Abstract
Background:
Episodic memory deficits occur in alcohol use disorder (AUD), but their anatomical substrates remain in question. Although persistent memory impairment is classically associated with limbic circuitry disruption, learning and retrieval of new information also relies on frontal systems. Despite AUD vulnerability of frontal lobe integrity, relations between frontal regions and memory processes have been under-appreciated.
Methods:
Participants included 91 AUD (49 with a drug diagnosis history) and 36 controls. Verbal and visual episodic memory scores were age- and education-corrected. Structural magnetic resonance imaging (MRI) data yielded regional frontal lobe (precentral, superior, orbital, middle, inferior, supplemental motor, and medial) and total hippocampal volumes.
Results:
AUD were impaired on all memory scores and had smaller precentral frontal and hippocampal volumes than controls. Orbital, superior, and inferior frontal volumes and lifetime alcohol consumption were independent predictors of episodic memory in AUD. Selectivity was established with a double dissociation, where orbital frontal volume predicted verbal but not visual memory, whereas inferior frontal volumes predicted visual but not verbal memory. Further, superior frontal volumes predicted verbal memory in AUD alone, whereas orbital frontal volumes predicted verbal memory in AUD+drug abuse history.
Conclusions:
Selective relations among frontal subregions and episodic memory processes highlight the relevance of extra-limbic regions in mnemonic processes in AUD. Memory deficits resulting from frontal dysfunction, unlike the episodic memory impairment associated with limbic dysfunction, may be more amenable to recovery with cessation or reduction of alcohol misuse and may partially explain the heterogeneity in episodic memory abilities in AUD.
Keywords: alcohol, episodic memory, frontal volumes, orbitofrontal, drug abuse, MRI
1. Introduction
Memory deficits occur in individuals with alcohol use disorder (AUD). Generally, AUD-related deficits are more often observed for episodic memory (past personally experienced events that occurred at a specific time and in a specific place) (Beatty, Katzung, Moreland, & Nixon, 1995; Fama, Le Berre, Sassoon, et al., 2019; Glenn & Parsons, 1992; Oscar-Berman et al., 2014; Parsons & Nixon, 1993; Pitel et al., 2007; Sullivan, Mathalon, Ha, Zipursky, & Pfefferbaum, 1992; Tivis, Beatty, Nixon, & Parsons, 1995) than for semantic (knowing “what”/facts that do not have a specific time or place associated with the memory) (Fama et al., 2011) or implicit (knowing “how” to perform a task or skill) memory (Fama, Pfefferbaum, & Sullivan, 2004; Fama, Rosenbloom, Sassoon, Pfefferbaum, & Sullivan, 2012). Episodic memory deficits are heterogeneous in pattern and severity, ranging from mild in uncomplicated AUD to profound with global amnesia marking alcoholic Wernicke-Korsakoff’s syndrome (WKS) (Kopelman, 1995; Victor, Adams, & Colllins, 1989). Factors potentially contributing to the heterogeneity in episodic memory performance in uncomplicated AUD include comorbid non-alcohol substance misuse, which is highly prevalent in AUD (Fein, Smith, & Greenstein, 2012; Grant et al., 2015; Mon et al., 2014; Schmidt, Pennington, Cardoos, Durazzo, & Meyerhoff, 2017).
Although there has been debate about whether memory deficits in AUD are a consequence of executive dysfunction rather than a primary mnemonic deficit, ample evidence supports a genuine episodic memory deficit in individuals with AUD) (Nixon, Tivis, Jenkins, & Parsons, 1998; Oscar-Berman, 1990; Pitel et al., 2007; Pitel, Eustache, & Beaunieux, 2014). A study assessing memory processes (i.e., learning, storage, encoding, and retrieval) and executive function processes (i.e., organization, inhibition, flexibility, updating and integration) in detoxified individuals with AUD reported that, although deficits were observed in both cognitive domains, memory deficits were statistically independent of executive function deficits (Pitel et al., 2007). That study did reveal a relation between fluency and learning, suggesting that executive functions may play a role in mnemonic performance albeit not a predominant one. Thus, despite the relevance of executive functions to cognitive processes in enhancing memory performance, especially retrieval and strategic recall, they did not fully account for the mnemonic deficits of AUD.
Episodic memory processes have classically been associated with integrity of the hippocampus and associated medial temporal and diencephalic structures (Aggleton, 2014; Aggleton & Morris, 2018; Milner, 1958). These limbic structures are integral to Papez circuit. Hippocampal volume deficits occur in AUD (Beresford et al., 2006; Pfefferbaum et al., 2018; Sawyer et al., 2020) and in alcohol-related Wernicke-Korsakoff syndrome (Sullivan & Marsh, 2003). The profound anterograde memory impairment associated with WKS and its underlying neuropathology is in most cases permanent with little to no recovery (Kopelman, 1995). Such severe and permanent episodic memory impairment can occur in other neurological conditions, including herpes simplex encephalitis (Cermak & O'Connor, 1983) and medial temporal lobe epilepsy (Scoville & Milner, 1957), that involve Papez circuit.
The frontal lobes are also relevant to episodic learning and retrieval (Buckner, Kelley, & Petersen, 1999; Kopelman, 1991). Indeed, human (Frey & Petrides, 2000, 2002) and nonhuman primate (Meunier, Bachevalier, & Mishkin, 1997) studies have demonstrated the relevance of orbitofrontal regions to information encoding. Similarly, superior frontal regions associated with working memory processes have been implicated in supporting episodic memory processes (Nissim et al., 2016).
Frontally-based systems may be the most vulnerable of all brain regions to AUD (in vivo: (Durazzo & Meyerhoff, 2020; Oscar-Berman & Hutner, 1993; Pfefferbaum, Sullivan, Mathalon, & Lim, 1997; Pfefferbaum et al., 2018; Sullivan et al., 2018); postmortem: (Courville, 1955; Harper & Kril, 1990). Volume deficits have consistently been reported in orbitofrontal cortex (Durazzo et al., 2011; Shields & Gremel, 2020; Wang et al., 2016) and in other frontal subregions including precentral, superior, middle, inferior, supplementary motor, and medial cortices (Sullivan et al., 2018) in AUD. Taken together, neuropsychological and neuroimaging results indicate a likelihood that frontally-based systems contribute to the genuine mnemonic deficits in AUD, thereby implicating a neural mechanism for selective mnemonic fragility that has been incompletely articulated to date. Further, AUD-drug abuse comorbidity exerts an additional toll on prefrontal cortical volumes, even in those who had been abstinent from substances for more than 2 years (Tanabe et al., 2009), posing an added source of degradation on associated mnemonic functions.
Recently, we reported a selective association between a memory composite score and frontal volumes in AUD, saliently in abstinent individuals with AUD who had a history of a drug abuse diagnosis (Fama, Le Berre, Sassoon, et al., 2019). Those findings were consistent with earlier studies suggesting that integrity of frontal cortical regions, particularly orbitofrontal regions (Frey & Petrides, 2000, 2002), may be critical for memory processes (Buckner et al., 1999). Although we found that AUD had smaller hippocampal volume, on average, compared with the control group, we did not identify an association between hippocampal volume and the memory composite score. Here we expand on our previous findings by separately examining the relations between verbal and visual stimuli and regional frontal gray matter and hippocampal volumes in AUD with and without a drug history for both immediate and delayed recall. We hypothesized that individuals with AUD, regardless of drug history, would show deficits for both verbal and visual stimuli, evident in both immediate and delayed recall, and that these deficits would be related to selective regional frontal volumes, namely orbital and superior frontal volumes. We speculated that based on previous studies on episodic memory in AUD relations between episodic memory and orbital frontal volumes would be greater in individuals with AUD who had a history of a drug diagnosis than those without such history and that this relation would be stronger for verbal than visual memory and immediate than delayed memory processes.
2. Methods and Materials
2.1. Participants
Participants included 91 individuals with alcohol use disorder (AUD: age 25-70 years; 71 men and 20 women) and 36 healthy controls (CTRL: age 25-73 years; 21 men and 15 women). AUD participants were almost exclusively recruited from local substance abuse treatment programs and sobriety support groups. All AUD participants were both treatment seeking and self-identified as having problems with alcohol misuse and met DSM-IV-TR criteria for alcohol dependence and DSM-5 criteria for AUD. Control participants were recruited from the local community. These participants were a subset of those reported in previous magnetic resonance imaging (MRI) studies (Pfefferbaum et al., 2018; Sullivan et al., 2018) and other reports published by our laboratory that examined the neurological and nutritional factors associated with cognitive and motor deficits in alcoholism (Fama, Le Berre, Hardcastle, et al., 2019; Pitel et al., 2011) and neural correlates of cognitive and motor domains (Fama, Le Berre, Sassoon, et al., 2019). The present paper extends previous reports by delving into specific episodic memory modalities (verbal vs. visual) and processes (immediate vs. delayed) and subregional volumes of the frontal lobe.
Screening for exclusion was based on the Structured Clinical Interview for DSM-IV (SCID) (First, Spitzer, Gibbon, & Williams, 1998) and questionnaires on health status, administered by calibrated research clinicians. Participants were excluded if they had fewer than 8 years of education or a significant history of medical (e.g., epilepsy, stroke, multiple sclerosis, uncontrolled diabetes, or loss of consciousness > 30 minutes), psychiatric (i.e., schizophrenia or bipolar I disorder) or neurological (e.g., neurodegenerative disease) disorder. An additional exclusion criterion for the control group was any DSM-IV-TR Axis I disorder. All participants also underwent a semi-structured timeline follow-back interview to quantify lifetime alcohol consumption (Skinner, 1982; Skinner & Sheu, 1982). Severity of depressive symptoms was assessed with the Beck Depression Inventory-II in all participants (Beck, Steer, & Brown, 1996). This research protocol was approved by the Institutional Review Boards of Stanford University and SRI International. Written informed consent was obtained from all participants, none of whom was clinically demented or conserved.
For the AUD group, the average age of onset of alcohol dependence was 24.1+8.7 years (range=12 to 48 years) and the average length of alcohol dependence was 23.8+11.6 years (range=3 to 51 years). AUD participants drank an average of 1340+1019 kg of alcohol over their lifetime (range=176 to 4711 kg; median=1040 kg). By contrast, controls drank on average 26+35 kg alcohol (range=0 to 136 kg; median=9.5 kg) over their lifetime. Of the 91 AUD participants, 49 (54%) met criteria in their lifetime for at least one non-alcohol substance abuse/dependence diagnosis (AUD+DrugHx), whereas 42 (46 %) had no substance abuse/dependence history besides 9 who had only a past marijuana diagnosis (AUD-noDrugHx).
In the AUD group (n=91) average days sober was 109+112 (range = 1 to 726 days); median was 83 days. Time since last met an alcohol diagnosis was on average 28 weeks (sd=42 weeks, range = 0 to 286 weeks, median=14 weeks). At the time of testing, all 91 participants met DSM-IV criteria for alcohol dependence with 65 participants being in early full remission, 7 participants meeting criteria for early partial remission, 1 being in sustained partial remission, 10 being sustained full remission, and 8 participants meeting criteria for current dependence (i.e., within the past month), and 18 reported drinking some amount of alcohol within the past month. Of the 91 AUD participants, 40 participants (44%) met criteria for DSM-5 diagnostic criteria for current AUD (i.e., within the past three months), whereas 51 participants met criteria for past AUD. No AUD participant had ever been diagnosed with Wernicke’s encephalopathy nor met criteria for alcohol-induced persisting amnestic disorder. Although the AUD+DrugHx group reported more days without drinking prior to testing than the AUD-noDrugHx group, the differences was not significant [t(89)=1.35, p=.181].
Investigation into individual drug classes indicated that of the 49 AUD participants who met DSM-IV-TR criteria for abuse/dependence for non-alcohol substances besides marijuana: 42 participants met criteria for a cocaine diagnosis, 17 for amphetamines, 13 for opioids, 6 for hallucinogens, 5 for sedatives, and 2 for other substance abuse/dependence. For those AUD participants with a history of cocaine, amphetamine, opioid, hallucinogen, or sedative misuse, all were in remission for at least one month, with an average time since remission of approximately 5.5 years. For nicotine, 46 (51%) of the 91 AUD participants were current tobacco smokers and 17 (19%) were past smokers, whereas 2 of 36 (6%) controls were current smokers and 1 (3%) was a past smoker.
In the AUD group (n=91), 10 participants (11.0%) identified as Hispanic, 2 (2.2%) identified as Native American, 41 (45.1%) identified as Black, and 41 (45.1%) identified as White. No AUD participant identified as either Asian or Islander. In the control group (n=36), 12 participants (33.3%) identified as Black, 16 (44.4%) identified as White, 7 (19.4%) identified as Asian, and 1 (2.8%) was unknown. No control participant identified as Native American or Islander. AUD and CTRL groups differed in percentage of Hispanic and Asian participants. Examination of AUD subgroups with and without a history of a drug diagnosis indicated that these subgroups did not differ significantly on ethnicity.
AUD and CTRL groups did not differ significantly in age or on an IQ estimate derived from the National Adult Reading Test (NART) (Nelson, 1982) (see Table 1). On average, the AUD group had fewer years of education and scored lower on a screening test of overall current cognitive level (Dementia Rating Scale-2) (Mattis, 2004) than the CRTL group; no AUD participant was clinically demented. The AUD group also endorsed a greater level of depressive symptoms (BDI-II) and as expected consumed far more alcohol over their lifetime than the CTRL group. There were significantly a greater proportion of women in the AUD group compared with the CTRL group (Chi-square=4.8, p=.03).
Table 1.
Demographic characteristics of Participant Groups: AUD, CTRL, and AUD subgroups with and without history of drug dx (mean, sd, range)
| Group | Group | Group | Group | Group | Group | Grou p |
DRS- 2c |
|---|---|---|---|---|---|---|---|
| consumption (kg) |
|||||||
| AUD | 20 F, 71 M | 48.5 | 13.0 | 106.7 | 1340 | 9.8 | 135.9 |
| (n=91) | (10.6) | (2.3) | (9.1) | (1019) | (6.7) | (5.2) | |
| 25 to 70 | 9 to 21 | 91 to 124 | 178 to 4783 | 0 to 38 | 121 to 144 | ||
| CTRL | 15 F, 21 M | 47.2 | 15.5 | 110.7 | 26 | 3.1 | 139.3 |
| (n=36) | (12.9) | (2.6) | (9.5) | (35) | (3.9) | (2.2) | |
| 25 to 73 | 11 to 21 | 92 to 126 | 0 to 136 | 0 to 16 | 135 to 144 | ||
| Group Differences |
p=.03 | p=.60 | p<.0001 | p=.09 | p<.0001 | p<.00 01 |
p=.00 02 |
| 95% CI | [5.6, −3.2] | [−1.6, −3.5] | [0.6, −8.6] | [1651, 977] | [9.1, 4.3] | [−1.6, −5.2] | |
| AUD subgroups: | |||||||
| AUD-noDrugHx | 12 F, 30 M | 46.3 | 13.2 | 108.3 | 1148 | 8.7 | 135.6 |
| (n=42) | (11.1) | (2.2) | (9.0) | (786) | (6.3) | (5.9) | |
| 25 to 69 | 11 to 21 | 91 to 124 | 181 to 3847 | 0 to 24 | 121 to 144 | ||
| AUD+DrugHx | 8 F, 41 M | 50.3 | 12.8 | 104.8 | 1505 | 10.7 | 136.2 |
| (n=49) | (9.8) | (2.3) | (9.1) | (1166) | (6.9) | (4.6) | |
| 26 to 67 | 9 to 21 | 91 to 124 | 178 to 4783 | 0 to 38 | 123 to 143 | ||
| Group Differences |
ns | p=.07 | p=.38 | p=.21 | p=.10 | p=.16 | p=.62 |
| 95% CI | [8.4, −0.4] | [0.5, −1.4] | [2.1, −9.1] | [779, −64] | [4.9, −0.9] | [2.8, −1.7] |
NART – National Adult Reading Test [AUD n=43, CTRL n=26, AUD-noDrugDx n=23, AUD+Drug Dx n=20]
BDI-II – Beck Depression Inventory – Second Edition
DRS-2 – Dementia Rating Scale – Second Edition
2.2. Neurocognitive Testing: Verbal and Visual Episodic Memory
Logical Memory Stories from the Wechsler Memory Scale – Revised (Wechsler, 1987).
The examinee is read a short narrative and asked to recall the story back to the examiner, using as close to the same words as were read aloud, both immediately after the story was read (LMI – Immediate Logical Memory) and then again after a 30-minute delay (LMII – Delayed Logical Memory). There are two narratives. The dependent score is the number of details recalled in the immediate and then the delayed conditions (maximum = 50 points for each condition).
Rey-Osterrieth Complex Figure Test (Rey, 1942).
The examinee is shown a complex figure and asked to copy it as accurately and as quickly as possible. After copy is complete, the examinee is asked to draw the complex figure from memory (Rey-O Imm). There is no time limit. The examinee is asked again to draw the complex figure from memory after a 30-minute delay (Rey-O Delay). Score is number of details recalled per standard scoring instructions on immediate and then delayed condition (maximum = 36 points for each condition).
2.3. Magnetic Resonance Image (MRI): Data Acquisition and Processing
MRI data were acquired on 3 Tesla GE whole body MR systems (General Electric Healthcare, Waukesha, WI) using an 8-channel phased-array head coil. T1-weighted Inversion-Recovery Prepared SPGR images (TR=6.55/5.92 ms, TE=1.56/1.93 ms, TI=300/300 ms, matrix = 256x256, thick=1.25 mm, skip=0 mm, 124 slices) were based on an axial structural sequence that was used for volumetric analysis. Drift was corrected by adjusting scanner calibration parameters when necessary to maintain spatial stability within manufacturer guidelines, and routine phantom data were used to evaluate spatial fidelity.
Preprocessing of the T1-weighted MRI data (124 slices, matrix=256x256, thickness=1.25mm, skip=0) involved noise removal (Coupe et al., 2008), correcting field inhomogeneity via N4ITK (Tustison, Avants, Siqueira, & Gee, 2011), and segmenting the brain mask by majority voting (Rohlfing, Brandt, Menzel, & Maurer, 2004). The voting was performed with respect to the maps generated by separately applying FSL BET (Smith, 2002), AFNI 3dSkullStrip (COX 1996), FreeSurfer mri_gcut (Sadananthan, Zheng, Chee, & Zagorodnov, 2010), and the Robust Brain Extraction (ROBEX) method (Iglesias, Liu, Thompson, & Tu, 2011) to the bias and non-bias corrected T1-weighted MRIs.
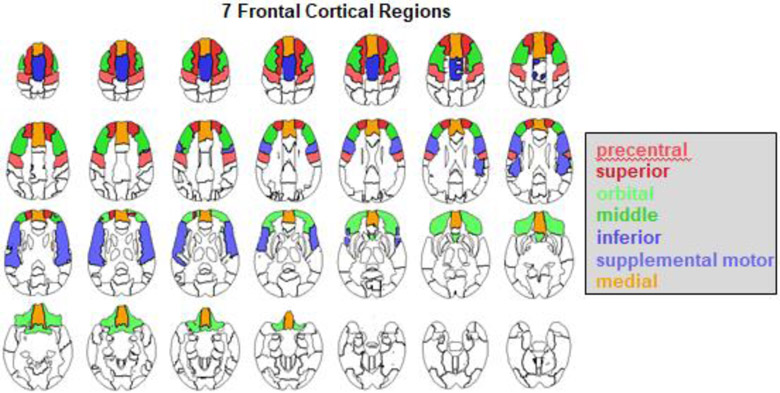
Brain tissue segmentation (gray matter, white matter, and cerebrospinal fluid) of the skull-stripped T1-weighted MRI was generated via Atropos (Avants et al., 2011). The label map was further parcellated into the regions defined by the SRI24 atlas (Rohlfing, Zahr, Sullivan, & Pfefferbaum, 2010) by non-rigidly registering the atlas to the MRI via ANTS (Avants, Epstein, Grossman, & Gee, 2008). Frontal gray matter was parcellated into seven regions of interest (ROIs): precentral, superior, orbital, middle, inferior, supplemental motor, and medial (Figure 1). Total hippocampal volume was also calculated. Automatic labeling was always visually inspected for accuracy by a trained research scientist. All brain volumes used in analyses were age- and head-size corrected.
Figure 1:
Color-coded atlas identifying frontal cortical subregions: precentral, superior, orbital, middle, inferior, supplementary motor, and medial.
2.4. Statistical analysis
Scores for the memory measures were age- and education-corrected and standardized on the CTRL group [Z-score of CTRL group: mean=0, standard deviation=±1]. CTRL men and women did not differ significantly on any memory score or brain volume measure and were thus collapsed into a single control group. Using Z-scores allowed for direct comparison across test scores within and between groups, which were assessed with 2-tailed t-tests. Cohen’s d was calculated for significant group differences. Correlational analyses were conducted to assess the relation between brain and behavioral measures. A False Discovery Rate was employed based on four comparisons (memory scores), requiring the smallest p-value across memory scores to be equal to or less than .0125 to be deemed significant (Benjamini & Hochberg, 1995). Multiple regression models were conducted to assess the amount of variance accounted for by brain ROIs that demonstrated a relation with a memory score based on the zero-order correlational analyses. Planned secondary analyses were conducted to test for differences in brain-behavior relations in individuals with AUD with and without a drug history. Post-hoc analyses, including nonparametric analyses and additional comparisons examining other subgroups of AUD participants (based on DSM-IV and DSM-5 criteria), were also conducted.
3. Results
Raw scores for verbal and visual immediate and delayed episodic memory measures for the AUD and CTRL groups are presented in Table 2.
Table 2.
Raw scores (mean, sd, range) of memory tests
| AUD | CTRL | |
|---|---|---|
| N=91 | N=36 | |
| Verbal Memory | ||
| Logical Memory - I (Immediate) | 20.6 (8.6) | 26.9 (7.1) |
| (max=50) | 2 to 37 | 8 to 39 |
| Logical Memory - II (Delayed) | 16.1 (8.7) | 22.9 (1.1) |
| (max=50) | 1 to 35 | 5 to 34 |
| Visual Memory | ||
| Rey-Osterrieth Figure - Immediate | 10.9 (5.3) | 15.0 (5.6) |
| (max=36) | 0 to 29 | 4.5 to 26.5 |
| Rey-Osterrieth Figure - Delayed | 11.3 (4.9) | 14.3 (5.8) |
| (max=36) | 2.5 to 23.5 | 5 to 24.5 |
3.1. Verbal and visual memory Z-scores (immediate and delayed)
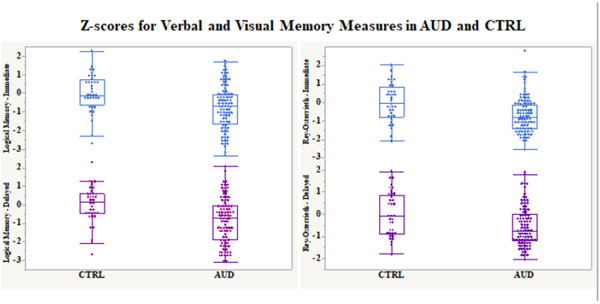
The AUD group scored lower than the CTRL group on all verbal and visual memory scores [LMI: t(125)=3.91, p=.0002, Cohen’s d=.70; LMII: t(125)=4.19, p<.0001, Cohen’s d=.75; Rey-O Imm: t(125)=3.89, p=.0002, Cohen’s d=.70; Rey-O Delay: t(125)=2.94, p=.004, Cohen’s d=.53] (Figure 2). Differences were significant with FDR correction for multiple comparisons.
Figure 2:
Box plots depicting verbal (Logical Memory Narratives) and visual (Rey-Osterrieth Complex Figure) immediate and delayed age- and education-corrected memory Z-scores for AUD and CTRL (mean=0, sd=1) groups.
3.2. Regional frontal and hippocampal volumes
A group difference emerged for precentral frontal and hippocampal volumes, with AUD having smaller precentral frontal [t(125)=3.13, p=.002, Cohen’s d=.63] and hippocampal [t(124)=2.10, p=.038, Cohen’s d=.39] volumes than CTRL. Group differences were not observed for superior, orbital, middle, inferior, supplementary motor, or medial frontal volumes (Table 3).
Table 3.
ICV- and Age-corrected Brain Volumes (cc) for CTRL, AUD, AUD-noDrugHx, and AUD+DrugHx (mean, sd)
| CT RL |
AUD | t, p- value |
AUD- noDrugH x |
AUD+ DrugH x |
t, p-value: AUD subgroups |
|
|---|---|---|---|---|---|---|
| Frontal ROIs | (n=36) | (n=91) | (n=42) | (n=49) | ||
| precentral | 18.51 | 17.38 | t=3.13, p=.002 | 17.86 | 16.96 | t=2.72, p=.026 |
| (1.65) | (1.91) | (1.70) | (2.00) | |||
| superior | 19.21 | 19.29 | t=0.24, p=.808 | 19.45 | 19.16 | t=0.76, p=.450 |
| (1.46) | (1.81) | (1.96) | (1.69) | |||
| orbital | 26.38 | 26.10 | t=0.69, p=.490 | 26.19 | 26.03 | t=0.35, p=.726 |
| (1.77) | (2.14) | (1.96) | (2.31) | |||
| middle | 25.92 | 25.18 | t=1.80, p=.074 | 25.23 | 25.14 | t=0.20, p=.840 |
| (1.88) | (2.16) | (2.05) | (2.26) | |||
| inferior | 24.14 | 23.78 | t=1.09, p=.277 | 24.16 | 23.45 | t=1.97, p=.052 |
| (1.49) | (1.75) | (1.57) | (1.84) | |||
| supplementary motor | 11.45 | 11.18 | t=1.04, p=.299 | 11.51 | 10.90 | t=2.21, p=.029 |
| (1.12) | (1.34) | (1.24) | (1.37) | |||
| medial | 24.91 | 24.69 | t=0.66, p=.511 | 24.99 | 24.44 | t=1.65, p=.102 |
| (1.85) | (1.59) | (1.54) | (1.61) | |||
| Hippocampus | 8.76 | 8.51 | t=2.10, p=.038 | 8.46 | 8.55 | t=0.82, p=.413 |
| (0.72) | (0.56) | (0.56) | (0.56) |
3.3. Correlations between verbal and visual memory scores and regional frontal and hippocampal volumes in AUD
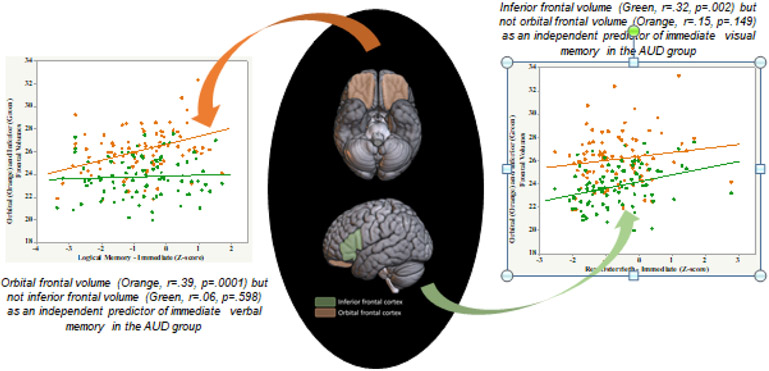
LMI and LMII scores correlated with orbital frontal volume [(n=91), LMI r=.39, p=.0001; LMII r=.43, p<.0001] (Table 4). By contrast, Rey-O Imm and Rey-O Delay scores correlated with inferior frontal volume [Rey-O Imm r=.32, p=.002; Rey-O Delay r=.30, p=.004] (Figure 3). Rey-O Delay score also correlated with superior [r=.28, p=.006] and orbital [r=.25, p=.015] frontal volumes. Scores on these memory tests did not correlate with hippocampal volume.
Table 4.
Correlations Between Memory Scores and Regional Frontal Volumes in AUD
| Log Mem I |
Log Mem II |
Rey-O Imm |
Rey-O Delay |
|
|---|---|---|---|---|
| Frontal subregions | r= | r= | r= | r= |
| precentral | −.10 | −.06 | −.05 | .16 |
| superior | .15 | .11 | .22 | .28 |
| orbital | .39 | .43 | .15 | .25 |
| middle | −.10 | −.10 | .06 | .09 |
| inferior | .06 | .13 | .32 | .30 |
| supplemental motor | .06 | .05 | −.01 | .02 |
| medial | −.09 | −.12 | .07 | .13 |
| Hippocampus | −.10 | −.07 | −.09 | −.04 |
Bold indicates correlations met False Discovery Rate with initial p value = .0125
Log Mem I = Logical Memory Immediate; Log Mem II = Logical Memory Delayed
Rey-O Imm = Rey-Osterrieth Figure Immediate; Rey-O Delay = Rey-Osterrieth Figure Delayed
Figure 3:
Dissociable structural brain correlates for immediate verbal and immediate visual memory: evidence of a double dissociation in AUD (n=91).
A multiple regression model predicting Rey-O Delay score from the 3 regions that showed significant zero-order correlations (i.e., inferior, superior, and orbital frontal volumes) indicated that although they accounted for 10.1% of the variance no single region was an independent predictor of this score.
3.4. Correlations between alcohol-related consumption variables and BDI-II and memory scores
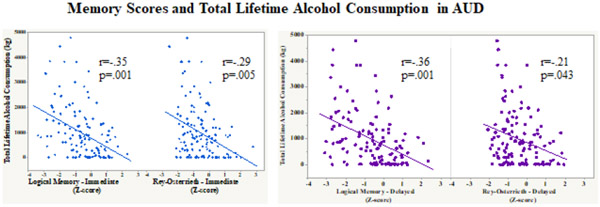
Total lifetime alcohol consumption (kg) correlated with each of the verbal and visual memory scores in AUD (n=91: LMI: r=−.35, p=.0008; LMII: r=−.36, p=.0005; Rey-O Imm: r=−.29, p=.005; Rey-O Delay: r=−.21, p=.043) (Figure 4). By contrast, age of alcohol onset, time since last met alcohol diagnosis, and days since last drink were not correlated with any of the memory scores. BDI-II scores were not significantly correlated with any of the memory scores.
Figure 4:
Scatterplots depicting the relation between total lifetime alcohol consumption (kg) and immediate and delayed verbal (Logical Memory) and visual (Rey-Osterrieth) memory scores in AUD.
Indeed, total lifetime alcohol consumption was an independent predictor of LMI, LMII, and Rey-O Imm scores, when entered into a model with relevant regional brain volumes (Table 5). Total lifetime alcohol consumption accounted for 7.5% of LMI score variance beyond the contribution of orbital frontal volume, which accounted for 11.2% of the variance. Total lifetime alcohol consumption accounted for 7.9% of LMII score variance beyond the contribution of orbital frontal volume, which accounted for 13.8% of the variance. Finally, total lifetime alcohol consumption accounted for 5.9% of Rey-O Imm score variance beyond the contribution of inferior frontal volume, which accounted for 7.4% of the variance.
Table 5.
Multiple regression models predicting memory scores from brain volumes and lifetime alcohol consumption in AUD
| t-Ratio | p-value | |
|---|---|---|
| LM-I | ||
| orbital | 3.58 | .001 |
| lifetime alcohol (kg) | −2.93 | .004 |
| LM-II | ||
| orbital | 4.07 | .000 |
| lifetime alcohol (kg) | −3.08 | .003 |
| Rey-O Immediate | ||
| inferior | 2.78 | .007 |
| lifetime alcohol (kg) | −2.48 | .015 |
| Rey-O Delayed | ||
| superior | 1.23 | .224 |
| orbital | 1.21 | .230 |
| inferior | 1.33 | .188 |
| lifetime alcohol (kg) | −1.43 | .155 |
Bold values denote significance
3.5.1. AUD subgroups based on lifetime drug diagnosis history
AUD+DrugHx (n=49) did not differ significantly from AUD-noDrugHx (n=42) in age, years of education, estimate of premorbid IQ, total lifetime alcohol consumed, severity of depressive symptoms reported, or current general cognitive ability (Table 6).
Table 6.
Correlations between Memory Scores and Regional Brain Volume
| No Drug Dx History N=42 |
Drug Dx History N=49 |
|||
|---|---|---|---|---|
| LMI | r | p-value | r | p-value |
| Frontal regions | ||||
| precentral | −.209 | .18 | −.019 | .89 |
| superior | .402 | .01 * | .115 | .43 |
| orbital | .304 | .05 * | .473 | .00 |
| No Drug Dx History N=42 |
Drug Dx History N=49 |
|||
| LMI | r | p-value | r | p-value |
| No Drug Dx History N=42 |
Drug Dx History N=49 |
|||
| LMI | r | p-value | r | p-value |
| Frontal regions | ||||
| precentral | −.209 | .18 | −.019 | .89 |
| superior | .402 | .01 * | .115 | .43 |
| orbital | .304 | .05 * | .473 | .00 |
| middle | −.047 | .77 | −.014 | .33 |
| inferior | .070 | .66 | .051 | .73 |
| supplemental motor | .101 | .52 | .038 | .80 |
| medial | −.104 | .51 | −.081 | .58 |
| Hippocampus | −.004 | .98 | −.194 | .18 |
| LMII | ||||
| Frontal regions | ||||
| precentral | −.165 | .30 | .008 | .95 |
| superior | .321 | .04 * | −.101 | .49 |
| orbital | .363 | .02 * | .491 | .00 |
| middle | .035 | .83 | −.201 | .17 |
| inferior | .111 | .48 | .133 | .36 |
| supplemental motor | .070 | .66 | .027 | .85 |
| medial | −.176 | .26 | −.087 | .55 |
| Hippocampus | .042 | .79 | −.163 | .26 |
| Rey-O Immediate | ||||
| Frontal regions | ||||
| precentral | −.212 | .18 | −.027 | .85 |
| superior | .236 | .13 | .176 | .23 |
| orbital | .037 | .82 | .253 | .08 |
| middle | −.051 | .75 | .170 | .26 |
| inferior | .245 | .12 | .322 | .02 |
| supplemental motor | −.152 | .34 | .019 | .90 |
| medial | −.067 | .68 | .132 | .37 |
| Hippocampus | −.045 | .78 | −.104 | .48 |
| Hippocampus | −.044 | .78 | −.010 | .94 |
Bold values denote significance:
p<.05
p<.01
3.5.2. Memory scores and regional frontal and hippocampal volumes
AUD+DrugHx had lower scores than AUD-noDrugHx on Rey-O Imm [t(89)=2.34, p=.02, Cohen’s d=.49], but the subgroups did not differ on LMI [t(89)=.07, p=.94], LMII [t(89)=.03, p=.80], or Rey-O Delay [t(89)=1.03, p=.31] scores. The AUD+DrugHx group had smaller precentral [t(89)=2.27, p=.026, Cohen’s d=.48] and supplementary motor [t(89)=2.21, p=.029, Cohen’s d=.47] frontal volumes and modestly smaller inferior frontal volumes [t(89)=1.97, p=.052, Cohen’s d=.42] than the AUD-noDrugHx group. AUD subgroups did not differ on hippocampal volume [t(89)=.82, p=.41].
3.5.3. Correlations between memory scores and regional frontal and hippocampal volumes
In the AUD-noDrugHx subgroup, LMI and LMII scores correlated with orbital (LMI r=.30, p=.05; LMII r=.36, p=.02) and superior (LMI r=.40, p=.008; LMII r=.32, p=.038) frontal volumes (Table 6). No significant correlations emerged between Rey-O Imm or Rey-O Delay scores and any regional frontal ROI in this subgroup. None of the memory scores correlated with hippocampal volume in the AUD-noDrugHx subgroup.
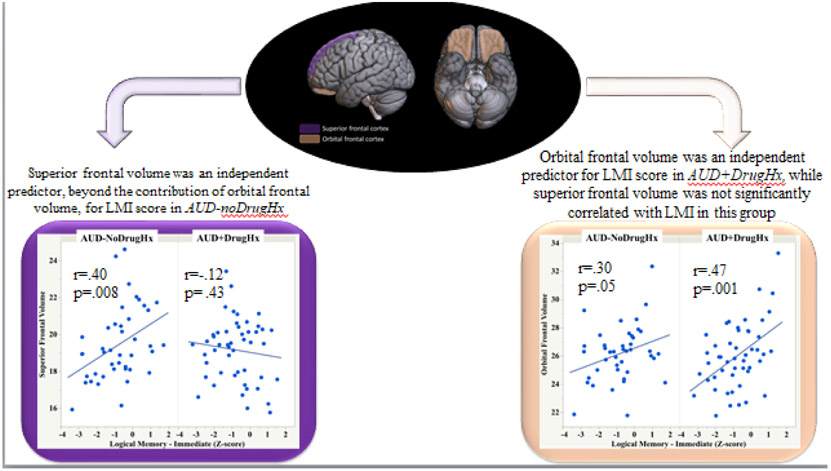
Multiple regression analyses examined the independent contributions of superior and orbital frontal volumes as predictors of verbal memory scores in AUD-noDrugHx (Table 7). Superior frontal volume was an independent predictor, beyond the contribution of orbital frontal volume, for LMI score, accounting for 11.7% of the score variance (Figure 5). By contrast, orbital frontal volume was an independent predictor, beyond the contribution of superior frontal volume, for LMII scores, accounting for 10.5% of the score variance.
Table 7.
Multiple regression models predicting memory score
| No Drug Diagnosis History |
||
|---|---|---|
| t-Ratio | p-value | |
| LMI | ||
| orbital | 1.47 | .151 |
| superior | 2.16 | .037 |
| total lifetime alcohol (kg) | −1.70 | .097 |
| LMII | ||
| orbital | 2.00 | .052 |
| superior | 1.51 | .141 |
| total lifetime alcohol (kg) | −1.24 | .223 |
| Drug Diagnosis History |
||
| t-Ratio | p-value | |
| LMI | ||
| orbital | 3.19 | .003 |
| total lifetime alcohol (kg) | −2.33 | .024 |
| LMII | ||
| orbital | 3.36 | .002 |
| total lifetime alcohol (kg) | −2.81 | .007 |
| ReyO-Imm | ||
| inferior | 2.22 | .031 |
| total lifetime alcohol (kg) | −1.53 | .132 |
| ReyO-Delay | ||
| orbital | 2.49 | .017 |
| inferior | 1.71 | .094 |
| total lifetime alcohol (kg) | −0.05 | .608 |
Bold values denote significance: p≤.05
Figure 5:
Immediate verbal memory scores and orbital frontal and superior frontal volumes in AUD with and without a drug diagnosis history - LMI: Logical Memory – Immediate score, Wechsler Memory Scale – Revised.
In the AUD+DrugHx, verbal memory scores correlated with orbital frontal volume (LMI: r=.47, p=.001; LMII: r=.49, p=.000) (Table 1). In contrast with AUD-noDrugHx, superior frontal volume was not an independent predictor of LMI in the AUD+DrugHx; indeed, superior frontal volume was not correlated with LMI in AUD+DrugHx and accounted for less than 1% of the variance in score after the contribution of orbitofrontal volume was taken into account (Figure 5). The Rey-O Imm score correlated with inferior frontal volume (r=.32, p=.024), and Rey-O Delay score correlated with orbital (r=.39, p=.005) and inferior (r=.29, p=.044) frontal volumes in AUD+DrugHx. Multiple regression analysis indicated that orbital frontal volume (p=.01), but not inferior frontal volume, was an independent predictor of Rey-O Delay score, accounting for 12.5% of the variance. None of the memory scores correlated significantly with hippocampal volume in the AUD+DrugHx subgroup.
Although the drug use in the AUD+DrugHx subgroup varied among participants, 42 of these participants (86%) had a lifetime cocaine diagnosis (37 had a diagnosis of past cocaine dependence and 5 had a diagnosis of past cocaine abuse). Post-hoc analyses for this subgroup of AUD+DrugHx who shared a history of cocaine misuse indicated that the pattern of results reported for the entire group of 49 AUD+DrugHx did not change when only these 42 AUD participants were included in the analyses [orbitofrontal volume and LMI r=.49, p=.0009; orbitofrontal volume and LMII r=.51, p=.0007; orbitofrontal volume and Rey-O Imm r=.33, p=.032; orbitofrontal volume and Rey-O Delay r=.43, p=.004]. In addition, post-hoc analyses indicated that the 9 people with AUD with only a lifetime marijuana diagnosis performed as well as controls on all memory measures; when these AUD participants were excluded from the analyses, group differences between AUD+DrugHx and AUD-noDrugHx and the brain-behavior relations reported endured.
3.5.4. Correlations between alcohol consumption and BDI-II scores and memory scores in AUD subgroups
Lifetime alcohol consumption (kg) correlated with LMI (r=−.32, p=.040) and Rey-O Imm (r=−.32, p=.038) scores in AUD-noDrugHx. In AUD+DrugHx, lifetime alcohol consumption correlated with LMI (r=−.39, p=.006) and LMII (r=−.44, p=.002) scores. Multiple regression indicated that lifetime alcohol consumption was an independent predictor of LMI and LMII scores in AUD+DrugHx, accounting for 8.2% of the variance in LMI score and 11.1% of the variance in LMII score. BDI scores were not correlated with any of the memory scores in either the AUD-noDrugHx or AUD+DrugHx subgroups.
4. Discussion
In support of our hypotheses and consistent with earlier studies (Fama, Rosenbloom, Nichols, Pfefferbaum, & Sullivan, 2009; Glenn & Parsons, 1992; Pitel et al., 2007; Tivis et al., 1995), verbal and visual episodic memory deficits were evident in individuals with AUD compared with healthy control participants. The current results further reveal that performance levels of AUD participants involving verbal and visual episodic memory were selectively related to volumes of orbital, inferior, and superior frontal regions but not to other frontal regions (precentral, middle, supplementary motor, or medial frontal). Selectivity between modalities revealed a double dissociation: orbital but not inferior frontal volumes predicted immediate verbal memory, whereas inferior but not orbital frontal volumes predicted immediate visual memory in AUD. Memory scores were not related to hippocampal volume in AUD.
4.1. Frontal systems of episodic memory processes
A critical and essential role for the frontal neocortex in encoding new experiences (Shallice et al., 1994; Takehara-Nishiuchi, 2020; Tulving, Markowitsch, Craik, Habib, & Houle, 1996) and retrieval of information (Eichenbaum, 2017) has been highlighted in human and animal studies. Among the frontal subregions examined herein, orbital frontal volume was related to episodic memory in AUD, comporting with other reports of the contribution of orbital frontal regions to processes involving consolidation and retrieval of episodic memory (Buckner et al., 1999; Frey & Petrides, 2000, 2002). This finding was robust and present even when post-hoc analyses were conducted on subsets of AUD participants divided by recency of drinking history: actively drinking participants according to DSM-5 (n=41), participants who were in early full remission according to DMS-IV (n=65) or early partial remission (n=7). These post-hoc analyses excluded 8 participants who were currently drinking and 10 participants who were in sustained full remission, having last met criteria for an alcohol diagnosis 2.5 to 12.3 months prior to testing or were in sustained partial remission.
Insofar as there is reported recovery of frontal lobe function with abstinence of curtailed drinking (Meyerhoff & Durazzo, 2020), the relation between orbital frontal volume and episodic memory in AUD raises the speculation that frontally-based episodic memory dysfunction in AUD may be amenable to recovery. Whereas the memory deficits associated with limbic dysfunction (medial temporal and diencephalic structures) have been reported to be relatively stable, memory deficits associated with frontal dysfunction have been shown to be more amenable to change over time. For instance, frontal lobe involvement has also been implicated in the profound anterograde episodic memory deficit associated with transient global amnesia (Guillery-Girard et al., 2004; Le Pira et al., 2005) with recovery of episodic memory processes generally within 24 hours, again supporting the role of extra-hippocampal regions as critical nodes of episodic memory function. The possibility of recovery in uncomplicated AUD is in contrast to the limited recovery observed in anterograde episodic memory arising from Papez circuit dysfunction as occurs in alcohol-related Wernicke-Korsakoff syndrome (Victor et al., 1989). Indeed, recovery of selective cognitive processes, including memory processes, in AUD can take place over years (Nixon & Lewis, 2020). Apart from acute recovery after initial abstinence, evidence for brain recovery from studies based on structural and functional imaging (Oscar-Berman et al., 2014; Pitel et al., 2014) and cognitive performance is documented well past the initial 30-days post abstinence period (Fein & Fein, 2013). Recovery of component cognitive processes involving episodic memory, including working memory as associated with prefrontal cortical integrity (Romanski, 2004), may contribute to the heterogeneity in severity of mnemonic deficits observed in AUD.
4.2. Alcohol and drugs
Total lifetime alcohol consumption was an independent predictor of verbal and visual episodic memory in AUD, accounting for upwards of 16% of the variance of memory scores. Although the relation between total lifetime alcohol consumption and severity of cognitive deficits in AUD has often been elusive, higher lifetime alcohol consumption was consistently related to poorer verbal and visual memory performance in this study and supports the assumption that alcohol was a principal agent exerting untoward effects on the brain and performance.
Occurrence of drug misuse in AUD was associated with worse immediate visual memory and smaller precentral, inferior, and supplementary motor frontal volumes than in AUD without a past drug abuse diagnosis. Orbitofrontal volume was related to immediate and delayed verbal episodic memory in both AUD with and without a history of a drug abuse diagnosis and to delayed visual memory in AUD with a history of a drug abuse diagnosis. This relation endured in post-hoc analyses including on those AUD with a history of a cocaine diagnosis. Similar relations were also reported in individuals with polysubstance use, specifically alcohol, cocaine, and amphetamine (Tanabe et al., 2009). These results extend reports of orbitofrontal dysregulation associated with general substance misuse (Moorman, 2018; Volkow & Fowler, 2000) yet with selective effects on verbal episodic memory.
Subgroup differences in brain-behavior relations did arise with superior frontal volume as an independent predictor of immediate verbal memory in AUD without a drug abuse diagnosis history, whereas orbital frontal volume was an independent predictor of immediate verbal memory in AUD with a history of a drug abuse diagnosis. Indeed, superior frontal regions have been associated with working memory (du Boisgueheneuc et al., 2006), a component process that supports episodic memory. This finding is consistent with others noting differential effects on brain structure of polysubstance misuse of just one substance (Meyerhoff, 2017) and provides evidence for a role of superior frontal regions in encoding of verbal information in AUD without complications of a drug abuse diagnosis history.
4.3. Limitations
Among the limitations of this study, examination of episodic memory processes was based on only single measures of verbal and one visual memory and requires replication using other or additional mnemonic measures of these processes. Absence of a relation between episodic memory scores and hippocampal volume in this study may be due to imaging limitations precluding measurement of hippocampal subfields. Imaging limitations also precluded us from examining possible relations between diencephalic structures, namely selective nuclei of the thalamus, which have been implicated in memory function in AUD (Pitel, Segobin, Ritz, Eustache, & Beaunieux, 2015), and memory scores. Other limitations include the absence of exact dosage of non-alcohol substances and pattern of use of these substances throughout a lifetime and differences in percentage of Asian and Hispanic participants between the groups. Further, restricted sample sizes constrained statistical exploration of the effects of specific drug misuse on the frontal-memory relations observed.
5. Conclusion
Taken together, this study highlights the role of selective frontal cortical sites in supporting encoding and retrieval processes of episodic memory in AUD, with a double dissociation observed between verbal and visual stimuli and regional frontal volumes. In addition, the pattern of brain-mnemonic relations in AUD differed with the presence versus absence of history of a drug abuse diagnosis. Both sets of results highlight the relevance and selectivity of frontal sites in disrupting episodic memory functions in AUD. Given the permanence of mnemonic impairment typically following limbic lesions, the extra-limbic, frontal substrate of the AUD-related impairment may have favorable implications for functional recovery with reduction in drinking (cf., Meyerhoff and Durazzo 2020).
Highlights:
Verbal and visual episodic memory are impaired in AUD
Frontal but not hippocampal regions were independent predictors of episodic memory
Frontal-memory relations in AUD differed with drug-abuse history
Lifetime alcohol consumption predicted episodic memory deficit severity
Frontally (rather than limbic)-based memory dysfunction in AUD may be remediable
Funding:
This work was supported by the National Institutes of Health (NIH)/National Institute on Alcohol Abuse and Alcoholism Grants AA005965, AA010723, AA017923, AA013521.
Footnotes
Portions of this data were presented at the Society for Neuroscience conference in Chicago, October 2019.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Disclosures:
No author on this manuscript has any disclosure to make in regard to this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- Aggleton JP (2014). Looking beyond the hippocampus: old and new neurological targets for understanding memory disorders. Proc Biol Sci, 281(1786). doi: 10.1098/rspb.2014.0565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggleton JP, & Morris RGM (2018). Memory: Looking back and looking forward. Brain Neurosci Adv, 2, 2398212818794830. doi: 10.1177/2398212818794830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants BB, Epstein CL, Grossman M, & Gee JC (2008). Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal, 12(1), 26–41. doi: 10.1016/j.media.2007.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants BB, Tustison NJ, Song G, Cook PA, Klein A, & Gee JC (2011). A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage, 54(3), 2033–2044. doi: 10.1016/j.neuroimage.2010.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty WW, Katzung VM, Moreland VJ, & Nixon SJ (1995). Neuropsychological performance of recently abstinent alcoholics and cocaine abusers. Drug Alcohol Depend, 37(3), 247–253. doi: 10.1016/0376-8716(94)01072-s [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, & Brown GK (1996). Manual for the Beck Depression Inventory-II. San Antonio: Psychological Corporation. [Google Scholar]
- Benjamini Y, & Hochberg Y (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B, 57, 289–300. [Google Scholar]
- Beresford TP, Arciniegas DB, Alfers J, Clapp L, Martin B, Du Y, … Davatzikos C (2006). Hippocampus volume loss due to chronic heavy drinking. Alcohol Clin Exp Res, 30(11), 1866–1870. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17067350 [DOI] [PubMed] [Google Scholar]
- Buckner RL, Kelley WM, & Petersen SE (1999). Frontal cortex contributes to human memory formation. Nat Neurosci, 2(4), 311–314. doi: 10.1038/7221 [DOI] [PubMed] [Google Scholar]
- Cermak LS, & O'Connor M (1983). The anterograde and retrograde retrieval ability of a patient with amnesia due to encephalitis. Neuropsychologia, 21(3), 213–234. doi: 10.1016/0028-3932(83)90039-8 [DOI] [PubMed] [Google Scholar]
- Coupe P, Yger P, Prima S, Hellier P, Kervrann C, & Barillot C (2008). An optimized blockwise nonlocal means denoising filter for 3-D magnetic resonance images. IEEE Trans Med Imaging, 27(4), 425–441. doi: 10.1109/TMI.2007.906087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courville CB (1955). Effects of Alcohol on the Nervous System of Man. Los Angeles: San Lucas Press. [Google Scholar]
- du Boisgueheneuc F, Levy R, Volle E, Seassau M, Duffau H, Kinkingnehun S, … Dubois B (2006). Functions of the left superior frontal gyrus in humans: a lesion study. Brain, 129(Pt 12), 3315–3328. doi: 10.1093/brain/awl244 [DOI] [PubMed] [Google Scholar]
- Durazzo TC, & Meyerhoff DJ (2020). Changes of frontal cortical subregion volumes in alcohol dependent individuals during early abstinence: associations with treatment outcome. Brain Imaging Behav, 14(5), 1588–1599. doi: 10.1007/s11682-019-00089-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Tosun D, Buckley S, Gazdzinski S, Mon A, Fryer SL, & Meyerhoff DJ (2011). Cortical thickness, surface area, and volume of the brain reward system in alcohol dependence: relationships to relapse and extended abstinence. Alcohol Clin Exp Res, 35(6), 1187–1200. doi: 10.1111/j.1530-0277.2011.01452.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H (2017). Prefrontal-hippocampal interactions in episodic memory. Nat Rev Neurosci, 18(9), 547–558. doi: 10.1038/nrn.2017.74 [DOI] [PubMed] [Google Scholar]
- Fama R, Le Berre AP, Hardcastle C, Sassoon SA, Pfefferbaum A, Sullivan EV, & Zahr NM (2019). Neurological, nutritional and alcohol consumption factors underlie cognitive and motor deficits in chronic alcoholism. Addict Biol, 24(2), 290–302. doi: 10.1111/adb.12584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fama R, Le Berre AP, Sassoon SA, Zahr NM, Pohl KM, Pfefferbaum A, & Sullivan EV (2019). Relations between cognitive and motor deficits and regional brain volumes in individuals with alcoholism. Brain Struct Funct, 224(6), 2087–2101. doi: 10.1007/s00429-019-01894-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fama R, Pfefferbaum A, & Sullivan EV (2004). Perceptual learning in detoxified alcoholic men: contributions from explicit memory, executive function, and age. Alcohol Clin Exp Res, 28(11), 1657–1665. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15547452 [DOI] [PubMed] [Google Scholar]
- Fama R, Rosenbloom MJ, Nichols BN, Pfefferbaum A, & Sullivan EV (2009). Working and episodic memory in HIV infection, alcoholism, and their comorbidity: baseline and 1-year follow-up examinations. Alcohol Clin Exp Res, 33(10), 1815–1824. doi: 10.1111/j.1530-0277.2009.01020.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fama R, Rosenbloom MJ, Sassoon SA, Pfefferbaum A, & Sullivan EV (2012). Differential effect of alcoholism and HIV infection on visuomotor procedural learning and retention. Alcohol Clin Exp Res, 36(10), 1738–1747. doi: 10.1111/j.1530-0277.2012.01790.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fama R, Rosenbloom MJ, Sassoon SA, Thompson MA, Pfefferbaum A, & Sullivan EV (2011). Remote semantic memory for public figures in HIV infection, alcoholism, and their comorbidity. Alcohol Clin Exp Res, 35(2), 265–276. doi: 10.1111/j.1530-0277.2010.01342.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, & Fein D (2013). Subcortical volumes are reduced in short-term and long-term abstinent alcoholics but not those with a comorbid stimulant disorder. Neuroimage Clin, 3, 47–53. doi: 10.1016/j.nicl.2013.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Smith S, & Greenstein D (2012). Gait and balance in treatment-naive active alcoholics with and without a lifetime drug codependence. Alcohol Clin Exp Res, 36(9), 1550–1562. doi: 10.1111/j.1530-0277.2012.01772.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, & Williams JBW (1998). Structured clinical interview for DSM-IV axis I disorders (SCID) version 2.0 New York: Biometrics Research Department, New York State Psychiatric Institute. [Google Scholar]
- Frey S, & Petrides M (2000). Orbitofrontal cortex: A key prefrontal region for encoding information. Proc Natl Acad Sci U S A, 97(15), 8723–8727. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10880572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey S, & Petrides M (2002). Orbitofrontal cortex and memory formation. Neuron, 36(1), 171–176. doi: 10.1016/s0896-6273(02)00901-7 [DOI] [PubMed] [Google Scholar]
- Glenn SW, & Parsons OA (1992). Neuropsychological efficiency measures in male and female alcoholics. J Stud Alcohol, 53(6), 546–552. doi: 10.15288/jsa.1992.53.546 [DOI] [PubMed] [Google Scholar]
- Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, … Hasin DS (2015). Epidemiology of DSM-5 Alcohol Use Disorder: Results From the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry, 72(8), 757–766. doi: 10.1001/jamapsychiatry.2015.0584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillery-Girard B, Desgranges B, Urban C, Piolino P, de la Sayette V, & Eustache F (2004). The dynamic time course of memory recovery in transient global amnesia. J Neurol Neurosurg Psychiatry, 75(11), 1532–1540. doi: 10.1136/jnnp.2003.024968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper CG, & Kril JJ (1990). Neuropathology of alcoholism. Alcohol Alcohol, 25(2-3), 207–216. doi: 10.1093/oxfordjournals.alcalc.a044994 [DOI] [PubMed] [Google Scholar]
- Iglesias JE, Liu CY, Thompson PM, & Tu Z (2011). Robust brain extraction across datasets and comparison with publicly available methods. IEEE Trans Med Imaging, 30(9), 1617–1634. doi: 10.1109/TMI.2011.2138152 [DOI] [PubMed] [Google Scholar]
- Kopelman MD (1991). Frontal dysfunction and memory deficits in the alcoholic Korsakoff syndrome and Alzheimer-type dementia. Brain, 114 (Pt 1A), 117–137. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/1998878 [PubMed] [Google Scholar]
- Kopelman MD (1995). The Korsakoff syndrome. Br J Psychiatry, 166(2), 154–173. doi: 10.1192/bjp.166.2.154 [DOI] [PubMed] [Google Scholar]
- Le Pira F, Giuffrida S, Maci T, Reggio E, Zappala G, & Perciavalle V (2005). Cognitive findings after transient global amnesia: role of prefrontal cortex. Appl Neuropsychol, 12(4), 212–217. doi: 10.1207/s15324826an1204_5 [DOI] [PubMed] [Google Scholar]
- Mattis S (2004). Dementia Rating Scale (DRS) professional manual. Odessa: Psychological Assessment Resources, Inc. [Google Scholar]
- Meunier M, Bachevalier J, & Mishkin M (1997). Effects of orbital frontal and anterior cingulate lesions on object and spatial memory in rhesus monkeys. Neuropsychologia, 35(7), 999–1015. doi: 10.1016/s0028-3932(97)00027-4 [DOI] [PubMed] [Google Scholar]
- Meyerhoff D (2017). Functionally relevant brain alterations in polysubstance users: differences to monosubstance users, study challenges, and implications for treatment. In Watson R & Zibadi S (Eds.), Addictive Substances and Neurological Disease: Academic Press. [Google Scholar]
- Meyerhoff D, & Durazzo TC (2020). Not all is lost for relapsers: Relapsers with low WHO risk drinking levels and complete abstainers have comparable gray matter volumes. Alcoholism Clinical and Experimental Research, 44(7), 1479–1487. [Google Scholar]
- Milner B (1958). Psychological defects produced by temporal lobe excision. Res Publ Assoc Res Nerv Ment Dis, 36, 244–257. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/13527787 [PubMed] [Google Scholar]
- Mon A, Durazzo TC, Abe C, Gazdzinski S, Pennington D, Schmidt T, & Meyerhoff DJ (2014). Structural brain differences in alcohol-dependent individuals with and without comorbid substance dependence. Drug Alcohol Depend, 144, 170–177. doi: 10.1016/j.drugalcdep.2014.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman DE (2018). The role of the orbitofrontal cortex in alcohol use, abuse, and dependence. Prog Neuropsychopharmacol Biol Psychiatry, 87(Pt A), 85–107. doi: 10.1016/j.pnpbp.2018.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson HE (1982). The National Adult Reading Test. Windsor: Nelson Publishing Company. [Google Scholar]
- Nissim NR, O'Shea AM, Bryant V, Porges EC, Cohen R, & Woods AJ (2016). Frontal Structural Neural Correlates of Working Memory Performance in Older Adults. Front Aging Neurosci, 8, 328. doi: 10.3389/fnagi.2016.00328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon SJ, & Lewis B (2020). Brain Structure and Function in Recovery. Alcohol Res, 40(3), 04. doi: 10.35946/arcr.v40.3.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon SJ, Tivis RD, Jenkins MR, & Parsons OA (1998). Effects of cues on memory in alcoholics and controls. Alcohol Clin Exp Res, 22(5), 1065–1069. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/9726274 [PubMed] [Google Scholar]
- Oscar-Berman M (1990). Learning and memory deficits in detoxified alcoholics. NIDA Res Monogr, 101, 136–155. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/2092212 [PubMed] [Google Scholar]
- Oscar-Berman M, & Hutner N (1993). Frontal lobe changes after chronic alcohol ingestion. In Hunt WA & Nixon SJ (Eds.), Alcohol-induced brain damage, NIAAA Research Monographs (Vol. 22, pp. 121–156). Rockville, MD: National Institutes of Health. [Google Scholar]
- Oscar-Berman M, Valmas MM, Sawyer KS, Ruiz SM, Luhar RB, & Gravitz ZR (2014). Profiles of impaired, spared, and recovered neuropsychologic processes in alcoholism. Handb Clin Neurol, 125, 183–210. doi: 10.1016/B978-0-444-62619-6.00012-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons OA, & Nixon SJ (1993). Neurobehavioral sequelae of alcoholism. Neurol Clin, 11(1), 205–218. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/8441371 [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Mathalon DH, & Lim KO (1997). Frontal lobe volume loss observed with magnetic resonance imaging in older chronic alcoholics. Alcohol Clin Exp Res, 21(3), 521–529. doi: 10.1111/j.1530-0277.1997.tb03798.x [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Zahr NM, Sassoon SA, Kwon D, Pohl KM, & Sullivan EV (2018). Accelerated and Premature Aging Characterizing Regional Cortical Volume Loss in Human Immunodeficiency Virus Infection: Contributions From Alcohol, Substance Use, and Hepatitis C Coinfection. Biol Psychiatry Cogn Neurosci Neuroimaging, 3(10), 844–859. doi: 10.1016/j.bpsc.2018.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitel AL, Beaunieux H, Witkowski T, Vabret F, Guillery-Girard B, Quinette P, … Eustache F (2007). Genuine episodic memory deficits and executive dysfunctions in alcoholic subjects early in abstinence. Alcohol Clin Exp Res, 31(7), 1169–1178. doi: 10.1111/j.1530-0277.2007.00418.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitel AL, Eustache F, & Beaunieux H (2014). Component processes of memory in alcoholism: pattern of compromise and neural substrates. Handb Clin Neurol, 125, 211–225. doi: 10.1016/B978-0-444-62619-6.00013-6 [DOI] [PubMed] [Google Scholar]
- Pitel AL, Segobin SH, Ritz L, Eustache F, & Beaunieux H (2015). Thalamic abnormalities are a cardinal feature of alcohol-related brain dysfunction. Neurosci Biobehav Rev, 54, 38–45. doi: 10.1016/j.neubiorev.2014.07.023 [DOI] [PubMed] [Google Scholar]
- Pitel AL, Zahr NM, Jackson K, Sassoon SA, Rosenbloom MJ, Pfefferbaum A, & Sullivan EV (2011). Signs of Preclinical Wernicke's Encephalopathy and Thiamine Levels as Predictors of Neuropsychological Deficits in Alcoholism without Korsakoff's Syndrome. Neuropsychopharmacology, 36(3), 580–588. doi:npp2010189 [pii] 10.1038/npp.2010.189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey A (1942). L’examen psychologique dans les cas d’encephalopathie traumatique. Arch Psychol, 28, 286–340. [Google Scholar]
- Rohlfing T, Brandt R, Menzel R, & Maurer CR Jr. (2004). Evaluation of atlas selection strategies for atlas-based image segmentation with application to confocal microscopy images of bee brains. Neuroimage, 21(4), 1428–1442. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15050568 [DOI] [PubMed] [Google Scholar]
- Rohlfing T, Zahr NM, Sullivan EV, & Pfefferbaum A (2010). The SRI24 multichannel atlas of normal adult human brain structure. Hum Brain Mapp, 31(5), 798–819. doi: 10.1002/hbm.20906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanski LM (2004). Domain specificity in the primate prefrontal cortex. Cogn Affect Behav Neurosci, 4(4), 421–429. doi: 10.3758/cabn.4.4.421 [DOI] [PubMed] [Google Scholar]
- Sadananthan SA, Zheng W, Chee MW, & Zagorodnov V (2010). Skull stripping using graph cuts. Neuroimage, 49(1), 225–239. doi: 10.1016/j.neuroimage.2009.08.050 [DOI] [PubMed] [Google Scholar]
- Sawyer KS, Adra N, Salz DM, Kemppainen MI, Ruiz SM, Harris GJ, & Oscar-Berman M (2020). Hippocampal subfield volumes in abstinent men and women with a history of alcohol use disorder. PLoS One, 15(8), e0236641. doi: 10.1371/journal.pone.0236641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt TP, Pennington DL, Cardoos SL, Durazzo TC, & Meyerhoff DJ (2017). Neurocognition and inhibitory control in polysubstance use disorders: Comparison with alcohol use disorders and changes with abstinence. J Clin Exp Neuropsychol, 39(1), 22–34. doi: 10.1080/13803395.2016.1196165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoville WB, & Milner B (1957). Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry, 20(1), 11–21. doi: 10.1136/jnnp.20.1.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shallice T, Fletcher P, Frith CD, Grasby P, Frackowiak RS, & Dolan RJ (1994). Brain regions associated with acquisition and retrieval of verbal episodic memory. Nature, 368(6472), 633–635. doi: 10.1038/368633a0 [DOI] [PubMed] [Google Scholar]
- Shields CN, & Gremel CM (2020). Review of Orbitofrontal Cortex in Alcohol Dependence: A Disrupted Cognitive Map? Alcohol Clin Exp Res, 44(10), 1952–1964. doi: 10.1111/acer.14441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner HA (1982). Development and validation of a lifetime alcohol consumption assessment procedure. Toronto: Addiction Research Foundation. [Google Scholar]
- Skinner HA, & Sheu WJ (1982). Reliability of alcohol use indices. The Lifetime Drinking History and the MAST. J Stud Alcohol, 43(11), 1157–1170. doi : 10.15288/jsa.1982.43.1157 [DOI] [PubMed] [Google Scholar]
- Smith SM (2002). Fast robust automated brain extraction. Hum Brain Mapp, 17(3), 143–155. doi: 10.1002/hbm.10062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, & Marsh L (2003). Hippocampal volume deficits in alcoholic Korsakoff's syndrome. Neurology, 61(12), 1716–1719. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=14694035 [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Mathalon DH, Ha CN, Zipursky RB, & Pfefferbaum A (1992). The contribution of constructional accuracy and organizational strategy to nonverbal recall in schizophrenia and chronic alcoholism. Biol Psychiatry, 32(4), 312–333. doi: 10.1016/0006-3223(92)90036-y [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Zahr NM, Sassoon SA, Thompson WK, Kwon D, Pohl KM, & Pfefferbaum A (2018). The Role of Aging, Drug Dependence, and Hepatitis C Comorbidity in Alcoholism Cortical Compromise. JAMA Psychiatry, 75(5), 474–483. doi: 10.1001/jamapsychiatry.2018.0021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takehara-Nishiuchi K (2020). Prefrontal-hippocampal interaction during the encoding of new memories. Brain Neurosci Adv, 4, 2398212820925580. doi: 10.1177/2398212820925580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe J, Tregellas JR, Dalwani M, Thompson L, Owens E, Crowley T, & Banich M (2009). Medial orbitofrontal cortex gray matter is reduced in abstinent substance-dependent individuals. Biol Psychiatry, 65(2), 160–164. doi: 10.1016/j.biopsych.2008.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tivis R, Beatty WW, Nixon SJ, & Parsons OA (1995). Patterns of cognitive impairment among alcoholics: are there subtypes? Alcohol Clin Exp Res, 19(2), 496–500. doi: 10.1111/j.1530-0277.1995.tb01537.x [DOI] [PubMed] [Google Scholar]
- Tulving E, Markowitsch HJ, Craik FE, Habib R, & Houle S (1996). Novelty and familiarity activations in PET studies of memory encoding and retrieval. Cereb Cortex, 6(1), 71–79. doi: 10.1093/cercor/6.1.71 [DOI] [PubMed] [Google Scholar]
- Tustison NJ, Avants BB, Siqueira M, & Gee JC (2011). Topological well-composedness and glamorous glue: a digital gluing algorithm for topologically constrained front propagation. IEEE Trans Image Process, 20(6), 1756–1761. doi: 10.1109/TIP.2010.2095021 [DOI] [PubMed] [Google Scholar]
- Victor M, Adams RD, & Colllins GH (1989). The Wernicke-Korsakoff Syndrome and Related Neurologic Disorders, 2nd Edition. Philadelphia: F. A. Davis Co. [Google Scholar]
- Volkow ND, & Fowler JS (2000). Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb Cortex, 10(3), 318–325. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10731226 [DOI] [PubMed] [Google Scholar]
- Wang J, Fan Y, Dong Y, Ma M, Ma Y, Dong Y, . . . Cui C (2016). Alterations in Brain Structure and Functional Connectivity in Alcohol Dependent Patients and Possible Association with Impulsivity. PLoS One, 11(8), e0161956. doi: 10.1371/journal.pone.0161956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (1987). Wechsler Memory Scale - Revised. San Antonio: The Psychological Corporation. [Google Scholar]