Abstract
Phthalates are a class of endocrine disruptors found in a variety of consumer goods, and offspring can be exposed to these compounds during gestation and lactation. Our laboratory has found that perinatal exposure to an environmentally relevant mixture of phthalates resulted in a decrease in cognitive flexibility and in neuron number in the adult rat medial prefrontal cortex (mPFC). Here, we examine effects of phthalate treatment on prenatal cellular proliferation and perinatal apoptosis in the mPFC. To examine the phthalate effects on cellular proliferation, dams consumed 0, 1, or 5mg/kg of the phthalate mixture daily from embryonic day 2 (E2) through the day of birth (P0), and on E16 and E17, they were injected with BrdU. The mPFC of offspring was analyzed on P5 and showed a decrease in labelled cells in the phthalate exposed groups. To examine whether changes in BrdU density observed on P5 were due to altered cell survival, cell death was measured on E18, P0, and P5 using a TUNEL assay in a separate cohort of prenatally exposed offspring. There was an increase in TUNEL labelled cells at E18 in the phthalate exposed groups. In the final experiment, dams consumed the phthalate mixture from E2 through P10, at which time mPFC tissue was stained with TUNEL. Phthalate treated subjects showed a higher density of apoptotic cells at P10. These results indicate both pre- and postnatal phthalate exposure increases apoptosis in the male and female rat mPFC. While the impact of phthalates on proliferation cannot be ruled out, these data do not allow for definitive conclusions.
Keywords: neurodevelopment, neurotoxicity, endocrine disruptor, TUNEL, BrdU
Graphical Abstract
2. Introduction
Phthalates are a class of endocrine-disrupting compounds primarily used to increase the durability and flexibility of plastics. They are found throughout the environment from a variety of sources including food packaging and processing equipment, as well as personal care, pharmaceutical, cosmetic and fragranced products. Phthalates are not covalently bound to plastic polymers and are readily released into the environment, with the primary source of human exposure being ingestion of contaminated food (Heudorf et al., 2007). Phthalates and their metabolites can bind to androgen and estrogen receptors (Chauvigné et al., 2009; Stroheker et al., 2005; Takeuchi et al., 2005) and act on gonadal hormone-producing cells (Parks et al., 2000). Importantly, phthalates can reach developing offspring through both placental and lactational transfer (Dostal et al., 1987; Mose et al., 2007), leading to exposure during the perinatal period when the cortex is particularly sensitive to hormones.
Correlational evidence from human studies suggests that prenatal exposure to phthalates is associated with alterations in prefrontal cortex (PFC) dependent behaviors in childhood (Ejaredar et al., 2015; Kobrosly et al., 2014; Radke et al., 2020), but establishing direct effects of phthalate exposure in humans is difficult given their ubiquitous presence in the environment. Therefore, rodent models are needed to examine causal effects of phthalates on neuroanatomy and behavior. Most previous studies have examined the effects of individual phthalates, often at higher doses that are less environmentally relevant. Studies in vitro have shown that exposure to individual phthalates can induce apoptosis via activation of caspase-3 and reduce cell proliferation in rodent cortical neurons (Chen et al., 2011; Lin et al., 2011; Wójtowicz et al., 2017). In vivo, administration of a very high dose (500mg/kg) of one phthalate (DBP, dibutyl phthalate) increased the density of TUNEL (Terminal deoxynucleotidyl transferase dUTP nick end labeling) positive cells and caspase-3 expression in the rat hippocampus and caused a deficit in spatial learning (Li et al., 2013). Prenatal administration of another phthalate (DEHP, di(2-ethylhexyl) phthalate) at two relatively high doses (10 and 750mg/kg) downregulated expression of the genes Ccnd1 and Cdc2, which play a role in neural proliferation (Lin et al., 2015). Additionally, prenatal exposure to this same phthalate showed dose-specific effects on measures of cell survival (Komada et al., 2016) where very high doses of 100mg/kg and 500mg/kg led to an increase in apoptosis and a decrease in neurogenesis in the mouse cerebral cortex. In summary, individual phthalates, at least at high doses, can decrease developmental neurogenesis and increase cell death, which could ultimately lead to a long-term decrease in the number of cortical neurons.
One limitation in investigating individual phthalates is that humans are simultaneously exposed to multiple, and it is not unprecedented for combinations of endocrine disruptors to have different effects on neuron number when compared to effects of individual compounds alone (Tanida et al., 2009). From a translational perspective, it is important to examine the net effects of an environmentally relevant combination of compounds, given their diverse mechanisms of action, along with the reality of human exposure to multiple phthalates. Previously, our laboratory found that low doses of a phthalate mixture reflective of human exposure, administered perinatally, resulted in a decrease in the number of neurons and synapses in the adult medial prefrontal cortex (mPFC) (Kougias et al., 2018b). Perinatal exposure to the mixture also reduced adolescent play behavior in males and decreased performance on an attentional set-shifting task in adults of both sexes (Kougias et al., 2018b, 2018a). The attentional set-shift task measures cognitive flexibility and is known to be dependent on the mPFC (Stefani and Moghaddam, 2005). Therefore, the decrease in neuron number along with their synapses appears to contribute to mPFC functional impairment. To investigate the cellular mechanisms by which phthalates decrease mPFC neuron number, the present study examined the effects of two doses of the same mixture on prenatal cellular proliferation and perinatal apoptosis in the male and female rat mPFC. Based on previous work, we hypothesized that perinatal phthalate exposure would reduce cell proliferation during peak periods of neurogenesis and increase postnatal apoptosis in the mPFC of both sexes.
3. Materials and Methods
3. 1. Animals and Housing
Young adult (2–3 months) male and female Long-Evans Hooded rats were purchased from Envigo (Indianapolis, IN) and habituated to the vivarium in same-sex pairs for a minimum of two weeks before breeding. Breeding occurred in suspended wire-bottom cages checked daily for sperm plugs. The day a plug was found was designated embryonic day (E)0, and the day of birth was designated as postnatal day (P)0. Animals were housed on a 12-hour light/dark cycle (lights on at 0500) with food and water available ad libitum. To minimize exposure to exogenous endocrine-disrupting compounds, all animals were housed in polysulfone cages with heat-treated hardwood beta chip bedding (Northeaster Product Corp., NY), fed a diet low in phytoestrogens (Harlan 2020X; Teklad Diets), and given water filtered by reverse osmosis in glass bottles. For every experimental endpoint, only one male and one female were used from each litter, resulting in a total of 267 animal subjects used in the experiments described below. All procedures were approved by the Institutional Care and Use Committee (IACUC) at the University of Illinois at Urbana-Champaign and all experimenters were blind to experimental condition.
3.2. Phthalate Mixture and Treatment
Previous studies (Kougias et al., 2018b, 2018a; Sellinger et al., 2020; Zhou et al., 2017) have used a mixture of phthalates derived from concentrations of phthalate metabolites in the urine of pregnant women in Champaign, Illinois (Strakovsky and Schantz, personal communication). This mixture is composed of six phthalates: 35% diethyl phthalate (DEP), 21% di(2-ethylhexyl) phthalate (DEHP), 15% dibutyl phthalate (DBP), 15% diisononyl phthalate (DiNP), 8% diisobutyl phthalate (DiBP) and 5% benzyl butyl phthalate (BBP) (all purchased from Sigma-Alrdrich, St. Louis, MO). This combination, while derived from pregnant women in the Champaign-Urbana area, has been shown to approximate exposure on a national level (Corbasson et al., 2016). Dams were weighed daily and fed half of a cookie (Newman’s Own Organic, vanilla) with 0, 1 or 5mg/kg of the phthalate mixture diluted in corn oil pipetted at a volume of 1μl/3g body weight. The 1 and 5mg/kg dose are lower than most of the rodent literature and have been shown to be comparable to human exposure (Heudorf et al., 2007; Wittassek et al., 2011). All rats readily consumed the cookie within several minutes each day.
3.3. Prenatal Phthalate Exposure: Proliferation
To examine whether phthalates inhibit prenatal cell proliferation in the mPFC, BrdU (5-bromo-2’-deoxyuridine) injections were given during maximal neurogenesis in the future mPFC (Bayer, 1990) and quantification was performed on P5. Dams were exposed to the phthalate mixture from E2 through the day of birth (P0) (Fig. 1). To label proliferating cells, dams were injected with 50mg/kg 5-bromo-2’-deoxyuridine (BrdU) (Sigma catalog # B5002) on E16 and E17. BrdU is a thymidine analogue that is incorporated into the DNA during the S-phase of the cell cycle and is a commonly used technique for assessing cellular genesis. This dose has been shown to induce optimal labeling of fetal cells during periods of cortical neurogenesis (Jahagirdar et al., 2012).
Fig. 1.
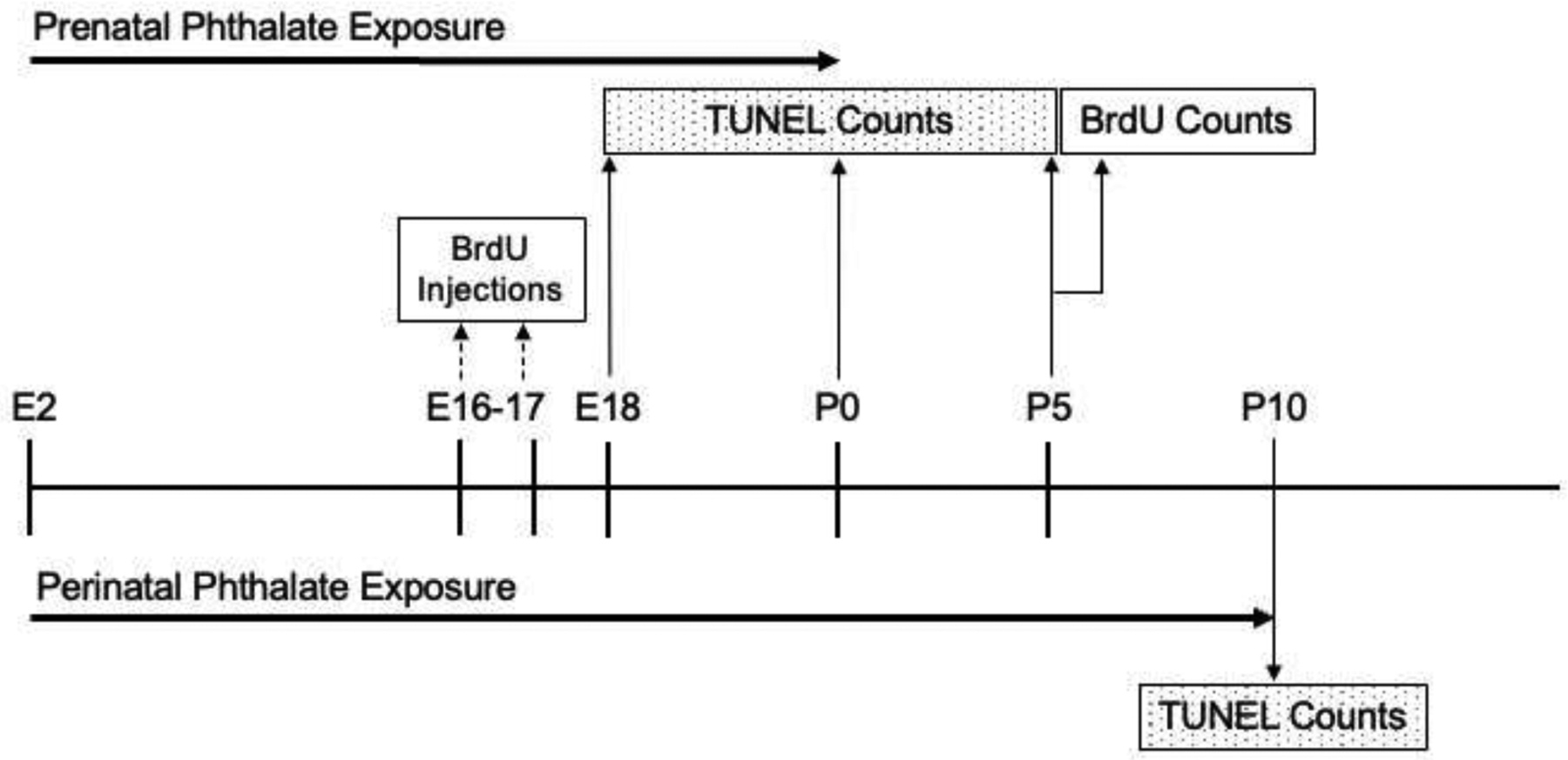
The timelines for investigating the effects of prenatal phthalate exposure on proliferation and assessing the effects of perinatal phthalate exposure on postnatal apoptosis.
A single male and female from each litter (9 males and 9 females per treatment group) were sacrificed on P5 to allow for BrdU-tagged cells to migrate out of the ventricular zone and into the developing mPFC. Subjects were given a lethal dose of sodium pentobarbital (100mg/kg i.p.) and were intracardially perfused with 0.1M phosphate-buffered saline (PBS, pH 7.4) followed by 4% paraformaldehyde in PBS. Brains were extracted and post-fixed in paraformaldehyde for 24 hours, then placed in 30% sucrose for 72 hours. Coronal sections were cut at 40 μm on a freezing microtome and stored in cryoprotectant at −20°C. After sectioning, anatomically matched mPFC sections were chosen for BrdU immunohistochemistry (Fig. 2A). At sacrifice, body and brain weight were also measured.
Fig. 2.
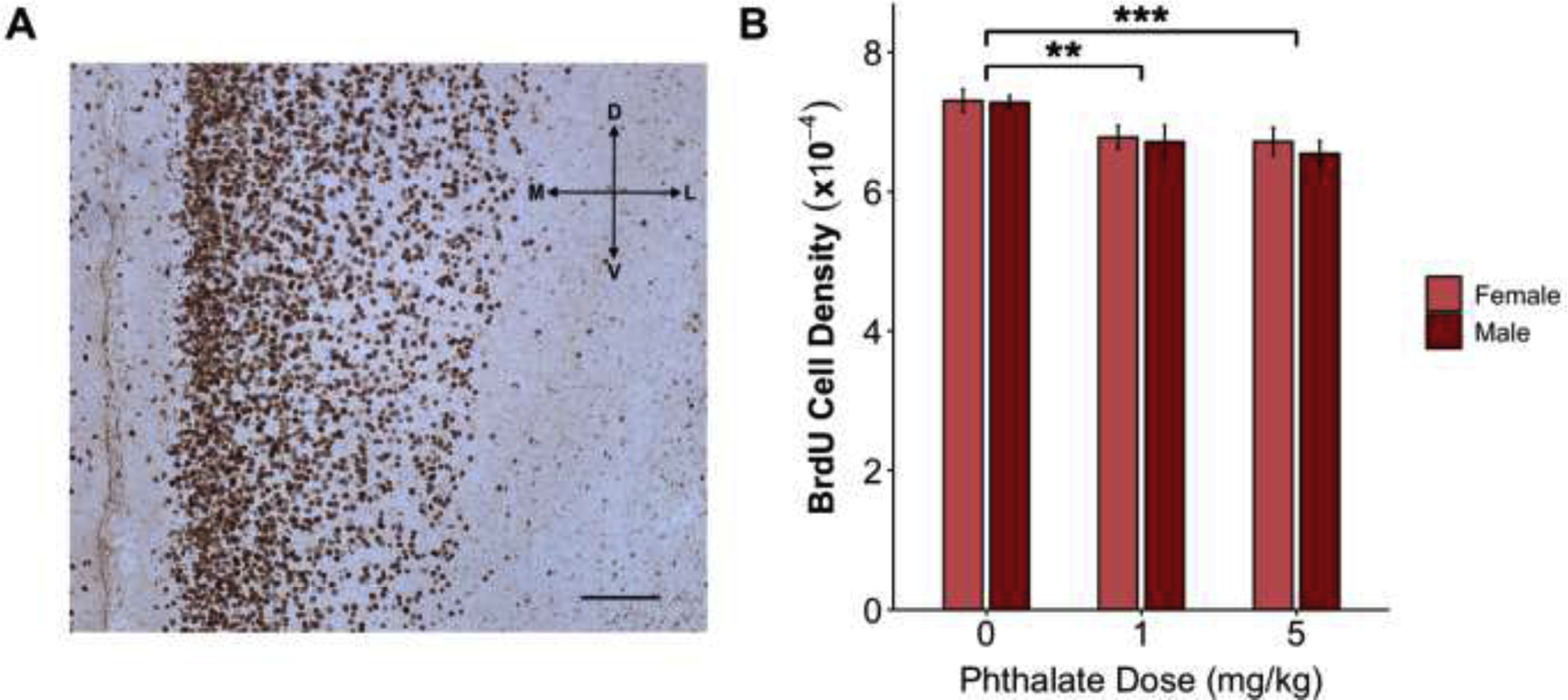
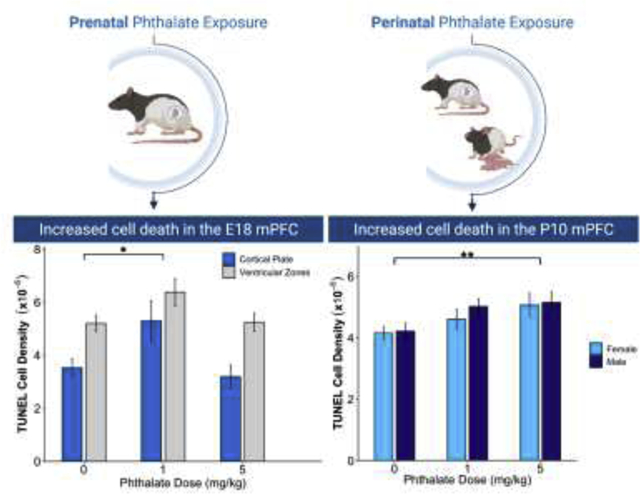
Prenatal phthalate treatment resulted in fewer cells by P5 (A) BrdU immunohistochemistry in the P5 rat mPFC under a 10x objective (scale bar 0.5mm). (B) Prenatal exposure (E2-P0) to both the 1mg/kg and 5mg/kg doses of the phthalate mixture decreased the density of BrdU immunoreactive cells in the mPFC of males and females. **p<0.01; ***p=0.001
3.3.1. BrdU Immunohistochemistry
Free-floating sections (2 per animal) were taken out of cryoprotectant and rinsed in 0.05M tris-buffered saline (TBS) (pH 7.6) 3 times for 5 min each. Sections were then incubated in 2N hydrochloric acid for 30 min at room temperature, followed by 3 rinses in TBS. Tissue was then blocked in TBS containing 20% normal goat serum (NGS), 1% H2O2, and 1% bovine serum albumin (BSA) for 30 min. Primary antibody for BrdU (mouse monoclonal, BD Biosciences, catalog #555627) was diluted in TBS containing 2% NGS and 0.1% Triton-X 100 (TTG) at a concentration of 1:200. Primary antibody incubation was conducted for 24 hours at room temperature. After 24 hours, sections were rinsed 3 times in TTG, then incubated in a biotinylated goat anti-mouse IgG secondary antibody (1:200; Vector Laboratories) for 90 min. After two rinses in TTG and two in TBS, sections were incubated in avidin biotin complex (ABC) (Vectastain Elite Kit, Vector Labs) in TBS for 1 hour. Sections were then rinsed 3 more times in TBS and reacted with 3,3’-diaminobenzidine (DAB) solution (Sigma Fast Tabs) for 2 minutes. Lastly, sections were rinsed in TBS 5 times, mounted on glass slides and coverslipped with Permount.
Because the borders of the mPFC cannot be precisely delineated by P5, the density rather than the total number of BrdU labelled cells was quantified. Using StereoInvestigator software (MicroBrightfield, Williston, VT), boundaries within the mPFC were drawn under a 2.5x objective, and cells were counted under a 63x objective. A counting frame of 40 × 40 microns with a 10-micron z-axis was used and cell counts were performed using the optical disector. For each animal, a minimum of 500 cells was manually counted to calculate the density of BrdU cells (number per cubed micron) (Fig. 2A).
3.4. Perinatal Phthalate Exposure: Apoptosis
Apoptosis was examined with a TUNEL assay in two groups of subjects. The first provided a control for our analysis of proliferation by measuring apoptosis in the late gestational to early perinatal period (E18, P0 and P5) when it may have occurred before the number of BrdU-tagged cells was quantified (Fig. 1). While cell death in the mPFC after the day of birth is elevated between P8 and P12 (Sellinger et al., 2021), apoptosis does occur as early at E14 in the developing rat cortex (Thomaidou et al., 1997). The other group of subjects was assessed for cell death on P10 which was meant to capture a time when postnatal cell death is elevated (Sellinger et al., 2021).
To collect tissue at E18, dams were exposed to carbon dioxide until unconscious (4–7 minutes) and decapitated. One fetus per litter was extracted, rapidly decapitated, and their brains removed (10 per treatment group). To collect postnatal tissue, litters were vaginally born and one male and one female per litter (9–11 males and 9–10 females per treatment group) were sacrificed by rapid decapitation on P0 and P5. For the analysis of P10 tissue, pregnant dams were treated with one of the three doses of the phthalate mixture from E2 continuing through lactation until P10 (Fig. 1), at which time one male and one female per litter (10 males and 10 females per treatment group) were given a lethal dose of sodium pentobarbital (100mg/kg i.p.). Following intracardial perfusion with 0.1M phosphate-buffered saline (PBS, pH 7.4) and 4% paraformaldehyde in PBS, brains were extracted. Brains from all age groups were placed immediately after extraction into 4% paraformaldehyde in PBS for 24 hours followed by 72 hours in 30% sucrose. Coronal sections were cut at 40 μm on a freezing microtome, stored in cryoprotectant at −20°C and anatomically matched mPFC sections were chosen for TUNEL processing.
3.4.1. TUNEL Assay
TUNEL kits were purchased from EMD Millipore (catalog # S7100). Sections (2 per subject) were rinsed three times (5 min each) with phosphate buffered saline (PBS) (pH 7.4) and post-fixed in an ethanol: acetic acid (2:1) solution for 5 min at −20°C. Sections were then rinsed twice in PBS, followed by an incubation in 3% hydrogen peroxide (in PBS) for 5 min. Following two more rinses in PBS, sections were placed in equilibration buffer for 10 seconds, then incubated in a solution containing TdT enzyme for 1 hour at 37°C. Then, tissue was placed in stop-wash buffer for 10 min and rinsed again three times in PBS. Lastly, sections were incubated in anti-digoxigenin conjugate for 30 min at room temperature, rinsed in PBS four times, and stained with 3,3’-diaminobenzidine (DAB) (Sigma Fast Tabs). Sections were thoroughly rinsed in PBS then mounted on glass slides and coverslipped with Permount.
The density rather than the number of TUNEL labelled cell across all developing layers of the mPFC was quantified with StereoInvestigator. Layer specific counts were not collected given that migration of cells into the rodent cortex occurs from E18 through P5, so exact delineation of layers cannot yet be made (Juraska and Fifková, 1979; Raedler and Sievers, 1975). TUNEL positive cells were counted within the mPFC under a 63X objective with a counting frame of 60 × 60 microns through a z-axis of 12 microns. This resulted in the number of TUNEL positive cells per cubed micron.
Before quantifying TUNEL-labeled cells in the E18 tissue, the cellular organization was visualized using a Nissl stain. This allowed for experimenters to divide the TUNEL-labeled mPFC into two regions and collect counts from each: the ventricular zones, which included the ventricular zone (VZ), subventricular zone (SZ), and intermediate zone (IZ), and separately, the cortical plate (CP). Briefly, the ventricular zones contain dividing cells within VZ and SZ and the migrating cells within IZ. The cortical plate contains the densely packed cells of the cortex that have finished migrating and are starting to grow dendrites. The sampling was conducted on the medial cortex which contains the future mPFC. These subregions are labeled on the lateral cortex in the Nissl-stained image where they are more apparent than on the thinner medial region (Fig. 3A). The VZ, SZ, and IZ were combined into one region (ventricular zones) for analysis. The density of TUNEL positive cells was analyzed within the ventricular and cortical plate regions separately and also pooled across these regions.
Fig. 3.
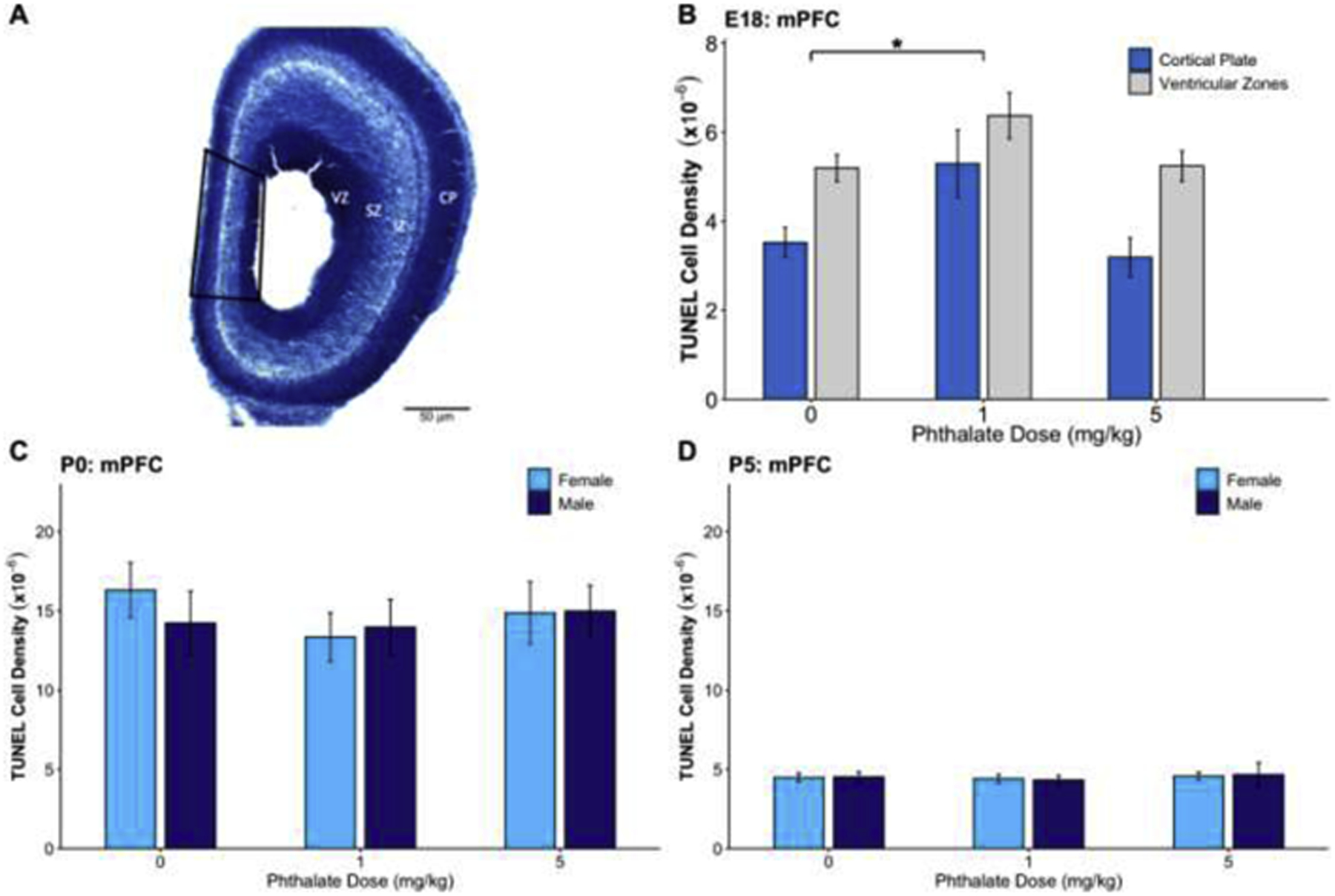
The effects of prenatal phthalate exposure on apoptosis. (A) A Nissl-stained section of the E18 cortex depicting the ventricular zone (VZ), subventricular zone (SZ), intermediate zone (IZ), and cortical plate (CP) along with the sampling region of mPFC outlined in black. (B) Prenatal phthalate exposure at the 1mg/kg dose led to increased apoptosis in the mPFC on E18 in the cortical plate and ventricular zones. There was no effect of phthalate exposure on TUNEL density in the mPFC on (E) P0 or (F) P5. *p < 0.05
3.5. Statistical Analysis
All datasets were tested for normal distributions and equal variances using Shapiro-Wilk and Levene tests. Since the data was found to be normally distributed with homogeneity of variance, subsequent analyses were performed in R using 2-way ANOVAs with dose and sex as factors. As tissue was collected in multiple breeding cohorts, cohort was included as a random factor. TUNEL positive cell density observed on E18 was analyzed using a 2-way ANOVA with dose as a between-subject factor and region (cortical plate or ventricular zones) as a within-subject factor. Sex was not included as it is not reliably discernable at this age. Following a significant main effect, Dunnett’s post hoc tests were used to correct for multiple comparisons, with each phthalate dose being compared to the control group. For each age and each measure, there were 9–11 males and 9–11 females in each phthalate dose group.
4. Results
4.1. Body and Brain Weight:
Body and brain weights were recorded for P5 (prenatal exposure only), as well as P10 (pre and postnatal exposure) animals. There was no effect of dose or sex on body weight at any age examined, nor were there any dose by sex interactions. On P5, there was a significant main effect of dose (F2,53 = 4.526, p=0.02) but not sex (F2,53 = 3.434, p=0.07) on brain weight. Bonferroni post hoc tests revealed that neither dose was significantly different from the control group. However, there was a non-significant trend toward a decrease in brain weight in the 1mg/kg group compared to controls (p=0.06). There were no main effects or interactions for brain weight on P10.
4.2. BrdU Density
There was a main effect of dose (F2,53 = 7.684, p=0.001) on the density of BrdU positive cells measured on P5. Dunnett’s post hoc tests revealed that both the 1mg/kg (p=0.008) and 5mg/kg (p=0.001) phthalate treated groups had a significantly lower density of BrdU cells than controls. There was no effect of sex (F1,53 = 0.358, p=0.553) and no interaction (F2,53 = 0.088, p=0.92) (Fig. 2B).
4.3. Perinatal TUNEL Density
TUNEL density was assessed after prenatal phthalate exposure on E18, P0, and P5 (Fig. 3). There was a main effect of phthalate exposure on TUNEL density in the cortical plate and ventricular zones (VZ, SV and IZ) on E18 (F2,27 = 4.922, p=0.015), and Dunnett’s post hoc testing showed a significantly higher density of TUNEL cells in the 1mg/kg (p=0.02) but not the 5mg/kg group, compared to controls (Fig. 3B). Additionally, there was a significant effect of the within-subject factor, region (F1,27 = 30.61, p <0.001) where TUNEL density was significantly higher in the subventricular zones compared to the cortical plate (p<0.001). There was no significant treatment by region interaction. There was no effect of phthalate exposure on TUNEL densities assessed on P0 (F2,55 = 0.458, p=0.63) (Fig. 3C) or P5 (F2,50 = 0.212, p=0.81) (Fig. 3D). Additionally, there was no effect of sex on P0 (F1,55 = 0.096, p=0.76) or P5 (F1,50 = 0.002, p=0.96), nor a sex by dose interaction at either age (F2,55 = 0.321, p=0.73; F2,50 = 0.036, p=0.964).
4.4. Postnatal TUNEL Density
There was a significant main effect of dose (F2,55 = 4.997, p=0.01) on the density of TUNEL positive cells in the P10 mPFC. A Dunnett’s post hoc test revealed significantly more TUNEL positive cells in the 5mg/kg phthalate dose (p=0.004) compared to controls. This pattern was also seen in the 1mg/kg phthalate group but did not reach statistical significance (p=0.08). There was no effect of sex (F1,55 = 0.535, p=0.47) and no sex by dose interaction (F2,55 = 0.189, p=0.83) (Fig. 4).
Fig. 4.
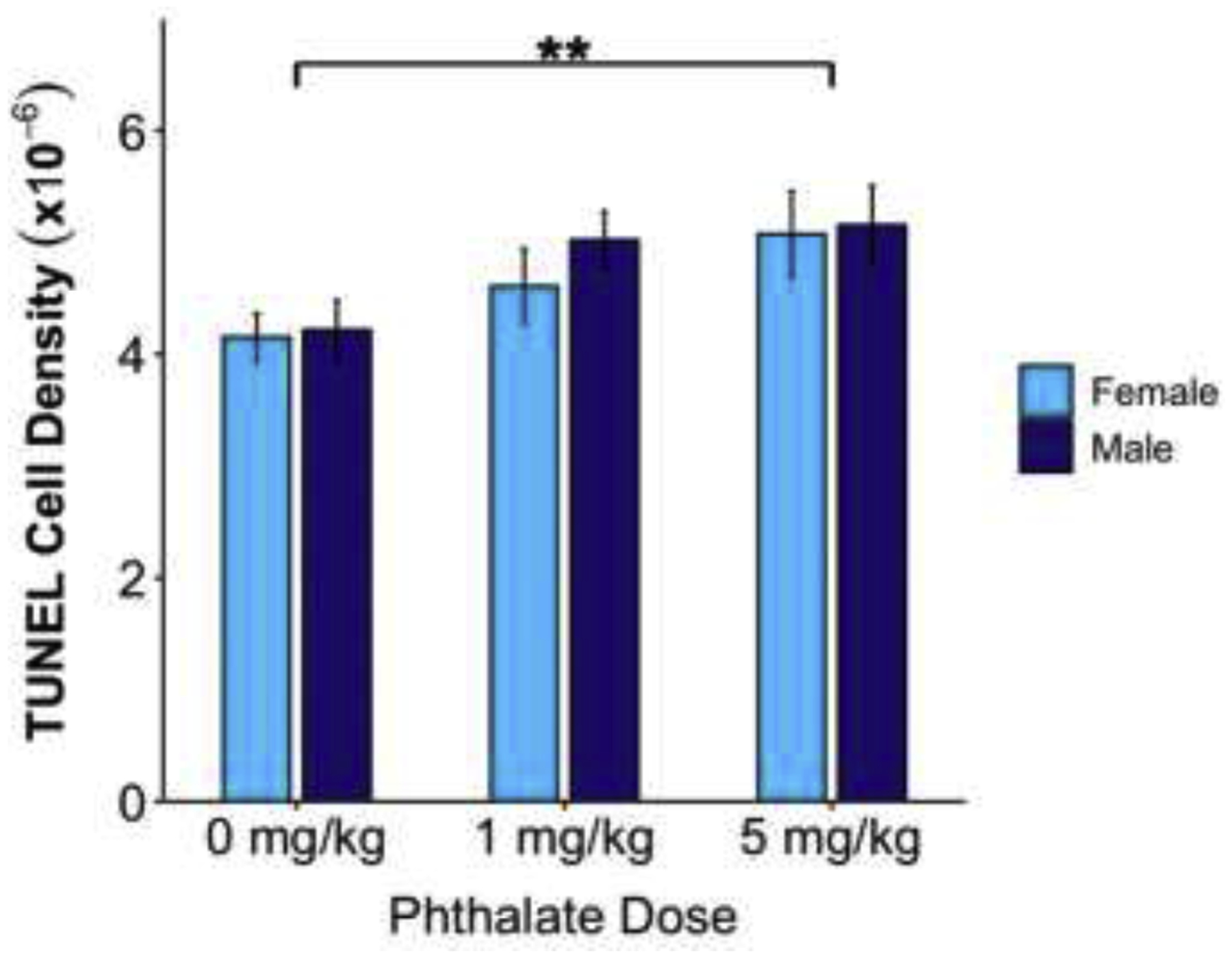
Combined pre and postnatal phthalate treatment increased apoptosis in the male and female rat medial prefrontal cortex in the 5mg/kg phthalate dose compared to controls on P10, but not between controls and the 1mg/kg phthalate group (p=0.08). **p<0.01
5.1. Discussion
The present study explores the developmental processes whereby exposure to an environmentally relevant mixture and dose of phthalates leads to fewer neurons in the adult mPFC. Pre- and postnatal exposure to the phthalate mixture increased apoptosis in both sexes. While it is possible phthalates may have also decreased cellular proliferation, this measure was confounded by both the notable presence of apoptosis and the observed effect of phthalate exposure on these levels before measures of BrdU were quantified. Finally, compared to controls, neither phthalate dose significantly altered body or brain weight suggesting their effects are not globally severe. These results indicate the cellular processes by which developmental exposure to this phthalate mixture decreases neuron number in the mPFC that are accompanied by decreases in cognitive flexibility and juvenile social play (Kougias et al., 2018b, 2018a).
Previous work from our laboratory has shown that perinatal treatment with this phthalate mixture at doses of 0.2 and 1mg/kg reduces neuron number in the adult mPFC (Kougias et al., 2018b). Addressing the potential role of altered proliferation in producing deficits in neuron number, we first showed both 1 and 5mg/kg doses of the phthalate mixture led to fewer BrdU positive cells detected on P5. These data suggested either a reduction in cellular proliferation and/or an increase in cell death occurring between BrdU administration and quantification. Therefore, levels of cell death in mPFC were analyzed during this window. Indeed, our results showed a significant degree of apoptosis occurring in the mPFC on the examined ages: E18, P0, and P5. In fact, our data replicate the surge in apoptosis occurring at birth in many forebrain regions (Mosley et al., 2017). Moreover, phthalate exposure at 1mg/kg led to increased apoptosis on E18 but did not change levels observed at the other ages. The high surge of apoptosis on P0 was not detectably affected by phthalate exposure. There also was not an effect of phthalates on apoptosis on P5, which was 5 days after the last phthalate exposure. Phthalates have a short half-life, ranging from 1–24 hours after oral exposure in rats (Domínguez-Romero and Scheringer, 2019). By P5, they have presumably been cleared, and while they might exert immediate effects, their influence over levels of apoptosis may not persist in their absence. In the future, an examination of BrdU labeling at several timepoints encapsulating cortical neurogenesis would clarify if levels or the time course of proliferation are altered, as has been shown in response to other endocrine-disrupting compounds in the hypothalamus (Hernandez Scudder et al., 2020; Nesan et al., 2021). Additionally, co-labeling with a neuronal marker to verify cellular identity would allow for conclusions on the effects of phthalates on neurogenesis specifically. Therefore, while the effects of phthalates on proliferation cannot be ruled out, our data reflect a role for apoptosis that interferes with our attempt to measure this process.
Apoptosis continues at least through the first two postnatal weeks in the rat mPFC in both sexes (Sellinger et al., 2021), but only the 5mg/kg significantly increased apoptosis on P10, although the means for 1 mg/kg dose were in the same direction. It is unclear why only the lower, 1mg/kg dose impacted apoptosis on E18 while only the higher 5mg/kg dose definitively altered apoptosis at P10, though non-linear dose response curves have been observed for phthalates previously (Andrade et al., 2006). A functional distinction between prenatal and postnatal apoptosis has been proposed (Blaschke et al., 1998) in that prenatal apoptosis, largely contained to the proliferative zones, targets the proliferating cell population after it has produced enough neurons (Blaschke et al., 1996) while postnatal surges primarily target post-mitotic neurons and are thought to contribute to appropriate circuit formation (Mosley et al., 2017). However, the effects of phthalates on E18 were seen both within the proliferative zones of the ventricular regions and in post-mitotic neurons – those that had successfully migrated to the cortical plate. As the receptors and signaling pathways phthalates target to induce apoptosis are unknown, it is difficult to propose an explanation for apoptosis at one age being more susceptible to different doses of phthalates compared to others.
It has been established that phthalates disrupt gonadal hormone signaling through anti-androgenic and either pro- or anti-estrogenic mechanisms (Takeuchi et al., 2005), and gonadal hormones have been shown to play a role in cortical development, specifically in the regulation of cell number. For example, androgens play a role in the regulation of postnatal apoptosis in the visual cortex (Nuñez et al., 2000) and estrogens have been shown to be involved in the proliferation of cortical cells (Martínez-Cerdeño et al., 2006). Phthalates can also alter the expression of steroid receptors in several brain regions (DeBartolo et al., 2016; Singh and Li, 2012) and inhibit production of enzymes necessary for steroidogenesis (David, 2006; Kim et al., 2004). Action through any of these mechanisms could interfere with developmental processes, including apoptosis. However, given the lack of sex differences in phthalate effects seen in the present study, as well as our previous work (Kougias et al., 2018b), non-steroid hormone related mechanisms must also be considered. For example, phthalates can increase oxidative stress and neuroinflammation (Ma et al., 2015; Win-Shwe et al., 2013), though our past work suggests a minimal inflammatory response after perinatal exposure to this mixture at low levels (Moody et al., 2019). Recent work in vitro suggests two phthalates used in the present mixture, DEHP and DBP, can alter aryl hydrocarbon receptor expression (Wójtowicz et al., 2019) and induce apoptosis through action at this receptor (Wójtowicz et al., 2017). Yet another potential mechanism of action, DEHP can also interfere with thyroid hormone signaling in rats (Dong et al., 2017). Thyroid hormone is critical to dendritic arborization and synaptogenesis and it also acts at the nucleus, activating transcription factors mediating cell survival (Horn and Heuer, 2010). Finally, several phthalates, including DEHP, have been shown to weakly inhibit the cannabinoid-1 receptor (CB1) in mouse cortical cell culture (Bisset et al., 2011) and their action at CB1 receptors has been shown to mediate apoptosis in mouse forebrain cultures (Tomiyama and Funada, 2013).
While this is the first examination of this combination of phthalates on these developmental processes, these data are also consistent with previous studies in rats involving exposure to individual phthalates, though the doses administered in previous studies were considerably higher. Exposure to DEHP or its metabolite (MEHP) decreases cell proliferation in vitro (Chen et al., 2011), and in vivo, decreases expression of genes (Ccnd1 and Ccnd2) involved in neurogenesis (Lin et al., 2015). Prenatal DEHP treatment also decreases neuronal proliferation in an undefined area of the mouse cortex (Komada et al., 2016). In terms of cell death, both DEHP and DBP have been shown to increase caspase-3 activity and apoptosis in cultured cells (Lin et al., 2011; Wójtowicz et al., 2019, 2017). In vivo, prenatal exposure to DBP and DEHP can increase apoptosis in the hippocampus (Li et al., 2013) and neocortex (Komada et al., 2016), though in both cases, the dose that yielded significant effects was high (500mg/kg). While the present study cannot determine whether effects on apoptosis were the result of individual or multiple phthalates, evidence strongly suggests that at least two phthalates in the mixture used (DBP and DEHP) can alter the generation and/or survival of cortical neurons.
This study indicates the importance of studying the effects of environmentally relevant mixtures of endocrine disrupting compounds from a translational perspective. It also provides a first step in understanding the cellular mechanisms leading to the lower number of neurons in adults that have been exposed to the phthalate mixture during the prenatal and early postnatal periods (Kougias et al., 2018b). PFC dysfunction has been implicated in a variety of clinical disorders, and alterations in the generation and/or survival of neurons could be a critical element in these disorders. For example, an excess of neurons in the PFC is commonly observed in autism spectrum disorder (Courchesne et al., 2011) while a decrease in PFC volume implicating apoptosis is seen in schizophrenia (Glantz et al., 2006). This aligns with correlations between maternal phthalate exposure and neurodevelopmental deficits common to psychiatric disorders in humans (Ejaredar et al., 2015).
5.2. Conclusions
In summary, phthalate exposure to an environmentally relevant mixture and dose leads to increases in apoptosis and potentially decreases in neurogenesis in both male and female rats, suggesting further work is needed to determine the extent of the effects of these environmental toxicants on human development.
Highlights.
Low doses of a phthalate mixture increase developmental apoptosis in the mPFC
Increased prenatal apoptosis after phthalates is prominent at embryonic day 18
Phthalates increase postnatal apoptosis in both sexes
Prenatal phthalate exposure leads to fewer BrdU-tagged cells in the mPFC on P5
Acknowledgements
We would like to thank the Division of Animal Resources Animal Care Staff in the Psychology Building. Microscopy for these experiments was conducted at the Microscopy Suite of the Beckman Institute for Advanced Science and Technology at the University of Illinois at Urbana-Champaign. The graphical abstract was created with biorender.com.
Funding Sources
This work was funded by National Institute of Environmental Health Sciences Grants R21 ES026896 and P01 ES002848-Project 3 (to JMJ), and U.S. Environmental Protection Agency Grant 83543401-Project 3 (to JMJ). JW and EPS were partially supported by National Institute of Environmental Health Sciences Grant T32 ES007326.
Abbreviations
- BrdU
5-bromo-2’-deoxyuridine
- BBP
benzyl butyl phthalate
- DBP
dibutyl phthalate
- DEP
diethyl phthalate
- DEHP
di(2-ethylhexyl) phthalate
- DiBP
diisobutyl phthalate
- DiNP
diisononyl phthalate
- PFC
prefrontal cortex
- mPFC
medial prefrontal cortex
- TUNEL
Terminal deoxynucleotidyl transferase dUTP nick end labeling
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Statement of Ethics
Animal experiments conform to internationally accepted standards and have been approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Illinois at Urbana-Champaign.
Declaration of Interest
The article has not been published previously (except in the form of an abstract), it is not under consideration for publication elsewhere, its publication is approved by all authors and tacitly or explicitly by the responsible authorities where the work was carried out and if accepted, it will not be published elsewhere in the same form, in English or in any other language, including electronically without the written consent of the copyright-holder.
References
- Andrade AJM, Grande SW, Talsness CE, Grote K, Chahoud I, 2006. A dose-response study following in utero and lactational exposure to di-(2-ethylhexyl)-phthalate (DEHP): Non-monotonic dose-response and low dose effects on rat brain aromatase activity. Toxicology 227, 185–192. 10.1016/j.tox.2006.07.022 [DOI] [PubMed] [Google Scholar]
- Bayer SA, 1990. Neurogenetic Patterns in the Medial Limbic Cortex of the Rat Related to Anatomical Connections with the Thalamus and Striatum. Experimental Neurology 107, 132–142. [DOI] [PubMed] [Google Scholar]
- Bisset KM, Dhopeshwarkar AS, Liao C, Nicholson RA, 2011. The G protein-coupled cannabinoid-1 (CB 1) receptor of mammalian brain: Inhibition by phthalate esters in vitro. Neurochemistry International 59, 706–713. 10.1016/j.neuint.2011.06.019 [DOI] [PubMed] [Google Scholar]
- Blaschke AJ, Staley K, Chun J, 1996. Widespread programmed cell death in proliferative and postmitotic regions of the fetal cerebral cortex. Development 122, 1165–1174. [DOI] [PubMed] [Google Scholar]
- Blaschke AJ, Weiner JA, Chun J, 1998. Programmed cell death is a universal feature of embryonic and postnatal neuroproliferative regions throughout the central nervous system. Journal of Comparative Neurology 396, 39–50. [DOI] [PubMed] [Google Scholar]
- Chauvigné F, Menuet A, Lesné L, Chagnon MC, Chevrier C, Regnier JF, Angerer J, Jégou B, 2009. Time- and dose-related effects of di-(2-ethylhexyl) phthalate and its main metabolites on the function of the rat retal testis in vitro. Environmental Health Perspectives 117, 515–521. 10.1289/ehp.11870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, Yang W, Li Y, Chen X, Xu S, 2011. Mono-(2-ethylhexyl) phthalate impairs neurodevelopment: Inhibition of proliferation and promotion of differentiation in PC12 cells. Toxicology Letters 201, 34–41. 10.1016/j.toxlet.2010.12.002 [DOI] [PubMed] [Google Scholar]
- Corbasson I, Hankinson SE, Stanek EJ, Reeves KW, 2016. Urinary bisphenol-A, phthalate metabolites and body composition in US adults, NHANES 1999–2006. International Journal of Environmental Health Research 26, 606–617. 10.1080/09603123.2016.1233524 [DOI] [PubMed] [Google Scholar]
- Courchesne E, Mouton PR, Calhoun ME, Semendeferi K, Ahrens-Barbeau C, Hallet MJ, Barnes CC, Pierce K, 2011. Neuron Number and Size in Prefrontal Cortex of Children With Autism. Jama 306, 2001–2010. [DOI] [PubMed] [Google Scholar]
- David RM, 2006. Proposed Mode of Action for In Utero Effects of Some Phthalate Esters on the Developing Male Reproductive Tract. Toxicologic Pathology 34, 209–219. 10.1080/01926230600642625 [DOI] [PubMed] [Google Scholar]
- DeBartolo D, Jayatilaka S, Siu NY, Rose M, Ramos RL, Betz AJ, 2016. Perinatal exposure to benzyl butyl phthalate induces alterations in neuronal development/maturation protein expression, estrogen responses, and fear conditioning in rodents. Behavioural Pharmacology 27, 77–82. 10.1097/FBP.0000000000000190 [DOI] [PubMed] [Google Scholar]
- Domínguez-Romero E, Scheringer M, 2019. A review of phthalate pharmacokinetics in human and rat: what factors drive phthalate distribution and partitioning? Drug Metabolism Reviews. 10.1080/03602532.2019.1620762 [DOI] [PubMed] [Google Scholar]
- Dong X, Dong J, Zhao Y, Guo J, Wang Z, Liu M, Zhang Y, Na X, 2017. Effects of long-term in vivo exposure to di-2-ethylhexylphthalate on thyroid hormones and the tsh/tshr signaling pathways in wistar rats. International Journal of Environmental Research and Public Health 14. 10.3390/ijerph14010044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dostal L, Weaver R, Schwetz A, 1987. Transfer of Di(2-ethylhexyl) Phthalate through Rat Milk and Effects on Milk Composition and the Mammary Gland. [DOI] [PubMed]
- Ejaredar M, Nyanza EC, ten Eycke K, Dewey D, 2015. Phthalate exposure and childrens neurodevelopment: A systematic review. Environmental Research 142, 51–60. 10.1016/j.envres.2015.06.014 [DOI] [PubMed] [Google Scholar]
- Glantz LA, Gilmore JH, Lieberman JA, Jarskog LF, 2006. Apoptotic mechanisms and the synaptic pathology of schizophrenia. Schizophrenia Research 81, 47–63. 10.1016/j.schres.2005.08.014 [DOI] [PubMed] [Google Scholar]
- Hernandez Scudder ME, Kunkel MN, Gore AC, 2020. Exposure to prenatal PCBs shifts the timing of neurogenesis in the hypothalamus of developing rats. Journal of Experimental Zoology Part A: Ecological and Integrative Physiology 333, 550–560. 10.1002/jez.2404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heudorf U, Mersch-Sundermann V, Angerer J, 2007. Phthalates: Toxicology and exposure. International Journal of Hygiene and Environmental Health 210, 623–634. 10.1016/j.ijheh.2007.07.011 [DOI] [PubMed] [Google Scholar]
- Horn S, Heuer H, 2010. Thyroid hormone action during brain development: More questions than answers. Molecular and Cellular Endocrinology. 10.1016/j.mce.2009.09.008 [DOI] [PubMed] [Google Scholar]
- Jahagirdar V, Zoeller TR, Tighe DP, Wagner CK, 2012. Maternal Hypothyroidism Decreases Progesterone Receptor Expression in the Cortical Subplate of Foetal Rat Brain. Journal of Neuroendocrinology 24, 1126–1134. 10.1111/j.1365-2826.2012.02318.x [DOI] [PubMed] [Google Scholar]
- Juraska JM, Fifková E, 1979. A Golgi study of the early postnatal development of the visual cortex of the hooded rat. Journal of Comparative Neurology 183, 247–256. 10.1002/cne.901830203 [DOI] [PubMed] [Google Scholar]
- Kim HS, Kim TS, Shin J-H, Moon HJ, Kang IH, Kim IY, Oh JY, Han SY, 2004. Neonatal exposure to di(n-butyl) phthalate (DBP) alters male reproductive-tract development. Journal of Toxicology and Environmental Health - Part A 67, 2045–2060. 10.1080/15287390490514859 [DOI] [PubMed] [Google Scholar]
- Kobrosly RW, Evans S, Miodovnik A, Barrett ES, Thurston SW, Calafat AM, Swan SH, 2014. Prenatal phthalate exposures and neurobehavioral development scores in boys and girls at 6–10 years of age. Environmental Health Perspectives 122, 521–528. 10.1289/ehp.1307063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komada M, Gendai Y, Kagawa N, Nagao T, 2016. Prenatal exposure to di(2-ethylhexyl) phthalate impairs development of the mouse neocortex. Toxicology Letters 259, 69–79. 10.1016/j.toxlet.2016.07.019 [DOI] [PubMed] [Google Scholar]
- Kougias DG, Cortes LR, Moody L, Rhoads S, Pan YX, Juraska JM, 2018a. Effects of perinatal exposure to phthalates and a high-fat diet on maternal behavior and pup development and social play. Endocrinology 159, 1088–1105. 10.1210/en.2017-03047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kougias DG, Sellinger EP, Willing J, Juraska JM, 2018b. Perinatal exposure to an environmentally relevant mixture of phthalates results in a lower number of neurons and synapses in the medial prefrontal cortex and decreased cognitive flexibility in adult male and female rats. Journal of Neuroscience 38. 10.1523/JNEUROSCI.0607-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XJ, Jiang L, Chen L, Chen HS, Li X, 2013. Neurotoxicity of dibutyl phthalate in brain development following perinatal exposure: A study in rats. Environmental Toxicology and Pharmacology 36, 392–402. 10.1016/j.etap.2013.05.001 [DOI] [PubMed] [Google Scholar]
- Lin CH, Chen TJ, Chen SS, Hsiao PC, Yang RC, 2011. Activation of Trim17 by PPARγ is involved in Di(2-ethylhexyl) phthalate (DEHP)-induced apoptosis on Neuro-2a cells. Toxicology Letters 206, 245–251. 10.1016/j.toxlet.2011.08.002 [DOI] [PubMed] [Google Scholar]
- Lin H, Yuan K, Li L, Liu S, Li S, Hu G, Lian QQ, Ge RS, 2015. In utero exposure to diethylhexyl phthalate affects rat brain development: A behavioral and genomic approach. International Journal of Environmental Research and Public Health 12, 13696–13710. 10.3390/ijerph121113696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma P, Liu X, Wu J, Yan B, Zhang Y, Lu Y, Wu Y, Liu C, Guo J, Nanberg E, Bornehag CG, Yang X, 2015. Cognitive deficits and anxiety induced by diisononyl phthalate in mice and the neuroprotective effects of melatonin. Scientific Reports 5, 1–14. 10.1038/srep14676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Cerdeño V, Noctor SC, Kriegstein AR, 2006. Estradiol stimulates progenitor cell division in the ventricular and subventricular zones of the embryonic neocortex. European Journal of Neuroscience 24, 3475–3488. 10.1111/j.1460-9568.2006.05239.x [DOI] [PubMed] [Google Scholar]
- Moody L, Kougias D, Jung PM, Digan I, Hong A, Gorski A, Chen H, Juraska J, Pan YX, 2019. Perinatal phthalate and high-fat diet exposure induce sex-specific changes in adipocyte size and DNA methylation. Journal of Nutritional Biochemistry 65, 15–25. 10.1016/j.jnutbio.2018.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mose T, Mortensen GK, Hedegaard M, Knudsen LE, 2007. Phthalate monoesters in perfusate from a dual placenta perfusion system, the placenta tissue and umbilical cord blood. Reproductive Toxicology 23, 83–91. 10.1016/j.reprotox.2006.08.006 [DOI] [PubMed] [Google Scholar]
- Mosley M, Shah C, Morse KA, Miloro SA, Holmes MM, Ahern TH, Forger NG, 2017. Patterns of cell death in the perinatal mouse forebrain. Journal of Comparative Neurology 525, 47–64. 10.1002/cne.24041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesan D, Feighan KM, Antle MC, Kurrasch DM, 2021. Gestational low-dose BPA exposure impacts suprachiasmatic nucleus neurogenesis and circadian activity with transgenerational effects, Sci. Adv [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuñez JL, Jurgens HA, Juraska JM, 2000. Androgens reduce cell death in the developing rat visual cortex. Developmental Brain Research 125, 83–88. 10.1016/S0165-3806(00)00126-7 [DOI] [PubMed] [Google Scholar]
- Parks LG, Ostby JS, Lambright CR, Abbott BD, Klinefelter GR, Barlow NJ, Gray LE, 2000. The plasticizer diethylhexyl phthalate induces malformations by decreasing fetal testosterone synthesis during sexual differentiation in the male rat. Toxicological Sciences 58, 339–349. [DOI] [PubMed] [Google Scholar]
- Radke EG, Braun JM, Nachman RM, Cooper GS, 2020. Phthalate exposure and neurodevelopment: A systematic review and meta-analysis of human epidemiological evidence. Environment International. 10.1016/j.envint.2019.105408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raedler A, Sievers J, 1975. The development of the visual system in the albino rat. Adv Anat Embryol Cell Bio 50, 3–88. 10.1007/978-3-642-45461-5 [DOI] [PubMed] [Google Scholar]
- Sellinger EP, Kougias DG, Drzewiecki CM, Juraska JM, 2020. Behavioral effects in adult rats exposed to low doses of a phthalate mixture during the perinatal or adolescent period. Neurotoxicology and Teratology 79, 106886. 10.1016/j.ntt.2020.106886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Li SSL, 2012. Epigenetic effects of environmental chemicals bisphenol A and phthalates. International Journal of Molecular Sciences 13, 10143–10153. 10.3390/ijms130810143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani MR, Moghaddam B, 2005. Systemic and prefrontal cortical NMDA receptor blockade differentially affect discrimination learning and set-shift ability in rats. Behavioral Neuroscience 119, 420–428. 10.1037/0735-7044.119.2.420 [DOI] [PubMed] [Google Scholar]
- Stroheker T, Cabaton N, Nourdin G, Régnier JF, Lhuguenot JC, Chagnon MC, 2005. Evaluation of anti-androgenic activity of di-(2-ethylhexyl)phthalate. Toxicology 208, 115–121. 10.1016/j.tox.2004.11.013 [DOI] [PubMed] [Google Scholar]
- Takeuchi S, Iida M, Kobayashi S, Jin K, Matsuda T, Kojima H, 2005. Differential effects of phthalate esters on transcriptional activities via human estrogen receptors α and β, and androgen receptor. Toxicology 210, 223–233. 10.1016/j.tox.2005.02.002 [DOI] [PubMed] [Google Scholar]
- Tanida T, Warita K, Ishihara K, Fukui S, Mitsuhashi T, Sugawara T, Tabuchi Y, Nanmori T, Qi WM, Inamoto T, Yokoyama T, Kitagawa H, Hoshi N, 2009. Fetal and neonatal exposure to three typical environmental chemicals with different mechanisms of action: Mixed exposure to phenol, phthalate, and dioxin cancels the effects of sole exposure on mouse midbrain dopaminergic nuclei. Toxicology Letters 189, 40–47. 10.1016/j.toxlet.2009.04.005 [DOI] [PubMed] [Google Scholar]
- Tomiyama K-I, Funada M, 2013. Cytotoxicity of synthetic cannabinoids on primary neuronal cells of the forebrain: the involvement of cannabinoid CB 1 receptors and apoptotic cell death. 10.1016/j.taap.2013.10.028 [DOI] [PubMed]
- Win-Shwe TT, Yanagisawa R, Koike E, Nitta H, Takano H, 2013. Expression levels of neuroimmune biomarkers in hypothalamus of allergic mice after phthalate exposure. Journal of Applied Toxicology 33, 1070–1078. 10.1002/jat.2835 [DOI] [PubMed] [Google Scholar]
- Wittassek M, Koch HM, Angerer J, Brüning T, 2011. Assessing exposure to phthalates - The human biomonitoring approach. Molecular Nutrition and Food Research 55, 7–31. 10.1002/mnfr.201000121 [DOI] [PubMed] [Google Scholar]
- Wójtowicz AK, Sitarz-Głownia AM, Szczęsna M, Szychowski KA, 2019. The Action of Di-(2-Ethylhexyl) Phthalate (DEHP) in Mouse Cerebral Cells Involves an Impairment in Aryl Hydrocarbon Receptor (AhR) Signaling. Neurotoxicity Research 35, 183–195. 10.1007/s12640-018-9946-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wójtowicz AK, Szychowski KA, Wnuk A, Kajta M, 2017. Dibutyl Phthalate (DBP)-Induced Apoptosis and Neurotoxicity are Mediated via the Aryl Hydrocarbon Receptor (AhR) but not by Estrogen Receptor Alpha (ERα), Estrogen Receptor Beta (ERβ), or Peroxisome Proliferator-Activated Receptor Gamma (PPARγ) in Mouse C. Neurotoxicity Research 31, 77–89. 10.1007/s12640-016-9665-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, Gao L, Flaws JA, 2017. Prenatal exposure to an environmentally relevant phthalate mixture disrupts reproduction in F1 female mice. Toxicology and Applied Pharmacology 318, 49–57. 10.1016/j.taap.2017.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]


