Abstract
WT2725 is a Wilms’ tumor gene 1 (WT1)-derived-oligopeptide vaccine designed to induce WT1-specific cytotoxic T-lymphocytes against WT1+ tumors in human leukocyte antigen (HLA)-A*0201+ and/or HLA-A*0206+ patients. Here, we report the results of a phase I study of WT2725. In this phase I, open-label, dose-escalation and expansion two-part study, the WT2725 dosing emulsion was administered as a monotherapy to patients with advanced malignancies known to overexpress WT1, including glioblastoma. In part 1, 44 patients were sequentially allocated to four doses: 0.3 mg (n = 5), 0.9 mg (n = 5), 3 mg (n = 6), and 9 mg (n = 28). In part 2, 18 patients were allocated to two doses: 18 mg (n = 9) and 27 mg (n = 9). No dose-limiting toxicities were observed, so the maximum tolerated dose was not reached. Median progression-free survival was 58 (95% confidence interval [CI] 56–81) days (~ 2 months) across all patients with solid tumors; median overall survival was 394 days (13.0 months) (95% CI 309–648). Overall immune-related response rate in solid tumor patients was 7.5% (95% CI 2.6–19.9); response was most prominent in the glioblastoma subgroup. Overall, 62.3% of patients were considered cytotoxic T-lymphocyte responders; the proportion increased with increasing WT2725 dosing emulsion dose. WT2725 dosing emulsion was well tolerated. Preliminary tumor response and biological marker data suggest that WT2725 dosing emulsion may exert antitumor activity in malignancies known to overexpress the WT1 protein, particularly glioblastoma, and provide a rationale for future clinical development.
Trial registration: NCT01621542.
Subject terms: Cancer immunotherapy, CNS cancer
Introduction
WT2725 is a Wilms’ tumor gene 1 (WT1)-derived-oligopeptide vaccine designed to induce WT1-specific cytotoxic T-lymphocytes (CTLs) against WT1+ tumors in human leukocyte antigen (HLA)-A*0201+ and/or HLA-A*0206+ patients.
WT1 encodes a zinc-finger transcription factor that is involved in cell proliferation, differentiation, apoptosis, and organ development, and is overexpressed in various malignancies1–4. WT1/WT1 is expressed/overexpressed in most (63–94%) glioblastoma samples, most (80–90%) acute myeloid leukemia (AML) patients, 96% of non-small-cell lung carcinoma (NSCLC) samples in one study (with expression markedly higher than in normal tissues across multiple studies), and 56–71% of ovarian cancers (when evaluated as a single set; 50–100% of serous carcinomas and 0–13% of non-serious ovarian tumors)5–22. Data demonstrate that WT1-derived peptides can trigger cellular and humoral immune responses in vivo; WT1-specific CTLs can lyse WT1-expressing tumor cells without harming normal tissue1. Following vaccination, WT2725 (a synthetic WT1 peptide) has potential to stimulate the host immune system to induce a CTL response against cancer cells that overexpress the WT1 protein, leading to cell lysis and preventing further tumor cell proliferation.
Previously studied WT1 vaccines have induced immunogenicity and antitumor responses in clinical trials of various malignancies2,23–36. However, injection of peptides in aqueous solutions alone is not always effective in stimulating a CTL response; different adjuvants can affect the body’s immune response to peptide antigens. For example, water-in-oil (W/O) emulsions enhance the immunogenicity of antigens by creating a depot effect that prevents the antigen from accessing tissue and blood-borne proteases but facilitates translocation into antigen-presenting cells37.
The WT2725 dosing emulsion comprises WT2725 (the acetate salt of a synthetic peptide with the same sequence as the naturally occurring peptide WT1126–134) diluted in a peptide-diluting solution, for administration with a novel W/O pre-emulsion adjuvant. In vitro studies using human peripheral blood mononuclear cells from an HLA-A*0201+ healthy donor confirmed that the WT2725 peptide binds to HLA-A*0201 (antigen-presenting molecule) and induces WT1-reactive CD8+ (cytotoxic) T-cells38. Furthermore, studies in HLA-A*0201-expressing transgenic mice confirmed that the WT2725 injection mixed with the novel W/O pre-emulsion induced HLA-A*0201-restricted, WT2725 peptide-specific CTLs. As observed with other WT1 peptide vaccines39,40, injection-site reactions were dose-limiting in preclinical toxicology studies.
Here, we report the results of a phase I study of WT2725. The WT2725 dosing emulsion was administered as a monotherapy to patients with advanced malignancies (glioblastoma, AML, NSCLC, and ovarian cancer) known to overexpress the WT1 protein in the majority of patients3,4,41. The study was conducted to determine dose levels for use in future clinical studies, and to evaluate safety, tolerability, and clinical and immunological responses.
Methods
Study design
This was a phase I, open-label, dose-escalation and expansion two-part study to define the maximum tolerated dose (MTD), and to evaluate the safety and tolerability of the WT2725 dosing emulsion in adults with advanced malignancies known to overexpress WT1 (registered 18/06/2012, clinicaltrials.gov NCT01621542).
A rolling-six design42 was used for enrollment into dose-escalation cohorts until the MTD was reached. When the MTD was reached, up to three expanded cohorts of 10 patients each were allowed, to investigate outcomes in patients with specific tumor types. In part 1, patients were treated with 0.3, 0.9, 3, or 9 mg WT2725 dosing emulsion monotherapy, subcutaneously once every week for 4 weeks (induction phase), then once every 2 weeks for 6 weeks (consolidation phase), and once every 4 weeks thereafter (maintenance phase) until progression or another discontinuation event (Online Resource Fig. 1). Each dose was administered at a single injection site. Since no MTD was established in part 1, the protocol was amended to add part 2, which used a dose- and frequency-intensified treatment schedule. In part 2, patients were treated with 18 or 27 mg WT2725 dosing emulsion monotherapy, subcutaneously once every week for 8 weeks (induction phase), then once every 2 weeks for 10 weeks (consolidation phase), and once every 4 weeks thereafter (maintenance phase) until progression or another discontinuation event (Online Resource Fig. 1). Each dose of the WT2725 dosing emulsion was administered at two injection sites. When possible, sites surrounding the regional lymph nodes in the upper arm, lower abdomen, or femoral area were selected; rotation of injection sites was permitted.
This open-label study involved no randomization or blinding; sequential cohorts were treated according to the dose-escalation scheme and stopping criteria. The study was conducted over ~ 5 years (from July 2012 to May 2017) at six clinical sites in the USA (Houston TX—2 sites, Tucson AZ, La Jolla CA, Chicago IL, Hershey PA). Sample size was based on clinical and practical considerations for this phase I dose-escalation study using the rolling-six design, and was outside of statistical considerations.
Patients
Patients with advanced-stage, measurable malignancies (that commonly overexpress the WT1 protein: glioblastoma, AML, NSCLC, or ovarian cancer), progressive or recurrent despite standard therapy, or for whom no standard therapy existed, were eligible to participate in part 1 of the study. Determination of WT1 expression was not assessed prior to patient enrollment, however, access to an archival tumor tissue sample or agreement to undergo biopsy after confirmation of study eligibility was required to enable subsequent evaluation of WT1 expression. Other major inclusion criteria were: age ≥ 18 years; Eastern Cooperative Oncology Group Performance Status score of 0–2; HLA-A*0201+ and/or HLA-A*0206+; adequate bone marrow and immune reserve (absolute neutrophil count ≥ 1000/µL; platelet count ≥ 10 × 104/µL, or ≥ 5 × 104/µL after stem cell transplant; hemoglobin ≥ 9 g/dL; and absolute lymphocyte count ≥ 1000/µL, or ≥ 500/µL after stem cell transplant); adequate renal function (serum creatinine ≤ 1.5 × the upper limit of normal); and adequate hepatic function (total bilirubin ≤ 2.0 mg/dL, or ≤ 3 mg/dL for patients with known Gilbert’s syndrome; and alanine aminotransferase and aspartate aminotransferase ≤ 3 × the upper limit of normal). Patients with glioblastoma or AML (including those who participated in part 1) were eligible to participate in part 2 of the study.
Endpoints
The primary endpoints were to determine the MTD of the WT2725 dosing emulsion, based on the incidence of dose-limiting toxicities (DLT), and to evaluate the overall safety profile.
DLT were defined as any ≥ grade III adverse events (AEs) that occurred after administration of the first dose of the WT2725 dosing emulsion but before receiving the fifth dose (Days 1–29), not related to underlying disease, intercurrent illness, or concomitant medications. Repeat assessment was required to confirm changes (a shift by ≥ 2 grades) in hematological parameters. Grade III AEs of nausea, vomiting, and fatigue (which are common and manageable in cancer patients) were not considered DLT if they could be reduced to < grade III with standard supportive care.
Secondary endpoints included: proportion of patients in each response category [based on immune-related (ir) response criteria for solid tumors, modified International Working Group response criteria in AML, and/or tumor markers]; and immune response evaluated by induction of WT1-specific CTLs in peripheral blood.
Progression-free survival (PFS) and overall survival (OS) in all patients and by malignancy type were exploratory endpoints.
Assessments and analyses
Safety assessments, efficacy assessments and analyses are described in Online Resource 1.
Ethics approval
This study was conducted in accordance with local laws and regulations, the protocol, International Council for Harmonisation Good Clinical Practice, International Council for Harmonisation guidelines, and in alignment with the ethical principles of the Declaration of Helsinki. The study protocol was approved by the UCSD Human Research Protections Program and the Penn State Health Milton S. Hershey Medical Center-Human Subjects Protection Office, and by the Institutional Review Boards at MD Anderson Cancer Center, Western, The University of Chicago, and The University of Texas, MD Anderson Cancer Center, before enrollment of patients into the study at each site.
Consent to participate
All patients provided informed consent.
Consent for publication
Not applicable. Personal identifying information are not disclosed and are removed before the use and publication of data.
Results
Patients
There were 62 patients in the safety population (part 1 n = 44, part 2 n = 18, Online Resource Fig. 2) and 52, 52, and 61 patients in the DLT, efficacy, and CTL populations, respectively.
In part 1, 44 patients were sequentially allocated to four doses: WT2725 dosing emulsion 0.3 mg (n = 5), 0.9 mg (n = 5), 3 mg (n = 6), and 9 mg (n = 28). In part 2, 18 patients were allocated to two doses: 18 mg (n = 9) and 27 mg (n = 9).
Demographics, baseline characteristics, and malignancy type are shown in Table 1. Median age was 62 years (range 26–76), 61.3% of patients being younger than 65 years. All patients had advanced-stage malignancies, and all but two had an Eastern Cooperative Oncology Group Performance Status of 0 or 1 at baseline. No differences in baseline demographic or clinical characteristics across dose cohorts were expected to influence the results of the study.
Table 1.
Demographics, patient characteristics, and cancer history at baseline.
| Safety population n = 62 |
|
|---|---|
| Males, n (%) | 29 (46.8) |
| Race/ethnicity, n (%) | |
| White | 53 (85.5) |
| Black or African American | 4 (6.5) |
| Asian | 2 (3.2) |
| Other | 3 (4.8) |
| Hispanic or Latino | 4 (6.5) |
| Age, years, median (range) | 62.0 (26–76) |
| Weight, kg, median (range) | 73.7 (50.3–127.4) |
| Height, cm, median (range) | 167.6 (145.5–188.0) |
| Body mass index, kg/m2, median (range) | 26.6 (18.5–46.8) |
| ECOG performance status, n (%) | |
| 0 | 24 (38.7) |
| 1 | 36 (58.1) |
| 2 | 2 (3.2) |
| Concomitant dexamethasone, n (%) | 18 (29.0) |
| Malignancy type, n (%) | |
| Glioblastoma | 20 (32.3) |
| Ovarian cancer | 21 (33.0) |
| Acute myeloid leukemia | 12 (19.4) |
| Non-small-cell lung cancer | 7 (11.3) |
| Other | 2 (3.2) |
ECOG Eastern Cooperative Oncology Group.
Safety
No DLT were observed (maximum dose 27 mg WT2725 dosing emulsion). The WT2725 dosing emulsions were well tolerated. Following completion of the planned dose escalation, the sponsor terminated the study for reasons not related to safety. Therefore, the MTD of the WT2725 dosing emulsion was not reached.
Most patients experienced one or more treatment-emergent AEs (TEAEs) during the study (Table 2). Approximately one-third of patients (31.0%) had a ≥ grade III TEAE, and approximately half (53.5%) had a TEAE that the investigator determined as possibly, probably, or definitely related to the study drug; nearly all TEAEs determined as related to study drug were injection-related reactions (Table 2). Serious AEs occurred in approximately one-quarter (23.9%) of patients. Four patients (5.6%) discontinued due to a TEAE (assessed by the investigator as not related to study drug) and no patients died due to causes not related to disease. One death occurred during the study due to disease progression; the death was not reportable as a TEAE, as it was related to progression of the malignancy being treated in the study.
Table 2.
Incidence of TEAEs.
| TEAE category, n (%) | Total, n = 71 | |||
|---|---|---|---|---|
| Any TEAE | 67 (94.4) | |||
| Any grade ≥ III TEAE | 22 (31.0) | |||
| Any treatment-relateda TEAE | 38 (53.5) | |||
| Any grade ≥ III treatment-related TEAE | 1 (1.4) | |||
| Any TEAE with outcome of death | 0 | |||
| Any SAE | 17 (23.9) | |||
| Any treatment-related SAE | 1 (1.4) | |||
| Any TEAE leading to treatment discontinuation | 4 (5.6) |
| Overall | Grade III | Grade IV/V | Treatment-related | |
|---|---|---|---|---|
| Individual TEAEs | ||||
| Injection-site erythema | 14 (19.7) | 0 | 0 | 14 (19.7) |
| Constipation | 13 (18.3) | 0 | 0 | 0 |
| Nausea | 12 (16.9) | 0 | 0 | 3 (4.2) |
| Decreased appetite | 10 (14.1) | 0 | 0 | 2 (2.8) |
| Vomiting | 9 (12.7) | 0 | 0 | 1 (1.4) |
| Cough | 9 (12.7) | 0 | 0 | 1 (1.4) |
| Injection-site pain | 8 (11.3) | 0 | 0 | 8 (11.3) |
| Hyponatremia | 8 (11.3) | 3 (4.2) | 0 | 0 |
| Headache | 8 (11.3) | 0 | 0 | 1 (1.4) |
| Dyspnea | 8 (11.3) | 1 (1.4) | 0 | 0 |
| Injection-site reaction | 7 (9.9) | 0 | 0 | 7 (9.9) |
| Fall | 7 (9.9) | 0 | 0 | 0 |
| Dizziness | 7 (9.9) | 0 | 0 | 0 |
| Anemia | 6 (8.5) | 3 (4.2) | 0 | 0 |
| Hypokalemia | 6 (8.5) | 1 (1.4) | 0 | 0 |
| Leukocytosis | 5 (7.0) | 3 (4.2) | 0 | 0 |
| Diarrhea | 5 (7.0) | 1 (1.4) | 0 | 0 |
| Pyrexia | 5 (7.0) | 1 (1.4) | 0 | 0 |
| Asthenia | 4 (5.6) | 0 | 0 | 0 |
| Contusion | 4 (5.6) | 0 | 0 | 1 (1.4) |
| Hyperglycemia | 4 (5.6) | 1 (1.4) | 0 | 0 |
| Hypoalbuminemia | 4 (5.6) | 0 | 0 | 0 |
| Dysgeusia | 4 (5.6) | 0 | 0 | 0 |
| Rash | 4 (5.6) | 1 (1.4) | 0 | 3 (4.2) |
| Hyperphosphatemia | 3 (4.2) | 0 | 0 | 0 |
| Muscular weakness | 3 (4.2) | 0 | 0 | 0 |
| Pneumonia | 2 (2.8) | 2 (2.8) | 0 | 0 |
| Vasogenic cerebral edema | 2 (2.8) | 0 | 0 | 0 |
| Confusional state | 2 (2.8) | 0 | 0 | 0 |
| Upper-airway cough syndrome | 2 (2.8) | 0 | 0 | 1 (1.4) |
Injection-related reactions are in bold font.
SAE serious adverse event, TEAE treatment-emergent adverse event.
aAssessed as possibly, probably, or definitely related to study drug.
Injection-related reactions were the most frequently reported TEAEs (Table 2, bold font): injection-site erythema 19.7%, injection-site pain 11.3%, injection-site reaction 9.9%. The majority were grade I and none were grade III or dose limiting; grade II injection-related reactions with itching, erythema, pain, and bruising and/or swelling were reported in three patients. No patients discontinued the study due to an injection-related reaction.
Efficacy
Survival
Median PFS was 58 (95% CI 56–81) days (~ 2 months) across all patients with solid tumors, 58 days (95% CI 56–81) in the NSCLC subgroup, 61.5 days (95% CI 56–112) in the ovarian cancer subgroup, and 59 days (95% CI 27–329) in the glioblastoma subgroup, respectively.
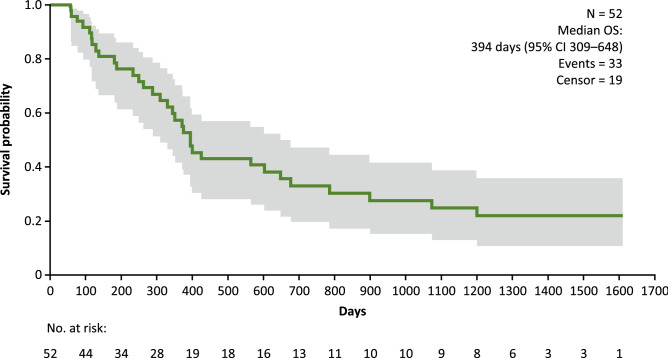
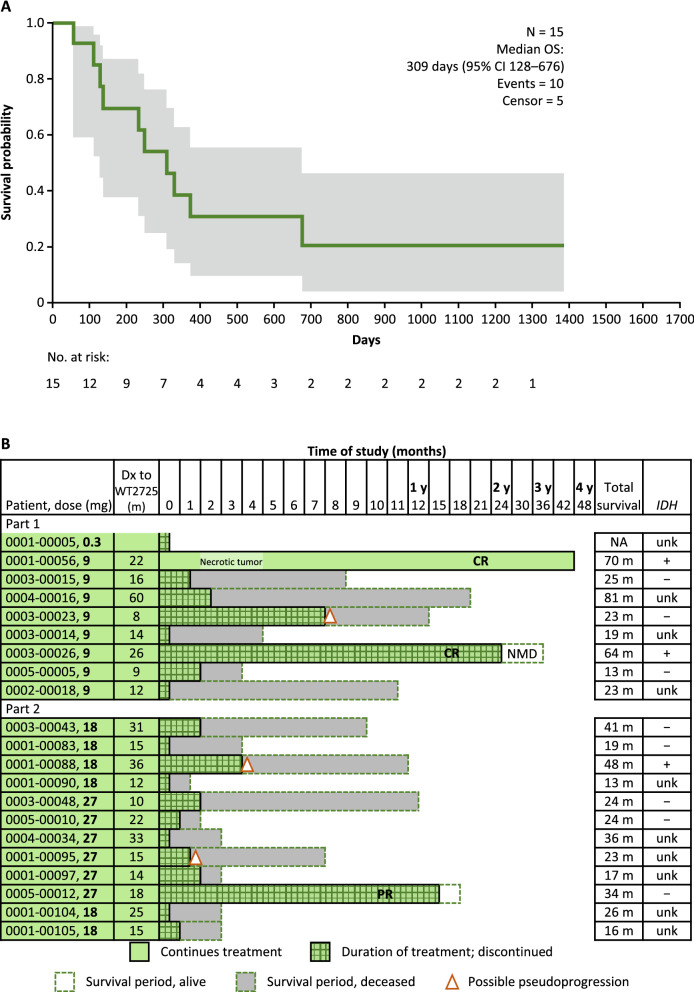
Overall, 63.5% of study participants died. Median OS was 394 days (13.0 months, 95% CI 309–648 days) across all patients (Fig. 1), 647 days (21.3 months, 95% CI 59 days–not calculable) in the AML subgroup, 275 days (9.0 months, 95% CI 117–351 days) in the NSCLC subgroup, 602 days (19.8 months, 95% CI 344–1200 days) in the ovarian cancer subgroup, and 309 days (10.2 months, 95% CI 128–676 days) in the glioblastoma subgroup (Fig. 2A, glioblastoma only).
Figure 1.
OS (efficacy population). All malignancy types combined. The shaded area represents the 95% CI of the survival probability at that day. CI confidence interval, OS overall survival.
Figure 2.
Patient survival in the glioblastoma subgroup. (A) OS. The shaded area represents the 95% CI of the survival probability at that day. (b) Individual patient survival. CI confidence interval, CR complete response, Dx diagnosis, IDH isocitrate dehydrogenase, m months, NMD no measurable disease, OS overall survival, PR partial response, unk unknown, y years.
One-third (n = 7, 33.3%) of glioblastoma patients survived for ≥ 1 year (Fig. 2B), three (14.3%) for ≥ 18 months, and two (9.5%) for ≥ 2 years. Both patients who survived for ≥ 2 years were in complete radiologic remission, although one discontinued before study termination. At time of analysis, two glioblastoma patients remained on treatment with WT2725 dosing emulsion; one had a complete response (CR) with no measurable disease for > 3 years, and one had a partial response (PR) after > 13 months of treatment.
Immune-related tumor response in solid tumor patients
Overall response rate (irCR + irPR) was 7.5% (95% CI 2.6–19.9). Two patients (5.0%) achieved an irCR; both were in the 9 mg dose cohort and the glioblastoma subgroup. One patient (2.5%) achieved an irPR; this patient was in the 27 mg dose cohort and the glioblastoma subgroup.
Ir-stable disease (irSD) was achieved in 12 of 40 evaluable patients (30.0%; 0.3 mg 5.0%, 0.9 mg 2.5%, 3.0 mg 5.0%, 9.0 mg 15.0%, 18 mg 2.5%). Disease control (irCR + irPR + irSD) was achieved in 15 of 40 patients (37.5%).
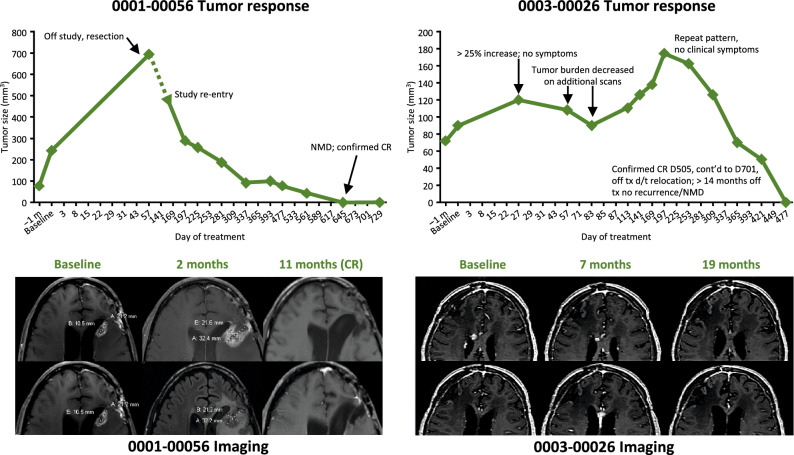
Response was most prominent in the glioblastoma subgroup. Two of 15 patients achieved irCR, one irPR, and two irSD. The time of maximum response was ~ 16 months (477 days). Figure 3 details tumor size over time (including imaging) for the two glioblastoma patients who achieved an irCR. Patient 0001-00056 underwent surgery for apparent tumor progression at Day 57. The apparent progression was in fact an immune response; the patient re-entered the study and experienced a delayed response. Patient 003-00026 appeared to have tumor progression (on imaging) between days 27 and 197. Treatment was continued and the immune response resolved on its own; a bimodal response was observed in this patient.
Figure 3.
Change in tumor size over time and imaging for the two glioblastoma patients who achieved an immune-related complete response. CR complete response, D day, m month, NMD no measurable disease, tx treatment.
There were no irCRs or irPRs in the ovarian cancer subgroup, but nine of 18 patients (50%) had irSD. Overall, 23 patients (57.5%) did not respond to treatment during the course of the study and were reported as either irPD (15.0%) or unconfirmed irPD (42.5%). An additional two patients (5.0%) were considered not evaluable.
Modified International Working Group response in AML
Four of nine evaluable patients (44.4%, 95% CI 18.9–73.3) achieved CR (cytogenic responses: 9 mg, n = 2; morphologic responses: 18 mg, n = 2). One additional patient in the 9 mg cohort achieved CR with persistence of cytopenias. Three patients were non-evaluable, due to either a missing baseline or on-treatment data point.
Tumor markers
None of the 19 evaluable ovarian cancer patients achieved a biological response (defined as a ≥ 50% reduction from baseline in CA-125 levels). Non-response/non-PD was reported for seven (36.8%) patients.
In AML patients, blood levels of the WT1 transcript decreased by 0.4% (mean % change from baseline, SD 2.47, range − 5.6 to 4.2) between baseline and maximum on-study measurement (n = 10), and by 1.7% (mean, SD 2.49, range − 5.6 to 0) between baseline and end of study (n = 5). Bone marrow levels of the WT1 transcript decreased by 1.4% (mean, SD 1.00, range − 2.1 to − 0.7) between baseline and maximum on-study measurement (n = 2).
CTL induction
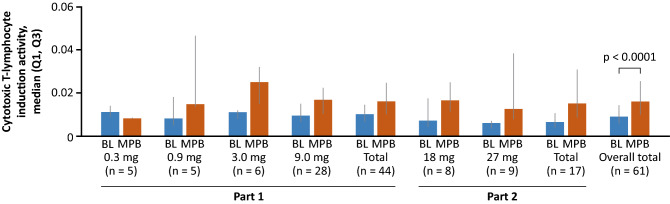
Overall, 62.3% of patients were considered CTL responders. The proportion of CTL responders increased with increasing dose, ranging from 0 in the 0.3 mg cohort to 88.9% in the 27 mg cohort. Representative baseline and post-baseline flow cytometry profiles from one individual are shown in Online Resource Fig. 3.
Mean (± standard deviation [SD]) CTL induction activity at baseline was 0.0228% ± 0.0739 and median CTL induction activity at baseline was 0.0090. For the mean of all post-baseline assessments of each patient, mean (SD) CTL induction activity was 0.0252% ± 0.0387 and median CTL induction activity was 0.0161 (Wilcoxon signed rank test for difference vs 0.0090 at baseline: p < 0.0001) (Fig. 4). When maximum post-baseline assessment values of each patient were assessed, mean (SD) CTL induction activity was 0.0827% ± 0.1776 and median CTL induction activity was 0.0290 (Wilcoxon signed rank test for difference vs 0.0090 at baseline: p < 0.0001).
Figure 4.
Baseline and post-baselinea CTL induction activity. All malignancy types combined. The CTLs in blood samples were measured by tetramer assay using flow cytometry; the evaluation of CTL induction is defined in the Efficacy assessments section of the Online Resource. P-values were calculated using the Wilcoxon signed-rank test (comparing post-baseline with baseline assessments). aBased on the mean of all post-baseline assessments of each patient. BL baseline, CTL cytotoxic T-lymphocyte, MPB mean of post-baseline.
Discussion
Subcutaneous injection with WT2725 dosing emulsion (0.3–27 mg) was generally well tolerated and had an acceptable safety profile in adult patients with advanced-stage malignancies known to overexpress the WT1 protein. An MTD could not be established, as no DLT were reported at any of the doses evaluated.
The frequency of the HLA-A*0201 allele ranges between ~ 2% and 44% across the USA; the frequency of the HLA-A*0206 allele is slightly lower (~ 3.2–16.5%)43. Therefore, WT2725 could be a feasible treatment option for a substantial proportion of patients (HLA-A*0201+ and/or HLA-A*0206+) in the USA.
After the initial dose-escalation phase of the study (part 1) and the subsequent dose expansion was completed without DLT, the protocol was amended to include two additional dose-escalation and expansion cohorts of 18 and 27 mg (part 2). These cohorts were restricted to patients with glioblastoma and AML, in order to accumulate more data in these types of malignancy, which typically have relatively high frequencies of WT1 expression.
Most patients had TEAEs during this study. As in previous clinical studies of WT1 peptide vaccines with the same peptide sequence as WT2725 (administered with various adjuvants and combination therapies)23,39,40,44–46, injection-related reactions were the most frequently reported type of TEAE. Most injection-related reactions were grade I, none were grade ≥ III, and no patients discontinued the study due to an injection-related reaction. Overall, approximately one-third of patients had a grade ≥ III TEAE, and approximately half had a TEAE judged as related to study drug; nearly all TEAEs related to study drug were injection-related reactions.
Immune-related tumor responses were most prominent in the glioblastoma subgroup, in which durable objective responses were observed. This finding is consistent with previous case reports and results of small studies, in which WT1 vaccine therapy produced clinical and immunological responses and improved clinical manifestations and quality of life in patients with glioblastoma and glioma28,47–50, warranting further investigation in this subgroup of patients. We therefore reported efficacy outcomes for the entire immune-related response criteria population, and separately for the glioblastoma subgroup, to provide more detail with regards to this malignancy type. PFS was comparable across solid tumor subgroups. PFS was 58 days in the full population and 59 days in the glioblastoma subgroup. OS was 394 days in the full population and 647, 275, 602, and 309 days in the AML, NSCLC, ovarian cancer, and glioblastoma subgroups, respectively. Three glioblastoma patients (14%) survived for ≥ 18 months, and two (10%) for ≥ 2 years. Both patients who survived for ≥ 2 years were in complete radiologic remission. As the study was conducted prior to the most recent World Health Organization Classification of Central Nervous System Tumors update, mutational status of isocitrate dehydrogenase was tested retrospectively.
There are challenges associated with monitoring disease progression during treatment with immune therapies51,52. Imaging of malignancies in patients treated with immune therapies can detect delayed responses, transient tumor enlargement, and the appearance of new lesions. We therefore used immune-related response criteria to assess response to WT2725 dosing emulsion in this study.
The overall immune-related tumor response rate was 7.5% among 40 evaluable patients. Two patients (5%) achieved an irCR; both were in the glioblastoma subgroup and receiving 9 mg WT2725 dosing emulsion. An irPR was achieved by 2.5% of patients, 30.0% achieved irSD, and 37.5% achieved disease control. Overall, 57.5% of patients did not respond to treatment during the course of the study and were reported as either irPD (15.0%) or unconfirmed irPD (42.5%). In the glioblastoma subgroup, 13.3% achieved irCR, 6.7% irPR, and 13.3% irSD.
Delayed response was noted in glioblastoma patients who responded. In the irCR + irPR population, time of maximum response was at ~ 16 months (477 days). One patient (Patient 0003-00026, glioblastoma treated with 9 mg WT2725 dosing emulsion) had a bimodal response with initial radiographic worsening followed by improvement, followed by recurrent worsening and improvement without any change in management. The driver for this type of radiographic response pattern is unknown. As our understanding of potential biomarkers for response to immunotherapies becomes refined53, prospective evaluation of specific biomarkers across various immunotherapeutic modalities will be of value. Furthermore, these two patients exhibited pseudoprogression between days 27 and 197. The RANO brain imaging criteria have recently been modified to take this into account54, and are currently being used in a trial of WT1 in glioblastoma (NCT03149003).
WT2725 dosing emulsion stimulated immune activation in 62% of patients, as evidenced by CTL response. CTL response increased with increasing WT2725 dose.
The clinical outcomes, tumor responses and biological marker data observed in our study confirm the promising results observed in other studies of WT1 vaccines2,23–36,55. Overall, these data suggest that WT1 vaccines are a potential future treatment option for patients with advanced malignancies, thus providing a rationale for the future clinical development of WT1 vaccines such as WT2725.
Limitations of our study include those typically associated with early phase studies, including the relatively small sample size (further divided into multiple types of advanced malignancy) and the lack of a comparator. However, the purpose of the study was achieved; we concluded that the WT2725 dosing emulsion was well tolerated. In combination with promising initial efficacy data, this study provides a solid foundation for the future development of WT2725.
A general limitation is that vaccination only targets one component of the immune response, and thus may not be effective if patients have deficiencies in other components. Therefore, future directions for the development of effective WT1-targeted treatments for advanced malignancies might involve WT1 vaccine delivery through RNA vaccines or the oncolytic virus system, potentially in combination with other immune therapeutics involving antigen presenting cells, immune checkpoint blockade, natural killer cells, or T cell activation. CAR-T cell therapy targeting WT1 could be another promising direction for the treatment of advanced malignancies.
Conclusions
WT2725 dosing emulsion was well tolerated in this first-in-human study. Our preliminary tumor response and biological marker data suggest that WT2725 dosing emulsion may exert antitumor activity in malignancies known to overexpress the WT1 protein, particularly glioblastoma, and provide a rationale for future clinical development.
Supplementary Information
Acknowledgements
Medical writing support was provided by Mallory Gough, PhD, ISMPP CMPPTM of FireKite, an Ashfield company, part of UDG Healthcare plc, and was funded by Sunovion Pharmaceuticals Inc.
Author contributions
D.P., K.Y., K.W., Y.Z., D.B. contributed to the conception and design of the study. S.F., D.P., H.L., R.L., D.A., K.W., Y.Z., Y.L.C., J.E., D.B. contributed to the collection and assembly of data. S.F., H.L., S.K., D.A., D.H., K.W., Y.Z., Y.L.C., N.P., D.B. contributed to the data analysis and interpretation. D.P., H.L., R.L., S.K., D.A., K.Y., K.W., Y.Z., N.P. contributed to drafting the manuscript. S.F., S.K., D.A., D.H., Y.L.C., D.B. contributed to writing the manuscript. All authors provided final approval of the manuscript and agree to be accountable for all aspects of the work.
Funding
This work was supported by Sunovion Pharmaceuticals Inc.
Data availability
Sunovion Pharmaceuticals Inc. is part of a clinical trial data-sharing consortium that facilitates access for qualified researchers to selected anonymized clinical trial data. For up-to-date information on data availability please visit: https://www.clinicalstudydatarequest.com/Study-Sponsors.aspx and click on Sunovion.
Competing interests
S.F. and D.A. have nothing to disclose. D.P. is part of the advisory council/committee for AbbVie and Tocagen. H.L. received honoraria from Agios and grants/funds from BMS and Karyopharm. R.L. served on a scientific advisory board for Novocure, Monteris and AbbVie; served on the speakers bureau for Novocure; received consulting fees from AbbVie, American Physician Institute, Medlink Neurology, EBSCO Publishing, Eisai, and New Link Genetics; received a BrainUp grant for Translational Approaches to Brain Tumors; and received research support (drug only) from Bristol Myers Squibb. S.K. is an employee of Pacific Neuroscience Institute, Providence Saint John’s Health Center, John Wayne Cancer Institute; owns stock/shares in Curtana and Nascent; received grants/funds from Aivita, Diffusion, Boston Biomedical, Medicenna, Aadi, BI, Sanofi, Spectrum, Novocure, Northwestern, Orbus Stellar, Prevlar (epicentrx), Stemedica, EORTC-CTCG, EIP Pharma, Caris, Amgen (Omniseq, Guardant, Biocept, LJIAI). D.H. owns stock/shares in OncoResponse Molecular Match (Advisor), OncoResponse (Founder), and Presagia Inc (Advisor); is part of the advisory council/committee and received consulting fees from Alpha Insights, Amgen, Axiom, Adaptimmune, Baxter, Bayer, Genentech, GLG, Group H, Guidepoint, Infinity, Janssen, Merrimack, Medscape, Numab, Pfizer, Prime Oncology, Seattle Genetics, Takeda, Trieza Therapeutics, WebMD; received honoraria from LOXO, miRNA, Genmab, AACR, ASCO, and SITC; received grants/funds from AbbVie, Adaptimmune, Aldi-Norte, Amgen, Astra-Zeneca, Bayer, BMS, Daiichi-Sankyo, Eisai, Fate Therapeutics, Genentech, Genmab, Ignyta, Infinity, Kite, Kyowa, Lilly, LOXO, Merck, Medimmune, Mirati, miRNA, Molecular Templates, Mologen, NCI-CTEP, Novartis, Pfizer, Seattle Genetics, Takeda, Turning Point Therapeutics. K.Y. is an employee of Sumitomo Dainippon Pharma Co., Ltd. K.W., Y.Z., and Y.L.C. are employees of Sunovion Pharmaceuticals Inc. N.P., J.E., and D.B. are former employees of Sunovion Pharmaceuticals Inc. D.B. is a paid consultant of Sunovion Pharmaceuticals Inc.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-01707-3.
References
- 1.Hutchings Y, et al. Immunotherapeutic targeting of Wilms' tumor protein. Curr. Opin. Mol. Ther. 2007;9:62–69. [PubMed] [Google Scholar]
- 2.Sugiyama H. Cancer immunotherapy targeting Wilms' tumor gene WT1 product. Expert Rev. Vaccines. 2005;4:503–512. doi: 10.1586/14760584.4.4.503. [DOI] [PubMed] [Google Scholar]
- 3.Nakatsuka S, et al. Immunohistochemical detection of WT1 protein in a variety of cancer cells. Mod. Pathol. 2006;19:804–814. doi: 10.1038/modpathol.3800588. [DOI] [PubMed] [Google Scholar]
- 4.Oji Y, et al. Overexpression of the Wilms' tumor gene WT1 in head and neck squamous cell carcinoma. Cancer Sci. 2003;94:523–529. doi: 10.1111/j.1349-7006.2003.tb01477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Netinatsunthorn W, Hanprasertpong J, Dechsukhum C, Leetanaporn R, Geater A. WT1 gene expression as a prognostic marker in advanced serous epithelial ovarian carcinoma: An immunohistochemical study. BMC Cancer. 2006;6:90. doi: 10.1186/1471-2407-6-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rhodes A, Vallikkannu N, Jayalakshmi P. Expression of WT1 and PAX8 in the epithelial tumours of Malaysian women with ovarian cancer. Br. J. Biomed. Sci. 2017;74:65–70. doi: 10.1080/09674845.2016.1220709. [DOI] [PubMed] [Google Scholar]
- 7.Kloudova K, et al. Expression of tumor antigens on primary ovarian cancer cells compared to established ovarian cancer cell lines. Oncotarget. 2016;7:46120–46126. doi: 10.18632/oncotarget.10028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamamoto S, et al. Clinicopathological significance of WT1 expression in ovarian cancer: A possible accelerator of tumor progression in serous adenocarcinoma. Virchows Arch. 2007;451:27–35. doi: 10.1007/s00428-007-0433-4. [DOI] [PubMed] [Google Scholar]
- 9.Waldstrom M, Grove A. Immunohistochemical expression of wilms tumor gene protein in different histologic subtypes of ovarian carcinomas. Arch. Pathol. Lab. Med. 2005;129:85–88. doi: 10.5858/2005-129-85-IEOWTG. [DOI] [PubMed] [Google Scholar]
- 10.Hwang H, Quenneville L, Yaziji H, Gown AM. Wilms tumor gene product: Sensitive and contextually specific marker of serous carcinomas of ovarian surface epithelial origin. Appl. Immunohistochem. Mol. Morphol. 2004;12:122–126. doi: 10.1097/00129039-200406000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Liliac L, et al. The value of PAX8 and WT1 molecules in ovarian cancer diagnosis. Rom. J. Morphol. Embryol. 2013;54:17–27. [PubMed] [Google Scholar]
- 12.Vermeij R, et al. Potential target antigens for a universal vaccine in epithelial ovarian cancer. Clin. Dev. Immunol. 2010;2010:891505. doi: 10.1155/2010/891505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hylander B, et al. Expression of Wilms tumor gene (WT1) in epithelial ovarian cancer. Gynecol. Oncol. 2006;101:12–17. doi: 10.1016/j.ygyno.2005.09.052. [DOI] [PubMed] [Google Scholar]
- 14.Kijima, N. et al. Functional roles of Wilms' Tumor 1 (WT1) in malignant brain tumors. In Wilms Tumor (ed. van den Heuvel-Eibrink, M. M.) 261–272 (Codon Publications, 2016). [PubMed]
- 15.Kijima N, et al. Wilms' tumor 1 is involved in tumorigenicity of glioblastoma by regulating cell proliferation and apoptosis. Anticancer Res. 2014;34:61–67. [PubMed] [Google Scholar]
- 16.Clark AJ, et al. Wilms tumor 1 expression in malignant gliomas and correlation of +KTS isoforms with p53 status. J. Neurosurg. 2007;107:586–592. doi: 10.3171/JNS-07/09/0586. [DOI] [PubMed] [Google Scholar]
- 17.Nakahara Y, Okamoto H, Mineta T, Tabuchi K. Expression of the Wilms' tumor gene product WT1 in glioblastomas and medulloblastomas. Brain Tumor Pathol. 2004;21:113–116. doi: 10.1007/BF02482185. [DOI] [PubMed] [Google Scholar]
- 18.Menssen HD, et al. Wilms' tumor gene (WT1) expression in lung cancer, colon cancer and glioblastoma cell lines compared to freshly isolated tumor specimens. J. Cancer Res. Clin. Oncol. 2000;126:226–232. doi: 10.1007/s004320050037. [DOI] [PubMed] [Google Scholar]
- 19.Oji Y, et al. Overexpression of the Wilms' tumor gene WT1 in de novo lung cancers. Int. J. Cancer. 2002;100:297–303. doi: 10.1002/ijc.10476. [DOI] [PubMed] [Google Scholar]
- 20.Xu C, et al. WT1 promotes cell proliferation in non-small cell lung cancer cell lines through up-regulating cyclin D1 and p-pRb in vitro and in vivo. PLoS ONE. 2013;8:e68837. doi: 10.1371/journal.pone.0068837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu C, et al. Wilms' tumor 1 enhances Cisplatin-resistance of advanced NSCLC. FEBS Lett. 2014;588:4566–4572. doi: 10.1016/j.febslet.2014.10.026. [DOI] [PubMed] [Google Scholar]
- 22.Rossi, G., Minervini, M. M., Carella, A. M., Melillo, L. & Cascavilla, N. Wilms' tumor gene (WT1) expression and minimal residual disease in acute myeloid leukemia. In Wilms Tumor (ed. van den Heuvel-Eibrink, M. M.) 273–285 (Codon Publications, 2016). [PubMed]
- 23.Rezvani K, et al. Leukemia-associated antigen-specific T-cell responses following combined PR1 and WT1 peptide vaccination in patients with myeloid malignancies. Blood. 2008;111:236–242. doi: 10.1182/blood-2007-08-108241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maslak PG, et al. Vaccination with synthetic analog peptides derived from WT1 oncoprotein induces T-cell responses in patients with complete remission from acute myeloid leukemia. Blood. 2010;116:171–179. doi: 10.1182/blood-2009-10-250993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dohi S, et al. WT1 peptide vaccine stabilized intractable ovarian cancer patient for one year: A case report. Anticancer Res. 2011;31:2441–2445. [PubMed] [Google Scholar]
- 26.Iiyama T, et al. WT1 (Wilms' tumor 1) peptide immunotherapy for renal cell carcinoma. Microbiol. Immunol. 2007;51:519–530. doi: 10.1111/j.1348-0421.2007.tb03940.x. [DOI] [PubMed] [Google Scholar]
- 27.Fujiki F, et al. A clear correlation between WT1-specific Th response and clinical response in WT1 CTL epitope vaccination. Anticancer Res. 2010;30:2247–2254. [PubMed] [Google Scholar]
- 28.Izumoto S, et al. Phase II clinical trial of Wilms tumor 1 peptide vaccination for patients with recurrent glioblastoma multiforme. J. Neurosurg. 2008;108:963–971. doi: 10.3171/JNS/2008/108/5/0963. [DOI] [PubMed] [Google Scholar]
- 29.Kawakami M, et al. Clinical and immunologic responses to very low-dose vaccination with WT1 peptide (5 microg/body) in a patient with chronic myelomonocytic leukemia. Int. J. Hematol. 2007;85:426–429. doi: 10.1532/IJH97.06194. [DOI] [PubMed] [Google Scholar]
- 30.Morita S, et al. A phase I/II trial of a WT1 (Wilms' tumor gene) peptide vaccine in patients with solid malignancy: Safety assessment based on the phase I data. Jpn. J. Clin. Oncol. 2006;36:231–236. doi: 10.1093/jjco/hyl005. [DOI] [PubMed] [Google Scholar]
- 31.Ohno S, et al. Wilms' tumor 1 (WT1) peptide immunotherapy for gynecological malignancy. Anticancer Res. 2009;29:4779–4784. [PubMed] [Google Scholar]
- 32.Oka Y, et al. Induction of WT1 (Wilms' tumor gene)-specific cytotoxic T lymphocytes by WT1 peptide vaccine and the resultant cancer regression. Proc. Natl. Acad. Sci. U S A. 2004;101:13885–13890. doi: 10.1073/pnas.0405884101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsuboi A, et al. Wilms tumor gene WT1 peptide-based immunotherapy induced a minimal response in a patient with advanced therapy-resistant multiple myeloma. Int. J. Hematol. 2007;86:414–417. doi: 10.1007/BF02983998. [DOI] [PubMed] [Google Scholar]
- 34.Liu H, et al. WT1 peptide vaccine in Montanide in contrast to poly ICLC, is able to induce WT1-specific immune response with TCR clonal enrichment in myeloid leukemia. Exp. Hematol. Oncol. 2018;7:1. doi: 10.1186/s40164-018-0093-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maslak PG, et al. Phase 2 trial of a multivalent WT1 peptide vaccine (galinpepimut-S) in acute myeloid leukemia. Blood Adv. 2018;2:224–234. doi: 10.1182/bloodadvances.2017014175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang W, et al. Phase I/II clinical trial of a Wilms' tumor 1-targeted dendritic cell vaccination-based immunotherapy in patients with advanced cancer. Cancer Immunol. Immunother. 2019;68:121–130. doi: 10.1007/s00262-018-2257-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Awate S, Babiuk LA, Mutwiri G. Mechanisms of action of adjuvants. Front. Immunol. 2013;4:114. doi: 10.3389/fimmu.2013.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sumitomo Dainippon Pharma. Data on file.
- 39.Keilholz U, et al. A clinical and immunologic phase 2 trial of Wilms tumor gene product 1 (WT1) peptide vaccination in patients with AML and MDS. Blood. 2009;113:6541–6548. doi: 10.1182/blood-2009-02-202598. [DOI] [PubMed] [Google Scholar]
- 40.Soeda A, et al. Long-term administration of Wilms tumor-1 peptide vaccine in combination with gemcitabine causes severe local skin inflammation at injection sites. Jpn. J. Clin. Oncol. 2010;40:1184–1188. doi: 10.1093/jjco/hyq112. [DOI] [PubMed] [Google Scholar]
- 41.Franko A, Magliocco AM, Duan Q, Duggan MA. WT1 immunoprofiling and comparison of malignant Mullerian mixed tumors of the female genital tract. Int. J. Gynecol. Pathol. 2010;29:452–458. doi: 10.1097/PGP.0b013e3181d55597. [DOI] [PubMed] [Google Scholar]
- 42.Skolnik JM, Barrett JS, Jayaraman B, Patel D, Adamson PC. Shortening the timeline of pediatric phase I trials: The rolling six design. J. Clin. Oncol. 2008;26:190–195. doi: 10.1200/JCO.2007.12.7712. [DOI] [PubMed] [Google Scholar]
- 43.Middleton, D., Menchaca, L., Rood, H. & Komerofsky, R. New allele frequency database: http://www.allelefrequencies.net. Tissue Antigens61, 403–407 (2003). [DOI] [PubMed]
- 44.Kuball J, et al. Pitfalls of vaccinations with WT1-, Proteinase3- and MUC1-derived peptides in combination with MontanideISA51 and CpG7909. Cancer Immunol. Immunother. 2011;60:161–171. doi: 10.1007/s00262-010-0929-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaida M, et al. Phase 1 trial of Wilms tumor 1 (WT1) peptide vaccine and gemcitabine combination therapy in patients with advanced pancreatic or biliary tract cancer. J. Immunother. 2011;34:92–99. doi: 10.1097/CJI.0b013e3181fb65b9. [DOI] [PubMed] [Google Scholar]
- 46.Gentilini C, et al. Vaccination with the WT-1 126–134 peptide in patients with acute myeloid leukemia after allogenic stem cell transplantation. Blood. 2006;108:3683. [Google Scholar]
- 47.Tsuboi A, et al. A phase I clinical study of a cocktail vaccine of Wilms' tumor 1 (WT1) HLA class I and II peptides for recurrent malignant glioma. Cancer Immunol. Immunother. 2019;68:331–340. doi: 10.1007/s00262-018-2274-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hashii Y, et al. Encouraging clinical evolution of a pediatric patient with relapsed diffuse midline glioma who underwent WT1-targeting immunotherapy: A case report and literature review. Front. Oncol. 2020;10:1188. doi: 10.3389/fonc.2020.01188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sakai K, et al. Clinical effect and immunological response in patients with advanced malignant glioma treated with WT1-pulsed dendritic cell-based immunotherapy: A report of two cases. Interdiscip. Neurosurg. 2017;9:24–29. [Google Scholar]
- 50.Sakai K, et al. Dendritic cell-based immunotherapy targeting Wilms' tumor 1 in patients with recurrent malignant glioma. J. Neurosurg. 2015;123:989–997. doi: 10.3171/2015.1.JNS141554. [DOI] [PubMed] [Google Scholar]
- 51.Eleneen Y, Colen RR. Cancer imaging in immunotherapy. Adv. Exp. Med. Biol. 2017;995:141–153. doi: 10.1007/978-3-319-53156-4_7. [DOI] [PubMed] [Google Scholar]
- 52.Okada H, et al. Immunotherapy response assessment in neuro-oncology: A report of the RANO working group. Lancet Oncol. 2015;16:e534–e542. doi: 10.1016/S1470-2045(15)00088-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao J, et al. Immune and genomic correlates of response to anti-PD-1 immunotherapy in glioblastoma. Nat. Med. 2019;25:462–469. doi: 10.1038/s41591-019-0349-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ellingson BM, Wen PY, Cloughesy TF. Modified criteria for radiographic response assessment in glioblastoma clinical trials. Neurotherapeutics. 2017;14:307–320. doi: 10.1007/s13311-016-0507-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zauderer MG, et al. A randomized phase II trial of adjuvant galinpepimut-S, WT-1 analogue peptide vaccine, after multimodality therapy for patients with malignant pleural mesothelioma. Clin. Cancer Res. 2017;23:7483–7489. doi: 10.1158/1078-0432.CCR-17-2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Sunovion Pharmaceuticals Inc. is part of a clinical trial data-sharing consortium that facilitates access for qualified researchers to selected anonymized clinical trial data. For up-to-date information on data availability please visit: https://www.clinicalstudydatarequest.com/Study-Sponsors.aspx and click on Sunovion.