Abstract
Common variable immunodeficiency (CVID) is characterized by hypogammaglobulinemia and/or a defective antibody response to T-dependent and T-independent antigens. CVID response to immunization depends on the antigen type, the vaccine mechanism, and the specific patient immune defect. In CVID patients, humoral and cellular responses to the currently used COVID-19 vaccines remain unexplored. Eighteen CVID subjects receiving 2-dose anti-SARS-CoV-2 vaccines were prospectively studied. S1-antibodies and S1-specific IFN-γ T cell response were determined by ELISA and FluoroSpot, respectively. The immune response was measured before the administration and after each dose of the vaccine, and it was compared to the response of 50 healthy controls (HC). The development of humoral and cellular responses was slower in CVID patients compared with HC. After completing vaccination, 83% of CVID patients had S1-specific antibodies and 83% had S1-specific T cells compared with 100% and 98% of HC (p = 0.014 and p = 0.062, respectively), but neutralizing antibodies were detected only in 50% of the patients. The strength of both humoral and cellular responses was significantly lower in CVID compared with HC, after the first and second doses of the vaccine. Absent or discordant humoral and cellular responses were associated with previous history of autoimmunity and/or lymphoproliferation. Among the three patients lacking humoral response, two had received recent therapy with anti-B cell antibodies. Further studies are needed to understand if the response to COVID-19 vaccination in CVID patients is protective enough. The 2-dose vaccine schedule and possibly a third dose might be especially necessary to achieve full immune response in these patients.
Keywords: Common variable immunodeficiency, Primary immunodeficiency diseases, SARS-CoV-2, COVID-19, Vaccination, Immunogenicity
Introduction
CVID is one of the most prevalent humoral immune defects. Patients with CVID frequently present multiple infections, mostly respiratory, but may also develop non-infectious inflammatory complications like autoimmunity, cytopenia, or lymphoproliferation. CVID patients show IgG hypogammaglobulinemia accompanied by absent or diminished IgM, IgA, or both. In addition, a low percentage of class switched IgD−CD27+ memory B lymphocytes is also frequent and associates with reduced antibody production capacity [1, 2].
In clinical practice, the assessment of specific antibody production after vaccination with T-dependent or T-independent antigens provides essential information about the severity of humoral alteration in CVID [3]. Patients with CVID exhibit different humoral response to vaccines [4]. This variability could depend on the antigen nature, the vaccine formulation, or the particular patient defect. Together with defective antibody production, CVID patients may also present altered cellular immune response after vaccination [5, 6]. Cellular response is crucial in the defense against viruses, including SARS-CoV-2.
In the vast majority of healthy individuals, COVID-19 vaccines induce strong cellular and humoral immune responses, with discrete variations depending on the vaccine technology [7–10]. However, the response to COVID-19 vaccination in patients with CVID is still unknown. In addition, given the novelty of mRNA-based vaccines, there is no information about their immunogenicity or protective capability in CVID subjects.
Gathering accurate data about the immunogenicity of COVID-19 vaccines in CVID patients is relevant to define adequate preventive strategies in the context of the pandemic. Moreover, the study of humoral and cellular immune responses to mRNA-based vaccines could lead to a better understanding of CVID and may help to design novel interventions to improve the quality of life of these patients. For these reasons, we aimed to characterize the cellular and humoral response to COVID-19 vaccines in patients with CVID.
Methods
Population and Sample Collection
We prospectively analyzed the immune response to COVID-19 vaccines in CVID patients followed up by the Clinical Immunology Unit at Hospital Universitario 12 de Octubre (Spain). All the patients included in the study strictly met diagnostic criteria for CVID according to the International Consensus [2, 3]. CVID subjects with previous documented SARS-CoV-2 infection were excluded. Patients who refused vaccination or refused to accomplish the immunogenicity testing protocol were also excluded. All patients were assigned to receive one of three different vaccines, BNT162b2 (Pfizer-BioNTech), mRNA-1273 (Moderna), or ChAdOx1 (Oxford-AstraZeneca), according to the National Vaccination Strategy between February and June 2021. For comparison purposes, we recruited a healthy control group (HC) of 50 volunteers (mean age 45 years, 42/50 females) vaccinated with BNT162b2.
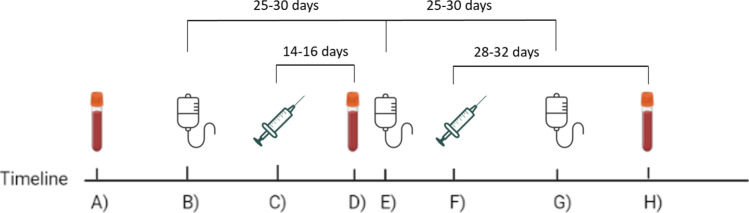
The response to COVID-19 vaccines was evaluated in peripheral blood samples obtained before vaccination, 14 to 16 days after the first dose and 28 to 32 after the second dose. Patients under immunoglobulin replacement therapy (IgRT) received the vaccine 1 or 2 weeks after the immunoglobulin infusion, depending on the treatment regimen (weekly or monthly, respectively) (Fig. 1). All samples were collected 0 to 4 days before the subsequent IgRT. Patients not receiving IgRT were scheduled to start this treatment after vaccination.
Fig. 1.
Study design and schedule. A 1st immune response test. B Immunoglobulin administration. C 1st vaccination dose. D 2nd immune response test. E Immunoglobulin administration. F 2nd vaccination dose. G Immunoglobulin administration. H 3rd immune response test
Determination of S1-Specific and NCP-Specific Antibodies by ELISA
Serum IgG antibodies targeting the S1 and NCP proteins were detected using the Euroimmun kits for ELISA (Anti-SARS-CoV-2 ELISA, Euroimmun AG, Lübeck, Germany) according to manufacturer’s instructions. Optical density (OD) values were measured at 450 nm using the PR 3100 microplate reader (Bio-Rad Life Science, Marnes-La-Coquette, France). In both cases, the results were semi-quantitatively evaluated by calculating the ratio of the OD value of the sample over the OD value of the calibrator (relative OD), with the following cut-off values: < 0.8, negative; ≥ 0.8 to < 1.1, borderline; and ≥ 1.1, positive.
Serum Neutralizing Antibodies Against S Protein
An hACE-2/spike antibody inhibition ELISA-based method was used to determine the neutralizing activity of the sera. Briefly, 96-well plates were coated with 8 ng/ul of a chimeric version of a monoclonal anti-foldon antibody [11]. After blocking with 1% BSA, purified Hexapro [12]-derived construct containing D614G substitution was captured by incubation at 1 ng/ul in blocking solution. Following protein incubation plates were washed with PBS, and incubated with hACE-2 monomeric-StreTag receptor (20 ng/ul) complexed with StrepTactin-HRP (1:2000). Sera were incubated with the receptor-StrepTactin-HRP and the OPD substrate (Sigma-Aldrich), and OD was measured at 493–620 nm. The background was determined in parallel using a close conformation spike protein unable to bind the hACE-2 receptor. A pool of anti-SARS-CoV-2 antibodies-negative sera and hACE-2 monomeric untagged receptor were used as negative and positive controls, respectively. After subtraction of the background, the percentage of neutralization was calculated as [1- (OD495-620 test serum / OD495-620 negative control)] × 100%. In all experiments, incubation of hACE-2 untagged receptor at 200 ng/ul achieved a neutralization rate higher than 85%. Serial dilutions of each sample were tested in duplicated. Neutralizing titer was defined as the serum dilution that resulted in 50% reduction in the absorbance compared with negative.
Determination of S1-Specific Cellular Immunity by FluoroSpot
Whole blood specimens were processed within 24 h from sampling. Peripheral blood mononuclear cells (PBMCs) were freshly isolated by density-gradient centrifugation using Ficoll-Paque and seeded at 300,000 cells/well onto IFN-γ FluoroSpotTM plates (MabTech, Nacka Strand, Sweden) with cell culture medium containing RPMI, 1% L-glutamine, 1% penicillin/streptomycin, 10% fetal bovine serum, and anti-CD28 monoclonal antibody (1 µg/ml). Tests were performed in duplicate and 15-mer overlapping peptides covering the S1 domain of the S protein (166 individual peptides) (SARS-CoV-2 S1 scanning pool, MabTech) were added at a final concentration of 1 µg/ml. Negative control wells lacked peptides, and positive control wells included anti-CD3 antibody (MabTech). Assays were incubated for 16–18 h at 37 °C. Spots were counted using an automated IRIS™ FluoroSpot Reader System (MabTech). To quantify specific cell-mediated responses, spots of the negative control wells were subtracted from the mean spots test wells. The results were expressed as IFN-γ-producing spot forming units (SFUs) per 106 PBMCs. Results were excluded if negative control wells had > 80 SFUs/106 PBMCs or positive control wells had < 400 SFUs/106 PBMCs. Responses were considered positive if the results were at least three times higher than the mean of the negative control wells and above the cut-off values previously reported [13].
Statistical Analysis
Qualitative variables were expressed as absolute and relative frequencies. Categorical variables were compared using the Fisher exact test. Quantitative data were reported as the median with interquartile range. Student’s t test or Mann–Whitney U test were used for continuous variables. Repeated measures were compared with the Wilcoxon signed rank test or the McNemar test, as appropriate. Statistical analysis was performed with GraphPad Prism 8.0 (GraphPad Software, Inc., San Diego, CA).
Ethical Approval
This study was approved by the Institutional Ethical Board (20/167).
Results
Patient Cohort
From a population of 32 patients that fulfilled diagnostic criteria for CVID and were being attended in our clinical unit, 14 patients were excluded. Eleven patients were not able to accomplish the immunogenicity testing protocol. Two patients had previous history of COVID-19: one was asymptomatic and had a positive PCR for SARS-CoV-2, and the other had mild clinical manifestations. Both patients had positive humoral and cellular immune responses against the SARS-CoV-2 S1-protein before vaccination. Finally, one patient refused to be vaccinated and consequently was excluded.
Eighteen patients met inclusion criteria and were further eligible for the analysis (Table 1). The mean age of the patients was 48.1 (22–72 years), and 67% (12/18) were females. Among these CVID patients, 83% (15/18) had a previous history of recurrent infections, which was the most prevalent clinical manifestation (Fig. 2), 66% (12/18) had autoimmune/lymphoproliferative complications, and 55% (10/18) had both recurrent infections and autoimmune/lymphoproliferative manifestations. Most of the patients (77%, 14/18) had some minor alteration of cellular immunity (such as transitory mild lymphopenia) but only 22% (P6, P7, P8, and P18) fulfilled late-onset combined cellular immunodeficiency criteria [14]. Fourteen patients were under IgRT, 9 with intravenous and 5 with subcutaneous immunoglobulins. Only P5, P6, and P14 received rituximab, but years after CVID diagnosis or hypogammaglobulinemia detection. P4, P5, P6, and P14 received corticosteroid therapy in the last years.
Table 1.
Description of the main clinical and laboratory characteristics of patients with common variable immunodeficiency included in the study
| P | Sex | Age | Clinical history | CVID group | Igs at diagnosis | Cellular phenotype | PSCv | PTv | Genetic study | IgRT | Immunosuppressant and/or therapy for tumor (last dose date) | Vaccine | Anti-SARS-COV-2 vaccine response (humoral/cellular) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P1 | F | 47 | Autoimmunity. Solid organ tumor | A/L |
IgG: 597 (low) IgA: 84 IgM: 32 (low) |
-LB: 230 cells/µI. Decreased naive and class switched memory B cells. Increased CD21low B cells -LT: 937 cells/µI. Augmented EM phenotype in CD8 + T cells |
No | No | TNFRSF13B* (c.198C > A/p.Cys66Ter, AR/AD, HE) | No | Surgical resection (2020) | BNT162b2 | Yes/Yes |
| P2 | F | 64 | RTI. Solid tumor | A/L |
IgG: 562 (low) IgA: 49 (low) IgM: 90 |
-LB: 192 cells/µI. Increased CD21low B cells -LT: 876 cells/µI. Augmented EM phenotype in CD8 + T cells |
No | No | Negative | SCIG | Surgical resection (2016) | BNT162b2 | Yes/Yes |
| P3 | F | 58 | Giardia infections. RTI. Autoimmunity. Splenectomized | A/L |
IgG: 326 (low) IgA: < 6.6 (low) IgM: 120 |
-LB: 1171 cells/µI. Decreased class switched and marginal zone memory B cells. Increased CD21low B cells -LT: 2049 cells/µI. Augmented EM activated phenotype in CD8 + T cells |
No | No | Negative | SCIG | No | BNT162b2 | Yes/Yes |
| P4 | F | 22 | RTI. Solid organ tumor. Bronchiectasis. Hodgkin lymphoma | A/L |
IgG: 260 (low) IgA: 31 (low) IgM: 160 |
-LB: 18 cells/µI. Increased CD21low B cells. Low isohemagglutinins -LT:1478 cells/µI. Increased EM T cells at the expense of naive lymphocytes |
No | Yes |
CARD11 (c.1528G > A/p.Ala510Thr, AR/AD, HE) VUS |
IVIG |
Bleomycin (2017) Etoposide (2017) Doxorubicin (2017) Cyclophosphamide (2017) Vincristine (2017) Procarbazine (2017) Corticoids (2017) |
BNT162b2 | Yes/No |
| P5 | M | 41 | RTI. Autoimmunity. Splenomegaly. Lymphadenopathies. GLILD. MALT lymphoma | A/L |
IgG: 426 (low) IgA: 44 (low) IgM: 23 (low) |
-LB: 64 cells/µI B cell lymphopenia. Increased CD21low B cells -LT: 1450 cells/µI. Increased EM T cells |
No | No | Negative | SCIG |
Corticoids (2020) Rituximab (2020) |
BNT162b2 | No/Yes |
| P6 | F | 45 | RTI. UTI. C. Jejuni infections. Fungal infections. Otitis. Autoimmunity | A/L |
IgG: 448 (low) IgA: < 6.6 (low) IgM: 25 (low) |
-LB: 20 cells/µI. Low levels of free light-chains and isohemagglutinins -LT: 1427 cells/µI. Th17 bias at the expense of Th1-like T cells |
No | No | NA | No |
Azatioprine (2011) Leflunomide (2011) Etarnecept (2013) Rituximab (2018) Corticoids (currently) Sulfasalazine (currently) |
BNT162b2 | No/Yes |
| P7 | M | 70 | RTI. Splenomegaly. Lymphadenopathies | A/L |
IgG: 534 (low) IgA: < 6.6 (low) IgM: 32 (low) |
-LB: 146 cells/µI -LT: 927 cells/µI. CD4 + lymphopenia |
No | No | NA | IVIG | No | BNT162b2 | Yes/Yes |
| P8 | F | 59 | Bell's palsy. Soft tissue infections. Hepatosplenomegaly | A/L |
IgG: 379 (low) IgA: 56 (low) IgM: 9 (low) |
-LB: 85 cells/µI. Increased CD21low B cells -LT: 776 cells/µI. CD4 + T cell lymphopenia. No Quantiferon-CMV responder |
No | No | NA | IVIG | No | BNT162b2 | No/No |
| P9 | F | 33 | RTI | Inf |
IgG: 521 (low) IgA: 94 IgM: 13 (low) |
-LB: 82 cells/µI. Decreased memory B cells -LT: 743 cells/µI |
NA | NA | NA | IVIG | No | BNT162b2 | Yes/Yes |
| P10 | M | 45 | RTI | Inf |
IgG: 371 (low) IgA: 43 (low) IgM: 121 |
-LB: 22 cells/µI -LT: 1014 cells/µI |
No | No | NA | IVIG | No | BNT162b2 | Yes/Yes |
| P11 | M | 30 | RTI. Zoster. Splenomegaly | Inf |
IgG: 491 (low) IgA: < 6.6 (low) IgM: 25 (low) |
-LB: 211 cells/µI. Decreased naive and class switched memory with increased transitional B cells -LT: 1736 cells/µI. Augmented activated EM phenotype in CD8 + T cells |
No | No | Negative | SCIG | No | BNT162b2 | Yes/Yes |
| P12 | F | 72 | RTI associated with sepsis | Inf |
IgG: 515 (low) IgA: 133 IgM: 26 (low) |
-LB: 249 cells/µI -LT: 1868 cells/µI |
No | No | NA | IVIG | No | mRNA-1273 | Yes/Yes |
| P13 | F | 36 | RTI. Hepatosplenomegaly Lymphadenopathies. Bronchiectasis | A/L |
IgG: 408 (low) IgA: 99 IgM: 15 (low) |
-LB: 208 cells/µI. Decreased class switched and marginal zone memory B cells. Increased transitional B cells. Increased CD21low B cells -LT: 828 cells/µI. Augmented EM phenotype in CD8 + T cell |
No | Yes | Negative | IVIG | No | mRNA-1273 | Yes/Yes |
| P14 | F | 24 | Autoimmunity. Lymphadenopathies | A/L |
IgG: 149 (low) IgA: < 6.6 (low) IgM: 360 (high) |
-LB: 170 cells/µI. Decreased class switched memory with increased CD21Low and transitional B cells -LT: 1001 cells/µI. CD4 + lymphopenia. Augmented EM phenotype in CD8 + T cells |
No | No |
2 ATM variants (c.4060C > A/ p.Pro1354Thr and c.5039C > T/ p.Pro1680Leu, AR, HE) VUS |
IVIG |
Rituximab (2010) Corticoids (currently) |
mRNA-1273 | Yes/Yes |
| P15 | F | 44 | Autoimmunity. Solid tumor | A/L |
IgG: 445 (low) IgA: 18 (low) IgM: 27 (low) |
-LB: 343 cells/µI. Absence of plasmablasts -LT: 948 cells/µI |
No | Yes |
PIK3R1 (c.889G > A/ p.Glu297Lys AR/AD,HE) VUS |
IVIG |
Surgical resection (2019) Corticoids (currently) |
mRNA-1273 | Yes/No |
| P16 | M | 56 | RTI | Inf |
IgG: 595 (low) IgA: 125 IgM: 31 (low) |
-LB: 224 cells/µI -LT: 1287 cells/µI. Augmented activated EM phenotype in CD8 + T cells |
Yes | Yes | NA | No | No | mRNA-1273 | Yes/Yes |
| P17 | F | 60 | RTI | Inf |
IgG: 345 (low) IgA: 115 IgM: 8 (low) |
-LB: 37 cells/µI -LT: 575 cells/µI. Augmented activated EM phenotype in CD8 + T cells |
No | Yes | NA | No | No | mRNA-1273 | Yes/Yes |
| P18 | M | 60 | RTI. Splenomegaly. Cytopenia | A/L |
IgG: 667 (low) IgA: < 6.6 (low) IgM: 15 (low) |
-LB: 63 cells/µI. Decreased naive and class switched memory B cells -LT: 696 cells/µI |
Yes | Yes | NA | SCIG | No | ChAdOx1 | Yes/Yes |
Immunoglobulins appointed in mg/dl. Normality gap: IgG 700–1600 mg/dl; IgA 70–400 mg/dl; IgM 40–230; LB 100–500 cells/µI; LT 850–2250 cells/µI. A/L autoimmune/lymphoproliferative (group); EM effector memory; F female; GLILD granulomatous-lymphocytic interstitial lung disease; HE heterozygosity; Igs immunoglobulin; IgRT immunoglobulin replacement treatment; Inf only infections (group); LB lymphocyte B; LT lymphocyte T; SCIG subcutaneous Immunoglobulin; IVIG intravenous Immunoglobulin; M male; MALT mucosa-associated lymphoid tissue; P patient; PSCv polysaccharide vaccination response; NA not assessed; PTv proteic vaccination response; RTI respiratory tract infections; UTI urinary tract infections; VUS variant of uncertain significance
*Among the genetic variants in this table, this is the only one described that has probably pathogenic according to previous publications [15, 16], online predictors [17], and genome databases [18]
Fig. 2.
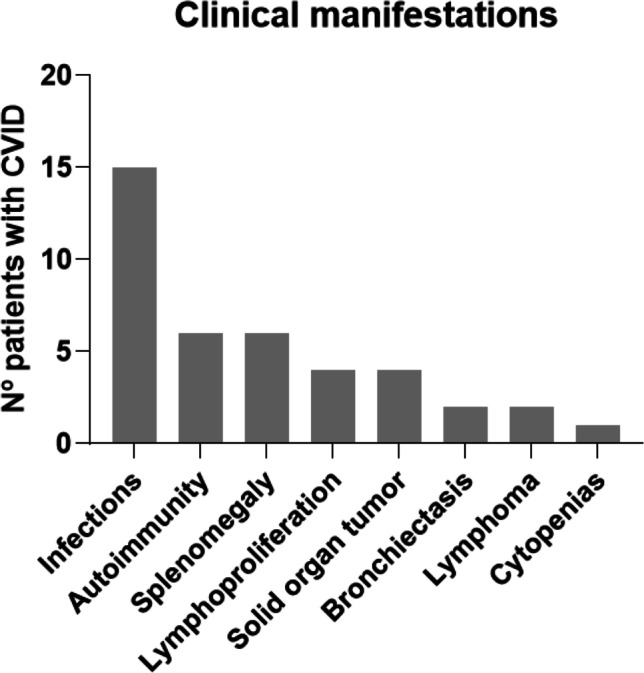
Clinical manifestations in CVID patients. Each category does not exclude other manifestations in the same patient. Number of patients by manifestation: infections = 15; autoimmunity = 6; splenomegaly = 6; lymphoproliferation = 4; solid organ tumor = 4; bronchiectasis = 2; lymphoma = 2; cytopenias = 1
Regarding SARS-CoV-2 vaccination, 17 received the 2-dose mRNA vaccines (11 received BNT162b2 and 6 mRNA-1273) and 1 patient received the 2 doses of the viral vector vaccine ChAdOx1. No serious adverse events were registered after vaccination. Pre-vaccination and post-second dose samples were available in all 18 patients. The sample after the first dose was obtained in 14 out of the 18 patients. As expected, all included CVID patients had negative humoral and cellular immune responses against SARS-CoV-2 pre-vaccination.
Immune Response Rate to Vaccination
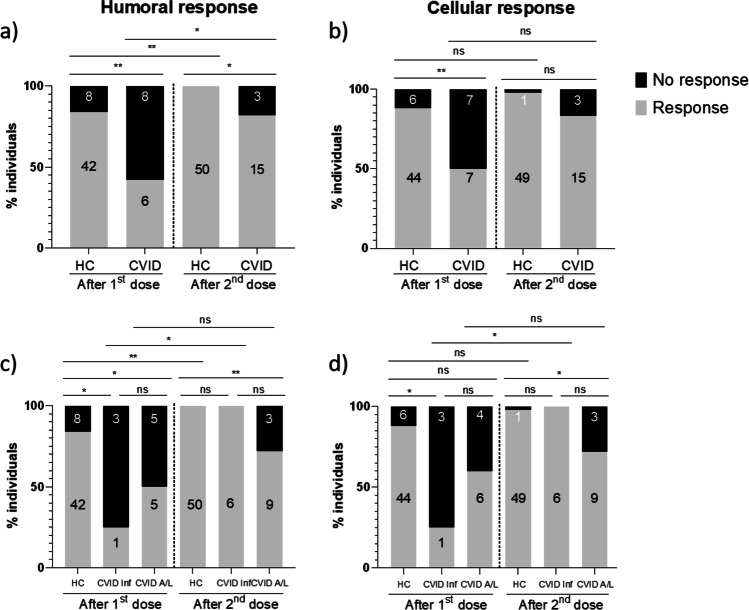
After the first dose, 42% of CVID subjects showed positive anti-SARS-CoV-2 antibody production compared with 84% of HC (p = 0.005) (Fig. 3a). After the second dose, 83% of CVID patients and 100% of HC became anti-S1 IgG positive (p = 0.016). Despite the increase in the antibody response rate observed after the second dose in CVID patients (42% vs 83%, p = 0.027), the percentage of patients who were positive for SARS-CoV-2 antibodies after complete vaccination remained lower than that observed in HC. Three patients (P5, P6, and P8) did not produce specific antibodies after full vaccination. To further assess the quality of the humoral response to vaccination, we evaluated the serum neutralization capacity in sera positive for anti-S1 antibodies. After the first vaccination dose, among 15 patients who made anti-S1 antibodies, 9 showed neutralization capacity. Three patients with positive, but non-neutralizing anti-S1 antibodies after the first dose (P2, P13, and P15), developed neutralizing capacity after the second dose. Six patients with anti-S1 antibodies (P3, P4, P10, P14, P17, and P18) were not able to develop neutralizing antibodies after completing the vaccination. In sum, among the global 18 CVID patients cohort, after full vaccination, 83% developed anti-S1 antibodies whereas neutralizing antibodies were only recorded in 50% of them.
Fig. 3.
Immune response rate in CVID patients. a S1-specific humoral response rate; b S1-specific cellular response rate; c S1-specific humoral response rate in CVID clinical subgroups; d S1-specific cellular response rate in CVID clinical subgroups. HC, healthy controls; CVID, common variable immunodeficiency; CVID Inf, CVID with only infections; CVID A/L, CVID with autoimmunity/lymphoproliferation. ns, not significant; * = p < 0.05; ** = p < 0.01; *** = p < 0.001
In order to evaluate a possible confounding role of the IgRT and/or previous asymptomatic infections in the measurement of anti-S1 antibodies, we tested for the presence of anti-NCP (nucleocapsid) antibodies in all the 15 anti-S1-positive sera obtained after full vaccination. Among them, only 1 sample, belonging to patient P7, was positive for anti-NCP antibodies. P7 did not refer previous COVID-19 symptoms or positive results for anti-SARS-CoV-2 tests. However, despite being negative for anti-S1 antibodies in the pre-vaccination sample, anti-NCP antibodies were already present.
Regarding the cellular immune response to COVID-19 vaccines, 50% of CVID patients had positive cellular response after the first dose, and 83% of them were positive after the second dose (p = 0.062) (Fig. 3b). The cellular response rate was decreased in CVID compared with HC, both after the first dose (50% vs 88%, p = 0.005) and after full vaccination (83% vs 98%, p = 0.054). Three patients (P4, P8, and P15) failed to develop a specific cellular immune response after complete vaccination.
The only two patients with positive antibody but no cellular response to vaccines (P4 and P15, Table 1) had a previous oncologic history at a young age. P4 showed hipogammaglobulinemia (IgG and IgA) with respiratory tract infections and bronchiectasis since 10 years old. In 2017, at 19 years old, she developed a Hodgkin lymphoma (stage IVB), which was treated with bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone with a complete remission. Lymphocyte count has been normal during the last 4 years except for a mild B cell lymphopenia (60 cells/µl). P15 was diagnosed with CVID and antiphospholipid syndrome in 2017. She presented a papillary thyroid microcarcinoma 2 years later which was surgically removed. She is currently under corticoids therapy.
Patients who exclusively lacked humoral response (P5 and P6) had a low CD19 + B lymphocyte count (< 100 cells/µl) after receiving rituximab in 2019 and 2018, respectively. P5 received rituximab as part of the treatment for a gastric mucosa-associated lymphoma developed 5 years after the CVID diagnosis, and because of the presence of a granulomatous-lymphocytic interstitial lung disease. P6 received rituximab due to rheumatoid arthritis plus systemic lupus erythematosus overlap syndrome which appeared 2 years after CVID diagnosis. Both P5 and P6 shared some other clinical characteristics like respiratory tract infection and autoimmunity.
P8 was the only patient who failed to produce humoral and cellular responses. She was a 59-year-old female with type 2 diabetes who had been under IgRT for 3 years, and was vaccinated with BNT162b2. At diagnosis, moderate hypogammaglobulinemia was reported (IgG 379 mg/dl, IgA 56 mg/dl, IgM 9 mg/dl). This patient lacked specific antibody response after tetanus toxoid and Salmonella typhi vaccination and did not respond to CMV-Quantiferon despite a positive serology. She showed cellular impairment (CD4 + lymphopenia of 122 cells/μl) and inflammatory manifestations (hepatosplenomegaly and elevated CD21low B cells). This patient had not received anti-B cell therapy (Table 1).
Finally, we found no differences in vaccine immunogenicity regarding age, gender, comorbidities, or the administered vaccine. IgRT did not affect the vaccination response, at least in our CVID patients’ schedule, nor did the immunoglobulin administration route (data not shown).
Immune Response to Vaccination According to Clinical Subsets of CVID
We further analyzed the response to vaccination in CVID patients with exclusively recurrent infections (n = 6) and in those CVID with autoimmune/lymphoproliferative manifestation (n = 12). Patients with only infectious diseases had a lower antibody production rate than HC after the first dose (25% vs 84%, p = 0.024) and a lower cellular response rate (25% vs 88%, p = 0.012) (Fig. 3c and d). Nevertheless, all six patients from this subgroup had positive SARS-CoV-2-specific antibodies and T cells after the second dose, which meant they had a similar response rate to HC after full vaccination (100% vs 100%, p = 1, for antibodies, and 100% vs 98%, p = 1, for cellular response). This result differed from the subgroup of CVID patients with autoimmune/lymphoproliferative manifestation. In this group, the humoral and the cellular response rates were lower than in HC after the first dose (50% vs 84%, p = 0.031, and 60% vs 88%, p = 0.052, respectively) (Fig. c and d). The decreased rate of response to vaccination in CVID patients with autoimmune/lymphoproliferative manifestation was also observed after the second dose, at the antibody level (75% vs 100%, p = 0.006) and at the cellular level (75% vs 98%, p = 0.021). Administration of the second vaccine dose significantly increased the humoral and cellular response rate in patients with only infectious diseases (p = 0.033 and p = 0.033, respectively); however, it did not have an incremental effect in the response rate in patients with autoimmune/lymphoproliferative manifestations (p = 0.372 and p = 0.651).
Magnitude of Immune Response to Vaccination
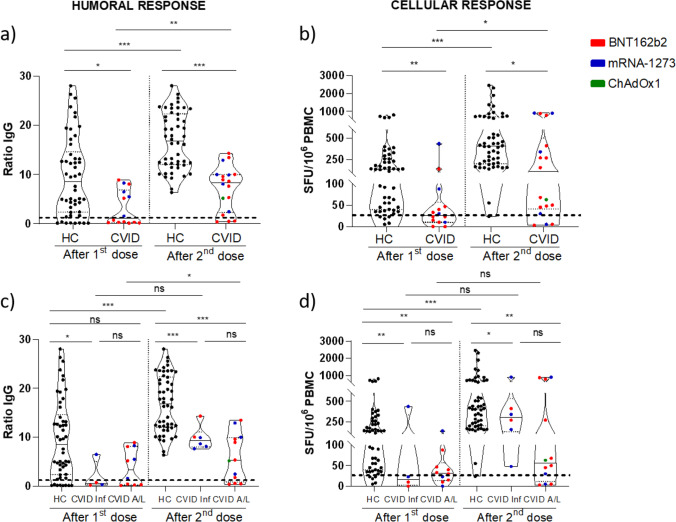
The strength of humoral and cellular responses was significantly lower in CVID patients compared to HC. Administration of the second dose increased the magnitude of the antibody response in CVID, from a level of anti-S1 antibodies of 1.2 to 8.4 (p = 0.002); however, these antibody levels were consistently lower than in HC, after the first dose (1.2 vs 8.6, p = 0.014) and after the second dose (8.4 vs 32.2, p = 0.0001) (Fig. 4a). Similarly, the second vaccine dose increased the magnitude of the cellular response in CVID, from a median of 27 to 113 SFU/106 PBMC (p = 0.018); however, the levels of specific T cells were lower than in HC, after the first dose (median of 27 vs 136 SFU/106 PBMC, p = 0.001) and after the second dose (median of 113 vs 408 SFU/106 PBMC, p = 0.010) (Fig. 4b).
Fig. 4.
Strength of immune response in CVID patients. a Anti-S1 antibodies; b anti-S1 IFN-γ T cells; c anti-S1 antibodies in CVID subgroups; d anti-S1 IFN-γ T cell in CVID subgroups. Dotted lines represent positivity cut-off: ≥ 1.1 ratio of OD for anti-S1 antibodies and > 25 IFN-γ SFU/106 PBMCs for S1-specific IFN-γ T cells response. HC, healthy controls; CVID, common variable immunodeficiency; CVID Inf, CVID with only infections; CVID A/L, CVID with autoimmunity/lymphoproliferation. ns, not significant; * = p < 0.05; ** = p < 0.01; *** = p < 0.001
When we analyzed by CVID patient subgroup, we observed a similar behavior. There was a discrete increase in antibody levels after the second dose in patients with only infectious diseases (from 0.5 to 9.3, p = 0.125) and in patients with autoimmune/lymphoproliferative manifestations (from 3.4 to 5.3, p = 0.018) (Fig. 4c). Nevertheless, the antibody response in both patient subgroups was significantly weaker than in HC after full vaccination (p = 0.0001 and p = 0.0001). Regarding the strength of the cellular response, there was a non-significant increase of specific T cells after the second dose in patients with only infectious diseases (from median of 17 to 306 SFU/106 PBMC, p = 0.125) and in patients with autoimmune/lymphoproliferative manifestations (from median of 32 to 57 SFU/106 PBMC, p = 0.084) (Fig. 4d). The cellular response in both patient subgroups was also significantly weaker than in HC after full vaccination (p = 0.046 and p = 0.008). When comparing the CVID subgroup of patients with only infectious diseases vs patients with autoimmune/lymphoproliferative manifestations, the differences in the magnitude of both humoral (9.3 vs 5.3 respectively, p = 0.151) and cellular (306 vs 57 SFU/106 PBMC respectively, p = 0.157) immune responses did not reach significance.
CVID Patients’ Follow-up After Vaccination
The evolution of all CVID patients was evaluated 3 months after all the patients completed the vaccination schedule. Only one patient (P10) referred contact or exposure to COVID-19 cases. P10 produced anti-S1 antibodies and cellular response after vaccination but he did not show neutralization capacity. Six days after the exposure, and 68 days after completing the vaccination schedule, this patient experienced COVID-19-related symptoms. He started referring intense myalgia and weakness, without fever or dyspnea. He was evaluated at the emergency room, showing 95% of oxygen saturation with adequate respiratory and heart rates. RT-PCR was positive for SARS-CoV-2 in a sample obtained via mucosal nasal swab. Ten days of quarantine at home was recommended without treatment. The symptom lasted 5 days and included cough, ageusia, and anosmia. Despite his background of obesity, smoking habit, and hypertriglyceridemia, he is now completely asymptomatic without sequels of the disease.
Discussion
In this work, we show a detectable SARS-CoV-2 specific humoral and cellular response in most CVID patients. Specifically, 83% of CVID patients had positive S1-specific antibodies and T cells after full vaccination. However, the development of these responses was slower, with a lower quality and less potent when compared to HC.
According to our results, COVID-19 vaccines may induce a high rate of detectable specific antibodies in CVID patients. Previous data has shown that 0–20% of patients had a humoral response to the influenza A vaccine [19–23]. The response rate was close to 20% after one dose of polysaccharide vaccines [5] and 64% after two doses of tetanus conjugated vaccine (compared with 93% in the general population) [24, 25]. Cellular responses after vaccination are much less explored. Diminished or normal IFN-γ T cell responses against influenza virus have been reported in CVID following vaccination [26, 27].
Regarding the immunogenicity of COVID-19 vaccines in CVID patients, the high response rate recorded in our work agrees with data from Squire and coworkers, who showed that all 6 CVID subjects analyzed by them developed specific anti-S1 antibodies after 2 doses of the vaccine [28]. Similarly, a second work studying a wider spectrum of immunodeficiencies showed that among 12 CVID subjects, 10 and 8 had adequate humoral and cellular response, respectively, after completing the vaccination schedule [29]. In addition, no serious adverse events were observed. Whether the observed immunogenicity of the COVID-19 vaccines may be related to the mRNA vaccine technology remains to be explored.
Despite the proportions of CVID patients responding to COVID-19 vaccination seem encouraging, the development of the immune response was slower in CVID than in HC, since most CVID patients needed 2 doses to become immunized. Moreover, we found a discordant capacity to produce virus-specific antibodies (83% of vaccinated patients) and virus-neutralizing antibodies (50% of vaccinated patients), suggesting that either one or both quantity and quality of the humoral immune response to COVID-19 vaccination may be suboptimal in CVID subjects. Interestingly, 2 out of the 3 patients who did not make antibodies after full vaccination had been receiving rituximab during the 2 previous years. In addition, after completing vaccination, CVID patients made significantly lower antibody levels and weaker cellular responses than HC. It has been suggested that some degree of isolated cellular response might provide protection against SARS-CoV-2 [30]. This concept might be related with our observation on patient P10 who contracted COVID-19 after vaccination but recovered at home without any treatment, despite not having made neutralizing antibodies and presenting severity risk factors. Further studies are needed to understand if the observed responses to vaccination in CVID patients are potent enough to provide adequate protection, or if CVID patients might benefit from a third vaccination dose.
During the first half of 2021, an elevation of anti-SARS-CoV-2 antibody levels has been reported in IgRT products [31]. By demonstrating the absence of anti-NCP antibodies in sera after vaccination, we discarded the possibility that the anti-S1 antibodies detected would come from the IgRT and/or previous asymptomatic SARS-CoV-2 infection. Only one patient who seroconverted for anti-S1 antibodies after vaccination showed also anti-NCP antibodies, which were already present in the pre-vaccination sample. Whether the concomitant presence of anti-S1 and anti-NCP antibodies in this patient could be related to a previous SARS-CoV-2 infection, other coronavirus species infection or transference of antibodies by IgRT remains unknown.
CVID patients with autoimmune/lymphoproliferative manifestations have the worst disease course [32]. Discordant or absent antibody and cellular responses appeared associated with this complications in our CVID cohort. Similarly, Rezaei and coworkers reported significantly increased rates of splenomegaly and autoimmunity among CVID patients who did not produce specific antibodies after the meningococcal polysaccharide vaccination [33].
The results of this study are limited by the relatively small number of patients included. The study was underpowered to evaluate vaccine immunogenicity in the different CVID subgroups, and it was not possible to evaluate the impact of different vaccine technologies or different IgRT strategies. These questions should be addressed studying wider multicentric cohorts.
In conclusion, in CVID patients, the 2-dose COVID-19 vaccine schedule seems to be especially necessary to develop specific antibodies and T cell response. Further studies may clarify the levels of specific antibodies and T cells which are needed to confer clinical protection. After vaccination, CVID subjects with autoimmune/lymphoproliferative manifestations may be at higher risk of SARS-CoV-2 infection due to the lower response in this particular population. Lack of antibody response was found associated with B lymphopenia after B cell depletion therapy, and absence of cellular response was observed in association with previous oncologic history and defects in the cellular immunity compartment. Compared to previous vaccines, the new mRNA vaccine technology could be useful for the prevention of future recurrent infections in CVID patients by inducing a stronger immune response.
Acknowledgements
We would like to thank all the patients and their families. Special thanks should be given to Miguel Moreno Batanero, for his technical collaboration in the cellular immunity quantification for this research.
Abbreviations
- CVID
Common variable immunodeficiency
- SFU
Spot forming units
- HC
Healthy control group
- IgRT
Immunoglobulins replacement therapy
Author Contribution
Conceptualization and methodology, O.C-M and E.P-A; funding acquisition, E.P-A; references and patient collection, D.P and D.A-S; laboratory measurement and laboratory methods, F.J.G-E, P.A-V, O.C, P.P-R, and P.S-F; statistical analysis, D.A-S.; writing, D.A-S and O.C-M; review and editing, A.S, R.L-G, and E.RdF; supervision, E.P-A and L.M.A. All authors have accepted responsibility for the entire content of this manuscript and approved its submission and publication.
Funding
This study was supported by the Instituto de Salud Carlos III, Spanish Ministry of Science and Innovation (COVID-19 research call COV20/00181)—cofinanced by the European Development Regional Fund “A way to achieve Europe,” Operative Program Intelligent Growth 2014/2020, and by Consejeria de Sanidad de la Comunidad de Madrid (CIVICO study 2020/0082). O.C-M and R.L-G hold a research contract “Rio Hortega” (CM19/00092 and CM19/00120, respectively) from the Instituto de Salud Carlos III, Spanish Ministry of Science and Innovation.
Data Availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Code Availability
This work complies with field standards.
Declarations
Ethics Approval
This study was approved by the Institutional Ethical Board (20/167).
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Consent for Publication
Patients signed informed consent regarding publishing their data.
Competing Interests
The authors declare no competing interests.
Footnotes
Daniel Arroyo-Sánchez and Oscar Cabrera-Marante share first authorship
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cunningham-Rundles C. Common variable immune deficiency: Dissection of the variable. Immunol Rev. 2019;287:145–161. doi: 10.1111/imr.12728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seidel MG, Kindle G, Gathmann B, Quinti I, Buckland M, van Montfrans J, et al. The European Society for Immunodeficiencies (ESID) Registry Working Definitions for the Clinical Diagnosis of Inborn Errors of Immunity. J Allergy Clin Immunol Pract. 2019;7:1763–1770. doi: 10.1016/j.jaip.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Bonilla FA, Barlan I, Chapel H, Costa-Carvalho BT, Cunningham-Rundles C, de la Morena MT, et al. International Consensus Document (ICON): Common Variable Immunodeficiency Disorders. J Allergy Clin Immunol Pract. 2016;4:38–59. doi: 10.1016/j.jaip.2015.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Herz W, McGeady SJ. Antibody response in common variable immunodeficiency. Ann Allergy Asthma Immunol. 2003;90:244–247. doi: 10.1016/S1081-1206(10)62149-7. [DOI] [PubMed] [Google Scholar]
- 5.Goldacker S, Draeger R, Warnatz K, Huzly D, Salzer U, Thiel J, et al. Active vaccination in patients with common variable immunodeficiency (CVID) Clin Immunol. 2007;124:294–303. doi: 10.1016/j.clim.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 6.Ko J, Radigan L, Cunningham-Rundles C. Immune competence and switched memory B cells in common variable immunodeficiency. Clin Immunol. 2005;116:37–41. doi: 10.1016/j.clim.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 7.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramasamy MN, Minassian AM, Ewer KJ, Flaxman AL, Folegatti PM, Owens DR, et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2021;396:1979–1993. doi: 10.1016/S0140-6736(20)32466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sahin U, Muik A, Vogler I, Derhovanessian E, Kranz LM, Vormehr M, et al. BNT162b2 vaccine induces neutralizing antibodies and poly-specific T cells in humans. Nature. 2021;595:572–577. doi: 10.1038/s41586-021-03653-6. [DOI] [PubMed] [Google Scholar]
- 11.Battles MB, Mas V, Olmedillas E, Cano O, Vazquez M, Rodriguez L, et al. Structure and immunogenicity of pre-fusion-stabilized human metapneumovirus F glycoprotein. Nat Commun. 2017;8:1528. doi: 10.1038/s41467-017-01708-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsieh CL, Goldsmith JA, Schaub JM, DiVenere AM, Kuo HC, Javanmardi K, et al. Structure-based design of prefusion-stabilized SARS-CoV-2 spikes. Science. 2020;369:1501–1505. doi: 10.1126/science.abd0826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Almendro-Vázquez PL-GR, RuizRuigomez M, Utrero-Rico A, Lalueza A, Maestro de la Calle G, Delgado P, Perez-Ordoño L, Muro E, Vila J, Zamarron I, Moreno-Batanero M, Chivite-Lacaba, M, Gil-Etayo FJ, Lumbreras C, Arellano I, Alarcon B, Allende LM, Aguado JM, Paz-Artal E. Longitudinal dynamics of SARS-CoV-2-specific adaptive immunity after natural infection or BNT162b2 vaccination. Submitted and under review 2021. [DOI] [PMC free article] [PubMed]
- 14.von Spee-Mayer C, Koemm V, Wehr C, Goldacker S, Kindle G, Bulashevska A, et al. Evaluating laboratory criteria for combined immunodeficiency in adult patients diagnosed with common variable immunodeficiency. Clin Immunol. 2019;203:59–62. doi: 10.1016/j.clim.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Pulvirenti F, Zuntini R, Milito C, Specchia F, Spadaro G, Danieli MG, et al. Clinical Associations of Biallelic and Monoallelic TNFRSF13B Variants in Italian Primary Antibody Deficiency Syndromes. J Immunol Res. 2016;2016:8390356. doi: 10.1155/2016/8390356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salzer U, Chapel HM, Webster AD, Pan-Hammarstrom Q, Schmitt-Graeff A, Schlesier M, et al. Mutations in TNFRSF13B encoding TACI are associated with common variable immunodeficiency in humans. Nat Genet. 2005;37:820–828. doi: 10.1038/ng1600. [DOI] [PubMed] [Google Scholar]
- 17.Kopanos C, Tsiolkas V, Kouris A, Chapple CE, Albarca Aguilera M, Meyer R, et al. VarSome: the human genomic variant search engine. Bioinformatics. 2019;35:1978–1980. doi: 10.1093/bioinformatics/bty897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karczewski KJ, Francioli LC, Tiao G, Cummings BB, Alfoldi J, Wang Q, et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature. 2020;581:434–443. doi: 10.1038/s41586-020-2308-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gardulf A, Abolhassani H, Gustafson R, Eriksson LE, Hammarstrom L. Predictive markers for humoral influenza vaccine response in patients with common variable immunodeficiency. J Allergy Clin Immunol. 2018;142(1922–31):e2. doi: 10.1016/j.jaci.2018.02.052. [DOI] [PubMed] [Google Scholar]
- 20.van Assen S, Holvast A, Telgt DS, Benne CA, de Haan A, Westra J, et al. Patients with humoral primary immunodeficiency do not develop protective anti-influenza antibody titers after vaccination with trivalent subunit influenza vaccine. Clin Immunol. 2010;136:228–235. doi: 10.1016/j.clim.2010.03.430. [DOI] [PubMed] [Google Scholar]
- 21.Mieves JF, Wittke K, Freitag H, Volk HD, Scheibenbogen C, Hanitsch LG. Influenza Vaccination in Patients with Common Variable Immunodeficiency (CVID) Curr Allergy Asthma Rep. 2017;17:78. doi: 10.1007/s11882-017-0749-3. [DOI] [PubMed] [Google Scholar]
- 22.Pedersen G, Halstensen A, Sjursen H, Naess A, Kristoffersen EK, Cox RJ. Pandemic influenza vaccination elicits influenza-specific CD4+ Th1-cell responses in hypogammaglobulinaemic patients: four case reports. Scand J Immunol. 2011;74:210–218. doi: 10.1111/j.1365-3083.2011.02561.x. [DOI] [PubMed] [Google Scholar]
- 23.Hanitsch LG, Lobel M, Mieves JF, Bauer S, Babel N, Schweiger B, et al. Cellular and humoral influenza-specific immune response upon vaccination in patients with common variable immunodeficiency and unclassified antibody deficiency. Vaccine. 2016;34:2417–2423. doi: 10.1016/j.vaccine.2016.03.091. [DOI] [PubMed] [Google Scholar]
- 24.Erdem SB, Gulez N, Genel F, Karaman S, Nacaroglu HT. Characteristics of the patients followed with the diagnosis of common variable immunodeficiency and the complications. Cent Eur J Immunol. 2019;44:119–126. doi: 10.5114/ceji.2019.87060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schlumberger M, Yvonnet B, Que HV, Chhem DB, Saliou P, Le Tu TC, et al. [Serological study carried out in Cambodia during a tetanus vaccination in adults] Bull Soc Pathol Exot. 2008;101:36–42. [PubMed] [Google Scholar]
- 26.van Assen S, de Haan A, Holvast A, Horst G, Gorter L, Westra J, et al. Cell-mediated immune responses to inactivated trivalent influenza-vaccination are decreased in patients with common variable immunodeficiency. Clin Immunol. 2011;141:161–168. doi: 10.1016/j.clim.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 27.Friedmann D, Goldacker S, Peter HH, Warnatz K. Preserved Cellular Immunity Upon Influenza Vaccination in Most Patients with Common Variable Immunodeficiency. J Allergy Clin Immunol Pract. 2020;8(2332–40):e5. doi: 10.1016/j.jaip.2020.04.019. [DOI] [PubMed] [Google Scholar]
- 28.Squire J, Joshi A. Seroconversion after coronavirus disease 2019 vaccination in patients with immune deficiency. Ann Allergy Asthma Immunol. 2021.10.1016/j.anai.2021.05.015. [DOI] [PMC free article] [PubMed]
- 29.Hagin D, Freund T, Navon M, Halperin T, Adir D, Marom R, et al. Immunogenicity of Pfizer-BioNTech COVID-19 vaccine in patients with inborn errors of immunity. J Allergy Clin Immunol. 2021 doi: 10.1016/j.jaci.2021.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sekine T, Perez-Potti A, Rivera-Ballesteros O, Stralin K, Gorin JB, Olsson A, et al. Robust T Cell Immunity in Convalescent Individuals with Asymptomatic or Mild COVID-19. Cell. 2020;183(158–68):e14. doi: 10.1016/j.cell.2020.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Romero C, Diez JM, Gajardo R. Anti-SARS-CoV-2 antibodies in healthy donor plasma pools and IVIG products. Lancet Infect Dis. 2021;21:765–766. doi: 10.1016/S1473-3099(21)00059-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cunningham-Rundles C. The many faces of common variable immunodeficiency. Hematology Am Soc Hematol Educ Program. 2012;2012:301–305. doi: 10.1182/asheducation-2012.1.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rezaei N, Aghamohammadi A, Siadat SD, Moin M, Pourpak Z, Nejati M, et al. Serum bactericidal antibody responses to meningococcal polysaccharide vaccination as a basis for clinical classification of common variable immunodeficiency. Clin Vaccine Immunol. 2008;15:607–611. doi: 10.1128/CVI.00489-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
This work complies with field standards.