Abstract
Rationale:
After myocardial ischemic injury, improper phagocytic clearance of dying cardiac cells and the ensuing lack of inflammation resolution results in adverse cardiac remodeling and dysfunction that might lead to heart failure. Therefore, therapeutic strategies to ameliorate immune cell phagocytic function is critical for augmenting cardiac repair after injury.
Objective:
To determine if mesenchymal stem cell-derived exosomes (MSC-Exo) act as opsonin for apoptotic cells and/or trigger “eat me” phagocytic signaling in resident/recruited phagocytes after myocardial ischemic injury.
Methods and Results:
We evaluated MSC-Exo-mediated opsonization of apoptotic cardiomyocytes; and invitro and invivo effects of milk fat globule- epidermal growth factor-factor VIII (MFGE8)-deficient mouse MSC-Exo on macrophage engulfment of apoptotic cardiomyocytes and its implications on cardiac remodeling, repair and function. Microscopy and FACS analyses show that opsonization of apoptotic cardiomyocytes with MSC-Exo enhances their engulfment by macrophages. Furthermore, pre-incubation of macrophages with MSC-Exo reprogrammed the signaling pathways involved in phagocytosis and expression of pro-reparative cytokines. Protein analysis of MSC-Exo reveals expression of MFGE8, a glycoprotein which bridges externalized phosphatidylserine (PS) on the apoptotic cell surface to alphaVbeta3 or alphaVbeta5 integrins on the phagocyte. Most intriguingly, siRNA inhibition of MFGE8 significantly reduced the MSC-Exo-mediated augmentation of dead cell engulfment, associated signaling and pro-reparative phenotype. After myocardial ischemic injury, intramyocardial administration of MSC-Exo increases macrophage uptake of apoptotic bodies in the border zone of infarct and is associated with reduced proinflammatory response, increase in neovascularization, lower infarct size and an improvement in cardiac function and MFGE8-deficient MSC-Exo administration failed to protect mice against MI.
Conclusions:
Our data demonstrates that exosome-associated MFGE8 on one hand enhances opsonization of dead cells and on the other activates phagocytic signaling thus augmenting removal of apoptotic cells, resolution of inflammation and therefore efficient cardiac recovery after injury.
Keywords: Basic Science Research, Inflammation, Ischemia, Myocardial Regeneration, Stem Cells
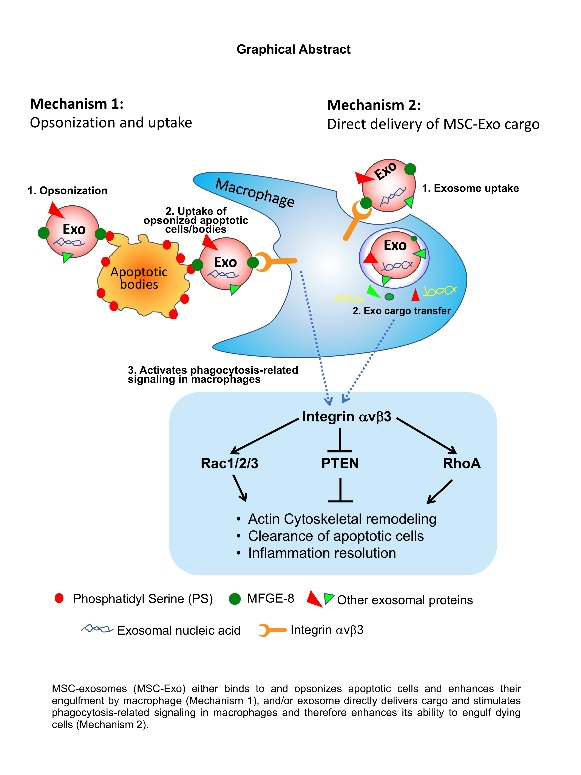
Graphical Abstract
INTRODUCTION
Recurrent myocardial infarction (MI) is common after a first acute MI and is associated with increased morbidity and mortality1. The immune cell response to acute MI, which comprises an initial pro-inflammatory reaction followed by an anti-inflammatory (resolution) phase, determines the final MI size and severity of post-MI remodeling2. However, persistent proinflammatory response can contribute to adverse post-MI LV remodeling, which might result in infarct extension and re-infarction. Interestingly, previous studies have shown that inhibition of innate immune cells is associated with adverse outcomes, post-MI3. In the infarcted heart, efficient phagocytic removal of dying cells (called as efferocytosis) by resident or infiltrating phagocyte before undergoing secondary necrosis reduces the potential damage to adjacent tissue. Most importantly, engulfment initiates signaling pathways within the phagocytic cell that act to dampen inflammation, promote secretion of factors that augments tissue regeneration and repair4. However, improper clearance of dying cells leads to secondary post-apoptotic necrosis, chronic inflammation and expansion of tissue damage therefore may precipitate the transition to heart failure. Previous studies have implicated defective apoptotic cell clearance to pathogenesis of numerous diseases, like atherosclerosis, lupus, infections and cancer5. Efferocytosis requires the recognition of ‘eat-me’ signals (for example, phosphatidylserine; PS) on apoptotic cells/bodies by specific engulfment receptors on phagocyte (MerTK, CD36, integrin αvβ3 and αvβ5), either directly or through bridging molecules like Gas6 or MFGE85. Studies have shown that MI-associated inactivation of myeloid-epithelial-reproductive tyrosine kinase (MERTK) in the myocardium or MFEG8 deficiency in monocyte/macrophages leads to delay in inflammation resolution and cardiac remodeling6,7. These studies further highlight the importance of targeting phagocytic signaling to mitigate adverse cardiac remodeling and dysfunction.
Cell-secreted extracellular vesicles like exosomes play an important role in intercellular communication, both in cardiac health and disease8,9. Stem cell-derived exosomes have been shown to mediate post-MI cardiac tissue repair through various mechanisms including- anti-inflammatory response, inducing angiogenesis, promoting proliferation, preventing apoptosis10. Recent studies have shown that macrophage mediates stem cell or its exosome induced cardioprotective benefits in acute myocardial infarction11,12. There is substantial accumulating evidence to show that MSCs exert a host of cardioprotective effects, including immunomodulation and inflammation suppression through exosomes13,14. However, it is not known whether exosomes modulate macrophage phagocytic activity in the heart and what are potential mechanisms. MSC-derived exosomes are shown to express or carry cargo containing miRNA, RNA or proteins including MFGE8, integrin, calreticulin, GAS615. Studies have shown that modulation of cardiac macrophages by phosphatidylserine-presenting liposomes improves infarct repair16. Furthermore, targeting and modulating infarct macrophages with hemin formulated in designed lipid-based particles, switches infarct macrophages toward M2 anti-inflammatory phenotype and improves angiogenesis, cardiac remodeling and function17. We hypothesize that mouse MSC-derived exosome enhances phagocytic clearance of dying cells in the heart through exosome opsonization of apoptotic cells [MFGE8 on exosome binds to phosphatidylserine (PS) on apoptotic cells], which then facilitates recognition by integrins αvβ3 or αvβ5 on phagocytes [through an RGD motif (Arg-Gly-Asp)], thus triggering rapid clearance of apoptotic cells/bodies. In the present study, we show that MSC-derived exosomes are enriched in MFGE8; MSC-exo opsonizes apoptotic CMs and/or activates phagocytic signaling in macrophages in culture. Interestingly, exosome from MFGE8-deficient MSCs (siRNA-mediated inhibition) failed to trigger phagocytic signaling, M2 macrophage phenotypic switch and showed lower ability to clear opsonized CMs after injection into the peritoneum. Furthermore, in a mouse model of myocardial infarction, as compared to mouse receiving intramyocardial injection of PBS, mouse receiving MSC exosome injection shows higher clearance of dead cells in the myocardium and is associated with lower proinflammatory response, lower infarct size and improvement in cardiac function post-MI.
METHODS
Data Availability.
The authors declare that all supporting data are available within the article and its online supplementary files. Detailed methods including list of resources and primers are provided in the online supplemental Material.
The data that support the findings of this study are available from the authors, Mallikarjun Patil (mpatil@uab.edu) and Sherin Saheera (Sherin.Saheera@umassmed.edu) upon reasonable request.
Vertebrate animals.
All experiments conform to the protocols approved by the Institutional Animal Care and Use Committee (IACUC) at The University of Alabama at Birmingham (Birmingham, AL). Mouse (6–8 weeks-old; C57BL/6J; male) were procured from Jackson Research Laboratory (Bar Harbor, ME).
Cell Culture and Reagents.
RAW 264.7 cells and H9c2 cells were cultured in DMEM media with 10% fetal bovine serum in a humidified chamber at 37°C with 5% CO2. List of major resources has been provided as a supplementary file (Online Table I). List of primers used in the study is shown in supplementary file (Online Table II).
Primary mouse MSC culture (MSCs).
Mouse Mesenchymal Stem Cells (MSC) were either commercially bought (Cyagen, Santa Clara, CA) or isolated from bone marrow as described previously18. MSCs were confirmed by flow cytometry and characterized using a differentiation kit (Cat# SC010; R&D systems).
Exosomes Isolation and Characterization.
Exosomes were isolated using total exosome isolation reagent (Cat# 4478359, Life Technologies) according to the manufacturer’s instructions and characterized as per our previous report8.
Engulfment of MSC-Exo-bound dead cells by macrophages (in vitro efferocytosis).
PKH26-labeled apoptotic H9c2 cells were incubated with MSC-Exo for 12-hours. The MSC-Exo-bound dead cells were then incubated with RAW 264.7 cells (macrophages). The number of macrophage cells that engulfed dead cells were counted as engulfment.
In vivo peritoneal efferocytosis of injected apoptotic cells.
PKH67-labeled apoptotic H9c2 cells were intraperitoneally injected into mice. Engulfed cells in the peritoneal lavage was analyzed by Amnis ImageStream Mark II Imaging Flow Cytometer (Luminex, Austin, TX).
Myocardial Infarction in mouse and MSC-exosome administration.
Male C57BL/6J mice were subjected to myocardial infarction (MI) as described in our previous studies19,20 followed by intramyocardial injection of either PBS or MSC exosomes (from 1×106 cultured MSCs). Study design is shown in Online Figure I.
Echocardiography.
Transthoracic two-dimensional B-mode echocardiogram was performed using Vevo 2100 (VisualSonics, Toronto, Canada). Details are provided in the online supplemental Material (Expanded Methods).
Histological analysis.
Histopathology studies were performed as described previously8. Details are provided in the online supplemental Material (Expanded Methods).
Statistical Analyses.
Summary statistics, means, standard deviation, median and inter-quartile ranges were computed. Nonparametric methods were utilized to assess differences amongst the treatment groups. Analyses were done in SAS version 9.4. Proc GLM was used to assess the responses over time with categorical variables of treatment group and day as factors. A treatment group by time interaction term was the independent variable with the p-values reported at the last visit by comparison of least squares means. P-values less than 0.05 were considered statistically significant as content consistency was important. That is, we expected the two active groups (MI-PBS and MI-MSC+exo) to be significantly different; that the MI-PBS would be different from Sham. Thus, needing to achieve these two simultaneously protected the Type I error and the comparison of the MI-MSC+exo was tested, but was more descriptive than hypothesis testing, since failing to reject the null hypothesis would not demonstrate no difference, only the lack of a statistically significant difference. When comparing only two groups, a Wilcoxon rank sum test was used generated from PROC NPAR1WAY in SAS. when n=3, a special case of the Wilcoxon rank-sum test was applied21. Survival curves were assessed using Log-rank (Mantel-cox) test.
RESULTS
MSC-derived exosome binds to and opsonizes apoptotic myoblast cells in vitro.
Mesenchymal stem cells (MSCs) are multipotent cells with the ability to differentiate into several cell lineages22,23. Mouse MSCs (MSCs) isolated from bone marrow showed a spindle-shaped morphology after day 8 in culture. Cell surface marker analyses by flow cytometry revealed that the cells were positive for CD44 and Sca-1 (MSC markers), and negative for endothelial, myeloid and hematopoietic cell lineage-specific antigens, such as CD31, CD11b and CD45 (Online Figure IIA). Furthermore, to evaluate its multipotent characteristics, we differentiated the cells into osteogenic and adipogenic cell lineage as assessed by staining with Alizarin Red and Oil Red O, respectively and further confirmed by staining with Osteopontin and FABP4 antibodies (Online Figure IIB).
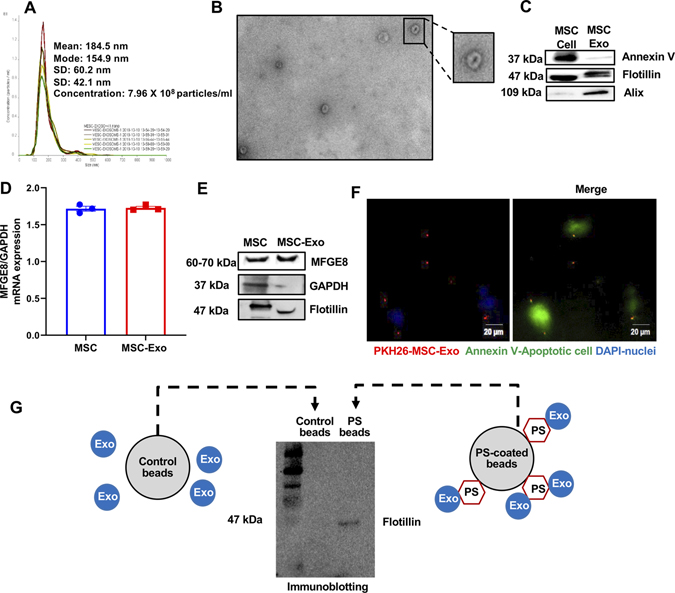
Exosomes isolated from MSC cell culture (72-hours in exosome-depleted FBS medium) were confirmed using dynamic light scatter (particle density and size; Figure 1A) and transmission electron microscopy (cup-shaped morphology, typical of exosomes; Figure 1B). Western blot analysis showed expression of common exosome markers such as Flotillin, Alix and Annexin V (Figure 1C; lysate from MSC cells is used for qualitative comparison).
Figure 1. MSCs-derived exosome contains MFGE8 mRNA and protein and opsonization of apoptotic cells.
A, MSC-exosome characterization by Nanosight dynamic light scattering analysis. B, Transmission electron microscopy showing exosomes. Inset represents a zoomed view of the indicated region, Scale bar, 100 μm. C, Immunoblotting for exosome markers like Annexin V, Flotillin and Alix (MSC cell lysate used for qualitative comparison). D, qRT-PCR data showing MFGE8 mRNA in MSC-Exo (normalized to GAPDH). E, Immunoblot showing presence of MFGE8 protein in MSC-Exo (GAPDH and Flotillin used as controls). F, Representative immunofluorescence staining image showing binding of PKH26-labeled MSC-Exo (red) to Annexin V FITC (green) stained apoptotic H9c2 cells. Scale bar, 20μm. G, Immunoblot showing exosome marker for MSC-exosomes (Exo) bound to phosphatidylserine (PS)-coated beads.
Exposure of phosphatidylserine on the outer leaflet of the plasma membrane is a surface change common to many apoptotic cells24. The phosphatidylserine-binding protein, MFGE8 is a major opsonin for apoptotic cells25 and is a crucial engulfment factor in the phagocytic “synapse” and clearance of apoptotic cells26. Interestingly, qRT-PCR and western blot analysis of exosome revealed enrichment of milk fat globule-EGF factor 8 (MFGE8) mRNA and protein (Figure 1D and 1E; MSC cells is used for qualitative comparison). We hypothesized that exosomes (as opsonins) might bridge apoptotic cells to phagocytes. First, to assess the binding of exosomes to dying cells, we incubated H2O2-induced apoptotic rat cardiomyoblast H9c2 cells with PKH26-labeled MSC-Exo for 12-hours. Microscopic analysis reveals binding of MSC-exo to apoptotic H9c2 cells (Figure 1F), suggesting its ability to act as opsonins on apoptotic cells. We next asked if MSC-exosome opsonization is specific to cardiomyocyte? Therefore, we determined the ability of exosome binding to apoptotic fibroblasts, another cell-type in the heart, by treating with 2μM H2O2 for 4-hours and incubated with exosomes. We found PKH26-labeled MSC-exosomes are capable of binding to apoptotic fibroblasts as well (Online Figure III). To further investigate if exosomes opsonize apoptotic cells through binding to phosphatidylserine (PS), we pulled down exosomes bound to beads coated with PS and performed immunoblotting for exosome marker, flotillin. Figure 1G demonstrates the presence of exosome marker in lysate from PS-coated beads (non-coated control beads did not show the presence of exosome marker).
Furthermore, to determine if MSC-exosomes are capable of binding to necrotic cells (in addition to apoptotic cells), we induced necrosis in H9c2 cells by treating with higher concentration of H2O2 (10μM) and incubated with exosomes overnight. Cells were stained with cell death detection kit containing annexin V and propidium iodide (PI) (Biolegend, cat# 640914). H2O2 (2 μM) mostly induced apoptosis [Annexin V+, Propidium iodide (PI)-] and higher H2O2 concentration (10μM) mostly induced necrosis [Annexin V+, PI-] (Online Figure IV). We found that PKH26-labeled exosomes are capable of binding to necrotic cells as well (Online Figure V).
MSC-Exo opsonization of apoptotic cardiomyoblast enhances its engulfment, both in vitro and in vivo following peritoneal injection.
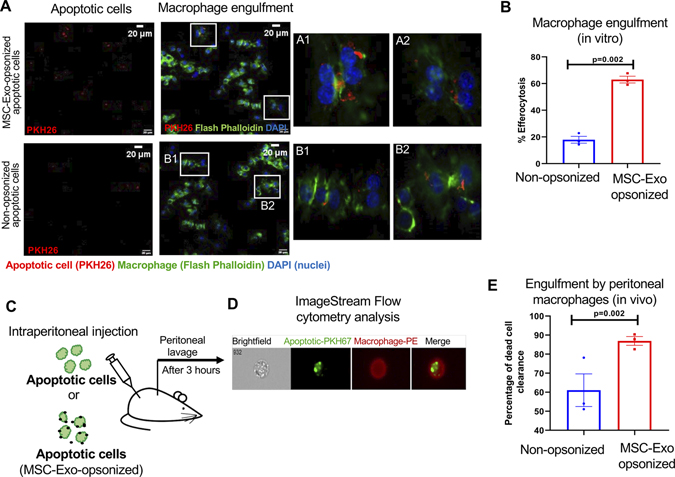
Opsonization has been shown to increase phagocytosis of dead cells by macrophages27. First, invitro, we evaluated the effect of MSC-exo binding to dead cells on engulfment by macrophage. Apoptotic H9c2 (PKH26-labeled, red) cells pre-incubated with MSC-Exo for 12-hours was fed to mouse macrophages (RAW 264.7) for 2-hours at 370C. The cells were fixed and stained with phalloidin-green for F-actin. Interestingly, we found that the phagocytic index (PKH26+ phalloidin-green+ double positive macrophages) was significantly higher when apoptotic cells were opsonized with MSC-Exo as compared to non-opsonized apoptotic cells (Figure 2A and B, P= 0.002).
Figure 2. Binding of MSC-Exo to dead cells enhances its engulfment by macrophages.
A, Immunofluorescence staining showing uptake of apoptotic H9c2 cells (PKH26-labeled, red) by macrophage cell line, RAW 264.7 cells (Flash Phalloidin, green). DAPI to stain nuclei of RAW 264.7 cells (blue); Scale bar, 20 μm; Insets (A1 through B2) showing magnified images of macrophages ingesting apoptotic cells opsonized with and without exosomes. B, Graph showing that MSC-Exo opsonization enhances efferocytosis of dead cells, P=0.002. C, Schematic representation of in vivo efferocytosis assay. D, Representative image-stream analysis images for the in vivo efferocytosis assay (apoptotic cells labelled with PKH26, green; macrophages were labelled with PE conjugated F4/80 antibody). E, Graph showing that MSC-Exo opsonization enhances engulfment of dead cells by peritoneal macrophages represented as % dead cell clearance. n=3, P=0.002.
Furthermore, to evaluate the biological significance of MSC-Exo-mediated opsonization on in vivo phagocytosis, we injected MSC-exo opsonized PKH67-labeled apoptotic cardiomyoblasts (green) into mouse peritoneum (Figure 2C). After 3-hours, cells from peritoneal lavage were isolated and labeled with PE-conjugated anti-F4/80 antibody (to identify macrophages). Phagocytosis was then evaluated by ImageStream Flow Cytometric analysis (Figure 2D). In vivo engulfment of MSC-Exo-bound apoptotic cells by resident peritoneal macrophages was significantly higher as compared to mice receiving non-opsonized apoptotic cell (Figure 2E, P=0.002). This is consistent with the in vitro phagocytosis data.
MSC-Exo treatment activates phagocytosis-related signaling in macrophages and promotes a switch to pro-healing phenotype.
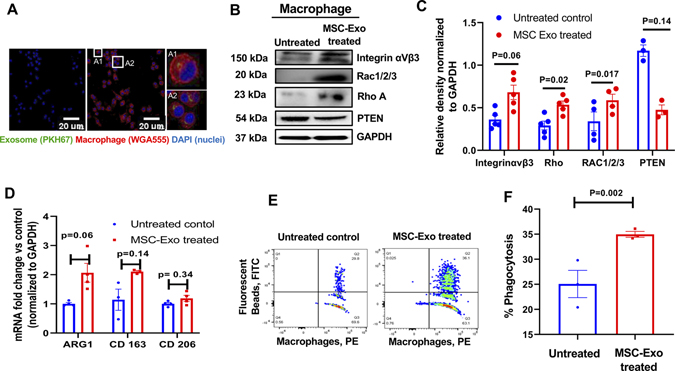
Next, in addition to the above opsonizing effect, we asked whether MSC-Exo binding to macrophage or uptake alone could stimulate macrophage efferocytosis signaling. First, to determine exosome uptake by macrophages, RAW 264.7 cells were incubated with PKH67-labeled exosomes for 24-hours. Immunofluorescence microscopy shows the uptake of MSC-Exo by RAW 264.7 cells (cell membrane stained with WGA Alexa Fluor 555; Figure 3A).
Figure 3. Uptake of MSC-exosomes activate and polarize macrophage cells.
A, Immunofluorescence staining showing uptake of PKH67-labeled MSC-exo (green) by RAW 264.7 cells (WGA Alexa Fluor 555, red). Inset (A1 and A2) represents magnified view of the boxed area, Scale bar, 20 μm. B and C, Immunoblots and corresponding densitometry analyses showing significant changes in proteins involved in phagocytic signaling in mouse macrophage cell line (RAW 264.7) after 24 hours of MSC-Exo uptake. Band intensity of individual protein was normalized to GAPDH loading control. n=3–5. D, qRT-PCR analysis showing upregulation of M2 macrophage phenotype-related mRNA expression when co-cultured with MSC-Exo for 24 hrs. The data was normalized to GAPDH gene and shown as fold change vs untreated control. E and F, Representative Flow cytometry histograms and bar graph showing percentage uptake of fluorescent beads (FITC) by MSC-Exo-pretreated RAW 264.7 cells (PE-anti-F4/80 antibody), P=0.002 vs untreated cells.
Second, we evaluated phagocytosis-related signaling after exosome uptake. Previous reports have shown that- in response to phagocytic stimuli, macrophages increase expression of integrin αVβ3, the receptor for MFGE8. Integrin αVβ3 then activates RhoA and RAC1/2/3 and inhibit expression of PTEN (negative regulator of Rac), which then results in downstream signaling events that facilitate extensive cytoskeletal rearrangement/polymerization of actin in the phagocyte to internalize dead cells28,29. Interestingly, we observed that pretreatment of macrophages with MSC-exosomes significantly increases the expression of integrin αVβ3 and its downstream effectors- upregulation of RhoA and RAC 1/2/3, and downregulation of PTEN, both at the level of mRNA (Online Figure VI, P<0.05), and proteins (Figure 3B and 3C, P<0.05), which was further confirmed in independent experiments by FACS analysis (Online Figure VII) and immunoblotting (Online Figures VIII, IX and X).
Third, to understand the functional outcome of macrophage uptake of MSC-Exo and upregulation of phagocytosis-related signaling, we evaluated it’s impact on macrophage biology specifically as it relates to its inflammatory response. Previous studies have shown that after processing of the ingested cargo, phagocytes elicit specific responses like secretion of anti-inflammatory mediators that help dampen the local immune response30,31. In our study, after treating macrophages with MSC exosomes, we did not observe a significant difference in M1-related genes such as TNF-α, NOS2 and IL6 (Online Figure XI). Interestingly, although not statistically significant we observed an upregulation of macrophage M2-related genes Arg1 (Figure 3D; P=0.06) and CD163 (Figure 3D; P=0.14) and no change in CD206 (Figure 3D; P=0.34). Furthermore, flow cytometry analyses demonstrate that macrophages pretreated with MSC-exo had significantly increased tendency to phagocytose fluorescent latex beads (FITC) as compared to untreated macrophages (Figure 3E and 3F; P=0.002).
MFGE8 deficiency in exosome diminishes its effect on macrophage engulfment and molecular reprogramming.
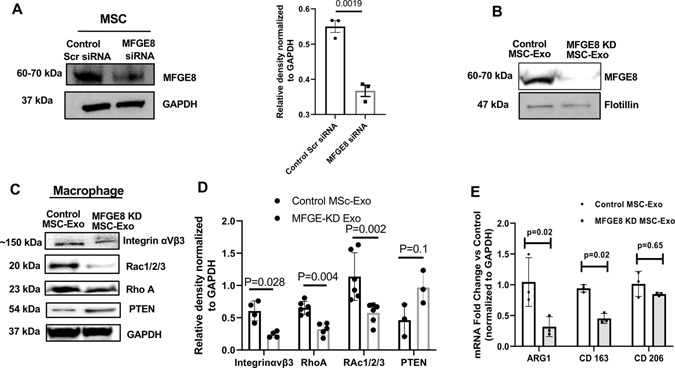
Next, to investigate how MSC-Exo promotes phagocytosis-related (integrin αVβ3 and its downstream molecular) signaling, we evaluated the role of MFGE8, ligand for integrin αVβ3. Previous studies have shown that MFGE8 has been shown to opsonize apoptotic cells for better phagocytosis by immature dendritic cells32, thus allowing us to speculate that exosomal MFGE8 has the potential to serve as bridge between phagocytes and dead cells. We tested the effect of MSC-exosome deficient in MFGE8 on macrophage phagocytic signaling. We generated MFGE8 knockdown (KD) MSCs using siRNA transfection for 48-hours and isolated exosomes (nonspecific scrambled siRNA served as controls). Immunoblotting shows siRNA-induced MFGE8 deficiency in both MSC cells (Figure 4A) and MSC-exosomes (Figure 4B). Next, RAW 264.7 cells were treated with control MSC-exo and MFGE8 KD-exo for 24-hours and phagocytosis-related signaling through integrin αVβ3 was evaluated. Interestingly, cells treated with MFGE8 deficient MSC-Exo showed a significant downregulation of integrin αVβ3, Rho-A and Rac1/2/3 and upregulation of PTEN as compared to cells treated with control-MSC-Exo (Figure 4C and D; Online Figures XII, XIII, XIV). Furthermore, there was a corresponding decrease in the mRNA expression of macrophage M2 phenotype-related genes such as ARG1 (Figure 4E; P=0.02) and CD163 (Figure 4E; P=0.02) and no changes in CD206 expression (Figure 4E; P=0.65). Also, phagocytosis analysis revealed reduced uptake of dead cells by macrophages pretreated with MFGE8 KD MSC-Exo (Online Figures XV; P=0.002 as compared to control-MSC-Exo pretreated macrophage cells).
Figure 4. MFGE8-deficient MSC-Exosome abrogates phagocytic response in macrophages.
A, Immunoblot and corresponding densitometry analysis showing efficient knockdown of MFGE8 in MSC after 48 hours of MFGE8 siRNA transfection [Scrambled siRNA transfected cells serve as control (control Scr siRNA)]. Band intensity of individual protein was normalized to GAPDH loading control. B, Immunoblot showing deficiency of MFGE8 in exosome derived from MFGE8-siRNA transfected MSCs (MFGE8 KD MSC-Exo). Flotillin, another exosome marker is shown for reference. C and D, Immunoblot and densitometry analysis showing changes in phagocytosis-related signaling proteins in macrophages treated with MFGE8 KD MSC-Exo (MFGE8-siRNA transfected) as compared to control exosomes (scrambled siRNA transfected). Band intensity of individual protein was normalized to GAPDH loading control. E, qRT-PCR analysis showing downregulation of mRNA related to M2 macrophage phenotype in macrophages co-cultured with exosomes from MFGE8 KD MSCs. Data normalized to GAPDH mRNA and represented as fold change vs. control MSC-Exo group. n=3.
Intramyocardial administration of MSC-exo increases in situ engulfment of apoptotic cardiac cells, enhances inflammation resolution and preserves cardiac function after myocardial ischemic injury.
Previous studies have shown that MI-associated inhibition of efferocytosis-related signaling in the myocardium leads to delay in inflammation resolution and cardiac remodeling6. To assess the in vivo effects of MSC-exosome on efferocytosis and repair after myocardial infarction, we administered MSC-Exo (from 1×10^6 cultured MSCs) intramyocardial or an equivalent volume of vehicle (PBS), immediately following LAD ligation. A second dose of MSC-Exo was also administered intravenously by tail vein injection 24-hours after MI surgery. We assessed in situ engulfment of dying cardiac cells and its effect on clearance of dead cells, infiltration of inflammatory cells and the impact on tissue remodeling and repair.
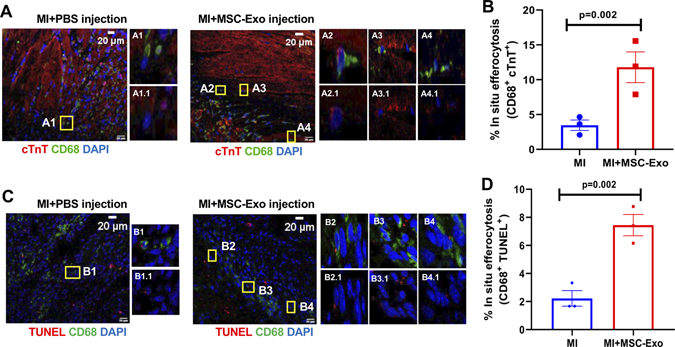
To evaluate in situ macrophage phagocytosis of cardiomyocytes, we assessed heart sections for the presence of cTnT positivity (cardiomyocyte marker) within cytoplasm of CD68+ macrophages in the infarct zone, at 7-days post-MI. Interestingly, MSC-Exo treated mouse group showed an increase in the number of CD68+ cells with cytoplasmic cTnT positivity as compared to MI mice receiving PBS (Figure 5A and B, P=0.002). Also, we assessed macrophage phagocytosis of apoptotic cells in cardiac tissue sections staining with TUNEL assay kit (for apoptotic cells) and CD68 (for macrophages), and we counted number of CD68+ cells with TUNEL positivity in the cytoplasm. We observed that MSC-Exo treated group showed a significant increase in the number of CD68+ cells showing TUNEL positivity in the cytoplasm as compared to mice receiving PBS, post-MI (Figure 5C and D, P=0.002).
Figure 5. MSC-Exo treatment enhances in situ efferocytosis in myocardium, 7-days post-MI.
A and B, Representative immunofluorescence images and bar graph showing increased efferocytosis of cardiomyocytes [cTnT positivity (red) inside CD68+ macrophages (green)] in the infarct myocardium of MSC-Exo treated mice, at 7-days post-MI. P=0.002, n=3, Scale bar, 20 μm. Insets represent magnified view of boxed areas (A1-A4: cTnT, CD68 and DAPI; A1.1-A4.1: cTnT and DAPI). C and D, Immunofluorescence images and bar graph showing increased efferocytosis of apoptotic cells in the myocardium of MSC-Exo treated mice [TUNEL positivity (red) within cytoplasm of CD68+ macrophages (green)], 7-day post MI. P=0.002, n=3, Scale bar, 20 μm. Insets represent magnified view of boxed areas (B1-B4: TUNEL, CD68 and DAPI; B1.1-B4.1 TUNEL and DAPI). We averaged the sample data that were derived from the same animal.
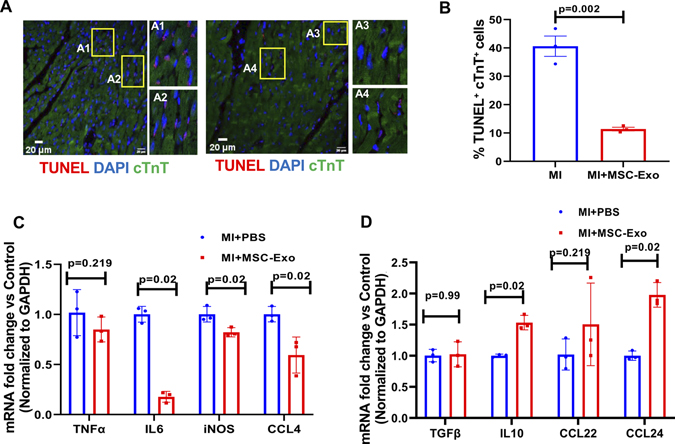
Furthermore, we evaluated the effect of efferocytosis on the number of apoptotic cells and inflammation resolution in the myocardium at 7-days post-MI. Immunofluorescence imaging shows that MSC-Exo administration significantly enhanced the clearance of dying cells [lower number of apoptotic cardiomyocytes (TUNEL+ cTnT+ cells)] in the myocardium as compared to mice receiving PBS (Figure 6A and B; P=0.002). Most importantly, increased engulfment and clearance of dying cardiomyocytes was associated with downregulation of mRNA expression of myocardial pro-inflammatory cytokines such as IL6, iNOS and CCL4 (Figure 6C; P=0.02), and upregulation of anti-inflammatory cytokine mRNA such as IL10, and CCL24 (Figure 6D; P=0.02). No changes were observed in expression of TNF-α, TGFβ, CCL22 (Figure 6D).
Figure 6. MSC-Exosome injection decreases the number of apoptotic cells in the myocardium and enhances inflammation resolution, 7-days post-MI.
Representative immunofluorescent images (A) and bar graph (B) showing reduced number of TUNEL positive cardiomyocyte cells in MSC-Exo treated mice as compared to PBS-treated mice. n=3, P=0.002. The inset in each panel represents magnified view of boxed area (A1-A4). Scale bar, 20 μm. C and D, qRT-PCR analysis showing lower mRNA expression of pro-inflammatory-related genes (C) and a corresponding increase in anti-inflammatory-related genes (D) in the myocardium of mice receiving MSC-Exo compared to PBS-treated mice. Data normalized to GAPDH mRNA expression and shown as fold change vs MI+PBS group. n=3. We averaged the sample data that were derived from the same animal.
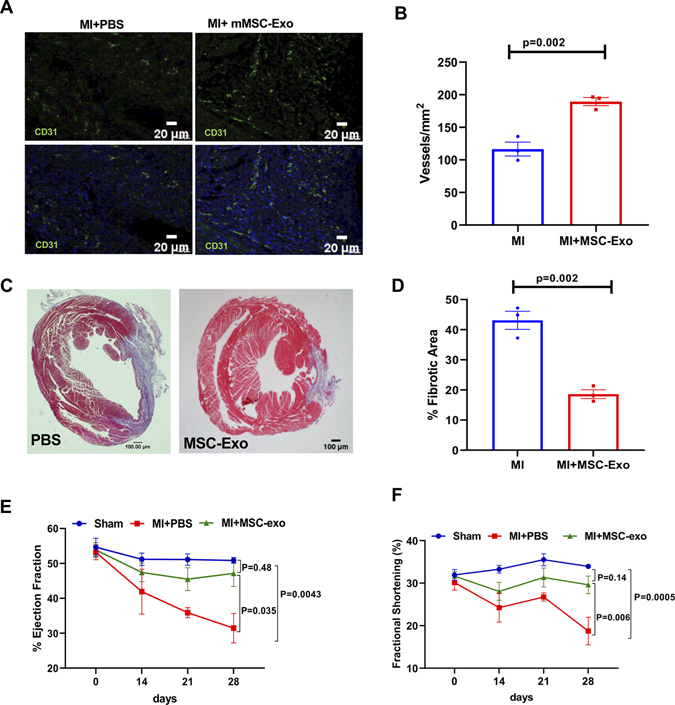
To demonstrate the impact of efficient clearance of apoptotic CMs on cardiac remodeling and function, we assessed neovascularization by CD31 staining, infarct size and LV function by trichrome staining and echocardiography at 4 weeks after MI. Immunofluorescence staining showed significantly increased number of CD31+ cells in the myocardium of mice receiving MSC-Exo as compared to PBS group (Figure 7A and B; P=0.002). Masson’s Trichrome staining of heart tissues and ImageJ analysis show a significant decrease in LV scar area/fibrosis following MSC-Exo administration (Figure 7C and D; P=0.002 vs PBS group). LV function was evaluated by echocardiography at baseline and at 14-, 21- and 28- days post-MI. At 28 days post MI, mice receiving MSC-exo showed preserved percent EF and %FS as compared to PBS-administered group (Figure 7E and 7F). LVEDV and LVESV are shown in Online Figure XVI.
Figure 7. Increased capillary density and improved heart function after myocardial infarction in mice receiving MSC-exosome, at 28-days post-MI.
A, Representative images showing CD31+ capillaries (green) and graphical representation (B) showing increased capillary density in the myocardium of mice receiving MSC-Exo compared to PBS-treated mice. C and D, Masson’s Trichrome staining (top) and ImageJ analysis (bottom) showing reduced fibrosis area in MSC-exo treated mice compared to PBS-treated mice. E and F, Echocardiography analysis showing improvement in % ejection fraction (E) and % fractional shortening (F) in mice treated with MSC-exo at 28-days after MI. n=3 sham, n =7–8 for MI+PBS and MI+mMSC-Exo groups (non-parametric test for multiple comparisons and a post hoc test; Sham vs MI+PBS, Sham vs MI+mMSC Exo and MI+PBS vs MI+MI mMSC Exo group). We averaged the sample readings that were derived from the same animal for each time-point.
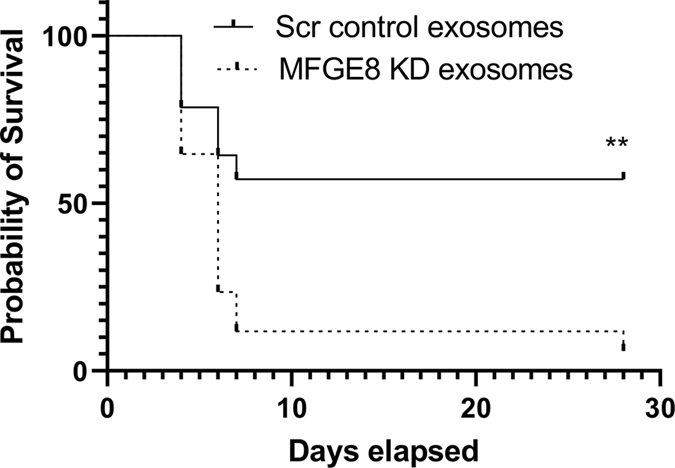
MFGE8-deficient MSC-Exo administration failed to protect mice against MI.
To investigate if MSC-exosome mediated myocardial repair and inflammation resolution was mediated through MFGE8, we injected exosomes from scramble control siRNA-transfected and MFGE8 siRNA-transfected (knockdown) mouse bone marrow-derived MSCs into myocardium after MI and evaluated its effect on survival and LV remodeling. Interestingly, of the total 17 mice (from 2 independent experiments) injected with MFGE8 KD-exosome, only one survived by day 28, whereas 9 of the 15 mice (from 2 independent experiments) survived by day 28 in scramble control exosome group. Survival curve analysis by log-rank (Mantel-Cox) test revealed significant difference between mice injected with scramble control exosome and MFGE8 KD-exosomes (Figure 8, P=0.0048). Histological analysis of heart from the surviving mouse revealed differences in scar size between control scramble exosome and MFGE8 KD MSC-exosome injected groups (Online Figure XVII). These findings prompted us to ask if MFGE8 knockdown affects MSC biology in terms of their angiogenesis and inflammatory response. We noticed that MFGE8 knockdown did not affect angiogenesis-related genes in MSCs (Online Figure XVIIIA). However, there was significant upregulation of pro-inflammatory cytokine IL6 and inhibition of anti-inflammatory cytokine IL10 in MFGE8 knockdown MSCs (Online Figure XVIIIB; P<0.05).
Figure 8. Reduced post-MI survival of mice receiving MFGE8-deficient MSC-exosomes.
Survival curve analysis by log-rank (Mantel-Cox) test showing percentile of mice surviving after MI surgery in mice receiving exosomes from Scrambled (Scr) control siRNA-transfected and MFGE8 siRNA-transfected mouse MSCs. n=15 (Scr, scrambled control exosome group) and n=17 (MFGE8 KD exosome). **P=0.0048.
DISCUSSION
Acute myocardial infarction and subsequent heart failure, are major causes of death and disability worldwide33. Inflammation, cardiomyocyte cell death, scar tissue formation and structural remodeling are the primary pathophysiological responses following MI. Infiltrating immune cells play a critical role in post-MI milieu and regulates these responses. The immune cell response to acute myocardial infarction (AMI), which transitions from an initial pro-inflammatory reaction to an anti-inflammatory phase is critical to the final MI size and post-AMI remodeling34–36. However, a persistent and severe pro-inflammatory reaction leads to adverse LV remodeling and dysfunction leading to heart failure2,36. Interestingly, animals with complete depletion of macrophages exhibit defective wound repair, suggesting that macrophages are critical players in the pathogenesis of MI37. Several studies have demonstrated the effect of cardiomyocyte apoptosis on LV remodeling, post-MI37; however, studies investigating the fate of apoptotic cardiomyocytes and its implications on myocardial repair are lacking. In this regard, previous studies have shown that removal of the dead cells by efferocytosis is a critical mechanism in tissue homeostasis and pathogenesis of several diseases38–42. An efficient clearance of apoptotic cell i) prevents secondary necrosis and consequent release of cell contents that further incite tissue damages; ii) triggers phagocytic signaling that subsequently induces an anti-inflammatory response and iii) contributes to the initiation of tissue repair signaling30,31. However, improper clearance of apoptotic cells leads to establishment and progression of a number of human chronic inflammatory diseases such as autoimmune and neurological disorders, inflammatory lung diseases, obesity, type 2 diabetes, or atherosclerosis25,38. Therefore, strategies that enhance efficient removal of dead cells, thereby resolving inflammation in the injured myocardium could lead to efficient cardiac healing and repair. In the present study, we demonstrate that exosomes (derived from mouse MSC) i) on one hand, binds to apoptotic CMs and enhances its engulfment by macrophages (thus acting as opsonin) and ii) on the other hand, macrophages when pretreated with exosomes is sufficient to activate phagocytic signaling (please see Graphical abstract). Furthermore, interestingly, we show that MFGE8-deficiency in exosomes fails to trigger phagocytic signaling, M2 macrophage phenotypic switch and a lower ability to clear apoptotic CMs, in vitro.
Efferocytosis requires the recognition of ‘eat-me’ signals (for example, phosphatidylserine; PS) on apoptotic cells/bodies by specific engulfment receptors on phagocyte (MerTK, CD36, integrin αvβ3 and αvβ5), either directly or through bridging molecules like Gas6 or MFGE85, which then results in downstream signaling events that facilitate extensive cytoskeletal rearrangement in the phagocyte to internalize dead cells30. Studies have shown that MI-associated inactivation of myeloid-epithelial-reproductive tyrosine kinase (MERTK) in the myocardium or MFGE8 deficiency in monocyte/macrophages leads to delay in inflammation resolution and cardiac remodeling6,7. It is not known whether exosomes modulate macrophage phagocytic activity in the heart and what are the potential mechanisms involved. In the present study, we hypothesized that peripheral membrane glycoprotein, MFGE8 on exosome binds to exposed phosphatidylserine (PS) on apoptotic cells and bridges to integrins αvβ3 or αvβ5 on phagocytes, thus facilitates recognition by phagocytes and triggers rapid clearance of apoptotic cells. In the present study, using PS-coated beads, we show that exosomes bind to PS. Our in vitro data shows that mouse MSC-derived-exosome (MSC-Exo) are enriched in MFGE8; binds to apoptotic cardiomyoblasts and exosome-coated apoptotic cells were much more readily engulfed by macrophages as compared to the non-coated dead cells, suggesting that exosomes acts as opsonins and promotes their engulfment. Furthermore, we found similar phenomenon when exosome-coated apoptotic cardiomyoblasts were injected into the peritoneum of mouse. Our data is consistent with previous report that opsonization of bacterium or dead cells enhances phagocytosis27, both invitro and in vivo43.
During acute ischemic injury, cardiomyocytes die by different mechanism such as apoptosis, necrosis etc44.
It is not clear if exosomes also bind to necrotic cells. Theoretically, cell death either due to apoptosis or necrosis is associated with externalization of PS on the cell surface. In the present study, we demonstrate that exosome also binds to necrotic cells. Therefore, our data suggests that exosome-mediated opsonization may not be specific only for apoptotic cells and that any form of cell death with externalization of PS might be subject to exosome opsonization.
In the present study, we used mouse MSCs to derive exosomes for opsonization, because previous studies have shown that MSCs are strong immunomodulators and alter immune response by means of direct cell-to-cell interactions or by secretion of extracellular vesicles45,46. However, the effect of MSC-derived exosome on efferocytosis (specifically apoptotic cardiomyocytes) and its implication on cardiac remodeling and repair has never been studied yet. In addition to the above findings, our in vitro studies demonstrate that macrophages pretreatment with exosome was sufficient to trigger engulfment receptors like integrin αvβ5 and the activation of downstream phagocytosis-related signaling such RhoA and RAC upregulation and inhibition of PTEN. Previous reports have shown that RhoA and RAC activation leads to actin polymerization, myosin contraction and phagosome formation whereas PTEN inhibits this process. Consistent with these reports, our study further demonstrates that exosome-pretreated macrophages had significantly higher capability to engulf apoptotic cells. It was not clear if the above effects are exclusively specific to MSC-derived exosomes or could be applicable to other stem cell types. While theoretically, all exosomes have PS on their surface, it may be a general effect than specific to MSC-exo, however, MSC exosome may have superior benefits due to other regenerative benefits in addition to promoting opsonization and engulfment of apoptotic cells. Future studies to evaluate such possibilities might have implications on other stem cell therapeutics currently under clinical investigation.
In order to identify molecular mechanism of MSC-derived exosome effects on efferocytosis, we focused our study on MFGE8, since our data confirmed the expression of MFGE8 in MSC-exosomes, which corroborates with earlier mass spectrometry study showing presence of MFGE8 in MSC-derived exosomes15. Previous study has shown that after MI, Mertk- and Mfge8-expressing monocyte/macrophages synergistically engage the clearance of injured cardiomyocytes, favoring the secretion of VEGFA to locally repair the dysfunctional heart7. MFGE8 is critical for apoptotic cell phagocytosis and in macrophage biology47,48. Studies using MFGE8−/− mice indicate that MFGE8 has an essential role in the process of phagocytosis49. MFGE8 administration in an ischemia-reperfusion injury model protects mice by promoting apoptotic cell engulfment50. In the present study, we demonstrate that MSC-Exo deficient in MFGE8 showed diminished capacity to trigger phagocytic activity and related signals, in vitro. In addition, interestingly, MSC-Exo administration post-MI, promotes in situ phagocytosis. Furthermore, we also investigated if MFGE8 knockdown affects MSC biology. While there was no significant difference in angiogenesis-related genes after MFGE8 KD in MSCs, we observed significant increase in pro-inflammatory cytokine IL6 and inhibition of anti-inflammatory cytokine IL10. Although our data does not conform with previous report that MSC from MFGE-8 knockout mice suppress tumor angiogenesis and growth through decreases in VEGF and ET-1 expression51; but our data concurs with published studies showing MFGE-8 knocking down in MSCs increase inflammatory cytokine production, which has been attributed to detrimental effect on MSCs biology and function47. Therefore, MSC-exosomes may also inhibit uncontrolled inflammation, therefore promoting efficient clearance and healing post-MI. All these observations support our findings that MFGE8 in MSC-exo plays an essential role in activating efferocytosis-related signaling via activation of integrin αVβ3, Rac1 and Rho A, resulting in efficient engulfment and clearance of apoptotic cells.
The final step is processing of the ingested cargo and efferocytosis triggers specific downstream intracellular signal transduction pathways, mainly the secretion of anti-inflammatory mediators that dampen the local immune response, macrophage M2 phenotype switch that elicits pro-healing effects, therefore favor the return to tissue homeostasis48. We determined the effect of MSC-Exo-MFGE8-mediated stimulation of efferocytosis on macrophage phenotype switch and inflammatory response. Our data shows that exosome pretreatment triggers efferocytosis-related signaling through integrin αVβ3/Rac1/Rho A activation, which was associated with downregulation of proinflammatory cytokine expression, and upregulation of anti-inflammatory cytokines and M2 phenotype markers. Interestingly, these effects were diminished in MFGE8-deficient exosome treated macrophages, suggesting that the effects are mediated through Exo-MFGE8. Previous studies have shown that MI associated inactivation of myeloid-epithelial-reproductive tyrosine kinase (MERTK) in the myocardium leads to delay in inflammation resolution and cardiac remodeling 6,7. To assess the in vivo effects MSC-exosome on efferocytosis and repair, in a mouse model of AMI, we evaluated the effect of MSC-exo on in situ clearance of dead cardiomyocytes, inflammation resolution, LV remodeling and repair, and its implications on LV function. Our data shows that MSC-Exo administration increases in situ macrophage uptake of apoptotic myocardial cells or cTnT+ cells and is associated with reduced proinflammatory response, increase in neovascularization, lower infarct size and an improvement in cardiac function. In addition, MFGE8 KD-exosomes failed to protect mice against MI-induced cardiac damage. Together, these data suggest that MSC-Exo-mediated efficient clearing of dead cells promote inflammation resolution, preservation of small scars, and prevented ventricular dilatation and remodeling, and most importantly, the exosome-mediated effects are partially mediated through MFGE8-mediated stimulation of phagocytosis-related signaling. However, it is possible that immunohistological analyses to evaluate in situ efferocytosis might over-estimate or under-estimate the observed phenomenon. Therefore, future studies should involve use of reporter mouse to label endogenous CMs and evaluate post-MI efferocytosis by FACS analyses of isolated macrophages from the heart.
In conclusion, the present study demonstrates a unique mechanism of MSC-Exo-mediated myocardial infarct repair through modulation of phagocytic activity in the myocardium. To the best of our knowledge, the present study is the first to demonstrate that MSC-exo, in addition to other beneficial effects, could enhance efferocytosis through distinct mechanisms that includes- both opsonization and presentation of apoptotic cells, and priming of the phagocytic signaling for efficient engulfment and clearance of dead cells. Therefore, we hope that our study will have broader implications on other diseases such as allergic and chronic inflammation, sepsis, atherosclerosis, stroke, obesity, rheumatoid arthritis and cancer (wherein efferocytosis plays a critical role), and we anticipate will potentially aid in applying this therapeutic strategy across the above disease platforms.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What Is Known?
Acute myocardial infarction (MI), also known as heart attack, is one of the leading causes of mortality in humans.
MI causes massive cell death, activation of inflammation and tissue injury.
Delayed inflammation resolution in injured myocardium leads to continued cardiac damage and dysfunction.
What New Information Does This Article Contribute?
Exosomes derived from mesenchymal stem cells (MSC-Exo) opsonizes dead cardiac cells and enhance their engulfment and removal by immune cells (also called as efferocytosis).
MSC-Exo activates macrophage phagocytosis ability through Milk Fat Globulin Epidermal Growth Factor VIII (MFGE-8)/Integrin signaling.
MSC-exo aids in cardiac repair by enhancing clearance of dead cells and resolution of inflammation.
Myocardial infarction is one of the leading causes of mortality across the globe. MI results in death of cardiac cells, inflammation and tissue injury. After MI, improper phagocytic clearance of dying cardiac cells and the ensuing lack of inflammation resolution results in adverse cardiac remodeling and dysfunction that might lead to heart failure. MSC-Exosome has been shown to play role in immunomodulation. However, it is not known whether MSC-Exo modulates efferocytosis and its implications on cardiac repair. Here we evaluated MSC-Exo-mediated opsonization of apoptotic cardiomyocytes; in vitro and in vivo effects of milk fat globule- epidermal growth factor-factor VIII (MFGE8)-deficient mouse MSC-Exo on macrophage engulfment of apoptotic cardiomyocytes; and its implications on cardiac remodeling, repair and function. To the best of our knowledge, this is the first study to show that exosome-associated MFGE8 on one hand enhances opsonization of dead cells and on the other activates phagocytic signaling, thus augmenting removal of apoptotic cells and inflammation resolution, leading to efficient cardiac recovery after injury. For broader translational implications, the molecular mechanisms identified in this study could be useful in therapeutics for conditions like allergic and chronic inflammation, sepsis, atherosclerosis, stroke, obesity, rheumatoid arthritis and cancer (wherein efferocytosis plays a critical role).
ACKNOWLEDGEMENT
We sincerely thank- Dr. Mary Flowers Braswell (for her generous donation of the Vevo 2100 VisualSonics Fujifilm Imaging System), Gary R. Cutter, PhD and Thanh Nguyen, PhD (for their help with Statistics).
SOURCES OF FUNDING
This work is supported, in part, by the National Institutes of Health (NIH) grants HL116729 (to P.K.), HL138023 (to P.K. and J.Z.) and American Heart Association Transformational Project Award 19TPA34850100 (to P.K.).
Nonstandard Abbreviations and Acronyms:
- MSC
Mesenchymal Stem Cells
- MSC-Exo
Mesenchymal Stem Cell derived Exosomes
- PS
Phosphatidylserine
- MFGE8
Milk Fat Globule Epidermal Growth Factor- Factor VIII
- CD
Cluster of Differentiation
- IL
Interleukin
- TNF
Tumor Necrosis Factor
- ARG
Arginase
- MI
Myocardial Infarction
- CCL
C-C motif chemokine ligand
- Echo
Echocardiography
Footnotes
DISCLOSURES
None. All the authors have reported “nothing to disclose”.
Publisher's Disclaimer: This article is published in its accepted form. It has not been copyedited and has not appeared in an issue of the journal. Preparation for inclusion in an issue of Circulation Research involves copyediting, typesetting, proofreading, and author review, which may lead to differences between this accepted version of the manuscript and the final, published version.
REFERENCES
- 1.thune jj, signorovitch je, kober l, mcmurray jjv, swedberg k, rouleau j, maggioni a, velazquez e, califf r, pfeffer ma, solomon sd. predictors and prognostic impact of recurrent myocardial infarction in patients with left ventricular dysfunction, heart failure, or both following a first myocardial infarction. european journal of heart failure. 2011;13:148–153. [DOI] [PubMed] [Google Scholar]
- 2.andreadou i, cabrera-fuentes ha, devaux y, frangogiannis ng, frantz s, guzik t, liehn ea, gomes cpc, schulz r, hausenloy dj. immune cells as targets for cardioprotection: new players and novel therapeutic opportunities. cardiovasc res. 2019;115:1117–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van amerongen mj, harmsen mc, van rooijen n, petersen ah, van luyn mja. macrophage depletion impairs wound healing and increases left ventricular remodeling after myocardial injury in mice. am j pathol. 2007;170:818–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.arienti s, barth nd, dorward da, rossi ag, dransfield i. regulation of apoptotic cell clearance during resolution of inflammation. front pharmacol [internet]. 2019. [cited 2020 apr 14];10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.poon ikh, lucas cd, rossi ag, ravichandran ks. apoptotic cell clearance: basic biology and therapeutic potential. nat rev immunol. 2014;14:166–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.wan e, yeap xy, dehn s, terry r, novak m, zhang s, iwata s, han x, homma s, drosatos k, lomasney j, engman dm, miller sd, vaughan de, morrow jp, kishore r, thorp eb. enhanced efferocytosis of apoptotic cardiomyocytes through myeloid-epithelial-reproductive tyrosine kinase links acute inflammation resolution to cardiac repair after infarction. circ res. 2013;113:1004–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.howangyin k-y, zlatanova i, pinto c, ngkelo a, cochain c, rouanet m, vilar j, lemitre m, stockmann c, fleischmann bk, mallat z, silvestre j-s. myeloid-epithelial-reproductive receptor tyrosine kinase and milk fat globule epidermal growth factor 8 coordinately improve remodeling after myocardial infarction via local delivery of vascular endothelial growth factor. circulation. 2016;133:826–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.govindappa pk, patil m, garikipati vns, verma sk, saheera s, narasimhan g, zhu w, kishore r, zhang j, krishnamurthy p. targeting exosome-associated human antigen r attenuates fibrosis and inflammation in diabetic heart. the faseb journal. 2020;34:2238–2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.patil m, henderson j, luong h, annamalai d, sreejit g, krishnamurthy p. the art of intercellular wireless communications: exosomes in heart disease and therapy. front cell dev biol. 2019;7: 315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.lai rc, arslan f, lee mm, sze nsk, choo a, chen ts, salto-tellez m, timmers, lee cn, el oakley rm, pasterkamp g, de kleijn dpv, lim sk. exosome secreted by msc reduces myocardial ischemia/reperfusion injury. stem cell res. 2010;4:214–222. [DOI] [PubMed] [Google Scholar]
- 11.de couto g, liu w, tseliou e, sun b, makkar n, kanazawa h, arditi m, marbán e. macrophages mediate cardioprotective cellular postconditioning in acute myocardial infarction. j clin invest. 2015;125:3147–3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de couto g, gallet r, cambier l, jaghatspanyan e, makkar n, dawkins jf, berman bp, marbán e. exosomal microrna transfer into macrophages mediates cellular postconditioning. circulation. 2017;136:200–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.spees jl, lee rh, gregory ca. mechanisms of mesenchymal stem/stromal cell function. stem cell res ther. 2016;7:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.chiossone l, conte r, spaggiari gm, serra m, romei c, bellora f, becchetti f, andaloro a, moretta l, bottino c. mesenchymal stromal cells induce peculiar alternatively activated macrophages capable of dampening both innate and adaptive immune responses. stem cells. 2016;34:1909–1921. [DOI] [PubMed] [Google Scholar]
- 15.lai rc, tan ss, teh bj, sze sk, arslan f, de kleijn dp, choo a, lim sk. proteolytic potential of the msc exosome proteome: implications for an exosome-mediated delivery of therapeutic proteasome. int j proteomics [internet]. 2012. [cited 2018 oct 15];2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.harel-adar t, ben mordechai t, amsalem y, feinberg ms, leor j, cohen s. modulation of cardiac macrophages by phosphatidylserine-presenting liposomes improves infarct repair. proc natl acad sci usa. 2011;108:1827–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.ben-mordechai t, kain, holbova r, landa n, levin l-p, elron-gross i, glucksam-galnoy y, feinberg ms, margalit r, leor j. targeting and modulating infarct macrophages with hemin formulated in designed lipid-based particles improves cardiac remodeling and function. j control release. 2017;257:21–31. [DOI] [PubMed] [Google Scholar]
- 18.soleimani m, nadri s. a protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. nature protocols. 2009;4:102–106. [DOI] [PubMed] [Google Scholar]
- 19.krishnamurthy p, rajasingh j, lambers e, qin g, losordo dw, kishore r. il-10 inhibits inflammation and attenuates left ventricular remodeling after myocardial infarction via activation of stat3 and suppression of hur. circ res. 2009;104:e9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.joladarashi d, garikipati vns, thandavarayan ra, verma sk, mackie ar, khan m, gumpert am, bhimaraj a, youker ka, uribe c, suresh babu s, jeyabal p, kishore r, krishnamurthy p. enhanced cardiac regenerative ability of stem cells after ischemia-reperfusion injury: role of human cd34+ cells deficient in microrna-377. j am coll cardiol. 2015;66:2214–2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.mann h. b., whitney d. r. on a test of whether one of two random variables is stochastically larger than the other. the annals of mathematical statistics. 1947;18:50–60. [Google Scholar]
- 22.visweswaran m, pohl s, arfuso f, newsholme p, dilley r, pervaiz s, dharmarajan a. multi-lineage differentiation of mesenchymal stem cells – to wnt, or not wnt. the international journal of biochemistry & cell biology. 2015;68:139–147. [DOI] [PubMed] [Google Scholar]
- 23.abedin moeen, tintut yin, demer linda l. mesenchymal stem cells and the artery wall. circulation research. 2004;95:671–676. [DOI] [PubMed] [Google Scholar]
- 24.fadok va, bratton dl, frasch sc, warner ml, henson pm. the role of phosphatidylserine in recognition of apoptotic cells by phagocytes. cell death & differentiation. 1998;5:551–562. [DOI] [PubMed] [Google Scholar]
- 25.akakura s, singh s, spataro m, akakura r, kim j-i, albert ml, birge rb. the opsonin mfg-e8 is a ligand for the alphavbeta5 integrin and triggers dock180-dependent rac1 activation for the phagocytosis of apoptotic cells. exp cell res. 2004;292:403–416. [DOI] [PubMed] [Google Scholar]
- 26.LAUBER K, KEPPELER H, MUNOZ LE, KOPPE U, SCHRÖDER K, YAMAGUCHI H, KRÖNKE G, UDERHARDT S, WESSELBORG S, BELKA C, NAGATA S, HERRMANN M. MILK FAT GLOBULE-EGF FACTOR 8 MEDIATES THE ENHANCEMENT OF APOPTOTIC CELL CLEARANCE BY GLUCOCORTICOIDS. CELL DEATH DIFFER. 2013;20:1230–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.HART SP, SMITH JR, DRANSFIELD I. PHAGOCYTOSIS OF OPSONIZED APOPTOTIC CELLS: ROLES FOR ‘OLD-FASHIONED’ RECEPTORS FOR ANTIBODY AND COMPLEMENT. CLIN EXP IMMUNOL. 2004;135:181–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.FREEMAN SA, GRINSTEIN S. PHAGOCYTOSIS: RECEPTORS, SIGNAL INTEGRATION, AND THE CYTOSKELETON. IMMUNOLOGICAL REVIEWS. 2014;262:193–215. [DOI] [PubMed] [Google Scholar]
- 29.KWIATKOWSKA K, SOBOTA A. SIGNALING PATHWAYS IN PHAGOCYTOSIS. BIOESSAYS. 1999;21:422–431. [DOI] [PubMed] [Google Scholar]
- 30.ARANDJELOVIC S, RAVICHANDRAN KS. PHAGOCYTOSIS OF APOPTOTIC CELLS IN HOMEOSTASIS. NAT IMMUNOL. 2015;16:907–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.HOCHREITER-HUFFORD A, RAVICHANDRAN KS. CLEARING THE DEAD: APOPTOTIC CELL SENSING, RECOGNITION, ENGULFMENT, AND DIGESTION. COLD SPRING HARB PERSPECT BIOL. 2013;5:A008748–A008748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.MIKSA M, WU R, DONG W, KOMURA H, AMIN D, JI Y, WANG Z, WANG H, RAVIKUMAR TS, TRACEY KJ, WANG P. IMMATURE DENDRITIC CELL-DERIVED EXOSOMES RESCUE SEPTIC ANIMALS VIA MILK FAT GLOBULE EPIDERMAL GROWTH FACTOR-FACTOR VIII [CORRECTED]. J IMMUNOL. 2009;183:5983–5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.SANCHIS-GOMAR F, PEREZ-QUILIS C, LEISCHIK R, LUCIA A. EPIDEMIOLOGY OF CORONARY HEART DISEASE AND ACUTE CORONARY SYNDROME. ANN TRANSL MED. 2016;4:256–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.HULSMANS M, SAM F, NAHRENDORF M. MONOCYTE AND MACROPHAGE CONTRIBUTIONS TO CARDIAC REMODELING. J MOL CELL CARDIOL. 2016;93:149–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.NAHRENDORF M, PITTET MJ, SWIRSKI FK. MONOCYTES: PROTAGONISTS OF INFARCT INFLAMMATION AND REPAIR AFTER MYOCARDIAL INFARCTION. CIRCULATION. 2010;121:2437–2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.SWIRSKI FK, NAHRENDORF M. LEUKOCYTE BEHAVIOR IN ATHEROSCLEROSIS, MYOCARDIAL INFARCTION, AND HEART FAILURE. SCIENCE. 2013;339:161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.MATSUMOTO K, OBANA M, KOBAYASHI A, KIHARA M, SHIOI G, MIYAGAWA S, MAEDA M, SAKATA Y, NAKAYAMA H, SAWA Y, FUJIO Y. BLOCKADE OF NKG2D/NKG2D LIGAND INTERACTION ATTENUATED CARDIAC REMODELING AFTER MYOCARDIAL INFARCTION. CARDIOVASC RES. 2018; [DOI] [PubMed] [Google Scholar]
- 38.ABDOLMALEKI F, FARAHANI N, GHEIBI HAYAT SM, PIRRO M, BIANCONI V, BARRETO GE, SAHEBKAR A. THE ROLE OF EFFEROCYTOSIS IN AUTOIMMUNE DISEASES. FRONT IMMUNOL. 2018;9:1645–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.KARAJI N, SATTENTAU QJ. EFFEROCYTOSIS OF PATHOGEN-INFECTED CELLS. FRONT IMMUNOL. 2017;8:1863–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.DORAN AC, YURDAGUL A, TABAS I. EFFEROCYTOSIS IN HEALTH AND DISEASE. NATURE REVIEWS IMMUNOLOGY. 2020;20:254–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.MORIOKA S, MAUERÖDER C, RAVICHANDRAN KS. LIVING ON THE EDGE: EFFEROCYTOSIS AT THE INTERFACE OF HOMEOSTASIS AND PATHOLOGY. IMMUNITY. 2019;50:1149–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.HORST AK, TIEGS G, DIEHL L. CONTRIBUTION OF MACROPHAGE EFFEROCYTOSIS TO LIVER HOMEOSTASIS AND DISEASE. FRONTIERS IN IMMUNOLOGY. 2019;10:2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.TAYLOR PR, CARUGATI, FADOK, COOK HT, ANDREWS M, CARROLL MC, SAVILL JS, HENSON PM, BOTTO M, WALPORT MJ. A HIERARCHICAL ROLE FOR CLASSICAL PATHWAY COMPLEMENT PROTEINS IN THE CLEARANCE OF APOPTOTIC CELLS IN VIVO. J EXP MED. 2000;192:359–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.CHIONG M, WANG ZV, PEDROZO Z, CAO DJ, TRONCOSO R, IBACACHE M, CRIOLLO A, NEMCHENKO A, HILL JA, LAVANDERO S. CARDIOMYOCYTE DEATH: MECHANISMS AND TRANSLATIONAL IMPLICATIONS. CELL DEATH DIS. 2011;2:E244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.WARD MR, ABADEH A, CONNELLY KA. CONCISE REVIEW: RATIONAL USE OF MESENCHYMAL STEM CELLS IN THE TREATMENT OF ISCHEMIC HEART DISEASE. STEM CELLS TRANSL MED. 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.KESHTKAR S, AZARPIRA N, GHAHREMANI MH. MESENCHYMAL STEM CELL-DERIVED EXTRACELLULAR VESICLES: NOVEL FRONTIERS IN REGENERATIVE MEDICINE. STEM CELL RES THER. 2018;9:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.UCHIYAMA A, MOTEGI S-I, SEKIGUCHI A, FUJIWARA C, PERERA B, OGINO S, YOKOYAMA Y, ISHIKAWA O. MESENCHYMAL STEM CELLS-DERIVED MFG-E8 ACCELERATES DIABETIC CUTANEOUS WOUND HEALING. J DERMATOL SCI. 2017;86:187–197. [DOI] [PubMed] [Google Scholar]
- 48.DAS A, GHATAK S, SINHA M, CHAFFEE S, AHMED NS, PARINANDI NL, WOHLEB ES, SHERIDAN JF, SEN CK, ROY S. CORRECTION OF MFG-E8 RESOLVES INFLAMMATION AND PROMOTES CUTANEOUS WOUND HEALING IN DIABETES. THE JOURNAL OF IMMUNOLOGY. 2016;196:5089–5100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.HANAYAMA R, TANAKA M, MIYASAKA K, AOZASA K, KOIKE M, UCHIYAMA Y, NAGATA S. AUTOIMMUNE DISEASE AND IMPAIRED UPTAKE OF APOPTOTIC CELLS IN MFG-E8-DEFICIENT MICE. SCIENCE. 2004;304:1147–1150. [DOI] [PubMed] [Google Scholar]
- 50.MATSUDA A, WU R, JACOB A, KOMURA H, ZHOU M, WANG Z, AZIZ MM, WANG P. PROTECTIVE EFFECT OF MILK FAT GLOBULE-EPIDERMAL GROWTH FACTOR-FACTOR VIII AFTER RENAL ISCHEMIA-REPERFUSION INJURY IN MICE. CRIT CARE MED. 2011;39:2039–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.YAMADA K, UCHIYAMA A, UEHARA A, PERERA B, OGINO S, YOKOYAMA Y, TAKEUCHI Y, UDEY MC, ISHIKAWA O, MOTEGI S-I. MFG-E8 DRIVES MELANOMA GROWTH BY STIMULATING MESENCHYMAL STROMAL CELL-INDUCED ANGIOGENESIS AND M2 POLARIZATION OF TUMOR-ASSOCIATED MACROPHAGES. CANCER RES. 2016;76:4283–4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that all supporting data are available within the article and its online supplementary files. Detailed methods including list of resources and primers are provided in the online supplemental Material.
The data that support the findings of this study are available from the authors, Mallikarjun Patil (mpatil@uab.edu) and Sherin Saheera (Sherin.Saheera@umassmed.edu) upon reasonable request.