We are experiencing an unprecedented growth in the number of older adults diagnosed with, and surviving from, cancer. By 2030, 70% of patients with cancer will be 65 years or older, and cancer incidence in this group will have increased nearly 70% from 2010 to 2030.1 It is estimated that there are currently 17 million cancer survivors alive in the United States and 64% are 65 years or older.2 By 2040, it is expected that the number of cancer survivors will grow to 26 million reflecting 73% of those 65 years and older and almost 50% of those over 75. Despite this substantial growth in the number of older cancer survivors, the impact of cancer and its treatment on older adults’ health, functional status, and underlying aging processes is not well understood.
In this issue of the Journal of the American Geriatrics Society, Siddique, et al. present data about the long-term impact of cancer on an individual’s physical function.3 Siddique, et al. evaluated the trajectory of functional status using three clinical measures (grip strength measured using a hydraulic handheld dynamometer, gait speed measured twice over a 6-m course, and Health, Aging, and Body Composition physical performance battery) over time. They compared these observations in subjects with and without a history of cancer. The authors found that cancer history was associated with 1.4-fold greater odds of a weak grip strength, and that the combination of older age (>65 years) and cancer history was associated with a 1.6-fold greater odds of slow gait speed and 0.11-unit lower physical performance than in younger (<65 years) cancer-free adults. Additionally, older individuals with history of cancer experienced steeper declines in grip strength and gait speed compared to cancer-free older adults. The authors concluded that the additive effect of cancer and aging appear to accelerate the onset and progression of functional decline.
While this study raises as many questions as it answers, it is one of the first glimpses into the impact of cancer on the aging trajectories in older adults. Even though older adults represent most individuals with cancer, they are severely underrepresented in clinical trials and research.4 Hence, most evidence that sets the standard of care for oncology treatment and survivorship is derived from studies of younger individuals. Only 5% of NIH-funded survivorship research specifically focuses on older adults.5 Additionally, cancer clinical trials to date primarily report oncological outcomes, such as overall survival and disease-free survival. However, health outcomes salient to the older adult population, including the impact of treatment on age-related comorbidities, function, and cognition, are lacking. Moreover, patients in cancer trials are typically not followed into their survivorship years when the long-term side effects of cancer therapy may become apparent. Hence, the ability to advance knowledge of the long-term effects of cancer on aging trajectories has been constrained. We applaud Siddiqui, et al. for taking advantage of a robust epidemiologic database with rich longitudinal data to compare the aging trajectories in survivors of adult-onset cancers with natural aging among cancer-free peers of the same age.
The research by Siddiqui, et al. highlights the interaction between cancer and aging. Aging is the leading risk factor for the development of cancer, yet how cancer and its treatment impact aging has received little attention. This is important because cancer survivors are living longer due to remarkable advances in early detection, treatment, and supportive care. Indeed, nearly 70% of cancer survivors will be alive 5 years or more from diagnosis and 18% will survive at least 20 years or longer.6 Yet with this great step forward, a significant problem has emerged - treatments that cure or control cancer can adversely accelerate the rate of fundamental aging processes in cancer survivors, manifesting as earlier onset and higher incidence of chronic health conditions compared to the general population.7–10
Emerging evidence suggests that these cancer survivors are at risk for accelerated or premature aging. Nearly two thirds of the 19 million cancer survivors today have at least one additional chronic health condition.11 Some of these conditions predate the cancer diagnosis (older cancer survivors may already have preexisting comorbidities), but are likely to be exacerbated by cancer and its treatment. Other conditions are new, or occur as a direct result of cancer and exposure to cancer treatment. Either way, persons with cancer or who have survived cancer are at risk for early onset of physiologic frailty, a phenotype characterized by reduced physiologic reserve and an accumulation of deficits, a sign of accelerated aging.
Most of the existing evidence for an accelerated aging state in cancer survivors comes from the pediatric literature. Numerous studies have shown that survivors of childhood cancers are more predisposed to the development of frailty12 as well as chronic health conditions such as cardiovascular disease13 and second cancers.14 At a median age of only 33 years (range 18–50), 8% of survivors of childhood cancers are frail, an additional 22% are pre-frail, rates seen in older adults.12 By age 50, a survivor of childhood cancers, on average, experiences >4 severe or life-threatening chronic conditions. These findings suggest that cancer and/or interventions to treat cancers increase risk for subsequent development of geriatric syndromes and multimorbidity, suggesting an accelerated aging state.7, 8
There is a smaller yet growing body of literature pointing towards similar findings in the geriatric population. Unlike children and young adults, older adults diagnosed with cancer usually carry a high burden of comorbid health conditions at diagnosis, making it a challenge to disentangle the contributions of aging and cancer as well as its treatment on posttreatment health-related outcomes. However, several studies, like the one in this issue by Siddiqui, et al., have shown that survivors of adult-onset cancers have a higher burden of frailty,15 mobility limitations,16 comorbid conditions,17 and fatigue,18 as well as greater risk of physical19 and cognitive impairment,20 compared to cancer-free, age-matched controls.
Understanding the biologic mechanisms driving the accelerated aging state in cancer survivors may point to targeted interventions to disrupt these processes. The emergence of accelerated aging phenotypes could be due, in part to the impact of cytotoxic and genotoxic treatments on normal cells.8, 21 Chemotherapeutic agents and radiation directly impact fundamental aging mechanisms (e.g., cellular senescence, inflammation, macromolecular/organelle dysfunction, stem cell and progenitor dysfunction) [Figure 1]. For example, chemotherapeutic agents and radiation induce cellular senescence, a cell state in which cells undergo growth arrest.21–26 Therapy-induced senescence (TIS) in tumor cells halts cancer growth. TIS in normal cells disrupts normal tissue. Senescent cells can secrete proinflammatory factors, collectively termed the senescence-associated secretory phenotype (SASP), which induce formation of other senescent cells and cause surrounding tissue damage. Senescence is linked to aging and frailty; common cellular stresses yield senescent cells that accumulate in various tissues over time and contribute to age-related tissue dysfunction. Transplantation of a small number of senescent cells into young animals induces frailty, and when these senescent cells are removed frailty is reversed.22, 24 Treatments that reduce senescent cells are now being tested in clinical trials, including adult survivors of childhood cancers (NCT04733534) and patients who have had bone marrow transplantation (NCT02652052). However, apart from these bone marrow transplant survivors, none to date are being tested in older cancer survivors that we are aware of. Targeted reduction of cellular senescence with senolytic agents represents a critical opportunity to ultimately reduce premature cellular aging and the risk for early-onset frailty and chronic health conditions that commonly occur in cancer survivors.27–30 Further work is warranted to better understand accelerated aging-like states and understand the mechanisms underlying them to develop alternative therapies (e.g., senolytics) and lifestyle interventions (e.g., exercise and diet) to prevent late complications and improve the health and wellbeing of cancer survivors.
Figure 1. Cancer therapeutics directly impact fundamental aging mechanisms.
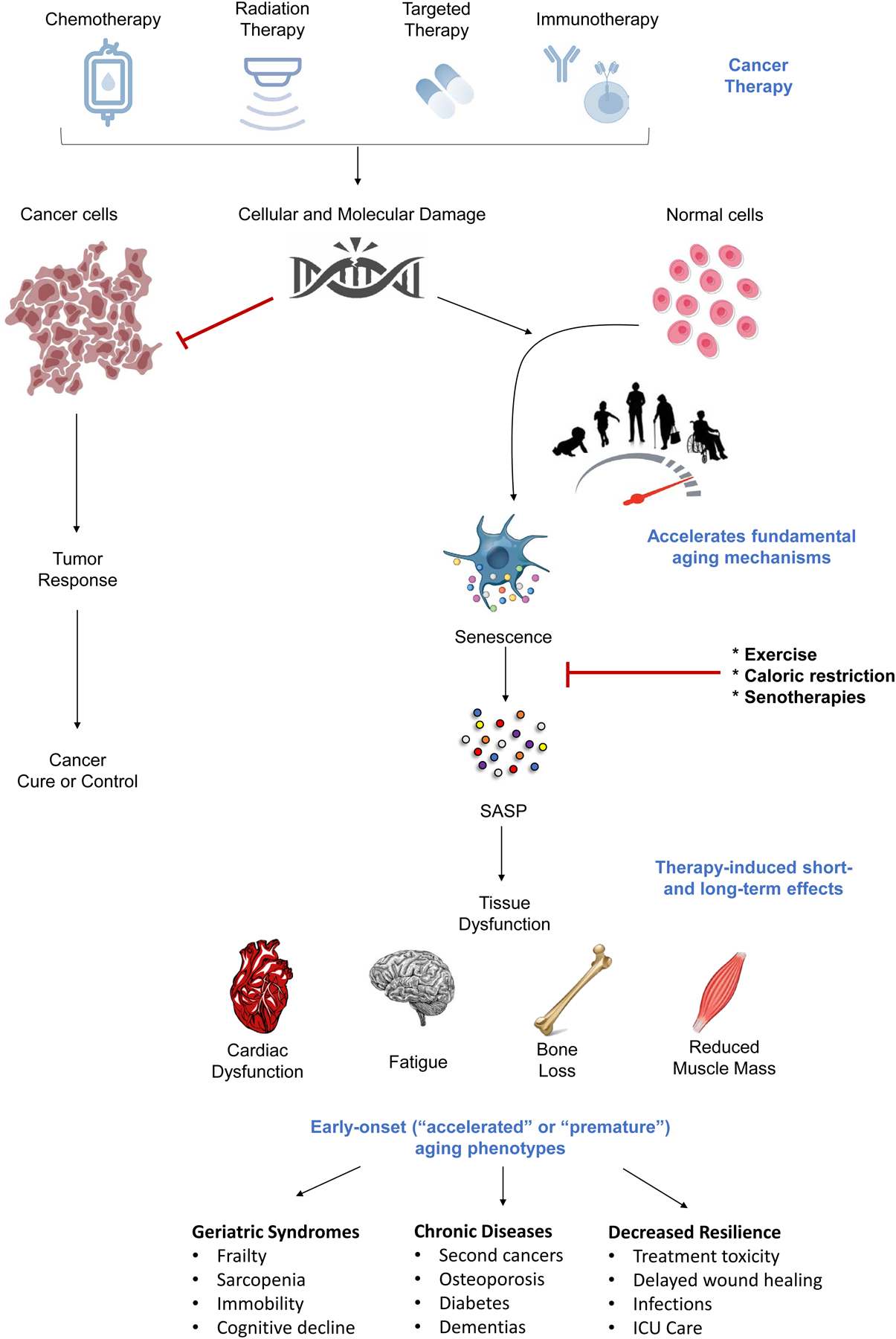
Cancer therapy is effective in inhibiting cancer progression but can induce accelerated or premature cellular aging in normal cells. Cancer therapy (e.g., chemotherapy) leads to cancer cell death. Cancer therapy also causes damage to normal cells through many of the same mechanisms that are thought to underlie the fundamental biological aging process (e.g., cellular senescence). Senescent cells can secrete a collection of proinflammatory factors collectively termed the senescence-associated secretory phenotype (SASP). SASP factors drive tissue dysfunction and can contribute to many of short- and long-term cancer treatment side effects, including cardiac dysfunction, fatigue, bone loss, and physical decline. The accumulation and persistence of therapy-induced senescent cells can promote the early onset of various age-related syndromes, chronic health conditions, and decreased resilience, suggesting an accelerated aging state. Exercise, diet, and pharmacological interventions (e.g., senotherapies) that can eliminate senescent cells or inhibit SASP production may alleviate these negative effects and represent novel strategies to prevent, mitigate, or reverse the adverse aging process-related effects of cancer treatments.
As the number of cancer survivors is growing because of the ever-improving cancer treatments, focus on treatment-induced accelerated aging has become even more imperative. Oncologists, geriatricians, and primary care providers will be challenged to provide timely and appropriate post-treatment care to a diverse population of older cancer survivors. The work by Siddique, et al. is an example of the type of research that is needed to fill gaps in knowledge around the survivorship issues facing older adults with cancer. However, this is just the beginning and many questions are yet to be fully answered. Studies on the pivotal molecular pathways shared between cancer and aging are needed to discover novel targets for future interventions that prevent, slow, or reverse the age-related toxicities of cancer and its treatment.
ACKNOWLEDGMENTS
The authors would like to acknowledge the support received from NIH grants R03 AG064377 (PI: Mina S. Sedrak); P01 AG62413 (PIs: James L. Kirkland, Sundeep Khosla); R33 AG61456 (Translational Geroscience Network; PI: James L. Kirkland, George A. Kuchel, Stephen B. Kritchevsky, Tamara Tchkonia); R37 AG13925 (PI: James L. Kirkland).
Footnotes
Conflict of Interest: Patents on senolytic drugs and their uses are held by Mayo Clinic.
REFERENCES
- 1.Bluethmann SM, Mariotto AB, Rowland JH: Anticipating the “Silver Tsunami”: Prevalence Trajectories and Comorbidity Burden among Older Cancer Survivors in the United States. Cancer Epidemiol Biomarkers Prev 25:1029–1036, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Office of Cancer Survivorship: Statistics, Graphs and Definitions [Internet]. Stat Graphs Defin [cited 2021 Aug 23] Available from: https://cancercontrol.cancer.gov/ocs/statistics [Google Scholar]
- 3.Siddique A, Simonsick EM, Gallicchio L: Functional decline among older cancer survivors in the Baltimore longitudinal study of aging. J Am Geriatr Soc jgs.17369, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sedrak MS, Freedman RA, Cohen HJ, et al. : Older adult participation in cancer clinical trials: A systematic review of barriers and interventions. CA Cancer J Clin 71:78–92, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacobsen PB, Rowland JH, Paskett ED, et al. : Identification of Key Gaps in Cancer Survivorship Research: Findings From the American Society of Clinical Oncology Survey. J Oncol Pract 12:190–193, 2016 [DOI] [PubMed] [Google Scholar]
- 6.Miller KD, Nogueira L, Mariotto AB, et al. : Cancer treatment and survivorship statistics, 2019. CA CANCER J CLIN 69:24, 2019 [DOI] [PubMed] [Google Scholar]
- 7.Ness KK, Kirkland JL, Gramatges MM, et al. : Premature Physiologic Aging as a Paradigm for Understanding Increased Risk of Adverse Health Across the Lifespan of Survivors of Childhood Cancer. J Clin Oncol 36:2206–2215, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cupit-Link MC, Kirkland JL, Ness KK, et al. : Biology of premature ageing in survivors of cancer. ESMO Open 2:e000250, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Armenian SH, Gibson CJ, Rockne RC, et al. : Premature Aging in Young Cancer Survivors. JNCI J Natl Cancer Inst 111:226–232, 2019 [DOI] [PubMed] [Google Scholar]
- 10.Guida JL, Ahles TA, Belsky D, et al. : Measuring Aging and Identifying Aging Phenotypes in Cancer Survivors. JNCI J Natl Cancer Inst 111:1245–1254, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ness KK, Wogksch MD: Frailty and aging in cancer survivors. Transl Res 221:65–82, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ness KK, Armstrong GT, Kundu M, et al. : Frailty in childhood cancer survivors: Frailty in Childhood Cancer Survivors. Cancer 121:1540–1547, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oeffinger KC, Kawashima T, Friedman DL, et al. : Chronic Health Conditions in Adult Survivors of Childhood Cancer. N Engl J Med 11, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Henderson TO, Moskowitz CS, Chou JF, et al. : Breast Cancer Risk in Childhood Cancer Survivors Without a History of Chest Radiotherapy: A Report From the Childhood Cancer Survivor Study. J Clin Oncol, 2016, 20;34(9):910–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoogendijk EO, Afilalo J, Ensrud KE, et al. : Frailty: implications for clinical practice and public health. The Lancet 394:1365–1375, 2019 [DOI] [PubMed] [Google Scholar]
- 16.Keating NL, Nørredam M, Landrum MB, et al. : Physical and Mental Health Status of Older Long-Term Cancer Survivors: HEALTH STATUS OF CANCER SURVIVORS. J Am Geriatr Soc 53:2145–2152, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Alfano CM, Peng J, Andridge RR, et al. : Inflammatory Cytokines and Comorbidity Development in Breast Cancer Survivors Versus Noncancer Controls: Evidence for Accelerated Aging? J Clin Oncol 35:149–156, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gresham G, Dy SM, Zipunnikov V, et al. : Fatigability and endurance performance in cancer survivors: Analyses from the Baltimore Longitudinal Study of Aging: Fatigability in Cancer Survivors. Cancer 124:1279–1287, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petrick JL, Reeve BB, Kucharska-Newton AM, et al. : Functional status declines among cancer survivors: Trajectory and contributing factors. J Geriatr Oncol 5:359–367, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mandelblatt JS, Small BJ, Luta G, et al. : Cancer-Related Cognitive Outcomes Among Older Breast Cancer Survivors in the Thinking and Living With Cancer Study. J Clin Oncol 36:3211–3222, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wyld L, Bellantuono I, Tchkonia T, et al. : Senescence and Cancer: A Review of Clinical Implications of Senescence and Senotherapies. Cancers 12:2134, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu M, Pirtskhalava T, Farr JN, et al. : Senolytics improve physical function and increase lifespan in old age. Nat Med 24:1246–1256, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prasanna PG, Citrin DE, Hildesheim J, et al. : Therapy-Induced Senescence: Opportunities to Improve Anticancer Therapy. JNCI J Natl Cancer Inst djab064, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Demaria M, O’Leary MN, Chang J, et al. : Cellular Senescence Promotes Adverse Effects of Chemotherapy and Cancer Relapse. Cancer Discov 7:165–176, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chandra A, Lagnado AB, Farr JN, et al. : Targeted Reduction of Senescent Cell Burden Alleviates Focal Radiotherapy-Related Bone Loss. J Bone Miner Res 35:1119–1131, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang B, Kohli J, Demaria M: Senescent Cells in Cancer Therapy: Friends or Foes? Trends Cancer 6:838–857, 2020 [DOI] [PubMed] [Google Scholar]
- 27.Guida JL, Agurs-Collins T, Ahles TA, et al. : Strategies to Prevent or Remediate Cancer and Treatment-Related Aging. JNCI J Natl Cancer Inst 113:112–122, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tchkonia T, Kirkland JL: Aging, Cell Senescence, and Chronic Disease: Emerging Therapeutic Strategies. JAMA 320:1319, 2018 [DOI] [PubMed] [Google Scholar]
- 29.Tchkonia T, Palmer AK, Kirkland JL: New Horizons: Novel Approaches to Enhance Healthspan Through Targeting Cellular Senescence and Related Aging Mechanisms. J Clin Endocrinol Metab 106:e1481–e1487, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kirkland JL, Tchkonia T: Senolytic drugs: from discovery to translation. J Intern Med 288:518–536, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]


