Abstract
The etonogestrel implant is the most effective contraceptive available. We report a case of ectopic pregnancy in a woman who had an etonogestrel implant inserted 15 months ago and the effective concentration of the implant was within normal limits.
Keywords: pregnancy, contraception, obstetrics and gynaecology
Background
The etonogestrel implant is a subdermal, single-rod, progestin-only contraceptive implant. It contains 68 mg etonogestrel, the biologically active metabolite of desogestrel.1
The implant is reported to maintain contraceptive efficacy for 36 months and, according to the most recent literature, is the most effective contraceptive available.2 Pregnancy while using the etonogestrel implant is rare and ectopic pregnancy (EP) even more so.3
We report a case of EP in a woman while using the etonogestrel implant.
Case presentation
A 29-year-old woman, gravida 6 para 4, who had an etonogestrel contraceptive implant inserted 15 months ago in our hospital, after the patient’s last delivery. The implant was correctly placed and was easily palpable in her left arm.
With the exception of being a 10 cigarette-per-day smoker, the patient had no relevant personal history, including obesity, history of pelvic inflammatory disease, surgeries and medication, or alternative therapy. All pregnancies had been conceived spontaneously and had resulted in four vaginal deliveries and a miscarriage.
The patient was admitted to the emergency department of a tertiary hospital—Hospital Professor Doutor Fernando Fonseca (district of Lisbon, Portugal)—for abdominal pain. The pain was more severe in the left iliac fossa, having started several hours before.
A clinical examination was performed which revealed a small amount of vaginal blood and severe pain on palpation of the left adnexal area. The patient was haemodynamically stable. The pelvic ultrasound showed the uterine cavity filled with 22 mm of blood and an image suggestive of a pseudo-sac. A 17 mm embryo (crown-rump length compatible with 8 weeks of gestation) with cardiac activity and surrounded by moderate haematoma was visualised in the left adnexa (figure 1). The serum beta-human chorionic gonadotropin level was 17.838 IU/L.
Figure 1.
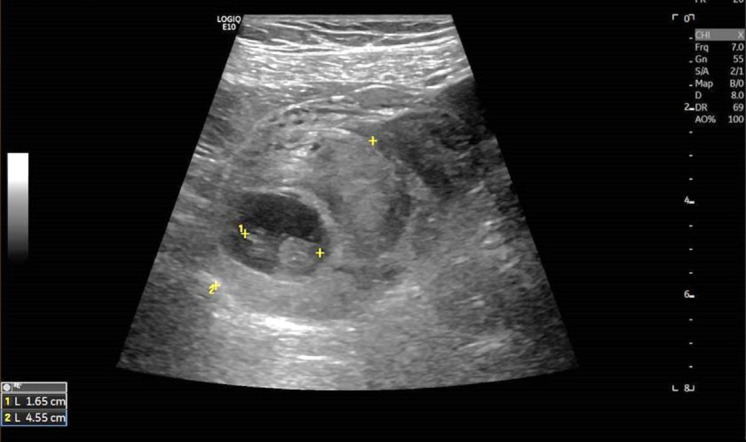
Pelvic ultrasound showed a 17 mm embryo surrounded by haematoma on the left adnexa.
The patient underwent surgical treatment laparotomy in which it was identified that the left tube was markedly dilated in the distal two-thirds portion of its length, with a rupture through which blood clots and spontaneous trophoblast emerged. A left salpingectomy was performed.
Histological examination confirmed the tubal pregnancy.
Subsequently, serum measurement of etonogestrel was performed in conjunction with Merck Sharp & Dohme (in Columbia University Medical Center – Clinical Pharmacology and Toxicology Laboratory; New York, USA) whose value was 190.7 pg/mL (reference range for effective etonogestrel implant: 150–800 pg/mL, according to this laboratory).
Discussion
The etonogestrel implant is a subdermal, single-rod, progestin-only contraceptive implant. It contains 68 mg etonogestrel, the biologically active metabolite of desogestrel. The mechanism of action is based on inhibition of ovulation, thickening the cervical mucus and endometrial changes causing unfavourable for blastocyst implantation.1 2 Etonogestrel implants are valid for 36 months and the release rate varies over time. At the end of the first year of use, the mean concentration of etonogestrel is 200 pg/mL (150–261 pg/mL) and by the end of the third year, it is 156 pg/mL (111–202 pg/mL).4 To suppress ovulation, a plasma etonogestrel level of at least 90 pg/mL is required.1
According to the most recent literature, the etonogestrel implant is the most effective contraceptive available.1 Pregnancy with the etonogestrel implant is rare and EP is even more so. The absolute incidence of EP with the etonogestrel implant is very low, amounting to 0.002 per 100 implants sold.3
Most cases of pregnancy resulted from non-detection or non-insertion of the implant at the time of intended insertion. Others occurred from conception prior to insertion and drug interaction with enzyme inducers and not due to a primary failure of the method.2
There are only five reported cases in the literature of EP with the etonogestrel implant3–7 and three of them due to primary failure of the etonogestrel implant.3 5 6 Mansour and Bouquier described cases that the patients had no risk factor, while Olowu reported a patient with a history of EP. The other two cases the EP probably occurred due to either enzyme-inducing medication (rifampicin and sertraline).4 7
We report a case of EP in a woman who had the etonogestrel implant inserted 15 months ago and the only risk factor for EP was that she was a smoker. The concentration of serum etonogestrel was within the limits needed to suppress ovulation. The report of this case is not intended to discredit the contraceptive efficacy of the etonogestrel implant, but to allow clinicians to be aware of the possibility of EP with the etonogestrel implant, since it is a potentially life-threatening condition.
Learning points.
The etonogestrel implant is the most effective contraceptive available.
The absolute incidence of ectopic pregnancy (EP) with the etonogestrel implant is very low, amounting to 0.002 per 100 implants sold.
Pregnancy and EP should still be considered in patients with an etonogestrel implant and pelvic pain.
Footnotes
Contributors: MLR managed the patient during admission and subsequent follow-up. MLR and RB wrote the article. EL contributed to the interpretation of the data, revision and final approval of the manuscript. All authors read and approved the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s)
References
- 1.Lazorwitz A, Aquilante CL, Sheeder J, et al. Relationship between patient characteristics and serum etonogestrel concentrations in contraceptive implant users. Contraception 2019;100:37–41. 10.1016/j.contraception.2019.03.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Callahan R, Yacobson I, Halpern V, et al. Ectopic pregnancy with use of progestin-only injectables and contraceptive implants: a systematic review. Contraception 2015;92:514–22. 10.1016/j.contraception.2015.08.016 [DOI] [PubMed] [Google Scholar]
- 3.Bouquier J, Fulda V, Bats A-S, et al. A life-threatening ectopic pregnancy with etonogestrel implant. Contraception 2012;85:215–7. 10.1016/j.contraception.2011.07.008 [DOI] [PubMed] [Google Scholar]
- 4.Henderson PMN, Gillespie MD. Ectopic pregnancy with Implanon. J Fam Plann Reprod Health Care 2007;33:125–6. 10.1783/147118907780254123 [DOI] [PubMed] [Google Scholar]
- 5.Mansour M, Louis-Sylvestre C, Paniel B-J. Grossesse extra-utérine survenue sous contraception par implant d’étonogestrel (Implanon®) : premier cas. J Gynecol Obstet Biol Reprod 2005;34:608–9. 10.1016/S0368-2315(05)82887-X [DOI] [PubMed] [Google Scholar]
- 6.Olowu O, Karunaratne J, Odejinmi F. Ectopic pregnancy with Implanon® as a method of contraception in a woman with a previous ectopic pregnancy - case report. Eur J Contracept Reprod Health Care 2011;16:229–31. 10.3109/13625187.2011.556278 [DOI] [PubMed] [Google Scholar]
- 7.Patni S, Ebden P, Kevelighan E, et al. Ectopic pregnancy with Implanon. J Fam Plann Reprod Health Care 2006;32:115. 10.1783/147118906776276404 [DOI] [PubMed] [Google Scholar]


