Keywords: cell cycle, proliferation, cancer biology, cyclin-dependent kinases
Abstract
The use of CDK4/6 inhibitors in the treatment of a wide range of cancers is an area of ongoing investigation. Despite their increasing clinical use, there is limited understanding of the determinants of sensitivity and resistance to these drugs. Recent data have cast doubt on how CDK4/6 inhibitors arrest proliferation, provoking renewed interest in the role(s) of CDK4/6 in driving cell proliferation. As the use of CDK4/6 inhibitors in cancer therapies becomes more prominent, an understanding of their effect on the cell cycle becomes more urgent. Here, we investigate the mechanism of action of CDK4/6 inhibitors in promoting cell cycle arrest. Two main models explain how CDK4/6 inhibitors cause G1 cell cycle arrest, which differ in their dependence on the CDK inhibitor proteins p21 and p27. We have used live and fixed single-cell quantitative imaging, with inducible degradation systems, to address the roles of p21 and p27 in the mechanism of action of CDK4/6 inhibitors. We find that CDK4/6 inhibitors can initiate and maintain a cell cycle arrest without p21 or p27. This work clarifies our current understanding of the mechanism of action of CDK4/6 inhibitors and has implications for cancer treatment and patient stratification.
1. Introduction
Cyclin-dependent kinase 4/6 (CDK4/6) inhibitors have garnered interest as cancer treatments due to their efficiency in inhibiting cell proliferation. Three small-molecule CDK4/6 inhibitors (palbociclib, abemabiclib and ribociclib) are clinically approved for the treatment of metastatic ER+/HER2− breast cancer, and their use in the treatment of other cancers is an area of active investigation [1–9]. However, not all patients respond to these drugs and it is unclear why. Understanding more about the mechanism of action of CDK4/6 inhibitors, and how they inhibit cell proliferation, will help to stratify patients for treatment based on biomarkers [1,10].
While the premise for the clinical use of CDK4/6 inhibitors is based on a ‘canonical’ model of CDK4/6 activity, recent work has highlighted gaps in our understanding of the role of CDK4/6 in cell cycle entry [11–13]. In this canonical model, cyclin D:CDK4/6 has a catalytic role, phosphorylating the transcriptional inhibitor retinoblastoma protein (Rb) during G1, and partially relieving its inhibition of E2F-mediated transcription. This initiates the expression of genes required for cell cycle entry, including cyclin E. Later in G1, increasing cyclin E:CDK2 activity results in the hyperphosphorylation and complete inhibition of Rb, allowing full activation of E2F-dependent transcription and entry into S-phase. More recent data have called this model into question, yet still support a primarily catalytic role for CDK4/6 in cell cycle entry [14]. Indeed, the catalytic activity of CDK4/6 towards Rb has been shown to be a major driver of proliferation [15–19]. However, CDK4/6 may also promote cell cycle entry through a non-catalytic role, sequestering the Cip/Kip Cdk inhibitors, p21 and p27, away from CDK2, thus promoting CDK2 activity [20,21]. While p21 and p27 inhibit CDK2 activity, they assist in functional cyclin D:CDK4/6 complex assembly [22–25]. In addition, p27 facilitates the phosphorylation of the T-loop in CDK4 by CDK activating kinase, which is required for CDK4 kinase activity [23]. However, p21/p27 binding can also inhibit cyclin D:CDK4/6 activity [23,25]. Other roles for CDK4/6 in cell cycle entry have been suggested [26–28]. For example, CDK4/6 substrates include proteins controlling mitochondrial function and glycolysis, coordinating the cell cycle and metabolism [26,28,29]. Other studies have reported that CDK4/6 are able to control transcription in a kinase-independent manner [27,30]. Thus, the precise mechanism by which CDK4/6 activity leads to increased CDK2 activity and cell cycle entry during G1 is unclear.
Our current understanding of CDK4/6 activity suggests two ways by which CDK4/6 inhibitors could act to block proliferation. Our first assumption, based on canonical models of cell cycle entry, is direct CDK4/6 kinase inhibition resulting in cell cycle arrest [4,15,31]. However, it has been reported that CDK4/6 inhibitors are able to arrest cell cycle progression even in the presence of catalytically inactive CDK4/6, although it should be noted that endogenous active CDK4/6 was still present in these experiments which may have been driving the arrest phenotypes [32]. Further, while RB1 (encoding Rb) status may be an important biomarker for CDK4/6 inhibitor sensitivity, some Rb-deficient tumour cells remain sensitive [1]. An alternative, indirect model of CDK4/6 inhibitor action resolves this issue, implicating CDK2 inhibition as the cause of G1 arrest. Recent experimental work suggests that the CDK4/6 inhibitor palbociclib can only bind to CDK4 monomers (or potentially also cyclin D:CDK4 dimers) but not to cyclin D:CDK4:p21/p27 trimers [23]. In this model, cell cycle arrest occurs through the inhibition of CDK2 activity by redistribution of p21 and p27 from CDK4 to CDK2 complexes [23]. Indeed, resistance to CDK4/6 inhibitors is linked to the amplification of cyclin E and CDK6 which may enable continued proliferation through increased CDK2 activity [1,13]. Increased CDK2 activity has also been reported to result from increased cyclin D expression, which sequesters p21 and p27 away from CDK2 [33]. This lack of CDK2 inhibition is proposed to drive proliferation in CDK4/6 inhibitor-treated cells.
CDK4/6 inhibitors are able to arrest cells in G1 despite continued mitogen stimulation [34], indicating that p21 is the most likely candidate for mediating an indirect inhibition of CDK2. Mitogen stimulation results in the abrogation of the inhibitory activity of p27 towards CDK4 due to phosphorylation of the Y74 residue by non-receptor tyrosine kinases (NRTK) such as Src [12,23,35–37]. Further, NRTK signalling can also result in Y88 phosphorylation of p27, which ejects the inhibitory 310 helix of p27 from the CDK2 active site, partially restoring CDK2 activity [36]. Tyrosine phosphorylation of the 310 helix of p21 does not appear to lead to helix ejection, which would allow p21 to retain its function as a CDK inhibitor despite mitogen signalling, which is vital for a robust DNA damage response [38,39].
The difference between the two models of CDK4/6 inhibitor action on cell cycle arrest is their dependence on p21 and p27 (figure 1a). To investigate whether CDK4/6 inhibitors require p21 and p27 to enter or maintain a G1 arrest in cells, we have generated new cell line models to manipulate p21 expression and used live-cell imaging to monitor cell cycle arrest in response to palbociclib. We find that palbociclib is able to initiate and maintain cell cycle arrest, even when p21 and p27 are removed. Our data call into question the essentiality of the indirect model of CDK4/6 inhibitor action in inhibiting proliferation and maintaining cell cycle arrest.
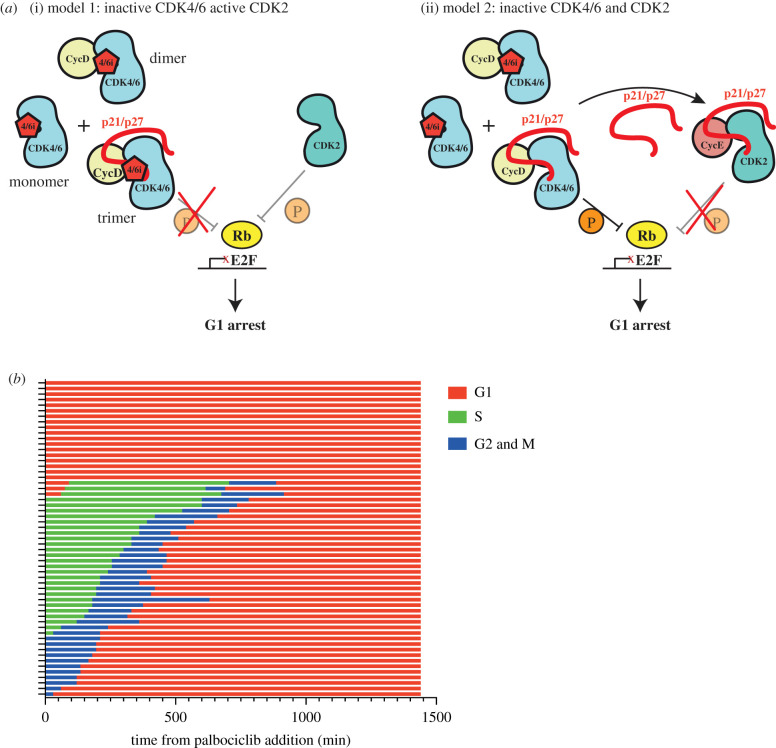
Figure 1.
(a) Models of palbociclib mechanism of action. (i) Model 1: direct inhibition of CDK4/6 catalytic activity by palbociclib. Palbociclib binds and inhibits CDK4/6 monomers, cyclinD:CDK4/6 dimers and Cip/Kip:cyclinD:CDK4/6 trimers. Depending when in the cell cycle palbociclib is added, CDK2 would be active or inactive depending on cyclin E/A expression. In this model, CDK4/6 complexes titrate p21/p27 from CDK2 complexes. (ii) Model 2: indirect inhibition of CDK2 activity through redistribution of Cip/Kip inhibitor proteins. Palbociclib binds and inhibits CDK4/6 monomers and cyclinD:CDK4/6 dimers but not Cip/Kip:cyclinD:CDK4/6 trimers. Cip/Kip redistribution from CDK4/6 complexes to CDK2 results in cell cycle arrest. (b) Graph shows how cell cycle stage affects response to palbociclib addition. hTert-RPE1 mRuby-PCNA cells were imaged after palbociclib addition at time 0 and cells were manually analysed to determine cell cycle phenotypes. Each row represents a single cell (n = 57 cells) and only one daughter cell was followed post-mitosis. See electronic supplementary material, movie S1 for an example of the imaging data.
2. Results
2.1. Palbociclib is only effective as a cell cycle inhibitor during G1 in RPE1 cells
For this study, we first used telomerase-immortalized hTERT-RPE1 cells (RPE1) as they are near diploid, non-transformed, have intact cell cycle control pathways and are sensitive to CDK4/6 inhibitors [34,40]. As such, we assume that cell cycle regulatory complexes will be present at the correct stoichiometries. Previous studies investigating the mechanism of action of CDK4/6 inhibitors have used cancer cells, where the extent to which cell cycle control networks are perturbed is poorly understood. We reasoned that by initially studying CDK4/6 inhibitor action in RPE1 cells, we could establish a baseline of how palbociclib modulates a well-controlled cell cycle. We then used this to understand the effects of mutations and perturbations observed in a cancer cell line. We focus on palbociclib here as it is the best characterized in terms of both its mechanism and its effect on RPE1 cells [23,34].
While RPE1 cells are not transformed, they do have reported mutations in CDKN2A and KRAS [41,42]. There is no clear link between KRAS mutations and palbociclib sensitivity, but CDKN2A (encoding p16) deletion has been reported to cause sensitivity to palbociclib [43]. We confirmed the expression of p16 protein in our RPE1 cells by western blot, indicating that it is not the loss of p16 protein which causes palbociclib sensitivity in these cells and that cells with functional p16 can still be sensitive to palbociclib (electronic supplementary material, figure S1) [43,44].
We have focussed the majority of our efforts on investigating the role of p21 in the cell cycle response to palbociclib. It has previously been shown that p21 protein is nuclear and expressed heterogeneously in RPE1 cells [38]. Together with previous data indicating that p21 and cyclin D levels are correlated in cycling cells [24,45,46], and that p27 would be largely tyrosine phosphorylated and degraded in growth factor-stimulated cells [35,36,39], suggests that p21, and not p27, is likely to be the primary regulator of cyclin D:CDK4/6 activity in cycling RPE1 cells. Additionally, recent work suggests that p21 is also more likely to mediate an indirect mechanism of action of palbociclib [47]. However, since the contribution of p27 cannot be discounted, we also perform our assays in the presence and absence of p27.
As it has been established that palbociclib is limited in its actions to G1 phase [13], we asked when RPE1 cells are sensitive to palbociclib during the cell cycle with respect to cell cycle arrest. We imaged asynchronous RPE1 cells following palbociclib addition and followed their cell cycle progression using endogenously tagged mRuby-PCNA [48] (electronic supplementary material, movie S1). We observed that cells in G1 phase of the cell cycle at the point of drug addition arrest immediately, while cells in S, G2 or mitosis complete their current cycle and re-enter G1 phase before arresting (figure 1b). A small fraction of G1 cells (14.3%) do enter S-phase in the presence of palbociclib and complete the current cycle before arresting in the next G1. All of these cells enter S-phase within 2 h of palbociclib addition and likely represent late G1 cells that were close to the G1/S transition at the time of drug addition. Thus, palbociclib can only induce cell cycle arrest in cells which are in early/mid G1 phase [15,46].
2.2. p21 and p27 are not required for entry into G1 arrest with palbociclib in RPE1 cells
We hypothesized two possible mechanisms for a G1 arrest response to palbociclib. One, that consistent with a direct model of palbociclib action, CDK4/6 activity is only essential for cell cycle progression during the early and mid G1 phase of the cell cycle. In this case, while CDK4/6 may be inhibited by palbociclib during the whole cell cycle, this does not affect progression until G1. Alternatively, this could be explained by the indirect model (figure 1a(ii)) as p21 is degraded abruptly at S-phase entry [38,49–51] and is therefore only present at high levels during G1 [13].
To test this indirect model of palbociclib action and determine if cell cycle arrest induced by palbociclib is dependent upon p21 and p27, we assayed cell cycle distribution by immunofluorescence in fixed cells following 48 h treatment with palbociclib, in the presence and absence of p21 and p27. We measured EdU incorporation, phospho-S807/811 Rb (P-Rb) levels and DNA content. Cells were pulse labelled with the nucleotide analogue 5-ethynyl-2′-deoxyuridine (EdU) 30 min before fixation and Click-iT chemistry used to assay the proportion of cells in S phase (see Methods, electronic supplementary material, figure S2a). While EdU incorporation enabled us to assess the percentage of cells in S phase, P-Rb and Hoechst staining was used to determine cell cycle phase distribution more specifically. Quantification of DNA content by Hoechst sum intensity allows the gating of cells into G1, S and G2/M phases (electronic supplementary material, figure S2b) [15]. P-Rb is bimodally distributed in a population of asynchronously cycling cells, reflecting the proliferation status of the population with G0 cells (and palbociclib-arrested cells) displaying hypophosphorylated Rb [52–54] (electronic supplementary material, figure S2c).
Assessing the cell cycle distribution of untreated p21 knockout (KO) cells [38] showed that p21KO does not appreciably alter the fraction of cells in G1 or S phase (figure 2a(ii,iii) columns 1 versus 5) and reduces the fraction of cells with hypophosphorylated Rb compared to p21 wild-type (WT) cells (electronic supplementary material, figure S2e). Assaying the proliferative status of p21KO cells following palbociclib treatment revealed an arrest in G1 to the same extent as for p21WT cells (figure 2a(ii,iii) columns 2 versus 6; electronic supplementary material figure, S2e).
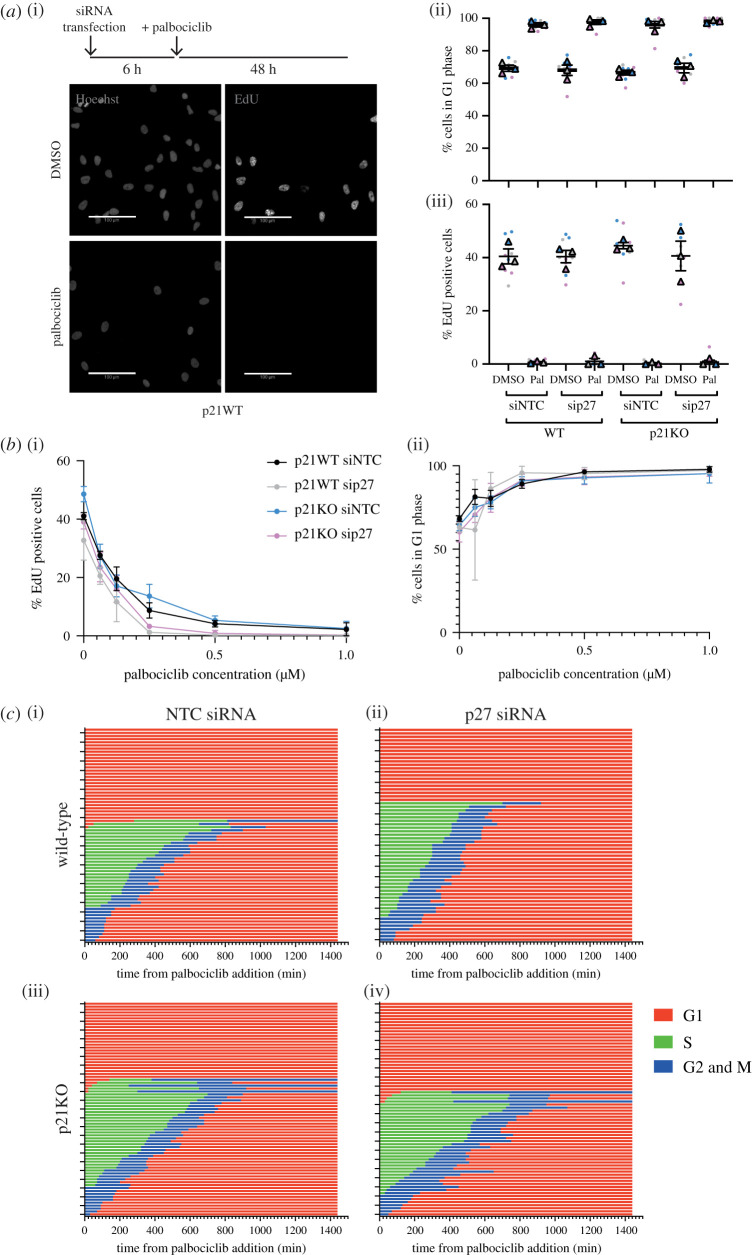
Figure 2.
p21 and p27 are not required for palbociclib-mediated arrest in hTert-RPE1 cells. (a) hTert-RPE1 WT and p21KO cells were reverse transfected with siNTC or sip27 and treated with DMSO or palbociclib 6 h after plating, as indicated. Cells were pulse labelled with 10 μM EdU for 30 min before fixation 48 h following drug treatment. EdU-positive cells quantified as cells with a nuclear : cytoplasmic ratio of EdU signal as greater than or equal to 1.2. (i) Representative images. Scale bar, 100 μm. (ii) Cells were classified in G1 phase according to their DNA content as quantified by Hoechst nuclear sum intensity. (iii) EdU positive cells quantified as cells with a nuclear : cytoplasmic ratio of EdU signal as greater than or equal to 1.2. Data plotted as a SuperPlot [55] and in subsequent figures: dots represent replicate wells, colour coded by experimental repeat, triangles represent mean values for each of n = 3 experimental repeats with mean and s.e.m. shown. One-way ANOVA with Sidak's multiple comparisons test was performed between mean values of palbociclib treated samples in comparison to WT siNTC palbociclib, all differences were non-significant. (b) hTert-RPE1 mRuby-PCNA p21 WT and p21KO cells were transfected with siNTC or sip27 and 6 h later treated with the indicated concentrations of palbociclib for 48 h before 30 min EdU pulse and fixation. Plot of percentage of EdU-positive cells, (i), and of cells in G1 phase, (ii). Mean and s.d. from one of n = 2 experimental repeats shown. One-way ANOVA with Sidak's multiple comparisons test was performed between WT and p21KO at each concentration of palbociclib; all differences were non-significant. (c) Graphs show timing of cell cycle arrest when palbociclib is added to asynchronous WT and p21KO cells (at 0 min) 6 h after transfection with the indicated siRNAs. Cell cycle phenotypes were monitored by live-cell imaging following drug addition and tracked manually over 24 h using mRuby-PCNA as a readout. Three fields of view were quantified per condition. Each row represents an individual cell. (i) WT NTC siRNA n = 68, (ii) WT p27 siRNA n = 61, (iii) p21KO NTC siRNA n = 73 and (iv) p21KO p27 siRNA n = 70 cells.
While we hypothesized that p21 would be more likely than p27 to mediate an indirect mechanism of G1 arrest in palbociclib in RPE1 cells, we wanted to ask if palbociclib could induce arrest in the absence of both Cip/Kip proteins, as p27 has also been implicated in this mechanism [20,21,23]. siRNA-mediated knockdown of p27 (electronic supplementary material, figure S2d,e) prior to palbociclib treatment did not affect proliferation (figure 2a(ii,iii) columns 1 versus 3 and 5 versus 7) or the ability of cells to arrest in G1 (columns 2 versus 4 and 6 versus 8) in p21WT or p21KO backgrounds. Further, this is true at a range of palbociclib concentrations, emphasizing that these Cip/Kips make little contribution to the mechanism of arrest in RPE1 cells even at low palbociclib concentrations (figure 2b; electronic supplementary material, figure S2f,g).
Our fixed cell analyses indicated that RPE1 cells arrest in G1 in response to palbociclib in the absence of p21 and/or p27. However, we wanted to test the hypothesis that it is the presence of p21 (and perhaps p27) during G1 that makes G1 cells sensitive to palbociclib [13]. We reasoned that, if this was the case, then in the absence of p21 and/or p27, a higher fraction of cells in G1 at the time of palbociclib addition may progress through S-phase and complete the cycle, before arresting in the next G1. Therefore, we repeated our live-imaging experiment in mRuby-PCNA labelled p21WT and p21KO cells treated with non-targeting control (NTC) or p27 targeting siRNA [38,48]. We observed that cells respond in the same way to palbociclib, arresting in G1, independent of the presence of p21 and p27 (figure 2c; fraction of G1 cells progressing into S-phase: p21WT NTC 9.4%, p21WT p27si 0%, p21KO NTC 16.1%, p21KO p27si 12.1%).
Together, these data suggest that p21 and p27 are not essential for entry into a palbociclib-mediated cell cycle arrest and that CDK4/6 activity is only required for cell cycle entry during early and mid G1.
2.3. p21 and p27 are not essential for CDK4/6 activity
While previous work suggests that p21 and p27 promote CDK4/6 activity and that p21/p27/p57 triple KO cells cannot assemble cyclin D1:CDK4 complexes [12], our data shows little effect of p21KO sip27 on the asynchronous cell cycle. This is in contrast with the depletion of CDK4 or CDK6 from RPE1 cells that leads to an increased fraction of cells arresting their cell cycle (electronic supplementary material, figure S3a), suggesting that CDK4 and CDK6 remain active in RPE1 cells after Cip/Kip loss. To confirm that CDK4/6 remains active in the absence of p21 and p27, we used a CDK4/6 sensor to measure CDK4/6 activity in our cells [19]. We did not observe a change in CDK4/6 activity 48 h following p21 and/or p27 knockdown, indicating that CDK4/6 remains active in these cells (electronic supplementary material, figure S3b). Our data indicate that CDK4/6 activity is important for timely cell cycle entry in RPE1 cells and that this activity is not affected by a lack of p21 and/or p27.
2.4. Generating p21-degron cell lines
In our previous experiments, the absence of p21 and p27 in RPE1 cells at the time of palbociclib addition suggests that palbociclib could bind directly to CDK4 and CDK6 to inhibit their activity [23] and that palbociclib could therefore act to arrest the cell cycle through a direct CDK4/6 inhibition mechanism. However, this does not address the question of whether, when present, p21 and p27 are required to mediate an indirect mechanism of cell cycle arrest?
One way to address this question is to allow cells to enter a palbociclib-mediated arrest in the presence of p21 and p27, and then remove the Cip/Kips and see if any cells re-enter the cell cycle. To be able to efficiently and inducibly degrade p21, we used a double degron system [56]. In this way, we could test if p21 is needed for maintaining a palbociclib-induced arrest in a system where p21 is normally present to assist in the assembly of functional cyclin D:CDK4/6 complexes, and where p21 could potentially relocalize upon palbociclib addition to cyclin:CDK2 complexes to mediate cell cycle arrest (figure 1a). We introduced an mVenus-mAID-SMASh tag at the C-terminus of p21 in RPE1 cells expressing myc-OsTIR1 under a doxycycline-inducible promoter (p21-degron cells, myc-OsTir1 RPE1 cells were a kind gift from H. Hochegger). Homozygous gene targeting was confirmed by PCR and western blot, and an siRNA directed against p21 was used to confirm the specificity of tagging (figure 3a(i); electronic supplementary material, figure S3c,d). We verified that this tag did not alter the function of p21 by confirming that there was no effect on cell growth, nuclear localization was retained and that a P-Rb low (G0) fraction of cells was still present in this population (electronic supplementary material, figure S3e,f,h). This G0 fraction is dependent upon the presence of p21 and is also present in RPE1 cells with p21 endogenously tagged with GFP (electronic supplementary material, figure S3h) [38]. We confirmed that tagged p21 is induced upon nutlin treatment and that tagged p21 can arrest the cell cycle in a p53-dependent manner (electronic supplementary material, figure S3h,i). There may be some basal degradation of p21 without DIA (doxycycline, indole-3-acetic acid (IAA) and asunaprevir (ASV)) addition, as p21-degron levels are decreased compared to endogenous p21 in WT cells (electronic supplementary material, figure S3h,i). Basal degradation due to leakiness of the AID system has been previously reported [57]. We verified that the addition of DIA resulted in the depletion of p21-mVenus-degron to undetectable levels by immunoblot and by live-cell imaging (figure 3a(ii); electronic supplementary material, figure S3d,g, movies S2 and S3).
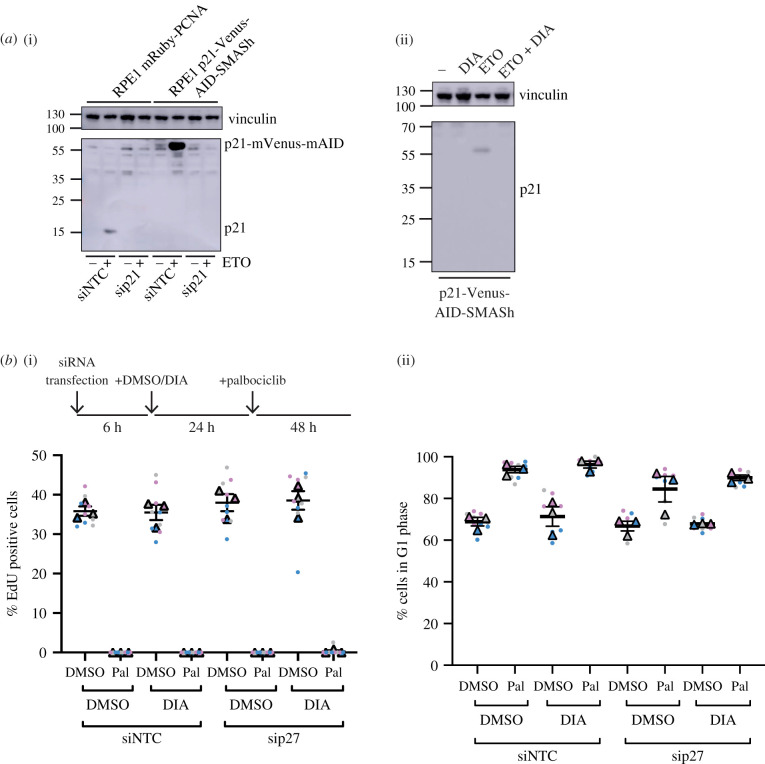
Figure 3.
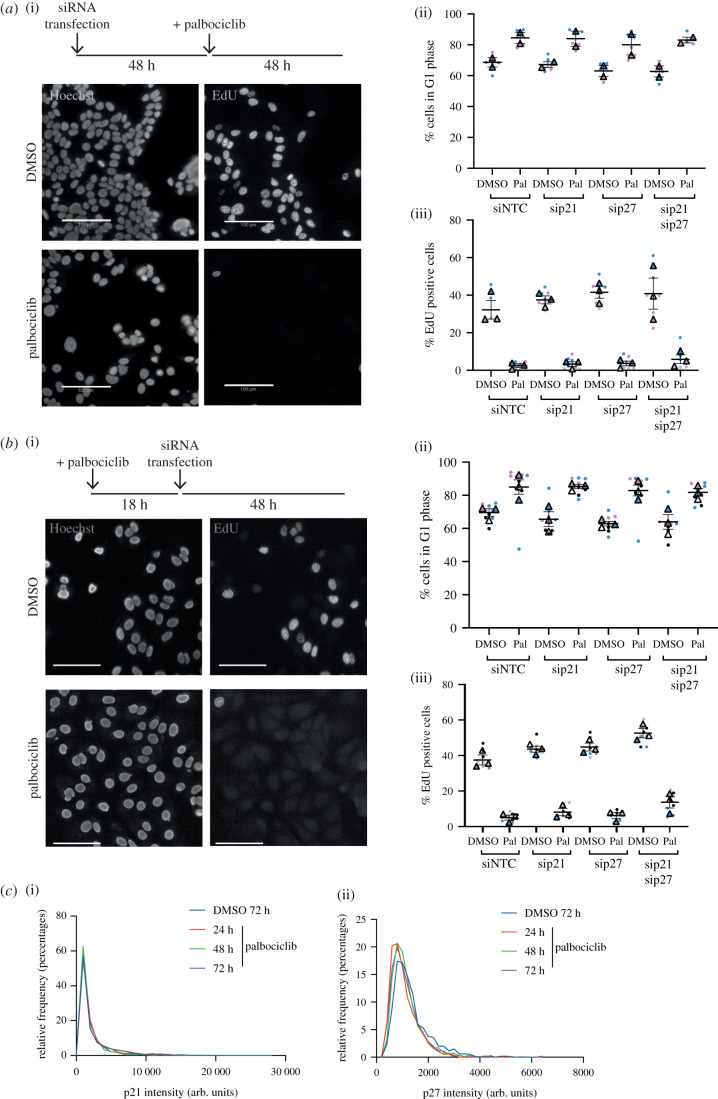
Generation of p21-degron lines. (a) Western blot of whole-cell extract from indicated cell lines probing for p21, indicating all p21 expressed in the hTert-RPE1 OsTIR1 mRuby-PCNA p21-mVenus-mAID-SMASh cell line is tagged with mVenus-mAID. (i) Cells were reverse transfected with the indicated siRNAs, NTC or p27 and collected after 48 h; 10 µM etoposide (ETO) was added 24 h prior to sample collection to induce DNA damage as p21 expression is low in untreated hTert-RPE1 cells. p21-mVenus-mAID has a predicted molecular weight of 55 kDa; no p21-mVenus-mAID-SMASh is detected as the SMASh tag self-cleaves from the protein. Vinculin was used as a loading control. (ii) Western blot of whole-cell extract indicating that p21-mVenus-AID-SMASh is degraded following DIA (doxycycline, IAA and ASV) addition after induction of p21 expression by ETO. ETO and DIA: doxycycline (1 µg ml−1), IAA (500 µM) and ASV (3 µM) were added 24 h before sample collection. (b) hTert-RPE1 mRuby-PCNA p21-Venus-AID-SMASh cells were reverse transfected with the indicated siRNAs, 6 h later DMSO or DIA were added as indicated and 24 h DMSO or palbociclib added for 48 h. Cells were pulse labelled with EdU before fixation and (i) EdU incorporation and (ii) G1 percentage were quantified. Data from n = 3 repeats plotted as SuperPlots. One-way ANOVA with Sidak's multiple comparisons test was used to compare palbociclib-treated samples with control (siNTC DMSO palbociclib), all differences were non-significant.
We first used the p21-degron cells to ask if acute depletion of p21 and/or p27 abrogates palbociclib-induced cell cycle arrest in a system where p21 is normally present to stabilize assembly of cyclin D:CDK4/6 complexes (in contrast with the p21KO cells which may also have adapted to the loss of p21). Cells were reverse transfected with siRNA, to deplete p27, and treated with DIA, to degrade p21, then treated with palbociclib 24 h later for 48 h. EdU incorporation, Hoechst and P-Rb staining were used to assess if (and at what cell cycle phase) cells were arrested. Degradation of p21 did not significantly affect the percentage of EdU-positive cells, the percentage of G1 phase cells or the distribution of P-Rb staining in DMSO and siNTC-treated cells (figure 3b column 1 versus 3; electronic supplementary material, figure S4a). p21 degradation also did not affect entry into cell cycle arrest mediated by palbociclib (figure 3b, column 2 versus 4; electronic supplementary material, figure S4a). Following p27 knockdown alone, proliferation was largely unaffected, similar to what we observed in p21WT and p21KO cells (figure 3b, columns 1 versus 5; electronic supplementary material, figure S4a). p27 knockdown also did not affect entry into cell cycle arrest in palbociclib, independently of DIA addition prior to treatment (figure 3b columns 2 versus 6 and 4 versus 8; electronic supplementary material, figure S4a).
In summary, neither p21 nor p27 are necessary for the initiation of a palbociclib-mediated arrest in RPE1 cells.
2.5. p21 and p27 are not required for maintenance of G1 arrest with palbociclib
To further clarify the importance of a potential indirect mechanism of palbociclib action in our system, we wanted to ask if maintenance of cell cycle arrest initiated in unperturbed conditions is dependent on p21 and p27. We reasoned that if an indirect mechanism maintains cell cycle arrest during palbociclib treatment then a decrease in p21 or p27 protein levels during arrest would result in cell cycle re-entry.
To test if removal of p21 and/or p27 promoted cell cycle re-entry in palbociclib-arrested cells, we first degraded p21 following 24 h palbociclib treatment in p21-degron cells. Assaying proliferation as before, we saw that cell cycle arrest was maintained following p21 degradation (figure 4a columns 5 versus 6; electronic supplementary material, figure S4b). Further, p27 knockdown following palbociclib treatment did not affect the arrest, independent of the presence of p21 (figure 4a columns 7 versus 8; electronic supplementary material, figure S4b). Additionally, in palbociclib-treated p21KO cells, the knockdown of p27 did not affect the arrest (figure 4b columns 7 versus 8; electronic supplementary material, figure S4c).
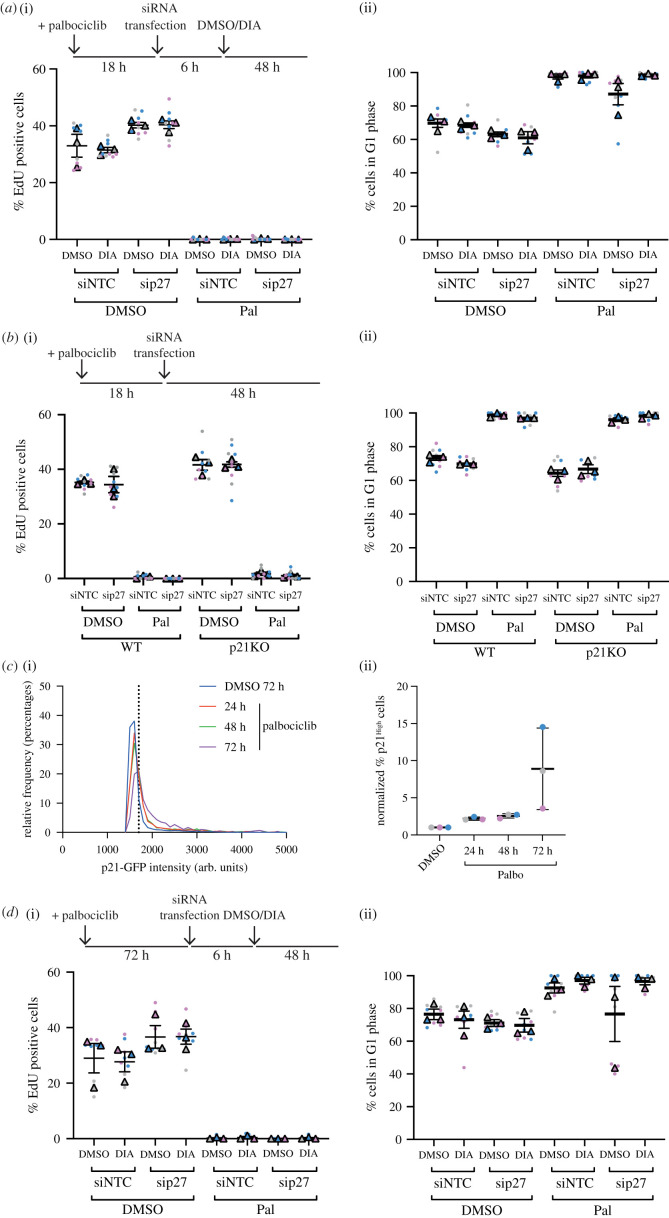
Figure 4.
Palbociclib-dependent arrest can be maintained in the absence of p21/p27. (a) hTert-RPE1 p21-degron cells were treated with DMSO/palbociclib, transfected with the indicated siRNAs 18 h later and then treated with DIA 6 h following transfection. Cells were pulse labelled for 30 min with 10 µM EdU and fixed 48 h following DIA addition. (b) hTert-RPE1 WT and p21KO cells were treated with DMSO/palbociclib and transfected with the indicated siRNAs 18 h after drug treatment; 48 h after transfection addition, cells were pulse labelled with 10 µM EdU for 30 min and fixed. (c) hTert-RPE1 mRuby-PCNA p21GFP cells were treated with DMSO for 72 h or palbociclib for the indicated times, and p21GFP levels quantified. (i) Representative frequency distribution of measured intensities from one experimental repeat shown, p21High threshold for this experiment shown as a dotted line. (ii), Percentage of cells classified as p21 high above a threshold of p21 intensity, n = 3 experiments shown. Data were normalized to 72 h DMSO treatment within each experimental repeat. (d) hTert-RPE1 p21-degron cells were treated with DMSO/Palbocilib for 72 h, transfected and then DMSO/DIA added 6 h later. Cells were pulse labelled with 10 µM EdU for 30 min 48 h after DIA addition. (a,b,d) Data from n = 3 repeats plotted as SuperPlots. One-way ANOVA with Sidak's multiple comparisons tests were used to compare palbociclib-treated samples, all differences were non-significant.
We observed that palbociclib treatment induces an increase in p21 and p27 protein levels in cells in a time-dependent manner, and that their localization remains exclusively nuclear. The largest increase in p21 protein occurs between 48 and 72 h palbociclib treatment (p21: figure 4c; p27: electronic supplementary material, figure S2e columns 1 versus 2). We hypothesized that this might reflect an increased dependence on p21 and p27 to maintain cell cycle arrest in the presence of palbociclib. To test this, we decreased p21 and p27 protein levels following a long-term palbociclib-mediated arrest, to ask if these proteins are necessary to maintain a prolonged arrest initiated in unperturbed conditions. We used p21-degron cells to degrade p21 72 h following palbociclib treatment. Assaying proliferation 48 h following the induction of p21 degradation revealed a similar extent of arrest, independent of the presence of p21 (figure 4d columns 5 versus 6; electronic supplementary material, figure S4d) or p27 (columns 5 versus 7 and 7 versus 8).
Together, our data demonstrate that RPE1 cells are not dependent on p21 or p27 for maintenance of a palbociclib-mediated cell cycle arrest.
2.6. p21 and p27 are not essential for palbociclib-mediated arrest in oestrogen receptor-positive (ER+) breast cancer cells
Our data do not rule out a potential role for p21/p27 during palbociclib-induced cell cycle arrest in some cells [23]. This could represent a difference between transformed and non-transformed cells in their dependence on Cip/Kip proteins for arrest. While ER+ MCF7 breast cancer cells are at least partly dependent on CDK4/6 activity for cell cycle entry [58], p21/p27 appear to mediate their palbociclib sensitivity [23]. We therefore investigated the dependence of MCF7 cells on p21 and p27 for palbociclib-mediated arrest.
We repeated our previous experiments using MCF7 cells, initially asking if cells are able to arrest in palbociclib following knockdown of p21 and/or p27. Assaying proliferation 48 h after palbociclib treatment revealed a minor dependence on p21 and p27 for palbociclib-mediated arrest (siNTC = 2.4% ±1.0 s.e.m. EdU positive, sip21 = 3.3% ±1.2 s.e.m., sip27 = 3.6% ±1.3 s.e.m. and sip21/p27 = 5.9% ±2.4 s.e.m.), although none of these effects was significant (figure 5a; electronic supplementary material, figure S5a). Similar results were observed when we assayed whether p21 and/or p27 were required for maintaining a palbociclib-induced cell cycle arrest, where we depleted the Cip/Kip proteins following arrest in palbociclib (figure 5b; electronic supplementary material, figure S5b). We reasoned that the small increase in the fraction of cells escaping palbociclib-mediated arrest after p21 and p27 depletion might be reflected in an increase in their expression following palbociclib treatment, as we had previously observed in RPE1 cells (figure 4c; electronic supplementary material, figure S2e). However, p21 and p27 protein levels do not increase following palbociclib addition (figure 5c).
Figure 5.
MCF7 cells do not require p21 or p27 for palbociclib-mediated cell cycle arrest. (a) MCF7 cells were transfected with the indicated siRNAs, NTC, sip21 and/or sip27 48 h before treatment with 1 µM palbociclib. Cells were pulse labelled for 30 min with 10 µM EdU and fixed 48 h following palbociclib addition. (b) MCF7 cells were treated with palbociclib for 18 h before transfection with the indicated siRNAs. Cells were fixed 48 h following palbociclib addition, after 30 min pulse labelling with 10 µM EdU. (a,b) Data plotted as SuperPlots; dots represent replicate wells; colour coded by experimental repeat. Triangles represent mean values for n = 3 (n = 2 for (a), ii) repeats with mean and s.e.m. shown. Scale bar = 100 µm. One-way ANOVA with Sidak's multiple comparisons test was performed between mean values of palbociclib-treated samples in comparison to WT siNTC palbociclib, all differences were non-significant. (c) MCF7 cells were treated with DMSO for 72 h or palbociclib for 24, 48 or 72 h and (i) p21 and (ii) p27 levels quantified by immunofluorescence. Representative frequency distributions of measured intensities from one experimental repeat (from n = 3) shown.
These data show that p21 and p27 may make a minor contribution to palbociclib-mediated cell cycle arrest in MCF7 ER + breast cancer cells, but are not essential to induce or maintain a G0/G1 arrest.
3. Discussion
In this study, we have established that a palbociclib-mediated cell cycle arrest is not dependent on the Cip/Kip inhibitor proteins p21 and p27 in RPE1 and MCF7 cells. We have demonstrated that cell cycle arrest in response to palbociclib can both be initiated and maintained without p21 or p27.
Importantly, in a system in which a ‘normal’ palbociclib-mediated arrest has been allowed to occur, the presence of p21 and p27 is not necessary for the maintenance of cell cycle arrest (figures 4 and 5). This indicates that an arrest initiated in the presence of cyclin D:CDK4/6 trimeric complexes with p21 (and potentially p27), which might be predicted to occur through an indirect mechanism of action of palbociclib, is not dependent on p21/p27. If the arrest were maintained through the indirect inhibition of CDK2, we would predict that the absence of p21/p27 would result in the release of, at least a fraction of, cells into the cell cycle despite the continued presence of palbociclib. While we are unable to rule out that in the presence of p21 and p27, palbociclib acts through an indirect mechanism to inhibit CDK2 to initiate arrest that is then maintained by direct palbociclib-mediated CDK4/6 inhibition, our data suggest that the presence of p21 and/or p27 is not essential for entry into cell cycle arrest or maintenance of that arrest.
This calls into question a solely indirect model of palbociclib-driven cell cycle arrest, which is dependent upon the presence of p21 and/or p27 both before and during the arrest (model 2; figure 1a(ii)). Our data support the direct inhibition of CDK4/6 by palbociclib to inhibit proliferation. This is supported by work assaying CDK4/6 and CDK2 activity in single non-transformed MCF10A cells using live-cell CDK4/6 and CDK2 activity reporters [19]. In both G1 and S phase cells released from synchronization in G0, palbociclib addition decreases CDK4/6 activity within 1 h, while CDK2 activity decreases at a much slower rate. Further, recent data from multiple cell line models suggested that in contrast with CDK2, CDK4 catalytic activity towards Rb is inhibited by palbociclib treatment [59]. Together, this suggests that palbociclib directly inhibits CDK4/6 catalytic activity and that this is sufficient for a G1 phase arrest.
While sensitivity to palbociclib is known to be restricted to G1, here we have reported that cells become insensitive to drug addition in late G1, at approximately 2 h before S phase entry (figure 1b). This corresponds with early reports of restriction point timing [60–62]. This could reflect an increasing rate of p21 degradation as cells approach the G1/S transition [50,51] or could be the result of a change in the dependency of cells on CDK4/6 activity for cell cycle progression at the restriction point. Since we see no change in sensitivity of G1 cells to palbociclib in the absence of p21 and/or p27, it is likely that it is the latter hypothesis that is correct here and that cells only require CDK4/6 activity in early and mid G1 to complete the cell cycle [15,46]. Interestingly, p21 has been implicated in cellular resistance mechanisms to CDK4/6 inhibitors, indicating it still has an important role in their mechanism of action in some contexts. The loss of p53, a major driver of p21 expression, has been implicated in resistance to the CDK4/6 inhibitor abemaciclib, with significant enrichment in TP53 mutations in resistant breast cancer [63,64]. Further, increasing p21 expression is linked to re-sensitizing resistant cells to palbociclib, indicating that low p21 levels may contribute to palbociclib resistance [33,65]. However, the loss of p53 does not prevent proliferative arrest induced by CDK4/6 inhibitors, supporting our hypothesis that CDK4/6 inhibitors are able to act through multiple potential mechanisms [64]. By contrast, Y88 phosphorylation of p27, a modification which prevents its inhibitory activity towards CDK2, correlates with sensitivity to palbociclib [66].
Our data does not rule out a potential role for p21/p27 during palbociclib-induced cell cycle arrest in some cells [23]. Indeed, the small decrease in arrest observed after p21/p27 depletion in MCF7 cells, while not significant, suggests that palbociclib is able to arrest the cell cycle through parallel direct and indirect mechanisms and that the dominant mechanism may depend upon the cellular context. A parallel pathways model explains how both RPE1 and MCF7 cells acutely depleted of p21/p27, and cells with impaired CDK4/6 or Rb activity are sensitive to palbociclib [23,32,67].
It seems likely that palbociclib is able to arrest cell cycle progression through both direct and indirect mechanisms, meaning the sensitivity of a cancer cell to palbociclib may be dependent upon both its reliance on CDK4/6 activity for cell cycle entry and the relative expression levels of p21/p27. As the activity of these pathways is often perturbed in cancer, this may alter the effect of palbociclib on the cell cycle, determining a cell's sensitivity to palbociclib and the mechanism by which it may cause cell cycle arrest. For example, the lack of sensitivity of some triple-negative breast cancer cells may reflect both a decreased dependence on CDK4/6 activity for cell cycle entry (due to high cyclin E expression and CDK2 activity) and low p21 and/or p27 levels [68]. The prediction of a cell's sensitivity to palbociclib may therefore require information about the balance between the activity of multiple cell cycle pathways (table 1). For example, we would predict that in Rb-deficient cells which remain sensitive to palbociclib would be sensitive to decreases in p21/p27. These different potential mechanisms of action of palbociclib may explain why there are no clear biomarkers for sensitivity.
Table 1.
Predictions of sensitivity to CDK4/6 inhibitors.
| Dependence on CDK4/6 | p21/p27 levels | CDK4/6 inhibitor sensitivity prediction |
|---|---|---|
| yes | high/normal | yes—both mechanisms |
| yes | low | yes—direct inhibition |
| no | high | yes—indirect mechanism |
| no | low | no |
4. Methods
4.1. Cell culture
hTert-RPE1 and MCF7 cells were from ATCC and were maintained in DMEM (Gibco) supplemented with 10% FBS and 1% penicillin–streptomycin at 37°C and 5% CO2. MCF7 cells were supplemented with 10 nM β-oestradiol (Sigma E8875). RPE1 mRuby-PCNA p21GFP cells, in which both alleles of the endogenous CDKN1A locus were labelled with GFP at the C-terminus and one allele of PCNA was labelled at the N-terminus with mRuby, were described previously [38]. RPE1 mRuby-PCNA p21 KO 1A cells were described previously [38].
Drugs used and working concentrations: etoposide 10 µM, doxycycline 1 µg ml−1, IAA 500 µM, ASV 3 µM, palbociclib 1 µM (unless otherwise stated) and nutlin 10 µM.
4.2. Generation of p21-Venus-AID-SMASh tagged hTert-RPE1 cell line
An mVenus-mAID-SMASh tag was introduced to the C terminus of the human CDKN1A gene using targeting vectors and gRNA/Cas9 cleavage.
For the homology donor plasmid primers used for the left and right homology arms were the same as in [38]. To PCR amplify mVenus, we used the following primers: forward, 5′-TCTTCTCCAAGAGGAAGCCCGGAGGAGGAGT GAGCAAGGGCGAGGAG-3′, reverse 5′-GCTGATGCCGCTGAGGCGCCCTTGTACAGCTCGTCCAT-3′. mAID-SMASh-Neomycin was amplified with the primers forward: 5′-GGCGCCTCAGCGGCATCAGCTGCAGGAGCTGGAGGTGCATC-3′ and reverse: 5′-GCAGGCTTCCTGTGGGCGGATCAGAAGAACTCGTCAAGAAG-3′. LHA, mVenus, mAID-SMASh-Neomycin, RHA PCR products were ligated into pAAV p21 vector by Gibson assembly at a ratio vector : inserts of 1 : 2 : 2 using T4 DNA ligase (NEB). All constructs were checked by sequencing before transfection into cells. To generate stable clones, hTERT-RPE1 OsTIR1 cells (a gift from Helfrid Hoechegger, [56]) were transfected with pX330 g21 gRNA plasmid [38] and the p21 homology donor plasmid at a ratio of 1 : 1 using Lipofectamine 2000, according to the manufacturer's instructions (Invitrogen). Cells were incubated for three weeks in media containing 0.5 µg ml−1 G418 and selected clones were screened by western blot and genomic DNA PCR.
4.3. siRNA transfection
Cells were transfected with siRNA at a final concentration of 20 nM using Lipofectamine RNAiMAX, according to the manufacturer's instructions (Invitrogen). Briefly, 40 nl of Lipofectamine RNAiMAX (Invitrogen) was mixed with siRNA in 10 µl OptiMEM (Gibco) per well of a 384-well plate. Twenty microlitres of cells at a density of 2.5 × 104 cells ml−1 were plated on top of this, and cells were incubated at 37°C. siRNAs used were Dharmacon ON-TARGETplus Non-targeting siRNA no. 1 (NTC) and CDKN1A (set of 4), Ambion Silencer Select siRNA CDKN1B (Cat. no. 4427038) and p16 siRNA sequence used: UACCGUAAAUGUCCAUUUAUA.
4.4. Immunofluorescence
Cells were grown on 384-well CellCarrierUltra (PerkinElmer) plates. For EdU staining, a final concentration of 10 µM EdU was added to the growth media 30 min prior to fixation. Cells were fixed in 4% paraformaldehyde in PBS for 15 min and washed three times with PBS. Permeabilization in PBS 0.2% Triton X-100 for 15 min was followed by blocking in 2% BSA in PBS for 1 h. Cells were incubated with primary antibodies diluted in blocking buffer at 4°C overnight and washed three times with PBS then incubated with a 1 : 1000 dilution of secondary antibodies for 1 h at room temperature. For EdU detection cells were incubated for 30 min in TBS 100 mM pH 7.5, CuSO4 4 mM, sulfo-cyanine 3 azide 5 µM and sodium ascorbate 100 mM. Cells were washed three times in PBS, incubated for 10 min with 1 µg ml−1 Hoechst, then washed a further three times in PBS.
Antibodies used p21 (Invitrogen MA5-14949 1 : 1000), p27 (CST 3688 1 : 1000), P-Rb S807/811 (CST 8516, 1 : 2000); secondary goat anti-rabbit IgG (H + L) and Alexa Fluor 647 (Invitrogen A21245, 1 : 1000). Plates were imaged using a 20× (NA 0.8) objective using an Operetta CLS microscope.
4.5. Western blot
Whole-cell extract of RPE1 cells was collected following aspiration of medium from culture plate, two washed in PBS and the addition of 1× Novex Tris-glycine SDS sample buffer (Invitrogen) and collection of cells by scraping. Samples were incubated at 95°C for 10 min before loading on 12–15% precast NuPAGE gels (Invitrogen). Primary antibodies used p16 (CST 80772, 1 : 1000), p21 (Invitrogen MA5-14949 1 : 1000), vinculin (CST 13901 1 : 1000); secondary antibodies HRP linked anti-rabbit IgG (CST 7074, 1 : 2000).
4.6. Growth curves
Cells were plated at a density of 20 000 cells per well in duplicate in six-well plates. Brightfield images were taken every 2 h for 5 days and the percentage confluency was calculated using an Incucyte Live-Cell analysis system (Sartorius).
4.7. Live imaging
hTert-RPE1 cells were seeded into 384-well CellCarrier Ultra plates (PerkinElmer) 1 day prior to imaging at a density of 1000 cells well−1 in 20 µl of phenol-red free DMEM:F12 with 10% FBS and 1% P/S. In cases where cells were transfected with siRNA, cells were plated onto siRNA:lipofectamine RNAiMax (Invitrogen) complexes (as described elsewhere). Prior to imaging, media was added to all wells to a final volume of 100 µl, with a final concentration of 1 µM palbociclib (where relevant). SiR-DNA (SC015, tebu-bio) was added at a final concentration of 10 nM 1 h before imaging. A breathable film was applied to the plate (ThermoFisher) to prevent media evaporation and cells were imaged on the Operetta CLS (PerkinElmer) at 37°C and 5% CO2, using a 20 × (N.A. 0.8) objective, every 10 (figure 2; electronic supplementary material, figure S3g) or 15 min (figure 1). Image analysis was performed in FIJI and NucliTrack [69]. Endogenously tagged mRuby-PCNA was used as previously described to determine cell cycle timing [48].
Acknowledgements
We would like to thank Adrian Saurin and Tony Ly for the critical reading and discussion of the manuscript and Hee Won Yang for the gift of the CDK4/6 and CDK2 sensor plasmid. We also thank the LMS/NIHR Imperial Biomedical Research Centre Flow Cytometry Facility for the support.
Data accessibility
This article has no additional data. The data are provided in the electronic supplementary material [70].
Authors' contributions
A.R.B. conceived the study. A.R.B. and B.R.P. designed, performed and analysed the experiments and wrote the manuscript.
Competing interests
We declare we have no competing interests.
Funding
B.R.P. and A.R.B. are funded by CRUK CDF: C63833/A25729 and MRC core funding to the London Institute of Medical Sciences (MC-A658-5TY60).
References
- 1.Álvarez-Fernández M, Malumbres M. 2020. Mechanisms of sensitivity and resistance to CDK4/6 inhibition. Cancer Cell 37, 514-529. ( 10.1016/j.ccell.2020.03.010) [DOI] [PubMed] [Google Scholar]
- 2.Dickler MN, et al. 2017. MONARCH 1, a phase II study of abemaciclib, a CDK4 and CDK6 inhibitor, as a single agent, N patients with refractory HR+ /HER2− metastatic breast cancer. Clin. Cancer Res. 23, 5218-5224. ( 10.1158/1078-0432.CCR-17-0754) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finn RS, et al. 2016. Palbociclib and letrozole in advanced breast cancer. New Engl. J. Med. 375, 1925-1936. ( 10.1056/NEJMoa1607303) [DOI] [PubMed] [Google Scholar]
- 4.Fry DW, et al. 2004. Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol. Cancer Therapeutics 3, 1427-1437. [PubMed] [Google Scholar]
- 5.Gelbert LM, et al. 2014. Preclinical characterization of the CDK4/6 inhibitor LY2835219: in-vivo cell cycle-dependent/independent anti-tumor activities alone/in combination with gemcitabine. Investigational New Drugs 32, 825-837. ( 10.1007/s10637-014-0120-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hortobagyi GN, et al. 2016. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. New Engl. J. Med. 375, 1738-1748. ( 10.1056/NEJMoa1609709) [DOI] [PubMed] [Google Scholar]
- 7.Rader J, et al. 2013. Dual CDK4/CDK6 inhibition induces cell-cycle arrest and senescence in neuroblastoma. Clin. Cancer Res. 19, 6173-6182. ( 10.1158/1078-0432.CCR-13-1675) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sledge GW, et al. 2017. MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2− advanced breast cancer who had progressed while receiving endocrine therapy. J. Clin. Oncol. 35, 2875-2884. ( 10.1200/JCO.2017.73.7585) [DOI] [PubMed] [Google Scholar]
- 9.Tripathy D, Bardia A, Sellers WR. 2017. Ribociclib (LEE011): mechanism of action and clinical impact of this selective cyclin-dependent kinase 4/6 inhibitor in various solid tumors. Clin. Cancer Res. 23, 3251-3262. ( 10.1158/1078-0432.CCR-16-3157) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spring LM, Wander SA, Andre F, Moy B, Turner NC, Bardia A. 2020. Cyclin-dependent kinase 4 and 6 inhibitors for hormone receptor-positive breast cancer: past, present, and future. Lancet 395, 817-827. ( 10.1016/S0140-6736(20)30165-3) [DOI] [PubMed] [Google Scholar]
- 11.Pennycook BR, Barr AR. 2020. Restriction point regulation at the crossroads between quiescence and cell proliferation. FEBS Lett. 594, 2046-2060. ( 10.1002/1873-3468.13867) [DOI] [PubMed] [Google Scholar]
- 12.Hume S, Dianov GL, Ramadan K. 2020. A unified model for the G1/S cell cycle transition. Nucleic Acids Res. 48, 12 485-12 501. ( 10.1093/nar/gkaa1002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rubin SM, Sage J, Skotheim JM. 2020. Integrating old and new paradigms of G1/S control. Mol. Cell 80, 183-192. ( 10.1016/j.molcel.2020.08.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Narasimha AM, Kaulich M, Shapiro GS, Choi YJ, Sicinski P, Dowdy SF. 2014. Cyclin D activates the Rb tumor suppressor by mono-phosphorylation. ELife 3, e02872. ( 10.7554/eLife.02872) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chung M, Liu C, Yang HW, Köberlin MS, Cappell SD, Meyer T. 2019. Transient hysteresis in CDK4/6 activity underlies passage of the restriction point in G1. Mol. Cell 76, 562-573. ( 10.1016/j.molcel.2019.08.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harbour JW, Luo RX, Dei Santi A, Postigo AA, Dean DC. 1999. Cdk phosphorylation triggers sequential intramolecular interactions that progressively block Rb functions as cells move through G1. Cell 98, 859-869. ( 10.1016/S0092-8674(00)81519-6) [DOI] [PubMed] [Google Scholar]
- 17.Lundberg AS, Weinberg RA. 1998. Functional inactivation of the retinoblastoma protein requires sequential modification by at least two distinct cyclin–cdk complexes. Mol. Cell. Biol. 18, 753-761. ( 10.1128/MCB.18.2.753) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Topacio BR, Zatulovskiy E, Cristea S, Xie S, Tambo CS, Rubin SM, Sage J, Kõivomägi M, Skotheim JM. 2019. Cyclin D-Cdk4,6 drives cell-cycle progression via the retinoblastoma protein's C-terminal helix. Mol. Cell 74, 758-770.e4. ( 10.1016/j.molcel.2019.03.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang HW, et al. 2020. Stress-mediated exit to quiescence restricted by increasing persistence in CDK4/6 activation. ELife 9, e44571. ( 10.7554/eLife.44571) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Polyak K, Kato JY, Solomon MJ, Sherr CJ, Massague J, Roberts JM, Koff A. 1994. p27(Kip1), a cyclin-Cdk inhibitor, links transforming growth factor-β and contact inhibition to cell cycle arrest. Genes Dev. 8, 9-22. ( 10.1101/gad.8.1.9) [DOI] [PubMed] [Google Scholar]
- 21.Sherr CJ, Roberts JM. 1999. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 13, 1501-1512. ( 10.1101/gad.13.12.1501) [DOI] [PubMed] [Google Scholar]
- 22.Cheng M, Olivier P, Diehl JA, Fero M, Roussel MF, Roberts JM, Sherr CJ. 1999. The p21(Cip1) and p27(Kip1) CDK ‘inhibitors’ are essential activators of cyclin D-dependent kinases in murine fibroblasts. EMBO J. 18, 1571-1583. ( 10.1093/emboj/18.6.1571) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guiley KZ, et al. 2019. p27 allosterically activates cyclin-dependent kinase 4 and antagonizes palbociclib inhibition. Science 366, 6471. ( 10.1126/science.aaw2106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Labaer J, Garrett MD, Stevenson LF, Slingerland JM, Sandhu C, Chou HS, Fattaey A, Harlow E. 1997. New functional activities for the p21 family of CDK inhibitors. Genes Dev. 11, 847-862. ( 10.1101/gad.11.7.847) [DOI] [PubMed] [Google Scholar]
- 25.Ray A, James MK, Larochelle S, Fisher RP, Blain SW. 2009. p27Kip1 inhibits cyclin D-cyclin-dependent kinase 4 by two independent modes. Mol. Cell. Biol. 29, 986-999. ( 10.1128/MCB.00898-08) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caillot M, et al. 2020. Cyclin D1 targets hexokinase 2 to control aerobic glycolysis in myeloma cells. Oncogenesis 9, 1-3. ( 10.1038/s41389-020-00253-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hydbring P, Malumbres M, Sicinski P. 2016. Non-canonical functions of cell cycle cyclins and cyclin-dependent kinases. Nat. Rev. Mol. Cell Biol. 17, 280-292. ( 10.1038/nrm.2016.27) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang H, et al. 2017. The metabolic function of cyclin D3-CDK6 kinase in cancer cell survival. Nature 546, 426-430. ( 10.1038/nature22797) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salazar-Roa M, Malumbres M. 2017. Fueling the cell division cycle. Trends Cell Biol. 27, 69-81. ( 10.1016/j.tcb.2016.08.009) [DOI] [PubMed] [Google Scholar]
- 30.Kollmann K, et al. 2013. A kinase-independent function of CDK6 links the cell cycle to tumor angiogenesis. Cancer Cell 24, 167-181. ( 10.1016/j.ccr.2013.07.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Toogood PL, et al. 2005. Discovery of a potent and selective inhibitor of cyclin-dependent kinase 4/6. J. Med. Chem. 48, 2388-2406. ( 10.1021/jm049354h) [DOI] [PubMed] [Google Scholar]
- 32.Schade AE, Oser MG, Nicholson HE, DeCaprio JA. 2019. Cyclin D–CDK4 relieves cooperative repression of proliferation and cell cycle gene expression by DREAM and RB. Oncogene 38, 4962-4976. ( 10.1038/s41388-019-0767-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vilgelm AE, et al. 2019. MDM2 antagonists overcome intrinsic resistance to CDK4/6 inhibition by inducing p21. Sci. Transl. Med. 11, 7171. ( 10.1126/scitranslmed.aav7171) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trotter EW, Hagan IM. 2020. Release from cell cycle arrest with Cdk4/6 inhibitors generates highly synchronized cell cycle progression in human cell culture. Open Biol. 10, 200200. ( 10.1098/rsob.200200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chu I, et al. 2007. p27 phosphorylation by Src regulates inhibition of cyclin E-Cdk2. Cell 128, 281-294. ( 10.1016/j.cell.2006.11.049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grimmler M, et al. 2007. Cdk-inhibitory activity and stability of p27Kip1 are directly regulated by oncogenic tyrosine kinases. Cell 128, 269-280. ( 10.1016/j.cell.2006.11.047) [DOI] [PubMed] [Google Scholar]
- 37.Tsytlonok M, et al. 2019. Dynamic anticipation by Cdk2/Cyclin A-bound p27 mediates signal integration in cell cycle regulation. Nat. Commun. 10, 1-13. ( 10.1038/s41467-019-09446-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barr AR, Cooper S, Heldt FS, Butera F, Stoy H, Mansfeld J, Novák B, Bakal C. 2017. DNA damage during S-phase mediates the proliferation-quiescence decision in the subsequent G1 via p21 expression. Nat. Commun. 8, 14728. ( 10.1038/ncomms14728) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Swadling JB, Warnecke T, Morris KL, Barr AR. 2021. Conserved Cdk inhibitors show unique structural responses to tyrosine phosphorylation. BioRxiv, 2021.02.04.429742. ( 10.1101/2021.02.04.429742) [DOI] [PMC free article] [PubMed]
- 40.Bodnar AG, et al. 1998. Extension of life-span by introduction of telomerase into normal human cells. Science 279, 349-352. ( 10.1126/science.279.5349.349) [DOI] [PubMed] [Google Scholar]
- 41.di Nicolantonio F, et al. 2008. Replacement of normal with mutant alleles in the genome of normal human cells unveils mutation-specific drug responses. Proc. Natl Acad. Sci. USA 105, 20 864-20 869. ( 10.1073/pnas.0808757105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Libouban MAA, et al. 2017. Stable aneuploid tumors cells are more sensitive to TTK inhibition than chromosomally unstable cell lines. Oncotarget 8, 38 309-38 325. ( 10.18632/oncotarget.16213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Young RJ, et al. 2014. Loss of CDKN2A expression is a frequent event in primary invasive melanoma and correlates with sensitivity to the CDK4/6 inhibitor PD0332991 in melanoma cell lines. Pigment Cell Melanoma Res. 27, 590-600. ( 10.1111/pcmr.12228) [DOI] [PubMed] [Google Scholar]
- 44.Wiedemeyer WR, et al. 2010. Pattern of retinoblastoma pathway inactivation dictates response to CDK4/6 inhibition in GBM. Proc. Natl Acad. Sci. USA 107, 11 501-11 506. ( 10.1073/pnas.1001613107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen JY, Lin JR, Tsai FC, Meyer T. 2013. Dosage of Dyrk1a shifts cells within a p21–cyclin D1 signaling map to control the decision to enter the cell cycle. Mol. Cell 52, 87-100. ( 10.1016/j.molcel.2013.09.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang HW, Chung M, Kudo T, Meyer T. 2017. Competing memories of mitogen and p53 signalling control cell-cycle entry. Nature 549, 404-408. ( 10.1038/nature23880) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pack LR, Daigh LH, Chung M, Meyer T. 2021. Clinical CDK4/6 inhibitors induce selective and immediate dissociation of p21 from cyclin D-CDK4 to inhibit CDK2. Nat. Commun. 12, 3356. ( 10.1038/s41467-021-23612-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zerjatke T, Gak IA, Kirova D, Fuhrmann M, Daniel K, Gonciarz M, Müller D, Glauche I, Mansfeld J. 2017. Quantitative cell cycle analysis based on an endogenous all-in-one reporter for cell tracking and classification. Cell Rep. 19, 1953-1966. ( 10.1016/j.celrep.2017.05.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bornstein G, Bloom J, Sitry-Shevah D, Nakayama K, Pagano M, Hershko A. 2003. Role of the SCFSkp2 ubiquitin ligase in the degradation of p21Cip1 in S phase. J. Biol. Chem. 278, 25 752-25 757. ( 10.1074/jbc.M301774200) [DOI] [PubMed] [Google Scholar]
- 50.Heldt FS, Barr AR, Cooper S, Bakal C, Novák B. 2018. A comprehensive model for the proliferation-quiescence decision in response to endogenous DNA damage in human cells. Proc. Natl Acad. Sci. USA 115, 2532-2537. ( 10.1073/pnas.1715345115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nathans JF, Cornwell JA, Afifi MM, Paul D, Cappell SD. 2021. Cell cycle inertia underlies a bifurcation in cell fates after DNA damage. Sci. Adv. 7, eabe3882. ( 10.1126/sciadv.abe3882) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stallaert W, et al. 2021. The structure of the human cell cycle. BioRxiv, 2021.02.11.430845. ( 10.1101/2021.02.11.430845) [DOI] [Google Scholar]
- 53.Crozier L, Foy R, Mouery BL, Whitaker RH, Corno A, Spanos C, Ly T, Cook JG, Saurin AT. 2021. CDK4/6 inhibitors induce replication stress to cause long-term cell cycle withdrawal. BioRxiv, 2021.02.03.428245. ( 10.1101/2021.02.03.428245) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spencer SL, Cappell SD, Tsai F-C, Overton KW, Wang CL, Meyer T. 2013. The proliferation-quiescence decision is controlled by a bifurcation in CDK2 activity at mitotic exit. Cell 155, 369-383. ( 10.1016/j.cell.2013.08.062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lord SJ, Velle KB, Mullins RD, Fritz-Laylin LK. 2020. SuperPlots: communicating reproducibility and variability in cell biology. J. Cell Biol. 219, e202001064. ( 10.1083/jcb.202001064) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hégarat N, et al. 2020. Cyclin A triggers mitosis either via the Greatwall kinase pathway or cyclin B. EMBO J. 39, 11. ( 10.15252/embj.2020104419) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yesbolatova A, et al. 2020. The auxin-inducible degron 2 technology provides sharp degradation control in yeast, mammalian cells, and mice. Nat. Commun. 11, 5701. ( 10.1038/s41467-020-19532-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grillo M, Bott MJ, Khandke N, McGinnis JP, Miranda M, Meyyappan M, Rosfjord EC, Rabindran SK. 2006. Validation of cyclin D1/CDK4 as an anticancer drug target in MCF-7 breast cancer cells: effect of regulated overexpression of cyclin D1 and siRNA-mediated inhibition of endogenous cyclin D1 and CDK4 expression. Breast Cancer Res. Treatment 95, 185-194. ( 10.1007/s10549-005-9066-y) [DOI] [PubMed] [Google Scholar]
- 59.Simoneschi D, et al. 2021. CRL4AMBRA1 is a master regulator of D-type cyclins. Nature 592, 789-793. ( 10.1038/s41586-021-03445-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Campisi J, Medrano EE, Morreo G, Pardee AB. 1982. Restriction point control of cell growth by a labile protein: evidence for increased stability in transformed cells. Proc. Natl Acad. Sci. USA 79, 436-440. ( 10.1073/pnas.79.2.436) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Foster DA, Yellen P, Xu L, Saqcena M. 2010. Regulation of G1 cell cycle progression: distinguishing the restriction point from a nutrient-sensing cell growth checkpoint(s). Genes Cancer 1, 1124-1131. ( 10.1177/1947601910392989) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yen A, Pardee AB. 1978. Exponential 3T3 cells escape in mid-G1 from their high serum requirement. Exp. Cell Res. 116, 103-113. ( 10.1016/0014-4827(78)90068-X) [DOI] [PubMed] [Google Scholar]
- 63.Patnaik A, et al. 2016. Efficacy and safety of abemaciclib, an inhibitor of CDK4 and CDK6, for patients with breast cancer, non-small cell lung cancer, and other solid tumors. Cancer Disc. 6, 740-753. ( 10.1158/2159-8290.CD-16-0095) [DOI] [PubMed] [Google Scholar]
- 64.Wander SA, et al. 2020. The genomic landscape of intrinsic and acquired resistance to cyclin-dependent kinase 4/6 inhibitors in patients with hormone receptor-positive metastatic breast cancer. Cancer Disc. 10, 1174-1193. ( 10.1158/2159-8290.CD-19-1390) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.AbuHammad S, et al. 2019. Regulation of PRMT5-MDM4 axis is critical in the response to CDK4/6 inhibitors in melanoma. Proc. Natl Acad. Sci. USA 116, 17 990-18 000. ( 10.1073/pnas.1901323116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gottesman SRS, Somma J, Tsiperson V, Dresner L, Govindarajulu U, Patel P, Blain SW. 2019. Tyrosine phosphorylation of p27KIP1 correlates with palbociclib responsiveness in breast cancer tumor cells grown in explant culture. Mol. Cancer Res. 17, 669-675. ( 10.1158/1541-7786.MCR-18-0188) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhao B, Burgess K. 2019. PROTACs suppression of CDK4/6, crucial kinases for cell cycle regulation in cancer. Chem. Commun. 55, 2704-2707. ( 10.1039/c9cc00163h) [DOI] [PubMed] [Google Scholar]
- 68.Asghar US, et al. 2017. Single-cell dynamics determines response to CDK4/6 inhibition in triple-negative breast cancer. Clin. Cancer Res. 23, 5561-5572. ( 10.1158/1078-0432.CCR-17-0369) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cooper S, Barr AR, Glen R, Bakal C. 2017. NucliTrack: an integrated nuclei tracking application. Bioinformatics 33, 3320-3322. ( 10.1093/bioinformatics/btx404) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pennycook BR, Barr AR. 2021. Palbociclib-mediated cell cycle arrest can occur in the absence of the CDK inhibitors p21 and p27. Figshare. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Pennycook BR, Barr AR. 2021. Palbociclib-mediated cell cycle arrest can occur in the absence of the CDK inhibitors p21 and p27. Figshare. [DOI] [PMC free article] [PubMed]
Data Availability Statement
This article has no additional data. The data are provided in the electronic supplementary material [70].