Abstract
Metastasis is a significant cause of the mortality resulting from solid malignancies. The process of metastasis is complex and is regulated by numerous cancer cell-intrinsic and - extrinsic factors. CXCR3 is a chemokine receptor that is frequently expressed by cancer cells, endothelial cells and immune cells. CXCR3A signaling in cancer cells tends to promote the invasive and migratory phenotype of cancer cells. Indirectly, CXCR3 modulates the anti-tumor immune response resulting in variable effects that can permit or inhibit metastatic progression. Finally, the activity of CXCR3B in endothelial cells is generally angiostatic, which limits the access of cancer cells to key conduits to secondary sites. However, the interaction of these activities within a tumor and the presence of opposing CXCR3 splice variants clouds the picture of the role of CXCR3 in metastasis. Consequently, thorough analysis of the contributions of CXCR3 to cancer metastasis is necessary. This review is an in-depth examination of the involvement of CXCR3 in the metastatic process of solid malignancies.
Keywords: CXCR3, cytokines, metastasis, cancer, tumor microenvironment
1. Introduction
Malignancies are the second leading cause of death in the United States [1]. Metastasis is not only a defining feature of cancer but is also a significant cause of cancer-related mortality. Metastatic disease contributes to organ dysfunction, which can be fatal depending on the secondary site and the extent of involvement. Moreover, metastases, almost categorically, preclude surgical management of cancer, thereby limiting utilization of the most effective treatment modality and necessitating the use of chemotherapies. The multifaceted nature of the contribution of metastases to cancer-related mortality makes it challenging to quantify the impact of metastasis on patient outcomes; however, conservative and liberal estimates indicate that between 66% and 90% of cancer deaths are related to metastatic disease progression, respectively [2–4]. These statistics highlight the crucial role that metastasis plays in cancer biology and patient outcomes.
Metastasis in solid malignancies proceeds through a long cascade involving invasion, intra- and extravasation, and colonization. This process begins with the invasion of cancer cells into the surrounding tissue [5]. As they invade, cancer cells encounter blood and lymphatic vessels, which act as conduits for cancer cells to secondary sites [5]. Upon entering the vessels, cancer cells must survive the loss of attachment [6, 7], fluid shear stress [8, 9], and the immune cell-rich environment [10, 11] and ultimately attach to the endothelium at a distant site. Once arrested at the distant site, cancer cells must escape from the vasculature through a process that is believed to mirror leukocyte extravasation [5]. Finally, cancer cells must adapt to the microenvironment of the secondary site and expand to produce a clinically detectable metastatic lesion [5]. Each of these steps of metastasis presents a unique set of challenges to the metastasizing cancer cells. Moreover, the complex nature of the metastatic process dictates that molecules may have competing effects during different phases of the metastatic process, making a thorough analysis of metastasis data both difficult and requisite for understanding the molecular mechanisms of metastasis.
CXCR3 is the receptor of the C-X-C domain-containing chemokines PF4 (CXCL4), PF4V1 (CXCL4L1), CXCL9, CXCL10, and CXCL11. Initially, CXCR3 was characterized as a chemokine receptor expressed predominantly on T-lymphocytes and subsets of natural killer (NK) cells [12]; however, subsequent studies revealed that CXCR3 is expressed on a wide variety of cells including endothelial cells and cancer cells. Like many other chemokine receptors, CXCR3 plays critical roles in the progression of numerous malignancies. These functions include modulating tumor immune infiltrate, promoting tumor growth, and influencing tumor angiogenesis, which collectively facilitate the metastatic process. However, because of the diversity in its expression and functions, the role of CXCR3 in metastasis is multifaceted, resulting in competing effects between the functions of CXCR3 splice variants and CXCR3 expression on different cell types. This review presents a detailed analysis of the nuances of direct and indirect involvement of CXCR3 in the metastatic processes of solid malignancies.
2. Biochemistry of CXCR3
An understanding of the biochemistry of a molecule is fundamental in developing an overall framework of its contributions to pathology. Currently, there are three described splice variants of CXCR3: CXCR3A, CXCR3B, and CXCR3 Alt. CXCR3A and CXCR3B constitute the vast majority of CXCR3 expression, and due to the lack of studies on CXCR3 alt, this review focuses solely on CXCR3A and B. Both CXCR3A and CXCR3B encode seven predicted transmembrane domains and both proteins couple with heterotrimeric G-proteins (HTGPs), which mediate their downstream signaling. In terms of sequence, the only difference between CXCR3A and B is in the N-terminus [13]. The coding sequence of CXCR3A includes three amino acids derived from exon 1, with the remainder being derived from exon 2. In contrast, the coding sequence of CXCR3B is derived from exon 2 and a retained intronic sequence that is contiguous with the 5’ end of exon 2 (Figure 1). By comparison, CXCR3B has 48 amino acids at the N-terminus that are not present in CXCR3A. Despite their sequence similarities, the behaviors of CXCR3A and B are vastly different, and appreciating these differences is paramount to understanding CXCR3 in the context of metastasis. For instance, CXCR3A is a high-affinity receptor for CXCL9, CXCL10, and CXCL11 [13], whereas CXCR3B has high affinity for CXCL4 and CXCL10 (roughly four-fold higher than its affinity for CXCL9 or 11) [14]. Interestingly, CXCL4 does not appear to bind or activate CXCR3A, but CXCL4L1 has intermediate affinities for both CXCR3A and B [14]. Despite having lower affinities for CXCL9 and CXCL11, CXCR3B can be activated by these ligands, though only at very high concentrations. It is essential to understand the differences in ligand binding between CXCR3A and B as these variants have different functions during metastasis, and thus, the ligands of each receptor produce different, sometimes conflicting, outcomes.
Figure 1.
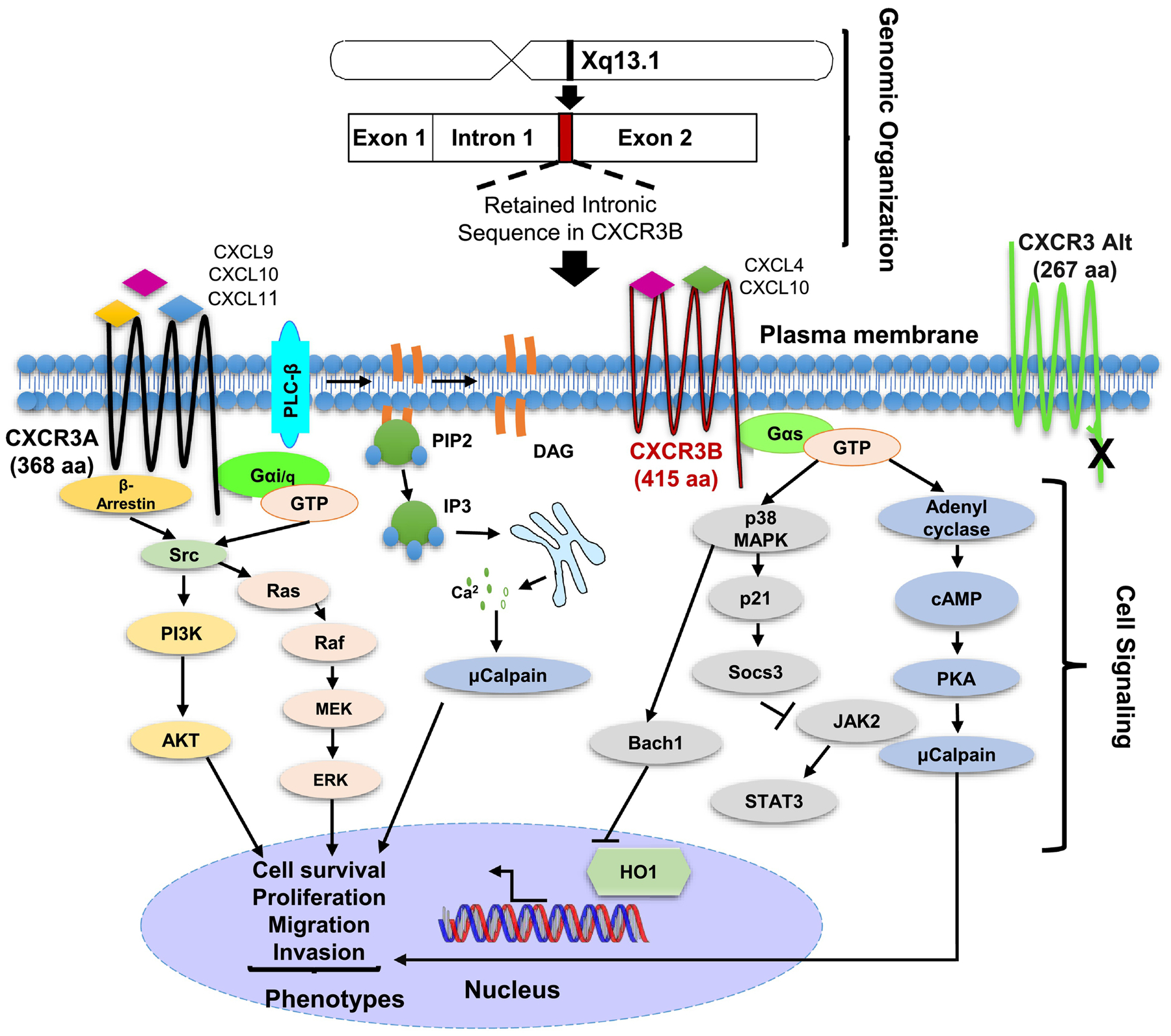
Schematic of CXCR3 chromosomal organization and signaling. Top). CXCR3 is located on the X chromosome and is composed of two exonic segments. Differential splicing of CXCR3 gives rise to three described splice variants A, B and Alt. CXCR3B is encoded entirely by a retained intronic sequence and exon 2. Bottom) CXCR3 A and B signaling is mediated by heterotrimeric G proteins (HTGP). CXCR3A couples with Gai/q and results in downstream activation of Ras/Raf/ Erk as well as PI3K/Akt signaling leading to cell proliferation, survival, and migration/invasion. CXCR3B signals through Gas resulting in activation of adenyl cyclase and PKA as well as p38 and p21 activation leading to sensitization of cells to death.
In addition to ligand binding, the differences in splicing between CXCR3A and B alter the coupling of the two receptors to HTGPs (Figure 1). CXCR3A is believed to couple to Gαi and Gαq (though credible data for Gαq coupling is limited) HTGPs on the basis that treatment of cells with pertussis toxin and knockout of Gαi both inhibit the cellular effects of activation of CXCR3A [14, 15]. Downstream of Gαi and Gαq, the understanding of CXCR3A signaling is fragmented. However, it is believed that activation of Gαi results in downstream activation of SRC kinase, and subsequently, MAPK and PI3K/AKT signaling, leading to increased proliferation of cells and resistance to apoptosis, respectively. Additionally, recruitment of β-arrestin 2 to activated CXCR3 contributes significantly to the non-canonical activation of SRC and subsequent activation of pro-proliferative and anti-apoptotic pathways. Activation of Gαq downstream of CXCR3A initiates phospholipase C-β (PLC-β) signaling leading to phosphatidylinositol 4,5-bisphosphate (PIP2) cleavage to form diacylglycerol (DAG) and inositol-triphosphate (IP3). DAG and IP3, in turn, stimulate release of calcium from the endoplasmic reticulum resulting in pleiotropic cellular effects, including activation of calpains, which can contribute to cell migration. While the signaling downstream of CXCR3A is mainly consistent across ligands, recent reports have suggested the possibility of biased signaling downstream of activation of this splice variant [16, 17].
In general, the signaling pathways downstream of CXCR3A appear to be consistent regardless of the cell type in which the signaling is occurring. However, the cellular response to the activation of these signaling pathways appears to be cell type specific. In cancer cells, signaling downstream of CXCR3A appears to promote proliferation, as well as migration and invasions thereby contributing to the metastatic cascade. In contrast, the signaling in T-cells largely culminates in the homing of T-cells to sites of inflammation or secondary lymphoid organs. The process of T-cell homing is multifaceted and within this process, CXCR3 has roles in both directing migration and facilitating arrest of T-cells at endothelium. Initial studies of the molecular mechanism of involvement of CXCR3 in T-cell homing demonstrated that the CXCR3-mediated migration was sensitive to pertussis toxin, suggesting the involvement of Gαi [18]. Further work by Thompson et al. demonstrated that knockout of Gαi2 in mice markedly decreased the migratory capacity of T-cell in response to CXCR3 ligands [15]. Additional studies have demonstrated that PLC activation is an additional factor in CXCR3-mediated chemotaxis, which suggests that Ca2+ signaling may be involved [18]. Interestingly, the activation of PLC appears to link CXCR3 activation with T-cell receptor signaling through ZAP70 and Lck [19, 20]. Finally, using biased CXCR3 agonists, Smith et al. were able to demonstrate that β-arrestin signaling is a functional requirement of CXCR3-mediated T-cell chemotaxis [21]. It is important to note that CXCR3 function in the immune system is not restricted to T-cells, and that new roles in B-cells, NK-cells as well as myeloid lineage cells are an area of active investigation. Similarly, recent studies have demonstrated that CXCR3 may have important functions in the maturation, polarization, and effector functions of T-cells [22]. However, the cellular and molecular basis of these functions remain poorly characterized and largely beyond the scope of this review.
In contrast to CXCR3 A, CXCR3B is thought to couple to Gαs based on findings that activation of CXCR3B in cells ectopically expressing CXCR3B, specifically, results in a rise in intracellular cAMP production, which is augmented in the presence of forskolin [14]. However, this evidence does not rule out the possibility that CXCR3B couples with Gαolf HTGPs, which also stimulate adenylate cyclase activity [23]. Given the opposing nature of the Gα subunits transmitting their respective signals, the signaling downstream of CXCR3B is quite different from that induced by CXCR3A. CXCR3B-mediated signaling proceeds through activation of PKA. While the roles of PKA have been widely researched as a whole, its contribution to metastasis is less well defined, though it is generally believed to suppress the malignant features of cancer cells. Additionally, CXCR3B activates p38, which, in turn, activates Bach1 and p21, thereby sensitizing cells to redox stress and suppressing progression through the cell cycle, respectively [24, 25]. Furthermore, the signaling downstream of CXCR3B activation is consistent irrespective of the activating ligand. It must also be mentioned that activation of any HTGP results in the release and activation of Gβγ subunits from the trimeric complex resulting in the activation of the PLC-β and, ultimately, the release of calcium from the endoplasmic reticulum. Consequently, both CXCR3A and B signal through this mechanism [26].
3. Role of CXCR3 in Metastasis
The expression of CXCR3 by diverse cell types in the tumor microenvironment allows CXCR3 to play several roles in the metastatic process. Malignant cells of numerous cancer types express both splice variants of CXCR3, and activation of CXCR3 directly modulates the metastatic behavior of these cells. In addition to this direct role, the expression of CXCR3A on lymphocytes indirectly affects the ability of malignant cells to metastasize. Similarly, the expression of CXCR3B on endothelial cells has profound effects on angiogenesis, which alters the availability of vessels for the transport of cancer cells to secondary sites. The following section reviews the data regarding the direct and indirect roles of CXCR3 in the metastatic processes of several malignancies, and Figure 2 presents a schematic of the trends in the roles of CXCR3 in the metastatic process.
Figure 2.

Schematic of the involvement of CXCR3 in the invasion-metastasis cascade. In the primary tumor CXCR3 ligands are primarily derived from surrounding stromal cells including macrophages and fibroblasts. Here, CXCR3A and B have opposing roles with respect to cancer cell invasion. CXCR3A promotes tumor cell invasion, migration, and intravasation largely through increased expression of matrix metalloproteinases. In contrast CXCR3B has an inhibitory effect on these processes and limits access of cancer cells to vasculature through its angiostatic activity. Additionally, CXCR3 is involved in the recruitment of T-cells and NK-cells to the TME as well as the differentiation of these cells into functionally important subsets. The effect of immune cell recruitment and differentiation on the overall metastatic process appears to context dependent. During the intravascular phase of metastasis, CXCR3 ligands are largely derived from platelets in the form of PF4. CXCR3B may augment cancer cell survival by augmenting stem-like phenotype in cancer cells. Finally, at the secondary site, the pro-invasive and immunomodulatory effects of CXCR3A can play critical roles in the establishment of a metastatic niche and ultimately colonization of the distant site.
3.1. Direct Contribution of CXCR3 to Metastasis
While the role of CXCR3 in metastasis is multifaceted, prostate cancer (PCa), melanoma, and colorectal cancer (CRC) serve as prime examples in which CXCR3 contributes directly to the metastatic process. In these settings, the activation of CXCR3 promotes the invasive phenotypes of cancer cells. Importantly CXCR3A is the key driver of this invasive phenotype, as demonstrated by a study of CXCL11-induced chemotaxis. Here, CXCR3 inhibition suppressed cancer cell chemotaxis, specifically in cancer cells that had comparatively high CXCR3A: CXCR3B ratios [27]. Interestingly, in those cells that had low CXCR3A: CXCR3B ratios, CXCL11 still promoted chemotaxis, which was augmented by inhibition of CXCR3 [27], thereby highlighting the importance of differentiating between CXCR3 splice variants. Corresponding to these changes in malignant cell phenotype, high expression of CXCR3, or its ligands, in cancer cells is associated with poor prognosis in patients and increased stage at diagnosis [28–35]. Moreover, this mechanism of CXCR3A’s involvement in metastasis has been observed in several other cancers. In pancreatic cancer (PC) [36, 37], oral squamous cell carcinoma [38], malignant glioma [39], and osteosarcoma [40], CXCR3 activation promotes the invasion and/or migration of cancer cells. Additionally, these studies independently demonstrate that CXCR3 functions to promote this activity through pathways including activation of AKT [38] and ERK [36], as well as increased expression of matrix metalloproteinases (MMPs) [40] further highlighting that these pathways are primary signaling events involved in CXCR3A’s function in cancer cells. In the settings of PC [36], osteosarcoma, and oral squamous cell carcinomas, CXCR3 was further connected to metastatic phenotypes through demonstration of robust CXCR3 expression in metastatic lesions, and suppressed metastasis in a tail vein injection model with CXCR3 loss [40], and association of primary tumor CXCR3 expression with the presence of metastatic disease at diagnosis [38].
Additionally, because of the role of CXCR3 in the homing of T-cells and its notable contribution to the dermato-tropism of cutaneous T-cell lymphomas, CXCR3 has long been suspected of being involved in the tropism of metastasizing tumor cells. Studies by Kawada et al. demonstrated that CXCR3 knockout significantly suppressed the formation lymph node metastasis in melanoma models [41]. Notably, lymph node metastasis was augmented by administration of adjuvants that culminated in the upregulation of CXCR3 A ligands. Similar findings were noted for CXCR3 in colorectal cancer by the same group. Here CXCR3 expression was associated with lymph node metastasis, but not with those of the liver or lung metastasis [29]. Cumulatively, these findings suggest that CXCR3 may be involved in the tropism of tumor cells to lymph nodes. Other studies have investigated the potential of CXCR3-mediated organotropism in hematogenous dissemination. Here, a study noted that inhibition of CXCR3 suppressed pulmonary metastasis in a tail vein injection model but did not affect liver metastasis following splenic injection [42]. The conclusions that can be drawn from this study are limited by the use of two different models to demonstrate a differential effect of CXCR3 inhibition on organ-specific metastasis. Finally, Doron et al. demonstrated that knockdown of CXCR3 in melanoma cells suppressed the formation of brain metastases in murine models. Importantly, they found that CXCL10 expressed by activated astrocytes was critical for driving the migration of melanoma cells [43]. These findings support the potential role of CXCR3 signaling in driving metastasis of CXCR3-positive cancer cells to inflamed sites. However, the extent to which this mechanism is involved in melanoma brain metastasis remains difficult to determine primarily due to a limited understanding of the underlying mechanism driving CXCL10 expression by astrocytes. Overall, the role of CXCR3 in organotropism will be a key area of future research to comprehensively define the functions of CXCR3 in metastasis.
Despite several studies providing strong evidence for CXCR3-mediated promotion of cancer cell invasion and/or migration, it must be noted that these effects were not observed in all studies, indicating that this role of CXCR3 is potentially context-dependent [44–46]. Such disparate findings regarding the function of CXCR3 in metastasis raises important considerations for studying and ultimately understanding the role of CXCR3 in complex biological systems (Table 1). Most notably, it is critical to differentiate between the expression profiles and function of the two CXCR3 splice variants. Cancer cells can express any combination of CXCR3A and B isoforms, ranging from no expression of CXCR3, to expression of a single splice variant, to mixed expression of both variants. This fact makes determining the expression pattern particularly critical for understanding the broader role of CXCR3 in an experimental system.
Table 1.
Summary of the Direct Roles of CXCR3 in Metastasis.
| Cancer | Splice Variant | Cellular Function | Citations |
|---|---|---|---|
| Prostate | CXCR3A | Augment invasion and proliferation | [46,47] |
| CXCR3B | Suppress invasion and proliferation | [46,47] | |
| Melanoma | CXCR3A | Augment invasion and brain metastasis in vivo | [41,50–52] |
| Colorectal | CXCR3A | Increased proliferation invasion, migration, lymph node and tail-vein metastasis | [29,42,59–62] |
| CXCR3B | Inhibition of proliferation, invasion and migration | [63] | |
| Breast | CXCR3A | Increased migration, and cardiac -injection bone metastasis | [72, 74, 94] |
| CXCR3B | Increased Cancer Stem Cell Features and in vivo metastasis | [65, 95] | |
| Ovarian | CXCR3 | Increased migration to malignant ascites | [98] |
| Gastric | CXCR3A | Increased migration, invasion, and liver metastases | [28, 102] |
| Hepatocellular Carcinoma | CXCR3 | Increased invasion, migration, tail-vein lung metastases | [103–105] |
| Pancreatic | CXCR3A | Increased invasion | [36,37] |
| Lung | CXCR3 | Stimulate migration | [125] |
| Renal Cell Carcinoma | CXCR3A | Induced migration | [32] |
| CXCR3B | Suppressed proliferation Increased Apoptosis | [24,129] |
3.1.1. Prostate Cancer
Most studies of CXCR3 in PCa have focused on CXCR3 function in malignant cells. In this context, the functions of CXCR3 in promoting metastatic spread are divided between the splice variants. One study showed that DU-145, a metastatic PCa cell line, had higher CXCR3A expression relative to CXCR3B as compared to RWPE-1, a non-cancerous, prostate epithelial cell line, and LNCaP, a primary tumor-derived PCa line [47]. For DU-145, CXCL10 and CXCL4 treatment increased cellular motility and invasiveness in vitro, which was not observed with RWPE-1 cells. Interestingly, overexpression of CXCR3B in DU-145 abrogated motility and invasiveness suggesting that the pro-metastatic effects of CXCR3 in PCa cells is mediated by CXCR3A and is opposed by CXCR3B signaling. Consistent with these findings, knockdown of CXCR3A in PC-3 PCa cells and overexpression of CXCR3B inhibited proliferation and invasion in vitro [48], further highlighting the disparate roles of CXCR3A and B in PCa metastasis. In patient samples, low CXCL4L1 expression, which can activate both CXCR3A and B, was associated with higher pathologic stage, greater Gleason score, and poor overall survival (OS) [49], suggesting that the anti-metastatic role of CXCR3B may predominate in human PCa; however, this conclusion is confounded by potential changes in tumor immune response and angiogenesis produced by CXCR3 activation.
The findings of the few studies conducted on CXCR3-mediated immune modulation provide important insights into the overall role of CXCR3 in the metastatic process, which may apply to multiple cancers. One study by Hu et al. demonstrated that PCa cells recruit CD4+ T-cells through the secretion of CXCL9. In turn, the recruitment of CD4+ cells resulted in the upregulation of FGF11 and downregulation of androgen receptor [50]. Together, the changes in the expression of these molecules increased the invasion and migration of PCa cell lines in vitro and increased the metastatic spread of cancer cell lines implanted in the prostates of nude mice co-transplanted with CD4+ T-cell lines. These findings are consistent with those from breast cancer, further suggesting that CXCR3 may promote the spread of cancer cells through an immune mechanism. However, it is difficult to know if this metastasis-promoting mechanism would outweigh the effect of an increased immune cell infiltrate in the presence of a complete host immune system.
3.1.2. Melanoma
Melanoma represents one of the most thoroughly researched malignancies in terms of the function of CXCR3. Interestingly, it is also a disease in which metastatic dissemination has a tremendous prognostic impact. Therefore, the contribution of CXCR3 to the metastatic process may be particularly critical in melanoma biology and patient outcomes. In a seminal study by Kawada et al., knockdown (KD) of CXCR3 in B16F10 melanoma cells followed by subcutaneous implantation showed that the CXCR3 KD cells produced fewer lymph node metastases compared to wild-type, CXCR3-expressing cells [41]. Consistently, treatment of mice with Freund’s adjuvant resulted in upregulation of CXCL9 and 10 in tumor-draining lymph nodes and, in turn, caused a dramatic increase in the number of tumor-positive lymph nodes, which was abrogated by the neutralization of CXCL9 and CXCL10 [41]. Subsequent studies showed that CXCR3 induced activation of the ERK signaling pathway, which is responsible for cellular invasion, as well as increased lymph node metastasis [51]. Similarly, another group studied highly metastatic and non-metastatic subclones of B16F10 cells and found a strong expression of CXCL10 in the metastatic subclones. Follow-up analyses demonstrated that silencing of CXCL10 or CXCR3 suppressed the metastatic ability of both sub-lines compared to non-targeted siRNA in tail vein models [52]. Finally, a very recent study suggested that CXCL10 expressed by glial cells of melanoma patients acts to promote brain-tropic metastasis of melanoma cells. Here, the knockdown of CXCR3 in melanoma cells abrogated brain metastasis in a murine model. Mechanistically, the function of CXCR3 in melanoma brain metastasis was theorized to occur through activation of integrins in a fashion parallel to that of T-cell migration. However, details of this mechanism and those regarding CXCL10 expression in the brain remain poorly characterized [43]. Analysis of correlations of clinicopathological features of melanoma patients with their CXCR3 expression levels may elucidate the potential effects that predominate in the human disease. In one study of melanoma cells, high CXCR3 expression in 82 patients was associated with increased Breslow depth and the presence of distant metastasis [53]. These findings suggest that CXCR3 function in melanoma cells may predominately function to promote metastatic dissemination.
In contrast, Antonicelli et al., demonstrated that treatment of B16F1 murine melanoma cells with CXCL10 modestly suppressed their invasive capacity in vitro [45]. Moreover, the investigators showed an association of high CXCL10 expressing peripheral blood mononuclear cells (PBMCs) with remission of melanoma, which was, in turn, associated with localized disease at time of diagnosis [45]. It is unclear, however, if this association is the result of CXCL10/CXCR3 signaling in cancer cells or immune cells as tumor-infiltrating lymphocytes were not assessed in these patients nor were similar studies conducted in mice lacking critical immune components. Similarly, treatment of lymphatic endothelial cells and melanoma cell lines with IFN-β caused marked upregulation of CXCL10 and CXCL11 [46]. In turn, CXCL10 suppressed melanoma proliferation and invasion in both IFN-β-sensitive and resistant cells [46]. Moreover, loss of CXCL10 through genetic ablation decreased the sensitivity of melanoma cells to IFN-β; notably, these effects were thought to be mediated by CXCR3B [46].
In addition, CXCR3 plays a crucial role in modulating the immune system in melanoma. Cell lines that expressed CXCR3 ligands recruited CD8+ T-cells both in vitro and when transplanted into mice [54]. Moreover, in CXCR3−/− mice bearing melanoma, the T-cell infiltration into tumors was diminished, and immune checkpoint therapy failed to reduce tumor progression [55]. Similar findings were confirmed by TCGA dataset analysis and histological correlations with cytokine expression in melanomas in which high CXCR3 ligand expression was associated with improved patient survival [56, 57]. These findings implicated CXCR3 in a robust anti-tumor immune response in melanoma. Subsequently, administration of a CXCL10 expression plasmid as DNA-based therapy to mice bearing B16F10 melanoma tumors caused loss of pulmonary metastases in a NK cell-dependent manner [58]. Finally, the treatment with human adipose mesenchymal stem cells ectopically expressing CXCL10 resulted in the disappearance of metastatic lesions from the lungs in a tail vein injection model. Here, CXCL10-overexpressing cells were shown to potentially affect numerous factors in the metastatic microenvironment, including augmented melanoma cell apoptosis, suppressed T-regulatory cell (T-reg) infiltration, increased activated T-cell infiltration, and reduced angiogenesis in lung colonies [59]. In the setting of established metastases, a combination of angiostatic effects and augmented immune response likely mediate these changes. However, CXCR3 expression in melanoma cells is associated with decreased lymphocytic infiltrate, which is consistent with CXCR3-mediated PD-L1 regulation, as observed in gastric cancer [16, 53]. This distinction highlights the significance of compartment-specific CXCR3 expression.
3.1.3. Colorectal Cancer (CRC)
As with melanoma, CXCR3 has been intensively studied for its involvement in CRC metastasis, however, most studies have focused on CXCR3 signaling in cancer cells. These studies, combined with analysis of multiple CRC patient cohorts, provide significant insight into the overarching roles of the CXCR3 axis in the CRC metastatic process. Initially, Kawada and colleagues demonstrated that CXCR3-overexpressing DLD-1 cells had more rapid dissemination through the lymphatic system in rectal transplantation models [29]. Interestingly, metastasis to the liver and lung were infrequent during the reported study and was not different between CXCR3 overexpressing and wild-type control cells. In patient tumors, CXCR3 expression was associated with lymph node metastasis and poor overall survival compared to absence of CXCR3 expression in the tumors [29]. A follow-up study demonstrated that CXCR3 and CXCR4 expression was higher in lymph node and liver metastases in patient samples and that activated CXCR3 cooperated with CXCR4 to increase migration in a manner that was dependent on CXCR4 [60, 61]. Later, the association of these two receptors was shown to occur through an atypical interaction in which CXCR3 increased CXCR4 surface retention, thereby mediating augmented signaling downstream of CXCR4 [61]. Under in vivo conditions, the loss of CXCR3, CXCR4, or both the receptors abrogated lymph node and liver metastasis in rectal transplantation models [60]. Similarly, knockdown of CXCL11 in CRC cell lines resulted in a loss of invasive/migratory potential corresponding to a loss of epithelial-mesenchymal transition (EMT) phenotype in vitro, which was mirrored in vivo with decreased tumor growth in subcutaneous models (in nude mice) as well as decreased metastasis in a tail-vein injection model [62]. These in vivo findings were shown to occur through the EMT phenotype as rescue of N-cadherin expression in CXCL11-knockdown cells reduced cell proliferation and invasion/migration in cell culture [62]. Partially consistent with these findings, another group demonstrated that treating CRC cells with CXCL9, 10, or 11 increased the proliferation and migration of these cells, which was lost with inhibition of CXCR3 [42, 63]. Together these findings support multiple metastasis-promoting roles for the CXCR3 axis in CRC, which may be important for the overall metastatic process in the early and the late phases of the disease.
Interestingly, in vivo studies using tail-vein and portal-vein injections of CRC cell lines with and without inhibition of CXCR3 demonstrated that CXCR3 inhibition resulted in suppression of pulmonary but not hepatic metastases [42]. While the authors interpreted this as CXCR3-mediated organotropism, this conclusion is not well supported by experimental data. Most notably, the use of different metastatic models to demonstrate organotropism is challenging. In these models, there are numerous differences, which include not only the final site but also the path of the cells to arrive at the indicated sites. For example, compared to a short journey through portal circulation for a cell to arrive in the liver, for a cell to colonize the lung, it must travel through the entire venous system, pass through the high-pressure system of the right heart, and finally reach the lung capillary bed. Thus, while it is explicitly clear that CXCR3 inhibition suppressed the colonies formed following tail vein injection, it is not clear that this was related specifically to the target tissue. To bolster such claims of CXCR3-mediated tropism, left ventricular cardiac injection may be a better model, and these studies would benefit greatly by demonstrating that cells expressing CXCR3 show a predilection for lung colonization (or fail to colonize the liver). In contrast, cells lacking CXCR3 either have no predilection regarding the final site of metastasis or favor the liver.
Finally, a single study has examined potential differences in the function of CXCR3 splice variants in CRC metastasis. Here, the authors demonstrated that while CXCR3 (total protein) was upregulated in CRC tissues, and positively associated with TNM staging, CXCR3B was under-expressed in CRC tissue (at the mRNA level) compared to adjacent normal tissue and correlated inversely with tumor stage [64]. Studies using overexpression of CXCR3 variants in vitro, showed decreased proliferation, invasion, and migration with CXCR3B expression and opposing effects for CXCR3A. While this study did not explicitly study metastasis in animal models, they did evaluate tumorigenicity, a characteristic feature of cancer stem cells (CSCs). In this setting, CXCR3A again was associated with increased tumorigenicity, whereas CXCR3B appeared to inhibit tumor formation [64]. These findings contrast with findings in breast cancer that suggested that CXCR3B-positive cells had CSC phenotypes. Though, in this report, investigation of CSC phenotype and association with known CSC markers was lacking [64, 65].
In addition to mechanistic studies, there are numerous reports of the associations of CXCR3 with clinical features in CRC patients. In multiple studies, CXCR3 expression was positively associated with recurrence [30, 66] as well as with the presence of lymph node and distant metastases [30]. CXCR3 ligands have also been thoroughly studied in human CRC tissues, but the outcomes of these studies are conflicting. One study demonstrated that CXCL9 was associated with decreased metastases as well as improved OS in Kaplan-Meier and Cox Proportional hazards analyses [67]. These findings were supported by additional studies of human CRC tissues, which demonstrated that low CXCL10 expression in stage II and III CRC patients was associated with an increased likelihood of recurrence as well as worse OS [68]. These findings suggest that CXCR3 ligands may have a metastasis-suppressing role in CRC, but molecular associations with CXCR3 were not investigated, leaving considerable questions regarding the biology underlying these associations. However, two studies focusing on CXCL10 showed that high CXCL10 expression in the tumor and levels in the blood were both positively associated with metastatic disease, indicating a potential metastasis-promoting role for CXCR3 ligands [31, 32]. The underlying reason for these discrepancies is unclear; however, further analysis of patient data is inevitably needed to better understand how the expression of these molecules and the resulting activation of CXCR3 affect the CRC metastatic process.
CXCR3 signaling is also involved in anti-CRC immune responses, which suppress the formation of metastasis. Initial studies by Musha et al. showed that CXCR3, along with CCR5, mRNA expression correlated with CD3+ T-cell infiltration in CRC tumors, particularly at the invasive edge of the tumor [69]. Subsequent flow cytometry of immune cells from patient tumors revealed that, on average, 75% of tumor-infiltrating CD8+ T-cells within tumors were CXCR3+, whereas 28% of CD4+ T-cells were CXCR3+ [69]. Similarly, CXCL10 and CCR5 ligands were associated with more robust Th1-type immune signatures in CRC tissues, and this high Th1-type response was associated with high expression of IFN-γ and Granzyme B in CD8+ T-cells. Moreover, high, compared to low, Th1 signature and associated changes in CD8+ T-cell gene expression were associated with reduced Tumor/Nodes/Metastases (TNM) stage, particularly the presence of distant metastases at time of diagnosis [70]. Finally, forced expression of CXCL10 in CT26 murine CRC cells suppressed the growth of subcutaneous tumors as well as metastases arising from splenic injection of cancer cells; these suppressive effects were abrogated by NK-cell depletion [71]. Overall, these findings suggest that CXCR3 is crucial for the recruitment and function of T-cells and NK-cells within tumors and that CXCR3-mediated immune modulation suppresses CRC metastasis.
3.2. Immunological Role of CXCR3 in Metastasis
Currently, the contributions of CXCR3 to the anti-tumor immune response and the resultant effects on metastasis are nebulous (Table 2). In breast [72–74], ovarian [35], and gastric cancer [75, 76], CXCR3 has been shown to have roles that promote the anti-tumor immune response, thereby limiting metastasis. Similarly, CXCR3 expression is associated with delayed progression of Barret’s esophagus to esophageal cancer through increasing Th1-type cell recruitment [77]. CXCR3 signaling promotes an anti-cancer immune response in the setting of sarcomas as well. In hemangiosarcoma, treatment of cancer cells with parvovirus modified for the transduction of CXCL10 increased survival and reduced the number of metastases formed in comparison to an unmodified form of the virus likely through an immune-mediated mechanism [78]. Similarly, in osteosarcoma patients, CXCR3 expression correlated positively with survival [79]. Additional analysis of the TCGA osteosarcoma dataset indicated that CXCR3 expression correlated strongly with immune cell-related pathways and, in CIBERSORT analysis, with increased CD8+ T-cell signatures.
Table 2.
Summary of Immunologic functions of CXCR3.
| Cancer | Function | Consequences | Citations |
|---|---|---|---|
| Prostate | CD4+ T-cell recruitment | Increased cancer cell invasion | [49] |
| Melanoma | CD8+ T-cell Recruitment, Suppress T-reg function | Decreased metastasis | [54–58] |
| Increased PD-L1 expression in cancer cells | Decreased immune infiltrate | [53] | |
| Colorectal | Associated with increased Th1 and CD8+ signatures | and associated with reduced TNM stage | [69–71] |
| Increased NK-cell infiltrate | Reduced tumor growth and metastasis in mice | [71] | |
| Breast | Increased NK-cell recruitment, Decreased myeloid cell infiltrate | Increased tail-vein metastasis | [72–74] |
| Increased CD4+ T-cell and NK-cell recruitment | Reduced tumor growth and metastasis | [89–91] | |
| Ovarian | Recruitment of Tregs | Undetermined | [99] |
| Associated with increased number of T-cells and NK cells | Associated with increased survival | [100] | |
| Gastric | Positively associated with increased CD4+ and CD8+ infiltrates | Associated with increased overall survival | [75, 76] |
| Increased PD-L1 expression in cancer cells | Undetermined | [16] | |
| Hepatocellular Carcinoma | Increased Treg recruitment | Increased likelihood of tumor recurrence | [81] |
| Increased alternative macrophage activation | Suppressed anti-tumor immune response | [82] | |
| Alternate macrophage polarization | Suppressed anti-tumor immune response | [83] | |
| Pancreatic Cancer | T-reg recruitment | Association with poor prognosis | [85,86] |
| Suppressed CD8+ T-cell function | Association with poor outcome and more rapid tumor progression in mice | [84, 87] |
However, CXCR3 also has immunosuppressive functions in many cancers, including breast [80], ovarian, gastric [16], hepatocellular carcinoma [81–83], and pancreatic cancer [84–87]. These disparate results regarding the immunological functions of CXCR3 highlight a natural balance present in the immune response. Moving forward, it will be critical to determine if and how CXCR3 and its signaling affects this balance. In pursuit of this goal, it will be essential to elucidate the differences in CXCR3-mediated recruitment of lymphocytes to tumors, non-recruitment functions of CXCR3 in lymphocytes, and finally, key differences between CXCR3 expression and activity in lymphocytes. Regarding the latter goal, recent findings have indicated that CXCR3-mediated T-cell recruitment may be, in part, independent of CXCR3 activation [88]. Regardless, further study of the CXCR3 concerning the cancer-specific immune response is required to understand the observed associations with this axis better.
3.2.1. Breast Cancer
In breast cancer, CXCR3 has robust expression on cancer cells as well as immune cells. However, there is controversy as to whether CXCR3 promotes or suppresses the metastatic dissemination of breast cancer cells. Inhibition of CXCR3 in mice reduced lung colonization in tail vein injection models of breast cancer metastasis [72, 73]. This suppression of metastatic colony formation caused by CXCR3 inhibition was slightly diminished by depletion of NK cells from recipient mice. However, this was a small percentage of the total change and the primary effect of CXCR3 in this model is likely mediated by signaling in other cell types besides NK cells [72, 73]. Similarly, CXCR3 inhibition did not abrogate metastasis in mice lacking IFN-γ further implicating the immune system [73]. Notably, high CXCR3 expression was associated with poor OS in a subset of breast cancer patients with local disease [73]. These findings in both humans and mice were confirmed by another study utilizing a 4T1 model of breast cancer in which the KO of CXCR3 form mice implanted with tumors resulted in decreased metastases, due to an augmented immune response secondary to a loss of myeloid cell-mediated immune suppression [74]. Again, this decrease in metastasis was dependent upon the function of IFN-γ.
In a subsequent study, however, 66.1 cells stably overexpressing CXCL9 had reduced metastasis and improved survival compared to parental cells with vector control. Moreover, these changes in metastasis and survival were strongly associated with increased infiltration of T-cells and NK cells, and the changes were not observed in genetically immunocompromised mice or mice depleted of NK cells [80]. Notably, in this model, CXCL9-overexpressing tumors were significantly smaller than non-overexpressing tumors, indicating that the suppression of metastasis may be related to the suppression of the primary tumor growth rather than directly affecting breast cancer metastasis. Because of these findings, gene therapies involving the overexpression of CXCR3A ligands have been considered as potential therapeutic avenues. Mice vaccinated with CXCL11-overexpressing 4T1 cells had reduced metastasis compared to mice that were administered vector control-transfected cells. The reduction of metastasis was linked to an augmented immune response against implanted tumors, as indicated by increased IFN-γ and TNF-α expression [89]. This immune response against tumors in vaccinated mice resulted in a significant improvement in survival. Similar findings were reported in a study where CXCL10, instead of CXCL11, was overexpressed [90, 91]. The metastatic suppression resulting from CXCR3 ligand overexpression was abrogated by depletion of CD8+ T-cells and partially reduced by the depletion of CD4+ T-cells and NK cells, suggesting the involvement of immune responses. Importantly, microvessel density was also decreased in this model, and thus, inhibition of angiogenesis may also contribute to the observed effect [91]. Nonetheless, there is substantial controversy regarding the immunological function of CXCR3A in terms of suppressing metastasis. Several studies point to a CXCR3-mediated immunosuppressive effect that, when activated, permits metastatic dissemination. However, overexpression of CXCR3 ligands suppresses both tumor growth and metastatic dissemination.
In addition to immunological functions, CXCR3 signaling in cancer cells plays a critical role in breast cancer metastasis. In this setting, the function of CXCR3 is more consistent. Treatment of breast cancer cell lines with CXCL9, 10, or 11 enhanced CXCR3 signaling, which promoted the migration of these cells in vitro [72, 74]. Moreover, inhibition of CXCR3 activity in cancer cells resulted in decreased expression of RANKL while activation of CXCR3 resulted in upregulation of cathepsin B; both of these molecules have been implicated in breast cancer metastasis [92, 93]. Additionally, and consistent with its regulation of RANKL, CXCL10 was found to be a critical mediator of osteoclastogenesis in breast cancer and melanoma bone metastasis [94]. Here the loss of CXCR3 from cancer cells, or neutralization of host-derived CXCL10, lead to fewer osteolytic lesions in cardiac injection models. Subsequently, CXCL10 signaling was shown to be critical for the recruitment of cancer cells to the bone and the resulting generation of osteoclasts [94]. Moreover, loss of CXCR3 from 4T1 cells inhibited the ability of these cells to produce lytic bone lesions.
With regard to CXCR3 splice variants, activation of CXCR3B suppressed bulk cancer cell invasion and proliferation; however, it promoted stemness properties, including mammosphere formation by breast CSCs [65]. Additional studies demonstrated increased expression of CXCR3B on breast CSCs, and that silencing of CXCR3B resulted in decreased aldehyde dehydrogenase activity and suppressed metastasis in an experimental model [95]. In human samples, a large cohort study showed that CXCR3B was associated with increased tumor grade, and expression of CXCR3B and CXCL4 were associated with poor prognosis [96]. In contrast to the metastasis promoting activities of CXCR3B, knockout of PF4 increased the formation of metastatic lung lesions [97]. This increase in metastasis was associated with increased vascular permeability and increased recruitment of myeloid suppressor cells to the pre-metastatic niche in the lungs, suggesting that the direct and indirect functions of CXCR3B in breast cancer may have conflicting outcomes. Consequently, more detailed studies with modulation of CXCR3B in multiple cell populations will be required to delineate the balance between the conflicting functions of CXCR3B in different cell compartments and phases of metastasis.
In sum, CXCR3 plays diverse roles in breast cancer metastasis. Immunologically, data from multiple groups suggest that CXCR3 may promote the suppression of anti-tumor immune responses, which permits metastatic dissemination. Curiously, CXCR3A ligands had the opposite effect. While the function of CXCR3 in the immune response is perplexing, the role of CXCR3 signaling in breast cancer cells is more apparent. In cancer cells, CXCR3A promotes the migration/invasion of breast cancer cells, and despite its opposite signaling effects, CXCR3B may also promote breast cancer metastasis through the promotion of stemness in cancer cells.
3.2.2. Ovarian Cancer
In ovarian cancer, expression of CXCR3 ligands is induced in cancer-associated fibroblasts by Lymphotoxin B/ Lymphotoxin B Receptor signaling [35]. The in vitro treatment of OVCAR3 or SKOV3 ovarian cancer cells with fluid from malignant ascites resulted in increased migration towards ascitic fluid, which was abrogated by CXCR3-neutralizing antibodies [98]. Moreover, in patient samples, high expression of CXCR3 was associated with high-grade tumor, positive lymph node status, and reduced OS and progression free survival (PFS) [35]. CXCR3 is also associated with a suppressive immunological role in ovarian cancer through the recruitment of T-regs to the primary tumor [99]. However, the immunosuppressive effect of CXCR3 in ovarian cancer may be counterbalanced by its immune-promoting effects, since high expression of CXCL9 and CXCL10 was associated with increased numbers of tumor-infiltrating T-cells and NK cells. Furthermore, these changes in immune infiltrate were associated with increased survival [100]. Importantly, these studies of the immunological role of CXCR3 in ovarian cancer did not assess the effects of CXCR3 on metastasis; such studies will be necessary for understanding the intricate balance of effects of CXCR3 on the immune response to ovarian cancer.
3.2.3. Gastric Cancer
The contribution of CXCR3 to the gastric cancer metastatic process has only recently been elucidated. Despite the limited number of studies, CXCR3’s functions in the anti-tumor immune response and direct promotion of metastasis have already been partially characterized. In immune cells, correlative studies in human gastric cancer samples demonstrated that both CXCR3A and CXCR3B were upregulated in tumor tissues compared to normal adjacent tissue [75, 76, 101]. Comparison of CXCR3 staining in tumors with infiltrating immune cells demonstrated a positive association of CXCR3 expression with the presence of dendritic cells, CD4+ T-cells, and CD8+ T-cells [75, 76]. Consistently, high CXCR3 expression was associated with decreased invasive depth, TNM stage, lymph node-negative disease, and well-differentiated histology [75, 76]. These associations translated into significantly improved overall survival for patients with high CXCR3 expression [75, 76]. Cumulatively, these findings suggest that CXCR3 may have a role in promoting metastasis-limiting immune responses. Interestingly, the outcomes of these studies contradict other data showing that CXCR3 signaling in gastric cancer cells promotes PD-L1 expression and, as a result, suppresses anti-tumor immune response [16]. Together, these findings highlight the differences in CXCR3 activity in different tissue compartments.
Initial studies of CXCR3 in gastric cancer cells demonstrated that treatment with CXCL10 increased MMP2 and MMP9 production in gastric cancer cells; knockdown of CXCR3 with siRNA attenuated this effect. Functionally, overexpression of CXCR3 resulted in increased invasion, migration, and MMP production in response to CXCL10. Overall, these effects of CXCL10 treatment were shown to be dependent upon PI3K/AKT signaling stimulated downstream of CXCR3 [28]. Interestingly, and in contrast to studies examining the association of CXCR3 expression with immune infiltrates and survival, this study found that CXCR3 staining in gastric cancer tissue was associated with poor OS and advanced stage at diagnosis [28]. This disparity is likely related to methodological differences in CXCR3 quantification and subsequent stratification of patients. While neither study reported IHC methods in sufficient detail to confirm this, presented IHC data suggests that these studies quantified CXCR3 staining in different tissue compartments.
While the above studies did not account for CXCR3 splice variants, this aspect of CXCR3’s function in gastric cancer was analyzed in a separate study. Here, the authors demonstrated that CXCR3A was upregulated in gastric cancer cell lines and tissues, whereas CXCR3B was downregulated [102]. Analysis of CXCR3 splice variant functions in a gastric cancer cell line showed that knockdown of CXCR3A inhibited CXCL10-stimulated migration and invasion, whereas knockdown of CXCR3B had little effect on the migration and invasion of gastric cancer cells. Furthermore, the loss of CXCR3A inhibited CXCL10-induced phosphorylation of ERK1/2, as well as expression of MMP13 and 16. Importantly, in a subcutaneous tumor model, mice bearing tumors derived from CXCR3A-knockdown cells had significantly fewer liver metastases compared to mice with WT tumors [102].
3.2.4. Hepatocellular Carcinoma (HCC)
As with gastric cancer, investigation of the role of CXCR3 in HCC is a recent development. The first study reporting the involvement of CXCR3 in HCC samples came from a study of tumor recurrence following liver transplantation [81]. In this study, patients who received small-for-size liver grafts had increased tumor recurrence accompanied by an increase in circulating endothelial progenitor cells and CXCL10. CXCL10- and CXCR3-KO animals that received similar liver transplants had significantly reduced recurrence. Most importantly, in an orthotopic nude animal model, administration of CXCL10 into the portal vein augmented the number of lung metastases formed. In this study, the authors claim that CXCL10 administration augmented angiogenesis in tumors, resulting in increased metastatic spread [81]. However, this contradicts the classical understanding of CXCR3’s functions, and while there were more CD34-positive cells within tumors of CXCL10-treated mice, these areas appeared to be smaller in comparison to untreated mice. Moreover, mice injected with endothelial progenitor cells had larger tumors and presence of multiple lesions in the liver but did not appear to have increased rates of metastases to the lungs. Because of these findings, the involvement of angiogenesis in metastases observed in this study is questionable and likely requires further investigation [81]. While this study was conducted in nude mice, thereby likely limiting the involvement of the immune system in mediating the effects of CXCL10, a separate study showed that CXCL10 in this same setting was associated with increased T-reg recruitment to the liver graft [82]. Similarly, the expression of CXCL10 by macrophages in HCC induced the differentiation of B-cells to IgG-secreting plasma cells, which, in turn, drove alternate activation of macrophages and suppression of the anti-tumor immune response [83]. Together, these studies compel further investigation of the role of CXCL10 in immune modulation as it relates to HCC metastasis.
Additional studies highlighted CXCR3 signaling in HCC cells and its effect on metastasis. These studies highlighted the ability of CXCR3 signaling to act through the AKT/PI3K pathways as well as ERK1/2 signaling to promote the invasive and migratory phenotype of HCC cells [103–105]. Notably, two studies found that CXCR3-mediated upregulation of MMP2 and MMP9 production, which augmented the in vitro invasive properties of cells and promoted colonization of the lung in tail-vein injection models [104, 105]. These findings parallel those observed in gastric cancer, suggesting that CXCR3-mediated regulation of MMPs may be an essential component of its function not only in metastasis but also in lymphocytes. Even though MMPs are an important part of the initial process of metastasis, additional studies are warranted to determine what fraction of CXCR3-induced invasion is mediated by MMPs. Finally, another study suggested that Gβγ-mediated activation of RAC downstream of CXCR3 increases expression of PREX2, leading to increased migratory/invasive behavior in cancer cells. This knockdown of PREX2 suppressed invasion in vitro, but it was not tested if, or to what extent, loss of PREX2 abrogates the invasion stimulated by activation of CXCR3 [103].
3.2.5. Pancreatic Cancer
Studies of the function of CXCR3 in PC are currently limited. However, from this work several important associations have emerged. In the setting of PC, it is hypothesized that CXCR3 ligands, mainly CXCL9 and CXCL10, are derived from the tumor stroma composed of activated pancreatic myofibroblast. This hypothesis is supported by observations of increased CXCR3 ligand expression, by RNA-Seq, in the stromal component of microdissected PC tumors [84]. Moreover, Lunardi and colleagues found that co-culture of PC cells with pancreatic myofibroblast resulted in expression of CXCL10 from the myofibroblast cells [85]. Further studies examining the function of a CXCR3A signaling axis in PC demonstrated clear associations between CXCR3 ligands and markers of immunosuppression and T-cell exhaustion [84–86]. Importantly, both studies found that high expression of CXCR3 A ligands were associated with worse OS in PC patients [84–86]. Consistent with these results, Gao and colleagues found that treatment of T-cells in vitro with CXCL9 reduced the activity of CD8+ T-cells and that this contributed to more rapid progression of PDAC in mouse models[87]. Cumulatively these results strongly suggest that activation of CXCR3 A signaling may result in suppressed immune function and worsened patient prognosis in PC. In contrast to CXCR3 Ligands, the expression of CXCR3 in PC samples had inconsistent effects across the two studies. The initial study demonstrated that CXCR3 was also associated with immunosuppression and worse overall outcomes [85]. In contrast, work from our lab in a larger number of patients demonstrated that high CXCR3 expression appeared to be associated more closely with increased markers of T-cell infiltrate with weaker associations with markers of immunosuppression suggestion the CXCR3 itself is a marker of more robust anti-tumor immune response. Accordingly, high CXCR3 expression in this study was associated with improved patient outcomes [84].
3.3. CXCR3-mediated Angiostasis as a Suppressor of Metastasis
Cancer, like any other human tissue, requires oxygen to carry out metabolic functions. The rapid growth rates and high metabolic demands of cancer cells require that they develop a vascular supply through angiogenesis [106]. In addition to hypoxia-driven VEGFA expression [107], cytokines and chemokines, including CXC chemokines, are important regulators of angiogenesis by acting as promoters or suppressors of the process [108, 109]. Mechanistically, it is crucial to understand the angiostatic effects of CXCR3 are mediated by CXCR3B [14]. Downstream of CXCR3B, the fact that forskolin augments the angiostatic effects of PF4 and CXCL10 treatment strongly suggests the involvement of Gαs [14]. Following Gαs activation, the angiostatic signaling of CXCR3B is enigmatic. Several studies suggest that PKA-induced endothelial cell apoptosis is the underlying mechanism [110, 111], while others cite CXCR3-induced P21 expression and resulting cell cycle arrest as the predominate signaling involved in the angiostatic activity of CXCR3 [112–114]. Consequently, the molecular mechanism of CXCR3-mediated angiostasis is a critical topic for future research.
Since access to lymphatic and blood vessels is an integral feature for metastatic dissemination, the ability of CXCR3 to suppress angiogenesis has important implications for metastasis and tumor growth. In lung cancer tumors derived from A549 cells, CXCL10 or CXCL4L1 treatment via intratumoral injection markedly reduced tumor angiogenesis, which was associated with decreased numbers and sizes of lung metastases, decreased numbers of cancer cells at secondary sites, and improved survival in treated mice [115–117]. While not conclusive, increased rates of cancer cell apoptosis in primary tumors, but not in cell culture or metastatic sites suggest that reduced metastasis may be a reflection of decreased access to vasculature and diminished malignant cell population at the primary site, which are expected due to intratumoral administration of CXCL10 and CXCL4L1 [117]. Similarly, Lewis lung carcinoma cells ectopically overexpressing PF4 produced fewer lung lesions and decreased lung mass following tail vein injections and a 28-day growth period; though, this study did not investigate associations with angiogenesis at the metastatic site [118]. Importantly the studies of intratumoral injection of CXCL10 and CXCL4L1 demonstrate that suppression of angiogenesis at the primary site is detrimental to the overall metastatic process. Similarly, the studies of PF4, which rely on ectopic overexpression of PF4 and tail-vein injection models of metastasis, show that angiogenesis is also critical at the end of the invasion-metastasis cascade.
Angiostasis as a mechanism of CXCR3’s metastasis suppressing activity is further supported by observations in RCC, PC, and CRC. In the former, intratumoral injection of CXCL9 induced necrosis in RCC tumors, which strongly suggests a hypoxic insult over immune-mediated cell death. In PDAC, CXCL4L1 was shown to inhibit both angiogenesis and lymphangiogenesis [36, 119]. While the role of CXCL4L1-mediated suppression of angiogenesis in the PC metastatic process was not specifically investigated, its suppression of lymphangiogenesis was shown to reduce the formation of lymph node metastasis in subcutaneous PC models [119]. Finally, treatment of CRC xenografts with DMXAA, a potent inducer of CXCL10 expression, results in vascular collapse within tumors and necrosis in 90% of the tumor area [120, 121]. Moreover, DMXAA suppressed bFGF-induced angiogenesis in Matrigel plugs in a CXCL10-dependent manner, further supporting the involvement of CXCL10-mediated angiostasis in the activity of the drug [120]. Finally, the direct administration of CXCL10 to CT26 derived colon cancer models suppressed alginate bead vascularization assays as well as CD31 expression in tumor samples [122].
3.3.1. Lung Cancer
In contrast to the previous studies which focused on the role of CXCR3 in malignant and immune cells, the studies of CXCR3 in lung cancer metastasis demonstrate yet another mechanism through which the CXCR3 signaling axis affects metastatic dissemination. Before the identification of CXCR3 as the receptor for CXCL4, 9, 10, and 11, the angiostatic functions of these chemokines were well characterized [112, 123]. In lung cancer, CXCL10 is highly expressed in squamous cell carcinomas compared to normal lung tissue and adenocarcinomas [116]. These findings are consistent with the strong association of squamous lung cancer with smoking and the profound inflammatory effect of cigarette smoke on lung tissue. In primary squamous lung cancer samples, neutralization of CXCL10 augmented angiogenic activities of cancer cells in vitro. Furthermore, tumor growth in a murine model of non-small cell lung cancer in severe combined immunodeficient showed that tumor growth was inversely correlated with plasma and tumor levels of CXCL10, although CXCL10 did not influence tumor cell proliferation in vitro. Importantly inhibition of CXCL10 augmented tumor growth and metastasis [116] and was associated with increased vascular density. Similarly, PF4-overexpressing Lewis lung carcinoma cells also demonstrated fewer lung metastasis in tail vein injection and subcutaneous models compared to vector controls, which was thought to occur through suppression of angiogenesis [118]. It should be noted that mice were sacrificed 28 days after tail vein injection of cancer cells. This time frame is consistent with detecting differences in the number of cells capable of successfully colonizing the lungs and being observed macroscopically. However, it is not sufficiently small to understand the effect of PF4 on the intravascular, extravasation, and early colonization phases. Moreover, this extended growth period allows tumors to become sufficiently large that they require angiogenesis. The result of this is that differences in the number of grossly observable metastatic lesions may be the product of decreased growth of lesions rather than a difference in the ability of cells to colonize the lungs successfully. Similar findings were demonstrated using subcutaneous injection models A549 and Lewis lung carcinoma cells that were treated with CXCL4L1. Notably, in these cases, both primary tumor growth and the number of metastatic cells in distant organs were suppressed by CXCR3 ligand treatment [115],[124]. Suppression of angiogenesis was suggested to be the mechanism by which CXCR3 inhibited metastasis by decreased intratumor microvessel density. However, rescue of angiogenesis in these tumors was not performed to confirm the anti-angiogenic activity CXCL4L1 as the underlying cause of reduced metastatic spread. Regardless, these findings are consistent with CXCR3-mediated suppression of angiogenesis as a predominant mechanism underlying CXCR3’s ability to suppress metastasis in lung cancer as well as with the current understanding of angiogenesis as a critical factor in metastatic dissemination.
While CXCR3 appears to inhibit lung cancer metastasis through signaling in endothelial cells, it is also expressed on malignant cells in ~90% of lung cancer patients. For these cells, the function of CXCR3 appears to oppose that for endothelial cells. For instance, A549 cells were shown to migrate towards CXCL10 in a CXCR3-dependent manner. Despite these in vitro findings and the expression of CXCR3 on lung cancer cells in patients, CXCR3 expression was not associated with lymph node status in these patients suggesting that of the two studied roles of CXCR3 in lung cancer the angiostatic role may predominate [125].
3.3.2. Renal Cell Carcinoma (RCC)
In RCC, CXCR3 plays roles in cancer cells, endothelial cells, and immune cells, and each of these functions may impact RCC metastasis. In a murine model of RCC, Pan et al. found that systemic administration of IL-2 induced the expression of CXCR3 and its ligands in PBMCs and serum, respectively and suppressed tumor growth over four weeks [126]. When the same study was conducted in CXCR3-null mice, there was minimal effect on tumor growth and a complete abrogation of tumor necrosis induced by IL-2 treatment [126]. Intratumoral injection of CXCL9, on the other hand, suppressed tumor growth, as a monotherapy and in combination with IL-2. Further analysis demonstrated that CXCL9 and combination therapy increased necrotic tumor area and decreased the percentage of cells positive for endothelial cell marker MECA-32. Despite these data, which convincingly implicate angiostasis as a mechanism of local tumor control in this model, CXCL9 also augmented the antitumor immune response [126]. Thus, it is unclear to what extent the effects of CXCL9 were mediated by angiostasis. The fact that CXCL9 treatment increases, and CXCR3 KO almost completely abrogates, the presence of necrosis in the tumor indicates the involvement of angiogenesis, since prominent necrosis is a salient feature of poor angiogenesis. Study of patient samples demonstrated that patients with advanced-stage disease had decreased CXCL10 expression in primary tumors compared to patients with local disease. These changes were in association with the loss of other cytokines with immunological functions, including SDF-1 (CXCL12) and IFN-γ, further suggesting an immunological mechanism [127]. However, a second study noted that metastatic RCC patients on high dose IL-2 had higher expression of CXCR3 ligands in PBMCs and, as a result, had a relative abundance of angiostatic cytokines in circulation [128]. Unfortunately, neither study examined changes in angiogenesis, or immune cell infiltrates associated with high CXCR3 ligand expression.
In contrast to the studies regarding CXCR3 in endothelial and/or immune cells, the studies of the CXCR3 axis in cancer cells delve into the cellular and molecular mechanisms that give rise to a metastatic phenotype. In RCC tissue, CXCR3 ligands were overexpressed, compared to normal tissue, and the CXCR3A: B ratio was 1.5 times higher in cancer compared to normal kidney tissues. Moreover, CXCL10 treatment of RCC cell lines induced migration and invasion in these cells, which was presumably mediated by CXCR3A [33]. These effects were later shown to occur through the activation of RhoA and downstream production of MMP9. Importantly, activation of HIF-1α through hypoxia or cobalt chloride upregulated CXCR3A expression. In contrast, treatment of the same cell lines with calcineurin inhibitors caused downregulation of CXCR3B, and this change augmented the invasive and proliferative capacity of these cells [129]. These results cumulatively suggest that CXCR3A expression and downstream signaling promote the initial stages of metastasis, while signaling mediated by CXCR3B may suppress them. Further studies confirmed the effect of CXCR3B as suppressing the malignant properties of RCC cell line by demonstrating that CXCR3B overexpression suppressed tumor cell proliferation and promoted apoptosis through downregulation of heme-oxygenase 1 [24]. Consistent with the findings that CXCR3A is the predominant form of CXCR3 expressed by RCC cells and that this variant of the protein promotes metastasis, two studies of RCC patient samples showed that CXCR3, specifically CXCR3A expression in cancer cells was significantly associated with metastatic RCC [33, 34]. Finally, it is essential to note that in the absence of animal models, and metastasis assays, it is difficult to understand how either CXCR3 splice variant or their ligands affect metastasis in general. Nevertheless, the in vitro studies and association of CXCR3 with patient clinicopathological features provide a framework for understanding that CXCR3A potentially promotes and CXCR3 B likely inhibits the metastatic spread of RCC.
4. Targeting CXCR3 in Cancer Therapy
The roles of CXCR3 in immune cell homing have made it an attractive target for therapy, especially in light of recent advances in immunotherapy. In this setting, augmentation of CXCR3 signaling through the administration of ligands or viral particles designed to induce expression of CXCR3 ligands has shown some promise. In melanoma, transfection with CXCL10 expressing plasmid or administration of mesenchymal stem cells overexpressing CXCl10 resulted in a regression of metastatic lesions in part through immune mediated and angiostatic mechanisms [58, 59]. Moreover, direct intratumoral administration of CXCL9 or 10 in combination with administration of anti-PD-1 antibody augmented immune response [130]. Similarly, expression of CXCL10 from melanoma cells and intratumoral injection of IFNγ was shown to augment the recruitment of adoptively transferred CXCR3 positive NK cells to tumors, resulting in decreased tumor size and increased survival of mice [131]. In RCC, intratumoral injection of inactivated sendai virus caused a marked increase in CXCL10 expression with a corresponding increase in NK cell infiltration leading to regression of tumor by 50 percent [132]. Furthermore, RCC tumors treated with CXCL9 showed immune and vascular mediated tumor regression. In this study the effects of CXCL9 were augmented by coadministration of IL-2, exceeding the effects of CXCL9 or IL2 alone. These findings suggest that CXCL9 acts synergistically with IL-2 [126]. Similar effects for CXCR3 ligand overexpression were observed in mesothelioma in which CXCL11-expressing vaccinia virus augmented the effects of CAR T-cell therapy in mice [133]. In contrast, the findings from CXCR3 targeted therapy in breast cancer are more conflicted. On the one hand, the overexpression of CXCL9, 10 and 11 produced markedly reduced metastasis in various breast cancer animal models [80, 89–91]. Moreover, CXCR3 signaling was required for the anti-tumor effect of a HER2/ CD3 bi-specific antibody [134]. In contrast to this, genetic ablation of CXCR3 significantly suppressed breast cancer metastasis [72, 74]. Consistently, Zhu et al. demonstrated that CXCR3 inhibition with AMG487 suppressed metastasis and augmented anti-tumor immune response [74]. This contrast in the setting of breast cancer highlights the complexity of the role of CXCR3 in cancer dissemination. Therapeutic approaches of targeting CXCR3 moving forward must account for the differential functions of CXCR3 in different tumors, as well as the significance of CXCR3 expression in different cell compartments within the same tumor. Finally, the development of splice variant-specific inhibitors and agonist of CXCR3 will be an essential component for unlocking the therapeutic potential of the CXCR3 axis.
5. Conclusion
The functions of CXCR3 in metastasis are incredibly nuanced. This complexity arises from the opposing roles of CXCR3 splice variants coupled with diverse patterns of expression in multiple different cell types (Figure 2). In experimentation, such complexity manifests itself in the form of seemingly incongruous findings from studies conducted in similar patient populations and models. However, analysis of the mechanism governing the observed phenotypes in a splice variant-, cell type-, and metastasis phase-specific manner reveals trends that appear to be consistent across cancers. From these studies, three guiding principles emerge. 1) CXCR3A signaling in cancer cells promotes the invasion and migration of these cells, thus representing a metastasis-promoting function of CXCR3, 2) CXCR3B-mediated inhibition of angiogenesis suppresses the formation of metastatic lesions, and 3) immune responses associated with CXCR3A attenuate the formation of metastasis. Thus, the role of CXCR3 in cancer metastasis is a balance of pro- and anti-metastatic activities and the extent of involvement of these activities in metastasis. This context-dependence is frequently magnified in model systems used to study metastasis. The presence of an intact immune system, use of tail-vein injection models versus spontaneous or orthotopic implantation models, and variable CXCR3 splice variant expression in cell lines exemplify features that may cause outcomes to differ based on the alterations of the balance of pro- and anti-metastatic effects of CXCR3 or host features that alter the relative importance of those effects.
Additional research on the contributions of CXCR3 to the metastatic process is still needed. For example, studies of the contribution of CXCR3 to tumor cell intravasation, survival in circulation, extravasation, and early events in colonization are underrepresented in the literature. The corollary of this is that CXCR3 may have roles in each of these aspects that represent critical aspects of its contribution to a cancer’s metastatic process. Similarly, the differential roles of CXCR3 splice variants remain poorly characterized in numerous cancers. As a result, the understanding of the roles of CXCR3 in those cancers suffers due to unexplained conflicting results.
Finally, one must ask if the CXCR3 axis is a therapeutic target. It is undeniable that the studies discussed here show some promise for targeting CXCR3 in the setting of malignancy. Despite this, CXCR3 and its ligands represent a complex biological system with pleiotropic effects, including pro- and anti-cancer activities. Moving forward, research must elucidate the specific contexts in which the pro- and anti-cancer properties of CXCR3 predominate. These studies must define not only the disease settings but also specific patient populations within a disease setting in which the CXCR3 activity acts to suppress malignant progression. Moreover, understanding the contributions of the CXCR3 splice variants is critical as targeting a specific variant will likely to increase therapeutic opportunities by promoting anti-cancer activity or suppressing pro-cancer activity while leaving the other functions of the signaling axis intact.
In conclusion, the CXCR3 signaling axis is intimately involved in several aspects of metastasis in a wide variety of cancers. Despite extensive study, there is no overarching conclusion as to whether CXCR3 promotes or inhibits metastasis. This ambiguity results from multiple factors that influence CXCR3 activity and the importance of those activities within tumors. Mechanistically, CXCR3 has several functions that appear consistently throughout the literature. These include the pro-invasive and/or migratory effects of CXCR3 that can promote malignant cell dissemination, while augmented anti-tumor immune response and suppression of angiogenesis may inhibit this same process. Moving forward, specific experimentation to further delineate the roles of CXCR3 splice variants in multiple phases of metastasis is required to further our understanding of the axis and reconcile seemingly opposing results present in the literature.
Acknowledgements:
The authors/work, in part, were supported by the NIH grants (F30 CA225117, F31 CA243469, R21 CA223429, R21 AA026428, R44 CA235991, P01 CA217798, P30 CA036727, U54 GM115458, R01 CA183459, R01 CA210637, P30 CA036727, and R01 CA228524).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: SKB is one of the co-founders of Sanguine Diagnostics and Therapeutics, Inc. The other authors declare no competing interests.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Siegel RL, Miller KD, Jemal A, Cancer statistics, 2020, CA Cancer J Clin 70(1) (2020) 7–30. [DOI] [PubMed] [Google Scholar]
- [2].Dillekas H, Rogers MS, Straume O, Are 90% of deaths from cancer caused by metastases?, Cancer Med 8(12) (2019) 5574–5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Steeg PS, Tumor metastasis: mechanistic insights and clinical challenges, Nat Med 12(8) (2006) 895–904. [DOI] [PubMed] [Google Scholar]
- [4].Gupta GP, Massague J, Cancer metastasis: building a framework, Cell 127(4) (2006) 679–95. [DOI] [PubMed] [Google Scholar]
- [5].Valastyan S, Weinberg RA, Tumor metastasis: molecular insights and evolving paradigms, Cell 147(2) (2011) 275–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Labelle M, Begum S, Hynes RO, Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis, Cancer Cell 20(5) (2011) 576–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Haemmerle M, Taylor ML, Gutschner T, Pradeep S, Cho MS, Sheng J, Lyons YM, Nagaraja AS, Dood RL, Wen Y, Mangala LS, Hansen JM, Rupaimoole R, Gharpure KM, Rodriguez-Aguayo C, Yim SY, Lee JS, Ivan C, Hu W, Lopez-Berestein G, Wong ST, Karlan BY, Levine DA, Liu J, Afshar-Kharghan V, Sood AK, Platelets reduce anoikis and promote metastasis by activating YAP1 signaling, Nat Commun 8(1) (2017) 310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Barnes JM, Nauseef JT, Henry MD, Resistance to fluid shear stress is a conserved biophysical property of malignant cells, PLoS One 7(12) (2012) e50973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Moose DL, Krog BL, Kim TH, Zhao L, Williams-Perez S, Burke G, Rhodes L, Vanneste M, Breheny P, Milhem M, Stipp CS, Rowat AC, Henry MD, Cancer Cells Resist Mechanical Destruction in Circulation via RhoA/Actomyosin-Dependent Mechano-Adaptation, Cell Rep 30(11) (2020) 3864–3874 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Palumbo JS, Talmage KE, Massari JV, La Jeunesse CM, Flick MJ, Kombrinck KW, Hu Z, Barney KA, Degen JL, Tumor cell-associated tissue factor and circulating hemostatic factors cooperate to increase metastatic potential through natural killer cell-dependent and-independent mechanisms, Blood 110(1) (2007) 133–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Nieswandt B, Hafner M, Echtenacher B, Mannel DN, Lysis of tumor cells by natural killer cells in mice is impeded by platelets, Cancer Res 59(6) (1999) 1295–300. [PubMed] [Google Scholar]
- [12].Sallusto F, Lenig D, Mackay CR, Lanzavecchia A, Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes, J Exp Med 187(6) (1998) 875–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Cole KE, Strick CA, Paradis TJ, Ogborne KT, Loetscher M, Gladue RP, Lin W, Boyd JG, Moser B, Wood DE, Sahagan BG, Neote K, Interferon-inducible T cell alpha chemoattractant (I-TAC): a novel non-ELR CXC chemokine with potent activity on activated T cells through selective high affinity binding to CXCR3, J Exp Med 187(12) (1998) 2009–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lasagni L, Francalanci M, Annunziato F, Lazzeri E, Giannini S, Cosmi L, Sagrinati C, Mazzinghi B, Orlando C, Maggi E, Marra F, Romagnani S, Serio M, Romagnani P, An alternatively spliced variant of CXCR3 mediates the inhibition of endothelial cell growth induced by IP-10, Mig, and I-TAC, and acts as functional receptor for platelet factor 4, J Exp Med 197(11) (2003) 1537–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Thompson BD, Jin Y, Wu KH, Colvin RA, Luster AD, Birnbaumer L, Wu MX, Inhibition of G alpha i2 activation by G alpha i3 in CXCR3-mediated signaling, J Biol Chem 282(13) (2007) 9547–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zhang C, Li Z, Xu L, Che X, Wen T, Fan Y, Li C, Wang S, Cheng Y, Wang X, Qu X, Liu Y, CXCL9/10/11, a regulator of PD-L1 expression in gastric cancer, BMC Cancer 18(1) (2018) 462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Berchiche YA, Sakmar TP, CXC Chemokine Receptor 3 Alternative Splice Variants Selectively Activate Different Signaling Pathways, Mol Pharmacol 90(4) (2016) 483–95. [DOI] [PubMed] [Google Scholar]
- [18].Smit MJ, Verdijk P, van der Raaij-Helmer EM, Navis M, Hensbergen PJ, Leurs R, Tensen CP, CXCR3-mediated chemotaxis of human T cells is regulated by a Gi- and phospholipase C-dependent pathway and not via activation of MEK/p44/p42 MAPK nor Akt/PI-3 kinase, Blood 102(6) (2003) 1959–65. [DOI] [PubMed] [Google Scholar]
- [19].Newton P, O’Boyle G, Jenkins Y, Ali S, Kirby JA, T cell extravasation: demonstration of synergy between activation of CXCR3 and the T cell receptor, Mol Immunol 47(2–3) (2009) 485–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Dar WA, Knechtle SJ, CXCR3-mediated T-cell chemotaxis involves ZAP-70 and is regulated by signalling through the T-cell receptor, Immunology 120(4) (2007) 467–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Smith JS, Nicholson LT, Suwanpradid J, Glenn RA, Knape NM, Alagesan P, Gundry JN, Wehrman TS, Atwater AR, Gunn MD, MacLeod AS, Rajagopal S, Biased agonists of the chemokine receptor CXCR3 differentially control chemotaxis and inflammation, Sci Signal 11(555) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bromley SK, Peterson DA, Gunn MD, Dustin ML, Cutting edge: hierarchy of chemokine receptor and TCR signals regulating T cell migration and proliferation, J Immunol 165(1) (2000) 15–9. [DOI] [PubMed] [Google Scholar]
- [23].Quan F, Thomas L, Forte M, Drosophila stimulatory G protein alpha subunit activates mammalian adenylyl cyclase but interacts poorly with mammalian receptors: implications for receptor-G protein interaction, Proc Natl Acad Sci U S A 88(5) (1991) 1898–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Datta D, Banerjee P, Gasser M, Waaga-Gasser AM, Pal S, CXCR3-B can mediate growth-inhibitory signals in human renal cancer cells by down-regulating the expression of heme oxygenase-1, J Biol Chem 285(47) (2010) 36842–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Balan M, Pal S, A novel CXCR3-B chemokine receptor-induced growth-inhibitory signal in cancer cells is mediated through the regulation of Bach-1 protein and Nrf2 protein nuclear translocation, J Biol Chem 289(6) (2014) 3126–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Clapham DE, Neer EJ, G protein beta gamma subunits, Annu Rev Pharmacol Toxicol 37 (1997) 167–203. [DOI] [PubMed] [Google Scholar]
- [27].Puchert M, Obst J, Koch C, Zieger K, Engele J, CXCL11 promotes tumor progression by the biased use of the chemokine receptors CXCR3 and CXCR7, Cytokine 125 (2020) 154809. [DOI] [PubMed] [Google Scholar]
- [28].Zhou H, Wu J, Wang T, Zhang X, Liu D, CXCL10/CXCR3 axis promotes the invasion of gastric cancer via PI3K/AKT pathway-dependent MMPs production, Biomed Pharmacother 82 (2016) 479–88. [DOI] [PubMed] [Google Scholar]
- [29].Kawada K, Hosogi H, Sonoshita M, Sakashita H, Manabe T, Shimahara Y, Sakai Y, Takabayashi A, Oshima M, Taketo MM, Chemokine receptor CXCR3 promotes colon cancer metastasis to lymph nodes, Oncogene 26(32) (2007) 4679–88. [DOI] [PubMed] [Google Scholar]
- [30].Wu Z, Han X, Yan J, Pan Y, Gong J, Di J, Cheng Z, Jin Z, Wang Z, Zheng Q, Wang Y, The prognostic significance of chemokine receptor CXCR3 expression in colorectal carcinoma, Biomed Pharmacother 66(5) (2012) 373–7. [DOI] [PubMed] [Google Scholar]
- [31].Toiyama Y, Fujikawa H, Kawamura M, Matsushita K, Saigusa S, Tanaka K, Inoue Y, Uchida K, Mohri Y, Kusunoki M, Evaluation of CXCL10 as a novel serum marker for predicting liver metastasis and prognosis in colorectal cancer, Int J Oncol 40(2) (2012) 560–6. [DOI] [PubMed] [Google Scholar]
- [32].Dimberg J, Skarstedt M, Lofgren S, Zar N, Matussek A, Protein expression and gene polymorphism of CXCL10 in patients with colorectal cancer, Biomed Rep 2(3) (2014) 340–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Utsumi T, Suyama T, Imamura Y, Fuse M, Sakamoto S, Nihei N, Ueda T, Suzuki H, Seki N, Ichikawa T, The association of CXCR3 and renal cell carcinoma metastasis, J Urol 192(2) (2014) 567–74. [DOI] [PubMed] [Google Scholar]
- [34].Rezakhaniha B, Dormanesh B, Pirasteh H, Yahaghi E, Masoumi B, Ziari K, Rahmani O, Immunohistochemical distinction of metastases of renal cell carcinoma with molecular analysis of overexpression of the chemokines CXCR2 and CXCR3 as independent positive prognostic factors for the tumorigenesis, IUBMB Life 68(8) (2016) 629–33. [DOI] [PubMed] [Google Scholar]
- [35].Lau TS, Chung TK, Cheung TH, Chan LK, Cheung LW, Yim SF, Siu NS, Lo KW, Yu MM, Kulbe H, Balkwill FR, Kwong J, Cancer cell-derived lymphotoxin mediates reciprocal tumour-stromal interactions in human ovarian cancer by inducing CXCL11 in fibroblasts, J Pathol 232(1) (2014) 43–56. [DOI] [PubMed] [Google Scholar]
- [36].Quemener C, Baud J, Boye K, Dubrac A, Billottet C, Soulet F, Darlot F, Dumartin L, Sire M, Grepin R, Daubon T, Rayne F, Wodrich H, Couvelard A, Pineau R, Schilling M, Castronovo V, Sue SC, Clarke K, Lomri A, Khatib AM, Hagedorn M, Prats H, Bikfalvi A, Dual Roles for CXCL4 Chemokines and CXCR3 in Angiogenesis and Invasion of Pancreatic Cancer, Cancer Res 76(22) (2016) 6507–6519. [DOI] [PubMed] [Google Scholar]
- [37].Hirth M, Gandla J, Hoper C, Gaida MM, Agarwal N, Simonetti M, Demir A, Xie Y, Weiss C, Michalski CW, Hackert T, Ebert MP, Kuner R, CXCL10 and CCL21 Promote Migration of Pancreatic Cancer Cells Toward Sensory Neurons and Neural Remodeling in Tumors in Mice, Associated With Pain in Patients, Gastroenterology (2020). [DOI] [PubMed] [Google Scholar]
- [38].Li Z, Liu J, Li L, Shao S, Wu J, Bian L, He Y, Epithelial mesenchymal transition induced by the CXCL9/CXCR3 axis through AKT activation promotes invasion and metastasis in tongue squamous cell carcinoma, Oncol Rep 39(3) (2018) 1356–1368. [DOI] [PubMed] [Google Scholar]
- [39].Pu Y, Li S, Zhang C, Bao Z, Yang Z, Sun L, High expression of CXCR3 is an independent prognostic factor in glioblastoma patients that promotes an invasive phenotype, J Neurooncol 122(1) (2015) 43–51. [DOI] [PubMed] [Google Scholar]
- [40].Pradelli E, Karimdjee-Soilihi B, Michiels JF, Ricci JE, Millet MA, Vandenbos F, Sullivan TJ, Collins TL, Johnson MG, Medina JC, Kleinerman ES, Schmid-Alliana A, Schmid-Antomarchi H, Antagonism of chemokine receptor CXCR3 inhibits osteosarcoma metastasis to lungs, Int J Cancer 125(11) (2009) 2586–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kawada K, Sonoshita M, Sakashita H, Takabayashi A, Yamaoka Y, Manabe T, Inaba K, Minato N, Oshima M, Taketo MM, Pivotal role of CXCR3 in melanoma cell metastasis to lymph nodes, Cancer Res 64(11) (2004) 4010–7. [DOI] [PubMed] [Google Scholar]
- [42].Cambien B, Karimdjee BF, Richard-Fiardo P, Bziouech H, Barthel R, Millet MA, Martini V, Birnbaum D, Scoazec JY, Abello J, Al Saati T, Johnson MG, Sullivan TJ, Medina JC, Collins TL, Schmid-Alliana A, Schmid-Antomarchi H, Organ-specific inhibition of metastatic colon carcinoma by CXCR3 antagonism, Br J Cancer 100(11) (2009) 1755–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Doron H, Amer M, Ershaid N, Blazquez R, Shani O, Lahav TG, Cohen N, Adler O, Hakim Z, Pozzi S, Scomparin A, Cohen J, Yassin M, Monteran L, Grossman R, Tsarfaty G, Luxenburg C, Satchi-Fainaro R, Pukrop T, Erez N, Inflammatory Activation of Astrocytes Facilitates Melanoma Brain Tropism via the CXCL10-CXCR3 Signaling Axis, Cell Rep 28(7) (2019) 1785–1798 e6. [DOI] [PubMed] [Google Scholar]
- [44].Goldberg-Bittman L, Sagi-Assif O, Meshel T, Nevo I, Levy-Nissenbaum O, Yron I, Witz IP, Ben-Baruch A, Cellular characteristics of neuroblastoma cells: regulation by the ELR--CXC chemokine CXCL10 and expression of a CXCR3-like receptor, Cytokine 29(3) (2005) 105–17. [DOI] [PubMed] [Google Scholar]
- [45].Antonicelli F, Lorin J, Kurdykowski S, Gangloff SC, Le Naour R, Sallenave JM, Hornebeck W, Grange F, Bernard P, CXCL10 reduces melanoma proliferation and invasiveness in vitro and in vivo, Br J Dermatol 164(4) (2011) 720–8. [DOI] [PubMed] [Google Scholar]
- [46].Kobayashi H, Nobeyama Y, Nakagawa H, Tumor-suppressive effects of natural-type interferon-beta through CXCL10 in melanoma, Biochem Biophys Res Commun 464(2) (2015) 416–21. [DOI] [PubMed] [Google Scholar]
- [47].Wu Q, Dhir R, Wells A, Altered CXCR3 isoform expression regulates prostate cancer cell migration and invasion, Mol Cancer 11 (2012) 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Shen D, Cao X, Potential role of CXCR3 in proliferation and invasion of prostate cancer cells, Int J Clin Exp Pathol 8(7) (2015) 8091–8. [PMC free article] [PubMed] [Google Scholar]
- [49].Zhang M, Guan J, Huo YL, Song YS, Chen LZ, Downregulation of serum CXCL4L1 predicts progression and poor prognosis in prostate cancer patients treated by radical prostatectomy, Asian J Androl 21(4) (2019) 387–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Hu S, Li L, Yeh S, Cui Y, Li X, Chang HC, Jin J, Chang C, Infiltrating T cells promote prostate cancer metastasis via modulation of FGF11-->miRNA-541-->androgen receptor (AR)-->MMP9 signaling, Mol Oncol 9(1) (2015) 44–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Jenkins MH, Brinckerhoff CE, Mullins DW, CXCR3 signaling in BRAFWT melanoma increases IL-8 expression and tumorigenicity, PLoS One 10(3) (2015) e0121140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Wightman SC, Uppal A, Pitroda SP, Ganai S, Burnette B, Stack M, Oshima G, Khan S, Huang X, Posner MC, Weichselbaum RR, Khodarev NN, Oncogenic CXCL10 signalling drives metastasis development and poor clinical outcome, Br J Cancer 113(2) (2015) 327–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Monteagudo C, Martin JM, Jorda E, Llombart-Bosch A, CXCR3 chemokine receptor immunoreactivity in primary cutaneous malignant melanoma: correlation with clinicopathological prognostic factors, J Clin Pathol 60(6) (2007) 596–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Harlin H, Meng Y, Peterson AC, Zha Y, Tretiakova M, Slingluff C, McKee M, Gajewski TF, Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment, Cancer Res 69(7) (2009) 3077–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Chheda ZS, Sharma RK, Jala VR, Luster AD, Haribabu B, Chemoattractant Receptors BLT1 and CXCR3 Regulate Antitumor Immunity by Facilitating CD8+ T Cell Migration into Tumors, J Immunol 197(5) (2016) 2016–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Xiong TF, Pan FQ, Liang Q, Luo R, Li D, Mo H, Zhou X, Prognostic value of the expression of chemokines and their receptors in regional lymph nodes of melanoma patients, J Cell Mol Med (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Kunz M, Toksoy A, Goebeler M, Engelhardt E, Brocker E, Gillitzer R, Strong expression of the lymphoattractant C-X-C chemokine Mig is associated with heavy infiltration of T cells in human malignant melanoma, J Pathol 189(4) (1999) 552–8. [DOI] [PubMed] [Google Scholar]
- [58].Keyser J, Schultz J, Ladell K, Elzaouk L, Heinzerling L, Pavlovic J, Moelling K, IP-10-encoding plasmid DNA therapy exhibits anti-tumor and anti-metastatic efficiency, Exp Dermatol 13(6) (2004) 380–90. [DOI] [PubMed] [Google Scholar]
- [59].Mirzaei H, Salehi H, Oskuee RK, Mohammadpour A, Mirzaei HR, Sharifi MR, Salarinia R, Darani HY, Mokhtari M, Masoudifar A, Sahebkar A, Salehi R, Jaafari MR, The therapeutic potential of human adipose-derived mesenchymal stem cells producing CXCL10 in a mouse melanoma lung metastasis model, Cancer Lett 419 (2018) 30–39. [DOI] [PubMed] [Google Scholar]
- [60].Murakami T, Kawada K, Iwamoto M, Akagami M, Hida K, Nakanishi Y, Kanda K, Kawada M, Seno H, Taketo MM, Sakai Y, The role of CXCR3 and CXCR4 in colorectal cancer metastasis, Int J Cancer 132(2) (2013) 276–87. [DOI] [PubMed] [Google Scholar]
- [61].Jin J, Zhang Z, Wang H, Zhan Y, Li G, Yang H, Fei Z, Xu Y, Li W, CXCR3 expression in colorectal cancer cells enhanced invasion through preventing CXCR4 internalization, Exp Cell Res 371(1) (2018) 162–174. [DOI] [PubMed] [Google Scholar]
- [62].Gao YJ, Liu L, Li S, Yuan GF, Li L, Zhu HY, Cao GY, Down-regulation of CXCL11 inhibits colorectal cancer cell growth and epithelial-mesenchymal transition, Onco Targets Ther 11 (2018) 7333–7343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Shin SY, Hyun J, Lim Y, Lee YH, 3’-Chloro-5,7-dimethoxyisoflavone inhibits TNFalpha-induced CXCL10 gene transcription by suppressing the NF-kappaB pathway in HCT116 human colon cancer cells, Int Immunopharmacol 11(12) (2011) 2104–11. [DOI] [PubMed] [Google Scholar]
- [64].Li H, Rong S, Chen C, Fan Y, Chen T, Wang Y, Chen D, Yang C, Yang J, Disparate roles of CXCR3A and CXCR3B in regulating progressive properties of colorectal cancer cells, Mol Carcinog 58(2) (2019) 171–184. [DOI] [PubMed] [Google Scholar]
- [65].Li Y, Reader JC, Ma X, Kundu N, Kochel T, Fulton AM, Divergent roles of CXCR3 isoforms in promoting cancer stem-like cell survival and metastasis, Breast Cancer Res Treat 149(2) (2015) 403–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Bai M, Chen X, Ba YI, CXCL10/CXCR3 overexpression as a biomarker of poor prognosis in patients with stage II colorectal cancer, Mol Clin Oncol 4(1) (2016) 23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Wu Z, Huang X, Han X, Li Z, Zhu Q, Yan J, Yu S, Jin Z, Wang Z, Zheng Q, Wang Y, The chemokine CXCL9 expression is associated with better prognosis for colorectal carcinoma patients, Biomed Pharmacother 78 (2016) 8–13. [DOI] [PubMed] [Google Scholar]
- [68].Jiang Z, Xu Y, Cai S, CXCL10 expression and prognostic significance in stage II and III colorectal cancer, Mol Biol Rep 37(6) (2010) 3029–36. [DOI] [PubMed] [Google Scholar]
- [69].Musha H, Ohtani H, Mizoi T, Kinouchi M, Nakayama T, Shiiba K, Miyagawa K, Nagura H, Yoshie O, Sasaki I, Selective infiltration of CCR5(+)CXCR3(+) T lymphocytes in human colorectal carcinoma, Int J Cancer 116(6) (2005) 949–56. [DOI] [PubMed] [Google Scholar]
- [70].Zumwalt TJ, Arnold M, Goel A, Boland CR, Active secretion of CXCL10 and CCL5 from colorectal cancer microenvironments associates with GranzymeB+ CD8+ T-cell infiltration, Oncotarget 6(5) (2015) 2981–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Kikuchi N, Ye J, Hirakawa J, Kawashima H, Forced Expression of CXCL10 Prevents Liver Metastasis of Colon Carcinoma Cells by the Recruitment of Natural Killer Cells, Biol Pharm Bull 42(1) (2019) 57–65. [DOI] [PubMed] [Google Scholar]
- [72].Walser TC, Rifat S, Ma X, Kundu N, Ward C, Goloubeva O, Johnson MG, Medina JC, Collins TL, Fulton AM, Antagonism of CXCR3 inhibits lung metastasis in a murine model of metastatic breast cancer, Cancer Res 66(15) (2006) 7701–7. [DOI] [PubMed] [Google Scholar]
- [73].Ma X, Norsworthy K, Kundu N, Rodgers WH, Gimotty PA, Goloubeva O, Lipsky M, Li Y, Holt D, Fulton A, CXCR3 expression is associated with poor survival in breast cancer and promotes metastasis in a murine model, Mol Cancer Ther 8(3) (2009) 490–8. [DOI] [PubMed] [Google Scholar]
- [74].Zhu G, Yan HH, Pang Y, Jian J, Achyut BR, Liang X, Weiss JM, Wiltrout RH, Hollander MC, Yang L, CXCR3 as a molecular target in breast cancer metastasis: inhibition of tumor cell migration and promotion of host anti-tumor immunity, Oncotarget 6(41) (2015) 43408–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Chen F, Yin S, Niu L, Luo J, Wang B, Xu Z, Yang G, Expression of the Chemokine Receptor CXCR3 Correlates with Dendritic Cell Recruitment and Prognosis in Gastric Cancer, Genet Test Mol Biomarkers 22(1) (2018) 35–42. [DOI] [PubMed] [Google Scholar]
- [76].Li K, Zhu Z, Luo J, Fang J, Zhou H, Hu M, Maskey N, Yang G, Impact of chemokine receptor CXCR3 on tumor-infiltrating lymphocyte recruitment associated with favorable prognosis in advanced gastric cancer, Int J Clin Exp Pathol 8(11) (2015) 14725–32. [PMC free article] [PubMed] [Google Scholar]
- [77].Kavanagh ME, Conroy MJ, Clarke NE, Gilmartin NT, Feighery R, MacCarthy F, O’Toole D, Ravi N, Reynolds JV, J OS, Lysaght J, Altered T Cell Migratory Capacity in the Progression from Barrett Oesophagus to Oesophageal Adenocarcinoma, Cancer Microenviron 12(1) (2019) 57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Giese NA, Raykov Z, DeMartino L, Vecchi A, Sozzani S, Dinsart C, Cornelis JJ, Rommelaere J, Suppression of metastatic hemangiosarcoma by a parvovirus MVMp vector transducing the IP-10 chemokine into immunocompetent mice, Cancer Gene Ther 9(5) (2002) 432–42. [DOI] [PubMed] [Google Scholar]
- [79].Tang Y, Gu Z, Fu Y, Wang J, CXCR3 from chemokine receptor family correlates with immune infiltration and predicts poor survival in osteosarcoma, Biosci Rep 39(11) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Walser TC, Ma X, Kundu N, Dorsey R, Goloubeva O, Fulton AM, Immune-mediated modulation of breast cancer growth and metastasis by the chemokine Mig (CXCL9) in a murine model, J Immunother 30(5) (2007) 490–8. [DOI] [PubMed] [Google Scholar]
- [81].Ling CC, Ng KT, Shao Y, Geng W, Xiao JW, Liu H, Li CX, Liu XB, Ma YY, Yeung WH, Qi X, Yu J, Wong N, Zhai Y, Chan SC, Poon RT, Lo CM, Man K, Post-transplant endothelial progenitor cell mobilization via CXCL10/CXCR3 signaling promotes liver tumor growth, J Hepatol 60(1) (2014) 103–9. [DOI] [PubMed] [Google Scholar]
- [82].Li CX, Ling CC, Shao Y, Xu A, Li XC, Ng KT, Liu XB, Ma YY, Qi X, Liu H, Liu J, Yeung OW, Yang XX, Liu QS, Lam YF, Zhai Y, Lo CM, Man K, CXCL10/CXCR3 signaling mobilized-regulatory T cells promote liver tumor recurrence after transplantation, J Hepatol 65(5) (2016) 944–952. [DOI] [PubMed] [Google Scholar]
- [83].Wei Y, Lao XM, Xiao X, Wang XY, Wu ZJ, Zeng QH, Wu CY, Wu RQ, Chen ZX, Zheng L, Li B, Kuang DM, Plasma Cell Polarization to the Immunoglobulin G Phenotype in Hepatocellular Carcinomas Involves Epigenetic Alterations and Promotes Hepatoma Progression in Mice, Gastroenterology 156(6) (2019) 1890–1904 e16. [DOI] [PubMed] [Google Scholar]
- [84].Cannon A, Thompson CM, Maurer HC, Atri P, Bhatia R, West S, Ghersi D, Olive KP, Kumar S, Batra SK, CXCR3 and Cognate Ligands are Associated with Immune Cell Alteration and Aggressiveness of Pancreatic Ductal Adenocarcinoma, Clin Cancer Res 26(22) (2020) 6051–6063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Lunardi S, Jamieson NB, Lim SY, Griffiths KL, Carvalho-Gaspar M, Al-Assar O, Yameen S, Carter RC, McKay CJ, Spoletini G, D’Ugo S, Silva MA, Sansom OJ, Janssen KP, Muschel RJ, Brunner TB, IP-10/CXCL10 induction in human pancreatic cancer stroma influences lymphocytes recruitment and correlates with poor survival, Oncotarget 5(22) (2014) 11064–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Lunardi S, Lim SY, Muschel RJ, Brunner TB, IP-10/CXCL10 attracts regulatory T cells: Implication for pancreatic cancer, Oncoimmunology 4(9) (2015) e1027473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Gao HF, Cheng CS, Tang J, Li Y, Chen H, Meng ZQ, Chen Z, Chen LY, CXCL9 chemokine promotes the progression of human pancreatic adenocarcinoma through STAT3-dependent cytotoxic T lymphocyte suppression, Aging (Albany NY) 12(1) (2020) 502–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Mikucki ME, Fisher DT, Matsuzaki J, Skitzki JJ, Gaulin NB, Muhitch JB, Ku AW, Frelinger JG, Odunsi K, Gajewski TF, Luster AD, Evans SS, Non-redundant requirement for CXCR3 signalling during tumoricidal T-cell trafficking across tumour vascular checkpoints, Nat Commun 6 (2015) 7458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Yang X, Chu Y, Wang Y, Guo Q, Xiong S, Vaccination with IFN-inducible T cell alpha chemoattractant (ITAC) gene-modified tumor cell attenuates disseminated metastases of circulating tumor cells, Vaccine 24(15) (2006) 2966–74. [DOI] [PubMed] [Google Scholar]
- [90].Taslimi Y, Zahedifard F, Habibzadeh S, Taheri T, Abbaspour H, Sadeghipour A, Mohit E, Rafati S, Antitumor Effect of IP-10 by Using Two Different Approaches: Live Delivery System and Gene Therapy, J Breast Cancer 19(1) (2016) 34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Zhang N, Yang Y, Cheng L, Zhang XM, Zhang S, Wang W, Liu SY, Wang SY, Wang RB, Xu WJ, Dai L, Yan N, Fan P, Dai LX, Tian HW, Liu L, Deng HX, Combination of Caspy2 and IP-10 gene therapy significantly improves therapeutic efficacy against murine malignant neoplasm growth and metastasis, Hum Gene Ther 23(8) (2012) 837–46. [DOI] [PubMed] [Google Scholar]
- [92].Jin WJ, Kim B, Kim D, Park Choo HY, Kim HH, Ha H, Lee ZH, NF-kappaB signaling regulates cell-autonomous regulation of CXCL10 in breast cancer 4T1 cells, Exp Mol Med 49(2) (2017) e295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Bronger H, Karge A, Dreyer T, Zech D, Kraeft S, Avril S, Kiechle M, Schmitt M, Induction of cathepsin B by the CXCR3 chemokines CXCL9 and CXCL10 in human breast cancer cells, Oncol Lett 13(6) (2017) 4224–4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Lee JH, Kim HN, Kim KO, Jin WJ, Lee S, Kim HH, Ha H, Lee ZH, CXCL10 promotes osteolytic bone metastasis by enhancing cancer outgrowth and osteoclastogenesis, Cancer Res 72(13) (2012) 3175–86. [DOI] [PubMed] [Google Scholar]
- [95].Kundu N, Ma X, Brox R, Fan X, Kochel T, Reader J, Tschammer N, Fulton A, The Chemokine Receptor CXCR3 Isoform B Drives Breast Cancer Stem Cells, Breast Cancer (Auckl) 13 (2019) 1178223419873628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Saahene RO, Wang J, Wang ML, Agbo E, Song H, The role of CXC chemokine ligand 4/CXC chemokine receptor 3-B in breast cancer progression, Biotech Histochem 94(1) (2019) 53–59. [DOI] [PubMed] [Google Scholar]
- [97].Jian J, Pang Y, Yan HH, Min Y, Achyut BR, Hollander MC, Lin PC, Liang X, Yang L, Platelet factor 4 is produced by subsets of myeloid cells in premetastatic lung and inhibits tumor metastasis, Oncotarget 8(17) (2017) 27725–27739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Windmuller C, Zech D, Avril S, Boxberg M, Dawidek T, Schmalfeldt B, Schmitt M, Kiechle M, Bronger H, CXCR3 mediates ascites-directed tumor cell migration and predicts poor outcome in ovarian cancer patients, Oncogenesis 6(5) (2017) e331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Redjimi N, Raffin C, Raimbaud I, Pignon P, Matsuzaki J, Odunsi K, Valmori D, Ayyoub M, CXCR3+ T regulatory cells selectively accumulate in human ovarian carcinomas to limit type I immunity, Cancer Res 72(17) (2012) 4351–60. [DOI] [PubMed] [Google Scholar]
- [100].Bronger H, Singer J, Windmuller C, Reuning U, Zech D, Delbridge C, Dorn J, Kiechle M, Schmalfeldt B, Schmitt M, Avril S, CXCL9 and CXCL10 predict survival and are regulated by cyclooxygenase inhibition in advanced serous ovarian cancer, Br J Cancer 115(5) (2016) 553–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Hu M, Li K, Maskey N, Xu Z, Yu F, Peng C, Li Y, Yang G, Overexpression of the chemokine receptor CXCR3 and its correlation with favorable prognosis in gastric cancer, Hum Pathol 46(12) (2015) 1872–80. [DOI] [PubMed] [Google Scholar]
- [102].Yang C, Zheng W, Du W, CXCR3A contributes to the invasion and metastasis of gastric cancer cells, Oncol Rep 36(3) (2016) 1686–92. [DOI] [PubMed] [Google Scholar]
- [103].Lan X, Xiao F, Ding Q, Liu J, Liu J, Li J, Zhang J, Tian DA, The effect of CXCL9 on the invasion ability of hepatocellular carcinoma through up-regulation of PREX2, J Mol Histol 45(6) (2014) 689–96. [DOI] [PubMed] [Google Scholar]
- [104].Ding Q, Xia Y, Ding S, Lu P, Sun L, Liu M, An alternatively spliced variant of CXCR3 mediates the metastasis of CD133+ liver cancer cells induced by CXCL9, Oncotarget 7(12) (2016) 14405–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Ren T, Zhu L, Cheng M, CXCL10 accelerates EMT and metastasis by MMP-2 in hepatocellular carcinoma, Am J Transl Res 9(6) (2017) 2824–2837. [PMC free article] [PubMed] [Google Scholar]
- [106].Potente M, Gerhardt H, Carmeliet P, Basic and therapeutic aspects of angiogenesis, Cell 146(6) (2011) 873–87. [DOI] [PubMed] [Google Scholar]
- [107].LaGory EL, Giaccia AJ, The ever-expanding role of HIF in tumour and stromal biology, Nat Cell Biol 18(4) (2016) 356–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Mehrad B, Keane MP, Strieter RM, Chemokines as mediators of angiogenesis, Thromb Haemost 97(5) (2007) 755–62. [PMC free article] [PubMed] [Google Scholar]
- [109].Belperio JA, Keane MP, Arenberg DA, Addison CL, Ehlert JE, Burdick MD, Strieter RM, CXC chemokines in angiogenesis, J Leukoc Biol 68(1) (2000) 1–8. [PubMed] [Google Scholar]
- [110].Kim S, Bakre M, Yin H, Varner JA, Inhibition of endothelial cell survival and angiogenesis by protein kinase A, J Clin Invest 110(7) (2002) 933–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Billottet C, Quemener C, Bikfalvi A, CXCR3, a double-edged sword in tumor progression and angiogenesis, Biochim Biophys Acta 1836(2) (2013) 287–95. [DOI] [PubMed] [Google Scholar]
- [112].Romagnani P, Annunziato F, Lasagni L, Lazzeri E, Beltrame C, Francalanci M, Uguccioni M, Galli G, Cosmi L, Maurenzig L, Baggiolini M, Maggi E, Romagnani S, Serio M, Cell cycle-dependent expression of CXC chemokine receptor 3 by endothelial cells mediates angiostatic activity, J Clin Invest 107(1) (2001) 53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Gentilini G, Kirschbaum NE, Augustine JA, Aster RH, Visentin GP, Inhibition of human umbilical vein endothelial cell proliferation by the CXC chemokine, platelet factor 4 (PF4), is associated with impaired downregulation of p21(Cip1/WAF1), Blood 93(1) (1999) 25–33. [PubMed] [Google Scholar]
- [114].Gartel AL, Tyner AL, The role of the cyclin-dependent kinase inhibitor p21 in apoptosis, Mol Cancer Ther 1(8) (2002) 639–49. [PubMed] [Google Scholar]
- [115].Struyf S, Burdick MD, Peeters E, Van den Broeck K, Dillen C, Proost P, Van Damme J, Strieter RM, Platelet factor-4 variant chemokine CXCL4L1 inhibits melanoma and lung carcinoma growth and metastasis by preventing angiogenesis, Cancer Res 67(12) (2007) 5940–8. [DOI] [PubMed] [Google Scholar]
- [116].Arenberg DA, Kunkel SL, Polverini PJ, Morris SB, Burdick MD, Glass MC, Taub DT, Iannettoni MD, Whyte RI, Strieter RM, Interferon-gamma-inducible protein 10 (IP-10) is an angiostatic factor that inhibits human non-small cell lung cancer (NSCLC) tumorigenesis and spontaneous metastases, J Exp Med 184(3) (1996) 981–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Arenberg DA, White ES, Burdick MD, Strom SR, Strieter RM, Improved survival in tumor-bearing SCID mice treated with interferon-gamma-inducible protein 10 (IP-10/CXCL10), Cancer Immunol Immunother 50(10) (2001) 533–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Yamaguchi K, Ogawa K, Katsube T, Shimao K, Konno S, Shimakawa T, Yoshimatsu K, Naritaka Y, Yagawa H, Hirose K, Platelet factor 4 gene transfection into tumor cells inhibits angiogenesis, tumor growth and metastasis, Anticancer Res 25(2A) (2005) 847–51. [PubMed] [Google Scholar]
- [119].Prats AC, Van den Berghe L, Rayssac A, Ainaoui N, Morfoisse F, Pujol F, Legonidec S, Bikfalvi A, Prats H, Pyronnet S, Garmy-Susini B, CXCL4L1-fibstatin cooperation inhibits tumor angiogenesis, lymphangiogenesis and metastasis, Microvasc Res 89 (2013) 25–33. [DOI] [PubMed] [Google Scholar]
- [120].Cao Z, Baguley BC, Ching LM, Interferon-inducible protein 10 induction and inhibition of angiogenesis in vivo by the antitumor agent 5,6-dimethylxanthenone-4-acetic acid (DMXAA), Cancer Res 61(4) (2001) 1517–21. [PubMed] [Google Scholar]
- [121].Rewcastle GW, Atwell GJ, Baguley BC, Boyd M, Thomsen LL, Zhuang L, Denny WA, Potential antitumor agents. 63. Structure-activity relationships for side-chain analogues of the colon 38 active agent 9-oxo-9H-xanthene-4-acetic acid, J Med Chem 34(9) (1991) 2864–70. [DOI] [PubMed] [Google Scholar]
- [122].Li G, Tian L, Hou JM, Ding ZY, He QM, Feng P, Wen YJ, Xiao F, Yao B, Zhang R, Peng F, Jiang Y, Luo F, Zhao X, Zhang L, Zhou Q, Wei YQ, Improved therapeutic effectiveness by combining recombinant CXC chemokine ligand 10 with Cisplatin in solid tumors, Clin Cancer Res 11(11) (2005) 4217–24. [DOI] [PubMed] [Google Scholar]
- [123].Angiolillo AL, Sgadari C, Taub DD, Liao F, Farber JM, Maheshwari S, Kleinman HK, Reaman GH, Tosato G, Human interferon-inducible protein 10 is a potent inhibitor of angiogenesis in vivo, J Exp Med 182(1) (1995) 155–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Struyf S, Burdick MD, Proost P, Van Damme J, Strieter RM, Platelets release CXCL4L1, a nonallelic variant of the chemokine platelet factor-4/CXCL4 and potent inhibitor of angiogenesis, Circ Res 95(9) (2004) 855–7. [DOI] [PubMed] [Google Scholar]
- [125].Maekawa S, Iwasaki A, Shirakusa T, Kawakami T, Yanagisawa J, Tanaka T, Shibaguchi H, Kinugasa T, Kuroki M, Kuroki M, Association between the expression of chemokine receptors CCR7 and CXCR3, and lymph node metastatic potential in lung adenocarcinoma, Oncol Rep 19(6) (2008) 1461–8. [PubMed] [Google Scholar]
- [126].Pan J, Burdick MD, Belperio JA, Xue YY, Gerard C, Sharma S, Dubinett SM, Strieter RM, CXCR3/CXCR3 ligand biological axis impairs RENCA tumor growth by a mechanism of immunoangiostasis, J Immunol 176(3) (2006) 1456–64. [DOI] [PubMed] [Google Scholar]
- [127].Romero JM, Aptsiauri N, Vazquez F, Cozar JM, Canton J, Cabrera T, Tallada M, Garrido F, Ruiz-Cabello F, Analysis of the expression of HLA class I, proinflammatory cytokines and chemokines in primary tumors from patients with localized and metastatic renal cell carcinoma, Tissue Antigens 68(4) (2006) 303–10. [DOI] [PubMed] [Google Scholar]
- [128].Reckamp KL, Figlin RA, Moldawer N, Pantuck AJ, Belldegrun AS, Burdick MD, Strieter RM, Expression of CXCR3 on mononuclear cells and CXCR3 ligands in patients with metastatic renal cell carcinoma in response to systemic IL-2 therapy, J Immunother 30(4) (2007) 417–24. [DOI] [PubMed] [Google Scholar]
- [129].Datta D, Contreras AG, Grimm M, Waaga-Gasser AM, Briscoe DM, Pal S, Calcineurin inhibitors modulate CXCR3 splice variant expression and mediate renal cancer progression, J Am Soc Nephrol 19(12) (2008) 2437–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Han X, Wang Y, Sun J, Tan T, Cai X, Lin P, Tan Y, Zheng B, Wang B, Wang J, Xu L, Yu Z, Xu Q, Wu X, Gu Y, Role of CXCR3 signaling in response to anti-PD-1 therapy, EBioMedicine 48 (2019) 169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Wennerberg E, Kremer V, Childs R, Lundqvist A, CXCL10-induced migration of adoptively transferred human natural killer cells toward solid tumors causes regression of tumor growth in vivo, Cancer Immunol Immunother 64(2) (2015) 225–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Fujihara A, Kurooka M, Miki T, Kaneda Y, Intratumoral injection of inactivated Sendai virus particles elicits strong antitumor activity by enhancing local CXCL10 expression and systemic NK cell activation, Cancer Immunol Immunother 57(1) (2008) 73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Moon EK, Wang LS, Bekdache K, Lynn RC, Lo A, Thorne SH, Albelda SM, Intra-tumoral delivery of CXCL11 via a vaccinia virus, but not by modified T cells, enhances the efficacy of adoptive T cell therapy and vaccines, Oncoimmunology 7(3) (2018) e1395997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Li J, Ybarra R, Mak J, Herault A, De Almeida P, Arrazate A, Ziai J, Totpal K, Junttila MR, Walsh KB, Junttila TT, IFNgamma-induced Chemokines Are Required for CXCR3-mediated T-Cell Recruitment and Antitumor Efficacy of Anti-HER2/CD3 Bispecific Antibody, Clin Cancer Res 24(24) (2018) 6447–6458. [DOI] [PubMed] [Google Scholar]


