Abstract
Hydroxy-epoxy- and trihydroxy derivatives of linoleic acid are proposed to play an essential function in formation of the mammalian skin permeability barrier, which could account for the essential nature of its precursor, linoleic acid. Recent literature suggests that a specific oxidized enone derivative of LA esterified in ceramides facilitates binding to proteins, potentially serving a structural role in formation of the epidermal skin barrier. However, it is still to be established if other linoleic acid derivatives are also required for skin barrier formation, and whether the essential role is performed exclusively by an esterified, structural lipid or as an unesterified, labile signaling lipid, or by some combination of these derivatives. Progress in this domain is limited by lack of availability of hydroxy-epoxy-and trihydroxy- and octadecenoate derivatives of linoleic acid and related compounds, and challenges in maintaining them in the unesterified lipid pool. Here we describe methods for the total synthesis of hydroxy-epoxy-octadecenoate derivatives of linoleic acid (HEL1), and stable analogs that are designed to be resistant to inactivation by: (a) acylation/esterification (thus trapping these lipids in the free acid pool), (b) dehydrogenation, and (c) analogs combining both modifications. We further provide a total synthesis of corresponding hydroxy-epoxy- derivatives of sebaleic acid (a regioisomer of linoleic acid present in skin), and of small molecule scaffolds containing the allylic and non-allylic epoxide 7-carbon substructures shared by both families of hydroxy-epoxy-and trihydroxy-octadecenoates. Finally, we demonstrate that 2,2-dimethyl analogs of hydroxy-epoxy-and trihydroxy- octadecenoates are resistant to esterification in in vitro assays and thus provide a novel template for stabilizing labile, bioactive lipids as free acids by preventing acylation/esterification.
Keywords: Esterification, Oxidized fatty acid, Synthesis, Stable analog, Linoleic acid, Sebaleic acid
1. Introduction
Recently we reported on oxidized derivatives of linoleic acid (LA) that are potential endogenous mediators of chronic pain and itch [1]. Linoleic acid, which is one of the most abundant fatty acids in the skin [2] is designated an “essential fatty acid” since a small amount (0.5% of energy) must be obtained in the diet to form a functional epidermal water barrier and to prevent clinical manifestations of “essential fatty acid deficiency” [3].
Lipoxygenase or oxygen radical catalyzed oxidation of LA produces hydroperoxy-octadecadienoates (9-HpODE, 13-HpODE), which can then undergo conversion to downstream products including hydroxy-octadecadienoates (HODEs), oxo-octadecadienoates (Oxo-ODEs) epoxy-octadecenoates (EpOMEs), dihydroxy-octadecenoates (DiHOMEs), trihydroxy-octadecenoates (TriHOMEs) [4], and hydroxy epoxide- and keto epoxide- octadecenoates (KODEs), compounds that are similar in structure to the 12-HpETE (12-hydroperoxy-eicosatetraenoic acid) derived hepoxilins [5]. The LA-derived peroxidation products that are the focus of this manuscript include the “hepoxilin-A”-like 13-hydroxy-9,10-trans-epoxy-(11E)-octadecenoate (13,9-HEL; 1), derived from 9-HpODE (Fig. 1), along with the corresponding acid-catalyzed epoxide hydrolysis product trihydroxy 9,10,13-trihydroxy-(11E)-octadecenoate (13,9,10-THL, with numbering that indicates the position of the precursor epoxide). The syntheses of a second “hepoxilin-A”-like regioisomer, 9-hydroxy-12,13-trans-epoxy-(10E)-octadecenoate (9,12-HEL), and of the “hepoxilin-B”-like 11-hydroxy epoxides (Fig 1) will be reported in a future manuscript.
Figure 1-. The 9-hydroperoxidation pathway of linoleic acid.
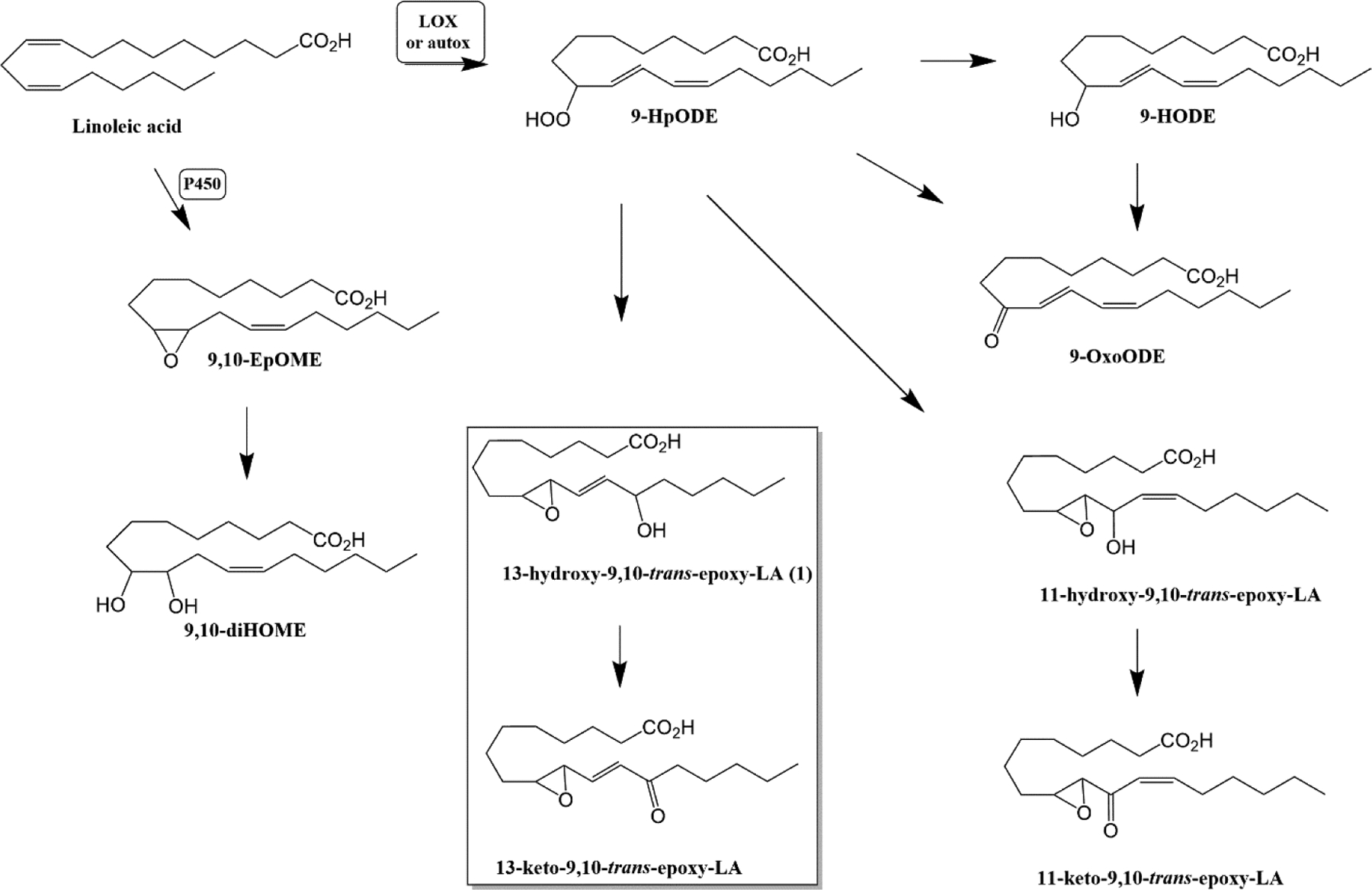
Enzymatic or oxygen radical catalyzed peroxidation of linoleic acid produces a variety of oxidized lipids including HpODEs, HODEs, oxoODEs, EpOMEs, DiHOMEs, as well as hydroxyl-epoxide and hydroxy-ketone derivatives. Hydroxy-epoxide derivatives of linoleic acid (not shown) can be converted to trihydroxy octadecenoates by soluble epoxide hydrolase or non-specific hydrolysis.
1.1. Endogenous synthesis of hydroxy-epoxy- and trihydroxy derivatives of linoleic acid
In 1985, Houtsmuller et al. [2] hypothesized that the conversion of LA in epidermal acyl-ceramides by an unspecified lipoxygenase(s) to an unknown oxidation product was essential for the formation of the epidermal water permeability layer. More recently, Brash et al [6],[7, 8] [9], [10] refined this hypothesis by proposing that the consecutive actions of two specific enzymes, 12-R-lipoxygenase and e-lipoxygenase-3, oxidized the LA esterified in acyl-ceramides to form a specific stereoisomer of 13,9-HEL (13-(R)-hydroxy-9(R),10(R)-trans-epoxy-(11E)-octadecenoate). 13,9-HEL can be further converted to trihydroxy octadecenoate derivatives by the actions of soluble epoxide hydrolase (sEH) or non-specific acid mediated hydrolysis. (Fig 2a). Brash et al proposed that one or more of these hydroxy-epoxy- or trihydroxy derivatives of linoleic acids play a critical role in formation of the corneocyte lipid envelope [11], which could potentially explain both the mechanism whereby small amounts of dietary LA are required to prevent the clinical manifestations of “essential fatty acid deficiency”, and the link between genetic mutations in 12-R-lipoxygenase (ALOX12B) and the hydroperoxide-isomerase e-lipoxygnease-3 (ALOXE3) with congenital ichthyosis [10], [12], [13]. In 2019, Takeichi et al published evidence showing that in an ichthyosis patient with mutations in a gene that codes for a short chain dehydrogenase (SDR9C7), and in a mouse Sdr9c7-KO model, there is a loss of covalent binding of ceramides to proteins in the corneocyte lipid envelope [14]. The authors suggest that the dehydrogenase coded for by SDR9C7 acts on the LOX-catalyzed product of CerEOS to produce an epoxy enone derivative of the linoleate ester (13-keto-9,10-epoxy-octadecenoate (E1) in Fig 2a depicts the free acid of this proposed structure) present in the ceramide, which can readily react with neighboring proteins to form covalent bonds [14]. Despite these observations, our understanding of the structural or signaling roles of 13,9-HEL or 13,9,10-THL as the ‘essential’ oxidized LA derivative(s) are incomplete. Although 13,9-HEL is mostly in the esterified pool in rodent and human skin, our previous results showed that small amounts are present in the free pool where they could potentially serve as signaling lipids [1].
Figure 2a-. Simplified model depicting relationships and structures of endogenous lipid mediators, stable analogs, and small molecules containing proposed pharmacophores.
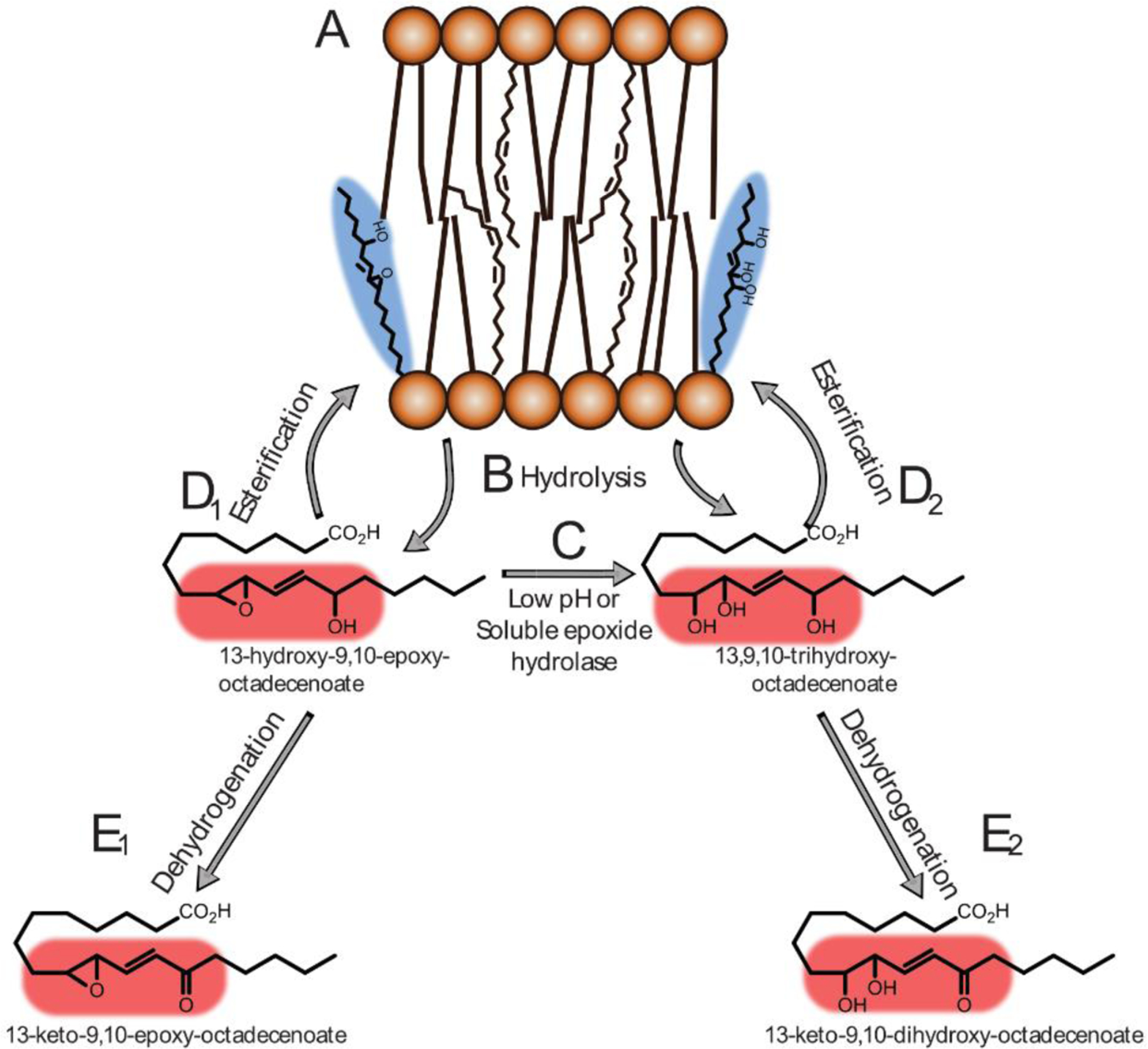
(A) Membranes are enriched in 13-hydroxy-9,10-epoxy-octadecenoate and 13,9,10-trihydroxy-octadecenoate; (B) These compounds are released as free acids via lipasemediated hydrolysis; (C) 13-hydroxy-9,10-epoxy-octadecenoate is converted to 13,9,10-trihydroxy-octadecenoate in low pH environment or by epoxide hydrolase; (D) Free 13-hydroxy-9,10-epoxy-octadecenoate (D1) and 13,9,10-trihydroxy-octadecenoate (D2) are inactivated by re-esterification; (E) Free 13-hydroxy-9,10-epoxy-octadecenoate and 13,9,10-trihydroxy-octadecenoate are dehydrogenated to produce 13-keto-9,10-epoxy-octadecenoate (E1) and 13-keto-9,10-dihydroxy-octadecenoate (E2).
1.2. Total synthesis of hydroxy-epoxy- and trihydroxy derivatives of linoleic acid
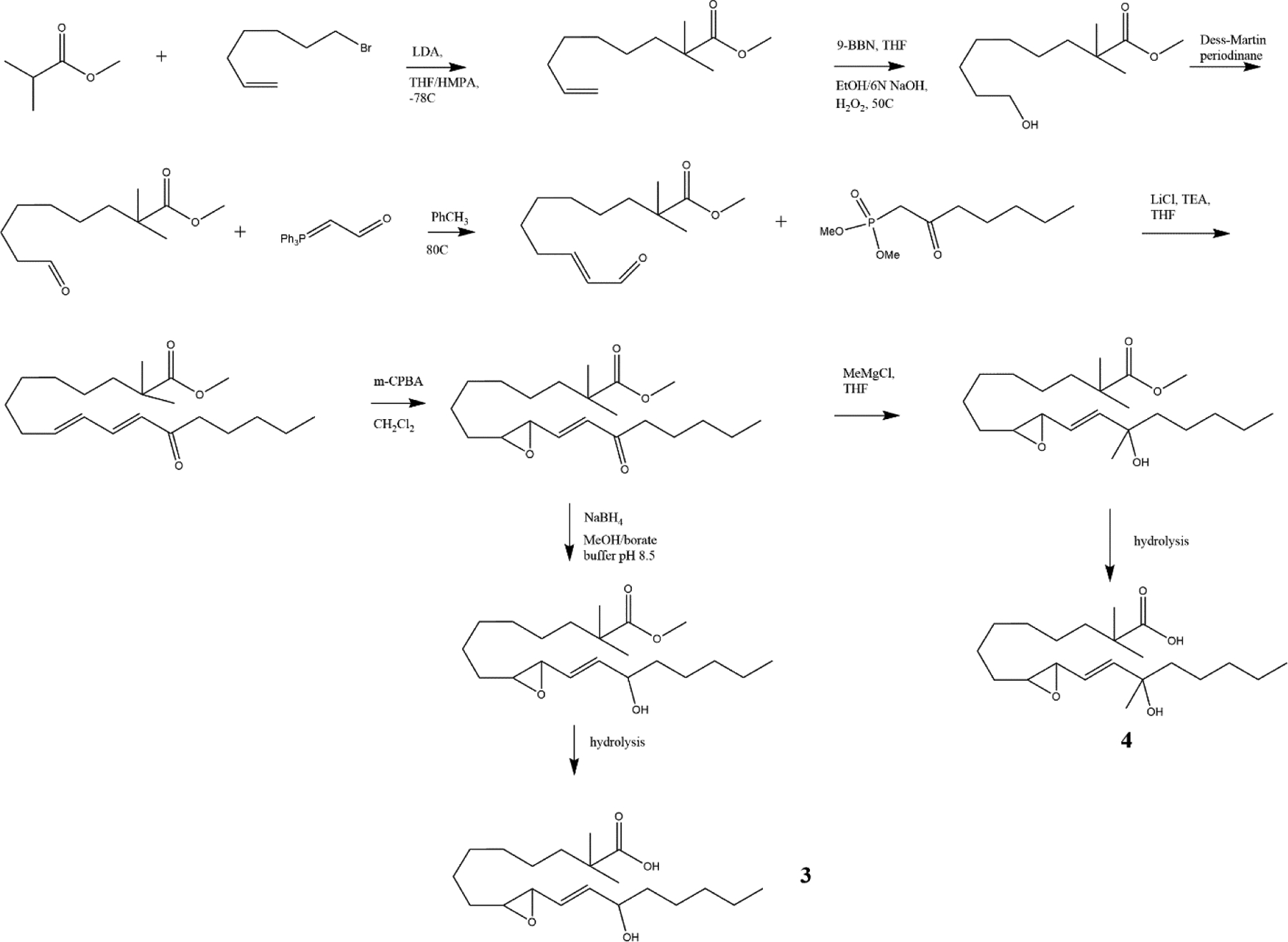
We determined that a rapid total synthesis of racemic hydroxy epoxide derivatives of linoleic acid would enable us to quickly have the tools in hand to explore their potential roles as structural and signaling lipids, without excluding any stereoisomer. The stereospecific synthesis of individual stereoisomers would follow as any of interest are determined. The synthetic scheme is shown in Fig 5.
Figure 5-.
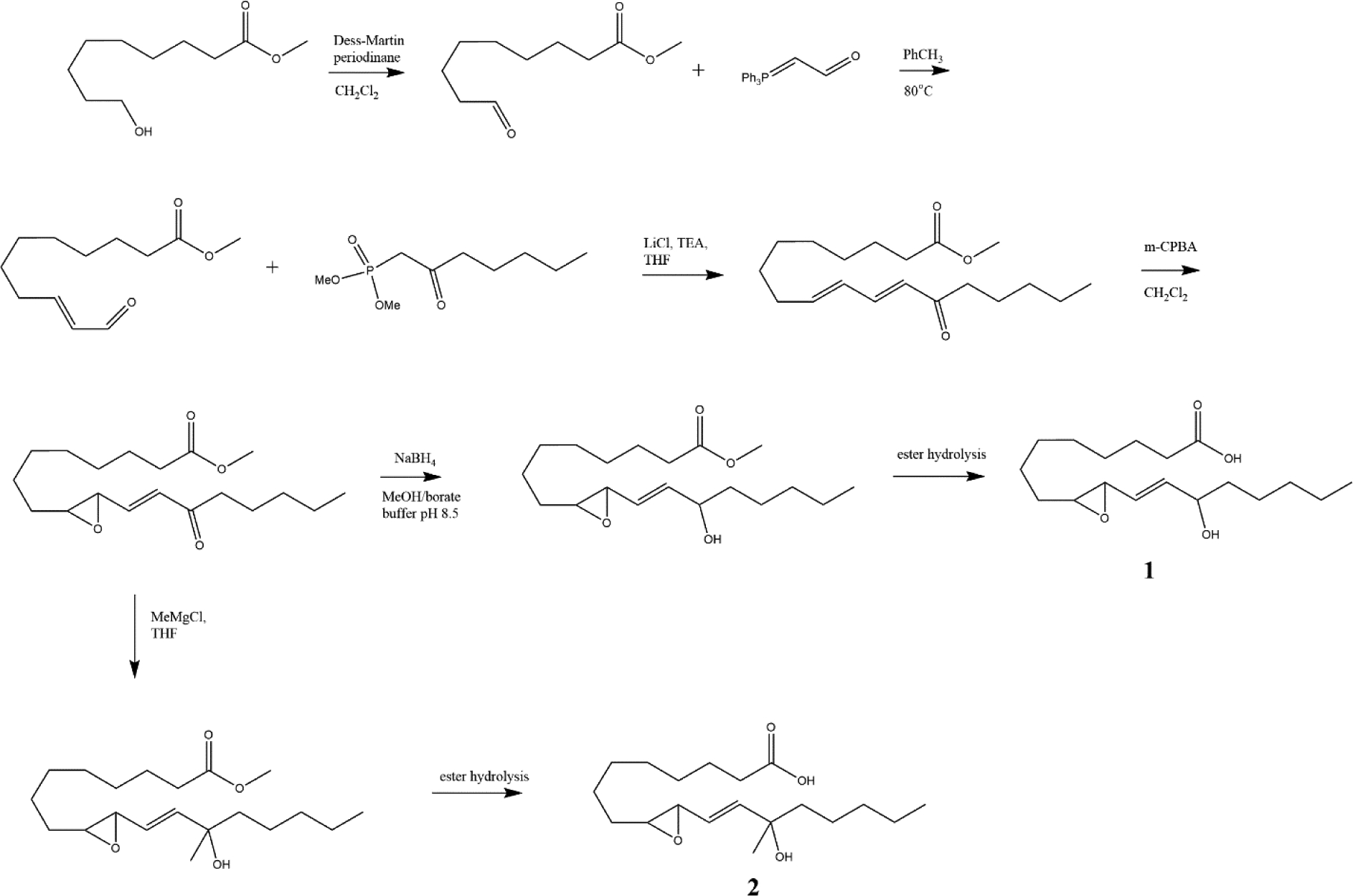
Synthesis of 13-hydroxy-9,10-(E)-LA (1) and 13-hydroxy-13-methyl-9,10-(E)-LA (2)
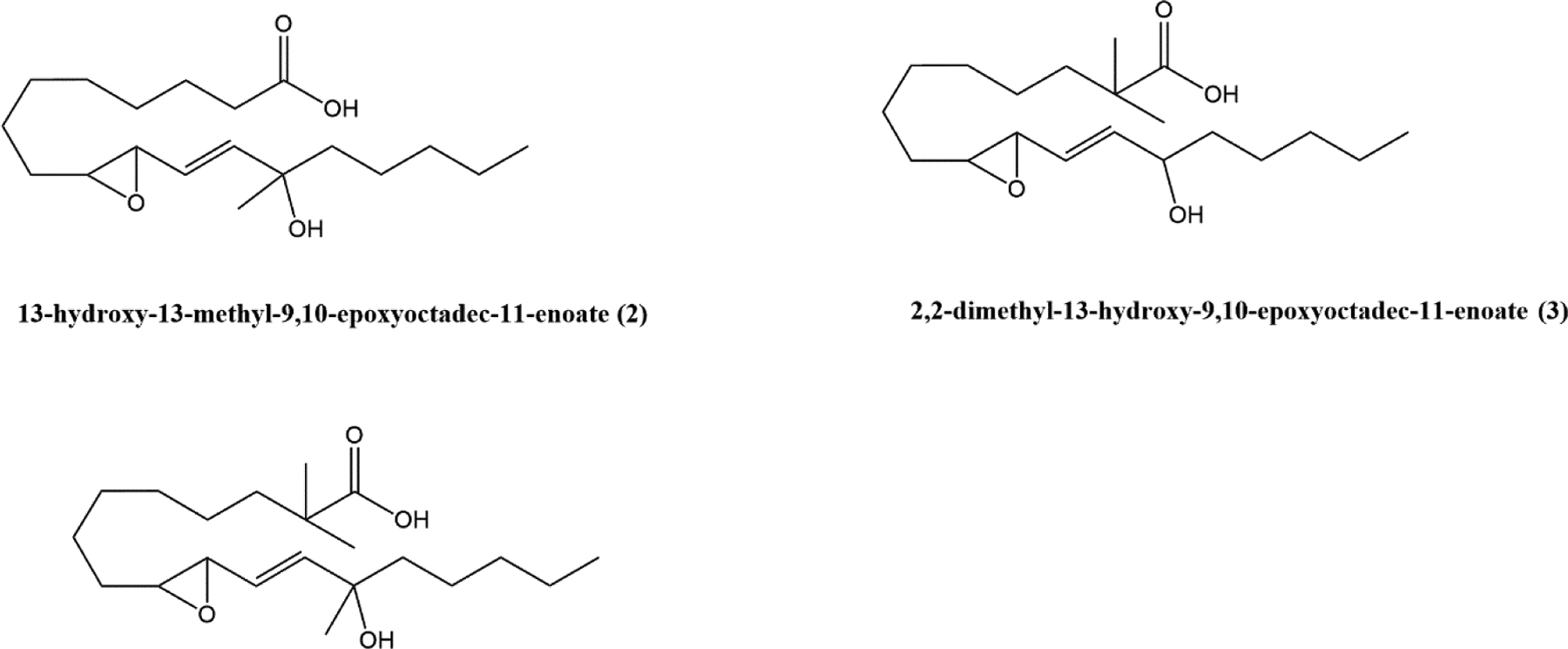
To investigate the possible roles of esterification and dehydrogenation on the function of these mediators, we .designed and synthesized three sets of stable analogs (sections F1, F2 and G, Fig 2b) utilizing (1) a 2,2-dimethyl moiety substitution to generate steric hindrance and prevent acylation/esterification (thus trapping these lipids in the free acid pool), (2) introduction of a methyl at the hydroxyl that serves to prevent oxidation to a ketone, and (3) a family of analogs containing both modifications.
Figure 2b-. Stable analogs and the seven-carbon pharmacophores incorporating allylic hydroxy epoxides.
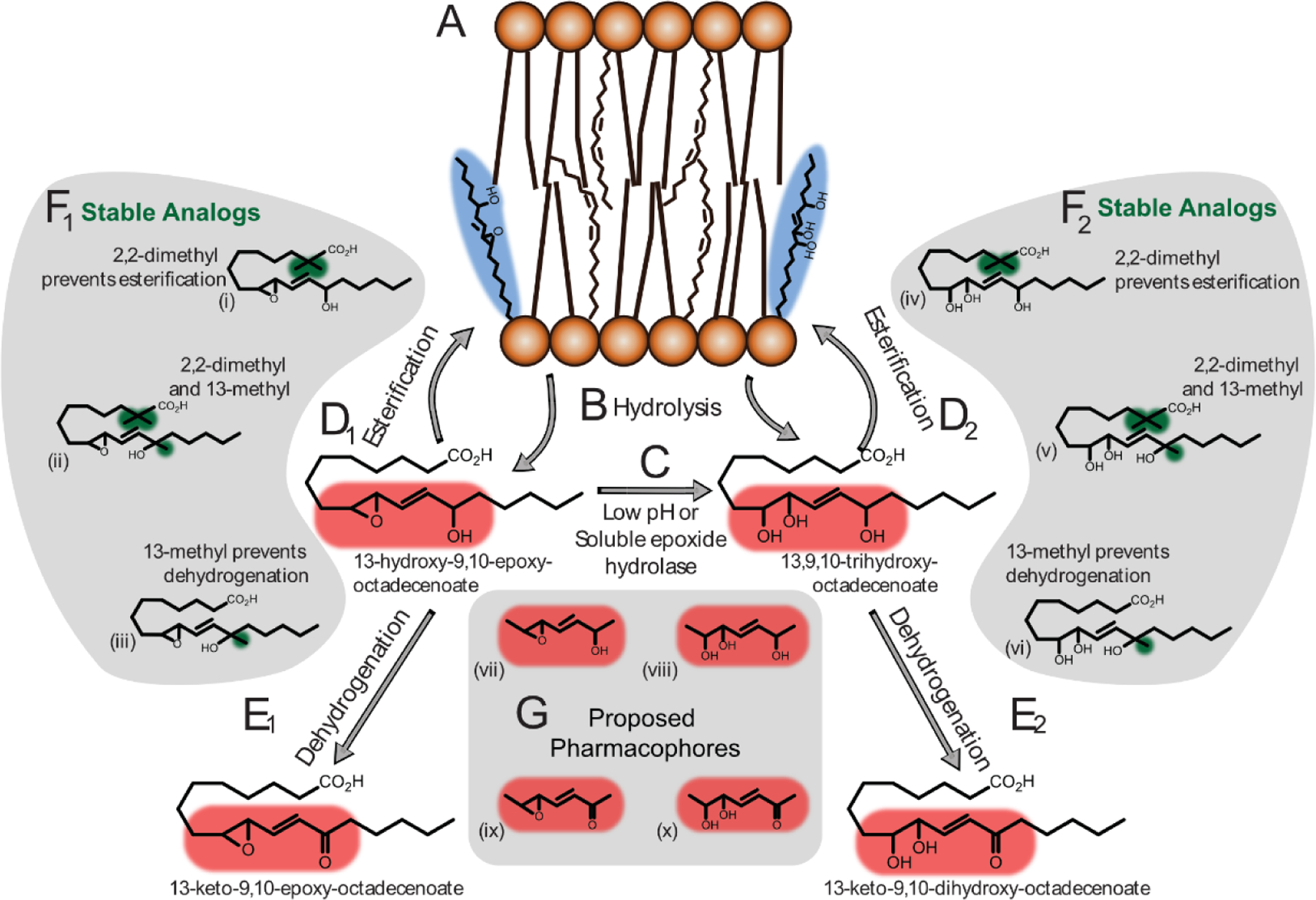
(F) Dimethyl addition at the C2 position of 13-hydroxy-9,10-epoxy-octadecenoate (F1-i and F1-ii), and 13,9,10-trihydroxy-octadecenoate (F2-i and F2-ii) prevents esterification. Methyl group addition at the C13 position prevents dehydrogenation (F1-ii, F1-iii & F2-ii, F2-iii); (G) Small molecules representing the pharmacophores of 13-hydroxy-9,10-epoxy-octadecenoate (G-vii), 13,9,10-trihydroxy-octadecenoate (G-viii), 13-keto-9,10-epoxy-octadecenoate (G-ix) and 13-keto-9,10-dihydroxy-octadecenoate (G-x).
1.3. Hydroxy-epoxy- and trihydroxy derivatives of sebaleic acid and small molecule pharmacophores
Sebaleic acid (SA; 18:2Δ5,8), formed from sapienic acid by elongation and fatty acid desaturase 1 (FADS1) catalyzed desaturation, is the predominant PUFA in the human sebaceous gland [15]. Sebaleic acid is an 18-carbon regioisomer of LA that differs only in the position of the two cis double bonds (C-5 and C-8 for SA vs C-9 and C12 for LA)[15]. In sebum, Powell et al. identified 5-HODE and 5-oxoODE as bioactive 5-LOX derivatives of SA which would require the 5-HpODE intermediate for their formation [15]. Here we predict that the 5-HpODE intermediate can alternatively rearrange to form 7-hydroxy and 9-hydroxy-5,6-epoxides (Fig 3), in a similar manner as observed for LA. We applied our rapid synthesis strategy to prepare the racemic hydroxy epoxides predicted to derive from SA shown in Fig 3. We have not determined whether these proposed mediators exist endogenously or whether they are bioactive.
Figure 3-.
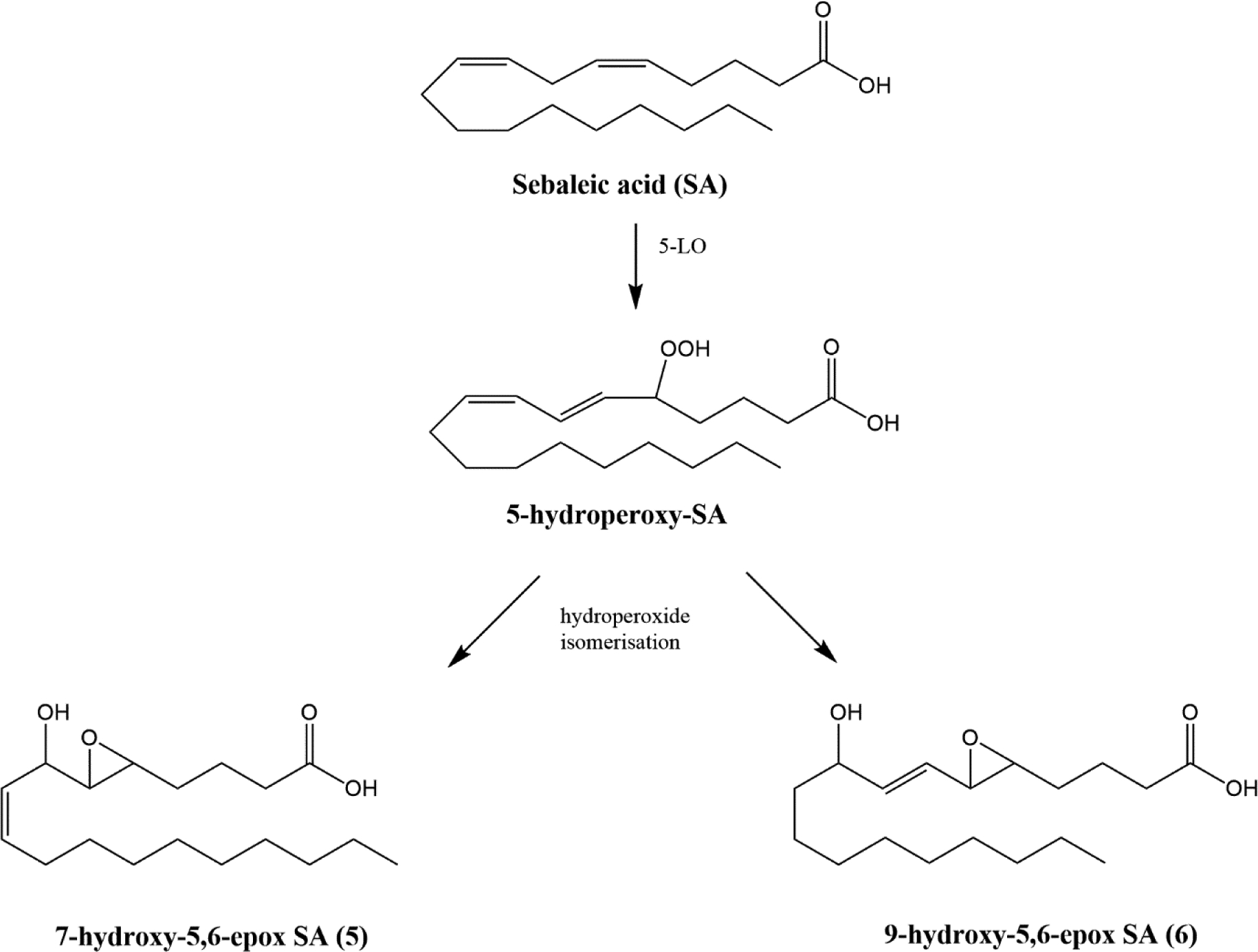
Endogenous oxidation of sebaleic acid
A retrosynthetic analysis of the two hydroxy-5,6-epoxy-derivatives of SA that are expected to form from the 5-hydroperoxide of SA is shown in Fig 7.
Fig 7-.
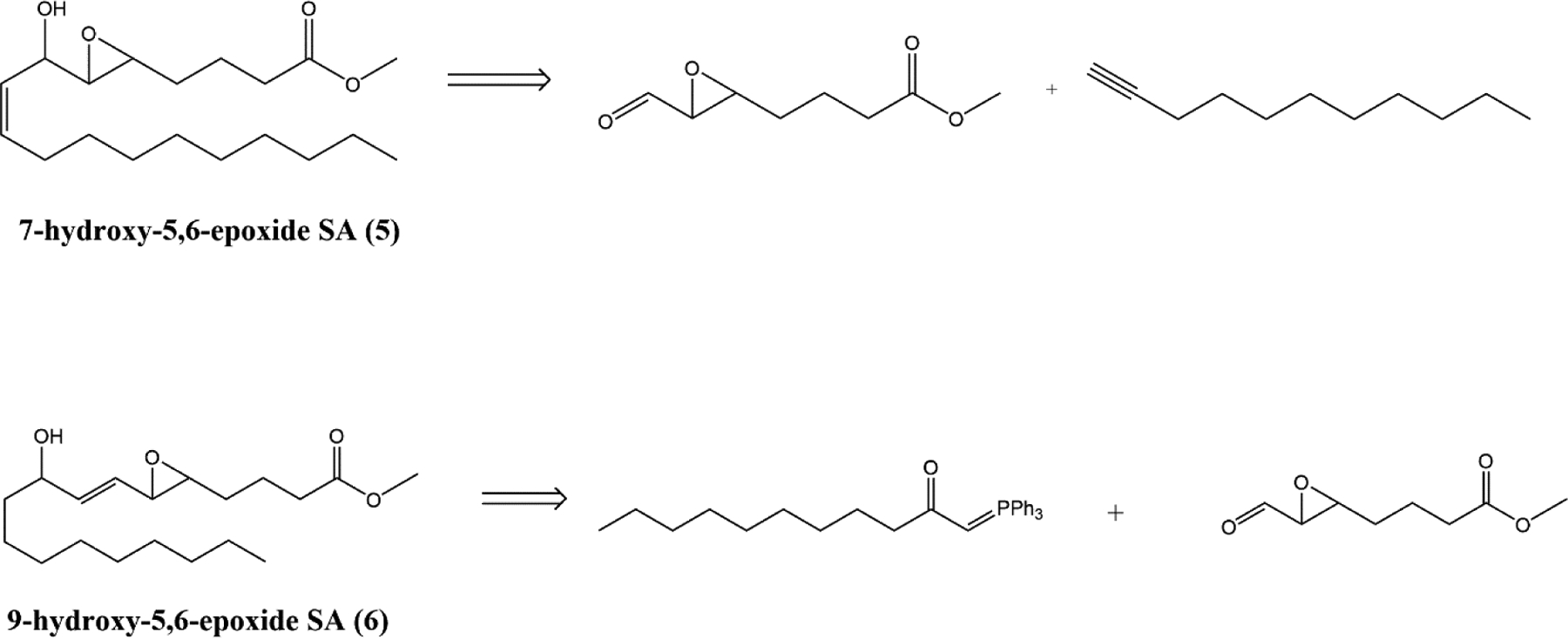
Retrosynthesis of 7-hydroxy-5,6-epoxide SA (5) and 9-hydroxy-5,6-epoxide SA (6)
We also provide a total synthesis of four small molecule pharmacophores containing the allylic and non-allylic epoxide, trihydroxy and keto epoxide 7-carbon substructures shared by both the LA and SA families of hydroxy-epoxy- hydroxy-epoxy-and trihydroxy- octadecenoates (Fig 4). Finally, we demonstrate that 2,2-dimethyl analogs of hydroxy-epoxy-and trihydroxy- trihydroxy- and hydroxy-epoxy-octadecenoates are resistant to esterification in in vitro assays.
Figure 4-.
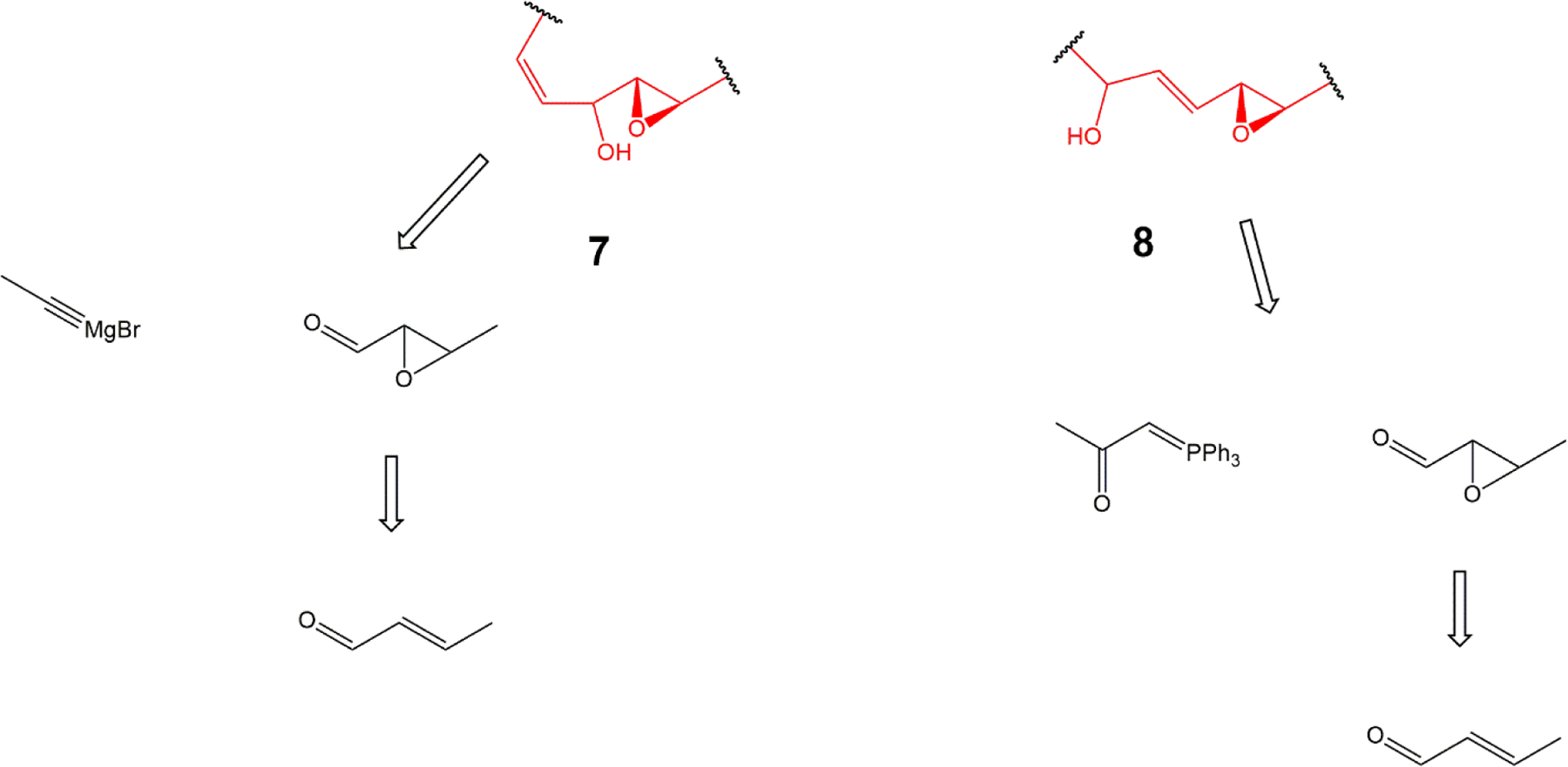
Retrosynthetic scheme of 2-epoxy-4-hydroxy-5-(Z)-heptene (7) and 2-epoxy-4-(E)-6-hydroxy-heptene (8) pharmacophores
2. Materials and Methods
2.1. Materials
Chemicals and solvents were used in the commercially available form in which they were supplied. All solvents were anhydrous grade, and reactions were conducted under inert atmosphere. Tetrahydrofuran (THF), methyl tert-butyl ether (MTBE), 3-chloroperoxybenzoic acid (m-CPBA), 9-borabicyclo[3.3.1]nonane solution (9-BBN), and methylmagnesium chloride (MeMgCl) were obtained from Sigma-Aldrich. Thin-layer chromatography (TLC) was performed on 250 μm silica gel plates with fluorescent indicator (254nm; Analtech). For 1H 400 MHz NMR spectra, tetramethylsilane or the solvent peak served as an internal standard for reporting chemical shifts, expressed on the δ scale. 1H NMR spectra acquired on a Varian Inova 400MHz NMR. Semi-preparative high performance liquid chromatography (HPLC) was performed on an Agilent 1200 purification system with a Luna silica (2) 5μm 250 × 10mm column (Phenomenex). LC-MS was recorded using an Agilent 1100 LC system equipped with diode array detector and 6120 quadrupole mass spectrometer with electrospray ionization in positive mode and in negative mode and capillary voltage set at 4000V (−3000V for negative ionization), nebulizer pressure at 45psig and gas flow at 12 L/min. The mobile phase flow rate was set at 0.4 mL/min and the injection volume was 15 μl. MS scans were analyzed using ChemStation software (Agilent). Chromatographic separation was achieved with a Zorbax XDB-C18 5μm C18 50 × 2.0mm column (Agilent) and a mobile phase gradient consisting of (A) 10mM NH4OAc, pH 7.4 (B) acetonitrile + 0.1% formic acid, increasing from 5% B to 90% B over 10 minutes, with UV monitoring at 254nm and 215nm.
2.2. Experimental
2.2.1. 13-hydroxy-9,10-trans-epoxy-(11E)-octadecenoate (13,9-HEL; 1)
To a solution of methyl 9-hydroxy nonanoate (1g) in dichloromethane (8ml) at 0°C was added Dess-Martin periodinane (1.7 equiv; 3.83g). The ice bath was removed after 10 minutes, and the reaction was allowed to proceed for up to 2 hours (until no starting material remained by TLC). The reaction was diluted with 200ml of 10% ethyl acetate/hexane, and it was immediately poured onto silica gel. Elution with the same solvent produced the product aldehyde in 90% yield (890mg).
A solution of 2.44g of (formylmethyl)triphenylphosphonium chloride in 12ml of toluene was treated with triethylamine (1.1 equiv; 1.11 ml) and stirred for one hour, then a solution of the aldehyde (890mg; 4.78mmol) in toluene was added and the reaction was stirred at 80°C for 2 hours. The mixture was then filtered through Celite and purified on silica gel (15% ethyl acetate/hexane) to yield 608mg of the en-al product.
The en-al was added slowly to a mixture of the phosphonate (3 equiv; 1.25g) and anhydrous LiCl (3 equiv; 237mg) in 12ml THF. Then triethylamine (3.3 equiv; 876μl) was added, and the reaction allowed to react for 3–4 hours (until TLC showed completion). The reaction mixture was diluted with ether and washed with water then brine. It was dried over sodium sulfate, filtered, and evaporated, then purified on silica gel using 10–15% ether/hexane to elute. There were 371mg (64%) of the dienone. This was further purified by normal phase HPLC (960/40 heptane/MTBE, 275nm) to yield 348mg of a white solid.
The dienone (348mg) was dissolved in 13ml of dichloromethane and cooled to 0°C, then 77% m-CPBA (1.5 equiv; 374mg) was added. After 15 minutes the ice bath was removed, and the reaction proceeded until completion (3 hours). It was diluted with dichloromethane and washed with saturated sodium thiosulfate, 10% sodium bicarbonate, then water. Dried over sodium sulfate and purified on silica gel (15–30% ether/hexane gradient elution) to produce 226mg (62%) of a white solid: 13-keto-9,10-trans-epoxy-(11E)-octadecenoic acid (13K-9E-LA): 1H NMR (400 MHz, CDCl3) δ 6.47–6.54 (m, 1H), 6.34–6.43 (m, 1H), 3.20 (dd, J=1.83, 6.86 Hz, 1H), 2.89 (dt, J=2.01, 5.58 Hz, 1H), 2.41–2.60 (m, 2H), 2.25–2.37 (m, 2H), 1.56–1.71 (m, 4H), 1.40–1.53 (m, 2H), 1.11–1.38 (m, 12H), 0.82–0.94 (m, 3H).
The 13-hydroxyl compound was produced from sodium borohydride reduction of the ketone (20mg) in 2ml of a 1:1 mixture of methanol and borate buffer (100mM, pH 8.5) at 0°C. The reaction was stopped with the addition of saturated NH4Cl and extracted with ether (3x), and the organic layers were washed with water and then with brine. After drying over sodium sulfate and purification on silica gel (10–15% ether/hexane gradient elution), the methyl ester was hydrolyzed over 3–5 hours at pH ~9 in 2ml of methanol with dilute potassium carbonate (0.5M K2CO3) to yield 13mg (68%) of 13-hydroxy-9,10-trans-epoxy-(11E)-octadecenoate (13,9-HEL; 1): 1H NMR (400 MHz, CDCl3) δ 5.91 (dd, J=6.40, 15.55 Hz, 1H), 5.40 (dd, J=7.87, 15.55 Hz, 1H), 3.90–4.17 (m, 1H), 3.65 (s, 3H), 3.08 (dd, J=2.10, 7.78 Hz, 1H), 2.80 (dt, J=2.01, 5.58 Hz, 1H), 2.29 (t, J=7.50 Hz, 2H), 1.69 (br. s., 1H), 1.48–1.63 (m,6H), 1.24–1.45 (m, 13H), 0.87 (t, J=6.59 Hz, 3H).
2.2.2. 13-hydroxy-13-methyl-9,10-trans-epoxy-(11E)-octadecenoate (2)
To a solution of 20mg of the enone in 3ml of THF at 0°C was added MeMgCl (3.0M, 1.3 equiv; 27μl). The reaction stirred as it reached room temperature until the starting material was mostly consumed. It was diluted with ether and washed rapidly with 1M HCl (to pH 6–7) followed by water and then brine, then dried over sodium sulfate and purified on silica gel (20–30% ether/hexane gradient elution) to yield 15mg (71%) of the 13-hydroxy-13-methyl-9,10-trans-epoxy-(11E)-octadecenoate (2): 1H NMR (400 MHz, CD3OD) δ 5.98 (d, J=15.74 Hz, 1H), 5.35 (dd, J=7.96, 15.65 Hz, 1H), 3.64 (s, 2H), 3.14 (dd, J=2.20, 7.87 Hz, 2H), 2.83 (dt, J=2.20, 5.58 Hz, 2H), 2.31 (t, J=7.41 Hz, 2H), 1.42–1.62 (m, 7H), 1.25–1.39 (m, 12H), 1.22–1.25 (m, 3H), 0.89 (t, J=6.95 Hz, 3H).
2.3. Lipase resistant 2,2-dimethyl analogs
2.3.1. 2,2-dimethyl-13-hydroxy-9,10-trans-epoxy-(11E)-octadecenoate
To a solution of methyl isobutyrate (2g; 19.60 mmol; Fig 8) in 25ml of THF at −78°C was added lithium diisopropylamide (2.0M, 1.2 equiv; 11.77ml) dropwise. After 30 minutes, a solution of 7-bromo-1-heptene (1.2 equiv; 4.14g) in 5ml of THF was added slowly. The reaction was stirred at room temperature for 1–2 hours (until TLC showed completion), then it was diluted with ether and washed with 1M HCl, water, and finally brine. It was dried over sodium sulfate and purified on silica gel (5% ethyl acetate/hexane gradient elution) to yield 3.25g (87%).
Figure 8-.
Synthesis of 2,2-dimethyl analogs of 13-hydroxy-9,10-trans-epoxy-(11E)-octadecenoate
To a stirred solution of 0.5M 9-BBN in THF (1 equiv; 20ml) was added a solution of 2,2-dimethyl methyl nonenoate (2g; 10.10 mmol) in THF. The solution was stirred for 2 hours and then it was cooled to 0°C and 6ml of ethanol was added followed by 2.2ml of 6N NaOH and 3.4ml of 30% hydrogen peroxide. The reaction was heated to 50°C and stirred for 1 hour, then cooled to room temperature and diluted with ethyl acetate and washed with water then brine. Dried over sodium sulfate and purified on silica gel to yield 1.61g (76%).
The rest of the synthesis was carried out as described for the endogenous compound. The ester was hydrolyzed with 1M LiOH in 2-propanol for 20 hours and purified on silica gel and eluted with 3–5% MeOH/CH2Cl2.
2,2-dimethyl-13-keto-9,10-trans-epoxy-(11E)-methyl-octadecenoate:
1H NMR (400 MHz, CDCl3) δ 6.63 (dd, J=6.59, 15.75 Hz, 1H), 6.50 (dd, J=6.96, 16.11 Hz, 1H), 6.34–6.41 (m, 1H), 3.64 (s, 3H), 3.37–3.46 (m, 1H), 3.18 (dd, J=1.83, 6.96 Hz, 1H), 2.84–2.92 (m, 1H), 2.51 (t, J=7.32 Hz, 2H), 2.38–2.45 (m, 1H), 2.22–2.33 (m, 1H), 1.53–1.63 (m, 4H), 1.38–1.50 (m, 4H), 1.16–1.37 (m, 11H), 1.14 (s, 6H), 0.87 (t, J=6.77 Hz, 3H).
2,2-dimethyl-13-hydroxy-9,10-trans-epoxy-(11E)-octadecenoate (DM-13,9-HEL):
1H NMR (400 MHz, CDCl3) δ 5.88–5.97 (m, 1H), 5.41 (dddd, J=1.01, 3.09, 7.88, 15.57 Hz, 1H), 4.05–4.20 (m, 1H), 3.06–3.13 (m, 1H), 2.79–2.86 (m, 1H), 2.04 (s, 1H), 1.33–1.61 (m, 10H), 1.20–1.32 (m, 9H), 1.18 (s, 5H), 0.81–0.96 (m, 3H).
The 2,2-dimethyl hydroxy methyl analog was prepared as described above.
2,2-dimethyl-13-hydroxy-13-methyl-9,10-trans-epoxy-(11E)-octadecenoate:
1H NMR (400 MHz, CDCl3) δ 5.95 (d, J=15.74 Hz, 1H), 5.39 (br dd, J=7.78, 15.65 Hz, 1H), 3.64 (s, 3H), 3.39–3.63 (m, 1H), 3.09 (br d, J=7.78 Hz, 1H), 2.78–2.90 (m, 1H), 1.32–1.67 (m, 13H), 1.17–1.32 (m, 20H), 1.15 (s, 6H), 0.87 (br t, J=6.59 Hz, 5H).
2.4. Lipase esterifications
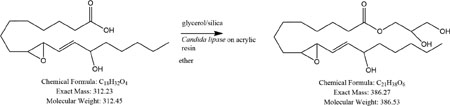
Approximately 30mg of silica gel was added to 25mg of glycerol, and it was then vortexed such that the silica was entirely coated with glycerol until the admixture was free flowing. Between 5–10mg of this material was added to a solution of the free acid (0.15mg) in 0.5ml diethyl ether containing 20mg of Candida antarctica lipase on acrylic resin (Sigma-Aldrich), and it was vortexed briefly. The reaction was left for 3 days, then the mixture was filtered and evaporated under nitrogen. The residue was reconstituted in ethanol and analyzed by LC-MS. Results for each compound reacted under these conditions are shown in Fig. 10.
Figure 10-.
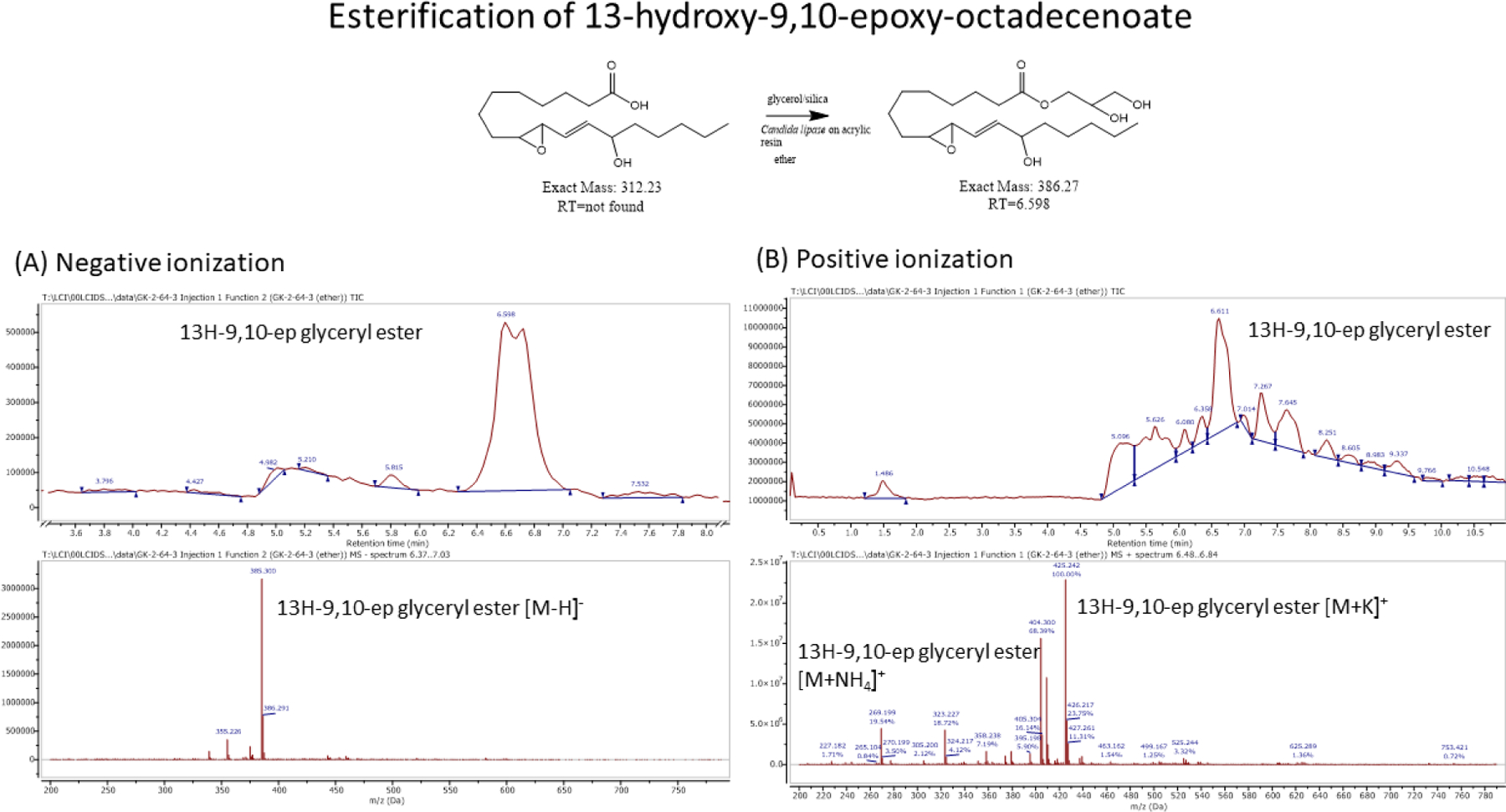
Lipase catalyzed glycerol esterification to 13,9-HEL (this Figure) and to DM-13,9-HEL (Fig 11). Schematic of the esterification reaction and structures is depicted at the top (see section 2 for conditions). Molar masses of free acid and expected glyceryl ester product are below the structures. Total ion chromatogram (TIC; top) and mass spectra (bottom) for positive mode (panel A) and for negative mode (panel B). Glyceryl ester product RT=6.5–6.61 min, (m/z) 385.30 [M-H]− (panel A); 404.3 [M+ NH4]+, 425.24 [M+K]+ (panel B).
3. Results
Previous reports on the synthesis of downstream oxidized linoleic acid compounds have included the epoxy ketones, described as EKODEs [16], used biogenic syntheses [17] or focused on the stereospecific synthesis of triol isomers [18]. Based on the structural similarities of these newly discovered mediators with the allylic epoxide hepoxilin A3 (derived from arachidonic acid), we reasoned that similar approaches could be adopted to prepare these compounds as racemic mixtures in amounts sufficient for multiple studies [19], [5]. Starting from commercially available methyl 9-hydroxynonanoate and phosphonate 9, the allylic epoxide 13-hydroxy-9,10-(E)-LA (1) was prepared in 6 steps (Fig 5). Wittig reaction of methyl 9-oxononanoate with the stabilized phosphorane provided the en-al which was then reacted with the keto phosphonate under trans selective Horner-Emmons conditions. Epoxidation of the dienone using standard peroxyacid mediated conditions [20] proceeded at the desired 9,10 position, maintaining the enone conjugation to provide the keto epoxide as a white solid after purification by HPLC. Rapid reduction with sodium borohydride in buffered conditions followed by careful ester hydrolysis yielded the hydroxy epoxide (1).
Standard acid hydrolysis (1M HCl in THF) provides access to each of the racemic trihydroxy regioisomers (13,9,10-THL and 9,12,13-THL) as a mixture (Fig 6). Initial attempts at separating these isomers by HPLC were not successful due to overlapping peaks with varying peak intensities and inconsistent retention times that prevented baseline separation.
Figure 6-.
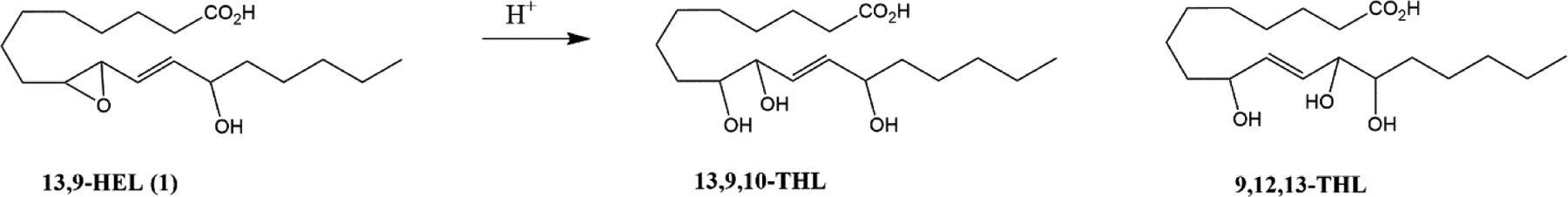
generation of triol mixture of isomers from 13-H-9,10-epoxide LA using acidic conditions
A similar approach was used for the synthesis of 2 oxidized derivatives of sebaleic acid (7-hydroxy-5,6-epoxy-SA (5); and 9-hydroxy-5,6-epoxy-SA (6); Fig 7).
As a precaution, these were stored as methyl esters to prevent hydrolysis of the 5,6-epoxide and subsequent formation of the γ-lactone.
As part of our ongoing investigations into the possible physiological and neurological roles of these lipid mediators, we also prepared stable analogs as shown in Fig 9. These analogs are expected to increase the half-life of the mediators by inhibiting esterification at the carboxylate by the introduction of a 2,2-dimethyl substitution. The increased steric bulk provided by two methyl groups at the C-2 carbon is expected to sharply reduce the rate of esterification of the oxidized fatty acid. The analog was obtained after the 2,2-dimethyl moiety was installed on the starting material using the strategy shown in Fig 8.
Figure 9-.
stable analogs of 13,9-HEL
Enolate displacement of the bromo-7-heptene followed by hydroboration proceeded in good yield (38% over two steps), allowing the rest of the synthesis to proceed as previously described for the endogenous compound.
Hydrolysis of the 2,2-dimethyl methyl ester required long reaction times; to minimize product loss to epoxide hydrolysis, a milder base (LiOH vs K2CO3) had to be used in combination with 2-propanol, followed by careful silica gel purification to isolate the 2,2-dimethyl free acid.
An in vitro assay using lipase catalyzed esterification of fatty acids to glycerol [21] clearly demonstrates that the 2,2-dimethyl substitution is an effective means of hindering and even blocking enzymatic esterification (Figs 10 and 11). Reaction conditions for the esterification of a free acid to glycerol utilizing a resin bound Candida antarctica lipase were used for both the 13,9-HEL (Fig 10) and for the DM-13,9-HEL (Fig 11). The results depicted in Figure 10 show the LC-MS data indicating near quantitative esterification of 13-H-9,10-epoxide LA while Figure 11 shows that the 2,2-dimethyl analog was not esterified in any detectable amount, with only unreacted starting 2,2-dimethyl free acid found.
Figure 11-.
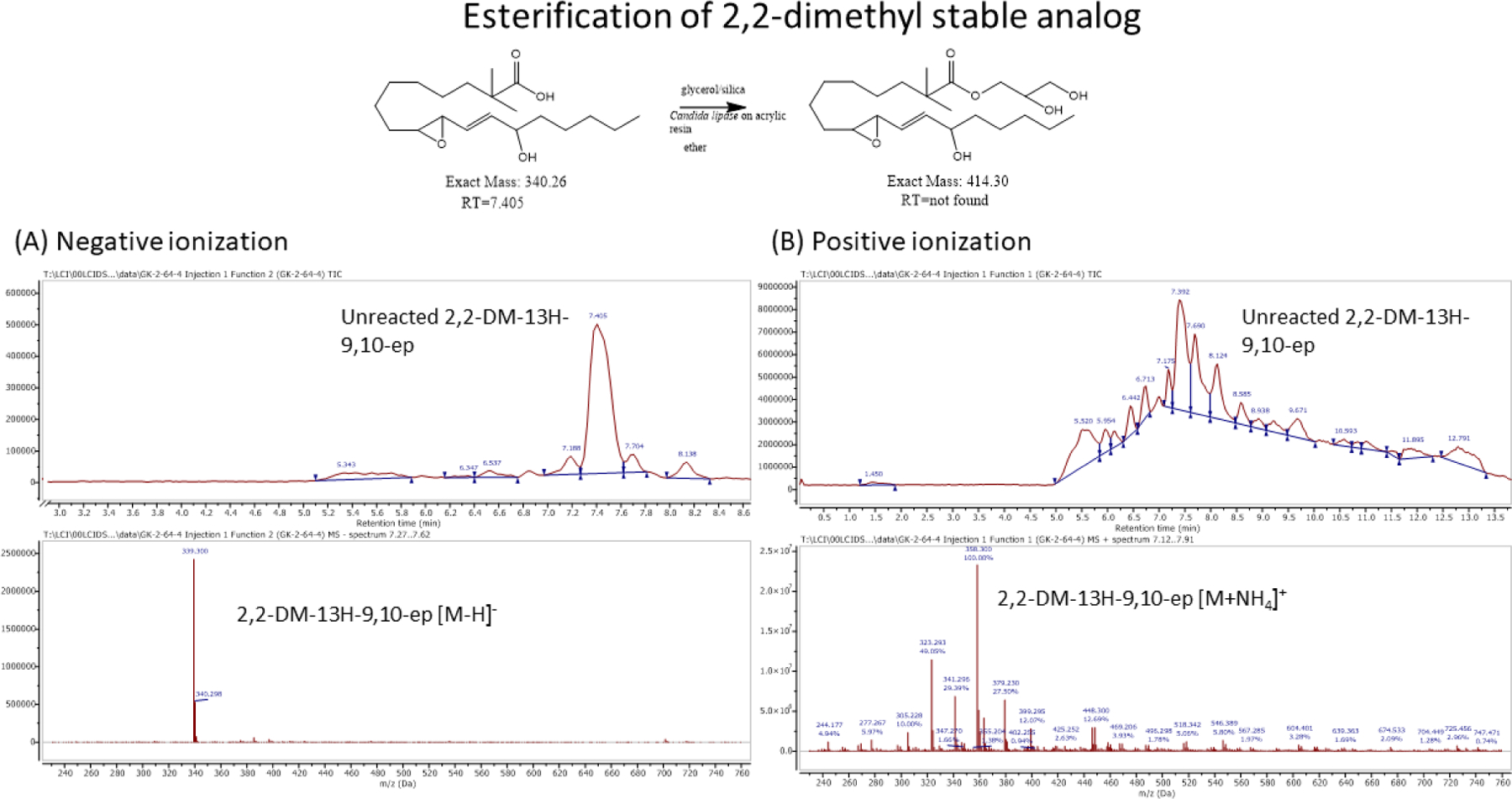
2,2-dimethyl analog provides resistance to lipase catalyzed esterification. Molar masses of free acid and expected glyceryl ester product are below the structures. Total ion chromatogram (TIC; top) and mass spectra (bottom) for positive mode (panel A) and for negative mode (panel B). No glyceryl ester product was observed, only unreacted 2,2-dimethyl free acid and adducts were found: (m/z) 339.30 [M-H]− (panel A); (m/z) 358.30 [M+NH4]+, 379.23 [M+K]+ (panel B).
Additionally, analogs designed to inhibit the action of dehydrogenases at the hydroxyl, preventing ketone formation by converting the secondary alcohol to a tertiary alcohol, were synthesized [22]. Grignard addition of methylmagnesium chloride to the enone with careful reaction monitoring and rapid work-up to minimize concurrent reduction of the ester produced the hydroxymethyl ester which was then hydrolyzed to 2 (Fig 5). The efficacy of this particular inhibition has not been tested by us.
Small molecules representing each configuration of hydroxy epoxide pharmacophore (structures 7 and 8 in Fig 12) were also synthesized from commercially available starting materials using procedures adapted from our syntheses of the endogenous oxylipins (Fig 4). These can be rapidly converted into enones or triols (Fig 12) and should be useful tools for further investigations on the potential actions of these oxylipins.
Figure 12-.
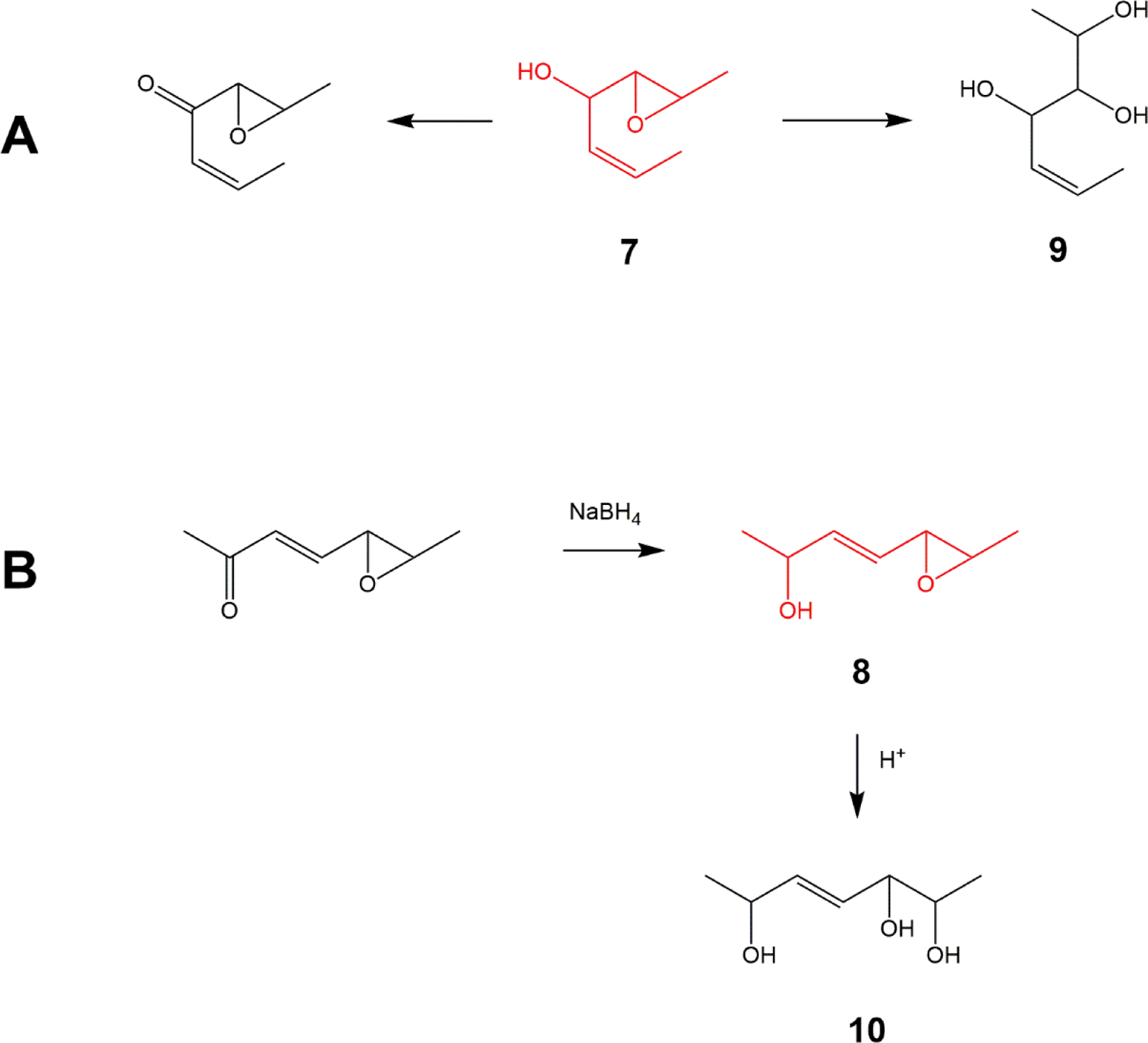
Pharmacophore 7 is rapidly converted to a ketone or to the trihydroxy compound 9 (A); Pharmacophore 8, prepared as the ketone precursor, is reduced with NaBH4 to hydroxy-epoxide 8 and subsequently converted to the trihydroxy 10 (B)
4. Discussion
Linoleic acid has been recognized as an “essential fatty acid” which must be obtained in the diet since 1929 [23]. Essential fatty acid deficiency (EFAD) is manifested by conditions such as dry skin, itch, eczema and atopic dermatitis [23, 24]. Studies published in the 1950s from Hansen and Burr demonstrated that skin abnormalities that were observed in infants fed a low fat diet were reversed when linoleic acid was introduced to the diet [23, 25]. Later studies reported similar findings when skin rashes and other abnormalities that developed in an adult male subject treated with a fat-free parenteral solution were alleviated when linoleic acid was added to the soybean emulsion solution [26]. LA is required to form the epidermal skin barrier as an O-acyl ceramide (Cer-EOS) [27] to prevent accelerated transepidermal water loss (TEWL), a condition that can lead to dermatitis and other skin diseases, some of which can be reversed with topical application of high-LA oils such as sunflower oil [28], [29]. Since an intact and functional epidermal barrier protects against age-associated systemic inflammation [30, 31], the role of lipids in barrier function could impact aging and age-associated diseases.
Recent literature has suggested that an oxidized derivative of LA (13-(R)-hydroxy-9(R),10(R)-trans-epoxy-(11E)-octadecenoate; 13,9-HEL), formed by the actions of 12(R)-lipoxygenase (12(R)-LO) and epidermal lipoxygenase 3 (eLOX3) on ceramide-bound LA causes lipid barrier disruption, which in turn leads to ceramide ester hydrolysis of the 13,9-HEL by an esterase, allowing the liberated omega-hydroxyl fatty acid of the ceramide to covalently bind with protein to form the epidermal skin barrier [11], [27], [32], [33], [14]. Our previous work suggested a potential role for hydroxy-epoxy-octadecenoates in chronic pain and itch that was worthy of future study [1]. However, progress in this domain is limited by lack of availability of hydroxyl-epoxy-and trihydroxy- and octadecenoate derivatives of linoleic acid and related compounds, and challenges in maintaining them in the unesterified lipid pool. To address these limitations, we developed methods to rapidly produce the tools that can help move this field forward. Specifically, (1) total synthesis of racemic mixtures of trihydroxy- and hydroxy-epoxy-octadecenoate derivatives of linoleic acid and sebaleic acid; (2) total synthesis of stable analogs that are designed to be resistant to inactivation via dehydrogenases and acylation/esterification; (3) demonstration that 2,2-dimethyl analogs of trihydroxy- and hydroxy-epoxy-octadecenoates are resistant to acylation/esterification in in vitro assays; (4) synthesis of small molecule scaffolds containing the 7-carbon substructures shared by both families of hydroxy-epoxy-octadecenoates for use as versatile probes.
As the specific bioactive stereoisomers have not been fully identified, we chose to prepare the compounds as racemic mixtures of regiospecific products using efficient non-stereospecific methods. We reasoned that the mixtures would contain the active stereoisomers and can be assayed as such, and stereospecific syntheses of the compounds of interest will follow as necessary.
4.1.2. Potential roles of endogenous compounds
The specific biological roles of these endogenous lipids as structural or signaling molecules are presently unclear. Preliminary evidence suggests an important role for 13,9-HEL and/or its trihydroxy derivatives in skin. Further studies are needed to confirm whether 13,9-HEL is the specific derivative of LA required to prevent essential fatty acid deficiency, and to characterize the structural and signaling roles of this lipid in relation to epidermal inflammation, keratinocyte differentiation and sensation. Specific actions of the other endogenous compounds included in this manuscript are currently speculative. Advancement in the field has been limited due to lack of commercial availability of these compounds and challenges in maintaining these compounds as free acids for testing.
4.1.3. 2,2-dimethyl moiety substitution as a novel template for stabilizing labile oxidized fatty acids
In the present manuscript we demonstrated that 2,2-dimethyl moiety substitution can effectively trap labile, bioactive oxidized fatty acids in the free pool. Previous work has shown that non-oxidized native omega-3 fatty acids modified at the 2-position with either mono-methyl, or dimethyl, or ethyl groups exhibit various bioactivities, including PPAR activation [34], enhanced hypolipidemic effects [35], or inhibition of platelet aggregation when compared to unmodified precursor omega-3 fatty acids [36], [37]. To our knowledge, the present work is the first demonstration for a 2,2-dimethyl substitution as a viable strategy for stabilizing labile, oxidized fatty acids by preventing acylation/esterification. The novel methods described in this work can be used to generate the materials needed to test the effects of stable free acids of the specific oxidized derivatives of LA and SA described in this paper. Moreover, the 2,2-dimethyl addition provides a novel template that can potentially be applied to stabilize any labile, bioactive fatty acid or derivative that is inactivated by acylation and esterification.
Our in vitro lipase esterification assay (Figures 10 and 11) demonstrates that the strategy of adding steric bulk at positions immediately adjacent to the carboxylate is an effective means of inhibiting esterification.
4.1.4. Hydroxy-methyl analogs
Oxidation by dehydrogenases is one of the modes of endogenous processing of hydroxy fatty acids [38]. Conversion of 5(S)-HETE to 5-oxo-ETE, for example, causes an increase in potency of the mediator [39], while a similar transformation of PGE2 to 15-keto-PGE2 causes inactivation [38]. To explore what effect oxidation of the hydroxyl in 13,9-HEL could have, we prepared analogs incorporating a methyl group at the hydroxyl-bearing carbon, in the process converting the secondary alcohol to a tertiary alcohol, preventing oxidation. This is a similar strategy that has been applied to bioactive oxylipins by others [22]. The availability of these analogs will enable the study of the effects of dehydrogenases on the endogenous compounds.
4.1.5. Small molecule pharmacophore probes
The pharmacophore compounds that we synthesized (7,8,9,10; Fig 12) are aqueous buffer soluble probes that could be used for further investigations into the actions of 13,9-HEL and other hydroxy epoxide compounds (possible receptor identification and characterization). Our synthesis provides each probe in high yield without the need for unnecessarily consuming potentially large amounts of precious oxidized fatty acid derivatives for biological assays.
4.1.6. Conclusion
Together, these synthetic methods provide the tools needed to investigate physiological and pathological functions of hydroxy-epoxy derivatives of LA and SA and their substructures, and provide a novel template for stabilizing labile, bioactive lipids by preventing acylation/esterification.
Highlights.
Oxidized derivatives of linoleic acid proposed to play essential role in formation of epidermal skin barrier
Total organic synthesis provides racemic mixtures of oxidized derivatives of linoleic acid useful for probing the essential nature of this fatty acid
Organic synthesis also provides stable analogs and buffer soluble small molecule probes containing key functional groups of oxidized linoleic acid derivatives, resistant to further oxidation or to esterification
Total synthesis of oxidized derivatives of sebaleic acid, a regioisomer of linoleic acid present in skin
Acknowledgements
Supported by the intramural programs of the National Institute on Aging and National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author statement
Gregory S. Keyes: Methodology, Investigation, Formal Analysis, Validation, Writing- Original draft preparation
Kristen Maiden: Investigation, Writing - Review & Editing
Christopher E. Ramsden: Conceptualization, Writing - Review & Editing
Competing interests
The NIA (NIH) has claimed intellectual property related to this discovery (PCT/US2018/041086;), with C.E.R. and G.S.K. named as inventors. All other authors declare that they have no competing interests.
HEL=hydroxy epoxide linoleic acid derivative
THL=trihydroxy linoleic acid derivative
SA=sebaleic acid
References
- 1.Ramsden CE, et al. , A systems approach for discovering linoleic acid derivatives that potentially mediate pain and itch. Sci Signal, 2017. 10(493). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bowser PA, et al. , Identification, isolation and characterization of epidermal lipids containing linoleic acid. Biochim Biophys Acta, 1985. 834(3): p. 419–28. [DOI] [PubMed] [Google Scholar]
- 3.Choque B, et al. , Dietary linoleic acid requirements in the presence of alpha-linolenic acid are lower than the historical 2 % of energy intake value, study in rats. Br J Nutr, 2015. 113(7): p. 1056–68. [DOI] [PubMed] [Google Scholar]
- 4.Gabbs M, et al. , Advances in Our Understanding of Oxylipins Derived from Dietary PUFAs. Adv Nutr, 2015. 6(5): p. 513–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pace-Asciak CR, Reynaud D, and Demin PM, Hepoxilins: a review on their enzymatic formation, metabolism and chemical synthesis. Lipids, 1995. 30(2): p. 107–14. [DOI] [PubMed] [Google Scholar]
- 6.Boeglin WE, Kim RB, and Brash AR, A 12R-lipoxygenase in human skin: mechanistic evidence, molecular cloning, and expression. Proc Natl Acad Sci U S A, 1998. 95(12): p. 6744–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brash AR, Boeglin WE, and Chang MS, Discovery of a second 15S-lipoxygenase in humans. Proc Natl Acad Sci U S A, 1997. 94(12): p. 6148–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu Z, et al. , The lipoxygenase gene ALOXE3 implicated in skin differentiation encodes a hydroperoxide isomerase. Proc Natl Acad Sci U S A, 2003. 100(16): p. 9162–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiba T, et al. , The Precise Structures and Stereochemistry of Trihydroxy-linoleates Esterified in Human and Porcine Epidermis and Their Significance in Skin Barrier Function: IMPLICATION OF AN EPOXIDE HYDROLASE IN THE TRANSFORMATIONS OF LINOLEATE. J Biol Chem, 2016. 291(28): p. 14540–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis RW, et al. , Stereocontrolled synthesis of four isomeric linoleate triols of relevance to skin barrier formation and function. Tetrahedron Lett, 2018. 59(52): p. 4571–4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng Y, et al. , Lipoxygenases mediate the effect of essential fatty acid in skin barrier formation: a proposed role in releasing omega-hydroxyceramide for construction of the corneocyte lipid envelope. J Biol Chem, 2011. 286(27): p. 24046–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Munoz-Garcia A, et al. , The importance of the lipoxygenase-hepoxilin pathway in the mammalian epidermal barrier. Biochim Biophys Acta, 2014. 1841(3): p. 401–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krieg P and Furstenberger G, The role of lipoxygenases in epidermis. Biochim Biophys Acta, 2014. 1841(3): p. 390–400. [DOI] [PubMed] [Google Scholar]
- 14.Takeichi T, et al. , SDR9C7 catalyzes critical dehydrogenation of acylceramides for skin barrier formation. J Clin Invest, 2020. 130(2): p. 890–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cossette C, et al. , Human neutrophils convert the sebum-derived polyunsaturated fatty acid Sebaleic acid to a potent granulocyte chemoattractant. J Biol Chem, 2008. 283(17): p. 11234–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin D, Zhang J, and Sayre LM, Synthesis of six epoxyketooctadecenoic acid (EKODE) isomers, their generation from nonenzymatic oxidation of linoleic acid, and their reactivity with imidazole nucleophiles. J Org Chem, 2007. 72(25): p. 9471–80. [DOI] [PubMed] [Google Scholar]
- 17.Thomas CP, et al. , Steric analysis of epoxyalcohol and trihydroxy derivatives of 9-hydroperoxy-linoleic acid from hematin and enzymatic synthesis. Chem Phys Lipids, 2013. 167–168: p. 21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamberg M, Regio- and stereochemical analysis of trihydroxyoctadecenoic acids derived from linoleic acid 9- and 13-hydroperoxides. Lipids, 1991. 26(6): p. 407–15. [DOI] [PubMed] [Google Scholar]
- 19.Vasiljeva LL, et al. , Synthesis, properties, and identification of epimeric hepoxilins (−)-(10R)-B3 and (+)-(10S)-B3. Tetrahedron, 1993. 49(19): p. 4099–4106. [Google Scholar]
- 20.Corey EJ and Su W.-g., Total synthesis of biologically active metabolites of arachidonic acid. The two 8-hydroxy-11,12(S,S)-epoxyeicosa-5,14(Z),9(E)-trienoic acids. Tetrahedron Lett, 1984. 25(45): p. 5119–5122. [Google Scholar]
- 21.Yesiloglu Y and Kilic I, Lipase-catalyzed esterification of glycerol and oleic acid. Journal of the American Oil Chemists’ Society, 2004. 81(3): p. 281–284. [Google Scholar]
- 22.Serhan CN, et al. , Design of lipoxin A4 stable analogs that block transmigration and adhesion of human neutrophils. Biochemistry, 1995. 34(44): p. 14609–15. [DOI] [PubMed] [Google Scholar]
- 23.Hansen AE, et al. , Essential fatty acids in infant nutrition. III. Clinical manifestations of linoleic acid deficiency. J Nutr, 1958. 66(4): p. 565–76. [DOI] [PubMed] [Google Scholar]
- 24.Mysliwiec H, et al. , Abnormal serum fatty acid profile in psoriatic arthritis. Arch Med Sci, 2019. 15(6): p. 1407–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spector AA and Kim HY, Discovery of essential fatty acids. J Lipid Res, 2015. 56(1): p. 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collins FD, et al. , Plasma lipids in human linoleic acid deficiency. Nutr Metab, 1971. 13(3): p. 150–67. [DOI] [PubMed] [Google Scholar]
- 27.Nugteren DH, et al. , Metabolism of linoleic acid and other essential fatty acids in the epidermis of the rat. Biochim Biophys Acta, 1985. 834(3): p. 429–36. [DOI] [PubMed] [Google Scholar]
- 28.Madison KC, Barrier function of the skin: “la raison d’etre” of the epidermis. J Invest Dermatol, 2003. 121(2): p. 231–41. [DOI] [PubMed] [Google Scholar]
- 29.Prottey C, Hartop PJ, and Press M, Correction of the cutaneous manifestations of essential fatty acid deficiency in man by application of sunflower-seed oil to the skin. J Invest Dermatol, 1975. 64(4): p. 228–34. [DOI] [PubMed] [Google Scholar]
- 30.Velarde MC, Epidermal Barrier Protects against Age-Associated Systemic Inflammation. J Invest Dermatol, 2017. 137(6): p. 1206–1208. [DOI] [PubMed] [Google Scholar]
- 31.Hu L, et al. , Epidermal Dysfunction Leads to an Age-Associated Increase in Levels of Serum Inflammatory Cytokines. J Invest Dermatol, 2017. 137(6): p. 1277–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamanashi H, et al. , Catalytic activities of mammalian epoxide hydrolases with cis and trans fatty acid epoxides relevant to skin barrier function. J Lipid Res, 2018. 59(4): p. 684–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wertz PW, Lipids and the Permeability and Antimicrobial Barriers of the Skin. J Lipids, 2018. 2018: p. 5954034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Larsen LN, et al. , Sulfur-substituted and alpha-methylated fatty acids as peroxisome proliferator-activated receptor activators. Lipids, 2005. 40(1): p. 49–57. [DOI] [PubMed] [Google Scholar]
- 35.Vaagenes H, et al. , Methylated eicosapentaenoic acid and tetradecylthioacetic acid: effects on fatty acid metabolism. Biochem Pharmacol, 1999. 58(7): p. 1133–43. [DOI] [PubMed] [Google Scholar]
- 36.Larsen LN, et al. , Alpha- and beta- alkyl-substituted eicosapentaenoic acids: incorporation into phospholipids and effects on prostaglandin H synthase and 5-lipoxygenase. Biochem Pharmacol, 1998. 55(4): p. 405–11. [DOI] [PubMed] [Google Scholar]
- 37.Willumsen N, et al. , On the effect of 2-deuterium- and 2-methyl-eicosapentaenoic acid derivatives on triglycerides, peroxisomal beta-oxidation and platelet aggregation in rats. Biochim Biophys Acta, 1998. 1369(2): p. 193–203. [DOI] [PubMed] [Google Scholar]
- 38.Hamberg M and Samuelsson B, On the metabolism of prostaglandins E 1 and E 2 in man. J Biol Chem, 1971. 246(22): p. 6713–21. [PubMed] [Google Scholar]
- 39.Powell WS, et al. , Stimulation of human neutrophils by 5-oxo-6,8,11,14-eicosatetraenoic acid by a mechanism independent of the leukotriene B4 receptor. J Biol Chem, 1993. 268(13): p. 9280–6. [PubMed] [Google Scholar]


