Abstract
Introduction
White matter (WM) energy supply is crucial for axonal function and myelin maintenance. An exogenous source of ketones, the brain's alternative fuel to glucose, bypasses the brain's glucose‐specific energy deficit and improves cognitive outcomes in mild cognitive impairment (MCI). How an additional supply of ketones affects glucose or ketone uptake in specific WM fascicles in MCI has not previously been reported.
Methods
This 6‐month interventional study included MCI participants randomized to a placebo (n = 16) or ketogenic medium chain triglyceride (kMCT; n = 17) drink. A neurocognitive battery and brain imaging were performed pre‐ and post‐intervention. WM fascicle uptake of ketone and glucose and structural properties were assessed using positron emission tomography and diffusion imaging, respectively.
Results
Ketone uptake was increased in the kMCT group by 2.5‐ to 3.2‐fold in all nine WM fascicles of interest (P < .001), an effect seen both in deep WM and in fascicle cortical endpoints. Improvement in processing speed was positively associated with WM ketone uptake globally and in individual fascicles, most importantly the fornix (r = +0.61; P = .014).
Discussion
A 6‐month kMCT supplement improved WM energy supply in MCI by increasing ketone uptake in WM fascicles. The significant positive association with processing speed suggests that ketones may have a role in myelin integrity in MCI.
Keywords: acetoacetate, Alzheimer's disease, beta‐hydroxybutyrate, brain metabolism, diffusion MRI, fascicle, glucose, ketone, medium chain triglyceride, mild cognitive impairment, positron emission tomography imaging, processing speed, tractography, tractometry, white matter
1. BACKGROUND
White matter (WM) undergoes axonal degeneration and demyelination in Alzheimer's disease (AD). 1 , 2 WM microstructural deterioration is also observed in specific brain regions in mild cognitive impairment (MCI). 3 , 4 AD is characterized by a chronic brain glucose deficit, which is already present in older people years before the onset of cognitive decline associated with AD. 5 , 6 WM energy supply is crucial for adequate axonal function in part because oligodendrocytes need a considerable amount of energy for dynamic remodeling of myelin throughout life. 7 Hence, the gradual brain glucose deficit that begins during aging may lead to declining myelin energy metabolism, myelin loss, and impaired network connectivity, thereby contributing to cognitive dysfunction. 8
Ketones (acetoacetate and β‐hydroxybutyrate) are the main alternative energy substrate to glucose for the brain. In infants, ketones are not only a brain energy substrate but are also the primary substrates for myelin synthesis. 9 Recent results show that WM metabolic deterioration in AD and MCI is specific to glucose and does not involve ketones. 10 Rescuing the brain energy deficit by providing ketones is an emerging therapeutic strategy for aging‐associated cognitive decline 11 and has shown promise in several randomized trials. 12 , 13 The Brain Energy Fitness, Imaging and Cognition (BENEFIC) trial conducted in individuals with MCI showed improved measures of episodic memory, language, executive function, and processing speed after the 6‐month ketogenic intervention 14 as well as improved cortical energy metabolism specific to increased ketone uptake. WM analysis and the association to cognitive data from the BENEFIC trial has not previously been reported and is the focus of the present report.
Specific WM fascicles were targeted instead of WM as a whole because WM glucose hypometabolism was reported to be specific to limbic regions in AD. 10 The primary aim of this study was to investigate in the BENEFIC trial imaging dataset whether a ketogenic supplement consumed for 6 months improved ketone metabolism by WM fascicles. The secondary aims were to assess whether WM glucose metabolism changed, whether WM ketone metabolism was associated with any improvement in cognitive function, and whether the ketogenic intervention impacted WM structural properties. WM energy metabolism was measured by positron emission tomography (PET) using 11C‐acetoacetate (for ketones) and 18F‐fluorodeoxyglucose (for glucose).
RESEARCH IN CONTEXT
Systematic review: We assessed all peer‐reviewed articles available on PubMed about ketogenic intervention and white matter (WM) analysis. No published studies assessing WM measurements after a ketogenic intervention in the Alzheimer's disease spectrum were found.
Interpretation: This randomized controlled trial demonstrated for the first time that a 6‐month ketogenic supplementation improved WM energy supply in mild cognitive impairment, with a nearly 3‐fold increase of ketone uptake. Several measures of processing speed improved directly with both the increase in WM ketone supply globally and in individual fascicles, most importantly the fornix. This association suggests that ketones may have a role in myelin integrity in older people.
Future directions: The impact of a long‐term ketogenic supplementation on myelin density should be investigated to better understand the role of ketones in myelin integrity and cognition in older people.
2. METHODS
2.1. Participants
The BENEFIC trial (identification number NCT02551419 at ClinicalTrials.gov) was approved by our institutional ethical committee (CIUSSS de l'Estrie–CHUS, Sherbrooke, Quebec, Canada) and informed written consent was obtained from all participants before enrollment (Table 1). Inclusion criteria were male or female aged ≥55 years and the presence of objective cognitive decline (MCI), based on the criteria of Petersen. 15 Specifically, MCI criteria were: subjective memory complaint plus cognitive impairment in one or more domains of a neuropsychological tests battery compared to appropriate normative data (≥1.5 standard deviation less than the mean), a Montreal Cognitive Assessment (MoCA) score of 18–26/30 or Mini‐Mental State Examination (MMSE) score of ≥24/30, no evidence of probable or possible AD or depression, and full autonomy for daily living activities. 16 Exclusion criteria are fully described in Fortier et al. 14 The present cohort included both amnestic and non‐amnestic MCI. Cognitive and depression scores, physical autonomy, and blood assessments were within the normal range at baseline as detailed in Fortier et al. 14
TABLE 1.
Clinical data of participants at enrollment
| Placebo (n = 16) | kMCT (n = 16–17)* | Intergroup P value† | |
|---|---|---|---|
| Age (y) | 75.4 ± 6.6 | 74.2 ± 6.3 | .600 |
| Sex (M/F) | 6/10 | 9/8 | .491 |
| APOE ε4 (+) (%) | 7/16 (44%) | 5/16 (31%) | .716 |
| Education (y) | 12.4 ± 3.5 | 13.1 ± 3.6 | .609 |
| MMSE (/30) | 26.7 ± 2.4 | 27.7 ± 2.3 | .126 |
| MoCA (/30) | 22.1 ± 2.4 | 23.1 ± 3.4 | .177 |
Abbreviations: APOE, apolipoprotein E; kMCT, ketogenic medium chain triglyceride; MMSE, Mini‐Mental State Examination; MoCA, Montreal Cognitive Assessment score.
* APOE genotype unavailable for one participant.
† Statistical analysis was made using Mann‐Whitney test or Fisher exact test (sex and APOE ε4 status).
2.2. Experimental design
Participants were randomized to the ketogenic medium chain triglyceride (kMCT) or placebo group. 14 Before starting the intervention, they underwent a dual‐tracer PET and separate magnetic resonance imaging (MRI) acquisition of the brain, plus a neurocognitive battery. A second and final dual‐tracer PET and MRI brain acquisition, and the neurocognitive battery were performed during the final week of the sixth month of the intervention (post‐intervention).
The kMCT and placebo drinks were prepared as previously described. 14 Briefly, the kMCT drink was an emulsion containing 60% caprylic acid and 40% capric acid in lactose‐free skim milk and provided 30 g/day of kMCT in a 250 mL bottle. The placebo drink contained high‐oleic acid sunflower oil and was indistinguishable from the kMCT drink. During the first 2 weeks of the intervention, the volume to be consumed was increased from 50 mL/day to the final dose of 250 mL/day to help minimize kMCT‐related gastrointestinal side effects.
2.3. Cognitive assessment
Testing and scoring cognition were double‐blinded. Tests for episodic memory, executive function, language, and attention and processing speed domains were reported in Fortier et al. 14 All test scores were normalized and averaged to generate four composite Z‐scores, 14 which were the first cognitive outcomes analyzed in the current study. Measures of processing speed were the Trail Making (visual scanning, number sequencing, letter sequencing, and motor speed) and Stroop Color and Word Interference tests from the Delis‐Kaplan Executive Function System, 17 and the Digit Symbol Substitution test from the Wechsler Adult Intelligence Scale, 18 all of which were used for secondary analysis in the current study. No learning effect was expected after the 6‐month period for most of the selected tests but alternative versions for the two memory tests were used for the post‐intervention assessment.
2.4. Image acquisition
The MRI protocol 10 was acquired on a 3 Tesla Philips Ingenia system and included a 3D T1‐weighted sequence (duration = 6 minutes, repetition time = 7.9 ms, echo time = 3.5 ms, matrix size = 240 × 240 × 160 mm, flip angle = 8°, and 1 mm isotropic voxels), followed by high angular resolution diffusion imaging acquisition with parallel imaging SENSE 2 (duration = 11 minutes, repetition time = 11 seconds, echo time = 100 ms, matrix size = 128 × 128 × 78 mm, 1.8 mm isotropic voxels, 60 directions, b = 1500 s/mm2), and, one blip‐up and one blip‐down b = 0 s/mm2 acquisition to correct for distortions.
The MRI protocol was followed on the same day by a dual‐tracer PET session 10 , 14 on a PET/CT Philips Gemini system, in dynamic list mode. First, 11C‐acetoacetate (AcAc) was injected for an acquisition of 10 minutes, followed by a 1‐hour wash‐out period to ensure residual 11C was negligible. 18F‐flurodeoxyglucose (FDG) was then injected and the acquisition started 30 minutes post‐injection and lasted for 30 minutes. A series of blood samples were taken during acquisitions and used to correct image‐derived input functions. 19 FDG plasma input functions were used also to verify baseline radioactivity after the AcAc scan. Plasma glucose was measured with a glucose assay (Siemens Healthcare Diagnostics) and plasma ketones were measured as previously described. 20
2.5. Image analysis
2.5.1. Diffusion MRI
All image processing steps were blinded. Preprocessing and processing of diffusion‐weighted images were performed as previously described 10 using the TractoFlow pipeline. 21 Diffusion tensor imaging (DTI) measures (fractional anisotropy, mean, radial and axial diffusivities) were computed and free‐water‐corrected 22 to reduce partial volume effects of the cerebrospinal fluid (CSF) associated with aging. 23 , 24 Fiber orientation distribution functions were computed 25 , 26 and the apparent fiber density, hereafter “fiber density,” at each fixel (specific fiber direction) was computed. The signal in a fixel is proportional to the volume of axons aligned in that direction. 27
WM masks were corrected for white matter hyperintensities (WMH) using an average T1 template registration strategy. 10 Local probabilistic tractography was performed to reconstruct whole‐brain tractograms, robust to crossing fibers. Nine fascicles of interest, the ones generally reported with microstructural alteration in AD, 3 , 10 were extracted. The WM query language method 28 was used to automatically extract the two posterior segments of the cingulum (parahippocampal and posterior cingulate). The RecoBundlesX algorithm 29 , 30 was used to extract the genu and splenium of the corpus callosum, arcuate, inferior fronto‐occipital, inferior longitudinal, and uncinate fasciculi. Due to its high curvature and location next to lateral ventricles contaminating voxels with CSF, the fornix was reconstructed using a separate automated fascicle‐specific approach. 10
2.5.2. PET
PET images were analyzed as previously described 10 , 31 using PMOD 3.807 and its kinetic modeling tool PXMOD. Briefly, the Patlak method 32 was used to quantify the cerebral metabolic rate (μmol/100 g/min; referred to hereafter as “uptake”), and voxel‐wise maps were generated for FDG and AcAc. Input functions and voxel‐wise uptake maps were partial volume‐corrected. Uptake maps were then co‐registered to diffusion MRI data as described previously 10 using Advanced Normalization Tools (ANTs).
2.5.3. Tractometry
The quantification of PET (Figure 1 and Video 1 in supporting information) and diffusion measures in each fascicle was done using a tractometry pipeline. 33 FDG and AcAc uptake, free‐water, fiber density, and free‐water‐corrected DTI maps were inputs for the pipeline. Mean values were calculated for all fascicles of interest. Fascicles were divided into five sections to produce a fascicle profile that better captured ketone and glucose uptake in the extremities, close to gray matter, compared to the middle of the fascicles. 10 , 34
FIGURE 1.
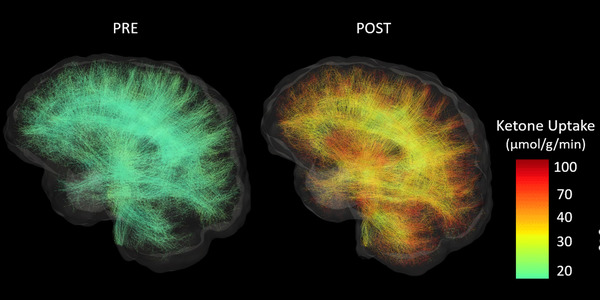
Whole‐brain white matter tractograms from a sample participant in the ketogenic medium chain triglyceride (kMCT) group before (PRE) and after (POST) the 6‐month intervention. Streamlines are colored according to their acetoacetate metabolic rate (μmol/g/min). Subsets of 10,000 streamlines per whole‐brain tractogram are shown.
2.6. Statistical analysis
Complete PET and diffusion MRIs were available for n = 16 placebo and n = 17 kMCT participants. Results are presented as the mean ± standard deviation, unless otherwise mentioned. Changes in imaging measures after the 6‐month intervention are expressed as percentage change from baseline. All statistical analyses were performed using SPSS 25.0 software. Because assumptions of homogeneity and normality of the variance were not met for some fascicles, non‐parametric tests were used without correction for multiple comparisons. Intragroup changes (pre‐ vs. post‐intervention) were analyzed with the Wilcoxon matched‐pairs signed rank test and the Mann‐Whitney test was used for intergroup comparison of changes in measures (placebo vs. kMCT). For fascicle profiles, pre‐ and post‐ketone uptake measures were set as repeated measures with a general linear model to assess the effect of the intervention along the sections of each fascicle. Correlations were performed to assess a possible association between fascicle ketone uptake and the four cognitive composite scores (episodic memory, language, executive function, and attention and processing speed combined), as well as between bothPET tracer uptake and fiber density. P < .05 was set as the threshold for statistical significance.
3. RESULTS
3.1. Clinical data
At baseline, both groups were of similar age, male‐to‐female ratio, apolipoprotein E ε4 status, and education. MMSE and MoCA scores were not significantly different between groups (Table 1). Details of the cognitive scores and protocol compliance were previously reported in Fortier et al. 14 Briefly, 75% of participants completed the intervention. All completers were protocol compliant, that is, consumed a mean of 90% of the planned daily dose as measured by return bottle count. No severe adverse effects were observed. Some gastrointestinal effects were noted for some participants but were mostly transitory. 14
3.2. Fascicle‐based ketone uptake
At baseline, plasma ketones and fascicle ketone uptake were similar between groups (Table 2). After the 6‐month intervention, mean ketone uptake was increased by 2.5‐ to 3.2‐fold in all nine fascicles of interest in the kMCT group (Figure 2), with the highest mean increase in ketone uptake in the fornix. Mean fascicle ketone uptake was unchanged in the placebo group, and the pre‐ to post‐intervention increase in ketone uptake was significantly higher in all fascicles in the kMCT group compared to placebo (P = .002 to .013; Table 2).
TABLE 2.
White matter fascicle‐based ketone uptake (AcAc) before (Pre) and after (Post) the 6‐month intervention
| Placebo (n = 16) | kMCT (n = 17) | Δ placebo vs. Δ kMCT | |||||
|---|---|---|---|---|---|---|---|
| Pre | Post | P value† | Pre | Post | P value† | P value‡ | |
| Plasma ketones (μM)* | |||||||
| AcAc | 136 ± 79 | 110 ± 52 | .515 | 124 ± 54 | 286 ± 142 | <.001 | <.001 |
| β‐HB | 212 ± 112 | 177 ± 89 | .525 | 210 ± 136 | 572 ± 325 | <.001 | <.001 |
| Fascicle AcAc metabolic rate (μmol/g/min) | |||||||
| PCC_L | 27.7 ± 20.6 | 21.8 ± 14.5 | .597 | 27.5 ± 17.6 | 49.8 ± 27.5 | .002 | .013 |
| PCC_R | 27.7 ± 17.6 | 22.9 ± 16.4 | .597 | 26.5 ± 15.6 | 55.2 ± 31.5 | <.001 | .002 |
| PHC_L | 24.5 ± 17.9 | 19.3 ± 14.1 | .495 | 22.7 ± 16.0 | 49.7 ± 27.3 | <.001 | .002 |
| PHC_R | 24.5 ± 17.3 | 19.8 ± 14.5 | .495 | 21.9 ± 13.2 | 47.1 ± 25.0 | <.001 | .002 |
| FX_L | 22.4 ± 12.3 | 19.5 ± 13.2 | .669 | 19.4 ± 10.8 | 43.7 ± 24.5 | <.001 | .004 |
| FX_R | 20.9 ± 13.7 | 18.1 ± 12.2 | .821 | 17.4 ± 10.0 | 39.9 ± 24.7 | <.001 | .002 |
| AF_L | 21.3 ± 15.9 | 18.1 ± 12.2 | .782 | 20.3 ± 12.7 | 44.7 ± 23.5 | <.001 | .003 |
| AF_R | 20.7 ± 13.7 | 18.3 ± 12.3 | .860 | 20.3 ± 12.8 | 43.6 ± 22.9 | <.001 | .004 |
| CC2 | 19.9 ± 12.9 | 18.4 ± 13.0 | .900 | 19.2 ± 11.4 | 43.2 ± 22.6 | <.001 | .002 |
| CC7 | 24.6 ± 17.0 | 19.5 ± 12.9 | .495 | 23.1 ± 14.0 | 47.5 ± 24.4 | <.001 | .004 |
| IFOF_L | 23.7 ± 16.5 | 20.2 ± 13.9 | .706 | 22.3 ± 13.7 | 48.3 ± 24.3 | <.001 | .004 |
| IFOF_R | 23.5 ± 16.1 | 20.6 ± 13.9 | .821 | 23.1 ± 14.4 | 50.0 ± 25.8 | <.001 | .004 |
| ILF_L | 23.7 ± 17.0 | 20.0 ± 13.6 | .706 | 22.1 ± 13.5 | 48.8 ± 25.0 | <.001 | .002 |
| ILF_R | 23.9 ± 16.1 | 20.5 ± 14.0 | .668 | 22.8 ± 14.0 | 49.9 ± 25.3 | <.001 | .003 |
| UF_L | 23.4 ± 15.5 | 20.9 ± 14.3 | .940 | 22.7 ± 14.1 | 49.6 ± 25.2 | <.001 | .004 |
| UF_R | 23.1 ± 15.9 | 20.9 ± 14.1 | >.999 | 23.2 ± 14.4 | 50.8 ± 26.9 | <.001 | .003 |
Abbreviations: AcAc, 11C‐acetoacetate; AF, arcuate fasciculus; β‐HB, β‐hydroxybutyrate; CC2, genu of the corpus callosum; CC7, splenium of the corpus callosum; FX, fornix; IFOF, inferior fronto‐occipital fasciculus; ILF, inferior longitudinal fasciculus; kMCT, ketogenic medium chain triglyceride; PCC, posterior cingulate segment of the cingulum; PHC, parahippocampal segment of the cingulum; UF, uncinate fasciculus.
* Plasma ketones were measured at baseline after a 6‐h fast (Pre) and 2 h after the kMCT supplement (Post).
† Statistical analysis using Wilcoxon matched‐pair signed rank test.
‡ Statistical analysis using Mann‐Whitney test.
FIGURE 2.
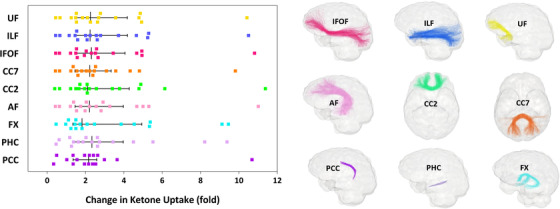
Left, White matter fascicle‐based changes (left hemisphere only) in ketone uptake in the kMCT group after the 6‐month intervention. Median ketone uptake was increased in all nine fascicles of interest after the intervention (refer to Table 2 for P values). Note that the median and interquartile range are illustrated for each fascicle, whereas mean values are reported in the text and tables. Right, Glass brain representations show the nine fascicles of interest. Fascicle colors correspond to the points in the left panel. AF, arcuate fasciculus; CC2, genu of the corpus callosum; CC7, splenium of the corpus callosum; FX, fornix; IFOF, inferior fronto‐occipital fasciculus; ILF, inferior longitudinal fasciculus; kMCT, ketogenic medium chain triglyceride; PCC, posterior cingulate segment of the cingulum; PHC, parahippocampal segment of the cingulum; UF, uncinate fasciculus
Ketone uptake along fascicle section profiles was significantly increased post‐intervention in the kMCT group (P < .001; Figure S1 in supporting information). This increase was similar in the middle section (section 3) compared to tail section (section 5) for all nine fascicles of interest (P = .610 to .946). The increase of ketone uptake in the kMCT group was similar in the total WM and total cortex, 2.9‐ versus 2.5‐fold, respectively (Table S1 in supporting information; P = .865). The change in plasma ketones was positively associated with the change in total WM ketone uptake (Figure S2 in supporting information; r = +0.84; P < .001).
3.3. Association of fascicle ketone uptake with processing speed
At baseline, processing speed Z‐score was similar between groups (P = .544). In the kMCT group after the 6‐month intervention, the composite Z‐score of processing speed was improved in direct relation to the increase in ketone uptake both in total WM (r = +0.52; P = .041) and in all fascicles individually (r = +0.46 to +0.61; P = .014 to .072), except for the posterior cingulate segment of the cingulum (Figure 3 and Table S2 in supporting information). In the placebo group, post‐intervention changes in processing speed were inversely related to ketone uptake in total WM (r = –0.53; P = .043) and in individual fascicles (Table S2), a relationship not found at baseline. Slopes between the two groups were significantly different for all nine fascicles of interest (P = .003 to .028). The strongest association between the improvement in processing speed Z‐score and the increase in ketone uptake in the kMCT group was in the fornix (r = +0.61; P = .014). Pre‐intervention, ketone uptake in the fornix also had the strongest association with processing speed Z‐score when both treatment groups were combined (r = +0.44; P = .010; data not shown).
FIGURE 3.
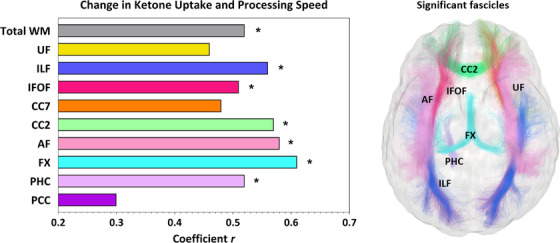
Left, Composite Z‐score of processing speed was improved in direct association with the mean increase of ketone uptake in total white matter (r = +0.52; P = .041) and in several fascicles in the kMCT group after the 6‐month intervention. Refer to Table S2 for P values. * = P < .05. Fascicles from the left hemisphere are presented. Right, The glass brain representation shows all fascicles for which the association between change in ketone uptake and processing speed was significant. Fascicle colors correspond to the bars in the panel on the left. AF, arcuate fasciculus; CC2, genu of the corpus callosum; CC7, splenium of the corpus callosum; FX, fornix; IFOF, inferior fronto‐occipital fasciculus; ILF, inferior longitudinal fasciculus; PCC, posterior cingulate segment of the cingulum; PHC, parahippocampal segment of the cingulum; UF, uncinate fasciculus; WM, white matter
Increased fascicle ketone uptake was not associated with episodic memory, language, or executive function composite scores. Therefore, further analyses were restricted to measures of processing speed. In the kMCT group, improved processing speed (decreased time taken) post‐intervention on the motor speed task of the Trail Making Test was significantly inversely associated with increased ketone uptake in total WM (r = –0.50; P = .043) and in all fascicles individually (Table S3 in supporting information). No such association was observed for the placebo group.
Post‐intervention, processing speed was 14% slower on the visual scanning task of the Trail Making Test for the placebo group (pre: 30 seconds and post: 34 seconds; P = .040) but remained unchanged in the kMCT group (P = .922). In the kMCT group, improvement on the visual scanning task of the Trail Making Test was also associated with increased ketone uptake in total WM (r = –0.53; P = .036) and in the fornix, genu of the corpus callosum, arcuate, inferior fronto‐occipital, and inferior longitudinal fasciculi (data not shown).
3.4. Fascicle‐based diffusion measures
No difference in diffusion measures pre‐ to post‐intervention was observed between both groups. After the 6‐month intervention, the fiber density was 8% to 14% lower in most fascicles of both groups, except for the fornix, and parahippocampal and posterior cingulate segments of the cingulum (Table S4 in supporting information). The splenium of the corpus callosum had the greatest decrease in fiber density. In contrast, fiber density was increased by 11% to 18% post‐intervention in both groups in the left parahippocampal and posterior cingulate segments of the cingulum, respectively. Free‐water‐corrected radial diffusivity was significantly increased in several fascicles post‐intervention in both groups but was reduced specifically in the posterior cingulate segment of the cingulum (P < .001 to .011; data not shown). Free‐water‐corrected fractional anisotropy was increased by 15% to 18% in the posterior cingulate segment of the cingulum in both groups (P < .001 to.003; data not shown). Post‐intervention, both groups had 4% to 23% higher free‐water in all fascicles, especially the parahippocampal segment of the cingulum, except for the left posterior cingulate segment of the cingulum and right fornix (Table S5 in supporting information).
3.5. Fascicle‐based glucose uptake and association with fiber density
No significant difference was found for fascicle glucose uptake between groups at baseline (Table S6 in supporting information). No difference in glucose uptake pre‐ to post‐intervention was found between the two treatments. Post‐intervention, mean glucose uptake was unchanged in all fascicles, except in the posterior cingulate segment of the cingulum where it was reduced by 9% to 11% in both groups (P = .009 to .044; Table S6). When combined across both groups, the change in glucose uptake was positively associated with change in fiber density in the uncinate fasciculus, fornix, and genu of the corpus callosum (r = +0.41 to +0.52; P = .003 to .028), but inversely in the posterior cingulate segment of the cingulum (r = –0.45; P = .012; Figure 4). For these significant associations, slopes were significantly non‐zero (P = .003 to .030; data not shown).
FIGURE 4.
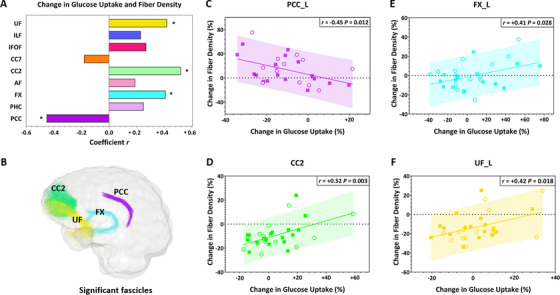
A, After the 6‐month intervention, change in mean glucose uptake was positively associated with change in fiber density in the uncinate fasciculus, fornix, and genu of the corpus callosum, but inversely associated in the posterior cingulate segment of the cingulum. Data from both the placebo (○) and kMCT (■) groups were combined because no significant treatment effect was found for these variables. * = P < .05. Fascicles from the left (L) hemisphere are presented. B, The glass brain representation shows all fascicles for which the association was significant. Fascicle colors correspond to the bars in panel A. C‐F, Scatter plots of significant associations between change in glucose uptake and fiber density. AF, arcuate fasciculus; CC2, genu of the corpus callosum; CC7, splenium of the corpus callosum; FX, fornix; IFOF, inferior fronto‐occipital fasciculus; ILF, inferior longitudinal fasciculus; PCC, posterior cingulate segment of the cingulum; PHC, parahippocampal segment of the cingulum; UF, uncinate fasciculus
4. DISCUSSION
This is the first study to report the impact of a ketogenic intervention on WM structure and energy metabolism in humans. We show that a 6‐month kMCT supplement improved WM energy supply with a nearly 3‐fold increase in ketone uptake but with no significant effect on glucose uptake. The increased ketone uptake was present in all WM fascicles assessed and was as important in the deep WM as in the fascicle endpoints in gray matter. Processing speed improvement was positively associated with increased WM ketone uptake globally and in individual fascicles, most importantly in the fornix, suggesting that ketones may have a role in myelin integrity in MCI. In MCI, increased fiber density was specific to the posterior cingulum, which also had declining glucose metabolism during the 6‐month study period.
In the kMCT group, ketone uptake increased over 6 months in all fascicles analyzed (Figure 1, 2, and Video 1). The fornix had the highest increase in mean ketone uptake post‐intervention, which may in part be linked to its lower ketone and glucose uptake at baseline. This increase in ketone availability to the fornix may potentially be beneficial to limit the early decline of structural integrity of the fornix during aging. 35 A global 2.5‐fold increase of ketone uptake was also found for the cortex (Table S1), an increase that was not significantly different from the 2.9‐fold increase in total WM. Clearly, in MCI, WM avidly consumes ketones when they are available.
In the kMCT group, processing speed composite Z‐score improved in association with the increase of ketone uptake in almost all individual fascicles as well as in WM as a whole (Figure 3). This suggests that information processing is probably linked to global structural connectivity in MCI, rather than to a specific fascicle, which agrees with previous studies in older adults. 36 , 37 For specific cognitive tests, the strongest association with increased ketone uptake was for the motor speed task of the Trail Making Test, a nearly pure measure of processing speed. Degeneration of myelin lipids associated with aging has been proposed to provide fatty acids or ketones as an energetic substrate for neurons. 38 Processing speed is directly linked to myelin integrity because conduction of action potentials is proportional to the thickness of myelin sheaths. 39 Declining processing speed is a key deficit in multiple sclerosis, 40 a disease with widespread demyelination of the WM. Hence, improved processing speed scores may have been a consequence of increased myelin density after the ketogenic intervention. Processing speed may also have improved due to increased intra‐axonal mitochondrial production of adenosine triphosphate, 41 resulting from the increase in ketone supply to the brain. An important part of the energy used by the brain is to maintain information processing speed through impulse propagation. 8 In the BENEFIC trial, 13 positive correlations between plasma ketones and tests of episodic memory, executive function, and language were found, but not with processing speed. This reinforces the essential role of WM in processing speed for which ketones may act as an important structural and energy substrate.
In contrast to the kMCT group, in the placebo group, an inverse association was found between WM ketone uptake and processing speed. Slower processing speed in the placebo group may be attributable to disease progression over the 6 months. Indeed, WM pathology in MCI is associated with impaired processing speed. 42 There was no significant association between ketone uptake by the fornix and memory, but there was a strong positive correlation with processing speed in the kMCT group. The microstructure of the fornix has previously been reported to be associated with processing speed but not with memory in older adults 43 and in epilepsy, 44 suggesting a role of the fornix beyond memory function.
The 6‐month kMCT supplement had no significant effect on structural WM measures. Physical activity interventions of a 1‐year duration also do not seem to impact WM microstructure in older adults 45 and MCI. 46 We show here that, as opposed to other fascicles, the fiber density of the posterior cingulum increased in both groups (Table S4). This supports the “network failure” theory, 47 , 48 which proposes that in the prodromal phase of AD, the posterior default mode network (a key processing hub) starts compensating for declining function of other brain networks, becomes overloaded, and starts to shift its processing load to related networks, resulting in increased connectivity between the posterior default mode network and other hubs. Structural connectivity changes associated with cognitive decline may therefore follow a non‐linear trajectory for some specific networks.
We show here a decline in glucose uptake over 6 months specifically in the posterior cingulate segment of the cingulum in both groups (Table S6). In the kMCT group, the increase of ketone uptake and decrease of glucose uptake in this fascicle were not significantly correlated (data not shown), so it seems possible that disease progression over the 6 months could have been linked specifically to declining glucose uptake in the posterior cingulate segment of the cingulum. The posterior cingulate segment of the cingulum had the highest glucose uptake at baseline (62% higher than the fornix), which may make this region more vulnerable to declining energy (glucose) metabolism during aging. The increase in ketone uptake post‐intervention was also the lowest in the posterior cingulate segment of the cingulum. This would make it more difficult for ketones to compensate for impaired glucose uptake in posterior cingulate segment of the cingulum during MCI. Other tractography studies show that the posterior cingulum has lower glucose uptake 10 and fiber density 3 in MCI compared to controls, suggesting it is a particularly vulnerable fascicle when MCI develops.
A limitation of the present study was the small sample size for assessing the relationships to cognitive outcomes. Although WMH were eliminated for robust fascicle reconstruction, another limitation was the inability to segment these lesions and establish whether both groups had a similar cerebrovascular burden. However, in terms of cardiovascular risk factors, both groups had similar blood pressure and plasma cholesterol at baseline. 14 WMH regional load may have affected the cerebral metabolic rate of PET tracers, but the relationship between WM ketone uptake and processing speed was still highly significant. In future studies, including fluid‐attenuated inversion recovery (FLAIR) images will be important for automated segmentation of WMH. 49 Because of the importance of myelin for information processing and its link to ketone metabolism, assessment of the myelin content would also be recommended in the future when ketogenic or other energetic interventions are used. The lack of specificity of radial diffusivity to myelin requires a more sophisticated MRI sequence, such as the inhomogeneous magnetization transfer data, 50 more specific to myelin.
In conclusion, we show that a 6‐month kMCT supplement increased ketone supply in WM fascicles in MCI, with an equivalent effect in deep WM as in fascicle gray matter endpoints. Several improved measures of processing speed were directly associated with increased WM ketone supply globally and in individual fascicles, most importantly in the fornix. These data also support the idea of a posterior cingulum vulnerability at the MCI stage. The impact of a long‐term ketogenic supplementation on myelin density should be investigated to better understand the role of ketones in myelin integrity and cognition in MCI.
CONFLICTS OF INTEREST
SCC has consulted for or received travel honoraria or test products from Nestlé Health Science, German Nutrition Society, Bulletproof, Cerecin, Abbott, Servier, Pro‐Diet and Abitec. SCC is the founder and director of Senotec Ltd. MD is CSO and shareholder of Imeka Solutions Inc and MR is consultant for Imeka Solutions Inc. MD and MR were part of advisory boards for INmune Bio Inc and MD received consulting fees from INmune Bio Inc. All other authors have nothing to disclose.
Supporting information
Supporting material
Supporting material
Supporting material
ACKNOWLEDGMENTS
The authors wish to acknowledge the PET and MRI clinical teams at the Sherbrooke Molecular Imaging Center, Matthieu Dumont, Camille Vandenberghe, Dr. Sébastien Tremblay, and Marie‐Christine Morin for technical assistance; the Sherbrooke Connectivity Imaging Lab team for their help on data processing; and Dr. Alexa Pichet Binette and Dr. Sylvia Villeneuve for their assistance on data interpretation. We would also like to acknowledge the participants of the BENEFIC trial.
Financial support for the BENEFIC trial was provided by the Alzheimer's Association USA (PCTR‐15‐328047), FRQS (FR40072), and Université de Sherbrooke. MR was funded by MITACS and Nestlé Health Science. TF was founded by Sanofi and Pfizer. The Université de Sherbrooke Institutional Chair in Neuroinformatics and NSERC also provided funding support. Abitec provided the kMCT (Captex 355) and placebo oil. The intervention drinks for both arms were prepared under contract at INAF, Université Laval, Québec, QC, Canada.
Roy M, Fortier M, Rheault F, et al. A ketogenic supplement improves white matter energy supply and processing speed in mild cognitive impairment. Alzheimer's Dement. 2021;7:e12217. 10.1002/trc2.12217
Maxime Descoteaux and Stephen C. Cunnane are co‐senior authors.
REFERENCES
- 1. Englund E. Neuropathology of white matter changes in Alzheimer's disease and vascular dementia. Dement Geriatr Cogn Disord. 1998;9:6‐12. Suppl 1. [DOI] [PubMed] [Google Scholar]
- 2. Sjobeck M, Haglund M, Englund E. Decreasing myelin density reflected increasing white matter pathology in Alzheimer's disease–a neuropathological study. Int J Geriatr Psychiatry. 2005;20:919‐926. [DOI] [PubMed] [Google Scholar]
- 3. Mito R, Raffelt D, Dhollander T, et al. Fibre‐specific white matter reductions in Alzheimer's disease and mild cognitive impairment. Brain. 2018;141:888‐902. [DOI] [PubMed] [Google Scholar]
- 4. Radanovic M, Pereira FR, Stella F, et al. White matter abnormalities associated with Alzheimer's disease and mild cognitive impairment: a critical review of MRI studies. Expert Rev Neurother. 2013;13:483‐493. [DOI] [PubMed] [Google Scholar]
- 5. Mosconi L, Brys M, Switalski R, et al. Maternal family history of Alzheimer's disease predisposes to reduced brain glucose metabolism. Proc Natl Acad Sci U S A. 2007;104:19067‐19072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cunnane SC, Courchesne‐Loyer A, St‐Pierre V, et al. Can ketones compensate for deteriorating brain glucose uptake during aging? Implications for the risk and treatment of Alzheimer's disease. Ann N Y Acad Sci. 2016;1367:12‐20. [DOI] [PubMed] [Google Scholar]
- 7. Saab AS, Nave KA. Myelin dynamics: protecting and shaping neuronal functions. Curr Opin Neurobiol. 2017;47:104‐112. [DOI] [PubMed] [Google Scholar]
- 8. Raz N, Daugherty AM. Pathways to Brain Aging and Their Modifiers: free‐Radical‐Induced Energetic and Neural Decline in Senescence (FRIENDS) Model ‐ A Mini‐Review. Gerontology. 2018;64:49‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cunnane SC, Crawford MA. Energetic and nutritional constraints on infant brain development: implications for brain expansion during human evolution. J Hum Evol. 2014;77:88‐98. [DOI] [PubMed] [Google Scholar]
- 10. Roy M, Rheault F, Croteau E, et al. Fascicle‐ and glucose‐specific deterioration in white matter energy supply in Alzheimer's Disease. J Alzheimers Dis. 2020;76(3):863‐881. 10.3233/JAD-200213 [DOI] [PubMed] [Google Scholar]
- 11. Cunnane SC, Trushina E, Morland C, et al. Brain energy rescue: an emerging therapeutic concept for neurodegenerative disorders of ageing. Nat Rev Drug Discov. 2020;19(9):609‐633. 10.1038/s41573-020-0072-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grammatikopoulou MG, Goulis DG, Gkiouras K, et al. To keto or not to keto? A systematic review of randomized controlled trials assessing the effects of ketogenic therapy on Alzheimer disease. Adv Nutr. 2020;11(6):1583‐1602. 10.1093/advances/nmaa073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fortier M, Castellano CA, St‐Pierre V, et al. A ketogenic drink improves cognition in mild cognitive impairment: Results of a 6‐month RCT. Alzheimers Dement. 2020;17(3):543‐552. 10.1002/alz.12206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fortier M, Castellano CA, Croteau E, et al. A ketogenic drink improves brain energy and some measures of cognition in mild cognitive impairment. Alzheimers Dement. 2019;15:625‐634. [DOI] [PubMed] [Google Scholar]
- 15. Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183‐194. [DOI] [PubMed] [Google Scholar]
- 16. Hebert R, Carrier R, Bilodeau A. The Functional Autonomy Measurement System (SMAF): description and validation of an instrument for the measurement of handicaps. Age Ageing. 1988;17:293‐302. [DOI] [PubMed] [Google Scholar]
- 17. Delis D, Kaplan E, Kramer J. Delis‐Kaplan Executive Function System (D‐KEFS). San Antonio, TX (USA): The Psychological Corporation; 2001. [Google Scholar]
- 18. Wechsler D. Wechsler Memory Scale. 3rd ed.. San Antonio, TX (USA): The Psychological Corporation; 1997. [Google Scholar]
- 19. Nugent S, Tremblay S, Chen KW, et al. Brain glucose and acetoacetate metabolism: a comparison of young and older adults. Neurobiol Aging. 2014;35:1386‐1395. [DOI] [PubMed] [Google Scholar]
- 20. Courchesne‐Loyer A, Fortier M, Tremblay‐Mercier J, et al. Stimulation of mild, sustained ketonemia by medium‐chain triacylglycerols in healthy humans: estimated potential contribution to brain energy metabolism. Nutrition. 2013;29:635‐640. [DOI] [PubMed] [Google Scholar]
- 21. Theaud G, Houde JC, Bore A, Rheault F, Morency F, Descoteaux M. TractoFlow: a robust, efficient and reproducible diffusion MRI pipeline leveraging Nextflow & Singularity. Neuroimage. 2020;218: 116889. [DOI] [PubMed] [Google Scholar]
- 22. Pasternak O, Sochen N, Gur Y, Intrator N, Assaf Y. Free water elimination and mapping from diffusion MRI. Magn Reson Med. 2009;62:717‐730. [DOI] [PubMed] [Google Scholar]
- 23. Concha L, Gross DW, Beaulieu C. Diffusion tensor tractography of the limbic system. AJNR Am J Neuroradiol. 2005;26:2267‐2274. [PMC free article] [PubMed] [Google Scholar]
- 24. Maier‐Hein KH, Westin CF, Shenton ME, et al. Widespread white matter degeneration preceding the onset of dementia. Alzheimers Dement. 2015;11:485‐493. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Descoteaux M, Deriche R, Knosche TR. Anwander A. Deterministic and probabilistic tractography based on complex fibre orientation distributions. IEEE Trans Med Imaging. 2009;28:269‐286. [DOI] [PubMed] [Google Scholar]
- 26. Tournier JD, Calamante F, Connelly A. Robust determination of the fibre orientation distribution in diffusion MRI: non‐negativity constrained super‐resolved spherical deconvolution. Neuroimage. 2007;35:1459‐1472. [DOI] [PubMed] [Google Scholar]
- 27. Raffelt DA, Tournier JD, Smith RE, et al. Investigating white matter fibre density and morphology using fixel‐based analysis. Neuroimage. 2017;144:58‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wassermann D, Makris N, Rathi Y, et al. The white matter query language: a novel approach for describing human white matter anatomy. Brain Struct Funct. 2016;221:4705‐4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Garyfallidis E, Cote MA, Rheault F, et al. Recognition of white matter bundles using local and global streamline‐based registration and clustering. Neuroimage. 2018;170:283‐295. [DOI] [PubMed] [Google Scholar]
- 30. Rheault F. Analyse et reconstruction de faisceaux de la matière blanche. Université de Sherbrooke; 2020. [Google Scholar]
- 31. Castellano CA, Paquet N, Dionne IJ, et al. A 3‐Month Aerobic Training Program Improves Brain Energy Metabolism in Mild Alzheimer's Disease: preliminary Results from a Neuroimaging Study. J Alzheimers Dis. 2017;56:1459‐1468. [DOI] [PubMed] [Google Scholar]
- 32. Patlak CS, Blasberg RG, Fenstermacher JD. Graphical evaluation of blood‐to‐brain transfer constants from multiple‐time uptake data. J Cereb Blood Flow Metab. 1983;3:1‐7. [DOI] [PubMed] [Google Scholar]
- 33. Cousineau M, Jodoin PM, Morency FC, et al. A test‐retest study on Parkinson's PPMI dataset yields statistically significant white matter fascicles. Neuroimage Clin. 2017;16:222‐233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yeatman JD, Dougherty RF, Myall NJ, Wandell BA, Feldman HM. Tract profiles of white matter properties: automating fiber‐tract quantification. PLoS One. 2012;7: e49790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Metzler‐Baddeley C, Mole JP, Sims R, et al. Fornix white matter glia damage causes hippocampal gray matter damage during age‐dependent limbic decline. Sci Rep. 2019;9:1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Haasz J, Westlye ET, Fjaer S, Espeseth T, Lundervold A, Lundervold AJ. General fluid‐type intelligence is related to indices of white matter structure in middle‐aged and old adults. Neuroimage. 2013;83:372‐383. [DOI] [PubMed] [Google Scholar]
- 37. Kuznetsova KA, Maniega SM, Ritchie SJ, et al. Brain white matter structure and information processing speed in healthy older age. Brain Struct Funct. 2016;221:3223‐3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Klosinski LP, Yao J, Yin F, et al. White Matter Lipids as a Ketogenic Fuel Supply in Aging Female Brain: implications for Alzheimer's Disease. EBioMedicine. 2015;2:1888‐1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Purves D, Augustine GJ, Fitzpatrick D, et al. Increased Conduction Velocity as a Result of Myelination. 2nd ed.. Sunderland, MA (USA): Sinauer Associates; 2001. Neuroscience. [Google Scholar]
- 40. Denney DR, Lynch SG, Parmenter BA, Horne N. Cognitive impairment in relapsing and primary progressive multiple sclerosis: mostly a matter of speed. J Int Neuropsychol Soc. 2004;10:948‐956. [DOI] [PubMed] [Google Scholar]
- 41. Micu I, Plemel JR, Caprariello AV, Nave KA, Stys PK. Axo‐myelinic neurotransmission: a novel mode of cell signalling in the central nervous system. Nat Rev Neurosci. 2018;19:49‐58. [DOI] [PubMed] [Google Scholar]
- 42. Kaskikallio A, Karrasch M, Koikkalainen J, et al. White Matter Hyperintensities and Cognitive Impairment in Healthy and Pathological Aging: a Quantified Brain MRI Study. Dement Geriatr Cogn Disord. 2019;48:297‐307. [DOI] [PubMed] [Google Scholar]
- 43. Burzynska AZ, Jiao Y, Knecht AM, et al. White Matter Integrity Declined Over 6‐Months, but Dance Intervention Improved Integrity of the Fornix of Older Adults. Front Aging Neurosci. 2017;9:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Alexander RP, Concha L, Snyder TJ, Beaulieu C, Gross DW. Correlations between Limbic White Matter and Cognitive Function in Temporal‐Lobe Epilepsy. Preliminary Findings Front Aging Neurosci. 2014;6:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Voss MW, Heo S, Prakash RS, et al. The influence of aerobic fitness on cerebral white matter integrity and cognitive function in older adults: results of a one‐year exercise intervention. Hum Brain Mapp. 2013;34:2972‐2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tarumi T, Thomas BP, Tseng BY, et al. Cerebral White Matter Integrity in Amnestic Mild Cognitive Impairment: a 1‐Year Randomized Controlled Trial of Aerobic Exercise Training. J Alzheimers Dis. 2020;73:489‐501. [DOI] [PubMed] [Google Scholar]
- 47. Jones DT, Knopman DS, Gunter JL, et al. Cascading network failure across the Alzheimer's disease spectrum. Brain. 2016;139:547‐562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jones DT, Graff‐Radford J, Lowe VJ, et al. Tau, amyloid, and cascading network failure across the Alzheimer's disease spectrum. Cortex. 2017;97:143‐159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schmidt P, Gaser C, Arsic M, et al. An automated tool for detection of FLAIR‐hyperintense white‐matter lesions in Multiple Sclerosis. Neuroimage. 2012;59:3774‐3783. [DOI] [PubMed] [Google Scholar]
- 50. Manning AP, Chang KL, MacKay AL, Michal CA. The physical mechanism of “inhomogeneous” magnetization transfer MRI. J Magn Reson. 2017;274:125‐136. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting material
Supporting material
Supporting material


