Abstract
Background/Aims
Many cancer survivors who received intensive treatment such as hematopoietic stem cell transplantation (HCT) experience posttraumatic stress disorder (PTSD) symptoms. PTSD is associated with lower quality of life and other symptoms that require clinical treatment. The iterative treatment decisions that happen in clinical practice are not adequately represented in traditional randomized controlled trials (RCT) of PTSD treatments. The proposed stepped-care SMART design allows for evaluation of initial response to the Cancer Distress Coach mobile app; adaptive stepped-care interventions; and precision treatment strategies that tailor treatment selection to patient characteristics.
Methods/Design
HCT survivors (N=400) reporting PTSD symptoms are being recruited at two cancer centers and randomly assigned to: 1) Cancer Distress Coach app or 2) Usual Care. The app includes educational and cognitive behavioral therapy (CBT)-based activities. Four weeks post-randomization, participants re-rate their PTSD symptoms and, based on intervention response, non-responders are re-randomized to receive video-conferenced sessions with a therapist: 3) coaching sessions in using the mobile app; or 4) CBT specific to HCT survivors. Participants complete outcome measures of PTSD, depression, and anxiety after Months 1, 3, and 6. Participant characteristics moderating intervention responses will be examined.
Conclusions
This novel adaptive trial design will afford evidence that furthers knowledge about optimizing PTSD interventions for HCT survivors. To our knowledge, this study is the first SMART design evaluating PTSD symptom management in cancer survivors. If successful, it could be used to optimize treatment among a range of cancer and other trauma survivors.
Keywords: SMART, novel trial designs, HCT, PTSD, mCBT, symptom management, mHealth, mobile apps
Introduction
Cancer survivors suffer from posttraumatic stress disorder (PTSD) symptoms as a result of their treatment more often than found in the general population. [1], [2] At highest risk for PTSD symptoms are cancer survivors who received hematopoietic cell transplantation (HCT) as part of an aggressive cancer therapy.[3]–[5] HCT is a physically and psychologically challenging treatment in which the patient’s immune system is suppressed using high doses of chemotherapy and/or radiation therapy, a procedure that places tremendous strain on patients and may lead to prolonged hospitalizations and significant treatment-related morbidity (e.g., infection, social isolation) and mortality. Approximately 20,000 cancer patients, most of who are diagnosed with a blood cancer, undergo HCT each year in hopes of long-term remission or cure. [6] Steep deteriorations in quality of life (QOL) and depression, anxiety, and PTSD symptoms have been reported in HCT patients. [7] PTSD symptoms such as nightmares, avoiding reminders of cancer, and heightened arousal are reported in 41% of HCT survivors up to 10 years post-transplant. [8]–[13] As our prior work demonstrates, we found among our lymphoma cohort that one in two who received a HCT were PTSD symptomatic. [1], [3], [14] In addition, about 20% of HCT survivors meet criteria for full PTSD. [15], [16]
Without treatment, PTSD symptoms may impair long term physical and mental health outcomes.[1], [15] PTSD treatment using traditional office-based cognitive behavioral therapies (CBTs) are effective,[2] yet survivors often lack of access due to cost and distance. Mobile health (mHealth) applications (apps) increasingly provide alternative treatment approaches to facilitate access to CBT.[17], [18] Some apps are self-guided and others require individualized, more intensive videoconferenced sessions with therapists. Thus, a wide range of mHealth CBT-based (mCBT) solutions with differing levels of intensity exist to support cancer survivors with PTSD symptoms.
On the continuum of mHealth solutions are three digital health interventions that have demonstrated reductions in PTSD symptoms in separate studies and are being administered in our trial. (1) First, use of the Cancer Distress Coach (CaDC) app was associated with significantly reduced PTSD symptoms after 4 weeks among 30 lymphoma, breast, and prostate cancer survivors.[19] (2) Second, with the addition of manualized clinician support (CS), the CS-PTSD Coach app facilitated uptake and led to significant improvements in PTSD symptoms in primary care patients after 8 weeks.[20] (3) Finally, a telephone-based CBT intervention for HCT survivors (1–3 years post-transplant) that includes education about cancer-related PTSD, self-monitoring and alteration of maladaptive beliefs, guided exposure to cues associated with PTSD, and relaxation training reduced PTSD symptoms after 10 weeks.[8]
Traditional CBT clinical trials apply fixed, randomized, “a priori” treatment decisions in which the findings may be mismatched with patient uptake or response, unlike the more fluid clinical practice wherein symptom treatment is adapted based on patient response during frequent reassessments. Adaptive treatment ensures optimal benefit without wasting resources, such as patient time and healthcare costs. Given these benefits, there has been a growth in the number and use of adaptive randomized controlled trial (RCT) designs attempting to optimize behavioral health interventions (i.e., develop the best decision rules based on research findings rather than single timepoint a priori treatment decisions).
Herein, we describe the rationale and methods for a multicenter testing of a sequential multiple assignment randomized trial (SMART) design for PTSD treatment of HCT survivors. The SMART design contains a stage (i.e., step) for each of the treatment decisions within the adaptive intervention and is designed to inform treatment decision rules and treatment effectiveness.[21] Study participants are randomly assigned to one treatment option at the initial stage, and the need for a subsequent randomization is determined by the effectiveness of the initial stage. The end result will be the development of the best decision rules that are based on patient characteristics and treatment responses rather than a priori decisions.
Methods
This study was approved by the Duke University and Memorial Sloan Kettering Cancer Center (MSK) Institutional Review Boards with all participants providing informed consent. Recruitment procedures comply with HIPAA guidelines.
Study Design
The study follows the SMART design displayed in Figure 1. Adult cancer survivors who received a HCT 1–5 years ago (N=400) at the Duke Cancer Institute and MSK and who enroll and have full or partial PTSD symptoms per the Diagnostic and Statistical Manual of Mental Disorders (DSM-5)[22] are randomly assigned to CaDC app versus Usual Care. Patients are identified by reviewing site Transplant Registries and clinic providers and then approached and screened to assess for PTSD symptoms. A computer-generated random number assignment procedure in REDCap [23] determines approach order. Randomization is stratified by site with equal allocation to each initial intervention. Participants randomized to the CaDC app will be encouraged to use it following download and not thereafter. Four weeks following randomization, participants are asked to re-rate their PTSD symptoms. Participants in the CaDC arm who show a reliable reduction in PTSD symptom severity (i.e., a reduction of 5 or more points on the PTSD Checklist; PCL5) are deemed responders and instructed to continue using the app.[22] Participants who do not respond (<5 point score reduction) are deemed non-responders and re-randomized to receive an increased intensity intervention (i.e., CaDC+mCoaching versus mCBT). Participants in the Usual Care arm who respond by reporting a ≥5 point reduction in their PTSD symptom severity are instructed to continue as before; participants who do not respond to Usual Care are re-randomized to receive an increased intensity intervention (i.e., CaDC+mCoaching versus mCBT). This second randomization is also 1:1 with equal allocation into one of the two increased intensity interventions. All participants who do not report a ≥5 point reduction in their PTSD symptom severity at four weeks will receive an evidence-based intervention that is expected to lead to PTSD symptom reduction.
Figure 1. SMART Design for the PTSD clinical trial.
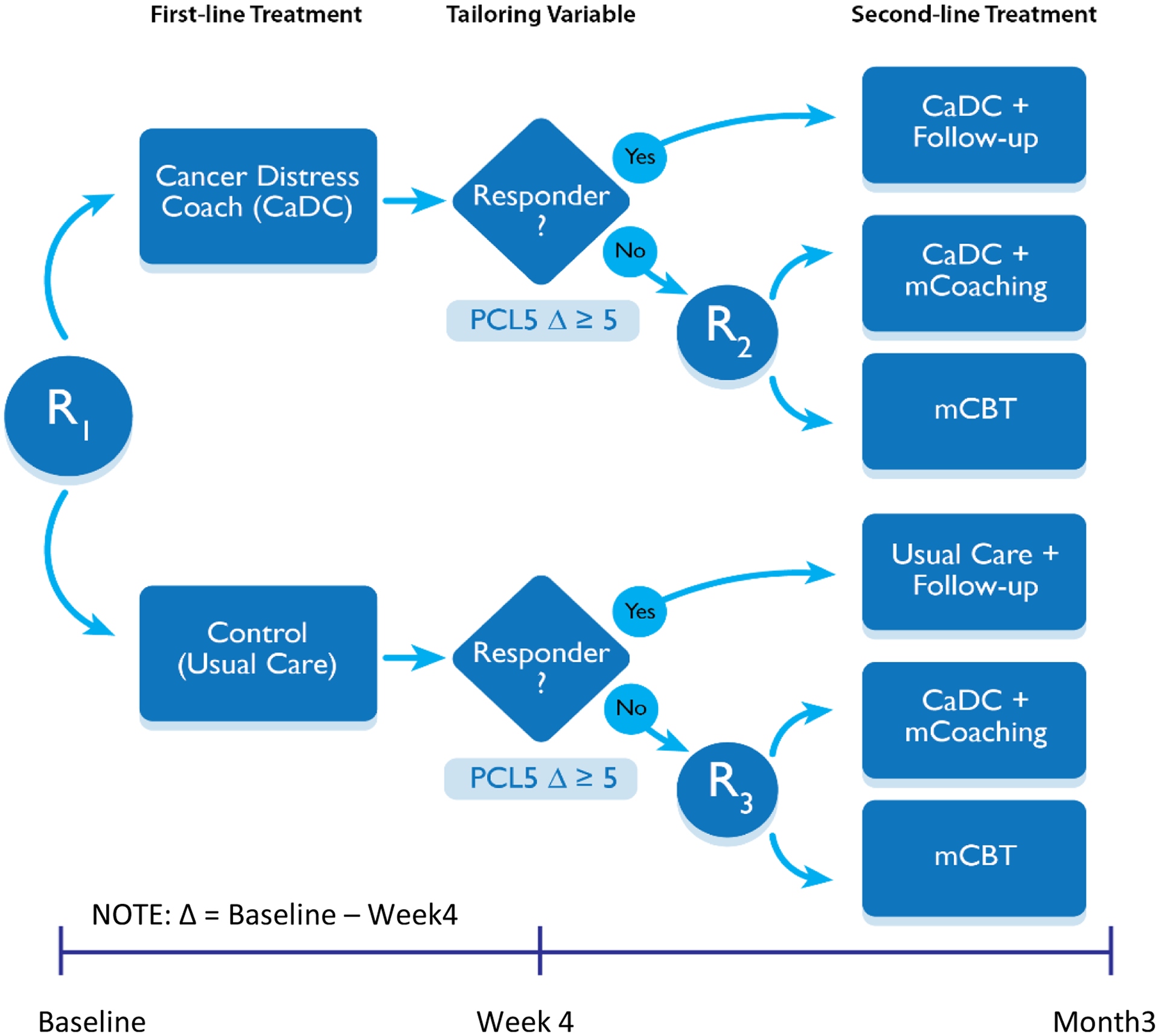
Response is based on a change in the PCL5 score of 5 points or greater. R indicates randomization, mCoaching (4 sessions) and mCBT (8 sessions) indicate videoconferencing with 2 levels of coaching intensity.
Study data are collected and managed using REDCap electronic data capture tools hosted at Duke University.[23] All participants complete assessments at baseline, 4 weeks, 3 months (post-intervention), and 6 months (follow-up). As described below, assessments include psychometrically strong measures of PTSD, depression, anxiety, pain interference, quality of life, and self-efficacy for managing chronic illness. The post-treatment and follow-up assessments also include a measure of perceived helpfulness and satisfaction with the intervention(s) that they received. As a means to record participant adherence, the study therapists are logging their sessions in a REDCap form and mobile app use is collected and stored within the Veteran Administration’s VAAppConnect interface.[24] These data will be considered as potential moderators in statistical analyses.
Eligibility criteria were chosen to minimize study attrition and to target survivors most able to benefit. For example, we include only cancer survivors who are in remission, which will minimize drop-outs due to relapse of malignancy, declining health, or death. In addition, our recruitment teams meet weekly to track each participant’s progress in the study. Finally, we send text messages via Twilio and REDCap survey reminders in unison over a ten-day period to participants that prompt them to complete assessments. Participants who fail to complete a survey after four weeks are withdrawn; no subsequent surveys will be sent. Text reminders for therapy appointments are available to participants who indicate that they would be helpful.
Participant Eligibility Criteria
Inclusion requirements are ≥18 and <85 years of age, completion of autologous or allogeneic HCT 1–5 years previously complete remission (i.e., no evidence of disease), absence of severe psychological impairment (e.g., hospitalization for suicidality), approved for contact by the patient’s oncologist, no CBT for PTSD within the last 6 months, no new cancer diagnosis since HCT completion, owns a smart device (i.e., iOS or Android smartphone or tablet), able to read and write English, and significant PTSD symptoms as indicated by at least one of the following two criteria: (1) probable cancer-related PTSD by a PCL5 score ≥31; and/or (2) subthreshold or partial PTSD symptoms as determined by endorsement of re-experiencing cluster and ≥1 other symptom cluster as recommended by the National Center for PTSD.[22], [25], [26] Only HCT survivors who meet criteria for full or partial PTSD are eligible to participate. In addition, HCT survivors with pre-existing mental health issues such as depression, anxiety, and PTSD are eligible for this trial while those with severe psychological impairment such as requiring hospitalization for suicidality are not eligible.
Interventions
Initial randomization
CaDC app.
As shown in Table 1, the lowest intensity and cost in terms of patient time and healthcare resources is CaDC, an mHealth app derived from the PTSD Coach mobile app.[27] The CaDC app was previously revised for cancer survivors by the National Center for PTSD in partnership with our research team.[19] CaDC can be used as a stand-alone education and symptom management tool, or to augment face-to-face care with a healthcare professional. This software uses 6th grade reading level text, and limits use of three-syllable words, making it ideally suited for use with low socioeconomic status populations. As shown in Figure 2, the Learn module provides psycho-education, with information derived from the National Center for PTSD and National Cancer Institute Physician Data Query (PDQ) resources. The Track Progress module administers a reliable and valid PTSD assessment measure (PCL5); participants receive interpretive feedback including symptom severity and information about their score relative to the last administration. Participants can view their historical data in graphical form and share their data with clinicians (e.g., at appointments or via MyChart) to encourage self-management of symptoms. The Manage Symptoms module provides participants with tools based on CBT principles (e.g., guided imagery, diaphragmatic breathing, stress inoculation techniques) to manage their stress in the moment they experience it. Before and after each tool is introduced, participants are asked to rate their distress from 0–10 on a thermometer.[28] Users selecting high distress are directed to the Get Support section of the app, which lists options for local and national cancer and non-cancer related professional support and crisis resources (e.g., National Suicide Prevention Lifeline).
Table 1.
Intervention Components
| Intervention | Sessions/Duration | Education | Activities | Coping Strategies | Relapse Prevention |
|---|---|---|---|---|---|
| CaDC app N=200 |
Self-directed as desired |
Learn module (text):
|
Manaae Symptoms module (audiovisual):
|
Manaae Symptoms module (text):
|
N/A |
| CaDC + mCoaching N=125 |
Self-directed app plus 4 × 30 min | Review the Learn module and discuss the topics presented |
Videoconference or phone:
|
Encouragement to use active coping skills as suggested in the CaDC app. | Advise continued use of CaDC app |
| mCBT N=125 |
1 × 90 min; then additional 7 × 60 min |
|
Videoconference or phone:
|
Homework:
|
|
Figure 2.
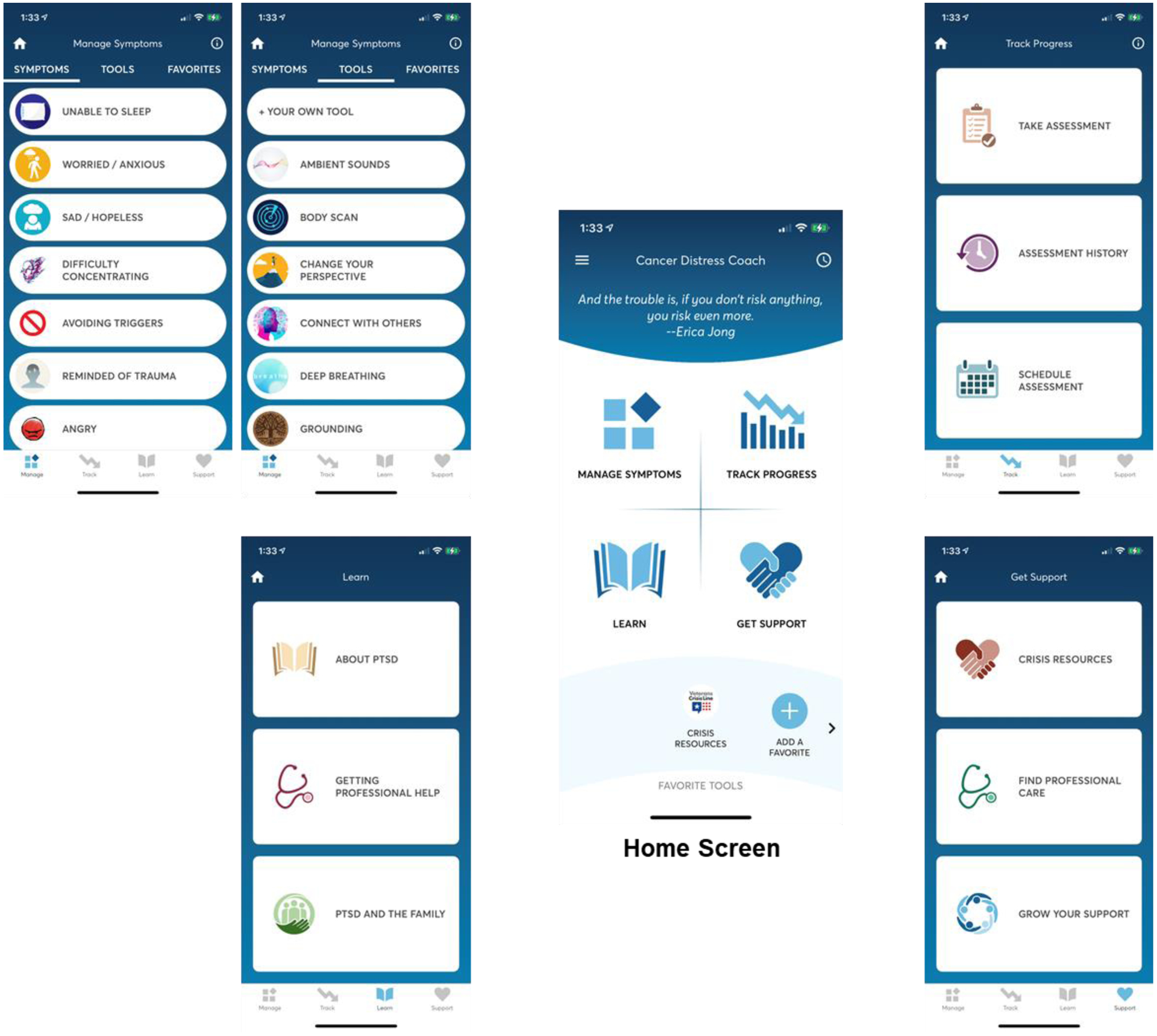
Cancer Distress Coach app. The four modules are Manage Symptoms, Track Progress, Learn, and Get Support.
Usual Care.
Participants randomized to the control arm have access to the mental health services that are available to all cancer patients at their respective institution.
Assessing Response to Initial Intervention:
Participants are asked to rate their PTSD symptoms via REDCap survey four weeks after completing the baseline assessment.
Second randomization
If participants do not respond to the CaDC app or Usual Care at the initial stage, they are randomized a second time (i.e., stepped care of increased intensity).
CaDC+mCoaching.
The second treatment modality of medium intensity combines CaDC with virtual clinician support (mCoaching). The manualized procedure consists of a 4-session (30 min each) biweekly intervention for PTSD that is delivered by phone or videoconferencing based on participant preference and has shown promise among primary care patients.[20] The purpose of these sessions is to: 1) address any technology-related concerns and provide support in navigating the app (e.g., finding local resources, selecting activities to manage symptoms) and encourage adherence to the use of the CaDC application; and, 2) provide guidance in choosing treatment strategies. The clinician identifies participants’ PTSD symptoms and guides them in how best to use CaDC to manage their specific symptoms. CaDC activities, such as diaphragmatic breathing, are suggested by the clinician and practiced in the session. Furthermore, the clinician and participant work collaboratively to schedule symptom-targeting homework.
mCBT.
The third and highest intensity treatment protocol is mCBT, which is derived from the HCT-specific telephone administered CBT protocol developed by colleagues at MSK, Mount Sinai, and Hackensack University Hospitals.[8] This eight-session manualized intervention is delivered weekly. The first session is 90 minutes and subsequent sessions are 60 minutes. The participant and therapist connect via videoconferencing or phone. The mCBT includes education regarding CBT and HCT- and PTSD-related symptoms, self-monitoring, alteration of maladaptive beliefs, guided exposure to cues associated with PTSD symptoms (de-sensitization), and relaxation training. Content overlap between the three interventions is surmised to be a strength as they are each evidence-based with CBT principles and the interventions will likely build on and reinforce each other (see Table 1).
Conceptual Model
This inquiry is guided by Bandura’s Social Cognitive Theory, which identifies external experiences and self-perception as influential in determining the outcome of many events and serves as a foundation for CBT.[29] Self-efficacy, which postulates that a person’s beliefs in his/her ability to succeed in a particular situation determine how he/she feels, thinks, and behaves, is central to this theory. The proposed project uses the CaDC app to address these beliefs in three modules (Learn, Track Progress, and Manage Symptoms) that promote symptom management. Receiving and understanding information, as experienced in CaDC Learn and Track Progress, contributes to mastery or a sense of competence. The Manage Symptoms module directly enhances coping skills through participation in various mind-body exercises (e.g., deep breathing, guided imagery). In addition, the mCBT protocol is informed by the Social Cognitive Theory such that coping self-statements, challenging unhelpful thoughts and facing triggering stimuli which were previously avoided, enhances self-efficacy in coping with these previously uncontrollable symptoms.
Measures
Participant characteristics.
REDCap surveys developed by our team are used to collect self-reported data: demographic (e.g., sex, race, age, income); medication (e.g., SSRI, benzodiazepine, corticosteroid); and, other mental health service use. HCT patients’ clinical information (e.g., diagnosis date, cancer type, number of remissions) is already being collected at both sites for the readily accessible Center for International Blood and Marrow Transplant Research database. This database also be used to retrieve healthcare utilization outcomes (e.g., readmissions, length of stay, relapse, death) to minimize survey burden.
Outcomes.
As shown in Table 2, this study employs seven standardized instruments and one investigator-developed survey to assess for intervention helpfulness and satisfaction. The primary outcome measure is the PTSD Checklist for DSM5 (PCL5) with items modified to key on cancer-related symptoms, as has been validated in the HCT population.[12], [22], [25] Secondary outcomes include the Distress Thermometer v.2018; it is a valid tool to detect cancer-related distress, depression, and anxiety.[28] The NIH PROMIS Global QOL is used to assess general perceptions of health.[30] Depression, anxiety, and pain interference is assessed with the PROMIS 8-item instruments.[31] The Self-efficacy for Managing Chronic Disease Scale assesses how capable one feels in managing symptoms and performing health-related tasks.[32] A post-intervention survey includes questions regarding the participant’s study experience (i.e., perceived helpfulness and satisfaction) to inform future improvements to the protocol and/or programs. In addition, CaDC clickstream activity (i.e., usage) is collected and reported via the VA AppConnect dashboard (https://vaappconnect.com).
Table 2.
Study Measures
| Construct | Instrument | Summary | Scoring | #Items |
|---|---|---|---|---|
| PTSD symptoms | PTSD Checklist (PCL5) [22] | Reliable (α=.94) measure of PTSD symptoms. | Continuous or symptom clustering. | 20 |
| Distress | Distress Thermometer v.2018 [28] | Validated measure of cancer-related distress. | One-item score (0–10). | 1 |
| Quality of Life | PROMIS Global QOL [30] | Reliable (α=.81, .86) measure of general health perceptions. | Physical and mental items are summed and converted to t-scores. | 10 |
| Depression | PROMIS [42] | Reliable (α=.91) measure of depression severity. | Items are summed and converted to t-scores. | 8 |
| Anxiety | PROMIS [42] | Reliable (α=.90) measure of anxiety severity. | Items are summed and converted to t-scores. | 8 |
| Pain Interference | PROMIS [43] | Reliable (α=.91) measure of pain interference. | Items are summed and converted to t-scores. | 8 |
| Self-efficacy | Self-efficacy for Chronic Disease [32] | Reliable (α=.91) measure of symptom self-efficacy. | Items are summed to a total score. | 6 |
| User satisfaction | Team-developed | Items assess perceived helpfulness of treatments. | Points are summed and totaled. | 20 |
Interventionist Adherence.
At each site, a licensed clinical psychologist who has extensive expertise in delivering behavioral health protocols will rate the therapists’ protocol adherence and competence. After each mCoaching call, the therapists will complete a Fidelity Checklist that includes all essential components of the protocol and session content. All mCoaching calls will also be audio-recorded and 20% will be randomly selected (with equal numbers being drawn from each of the four sessions of the protocol) for fidelity checks and adherence ratings. All sessions conducted by mCBT therapists will also be audio recorded and selected recordings reviewed in weekly supervision meetings. Initially, a random sample of 50% of mCBT sessions will undergo fidelity checks and adherence ratings with 20% reviewed following the first year.
Study Aims
Consistent with the goals of SMART designs, Aim 1 tests the first stage response, Aim 2 compares embedded adaptive interventions, and Aim 3 develops a deeply-tailored (optimized) adaptive intervention.[33] Aim 1 will evaluate the effectiveness of the CaDC app compared to Usual Care (i.e., control) to reduce PTSD symptoms among cancer survivors who received an HCT. We hypothesize that survivors who are randomized to the CaDC app will have less severe PTSD symptoms (i.e., lower mean PTSD Checklist for DSM5 [PCL5] score) after 3 months compared to those in the Usual Care group (defined as the mental health care received outside of the study protocol). Aim 2 will compare the pre-specified adaptive PTSD interventions that employ CaDC vs. Usual Care when augmented with mCoaching or mCBT for symptom reduction on the PCL5 after 3 months. We hypothesize that, among those requiring stepped up or more intensive care, the combination of CaDC+mCBT will lead to greater PTSD symptom reduction than CaDC+mCoaching at 12-weeks (post-intervention). Aim 3 will estimate tailored regimens for PTSD and other outcomes to generate hypotheses about if and how treatment should be tailored per individual patient characteristics including transplant type, race, sex, and income level. This hypothesis-generating aim specifies a more optimal sequence of treatments for each individual based on newly-discovered tailoring variables such as transplant type (i.e., autologous, allogeneic). Secondary analyses will also examine the sustained effects of the interventions after six months.
Statistical Analyses
Aim 1.
We will compare CaDC with Usual Care in terms of PTSD symptom reduction among cancer survivors from baseline to Month3. We will test the statistical hypothesis: (H1) mean symptom reduction is equal under CaDC and Usual Care. Thus, (H1) tests the overall effectiveness of CaDC relative to Usual Care across the patient population. Two sample t-test will be used to assess the overall effectiveness of the first line treatments between CaDC and Usual Care in terms of PTSD symptom reduction from baseline to Week4. Because we have repeated measures from baseline to Month1 and Month3, we will also build a linear mixed model to analyze longitudinal effects. The model will include fixed effects for the intercept, time, and a group-by-time interaction term as well as random effects for the intercept and time (slope) to account for possible correlation within each participant over time. We will test the hypothesis that the coefficient of the group-by-time interaction term is zero to assess the primary hypothesis.
Aim 2.
We will carry out secondary analyses to compare the four intervention sequences (see Figure 1) embedded in the SMART based in terms of average point reduction in PTSD symptoms achieved at Month3 (post-intervention) and Month 6 follow-up. We will also compute point and interval estimates for mean symptom reduction for each embedded regimen. Point estimates will be computed using an augmented inverse probability weighted estimator and interval estimates will be computed using the bootstrap.[34], [35] We will use multiple comparisons with the best to test for unique optimal embedded regimen.[36] In addition, we will compare the effects of each embedded treatment regimen among those who received an autologous HCT relative to those who completed allogeneic HCT. Any differences between these groups will be identified as a priority for follow-up study.
Aim 3.
We will use Q-learning, a regression-based approximate dynamic programming algorithm, to estimate optimal adaptive interventions for PTSD, distress, depression, anxiety, QOL, and personal characteristics.[37] Briefly, the Q-learning algorithm works by fitting a series of regressions, one for each decision point. Each regression estimates the conditional average treatment effect given current patient information if optimal interventions are assigned in the future.[38]–[40] From these regressions, one can then derive the estimated optimal intervention for each strategy at each decision point; for an introductory overview of Q-learning see Nahum-Shani, et al.[37] To ensure scientific interpretability, we will estimate regimes that are represented as a sequence of if-then statements.[41]
Sample Size and Power.
Sample size (N=400) is based on statistical power for Aim 1 (contrast of first line treatment) and Aim 2 (contrast of most vs. least intensive dynamic treatment regimen [DTR]). After Bonferroni adjustment, type I error is set to be 5%/2=2.5% for each of the two comparisons. Aim 1: Using a two-sided, two-sample t-test, a total of 314 participants are needed to detect an effect size of 0.35 to compare PTSD symptoms between the two first line treatments at the end of week 14 with 80% power. This postulated standardized effect size is reasonable given historical data.[17], [19] After accounting for an estimated attrition rate of 20%, a total of N=400 (314÷0.8) is needed. A difference in PCL5 score of 5 corresponds to a small-to-moderate standardized effect size of d=.35 in between groups change in PTSD (based on previous data, SD~14).[17]. This is a minimally clinically significant difference in change. Aim 2: Using a two-sided, two sample t-test, 121 participants in each DTR are needed to detect an effect size of 0.40 to compare PTSD symptoms between the most and least intensive DTR with 80% power. The effect size is proposed to be slightly bigger than 0.35 for first line treatment because we expect to see a bigger difference when comparing the most and least intensive DTR. Given an estimated response rate of 50% for the first line treatments, the total number of participants can be calculated by 121*8/3=323. Again, after accounting for an estimated attrition rate of 20%, a total of N=400 should be sufficient. Due to the exploratory nature of Aim 3, we will compute point and interval estimates for the interactions between embedded regimen and each moderator. Point estimates will be computed using an augmented inverse probability weighted estimator and interval estimates will be computed using the bootstrap.[34], [35] Any differences between groups will be identified as a priority for follow-up study.
Conclusions
To our knowledge, this is the first study to develop an evidence-based, adaptive stepped-care, mHealth protocol for personalized PTSD symptom management in HCT cancer survivors. For example, we will discover whether individuals who receive HCT should receive CaDC as a first-line treatment and if non-responders and non-adherers to initial CaDC should be switched to mCBT or mCoaching for enhanced outcomes. Potential limitations of this trial include the presence of COVID-19 as a stressor (i.e., potentially traumatic event) during the recruitment, intervention, and assessment phases, a demographically homogeneous sample limited to the US East Coast, and the absence of a longer follow-up time point.
While this study is designed to provide guidance related to treatment selection for managing PTSD symptoms, it will likely generate additional questions. We may find that these interventions are effective in managing related outcomes such as depression, anxiety, and pain. If found, optimization of newer protocols that take these important outcomes into account would be warranted.
Despite the implementation of distress management protocols at national cancer centers, many distressed cancer patients fall through the cracks due to miscommunication and lack of resources. If our hypotheses are supported, CaDC could be readily integrated into standard of care for HCT patients who report symptoms on the PCL5 and/or above the Distress Thermometer cutoff score given that it is moderately associated with the PCL5. For example, HCT patients would be screened for PTSD and, if relevant, receive instructions on downloading the CaDC app. The availability of this free app would be especially helpful to those at highest risk for persistent or worsening PTSD symptoms: low-income and/or nonwhite cancer survivors who received HCT as part of an aggressive cancer treatment.[3] Use of this free app as a first step could preserve evidence-based, video-conferenced CBT interventions more accessible for underserved populations who face barriers (e.g., cost, transportation, travel distance, time) to accessing traditional in person interventions. In addition, this work could serve as a national and international model for oncology distress management protocols and wide-spread clinical implementation of mHealth interventions. In summary, this SMART design clinical trial will provide critical data to inform tailored PTSD treatment regimens that will optimize resources, tailor intervention to need, and could be practically implemented into clinical cancer care, with the particular potential to reach underserved populations.
Acknowledgements
This study is funded through an NIH/NCI grant (1R01-CA244172) awarded to Dr. Smith and Dr. Applebaum.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Trials Registration: ClinicalTrials.gov, NCT04058795, registered 8/16/2019
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Smith SK et al. , “Post-traumatic stress symptoms in long-term non-Hodgkin’s lymphoma survivors: does time heal?,” J Clin Oncol, vol. 29, no. 34. pp. 4526–33, 2011. doi: 10.1200/JCO.2011.37.2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Cordova MJ, Riba MB, and Spiegel D, “Post-traumatic stress disorder and cancer,” The Lancet Psychiatry, vol. 4, no. 4. pp. 330–338, 2017. doi: 10.1016/S2215-0366(17)30014-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Smith SK, Zimmerman S, Williams CS, Preisser JS, and Clipp EC, “Post-traumatic stress outcomes in non-Hodgkin’s lymphoma survivors,” J Clin Oncol, vol. 26, no. 6. pp. 934–41, 2008. doi: 10.1200/JCO.2007.12.3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Fenech AL et al. , “Post-Traumatic Stress Symptoms in Hematopoietic Stem Cell Transplant Recipients.,” Transplant Cell Ther, vol. 27, no. 4, p. 341.e1–341.e6, Apr. 2021, doi: 10.1016/j.jtct.2021.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Liang J et al. , “Rates and Risk Factors for Post-Traumatic Stress Disorder Symptomatology among Adult Hematopoietic Cell Transplant Recipients and Their Informal Caregivers.,” Biol Blood Marrow Transplant, vol. 25, no. 1, pp. 145–150, Jan. 2019, doi: 10.1016/j.bbmt.2018.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].“Transplant Activity Report | Blood Stem Cell.” https://bloodstemcell.hrsa.gov/data/donation-and-transplantation-statistics/transplant-activity-report#numbers (accessed Aug. 27, 2021).
- [7].El-Jawahri AR et al. , “Quality of life and mood predict posttraumatic stress disorder after hematopoietic stem cell transplantation,” Cancer, vol. 122, no. 5. pp. 806–12, 2016. doi: 10.1002/cncr.29818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].DuHamel KN et al. , “Randomized clinical trial of telephone-administered cognitive-behavioral therapy to reduce post-traumatic stress disorder and distress symptoms after hematopoietic stem-cell transplantation,” J Clin Oncol, vol. 28, no. 23. pp. 3754–61, 2010. doi: 10.1200/JCO.2009.26.8722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Smith MY, Redd W, DuHamel K, Vickberg SJ, and Ricketts P, “Validation of the PTSD Checklist-Civilian Version in survivors of bone marrow transplantation,” J Trauma Stress, vol. 12, no. 3. pp. 485–99, 1999. doi: 10.1023/A:1024719104351. [DOI] [PubMed] [Google Scholar]
- [10].Jacobsen PB, Widows MR, Hann DM, Andrykowski MA, Kronish LE, and Fields KK, “Posttraumatic stress disorder symptoms after bone marrow transplantation for breast cancer,” Psychosom Med, vol. 60, no. 3. pp. 366–71, 1998. [DOI] [PubMed] [Google Scholar]
- [11].Mundy EA, Blanchard EB, Cirenza E, Gargiulo J, Maloy B, and Blanchard CG, “Posttraumatic stress disorder in breast cancer patients following autologous bone marrow transplantation or conventional cancer treatments,” Behav Res Ther, vol. 38, no. 10. pp. 1015–27, 2000. [DOI] [PubMed] [Google Scholar]
- [12].DuHamel KN et al. , “Construct validity of the posttraumatic stress disorder checklist in cancer survivors: analyses based on two samples,” Psychol Assess, vol. 16, no. 3. pp. 255–66, 2004. doi: 10.1037/1040-3590.16.3.255. [DOI] [PubMed] [Google Scholar]
- [13].Jacobsen PB, Sadler IJ, Booth-Jones M, Soety E, Weitzner MA, and Fields KK, “Predictors of posttraumatic stress disorder symptomatology following bone marrow transplantation for cancer,” J Consult Clin Psychol, vol. 70, no. 1. pp. 235–40, 2002. [DOI] [PubMed] [Google Scholar]
- [14].Smith SK, Williams CS, Zimmer CR, and Zimmerman S, “An exploratory model of the relationships between cancer-related trauma outcomes on quality of life in non-Hodgkin lymphoma survivors,” J Psychosoc Oncol, vol. 29, no. 1. pp. 19–34, 2011. doi: 10.1080/07347332.2011.534022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].El-Jawahri A et al. , “Effect of Inpatient Palliative Care During Hematopoietic Stem-Cell Transplant on Psychological Distress 6 Months After Transplant: Results of a Randomized Clinical Trial,” J Clin Oncol, vol. 35, no. 32. pp. 3714–3721, 2017. doi: 10.1200/JCO.2017.73.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Griffith S, Fenech AL, Nelson A, Greer JA, Temel JS, and El-Jawahri A, “Post-traumatic stress symptoms in hematopoietic stem cell transplant (HCT) recipients.,” JCO, vol. 38, no. 15_suppl, pp. 7505–7505, May 2020, doi: 10.1200/JCO.2020.38.15suppl.7505. [DOI] [Google Scholar]
- [17].Kuhn E, Kanuri N, Hoffman JE, Garvert DW, Ruzek JI, and Taylor CB, “A randomized controlled trial of a smartphone app for posttraumatic stress disorder symptoms,” J Consult Clin Psychol, vol. 85, no. 3. pp. 267–273, 2017. doi: 10.1037/ccp0000163. [DOI] [PubMed] [Google Scholar]
- [18].Rathbone AL, Clarry L, and Prescott J, “Assessing the Efficacy of Mobile Health Apps Using the Basic Principles of Cognitive Behavioral Therapy: Systematic Review.,” J Med Internet Res, vol. 19, no. 11, p. e399, Nov. 2017, doi: 10.2196/jmir.8598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Smith SK et al. , “Cancer distress coach: Pilot study of a mobile app for managing posttraumatic stress,” Psychooncology. 2016. doi: 10.1002/pon.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Possemato K et al. , “Using PTSD Coach in primary care with and without clinician support: a pilot randomized controlled trial,” Gen Hosp Psychiatry, vol. 38, no. Supplement C. pp. 94–8, 2016. doi: 10.1016/j.genhosppsych.2015.09.005. [DOI] [PubMed] [Google Scholar]
- [21].Murphy SA, “An experimental design for the development of adaptive treatment strategies.,” Stat Med, vol. 24, no. 10, pp. 1455–1481, May 2005, doi: 10.1002/sim.2022. [DOI] [PubMed] [Google Scholar]
- [22].“PTSD Checklist for DSM-5 (PCL-5) - PTSD: National Center for PTSD.” https://www.ptsd.va.gov/professional/assessment/adult-sr/ptsd-checklist.asp (accessed Aug. 27, 2021).
- [23].Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, and Conde JG, “Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support.,” J Biomed Inform, vol. 42, no. 2, pp. 377–381, Apr. 2009, doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].“VA AppConnect.” https://vaappconnect.com/login (accessed Aug. 27, 2021).
- [25].Blevins CA, Weathers FW, Davis MT, Witte TK, and Domino JL, “The Posttraumatic Stress Disorder Checklist for DSM-5 (PCL-5): Development and Initial Psychometric Evaluation,” J Trauma Stress, vol. 28, no. 6. pp. 489–98, 2015. doi: 10.1002/jts.22059. [DOI] [PubMed] [Google Scholar]
- [26].Blanchard EB, Hickling EJ, Taylor AE, Loos WR, and Gerardi RJ, “Psychological morbidity associated with motor vehicle accidents,” Behaviour Research and Therapy, vol. 32, no. 3, pp. 283–290, Mar. 1994, doi: 10.1016/0005-7967(94)90123-6. [DOI] [PubMed] [Google Scholar]
- [27].Kuhn E et al. , “Preliminary evaluation of PTSD Coach, a smartphone app for post-traumatic stress symptoms.,” Mil Med, vol. 179, no. 1, pp. 12–18, Jan. 2014, doi: 10.7205/MILMED-D-13-00271. [DOI] [PubMed] [Google Scholar]
- [28].Holland JC and Bultz BD, “The NCCN guideline for distress management: a case for making distress the sixth vital sign,” J Natl Compr Canc Netw, vol. 5, no. 1. pp. 3–7, 2007. [PubMed] [Google Scholar]
- [29].Bandura A, “Social foundations of thought and action: A social cognitive theory.” Prentice-Hall, Inc, Englewood Cliffs, NJ, US, 1986. [Google Scholar]
- [30].Hays RD, Bjorner JB, Revicki DA, Spritzer KL, and Cella D, “Development of physical and mental health summary scores from the patient-reported outcomes measurement information system (PROMIS) global items,” Qual Life Res, vol. 18, no. 7. pp. 873–80, 2009. doi: 10.1007/s11136-009-9496-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Cella D et al. , “The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008,” J Clin Epidemiol, vol. 63, no. 11. pp. 1179–94, 2010. doi: 10.1016/j.jclinepi.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lorig KR, Sobel DS, Ritter PL, Laurent D, and Hobbs M, “Effect of a self-management program on patients with chronic disease,” Eff Clin Pract, vol. 4, no. 6. pp. 256–62, 2001. [PubMed] [Google Scholar]
- [33].Almirall D, Nahum-Shani I, Sherwood NE, and Murphy SA, “Introduction to SMART designs for the development of adaptive interventions: with application to weight loss research,” Transl Behav Med, vol. 4, no. 3. pp. 260–74, 2014. doi: 10.1007/s13142-014-0265-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Zhang B, Tsiatis AA, Laber EB, and Davidian M, “A robust method for estimating optimal treatment regimes,” Biometrics, vol. 68, no. 4. pp. 1010–8, 2012. doi: 10.1111/j.1541-0420.2012.01763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Chakraborty B, Laber EB, and Zhao YQ, “Inference about the expected performance of a data-driven dynamic treatment regime,” Clin Trials, vol. 11, no. 4. pp. 408–417, 2014. doi: 10.1177/1740774514537727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hsu J, “Multiple comparisons: theory and methods.” CRC Press, Boca Raton, FL, 1996. [Google Scholar]
- [37].Nahum-Shani I et al. , “Q-learning: a data analysis method for constructing adaptive interventions,” Psychol Methods, vol. 17, no. 4. pp. 478–94, 2012. doi: 10.1037/a0029373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Clifton J and Laber E, “Q-Learning: Theory and Applications,” Annu. Rev. Stat. Appl, vol. 7, no. 1, pp. 279–301, Mar. 2020, doi: 10.1146/annurev-statistics-031219-041220. [DOI] [Google Scholar]
- [39].Schulte PJ, Tsiatis AA, Laber EB, and Davidian M, “Q- and A-learning Methods for Estimating Optimal Dynamic Treatment Regimes,” Stat Sci, vol. 29, no. 4. pp. 640–661, 2014. doi: 10.1214/13-STS450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Chakraborty B and Moodie Erica. E. M., “Statistical methods for dynamic treatment regimes.” Springer, New York, 2013. [Google Scholar]
- [41].Zhang Y, Laber EB, Davidian M, and Tsiatis AA, “Estimation of Optimal Treatment Regimes Using Lists,” Journal of the American Statistical Association, no. just-accepted. pp. 0–0, 2017. doi: 10.1080/01621459.2017.1345743. [DOI] [Google Scholar]
- [42].Schalet BD et al. , “Clinical validity of PROMIS Depression, Anxiety, and Anger across diverse clinical samples,” J Clin Epidemiol, vol. 73. pp. 119–27, 2016. doi: 10.1016/j.jclinepi.2015.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Broderick JE, Schneider S, Junghaenel DU, Schwartz JE, and Stone AA, “Validity and reliability of patient-reported outcomes measurement information system instruments in osteoarthritis,” Arthritis Care Res (Hoboken), vol. 65, no. 10. pp. 1625–33, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]


