Introduction
Disease-causing sequence variants in USH2A are the most common cause of Usher syndrome type 2 (USH2, a syndromic form of retinitis pigmentosa (RP) with congenital, mild to moderate hearing loss), the commonest cause of combined dual sensory impairment.1,2 Moreover, USH2A variants are also the commonest cause of autosomal recessive non-syndromic RP (ARRP, isolated RP with normal hearing at birth).1,3–7 Retinal degeneration associated with sequence variants in USH2A is characterized by slowly progressive rod, then cone, photoreceptor dysfunction and eventual photoreceptor death, resulting in escalating vision loss. It appears the combination of USH2A variants explains whether one has USH2 or non-syndromic RP.8–10 Retinal degeneration is more severe in patients with USH2 than USH2A-related non-syndromic RP.9 However, the reason is not clearly understood9 especially since there are many single variants in USH2A that have been associated with both Usher syndrome type 2 and non-syndromic RP.3,8 Therefore, the suggestion that retinal degeneration is more severe in patients with USH2 than USH2A-related non-syndromic RP may relate to other genetic modifiers and/or environmental influences.9 As new treatments for USH2A-related retinal degeneration are under development or in early clinical trials,11,12 a comprehensive understanding of the natural history of disease progression of USH2A-related retinal degeneration is essential.
Limited natural history data are available from patients with USH2A-related retinal degeneration. In general, the natural history studies to date reporting manual kinetic perimetry (KP) included USH2 patients not genetically characterized.13–16 None of the prior studies included longitudinal characterization of the retinal phenotype using current standard assessments, such as quantitative static perimetry (SP) employing the volumetric measure of the hill of vision (HOV).17 Previous studies were mostly retrospective with variable research approaches, such as visual acuity (VA) according to the Early Treatment of Diabetic Retinopathy Study (ETDRS) protocol,18 either within or across clinical centers. We do not know which structural/functional parameters provide sensitive and reliable outcome measures that reflect change and could be used to monitor progression or treatment effectiveness.
Because USH2A-related retinal degeneration is the commonest cause of USH2 and non-syndromic autosomal recessive RP, a multicenter, international, longitudinal natural history study of participants with retinal degeneration associated with USH2A sequence variants, the Rate of Progression of USH2A-related Retinal Degeneration (RUSH2A) study, was undertaken. The primary objective of the RUSH2A study was to characterize the natural history of retinal degeneration associated with USH2A biallelic disease-causing sequence variants over 4 years, using functional, structural, and patient-reported outcome measures, with the goal of identifying outcome measures that can be used to monitor disease progression and treatment response. Secondary study objectives included the evaluation of variability and possible risk factors (genotype, phenotype, environmental, and comorbidities) for progression of these outcome measures.
This report aims to: (1) describe the RUSH2A study design and methods, (2) summarize the baseline characteristics of the enrolled participants, including differences between those with USH2 and those with non-syndromic ARRP, and (3) summarize results of baseline visual fields, including the repeatability of the HOV derived from SP, and the relationships of clinical characteristics and other functional and structural measures with baseline HOV.
Methods
Study Design
This multicenter, longitudinal, international natural history study enrolled participants at 16 clinical sites in Canada, France, Germany, the United Kingdom, and the United States (US), adhering to the tenets of the Declaration of Helsinki and was approved by the ethics boards associated with each participating site. Informed consent was obtained from all participants. The RUSH2A protocol is listed on www.clinicaltrials.gov (NCT03146078), with registration completed prior to enrolling the first participant.
Eligibility criteria and genetic screening.
The inclusion and exclusion criteria are listed in e-Table 1. Participants were at least 8 years old with rod-cone degeneration associated with at least 2 disease-causing sequence variants in USH2A, based on existing genetic reports from Clinical Laboratory Improvement Amendments (CLIA)-certified laboratories (or equivalent, in non-US countries). Following initial eligibility assessment and enrollment, some participants without a history of hearing loss and presumed non-syndromic ARRP for whom the phase of alleles was unknown underwent additional genetic testing of first-degree relatives to confirm that inheritance of the mutant alleles was in trans. Ultimately, participants with (1) USH2 or (2) ARRP with either homozygous or compound heterozygous USH2A variants inherited in trans were enrolled into this natural history study. After enrollment, an independent audiologist reviewed both the history of hearing loss and the results of baseline audiology exams distinguishing USH2 from ARRP.
Study cohorts and sample size.
This study included two cohorts, one with vision of ETDRS letter score of 54 or more and one of ETDRS letter score of 53 or less. Due to the expected high degree of symmetry of retinal disease between eyes,14,19 most of the testing was performed in one “study eye” designated for each participant. The study eye was the eye with better baseline visual acuity. If both eyes had the same baseline visual acuity, the designation was made at investigator discretion as the eye with more stable fixation or clearer ocular media to permit ophthalmic imaging. The primary cohort included participants with study eye baseline ETDRS letter score of 54 or more (Snellen equivalent 20/80 or better) and stable fixation. Participants in the primary cohort were expected to have further deterioration in vision that could be measured reliably and will be followed in a longitudinal natural history study. A sample size of 100 for the primary cohort was selected to provide a 95% confidence interval half-width of approximately 4% for percentage change over 4 years in visual field area, assuming a mean decrease of 25% with a standard deviation (SD) of 20%.9,20 The study was also designed to enroll a secondary cohort of 20 participants with study eye baseline ETDRS letter score of 53 or less (Snellen equivalent 20/100 or worse), central visual field of less than 10 degrees diameter, or unstable fixation to complete a baseline visit only. The purpose of the secondary cohort was to obtain cross-sectional data on participants having disease spanning the full range of severity.
Visit schedule and testing procedures.
All participants completed a baseline visit. Primary cohort participants will return annually for visits through 4 years. The visit schedule and testing procedures are detailed in e-Table 2. In brief, in addition to medical history and demographic data, the RUSH2A study collected auditory and olfactory data at baseline to evaluate these as risk factors associated with baseline disease severity and progression of retinal degeneration based on all of the outcome measures, over the 4 year study duration. Visual function testing at baseline and follow up in the primary cohort included best-corrected visual acuity (BCVA), SP, fundus-guided microperimetry (MP), KP, full-field electroretinogram (ERG), and full-field stimulus threshold (FST) measures. Retinal structure was assessed using spectral-domain optical coherence tomography (SD-OCT) in all participants. All testing procedures were performed according to standardized procedures by study-certified technicians as noted in e-Table 2. Patient-reported outcomes were collected using the Veterans Affairs Low Vision Visual Functioning Questionnaire (VALVVFQ-48) in adults at least 18 years old and the L.V. Prasad-Functional Vision Questionnaire (LVP-FVQ-II) in children <18 years old. Adverse events and medications were collected for the study with the objective to provide historical control data for future clinical trials.
Outcome measures.
The RUSH2A study aims to evaluate progression of several main outcome measures over 4 years: (1) SP total HOV (VTOT, decibel-steradian (dB-sr)), (e-Figure 1A) graded by the Casey Reading Center (CRC; Casey Eye Institute, Oregon Health Sciences University, Portland, OR); (2) seeing area measured by KP using I4e, III4e, and V4e isopter targets, graded by CRC; (3) mean retinal sensitivity measured by MP, graded by the Duke Reading Center (DRC; Duke University, Durham, NC); (4) BCVA using the ETDRS protocol; (5) Ellipsoid Zone (EZ) area measured on SD-OCT, graded by DRC; (6) rod- and cone-mediated retinal sensitivity as measured by FST; and (7) retinal function measured with full-field rod and cone-mediated ERGs. DRC also graded SD-OCTs for central subfield thickness (CST) within the center 1mm and presence of intraretinal cysts defined as round or oval cavities within the retinal layers as additional measures of retinal structure. The primary focus of this paper is to characterize the baseline visual fields in detail; future papers will characterize the remaining outcome measures, (3) through (7) listed above.
Perimetry methods.
Full-field automated SP was performed on the Octopus 900 (Haag-Streit, Mason, Ohio) with a custom grid, using the German Adaptive Thresholding Estimation (GATE)21,22 strategy and a custom “RP 185 point” centrally condensed radial grid extending 65° nasally and superiorly, 67° inferiorly, and 80° temporally with a size V stimulus size (e-Figure 1A). Any participants found to have no measurable vision outside of 25 degrees at baseline were intended to be tested with only the central 30-degree grid (V30) at subsequent visits, but V30 was analyzed from the full grid for all participants at baseline (e-Figure 1B). Historical measures of SP are limited in the number of locations that can be tested in a reasonable time, but the full-threshold testing algorithm employed by the GATE strategy permits testing more locations over a shorter time, and is also better designed to identify and monitor visual field defects due to retinal disease compared to other algorithms, e.g. Swedish Interactive Thresholding Algorithm (SITA).21 Topographic analysis of SP using an approach called Visual Field Modeling and Analysis (VFMA) produces the three-dimensional, quantitative surface models of VTOT.22,23 The volume (in unit dB-sr) beneath the surface of the thin-plate spline representation of the HOV and within the external boundary of the grid was quantified (VTOT). The reliability factor (RF) measured subject performance as the sum of false positive and false negative answers divided by the total number of trial questions. False negative responses contribute more to the RF measure in patients with low retinal sensitivity due to RP.24 For the evaluation of KP, the Octopus perimetry EyeSuite software calculated areas in degree2 for each isopter automatically. Test vectors originating 10° outside the age-correlated normal isopter were presented every 15° with 4°per second angular velocity. Six reaction-time vectors were presented within seeing areas, with 1 repetition horizontally, vertically, and diagonally, originating from 10°and 30°eccentricity. Scotomas were mapped at 2°per second angular velocity originating from the assumed center and using at least 12 vectors. Blind spots were mapped with the I4e stimulus, or the smallest and least bright stimulus seen, at 2°per second angular velocity with a minimum of 8 vectors originating from the assumed center.
Statistical Methods
The distributions of baseline characteristics and measures of visual function and structure were summarized using means, standard deviations (SDs), medians, quartiles and ranges. SP was performed in the study eye, three times in the primary cohort to characterize within-visit variation in test responses, and only once in the secondary cohort. In the former case, the average of the three VTOT and three V30 tests for each of the participants was used for analyses of this measure. Intra-class correlation coefficients (ICC) and the methods of Bland and Altman for assessing agreement between measurements, the repeatability coefficient and Bland-Altman plots, were used to assess variability of SP on tests repeated three times per participant.25 General linear models adjusted for clinical diagnosis, disease duration, and age of enrollment were used to assess the association of baseline characteristics with VTOT. In addition, we evaluated the association between baseline VTOT and other functional and structural measures by calculating Spearman correlation coefficients for continuous factors and comparison of means with t-tests for categorical factors.
KP was performed in both eyes for all participants at baseline. Symmetry of left and right eyes areas at baseline was assessed using scatterplots and summarized with ICCs. Bland-Altman plots were used to assess the magnitude of differences and their association with the area size.
Missing data were treated as a separate category for discrete factors, and a missing indicator was created for continuous factors. Continuous covariates were included in all models in continuous form but were categorized for display and ease of interpretation in the tables. All reported P values were 2-sided. Statistical analyses were conducted using SAS software version 9.4 (SAS, Inc).
Results
Study Population
One hundred and forty-five participants consented to enroll into the RUSH2A study, of whom 127 were eligible after genetic screening and completed a baseline visit (e-Figure 2). Of these 127 participants, 105 (83%) were in the primary cohort, and 22 (17%) were in the secondary cohort. Key baseline characteristics of the participants are provided in Table 1 and stratified by clinical diagnosis (80 [63%] USH2 and 47 [37%] ARRP). Sixty-eight (54%) of participants were female, 113 (89%) were white. The median (interquartile range ([IQR]) age was 37 (27, 44) years in the USH2 group and 44 (36, 50) years in the ARRP group. The age of onset of disease reported by the participant was younger in the USH2 group than in the ARRP group (median 16 vs 32 years). Although median duration of disease was similar in the USH2 group (16 years) and the ARRP group (12 years), there was a higher percentage with duration ≥20 years in the USH2 group (44% [35 of 80] versus 17% [8 of 47]). Ninety-seven percent (73 of 75) of the USH2 participants had moderate or worse hearing loss, but 9% (4 of 47) of the ARRP participants had moderate hearing loss based on the 4 frequency pure tone average audiology test score (Table 1), and sites reported current hearing aid use in 6 of 47 with ARRP (13%). Hearing loss in subjects in the ARRP group was sensorineural and correlated with age (r2 = 0.53, P = <0.001), but there was no significant correlation between hearing loss and age in the USH2 group (r2 = 0.01, P = 0.46). A complete analysis of audiology results for participants in the RUSH2A study will be provided in a separate report. Additional baseline characteristics are summarized in e-Tables 3 and 4. Pre-existing conditions are summarized in e-Table 5. 29 (23%) of the 127 participants reported a pre-existing psychiatric disorder. Of these, depression and anxiety were the most commonly reported; 17 (59%) participants reported depression (12 in USH2 and 5 in ARRP), and 15 (52%) participants reported anxiety (7 participants in the USH2 group and 8 in ARRP).
Table 1.
Baseline Characteristics by Clinical Diagnosis
| Characteristic | Overall N=127 | Clinical Diagnosis | |
|---|---|---|---|
| USH2 N=80 | ARRP N=47 | ||
| Gender | |||
| Female | 68 (54%) | 44 (55%) | 24 (51%) |
| Male | 59 (46%) | 36 (45%) | 23 (49%) |
| Race/Ethnicity | |||
| White | 113 (89%) | 70 (88%) | 43 (91%) |
| Hispanic | 9 (7%) | 7 (8%) | 2 (4%) |
| Asian | 5 (4%) | 3 (4%) | 2 (4%) |
| Enrollment area | |||
| United States/Canada | 83 (65%) | 50 (62%) | 33 (70%) |
| Europe/UK | 44 (35%) | 30 (38%) | 14 (30%) |
| Age at enrollment, yrs a | |||
| Median (IQR) | 40 (30, 48) | 37 (27, 44) | 44 (36, 50) |
| [Min, Max] | [15, 80] | [15, 80] | [24, 75] |
| <35 | 44 (35%) | 36 (45%) | 8 (17%) |
| 35–45 | 44 (35%) | 25 (31%) | 19 (40%) |
| >=45 | 39 (30%) | 19 (24%) | 20 (43%) |
| Age of onset, yrs b | |||
| Median (IQR) | 19 (14, 30) | 16 (13, 22) | 32 (20, 41) |
| [Min, Max] | [5, 65] | [5, 46] | [7, 65] |
| <16 | 41 (32%) | 36 (45%) | 5 (11%) |
| [16, 25) | 40 (32%) | 30 (38%) | 10 (22%) |
| >=25 | 45 (36%) | 14 (18%) | 31 (67%) |
| Duration of Disease, yrs b | |||
| Median (IQR) | 15 (8, 23) | 16 (10, 27) | 12 (6, 18) |
| [Min, Max] | [1, 60] | [1, 60] | [1, 36] |
| <10 | 37 (29%) | 20 (25%) | 17 (37%) |
| [10, 19) | 46 (37%) | 25 (31%) | 21 (46%) |
| >=20 | 43 (34%) | 35 (44%) | 8 (17%) |
| Severity of hearing loss c | |||
| Normal | 35 (29%) | 0 | 35 (74%) |
| Mild | 10 (8%) | 2 (3%) | 8 (17%) |
| Moderate | 58 (48%) | 54 (72%) | 4 (9%) |
| Severe | 15 (12%) | 15 (20%) | 0 |
| Profound | 4 (3%) | 4 (5%) | 0 |
| Smoking status | |||
| Yes | 33 (26%) | 20 (25%) | 13 (28%) |
| No | 94 (74%) | 60 (75%) | 34 (72%) |
| Current use of dietary supplements | |||
| None | 53 (42%) | 41 (51%) | 12 (26%) |
| Vitamin A only | 11 (9%) | 5 (6%) | 6 (13%) |
| DHA only | 5 (4%) | 3 (4%) | 2 (4%) |
| Lutein only | 9 (7%) | 5 (6%) | 4 (9%) |
| Combination | 49 (38%) | 26 (33%) | 23 (49%) |
28 participants were not permitted to report date of birth due to regulatory restrictions. Therefore, only year of birth and categorical age was reported. For those participants, July 1st with the reported birth year was imputed as birth date to calculate continuous age
1 participant in the ARRP group was missing age of onset (a participant-reported field based on their awareness of visual symptoms) and duration of disease (computed based on age of onset and date of enrollment)
Composite score based on 4F-PTA (four frequency air conduction threshold pure-tone average based on 0.5, 1, 2, and 4 kHz). 5 participants in the USH2 group were missing baseline 4F-PTA (3 had cochlear implants in both ears, 2 missed their audiology exam for other reasons)
Functional and Structural Measures at Baseline
Functional and structural measures at baseline are summarized in Table 2. The median value for VTOT was twice as large in the ARRP participants as in the USH2 participants (32.8 versus 16.0 dB-sr, P<0.001), and both groups were lower than normal subjects (103 dB-sr).22 However, the median values for V30 were similar (9.3 versus 7.5 dB-sr, P=0.13) in both groups, although both groups were also lower than normal participants (27.4 dB-sr).22 The mean (SD) sensitivity on static perimetry was 9.3 (6.0) dB in USH2 participants, and 11.9 (6.0) dB in ARRP participants). Participants with ARRP had larger seeing areas for all 3 isopters (I4e, III4e, and V4e) compared to participants with USH2. Mean (SD) III4e area for left and right eyes was 4215 (4300) and 4561 (4426) squared degrees, respectively, showing high concordance (ICC=0.94; e-Figure 3A), but the seeing area was smaller than the lower limit of normal subjects (12799 squared degrees, data not published), in both groups. Bland-Altman plots (e-Figure 3B) show a mean difference (left minus right) between eyes equal to −346 squared degrees with limits of agreement −3340 to 2648 squared degrees. Mean sensitivity of microperimetry was 5.4 (4.9) dB in USH2 participants, and 6.7 (5.1) dB in ARRP participants. The median visual acuity score for all participants was 80 (Snellen equivalent 20/25) and similar in both diagnosis groups. Photopic ERG amplitudes were not measurable in 29% of participants with similar percentages in both diagnosis groups. Cysts were present in OCT scans from 49% of participants with USH2 and 34% of participants with ARRP. The central subfield thickness was similar in both diagnosis groups (overall median 253 microns).
Table 2.
Baseline Functional and Structural Measures
| Clinical Diagnosis | |||
|---|---|---|---|
| Overall N=127 | USH2 N = 80 | ARRP N = 47 | |
|
| |||
| VTOT (dB-sr) a | |||
| Median (IQR) | 20.6 (7.7, 46.3) | 16.0 (3.6, 35.2) | 32.8 (15.1, 54.6) |
| [Min, Max] | [0.2, 90.5] | [0.2, 81.4] | [2.5, 90.5] |
| Mean (SD) | 27.8 (23.7) | 22.5 (21.5) | 37.1 (24.7) |
| V30 (dB-sr) a | |||
| Median (IQR) | 8.3 (3.8, 12.8) | 7.5 (2.7, 12.7) | 9.3 (5.1, 13.3) |
| [Min, Max] | [0.2, 22.7] | [0.2, 21.6] | [1.4, 22.7] |
| Mean (SD) | 9.0 (5.9) | 8.4 (5.9) | 10.0 (5.9) |
| SP mean sensitivity (dB) a | |||
| Median (IQR) | 9.3 (5.2, 14.6) | 7.8 (4.3, 13.8) | 12.1 (7.0, 16.9) |
| [Min, Max] | [0.4, 24.6] | [0.4, 24.2] | [2.4, 24.6] |
| Mean (SD) | 10.2 (6.1) | 9.3 (6.0) | 11.9 (6.0) |
| I4e seeing area (deg 2 ) b | |||
| Median (IQR) | 85.8 (22.2, 607.0) | 61.4 (12.8, 289.2) | 187.7 (27.1, 1770.0) |
| [Min, Max] | [0.0, 8883.1] | [0.0, 5619.2] | [0.0, 8883.1] |
| III4e seeing area (deg 2 ) b | |||
| Median (IQR) | 2454.6 (431.6, 8064.4) | 1362.5 (226.1, 6465.6) | 5722.6 (2112.7, 9707.6) |
| [Min, Max] | [6.7, 13467.0] | [6.7, 13335.0] | [105.9, 13467.0] |
| V4e seeing area (deg 2 ) b | |||
| Median (IQR) | 8798.5(2619, 12344.0) | 5912.5 (842.4, 11521.0) | 11062.0 (7389.4, 13035.0) |
| [Min, Max] | [18.6, 15800.0] | [18.6, 15579.0] | [405.5, 15800.0] |
| VA ETDRS letter score c | |||
| Median (IQR) | 80.0 (75.0, 85.0) | 79.0 (73.5, 85.0) | 82.0(77.0, 87.0) |
| [Min, Max] | [18.0, 94.0] | [18.0, 92.0] | [41.0, 94.0] |
| Photopic ERG 30 Hz flicker | |||
| Amplitude (μV)d | |||
| N of unmeasurable (0) amplitudes | 37 (29%) | 25 (32%) | 12 (26%) |
| Median amplitude (IQR) | 2.0 (0.0, 7.7) | 1.5 (0.0, 5.5) | 3.1 (0.0, 20.0) |
| [Min, Max] | [0.0, 82.2] | [0.0, 82.2] | [0.0, 60.0] |
| MP mean retinal sensitivity e | |||
| Median (IQR) | 4.1 (2.5, 8.5) | 3.8 (2.2, 8.6) | 5.4 (2.7, 8.6) |
| [Min, Max] | [0.2, 22.8] | [0.2, 22.8] | [0.5, 19.2] |
| Mean (SD) | 6.0 (4.9) | 5.5 (4.9) | 6.6 (5.3) |
| Presence of cysts f | |||
| Yes | 55 (43%) | 39 (49%) | 16 (34%) |
| No | 70 (55%) | 39 (49%) | 31 (66%) |
| Ungradable | 2 (2%) | 2 (2%) | 0 |
| Central subfield thickness (microns) f | |||
| Median (IQR) | 253.0 (228.0, 285.0) | 247.0 (223.0, 280.0) | 261.0 (246.0, 288.0) |
| [Min, Max] | [137.0, 519.0] | [137.0, 519.0] | [175.0, 323.0] |
Static perimetry results were graded by a reading center. Results are based on the average of 3 fields when 3 tests were performed (primary cohort); otherwise they based on just the 1 test performed (secondary cohort). Static perimetry data is not included for 1 participant in the ARRP group (participant was not tested).
Kinetic perimetry results were graded by a reading center. Seeing area was calculated as isopter area minus scotoma. Scotoma not tested/measured was treated as 0 in the calculation. 49 participants in the USH2 group and 24 participants in the ARRP group have scotomas not tested/measured and treated as 0. 21 participants in the USH2group and 8 participants in the ARRP group have III4e scotomas not tested/measured and treated as 0 (1 subject was excluded for procedure issues). 28 participants in the USH2group and 14 participants in the ARRP group have V4e scotomas not tested/measured and treated as 0 (2 subjects were excluded for procedure issues).
5 sites used an ETDRS chart, 10 sites use an electronic visual acuity tester, and 1 site used both
Photopic ERG 30 Hz flicker amplitudes are not included for 1 participant in the USH2 group (participant was not tested)
Microperimetry mean retinal sensitivity results were graded by a reading center. Results are based on the average of first two (out of three) tests. Microperimetry mean retinal sensitivity data are not included for 25 participants in the USH2 and 10 participants in the ARRP group (reasons include: 22 not performed in secondary cohort per protocol; in the primary cohort, 10 were not performed because the site did not have the equipment, 2 were not done, 1 was ungradable).
Presence of any cyst and central subfield thickness on OCT were graded by a reading center. Central subfield thickness data are not included for 1 participant in the USH2 group (due to ungradable image).
Variability of Static Perimetry Testing
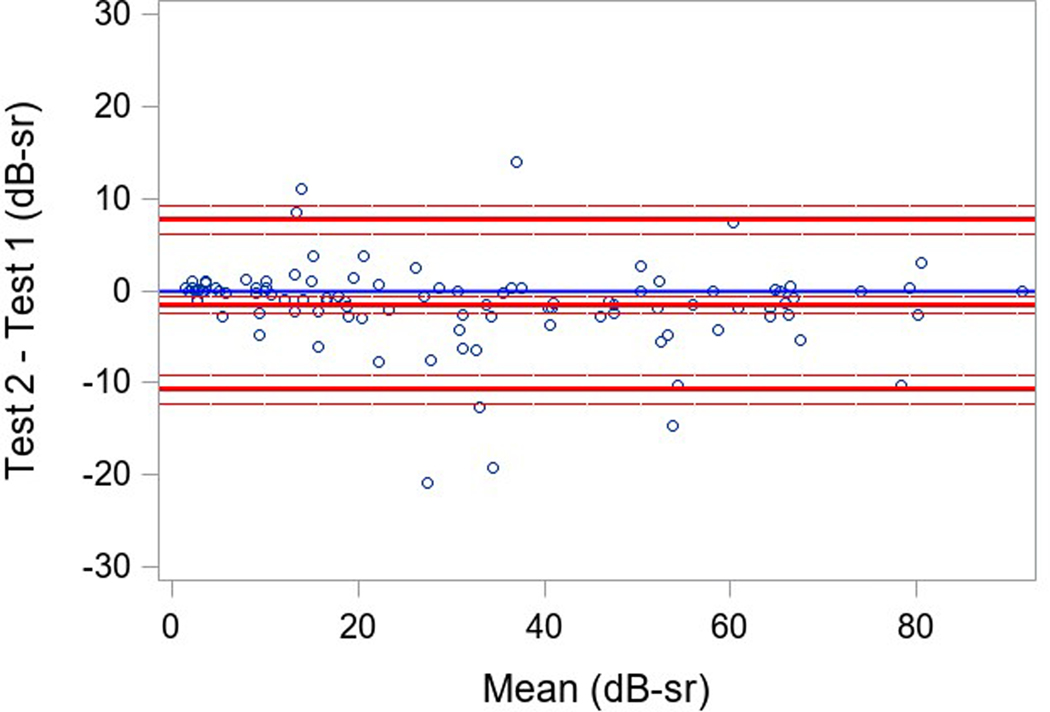
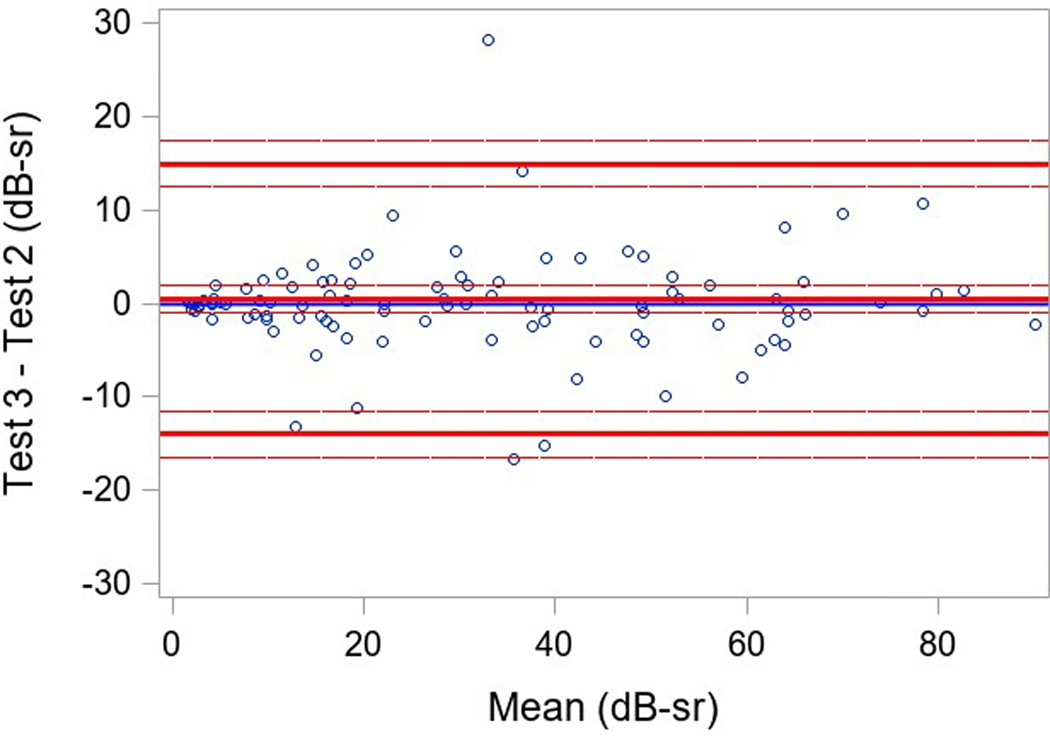
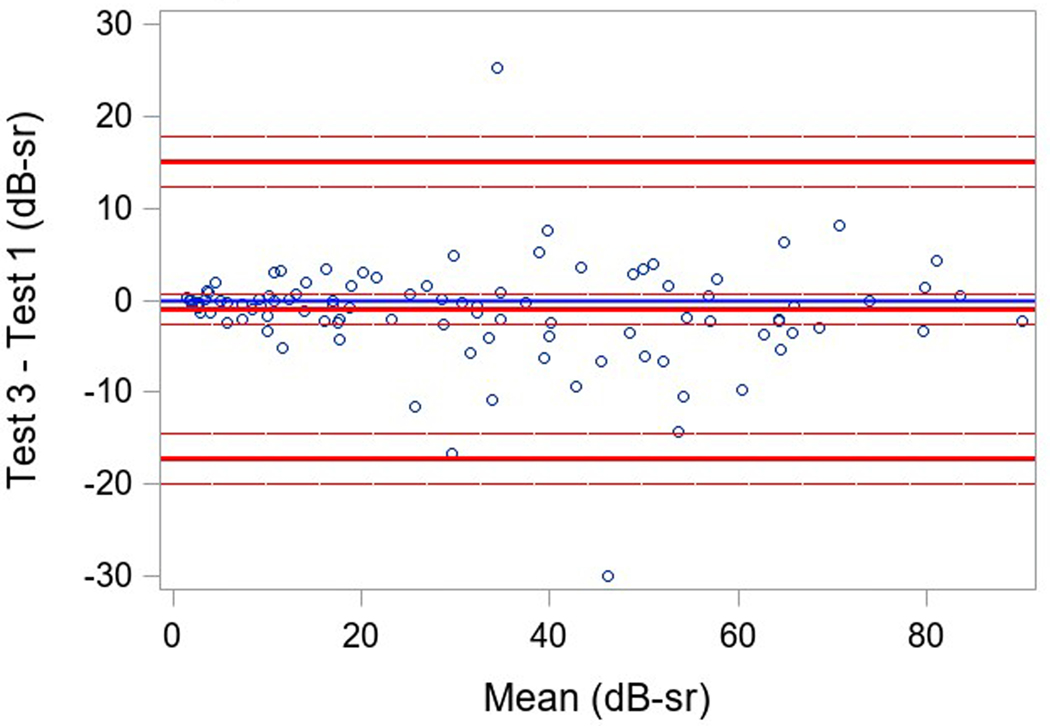
Measures of variability of results within a testing session (reliability factor, false positive rate, and false negative rate) and of variability in VTOT in a participant between testing sessions were examined. Three participants had only two SP tests, and one participant did not have baseline SP; the secondary cohort of participants with more severe disease had only a single baseline. Good reliability was found in both groups with a reliability factor (RF) median (IQR) over all tests of 5.2% (2.1%, 9.1%) in participants with USH2, and 5.1% (3.0%, 7.3%) in participants with ARRP (Table 3). The median (IQR) for the false positive rate over all tests was 1% (0%, 4%) and 2% (0%, 3%) and for the false negative rate was 8% (2%,15%) and 8% (5%,11%), respectively for the USH2 and ARRP groups. The overall repeatability for 101 participants with 3 available tests was high and similar when comparing the 3 pairs of tests results (ICC overall=0.96, ICC test 1 versus test 2 = 0.98, ICC test 2 versus test 3 = 0.95, test 1 versus test 3= 0.94; repeatability coefficient = 13.7) (Table 3). Bland-Altman plots showed mean differences near 0 (<1.5 with 95% limits of agreement of ± 16 dB-sr; Figure 1).
Table 3.
Baseline Static Perimetry –Reliability Measures Within Test Session and Variability Among Sessions Data
| Clinical Diagnosis | |||
|---|---|---|---|
| Overall N =126a | USH2 N=80 | ARRP N=46 | |
|
| |||
| Reliability Factor (%) b | |||
| Median (IQR) | |||
|
| |||
| Overall | 5.1 (2.6, 8.2) | 5.2 (2.1, 9.1) | 5.1 (3.0, 7.3) |
| Test 1 | 4.7 (1.9, 9.0) | 4.2 (1.7, 8.8) | 5.1 (2.8, 9.8) |
| Test 2 | 4.7 (2.5, 9.8) | 5.1 (2.4, 11.1) | 4.6 (2.6, 7.2) |
| Test 3 | 5.0 (2.4, 9.0) | 5.4 (2.3, 9.4) | 4.5 (2.6, 8.8) |
|
| |||
| False positives rate (%) b | |||
| Median (IQR) | |||
|
| |||
| Overall | 1 (0, 3) | 1 (0, 4) | 2 (0, 3) |
| Test 1 | 0 (0, 4) | 0 (0, 4) | 1 (0, 3) |
| Test 2 | 0 (0, 3) | 0 (0, 5) | 0 (0, 3) |
| Test 3 | 0 (0, 5) | 0 (0, 5) | 0 (0, 4) |
|
| |||
| False negatives rate (%) b | |||
| Median (IQR) | |||
|
| |||
| Overall | 8 (3, 14) | 8 (2, 15) | 8 (5, 11) |
| Test 1 | 6 (3, 15) | 6 (0, 13) | 8 (3, 15) |
| Test 2 | 7 (3, 16) | 8 (3, 19) | 7 (3, 13) |
| Test 3 | 7 (3, 17) | 7 (2, 17) | 7 (3, 14) |
|
| |||
| Intraclass correlation coefficient (95% confidence interval) c | |||
| Overall (tests 1,2, and 3) | 0.96 (0.94 0.97) | Not applicable | |
| Tests 1 and 2 | 0.98 (0.97 0.98) | Not applicable | |
| Tests 2 and 3 | 0.95 (0.93 0.97) | Not applicable | |
| Tests 1 and 3 | 0.94 (0.92 0.96) | Not applicable | |
|
| |||
| Repeatability coefficient (95% confidence interval) c | |||
| Overall (tests 1,2, and 3) | 13.7 (9.2, 16.3) | Not applicable | |
Static perimetry results were graded by a reading center. 1 participant in ARRP group was missing all SP tests and is excluded from this table.
Test 2 and Test 3 data are not included for 24 participants, respectively (22 secondary cohort participants only performed the test once, and 2 primary cohort participants were missing the second and the third test)
Variability analysis data are not included for 25 participants (22 secondary cohort participants only performed the test once, and 2 primary cohort participants were missing the second and the third test, and 1 participant was missing the third VTOT value.)
Figure 1 (a-c). Static Perimetry Bland-Altman Plots.
Legend: Bland-Altman plota of test 1, 2, and 3 pairwise. Only included participants with 3 fields(N=101). The differences between test 1 and 2, test 2 and 3, test 1 and 3 for VTOT are plotted on the y-axis against their averages on the x-axis.
aThe Bland-Altman plots only include participants with 3 fields(N=101, not included 22 secondary cohort participants only performed the test once, 1 participant was missing all SP tests, 2 primary cohort participants were missing the second and the third test, and 1 participant was missing the third VTOT value.)
Association of Baseline Characteristics with Total Hill of Vision (VTOT)
Mean VTOT values stratified by diagnosis and baseline characteristics are shown in Table 4. Among all participants and within each diagnosis group, mean VTOT decreased with increasing duration of disease. After adjustment for duration of disease and age of enrollment, USH2 participants had lower VTOT values compared with ARRP participants (mean difference estimated from linear regression: 13.4 dB-sr with 95% CI [4.2, 22.6], P= <0.001; Table 5). After adjustment for clinical diagnosis and age of enrollment, longer disease duration was associated with lower VTOT values (P<0.001), with a mean decrease of 0.45 [95% CI (0.03, 0.88)] dB-sr for each additional year of duration (Table 5). Age at enrollment was significantly associated with VTOT when adjusted for clinical diagnosis and disease duration. Older age of enrollment was associated with worse vision (P=0.02). The association of age of enrollment with VTOT remained similar in a sensitivity analysis with USH2 participants only (data not shown). No other baseline characteristic in Table 4 was found to be significantly associated with VTOT once clinical diagnosis and disease duration were accounted for.
Table 4.
Mean Full Field Hill of Vision (VTOT) Stratified by Clinical Diagnosis and Baseline Characteristicsa
| Characteristic | Overall | Clinical Diagnosis | ||||
|---|---|---|---|---|---|---|
| USH2 | ARRP | |||||
|
| ||||||
| N=126 | VTOT Mean (SD) | N=80 | VTOT Mean (SD) | N=46 | VTOT Mean (SD) | |
|
| ||||||
| Gender | ||||||
| Female | 68 | 27.4 (22.3) | 44 | 22.9 (20.8) | 24 | 35.5 (23.1) |
| Male | 58 | 28.4 (25.4) | 36 | 21.9 (22.6) | 22 | 38.9 (26.7) |
| Race/Ethnicity | ||||||
| White | 112 | 28.2 (23.7) | 70 | 21.9 (20.8) | 42 | 38.6 (24.8) |
| Hispanic | 9 | 26.4 (24.4) | 7 | 27.6 (25.6) | 2 | 22.4 (28.1) |
| Asian | 5 | 21.8 (27.1) | 3 | 23.0 (35.4) | 2 | 20.0 (20.1) |
| Enrollment area | ||||||
| United States | 83 | 25.7 (23.0) | 50 | 21.1 (21.2) | 33 | 32.5 (24.1) |
| Europe/UK | 43 | 32.0 (24.8) | 30 | 24.7 (22.1) | 13 | 48.8 (23.1) |
| Age at enrollment, yrs b | ||||||
| <35 | 44 | 35.7 (23.0) | 36 | 33.1 (20.0) | 8 | 47.6 (32.5) |
| 35–45 | 43 | 23.9 (22.4) | 25 | 18.3 (20.1) | 18 | 31.8 (23.6) |
| >= 45 | 39 | 23.1 (24.2) | 19 | 7.8 (15.3) | 20 | 37.8 (22.0) |
| Duration of Disease, yrs c | ||||||
| <10 | 36 | 40.5 (22.6) | 20 | 34.3 (20.0) | 16 | 48.2 (23.9) |
| [10,20) | 46 | 28.5 (21.9) | 25 | 28.7 (23.5) | 21 | 28.3 (20.4) |
| >=20 | 43 | 15.0 (18.2) | 35 | 11.2 (14.8) | 8 | 31.5 (23.4) |
| Smoking status | ||||||
| Yes | 33 | 31.2 (24.7) | 20 | 25.9 (24.2) | 13 | 39.3 (24.2) |
| No | 93 | 26.6 (23.4) | 60 | 21.3 (20.6) | 33 | 36.3 (25.2) |
| Current use of dietary supplements | ||||||
| None | 53 | 32.3 (23.9) | 41 | 25.6 (20.5) | 12 | 55.0 (20.7) |
| Vitamin A only | 11 | 14.9 (16.0) | 5 | 9.5 (12.7) | 6 | 19.5 (18.1) |
| DHA only | 5 | 15.8 (13.1) | 3 | 17.0 (15.4) | 2 | 13.9 (14.3) |
| Lutein only | 8 | 32.2 (23.5) | 5 | 24.4 (21.5) | 3 | 45.3 (24.4) |
| Combination | 49 | 26.4 (24.9) | 26 | 20.2 (24.6) | 23 | 33.4 (23.7) |
Static perimetry results were graded by a reading center. Results are based on the average of 3 fields when 3 tests were performed (primary cohort); otherwise they are based on the 1 test performed (secondary cohort). Static perimetry data is not included for 1 participant in the ARRP group (participant was not tested). Factors are presented categorically to show the data but were analyzed using a continuous version of the factor in the model. None of the other factors in the table were significantly associated with VTOT once disease duration, age of enrollment and clinical diagnosis were accounted for (P-value not shown).
28 participants were not permitted to report date of birth due to regulatory restrictions. Therefore, only year of birth and categorical age was reported. For those participants, July 1st with the reported birth year was imputed as birth date to calculate continuous age
1 participant in the ARRP group was missing age of onset (a participant-reported field based on their awareness of visual symptoms) and duration of disease (computed based on age of onset and date of enrollment)
Table 5.
Mean and Adjusted Mean Full Field Hill of Vision (VTOT)
| Models | N=126a | Mean (SD) – decibel steradians | Adjusted Mean (95% CI)- decibel steradiansb | Difference from Reference Group (95% CI) | P-valuec |
|---|---|---|---|---|---|
| Clinical Diagnosis | <0.001 | ||||
| USH2 | 80 | 22.5 (21.5) | 22.9 (17.9, 28.0) | Reference | |
| ARRP | 46 | 37.1 (24.7) | 36.3 (29.5, 43.1) | 13.4 (4.2, 22.6) | |
| Duration of disease, yrs d | <0.001 | ||||
| <10 | 36 | 40.5 (22.6) | 39.1 (31.9, 46.3) | Reference | |
| [10,20) | 46 | 28.5 (21.9) | 27.9 (21.7, 34.0) | −11.2 (−20.3, −2.1) | |
| >=20 | 43 | 15.0 (18.2) | 21.9 (13.8, 30.1) | −17.2 (−28.9, −5.4) | |
| Age of enrollment, yrs e | 0.02 | ||||
| <35 | 44 | 35.7 (23.0) | 35.4 (27.1, 43.6) | Reference | |
| 35–45 | 43 | 23.9 (22.4) | 27.7 (21.3, 34.1) | −7.6 (−18.4, 3.1) | |
| >=45 | 39 | 23.1 (24.2) | 25.8 (18.8, 32.7) | −9.6 (−21.4, 2.2) |
Static perimetry results were graded by a reading center. Results are based on the average of 3 fields when 3 tests were performed (primary cohort); otherwise they based on just the 1 test performed (secondary cohort). Static perimetry data is not included for 1 participant in the ARRP group (participant was not tested).
Simultaneous adjustment for duration of disease, clinical diagnosis, and age of enrollment
Factors are presented categorically to show the data but were analyzed using continuous version of the factor in the model.
1 participant in the ARRP group was missing age of onset (a participant-reported field based on their awareness of visual symptoms) and duration of disease (computed based on age of onset and date of enrollment)
28 participants were not permitted to report date of birth due to regulatory restrictions. Therefore, only year of birth and categorical age was reported. For those participants, July 1st with the reported birth year was imputed as birth date to calculate continuous age
Association of VTOT with Other Measures of Function and Structure
The association of baseline functional and structural measures with VTOT are summarized in Table 6. Better BCVA was associated with higher VTOT values (Spearman correlation coefficient r=0.59, P<0.001). Presence of cysts (well-defined round or oval cavities within the retinal layers) in OCT scans was associated with a lower VTOT (mean difference=9.1 dB-sr, P=0.03). Other factors including photopic ERG 30 Hz amplitudes, mean retinal sensitivity on MP, and central subfield thickness within the center 1mm on SD-OCT were all found to be moderately associated with VTOT, with correlation coefficients ranging from 0.48 to 0.55. KP III4e area was very strongly associated with VTOT (r=0.92, P <0.001). The correlation coefficients for V30 with the measures of function and structure were similar to the corresponding correlation coefficients for VTOT and similar between the two clinical diagnosis groups (r from 0.46 to 0.85; data not shown).
Table 6.
Correlation of VTOT with Other Baseline Functional and Structural Measures
| Overall | Clinical Diagnosis | Correlation Coefficientb | P Valueb | |||||
|---|---|---|---|---|---|---|---|---|
| USH2 | ARRP | |||||||
|
| ||||||||
| N=126a | VTOTa Mean (SD) |
N=80 | VTOTa Mean (SD) |
N=46 | VTOTa Mean (SD) |
|||
|
| ||||||||
| III4e seeing area (deg 2 ) c | 0.93 | <0.001 | ||||||
| <710 | 40 | 5.8 (6.1) | 36 | 5.8 (6.3) | 4 | 5.4 (4.0) | ||
| [710, 4000) | 33 | 19.0 (8.7) | 19 | 20.8 (9.0) | 14 | 17.0 (8.2) | ||
| [4000, 8000) | 25 | 38.7 (12.4) | 13 | 37.4 (11.4) | 12 | 40.1 (13.9) | ||
| >=8000 | 27 | 61.8 (15.5) | 12 | 58.8 (17.4) | 15 | 64.1 (13.9) | ||
| VA ETDRS letter score (approx. Snellen equivalent) d | 0.59 | <0.001 | ||||||
| <69 (<20/40) | 14 | 8.6 (12.6) | 11 | 3.3 (3.1) | 3 | 27.9 (16.4) | ||
| 69–73 (20/40) | 13 | 15.8 (19.4) | 9 | 12.7 (17.6) | 4 | 22.9 (23.9) | ||
| 74–78 (20/32) | 24 | 19.1 (15.8) | 17 | 17.8 (12.5) | 7 | 22.1 (22.8) | ||
| 79–83 (20/25) | 33 | 24.1 (20.9) | 18 | 19.2 (18.1) | 15 | 30.0 (23.1) | ||
| >=84 (>=20/20) | 42 | 45.8 (22.6) | 25 | 39.9 (23.1) | 17 | 54.5 (19.5) | ||
| Photopic ERG 30 Hz flicker amplitudes (μV)e | 0.54 | <0.001 | ||||||
| 0 | 37 | 16.9 (16.6) | 25 | 14.5 (16.7) | 12 | 22.1 (15.8) | ||
| (0, 1.8) | 20 | 13.9 (12.4) | 15 | 12.8 (13.0) | 5 | 17.2 (10.8) | ||
| [1.8, 6.8) | 34 | 27.1 (21.2) | 24 | 27.7 (22.9) | 10 | 25.7 (17.6) | ||
| >=6.8 | 34 | 48.9 (23.9) | 15 | 37.7 (24.2) | 19 | 57.8 (20.2) | ||
| MP mean retinal sensitivity (dB) f | 0.55 | <0.001 | ||||||
| <2 | 16 | 20.6 (18.6) | 12 | 14.4 (15.8) | 4 | 39.3 (14.0) | ||
| [2, 4) | 28 | 23.3 (19.2) | 17 | 20.9 (16.3) | 11 | 27.0 (23.4) | ||
| [4, 8) | 21 | 31.0 (23.6) | 11 | 24.4 (21.2) | 10 | 38.3 (25.0) | ||
| >=8 | 26 | 53.3 (20.5) | 15 | 47.1 (21.9) | 11 | 61.8 (15.4) | ||
| Presence of cysts g | N/A | 0.03 | ||||||
| Yes | 55 | 23.1 (23.0) | 39 | 20.2 (20.3) | 16 | 30.4 (28.0) | ||
| No | 69 | 32.2 (23.7) | 39 | 25.6 (22.8) | 30 | 40.7 (22.4) | ||
| Ungradable | 2 | 6.4 (6.2) | 2 | 6.4 (6.2) | 0 | NA | ||
| Central subfield thickness (μm) g | 0.48 | <0.001 | ||||||
| <230 | 32 | 11.7 (12.9) | 28 | 8.7 (10.4) | 4 | 32.2 (10.6) | ||
| [230, 250) | 22 | 28.8 (23.1) | 13 | 28.7 (24.6) | 9 | 28.9 (22.2) | ||
| [250, 280) | 33 | 30.2 (25.3) | 18 | 24.6 (23.4) | 15 | 36.8 (26.7) | ||
| >=280 | 38 | 39.2 (23.1) | 20 | 36.2 (19.6) | 18 | 42.6 (26.5) | ||
Static perimetry results were graded by a reading center. Results are based on the average of 3 fields when 3 tests were performed (primary cohort); otherwise they based on just the 1 test performed (secondary cohort). Static perimetry data is not included for 1 participant in the ARRP group (participant was not tested).
Correlation coefficients and p-values are based on analyses combining all participants (both USH2and ARRP groups). Factors are presented categorically to show the data but were analyzed using continuous version of the factor in the analysis.
Kinetic perimetry results were graded by a reading group and 8 participants in ARRP group have III4e scotoma not tested/measured and treated as 0 (1 subject was excluded for procedure issues).
5 sites used an ETDRS chart, 10 sites use an electronic visual acuity tester, and 1 site used both
Photopic ERG 30 Hz flicker amplitudes are not included for 1 participant in the USH2group (participant was not tested)
Microperimetry mean retinal sensitivity results were graded by a reading center. Results are based on the average of first two (out of three) tests. Microperimetry mean retinal sensitivity data are not included for 25 participants in the USH2and 10 participants in the ARRP group (reasons include: 22 not performed in secondary cohort per protocol; in the primary cohort, 10 were not performed because the site did not have the equipment, 2 were MP not done, 1 was ungradable).
Presence of any cyst and central subfield thickness on OCT were graded by a reading center. Central subfield thickness data are not included for 1 participant in the USH2group (due to ungradable image). The P-value for presence of any cyst was calculated using T-test.
Discussion
The RUSH2A study comprised roughly two-thirds of participants with USH2 and one-third with ARRP and represents a large, diverse population of patients with retinal degeneration due to USH2A variants, well-characterized genetically and phenotypically with a broad spectrum of disease severity. The main outcome measure, VTOT, differed between disease groups (USH2 and ARRP) and disease duration. VTOT results were repeatable over 3 repetitions at baseline separated by no more than 10 days in participants in the primary cohort, suggesting the learning effect was minimal and triplicate SP measures at baseline may not be necessary. Similar findings have been shown using VTOT and V30 in X-linked RP associated with RPGR.26 Furthermore, many common clinical measures including BCVA, ERG 30Hz flicker amplitudes, mean macular retinal sensitivity on microperimetry, III4e KP area, the presence of intraretinal cysts, and central subfield thickness correlated with the VTOT measured using standard SP protocols and common equipment among the 16 participating centers. The study results suggest that VTOT may provide a reliable outcome measure of disease progression for clinical trials of participants with USH2A-related retinal degeneration. V30 values were similar between USH2 and ARRP but provided a less sensitive measure of disease severity than VTOT. Greater disease duration significantly correlated with more severe visual field loss as measured by SP, consistent with the progressive nature of USH2A-related retinal degeneration.
Participants in the RUSH2A study reported anxiety (11%) and depression (9%) more commonly than other psychiatric disorders. Prior studies of participants with RP have shown significantly greater rates of anxiety and depression compared to controls,27 with anxiety in 36.5% and depression in 15.5%28 using a standard questionnaire to measure anxiety and depression. Other studies found significantly increased rates of depressive mood in RP patients (34.8%) compared to controls (17.1%),29 and depression scores indicative of clinical depression in 25.7% of RP patients.30 Rates reported in RUSH2A participants were lower than many studies in the literature. The present study relies on patient report of anxiety and depression, and therefore rates in the RUSH2A participants may under-represent the true prevalence of disease. Future studies will report results of quality of life test results using standard instruments at baseline and longitudinally in the RUSH2A study.
It is noteworthy that participants with USH2 had worse visual field sensitivity (VTOT and V30) than participants with ARRP, even after accounting for disease duration and age at enrollment. A previous study comparing participants with USH2 with ARRP due to biallelic USH2A sequence variants found that those with USH2 had more severe visual impairment measured by visual field and visual acuity, occurring at least a decade earlier than in those with ARRP.9 Similarly, in another study ERG 30 Hz flicker amplitudes were lower in participants with USH2 compared to ARRP.10 More severe truncating sequence variants have been reported in participants with USH2 than ARRP, and hearing loss is also more severe in those with truncating USH2A sequence variants compared to missense sequence variants.31 Genetic characteristics of the RUSH2A population will be reported in a future manuscript, but may provide further insight into the relationship between genotype and phenotype in patients with USH2A-related retinal degeneration.
In the RUSH2A study population, older age at enrollment into the study was associated with lower VTOT as measured by SP, after adjustment for clinical diagnosis and duration of disease. Due to congenital hearing loss, patients with USH2 may be diagnosed at earlier ages than patients with ARRP and similar loss of vision. Thus, the reported duration of vision loss for ARRP patients may be an underestimate of the true duration, so that the estimated mean of 13.4 dB-sr higher VTOT in the ARRP group relative to the USH2 group (Table 5) may be an underestimate.
USH2A-related retinal degeneration affects rods, then cones, so rod-mediated measures of retinal function may reflect disease severity earlier and potentially more sensitively than more cone-driven measures such as perimetry or BCVA.32 Due to the background illumination used in this study, the clinical measures that correlated with VTOT were most likely cone-mediated; but measures of rod function including FST, dark-adapted visual field sensitivity and rod ERGs are included in the RUSH2A study and will be described in future manuscripts.
In conclusion, VTOT interpolation of SP correlated significantly with diagnosis, disease duration and several clinical measures of retinal structure and function in the RUSH2A study population at baseline. Future work will evaluate genetic risk factors for disease severity, hearing loss, rod-mediated retinal function and the impact of disease on patient quality of life at baseline and during 4 years of longitudinal progression in the RUSH2A study.
Supplementary Material
Acknowledgment Permissions:
All persons give permission to be acknowledged.
a. Funding/Support: Foundation Fighting Blindness, Columbia, MD
b. Financial Disclosures: J. Cheetham reports personal fees to disclose from FFB. L. Erker reports grants from the NIH, FDA Orphan, Department of Defense Grant, and Wynn Gund grant from FFB during the conduct of the study. Other relevant conflict of interest from Sanofi, applied genetic technologies corporation Meira Gtx, NightstaRx, and Novelion. S. Degli Esposti reports grants and personal fees from Foundation Fighting Blindness during the conduct of the study and receives personal fees from GSK, Novartis, Bayer, Allergan, outside the submitted work. M. Maguire reports grants and personal fees from FFB via the Jaeb Center for Health Research. J. Duncan reports grants from Foundation Fighting Blindness, during the conduct of the study; personal fees from AGTC, Vedere, Horama, grants from Foundation Fighting Blindness, National Eye Institute, personal fees from Miller Medical Communications outside the submitted work. A. Iannaccone reports grants and personal fees from Foundation Fighting Blindness Consortium during the conduct of the study; personal fees from Alia Therapeutics, Trento, Italy, ClearViewHealthcare Partners, Newton, MA, Teladoc Health, Westwood, MA, GLG Group, San Francisco, CA, personal fees from Guidepoint, Boston, MA, personal fees from Astellas Institute for Regenerative Medicine, Marlborough, MA, Roivant Pharma, New York, NY, Editas Medicine, Cambridge, MA, Rhythm Pharmaceuticals, Boston, MA, personal fees from IQVIA, Durham, NC, Gyroscope, Stevenage, UK, Ocugen, Malvern, PA, grants from AGTC, Allergan, Acucela, ProQR, Retinagenix, Molecular Therapeutics outside the submitted work. D. Birch reports grants and personal fees from Foundation Fighting Blindness during the conduct of the study; personal fees from ProQR, Nacuity, AGTC, and Editas outside the submitted work. S.Farsiu reports grants from Foundation Fighting Blindness during the conduct of the study. Grants from Foundation Fighting Blindness outside the submitted work; and Duke University – He has developed DOCTRAP software, which a version is used for this study. This software is also used in other studies which are sponsored by external entities which pay Duke in form of research grants or contracts. Part of his compensation and research funds comes from such activities. J. Sahel reports grants from LabEx LIFESENSES (ANR-10-LABX-65), grants from IHU FOReSIGHT (ANR-18-IAHU-01) during the conduct of the study. J. Carroll reports grants from Foundation Fighting Blindness during the conduct of the study. M. Pennesi reports grants from Foundation Fighting Blindness during the conduct of the study. Consulting fees from Allergan/Editas, Spark Therapeutics, Wave Biosciences, Astellas Pharmaceuticals, RegenexBio, Iveric, other from Biogen, other from Novartis, other from Adverum, other from Gensight, other from ProQR, other from Horama, other from Eyevensys, other from Nayan, personal fees and other from Nacuity, personal fees and other from Ocugen, personal fees and other from Verede, other from Sparing Vision, other from AGTC, other from Sanofi outside the submitted work. M. Michaelides reports personal fees from MeiraGTx, MeiraGTx, Stargazer Pharma, Acucela, Astellas, ProQR, 2CTech, outside the submitted work and Stock options with MeiraGTx. G. Jaffe reports other from Jaeb Center for Health Research, other from Novarits during the conduct of the study.
c. Other Acknowledgments—Foundation Fighting Blindness (FFB) Consortium Contributors to the RUSH2A Protocol
Footnotes
The comprehensive list of FFB Consortium Investigator Group members participating in this protocol is included in the manuscript acknowledgements.
FFB Consortium Clinical Sites: Clinical sites participating in this protocol are listed in order by number of participants enrolled into the study. The number of participants enrolled is noted in parenthesis preceded by the site location and the site name. Personnel are listed as (I) for Study Investigator, (C) for Coordinator, (T) for Technician.
San Francisco, CA - UCSF Department of Ophthalmology (18): Jacque Duncan (I); Arshia Mian (C); Yiran Liu (C); Mary Lew (C, T); John Peterson (T); Tom Hernandez (T); Betty Hom (T); Hoover Chan (T)
Portland, OR - Oregon Health & Science University (14): Mark Pennesi (I); Paul Yang (I); Annelise Haft (C,T); Jordan Barth (T); Chiedozie Ukachukwu (T); Gareth Harman (T); Jocelyn Hui (T); Jennifer Hilgeman (T); Virinda Boyle (T); Scott Pickell (T); Darius Liseckas (T)
London, United Kingdom - Moorfields Eye Hospital (13): Michel Michaelides (I); Simona Delgi Esposti (I); Shweta Anand (I); William Tucker (I); Tabassum Master (C); Emma Treacy (C); Alexa King (C); Samiul Rahman (C); Tina Reetun (C); Emerson Tingco (C,T); Katherine Binstead (T); Sash Yoganand Jeetun (T); Nafaz Mohamed Illyias (T); Linda Burton (T); Selma Lewis (T); Graham Brown (T); Gareth Davey (T); Kanom Bibi (T); Vincent Rocco (T); Shabneez Laulloo (T); Tran Dang (T); Andrew Carter (T); Anne Georgiou (T); MinakoLisa Nathasingh (T)
Nijmegen, Netherlands - Radboud University Medical Center (13): Carel Hoyng (I); Jack Weeda (C,T); Esmee Runhart (C); Hans Hermens (T);
Paris, France - Centre Hospitalier National d’Ophtalmologie des Quinze-Vingts, INSERM-DGOS CIC1423 (13): Jose-Alain Sahel (I); Saddek Mohand-Said (I); Isabelle Audo (I); Pierre Queromes (I); Celine Devisme (C); Charlène Da Silveira (C); Carole Romand (C); Serge Sancho (T); Sarah Baranes (T); Mathias Chapon (T); Juliette Amaudruz (T); Céline Chaumette (T); Aurore Girmens (T); Victoria Ganem (T)
Dallas, TX - Retina Foundation of the Southwest (12): David Birch (I); Martin Klein (C,T); Kirsten Locke (C,T)
Tubingen, Germany - Center for Ophthalmology/Clinic for Hereditary Retina Degenerations, University of Tübingen (11): Katarina Stingl (I); Laura Kuehlewein (I); Andrea Rindtorff (C,T); Benjamin-Phillip Beier (C,T); Gudrun Härer (T); Michael Breuninger (T); Susanne Schweyer (T); Ulrike Fuchs (T); Susanne Kramer (T)
Ann Arbor, MI - University of Michigan, Kellogg Eye Center (9): Thiran Jayasundera (I); Naheed W. Khan (I); Abigail Fahim (I); Kari Branham (I); Munira Hussain (C,T); Cailyn Wolford (C,T); Lindsay Godsey (C,T); Laura Trebesh (T); Kit Morehead (T)
Boston, MA - Massachusetts Eye and Ear Infirmary (8): Rachel Huckfeldt (I); Tu Doan (C); Xiao-Hong Wen (C); John Hensel (T); Bethany Biron (T); Jasmeet Bhullar (T); Lisa Dennehy (T); Matthew DiRocco (T); Sarah Brett (T); Emorfily Potsidis (T); Yuji Che (T)
Gainesville, FL - Vitreo Retinal Associates, P.A. (7): Christine Kay (I); Jing Zhang (C,T)
Houston, TX - Baylor College of Medicine, Alkek Eye Center (7): John Timothy Stout (I); Tahira Scholle (I); Annika Joshi (C,T); Joseph Morales (T); Dana Barnett (T); Laine Maier (T)
Durham, NC - Duke University Medical Center (6): Alessandro Iannaccone (I); Magaly Guerrero (C,T); Amy Clark (C,T); Victoria Griffiths (T); Ryan Imperio (T); Malina Sexton (T); Alexandra Bosquet (T); Marina Kedrov (T); Brian Lutman (T)
Baltimore, MD - Johns Hopkins University Hospital, Wilmer Eye Institute (5): Mandeep Singh (I); Mary Frey (C,T); David Emmert (T); Mohamed Ahmed (T); Lisa Liberto (T); Jennifer Bassinger (T)
Salt Lake City, UT - University of Utah, John Moran Eye Center (4): Paul Bernstein (I); Kara Halsey (C); Donnell Creel (T); Kelliann Farnsworth (T); Danielle Wiscombe (T); William Hubbard (T)
Toronto, Canada - University of Toronto, Hospital for Sick Children (4): Elise Heon (I); Vaishnavi Batmanabane (C,T); Katelyn Macneill (T); Jeff Locke (T)
Milwaukee, WI - The Medical College of Wisconsin Eye Institute (1): Thomas Connor, Jr (I); William Wirostko (I); Katie McKenney (C,T); Amber Roberts (T)
FFB Consortium Coordinating Center: Jaeb Center for Health Research, Tampa, FL (staff as of 02/25/2020): Ken Arcieri; Allison Ayala; Elizabeth Caruana; Peiyao Cheng; Jennifer Kennedy; Nadine Label; Stephanie Lee; Wendi Liang; Olivia Parrillo; Rebecca Parsons; Elizabeth Smith
Casey Reading Center [CRC], Casey Eye Institute, Oregon Health Sciences University, Portland, OR (Electroretinography, Static Perimetry, and Kinetic Perimetry): Ellie Chegarnov; Laura Erker; Melissa Krahmer; Brandon Lujan; Audra Miller; Albert Romo; Edeleidys Sanchez Saucedo
Duke Reading Center [DRC], Duke University, Durham, NC (Optical Coherence Tomography and Microperimetry): Vivienne Fang; Sina Farsiu; Magaly Guerrero; Cynthia Heydary; Grace Huh; Glenn Jaffe; Leon Kwark; Eleonora Lad; Brittany Mullaney; Justin Myers; Jayne Nicholson; Katrina Winter
FFB Consortium Genetics Committee: Kari Branham; Stephen Daiger; Robert Hufnagel
FFB Consortium Executive Committee: Jacque Duncan (Chair); Allison Ayala; David Birch; Janet Cheetham; Todd Durham; Rick Ferris; Maureen Maguire; Mark Pennesi
FFB Consortium Audiology Reviewer: Carmen Brewer
References
- 1.McGee TL, Seyedahmadi BJ, Sweeney MO, Dryja TP, Berson EL. Novel mutations in the long isoform of the USH2A gene in patients with Usher syndrome type II or non-syndromic retinitis pigmentosa. J Med Genet. 2010;47(7):499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Le Quesne Stabej P, Saihan Z, Rangesh N, et al. Comprehensive sequence analysis of nine Usher syndrome genes in the UK National Collaborative Usher Study. J Med Genet. 2012;49(1):27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaiserman N, Obolensky A, Banin E, Sharon D. Novel USH2A mutations in Israeli patients with retinitis pigmentosa and Usher syndrome type 2. Arch Ophthalmol. 2007;125(2):219–224. [DOI] [PubMed] [Google Scholar]
- 4.Oishi M, Oishi A, Gotoh N, et al. Comprehensive molecular diagnosis of a large cohort of Japanese retinitis pigmentosa and Usher syndrome patients by next-generation sequencing. Invest Ophthalmol Vis Sci. 2014;55(11):7369–7375. [DOI] [PubMed] [Google Scholar]
- 5.Rivolta C, Sweklo EA, Berson EL, Dryja TP. Missense mutation in the USH2A gene: association with recessive retinitis pigmentosa without hearing loss. Am J Hum Genet. 2000;66(6):1975–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seyedahmadi BJ, Rivolta C, Keene JA, Berson EL, Dryja TP. Comprehensive screening of the USH2A gene in Usher syndrome type II and non-syndromic recessive retinitis pigmentosa. Exp Eye Res. 2004;79(2):167–173. [DOI] [PubMed] [Google Scholar]
- 7.Sun LW, Johnson RD, Langlo CS, et al. Assessing Photoreceptor Structure in Retinitis Pigmentosa and Usher Syndrome. Invest Ophthalmol Vis Sci. 2016;57(6):2428–2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lenassi E, Vincent A, Li Z, et al. A detailed clinical and molecular survey of subjects with nonsyndromic USH2A retinopathy reveals an allelic hierarchy of disease-causing variants. Eur J Hum Genet. 2015;23(10):1318–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pierrache LH, Hartel BP, van Wijk E, et al. Visual Prognosis in USH2A-Associated Retinitis Pigmentosa Is Worse for Patients with Usher Syndrome Type IIa Than for Those with Nonsyndromic Retinitis Pigmentosa. Ophthalmology. 2016;123(5):1151–1160. [DOI] [PubMed] [Google Scholar]
- 10.Sengillo JD, Cabral T, Schuerch K, et al. Electroretinography Reveals Difference in Cone Function between Syndromic and Nonsyndromic USH2A Patients. Sci Rep. 2017;7(1):11170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Slijkerman RW, Vache C, Dona M, et al. Antisense Oligonucleotide-based Splice Correction for USH2A-associated Retinal Degeneration Caused by a Frequent Deep-intronic Mutation. Mol Ther Nucleic Acids. 2016;5(10):e381. [DOI] [PubMed] [Google Scholar]
- 12.Fuster-Garcia C, Garcia-Garcia G, Gonzalez-Romero E, et al. USH2A Gene Editing Using the CRISPR System. Mol Ther Nucleic Acids. 2017;8:529–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iannaccone A. Usher syndrome: correlation between visual field size and maximal ERG response b-wave amplitude. In: LaVail MM, Hollyfield JG, Anderson RE, eds. Retinal Degenerations: Mechanisms and Experimental Therapy. Vol 533. New York: Plenum Publishers; 2003:123–131. [DOI] [PubMed] [Google Scholar]
- 14.Iannaccone A, Kritchevsky SB, Ciccarelli ML, et al. Kinetics of Visual Field Loss in Usher Syndrome Type II. Invest Ophthalmol Vis Sci. 2004;45(3):784–792. [DOI] [PubMed] [Google Scholar]
- 15.Walia S, Fishman GA, Hajali M. Prevalence of cystic macular lesions in patients with Usher II syndrome. Eye (Lond). 2009;23(5):1206–1209. [DOI] [PubMed] [Google Scholar]
- 16.Fishman GA, Bozbeyoglu S, Massof RW, Kimberling W. Natural course of visual field loss in patients with Type 2 Usher syndrome. Retina. 2007;27(5):601–608. [DOI] [PubMed] [Google Scholar]
- 17.Smith TB, Parker M, Steinkamp PN, et al. Structure-Function Modeling of Optical Coherence Tomography and Standard Automated Perimetry in the Retina of Patients with Autosomal Dominant Retinitis Pigmentosa. PLoS One. 2016;11(2):e0148022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferris FL 3rd, Kassoff A, Bresnick GH, Bailey I. New visual acuity charts for clinical research. Am J Ophthalmol. 1982;94(1):91–96. [PubMed] [Google Scholar]
- 19.Massof RW, Finkelstein D, Starr SJ, Kenyon KR, Fleischman JA, Maumenee IH. Bilateral symmetry of vision disorders in typical retinitis pigmentosa. Br J Ophthalmol. 1979;63(2):90–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Birch DG. Emerging Treatments for X-Linked Retinitis Pigmentosa. In. Retinal Physician. Vol 15. April 2018. ed. Ambler, PA: PentaVision LLC; 2018:46–48, 50, 52, 54. [Google Scholar]
- 21.Schiefer U, Pascual JP, Edmunds B, et al. Comparison of the new perimetric GATE strategy with conventional full-threshold and SITA standard strategies. Invest Ophthalmol Vis Sci. 2009;50(1):488–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weleber RG, Smith TB, Peters D, et al. VFMA: Topographic Analysis of Sensitivity Data From Full-Field Static Perimetry. Transl Vis Sci Technol. 2015;4(2):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith TB, Smith N, Weleber RG. Comparison of nonparametric methods for static visual field interpolation. Med Biol Eng Comput. 2017;55(1):117–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Birch DG, Bernstein PS, Iannacone A, et al. Effect of Oral Valproic Acid vs Placebo for Vision Loss in Patients With Autosomal Dominant Retinitis Pigmentosa: A Randomized Phase 2 Multicenter Placebo-Controlled Clinical Trial. JAMA Ophthalmol. 2018;136(8):849–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8(2):135–160. [DOI] [PubMed] [Google Scholar]
- 26.Tee JJL, Yang Y, Kalitzeos A, et al. Characterization of Visual Function, Interocular Variability and Progression Using Static Perimetry-Derived Metrics in RPGR-Associated Retinopathy. Invest Ophthalmol Vis Sci. 2018;59(6):2422–2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Azoulay L, Chaumet-Riffaud P, Jaron S, et al. Threshold Levels of Visual Field and Acuity Loss Related to Significant Decreases in the Quality of Life and Emotional States of Patients with Retinitis Pigmentosa. Ophthalmic Res. 2015;54:78–84. [DOI] [PubMed] [Google Scholar]
- 28.Chaumet-Riffaud AE, Chaumet-Riffaud P, Cariou A, et al. Impact of Retinitis Pigmentosa on Quality of Life, Mental Health, and Employment Among Young Adults. Am J Ophthalmol. 2017;177:169–174. [DOI] [PubMed] [Google Scholar]
- 29.Kim S, Shin DW, An AR, et al. Mental health of people with retinitis pigmentosa. Optom Vis Sci. 2013;90(5):488–493. [DOI] [PubMed] [Google Scholar]
- 30.Hahm B-J, Shin Y-W, Shim E-J, et al. Depression and the vision-related quality of life in patients with retinitis pigmentosa. Br J Ophthalmol. 2008;92(5):650–654. [DOI] [PubMed] [Google Scholar]
- 31.Hartel BP, Lofgren M, Huygen PL, et al. A combination of two truncating mutations in USH2A causes more severe and progressive hearing impairment in Usher syndrome type IIa. Hear Res. 2016;339:60–68. [DOI] [PubMed] [Google Scholar]
- 32.Calzetti G, Levy RA, Cideciyan AV, et al. Efficacy Outcome Measures for Clinical Trials of USH2A Caused by the Common c.2299delG Mutation. Am J Ophthalmol. 2018;193:114–129. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.