Abstract
Background
Aspirin is a key antiplatelet therapy for the prevention of thrombotic events in patients with cardiovascular disease. Studies suggest that ≈20% of patients with cardiac disease suffer from aspirin nonsensitivity, a phenomenon characterized by the inability of 81 mg aspirin to inhibit platelet aggregation and/or prevent adverse cardiovascular events.
Objectives
To investigate aspirin nonsensitivity in patients with vascular disease and assess the consequences of aspirin nonsensitivity.
Methods
One hundred fifty patients presenting to St. Michael’s Hospital’s outpatient clinics with evidence of vascular disease (peripheral arterial disease or carotid artery stenosis) and a previous prescription of 81 mg of aspirin were recruited in this study. Light transmission aggregometry with arachidonic acid induction was used to determine sensitivity to aspirin. Patients with a maximum aggregation ≥20% in response to arachidonic acid were considered aspirin nonsensitive, as per previous studies.
Results
Of the 150 patients recruited, 36 patients (24%) were nonsensitive to 81 mg of aspirin. Of these 36 nonsensitive patients, 30 patients provided a urine sample for urine salicyluric acid analysis (a major metabolite of aspirin). Urine analysis demonstrated that 14 patients were compliant and 16 were noncompliant with their aspirin therapy. Major adverse cardiovascular events and major adverse limb events were significantly higher in the nonsensitive patients compared to sensitive patients (hazard ratio, 3.68; P < 0.001).
Conclusion
These data highlight the high prevalence of aspirin nonsensitivity and noncompliance in patients with vascular disease and emphasizes the urgent need for improved medical management options for this patient population.
Keywords: antiplatelet, aspirin, blood platelets, platelet aggregation, thrombosis, vascular diseases
Essentials.
Aspirin nonsensitivity is documented in 20% to 30% of patients with cardiac disease.
Aspirin sensitivity was tested in patients with vascular disease using light transmission aggregometry.
Major adverse cardiovascular events were higher in nonsensitive patients.
Major adverse limb events were higher in nonsensitive patients.
1. INTRODUCTION
Acetylsalicylic acid, better known as aspirin, is a common antiplatelet agent used for the prevention of adverse cardiovascular events. The laboratory definition of aspirin nonsensitivity is the inability of low‐dose aspirin (81 mg) to inhibit platelet aggregation and is clinically defined as the inability of 81 mg of aspirin to prevent adverse cardiovascular events. 1 Specifically, patients who are aspirin nonsensitive have a 4‐fold higher risk of suffering from an adverse cardiovascular event, such as a myocardial infarction (MI), stroke, chronic limb‐threatening ischemia (CLTI), and cardiovascular‐related death. 1 Aspirin nonsensitivity is well documented in patients with cardiac disease with 20% to 30% of patients identified as aspirin nonsensitive 1 ; however, it remains a relatively uninvestigated issue within patients with vascular disease such as peripheral arterial disease (PAD) and carotid artery stenosis (CAS). Cardiovascular risk factors tend to be less intensively managed in the PAD population compared to their cardiac disease counterparts, despite there being a strong association between PAD and cardiovascular morbidity. 2 This, coupled with the lack of studies on aspirin nonsensitivity, puts this patient population at a higher risk of adverse cardiovascular events. 3 , 4 , 5 In this study, we assess the prevalence and consequences of aspirin nonsensitivity among patients with vascular disease.
2. METHODS
2.1. Ethics approval
This study was performed in accordance with the Declaration of Helsinki and approved by the Unity Health Toronto Research Ethics Board at St Michael’s Hospital in Toronto, Ontario, Canada (REB #16‐375). Informed consent was obtained from all participants. Approximately 10% of patients approached declined participation in this study.
2.2. Aspirin sensitivity testing by light transmission aggregometry
A physical examination was conducted on each patient by their treating physician before enrollment. Consecutive patients with PAD or asymptomatic CAS who were taking 81 mg of aspirin daily for at least 2 weeks presenting to the vascular clinic at St. Michael’s Hospital (Toronto, Ontario, Canada) between September 2018 and September 2019 were recruited. As previously described, PAD was defined an ankle brachial index < 0.9 or toe‐brachial index < 0.67, symptoms of claudication, and abnormal distal pulses. Asymptomatic CAS was defined as >50% stenosis of the internal carotid artery on duplex ultrasound in the absence of neurological symptoms. 3 Patients with arterial or venous disease who were not taking 81 mg of aspirin or any other antiplatelet were also recruited as a control. The following patients were excluded: patients with anemia, leukopenia, thrombocytopenia, gastrointestinal bleeding, or bleeding disorders/coagulopathy. Patients were also excluded if they were taking dual antiplatelet therapy, were taking any antiplatelet or anticoagulant other than aspirin, were currently pregnant, or were unable to provide written informed consent. Blood was drawn into Vacutainer tubes containing 3.2% sodium citrate. Light transmission aggregometry (LTA) analysis was performed on platelet‐rich plasma within 15 minutes of blood withdrawal using a Chrono‐log aggregometer (Chrono‐Log Corporation, Havertown, PA, USA) as we previously described. 3 , 7 Arachidonic acid (Bio/Data Corporation, Horsham, PA, USA) at a final concentration of 0.5 mg/mL was used to activate platelets. Participants with maximum aggregation in response to arachidonic acid ≥20% were considered aspirin nonsensitive, as this is the currently accepted laboratory definition of aspirin nonsensitivity when using the gold standard platelet function test LTA. 3 , 8 , 9 , 10 , 11
2.3. Aspirin compliance testing
On the same day of LTA analysis, urine samples were collected from each patient. Each sample was aliquoted and stored at −80°C before analysis. 3 Next, multisegment injection capillary electrophoresis mass spectrometry (MSI‐CE‐MS) was conducted as previously described. 3 Patients were considered aspirin compliant if urinary salicyluric acid levels were >5.25 μg/mL, as per previous studies on aspirin compliance. 12 , 13
2.4. Chart review and measured outcomes
Data were collected through retrospective chart review by the primary author and recorded on a standardized data collection form. Specifically, patients’ charts were reviewed over a 2‐year period (1 year before LTA analysis to 1 year after analysis). Information on the following variables were collected: (1) major adverse cardiovascular events (MACEs), defined as MI, stroke, or cardiovascular death; and (2) major adverse limb events (MALEs), defined as PAD‐specific outcomes such as development of acute or chronic limb‐threatening ischemia or all‐limb amputations.
2.5. Statistical analysis
Normally distributed continuous variables were reported as mean and standard deviation, whereas categorical variables were reported as frequencies and percentages. Median and interquartile range were calculated for nonnormally distributed data. Comparative analyses were carried out using Welch’s t test for continuous data or Fisher’s exact tests for categorical data. Event rates were calculated for each study group regarding MI, stroke, CLTI, MACEs, MALEs, limb amputation, and cardiovascular‐related death.
The event‐free curve was computed according to the Kaplan‐Meier method, and comparison of event‐free survival between subgroups was performed using the log‐rank test. A Cox proportional hazard analysis was performed to determine the independent predictor of any event for the entire population. Significant variables selected in univariate analysis were entered into the multivariate analysis. Hazard ratios with 95% confidence intervals were presented. Statistical significance was established at P <.05 (2‐sided). Statistical analysis was performed with Prism 8.4.2 (GraphPad Software, San Diego, CA, USA).
3. RESULTS
3.1. Aspirin sensitivity testing by LTA
For this study, 150 patients with PAD and CAS were recruited for aspirin sensitivity analysis. The patient population had a mean age of 69 years, primarily men (64%), and had a high prevalence of cardiovascular risk factors such as hypertension, hypercholesterolemia, and smoking (Table 1).
TABLE 1.
Demographics and clinical characteristics of patients taking 81 mg of aspirin daily and controls
| Characteristics | Control not taking aspirin (n = 10) | Patients taking 81 mg of aspirin daily (n = 150) |
|---|---|---|
| Mean (SD) | ||
| Age, y | 50.4 (25) | 69 (12) |
| Platelet count, 103/μL | 178 (23) | 222 (68) |
| Leukocyte count, 103/μL | 5.7 (2) | 7.4 (2) |
| Hematocrit | 37.1 (2) | 39.2 (4) |
| Frequency (%) | ||
| Sex, male | 4 (40) | 96 (64) |
| Hypertension | 2 (20) | 105 (70) |
| Hypercholesterolemia | 1 (10) | 119 (79) |
| Diabetes | 0 (0) | 58 (39) |
| Smoking history | 5 (50) | 114 (76) |
| CAS | 0 (0) | 35 (23) |
| PAD | 0 (0) | 119 (79) |
| CAD | 0 (0) | 45 (30) |
| Stroke | 0 (0) | 18 (12) |
| Statin | 1 (10) | 18 (12) |
| ACEi/ARB | 1 (10) | 79 (53) |
| Beta blocker | 1 (10) | 42 (28) |
| Calcium channel blocker | 1 (10) | 32 (21) |
Continuous variables are showing by mean (standard deviation), and categorical variables are shown in number (percent).
Abbreviations: ACEi, angiotensin‐converting enzyme (ACE) inhibitor; ARB, angiotensin receptor blocker; CAD, coronary artery disease; CAS, carotid artery stenosis; PAD, peripheral arterial disease.
Of the 150 patients analyzed, 36 patients (24%) had platelets with ≥20% residual platelet activity in response to arachidonic acid despite being advised to take 81 mg of aspirin. These patients were considered aspirin nonsensitive. There was no statistical difference between any of the clinical characteristics or demographics studied when comparing the aspirin‐sensitive to the aspirin‐nonsensitive patients (Table 2). The mean percent maximal aggregation in aspirin‐nonsensitive patients was 42% ± 24% compared to 10% ± 5% in those who were aspirin sensitive (Figure 1).
TABLE 2.
Demographics and clinical characteristics of aspirin‐sensitive versus aspirin‐nonsensitive patients
| Characteristics | Aspirin sensitive (n = 114) | Aspirin nonsensitive (n = 36) |
|---|---|---|
| Mean (SD) | ||
| Age, y, mean (SD) | 68 (13) | 69 (9) |
| Platelet count, 103/μL | 218 (64) | 234 (81) |
| Leukocyte count, 103/μL | 7.4 (2) | 7.9 (2) |
| Hematocrit | 39.4 (5) | 38.7 (5) |
| Frequency (%) | ||
| Sex, male | 70 (61) | 27 (75) |
| Hypertension | 82 (72) | 35 (97) |
| Hypercholesterolemia | 89 (78) | 32 (89) |
| Diabetes | 41 (36) | 17 (47) |
| Smoking history | 86 (75) | 29 (81) |
| Statin | 83 (72) | 28 (78) |
| ACEi/ARB | 62 (54) | 18 (50) |
| Beta blocker | 31 (27) | 11 (31) |
| Calcium channel blocker | 25 (23) | 7 (19) |
Continuous variables are showing by mean (standard deviation), and categorical variables are shown in number (percent). No significant difference between aspirin‐sensitive and aspirin‐nonsensitive patients in any of the characteristics with P >.05. Differences between groups were compared using chi‐square test for categorical variables, and Mann‐Whitney test for continuous variables.
Abbreviations: ACEi, angiotensin‐converting enzyme (ACE) inhibitor; ARB, angiotensin receptor blocker.
FIGURE 1.
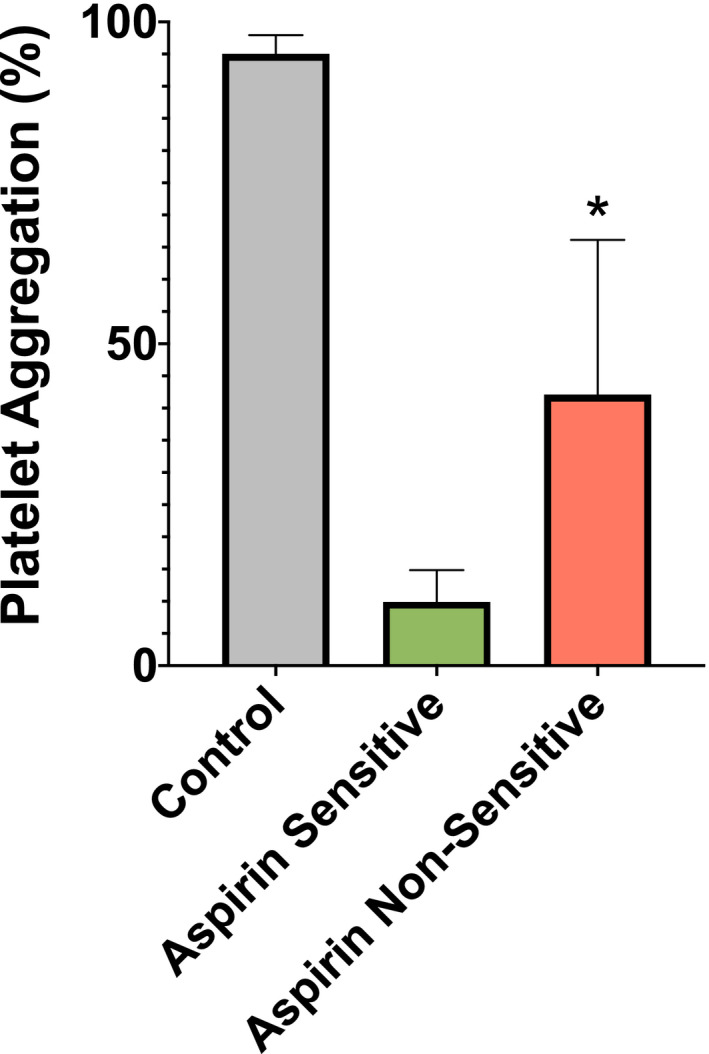
Mean percent platelet aggregation in aspirin‐sensitive (green) and aspirin‐nonsensitive (red) patients with vascular disease and control patients not taking aspirin (gray). Aspirin sensitivity was tested in patients with vascular disease taking 81 mg of aspirin daily using light transmission aggregometry. Bar chart depicts the mean percent maximal aggregation in response to 0.5 mg/ml lyophilized arachidonic acid arachidonic acid–induced platelet aggregation. Participants were grouped into aspirin‐sensitive (n = 114, mean = 10 ± 5), aspirin‐nonsensitive (n = 36, mean = 42 ± 24), and control patients not taking aspirin (n = 10, mean = 95 ± 3). Patients were grouped as aspirin nonsensitive if maximal aggregation in response to arachidonic acid was ≥20%. Error bars represent standard deviation of the mean. Significant difference in platelet aggregation between aspirin‐sensitive and aspirin‐nonsensitive patients is represented by (*) with P value = .001
3.2. Aspirin compliance testing
To determine why nonsensitive patients had platelet activity ≥20%, urinary salicyluric acid analysis was conducted by MSI‐CE‐MS. Of the 36 nonsensitive patients, 6 were unable to provide a urine sample and were excluded. Of the remaining 30 patients, it was determined that 14 patients had salicyluric acid values greater than the threshold for compliance. These 14 patients (9% of the 150 patients recruited) were considered compliant with their aspirin therapy. The remaining 16 patients (11% of the 150 patients recruited) had salicyluric acid level below the threshold for compliance and were considered noncompliant with their therapy.
3.3. Chart review and measured outcomes
To determine the consequences of aspirin nonsensitivity, a survival analysis was conducted on the 150 patients recruited to the study. Patient charts were retrospectively reviewed to determine rates of MACEs and/or MALEs. Median follow‐up time was 24 months, with 46 patients lost to follow‐up between 12 and 23 months. Of the 114 patients in the aspirin‐sensitive group, 2 (2%) had an MI, 2 (2%) had a stroke, 12 (11%) progressed to CLTI, 2 (2%) had a limb amputation, and 0 (0%) died of cardiovascular‐related events (Table 3). Of the 36 patients in the aspirin nonsensitive group, 4 (11%) had an MI, 3 (8%) had a stroke, 12 (33%) progressed to CLTI, 2 (6%) had a limb amputation, and 0 (0%) died of cardiovascular‐related events (Table 3).
TABLE 3.
Event rate comparison between aspirin sensitive and aspirin nonsensitive patients
| Event | Aspirin sensitive (n = 114) | Aspirin nonsensitive (n = 36) | P value |
|---|---|---|---|
| MI | 2 (2) | 4 (11) | .03* |
| Stroke | 2 (2) | 3 (8) | .09 |
| CLTI | 12 (11) | 12 (33) | .003* |
| Limb amputation | 2 (2) | 2 (6) | .24 |
| Cardiovascular related death | 0 (0) | 0 (0) | NA |
| MACE | 4 (4) | 7 (19) | .004* |
| MALE | 14 (12) | 14 (39) | .001* |
| MACE and/or MALE | 18 (16) | 21 (58) | .001* |
Variables are shown in number (percent).
Abbreviations: CLTI, chronic limb‐threatening ischemia; MACE, major adverse cardiovascular event; MALE, major adverse limb event; MI, myocardial infarction.
*Represents significant difference between aspirin‐sensitive and aspirin‐nonsensitive patients; P <.05; differences between groups were compared using Fisher’s exact test.
There was a significant difference in the event‐free rate (MACEs and/or MALEs) by Kaplan‐Meier analysis between aspirin‐nonsensitive and aspirin‐sensitive patients at 2 years (log rank, P = .001) (Figure 2). Overall risk of MACEs and/or MALEs was significantly higher for aspirin‐nonsensitive patients when compared to aspirin‐sensitive patients (hazard ratio, 3.68; 95% confidence interval [CI], 1.66‐8.17; P < .001). Among study variables, age, sex, smoking, and CAD were associated with subsequent MACE and/or MALE events using univariate analysis. Therefore, these 4 independent variables selected by the univariate analysis were entered into the multivariate analysis. Overall risk of MACEs and/or MALEs remained significantly higher for aspirin‐nonsensitive patients when compared to aspirin‐sensitive patients, after adjusting for these variables (hazard ratio, 2.92; 95% CI, 1.35‐6.32) (Table 4).
FIGURE 2.
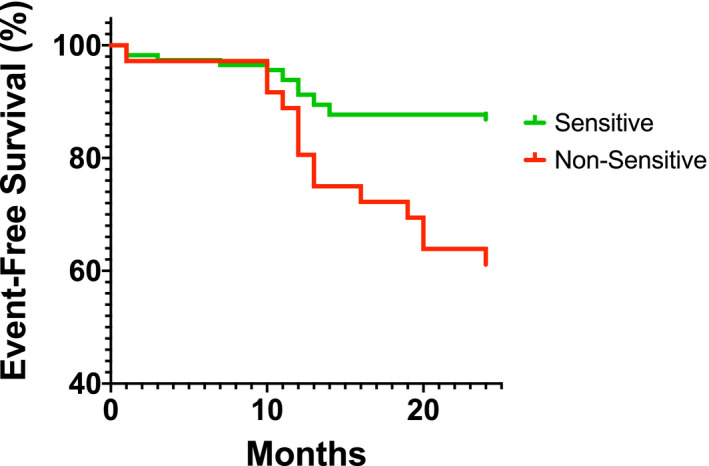
Overall event‐free survival analysis of patients of 2‐year period (1 year before aspirin sensitivity analysis to 1 year after analysis). When comparing event‐free survival between aspirin‐sensitive and aspirin‐nonsensitive patients, the hazard of death was 3.68 (3.68; 95% confidence interval, 1.66‐8.17; P = .001)
TABLE 4.
Cox regression hazard model evaluating the association between aspirin nonsensitivity and MACE and/or MALE
| Hazard ratio | 95% CI | |
|---|---|---|
| Unadjusted model (model 1) | 3.68 | 1.66‐8.17 |
| Model 1 + age + sex | 2.73 | 1.28‐5.80 |
| Model 1 + age + sex + smoking | 2.73 | 1.28‐5.82 |
| Model 1 + age + sex + smoking + CAD | 2.92 | 1.35‐6.32 |
Abbreviations: CAD, coronary artery disease; MACE, major adverse cardiovascular event; MALE, major adverse limb event.
4. DISCUSSION
Of 150 patients with vascular disease taking 81 mg of aspirin daily, 36 (24%) were aspirin nonsensitive according to the laboratory definition; however, ≈50% of these patients were noncompliant with their therapy based on urinary salicyluric acid levels.
Patients who are nonsensitive to aspirin are at a significantly higher risk of adverse cardiovascular events such as MI, stroke, CLTI, limb loss, and/or cardiovascular‐related death, with a hazard ratio of 3.68. This is a large proportion of patients, almost 1 in 4, who are at significantly higher risk. Patients with vascular disease, specifically patients with PAD, tend to be less intensively managed than patients with cardiac disease such as coronary artery disease. 2 Patients with PAD often are not treated with the best medical management, or there are delays in their treatment, putting them at a higher risk of adverse cardiovascular events. 3 , 6 This, in combination with a subset of patients being aspirin nonsensitive further exacerbates the risk of adverse events in these patients.
Recent studies indicate that modified treatment may be beneficial for patients who are non‐sensitive to aspirin. For example, Eikelboom et al 14 demonstrated the efficacy of combining aspirin with low‐dose rivaroxaban, a factor Xa inhibitor. They demonstrated a significant reduction in adverse cardiovascular events in patients who were on both low‐dose aspirin and rivaroxaban when compared to patients on aspirin alone (hazard ratio, 0.76). 14 Further research is required to determine if this combination therapy helps reduce adverse cardiovascular events in aspirin‐nonsensitive patients.
Currently, there is a lack of aspirin sensitivity tests that are accurate and easily accessible to physicians. The gold standard for aspirin sensitivity testing is LTA, and the generally accepted threshold is ≥20% platelet aggregation in response to arachidonic acid. 3 , 8 , 9 , 10 , 11 There are hurdles for LTA to become standard for testing, however, as it requires expensive machinery, trained personnel, and up to 2 hours per test. New point‐of‐care methods for detecting aspirin sensitivity in a cheap and timely manner is necessary to allow for routine aspirin sensitivity testing.
Some limitations to our study include a lack of standard measure for aspirin compliance testing. Also, some instances of MACE/MALE or cardiovascular‐related death may have been unaccounted in patients who were lost to follow‐up. Finally, a larger sample size may be beneficial to better evaluate the long‐term risks of aspirin nonsensitivity.
Our data suggests that ≈50% of our patients with a platelet aggregation ≥20% were noncompliant with their aspirin therapy. Physicians must take an active approach in speaking with their patients to better understand the underlying cause of noncompliance and educate patients on the importance of complying with their aspirin therapy. There is a high prevalence of aspirin nonsensitivity among patients with vascular disease taking 81 mg of aspirin, and this subgroup of patients are at a higher risk of MACEs and/or MALEs. New point‐of‐care tests that can detect aspirin nonsensitivity are needed, as this will help with making testing for aspirin nonsensitivity quicker and more widely available.
RELATIONSHIP DISCLOSURE
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
AUTHOR CONTRIBUTIONS
Conceptualization: MQ; methodology: HK, AZ, RCG, MLR, HN, MQ; formal analysis: HK, AZ, RCG, MQ; data interpretation: HK, AZ, RCG, MLR, HN, MA‐O, MQ; writing: HK, AZ, RCG, MLR, TLF, HN, MA‐O, MQ. All authors have read and approved the final manuscript and agree to the published version of the article.
ACKNOWLEDGMENTS
These data have not been previously published.
Khan H, Zamzam A, Gallant RC, et al. Aspirin nonsensitivity in patients with vascular disease: Assessment by light transmission aggregometry (aspirin nonsensitivity in vascular patients). Res Pract Thromb Haemost. 2021;5:e12618. 10.1002/rth2.12618
Handling Editor: Dr Neil Zakai
Funding information
This research was fully funded by the Blair Foundation
Contributor Information
Hamzah Khan, @hamzahkhan0606.
Mohammad Qadura, Email: mohammad.qadura@utoronto.ca.
REFERENCES
- 1. Hankey GJ, Eikelboom JW. Aspirin resistance. Lancet. 2006;367:606‐617. 10.1016/S0140-6736(06)68040-9 [DOI] [PubMed] [Google Scholar]
- 2. McDermott MM, Mehta S, Ahn H, Greenland P. Atherosclerotic risk factors are less intensively treated in patients with peripheral arterial disease than in patients with coronary artery disease. J Gen Intern Med. 1997;12:209‐215. 10.1046/j.1525-1497.1997.012004209.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Khan H, Gallant RC, Zamzam A, et al. Personalization of aspirin therapy ex vivo in patients with atherosclerosis using light transmission aggregometry. Diagnostics. 2020;10:871. 10.3390/diagnostics10110871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McDermott MM, Hahn EA, Greenland P, et al. Atherosclerotic risk factor reduction in peripheral arterial disease: results of a national physician survey. J Gen Intern Med. 2002;17:895‐904. 10.1046/j.1525-1497.2002.20307.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Clark AL, Byrne JC, Nasser A, McGroarty E, Kennedy JA. Cholesterol in peripheral vascular disease–a suitable case for treatment? QJM. 1999;92:219‐222. 10.1093/qjmed/92.4.219 [DOI] [PubMed] [Google Scholar]
- 6. Mukherjee D, Lingam P, Chetcuti S, et al. Missed opportunities to treat atherosclerosis in patients undergoing peripheral vascular interventions: insights from the University of Michigan Peripheral Vascular Disease Quality Improvement Initiative (PVD‐QI2). Circulation. 2002;106:1909‐1912. 10.1161/01.cir.0000035649.39669.ce [DOI] [PubMed] [Google Scholar]
- 7. Xu XR, Wang Y, Adili R, et al. Apolipoprotein A‐IV binds αIIbβ3 integrin and inhibits thrombosis. Nat Commun. 2018;9:3608. 10.1038/s41467-018-05806-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lordkipanidzé M, Pharand C, Schampaert E, Turgeon J, Palisaitis DA, Diodati JG. A comparison of six major platelet function tests to determine the prevalence of aspirin resistance in patients with stable coronary artery disease. Eur Heart J. 2007;28:1702‐1708. 10.1093/eurheartj/ehm226 [DOI] [PubMed] [Google Scholar]
- 9. Tantry US, Bliden KP, Gurbel PA. Overestimation of platelet aspirin resistance detection by thrombelastograph platelet mapping and validation by conventional aggregometry using arachidonic acid stimulation. J Am Coll Cardiol. 2005;46:1705‐1709. 10.1016/j.jacc.2005.05.090 [DOI] [PubMed] [Google Scholar]
- 10. Gum PA, Kottke‐Marchant K, Poggio ED, et al. Profile and prevalence of aspirin resistance in patients with cardiovascular disease. Am J Cardiol. 2001;88:230‐235. 10.1016/s0002-9149(01)01631-9 [DOI] [PubMed] [Google Scholar]
- 11. Pedersen SB, Grove EL, Nielsen HL, Mortensen J, Kristensen SD, Hvas A‐M. Evaluation of aspirin response by Multiplate® whole blood aggregometry and light transmission aggregometry. Platelets. 2009;20:415‐420. 10.1080/09537100903100643 [DOI] [PubMed] [Google Scholar]
- 12. Baxter GJ, Lawrence JR, Graham AB, Wiles D, Paterson JR. Identification and determination of salicylic acid and salicyluric acid in urine of people not taking salicylate drugs. Ann Clin Biochem. 2002;39:50‐55. 10.1258/0004563021901739 [DOI] [PubMed] [Google Scholar]
- 13. Lawrence JR, Peter R, Baxter GJ, Robson J, Graham AB, Paterson JR. Urinary excretion of salicyluric and salicylic acids by non‐vegetarians, vegetarians, and patients taking low dose aspirin. J Clin Pathol. 2003;56:651‐653. 10.1136/jcp.56.9.651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Eikelboom JW, Connolly SJ, Bosch J, et al. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med. 2017;377:1319‐1330. 10.1056/NEJMoa1709118 [DOI] [PubMed] [Google Scholar]


