Summary
The availability of genome-wide association studies (GWASs) for human blood metabolome provides an excellent opportunity for studying metabolism in a heritable disease such as migraine. Utilizing GWAS summary statistics, we conduct comprehensive pairwise genetic analyses to estimate polygenic genetic overlap and causality between 316 unique blood metabolite levels and migraine risk. We find significant genome-wide genetic overlap between migraine and 44 metabolites, mostly lipid and organic acid metabolic traits (FDR < 0.05). We also identify 36 metabolites, mostly related to lipoproteins, that have shared genetic influences with migraine at eight independent genomic loci (posterior probability > 0.9) across chromosomes 3, 5, 6, 9, and 16. The observed relationships between genetic factors influencing blood metabolite levels and genetic risk for migraine suggest an alteration of metabolite levels in individuals with migraine. Our analyses suggest higher levels of fatty acids, except docosahexaenoic acid (DHA), a very long-chain omega-3, in individuals with migraine. Consistently, we found a causally protective role for a longer length of fatty acids against migraine. We also identified a causal effect for a higher level of a lysophosphatidylethanolamine, LPE(20:4), on migraine, thus introducing LPE(20:4) as a potential therapeutic target for migraine.
Keywords: complex traits, cross-trait genetic analysis, gene-based analysis, genetic correlation, GWAS, headache, Mendelian randomization, metabolome, migraine, pleiotropy
Introduction
Migraine (MIGRAINE [MIM: 157300]) is a highly prevalent brain disorder of episodic severe headache imposing an enormous personal and socioeconomic burden.1,2 Migraine is the second cause of disability worldwide and co-occurs with other serious health conditions, such as cardiovascular diseases.1,2 The study of the blood metabolome (a collection of metabolites) in migraine can provide insights into the metabolism and co-morbidity underlying disease and identify new diagnostic and therapeutic targets. For example, higher levels of blood cholesterol and lower levels of high-density lipoprotein cholesterol (HDL-C)3 and its related metabolite, apolipoprotein A1,1 support the higher risk of cardiovascular diseases in migraine-affected individuals. Moreover, aberrant levels of 29 lipid metabolites in migraine-affected individuals suggest potential diagnostic and therapeutic roles for lipid metabolism.4
Twin studies estimated broad heritability of up to 80% for blood metabolite levels.5 Similarly, genome-wide association studies (GWASs) of metabolome identified common single-nucleotide polymorphisms (SNPs) significantly associated with metabolite levels6, 7, 8, 9, 10, 11, 12, 13 and identified significant SNP heritability for blood metabolome—i.e., significant variance explained by additive SNP effects. These findings show that blood metabolite levels are largely attributable to genetic factors.
Given GWAS has also identified significant SNP heritability (10.35%) for migraine,14 genetic overlap and pleiotropy of heritable metabolites on migraine can be investigated with GWAS data and statistical genetic approaches.15,16 We recently showed that shared genetic factors between clinical chemistry tests (highly related to metabolites) and migraine strongly reflect observed associations between the actual measurements and the risk of migraine.3 In the present study, we estimate the genetic overlap between migraine and blood metabolome at genome-wide and loci levels. Our gene-based analyses identify pleiotropic genes involved in the identified genetic overlaps. Lastly, we infer the genetic causal effects of the identified metabolic traits on migraine via a genome-wide analysis and then validate the findings by a Mendelian randomization method.
Material and methods
GWAS summary statistics
Publicly available GWAS summary statistics for 972 blood (including serum and plasma) metabolic traits from eight separate studies published between May 2014 and October 2019 were obtained.6, 7, 8, 9, 10, 11, 12, 13 Details on studies characteristics, participants, and metabolomics platforms are provided in Table S1. Where GWAS summary statistics have missing information, including rsIDs and non-effect allele, we used the matched human genome build (in this case GRCh37 [hg19]) as a reference to complete the GWAS summary statistics. We used the migraine GWAS summary statistics from the 2016 report of the International Headache Genetics Consortium (IHGC), consisting of 59,674 migraine-affected individuals and 316,078 migraine-free control individuals.14 This GWAS includes 173,612 individuals from 23andMe and provides association results for 8,049,884 SNPs. All GWAS datasets are from European ancestry populations.
Estimation of SNP heritability and filtration
We estimated polygenic SNP heritability for the 972 blood metabolic traits by using linkage disequilibrium score regression (LDSC).17 The European pre-calculated LD scores from the 1000 Genomes Project phase 3 (1000G) for HapMap3 SNPs provided by LDSC were used.18,19 SNPs with imputation quality < 0.9 and minor allele frequency (MAF) < 0.01 were filtered when the GWAS summary statistics include the relevant information. Estimated heritability for all 972 blood metabolic traits is provided in Table S2. We selected GWASs when Z score of is >1.64 (pone-sided value < 0.05), resulting in the selection of 405 of the 972 GWASs. For duplicated GWASs, the one with the highest Z score of was selected. To include only metabolic traits, we also removed body mass index (BMI) and four protein-level GWASs. In total, 316 unique blood metabolic traits GWASs were included for further analyses (Table S3).
Curation of metabolite names and categories
Metabolites were named differently across different studies (e.g., commercial names or chemical names). To find the actual metabolite and unify their names, we used PubChem20 and the Human Metabolome Database (HMDB).21 For categorizing them into superclass and class, we used the “chemical taxonomy” section of the HMDB. Lipoprotein lipids and lipid features (e.g., lipoprotein ratios) that are not reported in the HMDB were categorized into the “lipids and lipid-like molecules” superclass (Table S3).
Imputation of GWAS summary statistics
To have a consistent list of SNPs for all GWASs (316 metabolic traits GWASs and the migraine GWAS), HapMap3 SNPs were imputed with the robust and accurate imputation from summary statistics (RAISS).22 Here, we used 1000G LD reference to estimate Z scores of HapMap3-missing SNPs from neighboring HapMap3-observed SNPs. We limited our imputation to only HapMap3 SNPs that are reportedly well powered in most studies. The 1000G LD matrices for HapMap3 SNPs were computed for 1,703 predefined LD-independent loci.23 To include ambiguous SNPs (G/C and A/T) in our analyses, we removed them prior to imputation and then imputed them by using neighboring SNPs, making these SNPs uniformed across all studied GWASs. Standard errors for imputed SNPs were calculated via allele frequencies from 1000G and the reported sample size of the GWAS,24 and then imputed Z scores were converted to effect sizes. To increase imputation quality, we limited our imputation to only HapMap3 common SNPs (MAF ≥ 0.01) and filtered imputed SNPs with R2 < 0.6. However, as imputation quality is correlated with LD scores,15 we carried out LDSC and latent causal variable (LCV) model analyses (where LD scores are used) on the original (not RAISS-imputed) GWAS summary statistics.
Correlation between SNP effects
We estimated the Pearson correlation between Z scores (r) for independent SNPs from the migraine and blood metabolic traits GWASs, similar to the SNP effect concordant analysis (SECA) approach.25 The Z scores summarize the standardized magnitude and the direction of effect for each SNP, relative to the same reference allele, across the migraine and metabolite GWASs. We applied this approach to test “genetic overlap at the genome-wide level” between migraine risk and blood metabolites levels. This approach consists of two steps. First, to extract independent SNPs, we performed p-value-informed LD-clumping on the RAISS-imputed migraine GWAS by using PLINK 1.926 with --clump-r2 0.1 --clump-kb 10000 flags. This procedure identified 61,057 independent SNPs with the smallest p values at r2 < 0.1 within their 10 Mb LD blocks. We used the 1000G reference panel provided by the SECA software25 to compute LD. Second, we estimated Pearson correlation (r) between independent SNPs Z scores of migraine and the same set of SNPs for RAISS-imputed 316 metabolic traits GWASs. We adjusted the estimated correlation p values for 316 tests by using the “qvalue” R package27,28 to identify significant Pearson correlation at q (false discover rate [FDR]) < 0.05. All correlation results are available in Table S4.
LDSC genetic correlation
We estimated genetic correlations (rg) between migraine and metabolic traits by using cross-trait LDSC with original GWAS summary statistics (not RAISS-imputed).15 This analysis was limited to those metabolic traits with correlated independent SNPs (previous section) at FDR < 0.05 (Figure S1 and Table S4). The European pre-calculated LD scores from 1000G for HapMap3 SNPs were used. Because the migraine GWAS has no substantial sample overlap with metabolic traits GWASs, we constrained cross-trait (genetic covariance) intercepts to zero.
Pleiotropic-associated loci
To identify shared genomic loci containing a SNP within each locus influencing both migraine and a metabolic trait (“genetic overlap at the loci level”), we implemented pairwise GWAS (GWAS-PW) on RAISS-imputed GWASs.16 This software first divides the genome into 1,703 predefined LD-independent loci for GRCh37 human genome build23 and then estimates four posterior probabilities (PPAs) for each locus supporting four scenarios: the association only to migraine (PPA1), the association only to the metabolic trait (PPA2), shared association to the both via a SNP (PPA3), and shared association to them both but by two distinct SNPs (PPA4). We defined a locus as the pleiotropic-associated locus when its PPA3 is >0.9. Furthermore, we calculated the proportion of loci affecting the metabolic trait (when PPA2 > 0.9, PPA3 > 0.9, or PPA4 > 0.9) also affecting migraine (PPA3 > 0.9). This approach estimates genetic overlap between the risk of migraine and blood levels of metabolome at the loci level via a shared SNP. The SNP with the smallest p value in each identified locus for migraine GWAS was defined as the lead SNP, and then its Z score from migraine GWAS was multiplied by the Z score from the metabolic trait GWAS (Z1 × Z2), suggesting a concordant or discordant pleiotropic locus.
Gene-based analysis and pleiotropic genes
We used multi-marker analysis of genomic annotation (MAGMA v.1.07b)29 to perform gene-based analysis on RAISS-imputed GWASs. This analysis is limited to autosomal 18,563 protein-coding genes. SNPs were assigned to genes by addition of an annotation window of ± 500 kb flanking the gene boundaries with gene locations from the matched Ensembl build (Ensembl build GRCh37) and 1000G EUR data, and then Z scores (Zgene) and p values (pgene) for genes were computed. We call Zgene and pgene Zmigraine and pmigraine for migraine and Zmetabolite and pmetabolite for metabolic traits.
We performed a pairwise gene-based analysis to define the top three pleiotropic genes associated with migraine and metabolic traits identified in the genome-wide genetic overlap analysis. We first limited our analysis to migraine-associated genes (Benjamini and Hochberg adjusted pmigraine < 0.05). Second, we used the following equation to calculate “pleiotropy score,” pleiotropy score = loge rank (Zmigraine) × loge rank (Zmetabolite), where loge is natural logarithm and rank is a function returning the ranks of the Zgene (Zmigraine and Zmetabolite). Genes with higher pleiotropy scores are more likely to be associated with both risk of migraine and the blood level of the metabolite. The top three genes with the highest pleiotropy scores were defined as the top three pleiotropic genes.
Moreover, the lead gene in a pleiotropic locus (genetic overlap at the loci level identified by GWAS-PW) was the gene assigned to the lead SNP in that locus with the largest Zmigraine (smallest pmigraine).
Causal relationship
Finally, we aimed to test for causal relationships between migraine as the outcome and its genetically associated metabolic traits as exposures (those metabolic traits identified in genetic overlap at the genome-wide and loci levels analyses). The latent causal variable (LCV) model is a genome-wide analysis that estimates a genetic causality proportion (GCP) between metabolic traits and migraine.30 LCV model analysis was performed with original GWAS summary statistics (not RAISS-imputed) under the default parameters. The European pre-calculated LD scores from the 1000G for HapMap3 SNPs provided by LDSC were used.18,19 GCP of zero means no genetic causality, and GCP of one means full genetic causality. Briefly, the LCV model produces latent variables that mediate genetic correlation between two traits of interest. When a metabolite has a genetic correlation with the latent variables stronger than migraine does, migraine is genetically caused by the metabolite. All LCV model results are available in Table S5. As a causal relationship between two traits is a strong statement, we used a stringent correction methodology (Bonferroni) for multiple testing adjustment and used a Mendelian randomization method to validate LCV model significant findings.
The generalized summary data-based Mendelian randomization (GSMR),31 comprising a different statistical methodology from the LCV model, was applied to the LCV model significant findings (Bonferroni-adjusted p value < 0.05). GSMR provides important information about the direction and strength of the causal effects. Briefly, we used GSMR analysis to estimate the effect of a metabolic trait (exposure) on migraine (bxy) by using the effects of genetic instruments on the metabolic trait (bzx) and on migraine (bzy). As a Mendelian randomization approach, GSMR software only uses significant SNPs for exposure genetic instruments (unlike LCV model that uses genome-wide SNPs). This analysis was performed on RAISS-imputed GWASs, and the frequency of effect alleles required by the software was obtained from 1000G. Independent genetic instruments for each exposure (metabolic trait GWAS) were selected at GWAS p value < 1 × 10−5 and LD r2 < 0.1 (Tables S6–S10). This method leverages integrating multiple independent genetic instruments and removing instruments with a horizontal pleiotropic effect (if HEIDI-outlier p value < 0.01) to increase statistical power.31 GSMR results are available in Table S5.
Results
Metabolic traits with a significant polygenic SNP heritability
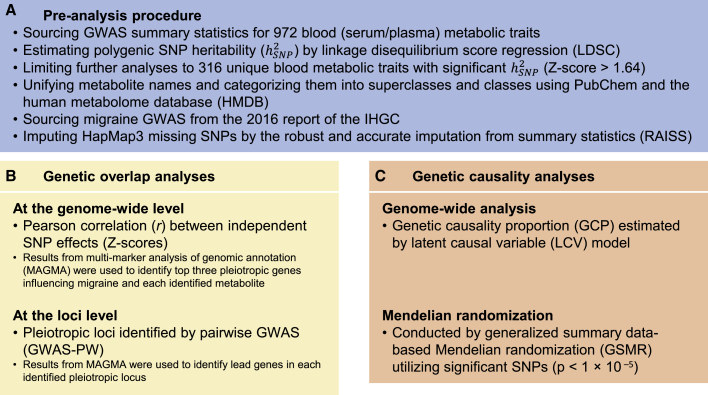
A summary of the analysis workflow is presented in Figure 1. We collected 972 publicly available GWAS summary statistics for blood metabolic traits from eight studies.6, 7, 8, 9, 10, 11, 12, 13 More details for included GWASs are available in Table S1. The median sample size across all collected GWASs was 7,824 individuals. We first estimated the polygenic for all 972 metabolic trait GWASs by using LDSC (Table S2).17 We observed that Z scores of are correlated with sample size (r = 0.17, 95% confidence interval [CI] = 0.11–0.23, p = 1 × 10−7) and the number of included SNPs (r = 0.12, 95% CI = 0.06–0.19, p = 9 × 10−5). To include only GWASs with sufficient statistical power and polygenic signal, we limited our analyses to GWASs with a nominally significant (Z score of > 1.64, pone-sided value < 0.05), resulting in 405 metabolic trait GWASs. For duplicated metabolic trait GWASs, the GWAS with the largest Z score of was retained for further analyses, resulting in 316 unique metabolic traits (Table S3). The included metabolic traits were categorized into ten superclasses and 24 classes according to the HMDB21 (Tables 1 and S3).
Figure 1.
Study workflow
(A) We first collected 972 publicly available GWAS summary statistics for blood metabolic traits from eight studies. We limited our analyses to GWASs with a nominally significant polygenic (Z score > 1.64; pone-sided value < 0.05), resulting in 316 unique metabolic traits. The included metabolic traits were categorized into ten superclasses and 24 classes according to the HMDB. For migraine, we used migraine GWAS summary statistics from the 2016 report of the International Headache Genetics Consortium (IHGC). To have a consistent list of SNPs for all GWASs (316 metabolic traits GWASs and a migraine GWAS), we imputed HapMap3 SNPs by using RAISS software.
(B) We conducted comprehensive genetic analyses estimating genetic overlap between the included 316 metabolic traits and migraine at two levels: (1) genome-wide (by estimating Pearson correlation between independent SNP effects) and (2) loci (applying GWAS-PW) levels. Moreover, we used MAGMA results to identify top three pleiotropic genes contributing to the identified genome-wide genetic overlaps and the lead migraine genes in the identified eight pleiotropic loci.
(C) The findings from genetic overlap analyses were followed-up for inferring causality implementing a genome-wide approach (LCV model) and then verified the LCV model statistically significant results via a Mendelian randomization method (GSMR).
Table 1.
Summary of the 316 unique metabolic traits (with a significant heritability) analyzed in this study per metabolite superclasses and classes
| Superclass (no. of metabolites) | Class (no. of metabolites) | Sample size | range | SE range | median | SE median |
|---|---|---|---|---|---|---|
| Lipids and lipid-like molecules (189) | lipoprotein lipids (82) | 6,263–24,925 | 0.05–0.38 | 0.02–0.12 | 0.12 | 0.03 |
| fatty acyls (39) | 7,478–24,925 | 0.07–0.57 | 0.04–0.14 | 0.21 | 0.07 | |
| glycerophospholipids (24) | 6,263–7,824 | 0.1–0.33 | 0.05–0.09 | 0.17 | 0.07 | |
| lipid features (24) | 6,263–24,925 | 0.05–0.36 | 0.03–0.12 | 0.14 | 0.03 | |
| steroids and steroid derivatives (10) | 6,263–7,824 | 0.16–0.51 | 0.06–0.3 | 0.26 | 0.09 | |
| lipid ratios (4) | 6,263 | 0.18–0.2 | 0.09–0.11 | 0.19 | 0.09 | |
| glycerolipids (3) | 7,824–24,925 | 0.12–0.35 | 0.03–0.08 | 0.18 | 0.07 | |
| sphingolipids (3) | 7,478–7,824 | 0.12–0.43 | 0.06–0.07 | 0.14 | 0.07 | |
| Organic acids and derivatives (42) | carboxylic acids and derivatives (32) | 6,263–24,925 | 0.06–1 | 0.02–0.12 | 0.34 | 0.07 |
| keto acids and derivatives (4) | 7,824–24,925 | 0.07–0.71 | 0.03–0.08 | 0.22 | 0.06 | |
| hydroxy acids and derivatives (3) | 7,824–24,925 | 0.04–0.36 | 0.02–0.08 | 0.11 | 0.06 | |
| organic sulfuric acids and derivatives (2) | 7,824 | 0.16–0.18 | 0.06–0.06 | 0.17 | 0.06 | |
| organic phosphoric acids and derivatives (1) | 7,824 | 0.15 | 0.07 | 0.15 | 0.07 | |
| Organic oxygen compounds (8) | organooxygen compounds (8) | 7,824–24,925 | 0.09–0.51 | 0.02–0.12 | 0.28 | 0.07 |
| Organoheterocyclic compounds (6) | imidazopyrimidines (3) | 7,824 | 0.16–0.21 | 0.06–0.08 | 0.2 | 0.07 |
| indoles and derivatives (2) | 7,824 | 0.14–0.35 | 0.06–0.07 | 0.25 | 0.07 | |
| oxepanes (1) | 7,824 | 0.22 | 0.13 | 0.22 | 0.13 | |
| Organic nitrogen compounds (3) | organonitrogen compounds (3) | 6,263–7,824 | 0.19–1 | 0.08–0.09 | 0.25 | 0.09 |
| Benzenoids (2) | benzene and substituted derivatives (1) | 7,824 | 0.39 | 0.07 | 0.39 | 0.07 |
| phenols (1) | 7,824 | 1 | 0.36 | 1 | 0.36 | |
| Nucleosides, nucleotides, and analogs (2) | purine nucleosides (2) | 7,824 | 0.48–0.71 | 0.05–0.1 | 0.59 | 0.08 |
| Homogeneous non-metal compounds (1) | non-metal oxoanionic compounds (1) | 7,824 | 0.35 | 0.05 | 0.35 | 0.05 |
| Phenylpropanoids and polyketides (1) | phenylpropanoic acids (1) | 7,824 | 0.23 | 0.06 | 0.23 | 0.06 |
| Unknown (62) | unknown (62) | 7,824 | 0.11–1 | 0.04–0.54 | 0.3 | 0.07 |
, SNP heritability; SE, standard error of SNP heritability.
We found several differences in the mean across different metabolite superclasses and classes. For example, lipids have a significantly lower mean than organic acids (0.19 versus 0.32, two-sided pt test = 3 × 10−8), supporting a previous study that estimated lower heritability for lipids than amino acids (organic acids).32 However, these results are not presented here because the focus of this study was to identify genetic relationships between migraine and blood metabolome rather than univariate heritability analysis of metabolites that has been investigated elsewhere.5
For migraine, the GWAS summary statistics from the IHGC 2016 study14 with a liability scale of 10.35% (standard error [SE] = 0.51%, population prevalence = 0.15) was analyzed.
Genetic overlap between migraine and metabolic traits at the genome-wide level
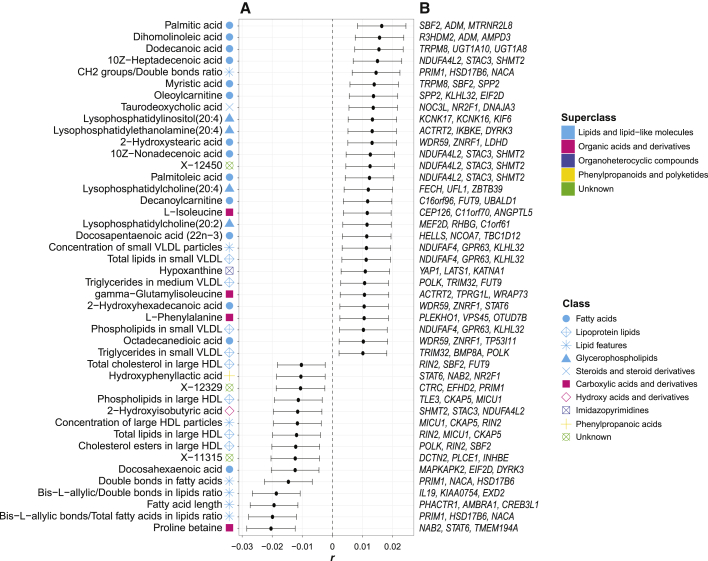
To identify the genome-wide genetic overlap between migraine and blood metabolic traits, we estimated Pearson correlation coefficients (r) between 61,057 independent SNP Z scores from the migraine GWAS and Z scores for the same set of SNPs for the 316 metabolic traits GWASs aligned to the same effect allele (full details are provided in the material and methods section). Significant correlations between SNP Z scores suggest a widespread shared etiology or a causal relationship between migraine and metabolic traits. All correlation results are provided in Table S4. Of the 316 correlation tests, we found SNP effects for 44 metabolites significantly correlated with SNP effects for increased migraine risk (Figure 2A) at FDR < 0.05. Genetic risk for migraine was twice as likely to be associated with a genetic risk for increased metabolite levels, and migraine risk was correlated with increased levels of 29 metabolites compared to 15 metabolites with decreased levels.
Figure 2.
Significant correlations between migraine and blood metabolite SNP effects (genetic overlap at the genome-wide level)
(A) Pearson correlation between Z scores (r) of 61,057 independent SNPs from migraine GWAS and from 316 blood metabolite GWASs produced significant results (FDR < 0.05) for 44 metabolic traits. Full correlation results for all 316 metabolic traits are available in Table S4. Circles represent the Pearson correlations (r), and the error bars indicate the 95% confidence interval.
(B) Our pairwise gene-based analysis with MAGMA results identified pleiotropic genes associated with both migraine and each metabolic trait. Here, we show the top three pleiotropic genes for migraine and each metabolic trait. Different colors and shapes represent the superclasses and classes defined by the HMDB, respectively.
The 44 significantly associated metabolic traits include 34 lipids, five organic acids (e.g., amino acids), an organoheterocyclic compound, a phenylpropanoid, and three unknown metabolites. For each class, the strongest correlations with migraine risk are proline betaine (carboxylic organic acid, r = –0.02, p = 7 × 10−7), Bis-L-allylic bonds/total fatty acids in lipids ratio (lipid feature, r = –0.02, p = 9 × 10−7), palmitic acid (lipid fatty acid, r = 0.02, p = 7 × 10−5), lysophosphatidylinositol(20:4) (glycerophospholipid, r = 0.01, p = 1 × 10−3), taurodeoxycholic acid (steroid, r = 0.01, p = 1 × 10−3), cholesterol esters in large HDL (lipoprotein lipid, r = –0.01, p = 3 × 10−3), 2-hydroxyisobutyric acid (hydroxy organic acid, r = –0.01, p = 5 × 10−3), hypoxanthine (organoheterocyclic imidazopyrimidine, r = 0.01, p = 8 × 10−3), and hydroxyphenyllactic acid (phenylpropanoic acid, r = –0.01, p = 0.01).
We also estimated genetic correlations (rg) between the 44 metabolic traits and migraine by using cross-trait LDSC.15 Both r between Z scores and rg estimate the genome-wide genetic overlap between a pair of polygenic traits. LDSC genetic correlation was performed on original GWASs because imputation from summary statistics may negatively affect the performance of LDSC.15 Results from the independent SNP effects correlation (where RAISS-imputed GWASs were used) reflect the LDSC genetic correlation results (Figure S1). Out of the 44 metabolic traits, we observed nominally significant rg (p value < 0.05) for 20 and marginally significant rg (0.05 < p value < 0.1) for 11 (Figure S1).
Genetic overlap between migraine and metabolic traits at the loci level
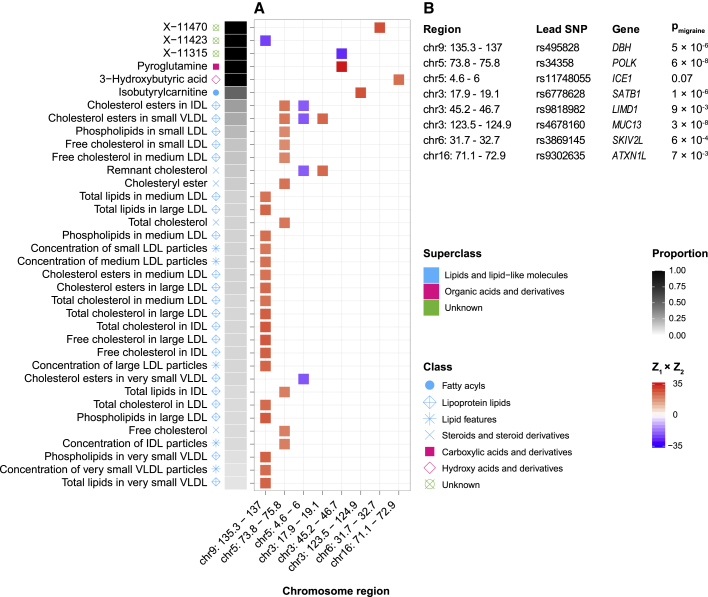
We used the GWAS-PW approach to identify pleiotropic genomic loci influencing both migraine and a metabolic trait via a SNP within each locus (genetic overlap at the loci level). As previously suggested,16 identifying pleiotropic loci complements genome-wide genetic overlap (r between Z scores and rg) findings. A significant genome-wide genetic overlap is exhibited when two traits have global shared genetic influences with a consistent direction; however, a pleiotropic locus is a genetic variant that affects both traits regardless of the direction of effects.15 This scan identified eight loci across five chromosomes influencing migraine and one or more metabolic traits. This connects migraine to 36 metabolic traits, including 31 lipids, two organic acids, and three unknown metabolites.
Consistent with the previous reports,15,16 our identified metabolites from GWAS-PW are distinct from genome-wide genetic overlap findings, except X-11315 (Figure 3A). Briefly, we believe that only widespread and strong pleiotropies with the consistent direction of effects produce a genome-wide genetic overlap between two traits. Despite the differences between the two methods, findings from both are significantly enriched for lipid metabolites (34 lipids out of 44 significant results in the genome-wide analysis, observed proportion = 34/44, null proportion = number of all included lipid metabolites/number of all metabolites [ = 189/316], two-sided pbinomial test = 0.02; and 31 lipids out of 36 significant results in the genetic overlap at the loci level analysis, observed proportion = 31/36, null proportion = 189/316, two-sided pbinomial test = 1 × 10−3). This highlights the importance of lipids in migraine biology.
Figure 3.
Pleiotropic-associated loci influencing migraine and blood metabolome (genetic overlap at the loci level)
(A) GWAS-PW identified eight independent pleiotropic loci across five chromosomes, influencing migraine risk and 36 metabolic traits levels (out of 316 tested metabolic traits) via a SNP within each locus. The concordance (red color) or discordance (blue color) of each locus is defined by the Z score of the lead migraine SNP from the migraine GWAS and from each metabolic trait GWAS (Z1 × Z2). The proportion of the independent loci influencing the metabolic trait also influencing migraine is shown in gray to black.
(B) For the identified pleiotropic loci, lead SNPs from the migraine GWAS (lead migraine SNPs) are shown. We used MAGMA results (pmigraine) to identify lead migraine genes in the identified pleiotropic loci. Different colors and shapes represent superclasses and classes, defined by the HMDB, respectively.
While pleiotropy is unsigned (unlike r between Z scores and rg), we tried to define the direction of the influence for each pleiotropic locus by using the product of Z scores (Z1 × Z2) from the lead migraine SNP located at the locus (see material and methods section for details). Detailed results are presented in Figure 3A. The identified pleiotropic loci include chromosome 9: 135.3–137 Mb (lead migraine SNP, rs495828; associated with migraine risk, higher levels of 18 lipids, and lower level of one unknown metabolite), chromosome 5: 73.8–75.8 Mb (lead migraine SNP, rs34358; associated with migraine risk and higher levels of ten lipids), chromosome 5: 4.6–6 Mb (lead migraine SNP, rs11748055; associated with migraine risk and lower levels of four lipids), chromosome 3: 17.9–19.1 Mb (lead migraine SNP, rs6778628; associated with migraine risk and higher levels of two lipids), chromosome 3: 45.2–46.7 Mb (lead migraine SNP, rs9818982; associated with migraine risk, a higher level of one organic acid, and a lower level of one unknown metabolite), chromosome 3: 123.5–124.9 Mb (lead migraine SNP, rs4678160; associated with migraine risk and a higher level of one lipid), chromosome 6: 31.7–32.7 Mb (lead migraine SNP, rs3869145; associated with migraine risk and a higher level of one unknown metabolite), and chromosome 16: 71.1–72.9 Mb (lead migraine SNP, rs9302635; associated with migraine risk and a higher level of one organic acid).
Moreover, we estimated the proportion of the independent loci that influence the metabolic trait also influence migraine by using GWAS-PW results (see material and methods section for details). The calculated proportion for three unknown metabolites and two organic acids (pyroglutamine and 3-hydroxybutyric acid) was one, meaning that the detected loci (in this case, only one locus) that affect the levels of the metabolites also affect migraine (Figure 3A).
Pleiotropic genes influencing migraine and metabolic traits
Our pairwise gene-based analysis identified pleiotropic genes contributing to the identified genome-wide genetic overlaps. We used the Zgene scores estimated by MAGMA29 for migraine (Zmigraine) and each metabolic trait (Zmetabolite) identified in the genome-wide genetic overlap analysis to define the top three pleiotropic genes (see material and methods section for details) associated with migraine risk and the metabolic trait level (Figure 2B). In total, 77 unique pleiotropic genes were identified, 27 of which were associated with migraine and more than one metabolic trait, including NDUFA4L2 (associated with migraine and five metabolites, pmigraine = 2 × 10−15, median pmetabolite = 9 × 10−3), SHMT2 (MIM: 138450, associated with migraine and five metabolites, pmigraine = 2 × 10−15, median pmetabolite = 9 × 10−3), and STAC3 (MIM: 615521, associated with migraine and five metabolites, pmigraine = 2 × 10−15, median pmetabolite = 9 × 10−3).
We also used MAGMA results to identify lead migraine genes in the identified eight pleiotropic loci (genetic overlap at the loci level). The results are shown in Figure 3B. This analysis revealed the strongest associated genes (with migraine) in the detected metabolite-migraine pleiotropic loci, including MUC13 (MIM: 612181, pmigraine = 3 × 10−8), POLK (MIM: 605650, pmigraine = 6 × 10−8), SATB1 (MIM: 602075, pmigraine = 1 × 10−6), DBH (MIM: 609312, pmigraine = 5 × 10−6), SKIV2L (MIM: 600478, pmigraine = 6 × 10−4), ATXN1L (MIM: 614301, pmigraine = 7 × 10−3), and LIMD1 (MIM: 604543, pmigraine = 9 × 10−3). ICE1 (MIM: 617958) that was assigned to chromosome 5: 4.6–6 Mb locus (lead SNP, rs11748055) is not quite statistically significant (pmigraine = 0.07).
Metabolic traits with causal effects on migraine
Our analyses of genetic overlap at the genome-wide and loci level identified 44 and 36 metabolic traits, respectively, having shared genetic components with migraine. These analyses genetically connect 79 “unique” metabolic traits to migraine risk. However, causal relationships between these metabolic traits and migraine remained to be found. Therefore, we tested for a causal role of the identified 79 unique metabolic traits on migraine by using two separate methodologies that are both reportedly immune to confounders, such as genetic correlation and horizontal pleiotropy. Here, we first estimated the GCP between metabolic traits and migraine by the LCV model30 and then verified the statistically significant results by using GSMR software.31 The LCV model and GSMR software interrogate causal relationships via all genome-wide SNPs and only genome-wide (suggestive) significant SNPs, respectively.
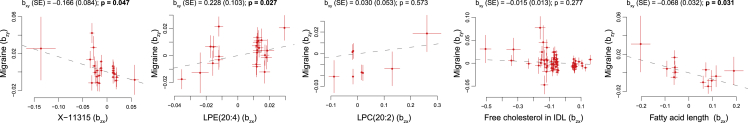
The LCV model found a genetic causality for five metabolic traits on migraine (not vice versa because GCP > 0), including X-11315 (GCP = 0.37, Bonferroni-adjusted p = 6 × 10−10), lysophosphatidylethanolamine(20:4) ([LPE(20:4)], GCP = 0.70, Bonferroni-adjusted p = 1 × 10−7), lysophosphatidylcholine(20:2) ([LPC(20:2)], GCP = 0.67, Bonferroni-adjusted p = 6 × 10−8), free cholesterol in IDL (GCP = 0.25, Bonferroni-adjusted p = 4 × 10−6), and fatty acid length (GCP = 0.45, Bonferroni-adjusted p = 2 × 10−5) (Table 2). Of these five traits, three traits reached nominal significant level (p value < 0.05) in GSMR analysis (Figure 4 and Table S5). Summary statistics for the instruments used in the GSMR analysis are provided in Tables S6–S10. GSMR results suggest that higher levels of LPE(20:4) (bxy = 0.23, p = 0.03), shorter length of fatty acid (bxy = –0.07, p = 0.03), and lower levels of X-11315 (bxy = –0.17, p = 0.05) causally increase migraine risk. Given causal relationship is a strong claim, we only discuss the three metabolic traits that also were replicated by GSMR analysis.
Table 2.
Metabolic traits with a causal effect on migraine identified by LCV model
| Metabolic trait | Superclass | Class | GCP | SE | Adjusted p |
|---|---|---|---|---|---|
| X-11315 | unknown | unknown | 0.37 | 0.05 | 8 × 10−12 |
| LPE(20:4) | lipids and lipid-like molecules | glycerophospholipids | 0.70 | 0.19 | 1 × 10−9 |
| LPC(20:2) | lipids and lipid-like molecules | glycerophospholipids | 0.67 | 0.07 | 6 × 10−8 |
| Free cholesterol in IDL | lipids and lipid-like molecules | lipoprotein lipids | 0.25 | 0.35 | 4 × 10−6 |
| Fatty acid length | lipids and lipid-like molecules | lipid features | 0.45 | 0.16 | 2 × 10−5 |
Full LCV model results for all 79 tested metabolic traits (identified in genetic overlap analyses) are available in Table S5. LPE, lysophosphatidylethanolamine; LPC, lysophosphatidylcholine; GCP and SE, posterior mean genetic causality proportion and posterior standard error estimated by the LCV model; Adjusted p, p values tested the null hypothesis of no genetic causality and were adjusted with the Bonferroni method.
Figure 4.
GSMR analysis testing the causal effect of X-11315, LPE(20:4), LPC(20:2), free cholesterol in IDL, and fatty acid length on migraine
The y and x axes show effect sizes of genetic instruments for each metabolic trait (bzx) and those for migraine (bzy), respectively. The lines represent standard errors for bzx and bzy. Independent genetic instruments (SNPs) were selected for each metabolic trait at GWAS p value < 1 × 10−5 and LD r2 < 0.1. After removing instruments with a horizontal pleiotropic effect (if HEIDI-outlier p value < 0.01), 26, 23, 8, 66, and 12 genetic instruments were used in GSMR analysis testing the causal effect of X-11315, lysophosphatidylethanolamine(20:4) (LPE(20:4)), lysophosphatidylcholine(20:2) (LPC(20:2)), free cholesterol in IDL, and fatty acid length, respectively. Summary statistics for the genetic instruments used in the GSMR analysis are provided in Tables S6–S10. Beta coefficient estimated by GSMR (bxy) is the effect of the metabolic trait on migraine. Nominally significant p values (<0.05) estimated by GSMR are shown in bold.
Discussion
We conducted comprehensive genetic analyses estimating genetic overlap between human blood metabolites with a polygenic and migraine by utilizing GWAS summary statistics. Such a genetic overlap suggests an alteration of metabolite levels in individuals with migraine, indicating a shared biological mechanism and/or a causal relationship. The genetic overlap between metabolic traits and migraine was explored at genome-wide and loci levels. Gene-based analyses suggested genes involved in the identified genetically overlapping traits. Lastly, the findings were followed-up for causality implementing a genome-wide approach (LCV model) and a Mendelian randomization method (GSMR). The study workflow is shown in Figure 1.
If migraine and a metabolic trait have a widespread shared etiology or a causal relationship, their SNP effects are likely to be correlated. We identified a significant r between SNP effect Z scores of migraine and 44 metabolic traits, mostly fatty acid lipids. Fatty acids are compartments of more complex lipids, such as triglycerides and phospholipids, contributing to cell signaling, cell membrane compositions, and gene expression, influencing a disease risk.33 We identified 14 fatty acids that are genetically associated with migraine risk, in which 12 metabolites are known as either medium- or long-chain fatty acids, and two metabolites are omega-3 fatty acids belonging to the very-long-chain fatty acids parent, including docosahexaenoic acid (DHA) and docosapentaenoic acid (22n-3) (DPA).21 Higher levels of all 12 fatty acids with a medium- or long-chain, including a rare omega-6 (dihomolinoleic acid), are genetically associated with a higher risk of migraine. As migraine is associated with inflammation and a higher risk for cardiovascular diseases,3,34 these findings may reflect adverse effects for medium- or long-chain fatty acids in health, as previously reviewed.33,35 Conversely, lower levels of omega-3 fatty acids (with a major contribution of DHA) have been associated with migraine risk among males.1 Here, we found a genetic overlap between migraine and a lower level of DHA, suggesting a protective role of this omega-3 in migraine, regardless of the sex. A lower level of DHA has been associated with inflammation, cardiovascular disorders, and brain disorders,36,37 such as depression (MDD [MIM: 608516]) and schizophrenia (SCZD [MIM: 181500]), which all were linked to migraine risk.3,34,38 Consistent with these findings, we found a genetic overlap between a shorter length of fatty acids and increased risk of migraine. Our tests for inferring causality identified a genetic causal effect of fatty acids with a shorter length on migraine. Interestingly, a clinical trial study showed that supplementation with fish oil rich in very long-chain omega-3 fatty acids (DHA and eicosapentaenoic acid) reduces headache frequency and severity among adolescent migraineurs.39 Moreover, it has been shown that resolvins (lipid mediators) derived from DHA and eicosapentaenoic acid remarkably benefit inflammation-related pains.40 We also found that the number of double bonds in fatty acids has a negative genome-wide genetic correlation (r between SNP Z scores) with migraine, suggesting lower levels of unsaturated fatty acids, such as omega-3 fatty acids, and higher levels of saturated fatty acids in migraine-affected individuals. Taken together, our study supports these findings and provides strong evidence for preventative or therapeutic implications of DHA, a very long-chain omega-3 fatty acid, in migraine. Conversely, we found a genome-wide genetic overlap between higher levels of DPA (another very long-chain fatty acid) and migraine, suggesting an unfavorable effect of this omega-3 on migraine or possibly other linked disorders. DPA has a complex metabolic relationship with DHA and eicosapentaenoic acid, and its role in health or disease remains to be found.41
Furthermore, we found a genome-wide genetic overlap between higher levels of four lysoglycerophospholipids and migraine risk. Lysoglycerophospholipids are mainly produced from the hydrolysis of membrane glycerophospholipids, and they contribute to signaling pathways and biosynthesis of other lipid molecules as intermediate precursors.42 Our causality inference showed that higher levels of a lysophosphatidylethanolamine (LPE), LPE(20:4), has a causal influence on migraine. LPEs are mainly derived from membrane phosphatidylethanolamines by a phospholipase A2 activity where one fatty acid is removed.42 Intriguingly, anandamide (N-arachidonoylethanolamine [AEA])—a primary lipid involved in the endocannabinoid system—is also derived from the common precursor as LPEs (phosphatidylethanolamines) by a phospholipase D activity.43 Lower levels of anandamide in cerebrospinal fluid and plasma have been linked to chronic migraine44,45 and associated with neurological pains.46 Inflammatory conditions trigger the production of anandamide that inhibits dural vessel dilation induced by calcitonin gene-related peptide (CGRP) and nitric oxide; however, the level of anandamide is rapidly decreased by fatty acid amide hydrolase (FAAH).46 Therefore, higher levels of anandamide may prevent pain facilitation among migraine-affected individuals; indeed, increasing the level of anandamide via inhibiting FAAH has been suggested as a promising therapy for migraine pain.46 We suggest that higher levels of LPE(20:4) in migraine-affected individuals may reduce levels of anandamide by two possible mechanisms (↑LPE(20:4) → ↓anandamide → ↑migraine). First, as LPEs and anandamide are both formed from membrane phosphatidylethanolamines, higher levels of LPE(20:4) may limit the resource required to produce anandamide. Second, LPEs inhibit phospholipase D activity47 that produces anandamide in neurons. These findings and our analyses highlight a causal role for LPEs, specifically LPE(20:4), indicating a potential therapeutic target for migraine.
The genome-wide genetic overlaps between migraine and lower levels of HDL and higher levels of very-low-density lipoprotein (VLDL) metabolites support the involvement of lipoproteins and cardiovascular disorders in migraine that were also shown in previous reports.1,3,48 However, consistent with our recent study,3 we found no causal influence of HDL and VLDL metabolites on migraine. We also found a positive genome-wide genetic correlation (r between SNP Z scores) but no causality for taurodeoxycholic acid level that is a bile acid metabolite derived from cholesterol. Bile acids contribute to the metabolism of dietary lipids and affect the function of several neurotransmitter receptors in the brain.49
Among organic acids, significant genome-wide genetic overlaps with migraine risk were identified for higher levels of three amino acids (isoleucine, gamma-glutamylisoleucine, phenylalanine) and lower levels of an amino acid (proline betaine) and an alpha hydroxy acid (2-hydroxyisobutyric acid). Amino acids are the main products of the dietary protein catabolism that contribute to almost every biological function. Although glutamate (as a crucial excitatory neurotransmitter in the brain) has been linked to neurological disorders, including migraine,50,51 our study found no significant genetic overlap between blood level of glutamate and migraine risk. However, gamma-glutamylisoleucine, a dipeptide of glutamate and isoleucine, has a positive genome-wide genetic correlation (r between SNP Z scores) with migraine risk. The involvement of the identified organic acids in migraine has not been reported before. We highlight that these genetic relationships can be either due to direct genetic overlaps between migraine and the identified organic acids or intermediated by other biological mechanisms and disorders that are linked to migraine. Here, we provide three examples for such intermediations. First, higher levels of isoleucine in blood might be associated with migraine via reducing the levels of neurotransmitters (such as dopamine and serotonin) in the brain.52 Second, a lower level of proline betaine in migraine (also known as stachydrine) can be due to its protective properties for inflammatory and cardiovascular disorders.53 Third, we found a genome-wide genetic overlap between lower levels of 2-hydroxyisobutyric acid and migraine risk. 2-hydroxyisobutyric acid is a metabolite produced during the elimination of methyl tert-butyl ether (an organic compound found in gasoline and contaminated water). Lower levels of 2-hydroxyisobutyric acid may reflect higher levels of methyl tert-butyl ether that has acute health adverse effects, including headache and nausea.54
We identified a genome-wide genetic overlap between a lower level of hydroxyphenyllactic acid (4-hydroxyphenyllactate) and migraine risk. This metabolite is a phenylpropanoic acid, and its role in disease has been rarely studied. Moreover, we found a significant genome-wide genetic overlap between aberrant levels of three unknown metabolites and migraine risk. Among these, lower levels of X-11315 has a causal effect on migraine. Identifying and characterizing these metabolites is a direction for further investigations.
Our pairwise gene-based analysis implicated the top three pleiotropic genes involved in both migraine risk and each metabolic trait identified in the genome-wide genetic overlap analysis. Among the implicated genes, three genes on chromosome 12 (NDUFA4L2, STAC3, and SHMT2) are common among combinations of migraine and five metabolic traits. The NDUFA4 mitochondrial complex associated like 2 (NDUFA4L2) gene is a subunit of the mitochondrial complex I (type I NADH dehydrogenase) that is a part of the electron transport chain (ETC). Any dysregulation of mitochondrial complex I compartments adversely affects tissues that require a high level of energy, such as brain and heart.55 The involvement of mitochondria and the ETC in migraine pathophysiology has been suggested previously.56 The SH3 and cysteine rich domain 3 (STAC3) gene is involved in neuromuscular transmission via Ca2+ release.57 Our finding may link the dysfunction of the neuromuscular transmission found in migraine-affected individuals to STAC3 rather than the calcium voltage-gated channel subunit alpha1 A (CACNA1A [MIM: 601011]) gene, implicated in a rare monogenic form of migraine (familial hemiplegic migraine, FHM1 [MIM: 141500]) that has been suggested previously.58 The serine hydroxymethyltransferase 2 (SHMT2) gene encodes a mitochondrial protein that generates glycine and 5,10-methylenetetrahydrofolate from serine and tetrahydrofolate. Interestingly, this gene recently has been associated with brain and heart abnormalities.59
Furthermore, we identified pleiotropic loci influencing migraine and metabolic traits (genetic overlap at the loci level). Our findings highlight shared biology between migraine and lipoproteins by identifying pleiotropic loci between migraine and 26 lipoprotein-related traits, including 16 low-density lipoprotein cholesterol (LDL), five very-low-density lipoprotein cholesterol (VLDL), and five intermediate-density lipoprotein cholesterol (IDL) metabolic traits. This analysis revealed eight distinct loci influencing migraine and blood metabolic traits. The strongest pleiotropic locus is in chromosome 9: 135.3–137 Mb that is shared between migraine and 19 metabolic traits, mostly LDL-related metabolic traits. Our gene-based analysis associates this region to the dopamine beta-hydroxylase (DBH) gene that catalyzes dopamine to norepinephrine. SNPs at this locus affect the activity and expression level of DBH60 and have been associated with migraine risk.61 Nevertheless, the identified findings are not always straightforward to interpret. For example, the second strongest pleiotropic region is in chromosome 5: 73.8–75.8 Mb, which is linked to the DNA polymerase kappa (POLK) gene encoding a DNA polymerase involved in DNA repair; hence, its relationship with migraine (and the identified lipid levels) remains unclear.
The present study has some potential limitations. First, given our GWAS summary statistics are from European ancestry, our findings are not necessarily generalized to other ancestries. Second, although the largest possible overlap of migraine GWAS individuals with metabolic traits GWASs individuals is <5%, it might lead to slightly overestimated findings, although we do not expect this to affect our conclusions. Third, although there are many other methods for detecting shared genetic influences between two traits, we applied the methods that are less sensitive to the metabolic traits GWASs small(er) sample sizes. However, these methods have their own limitations. For example, the Pearson correlations between independent SNPs effects are small and should not be interpreted as the magnitude of migraine-metabolite relationships—instead we point readers to the LDSC rg results presented in Figure S1. Fourth, given that we limited our analyses to metabolic traits with a significant polygenic and applied methods that require polygenic signals (especially the Pearson correlation between independent SNPs effects and the LCV model), our pipeline is more consistent with polygenicity than monogenicity. Therefore, the shared genetic effects between blood metabolome and migraine under the monogenic model remain to be studied. Fifth, the causal relationships of identified metabolic traits on migraine remain valid in a proportion of migraine-affected individuals who have the aberrant blood levels of the metabolites. Last, our findings will benefit from replication with future larger migraine and metabolite GWASs and validation in randomized clinical trials.
In the present study, we aimed to understand the relationships between human blood metabolome and migraine by using GWAS summary statistics and cross-trait genetic analyses. All analyses emphasize the importance of lipids in migraine biology. We also show that the identified genome-wide genetic overlaps between migraine and lipoproteins and migraine and organic acids are more consistent with shared biology than causality. Our pairwise gene-based analysis identified genes contributing most to the identified genome-wide genetic overlaps. Our scan for pleiotropic loci between migraine and blood metabolites identified eight distinct loci across chromosomes 9, 5, 3, 6, and 16. Overall, our genome-wide genetic overlap analysis showed higher levels of all studied fatty acids in migraine except DHA, a very long-chain omega-3. This suggests that consumption of DHA may benefit individuals with migraine. Consistently, we found a causally protective role for a longer length of fatty acids against migraine. Notably, we identified a causal effect of a higher level of a lysophosphatidylethanolamine, LPE(20:4), on migraine, thus introducing LPE(20:4) as a potential therapeutic target for migraine.
Consortia
The members of the IHGC are Verneri Anttila, Ville Artto, Andrea C. Belin, Anna Bjornsdottir, Gyda Bjornsdottir, Dorret I. Boomsma, Sigrid Børte, Mona A. Chalmer, Daniel I. Chasman, Bru Cormand, Ester Cuenca-Leon, George Davey-Smith, Irene de Boer, Martin Dichgans, Tonu Esko, Tobias Freilinger, Padhraig Gormley, Lyn R. Griffiths, Eija Hämäläinen, Thomas F. Hansen, Aster V.E. Harder, Heidi Hautakangas, Marjo Hiekkala, Maria G. Hrafnsdottir, M. Arfan Ikram, Marjo-Riitta Järvelin, Risto Kajanne, Mikko Kallela, Jaakko Kaprio, Mari Kaunisto, Lisette J.A. Kogelman, Espen S. Kristoffersen, Christian Kubisch, Mitja Kurki, Tobias Kurth, Lenore Launer, Terho Lehtimäki, Davor Lessel, Lannie Ligthart, Sigurdur H. Magnusson, Rainer Malik, Bertram Müller-Myhsok, Carrie Northover, Dale R. Nyholt, Jes Olesen, Aarno Palotie, Priit Palta, Linda M. Pedersen, Nancy Pedersen, Matti Pirinen, Danielle Posthuma, Patricia Pozo-Rosich, Alice Pressman, Olli Raitakari, Caroline Ran, Gudrun R. Sigurdardottir, Hreinn Stefansson, Kari Stefansson, Olafur A. Sveinsson, Gisela M. Terwindt, Thorgeir E. Thorgeirsson, Arn M.J.M. van den Maagdenberg, Cornelia van Duijn, Maija Wessman, Bendik S. Winsvold, and John-Anker Zwart.
Acknowledgments
We thank the participants and many researchers involved in metabolome GWASs. We thank the research participants and employees of 23andMe for making this work possible. H.M.T. is grateful for support from Queensland University of Technology through a QUT Postgraduate Research Scholarship.
Declaration of interests
The authors declare no competing interests.
Published: October 12, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ajhg.2021.09.011.
Contributor Information
Hamzeh M. Tanha, Email: hamzeh.mesriantanha@hdr.qut.edu.au.
Dale R. Nyholt, Email: d.nyholt@qut.edu.au.
Data and code availability
To express your interest in the IHGC migraine GWAS 2016 data (except 23andMe samples), please see the details provided at http://www.headachegenetics.org/content/datasets-and-cohorts. The migraine GWAS summary statistics for the 23andMe discovery dataset will be made available through 23andMe to qualified researchers under an agreement with 23andMe that protects the privacy of the 23andMe participants. Please visit https://research.23andme.com/collaborate/#dataset-access/ for more information and to apply to access the data. All other GWAS summary statistics except migraine are publicly available (Table S1). All codes used for performance of statistical analyses are available from the corresponding authors upon request.
Web resources
HMDB, https://hmdb.ca/
OMIM, https://www.omim.org/
PubChem, https://pubchem.ncbi.nlm.nih.gov/
The “qvalue” R package, https://github.com/StoreyLab/qvalue
Supplemental information
References
- 1.Onderwater G.L.J., Ligthart L., Bot M., Demirkan A., Fu J., van der Kallen C.J.H., Vijfhuizen L.S., Pool R., Liu J., Vanmolkot F.H.M., et al. BBMRI Metabolomics Consortium Large-scale plasma metabolome analysis reveals alterations in HDL metabolism in migraine. Neurology. 2019;92:e1899–e1911. doi: 10.1212/wnl.0000000000007313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nyholt D.R., Borsook D., Griffiths L.R. Migrainomics - identifying brain and genetic markers of migraine. Nat. Rev. Neurol. 2017;13:725–741. doi: 10.1038/nrneurol.2017.151. [DOI] [PubMed] [Google Scholar]
- 3.Tanha H.M., Martin N.G., Whitfield J.B., Nyholt D.R., International Headache Genetics Consortium (IHGC) Association and genetic overlap between clinical chemistry tests and migraine. Cephalalgia. 2021 doi: 10.1177/03331024211018131. 03331024211018131, 3331024211018131. [DOI] [PubMed] [Google Scholar]
- 4.Ren C., Liu J., Zhou J., Liang H., Wang Y., Sun Y., Ma B., Yin Y. Lipidomic analysis of serum samples from migraine patients. Lipids Health Dis. 2018;17:22. doi: 10.1186/s12944-018-0665-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hagenbeek F.A., Pool R., van Dongen J., Draisma H.H.M., Jan Hottenga J., Willemsen G., Abdellaoui A., Fedko I.O., den Braber A., Visser P.J., et al. BBMRI Metabolomics Consortium Heritability estimates for 361 blood metabolites across 40 genome-wide association studies. Nat. Commun. 2020;11:39. doi: 10.1038/s41467-019-13770-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shin S.Y., Fauman E.B., Petersen A.K., Krumsiek J., Santos R., Huang J., Arnold M., Erte I., Forgetta V., Yang T.P., et al. Multiple Tissue Human Expression Resource (MuTHER) Consortium An atlas of genetic influences on human blood metabolites. Nat. Genet. 2014;46:543–550. doi: 10.1038/ng.2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Draisma H.H.M., Pool R., Kobl M., Jansen R., Petersen A.K., Vaarhorst A.A.M., Yet I., Haller T., Demirkan A., Esko T., et al. Genome-wide association study identifies novel genetic variants contributing to variation in blood metabolite levels. Nat. Commun. 2015;6:7208. doi: 10.1038/ncomms8208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cornelis M.C., Kacprowski T., Menni C., Gustafsson S., Pivin E., Adamski J., Artati A., Eap C.B., Ehret G., Friedrich N., et al. Swiss Kidney Project on Genes in Hypertension (SKIPOGH) team Genome-wide association study of caffeine metabolites provides new insights to caffeine metabolism and dietary caffeine-consumption behavior. Hum. Mol. Genet. 2016;25:5472–5482. doi: 10.1093/hmg/ddw334. [DOI] [PubMed] [Google Scholar]
- 9.Kettunen J., Demirkan A., Würtz P., Draisma H.H., Haller T., Rawal R., Vaarhorst A., Kangas A.J., Lyytikäinen L.P., Pirinen M., et al. Genome-wide study for circulating metabolites identifies 62 loci and reveals novel systemic effects of LPA. Nat. Commun. 2016;7:11122. doi: 10.1038/ncomms11122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prins B.P., Kuchenbaecker K.B., Bao Y., Smart M., Zabaneh D., Fatemifar G., Luan J., Wareham N.J., Scott R.A., Perry J.R.B., et al. Genome-wide analysis of health-related biomarkers in the UK Household Longitudinal Study reveals novel associations. Sci. Rep. 2017;7:11008. doi: 10.1038/s41598-017-10812-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wittemans L.B.L., Lotta L.A., Oliver-Williams C., Stewart I.D., Surendran P., Karthikeyan S., Day F.R., Koulman A., Imamura F., Zeng L., et al. Assessing the causal association of glycine with risk of cardio-metabolic diseases. Nat. Commun. 2019;10:1060. doi: 10.1038/s41467-019-08936-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Locke A.E., Steinberg K.M., Chiang C.W.K., Service S.K., Havulinna A.S., Stell L., Pirinen M., Abel H.J., Chiang C.C., Fulton R.S., et al. FinnGen Project Exome sequencing of Finnish isolates enhances rare-variant association power. Nature. 2019;572:323–328. doi: 10.1038/s41586-019-1457-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gallois A., Mefford J., Ko A., Vaysse A., Julienne H., Ala-Korpela M., Laakso M., Zaitlen N., Pajukanta P., Aschard H. A comprehensive study of metabolite genetics reveals strong pleiotropy and heterogeneity across time and context. Nat. Commun. 2019;10:4788. doi: 10.1038/s41467-019-12703-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gormley P., Anttila V., Winsvold B.S., Palta P., Esko T., Pers T.H., Farh K.-H., Cuenca-Leon E., Muona M., Furlotte N.A., et al. International Headache Genetics Consortium Meta-analysis of 375,000 individuals identifies 38 susceptibility loci for migraine. Nat. Genet. 2016;48:856–866. doi: 10.1038/ng.3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bulik-Sullivan B., Finucane H.K., Anttila V., Gusev A., Day F.R., Loh P.R., Duncan L., Perry J.R., Patterson N., Robinson E.B., et al. ReproGen Consortium. Psychiatric Genomics Consortium. Genetic Consortium for Anorexia Nervosa of the Wellcome Trust Case Control Consortium 3 An atlas of genetic correlations across human diseases and traits. Nat. Genet. 2015;47:1236–1241. doi: 10.1038/ng.3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pickrell J.K., Berisa T., Liu J.Z., Ségurel L., Tung J.Y., Hinds D.A. Detection and interpretation of shared genetic influences on 42 human traits. Nat. Genet. 2016;48:709–717. doi: 10.1038/ng.3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bulik-Sullivan B.K., Loh P.R., Finucane H.K., Ripke S., Yang J., Patterson N., Daly M.J., Price A.L., Neale B.M., Schizophrenia Working Group of the Psychiatric Genomics Consortium LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat. Genet. 2015;47:291–295. doi: 10.1038/ng.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abecasis G.R., Auton A., Brooks L.D., DePristo M.A., Durbin R.M., Handsaker R.E., Kang H.M., Marth G.T., McVean G.A., 1000 Genomes Project Consortium An integrated map of genetic variation from 1,092 human genomes. Nature. 2012;491:56–65. doi: 10.1038/nature11632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Altshuler D.M., Gibbs R.A., Peltonen L., Altshuler D.M., Gibbs R.A., Peltonen L., Dermitzakis E., Schaffner S.F., Yu F., Peltonen L., et al. International HapMap 3 Consortium Integrating common and rare genetic variation in diverse human populations. Nature. 2010;467:52–58. doi: 10.1038/nature09298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim S., Thiessen P.A., Bolton E.E., Chen J., Fu G., Gindulyte A., Han L., He J., He S., Shoemaker B.A., et al. PubChem Substance and Compound databases. Nucleic Acids Res. 2016;44(D1):D1202–D1213. doi: 10.1093/nar/gkv951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wishart D.S., Feunang Y.D., Marcu A., Guo A.C., Liang K., Vázquez-Fresno R., Sajed T., Johnson D., Li C., Karu N., et al. HMDB 4.0: the human metabolome database for 2018. Nucleic Acids Res. 2018;46(D1):D608–D617. doi: 10.1093/nar/gkx1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Julienne H., Shi H., Pasaniuc B., Aschard H. RAISS: robust and accurate imputation from summary statistics. Bioinformatics. 2019;35:4837–4839. doi: 10.1093/bioinformatics/btz466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berisa T., Pickrell J.K. Approximately independent linkage disequilibrium blocks in human populations. Bioinformatics. 2016;32:283–285. doi: 10.1093/bioinformatics/btv546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pickrell J.K. Joint analysis of functional genomic data and genome-wide association studies of 18 human traits. Am. J. Hum. Genet. 2014;94:559–573. doi: 10.1016/j.ajhg.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nyholt D.R. SECA: SNP effect concordance analysis using genome-wide association summary results. Bioinformatics. 2014;30:2086–2088. doi: 10.1093/bioinformatics/btu171. [DOI] [PubMed] [Google Scholar]
- 26.Chang C.C., Chow C.C., Tellier L.C.A.M., Vattikuti S., Purcell S.M., Lee J.J. Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience. 2015;4:7. doi: 10.1186/s13742-015-0047-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Storey J.D. A direct approach to false discovery rates. J. R. Stat. Soc. Series B Stat. Methodol. 2002;64:479–498. doi: 10.1111/1467-9868.00346. [DOI] [Google Scholar]
- 28.Boca S.M., Leek J.T. A direct approach to estimating false discovery rates conditional on covariates. PeerJ. 2018;6:e6035. doi: 10.7717/peerj.6035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Leeuw C.A., Mooij J.M., Heskes T., Posthuma D. MAGMA: generalized gene-set analysis of GWAS data. PLoS Comput. Biol. 2015;11:e1004219. doi: 10.1371/journal.pcbi.1004219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Connor L.J., Price A.L. Distinguishing genetic correlation from causation across 52 diseases and complex traits. Nat. Genet. 2018;50:1728–1734. doi: 10.1038/s41588-018-0255-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu Z., Zheng Z., Zhang F., Wu Y., Trzaskowski M., Maier R., Robinson M.R., McGrath J.J., Visscher P.M., Wray N.R., Yang J. Causal associations between risk factors and common diseases inferred from GWAS summary data. Nat. Commun. 2018;9:224. doi: 10.1038/s41467-017-02317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rhee E.P., Ho J.E., Chen M.H., Shen D., Cheng S., Larson M.G., Ghorbani A., Shi X., Helenius I.T., O’Donnell C.J., et al. A genome-wide association study of the human metabolome in a community-based cohort. Cell Metab. 2013;18:130–143. doi: 10.1016/j.cmet.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Calder P.C. Functional Roles of Fatty Acids and Their Effects on Human Health. JPEN J. Parenter. Enteral Nutr. 2015;39(1, Suppl):18S–32S. doi: 10.1177/0148607115595980. [DOI] [PubMed] [Google Scholar]
- 34.Waeber C., Moskowitz M.A. Migraine as an inflammatory disorder. Neurology. 2005;64(10, Suppl 2):S9–S15. doi: 10.1212/wnl.64.10_suppl_2.s9. [DOI] [PubMed] [Google Scholar]
- 35.Innes J.K., Calder P.C. Omega-6 fatty acids and inflammation. Prostaglandins Leukot. Essent. Fatty Acids. 2018;132:41–48. doi: 10.1016/j.plefa.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 36.McNamara R.K., Almeida D.M. Omega-3 Polyunsaturated Fatty Acid Deficiency and Progressive Neuropathology in Psychiatric Disorders: A Review of Translational Evidence and Candidate Mechanisms. Harv. Rev. Psychiatry. 2019;27:94–107. doi: 10.1097/hrp.0000000000000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Swanson D., Block R., Mousa S.A. Omega-3 fatty acids EPA and DHA: health benefits throughout life. Adv. Nutr. 2012;3:1–7. doi: 10.3945/an.111.000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anttila V., Bulik-Sullivan B., Finucane H.K., Walters R.K., Bras J., Duncan L., Escott-Price V., Falcone G.J., Gormley P., Malik R., et al. Brainstorm Consortium Analysis of shared heritability in common disorders of the brain. Science. 2018;360:eaap8757. doi: 10.1126/science.aap8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harel Z., Gascon G., Riggs S., Vaz R., Brown W., Exil G. Supplementation with omega-3 polyunsaturated fatty acids in the management of recurrent migraines in adolescents. J. Adolesc. Health. 2002;31:154–161. doi: 10.1016/s1054-139x(02)00349-x. [DOI] [PubMed] [Google Scholar]
- 40.Xu Z.Z., Zhang L., Liu T., Park J.Y., Berta T., Yang R., Serhan C.N., Ji R.R. Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nat. Med. 2010;16:592–597. doi: 10.1038/nm.2123. 1p following 597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.von Schacky C., Harris W.S. Why docosapentaenoic acid is not included in the Omega-3 Index. Prostaglandins Leukot. Essent. Fatty Acids. 2018;135:18–21. doi: 10.1016/j.plefa.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 42.Tan S.T., Ramesh T., Toh X.R., Nguyen L.N. Emerging roles of lysophospholipids in health and disease. Prog. Lipid Res. 2020;80:101068. doi: 10.1016/j.plipres.2020.101068. [DOI] [PubMed] [Google Scholar]
- 43.Bisogno T., Howell F., Williams G., Minassi A., Cascio M.G., Ligresti A., Matias I., Schiano-Moriello A., Paul P., Williams E.J., et al. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J. Cell Biol. 2003;163:463–468. doi: 10.1083/jcb.200305129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sarchielli P., Pini L.A., Coppola F., Rossi C., Baldi A., Mancini M.L., Calabresi P. Endocannabinoids in chronic migraine: CSF findings suggest a system failure. Neuropsychopharmacology. 2007;32:1384–1390. doi: 10.1038/sj.npp.1301246. [DOI] [PubMed] [Google Scholar]
- 45.Rossi C., Pini L.A., Cupini M.L., Calabresi P., Sarchielli P. Endocannabinoids in platelets of chronic migraine patients and medication-overuse headache patients: relation with serotonin levels. Eur. J. Clin. Pharmacol. 2008;64:1–8. doi: 10.1007/s00228-007-0391-4. [DOI] [PubMed] [Google Scholar]
- 46.Greco R., Demartini C., Zanaboni A.M., Piomelli D., Tassorelli C. Endocannabinoid System and Migraine Pain: An Update. Front. Neurosci. 2018;12:172. doi: 10.3389/fnins.2018.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ryu S.B., Palta J.P. Specific inhibition of rat brain phospholipase D by lysophospholipids. J. Lipid Res. 2000;41:940–944. [PubMed] [Google Scholar]
- 48.Goulart A.C., Lotufo P.A., Santos I.S., Bittencourt M.S., Santos R.D., Blaha M.J., Jones S., Toth P.P., Kulkarni K., Benseñor I.M. The relationship between migraine and lipid sub-fractions among individuals without cardiovascular disease: A cross-sectional evaluation in the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil) Cephalalgia. 2018;38:528–542. doi: 10.1177/0333102417699181. [DOI] [PubMed] [Google Scholar]
- 49.Kiriyama Y., Nochi H. The Biosynthesis, Signaling, and Neurological Functions of Bile Acids. Biomolecules. 2019;9:E232. doi: 10.3390/biom9060232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Campos F., Sobrino T., Pérez-Mato M., Rodríguez-Osorio X., Leira R., Blanco M., Mirelman D., Castillo J. Glutamate oxaloacetate transaminase: a new key in the dysregulation of glutamate in migraine patients. Cephalalgia. 2013;33:1148–1154. doi: 10.1177/0333102413487444. [DOI] [PubMed] [Google Scholar]
- 51.Hoffmann J., Charles A. Glutamate and Its Receptors as Therapeutic Targets for Migraine. Neurotherapeutics. 2018;15:361–370. doi: 10.1007/s13311-018-0616-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sperringer J.E., Addington A., Hutson S.M. Branched-Chain Amino Acids and Brain Metabolism. Neurochem. Res. 2017;42:1697–1709. doi: 10.1007/s11064-017-2261-5. [DOI] [PubMed] [Google Scholar]
- 53.Cheng F., Zhou Y., Wang M., Guo C., Cao Z., Zhang R., Peng C. A review of pharmacological and pharmacokinetic properties of stachydrine. Pharmacol. Res. 2020;155:104755. doi: 10.1016/j.phrs.2020.104755. [DOI] [PubMed] [Google Scholar]
- 54.Silva L.K., Espenship M.F., Pine B.N., Ashley D.L., De Jesús V.R., Blount B.C. Methyl Tertiary-Butyl Ether Exposure from Gasoline in the U.S. Population, NHANES 2001-2012. Environ. Health Perspect. 2019;127:127003. doi: 10.1289/ehp5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abramov A.Y., Angelova P.R. Cellular mechanisms of complex I-associated pathology. Biochem. Soc. Trans. 2019;47:1963–1969. doi: 10.1042/bst20191042. [DOI] [PubMed] [Google Scholar]
- 56.Fila M., Pawłowska E., Blasiak J. Mitochondria in migraine pathophysiology - does epigenetics play a role? Arch. Med. Sci. 2019;15:944–956. doi: 10.5114/aoms.2019.86061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nelson B.R., Wu F., Liu Y., Anderson D.M., McAnally J., Lin W., Cannon S.C., Bassel-Duby R., Olson E.N. Skeletal muscle-specific T-tubule protein STAC3 mediates voltage-induced Ca2+ release and contractility. Proc. Natl. Acad. Sci. USA. 2013;110:11881–11886. doi: 10.1073/pnas.1310571110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Domitrz I., Kostera-Pruszczyk A., Kwieciñski H. A single-fibre EMG study of neuromuscular transmission in migraine patients. Cephalalgia. 2005;25:817–821. doi: 10.1111/j.1468-2982.2005.00961.x. [DOI] [PubMed] [Google Scholar]
- 59.García-Cazorla À., Verdura E., Juliá-Palacios N., Anderson E.N., Goicoechea L., Planas-Serra L., Tsogtbaatar E., Dsouza N.R., Schlüter A., Urreizti R., et al. SHMT2 Working Group Impairment of the mitochondrial one-carbon metabolism enzyme SHMT2 causes a novel brain and heart developmental syndrome. Acta Neuropathol. 2020;140:971–975. doi: 10.1007/s00401-020-02223-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Punchaichira T.J., Prasad S., Deshpande S.N., Thelma B.K. Deep sequencing identifies novel regulatory variants in the distal promoter region of the dopamine-β-hydroxylase gene. Pharmacogenet. Genomics. 2016;26:311–323. doi: 10.1097/fpc.0000000000000214. [DOI] [PubMed] [Google Scholar]
- 61.Lea R.A., Dohy A., Jordan K., Quinlan S., Brimage P.J., Griffiths L.R. Evidence for allelic association of the dopamine beta-hydroxylase gene (DBH) with susceptibility to typical migraine. Neurogenetics. 2000;3:35–40. doi: 10.1007/pl00022977. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
To express your interest in the IHGC migraine GWAS 2016 data (except 23andMe samples), please see the details provided at http://www.headachegenetics.org/content/datasets-and-cohorts. The migraine GWAS summary statistics for the 23andMe discovery dataset will be made available through 23andMe to qualified researchers under an agreement with 23andMe that protects the privacy of the 23andMe participants. Please visit https://research.23andme.com/collaborate/#dataset-access/ for more information and to apply to access the data. All other GWAS summary statistics except migraine are publicly available (Table S1). All codes used for performance of statistical analyses are available from the corresponding authors upon request.