Abstract
Non-alcoholic fatty liver disease (NAFLD) is considered one of the most serious public health problems affecting liver. The reported beneficial impact of raspberries on obesity and associated metabolic disorder makes it a suitable candidate against NAFLD. In the current study, the chemical profile of raspberry seed oil (RO) was characterized by analysis of fatty acid and tocopherol contents using high-performance liquid chromatography (HPLC) in addition to the determination of total phenolic and flavonoids. High levels of unsaturated fatty acids, linoleic acid (49.9%), α-linolenic acid (25.98%), and oleic acid (17.6%), along with high total tocopherol content (184 mg/100 gm) were detected in oil. The total phenolic and flavonoid contents in RO were estimated to be 22.40 ± 0.25 mg gallic acid equivalent (GAE)/100 mg oil and 1.34 ± 0.15 mg quercetin (QU)/100 mg, respectively. Anti-NAFLD efficacy of RO at different doses (0.4 and 0.8 mL) in a model of a high-fat diet (HFD) fed rats was assessed by estimating lipid profile, liver enzyme activity, glucose and insulin levels as well as adipokines and inflammatory marker. Peroxisome proliferator-activated receptor γ (PPARγ), which is a molecular target for NAFLD was also tested. Liver histopathology was carried out and its homogenate was used to estimate oxidative stress markers. Consumption of RO significantly improved lipid parameters and hepatic enzyme activities, reduced insulin resistance and glucose levels, significantly ameliorated inflammatory and oxidative stress markers. Furthermore, RO treatment significantly modulated adipokines activities and elevated PPARγ levels. Raspberry seed oil administration significantly improved these HFD induced histopathological alterations. Moreover, a molecular docking study was performed on the identified fatty acids and tocopherols. Among the identified compounds, oleic acid, α-linolenic acid and γ-tocopherol exhibited the highest docking score as PPARγ activator posing them as a potential anti-NAFLD drug leads. Study findings suggest RO as an effective therapeutic candidate for ameliorating NAFLD.
Keyword: Raspberry oil, NAFLD, HPLC profiling, HFD, PPARγ
1. Introduction
NAFLD is a pathological condition characterized by fat deposition in hepatocytes without history of excessive alcohol consumption (Pettinelli et al., 2011). Currently, NAFLD has become a big public-health issue, affecting more than 40 percent of countries' population (Ma et al., 2017). It is estimated that NAFLD global prevalence in adults is 7–37% and in children it is 20–30% (Mapfumo et al., 2020).
NAFLD causes continuous liver tissue damage ranging from steatosis to non-alcoholic steatohepatitis (NASH), that involves many inflammatory processes and may quickly proceed to develop liver fibrosis, cirrhosis, and subsequently liver cancer (Juárez-Hernández et al., 2015). Obesity and type 2 diabetes are strong risk factors for NAFLD (Wong et al., 2018). NAFLD is highly related to type 2 diabetes, insulin resistance, obesity, high blood pressure as well as high-serum lipoproteins (Iser and Ryan, 2013).
Unfortunately, there are no standard guidelines for NAFLD pharmacological therapy, therefore weight loss, dietary and lifestyle improvements are considered the most effective NAFLD therapy to date (Bril and Cusi, 2017). However, anti-obesity, anti-diabetic, antioxidants, and anti-hyperlipidemic synthetic agents are utilized as treatment for NAFLD and its related complications (Beaton, 2012). These synthetic drugs cause side effects and are expensive, thus most people in the world prefer to use plant-derived medicines for disease control (Mapfumo et al., 2020).
Edible oils are necessary macronutrients in foods that can promote human health by providing fat-soluble vitamins, essential fatty acids, and other phytochemicals (Gunstone, 2011). Berry seed oils, like red raspberry, are considered as special oil that is used in different food nutraceutical and cosmetic products. Raspberry seed oil (RO) is abundant in essential polyunsaturated fatty acids (PUFA), various anti-inflammatory, anticancer, antioxidant, and anti-atherosclerotic substances, like γ, α and δ-tocopherol, tocochromanols, phytosterols, flavonoids, carotenoids, and phenolic acids (Li et al., 2016, Parry et al., 2005).
Raspberry dietary supplementation has been reported to reduce skeletal muscle lipid accumulation (Zou et al., 2019), disrupt insulin resistance and leptin signaling (Attia et al., 2019), as well as modulate inflammatory condition, liver functions, and lipid metabolism in rats (Fotschki et al., 2015a). Red raspberry seed oil nanoemulsion showed a synergistic free radical scavenging effect between lipophilic and hydrophilic antioxidants in DPPH and ABTS test. The nanoemulsion carrier improved the effect of oil on HeLa cervical adenocarcinoma cells and showed a safety properties on normal human lung fibroblasts, MRC-5 (Gledovic et al., 2020). Red raspberry (Rubus idaeus) consumption restored the impaired vasoconstriction and vasorelaxation response in the aorta of the obese Zucker rat (VandenAkker et al., 2020). Hexane extracts from leaves and stems of Rubus idaeus showed antifungal activity against Candida albicans with IC50 at 500 and 250 μg/mL. Their bio-guided fractionation led to four active subfractions with IC50 between 62.5 and 125 μg/mL. Most of the components identified in active subfractions were fatty acids and terpenoids (Bernard et al., 2020). Rubusesquilin B1 isolated from the fruits of raspberries showed moderate neuroprotective effects against H2O2-induced neurotoxicity at the concentration of 25 μM (Lu et al., 2020). The various parts of raspberry (leaves, fruit pulp and seed extracts) demonstrated strong antioxidant activities and inhibitory effects on digestive enzymes (α-glucosidase and α-amylase), preventing oxidative damage and diabetes-related problems (Wu et al., 2019). Raspberry fruit extract decreased oxidation markers, improved lipid metabolism and reduced adipose tissue inflammation in hypertrophied 3 T3-L1 adipocytes (Kowalska et al., 2019). Red raspberry supplementation alleviated the effects of a high-fat diet on the brain and behavior in mice (Carey et al., 2019). Phenolic-enriched raspberry fruit extract resulted in decreased weight gain, increased ambulatory activity, and elevated hepatic lipoprotein lipase and heme oxygenase-1 expression in male mice fed a high-fat diet (Kshatriya et al., 2019). Higher antioxidant activity was detected in the leaf extracts than in the fruit extracts of wild raspberry populations, collected from the central Balkan region evaluated by measuring the scavenging capacity of the extracts on DPPH. Leaf extracts exhibited anticancer activity with IC50/24 h 162.38 μg mL−1 and IC50/48 h 95.69 μg mL−1 on human colorectal cancer cell line HCT-116 (Veljkovic et al., 2019). The beneficial features reported by RO make it the future prophylaxis of diet-induced NAFLD. However, more elaboration is needed to address the potential of oil in preventing NAFLD resulted from high-fat diet and its associated complications. Since excessive caloric consumption is an essential aspect of NAFLD clinical development, NAFLD induction by high-fat diet (HFD) animal model is considered one of the most established and reliable models used in NAFLD studies (Van Herck et al., 2017). HFD consumption causes animals to develop obesity, hyperglycemia, hyperinsulinemia, high blood pressure and liver damage close to the condition found in humans with NAFLD (Recena Aydos et al., 2019).
Hence, this study interrogated the potential of RO in controlling NAFLD associated with HFD. Due to the importance of chemical characterization of edible oil for assessing the efficacy of herbal medicine, the cold-pressed RO fatty acid and tocopherols composition were determined by HPLC in addition to total phenolic and total flavonoid contents determination. Moreover, Peroxisome proliferator-activated receptor gamma (PPARγ) is one of the potential therapeutic targets in ameliorating NAFLD. Several studies confirmed that the activation of PPARγ served as a useful strategy for preventing NAFLD (Shabalala et al., 2020, Zhong and Liu, 2018). It is highly expressed in adipose tissue where it controls adipocyte differentiation and its activation plays a major role in increasing insulin sensitivity as well as in promoting fatty acids uptake into adipocytes. The net effect of this process reduces FA delivery to the liver so it attenuates HFD-induced NAFLD (Feng et al., 2017, Tailleux et al., 2012). Consequently, the potential role of identified compounds as anti-NAFLD was predicted by molecular docking against PPARγ.
2. Materials and methods
2.1. Chemicals
Cold-pressed oil of red raspberry seed (RO, Rubus idaeus) was purchased from Makers Ingredients (York, North Yorkshire, UK). All fatty acids (FA) standards, tocopherol standards, Folin-Ciocalteau reagent, gallic acid, quercetin were obtained from Sigma Chemical Co. (St. Louis, MO, USA). Acetonitrile, hexane, 2-propanol and dimethyl propane of HPLC grade were obtained from Sigma Chemical Co. (St. Louis, MO, USA). Water purification was carried out via the Milli-Q system (Millipore, Bedford, MA, USA). N,N-dimethylformamide (DMF) was purchased from Loba Chemie (Colaba, Mumbai, India). Potassium carbonate (K2CO3) and pyridine were obtained from Sigma Chemical Co. (St. Louis, MO, USA) while chloroform was obtained from Andheri West, Mumbai, Maharashtra, India. The 2-(5-benzoacridine)ethyl-p-toluene sulfonate (BAETS) labeling reagent was synthesized in the laboratory. Unless mentioned, all reagents used were of analytical grade.
2.2. Fatty acid profile
2.2.1. Instrumentation
Free fatty acids analysis was carried out with an Agilent Technologies 1100 series HPLC (Germany) fluorescence detector (FLD) system. Separation of fatty acid derivatives was carried out via a reversed-phase ODS C18 column (250 × 4.6 mm, 5 μm, Netherlands).
2.2.2. Standard and sample solutions preparation
Individual standard fatty acid stock solution (1 × 10−2 mol L−1) was prepared in acetonitrile and diluted to a concentration of (1 × 10−4 mol L−1) with acetonitrile. The dissolution of 12.8 mg of BAETS into 10 mL of acetonitrile resulted in obtaining the derivatization solution. The cold-pressed RO was dissolved in acetonitrile for derivatization. All solutions were kept at 4 °C in the refrigerator when not in use.
2.2.3. Derivatization procedure
A 20 mg K2CO3, 200 μL DMF, 200 μL BAETS solution was applied to a vial containing standard fatty acid or a sample and sealed. Then, the reaction was conducted at 90 °C for 0.5 h in a water bath. Diluting the solution was performed via acetonitrile after cooling to room temperature and filtered via a 0.45 μm membrane filter for HPLC analysis.
2.2.4. HPLC conditions
HPLC analysis was carried out using solvent system: water (A) and acetonitrile (B). The elution was like the following: acetonitrile (45–83%) from 0 to 30 min; acetonitrile (83–92%) from 30 to 50 min and acetonitrile (92–100%) from 50 to 60 min. The injection volume was 10 μL at a flow rate 1 mL/min, and the column was maintained at 30 °C. Fluorescence excitation and emission wavelengths were set at 280 nm and 360 nm. Compared to standard peaks retention time, the peaks of fatty acids (FA) derivatives in a sample were determined. Results were expressed as FA group (gm) in 100 gm of total FA (% w/w).
2.3. Tocopherols analysis
Tocopherol content of RO was determined according to (Oomah et al., 2000) using normal phase HPLC. RO was diluted with hexane and filtered through 0.45 µm filter before HPLC analysis. Tocopherols were analyzed by HPLC system (Agilent technologies 1100 series, Germany) with a fluorescence detector. A Hypersil APS-2 normal phase column (250 × 4.6 mm, 5 µm, Chrompack, Netherlands) was used in combination with guard column (Hypersil APS, 10 × 4 mm, 5 µm, Chrompack, Netherlands). Operation of the system was performed isocratically using hexane/2-propanol/dimethyl propane (1000/5/1, v/v/v) as the mobile phase at a flow rate of 1 mL/min. Separations were carried out at 25 °C with the fluorescence detector set at excitation λex = 297 nm and emission λem = 325 nm. Quantitation of α-, β-, γ -, and δ-tocopherols was established by external standard method. The results are presented in the form of mg of tocopherols per 100 gm oil (mg/100 gm).
2.4. Total phenolic contents
The total phenolic contents in RO were determined via Folin-Ciocalteu method as described by and gallic acid as standard. The solution of RO was prepared by dissolving 1 mg of oil in 1 mL acetonitrile. An aliquot (0.5 mL) of the solution was mixed with 2.5 mL of 10% Folin-Ciocalteu reagent as well as 2.5 mL 7.5% sodium carbonate solution and was left at room temperature for 45 min. The absorbance was estimated at λmax760 nm. Total phenolic contents were calculated and expressed as mg gallic acid equivalents (GAE) for each 100 gm of oil.
2.5. Total flavonoid contents
Total flavonoid contents of RO were estimated via aluminum chloride colorimetric assay and quercetin as standard. RO (50 mg) was diluted with 10 mL acetonitrile. (50 μL) of the diluted solution was blended with 0.1 mL AlCl3 (0.1 mol/L), 0.1 mL potassium acetate (1 mol/L), 2.8 mL distilled water, and 2.15 mL ethanol, and then left for 30 min. The absorbance (A) was measured at 415 nm. The total flavonoids contents were presented as mg quercetin (QU) equivalents for each gm of oil.
2.6. Animals and experimental design
Forty male Wistar rats (150–180 gm) were obtained from Veterinary Unit for Laboratory Animals (Cairo, Egypt). Animals were kept in a 12:12-h artificial dark lightening cycle. The rats were housed in stainless steel cages with 10 animals each, with an excess of water and standard diet. All animal experiments were conducted in accordance with the Guide for the U.S. Public Health Service Policy on Humane Care and Use of Laboratory Animals (PHS Policy) and the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Guide). The experiment was approved by the Institutional Research Ethics Committee at the Faculty of Pharmacy, Cairo University, Cairo, Egypt (Approval no. PT-1621).
After one-week the conditioned rats were divided into four study groups, each group included 10 rats. Group 1: control group fed a normal chow diet. Group 2: rats fed HFD (58% rat show diet, 5% sucrose, 18% butter, 1% cholesterol, 10% corn oil, 0.2% methionine, 5% casein, 2% minerals mixture and 0.8% vitamins mixture). Group 3: rats fed HFD + RO (0.4 mL). Group 4: rats fed HFD + RO (0.8 mL). RO was administered orally 3 times weekly for 8 weeks (Pieszka et al., 2013). After 8 weeks, all rats were fasted for 12 h and euthanized under anesthesia by using ethyl ether. Blood samples were collected in heparinized tubes through the orbital sinus and liver tissue was excised and processed for other analyses.
2.6.1. Serum biochemical analysis
Fasting blood glucose level, total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), alanine aminotransferase (ALT), and aspartate aminotransferase (AST) were determined using Bio-diagnostic colorimetric kits (Cairo, Egypt) according to the manufacturer's protocol.
Nuclear factor-kappa B (NF-κB), levels of insulin, peroxisome proliferator activated receptor gamma (PPARγ), leptin and adiponectin were assessed using ELISA kits (abcam1 Kendall Square, Cambridge, MA 02139-1517, USA) according to manufactures' instructions. Insulin resistance was evaluated using the homeostasis model assessment index for insulin resistance (HOMA-IR) as follows (Matthews et al., 1985):
2.6.2. Estimation of biochemical parameters of liver tissue
Fresh liver tissue was rinsed with isotonic saline, weighed, and then homogenized in (10 % W/V) ice-cold 0.1 M phosphate buffer (pH 7.4) to determine the hepatic content of malondialdehyde (MDA) (Erdinçler et al., 1997), superoxide dismutase (SOD) (Kar et al., 2002) and nitric oxide (NO) (Ruiz-Larrea et al., 1994).
2.6.3. Histopathological assessment
Liver tissues were quickly dissected, sectioned, fixed in a solution of 10% formaldehyde saline and embedded in paraffin. After fixing the tissue sections, they were cut into 4 µm sections and stained with Hematoxylin-eosin (HE). The sections were imaged with light microscope using a digital camera (Bernard et al., 2020).
2.6.4. Statistical analysis
Data obtained from the study presented as mean ± SEM. Statistical analysis was carried out using SPSS statistical package, (release 16) for windows. Differences among groups were analyzed by student's t-test. Multiple comparisons were performed by one-way ANOVA tests. P < 0.05 was considered significant.
2.7. Molecular docking
The crystal structure of PPARγ was obtained from protein data bank (PDB ID: 2XKW) to make molecular docking analysis. Docking analysis was done using Molecular Operating Environment (MOE) software. Site finder tool of MOE was used to search for the active binding pocket of PPARγ. All identified compounds were docked into the binding pocket of PPARγ.
3. Results
3.1. Fatty acid profile
Palmitic, stearic, oleic, linoleic (ω6), α-linolenic (ω3), and arachidic acids were detected in RO (Table 1). Linoleic acid was found to be the most abundant fatty acid, contributing 49.9% of the total fatty acids. RO contained a high amount of polyunsaturated fatty acids (PUFA, 75.88%) compared with saturated fatty acids (SFA, 6.52%) and monounsaturated fatty acids (MUFA, 17.62%). Furthermore, RO contained significant amounts of α-linolenic acid (25.98%) resulting in a favorable ratio of ω6:ω3, 1.92. These findings are similar to the findings of other authors who described the RO fatty acid profile before (Oomah et al., 2000, Pieszka et al., 2013, Teng et al., 2016).
Table 1.
Free fatty acid profile and tocopherols contents of cold-pressed raspberry seed oil.
| Free fatty acids | Mean ± SD (%) | Tocopherols | Mean ± SD (mg/100 g) |
|---|---|---|---|
| Palmitic acid (C16:0) Stearic acid (C18:0) Oleic acid (C18:1 ω9) Linoleic acid (C18:2 ω6) α-Linolenic acid(C18:3 ω3) Arachidic acid (C20:0) Σ SFA Σ MUFA Σ PUFA ω6:ω3 ratio |
5.06 ± 0.69 0.78 ± 0.85 17.62 ± 0.51 49.90 ± 4.65 25.98 ± 4.39 0.68 ± 0.65 6.52 17.62 75.88 1.92 |
α-tocopherol β-tocopherol γ-tocopherol δ-tocopherol TTC |
42.73 ± 0.33 0.35 ± 0.18 134.62 ± 7.89 7.40 ± 0.52 185.1 ± 8.92 |
Values were presented as mean ± SD, n = 3. SFA, saturated fatty acids; MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids; and TTC, total tocopherol content.
3.2. Tocopherols analysis
Tocopherols concentrations in cold-pressed RO are shown in Table 1. In this analysis, RO was particularly rich in γ-tocopherol and α- tocopherol (134.62 and 42.73 mg/100 gm oil, respectively). RO exhibited a high concentration of total tocopherols (185.1 mg/100 gm oil).
3.3. Total phenolic and flavonoid contents
Total phenolic and flavonoid contents in RO were estimated to be 22.40 ± 0.25 mg GAE/100 mg oil and 1.34 ± 0.15 mg QU/100 mg, respectively.
3.4. Effect on serum lipid parameters
As outlined in Table 2, all parameters of the lipid profile changed due to the HFD; the serum TC, TG and LDL-C levels were increased, while HDL-C levels were significantly decreased when compared to the control group (P < 0.05). Treatment with RO significantly modulated these alterations, and thus improved lipid profile. RO oral administration significantly reduced TC, TG, and LDL-C levels with increased HDL-C as compared to the HFD group.
Table 2.
The effect of cold-pressed raspberry seed oil on lipid parameters and liver enzymes activity in rats fed with HFD.
| Groups | TG (mg/dl) | TC (mg/dl) | HDL-c (mg/dl) | LDL-c (mg/dl) | ALT (U/L) | AST (U/L) |
|---|---|---|---|---|---|---|
| Control | 82.5 ± 3.2 | 60.5 ± 2.1 | 35.0 ± 1.8 | 40.5 ± 4.0 | 33.7 ± 1.7 | 45.0 ± 2.0 |
| HFD control | 181.2 ± 4.2 a | 140.0 ± 5.4a | 17.7 ± 1.5 a | 82.5 ± 3.0a | 80.5 ± 2.1a | 108.7 ± 4.0 a |
| HFD + RO (0.4 mL) | 116.0 ± 6.2ab | 98.7 ± 4.2ab | 25.0 ± 0.8ab | 65.0 ± 2.2ab | 52.5 ± 1.4ab | 70.4 ± 2.8ab |
| HFD + RO (0.8 mL) | 101.0 ± 4.3abc | 78.5 ± 3.6abc | 31.0 ± 1.0abc | 49.7 ± 1.6abc | 43.5 ± 2.0abc | 57.5 ± 3.2abc |
Data are reported as means ± SEM, n = 10.
Differences among groups were analyzed via student's t-test. One-way ANOVA tests were carried out for multiple comparisons (p < 0.05).
Values with different superscripts are considered significantly different.
TG, triglycerides; TC, total cholesterol; HDL-c, high-density lipoprotein cholesterol; LDL-c, low-density lipoprotein cholesterol.
3.5. Effect on liver enzyme activity
Serum liver enzymes in group 2 were approximately two times higher than in group 1 (Table 2). RO treated groups showed a significant decrease in ALT and AST activities (P < 0.05) compared to the HFD group. RO (0.8 mL) per OS showed the most significant reduction in ALT and AST compared to the HFD group (43.5 ± 2.0 U/L and 57.5 ± 3.2 U/L vs. 80.5 ± 2.1 U/L and 108.7 ± 4.0 U/L, respectively).
3.6. Effect on fasting blood glucose, insulin and insulin resistance
With regard to group 2, the fasting blood glucose levels, as well as HOMA-IR (Table 3), were markedly elevated as compared to those of group 1. The RO per OS treatment led to an almost complete correction of the disturbed glucose homeostasis (P < 0.05). The blood glucose level of group 3 was 33.7% lower (p < 0.05) than that of group 2, which was remarkably reduced in group 4 by 44.6%. For the insulin resistance (HOMA-IR), it was significantly reduced in RO-treated groups compared to HFD fed rats. RO (0.8 mL) per OS showed the most significant reduction in HOMA-IR compared to the HFD group (2.7 ± 1.0 vs. 5.2 ± 0.02, respectively). There were no significant differences among the studied groups with respect to insulin level.
Table 3.
The impact of cold-pressed raspberry seed oil on fasting blood glucose, insulin, and insulin resistance index in rats fed with HFD.
| Groups | Glucose (mmol/L) | Insulin (mIU/L) | HOMA-IR index |
|---|---|---|---|
| Control | 3.9 ± 0.2 | 11.3 ± 0.34 | 1.9 ± 0.01 |
| HFD | 10.1 ± 0.9a | 11.8 ± 0.47 | 5.2 ± 0.02 a |
| HFD + RO (0.4 mL) | 6.7 ± 0.23ab | 11.5 ± 0.37 | 3.4 ± 0.8ab |
| HFD + RO (0.8 mL) | 5.6 ± 0.17abc | 11.1 ± 0.31 | 2.7 ± 1.0abc |
Data are reported as means ± SEM, n = 10.
Differences among groups were analyzed via student's t-test. One-way ANOVA tests were carried out for multiple comparisons (p < 0.05).
Values with different superscripts are considered significantly different.
3.7. Effects on SOD, MDA and NO in liver homogenate
The 8-week HFD triggered oxidative stress (Table 4) manifested by increased MDA and NO levels and lower SOD activity compared to control animals (P < 0.05). RO per OS treatment abated lipid peroxidation to reach its normal level. RO (0.4 mL) and (0.8 mL) significantly decreased hepatic MDA and NO levels and increased SOD activity compared with HFD group (2.7 ± 0.15 nmol/mg, 1.9 ± 0.09 μmol/mg and 30.0 ± 1.0 U/gm; 1.8 ± 0.14 nmol/mg, 1.3 ± 0.06 μmol/mg and 39.2 ± 1.7 U/gm vs. 3.5 ± 0.21 nmol/mg, 2.8 ± 0.1 μmol/mg and 21.2 ± 1.4 U/gm, respectively).
Table 4.
The effect of cold-pressed raspberry seed oil on levels of hepatic MDA, NO, and SOD in rats fed with HFD.
| Groups | MDA (nmol/mg) | NO (μmol/mg) | SOD (U/gm) |
|---|---|---|---|
| Control | 1.3 ± 0.09 | 0.85 ± 0.12 | 46.2 ± 1.7 |
| HFD | 3.5 ± 0.21a | 2.8 ± 0.1 a | 21.2 ± 1.4 a |
| HFD + RO (0.4 mL) | 2.7 ± 0.15ab | 1.9 ± 0.09ab | 30.0 ± 1.0ab |
| HFD + RO (0.8 mL) | 1.8 ± 0.14abc | 1.3 ± 0.06abc | 39.2 ± 1.7abc |
Data are reported as means ± SEM, n = 10.
Differences among groups were analyzed via student's t-test. One-way ANOVA tests were carried out for multiple comparisons (p < 0.05).
Values with different superscripts are considered significantly different.
3.8. Effects on serum leptin, adiponectin, PPARγ and NF-κB
RO (0.4 and 0.8 mL) oral administration showed a significant reduction in leptin as well as NF-κB levels, along with a significant rise in adiponectin and PPARγ levels compared with the HFD fed group (Fig. 1).
Fig. 1.
The effect of cold-pressed raspberry seed oil on NF-κB (A), leptin (B), adiponectin (C) and PPARγ (D) levels. Values are reported as mean ± SEM, n = 10. Bars with the different superscript letters are significantly different from each other, P < 0.05.
3.9. Effects on histopathology of liver
No histological problems in the liver have been detected in the control group where the hepatic parenchyma seemed normal and the hepatocytes around the central vein were organized. In contrast, the inflammation and rounded fat droplets within the cytoplasm and the nuclei were clearly observed in rats fed with HFD. Raspberry seed oil administration significantly improved these HFD induced alterations, especially in the animal group which received 0.8 mL of RO (Fig. 2).
Fig. 2.

Micrographs of liver sections. (A) Control group revealing normal parenchyma (B) HFD-provided control group revealing marked steatosis (C) RO (0.4 mL) provided HFD group revealing mild steatosis (D) RO (0.8 mL) provided HFD group revealing almost normal parenchyma.
3.10. Molecular docking
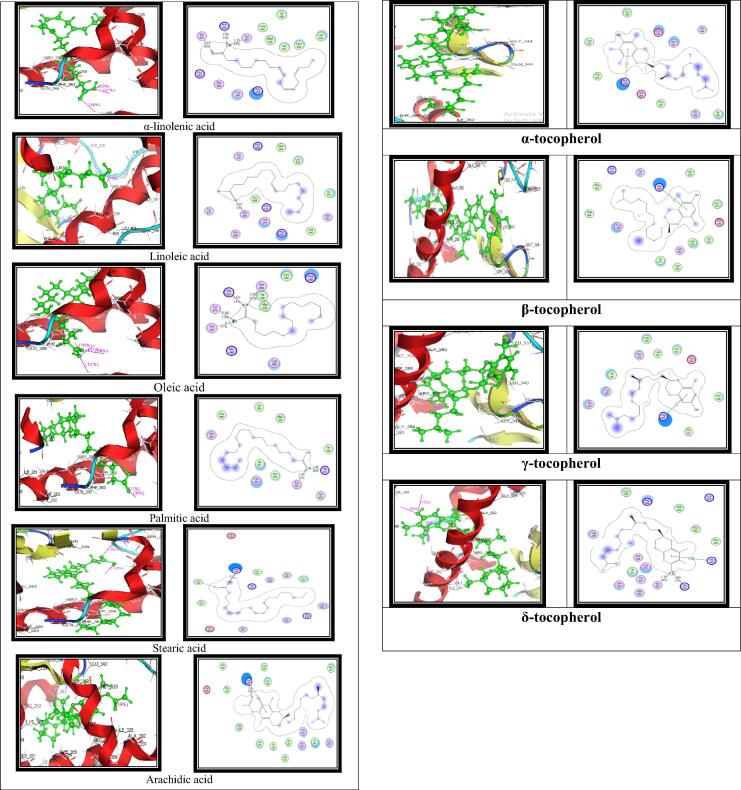
Molecular docking was conducted to predict the possible interaction between ligand and the target protein. All compounds identified as listed in Table 5 represent a significant interaction with PPARγ in comparable manner as represented by the PPARγ agonist Pioglitazone. However, some compounds especially oleic acid, α-linoleic acid and γ-tocopherol displayed better interaction with the PPARγ than with pioglitazone. Binding pocket of PPARγ with labeled amino acid residues present in the active site are shown in Fig. 3A. In addition, the interaction of pioglitazone (Fig. 3B) and all identified compounds (Fig. 4) are also displayed in Table 5.
Table 5.
Binding interactions between PPARγ and identified fatty acids and tocopherols.
| Compound | H-Bonding |
Arene-π interaction | ||
|---|---|---|---|---|
| Distance(oA) | Score (%) | Amino acid | ||
| α-linolenic acid | 2.72 2.58 2.33 |
38 31 33 |
Tyr 473 His 323 Tyr 327 |
|
| Linoleic acid | 2.63 | 13 | Ser 289 | |
| Oleic acid | 3 2.68 2.63 2.69 |
11 13 14 76 |
Tyr 327 Tyr 473 His 323 Ser 289 |
|
| Palmitic acid | 2.45 1.85 |
11 35 |
Tyr 327 His 323 |
|
| Stearic acid | 2.37 | 14 | Arg 288 | |
| Arachidic acid | 2.35 | 19 | Arg 288 | Arg 288 |
| Tocopherols | Arg 288 | |||
| Pioglitazone | 2.71 | 73 | Arg 288 | |
| 1.7 | 46 | His 323 | ||
Fig. 3.
(A) Binding pocket of PPARγ, (B) Binding interaction of Pioglitazone in the active pocket of PPARγ.
Fig. 4.
The putative binding mode analysis of all identified compounds in the binding pocket of PPARγ. Ligands are represented in green.
4. Discussion
NAFLD has become a noticeable global health issue, affecting large number of people in many countries. NAFLD causes continuous liver tissue damage that ranging from simple steatosis to non-alcoholic steatohepatitis (NASH), which involves many inflammatory processes and may rapidly progress to liver fibrosis, cirrhosis, and subsequently liver cancer (Uribe and Barbero-Becerra, 2015). Therefore, discovery of a new therapeutic agent for the management of NAFLD is a challenging goal. Raspberry is special oil that is used in the food, nutritional supplement, and cosmetic industries for various products. The reported beneficial features of raspberry seed oil contribute to its potential protective intervention for diet-induced NAFLD (Pieszka et al., 2013, Zou et al., 2019, Attia et al., 2019, Fotschki et al., 2015a). Nonetheless, the potential of RO to protect against NAFLD resulted from high-fat diet as well as its related complications need further investigation.
Nutrition is a vital contributing agent in the pathogenesis of NAFLD. High-fat foods have been proven to promote the intrahepatic deposition of triacylglycerols and the development of NAFLD (Nakamuta et al., 2008). However, nutritional components rich in essential fatty acids, vitamins, and flavonoids have a preventive role in the progression of NAFLD (Barrea et al., 2018). In the current study, HPLC analysis of raspberry seed oil revealed that it is a rich source of polyunsaturated fatty acids (PUFA) and tocopherols. These results are consistent with advanced researches, that reported high levels of unsaturated fatty acid and tocopherol contents from the RO (Oomah et al., 2000, Fotschki et al., 2015b). Furthermore, RO contains an adequate amount of phenolic compounds. Therefore, cold-pressed raspberry seed oil may be a useful strategy for preventing HFD induced NAFLD. High-fat diet triggered NAFLD is considered a useful animal model for evaluating the anti-NAFLD activity of a new test agent. In the current study, HFD-fed animals displayed marked hyperlipidemia, steatosis along with elevated HOMA-IR values, presenting almost all clinical manifestations of NAFLD observed in humans (Chang et al., 2014). However, HFD-fed animals provided with raspberry seed oil displayed a significant decline in the level of TG, TC, and LDL while suppressed plasma HDL levels were significantly elevated. The level of ALT and AST enzymes which are indicative of early liver damage was also elevated in HFD-provided group; however, their level was significantly suppressed in HFD-fed animals provided with RO.
HOMA-IR is considered an important predictor of NAFLD associated with liver fibrosis. Insulin resistance (IR) has been observed in nearly all NAFLD patients leading to obesity, diabetes mellitus, impaired glucose tolerance, dyslipidemia, and hypertension (Fujii et al., 2019). According to the results of the current study, group 2 provided with a high-fat diet exhibited a marked increase in HOMA-IR values, along with elevated glucose levels which can be considered as a contributing factor to the development of steatosis. Insulin has a marked inhibitory effect on lipolysis in adipose tissues. However, in the case of insulin resistance, the suppression of lipolysis is inhibited leading to efflux of fatty acids from adipose tissue and thus contributing to steatosis. Treatment of animals with RO significantly suppressed this HFD-induced increase in HOMA-IR, and thus contributing to prevent IR-induced steatosis. Hepatic steatosis is considered the first hit in the pathogenesis of NAFLD which sensitizes the liver to progress towards a second phase characterized by increased oxidative stress and pro-inflammatory cytokines formation which further exacerbating the condition (Li and Lu, 2018). Oxidative stress is an indicator of the imbalance which occurs between the production of reactive oxygen species (ROS) as well as the scavenging capacity of the antioxidant system (Takaki et al., 2013). MDA and NO, which are markers of ROS generation, are excessively produced in the liver of HFD provided group while this elevated level was significantly suppressed by the treatment of animals with raspberry seed oil. Interestingly, RO oral administration to group 3 and 4 revealed a significantly elevated level of SOD, which indicates the antioxidant potential of raspberry seed oil. The plant contains an abundance of tocopherols which may contribute to the antioxidant potential of raspberry seed oil as it has been documented previously that vitamin E possesses strong antioxidant potential (Podszun et al., 2020).
Histopathological examination revealed the presence of inflammation and rounded fat droplets within the cytoplasm and the nuclei in HFD-provided group. Raspberry seed oil administration significantly improved these HFD induced alterations, especially in the animal group which received 0.8 mL of RO.
Prolonged oxidative stress can lead to activation of redox-sensitive transcription factor like NF-κB, which is one of the main regulators of inflammation. Consequently, activation of NF-κB can activate pro-inflammatory mediators release especially TNF-α and IL-6. The increased level of these inflammatory cytokines diminishes the action of insulin via activation of serine-threonine kinase pathway, and thus contributing to insulin resistance (Evans et al., 2005). The results of the current study indicate that treatment with raspberry seed oil significantly decreased the level of NF-κB which was elevated by HFD treatment. Hence, the oil may exert its beneficial effect by modulating the level of NF-κB.
Leptin, a peptide hormone, released by adipocytes, plays a prominent role in developing insulin resistance, thus altering insulin signaling with resultant more production of fatty acids from hepatocytes. Leptin also has a role in regulating fat deposition, inflammation, and fibrogenesis in NAFLD patients (Elbadawy et al., 2006). Consequently, this hormone has been considered an important regulator of NAFLD development. Regarding the results of the present work, there was a significant elevation of leptin level in the HFD-provided group while this elevated level was significantly suppressed by RO treatment. Therefore, it might indicate that the oil might exert its anti-NAFLD attribute by modulating the level of leptin.
Adiponectin, an important adipokine secreted by adipocytes, has demonstrated protective implications in NAFLD. Adiponectin prevents the development of hepatic steatosis via attenuation of serum lipids as well as glucose production. Adiponectin also regulates HFD-induced insulin resistance by acting as an insulin sensitizer and also by inhibiting the inflammatory mediator TNF-α (Shabalala et al., 2020). The results of the study point out that treatment of RO significantly elevated the HFD-induced suppression of adiponectin level which also confirms the anti-NAFLD potential of raspberry seed oil.
PPARγ is a transcription factor, which is expressed in adipose tissues and the liver (Inoue et al., 2005). It contributes to regulating cholesterol homeostasis, and acts as an insulin sensitizer as well as a reservoir for excess FFAs, increases energy expenditure by induction of UCP-2 thereby potentially preventing lipotoxicity in liver and other tissues (Kahn et al., 2006, Kallwitz et al., 2008). In the current study, the level of PPARγ was significantly decreased in the HFD group compared with control group. The expression levels of PPARγ mRNA, as well as protein, were significantly decreased in the HFD fed group, which is compatible with the findings of the study (Zhu et al., 2020). RO treatment significantly elevates PPARγ levels.
Molecular docking has been used as a useful tool for predicting the possible interaction of new pharmacological agents with target protein thus, contributing to predicting possible mechanism of action. In this study, we attempted to investigate the possible interaction of the identified compounds with PPARγ. Several studies have confirmed that the activation of PPARγ served as a useful strategy to prevent NAFLD (Shabalala et al., 2020, Zhong and Liu, 2018). The binding pocket of PPARγ contains residues Gly 284, Arg 288, Ser 289, Ala 292, Leu 330, Phe 264, Leu 265, His 266, Ile 281, Met 329, and Ile 341 (Megantara et al., 2017). All identified compounds especially oleic acid, α-linolenic acid and γ-tocopherol showed an interaction with PPARγ in a manner similar to that of the agonist pioglitazone. Therefore, it might be possible that cold-pressed raspberry oil proved beneficial as anti-NAFLD due to its agonistic effect on PPARγ.
Concerning the results obtained, it is suggestive that raspberry seed oil possesses multimodal anti-NAFLD attributes. The plant exerts its beneficial effect possibly through amelioration of insulin resistance, oxidative stress, NF- κB, and leptin along with elevation of adiponectin and PPARγ levels.
5. Conclusion
Currently, NAFLD is regarded as the most common chronic liver disease in many parts of the world. This research revealed the protective and preventive implication of raspberry seed oil against HFD-induced NAFLD. RO treatment remarkably improved lipid parameters, reduced insulin resistance, and glucose levels, suppressed inflammation via inhibition of NF-κB concurrent with modulation of leptin, PPARγ and adiponectin levels. These effects might be attributed to the high content of polyunsaturated fatty acids, tocopherols in addition to an adequate amount of phenolic compounds. In conclusion, our findings suggest the potential anti-NAFLD of raspberry seed oil to be further tested clinically as a dietary supplement for NAFLD.
Author Contributions
OH and SH conceived and conducted the study. OH, HG, SH, SA, SQ, FA, AA and SA carried out the experiments, collected the data, analyzed the data and reviewed the manuscript. SH and SQ interpreted the data and wrote the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
References
- Attia R.T., Abdel-Mottaleb Y., Abdallah D.M., El-Abhar H.S., El-Maraghy N.N. Raspberry ketone and Garcinia Cambogia rebalanced disrupted insulin resistance and leptin signaling in rats fed high fat fructose diet. Biomed. Pharmacotherapy. 2019;110:500–509. doi: 10.1016/j.biopha.2018.11.079. [DOI] [PubMed] [Google Scholar]
- Barrea L., Di Somma C., Muscogiuri G., Tarantino G., Tenore G.C., Orio F., Colao A., Savastano S. Nutrition, inflammation and liver-spleen axis. Crit. Rev. Food Sci. Nutrit. 2018;58:3141–3158. doi: 10.1080/10408398.2017.1353479. [DOI] [PubMed] [Google Scholar]
- Beaton M.D. Current treatment options for nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Can. J. Gastroenterol. 2012;26 doi: 10.1155/2012/725468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard C., Juin C., Vitry M., Le V.T.D., Verdon J., Toullec A.-S., Imbert C., Girardot M. Can Leaves and Stems of Rubus idaeus L Handle Candida albicans Biofilms? Pharmaceuticals. 2020;13:477. doi: 10.3390/ph13120477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bril F., Cusi K. Management of nonalcoholic fatty liver disease in patients with type 2 diabetes: a call to action. Diabetes Care. 2017;40:419–430. doi: 10.2337/dc16-1787. [DOI] [PubMed] [Google Scholar]
- Carey A.N., Pintea G.I., Van Leuven S., Gildawie K.R., Squiccimara L., Fine E., Rovnak A., Harrington M. Red raspberry (Rubus ideaus) supplementation mitigates the effects of a high-fat diet on brain and behavior in mice. Nutrit. Neurosci. 2019:1–11. doi: 10.1080/1028415X.2019.1641284. [DOI] [PubMed] [Google Scholar]
- Chang C.J., Liou S.-S., Tzeng T.-F., Liu I.-M. The ethanol extract of Zingiber zerumbet Smith attenuates non-alcoholic fatty liver disease in hamsters fed on high-fat diet. Food Chem. Toxicol. 2014;65:33–42. doi: 10.1016/j.fct.2013.11.048. [DOI] [PubMed] [Google Scholar]
- Elbadawy R.A., Eleter E.A., Helmy A., Al Ghamdi A.S., Al-Mofleh I., Al Faleh F.Z., Al Freihi H., Al-Amri S. The role of leptin in non-alcoholic fatty liver disease. Saudi J. Gastroenterol. 2006;12:68. doi: 10.4103/1319-3767.27848. [DOI] [PubMed] [Google Scholar]
- Erdinçler D.S., Seven A., Inci F., Beǧer T., Candan G. Lipid peroxidation and antioxidant status in experimental animals: effects of aging and hypercholesterolemic diet. Clinica Chim. Acta. 1997;265:77–84. doi: 10.1016/s0009-8981(97)00106-x. [DOI] [PubMed] [Google Scholar]
- Evans J.L., Maddux B.A., Goldfine I.D. The molecular basis for oxidative stress-induced insulin resistance. Antioxidants Redox Signaling. 2005;7:1040–1052. doi: 10.1089/ars.2005.7.1040. [DOI] [PubMed] [Google Scholar]
- Feng X., Yu W., Li X., Zhou F., Zhang W., Shen Q.i., Li J., Zhang C., Shen P. Apigenin, a modulator of PPARγ, attenuates HFD-induced NAFLD by regulating hepatocyte lipid metabolism and oxidative stress via Nrf2 activation. Biochem. Pharmacol. 2017;136:136–149. doi: 10.1016/j.bcp.2017.04.014. [DOI] [PubMed] [Google Scholar]
- Fotschki B., Jurgoński A., Juśkiewicz J., Zduńczyk Z. Dietary supplementation with raspberry seed oil modulates liver functions, inflammatory state, and lipid metabolism in rats. J. Nutrit. 2015;145:1793–1799. doi: 10.3945/jn.115.212407. [DOI] [PubMed] [Google Scholar]
- Fotschki B., Juśkiewicz J., Sójka M., Jurgoński A., Zduńczyk Z. Ellagitannins and flavan-3-ols from raspberry pomace modulate caecal fermentation processes and plasma lipid parameters in rats. Molecules. 2015;20:22848–22862. doi: 10.3390/molecules201219878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii H., Imajo K., Yoneda M., Nakahara T., Hyogo H., Takahashi H., Hara T., Tanaka S., Sumida Y., Eguchi Y. HOMA-IR: An independent predictor of advanced liver fibrosis in nondiabetic non-alcoholic fatty liver disease. J. Gastroenterol. Hepatol. 2019;34:1390–1395. doi: 10.1111/jgh.14595. [DOI] [PubMed] [Google Scholar]
- Gledovic A., Janosevic Lezaic A., Krstonosic V., Djokovic J., Nikolic I., Bajuk-Bogdanovic D., Antic Stankovic J., Randjelovic D., Savic S.M., Filipovic M. Low-energy nanoemulsions as carriers for red raspberry seed oil: Formulation approach based on Raman spectroscopy and textural analysis, physicochemical properties, stability and in vitro antioxidant/biological activity. PloS One. 2020;15 doi: 10.1371/journal.pone.0230993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunstone F. John Wiley & Sons; 2011. Vegetable oils in food technology: composition, properties and uses. [Google Scholar]
- Inoue M., Ohtake T., Motomura W., Takahashi N., Hosoki Y., Miyoshi S., Suzuki Y., Saito H., Kohgo Y., Okumura T. Increased expression of PPARγ in high fat diet-induced liver steatosis in mice. Biochem. Biophys. Res. Commun. 2005;336:215–222. doi: 10.1016/j.bbrc.2005.08.070. [DOI] [PubMed] [Google Scholar]
- Iser D., Ryan M. Fatty liver disease: a practical guide for GPs. Austr. Family Physician. 2013;42:444. [PubMed] [Google Scholar]
- Juárez-Hernández E., Chávez-Tapia N.C., Uribe M., Barbero-Becerra V.J. Role of bioactive fatty acids in nonalcoholic fatty liver disease. Nutrit. J. 2015;15:72. doi: 10.1186/s12937-016-0191-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn S.E., Hull R.L., Utzschneider K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- Kallwitz E.R., McLachlan A., Cotler S.J. Role of peroxisome proliferators-activated receptors in the pathogenesis and treatment of nonalcoholic fatty liver disease. World J. Gastroenterol.: WJG. 2008;14:22. doi: 10.3748/wjg.14.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kar A., Panda S., Bharti S. Relative efficacy of three medicinal plant extracts in the alteration of thyroid hormone concentrations in male mice. J. Ethnopharmacol. 2002;81:281–285. doi: 10.1016/s0378-8741(02)00048-x. [DOI] [PubMed] [Google Scholar]
- Kowalska K., Olejnik A., Zielińska-Wasielica J., Olkowicz M. Raspberry (Rubus idaeus L.) fruit extract decreases oxidation markers, improves lipid metabolism and reduces adipose tissue inflammation in hypertrophied 3T3-L1 adipocytes. J. Funct. Foods. 2019;62:103568. doi: 10.1016/j.jff.2019.103568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kshatriya D., Li X., Giunta G.M., Yuan B.o., Zhao D., Simon J.E., Wu Q., Bello N.T. Phenolic-enriched raspberry fruit extract (Rubus idaeus) resulted in lower weight gain, increased ambulatory activity, and elevated hepatic lipoprotein lipase and heme oxygenase-1 expression in male mice fed a high-fat diet. Nutrit. Res. 2019;68:19–33. doi: 10.1016/j.nutres.2019.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Wang J., Shahidi F. Chemical characteristics of cold-pressed blackberry, black raspberry, and blueberry seed oils and the role of the minor components in their oxidative stability. J. Agric. Food Chem. 2016;64:5410–5416. doi: 10.1021/acs.jafc.6b01821. [DOI] [PubMed] [Google Scholar]
- Li W., Lu Y. Hepatoprotective effects of sophoricoside against fructose-induced liver injury via regulating lipid metabolism, oxidation, and inflammation in mice. J. Food Sci. 2018;83:552–558. doi: 10.1111/1750-3841.14047. [DOI] [PubMed] [Google Scholar]
- Lu L.-W., Hou Z.-L., Yao G.-D., Lin B., Huang X.-X., Song S.-J. Chiral-phase resolution of sesquilignans from raspberries (Rubus idaeus L.) and their neuroprotective effects. Fitoterapia. 2020;146 doi: 10.1016/j.fitote.2020.104655. [DOI] [PubMed] [Google Scholar]
- Ma Z., Chu L., Liu H., Wang W., Li J., Yao W., Yi J., Gao Y. Beneficial effects of paeoniflorin on non-alcoholic fatty liver disease induced by high-fat diet in rats. Sci. Rep. 2017;7:1–10. doi: 10.1038/srep44819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mapfumo M., Lembede B., Nkomozepi P., Ndhlala A., Chivandi E. Crude Moringa oleifera Lam. seed extract attenuates non-alcoholic fatty liver disease in growing Sprague-Dawley rats. South Afr. J. Bot. 2020;129:191–197. [Google Scholar]
- Matthews D., Hosker J., Rudenski A., Naylor B., Treacher D., Turner R. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- Megantara S., Utami D., Puspitasari L., Mustarichie R. Insilico Study of Thymoquinone as Peroxisome Proliferator Activated Receptor Gamma Agonist in the Treatment of Type 2 Diabetes Mellitus. J. Pharmaceut. Sci. Res. 2017;9:1478. [Google Scholar]
- Nakamuta M., Kohjima M., Higuchi N., Kato M., Kotoh K., Yoshimoto T., Yada M., Yada R., Takemoto R., Fukuizumi K. The significance of differences in fatty acid metabolism between obese and non-obese patients with non-alcoholic fatty liver disease. Int. J. Mol. Med. 2008;22:663–667. [PubMed] [Google Scholar]
- Oomah B.D., Ladet S., Godfrey D.V., Liang J., Girard B. Characteristics of raspberry (Rubus idaeus L.) seed oil. Food Chem. 2000;69:187–193. [Google Scholar]
- Parry J., Su L., Luther M., Zhou K., Yurawecz M.P., Whittaker P., Yu L. Fatty acid composition and antioxidant properties of cold-pressed marionberry, boysenberry, red raspberry, and blueberry seed oils. J. Agric. Food Chem. 2005;53:566–573. doi: 10.1021/jf048615t. [DOI] [PubMed] [Google Scholar]
- Pettinelli P., Obregón A., Videla L. Molecular mechanisms of steatosis in nonalcoholic fatty liver disease. Nutricion Hospitalaria. 2011;26:441–450. doi: 10.1590/S0212-16112011000300003. [DOI] [PubMed] [Google Scholar]
- Pieszka M., Tombarkiewicz B., Roman A., Migdał W., Niedziółka J. Effect of bioactive substances found in rapeseed, raspberry and strawberry seed oils on blood lipid profile and selected parameters of oxidative status in rats. Environ. Toxicol. Pharmacol. 2013;36:1055–1062. doi: 10.1016/j.etap.2013.09.007. [DOI] [PubMed] [Google Scholar]
- Podszun M.C., Alawad A.S., Lingala S., Morris N., Huang W.-C.-A., Yang S., Schoenfeld M., Rolt A., Ouwerkerk R., Valdez K. Vitamin E treatment in NAFLD patients demonstrates that oxidative stress drives steatosis through upregulation of de-novo lipogenesis. Redox Biol. 2020;37 doi: 10.1016/j.redox.2020.101710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recena Aydos, L., Aparecida do Amaral, L., Serafim de Souza, R., Jacobowski, A.C., Freitas dos Santos, E., Rodrigues Macedo, M.L., 2019. Nonalcoholic Fatty Liver Disease Induced by High-Fat Diet in C57bl/6 Models. Nutrients 11, 3067. [DOI] [PMC free article] [PubMed]
- Ruiz-Larrea M.B., Leal A.M., Liza M., Lacort M., de Groot H. Antioxidant effects of estradiol and 2-hydroxyestradiol on iron-induced lipid peroxidation of rat liver microsomes. Steroids. 1994;59:383–388. doi: 10.1016/0039-128x(94)90006-x. [DOI] [PubMed] [Google Scholar]
- Shabalala Samukelisiwe C., Dludla Phiwayinkosi V., Mabasa Lawrence, Kappo Abidemi P., Basson Albertus K., Pheiffer Carmen, Johnson Rabia. The effect of adiponectin in the pathogenesis of non-alcoholic fatty liver disease (NAFLD) and the potential role of polyphenols in the modulation of adiponectin signaling. Biomed. Pharmacotherapy. 2020;131:110785. doi: 10.1016/j.biopha.2020.110785. [DOI] [PubMed] [Google Scholar]
- Tailleux, A., Wouters, K., Staels, B., 2012. Roles of PPARs in NAFLD: potential therapeutic targets. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids 1821, 809–818. [DOI] [PubMed]
- Takaki A., Kawai D., Yamamoto K. Multiple hits, including oxidative stress, as pathogenesis and treatment target in non-alcoholic steatohepatitis (NASH) Int. J. Mol. Sci. 2013;14:20704–20728. doi: 10.3390/ijms141020704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng H., Chen L., Huang Q., Wang J., Lin Q., Liu M., Lee W.Y., Song H. Ultrasonic-assisted extraction of raspberry seed oil and evaluation of its physicochemical properties, fatty acid compositions and antioxidant activities. PLoS One. 2016;11 doi: 10.1371/journal.pone.0153457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uribe M., Barbero-Becerra V.J. Role of bioactive fatty acids in nonalcoholic fatty liver disease. Nutrit. J. 2015 doi: 10.1186/s12937-016-0191-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Herck M.A., Vonghia L., Francque S.M. Animal models of nonalcoholic fatty liver disease—a starter’s guide. Nutrients. 2017;9:1072. doi: 10.3390/nu9101072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VandenAkker N.E., Vendrame S., Tsakiroglou P., Klimis-Zacas D. Red raspberry (Rubus idaeus) consumption restores the impaired vasoconstriction and vasorelaxation in the aorta of the obese zucker rat, a model of the metabolic syndrome. J. Berry Res. 2020:1–13. [Google Scholar]
- Veljkovic B., Djordjevic N., Dolicanin Z., Licina B., Topuzovic M., Stankovic M., Zlatic N., Dajic-Stevanovic Z. Antioxidant and anticancer properties of leaf and fruit extracts of the wild raspberry (Rubus idaeus L.) Notulae Botanicae Horti Agrobotanici Cluj-Napoca. 2019;47:359–367. [Google Scholar]
- Wong V.W.S., Chan W.K., Chitturi S., Chawla Y., Dan Y.Y., Duseja A., Fan J., Goh K.L., Hamaguchi M., Hashimoto E. Asia-Pacific Working Party on Non-alcoholic Fatty Liver Disease guidelines 2017—part 1: definition, risk factors and assessment. J. Gastroenterol. Hepatol. 2018;33:70–85. doi: 10.1111/jgh.13857. [DOI] [PubMed] [Google Scholar]
- Wu Lingfeng, Liu Yufeng, Qin Yin, Wang Lu, Wu Zhenqiang. HPLC-ESI-qTOF-MS/MS characterization, antioxidant activities and inhibitory ability of digestive enzymes with molecular docking analysis of various parts of raspberry (Rubus ideaus L.) Antioxidants. 2019;8(8):274. doi: 10.3390/antiox8080274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong X., Liu H. Honokiol attenuates diet-induced non-alcoholic steatohepatitis by regulating macrophage polarization through activating peroxisome proliferator-activated receptor γ. J. Gastroenterol. Hepatol. 2018;33:524–532. doi: 10.1111/jgh.13853. [DOI] [PubMed] [Google Scholar]
- Zhu Ruyuan, Wei Junping, Liu Haixia, Liu Chenyue, Wang Lili, Chen Beibei, Li Lin, Jia Qiangqiang, Tian Yimiao, Li Rui, Zhao Dandan, Mo Fangfang, Li Yu, Gao Sihua, Wang Xiang-Dong, Zhang Dongwei. Lycopene attenuates body weight gain through induction of browning via regulation of peroxisome proliferator-activated receptor γ in high-fat diet-induced obese mice. J. Nutrit. Biochem. 2020;78:108335. doi: 10.1016/j.jnutbio.2019.108335. [DOI] [PubMed] [Google Scholar]
- Zou T., Kang Y., Wang B., de Avila J.M., You J., Zhu M.-J., Du M. Raspberry supplementation reduces lipid accumulation and improves insulin sensitivity in skeletal muscle of mice fed a high-fat diet. J. Funct. Foods. 2019;63 [Google Scholar]