Abstract
Accessibility is a key aspect for the presentation of research data. In palaeontology, new data is routinely obtained with computational techniques, such as finite-element analysis (FEA). FEA is used to calculate stress and deformation in objects when subjected to external forces. Results are displayed using contour plots in which colour information is used to convey the underlying biomechanical data. The Rainbow colour map is nearly exclusively used for these contour plots in palaeontological studies. However, numerous studies in other disciplines have shown the Rainbow map to be problematic due to uneven colour representation and its inaccessibility for those with colour vision deficiencies. Here, different colour maps were tested for their accuracy in representing values of FEA models. Differences in stress magnitudes (ΔS) and colour values (ΔE) of subsequent points from the FEA models were compared and their correlation was used as a measure of accuracy. The results confirm that the Rainbow colour map is not well suited to represent the underlying stress distribution of FEA models with other colour maps showing a higher discriminative power. As the performance of the colour maps varied with tested scenarios/stress types, it is recommended to use different colour maps for specific purposes.
Keywords: biomechanical analysis, digital visualization, fossils, three-dimensional models
1. Introduction
The last two decades have witnessed a surge in the use of computational techniques to study the anatomy and functional morphology of fossil organisms with the aim of reconstructing their palaeobiology [1–3]. Tools for the biomechanical analysis of fossils, such as finite-element analysis (FEA) [4,5], computational fluid dynamics (CFD) analysis [6,7] and multibody dynamics analysis (MDA) [8], are now routinely applied to investigate the form–function relationships of fossils. Of these, FEA has become a powerful and ubiquitous method to test hypotheses about the functional capabilities of extinct organisms, in particular for species for which no living analogues may exist.
Originally developed as an engineering technique, FEA predicts the deformation in objects with complex geometries and different materials subject to external load forces. Key to the technique is the subdivision (discretization) of the analysed object into numerous, small and geometrically simple elements connected by shared nodes, for which the deformation calculations are subsequently performed. This simplification allows a quick but generally accurate approximation to solve the problem for any given object and the calculation of biologically relevant performance measures, such as stress and strain [5]. Based on the discretization, discrete stress or strain values can be associated with each element and node in an FEA model. For the presentation of the results, these values can be reported quantitatively, for example as model averages or mean values [9,10], values of individually selected elements or sections [11,12], as stress intervals [13], or using a landmark-based approach on the deformed models [14]. However, reporting a large amount of numerical values may not intuitively convey the observed results to the reader. Therefore, it is common practice to present FEA results more qualitatively in the form of contour plots. For these colour-indexed (pseudo- or false-colour) plots, the numerical value of each element in an FEA model is represented by different colours. Such colour coding can be a powerful tool to differentiate and convey information. Although the use of different colour maps does not change the underlying results (e.g. stress and strain magnitudes), they have a substantial impact on the results' legibility and therefore accessibility of the same. This is particularly true for the use of FEA in a comparative context, which aims to identify (subtle) differences between models (e.g. species) [5].
Traditionally, and with very few exceptions [15–17], the colour scheme of choice for FEAs of palaeontological and biological specimens has been (and still is) the classic Rainbow colour map. It is based on the colours in the visible light spectrum from blue (usually lower values) via green, yellow and orange to red (usually higher values). It is one of the most common colour schemes for data visualization and the default option in many software toolkits. Despite its ubiquitous use and popularity, a number of studies in the last two decades have identified considerable problems with the Rainbow colour map [18–25]: (i) the perceived transitions between the individual colours of the Rainbow map are not uniform [25,26], with some colours (i.e. red, green) seemingly taking up a larger part of the colour map (figure 1a). This effect can simulate sharp transitions in sequential data, making small variations in the underlying data appear more important [27,28]. Similarly, yellow is the brightest colour in the Rainbow colour map. Although it is not at the extreme end of the colour map, it tends to attract the eye more than other colours in the spectrum [25,29,30]. (ii) While ordered from shorter (blue) to longer (red) wavelengths, the Rainbow map does not follow any naturally perceived order. This means that in contrast with greyscale or gradient colour maps (which can be arranged from dark to light or vice versa), there is no implicit order to the Rainbow colour map [23,26,31], making the comparison between two relative values difficult (figure 1b). (iii) Lastly, but importantly, the Rainbow colour map creates considerable accessibility problems for those with colour vision deficiencies (CVDs). Approximately 5–10% of the population may suffer from some form of CVD, such as red-green blindness (Deuteranopia), which renders data represented by the Rainbow colour map largely unreadable [32–34]. Furthermore, similar issues arise when results using the Rainbow colour map are converted to a greyscale format, such as for example for printing.
Figure 1.
Problems of the Rainbow colour scheme: (a) non-uniform distances between individual colours (adapted from [27]). (b) Lack of intuitive perceptual order. (c) Rainbow colour map as seen without and with colour vision deficiency (i.e. deuteranopia and protanopia type) and in greyscale.
Given these inherent problems with the Rainbow colour scheme, several disciplines, including oceanography [35], meteorology [36,37] and geosciences [25,38], have started to address this issue and proposed the use of alternative colour schemes. Here, different colour maps are tested and their effectiveness for the visualization of FEA results of palaeontological models is evaluated.
2. Material and methods
In order to evaluate their visual effect and accessibility, different colour maps were tested for a variety of FEA models of fossil specimens and different FEA stress measures. In addition to the traditional Rainbow map (see also [39]), nine further, established colour maps were selected (version numbers are provided where present): (a) The five sequential colour maps Batlow (7.0), Inferno, Parula, Viridis and YlGnBu [25,40–43]. Sequential colour maps vary between two colours ranging from dark to light (or vice versa) and are suitable for ordered data ranging gradually from low to high values (i.e. ratio data with an absolute zero value) [27]. (b) The three diverging colour maps Cork (7.0), Polar and Roma (7.0) [25,43]. Diverging colour maps range between two contrasting colours at either end separated by a neutral colour in the middle and are suitable for interval data that can have positive and negative values [27]. (c) As a further option, a variant of the classic Rainbow colour map known as Turbo was included in the analysis. Although Turbo similarly consists of a sequence of colours in the visible light spectrum, it has been suggested to represent a perceptually improved rainbow map with a uniform luminance [44,45]. All colour maps are non-proprietary, in some cases, versioned and available/defined via the respective references above. Not all colour maps are readily and equally available by default in all software but can be added in most cases (see also below),
Other colour maps, such as qualitative, categorical or cyclic colour maps, were not tested as these are not appropriate for FEA data. The colour maps tested here were selected following their use and popularity in different applications. However, not all of the colour maps are perceptually uniform (e.g. the difference between two colours as perceived by the human eye is proportional to the numerical distance within the given colour space). Batlow, Cork, Inferno, Roma, Viridis and YlGnBu are all perceptually uniform, whereas Parula, Polar, Rainbow and Turbo are not (see also [43]).
All colour maps used for this study consist of 24 individual colour values (definitions (order and HEX colour codes) are available in the electronic supplementary material), and all outputs presented here were generated in Abaqus and model views were saved as image files. Depending on the software, custom colour maps can be created. In this example, all colour maps were created in Abaqus via a command-line script detailing the colour components via HEX codes individually (see the script in electronic supplementary material). Alternatively, new colour maps (so-called spectra in Abaqus) can be created via a tools menu and selecting successive colours via a colour picker. This process will differ for individual software. However, specific pre-designed colour maps can be generated and accessed via online tools, such as https://colorbrewer2.org/#type=sequential&scheme=BuGn&n=3 [40].
Several FEA models of fossil specimens and different skeletal elements were used here to evaluate the perceptual effects of the tested colour maps with different three-dimensional morphologies: (a) a simplified, planar model of the mandible of the sabre-toothed cat Dinofelis cristata as used in [46] (figure 2). This model was chosen to represent a geometrically simple morphology as used for FEA models not derived from computed tomography (CT) or surface-based digitization methods [47,48]. For this model, contour plots displaying the distribution of von Mises stress were chosen as an example for ratio data. (b) A three-dimensional model of the mandible of Thrinaxodon liorhinus representing a geometrically more complex morphology compared to the model of Dinofelis and derived from CT scanning [49]. For the contour plots, tensile (positive) and compressive (negative) absolute stresses were displayed as an example for interval data. (c) A model of the skull of the therizinosaurian dinosaur Erlikosaurus andrewsi as used in [50]. In addition to the different contour plots displaying von Mises stress, the models were also displayed as perceived with deuteranopia-type CVDs. For this purpose, the images were converted accordingly using Adobe Photoshop CC 2020. (d) A model of the skull of the capitosaurian temnospondyl Parotosuchus helgolandicus [51] representing a dorsoventrally flattened skull morphology. In addition to the different contour plots displaying von Mises stress, the models were also displayed as perceived with a protanopia-type CVD. For this purpose, the images were converted accordingly using Adobe Photoshop CC 2020. (e) A model of a dorsal vertebra of the ornithischian dinosaur Stegosaurus stenops [47] representing a post-cranial skeletal element. In addition to the different contour plots displaying von Mises stress, the images of the contour plot models were also converted into greyscale using Adobe Photoshop CC (2020) (Image -> Adjustments -> Black & White and using the default setting for greyscale mode). (f) A model of the manual claw of the therizinosaurian dinosaur Nothronychus graffami [52] to illustrate the effect of colour maps against a different background colour. The boundary conditions for these models have no direct effect on the colour map interpretation. Therefore, please refer to the original publications for further details on the boundary conditions of the respective models.
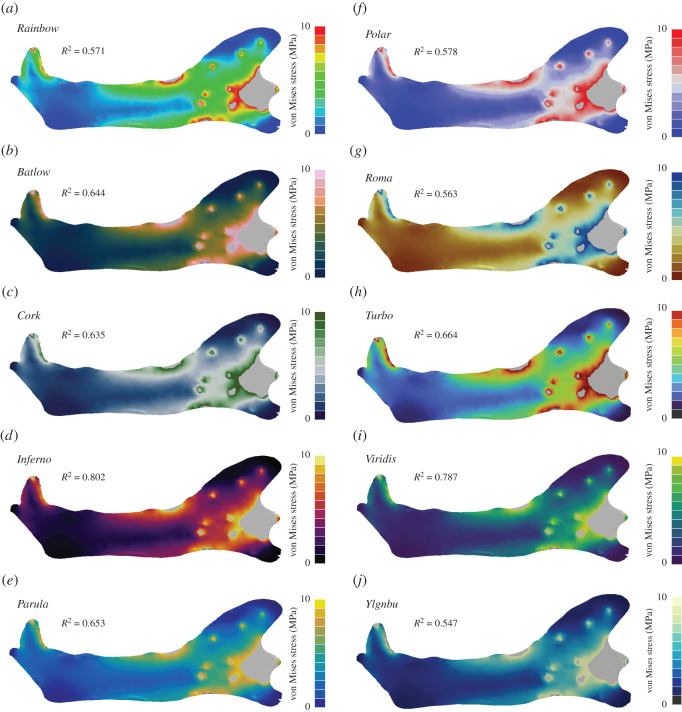
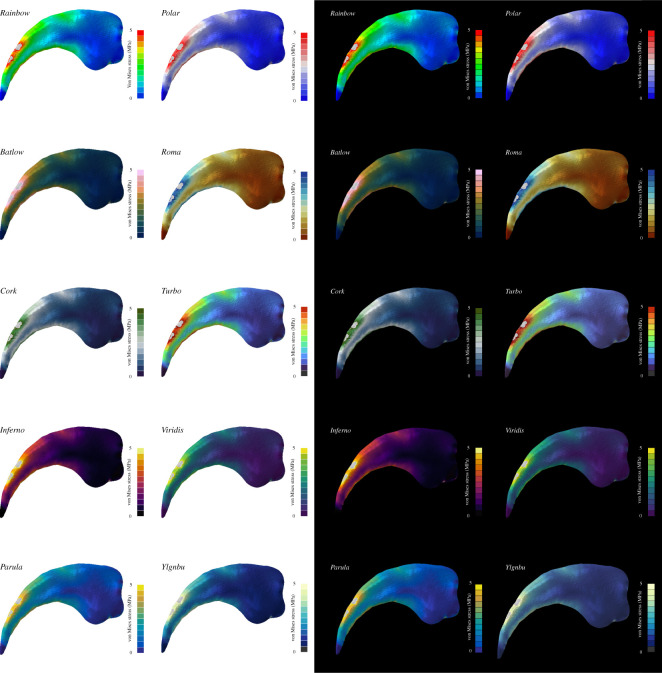
Figure 2.
Contour plots for different colour maps for Von Mises stress values are shown for the simplified planar mandible model of the sabre-tooth cat Dinofelis cristata. In addition to the standard Rainbow colour map (a), nine further colour maps were tested: Batlow (b), Cork (c), Inferno (d), Parula (e), Polar (f), Roma (g), Turbo (h), Viridis (i) and YlGnBu (j). Grey regions in the contour plots represent stress magnitudes beyond the applied scale limit. R2-values are given for each colour map (see electronic supplementary material for full correlation plots).
To quantify the discriminative power of the individual colour maps (i.e. relating individual colour values to their respective FEA stress magnitudes), the correlation between the colour maps and stress results of the FEA models was calculated. For consistency across the models, 20 points (i.e. elements) along with a line across the FEA model were selected that covered the morphology evenly. Stress magnitudes (von Mises and compressive/tensile stresses) were recorded for all sampled points. This approach follows the practice to sample a subset of elements of an FEA model [11,12,50] to quantify its biomechanical properties.
In the next step, the colour values for the sampled points for each tested colour map were recorded as RGB values. Although defining colour as RGB values is a common practice for many (web-based and digital) applications, they were specifically designed for use on monitors and do not reflect human colour perception as the RGB colour space is not uniform [53]. A solution to this problem is using the CIELAB (also known as CIE L*a*b*) colour space [54] which has been designed to be perceptually uniform. Here, the distance between two points defining individual colours is proportional to the perceptual difference between them [55,56]. Therefore, the collected RGB values were converted into CIELAB colour values. The collection of the RGB colours from images of the FEA models and subsequent conversion to CIELAB colour space was done via the convertColor function in R [57] (see electronic supplementary material). For a colour map to represent the underlying data correctly, it must reflect changes in magnitude between two sampled points accordingly. To test this correlation, the absolute difference ΔS in stress magnitude was calculated for subsequent points sampled for each model (equation (2.1)).
| 2.1 |
Similarly, the difference in colour value ΔE was determined for each colour map [53] by calculating the Euclidean distances between two subsequent points (equation (2.2))
| 2.2 |
In a final step, ΔE and ΔS were subjected to an ordinary least square regression and the R2-value was obtained as a measure for the discriminative power of the individual colour maps (see electronic supplementary material).
It should be noted that Abaqus applies a shading algorithm when displaying FEA contour plots in that it simulates an artificial light source positioned to the top left of the three-dimensional space. To avoid the effects of artificial shadows on the colour representation, all measurements were performed with the model exposed to the maximum light intensity (usually with models in left lateral or dorsal view).
3. Results
Overall, 10 different colour maps were tested for their accuracy to represent the underlying stress magnitudes of finite-element models in the form of contour plots. In addition to the default Rainbow map, nine further colour maps were tested and the R2-value was used as a measure for the correlation between stress magnitudes and colour maps.
Across the different colour maps, models, stress types and visual appearances, the R2 values range from nearly no (R2 = 0.008) to strong correlations (R2 = 0.967) (table 1). No single colour map was found to show consistently the strongest correlation for the different test settings, with rather more nuanced variations in representative performance for the different colour maps. It is noteworthy that the Rainbow colour map performed worse than most of the other colour maps.
Table 1.
R2-values for all tested colour maps stress and visual appearances. Score with the highest value italicized for each test setting.
| Batlow | Cork | Inferno | Parula | Polar | Rainbow | Roma | Turbo | Viridis | YlGnBu | |
|---|---|---|---|---|---|---|---|---|---|---|
| von Mises | 0.644 | 0.635 | 0.802 | 0.653 | 0.578 | 0.571 | 0.563 | 0.664 | 0.787 | 0.547 |
| tensile/compressive | 0.910 | 0.871 | 0.905 | 0.959 | 0.934 | 0.887 | 0.872 | 0.852 | 0.933 | 0.967 |
| deuteranopia | 0.890 | 0.696 | 0.693 | 0.460 | 0.604 | 0.504 | 0.476 | 0.117 | 0.475 | 0.511 |
| protanopia | 0.547 | 0.555 | 0.738 | 0.642 | 0.458 | 0.564 | 0.485 | 0.717 | 0.876 | 0.670 |
| greyscale | 0.476 | 0.440 | 0.578 | 0.360 | 0.348 | 0.086 | 0.098 | 0.008 | 0.306 | 0.500 |
For results in the form of ratio data, such as von Mises stress (figure 2; electronic supplementary material, figure S1), the sequential colour map Inferno produced the highest correlation (R2 = 0.802). By contrast, the commonly used Rainbow map showed only a weak correlation (R2 = 0.571), and only the colour maps Roma (R2 = 0.563) and YlGnBu (R2 = 0.547) had a weaker performance. The rainbow variant Turbo performed only moderately better than the classic Rainbow (R2 = 0.664).
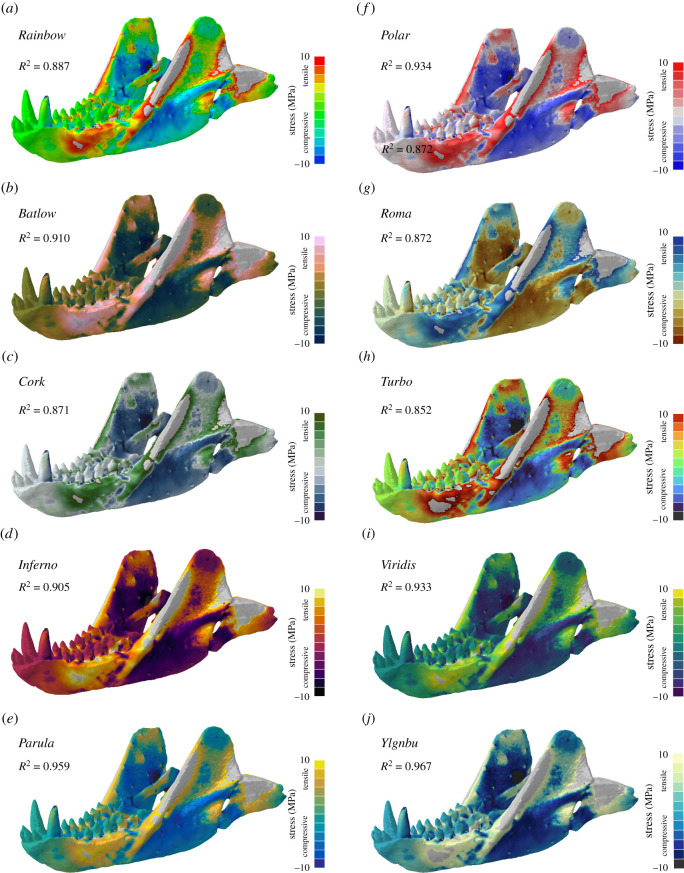
For interval data, such as compressive (i.e. negative) and tensile (i.e. positive) stresses plotted together (figure 3; electronic supplementary material, figure S2), the sequential colour maps YlGnBu (R2 = 0.967) and Parula (R2 = 0.959), as well as the diverging colour map Polar (R2 = 0.934) showed the highest correlation between stress magnitudes and colour representation. The Rainbow colour map produced a strong, although not the highest, correlation (R2 = 0.887), whereas Turbo performed worst in this scenario but with still a strong correlation (R2 = 0.852).
Figure 3.
Contour plots for different colour maps for compressive and tensile stress values shown for the mandible model of the cynodont Thrinaxodon liorhinus. In addition to the standard Rainbow colour map (a), nine further colour maps were tested: Batlow (b), Cork (c), Inferno (d), Parula (e), Polar (f), Roma (g), Turbo (h), Viridis (i) and YlGnBu (j). Grey regions in the contour plots represent stress magnitudes beyond the applied scale limit. R2-values are given for each colour map (see electronic supplementary material for full correlation plots).
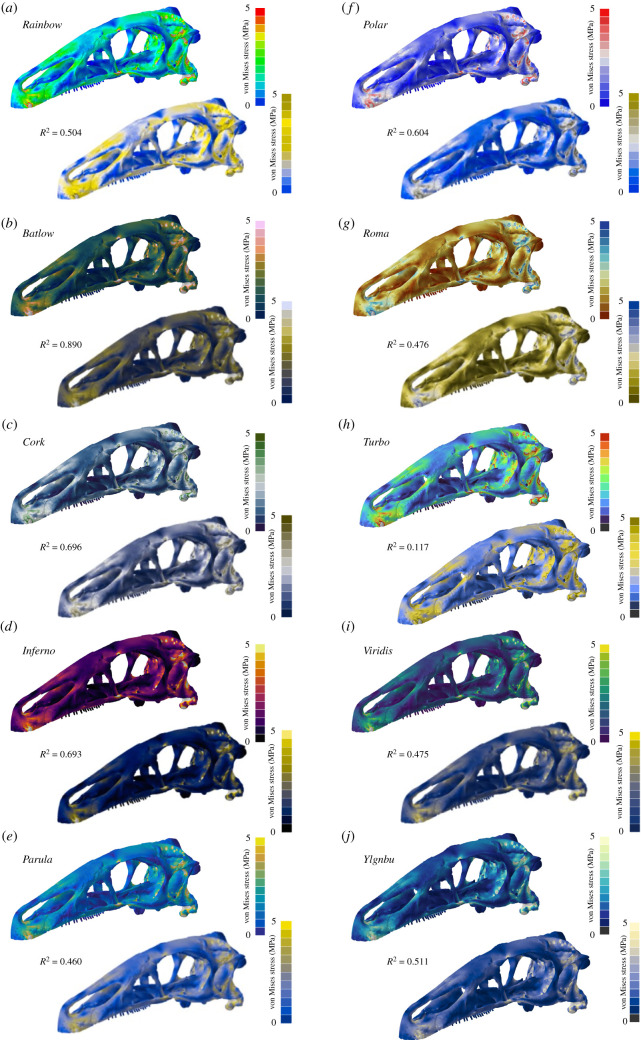
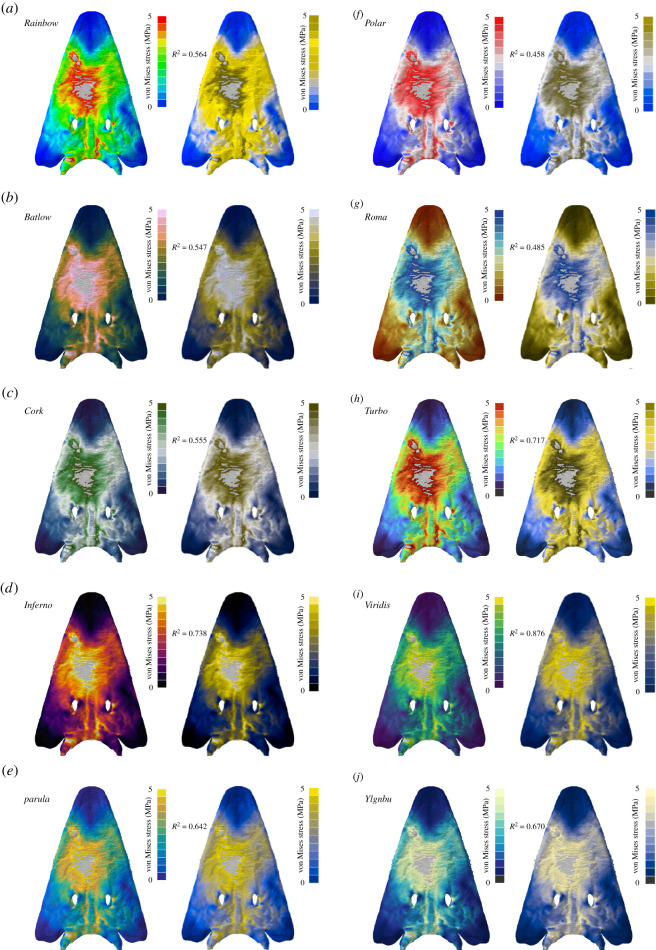
To test for the discriminative performance of the different colour maps when perceived with a CVD, contour plots were converted to deuteranopia- and protanopia-type appearances (figures 4 and 5; electronic supplementary material, figures S3 and S4). For the deuteranopia type, the sequential colour map Batlow (R2 = 0.89) and the diverging colour map Cork (R2 = 0.696) were found to represent the stress data the most accurately. Again, the Rainbow colour map (R2 = 0.504) was not able to represent the underlying stress results fully, while Turbo showed only a very weak correlation (R2 = 0.117). For the protanopia-type contour plots, Viridis (R2 = 0.876) and Inferno (R2 = 0.738) showed high correlation scores, whereas Polar (R2 = 0.458) recorded only a weak correlation.
Figure 4.
Contour plots as seen without and with deuteranopia-type colour vision deficiency for different colour maps. Von Mises stress are values shown for the cranium model of the dinosaur Erlikosaurus andrewsi. In addition to the standard Rainbow colour map (a), nine further colour maps were tested: Batlow (b), Cork (c), Inferno (d), Parula (e), Polar (f), Roma (g), Turbo (h), Viridis (i) and YlGnBu (j). Grey regions in the contour plots represent stress magnitudes beyond the applied scale limit. R2-values are given for each colour map (see electronic supplementary material for full correlation plots).
Figure 5.
Contour plots as seen without and with protanopia-type colour vision deficiency for different colour maps. Von Mises stress values are shown for the cranium model of the capitosaurian temnospondyl Parotosuchus helgolandicus. In addition to the standard Rainbow colour map (a), nine further colour maps were tested: Batlow (b), Cork (c), Inferno (d), Parula (e), Polar (f), Roma (g), Turbo (h), Viridis (i) and YlGnBu (j). Grey regions in the contour plots represent stress magnitudes beyond the applied scale limit. R2-values are given for each colour map (see electronic supplementary material for full correlation plots).
In a final analysis, contour plots were converted to greyscale and the discriminative performance of the colour maps was tested (figure 6; electronic supplementary material, figure S5). In this scenario, all colour maps produced only a moderate to no correlation (0.578 < R2 < 0.008). The best performance was found for Inferno (R2 = 0.578); Rainbow, Roma and Turbo showed the least correlation (R2 < 0.098).
Figure 6.
Contour plots as seen in full colour and greyscale for different colour maps. Von Mises stress values are shown for a vertebra of the ornithischian dinosaur Stegosaurus stenops. In addition to the standard Rainbow colour map (a), nine further colour maps were tested: Batlow (b), Cork (c), Inferno (d), Parula (e), Polar (f), Roma (g), Turbo (h), Viridis (i) and YlGnBu (j). Grey regions in the contour plots represent stress magnitudes beyond the applied scale limit. R2-values are given for each colour map (see electronic supplementary material for full correlation plots).
4. Discussion
The Rainbow colour map has been a ubiquitous tool in data visualization for decades [25,37]. Engineering techniques such as FEA, which has increasingly been used in palaeontological studies over the last 20 years, are no exception. Results from FEAs are routinely visualized in the form of contour plots using the Rainbow colour map. Contour plots typically display von Mises stress, a common measure to evaluate the stability of a model under loading conditions. However, as shown by the results from this study, the Rainbow colour map correlates only poorly with the underlying von Mises stress data (table 1 and figure 2), and its discriminative power is equally poor when perceived with different types of CVDs (figures 4 and 5). This should not come as a surprise as the Rainbow colour map has been considered problematic and misleading in other disciplines [18,19,22,23–25].
Other colour maps tested here performed considerably better. However, no one colour map was found to be optimally suited for all types of stress and visual perception. For interval-type stresses, such as compressive and tensile stresses plotted on the same model, the Rainbow colour map showed a high correlation (table 1, figure 3) similar to or even better than the diverging colour maps in this study. Interestingly, diverging colour maps did not necessarily perform better for interval data, whereas sequential colour maps were not always found to show the best correlation for ratio data (i.e. von Mises stress). Inferno, Batlow and Parula generally showed the highest discriminative power, but not consistently so (table 1). It is noteworthy that differences in the performance were recorded when colour maps were tested in CVD settings. The same colour maps (Inferno, Batlow and Parula, and to a lesser degree Polar and Viridis) represented the underlying stress values reasonably well despite the reduced colour information. However, this means that a single colour map cannot be used as a silver bullet to perform equally well under all conditions. Similar to the alternative text describing figures, the second set of contour plots with a different colour map could be provided in the electronic supplementary material accessible to those with CVD. More generally, the use of specific colour maps may have to be decided on a case to case basis using custom-made or existing colour maps (see, for example, [38] for available colour maps). For interval-type data, other considerations than the discriminative power (expressed as the R2 value here) may need to be considered. For such data, the central zero value can be an important identifier of stress-free regions in the model, which can be recognized more easily when diverging colour maps are used.
In this context, it should be noted that the correlation analysis used here to discriminate stress/colour changes is not perfect. Human colour perception is not uniform, often subjective and dependent on other factors such as age and individual variation and as such does not correspond to Euclidean distances in colour space [58]. The CIELAB colour space is an attempt to replicate human colour differentiation. As the correlation analysis only considers absolute changes along with a trajectory, the analysis may not record the exact correlation when non-monotonic changes on the stress scale are associated with changes in different directions in the CIELAB space. However, this is less likely to be a problem for the perceptually based colour maps.
The choice of an appropriate colour map may further depend on the nature of the results of an FEA. Models spanning a wide range of stress magnitudes, but with an uneven distribution of values will be biased towards certain regions of the colour map. This situation could result in a lower resolution of stress (and thereby colour) values towards the lower end of the colour map to encompass the full range of stress magnitudes present. Sequential colour maps will be a better option in such cases as their colour gradient is expressed along with the whole range of the colour map in comparison to divergent colour maps.
It is important to note that CVD is only one form of visual impairment and of course further improvements for accessibility should be aimed for when considering the presentation of results from FEA (and other analyses more broadly). The Web Content Accessibility Guidelines (WCAG) [59] provide further recommendations to improve accessibility, including appropriate contrast ratios between colours to allow their distinction. For example, Cork, Polar and Roma have high contrast ratios, whereas Inferno and Viridis have poorer contrast ratios. These ratios are, of course, lower if subsequent colours along with the gradient were to be tested, not just extreme and mid-point values. However, this goes to show that not only the uniform sequence of colours but also the contrast between them plays a role in making contour plots accessible.
This situation is further complicated in that FEA contour plots cannot be regarded in isolation but need to be considered in the context of background and environmental settings. In the simplest of cases, this could mean that the choice of background colour can influence the readability of the contour plots [25,60,61] (figure 7). Especially colour maps with a large amount of dark components can become invisible against a black background. Colourmaps with strong contrast and luminosity (e.g. Parula, Polar) can work well in such a case. For the presentation on a white background, colour maps with a decreasing chroma (=colour intensity), such as Batlow, Inferno and, in particular, YlGnBu are more appropriate to convey the results [25,61,62].
Figure 7.
Contour plots are depicted in the context of different background colours for the same model and colour maps. von Mises stress values are shown for a manual claw of the therizinosaurian dinosaur Nothronychus graffami. Grey regions in the contour plots represent stress magnitudes beyond the applied scale limit.
Within a digital, three-dimensional environment pseudo-colouring creates a further difficulty as the choice of colours interact with the shading and perception of spatial cues [24]. Properties such as the number, direction and intensity of light sources, specularity (i.e. reflectiveness of a surface) and other settings can have an impact on the appearance of colour maps as well. Further, different devices and display technologies display colours variably and for consistent perception colour calibration would have to be performed first.
Most FEA software allows turning off shading effects. However, this could possibly result in a reduced perception of the model morphology, especially for flattened surfaces with low topography (e.g. figure 5). Although not tested here, it should further be taken into consideration that different FEA software packages may use slightly different variations/colour definitions of the Rainbow colour map, further exacerbating comparisons between outputs from different software.
The eye-catching quality of the Rainbow colour map with its high luminance and contrast is likely the reason for its continued prevalence despite its problems with data distortion. Different reasons have been discussed in the past [24,25] for why the Rainbow colour map is still the visualization tool of choice for many studies and applications. For finite-element models, this has likely historic reasons and it is the default colour map in most software. Furthermore, the colour distribution of the Rainbow map has a very strong signalling function and communicative power: cold colours (i.e. blue) are associated with no or low stresses, whereas warm colours (i.e. yellow, red) indicate high-stress magnitudes. For von Mises stress, high magnitudes indicate possible material failure and an association with a colour such as red which is commonly used to convey danger is intuitive [63]. However, this concept can also be conveyed with other colour maps such as Inferno.
A recent study has used a variety of colour maps to display the results from FEAs [64]. Similarly, for palaeontological studies using other engineering tools, different colour maps have started to appear in publications. CFD, an engineering technique to simulate fluid flow within or around objects, uses a similar approach to FEA to represent data with pseudo-colour plots [7]. Although the Rainbow colour map is routinely used to visualize CFD results, different colour maps have been used recently in some studies [65].
5. Conclusion
Results from this study demonstrate that the Rainbow colour map is not well suited to represent the underlying stress distribution of FEA models. Although most of the other colour maps tested here showed a higher discriminative power, no single colour map was found to perform consistently well throughout all scenarios and for all stress types. It is therefore recommended that different colour maps without data distortion are used to present results. This could mean using different colour maps for ratio (e.g. von Mises stress) and interval data (e.g. compressive and tensile stresses). Alternatively, the second set of contour plots with a different colour map could be provided in the electronic supplementary material to increase accessibility.
The perception of colour is highly dependent on multiple factors, including display devices, colour standards for display and printing, and differences in the human visual apparatus. A variety of different colour maps displayed on different models of palaeontological specimens have been presented here. It is hoped that the reader will use these examples alongside the quantitative evaluation as guidance for their applications and studies. However, the tested colour maps in this study are far from exhaustive and a variety of tools exist to access pre-designed colourmaps (see [25], Box 2).
Supplementary Material
Acknowledgements
Emma Dunne and Sarah Greene kindly provided feedback on the draft manuscript. Fabio Crameri, Melisa Morales-Garcia, Eric Snively, Claus Wilke and Achim Zeileis are thanked for constructive comments and helpful suggestions improving earlier versions of the manuscript.
Ethics
No ethical issues arose in the course of this study.
Data accessibility
Raw measurements and code are included in the electronic supplementary material. FEA results files are available here: https://figshare.com/articles/dataset/FEA_models_from_True_colours_or_red_herrings_-_Colour_maps_for_finite_element_analysis_in_palaeontological_studies_and_how_they_can_enhance_interpretation_and_accessibility_/14905104. The data are provided in the electronic supplementary material [66].
Competing interests
I have no competing interests.
Funding
No funding source to report.
References
- 1.Cunningham JA, Rahman IA, Lautenschlager S, Rayfield EJ, Donoghue PC. 2014. A virtual world of paleontology. Trends Ecol. Evol. 29, 347-357. ( 10.1016/j.tree.2014.04.004) [DOI] [PubMed] [Google Scholar]
- 2.Sutton M, Rahman I, Garwood R. 2014. Techniques for virtual palaeontology. New York, NY: John Wiley Sons. [Google Scholar]
- 3.Lautenschlager S. 2016. Reconstructing the past: methods and techniques for the digital restoration of fossils. R. Soc. Open Sci. 3, 160342. ( 10.1098/rsos.160342) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rayfield EJ. 2007. Finite element analysis and understanding the biomechanics and evolution of living and fossil organisms. Annu. Rev. Earth Planetary Sci. 35, 541-576. ( 10.1146/annurev.earth.35.031306.140104) [DOI] [Google Scholar]
- 5.Bright JA. 2014. A review of paleontological finite element models and their validity. J. Paleontol. 88, 760-769. ( 10.1666/13-090) [DOI] [Google Scholar]
- 6.Bourke JM, Ruger Porter WM, Ridgely RC, Lyson TR, Schachner ER, Bell PR, Witmer LM. 2014. Breathing life into dinosaurs: tackling challenges of soft-tissue restoration and nasal airflow in extinct species. Anatomical Rec. 297, 2148-2186. ( 10.1002/ar.23046) [DOI] [PubMed] [Google Scholar]
- 7.Rahman IA. 2017. Computational fluid dynamics as a tool for testing functional and ecological hypotheses in fossil taxa. Palaeontology 60, 451-459. ( 10.1111/pala.12295) [DOI] [Google Scholar]
- 8.Lautenschlager S. 2020. Multibody dynamics analysis (MDA) as a numerical modelling tool to reconstruct the function and palaeobiology of extinct organisms. Palaeontology 63, 703-715. ( 10.1111/pala.12501) [DOI] [Google Scholar]
- 9.Farke AA. 2008. Frontal sinuses and head-butting in goats: a finite element analysis. J. Exp. Biol. 211, 3085-3094. ( 10.1242/jeb.019042) [DOI] [PubMed] [Google Scholar]
- 10.Lautenschlager S. 2017. Functional niche partitioning in Therizinosauria provides new insights into the evolution of theropod herbivory. Palaeontology 60, 375-387. ( 10.1111/pala.12289) [DOI] [Google Scholar]
- 11.Piras P, et al. 2015. Digging adaptation in insectivorous subterranean eutherians. The enigma of Mesoscalops montanensis unveiled by geometric morphometrics and finite element analysis. J. Morphol. 276, 1157-1171. ( 10.1002/jmor.20405) [DOI] [PubMed] [Google Scholar]
- 12.Attard MR, Wilson LA, Worthy TH, Scofield P, Johnston P, Parr WC, Wroe S. 2016. Moa diet fits the bill: virtual reconstruction incorporating mummified remains and prediction of biomechanical performance in avian giants. Proc. R. Soc. B 283, 20152043. ( 10.1098/rspb.2015.2043) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marcé-Nogué J, De Esteban-Trivigno S, Püschel TA, Fortuny J. 2017. The intervals method: a new approach to analyse finite element outputs using multivariate statistics. PeerJ 5, e3793. ( 10.7717/peerj.3793) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lautenschlager S, Brassey CA, Button DJ, Barrett PM. 2016. Decoupled form and function in disparate herbivorous dinosaur clades. Sci. Rep. 6, 1-10. ( 10.1038/srep26495) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Snively E, Russell A. 2002. The tyrannosaurid metatarsus: bone strain and inferred ligament function. Senckenbergiana Lethaea 82, 35. ( 10.1007/BF03043771) [DOI] [Google Scholar]
- 16.Kleinteich T, Maddin HC, Herzen J, Beckmann F, Summers AP. 2012. Is solid always best? Cranial performance in solid and fenestrated caecilian skulls. J. Exp. Biol. 215, 833-844. ( 10.1242/jeb.065979) [DOI] [PubMed] [Google Scholar]
- 17.van der Meijden A, Kleinteich T, Coelho P. 2012. Packing a pinch: functional implications of chela shapes in scorpions using finite element analysis. J. Anat. 220, 423-434. ( 10.1111/j.1469-7580.2012.01485.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herman GT, Levkowitz H. 1992. Color scales for image data. Comput. Graphics Appl. 12, 72-80. ( 10.1109/38.135915) [DOI] [Google Scholar]
- 19.Rogowitz BE, Treinish LA, Bryson S. 1996. How not to lie with visualization. Comput. Phys. 10, 268-273. ( 10.1063/1.4822401) [DOI] [Google Scholar]
- 20.Brewer CA. 1997. Spectral schemes: controversial color use on maps. Cartogr. Geogr. Information Syst. 24, 203-220. ( 10.1559/152304097782439231) [DOI] [Google Scholar]
- 21.Rogowitz BE, Treinish LA. 1998. Data visualization: the end of the rainbow. IEEE Spectr. 35, 52-59. ( 10.1109/6.736450) [DOI] [Google Scholar]
- 22.Light A, Bartlein PJ. 2004. The end of the rainbow? Color schemes for improved data graphics. Eos Trans. Am. Geophys. Union 85, 385-391. ( 10.1029/2004EO400002) [DOI] [Google Scholar]
- 23.Borland D, Taylor RM. 2007. Rainbow color map (still) considered harmful. IEEE Comput. Graph. Appl. 27, 14-17. ( 10.1109/MCG.2007.323435) [DOI] [PubMed] [Google Scholar]
- 24.Moreland K. 2016. Why we use bad color maps and what you can do about it. Electr. Imaging 2016, 1-6. ( 10.2352/ISSN.2470-1173.2016.16.HVEI-133) [DOI] [Google Scholar]
- 25.Crameri F, Shephard GE, Heron PJ. 2020. The misuse of colour in science communication. Nat. Commun. 11, 1-10. ( 10.1038/s41467-019-13993-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong B. 2010. Points of view: color coding. Nat. Methods 7, 573. ( 10.1038/nmeth0810-573) [DOI] [PubMed] [Google Scholar]
- 27.Hattab G, Rhyne TM, Heider D. 2020. Ten simple rules to colorize biological data visualization. PLoS Comput. Biol. 16, e1008259. ( 10.1371/journal.pcbi.1008259) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rhyne TM. 2017. Applying color theory to digital media and visualization. In Proc. of the 2017 CHI Conf. Extended Abstracts on Human Factors in Computing Systems, pp. 1264-1267. [Google Scholar]
- 29.Alexander KR, Shansky MS. 1976. Influence of hue, value, and chroma on the perceived heaviness of colors. Percept. Psychophys. 19, 72-74. ( 10.3758/BF03199388) [DOI] [Google Scholar]
- 30.Wolfe JM, Horowitz TS. 2017. Five factors that guide attention in visual search. Nat. Hum. Behav. 1, 1-8. ( 10.1038/s41562-017-0058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ware C. 2019. Information visualization: perception for design. San Francisco, CA: Morgan Kaufmann. [Google Scholar]
- 32.Sharpe LT, Stockman A, Jägle H, Nathans J. 1999. Opsin genes, cone photopigments, color vision, and color blindness. Color Vision 3-51. [Google Scholar]
- 33.Simunovic MP. 2010. Colour vision deficiency. Eye 24, 747-755. ( 10.1038/eye.2009.251) [DOI] [PubMed] [Google Scholar]
- 34.Wong B. 2011. Color blindness. Nat. Methods 8, 441-442. ( 10.1038/nmeth.1618) [DOI] [PubMed] [Google Scholar]
- 35.Samsel F, Petersen M, Geld T, Abram G, Wendelberger J, Ahrens J. 2015. Colormaps that improve perception of high-resolution ocean data. In Proc. of the 33rd Annual ACM Conf. Extended Abstracts on Human Factors in Computing Systems, pp. 703-710. [Google Scholar]
- 36.Sherman-Morris K, Antonelli KB, Williams CC. 2015. Measuring the effectiveness of the graphical communication of hurricane storm surge threat. Weather Clim. Soc. 7, 69-82. ( 10.1175/WCAS-D-13-00073.1) [DOI] [Google Scholar]
- 37.Stauffer R, Mayr GJ, Dabernig M, Zeileis A. 2015. Somewhere over the rainbow: how to make effective use of colors in meteorological visualizations. Bull. Am. Meteorol. Soc. 96, 203-216. ( 10.1175/BAMS-D-13-00155.1) [DOI] [Google Scholar]
- 38.Crameri F. 2018. Geodynamic diagnostics scientific visualisation and StagLab 3.0. Geoscient. Model Dev. 11, 2541-2562. ( 10.5194/gmd-11-2541-2018) [DOI] [Google Scholar]
- 39.Quinan PS, Padilla LM, Creem-Regehr SH, Meyer M. 2019. Examining implicit discretization in spectral schemes. Comput. Graphics Forum 38, 363-374. ( 10.1111/cgf.13695) [DOI] [Google Scholar]
- 40.Harrower M, Brewer CA. 2003. ColorBrewer.org: an online tool for selecting colour schemes for maps. Cartographic J. 40, 27-37. ( 10.1179/000870403235002042) [DOI] [Google Scholar]
- 41.Van der Walt S, Smith N. 2015. A better default colormap for Matplotlib SciPy 2015.
- 42.Van der Walt S, Smith N. 2020. MPL colour maps https://bids.github.io/colormap.
- 43.Crameri F. 2020. Scientific colour maps http://www.fabiocrameri.ch/colourmaps.
- 44.Mikhailov A. 2019. Turbo, an improved rainbow colormap for visualization. Google AI Blog. See https://ai.googleblog.com/2019/08/turbo-improved-rainbow-colormap-for.html (accessed 05.06.2021).
- 45.Reda K, Szafir DA. 2021. Rainbows revisited: modeling effective colormap design for graphical inference. IEEE Trans. Vis. Comput. Graph 27, 1032-1042. ( 10.1109/TVCG.2020.3030439) [DOI] [PubMed] [Google Scholar]
- 46.Lautenschlager S, Figueirido B, Cashmore DD, Bendel EM, Stubbs TL. 2020. Morphological convergence obscures functional diversity in sabre-toothed carnivores. Proc. R. Soc. B 287, 20201818. ( 10.1098/rspb.2020.1818) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rahman IA, Lautenschlager S. 2017. Applications of three-dimensional box modeling to paleontological functional analysis. Paleontol. Soc. Papers 22, 119-132. [Google Scholar]
- 48.Morales-García NM, Burgess TD, Hill JJ, Gill PG, Rayfield EJ. 2019. The use of extruded finite-element models as a novel alternative to tomography-based models: a case study using early mammal jaws. J. R. Soc. Interface 16, 20190674. ( 10.1098/rsif.2019.0674) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lautenschlager S, Gill PG, Luo ZX, Fagan MJ, Rayfield EJ. 2018. The role of miniaturization in the evolution of the mammalian jaw and middle ear. Nature 561, 533-537. ( 10.1038/s41586-018-0521-4) [DOI] [PubMed] [Google Scholar]
- 50.Lautenschlager S, Witmer LM, Altangerel P, Rayfield EJ. 2013. Edentulism beaks and biomechanical innovations in the evolution of theropod dinosaurs. Proc. Natl Acad. Sci. USA 110, 20 657-20 662. ( 10.1073/pnas.1310711110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lautenschlager S, Witzmann F, Werneburg I. 2016. Palate anatomy and morphofunctional aspects of interpterygoid vacuities in temnospondyl cranial evolution. Sci. Nat. 103, 79. ( 10.1007/s00114-016-1402-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lautenschlager S. 2014. Morphological and functional diversity in therizinosaur claws and the implications for theropod claw evolution. Proc. R. Soc. B 281, 20140497. ( 10.1098/rspb.2014.0497) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mokrzycki WS, Tatol M. 2011. Colour differenceΔ E-A survey. Mach. Graphics Vision 20, 383-411. [Google Scholar]
- 54.CIE Commission Intertnational de L'Eclairage. 1977. CIE recommendations on uniform color spaces color-difference equations and metric color terms. Color Res. Appl. 2, 5-6. ( 10.1002/j.1520-6378.1977.tb00102.x) [DOI] [Google Scholar]
- 55.Silva S, Santos BS, Madeira J. 2011. Using color in visualization: a survey. Comput. Graphics 35, 320-333. ( 10.1016/j.cag.2010.11.015) [DOI] [Google Scholar]
- 56.Kovesi P. 2015. Good colour maps: how to design them. arXiv preprint arXiv:1509.03700.
- 57.R Core Team. 2020. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. See https://www.R-project.org/. [Google Scholar]
- 58.Gravesen J. 2015. The metric of colour space. Graphical Models 82, 77-86. ( 10.1016/j.gmod.2015.06.005) [DOI] [Google Scholar]
- 59.Caldwell B, Cooper M, Reid LG, Vanderheiden G, Chisholm W, Slatin J, White J. 2008. Web content accessibility guidelines (WCAG) 2.0. WWW Consortium (W3C) 290, 1-34. [Google Scholar]
- 60.Schloss KB, Gramazio CC, Silverman AT, Parker ML, Wang AS. 2018. Mapping color to meaning in colormap data visualizations. IEEE Trans. Vis. Comput. Graph 25, 810-819. ( 10.1109/TVCG.2018.2865147) [DOI] [PubMed] [Google Scholar]
- 61.Sibrel SC, Rathore R, Lessard L, Schloss KB. 2020. The relation between color and spatial structure for interpreting colormap data visualizations. J. Vis. 20, 7. ( 10.1167/jov.20.12.7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zeileis A, Fisher JC, Hornik K, Ihaka R, McWhite CD, Murrell P, Stauffer R, Wilke CO. 2019. colorspace: a toolbox for manipulating and assessing colors and palettes. arXiv preprint arXiv 1903.06490.
- 63.Mayhorn CB, Wogalter MS, Bell JL, Shaver EF. 2004. What does code red mean? Ergonomics Design 12, 12-14. ( 10.1177/106480460401200404) [DOI] [Google Scholar]
- 64.Dutel H, Gröning F, Sharp AC, Watson PJ, Herrel A, Ross CF, Jones ME, Evans SE, Fagan MJ. 2021. Comparative cranial biomechanics in two lizard species: impact of variation in cranial design. J. Exp. Biol. 224, jeb234831. ( 10.1242/jeb.234831) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ferrón HG, Martínez-Pérez C, Rahman IA, Selles de Lucas V, Botella H, Donoghue PC. 2021. Functional assessment of morphological homoplasy in stem-gnathostomes. Proc. R. Soc. B 288, 20202719. ( 10.1098/rspb.2020.2719) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lautenschlager S. 2021. True colours or red herrings?: colour maps for finite-element analysis in palaeontological studies to enhance interpretation and accessibility. Figshare. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Lautenschlager S. 2021. True colours or red herrings?: colour maps for finite-element analysis in palaeontological studies to enhance interpretation and accessibility. Figshare. [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Raw measurements and code are included in the electronic supplementary material. FEA results files are available here: https://figshare.com/articles/dataset/FEA_models_from_True_colours_or_red_herrings_-_Colour_maps_for_finite_element_analysis_in_palaeontological_studies_and_how_they_can_enhance_interpretation_and_accessibility_/14905104. The data are provided in the electronic supplementary material [66].