SUMMARY
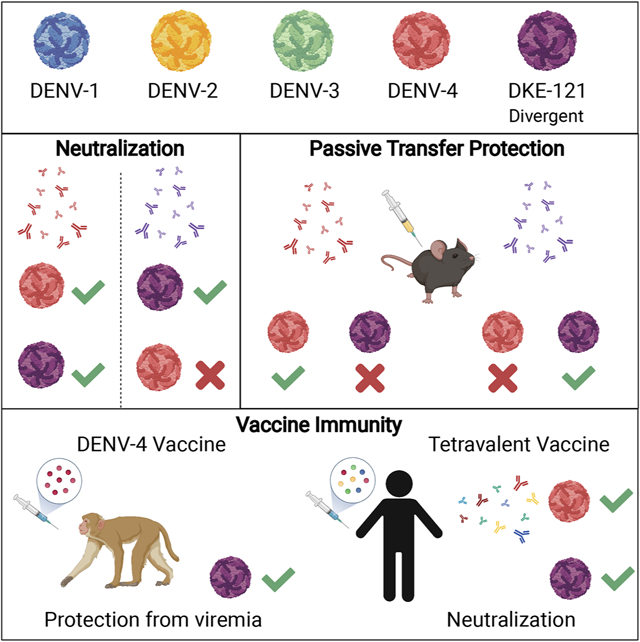
Although divergent dengue viruses (DENV) have been isolated in insects, non-human primates, and humans, their relationships to the four canonical serotypes (DENV 1-4) are poorly understood. One virus isolated from a Dengue patient, DKE-121, falls between genotype and serotype levels of sequence divergence to DENV-4. To examine its antigenic relationship to DENV-4, we assessed serum neutralizing and protective activity. Whereas DENV-4-immune mouse sera neutralize DKE-121 infection, DKE-121-immune sera inhibits DENV-4 less efficiently. Passive transfer of DENV-4 or DKE-121-immune sera protects mice against homologous, but not heterologous DENV-4 or DKE-121 challenge. Antigenic cartography suggests DENV-4 and DKE-121 are related but antigenically distinct. However, DENV-4 vaccination confers protection against DKE-121 in non-human primates, and serum from humans immunized with a tetravalent vaccine neutralize DENV-4 and DKE-121 infection equivalently. As divergent DENV strains like DKE-121 may meet criteria for serotype distinction, monitoring their capacity to impact dengue disease and vaccine efficacy appears warranted.
Graphical Abstract
eTOC Summary:
Chen et al. describe antigenic relationships between a highly variant Dengue virus (DENV) strain, DKE-121 and its closest serotype relative, DENV-4. A lack of serum antibody cross-neutralization and protection between DENV-4 and DKE-121 highlight limitations in existing definitions of Dengue serotypes and flavivirus taxonomy.
INTRODUCTION
Dengue virus (DENV), a member of the Flavivirus genus and Flaviviridae family, is transmitted by Aedes aegypti and Aedes albopictus mosquitoes in most tropical and sub-tropical regions of the world (Mattingly, 1957; Pierson and Diamond, 2013). Four billion people currently are at risk for DENV infection (Brady et al., 2012; Messina et al., 2019) with an estimated 100 million infections occurring annually (Bhatt et al., 2013). In most individuals, DENV infections are either subclinical without symptoms or result in mild, self-limiting illnesses characterized by fever, headache, and myalgia. For more symptomatic illness, the World Health Organization diagnostic criteria classifies dengue into (i) dengue with and without warning signs and (ii) severe dengue, which is associated with extravascular plasma leakage, visceral organ impairment, bleeding, and shock (2009).
There are four recognized DENV serotypes, which vary by ~25% to 40% in the envelope (E) protein amino acid sequence (Fried et al., 2010; Pierson and Diamond, 2013; VanBlargan et al., 2016). DENV serotypes historically were defined by a lack of cross-neutralization and cross-protection. Multiple genotypes, which have up to 3% amino acid variation, exist within each serotype (Rico-Hesse, 1990). For example, DENV-4 is comprised of five genotypes, with two (gI and gII) currently circulating in humans (Chen and Vasilakis, 2011; Gallichotte et al., 2018). The dominant serotype and genotype causing outbreaks in dengue-endemic regions changes over time (Holmes and Twiddy, 2003; Messina et al., 2014; Rico-Hesse et al., 1997). One source of genetic diversity for strains with epidemic potential is sylvatic dengue (Cardosa et al., 2009; Franco et al., 2011; Liu et al., 2016; Pollett et al., 2018; Pyke et al., 2016; Vasilakis et al., 2011; Weaver et al., 2008). DENV circulates in an epidemic cycle between humans and Aedes species mosquitoes, and also in an enzootic cycle with transmission between mosquitoes and non-human primates (NHPs) (Vasilakis et al., 2011; Wang et al., 2000). In NHPs, DENV infection with human and sylvatic strains generally causes subclinical disease (Halstead et al., 1973; Sabin, 1950; Sariol and White, 2014). Nonetheless, the sylvatic cycle is a source of emergent strains and has resulted in dengue outbreaks in humans (Cardosa et al., 2009; Franco et al., 2011; Weaver et al., 2008). Each DENV serotype arose from separate spillover events from the sylvatic to endemic cycle (Wang et al., 2000).
Epidemiological studies have linked dengue disease severity to a secondary DENV infection (Fried et al., 2010; Guzmán et al., 1990). Early studies observed that homotypic immunity against a given DENV serotype lasted durably, possibly for a person’s lifetime (Guzmán et al., 1990; Halstead, 2003). Heterotypic immunity, however, is less consistent in its protective activity, and in some cases, detrimental. The increased risk for severe dengue is associated with pre-existing, cross-reactive, poorly neutralizing DENV antibodies (Katzelnick et al., 2017). Antibody-dependent enhancement (ADE) has been hypothesized to cause the increased disease severity during secondary infection, whereby pre-existing cross-reactive antibodies promote DENV infection in Fcγ-receptor-expressing myeloid cells (Halstead, 2003; Halstead et al., 1970; Libraty et al., 2002; Taylor et al., 2015; Vaughn et al., 2000).
A successful DENV vaccine likely needs to produce robust immunity against all four serotypes to avoid potential ADE from poorly-neutralizing cross-reactive responses (Halstead, 2003; Porterfield, 1982; Screaton et al., 2015). Dengvaxia® (CYD-TDV), the only licensed dengue vaccine, exhibits complex protection phenotypes (Sridhar et al., 2018). After vaccination of DENV-naïve individuals, there was a higher risk of hospitalization and symptomatic dengue in children (Sridhar et al., 2018). In contrast, DENV-experienced individuals who received Dengvaxia® were protected against severe dengue (Hadinegoro et al., 2015; Sridhar et al., 2018). Two other live-attenuated tetravalent vaccines are currently in phase 3 clinical trials (Kallas et al., 2020; Tricou et al., 2020). One question with all of the tetravalent vaccines is whether they will protect efficiently against newly emerging strains or genotypes. Indeed, Dengvaxia® conferred less protection against heterologous genotypes within a serotype, especially for DENV-4 (Henein et al., 2017; Juraska et al., 2018; Rabaa et al., 2017).
In 2007, a novel DENV strain, DKE-121, that is distantly related to DENV-4, was isolated from a hospitalized 37-year-old farmer in Malaysia (Normile, 2013; Young et al., 2017). Here, we assess genetic, antigenic, and serological relationships between DENV-4 and DKE-121. Whereas DENV-4 immune sera from mice neutralize DKE-121 efficiently, DKE-121 immune sera do not always neutralize DENV-4 as effectively as DKE-121. In passive transfer experiments in mice, DENV-4 and DKE-121 immune sera protect against homologous but not heterologous DENV-4 and DKE-121 challenge. However, vaccination of NHPs with monovalent or trivalent DENV-4 vaccines reduces DKE-121 infection, and serum from human vaccine recipients of a live-attenuated tetravalent DENV vaccine neutralizes DKE-121 and DENV-4 equivalently. Despite clear antigenic differences between DENV-4 and DKE-121, adaptive immune responses induced after DENV-4 infection or vaccination confer at least some protection against DKE-121, a highly divergent strain.
RESULTS
Genetic divergence of DKE-121 from DENV-4.
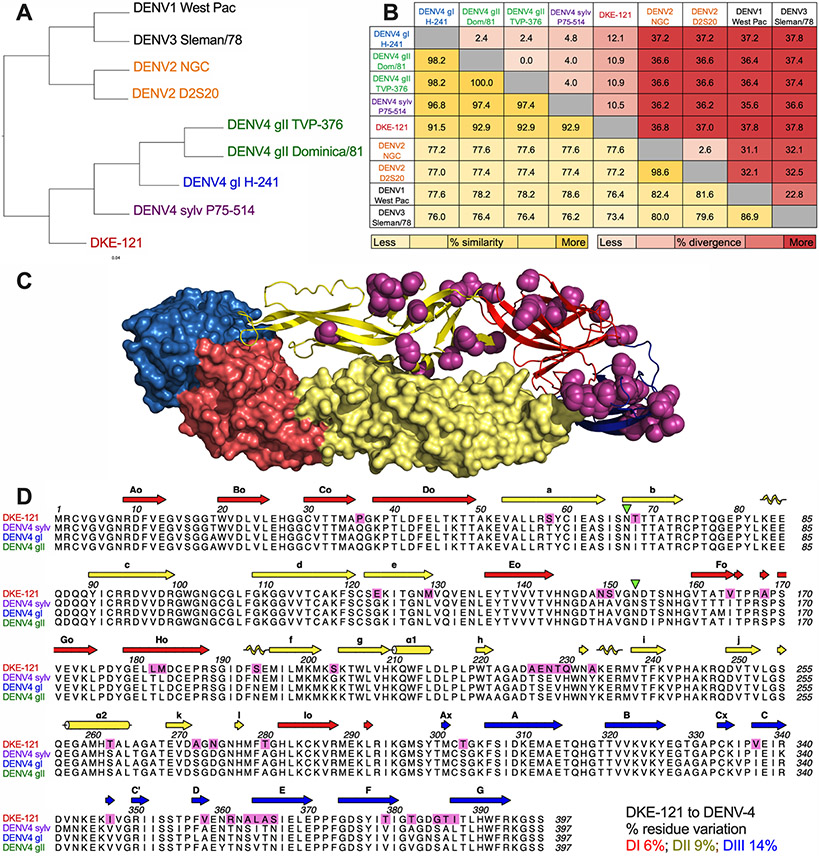
As variation exists within each of the four DENV serotypes, an intra-serotype genotype classification system was developed (Holmes and Twiddy, 2003; Rico-Hesse, 1990). The E protein amino acid sequences of the two DENV-4 genotypes (gI and gII) that currently circulate in humans are 2% divergent from each other and 4 to 5% divergent from the sylvatic genotype (Fig 1A-B). Sequencing analysis of DKE-121 E protein (accession number: PRJNA729800) revealed that it is more related to sylvatic DENV-4 (sylv) (10.5% amino acid divergence) than to circulating DENV-4 gI and gII, (12.1% and 10.9% divergence, respectively) (Fig 1B). These sequence relationships may be relevant to antigenicity, since the three ectodomains of DENV E protein (DI, DII, and DIII) are the principal targets of neutralizing antibodies, and the majority of the E protein residues differing between DKE-121 and DENV-4 lie in surface-accessible regions (Fig 1C-D). Within each domain there is 6% (DI: 8 of 129 residues), 9% (DII: 15 of 167 residues), and 14% (DIII: 14 of 98 residues) amino acid residue variation between DKE-121 and DENV-4 (Fig 1D).
Figure 1. Genetic divergence of DKE-121 from DENV-4.
Dendrogram (A) and amino acid divergence (red) and similarity (yellow) in the E proteins of DKE-121 compared with DENV-4 genotypes and DENV-1, DENV-2, and DENV-3 serotypes (B). Residues that differ between DKE-121 and DENV-4 are shaded in magenta on a reconstructed E dimer (C) and in an amino acid sequence alignment (D).
Serological relationship between DENV-4 and DKE-121 in mice.
To begin to understand the antigenic relationship between DKE-121 and DENV-4 genotypes, we compared the serological profiles of human STAT2 transgenic (hSTAT2-KI) C57BL/6 mice infected and boosted homologously with DENV-4 gI (strain H-241), DENV-4 gII (strain TVP-376), or DKE-121 (Fig 2A); hSTAT2-KI mice were used to promote infection and immunity, since DENV can antagonize human but not mouse STAT2 (Morrison and García-Sastre, 2014; Morrison et al., 2013). Boosting was required since DENV replicates inefficiently in C57BL/6 mice and generates poor antibody responses after primary infection. Two weeks after the final injections, sera were harvested and evaluated for inhibitory activity against different DENV-4 strains (gI, gII, and sylv) and DKE-121 using focus reduction neutralization tests (FRNT) (Brien et al., 2013). Differences in the display of E protein epitopes and neutralizing activity of antibodies can occur with cleavage of prM and virion maturation (Mukherjee et al., 2016; Nelson et al., 2008). As recent studies have suggested that DENV virions circulate in more mature forms in vivo (Dejnirattisai et al., 2010; Dejnirattisai et al., 2015; Raut et al., 2019), we performed FRNTs with viruses derived from Vero cells over-expressing the protease furin (Mukherjee et al., 2016), which promotes virus maturation (Mukherjee et al., 2016). Whereas DIII-specific mAb DV4-E88 inhibited Vero and Vero-furin cell-derived viruses similarly, mAb E60, which targets the fusion loop epitope that is poorly exposed on mature viruses (Nelson et al., 2008), neutralized Vero cell-derived viruses more efficiently than Vero-furin cell-derived viruses, confirming that viruses produced in Vero-furin cells are more mature than ones generated in standard Vero cells (Fig S1).
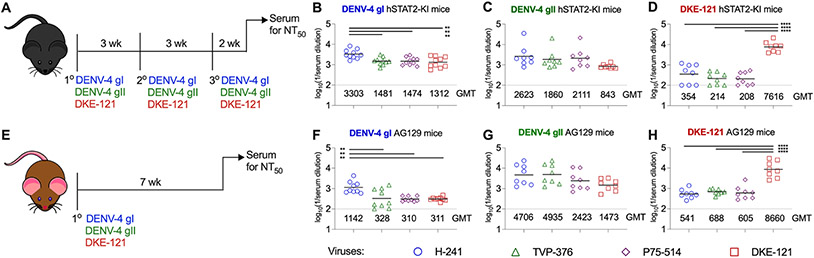
Figure 2. Serological relationship between DENV-4 and DKE-121 in hSTAT2-KI and AG129 mice.
Scheme to generate DENV-4 and DKE-121-immune sera in hSTAT2-KI (A) and AG129 (E) mice. hSTAT2-KI mice (3 to 4-week-old, males and females) were inoculated via intravenous route with DENV-4 gI H-241 (B), DENV-4 gII TVP-376 (C), or DKE-121 (D) three times and bled two weeks after boosting. AG129 mice (4-week-old, males and females) were infected via intraperitoneal route with DENV-4 gI H-241 (F), DENV-4 gII TVP-376 (G), or DKE-121 (H) once and bled seven weeks post-infection. Sera were assayed by FRNT against Vero-furin cell derived (DENV-4 gI H-241, DENV-4 gII TVP-376, DENV-4 sylv P75-514, or DKE-121) viruses. Bars represent mean values (n = 8-14 mice per group; one-way ANOVA with Tukey’s post-test; **, p < 0.01; ****, p < 0.0001). See also Fig S1-S2.
Serum from DENV-4 gI and gII-immunized hSTAT2-KI mice neutralized Vero-furin cell-derived DENV-4 strains (gI, gII, and sylv) and DKE-121 equivalently, except for a 2-fold difference against gI with sera from homologously immunized mice (Fig 2B-C). Sera from hSTAT2-KI mice immunized with DKE-121 showed 22 to 37-fold higher levels of inhibitory activity against DKE-121 than heterologous DENV-4 strains (gI, gII, and sylv) derived from Vero-furin cells (Fig 2D). Similar trends in neutralizing activity were observed when we tested serum from DENV-4 gI- or gII-immunized mice against standard Vero virus preparations (Fig S2A-C). As expected, all sera had substantially lower levels of neutralizing antibodies against DENV-2, a heterologous serotype (Fig S2A-C).
To corroborate these results, we evaluated neutralization profiles with sera from DENV-infected AG129 mice, which are deficient in receptors both for type I and II interferons (IFN) (Johnson and Roehrig, 1999) and commonly used in DENV research (Chen and Diamond, 2020; Sarathy et al., 2015; Zellweger and Shresta, 2014). AG129 were inoculated once with DENV-4 gI (strain H-241), DENV-4 gII (strain TVP-376), or DKE-121, and sera were harvested 7 weeks later for evaluation by FRNT with Vero and Vero-furin cell-derived viruses (Fig 2E and S2). Like the DENV-4 gI immune sera from hSTAT2-KI mice, DENV-4 gI immune sera from AG129 mice showed 3-fold greater neutralization of homologous DENV-4 gI than DENV-4 gII, DENV-4 sylv, or DKE-121 viruses derived from Vero-furin cells (Fig 2F). Sera from AG129 mice infected with DENV-4 gII had equivalent neutralizing activity against DENV-4 (gI, gII, and sylv) and DKE-121 (Fig 2G and S2E). Sera from DKE-121 immunized AG129 mice also showed the same trends as seen in hSTAT2-KI sera: preferential neutralization of DKE-121 compared to DENV-4 strains (gI, gII, and sylv) derived from Vero-furin or Vero cells (Fig 2H and S2F). Again, sera from DENV-4 or DKE-121 infected AG129 mice had markedly lower neutralizing activity against a strain of the heterologous DENV-2 serotype (Fig S2D-F). Thus, in mice, DENV-4 and DKE-121 elicit distinct serological responses: DENV-4 induces responses that neutralize both DENV-4 and DKE-121, whereas DKE-121 induces a more strain-specific response.
Serological differences between DENV-4 and DKE-121 in non-human primates.
To determine if the differential neutralization patterns seen in mice also occur in NHPs, four rhesus macaques were inoculated with DENV-4 gII (strain Dominica/814669) and bled at 60- and 365-days post-infection (dpi) (Fig 3A). Separately, four other NHPs were inoculated with DKE-121 and bled at 90 and 180 dpi (Fig 3D). Sera were tested for neutralization of Vero-furin and Vero cell derived viruses. DENV-4 gII immune sera from NHPs showed similar neutralization of all DENV-4 strains (gI, gII, and sylv) and DKE-121 derived from Vero-furin cells derived viruses at both time points (Fig 3B-C). Although NHPs inoculated with DENV-4 gII showed some strain-specific neutralization of Vero cell-derived viruses with ~4-fold less inhibitory activity against DENV-4 sylv and DKE-121 at 60 dpi, by 365 dpi there were no substantive differences in neutralization (Fig S3B-C).
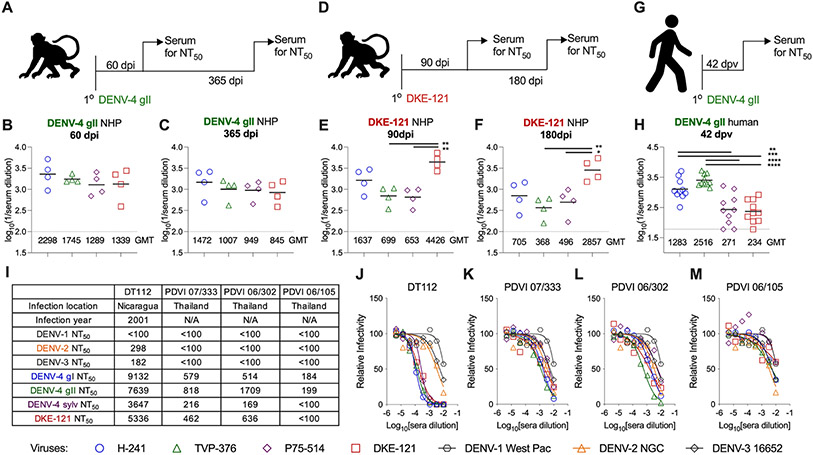
Figure 3. DENV-4 and DKE-121 neutralization by serum from NHPs and humans.
Scheme of NHPs infection with DENV-4 gII Dominica/814669 (A) or DKE-121 (D). Serum neutralizing titers from NHPs infected with DENV-4 gII Dominica/814669 at 60 dpi (B) and 365 dpi (C) or DKE-121 at 90 dpi (E) and 180 dpi (F) against Vero-furin cell derived (DENV-4 gI H-241, DENV-4 gII TVP-376, DENV-4 sylv P75-514, or DKE-121) viruses. Bars represent mean values (n = 4 NHPs per group; one-way ANOVA with Tukey’s post-test: *, p < 0.05; **, p < 0.01). Scheme for humans vaccinated with DENV-4 gII rDEN4Δ30 (G). Serum neutralization titers (NT50) by FRNT from human volunteers bled 42 days after rDEN4Δ30 vaccination against DENV-4 gI H-241, DENV-4 gII TVP-376, DENV-4 sylv P75-514, or DKE-121 viruses derived from Vero-furin cells (H). Bars represent mean values (n = 10 volunteers; one-way ANOVA with Tukey’s post-test: **, p < 0.005; ***, P < 0.001; ****, p < 0.0001). Subject infection history was adapted from a published study (Nivarthi et al., 2017) (I) with NT50 values derived by FRNT against Vero-furin cell-derived DENV-1 Western Pacific, DENV-2 NGC, DENV-3 16652, DENV-4 gI H-241, DENV-4 gII TVP-376, DENV-4 sylv P75-514, and DKE-121 viruses (J-M). FRNT samples were run in duplicate. See also Fig S1 and S3.
In comparison, sera from DKE-121 immunized NHPs showed greater strain specific neutralization. At 90 dpi, DKE-121 immune NHP sera inhibited infection of the homologous DKE-121 3 to 7-fold better than the DENV-4 strains (Fig 3E), and this preferential neutralization of DKE-121 using Vero-furin cell-derived viruses was also observed at 180 dpi (4 to 8-fold) (Fig 3F). However, when evaluated using Vero cell-derived viruses the strain-specificity of neutralization seen at 90 dpi was diminished by 180 dpi (Fig S3E-F).
Serological responses from DENV-4 vaccinated or infected humans.
We evaluated the neutralizing activity of sera from DENV-naïve humans that were immunized with an attenuated monovalent DENV-4 gII (strain Dominica/81, rDEN4Δ30) vaccine as part of phase II clinical studies building to a tetravalent vaccine (Durbin et al., 2005; Kallas et al., 2020). Ten individuals were immunized with the monovalent rDEN4Δ30 vaccine and phlebotomized 42 days later (Fig 3G). Sera were assessed for neutralization using an FRNT assay and Vero-furin and Vero cell-derived DENV-4 (gI, gII, and sylv) and DKE-121. Sera from DENV-4 gII-vaccinated humans showed strain-specific neutralization with 5 to 10-fold higher titers against DENV-4 gI and DENV-4 gII strains compared to DENV-4 sylv and DKE-121 strains (Fig 3H). Similar differences in neutralization were seen with DENV strains derived from Vero cells (Fig S3H).
We also tested sera from four returning travelers to dengue-endemic regions who experienced primary DENV-4 infections in Nicaragua or Thailand (Patel et al., 2017). These subjects were classified as having DENV-4 infection because their serum contained high levels of neutralizing antibodies against DENV-4 compared to the other three DENV serotypes (Fig 3I-M). While we observed no statistically significant differences in the ability of these sera to neutralize DENV-4 (gI, gII, and sylv) and DKE-121 derived in Vero or Vero-furin cells, some individuals (PDVI 07/333, PDVI 06/302, and PDVI 06/105) showed a trend towards less inhibitory activity against DKE-121 (Fig 3I, K-M).
Given that tetravalent DENV vaccines are being developed and deployed (Deng et al., 2020), we evaluated how vaccine-induced serum might neutralize DKE-121 compared to DENV-4. We obtained sera derived from individuals enrolled in a phase II trial of a tetravalent DENV vaccine developed by the Butantan Institute in Brazil based on the National Institutes of Health (NIH) TV003 vaccine (Kallas et al., 2020) with the same rDEN4Δ30 component. Sera obtained at days 28, 92, 180, and 365 days following a single dose of vaccine were tested for neutralization by FRNT against DENV-4 (gI, gII, sylv) and DKE-121 and also against DENV-4 gII and DKE-121 by a reporter virus particle (RVP)-based neutralization assay (Ansarah-Sobrinho et al., 2008). Vaccine recipients were separated into those who were naïve or had pre-existing DENV immunity at the time of vaccination. Regardless of the assay or time point of serum collection, we observed no significant difference in neutralization of the DENV-4 strains or DKE-121 (Fig S4). Of note, volunteers with pre-existing DENV immunity consistently had higher titers than those who were naïve upon vaccination. So, in humans, although monovalent DENV-4 vaccination produces a more strain-specific response, tetravalent vaccination induces a balanced, broadly neutralizing response.
DENV antigenic cartography relationships.
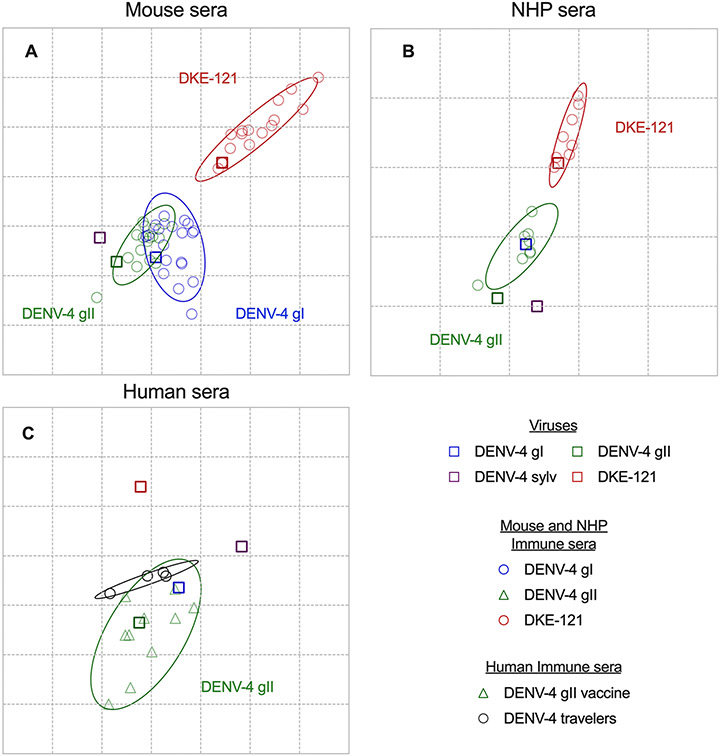
Using the neutralization data from mouse, NHP, and human sera, we created antigenic maps to visualize the relationships between DKE-121, DENV-2, and DENV-4 strains. Neutralization titers (Fig 2 and 3) were used to position the serum relative to each virus using multidimensional scaling, such that higher neutralization titers are represented by shorter distances between serum and the virus. As viruses derived from Vero-furin cells are antigenically different from those derived from Vero cells, we created separate maps for these data (Fig 4 and S5). Each gridline, or antigenic unit (AU), corresponds to a 2-fold difference in neutralization titer. In maps based on neutralization data obtained from mouse samples, the sera mapped more closely to the homologous viruses, such that DKE-121 immune sera clustered around DKE-121, and DENV-4 gI and DENV-4 gII immune sera clustered with DENV-4 gI and DENV-4 gII (Fig 4A and S5A). All immune sera clustered away from a heterologous serotype DENV-2, with DENV-4 immune sera being slightly closer to DENV-2 than DKE-121 immune sera (Fig S5A). In both maps, the sylvatic DENV-4 viral strain was located closer to DENV-4 gI and DENV-4 gII sera than to DKE-121 sera. Whereas the DENV-4 gI and DENV-4 gII serum clusters largely overlap, suggesting that they are similar antigenically, the overlap with DKE-121 serum was marginal. In both Vero-furin and Vero cell maps, the centers of the DKE-121 clusters are further away from DENV-4 gI and DENV-4 gII clusters (2.13-3.52 AU) than the DENV-4 gI and gII clusters are from each other (0.08-0.59 AU) (Fig 4A and S5A). We also generated antigenic maps using the few available DENV-4 gII and DKE-121 NHP immune sera (Fig 4B and S5B). Using neutralization data with Vero or Vero-furin derived viruses (Fig 3 and S3), DENV-4 gII and DKE-121 immune sera clusters showed minimal overlap. In addition, a cartography map using human serum neutralization titers also showed that DENV-4 immune sera clusters with DENV-4 gI and DENV-4 gII and away from DKE-121 (Fig 4C). Overall, the cartography analyses suggest that DENV-4 gI and gII are antigenically related as expected, but distinct from DKE-121.
Figure 4. DENV-4 and DKE-121 form separate clusters by antigenic cartography.
Antigenic maps of FRNT titers from mouse (A), NHP (B), or human (C) sera (from Figures 2 and 3) against viruses derived from Vero-furin cells. The length of each grid is equivalent to a two-fold dilution in neutralization titers. Each serum sample (circle) is plotted in relation to each virus (square) and the 95% confidence interval of each cluster is outlined. Error ellipses are shown for each serum cluster, with radii corresponding to two standard deviations. See also Fig S4.
Suboptimal in vivo cross-protection of antibodies derived from DENV-4 and DKE-121-infected mice.
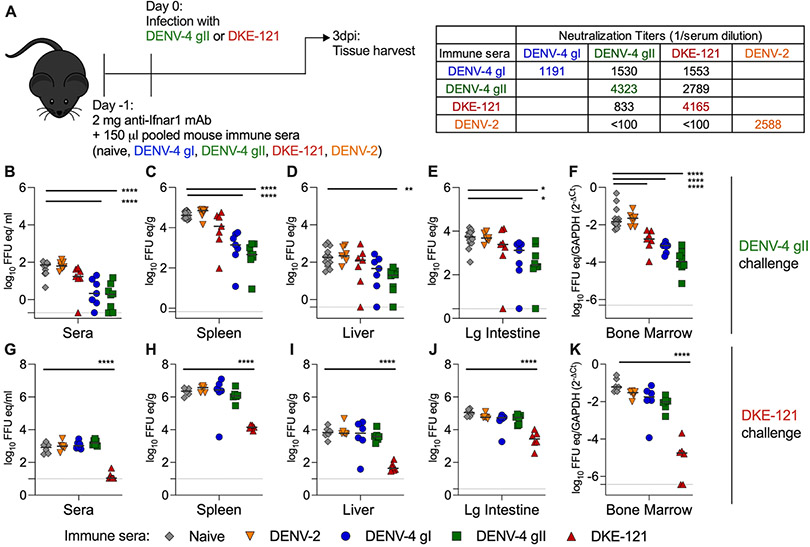
To begin to understand whether humoral immune responses protect against the reciprocal DENV strain, we performed passive transfer studies using sera from DENV-4 or DKE-121-infected mice (Fig 2). To examine the protective effects of serum transfer on viral outcome, we used three-week-old C57BL/6J mice and treated them with anti-Ifnar1 monoclonal antibody (mAb) to transiently attenuate innate immune responses and facilitate DENV infection. Animals were given 150 μl of pooled mouse sera with a reciprocal neutralizing titer of 1:1,000 against the respective homologous virus (DENV-4 gI, DENV-4 gII, DKE-121, or DENV-2) or naïve sera. Based on studies with a separate cohort of mice, we estimate that serum neutralizing titers decrease ~10 to 30-fold after transfer and redistribution (Table S1). One day later, mice were challenged with DKE-121 or DENV-4 gII (strain TVP-376) (Fig 5A). At 3 dpi mice were harvested, and viral RNA was recovered from tissues and quantified by qRT-PCR. Upon infection with DENV-4 gII, mice that received homologous DENV-4 gII immune sera had 10 to 100-fold less viral RNA in the serum, spleen, liver, large intestine, and bone marrow than mice that received naïve serum (Fig 5B-F). DENV-4 gI immune sera also had a similar protective effect and reduced DENV-4 gII viral RNA levels in the serum, spleen, large intestine, and bone marrow by 10 to 100-fold. DKE-121 immune sera reduced DENV-4 gII viral burden only in the bone marrow by 10-fold, whereas DENV-2 immune sera were not protective at all (Fig 5B-F). With DKE-121 infection, mice that received homologous DKE-121 immune sera had 100 to 1,000-fold lower DKE-121 viral RNA levels in the serum, spleen, liver, large intestine and bone marrow than animals receiving naïve serum (Fig 5G-K). In comparison, DENV-2, DENV-4 gI, and DENV-4 gII immune sera were not protective, and like naïve serum, did not reduce DKE-121 viral burden in any tissues (Fig 5G-K). Thus, there is a lack of cross-protection such that DENV-4-immune serum does not reduce DKE-121 viral replication in vivo and reciprocally, DKE-121 immune serum does not limit DENV-4 viral burden.
Figure 5. Passive transfer of DENV-4 and DKE-121-immune sera do not prevent respective DKE-121 and DENV-4 infection.
C57BL/6J mice (3-week-old males) were administered an intraperitoneal injection of 2 mg of anti-Ifnar1 mAb and 150 μl of pooled mouse sera (from mice immunized with DENV-2 D2S20, DENV-4 gI H-241, DENV-4 gII TVP-376, DKE-121, or naïve animals; homologous and heterologous NT50 values are shown (A)). One day later, mice were inoculated with 106 FFU of DENV-4 gII TVP-376 (B-F) or DKE-121 (G-K). At 3 dpi, mice were harvested, and viral RNA was extracted from sera (B and G), spleen (C and H), liver (D and I), large intestine (E and J), and bone marrow (F and K) and quantified by qRT-PCR. Bone marrow was normalized to Gapdh. Bars represent median values (n = 6-13 mice per group; one-way ANOVA with Dunnett’s post-test; *, p < 0.05; **, p <0.01; ***, p < 0.001; ****, p < 0.0001). See also Table S1.
Cross-protection conferred by DENV-2, DENV-4, and DKE-121 upon secondary challenge in mice.
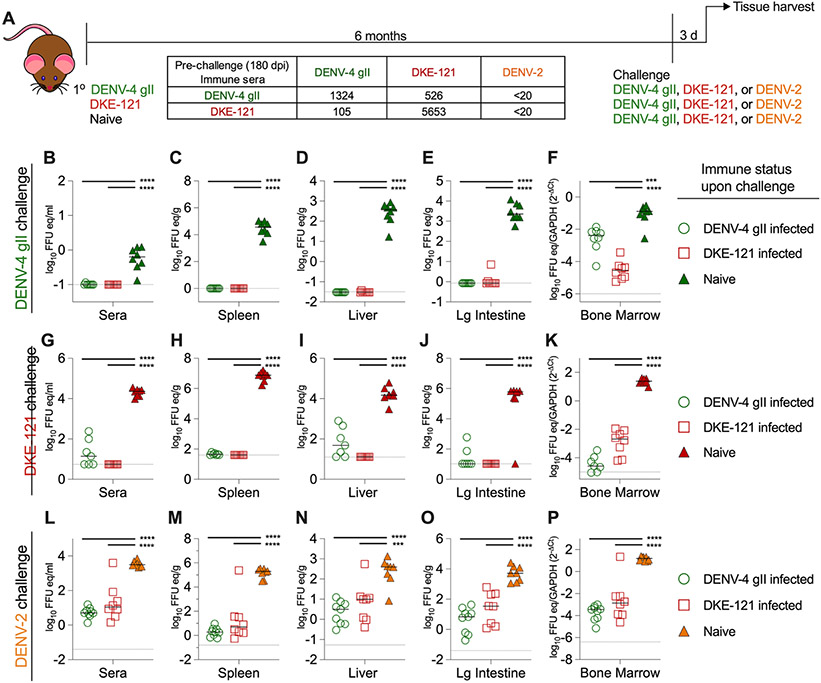
Our passive transfer data suggests that antibodies against DENV-4 strains and DKE-121 might not reciprocally protect against each other. However, apart from maternal IgG transfer and neonatal DENV infections, DENV immunity includes many aspects beyond antibodies, including anamnestic B and T cell responses. We initially utilized AG129 mice to study sequential infections, as these mice are permissive for infection with many different DENV strains. To assess protection of a DENV-primed immune system upon secondary infection, we inoculated AG129 mice by intraperitoneal injection of DENV-4 gII (strain TVP-376), DKE-121, or no virus (naïve). Six months later, mice were challenged with DENV-4 gII (strain TVP-376), DKE-121, or DENV-2 (strain D2S20) (Fig 6A). Three days later, viral load was quantified from harvested tissues by qRT-PCR. As expected, the naïve animals were permissive to challenge with DENV-4 gII, DKE-121, or DENV-2 and had high levels of viral RNA in the serum, spleen, liver, large intestine, and bone marrow (Fig 6B-P). In contrast, pre-existing immunity against DENV-4 gII or DKE-121 protected mice against DENV-4 gII and DKE-121, as viral RNA levels were near the limit of detection in all tissues (Fig 6B-P). Of note, mice that had recovered from DENV-4 gII or DKE-121 at 180 dpi also were protected against challenge with DENV-2, a heterologous serotype (Fig 6L-P). We also tested secondary DENV infection models in hSTAT2-KI mice and C57BL/6J mice treated with an anti-Ifnar1 mAb. Again, we observed protective effects between serotypes, even heterologous DENV-4 and DENV-2 viruses (Fig S6 and S7). Thus, mice exhibit heterologous DENV serotype protection for at least 6 months, which limits their utility to address questions of protection using sequential infection between more related DENV-4 and DKE-121 strains.
Figure 6. DENV-4 and DKE-121 immune AG129 mice are protected against DENV-2, DENV-4, and DKE-121 secondary infection.
Scheme with neutralization titers evaluated at 180 dpi (A). AG129 mice (4-week-old, males and females) were infected by intraperitoneal injection with 104 FFU of DENV-4 gII TVP-376, DKE-121, or not infected (naive control). After 180 dpi, secondary infection of 106 FFU of DENV-4 gII TVP-376 (B-F), 106 FFU of DKE-121 (G-K), or 105 FFU of DENV-2 (D2S20) (L-P) was administered by intraperitoneal injection. Three days post-challenge, mice were harvested, and viral RNA was extracted from serum (B, G, L), spleen (C, H, M), liver (D, I, N), large intestine (E, J, O), and bone marrow (F, K, P) and quantified by qRT-PCR. Bone marrow was normalized to Gapdh. Bars represent median values (n = 7-9 mice per group; one-way ANOVA with Dunnett’s post-test; ***, p < 0.001; ****, p < 0.0001). See also Fig S6-S7.
DENV-4 vaccination in NHPs protects against DKE-121 challenge.
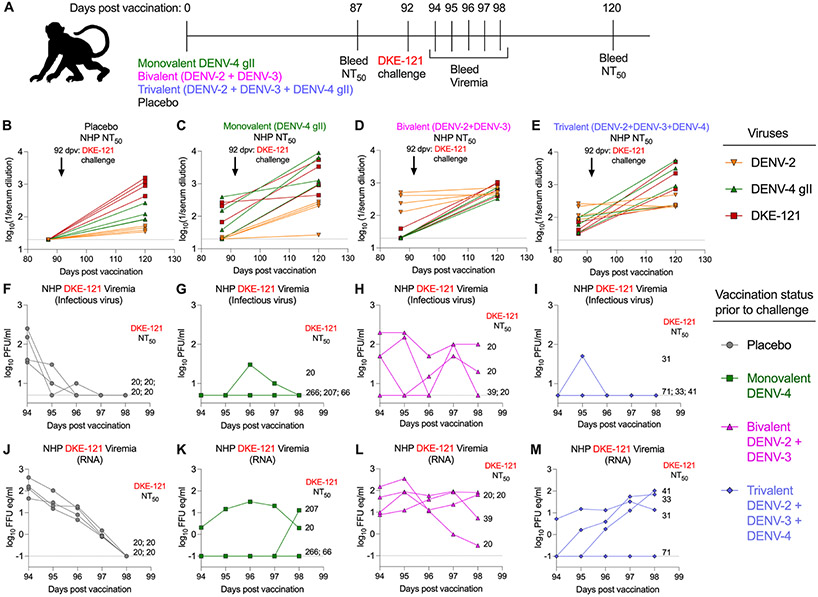
To understand further whether DENV vaccination would inhibit DKE-121 infections, we immunized NHPs with placebo, monovalent (DENV-4), bivalent (DENV-2 + DENV-3), or trivalent (DENV-2 + DENV-3 + DENV-4) formulations of the live-attenuated NIH vaccine. At 87 days post-vaccination, NHPs were bled, and five days later, animals were challenged subcutaneously with DKE-121 (Fig 7A). Starting at 2 dpi, NHPs were bled on five consecutive days, and viremia was assessed using plaque- and qRT-PCR-based assays. At 120 days post-vaccination (28 days post-challenge), NHPs also were bled to determine post-challenge neutralizing antibody titers. Sera harvested at 87- and 120-days post-vaccination were interrogated by FRNTs with DENV-2 (strain New Guinea C), DENV-4 gII (strain TVP-376), and DKE-121. The DENV-2 and DENV-4 strains used are homologous to the vaccine components. In NHPs in the placebo group, DKE-121 challenge resulted in increased DKE-121, DENV-4 gII, and DENV-2 neutralizing titers (Fig 7B). Three of the NHPs vaccinated with monovalent DENV-4 vaccine had neutralizing titers against DENV-4 and DKE-121 that were boosted upon DKE-121 challenge (Fig 7C). These three NHPs also showed an increase in DENV-2 neutralizing titers after DKE-121 challenge. NHPs that were immunized with the bivalent vaccine (DENV-2 and DENV-3) had higher anti-DENV-2 neutralizing titers prior to challenge that boosted only slightly after DKE-121 infection (Fig 7D). These bivalent immunized NHPs developed a neutralizing antibody response to DENV-4 and DKE-121 after DKE-121 challenge. The trivalent (DENV-2, DENV-3, and DENV-4) vaccine group was similar to the monovalent (DENV-4) vaccine group and had neutralizing titers against DENV-4 and DKE-121 that increased by >10-fold after DKE-121 challenge with smaller increases against DENV-2 (Fig 7E). Overall, serum from NHPs that had received a DENV-4 vaccine component showed baseline neutralizing activity against DENV-4 and DKE-121 that boosted upon DKE-121 challenge.
Figure 7. DENV-4 vaccination protects against DKE-121 challenge in NHPs.
Vaccination scheme (A). Sixteen NHPs were immunized subcutaneously with placebo or monovalent (DENV-4), bivalent (DENV-2+DENV-3), or trivalent (DENV-2+DENV-3+DENV-4) live-attenuated vaccines. Serum was harvested for neutralizing titers at 87 days post-vaccination (dpv). At 92 dpv, all NHPs were inoculated subcutaneously with 5 x 103 PFU of DKE-121. From 2 to 6 days post-challenge, serum was collected for viremia analyses. At 28 days post-challenge (120 dpv), serum was harvested for assessment of neutralizing titers using Vero cell-derived DENV-2 NGC, DENV-4 gII TVP-376, and DKE-121 (B-E). Viremia was assessed by plaque (F-I) or qRT-PCR (J-M) assays (n = 4 NHPs per group). For panels F-M, neutralizing Ab titers against DKE-121 at time of challenge are indicated as NT50 values.
To evaluate the effects of vaccination on viremia (NHPs did not get clinical disease after DKE-121 challenge), serum samples were analyzed by plaque and qRT-PCR assays. Placebo-vaccinated NHPs sustained the highest DKE-121 viremia (infectious virus and viral RNA) at 2 dpi that waned over the 5 days of sampling (Fig 7F and J). In comparison, two NHPs given the monovalent DENV-4 vaccine did not have detectable viremia in either assay throughout all time points (Fig 7G and K). One NHP immunized with monovalent DENV-4 vaccine had sustained viremia in both plaque and viral RNA assays; this NHP also had the lowest anti-DKE-121 and anti-DENV-4 neutralizing titers before challenge, suggesting this animal had a weak vaccine response. A second NHP in this cohort experienced breakthrough viremia (viral RNA only) on the last day of sampling (Fig 7K). In the NHPs immunized with the bivalent (DENV-2 and DENV-3) vaccine, all NHPs demonstrated DKE-121 viremia (both infectious virus and viral RNA), with some showing sustained viremia that lasted through the last final point and was noticeably higher than the placebo group (Fig 7H and L). In the NHPs immunized with the trivalent (DENV-2, DENV-3, and DENV-4) vaccine, 3 of 4 animals did not have detectable infectious DKE-121 virus in serum, whereas the animal with the lowest neutralizing antibody titers against DKE-121 had infectious viremia (Fig 7I). However, when measured by viral RNA, 3 out of 4 NHPs in this cohort experienced viremia that was rising on the last day of sampling (Fig 7M). In summary, DENV-4 vaccination in NHPs elicited neutralizing antibodies to DKE-121 that were boosted upon DKE-121 infection. Upon DKE-121 challenge, DENV-4 immunized NHPs were protected from infectious viremia. Thus, in NHPs, DENV-4 vaccination confers some protection against the divergent DKE-121 virus.
DISCUSSION
The discovery of variant DENV strains that infect humans has implications for sequential infections and severe disease as well as tetravalent vaccines. To begin to address how immunity is modulated by genetic diversity that is greater than typical genotypic or sylvatic variation, we evaluated the serological relatedness and functional implications of the highly variant strain, DKE-121 (Normile, 2013) and its most closely related serotype DENV-4. DKE-121 has ~85% whole genome amino acid identity to DENV-4 strains, which encroaches on what constitutes a DENV serotype.
We used infection and protection experiments in mice and NHPs, and serum from vaccinated humans to define relationships between DKE-121 and DENV-4. DENV strains historically were classified into serotypes based on serum neutralization patterns (Calisher et al., 1989) and differences in geographic origins. When first categorized, serum from DENV-4-infected individuals in the convalescent phase had at least 16-fold differences in the ratio of homologous (DENV-4) to heterologous (DENV-1, DENV-2, and DENV-3) neutralizing titers (Calisher et al., 1989). These differences later were explained by sequence distance (25 to 40% amino acid variation) in the E protein between serotypes (VanBlargan et al., 2016). Here, we observed the following: (a) Infection of mice or NHPs with DENV-4 generally induced neutralizing responses against both DENV-4 and DKE-121. However, infection with DKE-121 elicited more strain-specific neutralizing responses; (b) Serum neutralization in vitro did not translate directly into protection in vivo, as passive transfer of serum from DENV-4 or DKE-121-infected mice did not reciprocally protect against heterologous challenge with DKE-121 or DENV-4; (c) While serum from human subjects who received a live-attenuated monovalent DENV-4 vaccine demonstrated variable neutralizing activity against DENV-4 strains and DKE-121, serum from individuals vaccinated with a tetravalent DENV vaccine showed more balanced neutralizing responses; (d) The majority of NHPs immunized with DENV-4 as either monovalent or trivalent vaccine formulations had undetectable levels of DKE-121 infectious viremia after challenge. Although DENV-4 and DKE-121 generate neutralizing antibody responses that can be functionally distinguished from one another, a multivalent vaccine that includes DENV-4 generated neutralizing and protective immune responses against the variant DKE-121 strain.
Our findings do not place DKE-121 neatly into the historical definition of a distinct dengue serotype. We observed directionality in inhibitory capacity between DKE-121 and DENV-4; DKE-121 immune sera neutralized homologous DKE-121 far better (up to 35-fold) than the heterologous DENV-4 strains, whereas DENV-4 immune sera had relatively equivalent neutralizing activity against DKE-121. Further analyses incorporated antigenic cartography maps, which allow for visualization of strain relationships (Smith et al., 2004), where closer distances between groups suggest serum generated against one strain may recognize the other (Lapedes and Farber, 2001; Smith et al., 2004). These maps have more utility than genetic distances, as small genetic variations could result in large structural shifts in epitopes (Goo et al., 2017). This approach previously demonstrated that although the four DENV serotypes form different genetically-related clusters, they are not completely separate antigenically, and strains from one serotype can be more similar antigenically to another serotype than their own. (Katzelnick et al., 2015). Our antigenic cartography maps suggest DKE-121 is similar yet not identical to other DENV-4 strains.
Apart from serotype definitions by serum neutralization and antigenic cartography, data from cross-protection experiments in vivo must be considered. For example, while DENV-1 and DENV-2 cluster separately (Katzelnick et al., 2015), sera from DENV-1 infected individuals neutralized and protected against the 1995 Iquitos, Peru DENV-2 outbreak. Using mouse models, we evaluated for cross-protection between DENV-4 and DKE-121. In passive transfer experiments using sera from mice that recovered from DENV-4, DKE-121, or DENV-2, we did not observe protection against heterologous virus infections. In contrast, sera from DENV-4 gI and DENV-4 gII infected mice reduced DENV-4 gII infection, and sera from DKE-121 infected mice diminished viral loads in DKE-121-challenged mice. Thus, homologous, but not heterologous, protection was observed suggesting that antibodies lack cross-protection, which is part of the historical serotype definition. These results may have implications for situations of passive antibody transfer, such as during pregnancy; maternal antibodies from DENV-4 immune mothers might not protect infants against infection with variant viruses like DKE-121 or the reverse. Analogously, exogenous administration of type-specific therapeutic mAbs against DENV (Fibriansah and Lok, 2016; Lai et al., 2007; Salazar et al., 2017; Screaton et al., 2015) might not protect against divergent strains. One caveat to our passive transfer studies is the amount of antibody transferred; while the 150 μl dose of serum we used did not confer cross-protection against DENV-4 and DKE-121 strains, higher amounts might. Further studies are needed to assess whether serotype cross-reactive mAbs, such as those mapping to the E-dimer epitope (EDE) (Dejnirattisai et al., 2015) or fusion loop (Oliphant et al., 2006; Smith et al., 2013), would cross-neutralize and protect against variant DENV strains.
We also assessed cross-protection using models of sequential infection and vaccination. In humans, primary DENV infection confers cross-protection against heterologous DENV serotypes for a transient period (e.g., 2 to 6 months (Sabin, 1952)), but this wanes over time. To model this sequence in mice, we allowed a six-month interval between primary and secondary infections. However, even after 180 days, cross-protective immunity had not waned in three mouse models, and infection with all heterologous DENV serotypes was inhibited. Consistent with these results, a prior study in AG129 mice showed that cross-reactive immunity exists at 1 year between DENV-1 and DENV-2 (Kyle et al., 2008). The basis for this cross-protection in mice could reflect a combination of pre-existing cross-reactive serum antibodies, cross-reactive antibodies generated de novo from memory B cells, or cross-protective T cell responses. Given the extensive cross-protection observed between DENV serotypes in mice, these animals do not appear useful for studying the consequences of secondary infection between the closely related DENV-4 and DKE-121 strains.
To understand possible effects on DENV vaccination in the context of highly variant DKE-121, we immunized NHPs with placebo, monovalent (DENV-4), bivalent (DENV-2 + DENV-3), and trivalent (DENV-2 + DENV-3 + DENV-4) formulations of the NIH vaccine and challenged with DKE-121 three months later. NHPs that received DENV-4 in either monovalent or trivalent vaccine formulations did not develop infectious viremia after DKE-121 challenge. When viremia was measured by RNA levels, monovalently-vaccinated NHPs also were largely protected. While the viremia in placebo-immunized NHPs was high initially and cleared over time, animals immunized with the bivalent (DENV-2 + DENV-3) formulation sustained a more persistent DKE-121 viremia. Thus, pre-existing DENV-2 + DENV-3 immunity resulted in enhanced DKE-121 viremia, consistent with infection enhancement by poorly-neutralizing cross-reactive antibodies (Halstead, 2003; Porterfield, 1982). Although the trivalent (DENV-2 + DENV-3 + DENV-4) vaccinated NHPs were protected from DKE-121 viremia as measured by plaque assay, the DKE-121 RNA levels increased over time. Since our serum sampling was terminated 6 days after challenge, we were unable to determine if there was a delayed DKE-121 viremia peak, which could occur due to neutralization escape (Magnani et al., 2017) or possibly ADE from cross-reactive Abs generated from the trivalent vaccine. One possible explanation for the DKE-121 breakthrough is that the DENV-4 component of the vaccine is weakly immunogenic in NHPs. Persistent viral RNA in serum has been described in an immunosuppressed Dengue patient with neutralizing antibodies but undetectable antigen-specific T cells (Ng et al., 2019). Thus, the factors contributing to and the significance of the rising DKE-121 RNA levels in serum of the trivalent-vaccinated NHPs remains unclear. Vaccination studies in NHPs with even later DKE-121 challenge dates (e.g., 6 months or 1 year) along with detailed analysis of virus-specific T cells might elucidate further the extent of cross-protection conferred by DENV-4.
Clinical trials with the first licensed tetravalent DENV vaccine, Dengvaxia® (CYD-TDV), demonstrated that genotypic variation within a DENV serotype can affect vaccine efficacy. Whereas the vaccine included DENV-4 gII, the elicited neutralizing responses were less effective against DENV-4 gI (Henein et al., 2017; Juraska et al., 2018; Rabaa et al., 2017). Indeed, sera from volunteers who received monovalent DENV-4 and tetravalent NIH vaccines also demonstrated type-specificity with preferential neutralization of DENV-4 gII (Gallichotte et al., 2018). We tested sera from individuals who received the same monovalent DENV-4 and tetravalent NIH vaccines for neutralization capacity against DENV-4 and DKE-121 strains and observed similar trends in type-specificity. Nonetheless, our results suggest that a tetravalent vaccine including multiple DENV nonstructural genes with a DENV-4 gII component should induce a broad enough antibody response in humans to neutralize variant strains at comparable titers compared to vaccine strains.
DKE-121 is one of the most variant DENV strains described so far, with 15% whole genome and 11-12% E protein amino acid sequence divergence from DENV-4. Two divergent DENV-1 (Brun2014) (Pyke et al., 2016) and DENV-2 (QML22/2015) (Liu et al., 2016; Pyke et al., 2017) strains are distinct from sylvatic lineages and have 94% and 86% amino acid identity to historical and circulating DENV-1 and DENV-2 viruses, respectively. Our analyses, along with those from others (Gallichotte et al., 2018; Katzelnick et al., 2015; Martinez et al., 2020) challenge taxonomy assumptions that dengue viruses exist exclusively as four separate serotypes; instead, newly-emerging variant DENV strains may have more nuanced relationships. In contrast to the concept that infection creates long-lasting protective immunity against strains of the homologous serotype, symptomatic homotypic re-infections do occur (Forshey et al., 2016; Waggoner et al., 2016). Historical serotype definitions of cross-immunity may be less useful when applied to highly variant DENV strains like DKE-121. While our results in NHPs suggest that tetravalent vaccine immunity still shows promise for protection against highly variant DENV strains, protection generated by natural monotypic infection or via passive transfer of maternal or therapeutic mAbs (Budigi et al., 2018; Ong et al., 2017) may be incomplete and could sensitize some individuals to greater infection upon heterologous DENV challenge. Thus, greater surveillance is needed to determine whether DKE-121 or strains with similar levels of genetic divergence circulate and modulate DENV immunity.
Limitations of the study.
No animal infection model fully recapitulates human Dengue disease (Chen and Diamond, 2020; Coronel-Ruiz et al., 2020; Sarathy et al., 2015; Zellweger and Shresta, 2014). Thus, vaccine-induced immune responses and disease outcome may vary between animal models because of inherent differences in permissiveness of DENV strains and antibody responses. As we observed slightly different viral burdens in DENV-4 and DKE-121 infected mice, differences in infection levels could impact the relative protective efficacy of antibody responses. Also, while it would have been useful to compare directly the viremia of DENV-4-vaccinated and DENV-4-challenged NHPs to DENV-4-vaccinated and DKE-121-challenged NHPs, the former serum samples were exhausted in years prior, and all NHPs for current NIH research have been diverted to address the ongoing COVID-19 pandemic. Future NHP studies that assess both cellular and humoral immune responses against DENV-4 and DKE-121 after vaccination will be needed to corroborate and extend our findings.
STAR METHODS
RESOURCE AVAILABLITY
Lead Contact.
Further information and requests for resources and reagents should be directed to the Lead Contact, Michael S. Diamond (diamond@wusm.wustl.edu).
Materials Availability.
All requests for resources and reagents should be directed to the Lead Contact author. This includes mice and viruses. All reagents will be made available on request after completion of a Materials Transfer Agreement.
Data and code availability.
All data supporting the findings of this study are available within the paper.
This paper does not report original code
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Animal experiments.
Mouse experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. All animal protocols were approved by the Washington University School of Medicine Institutional Animal Care and Use Committee (IACUC assurance number A3381-01). Mice were maintained in normal light-dark cycles and fed standard chow. To minimize animal suffering, ketamine hydrochloride and xylazine was used for anesthesia and intravenous viral inoculations were performed under isoflurane. C57BL/6J (000664) mice were purchased from Jackson Laboratory. AG129 and hSTAT2-KI C57BL/6J mice (Gorman et al., 2018) were bred in a specific-pathogen-free facility at Washington University School of Medicine. The specific sex and age of mice uses are indicated in the Figure legends where they were used. In vivo studies were not blinded, and mice were randomly assigned to treatment groups. No sample-size calculations were performed to power each study. Instead, sample sizes were determined based on prior in vivo virus challenge experiments.
Some of the NHP experiments were performed at the Caribbean Primate Research Center, University of Puerto Rico and at the University of Texas Medical Branch (UTMB). These studies were reviewed by each institution’s IACUC. NHP facilities are accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC). Procedures were conducted with oversight of staff veterinarians and in accordance with USDA Animal Welfare Regulations, the Guide for the Care and use of Laboratory Animals and institutional policies. Steps were taken to minimize suffering, including use of anesthesia and method of sacrifice if appropriate, in accordance with the recommendations of the Guide for the Care and use of Laboratory Animals, Animal Welfare Act and the Public Health Service (PHS) Policy on Humane Care and Use of Laboratory Animals. NHPs were monitored continuously by trained veterinarians and evaluated twice daily for evidence of disease or injury. Feeding and drinking continued normally during this period. All NHPs were treated with an environmental-enrichment program, also approved by the IACUC.
The NHP study performed at the National Institutes of Health (NIH) was humanely conducted with the approval and oversight of the National Institute of Allergy and Infectious Diseases Division of Intramural Research Animal Care and Use Committee as part of the NIH Intramural Research Program (Animal Study Protocol LVD 9E). Rhesus macaques (Macaca mulatta) were housed and sustained in accordance with standards established by the Association for Assessment and Accreditation of Laboratory Animal Care. All animals were cared for at the National Institutes of Health Animal Center.
Cells.
Vero CCL81 (ATCC) and Vero-furin cells were maintained at 37°C and 5% CO2 in Dulbecco’s Modified Eagle medium (DMEM) supplemented with 5% fetal bovine serum (FBS), 100 U/ml penicillin-streptomycin, and 10 mM HEPES. Vero-furin cells were cultured with Blasticidin. C6/36 were maintained at 28°C and 5% CO2 in DMEM supplemented with 10% FBS, 100 U/ml penicillin-streptomycin, 1 mM sodium pyruvate, and 10 mM HEPES. HEK-293T/17 cells (ATCC) and Raji cells expressing the flavivirus attachment factor DC-SIGNR (Raji-DCSIGNR) (Davis et al., 2006) were maintained at 37°C and 7% CO2 in DMEM containing GlutaMAX (HEK-293T/17) or RPMI-1640 (Raji-DCSIGNR) medium supplemented with 7% FBS and 100 U/mL penicillin-streptomycin. All cells routinely tested negative for mycoplasma using a PCR-based assay. Cells were validated based on analysis of expression of key genes (DCSIGNR and furin) and behavior in culture.
Viruses.
DENV-1 Western Pacific has been described (Durbin et al., 2006). DENV-2 D2S20 was provided from S. Shresta (La Jolla, CA) (Makhluf et al., 2013). DENV-3 16652 originally was provided from R. Kinney (Centers for Disease Control and Prevention) (Brien et al., 2010). DENV4 H-241 and P75-514 were obtained from the World Reference Center for Emerging Viruses and Arboviruses at University of Texas Medical Branch. Additional viruses included DENV-2 NGC and DENV-4 TVP-376 (Sukupolvi-Petty et al., 2010; Sukupolvi-Petty et al., 2013). DKE-121 was isolated from a subject in Malaysia, passaged in Vero cells, and subjected to next generation sequencing (accession number: PRJNA729800). Viruses were propagated in Vero, Vero-furin, or C6/36 cells depending on the experiment. Cell supernatants were collected at 4 and 6 dpi and titered by focus forming assay (FFA) on Vero cells. To generate high titer DKE-121 stocks for in vivo studies, virus was amplified in Vero cells and collected at 3, 4, and 5 dpi. Cellular supernatants were concentrated on a 25% glycerol (+75% 10 mM Tris/150 mM NaCl/1mM EDTA (TNE) pH 8) cushion by ultracentrifugation for 30,000 rpm (Beckman Coulter SW32Ti) for 2 h at 4°C. Pellets were resuspended in TNE buffer, pooled, and titered by FFA on Vero cells.
METHOD DETAILS
Dendrogram and dimer structure.
Alignment of E protein amino acid sequences was done using Clustal Omega and phylogenetic tree was constructed with FigTree. Tree was rooted on DKE-121. The percent identity and similarity were determined using Ident and Sim. DKE-121 divergent residues were represented on a DENV E dimer (PDB: 1OAN) using PyMOL.
Focus-forming assay (FFA).
Vero cells were plated in 96-well flat bottom plates. One day later, viruses were serially diluted 10-fold and added to cell monolayers. After 1 hour at 37°C, 1% (w/v) methylcellulose in MEM overlay was added. Cells were incubated for 2 days, then fixed with 1% paraformaldehyde diluted in PBS for 1 h at room temperature. Cells were then stained with primary antibody: 1 μg/ml of E60 for either 2 h at room temperature or overnight at 4°C and then secondary antibody: 1:2000 of HRP-conjugated anti-mouse IgG whole molecule (Sigma) for 1 h at room temperature; both stains are diluted in PBS with 0.1% (w/v) saponin (Sigma) and 0.1% BSA. Foci were developed with TrueBlue peroxidase (KPL) and counted on a BioSpot reader (Cellular Technology Limited).
Focus reduction neutralization test (FRNT).
Vero cells were plated in 96-well flat bottom plates. One day later, serum or mAbs starting at 20 μg/ml were serially diluted 3-fold and mixed with an equal volume of virus (100 foci) and incubated for 1 h at 37°C. Serum-virus mixtures were then added to cell monolayers and after another incubation for 1 h at 37°C, 1% (w/v) methylcellulose in MEM was overlaid on plates. After 2 days, plates were fixed with 1% paraformaldehyde and stained and developed as described in FFA. Neutralization curves were generated and analyzed in GraphPad Prism.
Production and titration of DENV reporter virus particles (RVPs).
DENV RVPs were produced by genetic complementation of a green fluorescent protein-expressing West Nile virus (WNV) lineage II sub-genomic replicon with plasmids encoding DENV structural genes as previously described (Mukherjee et al., 2014; Pierson et al., 2006). To produce DENV-4 RVPs, HEK-293T/17 cells were co-transfected with the WNV replicon plasmid and a plasmid expressing the DENV-4 gII strain Dominica/81 C-prM-E sequence at a 1:3 ratio by mass using Lipofectamine 3000 reagent. To produce DENV strain DKE-121 RVPs, HEK-293T/17 cells were co-transfected with the WNV replicon plasmid, a plasmid expressing the DKE-121 capsid sequence, and a plasmid expressing the DKE-121 prM-E sequence at a 1:2.8:0.2 ratio by mass. The decreased amount of prM-E plasmid limits the potential for subviral particle generation (Lee et al., 2013; Pierson et al., 2006). To produce more homogeneously mature populations of DENV RVPs, transfections were performed with the addition of a plasmid expressing human furin (Dowd et al., 2014). Transfected cells were incubated at 30°C, and RVP-containing supernatants were collected on days 3-6 post-transfection, passed through 0.2 μm filters (Millipore), and stored at −80°C. To determine the infectious titer of each RVP, 5 x 104 Raji-DCSIGNR cells were incubated with 2-fold serial dilutions of RVP supernatants in technical duplicate in a 96-well plate format. Infections were carried out at 37°C, and two days later cells were fixed with 2% paraformaldehyde, and infectivity was measured by flow cytometry as a percentage of GFP-positive cells.
RVP Neutralization assays.
Neutralization assays were performed using previously described methods (Mukherjee et al., 2014; VanBlargan et al., 2013). Three-fold serial dilutions of heat-inactivated test sera were incubated with diluted DENV4 and DKE-121 RVPs in technical duplicate for 1 hour at 37°C to allow for steady-state binding. Resulting immune complexes were combined with 5 x 104 Raji-DCSIGNR cells per well in a 96-well plate and incubated at 37°C for 2 days. RVP stocks were diluted to ensure antibody excess at informative points of the dose-response curves. Infectivity was measured by flow cytometry as a percentage of GFP-positive cells, and the results were analyzed by non-linear regression analysis to estimate the serum dilution required to reduce RVP infectivity by half (NT50). The limit of detection of the assay was set at the starting reciprocal serum dilution of 1:60, and NT50 values predicted by non-linear regression to be below the LOD were assigned a value of half the LOD (a reciprocal titer of 30).
Mouse sera.
hSTAT2-KI mice (3 to 4-week-old, males and females) were inoculated intravenously by retro-orbital route with 106 FFU of DENV-4 gI (strain H-241), DENV-4 gII (strain TVP-376), or DKE-121. Mice were infected twice more with the same dose of virus at three-week intervals. Two weeks after the final infection, mice were sacrificed and serum was harvested. AG129 mice (4 to 5-week-old, males and females) were inoculated by intraperitoneal injection with 104 FFU of DENV-4 gI (strain H-241), DENV-4 gII (strain TVP-376), or DKE-121. At 7 weeks post-infection, mice were euthanized using ketamine/xylazine and serum was harvested.
NHP and human sera.
To obtain DENV-4-immune sera, four rhesus macaques were inoculated with DENV-4 gII (strain Dominica/814669) (105 PFU, intramuscular injection) and bled at 60 and 365 dpi. To obtain DKE-121-immune sera, four rhesus macaques were inoculated subcutaneously with 5 x 105 PFU of DKE-121 in the deltoid region diluted in 500 μL of PBS. Animals were bled at 90 and 180 dpi. All animals were male with an average age of 4.61 years old (4.67, 4.50, 4.67, 4.58).
Monovalent DENV-4 and tetravalent human sera were obtained from volunteers who received the NIH live-attenuated monovalent DENV-4 (Durbin et al., 2001; Durbin et al., 2005) or tetravalent vaccine (Kallas et al., 2020). Monovalent volunteers were bled at 42 days post-vaccination whereas tetravalent vaccinees were bled at 28, 91, 180, and 365 days post-vaccination (Kallas et al., 2020). Human sera from natural DENV infection were obtained from the Dengue Traveler collection at the University of North Carolina (gift of Aravinda de Silva) and described previously (Nivarthi et al., 2017).
Antigenic cartography.
Antigenic cartography was used to visualize and quantify antigenic distances between viral strains (Smith et al., 2004). Neutralization titers first were transformed into target map distances. For each serum/virus pair, the target distance was calculated as the difference between the log2 titer for that serum/virus pair and the maximum log2 titer of that serum for any virus. Multidimensional scaling methods were used to position sera and viruses on the map relative to each other, by minimizing the sum of squares of differences between target and map distances. When titers were beyond detection limits (e.g., <50), their positioning only contributed to map error when the measured map distance exceeded the target distance. Minimization was carried out using the minimize function from the Python library SciPy (Virtanen et al., 2020). We used the conjugate gradient method for minimization, with 500 restarts, randomly assigning starting coordinates each time to increase likelihood of finding a good minimum. The maps shown are those with the smallest error after minimization. Because we used a log2 transformation on neutralization titers, 1 antigenic unit, indicated with grid lines on maps, corresponds to a 2-fold change in titer. For sera developed against an identical viral strain, ellipses are calculated for the sera clusters such that the radii of the ellipses correspond to 2 standard deviations in coordinate data.
Mouse experiments.
For passive transfer experiments, mouse sera were pooled and assayed for neutralization. Serum (150 μl) from naïve mice or mice immunized with DENV-4 gI (strain H-241), DENV-4 gII (strain TVP-376), DKE-121, or DENV-2 (strain D2S20) (1:1,000 neutralizing titer against homologous virus) was administered to 3-week-old C57BL/6J mice at the same time as an injection of 2 mg of anti-Ifnar1 mAb (MAR1-5A3, Leinco I-1188). One day later, mice were injected by intraperitoneal route with 106 FFU of DENV-4 gII (strain TVP-376) or DKE-121. At 3 dpi, mice were euthanized, and organs were perfused extensively with PBS.
For sequential infection experiments, 4-week-old AG129 mice were inoculated via intraperitoneal route with 104 FFU of DENV-4 gII (strain TVP-376) or DKE-121, or not infected (naïve control). After 180 dpi, mice were challenged with 106 FFU of DENV-4 gII (TVP-376), 106 FFU of DKE-121 or 105 FFU of DENV-2 (strain D2S20) via intraperitoneal injection. Three days post challenge, mice were euthanized and tissues were harvested. For studies with WT C57BL/6J and hSTAT2-KI mice, 3-4-week-old mice were inoculated intravenously with 107 FFU of DENV-4 gII (strain TVP-376) or DKE-121 or not infected (naïve control). Three weeks later, mice were boosted a second time with the same dose and virus. 180 days later, hSTAT2-KI and C57BL/6J mice were inoculated with 106 FFU of DENV-4 gII (strain TVP-376), 106 FFU of DKE-121 or 105 FFU of DENV-2 (strain D2S20) by intraperitoneal injection with pre-administration of anti-Ifnar1 mAb one day prior to challenge. At 3 days post-challenge, mice were euthanized and tissues were harvested.
Viral RNA extraction and quantification.
Harvested tissues were weighed and homogenized in Dulbecco’s Modified Eagle’s Medium (DMEM) + 2% fetal bovine serum (FBS) with Zirconia/Silica beads using a MAgNA Lyser (Roche). Tissue homogenates were clarified, and RNA was extracted from tissue homogenate supernatant or serum using the MagMax viral RNA isolation kit (Thermo Scientific) with a Kingfisher RNA extraction machine (Thermo Scientific). RNA was quantified by one-step quantitative reverse transcriptase PCR (qRT-PCR) with strain-specific primers and probes (Integrated DNA Technologies) [DENV-2 (strain D2S20): Primer 1: 5'-ACA GTC AAC CCA ATC GTA ACA-3'; Primer 2: 5'-TTC AAT TGT CCC GGC TCT AC-3'; Probe: /56-FAM/AG GTT CTG C/ZEN/T TCT ATG TTG ACT GGG C/3IABkFQ/; DENV-4 (strain TVP-376): Primer 1: 5'-ACC AAC AGT GTA ACC AAC ATA GA-3'; Primer 2: 5'-TCC TGA ACC AAT GGA GTG TTA AT-3'; Probe: /56-FAM/AG TGA TAG G/ZEN/T GTT GGA AAC AGC GC/3IABkFQ/; DKE-121: Primer 1: 5'-GGC AAG CAT AGA GCT GGA A-3'; Primer 2: 5'-TTC TAA ACC AGT GGA GGG TAA TG-3'; Probe: /56-FAM/AC ATT ACC A/ZEN/T TGG AAC AGG AGA TGG GAC /3IABkFQ/]. For bone marrow, viral RNA was normalized to housekeeping gene Gapdh levels [Integrated DNA Technologies primer set (Mm.PT.39a.1): Primer 1: 5'-GTGGAGTCATACTGGAACATGTAG-3'; Primer 2: 5'-AATGGTGAAGGTCGGTGTG-3'; Probe: /56-FAM/TGCAAATGGCAGCCCTGGTG/36-TAMSp/].
NHP vaccination with DKE-121 challenge.
Sixteen juvenile rhesus macaques without prior-DENV exposure and confirmed to be DENV-serologically-naïve were enrolled in this study. NHPs were immunized subcutaneously with a single dose of formulations of the NIH live-attenuated vaccine: monovalent (rDEN4Δ30), bivalent (rDEN2/4Δ30 + rDEN3Δ30/31), or trivalent (rDEN4Δ30 + rDEN2/4Δ30 + rDEN3Δ30/31) or placebo diluent. All vaccine virus components were delivered at a potency of 104 FFU. NHPs were bled for serum neutralizing titers prior to vaccination and at 87 days post-vaccination. At 94 days post-vaccination, all NHPs were challenged subcutaneously with 104 FFU of DKE-121. At 2 to 6 days post-challenge, serum was collected and cryopreserved for analysis by plaque assay and viral RNA quantification by qRT-PCR. At 28 days post-challenge (120 days post-vaccination), serum was collected for FRNT assay.
Plaque assay.
To determine the presence and titer of infectious virus (viremia) in NHP serum samples, sera were serially diluted 10-fold and inoculated directly onto Vero cell monolayers for 1 h at 37°C and then overlaid with 1% methylcellulose in OptiMEM (Life Technologies) + 2% FBS. After five days at 37°C, cell monolayers were fixed in 80% methanol. Plaque foci were visualized after treatment with mouse monoclonal antibody 4G2 and peroxidase-labeled goat anti-mouse IgG (KPL, Gaithersburg, MD) and developing with TrueBlue peroxidase substrate (KPL, Gaithersburg, MD).
QUANTIFICATION AND STATISTICAL ANALYSIS
Data analysis and software.
All data were graphed and analyzed in GraphPad Prism v8.4.3. Dendrogram and alignment analyses were done using Clustal Omega (Sievers et al., 2011), FigTree v1.4.4 [http://tree.bio.ed.ac.uk/software/figtree/); Andrew Rambaut]), Indent and Sim (Stothard, 2000), and Aline (Bond and Schüttelkopf, 2009). Protein structure was created using MacPyMOL: PyMOL v2.4.1 (Schrödinger).
Statistical analysis.
Statistical significance was assigned when P values were < 0.05 using Prism Version 8 (GraphPad). The specific tests (one-way ANOVA with Tukey’s post-test or one-way ANOVA with Dunnett’s post-test), number of animals (n), median and mean values, and comparison groups are indicated in the Figure legends.
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| WNV E60 | Oliphant et al., 2006 | N/A |
| DV4 E88 | Sukupolvi-Petty et al., 2013 | N/A |
| Anti-mouse Ifnar1 mAb | Leinco | Clone MAR1-5A3; Product No I-1188 |
| HRP conjugated anti-mouse IgG (whole molecule) | Sigma | A8924; RRID:AB_258426 |
| Bacterial and virus strains | ||
| Dengue DKE-121 | Isolated from patient (this study) | N/A |
| DENV-1 Western Pacific | Durbin et al., 2006 | N/A |
| DENV-2 NGC | Sukupolvi-Petty et al., 2010 | N/A |
| DENV-2 D2S20 | Makhluf et al., 2013 | N/A |
| DENV-3 16652 | Brien et al., 2010 | N/A |
| DENV-4 H-241 | World Reference Center for Emerging Viruses and Arboviruses at UTMB | N/A |
| DENV-4 TVP-376 | Sukupolvi-Petty et al., 2013 | N/A |
| DENV-4 P75-514 | World Reference Center for Emerging Viruses and Arboviruses at UTMB | N/A |
| DENV-4 Dominica/81 | World Reference Center for Emerging Viruses and Arboviruses at UTMB | N/A |
| Deposited data | ||
| DKE-121 whole genome sequence | This paper | accession number: PRJNA729800 |
| Experimental models: Cell lines | ||
| Vero CCL81 | ATCC | CCL-81; RRID:CVCL_0059 |
| Vero-furin | Mukherjee et al., 2016 | N/A |
| Raji-DCSIGNR | Davis et al., 2006 | N/A |
| HEK-293T/17 | ATCC | CRL-11268; RRID:CVCL_1926 |
| C6/36 | ATCC | CRL-1660; RRID:CVCL_Z230 |
| Experimental models: Organisms/strains | ||
| C57Bl/6J | Jackson Laboratory | 000664; RRID:IMSR_JAX:000664 |
| AG129 | Johnson et al., 1999, Marshall BioResources | AG129 |
| hSTAT2-KI | Gorman et al., 2018; Jackson Laboratory | 031630; N/A |
| Macaca mulatta | NIHAC, Poolesville MD | N/A |
| Oligonucleotides | ||
| DENV-2 D2S20 qPCR Primer 1: 5'-ACA GTC AAC CCA ATC GTA ACA-3'; Primer 2: 5'-TTC AAT TGT CCC GGC TCT AC-3'; Probe: /56-FAM/AG GTT CTG C/ZEN/T TCT ATG TTG ACT GGG C/3IABkFQ/ | This paper | N/A |
| DENV-4 gII (TVP-376) qPCR: Primer 1: 5'-ACC AAC AGT GTA ACC AAC ATA GA-3'; Primer 2: 5'-TCC TGA ACC AAT GGA GTG TTA AT-3'; Probe: /56-FAM/AG TGA TAG G/ZEN/T GTT GGA AAC AGC GC/3IABkFQ/ | This paper | N/A |
| DKE-121 qPCR Primer 1: 5'-GGC AAG CAT AGA GCT GGA A-3'; Primer 2: 5'-TTC TAA ACC AGT GGA GGG TAA TG-3'; Probe: /56-FAM/AC ATT ACC A/ZEN/T TGG AAC AGG AGA TGG GAC /3IABkFQ/ | This paper | N/A |
| Software and algorithms | ||
| Clustal Omega | Sievers et al., 2011 | N/A |
| FigTree | Andrew Rambaut | http://tree.bio.ed.ac.uk/software/figtree/ |
| BioSpot | Cellular Technology Limited | N/A |
| Python SciPy | Virtanen et al., 2020 | N/A |
| Ident and Sim | Stothard, 2000 | |
| Aline | Bond and Schüttelkopf, 2009 | N/A |
| PyMOL | Schrödinger | https://pymol.org/2/ |
Highlights:
DKE-121 immune sera neutralize DENV-4 infection poorly
DENV-4 or DKE-121 immune sera do not protect against DKE-121 or DENV-4 infection
DENV-4 vaccination protects against DKE-121 infection
DKE-121 falls between genotype and serotype levels of sequence divergence
ACKNOWLEDGEMENTS
We thank Leran Wang, Ahmed Hassan, Arthur Kim, Michelle Elam-Noll, and Larissa Thackray for sequence submission, reagents, animal breeding, and experimental suggestions. We acknowledge Aravinda de Silva for providing human serum samples and manuscript comments. This work was supported by NIAID contract 75N93019C00062 and NIH grants R01 AI073755, R01 AI125202, R21 AI145012, and U01 AI115577, a pilot grant by the Institute for Human Infection and Immunity (to N.V.), and the Intramural Research Program of the National Institute of Allergy and Infectious Diseases (T.C.P. and S.S.W.). This work was also supported by the Caribbean Primate Research Center (Grants P40 OD012217 and 2U42OD021458 from ORIP/OD/NIH). E.S.W. is supported by T32 AI007163.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
COMPETING FINANCIAL INTERESTS
M.S.D. is a consultant for Inbios, Vir Biotechnology, and Carnival Corporation, and on the Scientific Advisory Boards of Moderna and Immunome. The Diamond laboratory has received unrelated funding support in sponsored research agreements from Moderna, Vir Biotechnology, and Emergent BioSolutions. The Vasilakis laboratory has received unrelated funding support in sponsored research agreements from Public Health Vaccines, LLC.
REFERENCES
- (2009). WHO Guidelines Approved by the Guidelines Review Committee. In Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control: New Edition (Geneva: World Health Organization; ). [PubMed] [Google Scholar]
- Ansarah-Sobrinho C, Nelson S, Jost CA, Whitehead SS, and Pierson TC (2008). Temperature-dependent production of pseudoinfectious dengue reporter virus particles by complementation. Virology 381, 67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, et al. (2013). The global distribution and burden of dengue. Nature 496, 504–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond CS, and Schüttelkopf AW (2009). ALINE: a WYSIWYG protein-sequence alignment editor for publication-quality alignments. Acta Crystallogr D Biol Crystallogr 65, 510–512. [DOI] [PubMed] [Google Scholar]
- Brady OJ, Gething PW, Bhatt S, Messina JP, Brownstein JS, Hoen AG, Moyes CL, Farlow AW, Scott TW, and Hay SI (2012). Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl Trop Dis 6, e1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brien JD, Austin SK, Sukupolvi-Petty S, O'Brien KM, Johnson S, Fremont DH, and Diamond MS (2010). Genotype-specific neutralization and protection by antibodies against dengue virus type 3. J Virol 84, 10630–10643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brien JD, Lazear HM, and Diamond MS (2013). Propagation, quantification, detection, and storage of West Nile virus. Curr Protoc Microbiol 31, 15D 13 11–15D 13 18. [DOI] [PubMed] [Google Scholar]
- Budigi Y, Ong EZ, Robinson LN, Ong LC, Rowley KJ, Winnett A, Tan HC, Hobbie S, Shriver Z, Babcock GJ, et al. (2018). Neutralization of antibody-enhanced dengue infection by VIS513, a pan serotype reactive monoclonal antibody targeting domain III of the dengue E protein. PLoS neglected tropical diseases 12, e0006209–e0006209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calisher CH, Karabatsos N, Dalrymple JM, Shope RE, Porterfield JS, Westaway EG, and Brandt WE (1989). Antigenic relationships between flaviviruses as determined by cross-neutralization tests with polyclonal antisera. J Gen Virol 70 (Pt 1), 37–43. [DOI] [PubMed] [Google Scholar]
- Cardosa J, Ooi MH, Tio PH, Perera D, Holmes EC, Bibi K, and Abdul Manap Z (2009). Dengue virus serotype 2 from a sylvatic lineage isolated from a patient with dengue hemorrhagic fever. PLoS neglected tropical diseases 3, e423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, and Vasilakis N (2011). Dengue--quo tu et quo vadis? Viruses 3, 1562–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen RE, and Diamond MS (2020). Dengue mouse models for evaluating pathogenesis and countermeasures. Curr Opin Virol 43, 50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coronel-Ruiz C, Gutiérrez-Barbosa H, Medina-Moreno S, Velandia-Romero ML, Chua JV, Castellanos JE, and Zapata JC (2020). Humanized Mice in Dengue Research: A Comparison with Other Mouse Models. Vaccines (Basel) 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis CW, Nguyen HY, Hanna SL, Sanchez MD, Doms RW, and Pierson TC (2006). West Nile virus discriminates between DC-SIGN and DC-SIGNR for cellular attachment and infection. J Virol 80, 1290–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejnirattisai W, Jumnainsong A, Onsirisakul N, Fitton P, Vasanawathana S, Limpitikul W, Puttikhunt C, Edwards C, Duangchinda T, Supasa S, et al. (2010). Cross-reacting antibodies enhance dengue virus infection in humans. Science 328, 745–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejnirattisai W, Wongwiwat W, Supasa S, Zhang X, Dai X, Rouvinski A, Jumnainsong A, Edwards C, Quyen NTH, Duangchinda T, et al. (2015). A new class of highly potent, broadly neutralizing antibodies isolated from viremic patients infected with dengue virus. Nature Immunology 16, 170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng SQ, Yang X, Wei Y, Chen JT, Wang XJ, and Peng HJ (2020). A Review on Dengue Vaccine Development. Vaccines (Basel) 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd KA, Mukherjee S, Kuhn RJ, and Pierson TC (2014). Combined effects of the structural heterogeneity and dynamics of flaviviruses on antibody recognition. J Virol 88, 11726–11737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durbin AP, Karron RA, Sun W, Vaughn DW, Reynolds MJ, Perreault JR, Thumar B, Men R, Lai CJ, Elkins WR, et al. (2001). Attenuation and immunogenicity in humans of a live dengue virus type-4 vaccine candidate with a 30 nucleotide deletion in its 3'-untranslated region. Am J Trop Med Hyg 65, 405–413. [DOI] [PubMed] [Google Scholar]
- Durbin AP, McArthur J, Marron JA, Blaney JE Jr., Thumar B, Wanionek K, Murphy BR, and Whitehead SS (2006). The live attenuated dengue serotype 1 vaccine rDEN1Delta30 is safe and highly immunogenic in healthy adult volunteers. Hum Vaccin 2, 167–173. [DOI] [PubMed] [Google Scholar]
- Durbin AP, Whitehead SS, McArthur J, Perreault JR, Blaney JE Jr., Thumar B, Murphy BR, and Karron RA (2005). rDEN4delta30, a live attenuated dengue virus type 4 vaccine candidate, is safe, immunogenic, and highly infectious in healthy adult volunteers. J Infect Dis 191, 710–718. [DOI] [PubMed] [Google Scholar]
- Fibriansah G, and Lok SM (2016). The development of therapeutic antibodies against dengue virus. Antiviral Res 128, 7–19. [DOI] [PubMed] [Google Scholar]
- Forshey BM, Reiner RC, Olkowski S, Morrison AC, Espinoza A, Long KC, Vilcarromero S, Casanova W, Wearing HJ, Halsey ES, et al. (2016). Incomplete Protection against Dengue Virus Type 2 Re-infection in Peru. PLoS Negl Trop Dis 10, e0004398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco L, Palacios G, Martinez JA, Vázquez A, Savji N, De Ory F, Sanchez-Seco MP, Martín D, Lipkin WI, and Tenorio A (2011). First Report of Sylvatic DENV-2-Associated Dengue Hemorrhagic Fever in West Africa. PLOS Neglected Tropical Diseases 5, e1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried JR, Gibbons RV, Kalayanarooj S, Thomas SJ, Srikiatkhachorn A, Yoon IK, Jarman RG, Green S, Rothman AL, and Cummings DA (2010). Serotype-specific differences in the risk of dengue hemorrhagic fever: an analysis of data collected in Bangkok, Thailand from 1994 to 2006. PLoS Negl Trop Dis 4, e617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallichotte EN, Baric TJ, Nivarthi U, Delacruz MJ, Graham R, Widman DG, Yount BL, Durbin AP, Whitehead SS, de Silva AM, et al. (2018). Genetic Variation between Dengue Virus Type 4 Strains Impacts Human Antibody Binding and Neutralization. Cell Rep 25, 1214–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goo L, VanBlargan LA, Dowd KA, Diamond MS, and Pierson TC (2017). A single mutation in the envelope protein modulates flavivirus antigenicity, stability, and pathogenesis. PLoS Pathog 13, e1006178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman MJ, Caine EA, Zaitsev K, Begley MC, Weger-Lucarelli J, Uccellini MB, Tripathi S, Morrison J, Yount BL, Dinnon KH 3rd, et al. (2018). An Immunocompetent Mouse Model of Zika Virus Infection. Cell Host Microbe 23, 672–685.e676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzmán MG, Kouri GP, Bravo J, Soler M, Vazquez S, and Morier L (1990). Dengue hemorrhagic fever in Cuba, 1981: a retrospective seroepidemiologic study. Am J Trop Med Hyg 42, 179–184. [DOI] [PubMed] [Google Scholar]
- Hadinegoro SR, Arredondo-García JL, Capeding MR, Deseda C, Chotpitayasunondh T, Dietze R, Hj Muhammad Ismail HI, Reynales H, Limkittikul K, Rivera-Medina DM, et al. (2015). Efficacy and Long-Term Safety of a Dengue Vaccine in Regions of Endemic Disease. New England Journal of Medicine 373, 1195–1206. [DOI] [PubMed] [Google Scholar]
- Halstead SB (2003). Neutralization and antibody-dependent enhancement of dengue viruses. Adv Virus Res 60, 421–467. [DOI] [PubMed] [Google Scholar]
- Halstead SB, Nimmannitya S, and Cohen SN (1970). Observations related to pathogenesis of dengue hemorrhagic fever. IV. Relation of disease severity to antibody response and virus recovered. Yale J Biol Med 42, 311–328. [PMC free article] [PubMed] [Google Scholar]
- Halstead SB, Shotwell H, and Casals J (1973). Studies on the pathogenesis of dengue infection in monkeys. I. Clinical laboratory responses to primary infection. J Infect Dis 128, 7–14. [DOI] [PubMed] [Google Scholar]
- Henein S, Swanstrom J, Byers AM, Moser JM, Shaik SF, Bonaparte M, Jackson N, Guy B, Baric R, and de Silva AM (2017). Dissecting Antibodies Induced by a Chimeric Yellow Fever-Dengue, Live-Attenuated, Tetravalent Dengue Vaccine (CYD-TDV) in Naive and Dengue-Exposed Individuals. The Journal of infectious diseases 215, 351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes EC, and Twiddy SS (2003). The origin, emergence and evolutionary genetics of dengue virus. Infect Genet Evol 3, 19–28. [DOI] [PubMed] [Google Scholar]
- Johnson AJ, and Roehrig JT (1999). New mouse model for dengue virus vaccine testing. J Virol 73, 783–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juraska M, Magaret CA, Shao J, Carpp LN, Fiore-Gartland AJ, Benkeser D, Girerd-Chambaz Y, Langevin E, Frago C, Guy B, et al. (2018). Viral genetic diversity and protective efficacy of a tetravalent dengue vaccine in two phase 3 trials. Proc Natl Acad Sci U S A 115, E8378–e8387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallas EG, Precioso AR, Palacios R, Thomé B, Braga PE, Vanni T, Campos LMA, Ferrari L, Mondini G, da Graça Salomão M, et al. (2020). Safety and immunogenicity of the tetravalent, live-attenuated dengue vaccine Butantan-DV in adults in Brazil: a two-step, double-blind, randomised placebo-controlled phase 2 trial. Lancet Infect Dis 20, 839–850. [DOI] [PubMed] [Google Scholar]
- Katzelnick LC, Fonville JM, Gromowski GD, Bustos Arriaga J, Green A, James SL, Lau L, Montoya M, Wang C, VanBlargan LA, et al. (2015). Dengue viruses cluster antigenically but not as discrete serotypes. Science (New York, NY) 349, 1338–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzelnick LC, Gresh L, Halloran ME, Mercado JC, Kuan G, Gordon A, Balmaseda A, and Harris E (2017). Antibody-dependent enhancement of severe dengue disease in humans. Science 358, 929–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyle JL, Balsitis SJ, Zhang L, Beatty PR, and Harris E (2008). Antibodies play a greater role than immune cells in heterologous protection against secondary dengue virus infection in a mouse model. Virology 380, 296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C-J, Goncalvez AP, Men R, Wernly C, Donau O, Engle RE, and Purcell RH (2007). Epitope determinants of a chimpanzee dengue virus type 4 (DENV-4)-neutralizing antibody and protection against DENV-4 challenge in mice and rhesus monkeys by passively transferred humanized antibody. Journal of virology 81, 12766–12774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapedes A, and Farber R (2001). The Geometry of Shape Space: Application to Influenza. Journal of Theoretical Biology 212, 57–69. [DOI] [PubMed] [Google Scholar]
- Lee PD, Mukherjee S, Edeling MA, Dowd KA, Austin SK, Manhart CJ, Diamond MS, Fremont DH, and Pierson TC (2013). The Fc region of an antibody impacts the neutralization of West Nile viruses in different maturation states. J Virol 87, 13729–13740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libraty DH, Endy TP, Houng HS, Green S, Kalayanarooj S, Suntayakorn S, Chansiriwongs W, Vaughn DW, Nisalak A, Ennis FA, et al. (2002). Differing influences of virus burden and immune activation on disease severity in secondary dengue-3 virus infections. J Infect Dis 185, 1213–1221. [DOI] [PubMed] [Google Scholar]
- Liu W, Pickering P, Duchêne S, Holmes EC, and Aaskov JG (2016). Highly Divergent Dengue Virus Type 2 in Traveler Returning from Borneo to Australia. Emerging infectious diseases 22, 2146–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnani DM, Ricciardi MJ, Bailey VK, Gutman MJ, Pedreño-Lopez N, Silveira CGT, Maxwell HS, Domingues A, Gonzalez-Nieto L, Su Q, et al. (2017). Dengue Virus Evades AAV-Mediated Neutralizing Antibody Prophylaxis in Rhesus Monkeys. Mol Ther 25, 2323–2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makhluf H, Buck MD, King K, Perry ST, Henn MR, and Shresta S (2013). Tracking the evolution of dengue virus strains D2S10 and D2S20 by 454 pyrosequencing. PloS one 8, e54220–e54220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez DR, Yount B, Nivarthi U, Munt JE, Delacruz MJ, Whitehead SS, Durbin AP, de Silva AM, and Baric RS (2020). Antigenic Variation of the Dengue Virus 2 Genotypes Impacts the Neutralization Activity of Human Antibodies in Vaccinees. Cell reports 33, 108226–108226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattingly PF (1957). Genetical aspects of the Aedes aegypti problem. I. Taxonom: and bionomics. Ann Trop Med Parasitol 51, 392–408. [PubMed] [Google Scholar]
- Messina JP, Brady OJ, Golding N, Kraemer MUG, Wint GRW, Ray SE, Pigott DM, Shearer FM, Johnson K, Earl L, et al. (2019). The current and future global distribution and population at risk of dengue. Nat Microbiol 4, 1508–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messina JP, Brady OJ, Scott TW, Zou C, Pigott DM, Duda KA, Bhatt S, Katzelnick L, Howes RE, Battle KE, et al. (2014). Global spread of dengue virus types: mapping the 70 year history. Trends Microbiol 22, 138–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison J, and García-Sastre A (2014). STAT2 signaling and dengue virus infection. Jakstat 3, e27715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison J, Laurent-Rolle M, Maestre AM, Rajsbaum R, Pisanelli G, Simon V, Mulder LC, Fernandez-Sesma A, and Garcia-Sastre A (2013). Dengue virus co-opts UBR4 to degrade STAT2 and antagonize type I interferon signaling. PLoS Pathog 9, e1003265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S, Pierson TC, and Dowd KA (2014). Pseudo-infectious reporter virus particles for measuring antibody-mediated neutralization and enhancement of dengue virus infection. Methods Mol Biol 1138, 75–97. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Sirohi D, Dowd KA, Chen Z, Diamond MS, Kuhn RJ, and Pierson TC (2016). Enhancing dengue virus maturation using a stable furin over-expressing cell line. Virology 497, 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson S, Jost CA, Xu Q, Ess J, Martin JE, Oliphant T, Whitehead SS, Durbin AP, Graham BS, Diamond MS, et al. (2008). Maturation of West Nile virus modulates sensitivity to antibody-mediated neutralization. PLoS Pathog 4, e1000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng KH, Zhang SL, Tan HC, Kwek SS, Sessions OM, Chan CY, Liu ID, Lee CK, Tambyah PA, Ooi EE, et al. (2019). Persistent Dengue Infection in an Immunosuppressed Patient Reveals the Roles of Humoral and Cellular Immune Responses in Virus Clearance. Cell Host Microbe 26, 601–605.e603. [DOI] [PubMed] [Google Scholar]
- Nivarthi UK, Kose N, Sapparapu G, Widman D, Gallichotte E, Pfaff JM, Doranz BJ, Weiskopf D, Sette A, Durbin AP, et al. (2017). Mapping the Human Memory B Cell and Serum Neutralizing Antibody Responses to Dengue Virus Serotype 4 Infection and Vaccination. Journal of virology 91, e02041–02016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normile D (2013). Surprising New Dengue Virus Throws a Spanner in Disease Control Efforts. Science 342, 415. [DOI] [PubMed] [Google Scholar]
- Oliphant T, Nybakken GE, Engle M, Xu Q, Nelson CA, Sukupolvi-Petty S, Marri A, Lachmi BE, Olshevsky U, Fremont DH, et al. (2006). Antibody recognition and neutralization determinants on domains I and II of West Nile Virus envelope protein. J Virol 80, 12149–12159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong EZ, Budigi Y, Tan HC, Robinson LN, Rowley KJ, Winnett A, Hobbie S, Shriver Z, Babcock GJ, and Ooi EE (2017). Preclinical evaluation of VIS513, a therapeutic antibody against dengue virus, in non-human primates. Antiviral Res 144, 44–47. [DOI] [PubMed] [Google Scholar]
- Patel B, Longo P, Miley MJ, Montoya M, Harris E, and de Silva AM (2017). Dissecting the human serum antibody response to secondary dengue virus infections. PLoS Negl Trop Dis 11, e0005554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson TC, and Diamond M (2013). Flaviviruses. In Fields Virology: Sixth Edition (Wolters Kluwer Health Adis (ESP)). [Google Scholar]
- Pierson TC, Sánchez MD, Puffer BA, Ahmed AA, Geiss BJ, Valentine LE, Altamura LA, Diamond MS, and Doms RW (2006). A rapid and quantitative assay for measuring antibody-mediated neutralization of West Nile virus infection. Virology 346, 53–65. [DOI] [PubMed] [Google Scholar]
- Pollett S, Melendrez MC, Maljkovic Berry I, Duchêne S, Salje H, Cummings DAT, and Jarman RG (2018). Understanding dengue virus evolution to support epidemic surveillance and counter-measure development. Infect Genet Evol 62, 279–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porterfield JS (1982). Immunological enhancement and the pathogenesis of dengue haemorrhagic fever. J Hyg (Lond) 89, 355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyke AT, Huang B, Warrilow D, Moore PR, McMahon J, and Harrower B (2017). Complete Genome Sequence of a Highly Divergent Dengue Virus Type 2 Strain, Imported into Australia from Sabah, Malaysia. Genome Announc 5, e00546–00517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyke AT, Moore PR, Taylor CT, Hall-Mendelin S, Cameron JN, Hewitson GR, Pukallus DS, Huang B, Warrilow D, and van den Hurk AF (2016). Highly divergent dengue virus type 1 genotype sets a new distance record. Scientific Reports 6, 22356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabaa MA, Girerd-Chambaz Y, Duong Thi Hue K, Vu Tuan T, Wills B, Bonaparte M, van der Vliet D, Langevin E, Cortes M, Zambrano B, et al. (2017). Genetic epidemiology of dengue viruses in phase III trials of the CYD tetravalent dengue vaccine and implications for efficacy. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raut R, Corbett KS, Tennekoon RN, Premawansa S, Wijewickrama A, Premawansa G, Mieczkowski P, Rückert C, Ebel GD, De Silva AD, et al. (2019). Dengue type 1 viruses circulating in humans are highly infectious and poorly neutralized by human antibodies. Proc Natl Acad Sci U S A 116, 227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rico-Hesse R (1990). Molecular evolution and distribution of dengue viruses type 1 and 2 in nature. Virology 174, 479–493. [DOI] [PubMed] [Google Scholar]
- Rico-Hesse R, Harrison LM, Salas RA, Tovar D, Nisalak A, Ramos C, Boshell J, de Mesa MT, Nogueira RM, and da Rosa AT (1997). Origins of dengue type 2 viruses associated with increased pathogenicity in the Americas. Virology 230, 244–251. [DOI] [PubMed] [Google Scholar]
- Sabin AB (1950). The dengue group of viruses and its family relationships. Bacteriol Rev 14, 225–232. [PubMed] [Google Scholar]
- Sabin AB (1952). Research on dengue during World War II. Am J Trop Med Hyg 1, 30–50. [DOI] [PubMed] [Google Scholar]
- Salazar G, Zhang N, Fu TM, and An Z (2017). Antibody therapies for the prevention and treatment of viral infections. NPJ Vaccines 2, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarathy VV, Milligan GN, Bourne N, and Barrett ADT (2015). Mouse models of dengue virus infection for vaccine testing. Vaccine 33, 7051–7060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sariol CA, and White LJ (2014). Utility, limitations, and future of non-human primates for dengue research and vaccine development. Front Immunol 5, 452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrödinger, L. The PyMOL Molecular Graphics System, Version 2.0. Schrödinger, LLC.; [Google Scholar]
- Screaton G, Mongkolsapaya J, Yacoub S, and Roberts C (2015). New insights into the immunopathology and control of dengue virus infection. Nat Rev Immunol 15, 745–759. [DOI] [PubMed] [Google Scholar]
- Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Söding J, et al. (2011). Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. In Mol Syst Biol, pp. 539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DJ, Lapedes AS, de Jong JC, Bestebroer TM, Rimmelzwaan GF, Osterhaus ADME, and Fouchier RAM (2004). Mapping the Antigenic and Genetic Evolution of Influenza Virus. Science 305, 371. [DOI] [PubMed] [Google Scholar]
- Smith SA, de Alwis AR, Kose N, Harris E, Ibarra KD, Kahle KM, Pfaff JM, Xiang X, Doranz BJ, de Silva AM, et al. (2013). The potent and broadly neutralizing human dengue virus-specific monoclonal antibody 1C19 reveals a unique cross-reactive epitope on the bc loop of domain II of the envelope protein. mBio 4, e00873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridhar S, Luedtke A, Langevin E, Zhu M, Bonaparte M, Machabert T, Savarino S, Zambrano B, Moureau A, Khromava A, et al. (2018). Effect of Dengue Serostatus on Dengue Vaccine Safety and Efficacy. New England Journal of Medicine 379, 327–340. [DOI] [PubMed] [Google Scholar]
- Stothard P (2000). The sequence manipulation suite: JavaScript programs for analyzing and formatting protein and DNA sequences. Biotechniques 28, 1102, 1104. [DOI] [PubMed] [Google Scholar]
- Sukupolvi-Petty S, Austin SK, Engle M, Brien JD, Dowd KA, Williams KL, Johnson S, Rico-Hesse R, Harris E, Pierson TC, et al. (2010). Structure and function analysis of therapeutic monoclonal antibodies against dengue virus type 2. Journal of virology 84, 9227–9239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukupolvi-Petty S, Brien JD, Austin SK, Shrestha B, Swayne S, Kahle K, Doranz BJ, Johnson S, Pierson TC, Fremont DH, et al. (2013). Functional analysis of antibodies against dengue virus type 4 reveals strain-dependent epitope exposure that impacts neutralization and protection. Journal of virology 87, 8826–8842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A, Foo S-S, Bruzzone R, Dinh LV, King NJC, and Mahalingam S (2015). Fc receptors in antibody-dependent enhancement of viral infections. Immunological reviews 268, 340–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricou V, Sáez-Llorens X, Yu D, Rivera L, Jimeno J, Villarreal AC, Dato E, Saldaña de Suman O, Montenegro N, DeAntonio R, et al. (2020). Safety and immunogenicity of a tetravalent dengue vaccine in children aged 2-17 years: a randomised, placebo-controlled, phase 2 trial. Lancet 395, 1434–1443. [DOI] [PubMed] [Google Scholar]
- VanBlargan LA, Goo L, and Pierson TC (2016). Deconstructing the Antiviral Neutralizing-Antibody Response: Implications for Vaccine Development and Immunity. Microbiology and molecular biology reviews : MMBR 80, 989–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanBlargan LA, Mukherjee S, Dowd KA, Durbin AP, Whitehead SS, and Pierson TC (2013). The type-specific neutralizing antibody response elicited by a dengue vaccine candidate is focused on two amino acids of the envelope protein. PLoS Pathog 9, e1003761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasilakis N, Cardosa J, Hanley KA, Holmes EC, and Weaver SC (2011). Fever from the forest: prospects for the continued emergence of sylvatic dengue virus and its impact on public health. Nat Rev Microbiol 9, 532–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn DW, Green S, Kalayanarooj S, Innis BL, Nimmannitya S, Suntayakorn S, Endy TP, Raengsakulrach B, Rothman AL, Ennis FA, et al. (2000). Dengue Viremia Titer, Antibody Response Pattern, and Virus Serotype Correlate with Disease Severity. J Infect Dis 181, 2–9. [DOI] [PubMed] [Google Scholar]
- Virtanen P, Gommers R, Oliphant TE, Haberland M, Reddy T, Cournapeau D, Burovski E, Peterson P, Weckesser W, Bright J, et al. (2020). SciPy 1.0: fundamental algorithms for scientific computing in Python. Nat Methods 17, 261–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waggoner JJ, Balmaseda A, Gresh L, Sahoo MK, Montoya M, Wang C, Abeynayake J, Kuan G, Pinsky BA, and Harris E (2016). Homotypic Dengue Virus Reinfections in Nicaraguan Children. J Infect Dis 214, 986–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E, Ni H, Xu R, Barrett AD, Watowich SJ, Gubler DJ, and Weaver SC (2000). Evolutionary relationships of endemic/epidemic and sylvatic dengue viruses. J Virol 74, 3227–3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver S, Tesh RB, and Vasilakis N (2008). Sylvatic Dengue Virus Type 2 Activity in Humans, Nigeria, 1966. Emerging Infectious Disease journal 14, 502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KI, Mundis S, Widen SG, Wood TG, Tesh RB, Cardosa J, Vasilakis N, Perera D, and Hanley KA (2017). Abundance and distribution of sylvatic dengue virus vectors in three different land cover types in Sarawak, Malaysian Borneo. Parasit Vectors 10, 406–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zellweger RM, and Shresta S (2014). Mouse Models to Study Dengue Virus Immunology and Pathogenesis. Front Immunol 5, 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data supporting the findings of this study are available within the paper.
This paper does not report original code
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.