Abstract
Women are prescribed opioids more often than men. Prescription opioid use among women of reproductive age is a public health concern because opioid use during pregnancy is associated with decreased prenatal care and increased risk of adverse perinatal and maternal outcomes. Recent prevalence estimates and correlates of prescription opioid use and long-term use among women of reproductive age are limited.
Using the 2003-2018 National Health and Nutrition Examination Survey (NHANES), we estimated the national prevalence, trend, and correlates of prescription opioid use, long-term use (≥ 90 days of use), and use of medications for opioid use disorder (MOUD) among women aged 15-44 (n=13,558). Prescription opioid use within the last 30 days and prescription duration were collected through interviews and identified using prescription codes. Trend analysis was conducted using the National Cancer Institute Joinpoint Trend Analysis Software.
The prevalence of prescription opioid use significantly decreased from 5.2% in 2003-2004 to 3.0% in 2017-2018 (p<.05). MOUD use increased significantly from 0.1% in 2005-2006 to 0.4% in 2011-2012. Long-term opioid use did not significantly change over time. Correlates of prescription opioid use and long-term use included ages 35-44, non-Hispanic White, public insurance, and women with poor or fair health status.
As policy makers and clinicians strive to reduce the negative impacts of the opioid epidemic, they should consider the demographic groups most likely to use prescription opioids long-term. Additionally, reductions in opioid prescribing should be balanced with increased availability of nonopioid therapies and monitoring for opioid use disorder.
Introduction
Prescription opioid use remains prevalent in the United States (US) and poses a threat to the health of women and families due to risks of opioid overdose, opioid dependency, and increased susceptibility to infectious diseases such as HIV and hepatitis C (2019; Centers for Disease Control and Prevention, 2018; National Institute on Drug Abuse, 2018; Rudd et al., 2016). From 2017-2018, 43% of opioid-related overdose deaths among women in the US involved a prescription opioid (Ko et al., 2020; Wilson et al., 2020). The Annual Surveillance Report of Drug-Related Risks and Outcomes found that 17% of females filled an opioid prescription compared to 13% of males possibly due to higher incidence of chronic and female specific pain conditions (Centers for Disease Control and Prevention, 2019; Darnall and Stacey, 2012). In 2018, 1 in 6 women of reproductive age (15-44 years old) were prescribed an opioid (Centers for Disease Control and Prevention, 2019). Many women are unaware of their pregnancy during the first few weeks, almost half of all pregnancies in the US are unplanned, and prescription opioid use during pregnancy is associated with decreased prenatal care and adverse pregnancy outcomes (Ailes et al., 2015; Clemans-Cope et al., 2019; Finer and Zolna, 2016; Johnson and Jones, 2018; Maeda et al., 2014; The American College of Obstetricians and Gynecologists, 2017). Therefore, it is important to examine prescription opioid use among women of reproductive age.
Chronic pain conditions common among women can lead to poor health and both have been associated with an increased risk of long-term prescription opioid use (Shah A, 2017). Long-term prescription opioid use, often defined as ≥90 days, increases the risk of opioid misuse and opioid use disorder (OUD) which is characterized by tolerance, craving, and continued use despite adverse consequences (Dowell et al., 2016; Ko et al., 2020; The American College of Obstetricians and Gynecologists, 2017). Medications for OUD (MOUD), including methadone and buprenorphine, are standard and effective treatments that prevent opioid withdrawal, overdoses, and reduce cravings while improving treatment retention (Administration, 2016; Patrick et al., 2020; The American College of Obstetricians and Gynecologists, 2017). Current studies have found that prescription opioid misuse, a common antecedent behavior to OUD, is fairly common among US women with 4% those ages 12 or older reporting misuse in the past year and 98,000 women of reproductive age reporting misuse in the past month (Centers for Disease Control and Prevention, 2019; Substance Abuse and Mental Health Services Administration, 2018). Women who use opioids long-term before pregnancy tend to continue use during pregnancy and nearly 9 of every 10 pregnancies among opioid dependent pregnant women were unintended (Clemans-Cope et al., 2019; Heil et al., 2011).
To inform interventions, it is important to identify sub-groups of women at higher risk of prescription opioid use and long-term use. A previous study using the national outpatient pharmacy data found that among women of reproductive age, those with public health insurance, non-Hispanic White, and older age were most likely to use prescription opioids (Ailes et al., 2015). However, this study was limited to women with employer-based private insurance or Medicaid from 2008-2012, which may not be generalizable to the entire US population. For correlates of long-term opioid use, a large multi-payer study found that patients who continued prescription opioid therapy for greater than 1 year were more likely to be older, female, and to be publicly insured compared to those that discontinued use before 1 year (Shah A, 2017). Therefore, risk factors for long-term opioid use among women of reproductive age are less understood. Lastly, previous studies have shown gaps in treatment access for women with OUD (Hodgins et al., 2019; Klaman et al., 2017; Patrick et al., 2020; The American College of Obstetricians and Gynecologists, 2017). Hence, understanding the correlates of MOUD use among women of reproductive age may be informative for drug treatment services.
Many policy and practice interventions have been implemented to reduce levels of opioid prescribing and improve access to MOUD but recent trend estimates of prescription opioid use among women of reproductive age are limited (Kiang et al., 2020; Lee et al., 2021). A national study of pharmacy data found that the annual opioid prescribing rates increased from 2006 to 2010, remained stable from 2010 to 2012, and then decreased until 2015 (Guy et al., 2017). However, the study did not determine trends for specific categories of prescription opioid use, such as type of opioid and length of use, or prevalence estimates for women of reproductive age (Guy et al., 2017). Current guidance from the CDC suggests serious consideration should be taken when prescribing opioids for longer than 7 days (Centers for Disease Control and Prevention, 2019). However, long-term prescription opioid use (≥ 90 days) significantly increased from 1999 to 2014 among US adults (Mojtabai, 2018). To our knowledge, no study has assessed the prevalence trends of long-term opioid use among women of reproductive age. Lastly, the National Survey of Substance Abuse Treatment Services data has found that the use of MOUD in treatment facilities and the number of clients receiving methadone or buprenorphine increased from 2003 to 2015 but trend estimates for MOUD use among women of reproductive age are insufficient (Alderks, 2017).
The purpose of this study was to determine the prevalence and trend of prescription opioid use, long-term use, and MOUD use among a nationally representative sample of women of reproductive age in the US. Based upon current evidence and national opioid prescribing rates, we hypothesized the prevalence of prescription opioid use may initially increase and then become stable before decreasing. While long-term prescription opioid use and MOUD use may increase over time. We predicted women who are non-Hispanic White, with poorer health, and public health insurance will have a greater odds of prescription opioid use, long-term use, and MOUD use.
Methods
Study Population
The National Health and Nutrition Examination Survey (NHANES), a cross-sectional survey of the US population conducted by the National Center for Health Statistics (NCHS),15,16 is used to monitor the health and nutritional status of the civilian, non-institutionalized population. To estimate the prevalence and trend of prescription opioid use over time, we combined NHANES data from eight 2-year cycles from 2003 through 2018 (n=80,312). After exclusions, our final study sample included 13,558 female participants of reproductive age, defined as 15-44 years old, of which 1,041 were pregnant at interview (Centers for Disease Control and Prevention, 2020). We excluded participants who were males (n=39,679), women <15 or >44 (n=26,943), and observations with missing data ((education (n=10), health insurance (n=115), and general health status (n=7)). Opioid use among excluded participants with missing data was rare (range, 0-6 women across three outcomes) and were spread across all survey years.
Outcome Assessment
The outcomes of interest included ‘any prescription opioid use’, ‘long-term opioid use’, and ‘MOUD use’. Survey participants were asked if they had taken a medication in the past month for which they needed a prescription and if answered “yes”, were asked to verify the medication by showing the prescription bottle or the prescription print out. Approximately 81% of prescription opioids and 70% of MOUD were verified in our sample but we did not exclude unverified prescriptions from the analyses. The interviewers entered the reported medication name on the container or pharmacy print out and if neither were available, entered the prescription name verbally reported. NHANES uses drug database software to classify reported medications into standardized generic prescription medication codes (Prevention, 2007). The medication code variable was used to identify the following opioids: hydrocodone, oxycodone, propoxyphene, codeine, tramadol, opium, morphine, fentanyl, hydromorphone, meperidine, pentazocine, oxymorphone, tapentadol. Among women who used opioids, the prescription duration reported as number of days was reclassified into short-term use (< 90 days) and long-term use (≥ 90 days) for consistency with previous studies (Mojtabai, 2018). If the participant reported more than one opioid prescription, the longest prescription length was used to quantify opioid duration. We also used the medication code variable to group methadone, buprenorphine, buprenorphine/naloxone, and naltrexone into the category of MOUD. Therefore, the category of ‘any prescription opioid use’ included both short-term use (< 90 days) and long-term use (≥ 90 days) but did not include MOUD.
Exposure Assessment
Demographic variables including age and race/ethnicity, correlates of prescription opioid use, and survey year were included as covariates in our study. Other correlates of prescription opioid use included were family income to poverty level (an NHANES calculated variable of the annual household income and household size as a ratio of the federal Health and Human Services’ poverty thresholds that were applicable at that wave), general health condition, health insurance, previous heroin use, and pregnancy status. Previous heroin use was included as a covariate because illicit opioid use may be related to prescription opioid use and MOUD use. Lastly, pregnancy status should be considered by clinicians when prescribing opioids or MOUD (The American College of Obstetricians and Gynecologists, 2017).
Statistical Analysis
Data from NHANES 2003-2018 was used to assess the trends of prescription opioid use, long-term prescription opioid use, and MOUD use within the last 30 days. To account for the complex survey design of NHANES, we stratified the data with masked variance pseudo-stratum, clustered with masked variance pseudo-PSU and used 16-year full sample interview weights to yield our weighted sample. Sample characteristics are presented as unweighted counts and weighted percentages. Per the NCHS guidelines for trend analysis using NHANES survey data, we used joinpoint regression to identify statistically significant changes in the temporal trends of the three outcomes (Ingram DD, 2018). Joinpoint regression can detect linear and non-linear changes in trends over time and is the preferred method for trend analysis using NHANES data. Joinpoint regression determines an annual percentage change to describe how rates changes within each time interval. Since NHANES is conducted in 2-year cycles, Joinpoint regression was used to determine bi-annual percentage changes (PC).
Chi square tests of independence were used to determine if prescription opioid use, long-term use, and MOUD use differed by characteristics of women of reproductive ages. Each prescription opioid outcome category was compared to participants with no opioid use. For further evaluation of the trend and correlates of prescription opioid use and long-term use, we performed survey logistic regressions through unadjusted bivariate models and adjusted models. To avoid small cell counts due to relatively low opioid use prevalence, survey year was dichotomized for the logistic regression models. Based on evidence that prescription opioid use peaked during 2012, as well as trends found in our analysis, survey year was dichotomized into 2003-2012 and 2013-2018 (Centers for Disease Control and Prevention, 2019). Survey logistic regression results are presented as crude and adjusted odds ratios and confidence intervals. Final multivariable models were chosen based upon preliminary analysis and epidemiological modeling with directed acyclic graphs. Logistic regression models were not conducted for the outcome of MOUD due to small sample size (N=27). Data management and data analyses were performed using SAS version 9.4, SUDAAN version 11.0, and NCI’s Joinpoint Trend Analysis Software version 4.7. This study is considered exempt from Institutional Review Board approval as NHANES data is publicly available.
Results
Sample Characteristics
The age groups of our sample were almost equally distributed and the majority of our sample were non-Hispanic White (59.4%), with more than a high school education (57.4%), and a family income to poverty ratio greater than 2.5 (45.6%) (Table 1). For all survey years combined, any prescription opioid use was reported by 4.5% (N=519) of participants, long-term prescription opioid use was reported by 2.1% (N=229), and 0.3% (N=27) reported MOUD use within the last 30 days (Table 1). Among women who used opioids long-term, the mean duration of use was 1,357 days (range: 91-9,855 days) compared to a mean duration of 17 days (range: 1-61 days) among women who used prescription opioids for less than 90 days. The mean duration of MOUD use was 1,052 days (range: 61-4,015 days).
Table 1:
Characteristics of Women of Reproductive Age by Any Prescription Opioid Use, Long-term Prescription Opioid Use, and use of Medications for Opioid Use Disorder; NHANES 2003-2018
| Characteristicsa | Total Sample (n=13,558) n (%)b |
Any Opioid Usec (n=519) n (%) |
P valued | Long Term Opioid Usee (n=229) n (%) |
P value | MOUD usef (n=27) n (%) |
P value |
|---|---|---|---|---|---|---|---|
| Total | 13,558 | 519 (4.5) | 229 (2.1) | 27 (0.3) | |||
| Survey Year | 0.02 | 0.73 | * | ||||
| 2003-2004 | 1792 (12.3) | 68 (5.2) | 24 (2.3) | 0 | |||
| 2005-2006 | 2027 (12.6) | 84 (6.1) | 28 (2.3) | 1 (0.1) | |||
| 2007-2008 | 1556 (12.4) | 64 (4.5) | 32 (2.0) | 2 (0.1) | |||
| 2009-2010 | 1773 (12.5) | 89 (5.2) | 39 (2.2) | 6 (0.4) | |||
| 2011-2012 | 1577 (12.4) | 64 (5.3) | 26 (2.7) | 6 (0.4) | |||
| 2013-2014 | 1748 (12.8) | 68 (4.2) | 37 (2.5) | 7 (0.6) | |||
| 2015-2016 | 1650 (12.6) | 42 (2.8) | 23 (1.7) | 3 (0.3) | |||
| 2017-2018 | 1435 (12.5) | 40 (3.0) | 20 (1.6) | 2 (0.2) | |||
| Age, years | <.01 | <.01 | 0.39 | ||||
| 15-24 | 5663 (32.9) | 130 (3.0) | 28 (0.8) | 3 (0.2) | |||
| 25-34 | 3988 (32.7) | 154 (4.3) | 60 (1.7) | 12 (0.3) | |||
| 35-44 | 3907 (34.4) | 235 (6.2) | 141 (3.9) | 12 (0.3) | |||
| Race/Ethnicity | <.01 | <.01 | <.01 | ||||
| Hispanic | 4016 (18.0) | 110 (3.1) | 39 (1.2) | 1 (0.1) | |||
| Non-Hispanic White | 4791 (59.4) | 265 (5.3) | 129 (2.6) | 23 (0.4) | |||
| Non-Hispanic Black | 3159 (13.8) | 115 (4.2) | 46 (1.8) | 2 (0.1) | |||
| Non-Hispanic Other | 1592 (8.7) | 29 (2.5) | 15 (1.8) | 1 (0.1) | |||
| Pregnant | <.01 | <.01 | * | ||||
| Yes | 1041 (4.6) | 18 (2.6) | 5 (1.0) | 0 | |||
| No | 10258 (83.3) | 459 (5.0) | 216 (2.5) | 27 (0.3) | |||
| Missing | 2259 (12.0) | 42 (2.2) | 8 (0.5) | 0 | |||
| Education Level | 0.16 | 0.06 | 0.26 | ||||
| Some High School | 4627 (23.7) | 150 (4.1) | 63 (1.9) | 10 (0.3) | |||
| High School Diploma | 2566 (18.8) | 108 (5.3) | 53 (3.0) | 7 (0.4) | |||
| More than High School | 6365 (57.4) | 261 (4.4) | 113 (2.0) | 10 (0.2) | |||
| Family Income-to-Poverty Ratio | 0.04 | <.01 | * | ||||
| 0.00-1.30 | 5959 (33.5) | 235 (5.0) | 118 (2.7) | 22 (0.6) | |||
| 1.31-1.85 | 1622 (10.8) | 65 (5.3) | 31 (2.9) | 3 (0.3) | |||
| 1.86-2.50 | 1325 (10.1) | 55 (5.4) | 25 (2.7) | 0 | |||
| >2.50 | 4652 (45.6) | 164 (3.8) | 55 (1.5) | 2 (0.1) | |||
| Health Insurance Type | <.01 | <.01 | <.01 | ||||
| Private | 6734 (59.7) | 244 (4.2) | 88 (1.7) | 4 (0.1) | |||
| Medicare, Medicaid, Medi-Gap or CHIP | 2408 (12.6) | 145 (8.0) | 85 (5.1) | 10 (0.7) | |||
| Other Insuranceg | 1169 (7.2) | 50 (4.5) | 24 (2.4) | 5 (0.6) | |||
| No Insurance | 3247 (20.5) | 80 (3.4) | 32 (1.6) | 8 (0.4) | |||
| General Health Condition | <.01 | <.01 | <.01 | ||||
| Excellent, Very Good, or Good | 11428 (86.9) | 345 (3.6) | 116 (1.3) | 14 (0.2) | |||
| Fair or Poor | 2130 (13.1) | 174 (10.8) | 113 (7.6) | 13 (1.0) | |||
| Ever Used Heroin h | <.01 | <.01 | <.01 | ||||
| Yes | 101 (1.0) | 7 (5.2) | 4 (2.8) | 9 (11.8) | |||
| No | 809 (7.8) | 68 (8.7) | 39 (5.5) | 6 (0.5) | |||
| Refused, Don’t know, or Missing | 12648 (91.2) | 444 (4.2) | 186 (1.9) | 12 (0.1) |
Total sample presented as column percentages and characteristics presented as row percentages
Unweighted sample counts and weighted sample percentages to account for NHANES survey design
At least 1 opioid prescription reported as used within the last month. Category includes short-term (<90 days) and long-term use (≥90 days) but does not include MOUD use. Participants with any opioid use are compared to those with no opioid use
P-values calculated for each covariate by chi-square test
At least 1 opioid prescription reported as used for ≥ 90 days and if multiple opioids were reported the longest frequency was used. Participants with long-term opioid use were compared to those with no opioid use (excluded short term opioid use (N=290))
At least 1 medication for treatment of opioid use disorder was reported as used within the last month. Participants with MOUD use were compared to those with no opioid use (excluded participants with non-MOUD opioid use (N=3098))
Other insurance includes other types of government insurance and single service plans
Respondents answer to question “Have you ever, even once, used heroin?”
Boldface indicates statistical significance (p<.05)
Chi-square p-value unavailable due to tables with 0 cells
Trend Analysis of Prescription Opioid Use
The prevalence of any prescription opioid use was 5.2% (95% CI: 4.2-6.1) in 2003-2004, peaked at 6.1% (95% CI: 5.4-6.8) in 2005-2006, and decreased to 3.0% in 2017-2018 (95% CI: 2.4-3.7) (Table 1, Figure 1). From 2003-2004 through 2017-2018, the prevalence of prescription opioid use decreased significantly (p<.05) by 9% (PC: −9.2) every two-years (Figure 1, Appendix Table 1). The prevalence of long-term prescription opioid use was 2.3% (95% CI: 1.8-2.6) in 2003-2004, peaked at 2.7% (95% CI: 2.0-3.3) in 2011-2012, and decreased to 1.6% (95% CI: 1.1-2.0) in 2017-2018 (Table 1). The trend of long-term prescription opioid use increased by 3% (PC: 2.6) from 2003-2004 through 2013-2014 and decreased by 20% (PC: −19.9) from 2013-2014 through 2017-2018; however, the prevalence changes were not statistically significant (Figure 2, Appendix Table 1). There were 0 observations with MOUD use during 2003-2004 so that survey wave was not included in the trend analysis for MOUD use. The trend of MOUD use increased by about 130% (PC: 129.7) every two-years from 2005-2006 through 2011-2012 and decreased from 2011-2012 through 2017-2018 by 32% (PC: −31.5) (Figure 3, Appendix Table 1). The increase in MOUD use from 2005-2006 through 2011-2012 was significant (p<.05) but the decrease from 2011-2012 through 2017-2018 was not.
Fig 1. Prevalence (with 95% Confidence Interval) of Any Prescription Opioid Use Among Women of Reproductive Age, NHANES 2003-2018.
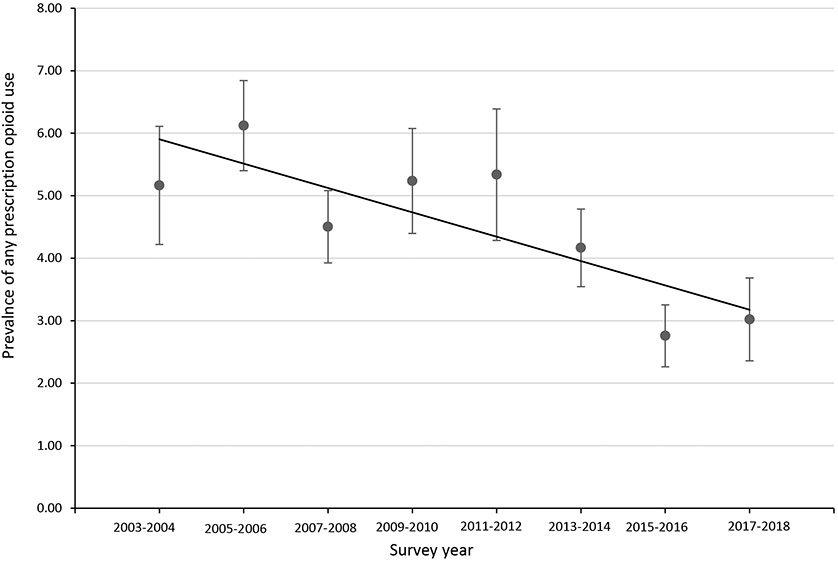
2003-2004 through 2017-2018 PC = −9.16* (P value=0.01)
*Indicates that the percentage change (PC) is significantly different from zero at the alpha=0.05 level
Fig 2. Prevalence (with 95% Confidence Interval) of Long-term Prescription Opioid Use Among Women of Reproductive Age, NHANES 2003-2018.
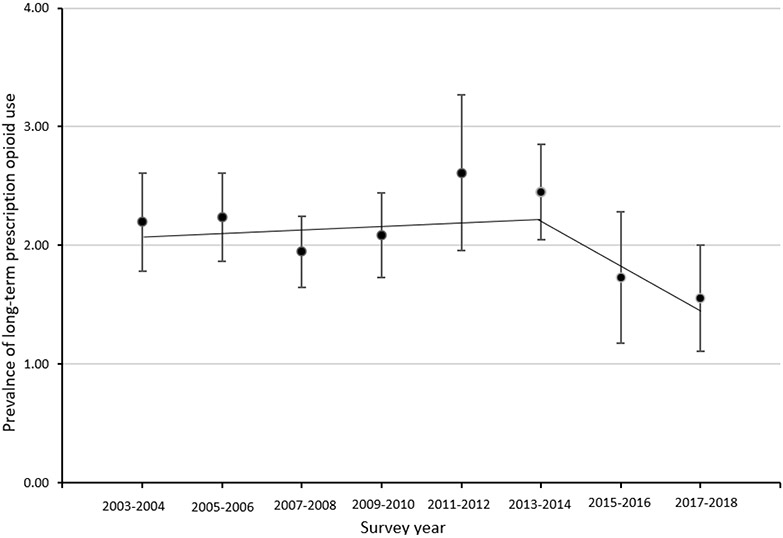
2003-2004 through 2013-2014 PC = 2.55 (P value=0.56)
2013-2014 through 2017-2018 PC = −19.89 (P value=0.45)
*Indicates that the percentage change (PC) is significantly different from zero at the alpha=0.05 level
Fig 3. Prevalence (with 95% Confidence Interval) of Medications for Opioid Use Disorder (MOUDs) Among Women of Reproductive Age, NHANES 2005-2018.
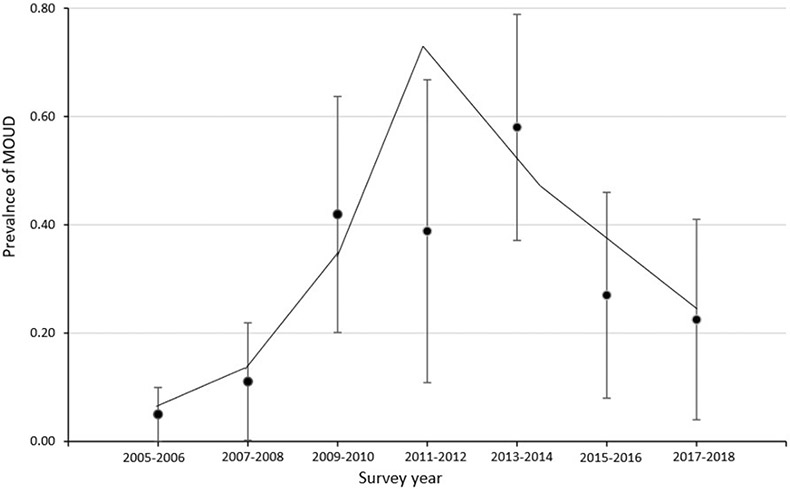
2005-2006 through 2011-2012 PC = 129.68* (P value=0.03)
2011-2012 through 2017-2018 PC = −31.51 (P value=0.09)
*Indicates that the percentage change (PC) is significantly different from zero at the alpha=0.05 level
Correlates of Prescription Opioid Use
Prevalence estimates of any prescription opioid use and long-term use varied significantly (p<.05) by age, race/ethnicity, pregnancy status, family income to poverty level, health insurance type, general health condition, and history of heroin use (Table 1). Approximately 2.6% of pregnant women reported any prescription opioid use and 0.9% reported long-term use but pregnant women were less likely than nonpregnant women to report both. Women ages 35-44 had higher odds of any prescription opioid use than those ages 15-24 (aOR: 1.8, 95% CI: 1.3-2.4, p<.01) and fair or poor health was positively associated with any prescription opioid use compared to those with excellent, very good, or good health (aOR: 3.2, 95% CI: 2.5-4.2, p<.01) (Table 2). All race/ethnicity categories were significantly associated with decreased odds of any prescription opioid use when compared to non-Hispanic Whites (p<.05). Compared to women with private health insurance, women with public insurance (i.e., Medicaid, Medicare, or CHIP) had a 76% higher odds of any prescription opioid use (aOR: 1.8, 95% CI: 1.3-2.5) (Table 2). Lastly, women with fair or poor health had 3 times the odds of prescription opioid use than those with excellent, very good, or good health (aOR: 3.2, 95% CI:2.5-4.2, p<.01) (Table 2).
Table 2:
Stratification of Prescription Opioid Use and Long-term Prescription Opioid Use Among Women of Reproductive Age, NHANES 2003-2018
| Characteristics | Any Opioid Usea (nb=519) vs. No Opioid Use (n=13,039) |
Long-Termc Opioid Use (n=229) vs. No Opioid Use (n=13,039) |
||
|---|---|---|---|---|
| cOR (95% CI)d | aOR (95% CI)e | cOR (95% CI)d | aOR (95% CI)e | |
| Survey Year | ||||
| 2003-2012 | Referent | Referent | ||
| 2013-2018 | 0.62 (0.48-0.80)** | 0.59 (0.45-0.78)** | 0.85 (0.61-1.19) | 0.79 (0.54-1.16) |
| Age, years | ||||
| 15-24 | Referent | Referent | ||
| 25-34 | 1.46 (1.08-1.95)* | 1.32 (0.95-1.85) | 2.13(1.19-3.84)* | 1.78 (0.95-3.36) |
| 35-44 | 2.14 (1.64-2.80)** | 1.75 (1.27-2.42)** | 4.95 (2.98-8.23)** | 3.71 (2.08-6.61)** |
| Race/Ethnicity | ||||
| Non- Hispanic White | Referent | Referent | ||
| Non-Hispanic Black | 0.78 (0.60-1.00) | 0.63 (0.48-0.84)** | 0.69 (0.49-0.95)* | 0.48 (0.33-0.70)** |
| Hispanic | 0.58 (0.44-0.75)** | 0.50 (0.37-0.67)** | 0.44 (0.29-0.66)** | 0.31 (0.19-0.51)** |
| Non-Hispanic Other | 0.45 (0.27-0.74)** | 0.46 (0.28-0.76)* | 0.71 (0.38-1.30) | 0.70 (0.38-1.30) |
| Pregnant | ||||
| Yes | Referent | Referent | ||
| No | 1.99 (1.04-3.83)* | 1.81 (0.94-3.47) | 2.60 (0.81-8.40) | 1.84 (0.56-6.00) |
| Missing | 0.87 (0.41-1.84) | 1.30 (0.60-2.81) | 0.52 (0.13-2.16) | 1.05 (0.25-4.48) |
| Education Level | ||||
| Some High School | Referent | Referent | ||
| High School Diploma | 1.30 (0.98-1.72) | 1.11 (0.82-1.50) | 1.56 (0.99-2.45) | 1.26 (0.78-2.06) |
| More than High School | 1.07 (0.85-1.36) | 1.05 (0.78-1.40) | 1.03 (0.69-1.53) | 1.04 (0.64-1.70) |
| Family Income-to-Poverty Ratio | ||||
| 0.00-1.30 | Referent | Referent | ||
| 1.31-1.85 | 1.06 (0.79-1.41) | 1.19 (0.87-1.63) | 1.08 (0.65-1.80) | 1.40 (0.80-2.46) |
| 1.86-2.50 | 1.09 (0.73-1.61) | 1.17 (0.76-1.79) | 1.01 (0.61-1.68) | 1.29 (0.72-2.31) |
| >2.50 | 0.74 (0.58-0.95)* | 0.80 (0.57-1.13) | 0.55 (0.37-0.80)** | 0.75 (0.45-1.23) |
| Health Insurance Type | ||||
| Private | Referent | Referent | ||
| Medicare, Medicaid, or CHIP | 1.97 (1.49-2.60)** | 1.76 (1.25-2.47)** | 3.17 (2.15-4.68)** | 2.43 (1.46-4.04)** |
| Other Insurancef | 1.08 (0.76-1.55) | 1.07 (0.74-1.54) | 1.43 (0.89-2.32) | 1.40 (0.83-2.37) |
| No Insurance | 0.80 (0.57-1.11) | 0.64 (0.44-0.94)* | 0.96 (0.59-1.56) | 0.71 (0.42-1.22) |
| General Health Condition | ||||
| Excellent, Very Good, or Good | Referent | Referent | ||
| Fair or Poor | 3.27 (2.63-4.07)** | 3.24 (2.53-4.15)** | 6.11 (4.67-8.00)** | 5.45 (3.76-7.89)** |
| Ever Used Heroin g | ||||
| Yes | Referent | Referent | ||
| No | 1.71 (0.71-4.15) | 2.38 (0.97-5.86) | 2.02 (0.62-6.62) | 3.21 (0.96-10.69) |
| Refused, Don’t know, or Missing | 0.79 (0.34-1.84) | 1.36 (0.57-3.24) | 0.66 (0.21-2.03) | 1.49 (0.47-4.72) |
At least 1 opioid prescription reported as used within the last month. Category includes short-term (<90 days) and long-term use (≥90 days) but does not include MOUD use.
Unweighted sample counts
At least 1 opioid prescription reported as used for ≥ 90 days and if multiple opioids were reported the longest frequency was used. Participants with long-term use were compared to those with no opioid use (excluded participants with short term opioid use (N=290))
Bivariate logistic regressions conducted for each variable separately. Results presented as crude odds ratio (cOR) and 95% wald confidence intervals (95% CI)
Multivariate logistic regression adjusted for survey year, age, race, pregnancy status, education level, and income-to-poverty ratio, health insurance type, general health condition, and history of heroin use. Results presented as adjusted odds ratios (aOR) and 95% wald confidence intervals (95% CI)
Other insurance includes other types of government insurance and single service plans
Respondents answer to question “Have you ever, even once, used heroin?”
Boldface indicates statistical significance
p<0.05
p<.01
Similarly, women of reproductive age that were older (35-44 years old), with public health insurance (i.e., Medicaid, Medicare, or CHIP), and with a health status as fair or poor had a higher odds of long-term prescription opioid use (Table 2). Participants ages 35-44 had almost 4 times higher odds of long-term prescription opioid use compared to ages 15-24 (aOR: 3.7, 95% CI: 2.1-6.6, p<.01), Hispanic and Non-Hispanic Black women had 69% and 52% lower odds of long-term prescription opioid use compared to non-Hispanic White women (aOR: 0.3, 95% CI: 0.2-0.5, p<.01; aOR:0.5, 95% CI: 0.3-0.7, p<.01; respectively), and those with fair or poor health had 5 times higher odds compared to those with excellent, very good, or good health (aOR: 5.5, 95% CI: 3.8-7.9, p<.01) (Table 2). Lastly, respondents with public insurance (i.e., Medicaid, Medicare, or CHIP) had a higher odds of long-term prescription opioid use (aOR: 2.4, 95% CI: 1.5-4.0, p<.01) (Table 2).
Discussion
Using a nationally representative sample from 2003-2018, we estimated that the overall prevalence of prescription opioid use within the last 30 days among women of reproductive age was 5%. This finding is supported by evidence showing the prevalence of prescription opioid use was also 5% among all adult NHANES participants from 1999-2014 (Mojtabai, 2018). Long-term prescription opioid use did not significantly change but the prevalence of MOUD use significantly increased from 2006-2006 through 2011-2012. Our findings of a decrease in prescription opioid use are consistent with recent estimates among the general US population (Centers for Disease Control and Prevention, 2019). Previous studies concluded that long-term prescription opioid use significantly increased from 1999-2014 (Mojtabai, 2018); however, we did not find a significant change in prevalence from 2003-2018. This difference may be due to implementation of a more rigorous NCHS methodology for trend analysis and/or exclusion of males and women of non-reproductive age. Few studies have estimated past-month prescription opioid use among women of reproductive age using a nationally representative sample, and therefore, our study is an important contribution to the literature.
We identified subgroups of women that had a higher odds of prescription opioid use and long-term use. Consistent with previous studies, the strongest predictors of prescription opioid use were a health status of fair or poor, older age, non-Hispanic White, and public health insurance (Ailes et al., 2015). These findings are also supported by studies showing privately insured women are less likely to use opioid prescriptions (Darnall and Stacey, 2012; Shah A, 2017). Samples for previous studies that identified predictors of opioid use were limited to women in certain health insurance systems and did not assess duration of opioid use. However, we found that the predictors of long-term opioid use were the same as those for general prescription opioid use among women of reproductive age. Our findings that long-term opioid use was most common among older women with public health insurance is consistent with a national study of US adults (Shah A, 2017).
The decreasing prevalence of prescription opioid use suggests changes in opioid prescribing guidelines, risk-assessment tools and policy interventions, such as the Prescription Drug Monitoring Program (PDMP), may have successfully reduced prescription opioid use among women of reproductive age (Grecu et al., 2019; Olfson et al., 2020). By 2014, 49 states implemented a PDMP which may explain the sharp decrease in prescription opioid use in 2015-2016 demonstrated in our study (Grecu et al., 2019). This hypothesis is supported by a recent national study that found mandatory state PDMP policies were associated with decreases in prescription opioid use (Lee et al., 2021). Changes in provider prescribing practices may also partially explain the decrease because most US healthcare providers were within recommended thresholds for opioid prescribing in 2017 (Kiang et al., 2020). However, broad policies focused on restricting prescription opioid access may have led to unintended consequences as PDMP policies have been associated with overdose deaths due to non-prescription opioids (Lee et al., 2021). Reductions in opioid prescribing should be balanced with increased availability of nonopioid therapies and improved monitoring for OUD (Olfson et al., 2020).
Our trend analysis demonstrated a decrease in long-term prescription opioid use after 2014 but the change was not statistically significant. However, we identified an alarmingly high mean duration of prescription opioid use among long-term users of 3.7 years. Given this and evidence from previous studies of an increasing prevalence of long-term opioid use, more attention should be given by clinicians and policy makers to the sub-groups of women at higher risk for long-term prescription opioid use identified by our study (Centers for Disease Control and Prevention, 2019; Mojtabai, 2018). Also, the 2017 study of opioid prescribing practices found that the top 1% of providers were responsible for 49% of all opioid doses (Kiang et al., 2020). Future research should identify providers most likely to prescribe opioids for long durations, alternative treatment options, and systematic barriers to providing evidence-based treatment for pain.
While our study results of MOUD use should be interpreted with caution due to the low sample size, our study found a considerably low prevalence of past-month MOUD use and no reported use among pregnant women. We found a significantly increasing trend of MOUD use from 2005-2006 through 2011-2012 but an insignificant decreasing trend from 2013-2014 through 2017-2018. Our findings are not consistent with results from the National Survey on Drug Use and Health (NSDUH) that MOUD use among women increased from 2016-2018 which may be related to social desirability bias of participants reporting MOUD use (Substance Abuse and Mental Health Services Administration, 2020). The low prevalence of MOUD use from 2015-2016 through 2017-2018 is concerning since previous studies show the prevalence of OUD among women of reproductive age has increased substantially (Gabrielson et al., 2020; Haight et al., 2018; Hirai et al., 2021; Patrick et al., 2015; Tolia et al., 2015). Previous studies have found significant barriers to medication treatment for OUD for women in the US (Guttmacher Institute, 2021; Hodgins et al., 2019; Klaman et al., 2017; Patrick et al., 2020; The American College of Obstetricians and Gynecologists, 2017). MOUD reduces the risk of overdose and in many states, pregnancy-associated and postpartum opioid related overdoses have significantly increased since 2010 (Gemmill et al., 2019; Nielsen et al., 2020). Improving access to MOUD and treatment services for women of reproductive age should be a public health priority to improve the health of women and families.
This study is subject to some limitations. First, prescription opioids were only reported if taken within the last 30 days. Prescriptions were self-reported during an in-person interview so there may be social-desirability bias, especially for MOUDs. Also, 19% of prescription opioids and 30% of MOUD were not verified by the survey respondent and may have been obtained by a source other than a doctor. However, we were not able to determine prescription opioid misuse, reasons for taking prescription opioids, or illicit use of opioids. Also, the low number of participants reporting long-term opioid use and MOUD use limited our ability to draw conclusions and the generalizability of our findings. We also excluded participants with missing covariate data (n=132) and found they were more likely to be younger, Hispanic or non-Hispanic other, and have lower income than those included in the study. Lastly, NHANES does not include institutionalized populations and is a highly clustered sample. However, use of eight cycles of data and complex survey weights provides an essential update on the national prevalence and trend of prescription opioid use and MOUD use among women of reproductive age. Many studies of prescription opioid use have been conducted using claims data so the inclusion of participants with all insurance types is an important strength of our study. Lastly, we used a rigorous trend analysis methodology outlined by the NCHS to determine the trend of prescription opioid use using aggregated data(Ingram DD, 2018).
Conclusion
Using a nationally representative sample of women of reproductive age in the US, our study provides an important update on the prevalence and trend of prescription opioid use. While any prescription opioid use significantly declined from 2003-2018, we found that MOUD increased significantly from 2005-2006 through 2011-2012 and long-term opioid use did not significantly change over time. As policy makers and clinicians strive to reduce the negative impacts of the opioid epidemic on women and children, they should consider the demographic groups most likely to use prescription opioids long-term and determine mechanisms to improve access to nonopioid therapies and treatment for OUD.
Highlights:
Prescription opioid use decreased from 2003 to 2018 among women of reproductive age
Long-term prescription opioid use remained stable from 2003 to 2018
Use of medications for opioid use disorder increased from 2005 to 2012
Women with public insurance had a higher odds of long-term prescription opioid use
Acknowledgements:
This work was supported in part by the National Institutes of Health (NIH-NIGMS) grant number T32-GM081740; and the Susan G. Komen training grant (GTDR17500160). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIGMS or NIH.
Appendix
Appendix Table 1:
Trend Analysis for Prescription Opioid Use, Long-term Prescription Opioid use, and Medications for Opioid Use Disorder among Women of Reproductive Age: NHANES 2003-2018
| Parameter Estimates for Joinpoint Model Fit Using NCI’s Joinpoint Software |
||||
|---|---|---|---|---|
| Percentage changea |
Slope | Standard error | P valueb | |
| Any Prescription Opioid Use | ||||
| 2003-2004 through 2017-2018 | −9.16 | −0.10 | 0.03 | 0.01 |
| Long-term Prescription Opioid Use | ||||
| Trend 1 (2003-2004 through 2013-2014) | 2.55 | 0.03 | 0.04 | 0.56 |
| Trend 2 (2013-2014 through 2017-2018) | −19.89 | −0.22 | 0.26 | 0.45 |
| Medications for Opioid Use Disorder c | ||||
| Trend 1 (2005-2006 through 2011-2012) | 129.68 | 0.83 | 0.16 | 0.03 |
| Trend 2 (2011-2012 through 2017-2018) | 31.51 | −0.38 | 0.12 | 0.09 |
Percentage change in the prevalence of prescription opioid or medication use per two years (survey cycle)
P value <0.05 indicates that the percentage change is significantly different from zero
Survey wave 2003-2004 had 0 observations and was excluded from joinpoint regression
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ailes EC, Dawson AL, Lind JN, Gilboa SM, Frey MT, Broussard CS, Honein MA, Centers for Disease, C., Prevention, 2015. Opioid prescription claims among women of reproductive age--United States, 2008-2012. MMWR Morb Mortal Wkly Rep 64:37–41. [PMC free article] [PubMed] [Google Scholar]
- Alderks CE, 2017. Trends in the Use of Methadone, Buprenorphine, and Extended-release Naltrexone at Substance Abuse Treatment Facilities: 2003-2015 (Update), The CBHSQ Report: August 22, 2017, Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration, Rockville, MD. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, 2007. National Health and Nutrition Examination Survey, Data Documentation, Codebook, and Frequencies-1988-2018, https://wwwn.cdc.gov/Nchs/Nhanes/1999-2000/RXQ_DRUG.htm.
- Centers for Disease Control and Prevention, 2018. U.S. Opioid Prescribing Rate Maps. [Google Scholar]
- Centers for Disease Control and Prevention, 2019. 2019 Annual Surveillance Report of Drug-Related Risks and Outcomes — United States Surveillance Special Report. Centers for Disease Control and Prevention, U.S. Department of Health and Human Services, https://www.cdc.gov/drugoverdose/pdf/pubs/2019-cdc-drug-surveillance-report.pdf. [Google Scholar]
- Centers for Disease Control and Prevention, N.C.f.H.S., 2020. Reproductive Health, https://www.cdc.gov/nchs/fastats/reproductive-health.htm.
- Clemans-Cope L, Lynch V, Howell E, Hill I, Holla N, Morgan J, Johnson P, Cross-Barnet C, Thompson JA, 2019. Pregnant women with opioid use disorder and their infants in three state Medicaid programs in 2013-2016. Drug Alcohol Depend 195:156–63. [DOI] [PubMed] [Google Scholar]
- Darnall BD, Stacey BR, 2012. Sex Differences in Long-term Opioid Use: Cautionary Notes for Prescribing in Women. JAMA Internal Medicine 172:431–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Department of Health and Human Services, 2019. What is the U.S Opioid Epidemic?, About the Epidemic, https://www.hhs.gov/opioids/about-the-epidemic/index.html. [Google Scholar]
- Dowell D, Haegerich TM, Chou R, 2016. CDC Guideline for Prescribing Opioids for Chronic Pain--United States, 2016. JAMA 315:1624–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finer LB, Zolna MR, 2016. Declines in Unintended Pregnancy in the United States, 2008-2011. N Engl J Med 374:843–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrielson SMB, Carwile JL, O'Connor AB, Ahrens KA, 2020. Maternal opioid use disorder at delivery hospitalization in a rural state: Maine, 2009-2018. Public Health 181:171–79. [DOI] [PubMed] [Google Scholar]
- Gemmill A, Kiang MV, Alexander MJ, 2019. Trends in pregnancy-associated mortality involving opioids in the United States, 2007-2016. Am J Obstet Gynecol 220:115–16. [DOI] [PubMed] [Google Scholar]
- Grecu AM, Dave DM, Saffer H, 2019. Mandatory Access Prescription Drug Monitoring Programs and Prescription Drug Abuse. J Policy Anal Manage 38:181–209. [PubMed] [Google Scholar]
- Guttmacher Institute, 2021. Substance Use During Pregnancy, State Laws and Policies, https://www.guttmacher.org/state-policy/explore/substance-use-during-pregnancy.
- Guy GP Jr., Zhang K, Bohm MK, Losby J, Lewis B, Young R, Murphy LB, Dowell D, 2017. Vital Signs: Changes in Opioid Prescribing in the United States, 2006-2015. MMWR Morb Mortal Wkly Rep 66:697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haight SC, Ko JY, Tong VT, Bohm MK, Callaghan WM, 2018. Opioid Use Disorder Documented at Delivery Hospitalization - United States, 1999-2014. MMWR Morb Mortal Wkly Rep 67:845–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil SH, Jones HE, Arria A, Kaltenbach K, Coyle M, Fischer G, Stine S, Selby P, Martin PR, 2011. Unintended pregnancy in opioid-abusing women. J Subst Abuse Treat 40:199–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai AH, Ko JY, Owens PL, Stocks C, Patrick SW, 2021. Neonatal Abstinence Syndrome and Maternal Opioid-Related Diagnoses in the US, 2010-2017. JAMA 325:146–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgins FE, Lang JM, Malseptic GG, Melby LH, Connolly KA, 2019. Coordinating Outpatient Care for Pregnant and Postpartum Women with Opioid Use Disorder: Implications from the COACHH Program. Matern Child Health J 23:585–91. [DOI] [PubMed] [Google Scholar]
- Ingram DD, M.D., Makuc DM, Kruszon-Moran D, Gindi RM, Albert M., 2018. National Center for Health Statistics Guidelines for Analysis of Trends, Vital Health Statistics, https://www.cdc.gov/nchs/data/series/sr_02/sr02_179.pdf. [PubMed]
- Johnson AJ, Jones CW, 2018. Opioid Use Disorders and Pregnancy. Obstet Gynecol Clin North Am 45:201–16. [DOI] [PubMed] [Google Scholar]
- Kiang MV, Humphreys K, Cullen MR, Basu S, 2020. Opioid prescribing patterns among medical providers in the United States, 2003-17: retrospective, observational study. BMJ 368:l6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaman SL, Isaacs K, Leopold A, Perpich J, Hayashi S, Vender J, Campopiano M, Jones HE, 2017. Treating Women Who Are Pregnant and Parenting for Opioid Use Disorder and the Concurrent Care of Their Infants and Children: Literature Review to Support National Guidance. J Addict Med 11:178–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko JY, D'Angelo DV, Haight SC, Morrow B, Cox S, Salvesen von Essen B, Strahan AE, Harrison L, Tevendale HD, Warner L, Kroelinger CD, Barfield WD, 2020. Vital Signs: Prescription Opioid Pain Reliever Use During Pregnancy - 34 U.S. Jurisdictions, 2019. MMWR Morb Mortal Wkly Rep 69:897–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, Zhao W, Yang KC, Ahn YY, Perry BL, 2021. Systematic Evaluation of State Policy Interventions Targeting the US Opioid Epidemic, 2007-2018. JAMA Netw Open 4:e2036687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda A, Bateman BT, Clancy CR, Creanga AA, Leffert LR, 2014. Opioid abuse and dependence during pregnancy: temporal trends and obstetrical outcomes. Anesthesiology 121:1158–65. [DOI] [PubMed] [Google Scholar]
- Mojtabai R, 2018. National trends in long-term use of prescription opioids. Pharmacoepidemiol Drug Saf 27:526–34. [DOI] [PubMed] [Google Scholar]
- National Institute on Drug Abuse, 2018. Medications to Treat Opioid Use Disorder, https://www.drugabuse.gov/download/21349/medications-to-treat-opioid-use-disorder-research-report.pdf?v=99088f7584dac93ddcfa98648065bfbe.
- Nielsen T, Bernson D, Terplan M, Wakeman SE, Yule AM, Mehta PK, Bharel M, Diop H, Taveras EM, et al. , 2020. Maternal and infant characteristics associated with maternal opioid overdose in the year following delivery. Addiction 115:291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olfson M, Wang S, Wall MM, Blanco C, 2020. Trends In Opioid Prescribing And Self-Reported Pain Among US Adults. Health Aff (Millwood) 39:146–54. [DOI] [PubMed] [Google Scholar]
- Patrick SW, Davis MM, Lehmann CU, Cooper WO, 2015. Increasing incidence and geographic distribution of neonatal abstinence syndrome: United States 2009 to 2012. J Perinatol 35:650–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick SW, Richards MR, Dupont WD, McNeer E, Buntin MB, Martin PR, Davis MM, Davis CS, Hartmann KE, et al. , 2020. Association of Pregnancy and Insurance Status With Treatment Access for Opioid Use Disorder. JAMA Netw Open 3:e2013456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd RA, Seth P, David F, Scholl L, 2016. Increases in Drug and Opioid-Involved Overdose Deaths - United States, 2010-2015. MMWR Morb Mortal Wkly Rep 65:1445–52. [DOI] [PubMed] [Google Scholar]
- Shah A, H.C., Martin BC, 2017. Characteristics of Initial Prescription Episodes and Likelihood of Long-Term Opioid Use — United States, 2006–2015, March 17, 2017 ed. MMWR Morb Mortal Wkly Rep, pp. 265–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration, 2016. A Collaborative Approach to the Treatment of Pregnant Women with Opioid Use Disorders, in: Substance Abuse and Mental Health Services Administration (Ed.). Substance Abuse and Mental Health Services Administration, Rockville, MD. [Google Scholar]
- Substance Abuse and Mental Health Services Administration, 2018. Clinical Guidance for Treating Pregnant and Parenting Women With Opioid Use Disorder and Their Infants. HHS Publication No. (SMA) 18–5054, Rockville, MD. [Google Scholar]
- Substance Abuse and Mental Health Services Administration, 2020. 2018 National Survey on Drug Use and Health: Women. U.S. Department of Health and Human Services, https://www.samhsa.gov/data/sites/default/files/reports/rpt23250/5_Women_2020_01_14.pdf. [PubMed] [Google Scholar]
- Terplan M, Longinaker N, Appel L, 2015. Women-Centered Drug Treatment Services and Need in the United States, 2002-2009. Am J Public Health 105:e50–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The American College of Obstetricians and Gynecologists, 2017. Opioid Use and Opioid Use Disorder in Pregnancy, Committee Opinion 711, Obstet Gynecol 2017, pp. 130: e81–94. [DOI] [PubMed] [Google Scholar]
- Tolia VN, Patrick SW, Bennett MM, Murthy K, Sousa J, Smith PB, Clark RH, Spitzer AR, 2015. Increasing incidence of the neonatal abstinence syndrome in U.S. neonatal ICUs. N Engl J Med 372:2118–26. [DOI] [PubMed] [Google Scholar]
- Wilson N, Kariisa M, Seth P, Smith H.t., Davis NL, 2020. Drug and Opioid-Involved Overdose Deaths - United States, 2017-2018. MMWR Morb Mortal Wkly Rep 69:290–97. [DOI] [PMC free article] [PubMed] [Google Scholar]


