Abstract
Prior studies with permanent lesion methods have demonstrated a role for the retrosplenial cortex (RSC) in the retrieval of remotely, but not recently, acquired delay fear conditioning. To extend the generalizability of these prior findings, the present experiments used chemogenetics to temporarily inactivate the RSC during either retrieval or encoding of delay auditory fear conditioning. Inactivation of the RSC at the time of test impaired retrieval of a remotely conditioned auditory cue, but not a recently conditioned one. In addition, inactivation of the RSC during encoding had no impact on freezing during later retrieval testing for both a remotely and recently conditioned auditory cue. These findings indicate that the RSC contributes to the retrieval, but not encoding, of remotely acquired auditory fear conditioning, and suggest it has less of a role in both retrieval and encoding of recently acquired auditory fear conditioning.
Keywords: retrosplenial, DREADDs, chemogenetics, fear conditioning, retrieval
The retrosplenial cortex (RSC) contributes to a variety of cognitive and behavior functions, most notably spatial navigation and contextual learning and memory (Vann, Aggleton, & Maguire, 2009; Corcoran, Yamawaki, Leaderbrand, & Radulovic, 2018). For instance, when rats are exposed to two distinct contexts, typically defined as the collection of static features of the experimental environment (e.g., black arena vs. white arena), neuronal responses in the RSC exhibit context-specific firing patterns (Miller, Serrichio, & Smith, 2021). This suggests that RSC activity represents environmental contexts, perhaps in the service of contextual memory (Miller et al., 2021). Indeed, this notion is consistent with prior studies in which manipulation of the RSC (i.e., permanent lesions, temporary inactivation) have been shown to disrupt retrieval of contextual fear conditioning (Keene and Bucci, 2008; Corcoran et al., 2011).
The RSC also contributes to learning and memory for phasic, discrete cues that are presented in the foreground relative to the contextual environment (for a review see Todd, Fournier, & Bucci, 2019). For example, Gabriel and colleagues have reported increased RSC neuronal activity in response to a brief tone stimulus that predicted shock in an avoidance paradigm with rabbits (for reviews see Gabriel, 1993; Smith, Miller, & Vedder, 2018). RSC manipulation has also been shown to impact learning and memory for phasic auditory cues. In particular, the RSC appears to have an important role in trace fear conditioning, in which there is a brief interval between offset of the auditory conditioned stimulus (CS) and onset of the shock unconditioned stimulus (US). Disruption of the RSC during conditioning, either via protein synthesis inhibition or optogenetic silencing, impairs memory formation during trace fear conditioning (Kwapis et al., 2015; Trask et al., 2021). Blocking RSC NMDA receptors at the time of test also impairs trace fear memory retrieval (Kwapis et al., 2014). Thus, there is evidence that the RSC is both active and necessary for some forms of learning and memory for auditory cues.
In contrast to trace fear conditioning, there is often little impact of RSC manipulation on delay fear conditioning, in which the offset of the CS coincides (or overlaps) with onset of the US. For example, pre-training permanent lesions of the RSC typically have no impact on delay fear conditioning to either an auditory (Keene & Bucci, 2008; Robinson et al., 2018) or visual cue (Jiang et al., 2018). Likewise, post-training permanent lesions of the RSC do not impact the retrieval / expression of delay fear conditioning (Keene & Bucci, 2008). More selective methods have produced similar results. Blocking protein synthesis in the RSC prior to conditioning does not impair acquisition of delay fear conditioning (Kwapis et al., 2015), and blocking NMDA receptors during retrieval also has no effect on retrieval / expression of delay fear conditioning in rats (Kwapis et al., 2014; 2015) or mice (Corcoran et al., 2011).
However, the role of the RSC in the retrieval of delay fear conditioning may be related to the age of the memory. For instance, in the studies mentioned previously, manipulation of the RSC during retrieval occurred shortly after initial conditioning, thus the delay fear - conditioned memory was considered ‘recently’ acquired. In these cases, manipulation of the RSC did not impact behavior (Corcoran et al., 2011; Kwapis et al., 2014; 2015). In contrast, we have shown that when lesions of the RSC occur 28 days after initial conditioning, what we consider ‘remote’ memory, the retrieval / expression of delay fear conditioning to either visual (Jiang et al., 2018) or auditory cues (Todd et al., 2016) is impaired. Thus, the role of the RSC in the retrieval of delay fear conditioning appears to be time-dependent, with the RSC contributing to the retrieval of remotely, but not recently, acquired memories.
All prior studies demonstrating a role for the RSC in the retrieval of remotely acquired delay fear conditioning utilized permanent lesion methods (Jiang et al., 2018; Todd et al., 2016). Thus, one purpose of the present experiments was to extend the generalizability of these prior studies by temporarily inactivating the RSC specifically at the time of retrieval. To do so, we used chemogenetics (DREADDs, designer receptors exclusively activated by designer drugs) to temporarily inhibit neural activity along the rostro-caudal extent of the RSC. In addition, this method allowed us to assess whether temporary inactivation of the RSC during the initial conditioning session only would later impair fear expression during a test of remote memory. Although we were primarily interested in remotely acquired delay fear conditioning, we also examined recently acquired delay fear conditioning to demonstrate the specificity of our manipulations on behavior.
Experiments 1a and 1b
The purpose of Experiments 1a and 1b was to assess the role of the RSC in the retrieval of remotely or recently acquired delay fear conditioning. All rats first underwent surgery and were infused with a virus containing either an inhibitory DREADD receptor (hM4Di) or a control virus targeting the RSC. Following recovery, all rats underwent delay fear conditioning, which consisted of three tone-shock pairings. Half of the rats had a 28-day retention interval before behavioral testing (to test “remote” memory; Experiment 1a), whereas the other half began behavioral testing after only 1 day (to test “recent” memory; Experiment 1b). All rats received injections of the DREADD agonist clozapine-n-oxide (CNO) just prior to the start of the tone test session.
Methods
Subjects
Subjects were 20 (Experiment 1a) and 32 (Experiment 1b) behaviorally naïve adult male Long Evans rats obtained from Envigo Laboratories (Indianapolis, IN, USA) that were ~60 days old upon arrival. All rats were pair-housed and allowed 6–17 days to acclimate to the vivarium before undergoing surgical procedures. Rats were individually housed after surgery and for the remainder of the experiment. Rats were allowed access to standard rat chow (Nestle Purina, St. Louis, MO, USA) and water ad libitum for the duration of the experiment. Rats were maintained on a 14:10 light-dark cycle and monitored and cared for in compliance with association for Assessment and Accreditation of Laboratory Animal Care guidelines and the Dartmouth College Institutional Animal Care and Use Committee.
Surgery
After the acclimation period, all rats underwent surgeries. Rats were anesthetized with isoflurane gas (1.5–3% in oxygen) and placed in a stereotaxic apparatus (Kopf Instruments, Tujunga, CA). The skin above the skull was retracted and a craniotomy was performed. Skull penetrating burr holes were drilled above the intended injection sites at the locations listed in Table 5.1. At each site, a 28-g Hamilton syringe was lowered into the brain and was used to infuse 0.8 μl (0.2 μl/min) of either the inhibitory DREADD virus pAAV8-hSyn-hM4Di-mCherry (abbreviated “hM4Di”) or a control virus pAAV8-hSyn-EGFP (abbreviated “GFP”; Addgene, Inc., Watertown, MA). After the syringe was lowered to the intended depth, it was left in place for one minute prior to starting the infusion and for two minutes after the termination of the infusion. Twenty-six rats (Experiment 1a, n = 10; Experimental 1b, n = 16) underwent infusions of the inhibitory DREADD virus, which contained the DNA for the inhibitory DREADD receptor, hM4Di. The remaining 26 (Experiment 1a, n= 10; Experimental 1b, n = 16) rats were infused with the control virus, that lacked the DNA for the hM4Di receptor but still expressed the fluorescent reporter eGFP. Rats were monitored after surgery and were allowed 33–52 days to recover before fear conditioning and behavioral testing.
Table 1.
Stereotaxic coordinates for Experiments 1, 2, and 4.
| Experiment | Anterior-Posterior | Medial-Lateral | Dorsal-Ventral |
|---|---|---|---|
| Exps 1, 2, & 4 | −2 | ±0.3 | −2.6 |
| −3.5 | ±0.3 | −2.4 | |
| −5.0 | ±0.3 | −2.6 | |
| −6.5 | ±1.0 | −2.4 | |
| −8.0 | ±1.5 | −2.5 |
Note. All anterior-posterior and medial-lateral measurements are derived from bregma and midline respectfully. Dorsal-ventral measurements were derived from the cortical surface. All measurements are in mm.
Behavioral Apparatus
Two sets of four conditioning chambers were used as Contexts A and B throughout the experiment. The first set of four conditioning chambers were of the same standard design (Med Associates, Inc., St. Albans, VT, USA, ENV-007; 24 cmW×30.5 cm L×29 cm H) and each chamber was housed in its own sound attenuating chamber (Med Associates, ENV-017 M; 66 cmW×56 cm H×56 cm D). Each sound attenuating chamber contained an exhaust fan to provide background noise (~68 dB) and provide steady airflow. The conditioning chambers had an acrylic plastic back wall, ceiling, and door, while the side walls were made of brushed aluminum. The grid floors were made up of stainless-steel rods (5-mm diameter) that were spaced 1.5-cm apart (center-to-center). Each conditioning chamber was outfitted with a food cup that was recessed within the center of the front wall (not used in this experiment), a retractable lever (Med Associates, ENV-112CM) to the right of the food cup (the lever remained retracted for the duration of the experiment), a panel light (Med Associates, ENV-221M) mounted ~16 cm above the grid floor centered above the food cup and a house light (Med Associates, ENV-215M) mounted ~24 cm above the grid floor on the back wall of the chamber. A speaker (Med Associates, ENV-224AM) was located in the top right corner of the same wall (~20 cm from the grid floor) that contained the recessed food cup. This set of conditioning chambers was only illuminated by a 2.8-W bulb with a red cover fitted to the interior wall of the sound attenuated chamber to allow for video recording, all other lights were off. The chambers were monitored by a video camera mounted to the back wall of the sound attenuating chamber. To provide a distinct olfactory cue, standard sawdust bedding (~1 cup) was used to fill the underlying metal tray. Lastly, manila folders were mounted to the exterior of the door and ceiling of the conditioning chamber.
The second set of four conditioning chambers was identical to the first set with the following exceptions. First, the grid floor was staggered so that odd- and even-numbered stainless-steel rods were mounted in two separate planes, 0.5 cm above the other. This arrangement provided a distinct tactile feature for this context. Second, in addition to the red light, the house and the panel lights were both illuminated for the duration of the experiment. Third, to provide a distinct visual cue, checkered wallpaper (1 cm black and white squares) was hung on the exterior of the door and ceiling. Fourth, a 10% anise (McCormick & Co Inc., Hunt Valley, MD, USA) in water solution was used as the unique olfactory cue. Approximately 5 mL of the anise solution was placed in a weigh boat and placed near the conditioning chamber. No sawdust was used. The two sets of conditioning chambers were counterbalanced so that half of the rats in each virus group (GFP & hM4Di) underwent conditioning in the first set and the remainder of rats in each group had conditioning in the second set. The set not used for conditioning was considered Context B and was used for tone testing.
The conditioned stimulus (CS) used in Experiment 1 was a 10-s, 1500 Hz tone. The unconditioned stimulus (US) was a 1-mA, 1-s footshock, generated by a Med Associates shock generator (ENV-414), and delivered through the metal rods of the grid floor. The US immediately followed the offset of the CS.
Behavioral Procedures
All rats underwent Pavlovian delay cue fear conditioning in Context A. This session consisted of 3 presentations of the CS, a 10-s tone, which co-terminated with the onset of the US, a 1-mA, 1-s shock. The first trial began three minutes after rats were placed in Context A. The time between shock and the next CS presentation (inter-trial interval; ITI) was 64-s. Rats were removed from the conditioning chamber ~4 minutes after the last tone-shock trial.
Further testing occurred either 28 days (Experiment 1a) or 1 day (Experiment 1b) following initial conditioning. After these respective retention intervals, rats were exposed to Context A in the absence of the discrete CS or US for a single 20-minute session. Twenty-four hours following the Context A test, rats were placed in Context B for a single 20-minute session in the absence of the CS or the US. The purpose of this session was to reduce fear generalization and familiarize rats with Context B.
The final test of auditory fear retrieval occurred twenty-four hours after the Context B session. All rats were injected intraperitoneally (i.p.) with the DREADD activating ligand CNO. Thirty minutes after the injection of CNO, all rats were returned to Context B for a 20-minute tone test session where the tone CS was presented 20 times (10-s each, 30-s ITI) beginning 3 minutes after the rat was placed in the chamber. The shock was not presented during the tone tests.
Drug Preparation and Administration
CNO solution was freshly prepared immediately before both the tone test session. CNO was weighed and dissolved into dimethyl sulfoxide (1% DMSO) followed by 0.9% sterile saline to obtain a final concentration of 2 mg/ml. CNO (4 mg/kg) was administered i.p. 30 minutes prior to the rats being placed in Context B for the tone test.
Behavioral Observations
Freezing was the primary variable of interest. Freezing was defined as the cessation of all movement except what is required for respiration (Blanchard & Blanchard, 1969). During the conditioning session, behavior for each rat was scored every 8-seconds during the 64-second period prior to the first conditioning trial (baseline freezing) and following every conditioning trial (post-shock freezing). Rats were scored every 8-seconds for the first 8 minutes of both the Context A and Context B exposure sessions. During the tone test session, behavior was scored every 8-seconds during the 64-seconds prior to the first tone presentation (baseline freezing). Rats were then scored every 2-seconds for the duration of the 10-second CS presentation. The frequency of freezing incidences was converted to a percentage of time spent freezing.
Data Analysis
Analyses of freezing behavior were conducted using analysis of variance (ANOVA) using virus type (GFP & hM4Di) as the between-subjects variables. The critical test session was divided into 4 blocks of 5 trials each. An alpha level of 0.05 was used for all analyses. SPSS was used to complete all statistical testing.
Virus Verification and Analysis
After the final behavioral testing session was complete, rats were deeply anesthetized with sodium pentobarbital and transcardially perfused with 0.9% saline (~250 ml) followed by 10% buffered formalin (~300 ml). Brains were removed and remained in formalin for 24 hours followed by 72 hours in 30% sucrose. A freezing microtome was used to collect 40 μm coronal brain sections throughout the RSC (and just anterior and just posterior to RSC), which were kept at 4° in phosphate buffer until being mounted onto gelatin-coated glass slides.
A compound fluorescent microscope (Axioskop I, Zeiss, Inc.) was used to visualize the fluorescent reporters, mCherry (hM4Di) and eGFP (control). The percentage of sections exhibiting virus expression in RSC was determined for each rat. In addition, the level of viral expression was assessed by rating the extent of expression on a 0 – 5 scale (0 = no expression, 5 = full structural expression) as in our prior studies (Fournier et al., 2019; Robinson et al., 2014; Todd et al., 2016). Virus expression in adjacent structures was also noted.
Results and Discussion
In Experiment 1a, one GFP rat was removed from analysis due to a mechanical failure during the conditioning session. Further, one hM4Di rat had adverse reactions (e.g., minor convulsions) to the CNO injection and was removed from the experiment. The final analyses consisted of 9 hM4Di and 9 GFP control rats. In Experiment 1b, two rats were removed from Group hM4Di due to adverse reactions to the CNO injection. The final analyses consisted of 14 hM4Di and 16 GFP control rats.
Histology
Virus expression for both hM4Di and GFP groups was visible throughout the rostro-caudal extent of the RSC and is illustrated in Figure 1. In Experiment 1a, the average percentage of RSC-containing sections (~23 sections) that had virus expression was 99% in rats in both of the virus groups (GFP and hM4Di). The average virus expression rating was 2.6 ± 0.1 in the hM4Di group and 2.4 ± 0.1 in the GFP group. The GFP virus had minor spread into the dorsal subiculum (n = 10), motor cortex (M2) (n = 1), cingulate cortex (n = 5), post-subiculum (n = 4), and secondary visual cortex (n = 4). The hM4Di-mcherry virus had minor spread into the dorsal subiculum (n = 14), post-subiculum (n = 4), and secondary visual cortex (n = 7).
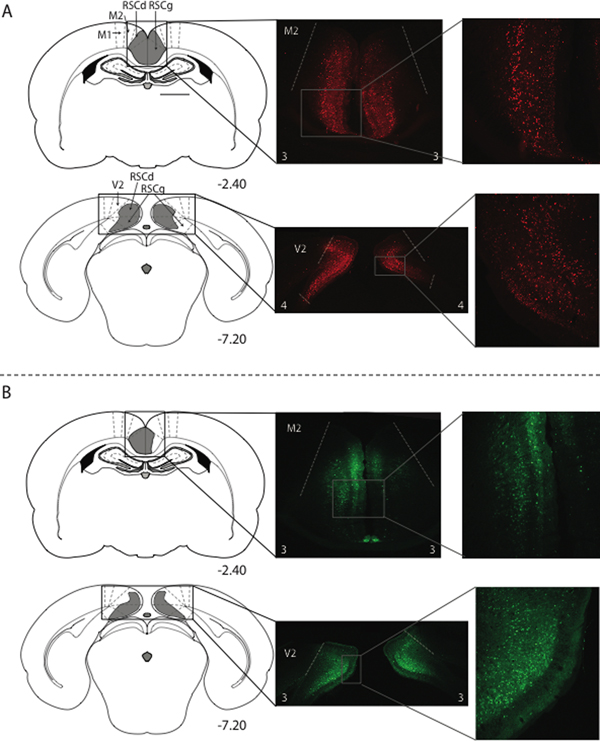
Figure 1.
Histology results from experiment 1a and 1b. Virus expression in anterior and posterior portions of the RSC in a rat from the hM4Di-mcherry group (A) and a rat from group GFP (B) Schematics in the left column depict the extent of virus expression within the RSC, with the numbers below each section indicating the A/P position in mm relative to bregma based on Paxinos and Watson (2009). Low magnification (middle column) and high magnification (right column) images show virus-expressing cells. The white numbers at the bottom of each photomicrograph in the middle column indicate the expression rating for that section. M2 = secondary motor cortex; RSCd = restrosplenial dysgranular; RSCg = retrosplenial granular, V2 = secondary visual cortex. GFP = AAV- hSyn-GFP and hM4Di = AAV-hSyn-hM4D(Gi)-mcherry.
In Experiment 1b the average percentage of RSC-containing sections (~24 sections) that had virus expression was 99.4% in hM4Di rats and 99.8% in GFP rats. The average virus expression rating was 2.3 ± 0.09 in the hM4Di group and 2.6 ± 0.06 in the GFP group. The GFP virus had minor spread into the dorsal subiculum (n = 7), motor cortex (n = 1), cingulate cortex (n = 9), post-subiculum (n = 5), and dorsal hippocampus (CA1 region) (n = 8). The hM4Di-mCherry virus had minor spread into the dorsal subiculum (n = 9), motor cortex (n = 1), cingulate cortex (n = 8), post-subiculum (n = 1), and secondary visual cortex (n = 2), and dorsal hippocampus (CA1-CA3 regions) (n = 1).
Behavior
The mean percentage freezing during all sessions (conditioning, Context A exposure, and Context B exposure) are presented in the left panel of Figure 2A for Experiment 1a (top) and 1b (bottom). The mean percent freezing during the tone test session is presented as 5 trial blocks in the right panel of Figure 2A.
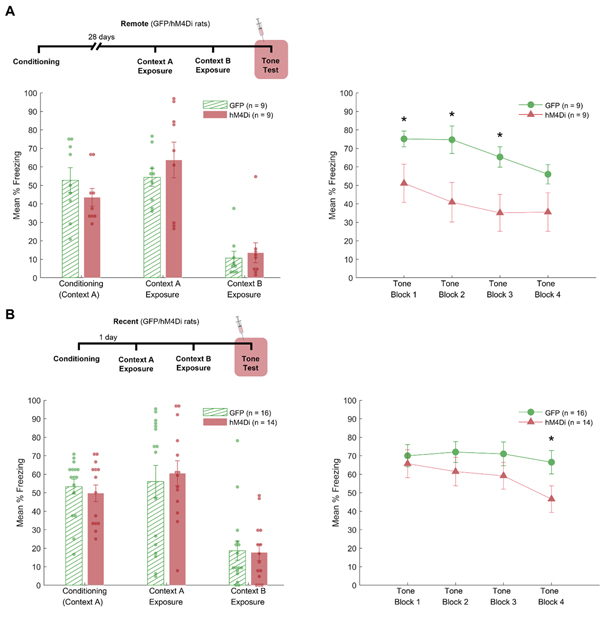
Figure 2.
Results for experiment 1. The figure shows the mean percentage freezing for all phases of the experiment for both remote (A) and recent (B) memory when RSC is inactivated during retrieval (tone test). The mean percentage freezing for the conditioning, context A and context B exposure sessions are shown on the left panel, while the right panel shows the mean percentage freezing during the tone test session in four 5-trials blocks. * = p < .05.
During the conditioning session of Experiment 1a, freezing during the baseline period was 0% in both virus groups. A one-way ANOVA comparing the average level of freezing after the three conditioning trials (post-shock freezing) was not significant between the two virus groups, F(1,16)= 1.26, p = .28. Likewise, freezing between the two virus groups was comparable when the rats were exposed to Context A and Context B, both Fs < 1.
During the final tone test in Context B, baseline freezing (64 s prior to the first tone) did not differ between groups, F < 1. Mean percent freezing during the baseline period was 8.3% (SEM = 4.2) for GFP and 13.8% (SEM = 5.7) for hM4Di. For freezing to the tone, a 2 (Group: GFP vs. hM4Di) × 4 (Trial Block) ANOVA revealed a significant main effect of group F(1,16) = 6.9, p = .02. Thus, freezing to the tone was significantly lower for Group hM4Di compared to Group GFP. There was also a significant effect of trial block, F(3,48) = 5.6, p = .002. Although the interaction between group and trial block was not significant, F < 1, planned comparisons on each of the 5-trial blocks revealed significant differences between groups during block 1 (F(1,16) = 4.6, p = .047), block 2 (F(1,16) = 6.7, p = .02), and block 3 (F(1,16) = 7.1, p = .02). There was no significant difference for block 4, F(1,16) = 3.1, p = .1).
In Experiment 1b, freezing during the baseline period of the conditioning session was 0% in both virus groups. During the conditioning session, post-shock freezing did not differ between groups, F < 1. Likewise, groups did not differ when exposed to either Context A or Context B, Fs < 1. During the tone test session, baseline freezing was not different between groups, F(1, 28) = 2.6, p = .12. The mean percent freezing during the baseline period was 18.8% (SEM = 6.4) for Group GFP and 7.1% (SEM = 2.5) for Group hM4Di. Freezing during tone presentations was analyzed with a 2 (Group) × 4 (Trial Block) ANOVA. This analysis revealed a significant main effect of trial block, F(3,84) = 3.5, p = .02. The main effect of group was not significant, F(1, 28) = 2.0, p = .17, and neither was the group × trial block interaction, F(3,84) =1.4, p = .26. Planned comparisons on each of the 5-trial blocks revealed a significant difference between groups only during block 4, F(1,28) = 4.4, p = .046. The group effect was not significant for block 1 (F < 1), block 2 (F(1,28) = 1.3, p = .27), or block 3 (F(1,28) = 1.5, p = .23). P
The results of Experiments 1a and 1b reveal that temporary inactivation of the RSC during retrieval testing impairs delay auditory fear conditioning that was acquired remotely, but not recently. In the remote condition (Exp. 1a), the main effect of group indicated freezing was overall lower for Group hM4Di, and planned comparisons revealed group differences in the first 3 trial blocks (15 total trials). In contrast, the main effect of group was not significant in the recent condition (Exp. 1b), although planned comparisons indicated that freezing in the recent condition was lower in the last 5-trial block of the retrieval session. This reduction in freezing at the end of the session might reflect changes in associative (e.g., extinction) or nonassociative (e.g., habituation) mechanisms (Myers & Davis, 2007). Nevertheless, the fact that freezing was not impaired during the initial 3 trial blocks (15 total trials) indicates that retrieval of recently acquired delay fear conditioning was not impaired. Finally, we note that there was little generalized fear to the novel context (Context B) for either recent or remote time points. This may have been unexpected given that remotely acquired contextual fear conditioning often generalizes to novel contexts (Ortiz et al., 2019; Wiltgen & Silva, 2007). However, the lack of generalization in the present experiments may have occurred because fear to Context A alone was first extinguished prior to exposure to Context B.
Experiments 2a and 2b
Although the RSC is necessary for the retrieval of delay fear conditioning, it is unknown if RSC activity during encoding is necessary for subsequent consolidation and/or retrieval. Therefore, we next examined the impact of transiently inactivating the RSC during delay fear conditioning on remote (Experiment 2a) and recent (Experiment 2b) retrieval. If RSC activity during conditioning is necessary for later retrieval, then it would be expected that the initial inactivation of the RSC during conditioning would impair subsequent memory retrieval.
Methods
Subjects
Subjects were 16 (Experiment 2a) and 20 (Experiment 2b) behaviorally naïve adult male Long Evans rats obtained from Envigo Laboratories (Indianapolis, IN, USA) and were ~60 days old upon arrival. All rats were pair-housed and allowed 4–9 days to acclimate to the vivarium before undergoing surgical procedures. Rats were otherwise maintained as in Experiment 1.
Surgery
Eighteen rats underwent infusions of the inhibitory DREADD virus, which contained the DNA for the inhibitory DREADD receptor, hM4Di. The remaining eighteen rats were infused with the control virus that lacked the DNA for the hM4Di receptor but still expressed the fluorescent reporter eGFP. Rats were monitored after surgery and were allowed 26–41 days to recover before fear conditioning and behavioral testing. All other surgical procedures were identical to Experiment 1.
Behavioral Apparatus and Procedures.
Rats were trained in the same behavioral apparatuses as were described in Experiment 1. The behavioral procedures were identical to Experiments 1, with the exception that all rats were injected intraperitoneally (i.p.) with CNO 30 minutes prior to Pavlovian delay cue fear conditioning. CNO was freshly prepared immediately before the conditioning session using the same procedure as Experiment 1.
Behavioral Observations, Data Analysis, and histology.
Behavioral observations, data analyses, and histological preparation and analyses were performed as described in Experiment 1.
Results and Discussion
Histology
In Experiment 2a, virus expression for Group hM4Di (n = 8) and GFP (n = 8) was visible throughout the rostro-caudal extent of the RSC and is illustrated in Figure 3. The average percentage of RSC-containing sections (~30 sections) that had virus expression was 99.6% in hM4Di rats and 97.8% in GFP rats. The average virus expression rating was 2.7 ± 0.12 in the hM4Di group and 2.3 ± 0.14 in the GFP group. The GFP virus had minor spread into the dorsal subiculum (n = 3) and secondary visual cortex (n = 4). The hM4Di-mcherry virus had minor spread into the dorsal subiculum (n = 8) and secondary visual cortex (n = 8).
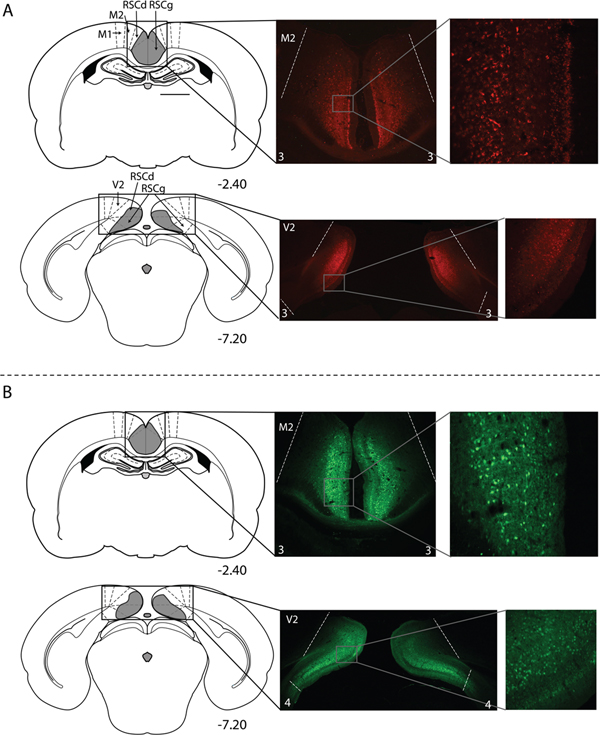
Figure 3.
Histology results from experiment 2a and 2b. Virus expression in anterior and posterior portions of the RSC in a rat from the hM4Di-mcherry group (A) and a rat from group GFP (B) Schematics in the left column depict the extent of virus expression within the RSC, with the numbers below each section indicating the A/P position in mm relative to bregma based on Paxinos and Watson (2009). Low magnification (middle column) and high magnification (right column) images show virus-expressing cells. The white numbers at the bottom of each photomicrograph in the middle column indicate the expression rating for that section. M2 = secondary motor cortex; RSCd = restrosplenial dysgranular; RSCg = retrosplenial granular, V2 = secondary visual cortex. GFP = AAV- hSyn-GFP and hM4Di = AAV-hSyn-hM4D(Gi)-mcherry.
In Experiment 2b, one rat died following surgery. The final analyses thus consisted of 10 hM4Di and 9 GFP control rats. The average percentage of RSC-containing sections (~24 sections) that had virus expression was 96.2% in hM4Di rats and 100% in GFP rats. The average virus expression rating was 2.1 ± 0.14 in the hM4Di group and 2.6 ± 0.12 in the GFP group. The GFP virus had minor spread into the dorsal subiculum (n = 1), motor cortex (n = 2), cingulate cortex (n = 5), post-subiculum (n = 2), and dorsal hippocampus (CA1 region) (n = 7). The hM4Di-mcherry virus had minor spread into the dorsal subiculum (n = 5), cingulate cortex (n = 4), post-subiculum (n = 3), and dorsal hippocampus (CA1 region) (n = 5).
Behavior
The mean percentage freezing during all sessions (Conditioning, Context A exposure, and Context B exposure are presented in the left panel of Figure 4 for Experiment 2a (top) and 2b (bottom). The mean percent freezing during the tone test session is presented as 4 blocks of 5 trials in the right panel of Figure 4.
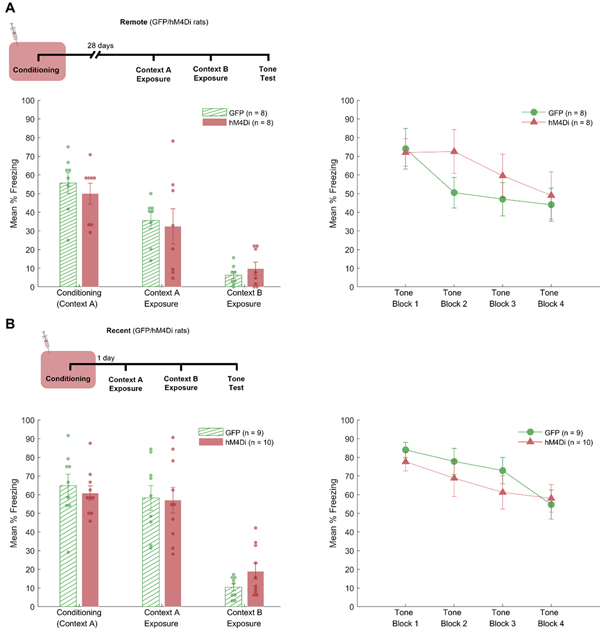
Figure 4.
Results for experiment 2. The figure shows the mean percentage freezing for all phases of the experiment for both remote (A) and recent (B) memory when RSC is inactivated during encoding (conditioning). The mean percentage freezing for the conditioning, context A and context B exposure sessions are shown on the left panel, while the right panel shows the mean percentage freezing during the tone test session in four 5-trials blocks.
For Experiment 2a (remote condition), freezing during the baseline period of the conditioning session was 0% in both virus groups. Post-shock freezing during the conditioning session did not differ between groups, F < 1. Likewise, there were no group differences when rats were exposed to Context A or Context B, Fs < 1. During the tone test session, baseline fear was low and did not differ between groups F < 1. Mean percent freezing during the baseline period was 3.1% (SEM = 2.0) for Group GFP and 6.3% (SEM = 3.3) for Group hM4Di. Freezing during the tone was analyzed with a 2 (Group) × 4 (Trial Block) ANOVA. This analysis revealed a significant effect of trial block, F(3,42) = 7.5, p < .001. The effect of group was not significant, F < 1, and neither was the interaction between group and trial block, F(3, 42) = 1.5, p = .22.
For Experiment 2b freezing during the baseline period of the conditioning session was 0% in both virus groups. Post-shock freezing did not differ between groups, F < 1. There was no difference in freezing levels when rats were re-exposed to Context A, F < 1, or when they were exposed to Context B, F(1,17) = 3.1, p = .1. During the tone test session, baseline freezing was low and did not differ between groups, F < 1. Mean percent freezing was 8.3% (SEM = 3.6) for Group GFP and 13.8% (SEM = 5.1) for group hM4Di. Freezing during tone presentations was analyzed with a (Group) × 4 (Trial Block) ANOVA. This analysis revealed a main effect of trial block, F(3, 51) = 12.4, p < .001. Neither the main effect of group (F < 1), nor the interaction between trial block and group were significant, F(3, 51) = 1.2, p = .31.
In the current experiment the RSC was inactivated during the initial encoding of delay fear conditioning. Memory retrieval was then tested 28 days later (Exp. 2a) or 1 day later (Exp. 2b) with the RSC back “online”. During the final test session, there was no apparent impact of the initial RSC inactivation on subsequent remote or recent memory retrieval. Coupled with the results of Experiments 1a and 1b, this suggest that the RSC is selectively necessary for the retrieval of delay auditory fear conditioning.
Experiments 1 and 2 also provide important information about the chemogenetic method used to silence the RSC. First, in both experiments, there were no group differences observed in the absence of CNO, indicating that hM4Di (mCherry) or GFP expression alone did not differentially impact freezing behavior. Second, silencing the RSC had no impact on post-shock freezing (Exp 2a/b) or retrieval of recently acquired delay fear conditioning (Exp 1b). Thus, even the combination of hM4Di receptor expression and CNO injections does not result in general freezing deficits; impaired freezing was only observed during the retrieval of remotely acquired delay auditory fear conditioning (Exp 1a). Nevertheless, the impact of CNO on behavior is further investigated in Experiments 3a and 3b.
Experiments 3a and 3b
Recent studies have shown that CNO is actively metabolized in vivo to clozapine, a psychoactive compound that can activate multiple endogenous receptors (Gomez et al., 2017; Ashby & Wang, 1996; Mahler & Jones, 2018). Furthermore, the active compound clozapine may affect and induce DREADD-independent changes in behaviors (Manvich et al., 2018; MacLauren et al., 2016). To control for these possibilities, in Experiments 1 and 2, both the GFP group and hM4Di group received CNO injections. A general effect of CNO alone would not predict the deficit in remote retrieval observed in Experiment 1a. Nevertheless, to directly assess the impact of CNO on behavior in our own laboratory, unoperated rats received either an injection of CNO or Vehicle prior to retrieval testing for a remotely (Exp 3a) or recently (Exp 3b) acquired delay fear conditioning.
Methods
Subjects
The subjects were 23 (Experiment 3a) and 24 (Experiment 3b) behaviorally naïve adult male Long Evans rats obtained from Envigo Laboratories (Indianapolis, IN, USA) and were ~60 days old upon arrival. All rats were pair-housed and allowed 13–20 days to acclimate to the vivarium before undergoing fear conditioning and behavioral testing.
Behavioral Apparatus and Procedures.
Rats were trained in the same behavioral apparatuses as were described in Experiment 1. The behavioral procedures were identical to Experiment 1, with the following exception. None of the rats underwent surgery, and rats were assigned to either receive CNO or Vehicle during the remote (Experiment 3a) or recent (Experiment 3b) tone test session. Groups were matched based on freezing during the initial conditioning session. Thirty minutes prior to the final tone test session, half of the rats (CNO group) were injected intraperitoneally (i.p.) with CNO, and the other half (Vehicle group) received injections of vehicle (1% DMSO in 0.9% physiological saline). Both CNO and vehicle were freshly prepared using the same procedure as Experiment 1. Behavioral observations and data analyses were performed as described in Experiment 1.
Results and Discussion
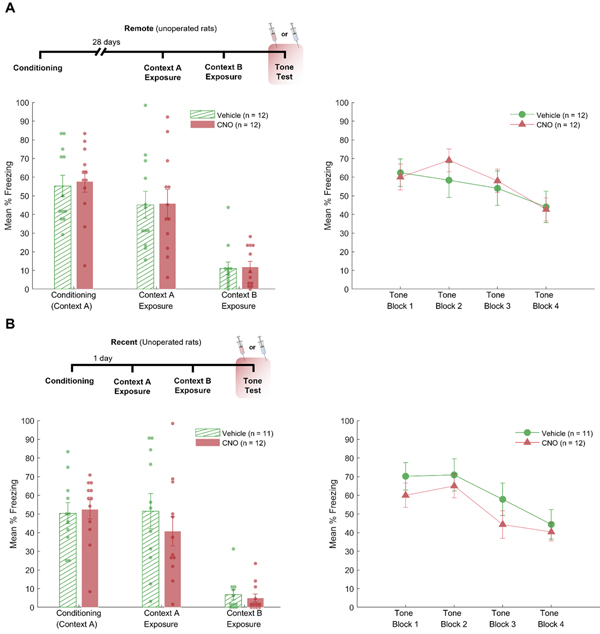
The results of Experiment 3a are depicted in Figure 5a. During the conditioning session, baseline freezing did not differ between groups, F < 1. Mean percent freeing was 2.1% (SEM = 1.4) for Group GFP and 1.0% (SEM = 1.0) for Group hM4Di. Post-shock freezing did not differ between groups, F(1,22) = 0.47, p = .5. The groups did not differ when they were exposed to Context A or Context B, both Fs < 1. During the final tone test, in which groups were first injected with either CNO or Vehicle, the level of baseline freezing between groups did not differ, F < 1. Baseline freezing was 3.1% (SEM = 2.2) for Group GFP and 1.0% (SEM = 1.0) for Group hM4Di. Freezing during the tone was analyzed with a 2 (Group) × 4 (Trial Block) ANOVA. This analysis revealed a significant main effect of trial block, F(3, 66) = 7.6, p < .001. Neither the main effect of group nor the interaction between group and trial block were significant, Fs < 1.
Figure 5.
Results for experiment 3. The figure shows the mean percentage freezing for all phases of the experiment for both remote (A) and recent (B) memory when CNO or vehicle is administered during the retrieval (tone test) phase in the absence of DREADD expression. The mean percentage freezing for the conditioning, context A and context B exposure sessions are shown on the left panel, while the right panel shows the mean percentage freezing during the tone test session in four 5-trials blocks.
The results of Experiment 3b are presented in Figure 5b. In the conditioning session, freezing during the baseline period was 0% for both groups. There were no significant differences between the two groups (CNO vs. Vehicle) during conditioning, Context A exposure, or Context B exposure (all Fs < 1). During the tone test session, baseline freezing did not differ between groups, F < 1. Mean percent freezing was 1.1% (SEM = 1.1) for Group GFP and 3.1% (SEM = 1.6) for Group hM4Di. Freezing to the tone was analyzed with a 2 (Group) × 4 (Trial Block) ANOVA. This revealed a significant effect of trial block, F(3, 63) = 15.8, p < .001. Neither the main effect of group nor the interaction with trial block was significant, Fs < 1.
In Experiments 3a and 3b the impact of CNO on freezing behavior was directly assessed by comparing CNO to vehicle injections during retrieval of remotely or recently acquired delay auditory fear conditioning in unoperated rats. In both experiments, there were no differences in freezing behavior for rats injected with CNO vs. Vehicle. This finding provides further support that the freezing deficit observed in Experiment 1a is not due to injections of CNO alone.
Experiment 4
The purpose for the final experiment was to verify the efficacy of the chemogenetic methods utilized in Experiments 1 and 2. To do this, we measured electrical responses of RSC GFP+ (control) and mCherry+ (hM4Di) neurons in vitro to depolarizing current injections before and after exposing them to bath-applied CNO (5 μM). If CNO acts only at M4Di receptors, our expectation is that its presence should selectively inhibit action potential generation in mCherry+ neurons and should not change the excitability of control cells expressing GFP.
Methods
Subjects
The subjects were four behaviorally naïve adult male Long Evans rats, obtained from Envigo Laboratories (Indianapolis, IN, USA) and were ~60 days old upon arrival. All rats were pair-housed and allowed 4–9 days to acclimate to the vivarium before undergoing surgical procedures. Rats were otherwise maintained as in Experiment 1.
Surgery
Surgical procedures were identical to Experiment 1a with two rats injected with GFP and two rats injected with hM4Di viruses respectively. Rats were monitored after surgery and were allowed 38–44 days to recover prior to the experiment.
Slice preparation.
Rats were anesthetized with vaporized isoflurane, decapitated, and brains rapidly removed into artificial cerebral spinal fluid (aCSF) composed of (in mM): 125 NaCl, 25 NaHCO3, 3 KCl, 1.25 NaH2PO4, 0.5 CaCl2, 6 MgCl2, and 25 glucose (saturated with 95% O2/5% CO2). Coronal brain slices (250 μm thick) of the RSC were cut using a Leica VT 1200 slicer and stored in a holding chamber containing aCSF adjusted to 2 mM CaCl2 and 1 mM MgCl2. Slices were maintained in the holding chamber for 1 hour at 35 °C and then at room temperature (~27 °C) until use in experiments.
Electrophysiology.
Slices were placed in a recording chamber perfused continuously with oxygenated aCSF heated to 35–36 °C. Pyramidal neurons expressing hM4Di-mCherry or GFP were identified using epifluorescence (470 or 530 nm excitation) and patched under visual control using a 60x water-immersion objective paired with a CMOS camera. Patch-pipettes (5–7 MΩ) contained a solution consisting of (in mM) 135 K-gluconate, 2 NaCl, 2 MgCl2, 10 HEPES, 3 Na2ATP, and 0.3 NaGTP, pH 7.2 with KOH. Data were acquired using Axograph software driving a BVC-700 amplifier (Dagan) via an ITC-18 digitizer (HEKA). Membrane potentials were sampled at 25 kHz, filtered at 10 kHz, and corrected for the liquid junction potential of +12 mV. Depolarizing current steps were adjusted to evoke ~10 action potentials in baseline conditions, and then triggered at 10 second intervals. Measurements were made of the number of action potentials generated by current steps before and after addition of 5 μM CNO. Slices of RSC were exposed to only a single application of CNO.
Results
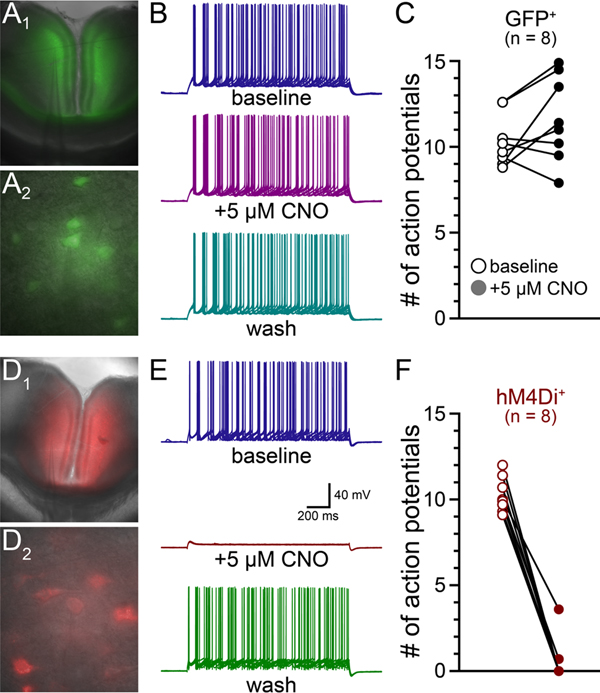
To confirm the efficacy of hM4Di-mediated inhibition of cortical neurons, in slices of RSC from control and hM4Di-injected rats we compared the effects of CNO (5 μM) on action potential generation in control (GFP+; n = 8) and hM4Di+ (mCherry+; n = 8) pyramidal neurons (Figure 6A, D). Current steps (1.5 s duration; applied at 0.1 Hz) were adjusted to evoke ~10 action potentials in baseline conditions. After 20 baseline trials, CNO (5 μM) was bath applied for 5 minutes (Figure 6B, E). In control (GFP+) neurons, current steps generated a similar number of action potentials in baseline (mean of 10.4 ± 1.5 spikes) and CNO (11.6 ± 2.5 spikes) conditions (p = 0.12; Figure 6C). On the other hand, CNO reduced the number of action potentials in hM4Di+ neurons from 10.3 ± 1.0 to 0.5 ± 1.3 spikes (p < 0.0001; Figure 6F). The effects of CNO were long-lasting, but when given sufficient time (>30 min after washout of CNO), inhibition by CNO was reversible (e.g., Figure 6E) with the number of action potentials returning to a mean of 9.6 ± 6.1 spikes (n = 4). In other neurons, in which recordings were held for less than 20 min after removal of CNO, little recovery was observed. These data confirm that CNO selectively inhibits pyramidal neurons expressing hM4Di in the rat RSC.
Figure 6.
Results from experiment 4. Representative images of control virus (GFP) expression at low (A1) and high (A2) magnification. Ten consecutive traces in response to identical depolarizing current steps in a GFP-expressing neuron in baseline conditions, in the presence of 5μM CNO, and after washout (B). Results of 5μM CNO application on the number of action potentials in GFP-expressing neurons (C). Representative images of hM4Di-mcherry expression at low (D1) and high (D2) magnification. Ten consecutive traces in response to identical depolarizing current steps in a hM4Di-expressing neuron in baseline conditions, in the presence of 5μM CNO, and after washout (E). Results of 5μM CNO application on the number of action potentials in hM4Di-expressing neurons (F).
General Discussion
The present series of experiments examined the contribution of the RSC to retrieval and encoding of delay auditory fear conditioning. In Experiment 1a, we found that temporary inactivation of the RSC at the time of retrieval impaired freezing to a remotely conditioned auditory CS. This finding is consistent with previous lesions studies (Jiang et al., 2018; Todd et al., 2016). However, in these prior studies, the use of permanent lesions resulted in the RSC being disrupted during both the Context A test and the CS test in Context B. Thus, the current experiments replicated and extended these findings by more selectively inactivating the RSC during only the final CS retrieval session. In contrast, temporary inactivation of the RSC had no observable impact on freezing to an auditory CS that underwent conditioning more recently (Exp 1b). Further, inactivation of the RSC during initial conditioning had no impact on freezing during later retrieval for either remotely (Exp. 2a) or recently (Exp. 2b) acquired delay fear conditioning. Taken together, this pattern of results suggests that the role of the RSC in delay fear conditioning is selective to the retrieval of remotely acquired memories.
As we have noted, prior lesion / inactivation studies have demonstrated that the RSC contributes to contextual learning (Keene and Bucci, 2008; Corcoran et al., 2011) and neurophysiological studies have demonstrated that contextual information in encoded within the RSC (Miller et al., 2021). One possibility is that the contribution of the RSC to retrieval of remotely acquired delay fear conditioning is related to its role in contextual memory (Todd et al., 2019). For example, with the current conditioning procedures, the auditory CS may be encoded as part of the entire conditioning episode, which includes temporal and spatial / contextual information. During testing, presentation of the CS is thus able to re-activate a representation of the entire conditioning episode. According to this framework, the RSC is engaged in the retrieval of remotely acquired delay fear conditioning because information about the CS is integrated with other information, such as the context where conditioning occurred (Todd et al., 2019). This conceptualization was suggested by Quinn et al. (2008) to explain why the hippocampus is sometimes involved in recently acquired delay fear conditioning. Indeed, this might suggest some form of interplay between the hippocampus and the RSC, with the hippocampus (and not RSC) supporting the retrieval of recently acquired delay fear conditioning episodes, and the RSC becoming more critical for successful retrieval over time. Future studies are necessary to determine if the role of the RSC in the retrieval of remotely acquired delay fear conditioning is related, or independent, of its role in contextual learning and memory.
The contribution of the RSC to the retrieval of remotely, but not recently, acquired delay auditory fear conditioning might also be related to differences in the quality of these memories at the time of test. For example, remotely acquired cued fear memories are often more general than recently acquired cued fear memories (Pollack et al., 2018; Thomas & Riccio, 1979). Although we did not test generalization to novel auditory cues in the current experiment, this notion raises the possibility that the RSC has a role in generalized fear memories. If so, it will be of interest to determine if the role of the RSC in memory retrieval extends to instances of generalization that are independent of time.
The present studies also indicate that inactivation of the RSC does not impair retrieval of delay fear conditioning that is more recently acquired, which is consistent with prior studies (Keene & Bucci, 2008; Kwapis et al., 2014). However, it is perhaps premature to fully exclude the RSC from contributing to delay conditioning in general. For instance, there is evidence of increased levels of c-fos in the RSC following pairings of whisker stimulation with mild tail shock in restrained mice (Radwanska et al., 2010), as well as neurophysiological evidence of RSC encoding of stimulus-reinforcer associations during appetitive discrimination learning in head-fixed rats (Yoshida et al., 2021). Although the procedures in these experiments are much different from the ones reported here, such findings raise the possibility that there are experimental procedures / parameters where damage or inactivation of the RSC would impair more recently acquired delay conditioning. Nevertheless, it is clear from the current and prior fear conditioning experiments that manipulation of the RSC typically has no impact on the retrieval of recently acquired delay fear conditioning (Corcoran et al., 2011; Kwapis et al., 2014).
In Exp. 2a and 2b, inactivation of the RSC during initial conditioning did not impair freezing during later exposure to Context A or B, or during tone testing in Context B. Coupled with the results of Exp. 1a, this suggests that the RSC is critical for retrieval, but not initial learning. Indeed, there is evidence from the human imaging literature that increased activity within the posterior cingulate cortex (which includes the RSC) during memory retrieval is positively correlated with successful retrieval (Daselaar et al., 2009). However, increased activity in these brain regions during encoding was correlated with worse performance during retrieval (Daselaar et al., 2009; for review see Huijbers et al., 2012). Therefore, RSC activity during retrieval, but not encoding, is positively correlated with successful memory retrieval.
Additionally, prior studies with rodents have also reported a dissociation between manipulation of the RSC during initial conditioning and later retrieval. For instance, Corcoran et al. (2011) observed that blocking NMDA receptors within the RSC impaired retrieval of contextual fear conditioning, but not initial encoding. In addition, Kwapis et al. (2015, Experiment 3) observed that infusions of anisomycin into the RSC prior to learning had no impact on behavior during the conditioning session, although contextual fear retrieval was impaired when tested the next day. Given that behavior during the conditioning session was unaffected, Kwapis et al. (2015) suggested that anisomycin infusions may have left initial encoding intact but disrupted early consolidation of contextual fear conditioning. Our findings are consistent with Kwapis et al. (2015), insofar as chemogenetic silencing of the RSC had no impact on behavior during the conditioning session, although our manipulation had no apparent impact on early consolidation. Taken together, the overall pattern of results suggests little role for the RSC in the initial encoding of delay fear conditioning. Nevertheless, in the present experiments, the absence of an effect does not necessarily rule out a role for the RSC in encoding of auditory fear conditioning. For instance, it may be the case that our manipulation of the RSC was strong enough to impact retrieval, but not strong enough to impact initial learning. A second possibility is that inactivation during initial conditioning may have resulted in compensation by another region, obscuring a possible role for the RSC. Thus, although the distinction between learning and retrieval might not be fully clear, our data does indicate that the RSC is involved in retrieval of remote auditory fear memory.
We have suggested that the results of these experiments are consistent with a role for the RSC in the retrieval of remotely acquired delay conditioning. However, there are additional issues to consider. First, these studies utilized the synthetic ligand CNO to activate the hM4Di receptor. There have been some reports that CNO, via its active metabolite clozapine, can alter the behavior of both rats and mice (MacLaren et al., 2016; Manvich et al., 2018). However, for several reasons, it seems unlikely that CNO alone produced the retrieval deficit observed in Exp. 1a. For example, both the hM4Di and the control (GFP) group received CNO injections 30 minutes prior to the tone test, but only the hM4Di group showed a reduction in freezing. In addition, in Exps. 3a and 3b, there were no differences in freezing behavior when injections of CNO and Vehicle were directly compared. Second, it is unlikely that the deficit observed in Exp 1a. was due to an inability of rats to perform the freezing response, given that RSC inactivation had no impact on freezing during retrieval of recently acquired conditioning or during initial encoding. Third, it is possible that inactivation of the RSC produced a change in the internal state of the animals that differed from initial conditioning and thus impaired retrieval. However, this seems unlikely given that the retrieval deficit observed was specific to remotely acquired conditioning, and that inactivation during encoding, which presumably could have produced a change in internal state, did not impair subsequent retrieval. Fourth, because the current experiments were conducted with male rats only, future experiments should examine RSC contributions to fear retrieval in female rats, and ideally make direct comparisons between male and females. Finally, the hM4Di receptor was expressed in RSC neurons using the hSyn promoter, which is neuronal specific, but not cell-type specific. Given that the hM4Di receptor may have been expressed on both excitatory and inhibitory neurons, we cannot be sure that activation of this receptor resulted in net inhibition. However, the cortex is thought to contain a higher proportion of excitatory than inhibitory neurons, making it likely that CNO injections resulted in generalized RSC inhibition in the hM4Di groups (Hendry et al., 1987).
In summary, the present experiments examined RSC contributions to the retrieval and encoding of delay auditory fear conditioning. Consistent with prior lesion studies (Jiang et al., 2018; Todd et al., 2016), we observed that inactivation of the RSC impaired retrieval of remotely, but not recently, acquired auditory fear conditioning. The RSC may act in concert with other regions that have also been implicated in retrieval of remotely acquired delay fear conditioning like the amygdala (Gale et al., 2004) and secondary sensory cortices (Sacco & Sachetti, 2010). In contrast, RSC inactivation during initial conditioning had no impact on later retrieval, indicating that the RSC has dissociable roles in encoding and retrieval of delay fear conditioned cues.
Highlights.
Chemogenetic inactivation of the retrosplenial cortex impaired retrieval of remotely acquired delay fear conditioning.
Inactivation of the retrosplenial cortex did not impair retrieval of recently acquired delay fear conditioning.
Inactivation during encoding had no impact on later retrieval of either recently or remotely acquired delay fear conditioning.
Funding:
NSF: IOS1353137 (D.J.B)
NIDA: T32DA037202 (D.I.F)
NIMH: K01MH116158 (T.P.T), R01MH118734 (T.P.T)
This work was supported by the National Institute of Mental Health of the National Institutes of Health under award numbers K01MH116158 and R01MH118734, and the National Institute of Drug Abuse grant T32DA037202 (D.I.F.). The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health. We dedicate this manuscript to the memory of David J. Bucci, a truly great teacher, mentor, and friend.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ashby CR Jr, & Wang RY (1996). Pharmacological actions of the atypical antipsychotic drug clozapine: a review. Synapse (New York, N.Y.), 24(4), 349–394. [DOI] [PubMed] [Google Scholar]
- Ashby CR Jr, & Wang RY (1996). Pharmacological actions of the atypical antipsychotic drug clozapine: a review. Synapse (New York, N.Y.), 24(4), 349–394. [DOI] [PubMed] [Google Scholar]
- Corcoran KA, Donnan MD, Tronson NC, Guzmán YF, Gao C, Jovasevic V, Guedea AL, & Radulovic J (2011). NMDA receptors in retrosplenial cortex are necessary for retrieval of recent and remote context fear memory. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 31(32), 11655–11659. 10.1523/JNEUROSCI.2107-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Yamawaki N, Leaderbrand K, & Radulovic J (2018). Role of retrosplenial cortex in processing stress-related context memories. Behavioral Neuroscience, 132(5), 388–395. 10.1037/bne0000223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daselaar SM (2009). Posterior midline and ventral parietal activity is associated with retrieval success and encoding failure. Frontiers in Human Neuroscience, 3. 10.3389/neuro.09.013.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel M (1993). Discriminative avoidance learning: A model system. In Vogt BA & Gabriel M (Eds.), Neurobiology of Cingulate Cortex and Limbic Thalamus: A Comprehensive Handbook (pp. 478–523). Birkhauser Boston. [Google Scholar]
- Gale GD, Anagnostaras SG, Godsil BP, Mitchell S, Nozawa T, Sage JR, ... & Fanselow MS (2004). Role of the basolateral amygdala in the storage of fear memories across the adult lifetime of rats. Journal of Neuroscience, 24(15), 38103815. 10.1523/JNEUROSCI.4100-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez JL, Bonaventura J, Lesniak W, Mathews WB, Sysa-Shah P, Rodriguez LA, Ellis RJ, Richie CT, Harvey BK, Dannals RF, Pomper MG, Bonci A, & Michaelides M (2017). Chemogenetics revealed: DREADD occupancy and activation via converted clozapine. Science, 357(6350), 503–507. 10.1126/science.aan2475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry S, Schwark H, Jones E, & Yan J (1987). Numbers and proportions of GABA-immunoreactive neurons in different areas of monkey cerebral cortex. The Journal of Neuroscience, 7(5), 1503–1519. 10.1523/JNEUROSCI.07-05-01503.1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijbers W, Vannini P, Sperling RA, Pennartz CM, Cabeza R, & Daselaar SM (2012). Explaining the encoding/retrieval flip: Memory-related deactivations and activations in the posteromedial cortex. 10.1016/j.neuropsychologia.2012.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang MY, DeAngeli NE, Bucci DJ, & Todd TP (2018). Retrosplenial cortex has a time-dependent role in memory for visual stimuli. Behavioral Neuroscience, 132(5), 396–402. 10.1037/bne0000229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene CS, & Bucci DJ (2008). Neurotoxic lesions of retrosplenial cortex disrupt signaled and unsignaled contextual fear conditioning. Behavioral Neuroscience, 122(5), 1070–1077. 10.1037/a0012895 [DOI] [PubMed] [Google Scholar]
- Kwapis JL, Jarome TJ, Lee JL, & Helmstetter FJ (2015). The retrosplenial cortex is involved in the formation of memory for context and trace fear conditioning. Neurobiology of Learning and Memory, 123, 110–116. 10.1016/j.nlm.2015.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwapis JL, Jarome TJ, Lee JL, Gilmartin MR, & Helmstetter FJ (2014). Extinguishing trace fear engages the retrosplenial cortex rather than the amygdala. Neurobiology of Learning and Memory, 113, 41–54. 10.1016/j.nlm.2013.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLaren DAA, Browne RW, Shaw JK, Krishnan Radhakrishnan S, Khare P, España RA, & Clark SD (2016). Clozapine N-Oxide Administration Produces Behavioral Effects in Long–Evans Rats: Implications for Designing DREADD Experiments. Eneuro, 3(5), ENEURO.0219–16.2016. 10.1523/ENEURO.0219-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, & Aston-Jones G (2018). CNO Evil? Considerations for the use of DREADDs in behavioral neuroscience. Neuropsychopharmacology, 43(5), 934–936. 10.1038/npp.2017.299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers KM, & Davis M (2007). Mechanisms of fear extinction. Molecular Psychiatry, 12, 120–150). [DOI] [PubMed] [Google Scholar]
- Miller AM, Serrichio AC, & Smith DM (2021). Dual-Factor Representation of the Environmental Context in the Retrosplenial Cortex. Cerebral Cortex, 31(5), 2720–2728. 10.1093/cercor/bhaa386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz S, Latsko MS, Founty JL, Dutta S, Adkins JM, & Jasnow AM (2019). Anterior cingulate cortex and ventral hippocamapal inputs to the basolateral amygdala selectively control generalized fear. Journal of Neuroscience, 39, 6526–6539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack GA, Bezek JL, Lee SH, Scarlatal MJ, Weingast LT, & Bergstrom HC (2018). Cued fear memory generalization increases over time. Learning & Memory, 25, 298–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn JJ, Wied HM, Ma QD, Tinsley MR, & Fanselow MS (2008). Dorsal hippocampus involvement in delay fear conditioning depends upon the strength of the tone-footshock association. Hippocampus, 18(7), 640–654. 10.1002/hipo.20424 [DOI] [PubMed] [Google Scholar]
- Radwanska A, Debowska W, Liguz-Lecznar M, Brzezicka A, Kossut M, & Cybulska-Klosowicz A (2010). Involvement of retrosplenial cortex in classical conditioning. Behavioural Brain Research, 214(2), 231–239. 10.1016/j.bbr.2010.05.042 [DOI] [PubMed] [Google Scholar]
- Robinson Siobhan., Adelman JS, Mogul AS, Ihle PCJ, & Davino GM (2018). Putting fear in context: Elucidating the role of the retrosplenial cortex in context discrimination in rats. Neurobiology of Learning and Memory, 148, 50–59. 10.1016/J.NLM.2017.12.009 [DOI] [PubMed] [Google Scholar]
- Sacco T, & Sacchetti B (2010). Role of secondary sensory cortices in emotional memory storage and retrieval in rats. Science (New York, N.Y.), 329(5992), 649–656. 10.1126/science.1183165 [DOI] [PubMed] [Google Scholar]
- Smith DM, Miller AMP, & Vedder LC (2018). The retrosplenial cortical role in encoding behaviorally significant cues. Behavioral Neuroscience, 132(5), 356–365. 10.1037/bne0000257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DA, & Riccio DC (1979). Forgetting of a CS attribute in a conditioned suppression paradigm. Animal Learning & Behavior, 7, 191–195. [Google Scholar]
- Todd TP, Fournier DI, & Bucci DJ (2019). Retrosplenial cortex and its role in cue-specific learning and memory. Neuroscience & Biobehavioral Reviews, 107, 713–728. 10.1016/j.neubiorev.2019.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd TP, Mehlman ML, Keene CS, DeAngeli NE, & Bucci DJ (2016). Retrosplenial cortex is required for the retrieval of remote memory for auditory cues. Learning & Memory (Cold Spring Harbor, N.Y.), 23(6), 278–288. 10.1101/lm.041822.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trask S, Pullins SE, Ferrara NC, & Helmstetter FJ (2021). The anterior retrosplenial cortex encodes event-related information and the posterior retrosplenial cortex encodes context-related information during memory formation. Neuropsychopharmacology, 46(7), 1386–1392. 10.1038/s41386-021-00959-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vann SD, Aggleton JP, & Maguire EA (2009). What does the retrosplenial cortex do? Nature Reviews Neuroscience, 10(11), 792–802. 10.1038/nrn2733 [DOI] [PubMed] [Google Scholar]
- Wiltgen BJ, & Silva AJ (2007). Memory for context becomes less specific with time. Learning & Memory, 14, 313–317. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Chinzorig C, Matsumoto J, Nishimaru H, Ono T, Yamazaki M, & Nishijo H (2021). Configural Cues Associated with Reward Elicit Theta Oscillations of Rat Retrosplenial Cortical Neurons Phase-Locked to LFP Theta Cycles. Cerebral Cortex. 10.1093/cercor/bhaa395 [DOI] [PubMed] [Google Scholar]