Abstract
Background
Patients with Parkinson’s disease (PD) are at higher risk of COVID-19 infection as most of them are at older age. The goal of this study is to update the pooled prevalence of COVID-19 infection in patients with PD.
Methods
Two researchers systematically searched PubMed, Scopus, EMBASE, Web of Science, Google Scholar, and also gray literature including references of the included studies which were published before September 2021. We extracted data regarding the total number of participants, first author, publication year, the country of origin, mean age, number with COVID-19, symptoms, hospitalization, and death.
Results
We found 1693 articles by literature search; after deleting duplicates, 798 remained. Thirty articles remained for meta-analysis. The pooled prevalence of COVID-19 infection in PD cases was 5% (95%CI: 4–6%) (I2 = 98.1%, P < 0.001). The pooled prevalence of fever in cases with PD was 4% (95%CI: 2–6%) (I2 = 96%, P < 0.001). The pooled prevalence of cough in cases with PD was 3% (95%CI: 2–4%) (I2 = 95.9%, P < 0.001). The pooled prevalence of hospitalization in cases with COVID-19 infection was 49% (95%CI: 29–52%) (I2: 93.5%, P < 0.001). The pooled prevalence of mortality in COVID-19 cases was 12% (95%CI: 10–14%) (I2 = 97.6%, P < 0.001).
Conclusion
The results of this systematic review and meta-analysis show that the pooled prevalence of COVID-19 infection in PD cases is 5% besides hospitalization and mortality rates which are 49% and 12%.
Keywords: COVID-19, Parkinson’s disease, Prevalence
Introduction
The new coronavirus was first introduced in December 2019, and now, it is in pandemic stage [1]. The most frequent manifestations are fever, cough, and malaise while underlying diseases, advanced age, and medications play an important role in prognosis [2]. Following Alzheimer’s disease, Parkinson’s disease (PD) is the second common neurodegenerative disease which could have a negative effect on COVID-19 prognosis in affected cases [3]. The prevalence of PD is between 113 and 873 cases per 100,000 people and is higher in Europe and lowest in Asia [4]. As the patients with PD are elderlies and they have underlying diseases, they are at higher risk of developing COVID-19 and quarantine will affect the presence of the symptoms [5].
Up to now, systematic reviews and meta-analysis have been done and reported various pooled prevalence of COVID-19 infection in patients with PD. The goal of this study is to update the pooled prevalence of COVID-19 infection in patients with PD.
Methods
Two researchers systematically searched PubMed, Scopus, EMBASE, Web of Science, Google Scholar, and also gray literature including references of the included studies which were published before September 2021.
The search strategy was as follows:
(“Idiopathic Parkinson Disease” OR “Lewy Body Parkinson Disease” OR (“Parkinson Disease” AND Idiopathic) OR (“Parkinson Disease” AND “Lewy Body”) OR (“Parkinson Disease” AND Idiopathic) OR “Parkinson Disease” OR “Idiopathic Parkinson Disease” OR “Lewy Body Parkinson Disease” OR “Primary Parkinsonism” OR (Parkinsonism AND Primary) OR “Paralysis Agitans” OR Parkinson) AND (“COVID 19” OR “COVID-19 Virus Disease” OR “COVID 19 Virus Disease*” OR “COVID-19 Virus Disease*” OR (Disease AND “COVID-19 Virus”) OR (“Virus Disease” AND COVID-19) OR “COVID-19 Virus Infection*” OR “COVID 19 Virus Infection” OR (Infection AND “COVID-19 Virus”) OR (“Virus Infection” AND COVID-19) OR “2019-nCoV Infection” OR “2019 nCoV Infection*” OR (Infection AND 2019-nCoV) OR “Coronavirus Disease-19” OR “Coronavirus Disease 19” OR “2019 Novel Coronavirus Disease” OR “2019 Novel Coronavirus Infection” OR “2019-nCoV Disease” OR “2019 nCoV Disease” OR “2019-nCoV Diseases” OR (Disease AND 2019-nCoV) OR “COVID19” OR “Coronavirus Disease 2019” OR (“Disease 2019” AND Coronavirus) OR “SARS Coronavirus 2 Infection” OR “SARS-CoV-2 Infection” OR (Infection AND SARS-CoV-2) OR “SARS CoV 2 Infection*” OR “COVID-19 Pandemic*” OR “COVID 19 Pandemic” OR (Pandemic AND COVID-19)).
Inclusion criteria were as follows:
We included cross-sectional studies which had reported the number of patients with Parkinson’s disease who had COVID-19 infection.
Exclusion criteria were as follows:
Letters to the editor, case–control, case reports, and cross-sectional studies which had no clear data.
We extracted data regarding the total number of participants, first author, publication year, the country of origin, mean age, number with COVID-19, symptoms, hospitalization, and death.
Risk of bias assessment
Using Newcastle–Ottawa Quality Assessment scale (adapted for cross-sectional studies), we evaluated the risk of bias [6].
Statistical analysis
All statistical analyses were performed using STATA (Version 14.0; Stata Corp LP, College Station, TX, USA). We used random effects.
To determine heterogeneity, Inconsistency (I2) was calculated.
Results
We found 1693 articles by literature search, after deleting duplicates 798 remained. Thirty articles remained for meta-analysis (Fig. 1).
Fig. 1.
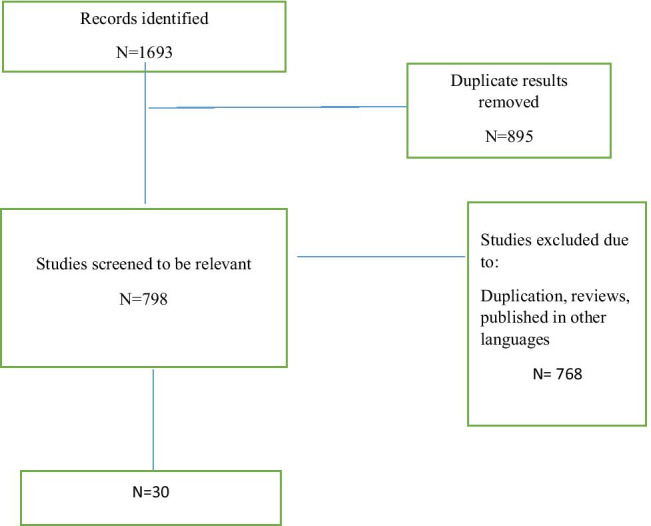
Flow diagram summarizing the selection of eligible studies
Thirty articles were included. The number of included patients differed between 10 and 64,434, and mean disease duration varied between 6 and 13 years. Most studies were conducted in the United States of America (USA). Minimum and maximum quality assessment scores were 4–9.
The basic characteristics of the included studies are shown in Table 1.
Table 1.
Basic characteristics of included studies
| Author | Year | Country | Type. Study | Total. PD | Number. Covid1 | Number confirm Number PCR2 | Ag Case3 | Age.SD_Case | Female Case | Male Case | Disease. Duration case | Disease. Duration. SD Case | Fever | Cough | Dyspnea | Hospitalized | Death | NOS Quality assessment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Heng Zhai [7] | 2021 | China | Retrospective cohort | 10 | 10 | 10 | 72.1 | 11.46 | 7 | 3 | 8.6 | 5.9 | 8 | 10 | 3 | 10 | 3 | 5/9 |
| Lynda Nwabuobi [8] | 2021 | USA | Retrospective cohort | 25 | 25 |
25 25 |
Median: 82 | IQR: 73–88 | 6 | 19 | NR | NR | 12 | 11 | NR | 17 | 8 | 5/9 |
| Raphael Scherbaum [9] | 2021 | Germany | Cross-sectional | 64,434 | 693 |
693 693 |
NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | 245 | 8/10 |
| Mehri Salari [10] | 2021 | Iran | Cross-sectional | 647 | 73 |
73 73 |
60.57 | 12.46 | NR | NR | NR | NR | 30 | 26 | 16 | 10 | NR | 6/10 |
| Ana C. Tahira [11] | 2021 | Brazil | Cross-sectional | 182 | 35 |
35 35 |
NR | NR | NR | NR | NR | NR | NR | NR | NR | 25 | 14 | 5/10 |
| Maria Buccafusca [12] | 2021 | Italy | Cohort | 12 | 12 |
12 12 |
73.33 | 10.73 | 6 | 6 | 13.5 | 6.34 | 4 | 2 | 1 | 9 | 0 | 5/9 |
| Raminder Parihar [13] | 2021 | USA | Cross-sectional | 70 | 53 |
53 53 |
Mean: 78.7 | IQR: 12 | 22 | 31 | NR | NR | NR | NR | NR | NR | 19 | 5/10 |
| Valentina Leta [14] | 2021 | UK | Cross-sectional | 27 | 27 | 27 | 59 | 12.7 | 11 | 16 | 9.2 | 7.8 | NR | NR | NR | 6 | NR | 5/10 |
| Mehri Salari [15] | 2021 | Iran | Cross-sectional | 87 | 87 | NR | 75.8 | 7.3 | NR | NR | NR | NR | NR | NR | NR | 31 | 31 | 5/10 |
| Ousseny Zerbo [16] | 2021 | USA | Cross -sectional | 9100 | 325 |
325 325 |
NR | NR | NR | NR | NR | NR | NR | NR | NR | 172 | 39 | 7/10 |
| Hossein Estiri [17] | 2021 | USA | Cross-sectional | 165 | 165 |
165 165 |
NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | 32 | 6/10 |
| Megan P. Feeney [18] | 2021 | USA | Cross-sectional | 1342 | 17 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | 6/10 |
| Christian Ineichen [19] | 2021 | Switzerland | Retrospective cohort | 264 | 4 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | 5/9 |
| Ladan Akbarian-Tefaghi [20] | 2020 | UK |
Cross-sectional (abstract) |
28 | 12 |
12 12 |
NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NA |
| Joy Antonelle de Mar [21] | 2020 | United States | Retrospective cohort | 21 | 21 |
21 21 |
75.19 | 5.41 | 8 | 13 | 9.95 | 6.45 | 10 | 9 | 14 | 16 | 6 | 5/9 |
| Jeanine J.S [22] | 2020 | Netherlands | Prospective cohort | 280 | 99 |
99 99 |
NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | 6/9 |
| Alfonso Fasano [23] | 2020 | Italy | Case–control | 1486 | 105 | NR | 70.5 | 10.1 | 50 | 55 | 9.9 | 6.4 | 74 | 62 | NR | 18 | 6 | 7/9 |
| Carlo Alberto Artusi [24] | 2020 | Italy | Cross-sectional | 1407 | 8 |
8 8 |
74 | 7.52 | 3 | 5 | 12.14 | 7.62 | 6 | 2 | 3 | 8 | 6 | 6/10 |
| Sainz-Amo [25] | 2020 | Spain | Case–control | 211 | 39 | NR | 75.9 | 9 | 16 | 23 | 8.9 | 6.2 | NR | NR | NR | 21 | 8 | 6/9 |
| Roberto Cilia [26] | 2020 | Italy | Case–control | 12 | 12 | NR | 65.5 | 8.9 | 7 | 5 | 6.3 | 3.6 | 10 | 9 | 4 | 1 | 0 | 5/9 |
| Santos García | 2020 | Spain | Cross-sectional | 568 | 15 | NR | 65.6 | 9.4 | 8 | 7 | 6.8 | 4.9 | NR | NR | NR | 5 | 0 | 6/10 |
| Mehri Salari | 2020 | Iran | Cross-sectional | 137 | 2 |
2 2 |
NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | 5/10 |
| Angelo Antonini | 2020 | Italy, UK | Case series | 10 | 10 | NR | 78.3 | 0.0847 | 4 | 6 | 12.7 | 8.09 | 6 | 8 | 2 | 10 | 4 | NA |
| Eleonora Del Prete | 2020 | Italy | Case-controlled | 740 | 7 |
7 7 |
75.71 | 8.9 | 3 | 4 | 9.29 | 3.59 | NR | NR | NR | 4 | 1 | 7/9 |
| Luca Vignatelli | 2020 | Italy | Cohort | 696 | 4 | NR | 76.5 | NR | 1 | 3 | NR | NR | NR | NR | NR | 4 | 1 | 6/9 |
| Alfonso Fasano | 2020 | Italy, Iran | Cohort | 2238 | 117 | NR | 71.4 | 10.8 | 43 | 74 | 9.4 | 5.8 | NR | NR | NR | 37 | 23 | 7/9 |
| Ethan G. Brown | 2020 | United States | Cross-sectional | 5429 | 51 |
22 17 |
65 | NR | 27 | 24 | NR | NR | 32 | 36 | NR | 5 | 0 | 6/10 |
| Qiang Zhang | 2020 | United States | Cross-sectional | 694 | 694 | NR | Median: 79 | NR | 276 | 418 | NR | NR | NR | NR | NR | NR | 148 | 6/10 |
1Number of PD patients who has effected by COVID-19
2Number of PD patients who has confirmed as COVID-19 case, by Polymerase chain reaction (PCR)
3Case means all PD patients who has effected by COVID-19
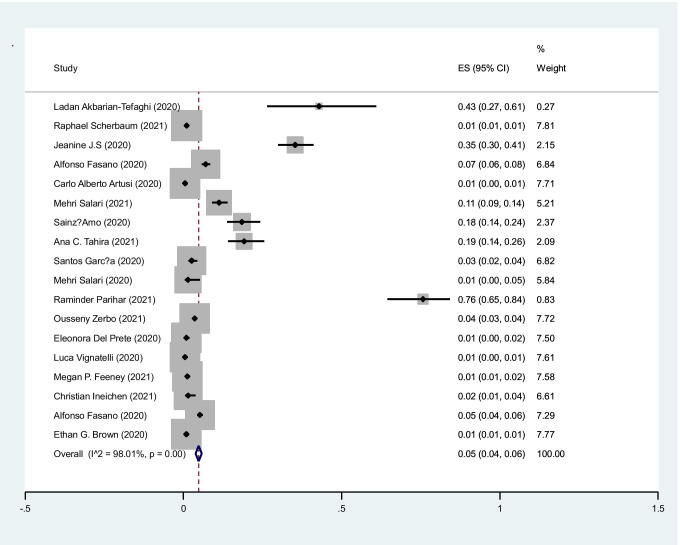
The pooled prevalence of COVID-19 infection in PD cases was 5% (95%CI: 4–6%) (I2=98.1%, P<0.001) (Figure 2).
Fig. 2.
The pooled prevalence of COVID-19 infection in PD cases
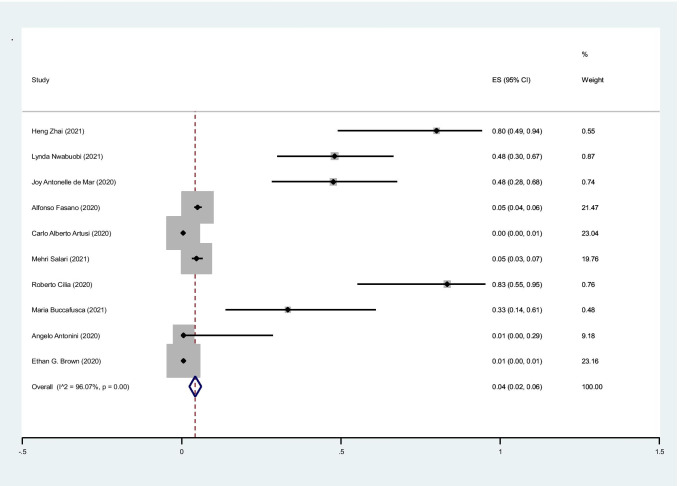
The pooled prevalence of fever in cases with PD was 4% (95%CI: 2–6%) (I2 = 96%, P < 0.001) (Fig. 3).
Fig. 3.
The pooled prevalence of fever in cases with PD
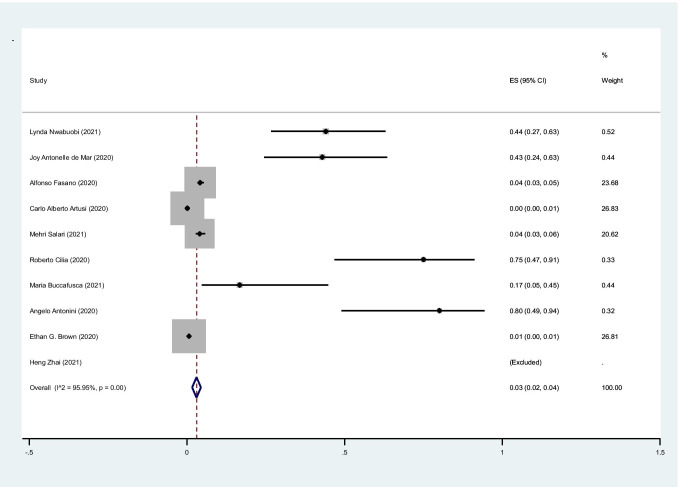
The pooled prevalence of cough in cases with PD was 3% (95%CI: 2–4%) (I2 = 95.9%, P < 0.001) (Fig. 4).
Fig. 4.
The pooled prevalence of cough in cases with PD
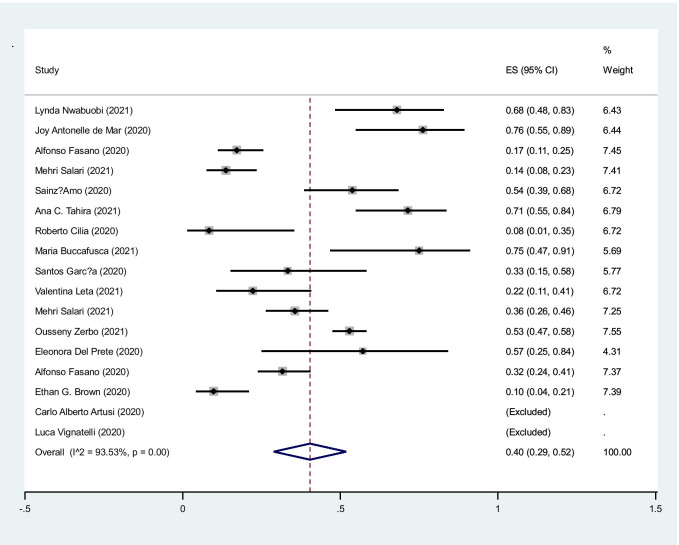
The pooled prevalence of hospitalization in cases with COVID-19 infection was 49% (95%CI: 29–52%) (I2: 93.5%, P < 0.001) (Fig. 5).
Fig. 5.
The pooled prevalence of hospitalization in cases with COVID-19
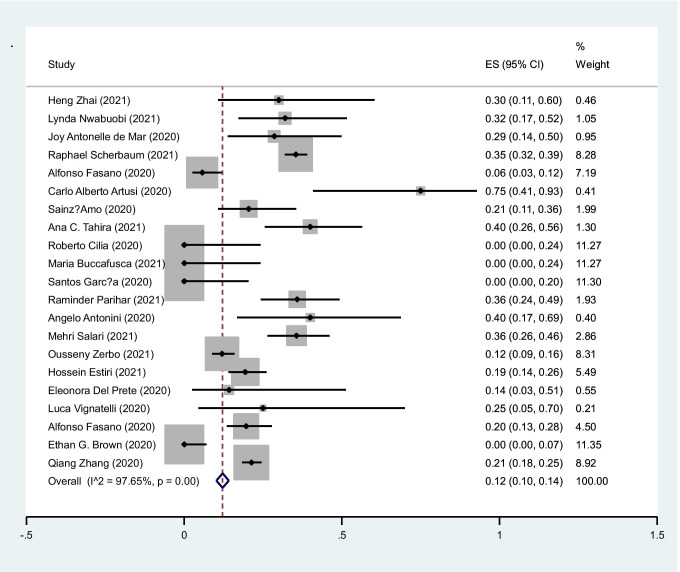
The pooled prevalence of mortality in COVID-19 cases was 12% (95%CI: 10–14%) (I2 = 97.6%, P < 0.001) (Fig. 6).
Fig. 6.
The pooled prevalence of death in COVID-19 cases
Discussion
The results of this systematic review and meta-analysis showed that the pooled prevalence of COVID-19 infection in patients with Parkinson’s disease is 5% which is higher than the pooled prevalence reported in previous systematic reviews. In two previous systematic reviews, the pooled prevalence of COVID-19 in PD reported was 2% [5, 27]. The difference could be due to the higher number of included studies. The prevalence of COVID-19 infection in PD cases ranged between 1 and 43% in different studies. We also found that the pooled hospitalization rate was 49% while the pooled mortality rate was 12%.
A recent systematic review and meta-analysis showed that the pooled prevalence of COVID-19 infection in patients with MS was 4% and pooled hospitalization rate was 10% [28]. The pooled hospitalization rate in this study for PD cases was 49% which is more higher than it was estimated for patients with MS. It could be due to higher age, advanced disease, and more comorbidities among patients with PD.
Zhang et al. found that patients with Alzheimer’s disease (AD) had significantly higher odds of dying from COVID-19 compared with the no AD group [29].
El‐Qushayri et al. published a systematic review and meta-analysis and reported the pooled hospitalization rate as 39.8% and the mortality rate as 25.1% [27].
Alberto Artusi et al. evaluated 1407 PD cases and reported COVID-19 infection in 8. They also found that six out of eight cases died due to COVID-19 infection while the fatality rate was 11.5%. The most common symptoms were fever and weakness [30].
Del Prete et al. enrolled 740 PD cases and reported COVID-19 infection in 7 (0.9%) and mortality rate as 0.13%. They reported hypertension and diabetes as predisposing factors of infection [31].
There are controversies regarding predisposing factors of COVID-19 infection in PD cases including higher susceptibility according to age and disease duration [23, 26, 32].
Patients with PD suffer from a wide range of comorbidities and they have twofold higher risk of hospitalization due to comorbidities [33]. On the other hand, PD may predispose cases to the risk of severe COVID-19 and higher rate of mortality based on disease complications such as delirium, drug adverse effects, syncope, aspiration pneumonia, falls, and fractures [34].
It should be considered that lock downs and social isolation during pandemic stage will result in reduction of outside activity which affects health being in PD cases. Being at home and having no activity affect motor function of patients with PD and also their psychological status. Literature shows that during COVID-19 pandemic stage, patients with PD suffer MORE from anxiety, and sleep disturbances [35–37].
On the other hand, case fatality rate is higher in PD cases after adjusting for different factors such as age, sex, and race and the mortality rate is reported as 30% which is more than general population [38].
SARS-CoV-2 enters the host through cellular receptor angiotensin-converting enzyme 2 (ACE2) which highly is expressed in human airway epithelia and also dopaminergic neurons [39]. On the other hand, brain angiotensin system has role in neurodegeneration in PD cases [36]. Literature also shows that antibodies against different forms of coronaviruses were detected in cerebrospinal fluid of PD patients [40]. All of these findings could show that PD patients are at higher risk of catching SARS-Co V-2 infection.
This study had some strength. First, the number of included studies is high. Second, we calculated the pooled prevalence of COVID-19 infection, hospitalization rate, and mortality in PD cases.
Conclusion
The results of this systematic review and meta-analysis show that the pooled prevalence of COVID-19 infection in PD cases is 5% besides hospitalization and mortality rates are 49% and 12%.
Declarations
Ethical approval
None.
Informed consent
Not applicable.
Conflict of interest
The authors declare no competing interests.
Footnotes
The original online version of this article was revised: Originally, affiliation 1 has been incorrectly presented online. Affiliation 1 should be change to “Functional Neurosurgery Research Center, Shohada Tajrish Neurosurgical Comprehensive Center of Excellence, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
11/24/2021
A Correction to this paper has been published: 10.1007/s10072-021-05781-3
References
- 1.Moghadasi AN, Mirmosayyeb O, Barzegar M, Sahraian MA, Ghajarzadeh M. The prevalence of COVID-19 infection in patients with multiple sclerosis (MS): a systematic review and meta-analysis. Neurol Sci. 2021;42(8):3093–3099. doi: 10.1007/s10072-021-05373-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li J, Huang DQ, Zou B, Yang H, Hui WZ, Rui F, et al. Epidemiology of COVID-19: A systematic review and meta-analysis of clinical characteristics, risk factors, and outcomes. J Med Virol. 2021;93(3):1449–1458. doi: 10.1002/jmv.26424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan. China Jama. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pringsheim T, Jette N, Frolkis A, Steeves TD. The prevalence of Parkinson’s disease: a systematic review and meta-analysis. Mov Disord. 2014;29(13):1583–1590. doi: 10.1002/mds.25945. [DOI] [PubMed] [Google Scholar]
- 5.Chambergo-Michilot D, Barros-Sevillano S, Rivera-Torrejón O, De la Cruz-Ku GA, Custodio N. Factors associated with COVID-19 in people with Parkinson’s disease: a systematic review and meta-analysis. Eur J Neurol. 2021;28(10):3467–3477. doi: 10.1111/ene.14912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Modesti PA, Reboldi G, Cappuccio FP, Agyemang C, Remuzzi G, Rapi S, et al. Panethnic differences in blood pressure in Europe: a systematic review and meta-analysis. PloS one. 2016;11(1):e0147601. doi: 10.1371/journal.pone.0147601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhai H, Lv Y, Xu Y, Wu Y, Zeng W, Wang T, et al. Characteristic of Parkinson’s disease with severe COVID-19: a study of 10 cases from Wuhan. J Neural Transm. 2021;128(1):37–48. doi: 10.1007/s00702-020-02283-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nwabuobi L, Zhang C, Henchcliffe C, Shah H, Sarva H, Lee A, et al. Characteristics and outcomes of Parkinson’s disease individuals hospitalized with COVID-19 in a New York City Hospital System. Mov Disord Clin Pract. 2021;8(7):1100–1106. doi: 10.1002/mdc3.13309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scherbaum R, Kwon EH, Richter D, Bartig D, Gold R, Krogias C, et al. Clinical Profiles and Mortality of COVID-19 Inpatients with Parkinson’s Disease in Germany. Mov Disord. 2021;36(5):1049–1057. doi: 10.1002/mds.28586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salari M, Etemadifar M, Zali A, Aminzade Z, Navalpotro-Gomez I, Fateh ST (2021) Covid-19 in Parkinson’s disease treated by drugs or brain stimulation. Neurologia (Engl Ed);S0213-4853(21)00123-7. 10.1016/j.nrl.2021.07.002 [DOI] [PubMed]
- 11.Tahira AC, Verjovski‐Almeida S, Ferreira ST (2021) Dementia is an age‐independent risk factor for severity and death in COVID‐19 inpatients. Alzheimers Dement;10.1002/alz.12352. 10.1002/alz.12352 [DOI] [PMC free article] [PubMed]
- 12.Buccafusca M, Micali C, Autunno M, Versace AG, Nunnari G, Musumeci O. Favourable course in a cohort of Parkinson’s disease patients infected by SARS-CoV-2: a single-centre experience. Neurol Sci. 2021;42(3):811–816. doi: 10.1007/s10072-020-05001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parihar R, Ferastraoaru V, Galanopoulou AS, Geyer HL, Kaufman DM. Outcome of hospitalized Parkinson’s disease patients with and without COVID-19. Mov Disord Clin Pract. 2021;8(6):859–867. doi: 10.1002/mdc3.13231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leta V, Rodríguez-Violante M, Abundes A, Rukavina K, Teo JT, Falup-Pecurariu C, et al. Parkinson’s Disease and Post–COVID-19 Syndrome: The Parkinson’s Long-COVID Spectrum. Mov Disord. 2021;36(6):1287. doi: 10.1002/mds.28622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salari M, Etemadifar M, Ashrafi F, Ommi D, Aminzade Z, Fateh ST. Parkinson’s disease patients may have higher rates of Covid-19 mortality in Iran. Parkinsonism Relat Disord. 2021;89:90–92. doi: 10.1016/j.parkreldis.2021.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zerbo O, Lewis N, Fireman B, Goddard K, Skarbinski J, Sejvar JJ et al (2021) Population‐based assessment of risks for severe COVID‐19 disease outcomes. IInfluenza Other Respir Viruses. 10.1111/irv.12901 [DOI] [PMC free article] [PubMed]
- 17.Estiri H, Strasser ZH, Klann JG, Naseri P, Wagholikar KB, Murphy SN. Predicting COVID-19 mortality with electronic medical records. NPJ digital medicine. 2021;4(1):1–10. doi: 10.1038/s41746-021-00383-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feeney MP, Xu Y, Surface M, Shah H, Vanegas-Arroyave N, Chan AK, et al. The impact of COVID-19 and social distancing on people with Parkinson’s disease: a survey study. npj Parkinson’s Disease. 2021;7(1):1–10. doi: 10.1038/s41531-020-00149-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ineichen C, Baumann-Vogel H, Sitzler M, Waldvogel D, Baumann CR. Worsened Parkinson’s disease progression: impact of the COVID-19 pandemic. J Parkinsons Dis. 2021;11(4):1579–1583. doi: 10.3233/JPD-212779. [DOI] [PubMed] [Google Scholar]
- 20.Lisa V, Jean-Claude L, Sonja R, Kurt B, Ingo B. Abstracts of the 16th International E-Congress of the European Geriatric Medicine Society: 7–9. Eur Geriatr Med. 2020;2020:1–309. [Google Scholar]
- 21.de Marcaida JA, Lahrmann J, Machado D, Bluth L, Dagostine M, Moro-de Casillas M, et al. Clinical characteristics of coronavirus disease 2019 (COVID-19) among patients at a movement disorders center. Geriatrics. 2020;5(3):54. doi: 10.3390/geriatrics5030054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rutten JJ, van Loon AM, van Kooten J, van Buul LW, Joling KJ, Smalbrugge M, et al. Clinical suspicion of COVID-19 in nursing home residents: symptoms and mortality risk factors. Journal of the American Medical Directors Association. 2020;21(12):1791–7.e1. doi: 10.1016/j.jamda.2020.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fasano A, Cereda E, Barichella M, Cassani E, Ferri V, Zecchinelli AL, et al. COVID-19 in Parkinson’s Disease Patients Living in Lombardy. Italy Movement Disorders. 2020;35(7):1089–1093. doi: 10.1002/mds.28176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Artusi CA, Romagnolo A, Imbalzano G, Marchet A, Zibetti M, Rizzone MG, et al. COVID-19 in Parkinson’s disease: Report on prevalence and outcome. Parkinsonism Relat Disord. 2020;80:7–9. doi: 10.1016/j.parkreldis.2020.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sainz-Amo R, Baena-Álvarez B, Pareés I, Sánchez-Díez G, Pérez-Torre P, López-Sendón J, et al. COVID-19 in Parkinson’s disease: what holds the key? J Neurol. 2021;268(8):2666–2670. doi: 10.1007/s00415-020-10272-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cilia R, Bonvegna S, Straccia G, Andreasi NG, Elia AE, Romito LM, et al. Effects of COVID-19 on Parkinson’s disease clinical features: a community-based case-control study. Mov Disord. 2020;35(8):1287–1292. doi: 10.1002/mds.28170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El‐Qushayri AE, Ghozy S, Reda A, Kamel AMA, Abbas AS, Dmytriw AA (2021) The impact of Parkinson’s disease on manifestations and outcomes of Covid‐19 patients: a systematic review and meta‐analysis. Rev Med Virol;e2278. 10.1002/rmv.2278 [DOI] [PMC free article] [PubMed]
- 28.Moghadasi AN, Mirmosayyeb O, Barzegar M, Sahraian MA, Ghajarzadeh M. The prevalence of COVID-19 infection in patients with multiple sclerosis (MS): a systematic review and meta-analysis. Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. 2021;42(8):3093–3099. doi: 10.1007/s10072-021-05373-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Q, Schultz JL, Aldridge GM, Simmering JE, Kim Y, Ogilvie AC et al (2021) COVID-19 case fatality and Alzheimer’s disease. J Alzheimers Dis. 10.3233/JAD-215161 [DOI] [PMC free article] [PubMed]
- 30.Artusi CA, Romagnolo A, Imbalzano G, Marchet A, Zibetti M, Rizzone MG, et al. COVID-19 in Parkinson’s disease: Report on prevalence and outcome. Parkinsonism Relat Disord. 2020;80:7–9. doi: 10.1016/j.parkreldis.2020.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Del Prete E, Francesconi A, Palermo G, Mazzucchi S, Frosini D, Morganti R, et al. Prevalence and impact of COVID-19 in Parkinson’s disease: evidence from a multi-center survey in Tuscany region. J Neurol. 2021;268(4):1179–1187. doi: 10.1007/s00415-020-10002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Antonini A, Leta V, Teo J, Chaudhuri KR. Outcome of Parkinson’s disease patients affected by COVID-19. Mov Disord. 2020;35(6):905–908. doi: 10.1002/mds.28104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Temlett J, Thompson P. Reasons for admission to hospital for Parkinson’s disease. Intern Med J. 2006;36(8):524–526. doi: 10.1111/j.1445-5994.2006.01123.x. [DOI] [PubMed] [Google Scholar]
- 34.Lubomski M, Rushworth RL, Tisch S. Hospitalisation and comorbidities in Parkinson’s disease: a large Australian retrospective study. J Neurol Neurosurg Psychiatry. 2015;86(3):324–330. doi: 10.1136/jnnp-2014-307822. [DOI] [PubMed] [Google Scholar]
- 35.Brown EG, Chahine LM, Goldman SM, Korell M, Mann E, Kinel DR, et al. The effect of the COVID-19 pandemic on people with Parkinson’s disease. J Parkinsons Dis. 2020;10(4):1365–1377. doi: 10.3233/JPD-202249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Del Prete E, Francesconi A, Palermo G, Mazzucchi S, Frosini D, Morganti R, et al. Prevalence and impact of COVID-19 in Parkinson’s disease: evidence from a multi-center survey in Tuscany region. J Neurol. 2021;268(4):1179–1187. doi: 10.1007/s00415-020-10002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Santos-García D, Oreiro M, Pérez P, Fanjul G, Paz González JM, Feal Painceiras MJ, et al. Impact of coronavirus disease 2019 pandemic on Parkinson’s disease: a cross-sectional survey of 568 Spanish patients. Mov Disord. 2020;35(10):1712–1716. doi: 10.1002/mds.28261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Q, Schultz JL, Aldridge GM, Simmering JE, Narayanan NS. Coronavirus Disease 201 9 Case Fatality and Parkinson’s Disease. Mov Disord. 2020;35(11):1914–1915. doi: 10.1002/mds.28325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rodriguez-Perez AI, Garrido-Gil P, Pedrosa MA, Garcia-Garrote M, Valenzuela R, Navarro G, et al. Angiotensin type 2 receptors: Role in aging and neuroinflammation in the substantia nigra. Brain Behav Immun. 2020;87:256–271. doi: 10.1016/j.bbi.2019.12.011. [DOI] [PubMed] [Google Scholar]
- 40.Fazzini E, Fleming J, Fahn S. Cerebrospinal fluid antibodies to coronavirus in patients with Parkinson’s disease. Movement disorders: official journal of the Movement Disorder Society. 1992;7(2):153–158. doi: 10.1002/mds.870070210. [DOI] [PMC free article] [PubMed] [Google Scholar]