Abstract
Iron (Fe) plays important roles in both essential cellular processes and virulence pathways for many bacteria. Consequently, Fe withholding by the human innate immune system is an effective form of defense against bacterial infection. In this perspective, we review recent studies that have established a foundation for our understanding of the impact of the metal sequestering host defense protein calprotectin (CP) on bacterial Fe homeostasis. We also discuss two recently uncovered strategies for bacterial adaptation to Fe withholding by CP. Together, these studies provide insight into how Fe sequestration by CP affects bacterial pathogens that include Pseudomonas aeruginosa, Staphylococcus aureus, and Acinetobacter baumannii. Overall, recent studies suggest that Fe withholding by CP may have implications for bacterial survival and virulence in the host, and further in vivo explorations that address this possibility present an important area for discovery.
Graphical Abstract
Introduction
Nutritional immunity describes the withholding of essential nutrient metals by the mammalian host in order to hinder microbial pathogen invasion.1, 2 The microbial requirement for transition metals makes nutritional immunity a key host defense process that pathogens must overcome in order to survive and replicate in the host. As part of nutritional immunity, the mammalian host releases metal sequestering proteins during the innate immune response to lower metal availability at infection sites.1, 2 Calprotectin (CP) is an important metal sequestering innate immune protein notable for its ability to limit the availability of multiple transition metal nutrients.3
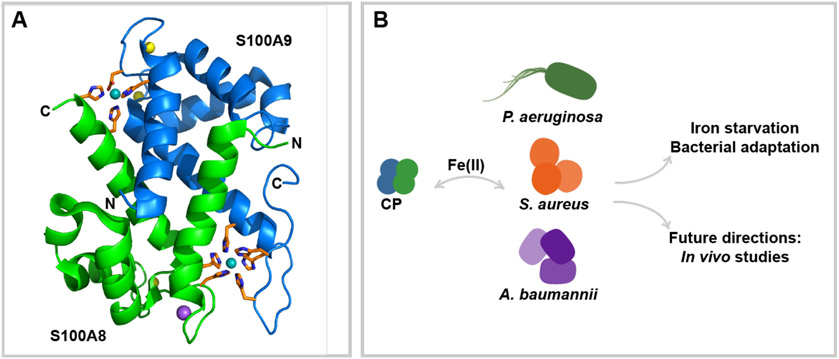
Human CP is a heterooligomer of two Ca(II)-binding S100 proteins, S100A8 (α, 10.8 kDa) and S100A9 (β, 13.2 kDa) (Figure 1a). Each CP αβ heterodimer has two transition metal binding sites at the S100A8/S100A9 interface: a His3Asp site that sequesters Zn(II) and a His6 site that sequesters Mn(II), Fe(II), Ni(II) and Zn(II).4-9 CP also houses two EF-hand domains in each subunit that bind Ca(II), and Ca(II) binding by CP causes self-association to form the (αβ)2 heterotetramer, promotes transition metal sequestration by increasing the metal binding affinities at each site, and enhances protease resistance.3, 10, 11 Consequently, Ca(II) binding plays a central role in the working model for how CP sequesters transition metal nutrients in the extracellular space. In this model, white blood cells release CP into the extracellular space where it encounters high Ca(II) levels (~2 mM) and binds Ca(II) ions, allowing it to become the metal sequestering heterotetramer and withhold essential transition metals from microbial pathogens.3, 12 Indeed, CP has been observed to withhold Mn, Fe, Ni, Cu, and Zn from microbial pathogens.3 In this way, CP imparts its growth inhibitory activity.3
Figure 1.
Overview of perspective. (A) Crystal structure of CP. A heterodimer from the crystal structure of Ni(II)-, Ca(II)- and Na(I)-bound CP-Ser [S100A8/(C42S)/S100A9(C3S) variant] (αβ)2 heterotetramer is presented to highlight the His3Asp and His6 sites (PDB 5W1F).9 S100A8 is shown in green, S100A9 is shown in blue. Transition metal binding residues are shown in orange. Bound Ni(II), Ca(II), and Na(I) are shown in teal, yellow, and purple, respectively. (B) CP competes with Gram-negative and Gram-positive bacterial pathogens for Fe. Current literature describes the responses of P. aeruginosa, S. aureus and A. baumannii to Fe withholding by CP.
Fe is an essential nutrient for the vast majority of bacterial pathogens, and is thus critical for bacterial pathogens to acquire in the host.13 Bacteria regulate multiple processes, including metabolism, DNA repair, respiration, and the production of virulence factors using Fe-responsive transcription factors such as the ferric uptake regulator Fur.14 Moreover, Fe is a cofactor of electron transport proteins and many enzymes involved in primary metabolic pathways, including heme and non-heme oxido-reductases.13 Thus, Fe starvation has the potential to disrupt multiple cellular processes that are important for bacterial replication in the host. Until recently, studies on Fe withholding by the mammalian innate immune system focused on Fe(III), which is expected to be the dominant redox form in aerobic environments. Transferrin, lactoferrin, and lipocalin-2 (siderocalin) are proteins that contribute to the nutritional immunity by sequestering Fe(III) or Fe(III)-siderophores.13, 15 CP is the first Fe(II) withholding protein that has been described.7 CP binds Fe(II) at its His6 site and the Ca(II)-bound heterotetramer displays subpicomolar affinity for this ion.7 These observations suggested a potential role for CP in withholding Fe in the reduced Fe(II) form from microbial pathogens, and that Fe(II) withholding may be an underappreciated form of nutritional immunity. Initially, the preference of CP for Fe(II) over Fe(III) suggested that CP would sequester Fe only in anaerobic or reducing environments that stabilize the reduced ferrous oxidation state.7 Nevertheless, further investigation revealed that CP coordinated Fe in microbial growth media under aerobic conditions and in the absence of an exogenous reductant, and that the protein can shift the redox speciation of Fe from Fe(III) to Fe(II) under aerobic conditions.16 These observations suggested that CP may also withhold Fe under aerobic and micro-aerobic conditions. Since Fe plays important roles in bacterial physiology, the implications of Fe(II) sequestration by CP on bacterial pathogens have become a point of interest in recent years (Figure 1b).
In this perspective, we consider several recent studies that have evaluated the biological impact of Fe withholding by CP on diverse bacterial pathogens, most notably Pseudomonas aeruginosa, Acinetobacter baumannii, and Staphylococcus aureus (Figure 1b). These studies highlight instances where CP has or has not inhibited bacterial Fe acquisition, cases where CP-mediated inhibition of Fe uptake causes robust bacterial Fe starvation responses, and examples of how bacterial pathogens can resist the effects of Fe(II) withholding by CP. We also revisit select investigations of the metal-sequestering activity of CP in murine infection models and pose the need for evaluation of Fe(II) withholding by CP in the host setting. While relatively recent, explorations of the impact of Fe(II) withholding by CP on bacterial pathogens have provided further insight into strategies bacteria employ to adapt to Fe limited environments.
Fe(II) withholding by calprotectin and bacterial responses
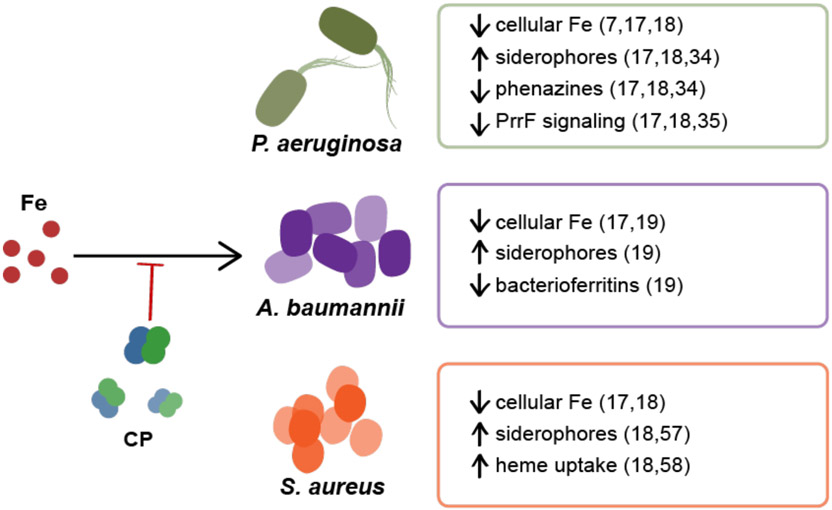
The discovery that CP sequesters Fe(II) afforded the hypothesis that CP contributes to Fe withholding by the host and motivated examination of the implications of its Fe sequestering ability for bacterial physiology and metal homeostasis. To date, these studies have described the responses of the Gram-negative pathogens Pseudomonas aeruginosa and Acinetobacter baumannii, and the Gram-positive pathogen Staphylococcus aureus, to Fe withholding by CP (Figure 2).17-19 While multiple metal starvation responses to CP have been observed for each of these pathogens, the following sections focus on the impact of CP-mediated Fe starvation responses and discuss adaptations of the bacteria to Fe withholding.17-19
Figure 2. CP induces Fe-starvation responses in both Gram-negative and Gram-positive bacteria.7, 17-19.
CP inhibits Fe uptake by P. aeruginosa, A. baumannii, and S. aureus, and various Fe starvation responses have been observed in each organism. For P. aeruginosa, a decrease in intracellular Fe levels has been observed as well as the upregulation of siderophore production and the downregulation of phenazine production. The downregulation of PrrF signaling in the form of repression of antR was also observed. For A. baumannii a decrease in cellular Fe and bacterioferritins accompanied by the upregulation of genes involved in siderophore production and utilization were observed. For S. aureus, lower cellular Fe levels, the upregulation of siderophore production, and the upregulation of genes involved in siderophore biosynthesis, siderophore transport, and heme uptake were observed.
Pseudomonas aeruginosa
Pseudomonas aeruginosa is a Gram-negative opportunistic pathogen that causes serious infections such as urinary tract infections, wound infections and respiratory infections, including cystic fibrosis (CF) lung infections.20 Prior studies have demonstrated the importance of Fe in the virulence of P. aeruginosa during CF lung infections.21, 22 Further, investigations of Fe homeostasis in P. aeruginosa have revealed central regulatory roles of Fe in P. aeruginosa metabolism and signaling.23 For example, the ferric Fe uptake regulator Fur contributes to increased expression of genes encoding anthranilate degradation enzymes in Fe replete conditions via repression of two small regulatory RNAs (PrrF RNAs).23 Anthranilate is a precursor of PQS, the pseudomonas quinolone signal, which is a quorum sensing molecule that is essential for multiple virulence functions.23, 24 P. aeruginosa employs several strategies to obtain Fe from the host, including Fe(II) uptake systems, Fe(III) uptake systems, and heme uptake systems.21 The FeoABC Fe uptake system allows P. aeruginosa to acquire Fe(II).25 Phenazines, which are redox-cycling secondary metabolites, can work in concert with the Feo system by reducing Fe(III) to Fe(II) to facilitate Fe(II) uptake by FeoABC.26 Fe(III) citrate may also serve as an Fe source for P. aeruginosa via a homolog of the Escherichia coli FecA system, which includes a Ton-B-dependent receptor (TBDR) paired with the Feo inner membrane transporter, following reduction to Fe(II) in the periplasm.27 Other Fe(III) uptake systems use siderophores to scavenge extracellular Fe(III) before transport via TBDRs.21, 28 P. aeruginosa synthesizes the siderophores pyoverdine (higher Fe affinity) and pyochelin (lower Fe affinity) and can also use siderophores biosynthesized by other microbes (xenosiderophores), such as enterobactin and ferrichrome.21 Heme is transported by the Phu system, where an outer-membrane TBDR can directly take up heme, or by the Has system, where a hemophore delivers heme to the HasR TBDR.29, 30 These Fe uptake strategies are vital for P. aeruginosa to infect the host and allow for its successful colonization, due to both the Fe requirement for central metabolism and regulatory roles of Fe for virulence traits such as biofilm formation.26, 30, 31
The extensive prior work on Fe homeostasis and signaling pathways in P. aeruginosa provided knowledge and tools to understand the consequences of Fe withholding by CP in this organism.32, 33 Moreover, P. aeruginosa is a clinically relevant case study of how CP affects bacterial Fe homeostasis because it co-localizes with high levels of CP in the CF lung.34 CP inhibits P. aeruginosa growth.7, 17 Two growth studies using medium depleted of one metal or combinations of metals demonstrated that Fe-depletion, but not Mn- or Zn-depletion, also inhibited P. aeruginosa growth, suggesting that the antibacterial activity of CP against this species may be linked to Fe withholding.7, 17 Examination of cell-associated metal levels of P. aeruginosa cultured in the presence of CP revealed a significant decrease in cell-associated Fe levels following growth in different media.17 Analysis using CP variants that lack one or both metal binding sites showed that CP-mediated reduction of cell-associated Fe levels in P. aeruginosa required the His6 site, in agreement with prior biophysical analyses of the high affinity Fe(II) binding at this site.7, 17 These observations provided a foundation for investigating whether CP induces Fe starvation responses in P. aeruginosa.
Siderophore production is a hallmark Fe starvation response. Two prior studies reported that the P. aeruginosa siderophore pyoverdine is produced in elevated quantities in the presence of CP.17, 34 Subsequent proteomic analysis of the response of P. aeruginosa to CP demonstrated increased expression of proteins involved in pyoverdine-mediated Fe uptake in the presence of CP.35 Moreover, PrrF sRNAs regulate an Fe sparing response in P. aeruginosa, and analyses of expression of the PrrF-regulated gene antR in response to CP indicated that Fe withholding by CP signals PrrF-mediated translational changes.17, 23, 36 While these findings support a CP-mediated Fe starvation and sparing responses in P. aeruginosa, the aforementioned proteomic analysis of P. aeruginosa indicated that CP did not affect many PrrF-dependent changes and thus appears to elicit an incomplete Fe-starvation response.35 In particular, CP treatment did not affect expression of many Fe-containing metabolic proteins, such as TCA cycle enzymes SdhABCD and AcnB, which are normally downregulated by PrrF sRNAs upon Fe limitation.35 It remains to be determined why CP appears to cause some Fe starvation responses but not others; however, it is possible that P. aeruginosa operates with tiered waves of Fe starvation responses based on Fe availability, as has been observed for Zn starvation responses in Bacillus subtilis.37 Overall, these findings support select Fe-dependent responses to CP in P. aeruginosa.
Two separate studies reported that CP inhibits phenazine production by P. aeruginosa.17, 34 Fe depletion of culture medium had a similar effect to CP on phenazine levels, whereas depletion of Zn or Mn did not, suggesting that CP inhibits phenazine production in an Fe-dependent manner.17 The proteomic analysis of the effect of CP on the P. aeruginosa proteome also supported these findings; growth of P. aeruginosa under CP-treated and Fe-depleted conditions resulted in a downregulation of proteins involved in phenazine biosynthesis.35 The mechanism by which CP inhibits phenazine production is unknown. It has been observed that phenazine production by P. aeruginosa is reduced in chronic CF lung infections,38 and whether or not the presence of CP contributes to this change warrants evaluation.
Collectively, these recent findings on how CP affects Fe homeostasis in P. aeruginosa provide a foundation for further investigation of the impact that CP may have on infections caused by this organism. For instance, one of the hallmarks of severe CF lung infection is the formation of P. aeruginosa biofilms, which is highly dependent on Fe availability, and normal biofilms cannot develop without sufficient Fe.39 Pyocyanin, a P. aeruginosa phenazine, aids biofilm formation by intercalating with DNA, increasing its viscosity.40 Together, the reduction of cellular Fe, promotion of Fe starvation responses, and reduction of phenazine production by CP suggest that CP may affect the ability of P. aeruginosa to form biofilms in the host, warranting further evaluation of this possibility.
Acinetobacter baumannii
Acinetobacter baumannii is a Gram-negative nosocomial pathogen that causes devastating infections in the lungs and wounds.41 It is able to colonize despite host-imposed stress conditions, including Fe limitation.42 A. baumannii has established Fe(III) uptake systems and putative ferrous and heme Fe uptake systems based on discovered feoABC and hemO gene clusters.25, 43-45 A. baumannii produces the siderophores fimsbactin, baumannoferrin, and acinetobactin.42 Based on the high conservation of the genes encoding machinery for acinetobactin biosynthesis in clinical isolates, it is considered the major siderophore used by A. baumannii and is currently understood to be the only one required for virulence.46 Heme utilization systems in A. baumannii are less well-characterized, but some strains have been shown to have conserved tonB genes related to the TonB-ExbB-ExbD energy-transducing complex used in other Gram-negative bacterial species for heme transport.42, 47 In addition, a recent study identified a hemO gene cluster in hypervirulent strains of A. baumannii, suggesting the importance of heme as a nutrient source during infection.48 Several pathogenic A. baumannii strains have homologs of the Feo system and upregulate a feoA homolog during infection.44 These data suggest that A. baumannii has multiple strategies for acquiring Fe in vivo.
A recent study reported a multi-metal response of A. baumannii to CP that included an Fe starvation response.19 Cellular Fe levels in A. baumannii were reduced by CP treatment, in agreement with a prior study.17, 19 Transcriptional analysis of the response of A. baumannii to CP revealed the upregulation of several genes involved in Fe-regulated processes, including those encoding the biosynthesis and transport of acinetobactin and fimsbactin.19 The genes feoAB and fhuE, which encode a Fe(II) receptor and a xenosiderophore receptor, respectively, were also upregulated.19, 49 Furthermore, genes encoding Fe storage systems, such as bacterioferritins, were downregulated, indicative of a potential Fe sparing response.19 Fe-dependent responses to CP treatment such as increased levels of proteins involved in siderophore biosynthesis were also obsesrved at the proteome level in this study.19 Together, these observations indicate that CP induces and Fe starvation response in A. baumannii.
Fe withholding from A. baumannii by CP may have important implications in the host environment. Prior work has demonstrated that A. baumannii strains defective in Fe uptake through the knockout of the Fur-regulated genes tonB3 (energy transducing complex) and feoB (ferrous Fe uptake) exhibited reduced growth in human serum.47 Another report showed that strains of A. baumannii isolated from patients with nosocomial infections exhibited greater Fe-uptake capability than non-human isolates.50 Thus, the possibility that Fe withholding by CP impacts virulence by A. baumannii during infection deserves further consideration.
Staphylococcus aureus
Staphylococcus aureus is a Gram-positive bacterial pathogen that causes many types of infections, including wound, respiratory, and bloodstream infections.51 The virulence of S. aureus is linked to Fe homeostasis. Fur modulates virulence traits such as host cell attachment and siderophore production, and the inactivation of heme transporters IsdE and HtsA attenuates the virulence of S. aureus.52, 53 Indeed, S. aureus uses multiple strategies to obtain Fe in the host with heme being the preferred source. It is capable of obtaining heme via the secretion of hemolysins which lyse red blood cells to release hemoglobin and the use of the dedicated Isd heme uptake system.53, 54 Moreover, S. aureus biosynthesizes two siderophores, staphyloferrin A and B, to sequester Fe and these siderophores can facilitate the removal of Fe from lactoferrin or transferrin.54, 55 S. aureus can also utilize xenosiderophores for iron uptake via the FhuCBG transporter.54, 56
Metal inventory analysis of several S. aureus laboratory strains and clinical isolates demonstrated that CP treatment reduces cellular Fe levels.18 This Fe withholding by CP has several metabolic and transcriptional effects on S. aureus that are indicative of Fe starvation. On the transcriptional level, two Fur-regulated genes that are upregulated under Fe starvation, sirA, encoding the staphyloferrin B receptor, and isdC, encoding a cell-wall anchored protein of the Isd heme transport system, display increased expression in S. aureus in the presence of CP.18 Additionally, two separate transcriptomics studies showed that transcripts for proteins involved in Fe-regulated processes such as siderophore biosynthesis (sbnC),57, 58 siderophore transport (sirA and sirB),57, 58 heme uptake (isdABCDEFG),58 and ferrichrome transport (fhuB)57, 58 are upregulated by CP, indicating an induction of Fe starvation responses by CP. Some genes involved in Fe storage and utilization in S. aureus were also shown to be downregulated by the presence of CP, namely one encoding a ferritin family protein and the alpha and beta subunits of an L-serine dehydratase Fe-S cluster protein, suggesting a putative Fe sparing response.57 On the level of metabolites, an increase in siderophore production by S. aureus in the presence of CP has been observed,18 echoing the enhanced siderophore production seen with P. aeruginosa and A. baumannii.17, 19 Together, these observations indicate that CP impacts S. aureus Fe homeostasis, and further studies are required to understand how CP affects Fe-regulated virulence traits in S. aureus. In particular, the Fe-regulated staphylococcal alpha-hemolysin plays an essential role in S. aureus virulence,59 and the possibility that CP alters production of alpha-hemolysin is one potential avenue of investigation.
Other bacterial pathogens
Observations of Fe withholding by CP have also been noted in some other bacterial species. Two studies that reported cellular metal levels in Escherichia coli (strains K-12 and uropathogenic UTI89) indicated a reduction of cellular Fe levels upon CP treatment.17, 60 Additionally, CP has been observed to reduce cell-associated Fe in the Gram-negative bacterial pathogens Salmonella enterica serovar Typhimurium and Klebsiella pneumoniae.17 We anticipate that further studies will show that CP has the capacity to sequester Fe from a wider range of bacteria. Case-by-case studies of the interplay between CP and various bacterial species will provide more insight into how Fe withholding – and more broadly metal withholding – affects bacterial growth and virulence when challenged with this innate immune protein.
Iron withholding by CP is organism- and medium-dependent
While the recent studies highlighted above uncovered the Fe withholding capabilities of CP, some earlier studies reported that CP does not withhold Fe from bacterial pathogens.61-63 These contrasting experimental outcomes – specifically whether or not a reduction in the cell-associated level of Fe or another metal is observed – most likely stem from different growth conditions. For example, an early analysis of cellular metal levels in A. baumannii reported that CP treatment reduced cellular levels of Mn and Zn, but not Fe.61 This study was performed using growth media containing a 5:1 ratio of Mn:Fe, which is not physiologically relevant and may have prevented Fe withholding responses. Two separate studies on S. aureus reported a reduction of cellular Mn levels, but negligible effect on cellular Fe levels.62, 63 These studies were performed in a Tryptic soy broth (TSB)-based medium, and two subsequent investigations reported that CP reduces cellular Fe levels in S. aureus during growth in Luria Broth (LB), but not in TSB-based medium.17, 18 Medium-specific effects of CP on cellular metal levels have also been observed for P. aeruginosa, for which a decrease in Mn uptake was observed in TSB-based media but not in LB or a chemically defined medium.17, 18 The origins of such medium dependence are unclear and may be attributable to variables such as differences in metal levels or metal speciation within the medium, or metabolic changes in the bacteria that arise from varied medium composition or nutrient availability. Along these lines, the host environment contains niches with highly varied chemical environments, and both the metal ions sequestered by CP and bacterial responses to CP may vary depending on the chemical composition at different infection sites.
Pathogen strategies to resist Fe withholding by CP
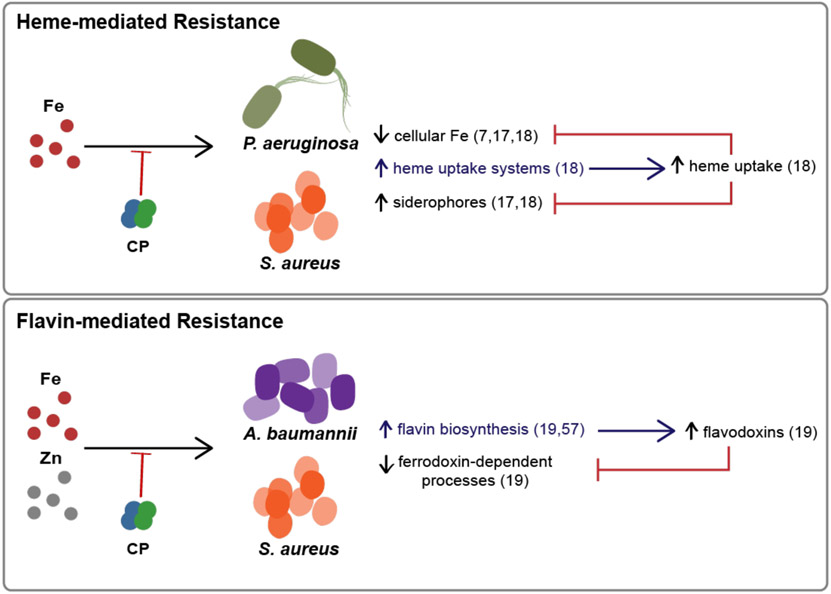
The studies that demonstrated Fe starvation responses in P. aeruginosa, A. baumannii and S. aureus also uncovered potential strategies that these pathogens use to adapt to Fe withholding by CP. In this section, we summarize two adaptations to Fe-withholding by CP that have been observed to date: the use of alternative Fe sources and reprogramming metabolism to reduce the utilization of Fe-dependent cofactors (Figure 3).
Figure 3. Models for how Pseudomonas aeruginosa, Staphylococcus aureus, and Acinetobacter baumannii resist Fe withholding by CP.
(Top) When experiencing Fe starvation by CP, P. aeruginosa and S. aureus upregulate heme uptake machinery and can use heme as an Fe source to resist CP-mediated depletion of cellular Fe and CP-mediated Fe starvation responses.18 (Bottom) When A. baumannii and S. aureus face metal starvation by CP, the pathogen upregulates enzymes involved in flavin biosynthesis, which could increase the use of flavodoxins. Flavodoxins can then be used as substitutes for ferredoxins, which reduces the cellular metabolic Fe requirement.19, 57.
Heme utilization
Heme is the most abundant Fe source in humans,64 and various bacterial pathogens can acquire heme from the host to fulfill their nutritional Fe requirements (Figure 3). A recent study evaluated how heme availability alters the responses of P. aeruginosa and S. aureus to CP.18 This work showed that these bacteria upregulate heme acquisition machinery when confronted with CP, and that the presence of heme prevents CP-induced reduction of cell-associated Fe levels in both organisms.18 In P. aeruginosa, Fe starvation responses to CP, including the upregulation of pyoverdine and repression of antR, were mitigated by the presence of heme.18 Heme utilization also protected S. aureus from CP-induced Fe-starvation responses, including upregulation of siderophore production.18 These data suggest that both P. aeruginosa and S. aureus can utilize heme to resist CP-mediated Fe starvation. Heme utilization as a means to resist the host Fe withholding factor transferrin has also been described,65 suggesting that heme utilization may represent a general response of bacterial pathogens to iron starvation.
Flavin biosynthesis
Recent work revealed that A. baumannii upregulates de novo flavin biosynthesis in response to Fe and Zn starvation by CP (Figure 3).19 Flavins are an essential cofactor that is involved in cellular redox processes and energy metabolism.66 Flavodoxins are used in place of ferredoxins by bacteria when non-metal substitutes are needed and it has been proposed that bacteria can substitute flavodoxins for ferredoxins as reduction agents in response to Fe starvation.67-69 In response to Fe and Zn starvation by CP, A. baumannii displays increased abundance of several enzymes of the flavin biosynthetic pathway, including RibBX and NusB.19 A study of the transcriptomic response of S. aureus to CP also revealed an upregulation of genes encoding proteins involved in riboflavin biosynthesis, such as ribH, ribBA, ribE, and ribD, with an ~1.5 fold increase in response to CP treatment.57 Thus, it is possible that bacteria with flavin biosynthesis pathways can lessen the metabolic impact of metal starvation by CP by utilizing flavin-dependent pathways.
Studies of microbial responses to CP in the host environment: a call for Fe analyses
The aformentioned observations of bacterial Fe starvation responses to CP provide compelling evidence for the role of CP in Fe withholding, but have not yet been evaluated in the host context. Various murine infection models have demonstrated the implications of metal withholding by CP in a mammalian host. Whereas all of the studies presented above employed human CP (hCP), investigations using murine models of infection provide information on the contributions of murine CP (mCP). mCP has been the subject of relatively few biochemical studies, and the available data indicates that hCP and mCP exhibit noteworthy similarities and differences.70, 71 For example, the C-terminal tail region of the S100A9 subunit of mCP (mS100A9) has a different amino acid composition than that of hCP (e.g., containing an HxHxH motif vs. an HHH motif, respectively). Nonetheless, consistent with hCP, mCP uses two His residues of the S100A9 C-terminal tail to form a His6 site and the protein depletes Fe from bacterial growth medium.70, 71 Thus, the His6 site is expected to bind Fe(II) with high affinity, though Fe coordination by mCP requires further characterization. In addition, hCP and mCP display different behavior in the presence of Ca(II) ions with mCP requiring higher Ca(II) equivalents to fully self-associate to the metal sequestering (αβ)2 heterotetramer. It is possible that hCP and mCP display disparate metal withholding activity and that the bacterial responses to hCP may vary from those observed for mCP, and further characterization of metal withholding by mCP will be required to explore this notion.
Murine infection studies addressing the role of CP in the host-pathogen interaction have been performed with S. aureus, A. baumannii, P. aeruginosa, S. Typhimurium, and Streptococcus species, and these contributions have provided an important foundation for our understanding of metal-withholding capabilities of CP during infection.61, 72-76 Some of these studies concluded that CP reduces bacterial viability via Zn(II) sequestration. For example, an A. baumannii pneumonia infection model found that CP-deficient mice were less protected against A. baumannii infection than wild-type mice, and that a strain impaired in Zn uptake via the knockout of znuB was more infectious in CP-deficient mice than it was in wild-type mice.61 A murine model of group A streptococcus infection model study indicated that the Zn(II)-responsive AdcR regulon was important for virulence in wild-type mice, but dispensable in mice lacking CP.72 Other murine model studies have indicated that the antimicrobial activity of CP is linked to Mn(II) withholding. S. aureus infection models have revealed that S. aureus mutants lacking Mn-dependent superoxide dismutases or the Mn-dependent phosphoglycerate mutase GpmA were less infectious in wild-type, but not in CP-deficient mice.73, 74 In contrast, studies performed using infection models of S. Typhimurimum indicated that expression of high affinity Mn(II) and Zn(II) transport systems facilitated the survival of this organism when challenged with CP.75, 76 To date, one investigation evaluated the effect of CP in co-infection.34 This study analyzed a murine model of S. aureus and P. aeruginosa respiratory infection and found that CP promoted their co-colonization in the murine lung. Each of these contributions provides valuable information to our understanding of how CP contributes to the defense against bacterial pathogens during infection. Most of the murine model studies performed to date focused on Mn and Zn withholding by CP because (i) many were performed before it was discovered that CP withholds Fe from bacterial pathogens, and (ii) all but the co-culture study focused on the contributions of specific Mn- or Zn-regulated virulence factors in bacterial pathogenesis. In light of recent characterization of Fe withholding by CP, it is worthwhile to evaluate how Fe withholding may contribute to CP-mediated defense against these pathogens and others in murine infection models. To evaluate this possibility, it will be important to analyze both markers of Fe starvation and the impact of Fe-uptake knockouts, as described for Mn- and Zn-uptake systems above, to identify a putative link between bacterial Fe homeostasis and susceptibility to growth inhibition by CP.
Conclusions and Perspectives
In this perspective, we describe several recent advances that have informed our current understanding of how CP affects Fe homeostasis in bacterial pathogens. From investigations of transcriptional, translational and metabolite responses, we learned that CP induces Fe starvation responses in bacterial pathogens. These studies provide a framework for continued investigations of Fe withholding by CP and its consequences for bacterial physiology and the host-microbe interaction. In closing, we consider how environmental factors of infection sites may influence Fe withholding by CP and other potential methods of bacterial adaptation to CP.
With access to multiple Fe sources in the host, a bacterial pathogen has the capability of prioritizing one source over the other in order to adapt to its environment. In general, bacterial pathogens prioritize Fe(II) and Fe(III) uptake based on the availability of each oxidation state. The availability of Fe(II) and Fe(III) is influenced by oxygen availability; Fe(II) predominates in anaerobic environments whereas Fe(III) is abundant in oxygenated environments. To adapt to changing oxygen availability, many bacteria utilize oxygen sensors to regulate expression of Fe uptake machinery. In general, bacteria employ oxygen sensor transcription factors (e.g. Fnr in E. coli, Anr in P. aeruginosa) which contain Fe-sulfur clusters to sense oxygen levels and activate gene expression.77, 78 Some infection sites are characterized as micro-aerobic, or even anaerobic environments, necessitating the use of these transcription factors to adapt to changing oxygen levels. In E. coli, Fe(II) uptake systems are under the control of Fur and Fnr such that their expression is controlled by Fe and oxygen levels.79 While dual activity of Fur and Anr in P. aeruginosa on Feo expression has not been characterized, it has been observed that P. aeruginosa adapts its Fe acquisition strategies to prioritize Fe(III) or Fe(II) uptake in aerobic or anaerobic settings, respectively.21 The relative availability of Fe(II) and Fe(III) is also influenced by pH. Like oxygen availability, the pH of infection sites is varied,25, 80-82 and in acidic environments the Fe(II):Fe(III) ratio is increased, causing bacteria prioritize Fe(II) uptake. At (near-)neutral pH, CP binds divalent metals at its His6 site with the relative affinities Kd,Mn > Kd,Fe > Kd,zn > Kd,Ni.9 Recent work has shown that CP does not effectively withhold Mn in mildly acidic environments.83 How other metal affinities are altered and whether Fe withholding, like Mn withholding, is impaired under low pH requires further study. It is important to note that all microbiology studies of metal withholding by CP discussed in this perspective were performed aerobically and at (near-)neutral pH, and thus further work is required to understand how CP impacts bacterial Fe homeostasis under oxygen-limited and acidic conditions.
Fe withholding host defense proteins can impact the relative availability of Fe(II) and Fe(III). Nonetheless, our understanding of whether bacteria shift their Fe uptake strategies in response to Fe sequestering host defense proteins is limited. Prior work in S. aureus has revealed that heme utilization is prioritized when Fe is limited by transferrin, and a study described above report a similar shift to heme utilization when S. aureus and P. aeruginosa are treated with CP.18, 65 While each of these studies provide insight into bacterial responses to a single Fe withholding host protein, at infection sites bacteria are most likely exposed to a combination of these proteins, including lactoferrin, lipocalin-2, CP, hemopexin and haptoglobin. Broadly, determining whether bacterial pathogens adapt their Fe acquisition strategies in response to these host factors both alone and in combination will inform our understanding of in vivo bacterial adaptation to host Fe withholding.
Many bacteria, including S. aureus, P. aeruginosa, and A. baumannii, are capable of forming biofilms, and Fe plays a central role in biofilm development for each of these pathogens.39, 84-86 Biofilms formed by these pathogens are a major health threat because once established, they are difficult to treat due to decreased antibiotic permeability.87 In addition to nutrient limitation, decreased oxygen availability forms microenvironments where Fe(II) may be a dominant Fe source.88 While we would expect CP to effectively sequester Fe(II), it is not clear whether CP and other host factors can penetrate biofilms, and if they can, whether Fe limitation is an effective form of antimicrobial activity for the metabolically disparate populations within biofilms. Thus, further studies that examine how bacterial Fe uptake machinery and CP affect biofilm formation by biofilm-forming pathogens would facilitate better understanding of how biofilms form in the host and potentially inform strategies to prevent them.
In addition to the adaptive mechanisms used by bacterial pathogens to resist CP described above, there are other Fe acquisition strategies used by bacterial pathogens as an adaptation to host metal withholding that have yet to be explored in the context of Fe withholding by CP. For example, some pathogens, like members of the Neisseriaceae and Pasteurellaceae families, acquire Fe in the host by stealing host Fe-binding proteins lactoferrin and transferrin.89 Neisseria species produce a CP-binding protein receptor, CbpA, as a means of zinc piracy.90, 91 It is unclear whether CP affects Fe starvation in Neisseria, and if so, whether CbpA would affect this activity. Many pathogens, such as P. aeruginosa, are able to intercept siderophores from neighboring bacteria when Fe starved.21 It is possible that the effects of CP on bacterial Fe homeostasis may be attenuated by using such strategies, and these possibilities warrant further evaluation. In general, the evaluation of strategies used by bacterial pathogens to resist host-mediated metal withholding is critical to the understanding of how they compete with the host immune response, and the informed design of therapeutics that account for these adaptations.
To conclude, these initial studies of the biological implications of Fe withholding by CP have formed a foundation for further discovery in a range of bacterial pathogens and in various infection models. In particular, these recent findings highlight the importance of considering distinct Fe sources and the possibility of bacterial adaptations to Fe withholding. Further evaluation of how environmental variations at infection sites and additional strategies of bacterial adapation impact Fe withholding by CP will better inform how CP may contribute to the Fe withholding response during infection. Finally, an assessment of how Fe withholding by CP contributes to its innate immune functions in the host environment is highly warranted based on the current evidence presented in this perspective and will advance our understanding of the host defense role of CP.
Acknowledgements
We thank our past and current lab members and collaborators for their invaluable contributions to elucidating metal sequestration by calprotectin. We thank the National Institutes of Health (R01 GM118695 and R01 GM126376) for supporting our current work on calprotectin. A.O.O. received support from the MIT Undergraduate Research Opportunities Program (UROP). E.M.Z. is a recipient of an NSF Graduate Research Fellowship and Whitaker Health Science Fund Fellowship.
References
- [1].Weinberg ED (1975) Nutritional immunity. Host's attempt to withold iron from microbial invaders, J. Am. Med. Assoc 231, 39–41. [DOI] [PubMed] [Google Scholar]
- [2].Hood MI, and Skaar EP (2012) Nutritional immunity: transition metals at the pathogen—host interface, Nat. Rev. Microbiol 10, 525–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zygiel EM, and Nolan EM (2018) Transition metal sequestration by the host-defense protein calprotectin, Annu. Rev. Biochem 87, 621–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Korndörfer IP, Brueckner F, and Skerra A (2007) The crystal structure of the human (S100A8/S100A9)2 heterotetramer, calprotectin, illustrates how conformational changes of interacting α-helices can determine specific association of two EF-hand proteins, J. Mol. Biol 370, 887–898. [DOI] [PubMed] [Google Scholar]
- [5].Damo SM, Kehl-Fie TE, Sugitani N, Holt ME, Rathi S, Murphy WJ, Zhang Y, Betz C, Hench L, Fritz G, Skaar EP, and Chazin WJ (2013) Molecular basis for manganese sequestration by calprotectin and roles in the innate immune response to invading bacterial pathogens, Proc. Natl. Acad. Sci. U.S.A 110, 3841–3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gagnon DM, Brophy MB, Bowman SEJ, Stich TA, Drennan CL, Britt RD, and Nolan EM (2015) Manganese binding properties of human calprotectin under conditions of high and low calcium: X-ray crystallographic and advanced electron paramagnetic resonance spectroscopic analysis, J. Am. Chem. Soc 137, 3004–3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Nakashige TG, Zhang B, Krebs C, and Nolan EM (2015) Human calprotectin is an iron-sequestering host-defense protein, Nat. Chem. Biol 11, 765–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Nakashige TG, Stephan JR, Cunden LS, Brophy MB, Wommack AJ, Keegan BC, Shearer JM, and Nolan EM (2016) The hexahistidine motif of host-defense protein human calprotectin contributes to zinc withholding and its functional versatility, J. Am. Chem. Soc 138, 12243–12251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Nakashige TG, Zygiel EM, Drennan CL, and Nolan EM (2017) Nickel sequestration by the host-defense protein human calprotectin, J. Am. Chem. Soc 139, 8828–8836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Nacken W, and Kerkhoff C (2007) The hetero-oligomeric complex of the S100A8/S100A9 protein is extremely protease resistant, FEBSLett. 581, 5127–5130. [DOI] [PubMed] [Google Scholar]
- [11].Stephan JR, and Nolan EM (2016) Calcium-induced tetramerization and zinc chelation shield human calprotectin from degradation by host and bacterial extracellular proteases, Chem. Sci 7, 1962–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Brini M, Ottolini D, Calì T, and Carafoli E (2013) Calcium in health and disease, Met. Ions Life Sci 20, 87–93. [DOI] [PubMed] [Google Scholar]
- [13].Cassat JE, and Skaar EP (2013) Iron in infection and immunity, Cell Host Microbe 13, 509–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Frawley ER, and Fang FC (2014) The ins and outs of bacterial iron metabolism, Mol. Microbiol 93, 609–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Golonka R, Yeoh BS, and Vijay-Kumar M (2019) The tug-of-war between bacterial siderophores and innate immunity, J. Innate Immun 11, 249–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Nakashige TG, and Nolan EM (2017) Human calprotectin affects the redox speciation of iron, Metallomics 9, 1086–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zygiel EM, Nelson CA, Brewer LK, Oglesby-Sherrouse AG, and Nolan EM (2019) The innate immune protein human calprotectin induces iron starvation responses in Pseudomonas aeruginosa, J. Biol. Chem 294, 3549–3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zygiel EM, Obisesan AO, Nelson CE, Oglesby AG, and Nolan EM (2021) Heme protects Pseudomonas aeruginosa and Staphylococcus aureus from calprotectin-induced iron starvation, J. Biol. Chem 296, 100160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wang J, Lonergan ZR, Gonzalez-Gutierrez G, Nairn BL, Maxwell CN, Zhang Y, Andreini C, Karty JA, Chazin WJ, Trinidad JC, Skaar EP, and Giedroc DP (2019) Multi-metal restriction by calprotectin impacts de novo flavin biosynthesis in Acinetobacter baumannii, Cell Chem. Biol 26, 745–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ahlgren HG, Benedetti A, Landry JS, Bernier J, Matouk E, Radzioch D, Lands LC, Rousseau S, and Nguyen D (2015) Clinical outcomes associated with Staphylococcus aureus and Pseudomonas aeruginosa airway infections in adult cystic fibrosis patients, BMC Pulm. Med 15, 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cornelis P, and Dingemans J (2013) Pseudomonas aeruginosa adapts its iron uptake strategies in function of the type of infections, Front. Cell Infect. Microbiol 3, 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Minandri F, Imperi F, Frangipani E, Bonchi C, Visaggio D, Facchini M, Pasquali P, Bragonzi A, and Visca P (2016) Role of iron uptake systems in Pseudomonas aeruginosa virulence and airway infection, Infect. Immun 84, 2324–2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Oglesby AG, Farrow JM 3rd, Lee J-H, Tomaras AP, Greenberg EP, Pesci EC, and Vasil ML (2008) The influence of iron on Pseudomonas aeruginosa physiology: a regulatory link between iron and quorum sensing, J. Biol. Chem 283, 15558–15567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lin J, Cheng J, Wang Y, and Shen X (2018) The pseudomonas quinolone signal (PQS): Not just for quorum sensing anymore, Front. Cell Infect. Microbiol 8, 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lau CK, Krewulak KD, and Vogel HJ (2015) Bacterial ferrous iron transport: the Feo system, FEMS Microbiol. Rev 40, 273–298. [DOI] [PubMed] [Google Scholar]
- [26].Wang Y, Wilks JC, Danhorn T, Ramos I, Croal L, and Newman DK (2011) Phenazine-1-carboxylic acid promotes bacterial biofilm development via ferrous iron acquisition, J. Bacteriol 193, 3606–3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Marshall B, Stintzi A, Gilmour C, Meyer J-M, and Poole K (2009) Citrate-mediated iron uptake in Pseudomonas aeruginosa: involvement of the citrate-inducible FecA receptor and the FeoB ferrous iron transporter, Microbiology 155, 305–315. [DOI] [PubMed] [Google Scholar]
- [28].Budzikiewicz H (2001) Siderophores of the human pathogenic fluorescent pseudomonads, Curr. Top. Med. Chem 1, 1–6. [DOI] [PubMed] [Google Scholar]
- [29].Ochsner UA, Johnson Z, and Vasil ML (2000) Genetics and regulation of two distinct heme-uptake systems, phu and has, in Pseudomonas aeruginosa, Microbiology 146, 185–198. [DOI] [PubMed] [Google Scholar]
- [30].Huang W, and Wilks A (2017) Extracellular heme uptake and the challenge of bacterial cell membranes, Annu. Rev. Biochem 86, 799–823. [DOI] [PubMed] [Google Scholar]
- [31].Caldwell CC, Chen Y, Goetzmann HS, Hao Y, Borchers MT, Hassett DJ, Young LR, Mavrodi D, Thomashow L, and Lau GW (2009) Pseudomonas aeruginosa exotoxin pyocyanin causes cystic fibrosis airway pathogenesis, Am. J. Pathol 175, 2473–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Konings AF, Martin LW, Sharples KJ, Roddam LF, Latham R, Reid DW, and Lamont IL (2013) Pseudomonas aeruginosa uses multiple pathways to acquire iron during chronic infection in cystic fibrosis lungs, Infect. Immun 81, 2697–2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Nguyen AT, O'Neill MJ, Watts AM, Robson CL, Lamont IL, Wilks A, and Oglesby-Sherrouse AG (2014) Adaptation of iron homeostasis pathways by a Pseudomonas aeruginosa pyoverdine mutant in the cystic fibrosis lung, J. Bacteriol 196, 2265–2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Wakeman CA, Moore JL, Noto MJ, Zhang Y, Singleton MD, Prentice BM, Gilston BA, Doster RS, Gaddy JA, Chazin WJ, Caprioli RM, and Skaar EP (2016) The innate immune protein calprotectin promotes Pseudomonas aeruginosa and Staphylococcus aureus interaction, Nat. Commun 7, 11951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Nelson CE, Huang W, Zygiel EM, Nolan EM, and Oglesby AG (2021) The human innate immune protein calprotectin elicits a multimetal starvation response in Pseudomonas aeruginosa, Microbiol. Spectr 9, e00519–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Nelson CE, Huang W, Brewer LK, Nguyen AT, Kane MA, Wilks A, and Oglesby-Sherrouse AG (2019) Proteomic analysis of the Pseudomonas aeruginosa iron starvation response reveals PrrF small regulatory RNA-dependent iron regulation of twitching motility, amino acid metabolism, and zinc homeostasis proteins, J. Bacteriol 201, e00754–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Shin J-H, and Helmann JD (2016) Molecular logic of the Zur-regulated zinc deprivation response in Bacillus subtilis, Nat. Commun 71, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hunter RC, Klepac-Ceraj V, Lorenzi MM, Grotzinger H, Martin TR, and Newman DK (2012) Phenazine content in the cystic fibrosis respiratory tract negatively correlates with lung function and microbial complexity, Am. J. Respir. Cell Mol. Biol 47, 738–45. [DOI] [PubMed] [Google Scholar]
- [39].Banin E, Vasil ML, and Greenberg EP (2005) Iron and Pseudomonas aeruginosa biofilm formation, Proc. Natl. Acad. Sci. U.S.A 102, 11076–11081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Das T, Kutty SK, Tavallaie R, Ibugo AI, Panchompoo J, Sehar S, Aldous L, Yeung AW, Thomas SR, Kumar N, Gooding JJ, and Manefield M (2015) Phenazine virulence factor binding to extracellular DNA is important for Pseudomonas aeruginosa biofilm formation, Sci. Rep 5, 8398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Dijkshoorn L, Nemec A, and Seifert H (2007) An increasing threat in hospitals: multidrug-resistant Acinetobacter baumannii, Nat. Rev. Microbiol 5, 939–951. [DOI] [PubMed] [Google Scholar]
- [42].Zimbler DL, Penwell WF, Gaddy JA, Menke SM, Tomaras AP, Connerly PL, and Actis LA (2009) Iron acquisition functions expressed by the human pathogen Acinetobacter baumannii, BioMetals 22, 23–32. [DOI] [PubMed] [Google Scholar]
- [43].Bohac TJ, Fang L, Giblin DE, and Wencewicz TA (2019) Fimsbactin and acinetobactin compete for the periplasmic siderophore binding protein BauB in pathogenic Acinetobacter baumannii, ACS Chem. Biol 14, 674–687. [DOI] [PubMed] [Google Scholar]
- [44].Álvarez-Fraga L, Vázquez-Ucha JC, Martinez-Guitián M, Vallejo JA, Bou G, Beceiro A, and Poza M (2018) Pneumonia infection in mice reveals the involvement of the feoA gene in the pathogenesis of Acinetobacter baumannii, Virulence 9, 496–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Runyen-Janecky LJ (2013) Role and regulation of heme iron acquisition in Gram-negative pathogens, Front. Cell Infect. Microbiol 3, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Sheldon JR, and Skaar EP (2020) Acinetobacter baumannii can use multiple siderophores for iron acquisition, but only acinetobactin is required for virulence, PLoS Pathog. 16, e10089995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Runci F, Gentile V, Frangipani E, Rampioni G, Leoni L, Lucidi M, Visaggio D, Harris G, Chen W, Stahl J, Averhoff B, and Visca P (2019) Contribution of active iron uptake to Acinetobacter baumannii pathogenicity, Infect. Immun 87, e00755–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Giardina BJ, Shahzad S, Huang W, and Wilks A (2018) Heme uptake and utilization by hypervirulent Acinetobacter baumannii LAC-4 is dependent on a canonical heme oxygenase (abHemO), Arch. Biochem. Biophys 672, 108066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Funahashi T, Tanabe T, Mihara K, Miyamoto K, Tsujibo H, and Yamamoto S (2012) Identification and characterization of an outer membrane receptor gene in Acinetobacter baumannii required for utilization of desferricoprogen, rhodotorulic acid, and desferrioxamine B as xenosiderophores, Biol. Pharm. Bull 35, 753–760. [DOI] [PubMed] [Google Scholar]
- [50].Antunes LC, Imperi F, Carattoli A, and Visca P (2011) Deciphering the multifactorial nature of Acinetobacter baumannii pathogenicity, PLos One 6, e22674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Tong SY, Davis JS, Eichenberger E, Holland TL, and Fowler VG Jr (2015) Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management, Clin. Microbiol. Rev 28, 603–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Hussain M, Becker K, von Eiff C, Schrenzel J, Peters G, and Herrmann M (2001) Identification and characterization of a novel 38.5-kilodalton cell surface protein of Staphylococcus aureus with extended-spectrum binding activity for extracellular matrix and plasma proteins, J. Bacteriol 183, 6778–6786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Mason WJ, and Skaar EP (2009) Assessing the contribution of heme-iron acquisition to Staphylococcus aureus pneumonia using computed tomography. , PLos One 4, e66668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Hammer ND, and Skaar EP (2011) Molecular mechanisms of Staphylococcus aureus iron acquisition, Annu. Rev. Microbiol 65, 129–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Madsen JLH, Johnstone TC, and Nolan EM (2015) Chemical synthesis of staphyloferrin B affords insight into the molecular structure, iron chelation, and biological activity of a polycarboxylate siderophore deployed by the human pathogen Staphylococcus aureus, J. Am. Chem. Soc 137, 9117–9127. [DOI] [PubMed] [Google Scholar]
- [56].Sebulsky MT, Hohnstein D, Hunter MD, and Heinrichs DE (2000) Identification and characterization of a membrane permease involved in iron-hydroxamate transport in Staphylococcus aureus, J. Bacteriol 182, 4394–4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Cho H, Jeong D-W, Liu Q, Yeo W-S, Vogl T, Skaar EP, Chazin WJ, and Bae T (2015) Calprotectin increases the activity of the SaeRS two component system and murine mortality during Staphylococcus aureus infections, PLoS Pathog. 11, 1005026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Peng H, Shen J, Edmonds KA, Luebke JL, Hickey AK, Palmer LD, Chang FJ, Bruce KA, Kehl-Fie TE, Skaar EP, and Giedroc DP (2017) Sulfide homeostasis and nitroxyl intersect via formation of reactive sulfur species in Staphylococcus aureus, mSphere 2, e00082–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Torres VJ, Attia AS, Mason WJ, Hood MI, Corbin BD, Beasley FC, Anderson KL, Stauff DL, McDonald WH, Zimmerman LJ, Friedman DB, Heinrichs DE, Dunman PM, and Skaar EP (2010) Staphylococcus aureus fur regulates the expression of virulence factors that contribute to the pathogenesis of pneumonia, Infect. Immun 78, 1618–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Besold AN, Culbertson EM, Nam L, Hobbs RP, Boyko A, Maxwell CN, Chazin WJ, Marques AR, and Culotta VC (2018) Antimicrobial action of calprotectin that does not involve metal witholding., Metallomics 10, 1728–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Hood MI, Mortensen BL, Moore JL, Zhang Y, Kehl-Fie TE, Sugitani N, Chazin WJ, Caprioli RM, and Skaar EP (2012) Identification of an Acinetobacter baumannii zinc acquisition system that facilitates resistance to calprotectin-mediated zinc sequestration, PLoS Pathog. 8, e1003068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Radin JN, Kelliher JL, Parraga Solórzano PK, and Kehl-Fie TE (2016) The two-component system ArlRS and alterations in metabolism enable Staphylococcus aureus to resist calprotectin-induced manganese starvation, PLoS Pathog. 12, e1006040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Radin JN, Zhu J, Brazel EB, McDevitt CA, and Kehl-Fie TE (2018) Synergy between nutritional immunity and independent host defenses contributes to the importance of the MntABC manganese transporter during Staphylococcus aureus infection, Infect. Immun 87, e00642–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Hooda J, Shah A, and Zhang L (2014) Heme, an essential nutrient from dietary proteins, critically impacts diverse physiological and pathological processes, Nutrients 6, 1080–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Skaar EP, Humayun M, Bae T, DeBord KL, and Schneewind O (2004) Iron-source preference of Staphylococcus aureus infections, Science 305, 1626–1628. [DOI] [PubMed] [Google Scholar]
- [66].Massey V (2000) The chemical and biological versatility of riboflavin, Biochem. Soc. Trans 28, 283–296. [PubMed] [Google Scholar]
- [67].Roche J, Boyd P, McKay R, and Geider R (1996) Flavodoxin as an in situ marker for iron stress in phytoplankton, Nature 382, 802–805. [Google Scholar]
- [68].Cisternas SISCJ, and García-Angulo VA (2018) Overview on the bacterial iron-riboflavin metabolic axis, Front. Microbiol 9, 1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Tognetti VB, Zurbriggen MD, Morandi EN, Fillat MF, Valle EM, Hajirezaei M-R, and Carrillo N (2007) Enhanced plant tolerance to iron starvation by functional substitution of chloroplast ferredoxin with a bacterial flavodoxin, Proc. Natl. Acad. Sci. U.S.A 104, 11495–11500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Hadley RC, Gu Y, and Nolan EM (2018) Initial biochemical and functional evaluation of murine calprotectin reveals Ca(II)-dependence and its ability to chelate multiple nutrient transition metal ions, Biochemistry 57, 2846–2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Hadley RC, Gagnon DM, Ozarowski A, Britt RD, and Nolan EM (2019) Murine calprotectin coordinates Mn(II) at a hexahistidine site with Ca(II)-dependent affinity, Inorg. Chem 58, 13578–13590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Makthal N, Do H, Wendel BM, Olsen RJ, Helmann JD, Musser JM, and Kumaraswami M (2020) Group a streptococcus AdcR regulon participates in bacterial defense against host-mediated zinc sequestration and contributes to virulence, Infect. Immun 88, e00097–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Kehl-Fie TE, Chitayat S, Hood MI, Damo S, Restrepo N, Garcia C, Munro KA, Chazin WJ, and Skaar EP (2011) Nutrient metal sequestration by calprotectin inhibits bacterial superoxide defense, enhancing neutrophil killing of Staphylococcus aureus, Cell Host Microbe 10, 158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Radin JN, Kelliher JL, Solorzano PKP, Grim KP, Ramezanifard R, Slauch JM, and Kehl-Fie TE (2019) Metal-independent variants of phosphoglycerate mutase promote resistance to nutritional immunity and retention of glycolysis during infection, PLoS Pathog 15, e1007971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Diaz-Ochoa VE, Lam D, Lee CS, Klaus S, Behnsen J, Liu JZ, Chim N, Nuccio S-P, Rathi SG, Mastroianni JR, Edwards RA, Jacobo CM, Cerasi M, Battistoni A, Ouellette AJ, Goulding CW, Chazin WJ, Skaar EP, and Raffatellu M (2016) Salmonella mitigates oxidative stress and thrives in the inflamed gut by evading calprotectin-mediated manganese sequestration, Cell Host Microbe 19, 814–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Liu JZ, Jellbauer S, Poe AJ, Ton V, Pesciaroli M, Kehl-Fie TE, Restrepo NA, Hosking MP, Edwards RA, Battistoni A, Pasquali P, Lane TE, Chazin WJ, Vogl T, Roth J, Skaar EP, and Raffatellu M (2012) Zinc sequestration by the neutrophil protein calprotectin enhances Salmonella growth in the inflamed gut, Cell Host Microbe 11, 227–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Sestok AE, Linkous RO, and Smith AT (2018) Toward a mechanistic understanding of Feo-mediated ferrous iron uptake, Metallomics 142, 685–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Winteler HV, and Haas D (1996) The homologous regulators Anr of Pseudomonas aeruginosa and Fnr of Escherichia coli have overlapping but distinct specificities for anaerobically inducible promoters, Microbiology 142, 685–693. [DOI] [PubMed] [Google Scholar]
- [79].Beauchene NA, Mettert EL, Moore LJ, Keles S, Willey ER, and Kiley PJ (2017) O2 availability impacts iron homeostasis in Escherichia coli, Proc. Natl. Acad. Sci. U.S A 114, 12261–12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Nugent S, Kumar D, Rampton D, and Evans D (2001) Intestinal luminal pH in inflammatory bowel disease: possible determinants and implications for therapy with aminosalicylates and other drugs, Gut 48, 571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Ravel K, Gajer P, Abdo Z, Schneider GM, Koenig SSK, McCulle SL, Karlebach S, Gorle R, Russell J, Tacket CO, Brotman RM, Davis CC, Ault K, Peralta L, and Forney LJ (2011) Vaginal microbiome of reproductive-age women, Proc. Natl. Acad. Sci. U.S.A 108, 4680–4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Elias PM (2007) The skin barrier as an innate immune element, Semin. Immunopathol 291, 3–14. [DOI] [PubMed] [Google Scholar]
- [83].Rosen T, and Nolan EM (2020) Metal sequestration and antimicrobial activity of human calprotectin are pH-dependent, Biochemistry 59, 2468–2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Gaddy JA, and Actis LA (2009) Regulation of Acinetobacter baumannii biofilm formation, Future Microbiol. 4, 273–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Mulcahy LR, Isabella VM, and Lewis K (2014) Pseudomonas aeruginosa biofilms in disease, Microb. Ecol 68, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Moormeier DE, and Beyles KW (2017) Staphylococcus aureus biofilm: a complex developmental organism, Mol. Microbiol 104, 365–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Sharma D, Misba L, and Kahn AU (2019) Antibiotics versus biofilm: an emerging battleground in microbial communities, Antimicrob. Resist. Infect. Control 8, 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Hunter RC, Asfour F, Dingemans J, Osuna BL, Samad T, Malfroot A, Cornelis P, and Newman DK (2013) Ferrous iron is a significant component of bioavailable iron in cystic fibrosis airways, MBio 4, e00557–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Gray-Owen SD, and Schryvers AB (1996) Bacterial transferrin and lactoferrin receptors, Trends Microbiol. 4, 185–191. [DOI] [PubMed] [Google Scholar]
- [90].Stork M, Grijpstra J, Bos MP, Torres CM, Devos N, Poolman JT, Chazin WJ, and Tommassen J (2013) Zinc piracy as a mechanism of Neisseria meningitidis for evasion of nutritional immunity, PLoS Pathog. 9, e1003733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Jean S, Juneau RA, Criss AK, and Cornelissen CN (2016) Neisseria gonorrhoeae evades calprotectin-mediated nutritional immunity and survives neutrophil extracellular traps by production of TdfH, Infect. Immun 84, 2982–2994. [DOI] [PMC free article] [PubMed] [Google Scholar]