Abstract
Polychlorinated biphenyls (PCBs) are persistent environmental contaminants that continue to be of concern due to their varied toxicities. Upon human exposure, many PCBs with lower numbers of chlorine atoms are metabolized to hydroxylated derivatives (OH-PCBs), and cytosolic sulfotransferases can subsequently catalyze the formation of PCB sulfates. Recent studies have indicated that PCB sulfates bind reversibly with a high affinity to human serum proteins, and that they are also taken up by cells and tissues. Since PCB sulfates might be hydrolyzed to the more toxic OH-PCBs, we have investigated the ability of human hepatic microsomal sulfatase to catalyze this reaction. Twelve congeners of PCB sulfates were substrates for the microsomal sulfatase with catalytic rates exceeding that of dehydroepiandrosterone sulfate as a comparison substrate for steroid sulfatase (STS). These results are consistent with an intracellular mechanism for sulfation and de-sulfation that may contribute to retention and increased time of exposure to OH-PCBs.
Keywords: Polychlorinated biphenyls, PCB, PCB sulfate, sulfotransferase, human microsomal sulfatase, steroid sulfatase, STS, hydroxylated polychlorinated biphenyls, OH-PCB
1. Introduction
Polychlorinated biphenyls (PCBs) comprise a class of environmental contaminants derived from industrial sources with both legacy and contemporary origins. Worldwide production and use of PCBs for many varied applications were common for much of the 20th century (Erickson and Kaley, 2011). Although an increased acknowledgment of their complex and serious adverse environmental and human health effects led to bans on their intentional commercial production and use (ATSDR, 2000), they continue to persist in the environment. While exposures to these legacy sources of PCBs remain in our environment, it is increasingly apparent that there are also current, non-legacy, sources of PCBs that are produced as inadvertent by-products during the manufacture of some paints, pigments, varnishes, and other commercial products (Herkert et al., 2018; Hombrecher et al., 2021; Hu and Hornbuckle, 2010; Jahnke and Hornbuckle, 2019; Shanahan et al., 2015). The PCBs found in these newer sources, as well as the many environmentally relevant legacy sources, are often semivolatile, and air samples are usually enriched in lower chlorinated PCBs (i.e., those that contain fewer than five chlorine atoms on the biphenyl ring) (Ampleman et al., 2015; Harrad et al., 2006; Herrick et al., 2004; Weitekamp et al., 2021; Wethington and Hornbuckle, 2005).
In addition to being major contributors to PCBs found in indoor and outdoor air, the lower chlorinated PCBs are more susceptible to metabolic reactions that convert them into molecules with both enhanced potency and diversity of toxic mechanisms (Dhakal et al., 2018; Grimm et al., 2015b; James, 2001; Ludewig et al., 2008). A major metabolic pathway for the initial metabolism of lower chlorinated PCBs is the formation of hydroxylated (phenolic) derivatives. These hydroxylated PCBs (OH-PCBs), and metabolites resulting from their further biotransformation, are of interest due to their roles in such human health effects as cancer, various neurotoxicities, and endocrine dysfunction related to alterations in steroid and thyroid hormones (Brouwer et al., 1998; Grimm et al., 2013; Kester et al., 2000; Ludewig and Robertson, 2013; Pessah et al., 2019; Quinete et al., 2014; Sethi et al., 2017).
It is increasingly apparent that the metabolic sulfation of OH-PCBs plays an important role in the toxicity of lower chlorinated PCBs and the OH-PCBs derived from them. Sulfation of OH-PCBs in humans is catalyzed by cytosolic sulfotransferases (SULTs) (Duffel, 2018; Grimm et al., 2015b; Wang et al., 2006). These SULTs display complex specificities for OH-PCBs as substrates and inhibitors that are dependent upon the structure of the congener and on the specific isoform of SULT (Ekuase et al., 2014; Ekuase et al., 2011; Liu et al., 2006; Schuur et al., 1998; Wang et al., 2006). For example, one OH-PCB congener may be a substrate for a particular isoform of SULT, while it is a potent inhibitor of another isoform. Such interactions can also lead to a complex interplay between the metabolism of the OH-PCB to a sulfate metabolite and/or interference with the metabolic homeostasis of a physiological molecule such as estradiol or dehydroepiandrosterone that is regulated via sulfation (Kester et al., 2000; Parker et al., 2018).
While there has often been a general assumption that sulfate metabolites of phenols are more water-soluble and more readily excreted in the urine, increasing evidence indicates that this is not always the case for many PCB sulfates. Indeed, studies in rats indicate that a dose of 0.6 mg/kg of 4-PCB 11 sulfate administered by intravenous injection is taken up by tissues and further metabolized, with only a small percentage excreted in the urine (Grimm et al., 2015a). Metabolism of PCB sulfates may proceed through hydrolysis of the sulfuric acid ester, regeneration of the OH-PCB, and subsequent oxidation and/or conjugation (Grimm et al., 2015a). For example, 4-PCB 52 sulfate is taken up by immortalized human liver-derived HepG2 cells, with subsequent metabolic conversion to 4-OH PCB 52 (Rodriguez et al., 2018). These results, along with the ability of PCB sulfates to exhibit high-affinity reversible binding to serum proteins such as human serum albumin (Rodriguez et al., 2016) and transthyretin (Grimm et al., 2013), lead to the hypothesis that retention of PCB sulfates, selective tissue uptake, and enzyme-catalyzed cycling between the PCB sulfate and OH-PCB may be important components in PCB toxicity.
Although the ability of some OH-PCBs to serve as substrates for human SULTs has been previously examined (Ekuase et al., 2011; Grimm et al., 2015b; Liu et al., 2006; Wang et al., 2006), there have not been direct studies of the ability of PCB sulfates to serve as substrates for intracellular sulfatases. Such sulfatases would be critical to regeneration/retention of the more toxic OH-PCBs. Furthermore, human hepatic microsomal sulfatase (also known either as STS, steroid sulfatase, or arylsulfatase C) catalyzes the hydrolysis of steroid sulfates as well as some xenobiotic sulfates (Daniel and Chang, 1990; Pang et al., 1994; Reed et al., 2005; Shankaran et al., 1991). These observations led us to hypothesize that a sulfatase or sulfatases in human hepatic microsomal fractions can catalyze the hydrolysis of PCB-sulfates, and that the kinetics of this de-sulfation reaction will vary with the PCB-sulfate congener. Thus, in the present study, we have examined the ability of human hepatic microsomal fractions to catalyze the hydrolysis of twelve PCB sulfates that are potential metabolites of PCBs that have been commonly measured in indoor air.
2. Materials and methods
2.1. Chemicals and reagents
All PCB sulfates were synthesized and characterized as described previously (Lehmler et al., 2013; Li et al., 2010; Rodriguez et al., 2016). Tris(hydroxymethyl)aminomethane hydrochloride (Tris-HCl; Molecular Biology grade) and anhydrous sodium sulfate (ACS grade) were obtained from Research Products International (Mt. Prospect, IL, USA). Hydrochloric acid (37%, ACS grade), sodium dehydroepiandrosterone sulfate (98%), chloroform (ACS grade), and methylene blue were purchased from Sigma-Aldrich (St. Louis, MO, USA). Concentrated sulfuric acid (ACS grade) was obtained from Fisher Scientific (Pittsburgh, PA, USA).
2.2. Human hepatic microsomes
A mixed-gender pool of human hepatic microsomes (pool of 31 male, 19 female; ages 25-78; lot number 1110473), a pooled preparation of microsomes from 10 male donors (ages 21-58; lot number 0710494), and a pooled preparation of microsomes from 10 female donors (ages 31-72; lot number 1210079) were obtained from Sekisui Xenotech (Lenexa, KS, USA). Microsomes were stored at −80 °C in 0.25 M sucrose at a protein concentration of at least 20 mg/mL before thawing at 4 °C and subsequent use in assays for sulfatase activity. The concentration of microsomal protein was determined by the modified Lowry method with bovine serum albumin as standard (Bensadoun and Weinstein, 1976).
2.3. Sulfatase assays and kinetic analysis
The catalytic activity of the microsomal sulfatase with PCB sulfates as substrates was determined using a modification of the methylene blue paired ion extraction assay previously described for hydrolysis of various steroid sulfates and phenyl sulfates catalyzed by a sulfatase (Roy, 1987). The method employs extraction of the paired ion formed between methylene blue and an organic sulfate into chloroform, with subsequent quantitation of the paired ion by absorbance at 651 nm.
Briefly, assays for human hepatic sulfatase activity with PCB sulfates were conducted in 0.1 M Tris-HCl buffer, pH 7.4, in a total reaction volume of 0.4 mL at 37°C. Reaction mixtures contained the indicated concentration of PCB sulfate that had been prepared as a stock solution in Tris-HCl buffer at pH 7.4. Reactions were initiated by adding 0.1 to 0.5 mg of microsomal protein and incubated for 0, 3, 5, and/or 10 min as appropriate to obtain initial velocities of sulfatase activity. The t=0 assay mixture (methylene blue/chloroform added immediately after addition of microsomes) served as a control for the indicated concentration of PCB sulfate carried through the reaction. A reagent control (all components except PCB sulfate) was carried through the procedure, and the final absorbance was subtracted to obtain the absorbance at 651 nm related to the paired ion between methylene blue and the PCB sulfate. The concentrations of PCB sulfate in the original reaction mixture were calculated based upon previous determinations that 10 nmol of either 2-naphthyl sulfate or DHEA sulfate in a 0.4 ml assay carried through the methylene blue/chloroform extraction procedure with a final volume of 2 ml of chloroform yields a value of 0.3 absorbance units at 651 nm (Sekura et al., 1981; Sheng et al., 2001). Initial determinations of substrate specificity were determined at a concentration of 50 μM PCB sulfate and carried out in triplicate. Since the microsomes were pooled preparations, the resulting standard deviations represent the technical reproducibility of the assay method; they do not imply a measure of biological variability.
Kinetic constants for the microsomal sulfatase-catalyzed hydrolysis of PCB sulfates were determined by non-linear least-squares fit of initial velocities to the Michaelis Menten equation using the Enzyme Kinetics module of SigmaPlot 14.0 (Systat Software; San Jose, CA, USA). Concentrations of PCB sulfates or DHEA sulfate were chosen to provide values both above and below the final calculated apparent Km. Standard deviations calculated for Km(app) and Vmax values of the PCB sulfates represent the fit of all initial velocity vs. substrate data for sulfatase-catalyzed hydrolysis of all concentrations of each PCB sulfate.
3. Results
3.1. Hydrolysis of PCB sulfates catalyzed by human hepatic microsomal sulfatase
The choice of PCB sulfate congeners for this study (structures shown in Fig. 1) was based on those that would be metabolically derived from environmentally relevant lower chlorinated PCBs that are most commonly seen by indoor air sampling (Grimm et al., 2015b). Moreover, four PCB sulfates (i.e., 4’-PCB 3 Sulfate, 4-PCB 11 Sulfate, 4’-PCB 25 Sulfate, and 4’-PCB 26 Sulfate) have been recently detected in a pooled sample of human serum (Zhang et al., 2021). Dehydroepiandrosterone (DHEA) sulfate was used as a standard comparison substrate to determine the STS activity of the human hepatic microsomes.
Fig. 1.
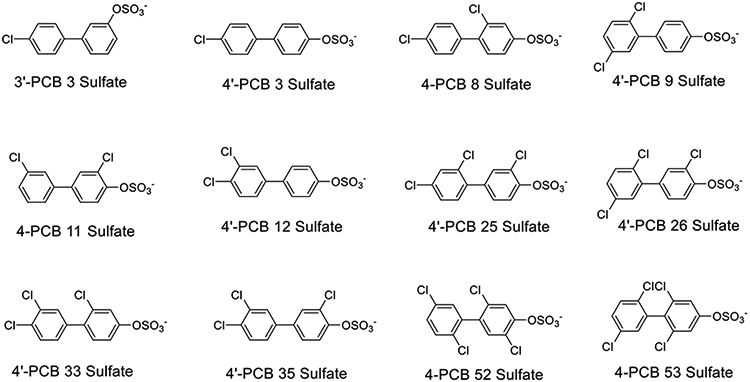
Chemical structures of the PCB sulfates utilized for studies of sulfatase activity with human hepatic microsomal preparations.
A preliminary assessment of the ability of human microsomal sulfatase to catalyze the hydrolysis of PCB sulfates was carried out using a mixed-gender pool of human hepatic microsomes. As seen in Fig. 2., the rates of hydrolysis of all twelve PCB sulfates (initial concentration of 50 μM) were higher than the rate of hydrolysis of 50 μM DHEA sulfate. Additionally, the rate of hydrolysis depended upon the structure of the PCB sulfate but not the total number of chlorine atoms in the PCB sulfate.
Fig. 2.

Sulfatase activity of a mixed-gender pool of human hepatic microsomes catalyzing the hydrolysis of DHEA sulfate and twelve PCB sulfate congeners. All rates are initial velocities measured at 50 μM concentrations of the indicated sulfates. Values indicated are the means and standard deviations of three technical replicates.
While this microsomal preparation was a pool of 50 mixed gender donors (31 male and 19 female), we also examined the potential for any differences in microsomes derived from male and female donors. Thus, as seen in Fig. 3, a similar set of assays were performed comparing a pooled preparation of hepatic microsomes from 10 male donors with a pooled preparation from 10 female donors. Six representative mono-, di-, tri-, and tetra-chlorinated PCB sulfates were examined at a concentration of 50 μM. The results indicated that there were no significant differences between the rates of hydrolysis of these PCB sulfates seen with the donor pools from males and females.
Fig. 3.

Comparison of the sulfatase activity of male and female pooled samples of human hepatic microsomes catalyzing the hydrolysis of DHEA sulfate and six PCB sulfate congeners. All rates are initial velocities measured at 50 μM concentrations of the indicated sulfates. Values indicated are the means and standard deviations of three technical replicates.
3.2. Kinetic analysis of the hydrolysis of PCB sulfates catalyzed by human hepatic microsomes
The response of the rate of PCB sulfate hydrolysis to the concentration of PCB sulfate is important for understanding the relevance of the hydrolytic reaction to concentrations that may be present in the environment of the sulfatase. Within the limitations of the assay method utilized, it was possible to determine kinetic constants for the reaction of six PCB sulfates catalyzed by the microsomal sulfatase and compare them with the kinetic constants for hydrolysis of DHEA sulfate. Initial velocities were measured at multiple concentrations of sulfate substrate, and values for apparent Km and Vmax were determined by non-linear regression fit to the Michaelis-Menten equation (Table 1).
Table 1.
Kinetic constants calculated for the human hepatic microsomal sulfatase-catalyzed hydrolysis of DHEA sulfate and six PCB-sulfates
| Substrate | Vmax nmol(min)−1(mg)−1 |
Km(app) μM |
Catalytic Efficiency Vmax/Km(app) nmol(min)−1(mg)−1(μM)−1 |
|---|---|---|---|
| DHEA Sulfate | 2.0 ± 0.2 | 18.1 ± 3.1 | 0.1 |
| 4’-PCB 12 Sulfate | 2.1 ± 0.2 | 16.6 ± 3.2 | 0.1 |
| 4’-PCB 3 Sulfate | 16.8 ± 3.8 | 41.0 ± 18.4 | 0.4 |
| 4-PCB 8 Sulfate | 10.7 ± 0.9 | 9.1 ± 2.2 | 1.2 |
| 4-PCB 11 Sulfate | 18.0 ± 2.8 | 15.1 ± 7.1 | 1.2 |
| 4’-PCB 25 Sulfate | 33.8 ± 3.0 | 27.9 ± 4.7 | 1.2 |
| 4-PCB 52 Sulfate | 7.6 ± 0.2 | 3.4 ± 0.4 | 2.3 |
The catalytic efficiency of an enzyme-catalyzed reaction with different substrates is an effective way to compare the specificity of an enzyme with various substrates in a way that would be relevant at low concentrations of the substrate. As seen in Table 1, 4’-PCB 12 sulfate exhibited a catalytic efficiency with the microsomal sulfatase that was the same as DHEA sulfate, but all of the other PCB sulfates displayed higher catalytic efficiencies as substrates for the microsomal sulfatase. Catalytic efficiency was over ten-fold higher than DHEA sulfate for four out of the six PCB sulfates examined, and 4-PCB 52 sulfate had a value over 20 times greater than DHEA sulfate. This finding indicates that PCB sulfates are hydrolyzed efficiently by human hepatic microsomal sulfatase, and it would be expected that this would occur at low concentrations of the PCB sulfate. It is also clear that at the low concentrations of PCB sulfate that would be expected (e.g., less than micromolar concentrations), the rate of conversion of the PCB sulfate to the OH-PCB would be dependent upon both the concentration of sulfatase and the intracellular concentration of PCB sulfate.
4. Discussion
Multiple toxicities of lower-chlorinated PCBs are often associated with the corresponding OH-PCB metabolites (Dhakal et al., 2018). Concerns about the toxicologic effects of these metabolites are exemplified by their conversion to reactive electrophilic quinones that can undergo reaction with cellular macromolecules to produce genotoxicity and initiate carcinogenesis (Ludewig and Robertson, 2013). Additionally, PCB quinones derived from OH-PCBs may undergo redox cycling to produce reactive oxygen species with varied toxic effects (Song et al., 2008). Many congeners of OH-PCBs are also potent inhibitors of estrogen sulfotransferase (SULT1E1), and this inhibition results in endocrine disruption via undesired estrogenic effects (Kester et al., 2000; Parker et al., 2018).
In view of these toxic responses to OH-PCBs, their biological persistence is of significant concern. While there is often the assumption that lower-chlorinated OH-PCBs would be relatively easily eliminated as conjugates, some lower-chlorinated OH-PCBs have unusually long half-lives in relation to what might be expected for these phenolic metabolites. For example, studies on a human cohort exposed to PCBs indicated that half-lives of the tri-chlorinated PCB 28 and its OH-PCB metabolites in plasma were on the order of several years (Quinete et al., 2017). Specifically, the plasma half-life for PCB 28 was calculated as 4.32 years, while the half-life for 4’-OH-PCB 25, a metabolite formed by cytochrome P450-mediated oxidation with an NIH-shift, was calculated as 6.46 years (Quinete et al., 2017). While other OH-PCB metabolites derived from PCB 28 had either longer or shorter elimination half-lives than the parent PCB 28 (Quinete et al., 2017), the observation that some of these OH-PCB metabolites had significantly longer elimination half-lives than PCB 28 raises the question of potential mechanisms for their retention.
A conjugation reaction such as sulfation might be expected to aid in the elimination of lower-chlorinated OH-PCBs, and previous studies have confirmed the ability of human cytosolic sulfotransferases to catalyze their congener-selective sulfation (Ekuase et al., 2014; Ekuase et al., 2011; Liu et al., 2006; Wang et al., 2006). As outlined in the Introduction, the ability of PCB sulfates to bind reversibly with high affinity to human serum proteins such as albumin and transthyretin, as well as their ability to be taken up by cells/tissues, suggests that sulfated metabolites of PCBs may be retained. For this to be a mechanism for retention of OH-PCBs, however, there would have to be intracellular hydrolysis of the PCB sulfate, and our current results indicate that a human hepatic microsomal sulfatase catalyzes congener-selective hydrolysis of PCB sulfates. As illustrated in Figure 4, the combination of serum transport of PCB sulfates and OH-PCBs, cellular uptake, and the SULT- and sulfatase-mediated cycling between the PCB sulfate and its OH-PCB, provides a potential mechanism for retention of the OH-PCB.
Fig. 4.
Proposed enzymatic interconversion of PCB sulfates and OH-PCBs with transport and retention by serum proteins (e.g., serum albumin, transthyretin, and others). The roles of congener- and tissue-specific uptake and efflux transporters for PCB sulfates and OH-PCBs remain to be determined.
The roles of sulfation and de-sulfation in the retention of both endogenous molecules and xenobiotics have ample precedence. For example, this process is important for the modulation of steroid hormone activity in tissues (Mueller et al., 2015), and it has been previously recognized as a key factor in the metabolic kinetics of drugs and other xenobiotics (Falany and Falany, 2007; Maier-Salamon et al., 2013; Pang et al., 1994). An additional aspect of a model such as that shown in Figure 4 is that toxicity of lower-chlorinated PCBs is tissue- and congener-specific. Changes in the expression of relevant SULTs and STS are age- and tissue-dependent (Dooley, 2000; Dubaisi et al., 2019; Ladumor et al., 2019; Miki et al., 2002), as is the specificity of these enzymes for OH-PCBs and PCB sulfates. Although the current study focused on the hepatic microsomal enzyme, STS is also present in extrahepatic tissues that include reproductive organs, the gastrointestinal tract, brain, kidney, lung, and others (Reed et al., 2005). Thus, further exploration of the potential role of PCB sulfates as mediators of extrahepatic toxicities through their retention and STS-mediated conversion to OH-PCBs is warranted. Moreover, the presence of relevant transporters that regulate the uptake and efflux of PCB sulfates and OH-PCBs would be important in tissue-specific toxicity. While the roles of such transporters involved in uptake and efflux of steroid hormones and their sulfated conjugates have received attention (Mueller et al., 2015), the congener-selectivity of these and other tissue-specific transporters for OH-PCBs and PCB sulfates remain to be more fully explored. Such an analysis of these transporters would also be important in understanding the relationships among the various cellular compartments represented in this model. For example, SULTs in the cytoplasm catalyze the formation of PCB sulfates in proximity to efflux transporters in the cell membrane. On the other hand, those PCB sulfates that reach the endoplasmic reticulum would be subject to the sulfatase-catalyzed formation of OH-PCBs. Those OH-PCBs would then be located in the same subcellular organelle as the cytochrome P450s that are responsible for formation of the catechol and hydroquinone precursors to the more toxic PCB quinones.
Finally, it should be noted that we have not conclusively determined that a single sulfatase in the microsomal preparations is responsible for the hydrolysis of the PCB sulfates. Indeed there is evidence for the presence of two forms of arylsulfatase C (STS) in humans (Shankaran et al., 1991). While hepatic STS is the most likely candidate based upon earlier characterization of the enzyme with xenobiotic sulfates (Pang et al., 1994), further studies will be necessary to determine the specific sulfatase(s) involved in these reactions with PCB sulfates.
5. Conclusions
Human hepatic sulfatase in a mixed-gender pool of microsomal fractions catalyzed the hydrolysis of structurally diverse lower-chlorinated PCB sulfates, with the catalytic efficiency of the reaction dependent upon the structure of the PCB metabolite. Studies with separate pooled fractions from males and females showed that although rates of reaction of six of these PCB sulfates depended upon the structure of the congener, there were no major gender differences in the rates of hydrolysis of 50 μM concentrations of six PCB sulfates that were examined. These results provide important supporting evidence that intracellular hydrolysis of PCB sulfates may participate in a cycle of sulfation and de-sulfation that could lead to retention of these PCB metabolites and continued exposure to the more toxic OH-PCBs.
Highlights.
Human hepatic microsomes catalyze the hydrolysis of PCB Sulfates.
Catalytic efficiency of the sulfatase depends upon the structure of the PCB sulfate.
No apparent gender difference was observed for pooled microsomal preparations.
A cycle of sulfation and de-sulfation may lead to the retention of toxic OH-PCBs.
Acknowledgment:
The authors thank Dr. Xueshu Li of the Synthesis Core of the Iowa Superfund Research Program for providing several PCB sulfates used in these experiments.
Funding:
This work was supported by NIH grants P42 ES013661 and P30 ES005605 from the National Institute of Environmental Health Sciences.
Abbreviations
- DHEA
dehydroepiandrosterone
- OH-PCB
hydroxylated polychlorinated biphenyl
- PCB 28
2,4,4’-trichlorobiphenyl
- 4’-OH PCB 25
2,3’,4-trichloro-4’-hydroxybiphenyl
- 4’-PCB 3 sulfate
4-chloro-4’-biphenylsulfate
- 4’-PCB 3 sulfate
4-chloro-3’-biphenylsulfate
- 4-PCB 8 sulfate
2,4’-dichloro-4-biphenylsulfate
- 4’-PCB 9 sulfate
2,5-dichloro-4’-biphenylsulfate
- 4-PCB 11 sulfate
3,3’-dichloro-4-biphenylsulfate
- 4’-PCB 12 sulfate
3,4-dichloro-4’-biphenylsulfate
- 4’-PCB 25 sulfate
2,3’,4-trichloro-4’-biphenylsulfate
- 4’-PCB 26 sulfate
2,3’,5-trichloro-4’-biphenylsulfate
- 4’-PCB 33 sulfate
2’,3,4-trichloro-4’-biphenylsulfate
- 4’-PCB 35 sulfate
3,3’,4-trichloro-4’-biphenylsulfate
- 4-OH PCB 52
2,2’,5,5’-tetrachloro-4-hydroxybiphenyl
- 4-PCB 52 sulfate
2,2’,5,5’-tetrachloro-4-biphenylsulfate
- 4-PCB 53 sulfate
2,2’,5’,6-trichloro-4-biphenylsulfate
- STS
human steroid sulfatase or arylsulfatase C
- SULT
human cytosolic sulfotransferase
- SULT1E1
human estrogen sulfotransferase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References Cited
- Ampleman MD, Martinez A, DeWall J, Rawn DF, Hornbuckle KC, Thorne PS, 2015. Inhalation and dietary exposure to PCBs in urban and rural cohorts via congener-specific measurements. Environ. Sci. Technol 49, 1156–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATSDR, 2000. Toxicological profile for polychlorinated biphenyls (PCBs). U.S. Department of Health and Human Services, Public Health Service; https://www.atsdr.cdc.gov/ToxProfiles/tp17.pdf (accessed 8/5/2021). [Google Scholar]
- Bensadoun A, Weinstein D, 1976. Assay of proteins in the presence of interfering materials. Anal. Biochem 70, 241–250. [DOI] [PubMed] [Google Scholar]
- Brouwer A, Morse DC, Lans MC, Schuur AG, Murk AJ, Klasson-Wehler E, Bergman A, Visser TJ, 1998. Interactions of persistent environmental organohalogens with the thyroid hormone system: mechanisms and possible consequences for animal and human health. Toxicol. Ind. Health 14. [DOI] [PubMed] [Google Scholar]
- Daniel WL, Chang PL, 1990. Comparison of arylsulfatase C and steroid sulfatase from human placenta and liver. Enzyme 43, 212–222. [DOI] [PubMed] [Google Scholar]
- Dhakal K, Gadupudi GS, Lehmler HJ, Ludewig G, Duffel MW, Robertson LW, 2018. Sources and toxicities of phenolic polychlorinated biphenyls (OH-PCBs). Environ. Sci. Pollut. Res. Int 25, 16277–16290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooley TP, Haldeman-Cahill R, Joiner J, and Wilborn TW, 2000. Expression profiling of human sulfotransferase and sulfatase gene superfamilies in epithelial tissues and cultures cells. Biochem Biophys Res Commun. 277, 236–245. [DOI] [PubMed] [Google Scholar]
- Dubaisi S, Caruso JA, Gaedigk R, Vyhlidal CA, Smith PC, Hines RN, Kocarek TA, Runge-Morris M, 2019. Developmental Expression of the Cytosolic Sulfotransferases in Human Liver. Drug Metab Dispos 47, 592–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffel MW, 2018. Sulfotransferases, in: McQueen CA (Ed.), Comprehensive Toxicology, 3rd ed. Elsevier, Oxford, pp. 407–428. [Google Scholar]
- Ekuase EJ, Lehmler HJ, Robertson LW, Duffel MW, 2014. Binding interactions of hydroxylated polychlorinated biphenyls (OHPCBs) with human hydroxysteroid sulfotransferase hSULT2A1. Chemico-biological Interactions 212, 56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekuase EJ, Liu Y, Lehmler HJ, Robertson LW, Duffel MW, 2011. Structure-activity relationships for hydroxylated polychlorinated biphenyls as inhibitors of the sulfation of dehydroepiandrosterone catalyzed by human hydroxysteroid sulfotransferase SULT2A1. Chemical Research in Toxicology 24, 1720–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson MD, Kaley RG 2nd, 2011. Applications of polychlorinated biphenyls. Environ. Sci. Pollut. Res. Int 18, 135–151. [DOI] [PubMed] [Google Scholar]
- Falany JL, Falany CN, 2007. Interactions of the human cytosolic sulfotransferases and steroid sulfatase in the metabolism of tibolone and raloxifene. J Steroid Biochem Mol Biol 107, 202–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm FA, He X, Teesch LM, Lehmler HJ, Robertson LW, Duffel MW, 2015a. Tissue Distribution, Metabolism, and Excretion of 3,3'-Dichloro-4'-sulfooxy-biphenyl in the Rat. Environ. Sci. Technol 49, 8087–8095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm FA, Hu D, Kania-Korwel I, Lehmler HJ, Ludewig G, Hornbuckle KC, Duffel MW, Bergman A, Robertson LW, 2015b. Metabolism and metabolites of polychlorinated biphenyls. Crit. Rev. Toxicol 45, 245–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm FA, Lehmler HJ, He X, Robertson LW, Duffel MW, 2013. Sulfated metabolites of polychlorinated biphenyls are high-affinity ligands for the thyroid hormone transport protein transthyretin. Environ. Health Perspect 121, 657–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrad S, Hazrati S, Ibarra C, 2006. Concentrations of polychlorinated biphenyls in indoor air and polybrominated diphenyl ethers in indoor air and dust in Birmingham, United Kingdom: implications for human exposure. Environ Sci Technol 40, 4633–4638. [DOI] [PubMed] [Google Scholar]
- Herkert NJ, Jahnke JC, Hornbuckle KC, 2018. Emissions of Tetrachlorobiphenyls (PCBs 47, 51, and 68) from Polymer Resin on Kitchen Cabinets as a Non-Aroclor Source to Residential Air. Environ. Sci. Technol 52, 5154–5160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrick RF, McClean MD, Meeker JD, Baxter LK, Weymouth GA, 2004. An unrecognized source of PCB contamination in schools and other buildings. Environ Health Perspect 112, 1051–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hombrecher K, Quass U, Leisner J, Wichert M, 2021. Significant release of unintentionally produced non-Aroclor polychlorinated biphenyl (PCB) congeners PCB 47, PCB 51 and PCB 68 from a silicone rubber production site in North Rhine-Westphalia, Germany. Chemosphere 285, 131449. [DOI] [PubMed] [Google Scholar]
- Hu D, Hornbuckle KC, 2010. Inadvertent polychlorinated biphenyls in commercial paint pigments. Environ. Sci. Technol 44, 2822–2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahnke JC, Hornbuckle KC, 2019. PCB Emissions from Paint Colorants. Environ. Sci. Technol 53, 5187–5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James MO, 2001. Polychlorinated biphenyls: Metabolism and metabolites, in: Robertson LW, Hansen LG (Eds.), PCBs: Recent Advances in Environmental Toxicology and Health Effects. The University Press of Kentucky, Lexington, KY, pp. 35–46. [Google Scholar]
- Kester MH, Bulduk S, Tibboel D, Meinl W, Glatt H, Falany CN, Coughtrie MW, Bergman A, Safe SH, Kuiper GG, Schuur AG, Brouwer A, Visser TJ, 2000. Potent inhibition of estrogen sulfotransferase by hydroxylated PCB metabolites: a novel pathway explaining the estrogenic activity of PCBs Endocrinology 141, 1897–1900. [DOI] [PubMed] [Google Scholar]
- Ladumor MK, Bhatt DK, Gaedigk A, Sharma S, Thakur A, Pearce RE, Leeder JS, Bolger MB, Singh S, Prasad B, 2019. Ontogeny of Hepatic Sulfotransferases and Prediction of Age-Dependent Fractional Contribution of Sulfation in Acetaminophen Metabolism. Drug Metab Dispos 47, 818–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmler HJ, He XR, Li XS, Duffel MW, Parkin S, 2013. Effective synthesis of sulfate metabolites of chlorinated phenols. Chemosphere 93, 1965–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Parkin S, Duffel MW, Robertson LW, Lehmler H-J, 2010. An efficient approach to sulfate metabolites of polychlorinated biphenyls. Environ. Int. 36, 843–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Apak TI, Lehmler HJ, Robertson LW, Duffel MW, 2006. Hydroxylated polychlorinated biphenyls are substrates and inhibitors of human hydroxysteroid sulfotransferase SULT2A1. Chem. Res. Toxicol 19, 1420–1425. [DOI] [PubMed] [Google Scholar]
- Ludewig G, Lehmann L, Esch H, Robertson LW, 2008. Metabolic Activation of PCBs to Carcinogens in Vivo - A Review. Environ. Toxicol. Pharmacol 25, 241–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludewig G, Robertson LW, 2013. Polychlorinated biphenyls (PCBs) as initiating agents in hepatocellular carcinoma. Cancer Lett 334, 46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier-Salamon A, Bohmdorfer M, Riha J, Thalhammer T, Szekeres T, Jaeger W, 2013. Interplay between metabolism and transport of resveratrol. Ann N Y Acad Sci 1290, 98–106. [DOI] [PubMed] [Google Scholar]
- Miki Y, Nakata T, Suzuki T, Darnel AD, Moriya T, Kaneko C, Hidaka K, Shiotsu Y, Kusaka H, Sasano H, 2002. Systemic distribution of steroid sulfatase and estrogen sulfotransferase in human adult and fetal tissues. Journal of Clinical Endocrinology & Metabolism. 87, 5760–5768. [DOI] [PubMed] [Google Scholar]
- Mueller JW, Gilligan LC, Idkowiak J, Arlt W, Foster PA, 2015. The Regulation of Steroid Action by Sulfation and Desulfation. Endocr Rev 36, 526–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang KS, Schwab AJ, Goresky CA, Chiba M, 1994. Transport, binding, and metabolism of sulfate conjugates in the liver. Chem Biol Interact 92, 179–207. [DOI] [PubMed] [Google Scholar]
- Parker VS, Squirewell EJ, Lehmler HJ, Robertson LW, Duffel MW, 2018. Hydroxylated and sulfated metabolites of commonly occurring airborne polychlorinated biphenyls inhibit human steroid sulfotransferases SULT1E1 and SULT2A1. Environ. Toxicol. Pharmacol 58, 196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessah IN, Lein PJ, Seegal RF, Sagiv SK, 2019. Neurotoxicity of polychlorinated biphenyls and related organohalogens. Acta Neuropathol. 138, 363–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinete N, Esser A, Kraus T, Schettgen T, 2017. PCB 28 metabolites elimination kinetics in human plasma on a real case scenario: Study of hydroxylated polychlorinated biphenyl (OH-PCB) metabolites of PCB 28 in a highly exposed German Cohort. Toxicol. Lett 276, 100–107. [DOI] [PubMed] [Google Scholar]
- Quinete N, Schettgen T, Bertram J, Kraus T, 2014. Occurrence and distribution of PCB metabolites in blood and their potential health effects in humans: a review. Environ. Sci. Pollut. Res. Int 21, 11951–11972. [DOI] [PubMed] [Google Scholar]
- Reed MJ, Purohit A, Woo LWL, Newman SP, Potter BVL, 2005. Steroid sulfatase: molecular biology, regulation, and inhibition. Endocrine reviews 26, 171–202. [DOI] [PubMed] [Google Scholar]
- Rodriguez EA, Li X, Lehmler HJ, Robertson LW, Duffel MW, 2016. Sulfation of Lower Chlorinated Polychlorinated Biphenyls Increases Their Affinity for the Major Drug-Binding Sites of Human Serum Albumin. Environ. Sci. Technol 50, 5320–5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez EA, Vanle BC, Doorn JA, Lehmler H-J, Robertson LW, Duffel MW, 2018. Hydroxylated and sulfated metabolites of commonly observed airborne polychlorinated biphenyls display selective uptake and toxicity in N27, SH-SY5Y, and HepG2 cells. Environ. Toxicol. Pharmacol 62, 69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy AB, 1987. Sulfatases from Helix pomatia. Methods Enzymol 143, 361–366. [DOI] [PubMed] [Google Scholar]
- Schuur AG, van Leeuwen-Bol I, Jong WM, Bergman A, Coughtrie MW, Brouwer A, Visser TJ, 1998. In vitro inhibition of thyroid hormone sulfation by polychlorobiphenylols: isozyme specificity and inhibition kinetics. Toxicological Sciences 45, 188–194. [DOI] [PubMed] [Google Scholar]
- Sekura RD, Duffel MW, Jakoby WB, 1981. Aryl sulfotransferases. Methods Enzymol. 77, 197–206. [DOI] [PubMed] [Google Scholar]
- Sethi S, Keil KP, Chen H, Hayakawa K, Li X, Lin Y, Lehmler HJ, Puschner B, Lein PJ, 2017. Detection of 3,3'-Dichlorobiphenyl in Human Maternal Plasma and Its Effects on Axonal and Dendritic Growth in Primary Rat Neurons. Toxicol Sci 158, 401–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanahan CE, Spak SN, Martinez A, Hornbuckle KC, 2015. Inventory of PCBs in Chicago and Opportunities for Reduction in Airborne Emissions and Human Exposure. Environ Sci Technol 49, 13878–13888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankaran R, Ameen M, Daniel WL, Davidson RG, Chang PL, 1991. Characterization of arylsulfatase C isozymes from human liver and placenta. Biochimica et biophysica acta 1078, 251–257. [DOI] [PubMed] [Google Scholar]
- Sheng JJ, Sharma V, Duffel MW, 2001. Measurement of aryl and alcohol sulfotransferase activity. Curr. Protocols Toxicol Vol. 8, Chapter 4, Unit4 5. [DOI] [PubMed] [Google Scholar]
- Song Y, Wagner BA, Lehmler HJ, Buettner GR, 2008. Semiquinone radicals from oxygenated polychlorinated biphenyls: electron paramagnetic resonance studies. Chem Res Toxicol 21, 1359–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LQ, Lehmler HJ, Robertson LW, James MO, 2006. Polychlorobiphenylols are selective inhibitors of human phenol sulfotransferase 1A1 with 4-nitrophenol as a substrate. Chemico-biological Interactions 159, 235–246. [DOI] [PubMed] [Google Scholar]
- Weitekamp CA, Phillips LJ, Carlson LM, DeLuca NM, Hubal EAC, Lehmann GM, 2021. A state-of-the-science review of polychlorinated biphenyl exposures at background levels: Relative contributions of exposure routes. Science of the Total Environment 776, 145912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wethington DM 3rd, Hornbuckle KC, 2005. Milwaukee, WI, as a source of atmospheric PCBs to Lake Michigan. Environ. Sci. Technol 39, 57–63. [DOI] [PubMed] [Google Scholar]
- Zhang D, Saktrakulkla P, Tuttle K, Marek RF, Lehmler HJ, Wang K, Hornbuckle KC, Duffel MW, 2021. Detection and Quantification of Polychlorinated Biphenyl Sulfates in Human Serum. Environ Sci Technol 55, 2473–2481. [DOI] [PMC free article] [PubMed] [Google Scholar]



