Abstract
Introduction
Preterm neonates have under-developed immune-regulatory system; consequently, there is a risk for developing chronic inflammation. Necrotizing enterocolitis (NEC) is an acute devastating neonatal intestinal inflammatory disorder. Due to the obscure multifactorial etiology, early diagnosis and effective treatment of NEC are limited. Consequently, effective strategies in the prevention of NEC, including nutritional approaches, are critically needed. The current study was conducted to assess the potential immunomodulatory effect of Docosahexaenoic Acid (DHA) supplementation in preterm neonates at neonatal intensive care unit (NICU) and subsequently its effect on preventing or reducing NEC incidence.
Methods
This was a prospective randomized controlled study. A total of 67 neonates, with gestational age equal or less than 32 weeks at birth and weight less than or equal 1500 g, were randomly assigned to either DHA group or the control group. Modified Bell’s staging criteria for NEC was used as an objective tool for diagnosis and staging of NEC. Levels of Interleukin 1 beta (IL-1β) were measured at baseline and after 10 days. Mortality and NICU length of stay (LOS) were also monitored.
Results
Thirty neonates of each group completed the study. A statistically significant difference was observed between the two groups regarding diagnosis and staging of NEC (p = 0.0001). There was also a statistically significant difference between DHA group 22(73.3), 95% CI [55.9, 86.5] and the control group 8 (26.7), 95% CI [13.5, 44.1] in the percentage change in IL-1β levels (p = 0.0001).
A statistically significant association was found between IL and 1 β change and NEC diagnosis (p = 0.001). NICU LOS was significantly lower among DHA group 21.63 ± 6.67 compared to the control group 25.07 ± 4.67 (p = 0.025). Mortality n (%) among the control group 4 (11.8) was higher than DHA group 3 (9.1), however, no significant difference was detected (p = 1.0).
Conclusion
Findings of this study suggest that enteral DHA supplementation can reduce NEC incidence in preterm neonates through its immunoregulatory effect that modulates production of regulatory cytokines.
Trial registration: Registered at clinical trials.gov (NCT03700957), 6 October 2018.
Keyword: Neonatal intensive care unit, Prematurity, Inflammation, Long-chain polyunsaturated fatty acids, Immunomodulation, Interleukin 1 beta
Abbreviations: NICU, Neonatal intensive care unit; NEC, Necrotizing enterocolitis; DHA, Docosahexaenoic acid; IL-1β, Interleukin 1 beta; n-3 LCPUFA, Omega-3 long-chain polyunsaturated; LOS, Length of stay
1. Introduction
Preterm birth is defined as birth that occurs at less than 37 completed weeks’ gestation. It takes place in approximately 12% of deliveries globally and can significantly influence child’s long-term health. Over the last few decades, advancements in medical care have substantially contributed to reduce preterm neonates’ mortality rates. Nevertheless, morbidity rates, especially in early preterm neonate (born at less than 28 weeks’ gestation) have continued to rise (Beck et al., 2010).
Preterm neonates have under-developed immune-regulatory system; consequently, there is a risk for developing chronic inflammation. Moreover, dysregulation of inflammatory responses plays a principal role in the etiology of many fatal neonatal inflammatory disorders and therefore represents an ongoing challenge to healthcare workers involved in neonatal care (Fink et al., 2016).
Necrotizing enterocolitis (NEC) is an acute devastating neonatal intestinal inflammatory disorder primarily described in 1965 by Mizrahi et al (Mizrahi et al., 1965). It involves gut wall inflammation and injury that may proceed to necrosis and, eventually, gut perforation (Good et al., 2014, Rasiah et al., 2014). The incidence of NEC in developed countries is 5–12%. The prevalence increases in preterm neonates; being higher in early-preterm (born less than 35 weeks’ gestation) than late-preterm (35–36 weeks’ gestation) or term infants (37–42 weeks’ gestation). Incidence is also considerably higher in neonates with a birth weight less than 1500 g (Shulhan et al., 2017). Mortality rates ranging from 15% to 30% have been reported (Lin and Stoll, 2006). In Egypt, a NEC incidence of 9.6% and a mortality rate of 20–30% have been reported among preterm neonates suffering from feeding intolerance (Khashana and Moussa, 2016).
The pathogenesis of NEC has not been clearly elucidated yet, but it is thought to be due to involvement of multiple factors including premature birth, low birth weight, ischemia/reperfusion (I/R) injury, abnormal gut bacterial colonization and inappropriate enteral feeding (Chen et al., 2014).
Compelling evidence suggests that immaturity of innate immunity mediated via Toll-like receptors signaling pathway may contribute to excessive intestinal inflammation in NEC (Lee et al., 2003). Furthermore, several cross-sectional studies have shown increased expression of inflammatory cytokines including, tumor necrosis factor (TNF), interleukin (IL)-1β and IL-6 in both plasma and affected tissues of NEC patients (Maheshwari et al., 2014).
Due to the obscure multifactorial etiology, early diagnosis and effective treatment of NEC are limited. Consequently, effective strategies in the prevention of NEC, including nutritional approaches, are critically needed. A growing body of evidence suggests the imperative role of immune modulatory nutrients in primary prevention of NEC including probiotics, prebiotics, long-chain polyunsaturated fatty acids (LCPUFA) and amino acids (glutamine, cysteine, L-arginine, and N-acetylcysteine) (Ilardi et al., 2021, Zhou et al., 2015).
LCPUFA including Docosahexaenoic acid (DHA), are essential for normal health and neurodevelopment (Klevebro et al., 2020). Additionally, there is increasing evidence that Omega-3 (n-3) LCPUFA may reduce the incidence or severity of the common inflammatory disorders and comorbidities of prematurity by influencing various steps of the immune and anti-inflammatory response (Lapillonne and Moltu, 2016).
LCPUFA are progressively transferred from mother to fetus late in pregnancy, reaching peak accretion rates at 35–40 weeks’ gestation. Premature neonates, born before this process is complete, are at risk of deficiency. Moreover, very low birth weight (VLBW) neonates have poor DHA status. This can be attributed to the inadequate fat stores, the inability to convert precursor fatty acids and the limited postnatal nutritional supply (Baack et al., 2016, Harris and Baack, 2015).
The lack of exposure of premature infant’s gut to LCPUFA is associated with their risk for NEC. n-3 LCPUFA including DHA can directly or indirectly suppress the activity of nuclear transcription factors and decrease the production of pro-inflammatory enzymes and cytokines, including COX-2, tumor necrosis factor (TNF)-α, and interleukin (IL-1β) (Kang and Weylandt, 2008). DHA also supports the colonization of beneficial bacteria and protects against growth of pathogenic bacteria (Lee et al., 2004). Overall, rising evidence supports the benefits of DHA in NEC prevention (Harris and Baack, 2015).
To date, limited studies have assessed the effects of n-3 LCPUFA on inflammatory biomarkers as a primary outcome in preterm infants. Accordingly, the present study was conducted to assess the potential immunomodulatory effect of DHA supplementation in preterm neonates at neonatal intensive care unit (NICU) and subsequently its effect on preventing or reducing the incidence of NEC as one of the most common inflammatory disorders in the neonatal period.
2. Materials and Methods
2.1. Study design and setting
The current study was a prospective randomized controlled trial conducted from October 2018 to November 2019 in the NICU of Ain Shams University Children Hospital, Cairo Egypt.
2.2. Ethical considerations
The ethical approval for both the scientific and ethical aspects to conduct the study was obtained from the committee of ethics of Faculty of Pharmacy, Ain Shams University (serial number of protocol: Ph.D. No.73) and the Joint Committee for the Protection of Human Subjects in Research of the Health Science Centre, Faculty of Pharmaceutical Sciences and Pharmaceutical Industries, Future University in Egypt (serial number of protocol: REC-FPSPI-13/97).
In consonance with the Declaration of Helsinki for protecting human subjects, a written informed consent was obtained from the participating neonate’s parents or primary caregiver.
2.3. Methodology
2.3.1. Study population
Preterm neonates admitted to the NICU were screened for eligibility. Neonates from both genders with gestational age equal or less than 32 weeks at birth, weight less than or equal 1500 g and clinically stable to begin enteral feeding were enrolled. Exclusion criteria included bleeding disorders, receiving anticoagulants, persistent vomiting, gastrointestinal malformations and maternal use of omega-3 supplements.
2.3.2. Study intervention
Eligible neonates were randomly assigned to one of the two study groups. A computer random number generator program (Stattrek.com/statistics/random-number-generator) was used, where a list of random numbers for allocating participants was generated and a unique number was assigned to each neonate. Participant randomization assignment was then kept in sealed, signed and dated envelopes. Clinicians and NICU staff responsible for assessments remained blind from randomization. According to standard operating procedures, all other operating personnel, including lab technicians and staff who performed lab analysis and blood sampling, were blinded to group assignment.
The study groups comprised DHA group, who received 100 mg/day Docosahexaenoic acid (DHA) for 14 days administered by enteral route with the standard neonatal feeding, and the control group who received only the standard neonatal feeding (López-Alarcón et al., 2012).
The product used in the DHA group was Mom and Baby Pure DHA, a 100% Vegetarian liquid DHA supplement, EAN 7425619560524, manufactured by Hearts and Minds Pure Health (NSF certified, FDA and cGMP compliant facility), USA.
All the participants received the standard neonatal care according to the protocol of the NICU of Ain Shams University Children Hospital and were followed-up from birth until reaching 37 weeks corrected gestational age, discharge or death whichever came first.
Feeding protocol:
Trophic feeding was begun as early as the neonate could tolerate, when hemodynamically stable. All participating neonates received maternal milk and the feeding method was individualized based on gestational age, clinical condition, and feeding tolerance.
Feeding was started with low volume 0.5 to 1 ml/6 h, increased gradually according to the tolerance, then feds were deduced from total fluid intake thereafter. Increments were from 10 to 20 ml/kg/day. Trophic feeding was not used in patients with severe hemodynamic instability, suspected or confirmed NEC (Ellard et al., 2012).
2.3.3. Study procedure
All participating neonates were subjected to the following
Careful perinatal history taking
Antenatal history on rupture of membrane, chorioamnionitis, urinary tract infection and pre-eclampsia was taken. Natal history included mode and place of delivery, the need for resuscitation and Apgar score at 1 and 5 min, while postnatal history included age of admission to NICU and symptoms suggesting infection.
Clinical assessment
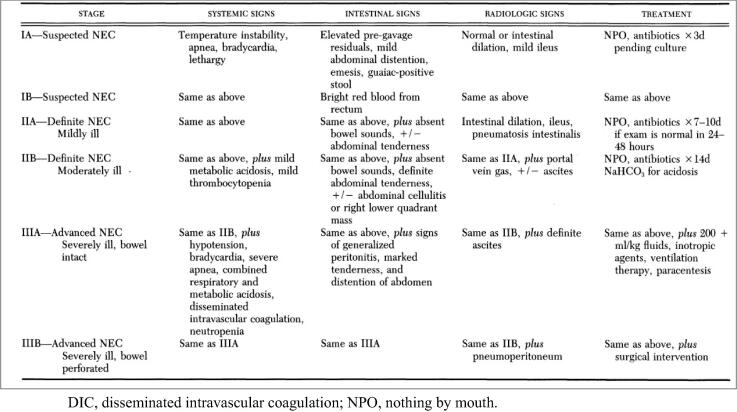
Anthropometric measurements, including weight, length and occipitofrontal circumference (OFC) were recorded. Abdominal examination was done focusing on signs of feeding intolerance, including measurement of abdominal girth, intestinal sounds, passage of stool, presence of gastric residual, abdominal tenderness and rigidity. Modified Bell’s staging criteria for NEC was used as an objective tool for diagnosis and staging of NEC (Bell et al., 1978, Walsh and Kliegman, 1986) (Fig. 1).
Fig. 1.
Modified Bell’s Staging Criteria for NEC.
Laboratory investigations and biomarker assay
Routine blood chemistry was done, including complete blood picture and coagulation profile at baseline and after 10 days. Levels of Interleukin 1 beta (IL-1β) were measured, where blood samples withdrawn at baseline and on day 10 were centrifuged for 15 min, serum was aliquoted and stored frozen at − 80 ˚C until time of assay.
IL-1β levels were assayed by Human IL-1β ELISA Kit (Elabscience Biotechnology Ltd., USA) and were measured according to the manufacturer by an ELISA Tecan Sunrise microplate reader.
2.3.4. Measurable outcomes
The primary outcome of the present study was the evidence for development of NEC. This was assessed clinically by using modified bell’s staging criteria for diagnosis of NEC and was supplemented by assessment of pro-inflammatory cytokine IL-1β levels which is considered a key mediator of inflammatory response in neonatal inflammatory disorders including NEC.
The secondary outcomes included NICU length of stay (LOS), reported adverse effects (if any) and mortality in each of the two groups.
2.4. Data management and analysis
The collected data was revised, coded, tabulated and introduced to a PC using IBM SPSS Statistics for Windows, Version 19.0.. Data was presented and suitable analysis was done according to the type of data obtained for each parameter.
For descriptive statistics Shapiro wilk test was used to evaluate normal distribution of continuous data. Continuous variables are expressed as Mean, Standard deviation (±SD) for parametric data, and Median and Interquartile range (IQR) for non-parametric data. Frequency and percentage were used for non-numerical data. Percent change was calculated as ((Last reading- baseline reading)/baseline reading)*100.
For analytical statistics, Student T-Test was used to assess the statistical significance of the difference between two study group means, Mann Whitney Test (U test) was used to assess the statistical significance of the difference of a non-parametric variable between two study groups, Chi-Square test was used to examine the relationship between two qualitative variables and Fisher’s exact test was used to examine the relationship between two qualitative variables when the expected count is less than 5 in more than 20% of cells. P less than 0.05 was considered to be statistically significant.
Sample size was calculated using PASS® program, setting the type-1 error (α) at 0.05 and the power (1-β) at 0.8. Results from a previous study by Carleson et al (Carlson et al., 1998) showed that among neonates fed with experimental formula only 2.9% developed NEC while among controls it is assumed to be 25%. Calculation according to these values produced a minimal sample size of 30 preterm neonates per each study group.
3. Results
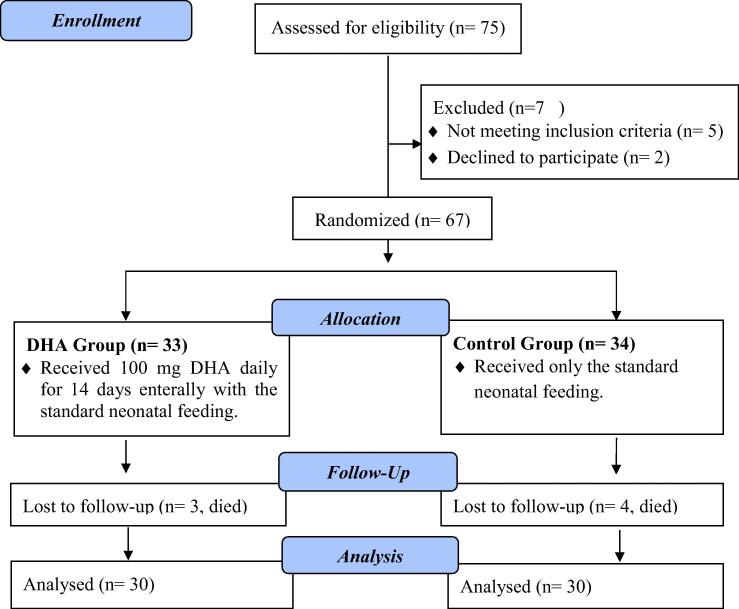
The study was conducted from October 2018 to November 2019. A total of 75 neonates were screened for eligibility and only 67 fulfilled the inclusion criteria and were randomly assigned to one of the two groups. 33 neonates were assigned to DHA group and 34 to the control group. After dropouts, due to death, 30 neonates of each group were finally analyzed (Fig. 2).
Fig. 2.
CONSORT flow chart diagram of study selection.
Baseline evaluation, with regards to gender, gestational age, age on admission, anthropometric measurements and natal history showed sufficient balancing across the groups. The mean gestational age among DHA group neonates was 31.3 ± 1.5 weeks, with a mean age on admission equal to 1.06 ± 0.36 days, versus 31.4 ± 1.61 weeks’ gestational age and 1.27 ± 0.5 days of age on admission in the control group. About 63% of the DHA group neonates had positive maternal risk factors compared to 73.3% in the control group. The two groups were comparable regarding gender (p = 0.787), gestational age (p = 0.934), age of admission (p = 0.07), natal history and anthropometric measures, except for length, where DHA group showed significantly higher mean length (p = 0.016) (Table 1).
Table 1.
Baseline evaluation.
| Parameter | DHA group (n = 30) | Control group (n = 30) | p-value |
|---|---|---|---|
| Gender; n (%) | |||
| Male | 11(36.7) | 10(33.3) | 0.787** |
| Female | 19(63.3) | 20(66.7) | |
| Gestational age (days); mean ± SD | 31.37 ± 1.5 | 31.40 ± 1.61 | 0.934* |
| Age of admission (days); mean ± SD | 1.06 ± 0.36 | 1.27 ± 0.5 | 0.07* |
| Anthropometric measures | |||
| Baseline weight (g); mean ± SD | 1306.33 ± 152.94 | 1346.67 ± 123.13 | 0.265* |
| Baseline length (cm); mean ± SD | 40.33 ± 1.97 | 39.27 ± 1.26 | 0.016* |
| Baseline OFC (cm); mean ± SD | 28.30 ± 2 | 28.07 ± 0.98 | 0.570* |
| Natal history | |||
| Mode of delivery; n (%) | |||
| LSCS | 18(60) | 20(66.7) | 0.592** |
| VD | 12(40) | 10(33.3) | |
| Maternal risk factors; n (%) | |||
| Absent | 11(36.7) | 8(26.7) | 0.405** |
| Present | 19(63.3) | 22(73.3) | |
| Need for resuscitation; n (%) | |||
| No | 9(30) | 10(33.3) | 0.781** |
| Yes | 21(70) | 20(66.7) | |
cm, centimeters; DHA, Docosahexaenoic acid; g, grams; LSCS, Lower segment caesarian section; OFC, Occipitofrontal circumference; SD, standard deviation; VD, Vaginal delivery.
Student t test; **Chi-Square Test.
Primary outcome:
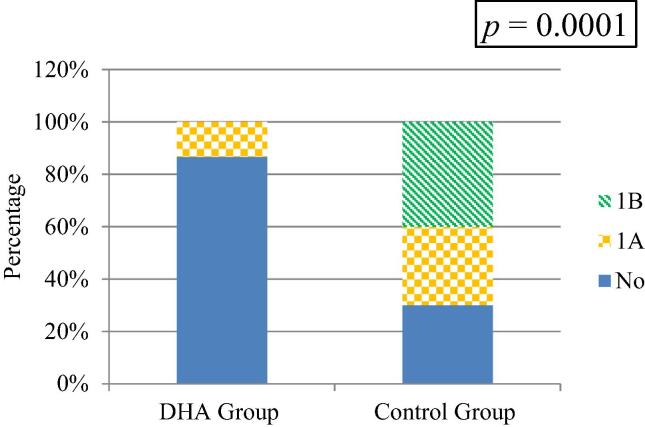
There was a statistically significant difference between the two groups regarding diagnosis and staging of NEC on day 10 (p = 0.0001). Among DHA group only 13.3% of the neonates were clinically diagnosed with NEC (stage IA), 95% CI [4.7, 28.7], whereas 30% of the control group were clinically diagnosed with NEC (stage IA), 95% CI [16, 47.7] and 30% were clinically diagnosed with NEC (stage IB), 95% CI [24, 57.8] (Fig. 3).
Fig. 3.
Diagnosis and staging of NEC on day 10 for both groups.
There was no significant difference between the DHA and the control group in baseline IL-1β levels (p = 0.09), however a significant difference between the two groups was found in IL-1β levels after 10 days (p = 0.001). Regarding percentage of change (decrease) in IL-1β levels, there was a statistically significant difference between DHA group 22(73.3), 95% CI [55.9, 86.5] and the control group 8 (26.7), 95% CI [13.5, 44.1] (p = 0.0001) (Table 2).
Table 2.
Comparison between both study groups regarding baseline, day 10 and percentage change in IL-1β levels.
| Parameter | DHA Group | Control Group | p-value |
|---|---|---|---|
| IL-1β Baseline (pg/ml); median [IQR] | 3.7 [2.4–8.8] | 8.9 [3.3–20.4] | 0.09** |
| IL-1β 10 days (pg/ml); median [IQR] | 2.9 [1.9–4.7] | 20.9 [3.4–37.2] | 0.001** |
| IL-1β change; n (%) | |||
| Decreased | 22(73.3) | 8(26.7) | 0.0001‡ |
DHA, Docosahexaenoic acid; IL-1β, Interleukin 1 beta; IQR, Interquartile range; pg/ml, picograms per millilitre.
Mann-Whitney Test; ‡Chi-Square Test.
The association between IL and 1 β change and NEC diagnosis was examined and was found to be statistically significant (p = 0.001). Only 6.7% of neonates with decreased IL-1 β levels were diagnosed with NEC (stage IA only) compared to 76.7% of neonates with increased IL-1 β levels, including 36.7 % (stage IA) and 40% (stage IB) (Table 3).
Table 3.
Relationship between IL and 1 β change and NEC diagnosis.
| IL-1 β Change |
p-value | ||
|---|---|---|---|
| Increased | Decreased | ||
| NEC diagnosis on day 10; n (%) | |||
| No | 7(23.3) | 28(93.3) | 0.001 |
| 1A | 11(36.7) | 2(6.7) | |
| 1B | 12(40.0) | 0(0) | |
IL-1β, Interleukin 1 beta; NEC, Necrotizing Enterocolitis.
Chi-Square Test.
Secondary outcomes:
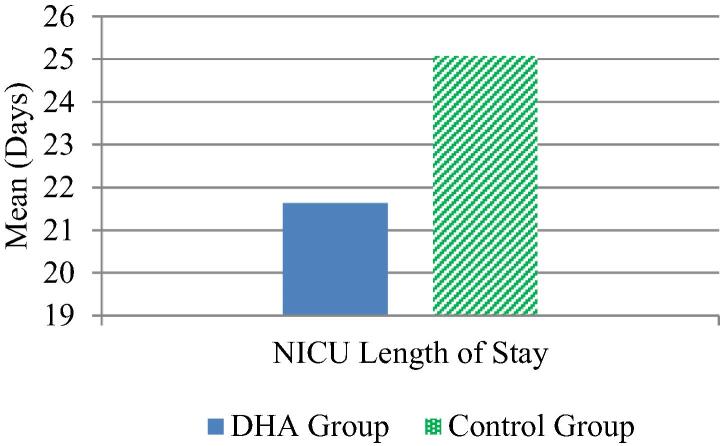
A significant difference was observed between DHA group 21.63 ± 6.67 and the control group 25.07 ± 4.67 in NICU LOS, (p = 0.025) (Fig. 4).
Fig. 4.
NICU length of stay.
There was no significant difference between the two groups regarding change in Hb (p = 0.79). However, there was a statistically significant difference between the two groups regarding changes in White blood cells (WBCS) (p = 0.002), platelets (PLT) (p = 0.001), Prothrombin time (PT) (p = 0.001), Partial thromboplastin time (PTT) (p = 0.0001) and International normalized ratio (INR) (p = 0.001) (Table 4).
Table 4.
Comparison between both study groups regarding percentage change in CBC and coagulation profile.
| Parameter | DHA Group | Control Group | p-value |
|---|---|---|---|
| Hb change; median [IQR] | 0 [−9.1–8.3] | 0 [−18.2–14.3] | 0.79 |
| WBCs change; median [IQR] | 6.3 [−41.5–29.5] | −39.8 [−52.9--10.7] | 0.002 |
| PLT change; median [IQR] | −74.4 [−157.1–3.0] | 43.5 [30.8–61.9] | 0.001 |
| Coagulation Profile | |||
| PT change; median [IQR] | 2.5 [−8.2–13.0] | −13.0 [−23.5--2.2] | 0.001 |
| PTT change; median [IQR] | 15.5 [−9.6–32.4] | −58.4 [−147.1--9.6] | 0.0001 |
| INR change; median [IQR] | 3.3 [−8.3–18.8] | −20.0 [−36.4--9.1] | 0.0001 |
DHA, Docosahexaenoic acid; Hb, Hemoglobin; WBC, White blood cells, PLT, platelets; PT, Prothrombin time; PTT, Partial thromboplastin time; INR, International normalized ratio.
Mann Whitney test.
Mortality n (%) was higher in the control group 4 (11.8) than DHA group 3 (9.1), however, no significant difference was detected (p = 1.0). Finally, on monitoring safety, DHA was well tolerated by the neonates in DHA group and no significant adverse effects were reported.
4. Discussion
To the best of our knowledge, this is the first prospective randomized controlled study that primarily assessed the potential immunoregulatory role of DHA in the prevention of NEC in preterm neonates.
Preterm neonates are a vulnerable population characterized by under-developed immune-regulatory system that fails to down regulate immune responses and the resulting pro-inflammatory cytokine cascade initiated by variable stimuli. This results in a higher risk of developing inflammatory disorders, particularly in the postnatal period.
Increasing evidence suggests that enteral supplementation of n-3 LCPUFA, including DHA, early in life can provide an immunomodulatory effect. The potential for n-3 LCPUFA to regulate the immune response is well known and has been extensively reviewed in adult as well as animal models (Calder, 2012). Results from recent studies have reported the anti-inflammatory effect of DHA manifested by suppressing the secretion of IL-1β and IL-6 (Kawano et al., 2020, Xu et al., 2019, Yuan et al., 2020).
IL-1β is a key cytokine for controlling immune tolerance during inflammation (Gagliani et al., 2014). Findings of studies including surgical and septic adults have demonstrated lower production and plasma concentration of IL-1β in the n-3 LCPUFAs group when compared to a control group (Barbosa et al., 2010, Bernabe-Garcia et al., 2014).
Results from previous human studies reported the effectiveness of high DHA fish oil in lowering proinflammatory cytokines’ levels (Ramirez-Ramirez et al., 2013, Vedin et al., 2008). Additionally, findings from animal studies have reported lower levels of pro-inflammatory mediators and/or higher levels of regulatory cytokines, thus emphasizing the anti-inflammatory capacity of n-3 LCPUFAs (Myles et al., 2014, Turner et al., 2016).
Despite the extensive literature discussing the immunomodulatory effect of n-3 LCPUFA in adults, limited studies have assessed their effects on inflammatory biomarkers as a primary outcome in preterm infants. The available data is therefore not conclusive (Richard et al., 2016, Skouroliakou et al., 2016) and given the basic differences in their immune systems, results obtained from adults cannot be extrapolated to preterm infants (Sharma et al., 2012, Strunk et al., 2011). Consequently, a considerable gap exists in the current knowledge related to the immunomodulatory effect of n-3 LCPUFA in neonates, and more specifically, in preterm.
In pursuit of addressing this gap in knowledge, the primary outcome of the present study was to assess the potential immunomodulatory effect of DHA supplementation in preterm and subsequently its effect on preventing or reducing the incidence of NEC as one of the most common inflammatory disorders in the neonatal period. The results of the present study revealed a significant difference between DHA group and the control group regarding the percentage change of IL-1β after 10 days, where 73.3% of DHA group versus 26.7% of the control group demonstrated a reduction in IL-1β levels.
These findings are in accordance with the available data from studies on preterm infants which endorse the role of DHA as a protective anti-inflammatory agent that exerts its effect while maintaining the normal development and function of underdeveloped organs (Smithers et al., 2008, Zhang et al., 2014).
In a double-blinded, randomized controlled clinical trial in preterm infants, levels of pro-inflammatory cytokines were significantly reduced in the n-3 LCPUFA group compared to soy (omega-6) group, resulting in an attenuated inflammatory response (Skouroliakou et al., 2016).
Similarly, enteral DHA supplementation of preterm and term infants with confirmed sepsis, for 14 days resulted in an attenuation in IL-1β levels and a less severe course of sepsis (López-Alarcón et al., 2012). Findings of an intervention study also revealed that enteral administration of DHA regulates the pro- and anti-inflammatory cytokines levels (Bernabe-Garcia et al., 2016).
In another previous study, mononuclear cells obtained from neonatal cord blood, initially pretreated with DHA and then stimulated with LPS endotoxin, yielded a concentration-dependent inhibition of the secretion of IL-1β, IL-6 and TNF-α (Espiritu et al., 2016). Likewise, in a study conducted on infants who underwent cardiopulmonary bypass, infants who received intravenous fish oil had significantly lower circulating concentrations of IL-1β, IL-6 and TNF-α in comparison to control infants (Larsen et al., 2012).
Moreover, findings from previous randomized trials suggest that maternal DHA supplementation, particularly throughout the third trimester of pregnancy and/or during lactation, can modulate cytokine production, increasing levels of anti-inflammatory cytokines and suppressing pro-inflammatory cytokines expression in both the mother and the infant (Rodriguez-Santana et al., 2017, Valentine et al., 2019).
By contrast, findings from multicenter N3RO randomized controlled trial suggest that daily supplementation of 60 mg/kg of DHA does not modulate the release of pro-inflammatory or regulatory mediators in preterm infants born at less than 29 weeks’ gestation. The discrepancy between the results of N3RO trial and other studies, including ours, may imply that the effect of DHA on cytokine production in preterm infants can be influenced by factors as dose, duration, gestational age, and route of administration (Collins et al., 2017).
Data from term infants also substantiate the immunoregulatory effect of DHA. Findings of a previous study on term neonates suggest that n-3 LCPUFA can impact the extent of T-cell maturation as well as the effector memory T-cells development (Field et al., 2008). In another study, including immune cells from term infants with history of maternal fish oil supplementation during pregnancy, lower levels of pro-inflammatory cytokines following in vitro stimulation by allergens were reported (Dunstan et al., 2003).
A robust immune response resulting in an excessive release of inflammatory mediators is considered a hallmark of neonatal inflammatory disorders such as sepsis, NEC, bronchopulmnary dysplasia (BPD) and retinopathy of prematurity (ROP) (Sharma et al., 2012). The pathogenesis of these disorders is multi-factorial. Accordingly, a comprehensive treatment or a single medication is not available. Minimizing the injury resulting from unregulated inflammation is thus a priority for clinicians while infants are in NICU. An anti-inflammatory nutritional intervention in the early postnatal period is a compelling option.
A growing body of evidence suggests that n-3 LCPUFA may reduce the incidence or severity of the common inflammatory disorders and comorbidities of prematurity by influencing various steps of the immune and anti-inflammatory response (Lapillonne and Moltu, 2016).
Results of the present study demonstrated a significant difference between DHA group and the control group regarding NEC development, as diagnosed clinically using modified bell’s staging criteria. 13.3% of DHA group were diagnosed as stage IA NEC on day 10 whereas in the control group 30% were diagnosed as stage IA NEC and 30% as stage IB NEC. According to these findings, none of the NEC diagnosed cases in the DHA group proceeded to stage IB unlike the control group where half the NEC diagnosed cases were stage IB. This may imply that DHA supplementation can affect the incidence as well as the severity of NEC. However, further powered studies are needed to confirm these findings. The current study also revealed a statistically significant association between IL and 1 β change and NEC diagnosis. Only 6.7% of neonates with decreased IL-1 β levels were diagnosed with stage IA NEC compared to 76.7% of neonates with increased IL-1 β levels, including 36.7 % stage IA NEC and 40% stage IB NEC.
The study findings agree with data reported regarding the protective effect of n-3 LCPUFA against sepsis (Al-Biltagi et al., 2017). The findings are also consistent with those of a recent study suggesting that daily enteral DHA supplementation can prevent NEC in preterm infants (Bernabe-García et al., 2021). Unlike our study, results from that study only took into consideration neonates with confirmed NEC (evaluated with Bell’s scale from stage ≥ IIA). Also, the study did not assess markers of immune function or inflammation. The protective effect of n-3 supplementation against development of NEC and mucosal inflammation has also been reported in animal models (Andersen et al., 2011, Ohtsuka et al., 2011). Data are promising but further large-scale human studies should be conducted to demonstrate the effect of DHA on NEC incidence and to elucidate the potential mechanism of action.
Moreover, when randomized controlled trials targeting only preterm infants born at 32 weeks’ gestation were examined in a systematic review, evidence for the potential protective effect of n-3 LCPUFA supplementation on NEC and BPD was suggested (Zhang et al., 2014).
This finding is of paramount clinical significance because early preterm neonates are born before the process of transfer of n-3 LCPUFA from mother to fetus is complete and consequently they are at high risk for deficiency of prematurity, potentially predisposing them to adverse neonatal outcomes. These results are also consistent with results from observational studies, signifying an association between DHA levels and adverse neonatal outcomes in preterm infants (Martin et al., 2011, Skouroliakou et al., 2012).
NEC is associated with multifarious consequences. Studies revealed substantially longer length of NICU LOS for NEC patients compared to neonates without NEC (Ganapathy et al., 2012, Johnson et al., 2015). Extended NICU stay is commonly associated with increased risk of infection, poor neurodevelopmental outcomes and higher costs (DeSena et al., 2015).
Reducing the length of the NICU stay is thus a major concern. Findings of the present study suggest that DHA supplementation can have a positive impact on reducing the length of NICU stay in DHA group 21.63 ± 6.67 compared to the control group 25.07 ± 4.67. This agrees with the findings of an intervention study on neonates undergoing cardiovascular surgery where shorter NICU stay was observed in the DHA group (Bernabe-Garcia et al., 2016). Findings are also in consonance with data from a meta-analysis reporting shorter stay in ICUs for surgical adults who received n-3 LCPUFA (Chen et al., 2010).
Interestingly, DHA was well tolerated by the neonates in the intervention group and no significant adverse effects were reported. These findings added to the increasing evidence that daily enteral DHA supplementation, at a wide range of doses, is well tolerated (Collins et al., 2015, Henriksen et al., 2008). Generally, the Food and Drug Administration (FDA) does not evaluate nutritional supplements, however, the agency has verified the overall safety of fish/DHA oil, with no substantial adverse effects being reported in humans at doses from 25 to 5900 mg/kg/day (Lien and Clandinin, 2009). The safety profile also spreads to the infant population, including preterm (Baack et al., 2016).
The current study has several strengths. It was a randomized double-blind study. It is considered one of the limited studies whose primary outcome was clinical diagnosis of NEC as well as assessment of proinflammatory cytokines levels in preterm neonates.Also the loss to follow-up was limited. Nevertheless, there are some Limitations. Since the study was conducted at a single NICU, the results cannot be generalized. The small blood volume that could be safely and ethically obtained from preterm for research purposes is an unavoidable limitation. The limited number of neonates hindered the detection of small group differences; thus, further large, multicenter, randomized controlled trials must be conducted to confirm our findings.
5. Conclusions
Findings of this study suggest that enteral DHA supplementation can reduce NEC nincidence in preterm neonates. The study also adds to the growing evidence on the role of n-3 LCPUFA in suppressing the release of proinflammatory cytokines and thus protecting against inflammatory disorders in neonates.
Funding Source
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to thank all the neonatal intensive care unit staff for their help and support.
Footnotes
Peer review under responsibility of King Saud University.
References
- Al-Biltagi M.A.M., Abo-Elezz A.A.E., Abd-Elhafez M.A., Mabrouk M.M., Suliman G.A. Beneficial Effects of Omega-3 Supplement to the Enteral Feeding in Children with Mild to Moderate Sepsis. J. Intensive Care Med. 2017 doi: 10.1177/0885066615623927. [DOI] [PubMed] [Google Scholar]
- Andersen, A.D., Mølbak, L., Thymann, T., Michaelsen, K.F., Lauritzen, L., 2011. Dietary long-chain n-3 PUFA, gut microbiota and fat mass in early postnatal piglet development-exploring a potential interplay. Prostaglandins Leukot. Essent. Fat. Acids. 10.1016/j.plefa.2011.08.004. [DOI] [PubMed]
- Baack M.L., Puumala S.E., Messier S.E., Pritchett D.K., Harris W.S. Daily Enteral DHA Supplementation Alleviates Deficiency in Premature Infants. Lipids. 2016 doi: 10.1007/s11745-016-4130-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa V.M., Miles E.A., Calhau C., Lafuente E., Calder P.C. Effects of a fish oil containing lipid emulsion on plasma phospholipid fatty acids, inflammatory markers, and clinical outcomes in septic patients: A randomized, controlled clinical trial. Crit. Care. 2010 doi: 10.1186/cc8844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck, S., Wojdyla, D., Say, L., Betran, A.P., Merialdi, M., Requejo, J.H., Rubens, C., Menon, R., Van Look, P.F.A., 2010. 1. Owen J, Mancuso M. Cervical Cerclage for the Prevention of Preterm Birth. Vol. 39, Obstetrics and Gynecology Clinics of North America. 2012. p. 25–33. Bull. World Health Organ. [DOI] [PubMed]
- Bell, M.J., Ternberg, J.L., Feigin, R.D., Keating, J.P., Marshall, R., Barton, L., Brotherton, T., 1978. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann. Surg. 10.1097/00000658-197801000-00001. [DOI] [PMC free article] [PubMed]
- Bernabe-García M., Calder P.C., Villegas-Silva R., Rodríguez-Cruz M., Chávez-Sánchez L., Cruz-Reynoso L., Mateos-Sánchez L., Lara-Flores G., Aguilera-Joaquín A.R., Sánchez-García L. Efficacy of Docosahexaenoic Acid for the Prevention of Necrotizing Enterocolitis in Preterm Infants: A Randomized Clinical Trial. Nutrients. 2021;13:648. doi: 10.3390/nu13020648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernabe-Garcia, M., Lopez-Alarcon, M., Mansilla- Olivares, A., Maldonado-Hernandez, J., Blanco-Favela, F., Chavez-Sanchez, L., Chavez-Rueda, K., Mancilla-Ramirez, J., Arriaga-Pizano, L., Riera-Kinkel, C., 2014. Oral administration of n-3 long-chain fatty acids reduce inflammatory response and improve clinical outcomes in patients with cardiovascular surgery. Exp. Clin. Cardiol.
- Bernabe-Garcia M., Lopez-Alarcon M., Villegas-Silva R., Mancilla-Ramirez J., Rodriguez-Cruz M., Maldonado-Hernandez J., Chavez-Rueda K.A., Blanco-Favela F., Espinoza-Garcia L., Lagunes-Salazar S. Beneficial Effects of Enteral Docosahexaenoic Acid on the Markers of Inflammation and Clinical Outcomes of Neonates Undergoing Cardiovascular Surgery: An Intervention Study. Ann. Nutr. Metab. 2016 doi: 10.1159/000447498. [DOI] [PubMed] [Google Scholar]
- Calder P.C. Mechanisms of action of (n-3) fatty acids. J. Nutr. 2012 doi: 10.3945/jn.111.155259. [DOI] [PubMed] [Google Scholar]
- Carlson S.E., Montalto M.B., Ponder D.L., Werkman S.H., Korones S.B. Lower incidence of necrotizing enterocolitis in infants fed a preterm formula with egg phospholipids. Pediatr. Res. 1998 doi: 10.1203/00006450-199810000-00005. [DOI] [PubMed] [Google Scholar]
- Chen A.C., Chung M.Y., Chang J.H., Lin H.C. Pathogenesis implication for necrotizing enterocolitis prevention in preterm very-low-birth-weight infants. J. Pediatr. Gastroenterol. Nutr. 2014 doi: 10.1097/MPG.0b013e3182a7dc74. [DOI] [PubMed] [Google Scholar]
- Chen B., Zhou Y., Yang P., Wan H.W., Wu X.T. Safety and efficacy of fish oil-enriched parenteral nutrition regimen on postoperative patients undergoing major abdominal surgery: A meta-analysis of randomized controlled trials. J. Parenter. Enter. Nutr. 2010 doi: 10.1177/0148607110362532. [DOI] [PubMed] [Google Scholar]
- Collins, C.T., Gibson, R.A., Makrides, M., McPhee, A.J., Sullivan, T.R., Davis, P.G., Thio, M., Simmer, K., Rajadurai, V.S., Team, N.I., Ryan, P., Morris, S., Stark, M., Travadi, J., Wright, I., Tan, K., Holberton, J., Opie, G., Callander, I., Stack, J., Shein, D., Bellhouse, S., Bolisetty, S., Lui, K., Liley, H., Berry, M., Harris, D., Chua, M.C., Agarwal, P., 2017. Docosahexaenoic acid and bronchopulmonary dysplasia in preterm infants. N. Engl. J. Med. [DOI] [PubMed]
- Collins, C.T., Sullivan, T.R., McPhee, A.J., Stark, M.J., Makrides, M., Gibson, R.A., 2015. A dose response randomised controlled trial of docosahexaenoic acid (DHA) in preterm infants. Prostaglandins Leukot. Essent. Fat. Acids. 10.1016/j.plefa.2015.04.003. [DOI] [PubMed]
- DeSena H.C., Nelson D.P., Cooper D.S. Cardiac intensive care for the neonate and child after cardiac surgery. Curr. Opin. Cardiol. 2015 doi: 10.1097/HCO.0000000000000127. [DOI] [PubMed] [Google Scholar]
- Dunstan J.A., Mori T.A., Barden A., Beilin L.J., Taylor A.L., Holt P.G., Prescott S.L. Fish oil supplementation in pregnancy modifies neonatal allergen-specific immune responses and clinical outcomes in infants at high risk of atopy: A randomized, controlled trial. J. Allergy Clin. Immunol. 2003 doi: 10.1016/j.jaci.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Ellard, D.M., Anderson, D.M., Cloherty, J.P., Eichenwald, E.C., Stark, A.R., 2012. Manual of neonatal care.
- Espiritu M.M., Lin H., Foley E., Tsang V., Rhee E., Perlman J., Cunningham-Rundles S. Omega-3 fatty acids modulate neonatal cytokine response to endotoxin. J. Perinat. Med. 2016 doi: 10.1515/jpm-2015-0248. [DOI] [PubMed] [Google Scholar]
- Field C.J., Van Aerde J.E., Robinson L.E., Clandinin M.T. Effect of providing a formula supplemented with long-chain polyunsaturated fatty acids on immunity in full-term neonates. Br. J. Nutr. 2008 doi: 10.1017/S0007114507791845. [DOI] [PubMed] [Google Scholar]
- Fink N.H., Collins C.T., Gibson R.A., Makrides M., Penttila I.A. Targeting inflammation in the preterm infant: The role of the omega-3 fatty acid docosahexaenoic acid. J. Nutr. Intermed. Metab. 2016 doi: 10.1016/j.jnim.2016.03.004. [DOI] [Google Scholar]
- Gagliani N., Palm N.W., de Zoete M.R., Flavell R.A. Inflammasomes and intestinal homeostasis: Regulating and connecting infection, inflammation and the microbiota. Int. Immunol. 2014 doi: 10.1093/intimm/dxu066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganapathy, V., Hay, J.W., Kim, J.H., 2012. Costs of necrotizing enterocolitis and cost-effectiveness of exclusively human milk-based products in feeding extremely premature infants. Breastfeed. Med. 10.1089/bfm.2011.0002. [DOI] [PubMed]
- Good, M., Sodhi, C.P., Hackam, D.J., 2014. Evidence-based feeding strategies before and after the development of necrotizing enterocolitis. Expert Rev. Clin. Immunol. 10.1586/1744666X.2014.913481. [DOI] [PMC free article] [PubMed]
- Harris W.S., Baack M.L. Beyond building better brains: Bridging the docosahexaenoic acid (DHA) gap of prematurity. J. Perinatol. 2015 doi: 10.1038/jp.2014.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen C., Haugholt K., Lindgren M., Aurvåg A.K., Rønnestad A., Grønn M., Solberg R., Moen A., Nakstad B., Berge R.K., Smith L., Iversen P.O., Drevon C.A. Improved cognitive development among preterm infants attributable to early supplementation of human milk with docosahexaenoic acid and arachidonic acid. Pediatrics. 2008 doi: 10.1542/peds.2007-1511. [DOI] [PubMed] [Google Scholar]
- Ilardi L., Proto A., Ceroni F., Morniroli D., Martinelli S., Mosca F., Giannì M.L. Overview of Important Micronutrients Supplementation in Preterm Infants after Discharge: A Call for Consensus. Life. 2021;11:331. doi: 10.3390/life11040331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson T.J., Patel A.L., Bigger H.R., Engstrom J.L., Meier P.P. Cost savings of human milk as a strategy to reduce the incidence of necrotizing enterocolitis in very low birth weight infants. Neonatology. 2015 doi: 10.1159/000370058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J.X., Weylandt K.H. Modulation of inflammatory cytokines by omega-3 fatty acids. Subcell. Biochem. 2008 doi: 10.1007/978-1-4020-8831-5_5. [DOI] [PubMed] [Google Scholar]
- Kawano, A., Ariyoshi, W., Yamasaki, R., Yoshioka, Y., Kashiwagi, K., Namikawa, D., Nishihara, T., Okinaga, T., 2020. Docosahexaenoic acid attenuates cell death and interleukin-1beta secretion in THP-1 cells responded to Aggregatibacter actinomycetemcomitans invasion.
- Khashana, A., Moussa, R., 2016. Incidence of feeding intolerance in preterm neonates in neonatal intensive care units, Port Said, Egypt. J. Clin. Neonatol. 10.4103/2249-4847.194165.
- Klevebro S., Juul S.E., Wood T.R. A More Comprehensive Approach to the Neuroprotective Potential of Long-Chain Polyunsaturated Fatty Acids in Preterm Infants Is Needed—Should We Consider Maternal Diet and the n-6: n-3 Fatty Acid Ratio? Front. Pediatr. 2020;7:533. doi: 10.3389/fped.2019.00533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapillonne A., Moltu S.J. Long-chain polyunsaturated fatty acids and clinical outcomes of preterm infants. Ann. Nutr. Metab. 2016 doi: 10.1159/000448265. [DOI] [PubMed] [Google Scholar]
- Larsen B.M.K., Goonewardene L.A., Joffe A.R., Van Aerde J.E., Field C.J., Olstad D.L., Clandinin M.T. Pre-treatment with an intravenous lipid emulsion containing fish oil (eicosapentaenoic and docosahexaenoic acid) decreases inflammatory markers after open-heart surgery in infants: A randomized, controlled trial. Clin. Nutr. 2012 doi: 10.1016/j.clnu.2011.11.006. [DOI] [PubMed] [Google Scholar]
- Lee J.Y., Plakidas A., Lee W.H., Heikkinen A., Chanmugam P., Bray G., Hwang D.H. Differential modulation of Toll-like receptors by fatty acids: Preferential inhibition by n-3 polyunsaturated fatty acids. J. Lipid Res. 2003 doi: 10.1194/jlr.M200361-JLR200. [DOI] [PubMed] [Google Scholar]
- Lee J.Y., Zhao L., Youn H.S., Weatherill A.R., Tapping R., Feng L., Lee W.H., Fitzgerald K.A., Hwang D.H. Saturated Fatty Acid Activates but Polyunsaturated Fatty Acid Inhibits Toll-like Receptor 2 Dimerized with Toll-like Receptor 6 or 1. J. Biol. Chem. 2004 doi: 10.1074/jbc.M312990200. [DOI] [PubMed] [Google Scholar]
- Lien V.W., Clandinin M.T. Dietary assessment of arachidonic acid and docosahexaenoic acid intake in 4–7 year-old children. J. Am. Coll. Nutr. 2009 doi: 10.1080/07315724.2009.10719755. [DOI] [PubMed] [Google Scholar]
- Lin P.W., Stoll B.J. Necrotising enterocolitis. Lancet. 2006 doi: 10.1016/S0140-6736(06)69525-1. [DOI] [PubMed] [Google Scholar]
- López-Alarcón M., Bernabe-García M., Del Valle O., González-Moreno G., Martínez-Basilea A., Villegas R. Oral administration of docosahexaenoic acid attenuates interleukin-1β response and clinical course of septic neonates. Nutrition. 2012 doi: 10.1016/j.nut.2011.07.016. [DOI] [PubMed] [Google Scholar]
- Maheshwari A., Schelonka R.L., Dimmitt R.A., Carlo W.A., Munoz-Hernandez B., Das A., McDonald S.A., Thorsen P., Skogstrand K., Hougaard D.M., Higgins R.D. Cytokines associated with necrotizing enterocolitis in extremely-low-birth- weight infants. Pediatr. Res. 2014 doi: 10.1038/pr.2014.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C.R., Dasilva D.A., Cluette-Brown J.E., Dimonda C., Hamill A., Bhutta A.Q., Coronel E., Wilschanski M., Stephens A.J., Driscoll D.F., Bistrian B.R., Ware J.H., Zaman M.M., Freedman S.D. Decreased postnatal docosahexaenoic and arachidonic acid blood levels in premature infants are associated with neonatal morbidities. J. Pediatr. 2011 doi: 10.1016/j.jpeds.2011.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizrahi A., Barlow O., Berdon W., Blanc W.A., Silverman W.A. Necrotizing enterocolitis in premature infants. J. Pediatr. 1965 doi: 10.1016/S0022-3476(65)80003-8. [DOI] [PubMed] [Google Scholar]
- Myles I.A., Pincus N.B., Fontecilla N.M., Datta S.K. Effects of parental omega-3 fatty acid intake on offspring microbiome and immunity. PLoS One. 2014 doi: 10.1371/journal.pone.0087181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuka Y., Okada K., Yamakawa Y., Ikuse T., Baba Y., Inage E., Fujii T., Izumi H., Oshida K., Nagata S., Yamashiro Y., Shimizu T. ω-3 fatty acids attenuate mucosal inflammation in premature rat pups. J. Pediatr. Surg. 2011 doi: 10.1016/j.jpedsurg.2010.07.032. [DOI] [PubMed] [Google Scholar]
- Ramirez-Ramirez, V., Macias-Islas, M.A., Ortiz, G.G., Pacheco-Moises, F., Torres-Sanchez, E.D., Sorto-Gomez, T.E., Cruz-Ramos, J.A., Orozco-Aviña, G., Celis De La Rosa, A.J., 2013. Efficacy of fish oil on serum of TNF α, IL-1 β, and IL-6 oxidative stress markers in multiple sclerosis treated with interferon beta-1b. Oxid. Med. Cell. Longev. 10.1155/2013/709493. [DOI] [PMC free article] [PubMed]
- Rasiah V., Yajamanyam P.K., Ewer A.K. Necrotizing enterocolitis: current perspectives. Res. Reports Neonatol. 2014 doi: 10.2147/rrn.s36576. [DOI] [Google Scholar]
- Richard C., Lewis E.D., Field C.J. Evidence for the essentiality of arachidonic and docosahexaenoic acid in the postnatal maternal and infant diet for the development of the infant’s immune system early in life. Appl. Physiol. Nutr. Metab. 2016 doi: 10.1139/apnm-2015-0660. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Santana, Y., Ochoa, J.J., Lara-Villoslada, F., Kajarabille, N., Saavedra-Santana, P., Hurtado, J.A., Peña, M., Diaz-Castro, J., Sebastian-Garcia, I., Machin-Martin, E., Villanueva, M., Ramirez-Garcia, O., Peña-Quintana, L., 2017. Cytokine distribution in mothers and breastfed children after omega-3 LCPUFAs supplementation during the last trimester of pregnancy and the lactation period: A randomized, controlled trial. Prostaglandins Leukot. Essent. Fat. Acids. 10.1016/j.plefa.2017.09.006. [DOI] [PubMed]
- Sharma A.A., Jen R., Butler A., Lavoie P.M. The developing human preterm neonatal immune system: A case for more research in this area. Clin. Immunol. 2012 doi: 10.1016/j.clim.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulhan, J., Dicken, B., Hartling, L., Larsen, B.M.K., 2017. Current knowledge of necrotizing enterocolitis in preterm infants and the impact of different types of enteral nutrition products. Adv. Nutr. 10.3945/an.116.013193. [DOI] [PMC free article] [PubMed]
- Skouroliakou, M., Konstantinou, D., Agakidis, C., Delikou, N., Koutri, K., Antoniadi, M., Karagiozoglou-Lampoudi, T., 2012. Cholestasis, bronchopulmonary dysplasia, and lipid profile in preterm infants receiving MCT/ω-3-PUFA-containing or soybean-based lipid emulsions. Nutr. Clin. Pract. 10.1177/0884533612454547. [DOI] [PubMed]
- Skouroliakou, M., Konstantinou, D., Agakidis, C., Kaliora, A., Kalogeropoulos, N., Massara, P., Antoniadi, M., Panagiotakos, D., Karagiozoglou-Lampoudi, T., 2016. Parenteral MCT/ω-3 Polyunsaturated Fatty Acid-Enriched Intravenous Fat Emulsion is Associated with Cytokine and Fatty Acid Profiles Consistent with Attenuated Inflammatory Response in Preterm Neonates: A Randomized, Double-Blind Clinical Trial. Nutr. Clin. Pract. 10.1177/0884533615602011. [DOI] [PubMed]
- Smithers, L.G., Gibson, R.A., McPhee, A., Makrides, M., 2008. Effect of long-chain polyunsaturated fatty acid supplementation of preterm infants on disease risk and neurodevelopment: A systematic review of randomized controlled trials. Am. J. Clin. Nutr. 10.1093/ajcn/87.4.912. [DOI] [PubMed]
- Strunk T., Currie A., Richmond P., Simmer K., Burgner D. Innate immunity in human newborn infants: Prematurity means more than immaturity. J. Matern. Neonatal Med. 2011 doi: 10.3109/14767058.2010.482605. [DOI] [PubMed] [Google Scholar]
- Turner J.M., Josephson J., Field C.J., Wizzard P.R., Ball R.O., Pencharz P.B., Wales P.W. Liver Disease, Systemic Inflammation, and Growth Using a Mixed Parenteral Lipid Emulsion, Containing Soybean Oil, Fish Oil, and Medium Chain Triglycerides, Compared with Soybean Oil in Parenteral Nutrition-Fed Neonatal Piglets. J. Parenter. Enter. Nutr. 2016 doi: 10.1177/0148607115579711. [DOI] [PubMed] [Google Scholar]
- Valentine C.J., Dingess K.A., Kleiman J., Morrow A.L., Rogers L.K. A Randomized Trial of Maternal Docosahexaenoic Acid Supplementation to Reduce Inflammation in Extremely Preterm Infants. J. Pediatr. Gastroenterol. Nutr. 2019 doi: 10.1097/MPG.0000000000002375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedin I., Cederholm T., Levi Y.F., Basun H., Garlind A., Irving G.F., Jönhagen M.E., Vessby B., Wahlund L.O., Palmblad J. Effects of docosahexaenoic acid-rich n-3 fatty acid supplementation on cytokine release from blood mononuclear leukocytes: The OmegAD study. Am. J. Clin. Nutr. 2008 doi: 10.1093/ajcn/87.6.1616. [DOI] [PubMed] [Google Scholar]
- Walsh M.C., Kliegman R.M. Necrotizing enterocolitis: Treatment based on staging criteria. Pediatr. Clin. North Am. 1986 doi: 10.1016/S0031-3955(16)34975-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, F., Song, Y., Guo, A., 2019. Anti-apoptotic effects of docosahexaenoic acid in il-1β-induced human chondrosarcoma cell death through involvement of the mapk signaling pathway. Cytogenet. Genome Res. 10.1159/000500290. [DOI] [PubMed]
- Yuan, S., Li, H., Yang, C., Xie, Wenyi, Wang, Y., Zhang, J., Mao, Z., Xie, Weibing, Lü, T., 2020. DHA attenuates Aβ-induced necroptosis through the RIPK1/RIPK3 signaling pathway in THP-1 monocytes. Biomed. Pharmacother. 126, 110102. [DOI] [PubMed]
- Zhang P., Lavoie P.M., Lacaze-Masmonteil T., Rhainds M., Marc I. Omega-3 long-chain polyunsaturated fatty acids for extremely preterm infants: A systematic review. Pediatrics. 2014 doi: 10.1542/peds.2014-0459. [DOI] [PubMed] [Google Scholar]
- Zhou P., Li Y., Ma L.Y., Lin H.C. The role of immunonutrients in the prevention of necrotizing enterocolitis in preterm very low birth weight infants. Nutrients. 2015 doi: 10.3390/nu7095334. [DOI] [PMC free article] [PubMed] [Google Scholar]