Abstract
Patient: Female, 12-year-old
Final Diagnosis: Ulcerative
Symptoms: Bloody diarrhea
Medication:—
Clinical Procedure: —
Specialty: Gastroenterology and Hepatology
Objective:
Rare disease
Background:
Ulcerative colitis (UC) is a chronic autoimmune inflammatory disease of the colon that infrequently affects children. The disease requires immunosuppressive therapy to achieve remission and keep the disease in remission. Currently, many therapies are approved for use in pediatric patients with UC, including steroid, 5-aminosalicylic acid (5-ASA), azathioprine, and biologic therapy with anti-tumor necrosis factor (TNF) inhibitors. Despite their efficacy, many patients have refractory severe disease that fails therapy and may require surgical interventions. Recently, the small molecule Janus Kinase (JAK) inhibitor tofacitinib has been approved for moderate to severe UC that fails biologic therapy in adults. However, the safety and efficacy of this drug has not been tested in pediatric UC patients.
Case Report:
We describe a case of a 13-year-old girl with 2-year history of severe UC who had secondary loss response to both infliximab and adalimumab over 2 years, despite adequate trough serum drug levels and the concomitant use of azathioprine. She was also dependent on steroid to control her disease. Infectious work-ups were always negative for infectious organisms. She was then successfully treated with tofacitinib 5 mg orally twice daily. She went into complete clinical, endoscopic, and steroid-free remission.
Conclusions:
This case report highlights the safety and efficacy of tofacitinib in pediatric patients with severe refractory UC, potentially avoiding proctocolectomy in this young patient population. Future research should study the role of tofacitinib in patients with moderate to severe UC in children.
Keywords: JAK1 Protein, Human; Pediatric Ulcerative Colitis; Tofacitinib
Background
Ulcerative colitis (UC) is a chronic, inflammatory, autoimmune, potentially progressive disorder of the colon that has no cure. Patients often need to be on long-term medical therapy to keep the disease in remission. The etiology of the disease is multifactorial, with genetic and environmental factors believed to be involved. The mean age of onset is usually in early adulthood (15–30 years of age); however, it can start in children or in the elderly, but it is less common in these populations [1]. Treatment of UC varies depending on severity of illness. Both 5-aminosalicylic acid (5-ASA) and azathioprine are recommended for mild to moderate disease, while steroids are used to treat acute exacerbation of the disease. In addition, biological therapies, such as tumor necrosis factor inhibitor (anti-TNF), vedolizumab, and ustekinumab, are recommended for moderate to severe ulcerative colitis in adults. The options in children are more limited since neither vedolizumab nor ustekinumab are approved for use in this population. Moreover, Tofacitinib, a Janus kinase (JAK) inhibitor, is recommended by the American Gastroenterology Association (AGA) as second- or third-line therapy for those who failed at least one class of biologic therapy [2]. However, it has not been approved for use in patients below the age of 18 years. In this case report, we describe a 13-year-old girl who responded clinically and endoscopically to tofacitinib after failing 2 anti-TNFs, had been steroid-dependent, and was planned to undergo proctocolectomy as a salvage therapy. We highlight the importance of considering tofacitinib as a treatment option in children.
Case Report
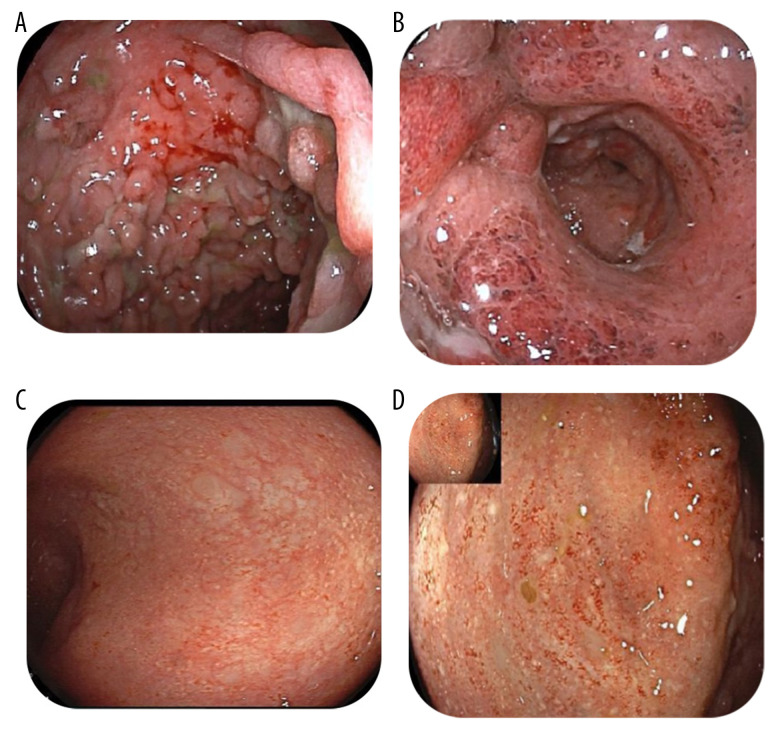
A 13-year-old girl with 2-year history of severe UC was assessed for worsening symptoms. She was originally diagnosed with severe UC that was steroid-refractory and only responded to induction with infliximab (10 mg/kg). The patient’s pediatric ulcerative colitis index (PUCAI) was 80 points, her body weight was 40 kg, and her body mass index (BMI) was 17.3 kg/m2 (Figure 1A). She had an initial response and remained in steroid-free remission for about 6 months on combination therapy (infliximab 10 mg/kg every 8 weeks and azathioprine 2.5 mg/kg). Her PUCAI score was 0 and her BMI increased to 19.3 kg/m2 (Figure 1B). Unfortunately, after 6 months of infliximab therapy, she had an exacerbation of her disease and was readmitted. An infectious disease work-up was negative for infectious organisms, including clostridium difficile and CMV colitis. Flexible sigmoidoscopy confirmed severe extensive colitis (Mayo 3). Her infliximab drug level was undetectable and antibodies against infliximab were high, at 30 AU/ml (normal <5 AU/ml), confirming secondary loss of response due to immunogenicity. Her PUCAI score was 80 and her BMI dropped again to 17.3 kg/m2 (Figure 1C). She received a prednisone 40 mg tapering course and clinical remission was achieved. She was switched to adalimumab 40 mg every 2 weeks subcutaneously with azathioprine (2.5 mg/kg) for maintenance. She remained in clinical and biochemical remission for 9 months before having another relapse. At this stage, the infectious diseases work-up was negative and sigmoidoscopy confirmed severe extensive colitis (Mayo 3) with multiple pseudopolyps (Figure 2A, 2B). Given that her drug level was sub-optimal, with adalimumab levels of 5 ug/ml (therapeutic level >7.5 ug/ml) and adalimumab antibodies of 5 (normal range <10 AU/ml), the dose of adalimumab was increased to 40 mg weekly and eventually to 80 mg weekly based on therapeutic drug monitoring (DMT). She received another tapering course of prednisone 40 mg. Unfortunately, the patient was not able to wean off steroid completely, with rapid recurrence of symptoms after the prednisone dose was decreased to 15 mg/day, despite a therapeutic adalimumab level (12 ug/ml) but adalimumab antibodies of 20 (normal range <10 AU/ml). Her laboratory results showed the following: WBC: 4.6 (reference 3.9–10×109 cells/L), hemoglobin: 81 g/L (reference 120–150 g/L), MCV: 71.6 fL (reference 83–101fL), platelets 362 000 mcL (reference 130 000–430 000 mcL), albumin 28 g/L (reference 35–50 g/L), C-reactive protein 110 mg/L (reference 0–10 mg/L), and ESR 74 mm/hr (reference 0–20 mm/h). She was also seen by our dietician, who tried a total exclusive enteral nutrition (EEN) diet with no satisfactory clinical response. She was assessed by our colorectal surgical team, who offered proctocolectomy but the patient’s parents refused that option. Her BMI was still at 17.3 kg/m2 and the PUCAI score was 80.
Figure 1.
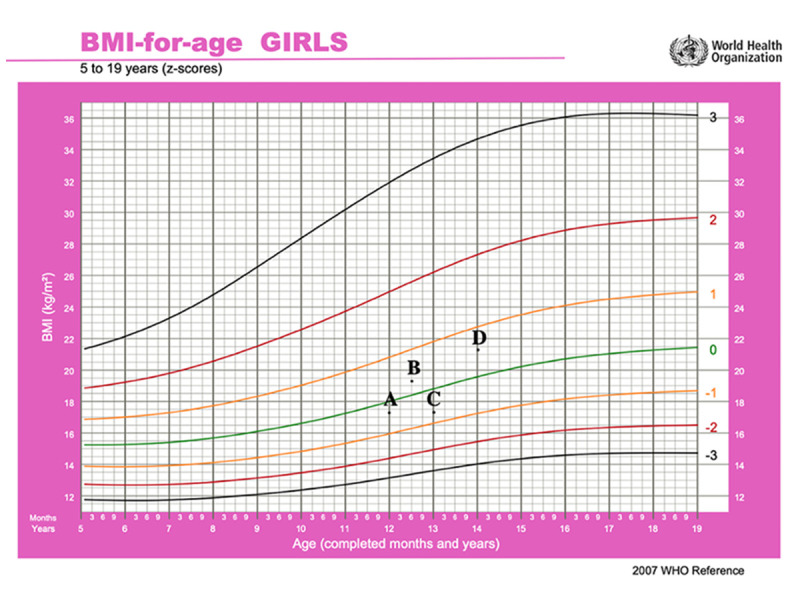
This graph represents the body mass index (BMI-for-age) based on the World Health Organization method. (A) At the onset of the disease with BMI of 17.3 kg/m2; (B) When she was on infliximab 10 mg/kg every 8 weeks and azathioprine 2.5 mg/kg), her BMI was 19.3 kg/m2. (C) When she relapsed after failing infliximab with BMI of 17.3 kg/m2. (D) The current body mass index BMI is 21.2 kg/m2.
Figure 2.
PicturesA and B showing the rectum and sigmoid colon, respectively, before starting tofacitinib, with severe ulcerations, inflammation, loss of vascularity, pseudopolyps, and severely deformed lumen. Pictures C and D showing the rectum and sigmoid, respectively, 9 months after starting tofacitinib, with no ulcerations or erosions except for mild patchy erythema.
At this stage, other medical options were discussed for off-label use. Unfortunately, both vedolizumab and ustekinumab were declined by her medical insurance company given their non-approved status for pediatric UC. The patient and her family were offered tofacitinib as a potential salvage therapy. The patient and her parents were informed that the medication is not licensed yet for children with UC, but it is licensed for adults with ulcerative colitis and children with juvenile idiopathic arthritis. The patient and her parents agreed to try tofacitinib with informed consent, understanding its benefits, risks, potential complications, and side effects. She was approved to receive it on compassionate grounds, funded by a patient funding organization. The patient had no contraindications to tofacitinib use, with normal lipid profile and cardio-pulmonary status. She had varicella zoster infection at the age of 5 and was fully vaccinated up to her age. Currently, she is waiting to receive the herpes zoster vaccine as it is not readily available. She was started on tofacitinib 5 mg orally twice daily. Remarkably, she had complete clinical response and went into clinical remission and was able to wean off steroids with no active symptoms (eg, bloody diarrhea or abdominal pain). At 9-month follow-up, she was in complete clinical, biochemical, and endoscopic remission. Her C-reactive protein decreased from 110 mg/L to 5 mg/L (reference 0–10 mg/L) and fecal calprotectin decreased from >1000 ug/g to 110 ug/g. Her repeat colonoscopy showed completely healed mucosa in the whole colon (Mayo 0) with mild loss of erythema in the sigmoid colon (Mayo 1). Her colonic biopsies showed inactive chronic colitis in the rectum (Figure 2C, 2D). Her pediatric ulcerative colitis index (PUCAI) was 0 and her BMI increased to 21.2 kg/m2 (Figure 1D).
Discussion
In this case we used tofacitinib for a 13-year-old girl with refractory UC, resulting in significant improvement, with clinical response at week 8 and clinical/endoscopic remission at week 36, after exhausting all therapeutic options and excluding surgery due the patient’s and parents’ wishes. To date, tofacitinib is not yet licensed to be used in pediatric patients with UC, and limited evidence addresses its use exclusively for induction of remission/maintenance in such patients. However, it is licensed to be used in patients with other autoimmune diseases such as rheumatoid arthritis, psoriasis, and juvenile idiopathic arthritis (JIA) [3–5]. Therefore, the safety of tofacitinib in pediatric patients is well-established in these diseases.
It is worth mentioning the current management of UC in pediatrics who are steroid-dependent, which includes using thiopurines and biologics, including infliximab, adalimumab, golimumab and vedolizumab [6].
With regards to the treatment of moderate to severe UC in adults, tofacitinib has shown efficacy. This was clear in the OCTAVE trials (1 and 2), where tofacitinib was given to patients with moderate to severe UC who failed at least 1 class of medical therapy [3]. These studies have shown that high-dose tofacitinib (10 mg twice daily) is significantly superior to placebo at inducing remission at 8 weeks [3]. The OCTAVE SUSTAIN study confirmed the efficacy of tofacitinib in maintaining remission using low-dose tofacitinib (5 mg twice daily) [3]. Comparable outcomes were also seen in real-world experience data [7]. In concordance with these studies, we were able to achieve clinical and endoscopic remission (Mayo 0–1) using low-dose tofacitinib for both induction and maintenance.
Despite the limited evidence on the use of tofacitinib in children with UC, there are some case series that reported similar outcomes. The first study involved 5 children with severe refractory UC who received tofacitinib at a dose of 10 mg twice daily over a mean observation period of 9.7 weeks. This study showed that high-dose tofacitinib resulted in clinical response and/or steroid-free remission in all UC patients [8]. Another study used dual therapy including tofacitinib. The study included 8 children under age 18 years, all with refractory UC and who failed 2 or more biologic therapy and never achieved steroid-free remission. This trial’s primary outcome was to achieve steroid-free remission at 6 months, which is defined as a partial Mayo score of <2 and steroid-free response for at least 4 weeks. In the trial, 4 out 5 UC patients who were treated with vedolizumab/tofacitinib achieved 6-month steroid-free remission. Moreover, the other 3 UC patients were treated with ustekinumab/tofacitinib and 2 out of 3 achieved 6-month steroid-free remission. Adverse events were seen in 1 patient only who was on vedolizumab/tofacitinib and developed right knee arthritis and then right leg deep vein thrombosis (DVT), which was managed accordingly. The patient’s dose of tofacitinib was reduced from 10 mg to 5 mg twice daily, similar to the dose we used, and eventually achieved remission. Apart from that, the lipid profile for all patients was normal throughout, and clinical response was reached with a decrease in C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR). Despite the difference from the present study, in that tofacitinib was used in combination with other drugs, outcomes were promising and once again remission was achieved in refractory UC patients [9].
The efficacy and safety of tofacitinib in children is evident in the FDA approval of the drug to be used in patients with juvenile idiopathic arthritis and psoriatic arthritis. In a phase-3 clinical trial that compared tofacitinib with placebo in pediatric patients, toafacinib resulted in a significant reduction in the rate of disease exacerbations compared to placebo [10]. Furthermore, an open-label phase 3 study for pediatric patients with moderately to severely active UC started in July 2021 with expected primary completion in August 2026 [11]. This study will assess the efficacy of tofacitinib in both induction and maintenance phases, and will ultimately provide strong evidence for the use of tofacitinib in pediatric patients with UC.
Regarding safety concerns, physicians should take into consideration the adverse effects associated with the use of tofacitinib, including headache, nausea, arthralgia, increased low-density lipoprotein (LDL) levels, infections (especially varicella zoster), and thrombosis [12]. In February 2021 the FDA added to the list of adverse effects associated with the drug use an increased risk of cardiovascular events, malignancy, thrombosis, and death. This was based on a randomized control trial that included patients with rheumatoid arthritis on methotrexate, with inclusion criteria of 50 years of age or older and having at least 1 cardiovascular risk factor such as smoking, hypertension, diabetes mellitus, or previous heart attack. Tofacitinib (5 mg or 10 mg bid) was compared to TNF (tumor necrosis factor) inhibitor resulting in greater risk of developing the above-mentioned adverse events and obligating the health care providers to weigh risks versus benefits when prescribing tofacitinib with the provision of patients with education about such adverse effects [13,14]. Our patient was less than 50 years old, with no cardiovascular risk factors. In addition, she was due to receive her shingles vaccine and her lipid profile was regularly monitored, which was normal throughout and she had no adverse effects related to the drug. In fact, our case provides some evidence to support that low-dose tofacitinib monotherapy may be sufficient to achieve clinical response and remission, hence avoiding the need for high-dose tofacitinib and/or combining it with other biological therapies that may increase the risk of adverse events in young patients with no other comorbidities.
Conclusions
Tofacitinib is an important and relatively new drug in the management of refractory ulcerative colitis. It can be considered as a salvage therapy for refractory UC in pediatric patients if they fail biological therapy on an off-label use. Randomized control trials are needed to better establish the efficacy and safety in this population.
Footnotes
Department and Institution Where Work Was Done
This work was done at the Department of Internal Medicine, Mubarak Alkabeer Hospital, Al-Jabreyah, Kuwait.
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
References:
- 1.Kappelman MD, Rifas-Shiman SL, Kleinman K, et al. The prevalence and geographic distribution of Crohn’s disease and ulcerative colitis in the United States. Clin Gastroenterol Hepatol. 2007;5(12):1424–29. doi: 10.1016/j.cgh.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 2.Kornbluth A, Sachar DB. Ulcerative colitis practice guidelines in adults: American College of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2010;105(3):501–23. doi: 10.1038/ajg.2009.727. [DOI] [PubMed] [Google Scholar]
- 3.Sandborn WJ, Su C, Sands BE, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2017;376(18):1723–36. doi: 10.1056/NEJMoa1606910. [DOI] [PubMed] [Google Scholar]
- 4.Tseng B, Amighi A, Bradford K, et al. Tofacitinib response in juvenile idiopathic arthritis (JIA) and collagenous colitis. J Clin Rheumatol. 2016;22(8):446–48. doi: 10.1097/RHU.0000000000000456. [DOI] [PubMed] [Google Scholar]
- 5.Ruperto N, Brunner HI, Zuber Z, et al. Pharmacokinetic and safety profile of tofacitinib in children with polyarticular course juvenile idiopathic arthritis: Results of a phase 1, open-label, multicenter study. Pediatr Rheumatol. 2017;15(1):86. doi: 10.1186/s12969-017-0212-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turner D, Ruemmele FM, Orlanski-Meyer E, et al. Management of paediatric ulcerative colitis, part 1: Ambulatory care-an evidence-based guideline from European Crohn’s and Colitis Organization and European Society of Paediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2018;67(2):257–91. doi: 10.1097/MPG.0000000000002035. [DOI] [PubMed] [Google Scholar]
- 7.D’Amico F, Parigi TL, Fiorino G, et al. Tofacitinib in the treatment of ulcerative colitis: Efficacy and safety from clinical trials to real-world experience. Therap Adv Gastroenterol. 2019;12:1756284819848631. doi: 10.1177/1756284819848631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dolinger MT, Rolfes P, Phan BL, Dubinsky MC. Letter: Tofacitinib use for biologic-refractory paediatric inflammatory bowel disease. Aliment Pharmacol Ther. 2019;50(8):966–67. doi: 10.1111/apt.15496. [DOI] [PubMed] [Google Scholar]
- 9.Dolinger MT, Spencer EA, Lai J, et al. Dual biologic and small molecule therapy for the treatment of refractory pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2021;27(8):1210–14. doi: 10.1093/ibd/izaa277. [DOI] [PubMed] [Google Scholar]
- 10.Efficacy study of tofacitinib in pediatric JIA population – full text view – ClinicalTrials.gov. ClinicalTrials.gov. Published February 21, 2020. Accessed August 17, 2021. https://www.clinicaltrials.gov/ct2/show/NCT02592434.
- 11.Evaluation of oral tofacitinib in children aged 2 to 17 years old suffering from moderate to severe ulcerative colitis – full text view – ClinicalTrials.gov. ClinicalTrials.gov. Published November 10, 2020. Accessed August 17, 2021. https://clinicaltrials.gov/ct2/show/NCT04624230.
- 12.López-Sanromán A, Esplugues JV, Domènech E. Pharmacology and safety of tofacitinib in ulcerative colitis. Gastroenterol Hepatol. 2021;44(1):39–48. doi: 10.1016/j.gastrohep.2020.04.012. [DOI] [PubMed] [Google Scholar]
- 13.Serious heart events, cancer, blood clots for certain JAK inhibitors. U.S. Food and Drug Administration. Published 2021. Accessed October 2, 2021. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clotsand-death.
- 14.Safety study of tofacitinib versus tumor necrosis factor (TNF) inhibitor in subjects with rheumatoid arthritis – full text view – ClinicalTrials.gov. Clinicaltrials.gov. Published 2021. Accessed October 2, 2021 https://clinicaltrials.gov/ct2/show/NCT02092467.