Key Points
Question
What is the effect of sacubitril/valsartan compared with standard medical therapy on plasma N-terminal pro–brain natriuretic peptide (NT-proBNP) concentration and submaximal exercise capacity in patients with heart failure and preserved ejection fraction?
Findings
This randomized clinical trial included 2572 participants with heart failure and left ventricular ejection fraction higher than 40%. Treatment with sacubitril/valsartan vs standard treatment with enalapril, valsartan, or placebo resulted in a statistically significant reduction in plasma NT-proBNP levels at 12 weeks (adjusted geometric mean ratio, 0.84) but did not significantly change the 6-minute walk distance at 24 weeks (9.7 m vs 12.2 m).
Meaning
Compared with standard medical therapies, sacubitril/valsartan resulted in a significantly greater decrease in plasma N-terminal pro–brain natriuretic peptide at 12 weeks but did not improve submaximal exercise capacity at 24 weeks.
Abstract
Importance
There is limited evidence on the benefits of sacubitril/valsartan vs broader renin angiotensin system inhibitor background therapy on surrogate outcome markers, 6-minute walk distance, and quality of life in patients with heart failure and mildly reduced or preserved left ventricular ejection fraction (LVEF >40%).
Objective
To evaluate the effect of sacubitril/valsartan on N-terminal pro–brain natriuretic peptide (NT-proBNP) levels, 6-minute walk distance, and quality of life vs background medication–based individualized comparators in patients with chronic heart failure and LVEF of more than 40%.
Design, Setting, and Participants
A 24-week, randomized, double-blind, parallel group clinical trial (August 2017-October 2019). Of 4632 patients screened at 396 centers in 32 countries, 2572 patients with heart failure, LVEF of more than 40%, elevated NT-proBNP levels, structural heart disease, and reduced quality of life were enrolled (last follow-up, October 28, 2019).
Interventions
Patients were randomized 1:1 either to sacubitril/valsartan (n = 1286) or to background medication–based individualized comparator (n = 1286), ie, enalapril, valsartan, or placebo stratified by prior use of a renin angiotensin system inhibitor.
Main Outcomes and Measures
Primary end points were change from baseline in plasma NT-proBNP level at week 12 and in the 6-minute walk distance at week 24. Secondary end points were change from baseline in quality of life measures and New York Heart Association (NYHA) class at 24 weeks.
Results
Among 2572 randomized patients (mean age, 72.6 years [SD, 8.5 years]; 1301 women [50.7%]), 2240 (87.1%) completed the trial. At baseline, the median NT-proBNP levels were 786 pg/mL in the sacubitril/valsartan group and 760 pg/mL in the comparator group. After 12 weeks, patients in the sacubitril/valsartan group (adjusted geometric mean ratio to baseline, 0.82 pg/mL) had a significantly greater reduction in NT-proBNP levels than did those in the comparator group (adjusted geometric mean ratio to baseline, 0.98 pg/mL) with an adjusted geometric mean ratio of 0.84 (95% CI, 0.80 to 0.88; P < .001). At week 24, there was no significant between-group difference in median change from baseline in the 6-minute walk distance with an increase of 9.7 m vs 12.2 m (adjusted mean difference, −2.5 m; 95% CI, −8.5 to 3.5; P = .42). There was no significant between-group difference in the mean change in the Kansas City Cardiomyopathy Questionnaire clinical summary score (12.3 vs 11.8; mean difference, 0.52; 95% CI, −0.93 to 1.97) or improvement in NYHA class (23.6% vs 24.0% of patients; adjusted odds ratio, 0.98; 95% CI, 0.81 to 1.18). The most frequent adverse events in the sacubitril/valsartan group vs the comparator group were hypotension (14.1% vs 5.5%), albuminuria (12.3% vs 7.6%), and hyperkalemia (11.6% vs 10.9%).
Conclusions and Relevance
Among patients with heart failure and left ventricular ejection factor of higher than 40%, sacubitril/valsartan treatment compared with standard renin angiotensin system inhibitor treatment or placebo resulted in a significantly greater decrease in plasma N-terminal pro–brain natriuretic peptide levels at 12 weeks but did not significantly improve 6-minute walk distance at 24 weeks. Further research is warranted to evaluate potential clinical benefits of sacubitril/valsartan in these patients.
Trial Registration
ClinicalTrials.gov Identifier: NCT03066804
This trial tested the efficacy of sacubitril/valsartan vs standard care in reducing NT-proBNP levels and improving exercise capacity and quality of life in patients with chronic heart failure.
Introduction
More than half of all patients with heart failure have preserved or mildly reduced left ventricular ejection fraction (LVEF >40%),1 which is associated with substantial morbidity and mortality.2 Clinical trials of renin-angiotensin-aldosterone system antagonists have not demonstrated similar benefits among patients with heart failure with reduced ejection fraction (HFrEF) that was 40% or lower.3,4,5,6 However, post hoc analyses indicated that patients whose LVEF levels that ranged from 40% to 50% while being treated with either candesartan7 or spironolactone8 were admitted to the hospital less often.
In the PARAGON-HF (Prospective Comparison of ARNI [angiotensin receptor–neprilysin inhibitor] with ARB [angiotensin-receptor blockers] Global Outcomes in HFpEF) trial8 involving patients with heart failure and LVEF levels of 45% or higher, sacubitril/valsartan compared with valsartan resulted in a rate ratio (RR) of 0.87 (95% CI, 0.75-1.01; P = .06)9 for heart failure hospitalization and cardiovascular death. However, there is no evidence-based recommended therapy for renin-angiotensin-aldosterone system blockade in heart failure with LVEF higher than 40%,10 with a wide variation in background therapy in routine practice.11,12
In the phase 2 PARAMOUNT (Prospective Comparison of ARNI With ARB on Management of Heart Failure With Preserved Ejection Fraction) trial13 involving patients with heart failure and an LVEF level of 45% or higher, sacubitril/valsartan compared with valsartan reduced N-terminal pro–brain natriuretic peptide (NT-proBNP), a prognostic biomarker in heart failure, and left atrial volume. In PARAGON-HF, reduction in NT-proBNP was associated with lower subsequent risk of heart failure hospitalizations and cardiovascular death.14
Exercise capacity, quality of life, and routine daily activities are impaired in heart failure and are regarded as important treatment targets.15 Effects of pharmacotherapies on these outcomes in heart failure with preserved ejection fraction (HFpEF) have been neutral,15 and the effects of sacubitril/valsartan on exercise capacity and quality of life in patients with heart failure and LVEF higher than 40% are incompletely understood.16
This trial was designed to test the hypothesis that sacubitril/valsartan is more effective than background medication–based individualized comparators in reducing NT-proBNP levels and improving submaximal exercise capacity and quality of life in patients with chronic heart failure and an LVEF higher than 40%.
Methods
Study Design and Eligibility
The PARALLAX (Prospective Comparison of ARNI vs Comorbidity-Associated Conventional Therapy on Quality of Life and Exercise Capacity) trial was a 24-week, randomized, double-blind, parallel group, active-controlled (for patients taking angiotensin-converting enzyme [ACE] inhibitors or angiotensin II receptor blockers [ARBs] prior to recruitment) or placebo-controlled (for patients who were naive to a renin angiotensin system [RAS] inhibitors prior to recruitment) trial. Details of the rationale and trial design have been published17 and are available in the trial protocol (Supplement 1). This study was conducted between August 2017 and October 2019. Ethics committee approval was provided at each trial center. An independent data and safety monitoring committee monitored trial conduct and patient safety. All patients provided written informed consent.
Patients 45 years or older, with symptomatic heart failure requiring the use of diuretics, New York Heart Association (NYHA) functional class II through IV, elevated plasma NT-proBNP levels (>220 pg/mL for patients in sinus rhythm, and >600 pg/mL for patients with atrial fibrillation or atrial flutter); with evidence of structural heart disease (either left atrial enlargement or left ventricular hypertrophy) as demonstrated by echocardiography at screening or within 6 months of randomization; with an LVEF of 40% or higher (initially defined as LVEF ≥45%, but we changed it to >40% on September 12, 2018, Amendment 2 in the trial protocol); and with an impaired health–related quality of life (Kansas City Cardiomyopathy Questionnaire Clinical Summary Score [KCCQ-CSS] <75 [range, 0-100; higher scores indicate a better quality of life]) were included. Patients taking an ACE inhibitor or ARB (background therapy) were required to have a history of hypertension.17 Detailed inclusion and exclusion criteria are listed in eTable 1 in Supplement 2. To assess potential differences in demographic data and treatment effects, patients self-reported race based on an open-ended question that was included in the data collection.
Randomization
Eligible patients were stratified via interactive response technology into 1 of 3 strata according to RAS inhibitor treatment modality as prescribed by their treating physicians prior to trial recruitment: ACE inhibitor (n = 1066), ARB (n = 1174), or neither (n = 326). Each patient was assigned a randomization number using a validated system that automates the random assignment. Patients in each stratum were randomized in a 1:1 ratio to receive either sacubitril/valsartan or the background medication–based individualized comparator (Figure 1). Patients, investigator staff, persons performing the assessments, and data analysts were blinded to the identity of the treatment.
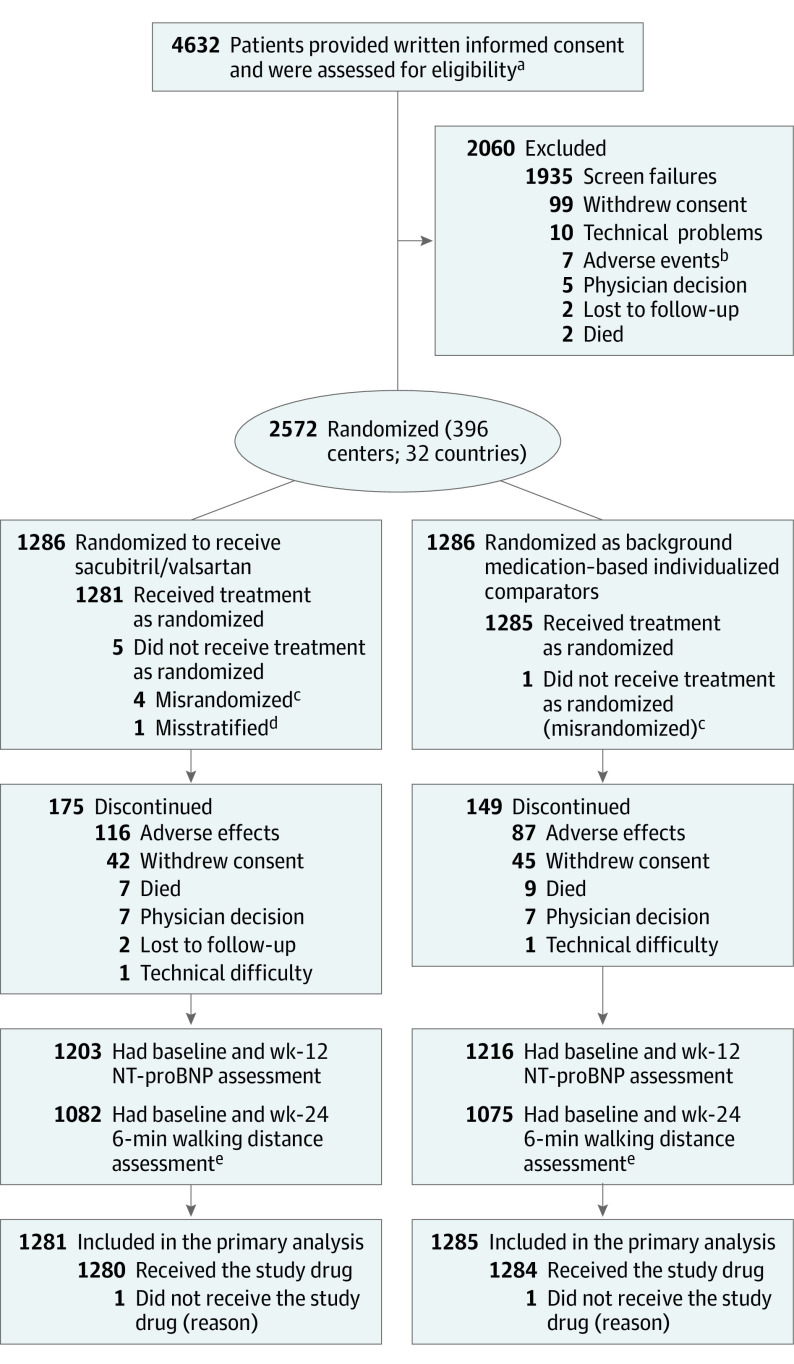
Figure 1. Patient Flow Through the PARALLAX -HF Trial.
aFour hundred fifty-one patients were screened more than once; rescreened patients were included only once.
bDefined as any untoward medical occurrence or worsening of preexisting medical conditions or diseases at the time of signing the informed consent form.
cPatients did not qualify for randomization and did not receive study treatment but were inadvertently randomized into the study.
dPatients were inadvertently randomized within a wrong treatment stratum and did not receive the study treatment. Rerandomized patients were included only once.
ePatients with baseline 6-minute walk distance of 100 to 450 m.
NT-proBNP indicates N-terminal pro–brain natriuretic peptide.
Intervention
Patients in each stratum received twice daily either sacubitril/valsartan at a target dose of 97 mg/103 mg; background medication–based individualized comparator: enalapril at a target dose of 10 mg (ACE inhibitors stratum), valsartan at a target dose of 160 mg (ARB stratum); or placebo (no RAS inhibitor stratum). Sites were instructed to uptitrate study medication within 4 weeks to a maximally tolerated dose. Study visits were conducted every 2 to 4 weeks until week 24. Details of the study procedures are provided in Supplement 1 and the statistical analysis plan in Supplement 3. Both have been published.17 Training was provided to sites to ensure standardization of the 6-minute walk distance test.
Trial Outcomes
The 2 primary end points for this study were change in plasma NT-proBNP concentrations from baseline to week 12 and change in the 6-minute walk distance from baseline to week 24 (in the subgroup of patients with a baseline ability of walk between 100 m and 450 m). We chose these cutoffs because walk less than 450 m during the 6-minute walk distance test could indicate impaired exercise capacity18,19,20 and less than 100 m could indicate nonmodifiable causes of exercise limitation. We changed the 6-minute walk distance measure from a secondary end point to a coprimary end point on September 12, 2018 (Amendment 2, Supplement 1) reflecting the importance of functional improvement as a therapeutic target in patients with HFpEF. An increase by 30 m was considered as a minimal clinically important difference.21
Secondary end points included the mean change from baseline in KCCQ-CSS at week 24, proportion of patients with a 5-point score change in deterioration or improvement at week 24, and change in NYHA functional class. A 5-point increase or decrease in the KCCQ-CSS score is considered a minimal clinically important difference.22 NYHA class was graded based on functional capacity; dyspnea with mild exertion, class II; with moderate exertion, class III; and at rest, class IV.
Exploratory end points included the change in NT-proBNP levels at week 24 and the rate of change in the estimated glomerular filtration rate (eGFR) from baseline.
Safety was based on adverse events reported during the study. Suspected cases of angioedema were adjudicated by a blinded and independent angioedema adjudication committee.
Sample Size Estimation
A sample size of 2500 patients was estimated to provide adequate power to evaluate primary and secondary end points.17 The significance level of a 1-sided α of .025 (2-sided .05) was split between the 2 primary end points for treatment comparisons: 90% for NT-proBNP and 10% for the 6-minute walk distance. The 1-sided α was significant at .025 and the 2-sided α, at .05. With a 1-sided α of .0225, based on results of the PARAMOUNT study, the power for the NT-proBNP end point was at least 92% to detect a relative reduction from baseline of at least 11% at week 12, assuming an SD of 0.81 for change from baseline in the log-transformed NT-proBNP and an overall dropout rate of 10%.13 With a 1-sided α of .0025, the power for the 6-minute walk distance was at least 90% to detect a mean difference of 22 m or more in change from baseline to week 24, assuming an SD of 120 m, an overall dropout rate of 10%, and an overall proportion of 88% for patients with a baseline walk distance of between 100 m and 450 m.17,23
Statistical Analysis
Analysis Sets
Efficacy variables were analyzed based on the full analysis set. The full analysis set included all validly randomized patients except for those who were misrandomized or misstratified and did not receive any study drug. Patients were analyzed according to the treatment to which they were assigned at randomization. The safety set, which included all randomized patients receiving at least 1 dose of study drug, was used for the analyses of adverse effects.
Testing Strategy
To control the family-wise type I error rate at the 1-sided .025 significance level, a sequential rejective multiple testing procedure was used.17,24At first, between the 2 primary end points, the overall α level was split in a 9:1 ratio. When either of them was rejected, the corresponding assigned α was propagated and accumulated to test the KCCQ-CSS. If the 6-minute walk distance was not rejected but the KCCQ-CSS was rejected at the α level inherited from the rejection of NT-proBNP change, the corresponding α level was propagated to 6-minute walk distance, together with the original assigned α, to test the 6-minute walk distance again. If both the KCCQ-CSS and 6-minute walk distance measures were rejected, the NYHA class was tested at the full level of α. Otherwise, the testing procedure was stopped. Based on this sequential rejective strategy, a significant treatment effect on either of the 2 primary end points was sufficient to consider the study successful.
Analyses of Primary and Secondary End Points
The primary end point NT-proBNP was analyzed using a mixed model for repeated measures,17 from which the estimates and the 2-sided 95% CIs were provided for the adjusted geometric mean ratios for plasma NT-proBNP concentration at week 12 to the baseline in the 2 treatment groups and for sacubitril/valsartan in comparison to background medication–based individualized comparators. Similarly, the 6-minute walk distance was analyzed using the mixed model for repeated measures in the subgroup of patients with baseline walk distance ranging from 100 m to 450 m.17 The secondary end point, change in the KCCQ-CSS score at 24 weeks from baseline, was analyzed using a similar mixed model for repeated measures. The NYHA class change (improved, unchanged, and worsened) at week 24 from baseline was analyzed using a proportional cumulative odds model.17 Patients experiencing a 5-point or more improvement or deterioration in KCCQ-CSS scores were summarized by treatment group and visit, using the number of patients showing more than a 5-point change and the proportion of patients with a 5-point or more change. and analyzed using binary logistic regression models.
In the analyses of NT-proBNP concentration, missing data were handled using the likelihood method, with the assumption that they were missing at random. In the analyses of the 6-minute walk distance, KCCQ-CSS, and NYHA class change, death was considered the worst possible outcome and scheduled visits after death were imputed to the worst value accordingly. Other missing data were assumed to be missing at random.17
Statistical analyses were performed using SAS version 9.4 or higher (SAS Institute Inc).
Sensitivity and Subgroup Analyses
A sensitivity analysis was carried out to assess cases for which data were missing not at random; in particular, the sensitivity analysis assumed that patients treated with sacubitril/valsartan who had discontinued due to adverse events, death, or lack of efficacy would not have adhered to therapy if they had stayed in the study.
To assess consistency of treatment effects across key clinically relevant subgroups, subgroup analyses were performed using the corresponding analysis model used for the primary and secondary end points, after adding factors of subgroup and subgroup-treatment interaction to the model. However, to allow for an unbiased estimation of within-subgroup treatment effects and an assessment of homogeneity of treatment effects at the specific target visit, post hoc subgroup analyses were instead conducted by adding fixed-effect factors of subgroup, subgroup × treatment interaction, subgroup × visit interaction, and subgroup × treatment × visit interaction into the original primary analysis model. The estimated treatment effect, 2-sided 95% CIs, and interaction P values are reported for each of the subgroups.
Results
Between August 22, 2017, and May 7, 2019, 4632 patients were screened at 396 centers in 32 countries, and 2572 patients were randomized. The study ended as planned after the last patient completed the 24-week follow-up visit on October 28, 2019. Five patients who were misrandomized and 1 patient who was mistakenly assigned to a wrong stratum were withdrawn from the trial without having received study medication and excluded from primary analysis. The primary intention-to-treat population consisted of 2566 patients (1281 randomized to the sacubitril/valsartan group and 1285 randomized to background medication–based individualized comparator group; Figure 1). Before randomization, 1066 patients (41.5%) were taking ACE inhibitors (ACE inhibitor stratum), 1174 patients (45.8%) were taking ARBs (ARB stratum), and 326 patients (12.7%) were not taking any RAS inhibitors. Baseline demographic and clinical characteristics were balanced between the treatment groups (Table 1 and Table 2). A comparison of clinical characteristics among the 3 strata has been described previously,17 with fewer patients who were not taking a RAS inhibitors reporting a history of hypertension, hyperlipidemia, or coronary artery disease and who had slightly lower blood pressure at entry into the study than those in the other 2 strata.
Table 1. Baseline Characteristics of Patients Enrolled in PARALLAX-HF.
| Characteristics | No. (%) of patients | |
|---|---|---|
| Sacubitril/valsartan (n = 1281) | Background medication–based individualized comparator (n = 1285) | |
| Age, y | ||
| Mean (SD) | 72.9 (8.4) | 72.4 (8.6) |
| ≥65 | 1085 (84.7) | 1064 (82.8) |
| Sex | ||
| Women | 643 (50.2) | 658 (51.2) |
| Men | 638 (49.8) | 627 (48.8) |
| Racea | ||
| Asian | 56 (4.4) | 59 (4.6) |
| Black | 11 (0.9) | 16 (1.3) |
| Native American | 39 (3.0) | 33 (2.6) |
| White | 1112 (86.8) | 1117 (86.9) |
| Otherb | 63 (4.9) | 59 (4.6) |
| Geographical region | ||
| Europe | 970 (75.7) | 969 (75.4) |
| Latin America | 177 (13.8) | 179 (13.9) |
| Asia-Pacific or other | 69 (5.4) | 70 (5.5) |
| North America | 65 (5.1) | 67 (5.2) |
| BMI, mean (SD) | 30.6 (5.0) | 30.5 (4.8) |
| Blood pressure, mean (SD), mm Hg | ||
| Systolic | 132.6 (13.9) | 134.2 (14.5) |
| Diastolic | 77.1 (10.3) | 77.3 (10.2) |
| eGFR, mean (SD), mL/min/1.73 m2 | 62.5 (20.2) | 62.7 (19.6) |
| 6-min walk distance, m | ||
| Median (IQR) | 305.0 (225.5-372.5) | 308.0 (240.0-375.0) |
| <100 | 35 (2.7) | 31 (2.4) |
| 100-450 | 1154 (90.1) | 1159 (90.2) |
| >450 | 91 (7.1) | 90 (7.0) |
| KCCQ CSS, mean (SD)c | 52.7 (16.7) | 53.3 (16.9) |
| KCCQ, mean (SD) | ||
| Total symptom score | 56.3 (18.4) | 56.6 (18.7) |
| Physical limitation score | 49.1 (19.2) | 50.0 (19.1) |
Abbreviations: BMI, body mass index, calculated as weight in kilograms divided by height in meters squared; eGFR, estimated glomerular filtration rate; KCCQ CSS, Kansas City Cardiomyopathy Questionnaire clinical summary score.
Information was collected as part of the routine demographic data; race as reported by the patients.
Includes multiaracial individuals or of any group not covered by the listed categories.
Score range, 0-100 (0, worst health; 100, excellent health). Measures quality of life of patients with HF using 7 domains: physical limitations; symptom stability, burden, and frequency; self-efficacy; quality of life; and social limitation. Physical limitations measures the limitations patients experience from HF in performing routine activities. The total symptom score comprises the symptom frequency and symptom burden domains; CSS comprises physical limitation and total symptom score.
Table 2. Baseline Clinical Features of Patients Enrolled in PARALLAX-HF .
| No. (%) of patients | ||
|---|---|---|
| Sacubitril/valsartan (n = 1281) | Background medication–based individualized comparator (n = 1285) | |
| Clinical features of HF | ||
| NYHA class at randomizationa | ||
| Class I | 1 (0.1) | 4 (0.3) |
| Class II | 858 (67.0) | 876 (68.2) |
| Class III | 416 (32.5) | 401 (31.2) |
| Class IV | 5 (0.4) | 4 (0.3) |
| NT-proBNP, median (IQR), pg/mL | 786.0 (415.0-1401.0) | 760 (380.0-1398.0) |
| LVEF, % (SD) | 56.7 (8.3) | 56.2 (8.0) |
| Ischemic HF etiology | 444 (34.7) | 448 (34.9) |
| LVEF subgroups | ||
| Type of HF | ||
| HFpEF (EF≥50%) | 1029 (80.3) | 1036 (80.6) |
| HFmrEF (40%<EF<50%) | 252 (19.7) | 249 (19.4) |
| Type of HF among women | ||
| HFpEF (EF≥50%) | 566/643 (88.0) | 581/658 (88.3) |
| HFmrEF (40%<EF<50%) | 77/643 (12.0) | 77/658 (11.7) |
| Type of HF among men | ||
| HFpEF (EF≥50%) | 463/638 (72.6) | 455/627 (72.6) |
| HFmrEF (40%<EF<50%) | 175/638 (27.4) | 172/627 (27.4) |
| Medical history | ||
| Hypertension | 1241 (96.9) | 1251 (97.4) |
| Dyslipidemia | 869 (67.8) | 850 (66.2) |
| Atrial fibrillation or flutter | 699 (54.6) | 692 (53.9) |
| Coronary artery disease | 686 (53.6) | 682 (53.1) |
| Chronic kidney diseaseb | 636 (49.7) | 626 (48.7) |
| Diabetesc | 566 (44.2) | 589 (45.8) |
| Prior HF hospitalization | 446 (34.9) | 459 (35.7) |
| Myocardial infarction | 295 (23.0) | 306 (23.8) |
| Treatment | ||
| Diureticsd | 1277 (99.8) | 1282 (99.8) |
| ACE/ARB (prestudy) | 1115 (87.1) | 1124 (87.5) |
| β-Blockers | 1071 (83.7) | 1066 (83.0) |
| Calcium channel blockers | 444 (34.7) | 479 (37.3) |
| MRAs | 419 (32.7) | 392 (30.5) |
| SGLT-2 inhibitors | 34 (2.7) | 26 (2.0) |
Abbreviations: ACE, angiotensin converting enzyme; ARB, angiotensin II receptor blocker; HF, heart failure; HFmrEF, HF with mildly reduced ejection fraction; HFpEF, HF with preserved ejection fraction; MRA, mineralocorticoid receptor antagonist; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; SGLT-2, sodium glucose co-transporter-2.
NYHA is a graded class of functional capacity. Class I indicates no symptoms or limitations in ordinary physical activity; class II, dyspnea with moderate exertion; class III, dyspnea with mild exertion; and class IV, dyspnea at rest.
Estimated glomerular filtration rate of 60 mL/min/1.73 m2 or lower.
Hemoglobin A1c 6.5% or higher at screening.
Includes MRAs.
Primary Outcomes
At week 12, NT-proBNP levels were significantly reduced in the sacubitril/valsartan group vs the comparator group. The adjusted geometric mean ratio was 0.84 (95% CI, 0.80-0.88; P < .001; Figure 2). Reductions in NT-proBNP levels in the sacubitril/valsartan group were evident as early as week 4 and remained lower than levels in the background medication–based individualized comparator group at week 24 (adjusted geometric mean ratio to baseline, 0.82 vs 0.98 pg/mL; Figure 2). Furthermore, reductions in NT-proBNP levels in the sacubitril/valsartan group was not affected by prior treatment, with no significant interactions among strata (Figure 3). The reduction in NT-proBNP levels at week 12 was consistent across all prespecified subgroups with no significant subgroup by treatment interaction (Figure 3).
Figure 2. N-Terminal Pro–Brain Natriuretic Peptide and 6-Minute Walk Distance Primary Outcomes.
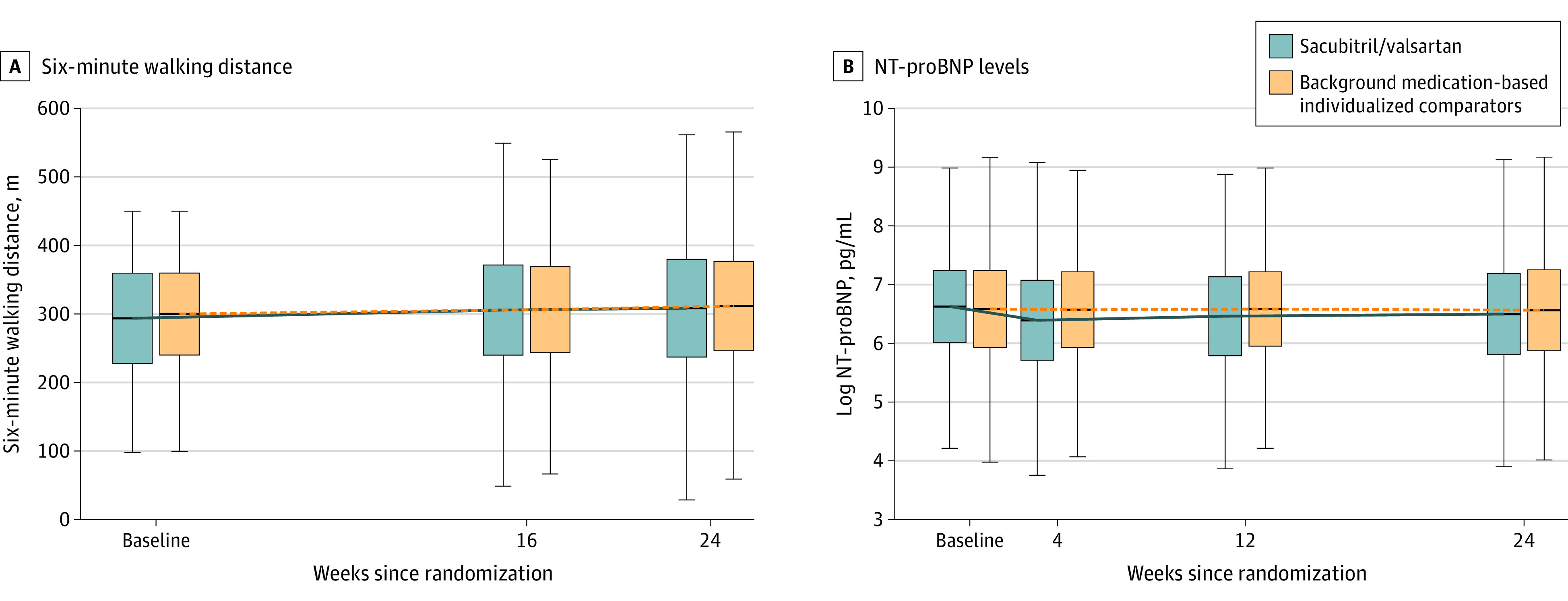
Median values for N-terminal pro–brain natriuretic peptide (NT-proBNP) and 6-minute walk distance. Patients included had baseline 6-minute walk distances of 100 m to 450 m.
Box edges indicate the 25th and 75th percentiles, the horizontal line inbetween the edges, the median. Whiskers extend to the furthest point with 1.5 × IQR of the box ends.
Figure 3. Effect of Sacubitril/Valsartan and Individualized Medical Therapy on NT-proBNP in Predefined Subgroups.
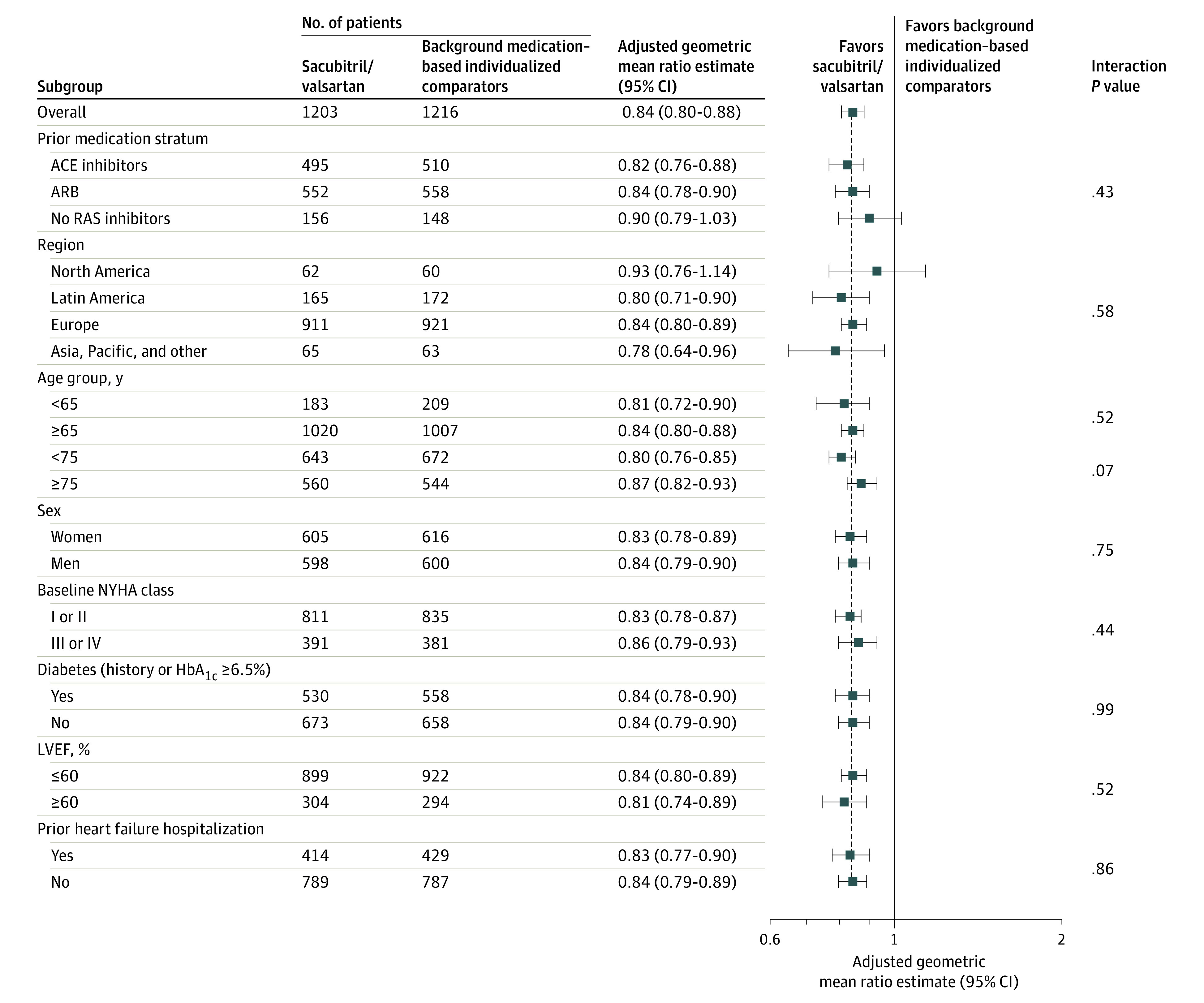
An adjusted geometric mean ratio lower than 1 favors sacubitril/valsartan. The interaction P value is for the subgroup variable × the treatment interaction at week 12. The mixed model for the repeated-measures model includes stratum angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor blockers (ARBs), no renin angiotensin system inhibitors, region, treatment (sacubitril/valsartan, background medication–based individualized comparators), visit, treatment × visit interaction, subgroup, subgroup × visit interaction, treatment × subgroup interaction, and treatment × subgroup × visit interaction as fixed-effect factors; baseline log-transformed N-terminal pro–brain natriuretic peptide (NT-proBNP), stratum × baseline log-transformed NT-proBNP, and visit × baseline log-transformed NT-proBNP interactions as covariates; and models the within-patient covariance using an unstructured covariance matrix (a common matrix for the 2 treatment groups). The analysis includes data observed up to week 12. Test values below lower or above the upper limit of quantification are imputed by 0.5 × the lower limit of quantification × 1.5 × upper limit of quantification.
ACE indicates angiotensin-converting enzyme; ARB, angiotensin II blocker; HbA1c, hemoglobin A1c; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; RAS, renin angiotensin system.
At week 24, no significant difference existed between study groups in the 6-minute walk distance from baseline (a mean increase, 9.7 m for the sacubitril/valsartan group vs 13.2 m for the comparator group). The adjusted mean difference was −2.5 m (95% CI, −8.5 to 3.5 m; P = .42; Figure 2). This finding was consistent across subgroups and prestudy treatment strata, except for a modest effect modification by geographical region and sex: among patients in the sacubitril/valsartan group compared with the background medication–based individualized comparator group, walking distance improved among women but decreased among men and decreased among those living in Latin America; however, the effect sizes remained small (eFigure in Supplement 2). A similar proportion of patients improved their walk distance by 30 m or more: 36.0% in the in the sacubitril/valsartan group and 35.4% in the comparator group. A prespecified sensitivity analysis that included all randomized patients (irrespective of their baseline 6-minute walk distance) confirmed the results of the primary analysis (adjusted mean difference, −3.0 m; 95% CI, −8.8 to 2.8 m; P = .31).
Secondary Outcomes
There was no significant difference between groups in the KCCQ-CSS score at week 24 compared with baseline (12.3 points in the sacubitril/valsartan group and 11.8 points in the comparator group, P = .24; Table 3). The proportion of patients with an improvement or decrease by 5 or more points were not significantly different in the treatment groups. Changes in NYHA class were also not significantly different between treatment groups. Change from baseline in KCCQ-CSS and NYHA class at week 24 by strata are presented in eTable 2 in Supplement 2.
Table 3. Secondary Efficacy Outcomes.
| Outcomes | Sacubitril/valsartan | Background medication based–individualized comparator | Adjusted difference (95% CI) |
|---|---|---|---|
| Change from baseline in KCCQ CSS at week 24 a , b | |||
| KCCQ-CSS, mean (SD) | |||
| Week 0 | 52.70 (16.7) | 53.32 (16.9) | |
| No. | 1281 | 1285 | |
| Week 24 | 66.68 (19.0) | 66.15 (18.6) | |
| No. | 1207 | 1210 | |
| Adjusted mean change from baseline (95% CI) | 12.3 (11.3 to 13.4) | 11.8 (10.8 to 12.8) | Mean difference, 0.52 (−0.93 to 1.97) |
| ≥5 points, No. (%) | |||
| Improvement | 820 (67.9) | 795 (65.7) | OR, 1.11 (0.83 to 1.48) |
| Deterioration | 187 (15.5) | 202 (16.7) | OR, 0.90 (0.65 to 1.25) |
| Change from baseline in NYHA class at week 24 a , c | |||
| NYHA class, No. (%)d | |||
| Week 0, No. | 1281 | 1285 | |
| I | 1 (0.1) | 4 (0.3) | |
| II | 858 (67.0) | 876 (68.2) | |
| III | 416 (32.5) | 401 (31.25) | |
| IV | 5 (0.4) | 4 (0.3) | |
| Week 24 | 1228 | 1229 | |
| I | 104 (8.6) | 120 (9.9) | |
| II | 887 (73.3) | 868 (71.4) | |
| III | 209 (17.3) | 221 (18.2) | |
| IV | 5 (0.4) | 3 (0.3) | |
| Adjusted OR to be in favorable NYHA class (95% CI)e | 0.98 (0.81 to 1.18) | ||
| No. | 1228 | 1229 | |
| Improved, No. (%) | 290 (23.6) | 295 (24.0) | |
| Unchanged, No. (%) | 887 (72.2) | 881 (71.7) | |
| Worsened, No. (%) | 51 (4.2) | 53 (4.3) | |
Abbreviations: KCCQ-CSS, Kansas City Cardiomyopathy Questionnaire clinical summary score; NYHA, New York Heart Association; OR, odds ratio.
The mixed model for repeated measures includes stratum (angiotensin-converting enzyme inhibitors, angiotensin II receptor blocker, no renin angiotensin system inhibitors), region, treatment (sacubitril/valsartan and background medication–based individualized comparator), visit, and treatment × visit as fixed-effect factors; baseline values, baseline systolic blood pressure, stratum × baseline value, stratum × baseline systolic blood pressure, and visit × baseline value interactions as covariates.
KCCQ CSS is a combined score (range, 0-100; 0, worst health; 100, excellent health) based on the clinical symptoms and physical function domains of the questionnaire.
Due to the predefined sequentially rejective multiple testing procedure, change in NYHA class was not formally tested.
NYHA is a graded class of functional capacity. Class I indicates no symptoms or limitations in ordinary physical activity; class II, dyspnea with moderate exertion; class III, dyspnea with mild exertion; and class IV, dyspnea at rest.
Favorable NYHA class indicates improvement from higher to lower NYHA class.
Exploratory End Points
At week 24, The sacubitril/valsartan group had a significantly lower rate of monthly change from baseline (slope) in eGFR than did the background medication–based individualized comparator group (−0.25 vs −0.43 mL/min/1.73 m2, respectively, P = .02). The annualized rate of eGFR decline was −2.95 mL/min/1.73 m2 in the sacubitril/valsartan group and −5.14 mL/min/1.73 m2 in the background medication–based individualized comparator group, corresponding to an annualized adjusted mean treatment difference of 2.19 mL/min/1.73 m2 (95% CI, 0.41 to 3.97; P = .01).
Adverse Events
Permanent study drug discontinuation was reported in 175 patients (13.6%) in the sacubitril/valsartan group and 147 patients (11.6%) in the comparator group. Adverse events were the main reason for permanent study drug discontinuation: 116 patients (9.0%) in the sacubitril/valsartan group and 87 (6.8%) in the comparator group.
A total of 1087 patients (84.9%) in the sacubitril/valsartan group and 1030 patients (80.2%) in the background medication–based individualized comparator group had at least 1 adverse event (P = .002). Overall, a higher proportion of patients in the sacubitril/valsartan group reported study drug–related adverse events vs patients in the background medication–based individualized comparator group (30.5% vs 22.6%; P < .001; eTable 3 in Supplement 2). Hypotension (14.1% vs 5.5%), albuminuria (12.3% vs 7.6%), and hyperkalemia (11.6% vs 10.9%) were the most commonly reported adverse events in the sacubitril/valsartan group, especially in the no RAS inhibitor stratum (eTable 4 and eTable 5 in Supplement 2). A higher proportion of patients in the sacubitril/valsartan group had a systolic blood pressure less than 90 mm Hg and higher than a 20 mm Hg decrease from baseline, notably in the no RAS inhibitor stratum (eTable 5 in Supplement 2).
Seventy patients (5.5%) in the sacubitril/valsartan group had cardiac disorders reported as serious adverse events compared with 94 (7.3%) in the comparator group.
Discussion
In this trial involving patients with heart failure and LVEF higher than 40%, sacubitril/valsartan was more effective in reducing plasma NT-proBNP levels but did not improve 6-minute walk distance, quality of life (as assessed by KCCQ-CSS), or NYHA functional class compared with background medication–based individualized comparators.
The reduction in NT-proBNP observed with sacubitril/valsartan was consistent across 9 prespecified subgroups. This observation corroborates previous findings from the PARAMOUNT13 and PARAGON-HF9 trials involving patients with an LVEF of 45% or higher. Although there is robust evidence showing NT-proBNP reduction with sacubitril/valsartan in patients with HFrEF and HFpEF (LVEF ≥45%), the results of this study extend these findings to patients in the LVEF range higher than 40%, encompassing both heart failure with mildly reduced ejection fraction (HFmrEF) and HFpEF, without a trial run-in period and compared with different RAS inhibitor blockers or placebo. The reduction of NT-proBNP levels with sacubitril/valsartan in this study may primarily be attributed to neprilysin inhibition because the ARB irbesartan had a negligible effect on NT-proBNP levels in the Irbesartan in Heart Failure With Preserved Systolic Function (I-Preserve) trial,4 and the reduction was seen across all 3 strata in the current study.
The second primary end point, change from baseline in 6-minute walk distance at week 24, was not significantly different between groups. The observed sex interaction for the 6-minute walk distance with women deriving more benefit than men may need further consideration because women benefited more from angiotensin receptor neprilysin inhibitors than did men in the PARAGON-HF trial.9
Prior randomized trials of heart failure pharmacotherapies have failed to show benefits for exercise capacity in patients with HFpEF, with 6-minute walk distance improving in both the control and active intervention groups in several trials.15 Specifically, there was no improvement in 6-minute walk distance with vericiguat,25 praliciguat,26 sildenafil,27 and neladonoson,28 and submaximal exercise capacity decreased slightly with spironolactone29 compared with placebo. One explanation may be that the 6-minute walk distance measure is not sufficiently sensitive to detect differences with pharmacological interventions in heart failure compared with implantable electronic devices30 or a ventricular assist device.31 Additionally, therapies might reduce heart failure hospitalizations without improving exercise capacity. In HFrEF, changes in 6-minute walk distance over time bear prognostic information.32 The sodium-glucose co-transporter-2 inhibitor empagliflozin reduced heart failure hospitalizations33 but had no effect on exercise capacity in HFrEF or HFpEF.34 Similarly, sacubitril/valsartan reduced heart failure hospitalizations and cardiovascular mortality in HFrEF35 but did not improve 6-minute walk distance.36
Alternatively, the lack of improvement of 6-minute walk distance with sacubitril/valsartan may be explained by the heterogeneity of heart failure, with distinct pathophysiological pathways underlying reduced exercise capacity. Better phenotyping37 might help to tailor therapy to specific subsets of patients with heart failure that benefit from a specific intervention. In addition, patients in this trial were older (>80% >65 years), with considerable comorbidity, and the heart failure syndrome may not have been the sole limiting factor for submaximal exercise capacity. Future research should test the combination of therapies with beneficial effects on cardiac remodeling (eg, sacubitril/valsartan9 or spironolactone29) with therapies beneficial for quality of life (eg, exercise training).38
The adverse event profile of sacubitril/valsartan was consistent with previous trials.9,13,35 Hypotension was more common with sacubitril/valsartan, particularly in the no RAS inhibitor stratum, in which patients treated with sacubitril/valsartan more commonly had hyperkalemia and kidney impairment than did patients treated with placebo. Hence, initiation of sacubitril/valsartan at low dosages and a longer, gradual uptitration may result in improved tolerability in RAS inhibitor–naive patients.39
Unlike many other clinical trials, this study recruited only patients with reduced quality of life at screening. This approach was chosen because patients with lower quality of life may have more room for improvement. In line with this, large improvements from baseline in mean KCCQ scores were observed at week 24 in both treatment groups (with no significant differences between groups). However, because low KCCQ scores in patients with heart failure are often multifactorial, it may be that (similarly to 6-minute walk distance) further improvements in KCCQ are affected by persistent comorbidities.
Limitations
This study has several limitations. First, the study did not have a run-in phase as used in previous trials. The advantage of this design is that the data are more likely to reflect clinical practice; the disadvantage is that differences between groups are smaller because patients who are intolerant of target drug dosages dilute the difference between groups.
Second, the comparator therapy was stratified based on the use of RAS inhibitor therapy prior to randomization. This approach was justified by the lack of data supporting routine use of RAS inhibitor therapy in patients with heart failure and LVEF higher than 40%. In this trial, nearly 90% of patients were taking either an ACE inhibitor or ARB prior to randomization, reflecting routine practice. These data complement the PARAMOUNT13 and PARAGON-HF9 trials, which showed that NT-proBNP plasma levels are reduced by sacubitril/valsartan in comparison with an ARB.
Third, although plasma natriuretic peptides have been shown to be associated with heart failure outcomes, this trial was of relatively short duration (24 weeks). Whether a longer treatment duration would result in significant differences between treatment groups in outcomes such as 6-minute walk distance, KCCQ scores, or both remains to be elucidated.
Fourth, obesity is a frequent comorbidity in patients with HFpEF, and obese patients have lower NT-proBNP levels. This study included patients with heart failure and a body mass index (BMI, calculated as weight in kilograms divided by height in meters squared) of 40 or lower and elevated NT-proBNP levels. Although patients with BMIs higher than 40 were excluded from this study because of its possible effect on the walk distance measure, the median BMI of the patients enrolled was 30.4 (IQR, 27.0-34.1) demonstrating that the majority of the patients were overweight or obese.
Conclusions
Among patients with heart failure and left ventricular ejection fraction higher than 40%, sacubitril/valsartan treatment compared with standard renin angiotensin system inhibitor treatment or placebo resulted in a significantly greater decrease in plasma N-terminal pro–brain natriuretic peptide levels at 12 weeks but did not significantly improve 6-minute walk distance at 24 weeks. Further research is warranted to evaluate potential clinical benefits of sacubitril/valsartan in these patients.
Trial Protocol
eTable 1. Key eligibility criteria
eTable 2. Incidence of death, key serious AEs and AEs (safety set)
eTable 3. Incidence of key adverse events by stratum
eTable 4. Incidence of low SP, abnormal renal parameters and angioedema at any time postbaseline by stratum
eTable 5. Incidence of low SBP, abnormal renal jparameters and angioedema at any time poste-baseline, by stratum
eFigure. Effect of sacubitril/valsartan and background-medication based individualized comparator on 6-minute walk distance in various predefined subgroups
Statistical Analysis Plan
Nonauthor Collaborators. The PARALLAX investigators and committee members
Data Sharing Statement
References
- 1.Groenewegen A, Rutten FH, Mosterd A, Hoes AW. Epidemiology of heart failure. Eur J Heart Fail. 2020;22(8):1342-1356. doi: 10.1002/ejhf.1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Redfield MM. Heart failure with preserved ejection fraction. N Engl J Med. 2016;375(19):1868-1877. doi: 10.1056/NEJMcp1511175 [DOI] [PubMed] [Google Scholar]
- 3.Yusuf S, Pfeffer MA, Swedberg K, et al. ; CHARM Investigators and Committees . Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet. 2003;362(9386):777-781. doi: 10.1016/S0140-6736(03)14285-7 [DOI] [PubMed] [Google Scholar]
- 4.Massie BM, Carson PE, McMurray JJ, et al. ; I-Preserve Investigators . Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359(23):2456-2467. doi: 10.1056/NEJMoa0805450 [DOI] [PubMed] [Google Scholar]
- 5.Pitt B, Pfeffer MA, Assmann SF, et al. ; TOPCAT Investigators . Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370(15):1383-1392. doi: 10.1056/NEJMoa1313731 [DOI] [PubMed] [Google Scholar]
- 6.Cleland JG, Tendera M, Adamus J, Freemantle N, Polonski L, Taylor J; PEP-CHF Investigators . The Perindopril in Elderly People With Chronic Heart Failure (PEP-CHF) study. Eur Heart J. 2006;27(19):2338-2345. doi: 10.1093/eurheartj/ehl250 [DOI] [PubMed] [Google Scholar]
- 7.Lund LH, Claggett B, Liu J, et al. Heart failure with mid-range ejection fraction in CHARM: characteristics, outcomes and effect of candesartan across the entire ejection fraction spectrum. Eur J Heart Fail. 2018;20(8):1230-1239. doi: 10.1002/ejhf.1149 [DOI] [PubMed] [Google Scholar]
- 8.Solomon SD, Claggett B, Lewis EF, et al. ; TOPCAT Investigators . Influence of ejection fraction on outcomes and efficacy of spironolactone in patients with heart failure with preserved ejection fraction. Eur Heart J. 2016;37(5):455-462. doi: 10.1093/eurheartj/ehv464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Solomon SD, McMurray JJV, Anand IS, et al. ; PARAGON-HF Investigators and Committees . Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. 2019;381(17):1609-1620. doi: 10.1056/NEJMoa1908655 [DOI] [PubMed] [Google Scholar]
- 10.Ferrari R, Fucili A, Rapezzi C. Understanding the results of the PARAGON-HF trial. Eur J Heart Fail. 2020;22(9):1531-1535. doi: 10.1002/ejhf.1797 [DOI] [PubMed] [Google Scholar]
- 11.Anker SD, Butler J, Filippatos G, et al. ; EMPEROR-Preserved Trial Committees and Investigators . Baseline characteristics of patients with heart failure with preserved ejection fraction in the EMPEROR-Preserved trial. Eur J Heart Fail. 2020;22(12):2383-2392. doi: 10.1002/ejhf.2064 [DOI] [PubMed] [Google Scholar]
- 12.Uijl A, Veenis JF, Brunner-La Rocca HP, et al. Clinical profile and contemporary management of patients with heart failure with preserved ejection fraction: results from the CHECK-HF registry. Neth Heart J. 2021;29(7-8):370-376. doi: 10.1007/s12471-020-01534-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Solomon SD, Zile M, Pieske B, et al. ; Prospective Comparison of ARNI with ARB on Management of Heart Failure With Preserved Ejection Fraction (PARAMOUNT) Investigators . The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. Lancet. 2012;380(9851):1387-1395. doi: 10.1016/S0140-6736(12)61227-6 [DOI] [PubMed] [Google Scholar]
- 14.Cunningham JW, Vaduganathan M, Claggett BL, et al. Effects of sacubitril/valsartan on N-terminal Pro-B-type natriuretic peptide in heart failure with preserved ejection fraction. JACC Heart Fail. 2020;8(5):372-381. doi: 10.1016/j.jchf.2020.03.002 [DOI] [PubMed] [Google Scholar]
- 15.von Haehling S, Arzt M, Doehner W, et al. Improving exercise capacity and quality of life using non-invasive heart failure treatments: evidence from clinical trials. Eur J Heart Fail. 2021;23(1):92-113. doi: 10.1002/ejhf.1838 [DOI] [PubMed] [Google Scholar]
- 16.Solomon SD, Vaduganathan M, L Claggett B, et al. Sacubitril/valsartan across the spectrum of ejection fraction in heart failure. Circulation. 2020;141(5):352-361. doi: 10.1161/CIRCULATIONAHA.119.044586 [DOI] [PubMed] [Google Scholar]
- 17.Wachter R, Shah SJ, Cowie MR, et al. Angiotensin receptor neprilysin inhibition versus individualized RAAS blockade: design and rationale of the PARALLAX trial. ESC Heart Fail. 2020;7(3):856-864. doi: 10.1002/ehf2.12694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abraham WT, Zile MR, Weaver FA, et al. Baroreflex activation therapy for the treatment of heart failure with a reduced ejection fraction. JACC Heart Fail. 2015;3(6):487-496. doi: 10.1016/j.jchf.2015.02.006 [DOI] [PubMed] [Google Scholar]
- 19.Bittner V, Weiner DH, Yusuf S, et al. ; SOLVD Investigators . Prediction of mortality and morbidity with a 6-minute walk test in patients with left ventricular dysfunction. JAMA. 1993;270(14):1702-1707. doi: 10.1001/jama.1993.03510140062030 [DOI] [PubMed] [Google Scholar]
- 20.Zugck C, Krüger C, Dürr S, et al. Is the 6-minute walk test a reliable substitute for peak oxygen uptake in patients with dilated cardiomyopathy? Eur Heart J. 2000;21(7):540-549. doi: 10.1053/euhj.1999.1861 [DOI] [PubMed] [Google Scholar]
- 21.Shoemaker MJ, Curtis AB, Vangsnes E, Dickinson MG. Clinically meaningful change estimates for the six-minute walk test and daily activity in individuals with chronic heart failure. Cardiopulm Phys Ther J. 2013;24(3):21-29. doi: 10.1097/01823246-201324030-00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spertus JA, Jones PG, Sandhu AT, Arnold SV. Interpreting the Kansas City Cardiomyopathy Questionnaire in Clinical Trials and Clinical Care: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;76(20):2379-2390. doi: 10.1016/j.jacc.2020.09.542 [DOI] [PubMed] [Google Scholar]
- 23.Ingle L, Shelton RJ, Rigby AS, Nabb S, Clark AL, Cleland JG. The reproducibility and sensitivity of the 6-min walk test in elderly patients with chronic heart failure. Eur Heart J. 2005;26(17):1742-1751. doi: 10.1093/eurheartj/ehi259 [DOI] [PubMed] [Google Scholar]
- 24.Bretz F, Maurer W, Brannath W, Posch M. A graphical approach to sequentially rejective multiple test procedures. Stat Med. 2009;28(4):586-604. doi: 10.1002/sim.3495 [DOI] [PubMed] [Google Scholar]
- 25.Armstrong PW, Lam CSP, Anstrom KJ, et al. ; VITALITY-HFpEF Study Group . Effect of Vericiguat vs Placebo on Quality of Life in Patients With Heart Failure and Preserved Ejection Fraction: the VITALITY-HFpEF randomized clinical trial. JAMA. 2020;324(15):1512-1521. doi: 10.1001/jama.2020.15922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Udelson JE, Lewis GD, Shah SJ, et al. Effect of Praliciguat on Peak Rate of Oxygen Consumption in Patients With Heart Failure With Preserved Ejection Fraction: the CAPACITY HFpEF Randomized Clinical Trial. JAMA. 2020;324(15):1522-1531. doi: 10.1001/jama.2020.16641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Redfield MM, Chen HH, Borlaug BA, et al. ; RELAX Trial . Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2013;309(12):1268-1277. doi: 10.1001/jama.2013.2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shah SJ, Voors AA, McMurray JJV, et al. Effect of neladenoson bialanate on exercise capacity among patients with heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2019;321(21):2101-2112. doi: 10.1001/jama.2019.6717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Edelmann F, Wachter R, Schmidt AG, et al. ; Aldo-DHF Investigators . Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the Aldo-DHF randomized controlled trial. JAMA. 2013;309(8):781-791. doi: 10.1001/jama.2013.905 [DOI] [PubMed] [Google Scholar]
- 30.De Marco T, Wolfel E, Feldman AM, et al. Impact of cardiac resynchronization therapy on exercise performance, functional capacity, and quality of life in systolic heart failure with QRS prolongation: COMPANION trial sub-study. J Card Fail. 2008;14(1):9-18. doi: 10.1016/j.cardfail.2007.08.003 [DOI] [PubMed] [Google Scholar]
- 31.Gustafsson F, Shaw S, Lavee J, et al. Six-month outcomes after treatment of advanced heart failure with a full magnetically levitated continuous flow left ventricular assist device: report from the ELEVATE registry. Eur Heart J. 2018;39(37):3454-3460. doi: 10.1093/eurheartj/ehy513 [DOI] [PubMed] [Google Scholar]
- 32.Ferreira JP, Metra M, Anker SD, et al. Clinical correlates and outcome associated with changes in 6-minute walking distance in patients with heart failure: findings from the BIOSTAT-CHF study. Eur J Heart Fail. 2019;21(2):218-226. doi: 10.1002/ejhf.1380 [DOI] [PubMed] [Google Scholar]
- 33.Packer M, Anker SD, Butler J, et al. ; EMPEROR-Reduced Trial Investigators . Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383(15):1413-1424. doi: 10.1056/NEJMoa2022190 [DOI] [PubMed] [Google Scholar]
- 34.Abraham WT, Lindenfeld J, Ponikowski P, et al. Effect of empagliflozin on exercise ability and symptoms in heart failure patients with reduced and preserved ejection fraction, with and without type 2 diabetes. Eur Heart J. 2021;42(6):700-710. doi: 10.1093/eurheartj/ehaa943 [DOI] [PubMed] [Google Scholar]
- 35.McMurray JJ, Packer M, Desai AS, et al. ; PARADIGM-HF Investigators and Committees . Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371(11):993-1004. doi: 10.1056/NEJMoa1409077 [DOI] [PubMed] [Google Scholar]
- 36.Piepoli MF, Hussain RI, Comin-Colet J, et al. OUTSTEP-HF: randomised controlled trial comparing short-term effects of sacubitril/valsartan versus enalapril on daily physical activity in patients with chronic heart failure with reduced ejection fraction. Eur J Heart Fail. 2021;23(1):127-135. doi: 10.1002/ejhf.2076 [DOI] [PubMed] [Google Scholar]
- 37.Shah SJ, Katz DH, Selvaraj S, et al. Phenomapping for novel classification of heart failure with preserved ejection fraction. Circulation. 2015;131(3):269-279. doi: 10.1161/CIRCULATIONAHA.114.010637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nolte K, Herrmann-Lingen C, Wachter R, et al. Effects of exercise training on different quality of life dimensions in heart failure with preserved ejection fraction: the Ex-DHF-P trial. Eur J Prev Cardiol. 2015;22(5):582-593. doi: 10.1177/2047487314526071 [DOI] [PubMed] [Google Scholar]
- 39.Ambrosy AP, Braunwald E, Morrow DA, et al. ; PIONEER-HF Investigators . Angiotensin receptor-neprilysin inhibition based on history of heart failure and use of renin-angiotensin system antagonists. J Am Coll Cardiol. 2020;76(9):1034-1048. doi: 10.1016/j.jacc.2020.06.073 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Key eligibility criteria
eTable 2. Incidence of death, key serious AEs and AEs (safety set)
eTable 3. Incidence of key adverse events by stratum
eTable 4. Incidence of low SP, abnormal renal parameters and angioedema at any time postbaseline by stratum
eTable 5. Incidence of low SBP, abnormal renal jparameters and angioedema at any time poste-baseline, by stratum
eFigure. Effect of sacubitril/valsartan and background-medication based individualized comparator on 6-minute walk distance in various predefined subgroups
Statistical Analysis Plan
Nonauthor Collaborators. The PARALLAX investigators and committee members
Data Sharing Statement