen L, Wang DQ, Lo CM, Tso P, Davidson WS, Woods SC & Liu M (2010). Estradiol increases the anorectic effect of central apolipoprotein A-IV. Endocrinology 151, 3163–3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Wang DQH, Xu M, Woods SC & Liu M (2017). BDNF/TrkB signaling mediates the anorectic action of estradiol in the nucleus tractus solitarius. Oncotarget 8, 84028–84038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small DM (1991). The effects of glyceride structure on absorption and metabolism. Annu Rev Nutr 11, 413–434. [DOI] [PubMed] [Google Scholar]
- Smith JD (2010). Apolipoprotein A-I and its mimetics for the treatment of atherosclerosis. Curr Opin Investig Drugs 11, 989–996. Available at: https://pubmed.ncbi.nlm.nih.gov/20730693/ [Accessed January 19, 2021]. [PMC free article] [PubMed] [Google Scholar]
- Soker S, Takashima S, Miao HQ, Neufeld G & Klagsbrun M (1998). Neuropilin-1 is expressed by endothelial and tumor cells as an isoform- specific receptor for vascular endothelial growth factor. Cell 92, 735–745. [DOI] [PubMed] [Google Scholar]
- Stremmel W (1988). Uptake of fatty acids by jejunal mucosal cells is mediated by a fatty acid binding membrane protein. JClinInvest 82, 2001–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson AB, Keelan M, Garg ML & Clandinin MT (1989). Intestinal aspects of lipid absorption: in review. Can J Physiol Pharmacol 67, 179–191. [DOI] [PubMed] [Google Scholar]
- Tso P, Balint JA & Rodgers JB (1980). Effect of hydrophobic surfactant (Pluronic L-81) on lymphatic lipid transport in the rat. AmJ Physiol 239, G348–G353. [DOI] [PubMed] [Google Scholar]
- Wang DQ-H (2007). Regulation of Intestinal Cholesterol Absorption. Annu Rev Physiol 69, 221–248. [DOI] [PubMed] [Google Scholar]
- Wang TY, Liu M, Portincasa P & Wang DQ-H (2013). New insights into the molecular mechanism of intestinal fatty acid absorption. Eur J Clin Invest n/a–n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasan KM, Brocks DR, Lee SD, Sachs-Barrable K & Thornton SJ (2008). Impact of lipoproteins on the biological activity and disposition of hydrophobic drugs: Implications for drug discovery. Nat Rev Drug Discov 7, 84–99. [DOI] [PubMed] [Google Scholar]
- Wilmot KA, O’Flaherty M, Capewell S, Ford ES & Vaccarino V (2015). Coronary Heart Disease Mortality Declines in the United States From 1979 Through 2011: Evidence for Stagnation in Young Adults, Especially Women. Circulation 132, 997–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson FA & Dietschy JM (1972). Characterization of bile acid absorption across the unstirred water layer and brush border of the rat jejunum. J Clin Invest 51, 3015–3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Zarkada G, Han J, Li J, Dubrac A, Ola R, Genet G, Boyé K, Michon P, Künzel SE, Camporez JP, Singh AK, Fong GH, Simons M, Tso P, Fernández-Hernando C, Shulman GI, Sessa WC & Eichmann A (2018). Lacteal junction zippering protects against diet-induced obesity. Science (80-) 361, 599–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analysed during the present study are available from the corresponding author upon reasonable request. No novel applicable resources were generated or analysed during the study.


