Abstract
Hawthorn (Crataegus spp.) has been used for the treatment of several heart diseases and hypertension. The studies carried out on several hawthorn species have led to the development of standardized extracts useful in the cure of mild chronic cardiac diseases.
In Mexico, the most common Crataegus species are C. mexicana and C. gracilior. Decoctions prepared from the fruits and leaves of these species have been employed to the treat respiratory diseases, tachycardia and to improve coronary blood flow. Considering that to date there are no reports of the use of Mexican Crataegus species to treat cardiovascular diseases, we propose an analytical method to obtain a quantified extract of Crataegus mexicana leaves for the development of a standardized extract with therapeutic value in cardiovascular diseases as an alternative source to the extracts obtained from Crataegus species of European and Asian origin.
Therefore, the aim of this study was to obtain an extract prepared from C. mexicana leaves with the highest vasodilator activity to select the optimal chemical marker to stablish and validate a reversed-phase high-performance liquid chromatography (RPHPLC-DAD) analytical method for obtaining a quantified extract with vasodilator effect.
The results obtained from the analytical method validation, which was carried out according to the guidelines stablished in the Eurachem Guide and the ICH guidelines proved that the RPHPLC-DAD method we developed was specific, precise, accurate, and showed good linearity over the concentration range of 3 – 21 µg/ml for (-)-epicatechin and rutin, which were selected as chemical markers.
Keywords: Cardiovascular diseases, Crataegus mexicana, Quantified extract, Validation, Vasorelaxant effect
1. Introduction
Cardiovascular diseases (CVD) are the main cause of death globally. According to World Health Organization (WHO) in 2016 almost 18 million people died from CVDs, representing 31% of all global deaths (World Health Organization, 2020).
Hypertension is considered the major risk factor for cardiovascular death (Qamar and Braunwald, 2018, Yano et al., 2018) and its global trend frequency is growing, particularly in low- and middle-income countries (Forouzanfar et al., 2017) because of the high number of people undiagnosed, untreated and uncontrolled (Haldar, 2013). Therefore, it is necessary to implement new strategies in the search for new therapeutic alternatives for the prevention and treatment of hypertension.
Considering that approximately 80% of the world’s population rely on plants from traditional medicine to solve their health needs (Cloud et al., 2020), especially in developing countries where many people do not have access to other health systems, standardized extracts prepared from medicinal plants could represent a very valuable alternative to address the treatment of hypertension and other cardiovascular diseases. Furthermore, in developed countries there has been an increased interest in the use of therapies of natural origin to achieve healthier and more balanced lives (Kunle, 2012).
Although herbal preparations seem to be an excellent alternative to “allopathic” drugs, it is necessary to consider that this kind of medicinal preparations could show variability in their constituents due to cultural and environmental factors such as growth, geographical location, climate, soil nutritional conditions, time of harvesting and post-harvest storage (Tsai et al., 2015). Hence, the profile of constituents is variable from batch to batch with obvious implications in efficacy and safety (Kunle, 2012). Consequently, one of the most important features in the development of standardized herbal extracts is to determine a compound as chemical marker that guarantees a well-defined and constant composition in order to produce a drug of defined quality (Kunle, 2012).
Regarding hawthorn (Crataegus spp.), these species have been employed in many parts of the world for the treatment of several heart diseases and hypertension, particularly in Europe, China, and North America (Dahmer and Scott, 2010, Edwards et al., 2012, Orhan, 2016, Yang and Liu, 2012). Some Crataegus species have been thoroughly investigated, such as C. pinnatifida (Chang et al., 2002, Jurikova et al., 2012, Shao et al., 2017, Wu et al., 2014); C. monogyna (Bardakci et al., 2019, Barros et al., 2011, Nabavi et al., 2015), C. laevigata (Dahmer and Scott, 2010, Edwards et al., 2012) and C. oxyacantha (Orhan, 2016, Ranjbar et al., 2018, Rastogi et al., 2016, Sadek et al., 2018, Wang et al., 2013). However, since Crataegus is a highly variable genus, the chemical composition of its many species may significantly vary (Edwards et al., 2012, Venskutonis, 2018).
In vitro and in vivo studies have showed that Crataegus spp. extracts, obtained from the leaves, flowers and fruits, induce anti-ischemia/reperfusion-injury, anti-arrhythmic, hypolipidemic, vasorelaxant, anti-inflammatory, hypotensive and cardioprotective effects (Bujor et al., 2020, Nazhand et al., 2020, Negi et al., 2018, Ranjbar et al., 2018, Rastogi et al., 2016, Sadek et al., 2018, Shao et al., 2017, Shatoor et al., 2019, Wang et al., 2013, Wu et al., 2014). Moreover, there are evidences that hawthorn can significantly lower blood pressure in patients with mild hypertension in longer-term treatments (Cloud et al., 2020).
Studies concerning the chemical composition and pharmacological effect of Crataegus species have led to the development of standardized extracts and herbal medicines useful in the treatment of mild chronic cardiac diseases (Koch & Malek, 2011).
At least thirteen species belonging to the genus Crataegus have been identified in Mexico and the most common species are C. mexicana and C. gracilior (Banderas-Tarabay et al., 2015). All Crataegus species are commonly known in Mexico as Tejocote, a word originated from the nahuatl term tetl-xocotl, which means wild or hard sour fruit (Martínez, 1991). The fruits of these species are eaten fresh, in jam, compote or in decoctions to obtain hot beverages (García-Mateos et al., 2013). Decoctions prepared from the leaves, fruits and flowers of Crataegus spp have been used in Mexican traditional medicine for the treatment of several respiratory diseases such as cough, cold, bronchitis, as diuretic and to improve coronary blood flow (Arrieta et al., 2010, Hernández-Pérez et al., 2014, Martínez, 1991, UNAM, 2009).
Regarding the chemical composition of Mexican Crataegus species, chlorogenic acid, (+)-catechin, (-)-catechin, quercetin, kaempferol and apigenin glycosides have been identified in the ethanolic extracts obtained from the fruits of C. stipulosa, C. mexicana, C. nelsoni (García-Mateos et al., 2013) and C. pubescens (González-Jiménez et al., 2018). Additionally, ursolic, corosolic and euscapic acids have been identified in the methanolic extracts obtained from the leaves and flowers of C. gracilior (Hernández-Pérez et al., 2014, Torres-Ortiz et al., 2019). Both extracts showed antioxidant effect and elicited a significant vasodilator activity. The results derived from these studies indicate that Crataegus species growing in México could be employed for the development of phytomedicines useful to prevent and treat cardiovascular diseases in the same way as European and Asiatic Crataegus species.
To date there is not information about the use of Mexican Crataegus species for the treatment of cardiovascular diseases, therefore, in the present study we propose an analytical method to obtain a quantified extract of Crataegus mexicana leaves in order to develop a standardized extract from this plant as an alternative source to the extracts obtained from Crataegus species from Europe and Asia.
2. Materials and methods
2.1. Chemical and reagents
Gallic acid, chlorogenic acid, caffeic acid, p-coumaric acid, vanillic acid, (-)-epicatechin, quercetin, rutin, kaempferol, epicatechin gallate, catechin gallate, 2,2́-diphenil-1-picrilhidrazil, 6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid, 2,4,6-Tris(2-pyridyl)-s-triazine and the Folin-Ciocalteau reagent were obtained from Sigma-Aldrich (St. Louis, MO, USA). Sodium carbonate, sodium nitrite, aluminum chloride, sodium hydroxide, ferric chloride, sodium acetate were obtained from J.T. Baker (Mexico).
Hexane, methanol, acetone, ethanol, hydrochloric acid, acetic acid reagent grade were obtained from J.T. Baker, methanol HPLC grade was obtained from J.T. Baker (Mexico).
2.2. Experimental animals
All experimental procedures were performed in accordance with guidelines of the Mexican Official Standard NOM-062-ZOO-1999, “Technical specifications for the production, care, and use of laboratory animals” and approved by the Bioethics Committee of the Faculty of Chemistry of the Autonomous University of Querétaro.
Wistar male rats (250–300 g) were provided by the Institute of Neurobiology of the National Autonomous University of Mexico, Campus Juriquilla. Animals were housed in standard cages under controlled temperature conditions with a 12:12 h light–dark cycle. Water and food were provided ad libitum.
2.3. Materials and sample preparation
Four samples of Crataegus mexicana leaves were acquired in Querétaro, Mexico during the summers of 2016, 2018 and 2019.
2.3.1. Preparation of the crude extracts from C. mexicana leaves
Air dried C. mexicana leaves were ground to a fine powder and defatted through maceration with hexane for 24 h. Thereafter, the solvent was removed by rotary evaporation. Four solvent mixtures were used to obtain the respective extracts from each of the four leaf samples of C. mexicana: a) methanol 100% (MeOH), b) ethanol 60% (EtOH), c) acetone, water, acetic acid (AWAc, 80:18.5:1.5), and d) acetone, methanol, water, acid acetic (AMWAc, 40:40:18.5:1.5).
The extracts were prepared by sonication during 20 min of the defatted material and subsequent stirring for 24 h at 50 rpm. The material: solvent ratio was 1:10. This process was repeated three times with fresh solvent. Later, the plant material was filtered, and the solvents were removed by rotary evaporation.
2.3.2. Extraction of phenolic compounds from the concentrated crude extracts
The extraction of phenolic compounds was carried out in triplicate from the concentrated crude extracts according to Kähkönen et al. (Kähkönen et al., 1999) with some modifications: each extract sample (250 mg) was re-dissolved in 2.5 mL of its respective solvent by vortexing for 30 s and sonication for 30 min. The dissolved extracts were centrifuged for 15 min and the supernatant was transferred to a 10 mL flask. This extraction procedure was repeated three times. The extracts thus obtained were stored at −70 °C until further analysis.
2.4. Determination of the content of phenolic compounds in the extracts
The concentration of total phenolics was determined using the Folin-Ciocalteu’s method reported by García-Solís (García-Solís et al., 2009). The calibration curve was prepared from 0.04 to 0.24 mg/mL with a methanolic solution of gallic acid and the content of phenolics in the extracts was expressed in gallic acid equivalent as mg of GA/g of extract.
2.5. Determination of flavonoid content in the extracts
Flavonoid content was determined by the Zhishen’s method as reported by Cantín (Cantín et al., 2009). The calibration curve was prepared from 0.2 to 1.0 g/mL with a methanolic solution of (+) catechin and the content of flavonoids in the extracts was expressed in (+) catechin equivalent as mg of CA/g of extract.
2.6. Determination of the vasodilator effect using isolated rat aorta assay
The isolated rat aorta assay was employed to evaluate the vasodilator activity of the crude extracts, according to the method previously reported (Castro-Ruiz et al., 2017, Ibarra-Alvarado et al., 2010, Luna-Vázquez et al., 2018, Medina-Ruiz et al., 2019). Rats were sacrificed by decapitation (NOM-062-ZOO-1999, section 9.5.3.3). and the thoracic aorta was surgically removed and placed in a cold Krebs-Henseleit solution with the following composition (mM): 126.8 NaCl; 5.9 KCl; 1.2 KH2PO4; 1.2 MgSO4; 5.0 D-glucose; 30 NaHCO3; 2.5 CaCl2 (pH 7.4), bubbled with carbogen (95% O2 and 5% CO2). The intraluminal space of the aorta was rinsed with Krebs-Heinseleit fresh solution to prevent clot formation and cleaned from surrounding adipose and connective tissues. Thereafter, aorta was cut into 4–5 mm rings. These rings were mounted in 5 mL incubation chambers containing pre-warmed Krebs-Henseleit solution (37 °C) gassed with carbogen.
Tissues were stabilized for 30 min under a tension of 1.5 g at 37 °C. During this period, the bathing medium was changed every ten minutes. To stimulate the vascular smooth muscle, the tissues were contracted with 100 mM KCl, when a stable contractile tone had been reached, the bathing medium was changed every ten minutes to restore the initial resting tension.
Thereafter, aortic rings were challenged with 1 µM L-phenylephrine. The contractile force induced was defined as 100 %, and as soon as the plateau was reached, the extracts were cumulatively tested. Acetylcholine was used as positive control.
Changes in tension caused by the different concentrations of the tested extracts were detected by Grass FT03 force transducers coupled to a Grass 7D Polygraph (Grass Instrument Co, Quincy, MA, USA); they were expressed as percentages of relaxation based on the contraction generated by adding L-phenylephrine (Ibarra-Alvarado et al., 2010).
2.7. Determination of the chemical marker from C. mexicana leaves
Selection of the optimal chemical marker to establish and validate an analytical method for obtaining a quantified extract from C. mexicana leaves was carried out using the following strategy: a) identification of the phenolic compounds present in the different crude extracts prepared from C. mexicana leaves; b) quantification of the main phenolic compounds identified in the extracts and c) determination of the correlation between the concentration of each phenolic compound and the vasodilator activity of the extract. Finally, the vasodilator activity of the selected chemical marker was determined.
2.7.1. Phenolic compound profile by HPLC analysis
Performed by high-performance liquid chromatography–photodiode array detection (HPLC–PDA) using a Waters HPLC system (Millipore Corp., Waters Chromatography Division, Milford, MA, USA) consisting of a 600S controller and 2998 PDA detector. A ZORBAX ECLIPSE XDB-C18 column (150 mm × 4.5,3.5 um particle size) was used and the mobile phases were 1% formic acid in HPLC-grade water (A) and acetonitrile (B) with the following gradient conditions: a) start from time zero to four minutes (A 95% and B 5%), b) 4–7 min (A 90% and B 10%), c) 7–25 min (A 50% and B 50%), and d) 25–40 min (A 95% and B 5%) at a flow rate of 0.9 mL/ min. The injection volume was 20 μL, and the total analysis time was 40 min. The detection wavelengths were fixed as 254, 280 and 360 nm.
Identification of the phenolic compounds present in the extracts was carried out by comparison of retention times and UV spectra of chromatogram peaks with those of standards, including gallic, chlorogenic, caffeic, p-coumaric, and vanillic acids, (-)-epicatechin, epicatechin gallate, catechin gallate, (+)-catechin, quercetin, rutin and kaempferol. A calibration curve was constructed for each standard in order to quantify the phenolic compounds that could be detected in the extracts.
The extract samples used for this analysis were prepared according to the methodology described in section 2.3.2 employing 1:40 and 1:60 dilutions.
2.8. Method validation
Once the chemical marker was selected and the conditions for the HPLC analysis were determined, validation of the analytical method was carried out according to the criteria established by the Eurachem Guide: The Fitness for Purpose of Analytical Methods (Magnusson, 2014) and the ICH Guidelines (ICH, 2005). The parameters evaluated were specificity, linearity (working range), precision (repeatability and intermediate precision), and accuracy (trueness).
2.8.1. Specificity
Specificity of the method was carried out by comparing the maximum wavelengths of the standards with those maximum wavelengths obtained from C. mexicana leaves extract.
2.8.2. Linearity
This parameter was evaluated from seven points of calibration standards to make a calibration curve between 3 and 21 ug/mL, each concentration was analyzed in six replicates. The calibration curve was plotted representing the peak area as a function of the standard concentration. The slope (b1), y-intercept (b0), correlation coefficient (r), determination coefficient (r2), and confidence interval (IC β1) were obtained using the least squares regression method.
2.8.3. Precision
Precision was estimated by evaluating the within-day (intraday, repeatability) and between-day (interday, intermediate precision) results of analyses carried out on three different and consecutive days.
2.8.3.1. Repeatability
Repeatability was determined by calculating the percentage of relative standard deviation (% RSD) for three independent determinations at three concentrations (3.0, 12.0 and 21.0 μg/mL, for (-)-epicatechin and rutin.
2.8.3.2. Intermediate precision
Intermediate precision was carried out by calculating % RSD for three independent 3 μg/mL (-)-epicatechin and rutin solutions analyzed in three days. One-way ANOVA was made to determine differences between everyday analysis.
2.8.4. Accuracy
Accuracy was evaluated by determining the average % recovery of the analytes (% R). This analysis was performed by adding known amounts of standard in 1:266 dilution extracts to obtain 3, 9 and 15 μg/mL concentrations. A sample of extract solution diluted 1: 266 was used as blank. Each concentration was analyzed in triplicate.
% R was calculated according to the following equation:
where x’ is the value of the spiked sample, x is the value of the blank and ‘x spike’ is the added concentration.
Average, %R, IC β1, and variation coefficient values were calculated to determine the accuracy of the system. Acceptance criteria are %R from 98 to 102%, IC β1 including 100% and CV < 3%.
2.9. Statistical analysis
Total phenolic compounds and flavonoid content were performed in triplicate and the results were reported as the mean ± standard error mean (SEM). Analyses for identification and quantification of phenolic compounds was performed in triplicate and the results were reported as the mean ± standard deviation (SD). Statistical comparisons were carried out using one-way ANOVA with Tukey and Dunnett post hoc tests. Statistical significance was accepted within the 95% confidence limit (p < 0.05).
Ex vivo assays were performed in quadruplicate and the results were reported as mean ± SEM. Experimental data were fitted to a sigmoidal equation, plotted and analyzed to calculate EC50 and Emax values (GraphPad Prism 7.02, San Diego, CA, USA). These results were subjected to one-way ANOVA analysis, followed by Dunnett’s post hoc test, using the statistical program GraphPad Prism 7.02.
3. Results and discussion
Numerous analytical methods have been proposed for quantifying phenolic compounds and flavonoids in herbal extracts (Bishnoi et al., 2018, Costa et al., 2015, Miguel et al., 2015, Seo et al., 2016, Suganthi and Ravi, 2019). Particularly, some techniques have been reported for analyzing phenolic compounds in Crataegus species (Bardakci et al., 2019, Hellenbrand et al., 2015, Koch and Malek, 2011, Orhan, 2016, Venskutonis, 2018). However, every single extract plant possesses its own complexity and variations in its chemical composition, therefore, it is necessary to identify a suitable chemical marker and develop an analytical method to quantify it in order to carry out quality control analyzes of medicinal products based on it (Souza et al., 2017).
3.1. Extract preparation
In order to find an extract from C. mexicana leaves that contained a high concentration of phenolic compounds and induced a significant vasodilator effect, four extracts were prepared using different solvent mixtures.
Several authors have reported that solvent systems containing acetone and acidulated water are very efficient in extracting oligomeric and polymeric procyanidins (Chen et al., 2016, Díaz-de-Cerio et al., 2017, Prior et al., 2005, Rohr et al., 2000). These types of compounds are considered responsible for the therapeutic activities attributed to Crataegus species. Although, other type of solvents such as aqueous methanol and aqueous ethanol have also been used in the extraction of procyanidins and other phenolic compounds (Luca et al., 2019, Rue et al., 2018, Sui et al., 2016).
3.2. Total phenolic and flavonoid contents
The results obtained from the determination of phenolic compounds and flavonoids are shown in Table 1. We found that the extracts obtained using methanol (MeOH) and acetone: methanol: water: acetic acid (AMWAc, 40:40:18.5:1.5) contained the highest yield of phenolic compounds, while the extract prepared with acetone, water, acetic acid (AWAc, 80:18.5:1.5) showed the highest flavonoid content.
Table 1.
Content of total phenolic compounds and flavonoids in extracts of C. mexicana leaves obtained by using four different systems of extraction solvents.
| System of extraction solvents | Total phenolics (mg of GAE/ g of dw5) |
Flavonoids (mg of CE/ g of dw6) |
|---|---|---|
| AMWAc1 | 188.76 ± 13.27b | 143.22 ± 5.87b |
| AWAc2 | 174.23 ± 16.6a | 159.57 ± 6.54a |
| EtOH3 | 169.18 ± 3.96a | 153.80 ± 6.45a |
| MeOH4 | 190.42 ± 11.95b | 154.87 ± 5.94a |
AMWAc, acetone: methanol: water: acetic acid (40:40:18.5:1.5); 2AMAc, acetone: water: acetic acid (80:18.5:1.5); 3EtOH, ethanol (60% aqueous); 4MeOH, methanol (100%).
mg of GAE/ g of dw: milligrams of gallic acid equivalents for grams of dry weight
mg of CE/ g of dw: milligrams of catechin equivalents for grams of dry weight
and b: values in the same column followed by the same letter are not significantly different (p < 0.05)
3.3. Pharmacological evaluation of the crude extracts obtained from C. mexicana leaves
Several studies have shown that species of the genus Crataegus have a high content of phenolic compounds, particularly oligomeric procyanidins, such as catechin and epicatechin (Cloud et al., 2020, Edwards et al., 2012, Hellenbrand et al., 2015), which have pharmacological effects on the cardiovascular system (Brixius et al., 2006, Bujor et al., 2020, Orhan, 2016). In this study, we evaluated the vasorelaxant effect of the crude extracts obtained from C. mexicana leaves, employing the rat aorta model.
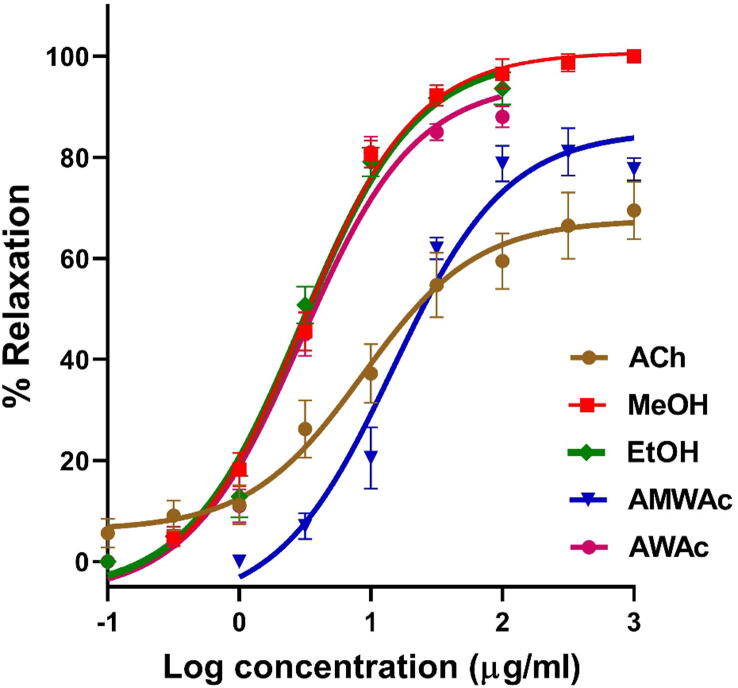
The results of the pharmacological evaluation indicated that all extracts elicited a concentration-dependent relaxation of aortic rings (Fig. 1). AWAc (Emax = 95.02 ± 2.89%, EC50 = 2.98 ± 0.10 μg/mL), MeOH (Emax = 100.08 ± 3.33%, EC50 = 3.00 ± 0.09 μg/mL), and EtOH (Emax = 99.98 ± 1.43%, EC50 = 2.99 ± 0.07 μg/mL) displayed a similar maximum vasorelaxant response, which was higher than that elicited by AMWAc (Emax = 85.20 ± 3.35%, EC50 = 14.66 ± 0.64 μg/mL).
Fig. 1.
Concentration-response curves of the vasodilator effect induced by the crude extracts obtained from C. mexicana leaves: AMWAc, AcMA, EtOH, MeOH (abbreviations are described in section 2.3.1); ACh: acetylcholine (positive control).
MeOH, EtOH and AWAc were approximately three-fold more potent than acetylcholine, which was used as positive control (Emax = 67.61 ± 1.43% and EC50 = 8.67 ± 1.14 μg/mL). Moreover, these extracts obtained from C. mexicana leaves elicited a maximum vasorelaxant effect larger than that of this positive control.
Our research group had previously demonstrated that the methanolic extract obtained from C. gracilior leaves displayed a vasodilator effect and some of the phenolic compounds contained in the extract, such as chlorogenic acid, rutin, quercetin, kaempferol and (+)-catechin were identified by HPLC-DAD (Hernández-Pérez et al., 2014). These compounds have been shown to induce vasorelaxant and antihypertensive effects (Aggio et al., 2013, Novakovic et al., 2017, Patel et al., 2018, Sánchez et al., 2018, Vechi et al., 2019). Therefore, it was very likely that these secondary metabolites could significantly contribute to the vasodilation elicited by the crude extracts obtained from C. mexicana leaves, especially considering that these compounds have been identified in fruits and leaves of other Mexican Crataegus species (Banderas-Tarabay et al., 2015, García-Mateos et al., 2013).
Taking into account the content of phenolic compounds and the significant vasodilator effect of the methanolic extract obtained from the leaves of C. mexicana, we assumed that this extract could be used for the development of a standardized herbal extract with beneficial effects on the cardiovascular system.
3.4. Identification of phenolic compounds by HPLC-DAD
Three phenolic compounds were identified, by comparing their retention time and maximum absorbance wavelength (200 to 400 nm) with those of standards: chlorogenic acid, (-)-epicatechin and rutin. All of them, had been previously identified in the leaves, flowers and fruits from other species of Crataegus and had shown beneficial effects on the cardiovascular system (Bujor et al., 2020, Cloud et al., 2020, Koch and Malek, 2011, Orhan, 2016, Venskutonis, 2018).
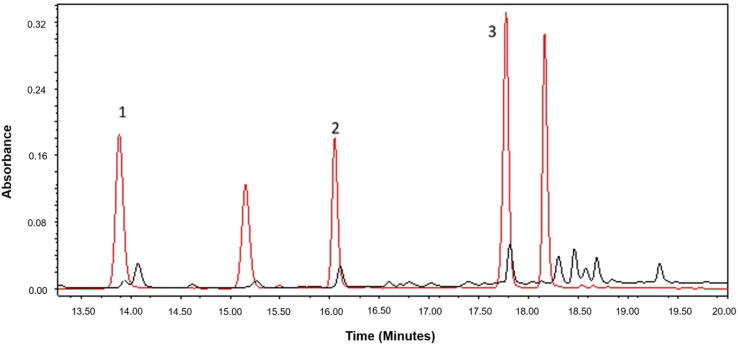
Fig. 2 shows the chromatogram obtained from the methanolic extract compared with a standard mix chromatogram (chlorogenic acid, caffeic acid, (-)-epicatechin, rutin, hyperoside, quercetin, and kaempferol). Retention times were 14.1, 16.08 and 17.79 min for chlorogenic acid, (-)-epicatechin and rutin, respectively. The wavelengths of maximum absorption were 240 and 326 nm for chlorogenic acid, 242 and 278 nm for (-)-epicatechin, and 254 and 354 nm for rutin.
Fig. 2.
HPLC-DAD chromatograms obtained from standard mix (red) and the methanolic extract of C. mexicana leaves (black). 1) Chlorogenic acid 2) (-)-epicatechin 3) Rutin.
These results indicated that these chromatographic conditions did not allow a good resolution for chlorogenic acid. Moreover, the peak corresponding to rutin was not properly separated and showed a baseline variation. Therefore, considering that the wavelengths of maximum absorption for rutin are 254 and 354 nm, the chromatograms were recorded using these wavelengths and, as expected, a good resolution was obtained for this flavone. Since the baseline was not stable at 254 nm, we selected the 354 nm wavelength for rutin quantification. These same chromatographic conditions were adequate for (-)-epicatechin.
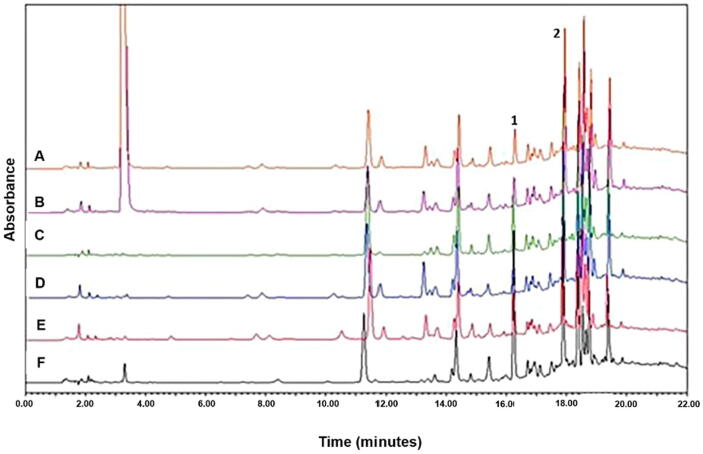
Considering that one of the criteria for selecting a chemical marker in a plant extract is the fact that this compound is responsible for the pharmacological effect elicited by the extract (Ding et al., 2017, Kunle, 2012, Ong, 2004), we chose (-)-epicatechin and rutin as potential chemical markers of C. mexicana leaves extracts. In order to identify these phenolic compounds in specimens of C. mexicana from different collections, methanolic and ethanolic leaf extracts obtained from two different years (2018 and 2016) were analyzed in addition to the extracts prepared from C. mexicana leaves (batch 2019).
Fig. 3 shows the chromatograms of the extracts obtained from C. mexicana leaves collected in 2016, 2018 and 2019. (-)-epicatechin and rutin were identified in all the analyzed extracts. Considering the presence of both compounds in all C. mexicana leaves extracts and their previously demonstrated vasodilator effect (Fraga et al., 2018, Kluknavsky et al., 2020, Maaliki et al., 2019, Sharma et al., 2013), we propose (-)-epicatechin and rutin as chemical markers for C. mexicana leaves extracts.
Fig. 3.
Chromatograms obtained at 280 nm from AWAc(a), AMWAc (b), EtOH (c), MeOH (d) (abbreviations, see the text) extracts from C. mexicana leaves batch 2019, ethanolic extract and methanolic extract obtained from C. mexicana leaves batches 2018 (e) and 2016 (f). 1) (-)-epicatechin 2) Rutin.
3.4.1. Quantification of (-)-epicatechin and rutin
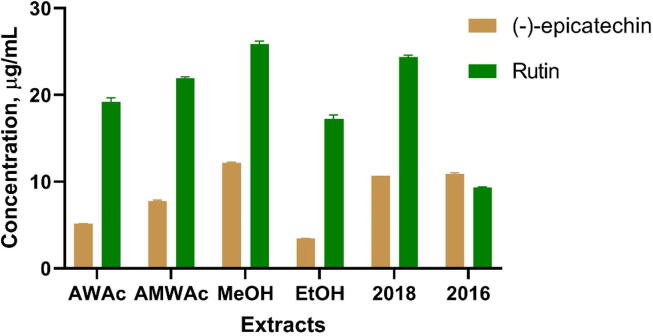
In order to determine the quantity of (-)-epicatechin and rutin present in the extracts obtained from the three analyzed batches of C. mexicana, we obtained the calibration curves for both compounds.
The calibration curves showed good linearity in the range from 3 to 21 µg/mL (Table 2.). Thereafter, we quantified the content of (-)-epicatechin and rutin in AWAc, AMWAc, EtOH, and MeOH obtained from C. mexicana leaves (batch 2019) and in the methanolic extracts obtained from C. mexicana leaves (batches 2016 and 2018). Fig. 4 shows that all evaluated extracts have quantifiable amounts of both flavonoids. It was found that the methanolic extracts (batches 2019 and 2018) had the largest content of rutin and (-)-epicatechin. The cardiovascular activity previously reported for these flavonoids (Bujor et al., 2020, Cloud et al., 2020, Orhan, 2016, Venskutonis, 2018) and the vasorelaxant activity elicited by the methanolic extract of C. mexicana leaves, support the choice of this extract for the validation of an analytical method for the development of a standardized extract of C. mexicana leaves potentially valuable in the treatment of cardiovascular diseases.
Table 2.
Analytical parameters for the calibration curves obtained for quantification of (-)-epicatechin and rutin standards.
| Parameter | Acceptance criteria | (-)-epicatechin | Rutin |
|---|---|---|---|
| Equation | y = mx + b | y = 15940*x + 7978 | y = 36939*x + 4175 |
| Slope | b1 ≠ 0 | 15940 ± 104.10 | 36939 ± 257.50 |
| Correlation coefficient | r ≥ 0.99 | 0.9991 | 0.9990 |
| Determination coefficient | r2 ≥ 0.98 | 0.9983 | 0.9981 |
| Confidence interval (β1) | IC (β1) no zero included | 15730–16151 | 36,418 – 37,459 |
Fig. 4.
Content of (-)-epicatechin and rutin in the different extracts evaluated obtained from three different batches of Crataegus mexicana leaves.
The results indicated that methanolic extract presented the largest content in (-)-epicatechin and rutin, hence this extract was used in the validation of analytical method to develop a Crataegus mexicana leaves quantified extract.
3.5. Validation of the analytical method for quantification of rutin and (-)-epicatechin
3.5.1. Specificity
Specificity of the method was verified by comparing the wavelengths of maximum absorption between the standards (242 and 278 nm for (-)-epicatechin and 253 and 354 nm for rutin) and C. mexicana leaf extracts.
3.5.2. Linearity
Linearity of the method was demonstrated by evaluating slope, correlation and determination coefficients and confidence interval (β1) obtained by linear regression analysis of data. The statistical data of linearity agreed with ICH guidelines for analytical methods (Table 2).
3.5.3. Precision
Table 3 shows the repeatability results for (-)-epicatechin and rutin evaluated at three different concentrations (3, 12 and 21 µg/mL) for three replicates. In all cases, % RSD was within acceptance criteria (<2.5%).
Table 3.
Results of repeatability for the quantification of (-)-epicatechin and rutin standards.
| Concentration (µg/mL) | (-)-epicatechin |
Rutin |
||||
|---|---|---|---|---|---|---|
| Mean peak area | SD1 | % RSD2 | Mean peak area | SD | % RSD | |
| 3 | 52,762 | 912 | 1.729 | 102,837 | 1813 | 1.7635 |
| 12 | 198,519 | 3512 | 1.770 | 451,593 | 7037 | 1.558 |
| 21 | 335,043 | 6511 | 1.943 | 773,467 | 14,656 | 1.895 |
Standard deviation
Deviation standard relative percentage (coefficient of variation)
Results of inter-day variability corresponding at 3 µg/mL measured at three different days are shown in Table 4. for (-)-epicatechin and rutin. According to the obtained % RSD (<3.0%), all results were under the limit as per recommendations of the International Conference for Harmonisation (ICH) guidelines. However according to the statistical analysis (one-way ANOVA), even though there was no significant difference among the inter-day analysis for rutin, there was significance difference in the inter-day analysis for (-)-epicatechin. This last result may be due to variations in room temperature recorded during the different days of the test since the equipment does not have a temperature controller for the chromatographic column.
Table 4.
Results of inter-day variability for (-)-epicatechin and rutin at 3 µg/mL.
| (-)-epicatechin |
Rutin |
|||||
|---|---|---|---|---|---|---|
| Day of analysis | 1 | 2 | 3 | 1 | 2 | 3 |
| Intraday mean peak area | 55,563 | 52,552 | 54,567 | 102,401 | 105,704 | 102,566 |
| Intraday % RSD1 | 0.99 % | 2.05 % | 1.48 % | 0.80 % | 1.55% | 1.34 % |
| Interday mean | 54,228 | 103,557 | ||||
| Interday % RSD | 2.78% | 1.95% | ||||
| p (one way ANOVA) | 0.03 | 0.08 | ||||
Deviation standard relative percentage (coefficient of variation).
3.5.4. Accuracy
According to the ICH Q2(R1), the accuracy of an analytical method refers to the closeness of agreement between the accepted reference value and the value found and could be reported as the percent recovery of a known amount of analyte added to the sample (ICH, 2005).
In order to evaluate the accuracy of this method, (-)-epicatechin and rutin standards were added at three concentrations (3, 9 and 15 µg/mL) to the methanolic extracts of C. mexicana leaves, which were then analyzed using the proposed HPLC method. The recoveries of the added standards were from 97.26 to 101.73% for (-)-epicatechin and from 97.30 to 101.99% for rutin, with a variation coefficient of 2.04% and 2.07 for (-)-epicatechin and rutin, respectively. IC β1 parameters were 0.9931 – 1.0070 for (-)-epicatechin and 0.9789 – 1.0170 for rutin. These results show that interferences by other matrix components are not significant, and the HPLC conditions are suitable to obtain an adequate method accuracy.
4. Conclusions
In this study, a quantified methanolic extract with vasodilator activity was developed from Crataegus mexicana leaves. (-) - epicatechin and rutin were selected as chemical markers, considering their pharmacological activity and their presence in C. mexicana specimens collected in different years. Additionally, an analytical method, using HPLC-DAD, was developed and validated for identification and quantification of the chemical markers. The results derived from this work constitute the bases for obtaining a standardized extract from C. mexicana leaves, which could be employed for the development of herbal medicinal products useful for the treatment of cardiovascular diseases.
Funding sources
This work was funded by grant 316,849 from “Fondo de Desarrollo Científico 2 (FOP02-2021–04)” of the Consejo Nacional de Ciencia y Tecnología (CONACYT)
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
References
- Aggio A., Grassi D., Onori E., D’Alessandro A., Masedu F., Valenti M., Ferri C. Endothelium/nitric oxide mechanism mediates vasorelaxation and counteracts vasoconstriction induced by low concentration of flavanols. Eur. J. Nutr. 2013;52(1):263–272. doi: 10.1007/s00394-012-0320-x. [DOI] [PubMed] [Google Scholar]
- Arrieta J., Siles-Barrios D., García-Sánchez J., Reyes-Trejo B., Sánchez-Mendoza M.E. Relaxant effect of the extracts of Crataegus mexicana on guinea pig tracheal smooth muscle. Pharmacognosy Journal. 2010;2(17):40–46. doi: 10.1016/S0975-3575(10)80008-2. [DOI] [Google Scholar]
- Banderas-Tarabay J.A., Cervantes-Rodríguez M., Méndez-Iturbide D. Biological Properties and Antioxidant Activity of Hawthorn Crataegus mexicana. J. Pharm. Pharm. 2015;06(04) doi: 10.4172/2153-0645.1000153. [DOI] [Google Scholar]
- Bardakci H., Celep E., Gözet T., Kan Y., Kırmızıbekmez H. Phytochemical characterization and antioxidant activities of the fruit extracts of several Crataegus taxa. S. Afr. J. Bot. 2019;124:5–13. doi: 10.1016/j.sajb.2019.04.012. [DOI] [Google Scholar]
- Barros L., Carvalho A.M., Ferreira I.C.F.R. Comparing the composition and bioactivity of Crataegus monogyna flowers and fruits used in folk medicine. Phytochem. Anal. 2011;22(2):181–188. doi: 10.1002/pca.v22.210.1002/pca.1267. [DOI] [PubMed] [Google Scholar]
- Bishnoi R.S., Kumar M., Shukla A.K., Jain C.P. Development and validation of novel HPLC method for the estimation of Rutin in crude hydromethanolic leaf extract of Prosopis cineraria. J. Drug Delivery Therap. 2018;8(6):68–73. doi: 10.22270/jddt.v8i6.2016. [DOI] [Google Scholar]
- Brixius K., Willms S., Napp A., Tossios P., Ladage D., Bloch W., Mehlhorn U., Schwinger R.H.G. Crataegus special extract WS® 1442 induces an endothelium-dependent, NO-mediated vasorelaxation via eNOS-phosphorylation at serine 1177. Cardiovasc. Drugs Ther. 2006;20(3):177–184. doi: 10.1007/s10557-006-8723-7. [DOI] [PubMed] [Google Scholar]
- Bujor A., Miron A., Luca S.V., Skalicka-Wozniak K., Silion M., Trifan A., Girard C., Demougeot C., Totoson P. Vasorelaxant effects of Crataegus pentagyna: Links with arginase inhibition and phenolic profile. J. Ethnopharmacol. 2020;252:112559. doi: 10.1016/j.jep.2020.112559. [DOI] [PubMed] [Google Scholar]
- Cantín C.M., Moreno M.A., Gogorcena Y. Evaluation of the antioxidant capacity, phenolic compounds, and vitamin C content of different peach and nectarine [Prunus persica (L.) batsch] breeding progenies. J. Agric. Food. Chem. 2009;57(11):4586–4592. doi: 10.1021/jf900385a. [DOI] [PubMed] [Google Scholar]
- Castro-Ruiz J., Rojas-Molina A., Luna-Vázquez F., Rivero-Cruz F., García-Gasca T., Ibarra-Alvarado C. Affinin (Spilanthol), isolated from heliopsis longipes, induces vasodilation via activation of gasotransmitters and prostacyclin signaling pathways. Int. J. Mol. Sci. 2017;18(1):218. doi: 10.3390/ijms18010218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, Q., Zuo, Z., Harrison, F., Sing, M., & Chow, S. (2002). Qi Chang, PhD, Zhong Zuo, PhD, Francisco Harrison, MD, and Moses Sing Sum Chow, PharmD. 605–612.
- Chen M.H., McClung A.M., Bergman C.J. Concentrations of oligomers and polymers of proanthocyanidins in red and purple rice bran and their relationships to total phenolics, flavonoids, antioxidant capacity and whole grain color. Food Chem. 2016;208:279–287. doi: 10.1016/j.foodchem.2016.04.004. [DOI] [PubMed] [Google Scholar]
- Cloud, A., Vilcins, D., McEwen, B. (2020). The effect of hawthorn (Crataegus spp.) on blood pressure: A systematic review. Adv. Integrative Med. https://doi.org/10.1016/j.aimed.2019.09.002.
- Costa D.C., Costa H.S., Albuquerque T.G., Ramos F., Castilho M.C., Sanches-Silva A. Advances in phenolic compounds analysis of aromatic plants and their potential applications. Trends Food Sci. Technol. 2015;45(2):336–354. [Google Scholar]
- Dahmer S., Scott E. Health effects of hawthorn. Am. Fam. Physician. 2010;81(4):465–468. [PubMed] [Google Scholar]
- Díaz-de-Cerio E., Pasini F., Verardo V., Fernández-Gutiérrez A., Segura-Carretero A., Caboni M.F. Psidium guajava L. leaves as source of proanthocyanidins: Optimization of the extraction method by RSM and study of the degree of polymerization by NP-HPLC-FLD-ESI-MS. J. Pharm. Biomed. Anal. 2017;133:1–7. doi: 10.1016/j.jpba.2016.10.011. [DOI] [PubMed] [Google Scholar]
- Ding G., Wang Y., Liu A., Hou Y., Zhang T., Bai G., Liu C. From chemical markers to quality markers: An integrated approach of UPLC/Q-TOF, NIRS, and chemometrics for the quality assessment of honeysuckle buds. RSC Adv. 2017;7(36):22034–22044. doi: 10.1039/c6ra28152d. [DOI] [Google Scholar]
- Edwards J.E., Brown P.N., Talent N., Dickinson T.A., Shipley P.R. A review of the chemistry of the genus Crataegus. Phytochemistry. 2012;79:5–26. doi: 10.1016/j.phytochem.2012.04.006. [DOI] [PubMed] [Google Scholar]
- Forouzanfar M.H., Liu P., Roth G.A., Ng M., Biryukov S., Marczak L., Alexander L., Estep K., Hassen Abate K., Akinyemiju T.F., Ali R., Alvis-Guzman N., Azzopardi P., Banerjee A., Bärnighausen T., Basu A., Bekele T., Bennett D.A., Biadgilign S., Catalá-López F., Feigin V.L., Fernandes J.C., Fischer F., Gebru A.A., Gona P., Gupta R., Hankey G.J., Jonas J.B., Judd S.E., Khang Y.-H., Khosravi A., Kim Y.J., Kimokoti R.W., Kokubo Y., Kolte D., Lopez A., Lotufo P.A., Malekzadeh R., Melaku Y.A., Mensah G.A., Misganaw A., Mokdad A.H., Moran A.E., Nawaz H., Neal B., Ngalesoni F.N., Ohkubo T., Pourmalek F., Rafay A., Rai R.K., Rojas-Rueda D., Sampson U.K., Santos I.S., Sawhney M., Schutte A.E., Sepanlou S.G., Shifa G.T., Shiue I., Tedla B.A., Thrift A.G., Tonelli M., Truelsen T., Tsilimparis N., Ukwaja K.N., Uthman O.A., Vasankari T., Venketasubramanian N., Vlassov V.V., Vos T., Westerman R., Yan L.L., Yano Y., Yonemoto N., Zaki M.E.S., Murray C.J.L. Global burden of hypertension and systolic blood pressure of at least 110 to 115mmHg, 1990–2015. JAMA - Journal of the American Medical Association. 2017;317(2):165. doi: 10.1001/jama.2016.19043. [DOI] [PubMed] [Google Scholar]
- Fraga C.G., Oteiza P.I., Galleano M. Plant bioactives and redox signaling: (–)-Epicatechin as a paradigm. Mol. Aspects Med. 2018;61:31–40. doi: 10.1016/j.mam.2018.01.007. [DOI] [PubMed] [Google Scholar]
- García-Mateos R., Ibarra-Estrada E., Nieto-Angel R. Antioxidant compounds in hawthorn fruits (Crataegus spp.) of Mexico. Revista Mexicana de Biodiversidad. 2013;84(4):1298–1304. doi: 10.7550/rmb.35675. [DOI] [Google Scholar]
- García-Sols P., Yahia E.M., Morales-Tlalpan V., Díaz-Muñoz M. Screening of antiproliferative effect of aqueous extracts of plant foods consumed in México on the breast cancer cell line MCF-7. Int. J. Food Sci. Nutr. 2009;60(SUPPL. 6):32–46. doi: 10.1080/09637480802312922. [DOI] [PubMed] [Google Scholar]
- González-Jiménez F.E., Salazar-Montoya J.A., Calva-Calva G., Ramos-Ramírez E.G. Phytochemical Characterization, in Vitro Antioxidant Activity, and Quantitative Analysis by Micellar Electrokinetic Chromatography of Hawthorn (Crataegus pubescens) Fruit. J. Food Qual. 2018;2018:1–11. doi: 10.1155/2018/2154893. [DOI] [Google Scholar]
- Haldar, R. N. (2013). Global Brief on Hypertension: Silent Killer, Global Public Health Crisis. Indian J. Phys. Med. Rehabilitation, 24(1), 2–2. https://doi.org/10.5005/ijopmr-24-1-2.
- Hellenbrand N., Sendker J., Lechtenberg M., Petereit F., Hensel A. Isolation and quantification of oligomeric and polymeric procyanidins in leaves and flowers of Hawthorn (Crataegus spp.) Fitoterapia. 2015;104:14–22. doi: 10.1016/j.fitote.2015.04.010. [DOI] [PubMed] [Google Scholar]
- Hernández-Pérez A., Bah M., Ibarra-Alvarado C., Rivero-Cruz J.F., Rojas-Molina A., Rojas-Molina J.I., Cabrera-Luna J.A. Aortic relaxant activity of Crataegus gracilior phipps and identification of some of its chemical constituents. Molecules. 2014;19(12):20962–20974. doi: 10.3390/molecules191220962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibarra-Alvarado C., Rojas A., Mendoza S., Bah M., Gutiérrez D.M., Hernández-Sandoval L., Martínez M. Vasoactive and antioxidant activities of plants used in Mexican traditional medicine for the treatment of cardiovascular diseases. Pharm. Biol. 2010;48(7):732–739. doi: 10.3109/13880200903271280. [DOI] [PubMed] [Google Scholar]
- ICH. (2005). ICH Harmonised Tripartite Guideline: Validation of Analytical Procedures: Text and Methodology Q2 (R1).
- Jurikova T., Sochor J., Rop O., Mlcek J., Balla S., Szekeres L., Adam V., Kizek R. Polyphenolic profile and biological activity of chinese hawthorn (Crataegus pinnatifida BUNGE) fruits. Molecules. 2012;17(12):14490–14509. doi: 10.3390/molecules171214490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kähkönen M.P., Hopia A.I., Vuorela H.J., Rauha J.-P., Pihlaja K., Kujala T.S., Heinonen M. Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food. Chem. 1999;47(10):3954–3962. doi: 10.1021/jf990146l. [DOI] [PubMed] [Google Scholar]
- Kluknavsky M., Balis P., Skratek M., Manka J., Bernatova I. (–)-Epicatechin reduces the blood pressure of young borderline hypertensive rats during the post-treatment period. Antioxidants. 2020;9(2):96. doi: 10.3390/antiox9020096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch E., Malek F.A. Standardized extracts from hawthorn leaves and flowers in the treatment of cardiovascular disorders preclinical and clinical studies. Planta Med. 2011;77(11):1123–1128. doi: 10.1055/s-0030-1270849. [DOI] [PubMed] [Google Scholar]
- Kunle Standardization of herbal medicines - A review. Int. J. Biodivers. Conserv. 2012;4(3):101–112. doi: 10.5897/ijbc11.163. [DOI] [Google Scholar]
- Luca, S. V., Bujor, A., Miron, A., Aprotosoaie, A. C., Skalicka-Woźniak, K., & Trifan, A. (2019). Preparative separation and bioactivity of oligomeric proanthocyanidins. In Phytochemistry Reviews (Vol. 5). https://doi.org/10.1007/s11101-019-09611-5.
- Luna-Vázquez F., Ibarra-Alvarado César, Camacho-Corona M., Rojas-Molina A., Rojas-Molina J., García A., Bah M. Vasodilator activity of compounds isolated from plants used in Mexican traditional medicine. Molecules. 2018;23(6):1474. doi: 10.3390/molecules23061474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maaliki D., Shaito A.A., Pintus G., El-Yazbi A., Eid A.H. Flavonoids in hypertension: a brief review of the underlying mechanisms. Curr. Opin. Pharmacol. 2019;45:57–65. doi: 10.1016/j.coph.2019.04.014. [DOI] [PubMed] [Google Scholar]
- Magnusson, B. (2014). The fitness for purpose of analytical methods: a laboratory guide to method validation and related topics (2014). Eurachem.
- Martínez M. (7th ed.). Editorial Botas; 1991. Plantas Medicinales de México. [Google Scholar]
- Medina-Ruiz D., Erreguin-Luna B., Luna-Vázquez F.J., Romo-Mancillas A., Rojas-Molina A., Ibarra-Alvarado C. Vasodilation elicited by isoxsuprine, identified by high-throughput virtual screening of compound libraries, involves activation of the NO/cGMP and H<inf>2</inf>S/K<inf>ATP</inf> pathways and blockade of α<inf>1</inf>-adrenoceptors and calcium channels. Molecules. 2019;24(5) doi: 10.3390/molecules24050987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel F.G., Cavalheiro A.H., Spinola Nathália.F., Ribeiro D.L., Barcelos G.R.M., Antunes L.M.G., Hori J.I., Marquele-Oliveira F., Rocha B.A., Berretta A.A. Validation of a RP-HPLC-DAD method for chamomile (Matricaria recutita) preparations and assessment of the marker, apigenin-7-glucoside, safety and anti-inflammatory effect. Evidence-Based Complementary and Alternative Medicine. 2015;2015:1–9. doi: 10.1155/2015/828437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabavi S.F., Habtemariam S., Ahmed T., Sureda A., Daglia M., Sobarzo-Sánchez E., Nabavi S.M. Polyphenolic composition of Crataegus monogyna jacq.: From chemistry to medical applications. Nutrients. 2015;7(9):7708–7728. doi: 10.3390/nu7095361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazhand A., Lucarini M., Durazzo A., Zaccardelli M., Cristarella S., Souto S.B., Silva A.M., Severino P., Souto E.B., Santini A. Hawthorn (Crataegus spp.): An updated overview on its beneficial properties. Forests. 2020;11(5):1–21. doi: 10.3390/F11050564. [DOI] [Google Scholar]
- Negi P.S., Singh R., Dwivedi S.K. Evaluation of Antihypertensive Effect of Fruit Beverage of Crataegus crenulata Roxb. : A wild Shrub of Himalayan Hills. Defence Life Sci. J. 2018;3(2):146. doi: 10.14429/dlsj.3.12571. [DOI] [Google Scholar]
- Novakovic A., Marinko M., Jankovic G., Stojanovic I., Milojevic P., Nenezic D., Kanjuh V., Yang Q., He G.W. Endothelium-dependent vasorelaxant effect of procyanidin B2 on human internal mammary artery. Eur. J. Pharmacol. 2017;807(April):75–81. doi: 10.1016/j.ejphar.2017.04.015. [DOI] [PubMed] [Google Scholar]
- Ong E.S. Extraction methods and chemical standardization of botanicals and herbal preparations. J. Chromatogr., B: Anal. Technol. Biomed. Life Sci. 2004;812(1–2 SPEC. ISS.):23–33. doi: 10.1016/j.jchromb.2004.07.041. [DOI] [PubMed] [Google Scholar]
- Orhan I.E. Phytochemical and Pharmacological Activity Profile of Crataegus oxyacantha L. (Hawthorn) - A Cardiotonic Herb. Curr. Med. Chem. 2016;25(37):4854–4865. doi: 10.2174/0929867323666160919095519. [DOI] [PubMed] [Google Scholar]
- Patel R.V., Mistry B.M., Shinde S.K., Syed R., Singh V., Shin H.-S. Therapeutic potential of quercetin as a cardiovascular agent. Eur. J. Med. Chem. 2018;155:889–904. doi: 10.1016/j.ejmech.2018.06.053. [DOI] [PubMed] [Google Scholar]
- Prior R.L., Wu X., Schaich K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food. Chem. 2005;53(10):4290–4302. doi: 10.1021/jf0502698. [DOI] [PubMed] [Google Scholar]
- Qamar A., Braunwald E. Treatment of Hypertension: Addressing a Global Health Problem. JAMA. 2018;320(17):1751–1752. doi: 10.1001/jama.2018.16579. [DOI] [PubMed] [Google Scholar]
- Ranjbar, K., Zarrinkalam, E., Salehi, I., Komaki, A., & Fayazi, B. (2018). Cardioprotective effect of resistance training and Crataegus oxyacantha extract on ischemia reperfusion–induced oxidative stress in diabetic rats. Biomed. Pharm. 100(November 2017), 455–460. https://doi.org/10.1016/j.biopha.2018.02.021 [DOI] [PubMed]
- Rastogi S., Pandey M.M., Rawat A.K.S. Traditional herbs: a remedy for cardiovascular disorders. Phytomedicine. 2016;23(11):1082–1089. doi: 10.1016/j.phymed.2015.10.012. [DOI] [PubMed] [Google Scholar]
- Rohr G.E., Meier B., Sticher O. Analysis of procyanidins. In Studies in natural products chemistry. 2000;Vol. 21:497–570. [Google Scholar]
- Rue E.A., Rush M.D., van Breemen R.B. Procyanidins: a comprehensive review encompassing structure elucidation via mass spectrometry. Phytochem. Rev. 2018;17(1):1–16. doi: 10.1007/s11101-017-9507-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadek M., Mousa S., El-Masry S., Demain A. Influence of Hawthorn (Crataegus oxyacantha) Leaves Extract Administration on Myocardial Infarction Induced by Isoproterenol in Rats. Cardiol. Angiol. Int. J. 2018;7(1):1–12. doi: 10.9734/ca/2018/39025. [DOI] [Google Scholar]
- Sánchez M., Romero M., Gómez-Guzmán M., Tamargo J., Pérez-Vizcaino F., Duarte J. Cardiovascular Effects of Flavonoids. Curr. Med. Chem. 2018;26(39):6991–7034. doi: 10.2174/0929867326666181220094721. [DOI] [PubMed] [Google Scholar]
- Seo J.H., Kim J.E., Shim J.H., Yoon G., Bang M.A., Bae C.S., Lee K.J., Park D.H., Cho S.S. HPLC analysis, Optimization of extraction conditions and biological evaluation of corylopsis coreana uyeki flos. Molecules. 2016;21(1):1–13. doi: 10.3390/molecules21010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao F., Gu L., Chen H., Liu R., Huang H., Chen L., Yang M. Evaluation of hypolipidemic and antioxidant effects in phenolrich fraction of Crataegus pinnatifida fruit in hyperlipidemia rats and identification of chemical composition by ultra-performance liquid chromatography coupled with quadropole time-of-flight m. Pharmacognosy Magazine. 2017;13(52):725. doi: 10.4103/pm.pm_402_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S., Ali A., Ali J., Sahni J.K., Baboota S. Rutin: Therapeutic potential and recent advances in drug delivery. Expert Opin. Invest. Drugs. 2013;22(8):1063–1079. doi: 10.1517/13543784.2013.805744. [DOI] [PubMed] [Google Scholar]
- Shatoor A.S., Al Humayed S., Alkhateeb M.A., Shatoor K.A., Aldera H., Alassiri M., Shati A.A. Crataegus aronia protects and reverses vascular inflammation in a high fat diet rat model by an antioxidant mechanism and modulating serum levels of oxidized low-density lipoprotein. Pharm. Biol. 2019;57(1):38–48. doi: 10.1080/13880209.2018.1564930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Souza V.G., De Andrade F.H.D., De Souza F.S., Macedo R.O. Analytical Method By Hplc-Dad Allows Quantification of Quercetin Marker in Standardized Extract of Anadenanthera Colubrina Var Cebil. Int. J. Pharm. Pharm. Sci. 2017;9(8):47. doi: 10.22159/ijpps.2017v9i8.16468. [DOI] [Google Scholar]
- Suganthi A., Ravi T.K. Chemical Methodologies Estimation of Anti-dengue Phytochemical Markers Gallic acid, Rutin and Quercetin in Methanolic Extract of Euphorbia hirta (L.) and Tawa-Tawa Capsule Formulation by Validated RP-HPLC Method. Chem. Methodolog. 2019;3:43–54. doi: 10.22034/chemm.2018.129381.1051. [DOI] [Google Scholar]
- Sui Y., Zheng Y., Li X., Li S., Xie B., Sun Z. Characterization and preparation of oligomeric procyanidins from Litchi chinensis pericarp. Fitoterapia. 2016;112:168–174. doi: 10.1016/j.fitote.2016.06.001. [DOI] [PubMed] [Google Scholar]
- Torres-Ortiz D.A., Eloy R.-D., Moustapha B., César I.-A., Edmundo M.-S., Jesús Eduardo C.-R., Dulce María R.-P. Vasorelaxing effect and possible chemical markers of the flowers of the Mexican. Crataegus gracilior Nat. Prod. Res. 2019;34(24):3522–3525. doi: 10.1080/14786419.2019.1577833. [DOI] [PubMed] [Google Scholar]
- Tsai C.H., Tzeng S.F., Hsieh S.C., Lin C.Y., Tsai C.J., Chen Y.R., Yang Y.C., Chou Y.W., Lee M.T., Hsiao P.W. Development of a standardized and effect-optimized herbal extract of Wedelia chinensis for prostate cancer. Phytomedicine. 2015;22(3):406–414. doi: 10.1016/j.phymed.2015.01.013. [DOI] [PubMed] [Google Scholar]
- UNAM. (2009). Biblioteca Digital de la Medicina Tradicional Mexicana. http://www.medicinatradicionalmexicana.unam.mx/apmtm/termino.php?l=3&t=crataegus-pubescens.
- Vechi G., Fonseca R.C.M.V.A., de Souza P., da Silva L.M., de Andrade S.F., Cechinel Filho V. Cryptostrobin and catechin isolated from Eugenia mattosii D. Legrand leaves induce endothelium-dependent and independent relaxation in spontaneously hypertensive rat aorta. Pharmacol. Rep. 2019;71(5):950–957. doi: 10.1016/j.pharep.2019.05.006. [DOI] [PubMed] [Google Scholar]
- Venskutonis P.R. Phytochemical composition and bioactivities of hawthorn (Crataegus spp.): review of recent research advances. J. Food Bioactives. 2018;4:69–87. doi: 10.31665/jfb.2018.4163. [DOI] [Google Scholar]
- Wang J., Xiong X., Feng B. Effect of Crataegus usage in cardiovascular disease prevention: An evidence-based approach. Evid. Based Complementary Alternative Med. 2013;2013:1–16. doi: 10.1155/2013/149363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. (2020). The top 10 causes of death. https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death
- Wu J., Peng W., Qin R., Zhou H. Crataegus pinnatifida: Chemical Constituents, Pharmacology, and Potential Applications. Molecules. 2014;19(2):1685–1712. doi: 10.3390/molecules19021685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B., Liu P. Composition and health effects of phenolic compounds in hawthorn (Crataegus spp.) of different origins. J. Sci. Food Agric. 2012;92(8):1578–1590. doi: 10.1002/jsfa.v92.810.1002/jsfa.5671. [DOI] [PubMed] [Google Scholar]
- Yano Y., Reis J.P., Colangelo L.A., Shimbo D., Viera A.J., Allen N.B., Gidding S.S., Bress A.P., Greenland P., Muntner P., Lloyd-Jones D.M. Association of Blood Pressure Classification in Young Adults Using the 2017 American College of Cardiology/American Heart Association Blood Pressure Guideline with Cardiovascular Events Later in Life. JAMA – J. Am. Med. Assoc. 2018;320(17):1774–1782. doi: 10.1001/jama.2018.13551. [DOI] [PMC free article] [PubMed] [Google Scholar]