Abstract
Accumulating evidence suggests that the molecular circadian clock is crucial in blood pressure (BP) control. Circadian rhythms are controlled by the central clock, which resides in the suprachiasmatic nucleus (SCN) of the hypothalamus, and peripheral clocks throughout the body. Both light and food cues entrain these clocks but whether these cues are important for the circadian rhythm of BP is a growing area of interest. The peripheral clocks in the smooth muscle, perivascular adipose tissue, liver, adrenal gland, and kidney have been recently implicated in the regulation of BP rhythm. Dysregulation of the circadian rhythm of BP is associated with adverse cardiorenal outcomes and increased risk of cardiovascular mortality. In this review, we summarize the most recent advances in peripheral clocks as BP regulators, highlight the adverse outcomes of disrupted circadian BP rhythm in hypertension, and provide insight into potential future work in areas exploring the circadian clock in BP control and chronotherapy. A better understanding of peripheral clock function in regulating the circadian rhythm of BP will help pave the way for targeted therapeutics in the treatment of circadian BP dysregulation and hypertension.
Keywords: Non-dipping, ambulatory blood pressure monitoring, chronic kidney disease, BMAL1, PER1
Introduction
Hypertension is the leading modifiable risk factor for all-cause mortality, with the global burden estimated at 1.4 billion, ~31% of the global adult population1–3. Diagnosis mainly relies on in-office or clinic blood pressure (BP) measurements, which do not account for variations in the circadian rhythm of BP1. Circadian rhythms are 24-hour oscillations that are imperative coordinators to many physiological functions including BP regulation (reviewed in 4). The daily BP rhythm is characterized by a morning surge on waking with a plateau during the day and a nocturnal decline (a decrease of 10–20% from the average daytime BP). Ambulatory BP monitoring is essential for accounting for this 24-hour BP variation1,5,6. Disruption of this 24-hour BP variation is more likely in hypertensive and chronic kidney disease (CKD) patients and is associated with adverse cardiorenal outcomes7,8. Abnormal BP rhythms include non-dipping BP (defined as a <10% decrease from daytime to nocturnal BP), reverse dipping (nocturnal risers), and extreme dippers (≥20% between nocturnal and daytime BP). Isolated nocturnal hypertension, defined as nighttime systolic BP ≥120 mmHg and diastolic BP ≥70 mmHg with daytime <135/85 mmHg9, is usually associated with non-dipping or reverse dipping but can be found in dippers1. Nocturnal hypertension and a non-dipping pattern have been shown to increase the risk of hypertension-induced organ damage10, with the worst outcomes seen in individuals who have both features11.
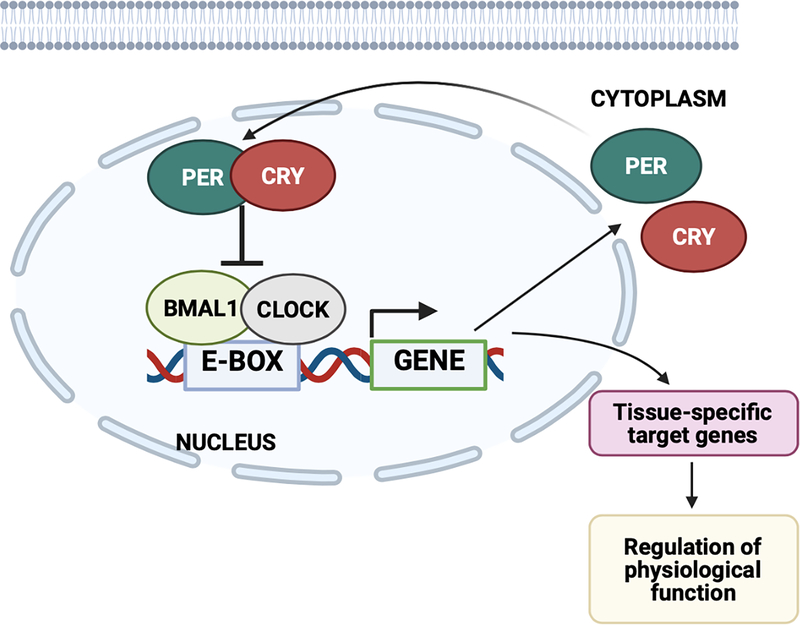
Circadian rhythms are controlled by both the central clock, which resides in the suprachiasmatic nucleus (SCN) of the hypothalamus, and peripheral clocks throughout the body. The contribution of peripheral clocks, particularly the kidney clock, to BP rhythm is an active area of research (discussed in detail below). At the molecular level, this is controlled by a cellular clock, which is comprised of four key core clock proteins (CLOCK, BMAL1, PER, and CRY; Figure 1). CLOCK (and its paralogue NPAS2) and BMAL1 (also known as ARNTL) heterodimerize and bind E-box response elements within promoter regions of target genes, including Period and Cryptochrome (encoding PER1/2/3 and CRY1/2, respectively), and Ror and Nrd1d1/2 (encoding RORα/β/γ and REV-ERBα/β, respectively). In the negative feedback loops, PER and CRY heterodimerize and repress the activity of BMAL1 and CLOCK. ROR and REV-ERB mediate opposing actions on Bmal1 gene expression12. Preclinical research has established that these circadian clock proteins regulate 43% of all expressed genes in mice13, with >80% of protein-coding genes displaying 24-hour rhythms in expression in non-human primates14. Work by various investigators using knockout (KO) rodent models of these core clock genes has brought us closer to understanding how the circadian mechanism contributes to BP regulation. However, the mechanisms underlying non-dipping and abnormally elevated nocturnal BP remain to be fully elucidated.
Figure 1. Core components of the circadian clock.
CLOCK and BMAL1 heterodimerize and bind E-box response elements within promoter regions of target genes, including Period and Cryptochrome (encoding PER1/2/3 and CRY1/2, respectively). In the negative feedback loops, PER and CRY heterodimerize and repress the activity of BMAL1 and CLOCK. This clock mechanism regulates tissue-specific target genes to regulate many physiological processes. Diagram created with Biorender.com.
The purpose of this review is to summarize the most recent advances in both basic and clinical research relating to circadian rhythms, clock genes, and hypertension. Furthermore, we highlight the adverse outcomes of altered circadian rhythm of BP in hypertension and consider future directions from these important findings.
Adverse outcomes of a non-dipping blood pressure profile
High nocturnal BP is often accompanied by a non-dipping profile, but both independently have significance for target organ damage, particularly cardiovascular and renal damage. This has been evident in recent basic and clinical studies published between 2020 and 2021. Wang et al. assessed isolated nocturnal hypertension in CKD patients (n=1484) and found a high proportion of these individuals were non-dippers (individuals with nocturnal systolic hypertension, 86%; nocturnal diastolic, 71%; nocturnal systolic-diastolic, 86%). Interestingly, there was a higher prevalence of non-dipping with nocturnal systolic and nocturnal systolic-diastolic hypertension. Furthermore, they observed an association between nocturnal systolic hypertension, either alone or in combination with nocturnal diastolic hypertension, and increased risk for cardiovascular events and renal failure in CKD patients15. Non-dipping BP has also been associated with proteinuria and progression of renal injury in a rat model of salt-sensitive hypertension16. In support of this, Cho et al. reported that patients with controlled hypertension, described in this study as BP managed within a normal range (<140/90 mmHg) by antihypertensive treatment, who were reverse dippers/non-dippers had an increased risk of albuminuria and decreased renal function compared with normal/extreme dippers. Therefore, suggesting monitoring diurnal and nocturnal BP may predict CKD progression, which has been previously reported by Timio et al.17 and Davidson et al.7. However, a limitation of these studies described above was that ambulatory BP was only measured for one 24-hour period. One 24-hour recording with BP measurements at 30 min intervals with ≥70% of expected measurements (20 valid wake (9 AM – 9 PM) and 7 valid asleep (1 AM – 6 AM)) has been reported to validate ambulatory BP data. However, for research purposes, ≥ 2 valid daytime and 1 valid nighttime measurement per hour have been advised18.
Primary aldosteronism (PA) is reported in 5–10% of hypertensive patients. Patients with PA have been shown to be at high risk of left ventricular hypertrophy due to excessive aldosterone which is at least partly BP independent19. Although, BP rhythm in PA has only been recently investigated by Wu et al. who demonstrated that PA patients (n=385) had higher nocturnal systolic BP, along with a non-dipping pattern, compared to patients with essential hypertension (n=385). This non-dipping profile was suggested to be due to excessive aldosterone. There was no difference between age, sex, body mass index, 24-hour BP, daytime BP, or duration of hypertension between these groups. Importantly, the higher nocturnal systolic BP was strongly associated with cardiac damage in PA compared with essential hypertensive patients, emphasizing the importance of measuring ambulatory BP in this case20.
In contrast, the STANISLAS Cohort study did not find an association between non-dipping BP profile (in normotensive or hypertensive patients) and increased cardiovascular or renal damage21. Normotensive and hypertensive individuals who were non-dippers showed no differences between cardiovascular and renal parameters (including carotid intima-media thickness, pulse-wave velocity, left ventricular mass index, left ventricular hypertrophy, eGFR, microalbuminuria, and albuminuria/creatinine ratio) compared with their dipper counterparts21. However, the mean 24-hour ambulatory BP in the “hypertensive” participants was <130/90 mmHg. Thus, this group is representative of controlled hypertension by antihypertensive treatment, and the lack of an association between non-dipping BP and detrimental cardiorenal outcomes could be dependent on the level of BP control and whether the individual is truly hypertensive (>130/90 mmHg). It could be hypothesized that good BP control even in non-dipping hypertensive patients could overcome the potential adverse effects of non-dipping BP on target organ damage.
Many studies support this association that nocturnal systolic BP increases the risk of a decline in renal function and increased risk for CV events22–25, for an extensive review on this association see Hansen et al.26. Although differing results have been reported from these studies regarding the link between the non-dipping profile and worsening cardiorenal outcomes. There were no differences in the mean age range (between 50 and 70) in these studies. However, in Redon et al.22, where non-dipping was not associated with worsening of cardiorenal outcomes, the enrolled participant number was far smaller than other studies and had a shorter follow-up period. The association between non-dipping BP and target organ damage remains controversial due to the populations studied, sample size, and the assessment of specific target organ damage. There is a need for larger analysis addressing the potential association between non-dipping BP and target organ damage, involving ambulatory BP measurements over a longer period of time in a variety of populations spanning various age groups and taking into account whether their hypertension is managed. This is essential to clarify the influence of ethnicity, age, and antihypertensive treatment on the predictive value of non-dipping BP. Overall, these studies highlight the importance of monitoring nocturnal BP and BP rhythm using ambulatory BP measurements and nocturnal BP control for reducing the risk of target organ damage.
A role for peripheral clocks in regulating blood pressure rhythm
Smooth muscle:
The mechanisms regulating BP rhythm are not fully understood. Numerous studies have examined the role of circadian clocks using rodent models of null core clock gene mutations, with a particular interest in BMAL1. Male global Bmal1 KO mice exhibit non-rising BP during the active period, resulting in an overall lower 24-hour BP and loss of diurnal BP rhythm27. Efforts have been made to explore the tissue-specific mechanisms underlying the non-rising BP phenotype. Xie et al. demonstrated that smooth muscle BMAL1 was essential for time-of-day variations in phenylephrine- and serotonin-induced vasoconstriction of renal and mesenteric arteries thought to reflect the dampened BP rhythm in smooth muscle-specific Bmal1 KO male mice41. The rhythmicity of the central clock in the SCN remained intact. Although, the extent of the loss of the diurnal BP rhythm was less than the global KO, raising the question - what else is contributing to this non-rising phenotype? It remains unknown what the effect of Bmal1 deletion in smooth muscle of female mice is.
Perivascular adipose tissue (PVAT):
PVAT regulates vascular tone via contractile and anticontractile effects and has been shown to modulate BP in vivo, reviewed in 28,29. Chang et al. investigated the role of the PVAT clock on BP regulation, specifically in brown adipocyte-specific Bmal1 KO male mice. These mice also had altered expression of other clock genes, including increased Cry1 and Npas2, with decreases in Per3 and Rev-erbα. Brown adipocyte-specific Bmal1 KO male mice displayed reduced daytime BP, resulting in an extreme-dipper phenotype. The mechanisms behind this are thought to be via BMAL1 regulating transcription of angiotensinogen, leading to increases in angiotensin II levels in PVAT which act on smooth muscle cells within the vasculature to regulate vascular tone and BP rhythm30. Again, it is unknown what the role of PVAT BMAL1 in females is, which merits investigation. Obesity is known to cause increased PVAT mass and PVAT dysfunction which correlates with increased BP (reviewed in 31). Therefore, it would be of interest to investigate whether the PVAT clock is altered in animal models of obesity and if this impacts BP rhythm.
Liver:
Type 2 diabetic patients have increased prevalence of BP rhythm disruption, with up to 70% reported as non-dippers in a cross-sectional study of 20,000 diabetic patients, and a role of the circadian clock has been indicated in the pathogenesis of diabetes32,33. Although, little is known about the role of the clock genes in disruption of BP rhythm in diabetes. Hou et al. used the db/db mouse (shown to have a non-dipping phenotype34) crossed with PERIOD2::LUCIFERASE knock-in mouse to measure PER2 protein oscillation by bioluminescence in a diabetic mouse model35. These diabetic mice were also non-dippers which was suggested to, in part, be due to the advanced phase shift of PER2 in peripheral tissues including the liver, as well as the kidney, and not the central clock in the SCN. Limited studies have explored whether specifically the liver circadian clock plays a role in BP regulation. A recent study has shown altered PVAT-mediated endothelial vascular function in hepatic-specific Bmal1 KO male mice, with lower systolic BP during the inactive period but no changes in either diastolic BP or heart rate (HR)36. This was thought to be due to changes in circulating levels of liver derived mediators, including β-HB and IGF-1.
Adrenal gland:
A previous study found an adrenal disorder, characterized by Hsd3b6-dependent aldosterone overproduction and differing aldosterone rhythm, in global double Cry1/2 KO mice compared with controls, which caused these mice to display salt-sensitive hypertension37. Whether BP rhythm was affected in these mice and if this is associated with the adrenal clock remains in question. However, it emphasizes the importance of maintenance of daily expression of adrenal Hsd3b6 by the circadian clock in BP control and its response to a dietary salt challenge. A more recent study by Tanaka et al. investigated the role of the adrenal gland circadian clock in spontaneous hypertensive rats. Several adrenal gland circadian clock genes, such as Bmal1, Per2, Per3, and Cry1 were phase-advanced compared with control rats. A major role of the adrenal gland is the steroidogenesis of glucocorticoids, mineralocorticoids, and androgens. The circadian expression profile of StAR, the rate-limiting enzyme for steroidogenesis, was also found to be phase advanced, as well as serum corticosterone and aldosterone38. Both of these steroids have been implicated in BP rhythm20,39. This study was observational so further research into the direct effect of the adrenal gland circadian clock on BP rhythmicity would be of interest.
Kidney:
Numerous studies have examined the role of the intrinsic renal circadian clock in renal sodium handling to better understand its contributions to BP rhythm. For an excellent and extensive review on the role of the central/renal clock and reactive systems in regulating the rhythm of renal sodium reabsorption to influence BP rhythm, see 40. As previously mentioned, global Bmal1 KO mice are non-risers27, which could be due in part to smooth muscle BMAL141. The global Bmal1 KO mice also exhibited a loss in diurnal sodium excretion42. Efforts have been made to explore the mechanisms behind the lost diurnal sodium excretion in global Bmal1 KO mice by utilizing mouse models with deletions of Bmal1 in different regions of the nephron of the kidney. Deleting Bmal1 in the collecting duct of mice resulted in lowered BP but with no changes in either the diurnal rhythm of BP or sodium excretion. Furthermore, this phenotype was only seen in males, with females protected42. This finding was supported by our own study where we generated a distal nephron-specific Bmal1 KO mouse model and found lowered BP, with no changes in rhythmicity, in a sex-dependent manner43. However, we did find that male mice had reduced renal sodium retention when challenged with a potassium-restricted diet43. Overall, suggesting BMAL1 in the distal portions of the kidney of mice contribute to BP regulation independent of changes in BP rhythm in a sex-dependent manner.
Overall, still little is known about the contribution of BMAL1 on diurnal control of renal sodium handling. Using a global Bmal1 KO rat, Johnston et al. found that male Bmal1 KO rats had lost the diurnal rhythm of sodium excretion although BP rhythm was intact (unlike the Bmal1 KO mouse)54. Like previous studies, females were protected from this phenotype. This suggests BMAL1 is important for the control of diurnal sodium excretion specifically in male rats, which interestingly appeared dissociated from BP rhythm.
There appears to be species differences with the role of BMAL1, increasing the complexity of understanding its contribution to BP control and rhythm. However, what has been continually reported is that females are protected from BP and/or renal sodium handling changes in Bmal1 KO rodent models. This has also been demonstrated in female Per1 KO mice44. This leads to questioning whether ovarian sex hormones play a role in the protection of this phenotype. Premenopausal women have a reduced prevalence of hypertension than men and are less likely to present with blunted BP rhythm45–47. The prevalence for non-dipping BP was 16 times higher in postmenopausal, compared with premenopausal woman48. Therefore, future work to explore the role of ovarian sex hormones in BP rhythm would be of interest.
An alternative approach was conducted by Murata et al. where they suggested the potential contribution of renal clock-regulated proteins, FXR1 and PPAT, in BP rhythm in spontaneous hypertensive rats (SHR)49. FXR1 gene and protein levels were reduced in SHR compared with Wistar Kyoto rats (WKY) as controls, which was suggested to impact the proliferation and growth of renal tubular cells. Ppat expression was increased in these rats, with no changes in protein levels, and has been linked with vascular toxicity via altering uric acid metabolism. This study was speculative in the role of FXR1 and PPAT in the diurnal variation of electrolyte and water handling, impacting BP rhythm. A caveat of this study was that the SHR model does not have an appropriate genetic control. WKY are often used as controls for this model, as they were derived from the same colony as the SHR. However, it has been reported that WKY may not constitute a single inbred strain50. Future studies would be necessary to determine a causal link between altered FXR1 and/or PPAT levels and disruption of BP circadian rhythm and assess strain to strain variability. Furthermore, whether this is sex-dependent would be of interest as the sex of the rats was not provided.
Together, these studies provide evidence for the potential role of the peripheral clock in the regulation of BP rhythm, and for disruption of peripheral clock genes contributing to the non-dipping BP pattern. However, this illustrates the complexity of control of BP rhythm and that, like the pathogenesis of hypertension, this likely involves complex crosstalk between these multiple systems and neurohormonal controllers.
Regulating blood pressure rhythm: the timing of food intake
The central clock receives light input directly from the retina for entrainment of time-of-day and remains resistant to phase perturbations from internal cues, unlike peripheral clocks that are susceptible to adjustments to reflect local metabolic demand (reviewed in 51). Peripheral clocks have been shown to be entrained by food cues. The question remains, are light and/or food cues important for the circadian rhythm of BP? Zhang et al. addressed this by restricted feeding mice during their inactive phase, causing the BP rhythm to be inverted with no change in average 24-hour BP52. Food consumption, over light, entrained BP rhythm in mice as their BP peak was during the feeding period even when fed at various times in constant darkness. Furthermore, the rhythm of PER2 was assessed in tissues following restricted feeding, using the PERIOD2::LUCIFERASE knock-in mouse. PER2 in the SCN (central clock) was not affected, but restricted feeding caused a phase shift in the liver, renal inner medulla, and adrenal gland peripheral clocks. Changes in metabolism via restricted feeding have been previously shown to uncouple peripheral clocks from the central clock in the SCN53. Interestingly, the timing of food intake did not entrain renal excretion in mice as urine volume and sodium excretion were unaffected, suggesting a dissociation between BP rhythm and sodium excretion (previously discussed by Johnston et al.54). These findings were shown to be independent of BMAL1. However, the role of other clock genes still needs to be explored. This study also observed an inactive phase (day) surge in plasma insulin and an overall increase in inactive phase plasma leptin compared with the active phase in restricted feeding. The contribution of increased nocturnal plasma insulin and leptin levels to the diurnal BP pattern warrants investigation.
What are the implications of this for human health? The benefits of time-restricted feeding (or intermittent fasting) have focussed on weight loss, but recently more benefits have been discovered that are dependent on the time-of-day of the feeding window. As previously mentioned, type 2 diabetic individuals have an increased prevalence of BP rhythm disruption32,33 along with sleep disturbances55, possibly in part due to clock gene rhythm disruptions35. In db/db mice, restricted feeding to the active phase (between 10 AM and 6 PM) improved their sleep-wake cycle and suggested sleep homeostatic function improvement56. Eating during a 6-hour period during the daytime, with a diet provided to ensure maintenance of body weight, in pre-diabetic men with their last meal consumed before 3 PM (to align with circadian rhythms of metabolism) has been shown to improve insulin sensitivity and lower BP, although BP rhythm was not assessed57. This lowering of BP was supported by another study following 10-hour restricted feeding in both male and female metabolic syndrome patients58. These participants were not restricted to what they could eat during this time and were instructed to continue their regular diet, which had to be recorded during this study. Sex differences were not explored in either of these studies. Interestingly, restricted feeding to the late afternoon/evening, with a diet to ensure maintenance of body weight, increased BP59. These studies highlight the potential benefits of time-restricted feeding, specifically during the day, on BP regulation, as well as sleep-wake cycles and insulin sensitivity. However, these studies did not assess the effect of time-restricted feeding on BP rhythm. Therefore, future work in determining the effect of restricted food consumption late at night/during the night compared to during the day on BP rhythm and nocturnal BP changes in both men and women would be of interest, especially for shift workers.
What can your gut tell you about blood pressure rhythm?
Recent evidence has highlighted a relationship between the circadian clock and the gut microbiota - where the circadian clock can modulate the composition and time-of-day variation of gut microbiota, and microbiota can contribute to the maintenance of clock function (reviewed in 60). Diurnal changes in the composition and function of the gut microbiota can be modulated by time of feeding and diet61,62, and diurnal oscillations are abolished in certain gut microbiota in global Bmal1 KO mice which were sex-dependent63. Gut microbiota dysbiosis has been shown to contribute to the development of hypertension64. Chakraborty et al. hypothesized that diurnal alterations in gut microbial composition could contribute to a salt-sensitive hypertensive phenotype. This study illustrated that the composition of microbial communities exhibits circadian rhythms that were aligned with BP rhythm in male Dahl salt-sensitive rats. This reshaping of microbiota could be an evolutionary adaptation to ensure the survival of bacterial species, increasing their ability to survive under differing food availability during a 24-hour period. However, it has been suggested that this reshaping alters microbial function, impacting the BP of the host. Furthermore, a high salt diet in these rats elicited a time-of-day variation in specific gut microbes65. Future work is needed to test a causal link between gut microbiota rhythm and BP variation. With time of feeding impacting diurnal alterations in the gut microbiota, it would also be of interest to determine if the link between time of restricted feeding and BP rhythm (previously discussed) is associated with diurnal alterations in gut microbiota.
Inflammation
The cellular clock is present in immune cells with many aspects of the immune response exhibiting daily oscillations. These oscillations of immune cell recruitment to tissues are thought to promote tissue recovery66. This has been implicated in a rat model of sepsis, where the absence of circadian light cues reduced survival compared with a 12-hour light/dark cycle67. This study raises the question of whether there would be a benefit of daily lighting cycles in intensive care units for improving the recovery of patients with sepsis. The circadian rhythm of the immune response can also impact disease development66. Clock gene expression has been linked to the expression of pro-inflammatory cytokines and implicated in the development of cancer68,69. Over the last decade, there has been increasing evidence for the contribution of the immune system in the pathogenesis of hypertension, recently reviewed by Drummond et al70. Although, little is known if there is a role for the clock in different immune cells in the regulation of BP rhythm. A recent study by Yang et al. utilized a mouse model of myeloid cell-specific Bmal1 deletion showed to have unaltered BP at Zeitgeber times (ZT) 4–5 (~11 am, during the mouse rest phase), using tail-cuff system, and therefore, BP rhythm was not assessed71. With previous studies implicating a link between circadian rhythms and the immune response, and the contribution of the immune system in hypertension, improved understanding of rhythms in the immune response and if these rhythms impact BP rhythms would be of great interest.
Autonomic nervous system
Several studies have investigated the role of the autonomic nervous system in non-dipping BP. Studies have suggested a failure to reduce sympathetic and increase parasympathetic activity contributes to a non-dipping BP phenotype72–74. However, patients with primary autonomic failure, so very low sympathetic and parasympathetic activities have a high incidence of non-dipping75. This suggests that it is the lack of autonomic tone modulation that contributes to non-dipping. The autonomic nervous system also plays a role in renal solute handing76 and it is postulated that this autonomic control of renal function plays a role in diurnal BP rhythms, although further work is needed to explore these interactions directly. This has been recently reviewed by Becker et al. and highlights future directions in this area including the recognition of time-of-day which can influence renal function and autonomic control outcomes77.
Recent and ongoing chronotherapy clinical trials
Importantly, the majority (~82%) of U.S. FDA-approved drugs target the products of rhythmic genes found in the mouse13 and non-human primate14 and therefore, there may be benefits of timed dosing. Theoretically, chronotherapy has the potential to offer these benefits, although many remain skeptical. The clinical relevance of chronotherapy has been demonstrated in rheumatoid arthritis, as administration of glucocorticoid treatment at night when IL-6 levels peak has been shown to improve the pronounced joint pain seen in the morning in these patients78, reduce inflammation and improve sleep quality (reviewed in 79). Furthermore, chronotherapy clinical trials with various cancer treatments, including oxaliplatin, 5-fluorouracil, and folinic acid in metastatic colorectal cancer80, have shown to be beneficial and less toxic. Over the last decade, Hermida and colleagues have published several articles emphasizing the importance of ambulatory BP monitoring and demonstrating the cardiovascular benefits of nighttime dosing of antihypertensive drugs to patients with non-dipping hypertension81–83. A recent report of the Hygia Chronotherapy Trial (ClinicalTrials.gov, NCT00741585) has been published illustrating that routine ingestion of 1 or more antihypertensive drugs (ARB, ACEI, CCB, β-blocker, and/or diuretic) at bedtime in hypertensive patients (n=9552) improved dipper profile and reduced the occurrence of major cardiovascular events, compared with ingestion upon awaking (n=9532)84. However, limitations of this study were the use of the PROBE (prospective, randomized, open-label, blinded-end point) design, which can create a source of bias as both the treating physician and participant are aware of their assigned group. Also, participants could be prescribed any and as many medications of the antihypertensive drug classes, potentially for treatment benefit over nighttime dosing benefit. Concerns have been raised over the effect size and conduct of this trial and the European Heart Journal has reviewed and published these in the Discussion Forum in its 21 April 2020 issue, including responses from the investigators85–92. It is also worth noting that the recent HARMONY trial showed no benefits of nighttime dosing with anti-hypersensitive treatment93, see Table 1. Furthermore, some studies have shown no benefits of nighttime dosing with specific antihypertensive treatment94 or in specific ethnic groups95.
Table 1.
Recent and in progress clinical trials for chronotherapy in hypertension.
| Clinical trial | Participants details | Duration | Study design | In progress? | Results | Reference |
|---|---|---|---|---|---|---|
| Hellenic-Anglo Research into Morning or Night Antihypertensive Drug Delivery (HARMONY; NCT01669928) | 103 hypertensive patients (59 men/44 women, 61.8 ± 10.3 years of age) | 24 weeks | Patients assigned to ingest ≥ 1 hypertension medication in the morning (6AM-11AM; n=51) or in the evening (6 PM-11 PM; n=52) for 12 weeks then crossed over for remaining 12 weeks. 24 h ambulatory BP monitoring. | No | No significant differences in 24 h, daytime, or nighttime SBP between morning and evening administration of antihypertensive medication. | 93 |
| Hygia Chronotherapy (NCT00741585) |
19084 hypertensive patients (10614 men/8470 women, 60.5 ± 13.7 years of age) | 6.3 years median patient follow-up | Patients assigned to ingest ≥ 1 hypertension medication at bedtime (n=9552) or upon awakening (n=9532). 48 h ambulatory BP monitoring. | No | Reduced nighttime SBP, lower prevalence of non-dipping BP and 45% reduction in primary cardiovascular disease outcome in bedtime ingestion patients, compared with morning dosing. | 84 |
| Treatment in Morning versus Evening (TIME; UKCRN1707) | 21116 hypertensive patients | 4 years | Patients assigned to ingest ≥ 1 hypertension medication in the morning or evening. No ambulatory BP monitoring. Primary end point is hospitalization for the composite end point of non-fatal MI/stroke or vascular death. | Yes | 96 | |
| Effect of Antihypertensive Medication Timing on Morbidity and Mortality (BedMed; NCT02990663) | 3440 hypertensive patients (estimated enrollment) | 4 years | Patients assigned to ingest ≥ 1 hypertension medication in the morning or evening. | Yes | ||
| BedMed Frail (NCT02990663) | 1200 hypertensive patients who are residents in a participating long term care facility (estimated enrollment) | 2 years (estimate) | Patients assigned to ingest ≥ 1 hypertension medication in the morning or evening. | Yes |
Currently, the Treatment in Morning versus Evening (TIME) clinical study (recruitment details published in 96,97) based in the United Kingdom is in progress, involving the PROBE based design. Recruitment includes more than 20000 participants already on antihypertensive treatment instructed to take their medication either in the morning or at night over a 4-year period, with cardiovascular outcomes assessed. Although, ambulatory BP monitoring will not be reported in this trial, and therefore unlikely to provide further evidence for a benefit for chronotherapy for hypertension. In the Effect of Antihypertensive Medication Timing on Morbidity and Mortality (BedMed) trial (ClinicalTrials.gov, NCT02990663), based in Alberta Canada, hypertensive patients (estimated n=3400) are again instructed to take their antihypertensive medication either in the morning or at night over an average of a 4-year period. Interestingly, the same group of investigators are completing a similar clinical trial as above, however specifically in an elderly population of 1200 participants (BedMed-Frail, NCT04054648). The completion of both BedMed trials is due by the end of 2022. Recent and ongoing clinical trials for chronotherapy in hypertension have been summarized in Table 1.
There are studies, although with small sample sizes, that have explored morning versus evening dosing of specific antihypertensive drugs or combination antihypertensive therapy, reviewed in 98. The bulk of these studies suggested benefits of evening dosing over morning, but some highlighted benefits of morning administration or no differences with timing of dosage. These differences could relate to the specific pharmacokinetic profile of antihypertensive drugs. Therefore, the pharmacokinetics of drugs for chronotherapy need to be considered99. Previous studies have investigated the pharmacokinetic profile of antihypertensive drugs after morning or evening administration, reviewed in100. The calcium channel blocker, amlodipine, has been shown to have a shorter time to reach maximum plasma concentration (Tmax), greater mean peak plasma concentration (Cmax), and longer half-life after evening oral dosing compared with morning dosing in both normotensive and hypertensive subjects, indicating enhanced absorption of amlodipine when administered at night. This pharmacokinetic profile correlated with significant reductions in BP and HR in hypertensive patients following evening dosing101. There have also been reports highlighting sex-specific differences in pharmacokinetics of antihypertensive drugs102–104. For example, women have been shown to exhibit higher Cmax to β-blockers metoprolol and propranolol due to increased absorption and slower clearance via CYP2D6105,106.
Although these trials provide promise, uncertainty remains over the benefit of chronotherapy for hypertension, and larger trials across multiple ethnic groups with specific antihypertensive drug classes and gender differences investigated are needed to determine who will benefit from chronotherapy. If chronotherapy is not deemed to be superior to the current standard of care for hypertension, it may be beneficial in correcting loss of BP rhythm, which alone is a risk factor for cardiovascular mortality107. Overall, the optimal method to test chronotherapy in hypertension is using ambulatory BP monitoring and using drugs with half-lives that are appropriate for nighttime use.
Shift working
Shift work, defined by working hours out with the typical working time of 7 AM – 6 PM, has been associated with increased risk of hypertension, as well as cardiovascular disease and type 2 diabetes108. A meta-analysis of 27 observational studies found a significant association between shift work and hypertension, especially in male shift workers. There was no association between specifically shift work at night, i.e., the Graveyard shift, and a higher risk of hypertension however, data for this was limited109. Although, this was not the case when assessing the risk of hypertension in a cohort of 2151 workers from US shift/night workers in manufacturing facilities. Workers with mostly night work with frequent rotations had a 4-fold high risk for hypertension, with the highest rates of hypertension in individuals who worked 95–100% night work110. Regarding the risk of CKD in shift workers, the KNHANES study explored the association between shift work and CKD in both male and female manual labor daytime and shift workers (n=3504). This study illustrated female shift workers had an increased risk of CKD, with no association in male workers111. BP was measured in these individuals with no significant difference between daytime and shift workers. The BP rhythm was not assessed in these individuals. Hill et al. used a pre-clinical model to investigate how circadian disruption affects kidney function and found that shifting the light cycle to mimic shift work in male hypertensive rats disrupted rhythms in renal excretion and caused an acceleration of renal injury marker excretion112. Whether this could influence the pathogenesis of hypertension and CKD remains to be explored.
There have been limited studies on the impact of acute or long-term shift work on BP rhythm. A recent meta-analysis of 50 publications between 1980 and 2018 revealed BP dipped during the sleep period in shift workers but these studies varied widely of shift work type, shift schedules, and regularity of BP monitoring113. Further research is needed to investigate the impact of acute and long-term shift work on ambulatory BP in shift workers with and without hypertension.
Conclusion and future perspectives
Together these findings prompt many ongoing questions. Whether non-dipping BP worsens cardiorenal outcomes remains in debate, but the recent studies illustrated in this review have provided evidence for adverse outcomes in patients that have non-dipping nocturnal hypertension. This was particularly evident in CKD patients, suggesting the circadian BP profile could predict the progression of CKD. Therefore, highlighting the importance of monitoring 24-hour BP rhythm using ambulatory BP monitoring and that restoring a BP dip should be recognized as an important aspect of BP control. Although, the mechanisms behind the circadian rhythm of BP are not fully understood, what is clear is that it involves multiple organ systems, illustrated in Figure 2. A better understanding of clock function in various peripheral clocks will help pave the way for targeted therapeutics in the treatment of hypertension. Time of feeding and what we are eating, which can affect our gut microbiota composition, could have long-term impacts on our BP rhythm. A consideration for future research would be whether the timing of the consumption of specific foods would have differing effects on BP. Does a higher salt load at night have implications on non-dipping BP? Considering the findings regarding time-restricted feeding, it would be of interest to investigate whether the timing of the consumption of high salt meals at lunchtime rather than dinnertime had influences on BP. This would also be particularly relevant for shift workers where late-night eating is more common.
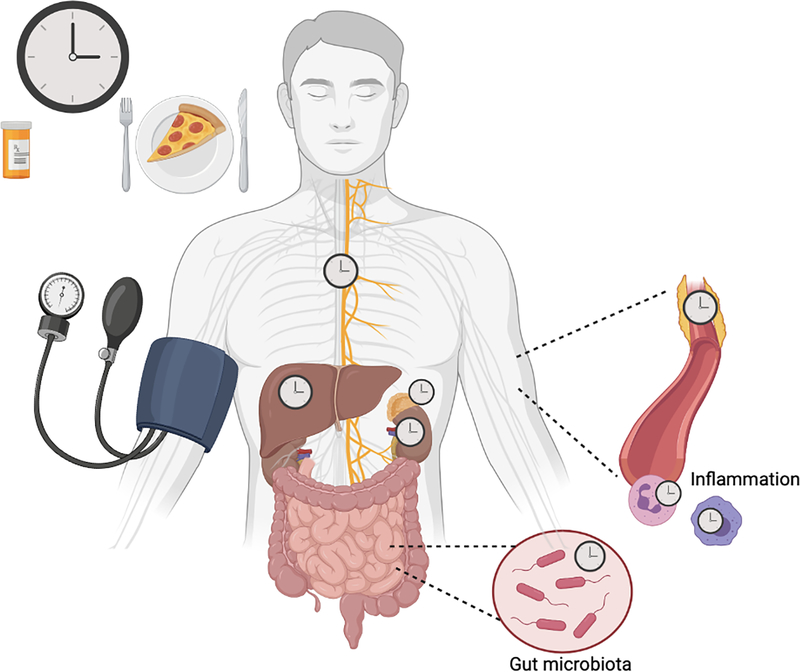
Figure 2. Cellular circadian clocks throughout the body, entrained by food cues, contributing to the circadian rhythm of blood pressure.
Blood pressure has a 24-hour cycle, peaking during the day and dipping by 10–20% during the night. Studies in rodents and humans suggest peripheral clocks within the vasculature, liver, adrenal glands, kidneys, microbiota in the gut, immune system, and autonomic nervous system contribute to regulation of the circadian rhythm of blood pressure (BP). These peripheral clocks can be entrained by food cues therefore, time-of-day feeding could be important for BP rhythm. Dysregulation of the circadian rhythm of BP is associated with adverse cardiorenal outcomes and increased risk of cardiovascular mortality. There are ongoing clinical trials to determine if chronotherapy will be beneficial for hypertension management and this should be expanded to whether it can correct any losses of BP rhythm. Diagram created with Biorender.com.
Sources of Funding:
This work was supported by R01DK109570 and American Heart Association Established Investigator Award to M.L. Gumz, R01DK123078 (M.L. Gumz co-investigator).
Footnotes
Disclosures: None
References
- 1.Authors/Task Force Members, Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Böhm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34(28):2159–2219. [DOI] [PubMed] [Google Scholar]
- 2.Whelton PK. Epidemiology of Hypertension. Lancet. 1994;344(8915):101–106. [DOI] [PubMed] [Google Scholar]
- 3.Egan BM, Kjeldsen SE, Grassi G, Esler M, Mancia G. The global burden of hypertension exceeds 1.4 billion people: should a systolic blood pressure target below 130 become the universal standard? J Hypertens. 2019;37(6):1148–1153. [DOI] [PubMed] [Google Scholar]
- 4.Douma LG, Gumz ML. Circadian clock-mediated regulation of blood pressure. Free Radic Biol Med. 2018;119:108–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giuseppe M, Gianfranco P. Ambulatory Blood Pressure Monitoring and Organ Damage. Hypertension. 2000;36(5):894–900. [DOI] [PubMed] [Google Scholar]
- 6.Clement DL, De Buyzere ML, De Bacquer DA, de Leeuw PW, Duprez DA, Fagard RH, Gheeraert PJ, Missault LH, Braun JJ, Six RO, Van Der Niepen P, O’Brien E. Prognostic Value of Ambulatory Blood-Pressure Recordings in Patients with Treated Hypertension. N Engl J Med. 2003;348(24):2407–2415. [DOI] [PubMed] [Google Scholar]
- 7.Davidson MB, Hix JK, Vidt DG, Brotman DJ. Association of impaired diurnal blood pressure variation with a subsequent decline in glomerular filtration rate. Arch Intern Med. 2006;166(8):846–852. [DOI] [PubMed] [Google Scholar]
- 8.Mojón A, Ayala DE, Piñeiro L, Otero A, Crespo JJ, Moyá A, Bóveda J, de Lis JP, Fernández JR, Hermida RC. Comparison of Ambulatory Blood Pressure Parameters of Hypertensive Patients With and Without Chronic Kidney Disease. Chronobiol Int. 2013;30(1–2):145–158. [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Staessen JA, Lu L, Li L-H, Wang G-L, Wang J-G. Is Isolated Nocturnal Hypertension a Novel Clinical Entity? Hypertension. 2007;50(2):333–339. [DOI] [PubMed] [Google Scholar]
- 10.Verdecchia P, Porcellati C, Schillaci G, Borgioni C, Ciucci A, Battistelli M, Guerrieri M, Gatteschi C, Zampi I, Santucci A, Santucci C, Reboldi G. Ambulatory blood pressure. An independent predictor of prognosis in essential hypertension. Hypertension. 1994;24(6):793–801. [DOI] [PubMed] [Google Scholar]
- 11.De La Sierra A, Gorostidi M, Banegas JR, Segura J, De La Cruz JJ, Ruilope LM. Nocturnal hypertension or nondipping: Which is better associated with the cardiovascular risk profile? Am J Hypertens. 2014;27(5):680–687. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi JS. Transcriptional architecture of the mammalian circadian clock. Nat Rev Genet. 2017;18(3):164–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB. A circadian gene expression atlas in mammals: Implications for biology and medicine. Proc Natl Acad Sci U S A. 2014;111(45):16219–16224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mure L, Le H, Benegiamo G, Chang M, Rios L, Jillani N, Ngotho M, Kariuki T, Dkhissi-Benyahya O, Cooper H, Panda S. Diurnal transcriptome atlas of a primate across major neural and peripheral tissues. Science. 2018;359(6381). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Q, Wang Y, Wang J, Zhang L, Zhao M-H. Nocturnal Systolic Hypertension and Adverse Prognosis in Patients with CKD. Clin J Am Soc Nephrol. 2021;16(3):356–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sufiun A, Rahman A, Rafiq K, Fujisawa Y, Nakano D, Kobara H, Masaki T, Nishiyama A. Association of a disrupted dipping pattern of blood pressure with progression of renal injury during the development of salt-dependent hypertension in rats. Int J Mol Sci. 2020;21(6):2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Timio M, Venanzi S, Lolli S, Lippi G, Verdura C, Monarca C, Guerrini E. ‘Non-dipper’ hypertensive patients and progressive renal insufficiency: a 3-year longitudinal study. Clin Nephrol. 1995;43(6):382–387. [PubMed] [Google Scholar]
- 18.O’Brien E, Parati G, Stergiou G. Ambulatory Blood Pressure Measurement. Hypertension. 2013;62(6):988–994. [DOI] [PubMed] [Google Scholar]
- 19.Savard S, Amar L, Plouin P, Steichen O. Cardiovascular Complications Associated With Primary Aldosteronism. Hypertension. 2013;62(2):331–336. [DOI] [PubMed] [Google Scholar]
- 20.Wu Q, Hong M, Xu J, Tang X, Zhu L, Gao P, Wang J. Diurnal blood pressure pattern and cardiac damage in hypertensive patients with primary aldosteronism. Endocrine. 2021;72(3):835–843. [DOI] [PubMed] [Google Scholar]
- 21.Lopez-Sublet M, Girerd N, Bozec E, Machu JL, Ferreira JP, Zannad F, Mourad JJ, Rossignol P. Nondipping Pattern and Cardiovascular and Renal Damage in a Population-Based Study (The STANISLAS Cohort Study). Am J Hypertens. 2019;32(7):620–628. [DOI] [PubMed] [Google Scholar]
- 22.Redon J, Plancha E, Swift PA, Pons S, Muñoz J, Martinez F. Nocturnal blood pressure and progression to end-stage renal disease or death in nondiabetic chronic kidney disease stages 3 and 4. J Hypertens. 2010;28(3):602–607. [DOI] [PubMed] [Google Scholar]
- 23.Minutolo R, Agarwal R, Borrelli S, Chiodini P, Bellizzi V, Nappi F, Cianciaruso B, Zamboli P, Conte G, Gabbai FB, De Nicola L. Prognostic Role of Ambulatory Blood Pressure Measurement in Patients With Nondialysis Chronic Kidney Disease. Arch Intern Med. 2011;171(12):1090–1098. [DOI] [PubMed] [Google Scholar]
- 24.Fagard RH, Celis H, Thijs L, Staessen J, Clement D, De Buyzere M, De Bacquer D. Daytime and Nighttime Blood Pressure as Predictors of Death and Cause-Specific Cardiovascular Events in Hypertension. Hypertension. 2008;51(1):55–61. [DOI] [PubMed] [Google Scholar]
- 25.Salles G, Reboldi G, Fagard R, Cardoso C, Pierdomenico S, Verdecchia P, Eguchi K, Kario K, Hoshide S, Polonia J, de la Sierra A, Hermida R, Dolan E, O’Brien E, Roush G. Prognostic Effect of the Nocturnal Blood Pressure Fall in Hypertensive Patients. Hypertension. 2016;67(4):693–700. [DOI] [PubMed] [Google Scholar]
- 26.Hansen T, Li Y, Boggia J, Thijs L, Richart T, Staessen J. Predictive Role of the Nighttime Blood Pressure. Hypertension. 2011;57(1):3–10. [DOI] [PubMed] [Google Scholar]
- 27.Curtis AM, Cheng Y, Kapoor S, Reilly D, Price TS, Fitzgerald GA. Circadian variation of blood pressure and the vascular response to asynchronous stress. Proc Natl Acad Sci U S A. 2007;104(9):3450–3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saxton SN, Clark BJ, Withers SB, Eringa EC, Heagerty AM. Mechanistic Links Between Obesity, Diabetes, and Blood Pressure: Role of Perivascular Adipose Tissue. Physiol Rev. 2019;99(4):1701–1763. [DOI] [PubMed] [Google Scholar]
- 29.Chang L, Garcia-Barrio MT, Chen YE. Perivascular Adipose Tissue Regulates Vascular Function by Targeting Vascular Smooth Muscle Cells. Arterioscler Thromb Vasc Biol. 2020;40(5):1094–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang L, Xiong W, Zhao X, Fan Y, Guo Y, Garcia-Barrio M, Zhang J, Jiang Z, Lin J, Chen Y. Bmal1 in perivascular adipose tissue regulates resting phase blood pressure through transcriptional regulation of angiotensinogen. Circulation. 2018;138(1):67–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xia N, Li H. The role of perivascular adipose tissue in obesity-induced vascular dysfunction. Br J Pharmacol. 2017;174(20):3425–3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gorostidi M, Sobrino J, Segura J, Sierra C, de la Sierra Á, Hernández del Rey R, Vinyoles E, Galcerán JM, López-Eady MD, Marín R, Banegas JR, Sarría A, Coca A, Ruilope LM, Investigators on behalf of the SS of HAR. Ambulatory blood pressure monitoring in hypertensive patients with high cardiovascular risk: a cross-sectional analysis of a 20 000-patient database in Spain. J Hypertens. 2007;25(5):977–984. [DOI] [PubMed] [Google Scholar]
- 33.Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. 2010;330(6009):1349–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Su W, Guo Z, Randall DC, Cassis L, Brown DR, Gong MC. Hypertension and disrupted blood pressure circadian rhythm in Type 2 diabetic db/db mice. Am J Physiol Circ Physiol. 2008;295(4):H1634–H1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hou T, Su W, Guo Z, Gong MC. A novel diabetic mouse model for real-time monitoring of clock gene oscillation and blood pressure circadian rhythm Tianfei. J Biol Rhythm. 2019;34(1):51–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pati P, Valcin JA, Zhang D, Neder TH, Millender-Swain T, Allan JM, Sedaka RS, Jin C, Becker BK, Pollock DM, Bailey SM, Pollock JS. Liver circadian clock disruption alters perivascular adipose tissue gene expression and aortic function in mice. Am J Physiol Integr Comp Physiol. 2021;320:R960–R971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doi M, Takahashi Y, Komatsu R, Yamazaki F, Yamada H, Haraguchi S, Emoto N, Okuno Y, Tsujimoto G, Kanematsu A, Ogawa O, Todo T, Tsutsui K, Van Der Horst GTJ, Okamura H. Salt-sensitive hypertension in circadian clock-deficient Cry-null mice involves dysregulated adrenal Hsd3b6. Nat Med. 2010;16(1):67–74. [DOI] [PubMed] [Google Scholar]
- 38.Tanaka S, Ueno T, Tsunemi A, Nagura C, Tahira K, Fukuda N, Soma M, Abe M. The adrenal gland circadian clock exhibits a distinct phase advance in spontaneously hypertensive rats. Hypertens Res. 2019;42(2):165–173. [DOI] [PubMed] [Google Scholar]
- 39.Ivy JR, Oosthuyzen W, Peltz TS, Howarth AR, Hunter RW, Dhaun N, Al-Dujaili EAS, Webb DJ, Dear JW, Flatman PW, Bailey MA. Glucocorticoids induce nondipping blood pressure by activating the thiazide-sensitive cotransporter. Hypertension. 2016;67(5):1029–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ivy JR, Bailey MA. Nondipping Blood Pressure: Predictive or Reactive Failure of Renal Sodium Handling? Physiology. 2020;36(1):21–34. [DOI] [PubMed] [Google Scholar]
- 41.Xie Z, Su W, Liu S, Zhao G, Esser K, Schroder EA, Lefta M, Stauss HM, Guo Z, Gong MC. Smooth-muscle BMAL1 participates in blood pressure circadian rhythm regulation. J Clin Invest. 2015;125(1):324–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang D, Jin C, Obi IE, Rhoads MK, Soliman RH, Sedaka RS, Allan JM, Tao B, Speed JS, Pollock JS, Pollock DM. Loss of circadian gene Bmal1 in the collecting duct lowers blood pressure in male, but not female, mice. Am J Physiol Physiol. 2020;318(3):F710–F719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Crislip GR, Douma LG, Masten SH, Cheng K-Y, Lynch IJ, Johnston JG, Barral D, Glasford KB, Holzworth MR, Verlander JW, Wingo CS, Gumz ML. Differences in renal BMAL1 contribution to Na+ homeostasis and blood pressure control in male and female mice. Am J Physiol Physiol. 2020;318(6):F1463–F1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Douma LG, Solocinski K, Holzworth MR, Crislip GR, Masten SH, Miller AH, Cheng K-Y, Lynch IJ, Cain BD, Wingo CS, Gumz ML. Female C57BL/6J mice lacking the circadian clock protein PER1 are protected from nondipping hypertension. Am J Physiol Integr Comp Physiol. 2018;316(1):R50–R58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sherwood A, Thurston R, Steffen P, Blumenthal JA, Waugh RA, Hinderliter AL. Blunted nighttime blood pressure dipping in postmenopausal women*. Am J Hypertens. 2001;14(8):749–754. [DOI] [PubMed] [Google Scholar]
- 46.Perez-Lloret S, Toblli JE, Cardinali DP, Milei J. Gender differences in age-related increase of asleep blood pressure. Arch Gerontol Geriatr. 2010;50(3):319–322. [DOI] [PubMed] [Google Scholar]
- 47.Muntner P, Carey RM, Gidding S, Jones DW, Taler SJ, Wright JT, Whelton PK. Potential US Population Impact of the 2017 ACC/AHA High Blood Pressure Guideline. Circulation. 2018;137(2):109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Routledge FS, McFetridge-Durdle JA, Dean CR. Stress, menopausal status and nocturnal blood pressure dipping patterns among hypertensive women. Can J Cardiol. 2009;25(6):e157–e163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Murata Y, Ueno T, Tanaka S, Kobayashi H, Okamura M, Hemmi S, Fuke Y, Matsumoto Y, Abe M, Fukuda N. Identification of Clock Genes Related to Hypertension in Kidney from Spontaneously Hypertensive Rats. Am J Hypertens. 2020;33(12):1136–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kurtz TW, Morris RC. Biological variability in Wistar-Kyoto rats. Implications for research with the spontaneously hypertensive rat. Hypertension. 1987;10(1):127–131. [DOI] [PubMed] [Google Scholar]
- 51.Partch C, Green C, Takahashi J. Molecular Architecture of the Mammalian Circadian Clock Carrie. Trends Cell Biol. 2014;24(2):90–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang D, Colson JC, Jin C, Becker BK, Rhoads MK, Pati P, Neder TH, King MA, Valcin JA, Tao B, Kasztan M, Paul JR, Bailey SM, Pollock JS, Gamble KL, Pollock DM. Timing of Food Intake Drives the Circadian Rhythm of Blood Pressure. Function. 2021;2(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Damiola F, Le Minli N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000;14(23):2950–2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Johnston JG, Speed JS, Becker BK, Kasztan M, Soliman RH, Rhoads MK, Tao B, Jin C, Geurts AM, Hyndman KA, Pollock JS, Pollock DM. Diurnal Control of Blood Pressure Is Uncoupled from Sodium Excretion. Hypertension. 2020;75(6):1624–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nakanishi-Minami T, Kishida K, Funahashi T, Shimomura I. Sleep-wake cycle irregularities in type 2 diabetics. Diabetol Metab Syndr. 2012;4(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hou T, Wang C, Joshi S, O’Hara BF, Gong MC, Guo Z. Active Time-Restricted Feeding Improved Sleep-Wake Cycle in db/db Mice. Front Neurosci. 2019;13(September):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sutton E, Beyl R, Early K, Cefalu W, Ravussin E, Peterson C. Early Time-Restricted Feeding Improves Insulin Sensitivity, Blood Pressure, and Oxidative Stress Even Without Weight Loss in Men with Prediabetes. Cell Metab. 2018;27(6):1212–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wilkinson MJ, Manoogian ENC, Zadourian A, Lo H, Fakhouri S, Shoghi A, Wang X, Fleischer JG, Navlakha S, Panda S, Taub PR. Ten-Hour Time-Restricted Eating Reduces Weight, Blood Pressure, and Atherogenic Lipids in Patients with Metabolic Syndrome. Cell Metab. 2020;31(1):92–104.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stote KS, Baer DJ, Spears K, Paul DR, Harris GK, Rumpler W V, Strycula P, Najjar SS, Ferrucci L, Ingram DK, Longo DL, Mattson MP. A controlled trial of reduced meal frequency without caloric restriction in healthy, normal-weight, middle-aged adults. Am J Clin Nutr. 2007;85(4):981–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Murakami M, Tognini P. The Circadian Clock as an Essential Molecular Link Between Host Physiology and Microorganisms. Front Cell Infect Microbiol. 2020;9:469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zarrinpar A, Chaix A, Yooseph S, Panda S. Diet and Feeding Pattern Affect the Diurnal Dynamics of the Gut Microbiome. Cell Metab. 2014;20(6):1006–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thaiss CA, Zeevi D, Levy M, Zilberman-Schapira G, Suez J, Tengeler AC, Abramson L, Katz MN, Korem T, Zmora N, Kuperman Y, Biton I, Gilad S, Harmelin A, Shapiro H, Halpern Z et al. Transkingdom Control of Microbiota Diurnal Oscillations Promotes Metabolic Homeostasis. Cell. 2014;159(3):514–529. [DOI] [PubMed] [Google Scholar]
- 63.Liang X, Bushman FD, FitzGerald GA. Rhythmicity of the intestinal microbiota is regulated by gender and the host circadian clock. Proc Natl Acad Sci. 2015;112(33):10479 LP–10484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Adnan S, Nelson JW, Ajami NJ, Venna VR, Petrosino JF, Bryan RM, Durgan DJ. Alterations in the gut microbiota can elicit hypertension in rats. Physiol Genomics. 2017;49(2):96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chakraborty S, Mandal J, Cheng X, Galla S, Hindupur A, Saha P, Yeoh BS, Mell B, Yeo JY, Vijay-Kumar M, Yang T, Joe B. Diurnal Timing Dependent Alterations in Gut Microbial Composition Are Synchronously Linked to Salt-Sensitive Hypertension and Renal Damage. Hypertension. 2020;76(1):59–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Scheiermann C, Kunisaki Y, Frenette PS. Circadian control of the immune system. Nat Rev Immunol. 2013;13(3):190–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Carlson DE, Chiu WC. THE ABSENCE OF CIRCADIAN CUES DURING RECOVERY FROM SEPSIS MODIFIES PITUITARY-ADRENOCORTICAL FUNCTION AND IMPAIRS SURVIVAL. Shock. 2008;29(1):127–132. [DOI] [PubMed] [Google Scholar]
- 68.Narasimamurthy R, Hatori M, Nayak SK, Liu F, Panda S, Verma IM. Circadian clock protein cryptochrome regulates the expression of proinflammatory cytokines. Proc Natl Acad Sci. 2012;109(31):12662–12667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fu L, Pelicano H, Liu J, Huang P, Lee CC. The Circadian Gene Period2 Plays an Important Role in Tumor Suppression and DNA Damage Response In Vivo. Cell. 2002;111(1):41–50. [DOI] [PubMed] [Google Scholar]
- 70.Drummond GR, Vinh A, Guzik TJ, Sobey CG. Immune mechanisms of hypertension. Nat Rev Immunol. 2019;19(8):517–532. [DOI] [PubMed] [Google Scholar]
- 71.Yang G, Zhang J, Jiang T, Monslow J, Tang SY, Todd L, Puré E, Chen L, Fitzgerald GA. Bmal1 Deletion in Myeloid Cells Attenuates Atherosclerotic Lesion Development and Restrains Abdominal Aortic Aneurysm Formation in Hyperlipidemic Mice. Arterioscler Thromb Vasc Biol. 2020;(June):1523–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kohara K, Nishida W, Maguchi M, Hiwada K. Autonomic Nervous Function in Non-dipper Essential Hypertensive Subjects. Hypertension. 1995;26(5):808–814. [DOI] [PubMed] [Google Scholar]
- 73.Sherwood A, Steffen PR, Blumenthal JA, Kuhn C, Hinderliter AL. Nighttime blood pressure dipping: the role of the sympathetic nervous system*. Am J Hypertens. 2002;15(2):111–118. [DOI] [PubMed] [Google Scholar]
- 74.Grassi G, Seravalle G, Quarti-Trevano F, Dell’Oro R, Bombelli M, Cuspidi C, Facchetti R, Bolla G, Mancia G. Adrenergic, Metabolic, and Reflex Abnormalities in Reverse and Extreme Dipper Hypertensives. Hypertension. 2008;52(5):925–931. [DOI] [PubMed] [Google Scholar]
- 75.Mann S, Altman DG, Raftery EB, Bannister R. Circadian variation of blood pressure in autonomic failure. Circulation. 1983;68(3 I):477–483. [DOI] [PubMed] [Google Scholar]
- 76.Johns EJ, Kopp UC, DiBona GF. Neural Control of Renal Function. Compr. Physiol. 2011;:731–767. [DOI] [PubMed] [Google Scholar]
- 77.Becker BK, Zhang D, Soliman R, Pollock DM. Autonomic nerves and circadian control of renal function. Auton Neurosci. 2019;217:58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Arvidson NG, Gudbjörnsson B, Larsson A, Hällgren R. The timing of glucocorticoid administration in rheumatoid arthritis. Ann Rheum Dis. 1997;56(1):27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Buttgereit F, Smolen JS, Coogan AN, Cajochen C. Clocking in: chronobiology in rheumatoid arthritis. Nat Rev Rheumatol. 2015;11(6):349–356. [DOI] [PubMed] [Google Scholar]
- 80.Lévi F, Zidani R, Misset J-L. Randomised multicentre trial of chronotherapy with oxaliplatin, fluorouracil, and folinic acid in metastatic colorectal cancer. Lancet. 1997;350(9079):681–686. [DOI] [PubMed] [Google Scholar]
- 81.Ayala DE, Hermida RC, Mojón A, Fernández JR. Cardiovascular Risk of Resistant Hypertension: Dependence on Treatment-Time Regimen of Blood Pressure–Lowering Medications. Chronobiol Int. 2013;30(1–2):340–352. [DOI] [PubMed] [Google Scholar]
- 82.Hermida RC, Ayala DE, Mojón A, Fernández JR. Decreasing Sleep-Time Blood Pressure Determined by Ambulatory Monitoring Reduces Cardiovascular Risk. J Am Coll Cardiol. 2011;58(11):1165–1173. [DOI] [PubMed] [Google Scholar]
- 83.Hermida RC, Smolensky MH, Ayala DE, Portaluppi F, Crespo JJ, Fabbian F, Haus E, Manfredini R, Mojón A, Moyá A, Piñeiro L, Ríos MT, Otero A, Balan H, Fernández JR. 2013 Ambulatory Blood Pressure Monitoring Recommendations for the Diagnosis of Adult Hypertension, Assessment of Cardiovascular and other Hypertension-associated Risk, and Attainment of Therapeutic Goals. Chronobiol Int. 2013;30(3):355–410. [DOI] [PubMed] [Google Scholar]
- 84.Hermida RC, Crespo JJ, Domínguez-Sardiña M, Otero A, Moyá A, Ríos MT, Sineiro E, Castiñeira MC, Callejas PA, Pousa L, Salgado JL, Durán C, Sánchez JJ, Fernández JR, Mojón A, Ayala DE et al. Bedtime hypertension treatment improves cardiovascular risk reduction: the Hygia Chronotherapy Trial. Eur Heart J. 2020;41(48):4565–4576. [DOI] [PubMed] [Google Scholar]
- 85.Guthrie G, Poulter (Neil, Macdonald T, Ford I, Mackenzie I, Findlay E, Williams B, Brown M, Lang C, Webb D. Chronotherapy in hypertension: the devil is in the details. Eur Heart J. 2020;41(16):1606–1607. [DOI] [PubMed] [Google Scholar]
- 86.Relates to: ‘Bedtime Hypertension Treatment Improves Cardiovascular Risk Reduction: Hygia Chronotherapy Trial’. Eur Heart J. 2020;41(16):1600. [DOI] [PubMed] [Google Scholar]
- 87.Lüscher TF. The Hygia trial: Discussions about surprising results. Eur Heart J. 2020;41(16):1600. [DOI] [PubMed] [Google Scholar]
- 88.Sánchez-Sánchez C, López-Caballero C, Contreras I, Puerto B, Blazquez-Bermejo Z. Is bedtime treatment appropriate for all hypertensive patients? Eur Heart J. 2020;41(16):1604. [DOI] [PubMed] [Google Scholar]
- 89.Şen S, Kaşkal M, Üresin Y. Chrono-pharmacological effects of antihypertensive drugs. Eur Heart J. 2020;41(16):1601. [DOI] [PubMed] [Google Scholar]
- 90.Hermida RC, Mojón A, Fernández JR, Investigators for the HP. Comparing the design of the primary-care based Hygia Chronotherapy Trial and the Internet-Based TIME Study. Eur Heart J. 2020;41(16):1608. [DOI] [PubMed] [Google Scholar]
- 91.Hermida RC, Fernández JR, Mojón A, Investigators for the HP. Chronotherapy of hypertension, asleep ambulatory blood pressure, and glaucoma. Eur Heart J. 2020;41(16):1605. [DOI] [PubMed] [Google Scholar]
- 92.Hermida RC, Crespo JJ, Domínguez-Sardiña M, Investigators for the HCT. Improved reduction of cardiovascular risk by bedtime ingestion of ARB and ACEI medication class therapies. Eur Heart J. 2020;41(16):1602–1603. [DOI] [PubMed] [Google Scholar]
- 93.Poulter NR, Savopoulos C, Anjum A, Apostolopoulou M, Chapman N, Cross M, Falaschetti E, Fotiadis S, James RM, Kanellos I, Szigeti M, Thom S, Sever P, Thompson D, Hatzitolios AI. Randomized Crossover Trial of the Impact of Morning or Evening Dosing of Antihypertensive Agents on 24-Hour Ambulatory Blood Pressure. Hypertension. 2018;72(4):870–873. [DOI] [PubMed] [Google Scholar]
- 94.Asmar R, Gosse P, Queré S, Achouba A. Efficacy of morning and evening dosing of amlodipine/valsartan combination in hypertensive patients uncontrolled by 5 mg of amlodipine. Blood Press Monit. 2011;16(2):80–86. [DOI] [PubMed] [Google Scholar]
- 95.Rahman M, Greene T, Phillips RA, Agodoa LY, Bakris GL, Charleston J, Contreras G, Gabbai F, Hiremath L, Jamerson K, Kendrick C, Kusek JW, Lash JP, Lea J, Miller ER, Rostand S et al. A trial of 2 strategies to reduce nocturnal blood pressure in blacks with chronic kidney disease. Hypertens (Dallas, Tex 1979). 2013;61(1):82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rorie DA, Rogers A, Mackenzie IS, Ford I, Webb DJ, Willams B, Brown M, Poulter N, Findlay E, Saywood W, MacDonald TM. Methods of a large prospective, randomised, open-label, blinded end-point study comparing morning versus evening dosing in hypertensive patients: the Treatment In Morning versus Evening (TIME) study. BMJ Open. 2016;6(2):e010313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rorie DA, Flynn RWV, Mackenzie IS, MacDonald TM, Rogers A. The Treatment In Morning versus Evening (TIME) study: analysis of recruitment, follow-up and retention rates post-recruitment. Trials. 2017;18(1):557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.De Giorgi A, Mallozzi Menegatti A, Fabbian F, Portaluppi F, Manfredini R. Circadian rhythms and medical diseases: Does it matter when drugs are taken? Eur J Intern Med. 2013;24(8):698–706. [DOI] [PubMed] [Google Scholar]
- 99.Dong D, Yang D, Lin L, Wang S, Wu B. Circadian rhythm in pharmacokinetics and its relevance to chronotherapy. Biochem Pharmacol. 2020;178:114045. [DOI] [PubMed] [Google Scholar]
- 100.Hermida RC, Ayala DE, Calvo C, Portaluppi F, Smolensky MH. Chronotherapy of hypertension: Administration-time-dependent effects of treatment on the circadian pattern of blood pressure. Adv Drug Deliv Rev. 2007;59(9):923–939. [DOI] [PubMed] [Google Scholar]
- 101.Khodadoustan S, Nasri Ashrafi I, Vanaja Satheesh K, Kumar C, HS S, S C. Evaluation of the effect of time dependent dosing on pharmacokinetic and pharmacodynamics of amlodipine in normotensive and hypertensive human subjects. Clin Exp Hypertens. 2017;39(6):520–526. [DOI] [PubMed] [Google Scholar]
- 102.Ueno K, Sato H. Sex-related differences in pharmacokinetics and pharmacodynamics of anti-hypertensive drugs. Hypertens Res. 2012;35(3):245–250. [DOI] [PubMed] [Google Scholar]
- 103.Tamargo J, Rosano G, Walther T, Duarte J, Niessner A, Kaski JC, Ceconi C, Drexel H, Kjeldsen K, Savarese G, Torp-Pedersen C, Atar D, Lewis BS, Agewall S. Gender differences in the effects of cardiovascular drugs. Eur Hear J - Cardiovasc Pharmacother. 2017;3(3):163–182. [DOI] [PubMed] [Google Scholar]
- 104.Kalibala J, Pechère-Bertschi A, Desmeules J. Gender Differences in Cardiovascular Pharmacotherapy—the Example of Hypertension: A Mini Review. Front Pharmacol. 2020;11:564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Luzier AB, Killian A, Wilton JH, Wilson MF, Forrest A, Kazierad DJ. Gender-related effects on metoprolol pharmacokinetics and pharmacodynamics in healthy volunteers. Clin Pharmacol Ther. 1999;66(6):594–601. [DOI] [PubMed] [Google Scholar]
- 106.Walle T, Walle UK, Cowart TD, Conradi EC. Pathway-selective sex differences in the metabolic clearance of propranolol in human subjects. Clin Pharmacol Ther. 1989;46(3):257–263. [DOI] [PubMed] [Google Scholar]
- 107.Ohkubo T, Hozawa A, Yamaguchi J, Kikuya M, Ohmori K, Michimata M, Matsubara M, Hashimoto J, Hoshi H, Araki T, Tsuji I, Satoh H, Hisamichi S, Imai Y. Prognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24-h blood pressure: the Ohasama study. J Hypertens. 2002;20(11):2183–2189. [DOI] [PubMed] [Google Scholar]
- 108.Puttonen S, Härmä M, Hublin C. Shift work and cardiovascular disease – pathways from circadian stress to morbidity. Scand J Work Environ Health. 2010;(2):96–108. [DOI] [PubMed] [Google Scholar]
- 109.Manohar S, Thongprayoon C, Cheungpasitporn W, Mao MA, Herrmann SM. Associations of rotational shift work and night shift status with hypertension: A systematic review and meta-analysis. J Hypertens. 2017;35(10):1929–1937. [DOI] [PubMed] [Google Scholar]
- 110.Ferguson JM, Costello S, Neophytou AM, Balmes JR, Bradshaw PT, Cullen MR, Eisen EA. Night and rotational work exposure within the last 12 months and risk of incident hypertension. Scand J Work Environ Heal. 2019;45(3):256–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Uhm JY, Kim HR, Kang GH, Choi YG, Park TH, Kim SY, Chang SS, Choo WO. The association between shift work and chronic kidney disease in manual labor workers using data from the Korea National Health and Nutrition Examination Survey (KNHANES 2011–2014). Ann Occup Environ Med. 2018;30(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hill AM, Crislip GR, Stowie A, Ellis I, Ramsey A, Castanon-Cervantes O, Gumz ML, Davidson AJ. Environmental circadian disruption suppresses rhythms in kidney function and accelerates excretion of renal injury markers in urine of male hypertensive rats. Am J Physiol Physiol. 2021;320(2):F224–F233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Patterson PD, Mountz KA, Budd CT, Bubb JL, Hsin AU, Weaver MD, Turner RL, Platt TE, Guyette FX, Martin-Gill C, Buysse DJ, Callaway CW. Impact of shift work on blood pressure among emergency medical services clinicians and related shift workers: A systematic review and meta-analysis. Sleep Heal. 2020;6(3):387–398. [DOI] [PubMed] [Google Scholar]