Abstract
Aging is an inescapable complex physiological but extendable process, and all cells, including stem cells, are altered over time. Diverse mechanism(s) could modulate stem cell number, their proliferation rate, and promote tissue repair during aging that leads to longevity. However, the factors that could restore aging stem cell potency and would lead to healthy aging are not fully identified. Here we show that maintaining cytosolic Ca2+ levels was essential for modulating stem cells function in aged mesenchymal stem cells (MSCs). Increasing external Ca2+ induced spindle shape stem cell morphology and maintained stem cell surface marker expression in aged bone marrow-derived MSCs. Similarly, stem cell survival and proliferation of aged MSCs was dependent on cytosolic Ca2+ levels. Importantly, Ca2+ entry potentiated cell cycle progression, and stem cell potential was increased in cells incubated with higher external Ca2+. Moreover, blocking Ca2+ entry using SKF 96365, decreased stem cell survival and its proliferation but, treatment with 2-APB did not significantly affected cell proliferation, rather only modulated cell viability. Evaluation of Ca2+ entry channels, showed that TRPC1/Orai1/Orai3 and their regulator STIM1 was essential for MSCs proliferation/viability as gene silencing of Orai1/Orai3/TRPC1/STIM1 significantly inhibited stem cell viability. Finally, MSCs isolated from aged mice that were subjected to higher Ca2+ levels, were able to rescue age-induced loss of MSCs function. Together these results suggest that Ca2+ entry is essential for preventing the loss of aged stem cell function and supplementing Ca2+ not only restored their proliferative potential but, allowed them to develop into younger stem cell lineages that could be critical for regenerative medicine.
Keywords: Stem cell function, Cell viability, Cell proliferation/cell cycle, Calcium channels, Aged stem cells and potency
1. Introduction
Stem cell’s self-renewal and differentiation power is restricted during aging and loss of stem cell function is the most significant aspect that leads to age-related human diseases. Moreover, due to their indefinite self-renewable capability, stem cells are essential for regenerative medicine. However, factors that modulate their pluripotency and self-renewal ability especially in aging stem cells are not well established. Aging is a complex process, and all cells, tissues, organs, and organisms are affected with age. Diverse factors drive aging, and among the most significant known hazard factors for most human diseases, about two-thirds die from age-related problems (Ahmed et al., 2017; Ullah and Sun, 2018). Aging and longevity are defined by a complicated sequence of various processes that modulate gene expression changes leading to a loss in stem cell number and their proliferation rate. Similarly, epigenetic mechanisms, ROS levels, metabolic pathways, diet or caloric restriction, and telomeres shortening are also some of the mechanism that modulate stem cell function. Interestingly, although all these diverse mechanisms together determine aging, they are regulated by common factors such as calcium signaling. Furthermore, loss of signaling mechanisms has been shown in several progressing disorders that are rendered at an earlier age than expected (Pichard et al., 2020). Thus, identifying these critical mechanisms may prevent many of the age-related disorders.
Stem cells are special and hold two unique properties, the indefinite self-renewable capability, and that they can be differentiated into all major cell lineages, thus, are unique and ideal candidates for regenerative therapy (Bishop et al., 2002; Lerou and Daley, 2005). However, stem cell’s self-renewal and their differentiation power is also restricted during aging, and reduction of overall stem cell number happens with increasing age (Sharpless and DePinho, 2007; Ullah and Sun, 2018). The bone marrow is the most extensive storage that comprises various stem cells such as hematopoietic stem cells (HSCs), multipotent adult progenitor stem cells (MAPCs), and mesenchymal stem cells (MSCs) (Ahamad and Rath, 2019; Murphy et al., 2010; Pleyer et al., 2016). Bone marrow also exhibit variation with aging, including decreased stem cell potential and their proliferation. Similarly, bone marrow cellularity, fat cell deposition, anemia, and decline immunity, osteoporosis, and weak bone mass are also some of the few features during aging (Prabhakar et al., 2009). Almost all stem cells, including HSCs, MAPCs, and MSCs, showed a decline in homing efficiency with age (Ergen and Goodell, 2010; Liang et al., 2005). Although growth hormone production that modulates cellular function are decreased with age, the exact mechanism as why stem cell loose its potency is still unclear. Dysregulation of cellular signaling such as insulin growth factor, calcium signaling, and changes in the extracellular matrix composition (Kovtonyuk et al., 2016), are some of the most common mechanisms that could contribute towards the loss of stem cell function.
Tissue-specific stem cells differentiate and sustain the homeostat in the tissue by producing the required cell type. Stem cells are also required to repair the tissues, protect against tissue or organ failure. However, the functional ability of tissue-specific stem cells is not constant during age (Ergen and Goodell, 2010; Gross, 2007). Demand for controlling aging and tissue regeneration by the stem cells, especially for the regeneration of damaged tissues due to aging, is developing. Today, stem cells, are recognized among the “Seven Pillars of Aging” and the nine hallmarks of aging, play a central role in the aging process (Le Couteur et al., 2017). Thus, there is a crucial need to know the factors that could change the stemness/proliferation of stem cells (Pleyer et al., 2016). Although MSCs sustain their stemness, it is unclear how steady-state function and homeostatic responses are regulated. Ca2+ is a ubiquitous intracellular messenger that participates in almost all cellular processes such as cell signaling, apoptosis, exocytosis, cell death, protein secretion, and cell growth/proliferation. Stem cells use Ca2+ signaling pathways in controlling the self-renewal and differentiation process (Brini et al., 2013; Ermakov et al., 2018). Binding of the ligand to G-protein coupled receptors (GPCR) leads to the breakdown of PIP2 into two intracellular messengers IP3 and DAG. IP3-binding to the IP3Rs begins to discharge Ca2+ from the internal (ER) stores that promote Ca2+ entry (Bollimuntha et al., 2011; Sun et al., 2014).
Two distinct Ca2+ entry channels such as Orai’s and transient receptor potential canonical (TRPCs) are responsible for Ca2+ influx into the cytoplasm from extracellular space. Importantly both these channels are activated by Stromal interaction molecule 1 (STIM1) that is present in the ER (Sun et al., 2020), but the role of these channels in the aging of stem cell function is not yet identified. The EF-hand in the c-terminus of STIM1 senses a decrease in ER Ca2+ concentration, leads to a conformational change that allows it to multimerize, translocate and ER-PM junctions, and physically interact with Orai’s/TRPC’s channels to stimulate Ca2+ entry. This rise in Ca2+ entry is necessary for cell fate choices required during stem cell growth and function. Ca2+ entry is crucial for numerous cellular processes, such as Ca2+ stimulate calmodulin-dependent protein kinase II that control the regeneration of hematopoietic stem and progenitor cells. Moreover, Ca2+ channel blockers such as SKF 96365 and 2-APB decreased mESC proliferation and down-regulated pluripotency factors (Sox-2, Klf-4, and Nanog), supporting that Ca2+ entry is associated with self-renewal and proliferation of mESC (Lam et al., 2011; Wong et al., 2012). Ca2+ entry is also involved in switching stem cell state (from quiescence to cell cycle activation state) and Ca2+ dependent signaling pathways modulate stem cell division (Broxmeyer, 2019; Racioppi et al., 2017; Umemoto et al., 2018). In contrast, low Ca2+ levels inhibits calpain proteases, thereby enhancing stem cell maintenance in vitro, suggesting that tight regulation of Ca2+ is required for supporting stem cell function (Yajima and Kawashima, 2002). However, it remains inadequately explained how Ca2+ and Ca2+-induced signaling pathways change stem self-renewal/function in aged stem cells. Our data shows that Ca2+ is critical in modulating stem cell function and improve aged stem cell morphology and function. Importantly, Ca2+ entry increases the CD44 surface marker expression, which is essential for MSCs migration. Finally, aged stem cells and stem cells from aged mice propagating in higher Ca2+ were able to rescue defects in age-related loss of their function, suggesting that Ca2+ entry is essential for stem cell function restoring Ca2+ could prevent age-related loss of stem cell function.
2. Materials and methods
2.1. Isolation, culture, enrichment of mesenchymal stem cells (MSCs) and gene silencing and Immunophenotypic characterization of MSCs
Primary MSCs of young and old mice were isolated from the bone marrow of the femur and the humeri of C57BL/J6 mice, as described earlier1. In brief, young, and old mice (4 weeks and 48 weeks old respectively) were anesthetized, sacrificed by cervical dislocation. The mice’s external body surface was sterilized with 70% ethanol, and the femur and tibiae-fibulae were dissected. All associated tissues were removed, the epiphysis of each bone was removed with sterile scissors, and bones were collected in a 0.5 ml tube containing holes at the bottom. Tubes having bones were placed in a 15 ml polypropylene culture tube, centrifuged at 10000 rpm, 5 min at 37 °C, and BMCs palette were dissociated by vertexing to make a single-cell suspension. Five ml complete culture medium, [(Dulbecco’s Modified Eagle’s Medium (DMEM, Cat no. D5796, Sigma), 15% heat-inactivated fetal bovine serum, FBS (Cat no. F4135, Sigma) and 1 × anti-anti solution (Cat no. 15240–062, Gibco)] was added into the tube. The cell suspension was filtered through a 70 μm cell strainer to exclude any cell clump, bone spicules, muscle, and debris from the single-cell suspension. The cell number and cell viability were evaluated by trypan blue assay, and Cells (1×106 cells/cm2) were seeded in T-75 cell culture flasks and placed at 37 °C in a 5% CO2 humidified incubator. Non-adherent and dead cells were removed during the replacement of fresh media after 48 h. Afterward, 2/3 of old media was substituted with fresh media every three days until the adherent cells reached 80% to 90% confluency. Adherent cells were harvested by trypsinization. Culture media was aspirated and added pre-warmed 5 ml of 0.25% trypsin/1 mM EDTA (Cat no. 25200–056, Gibco) at 37 °C for 2 min trypsinization process was stopped by adding 10 ml complete medium. The cell suspension was centrifuged at 500 × g for 5 min, 37 °C, and the supernatant was discarded. The cell pellet was vortexed, resuspended in a complete medium, seeded into three flasks, and cultured up to MSC-P20. Young mice MSC-P2–4, and Old mice MSC-P18–20 cells were used for all the experiments.
For MSC characterization, media of MSC-P3/4 and MSC-P19/20 cultured flask was removed and washed with 1xPBS. Cells were harvested by trypsinization and counted by trypan blue assay. MSC cells (one million) were resuspended in ice-cold neutralizing buffer (10% FBS, I% sodium azide in 1xPBS), centrifuged at 500 × g, 5 min, at 4 °C, discarded supernatant and Cell pellets were resuspended in staining buffer (Cat no. 00–4222–26, Invitrogen). Antibodies [PE-conjugated antibodies CD29 (Cat no. 12–0291–82), CD44 (Cat no. 12–0441–82), Ly-6A/E (Sca-1) (Cat no. 12–5981–82), FITC-conjugated antibodies CD11b (Cat no. 11–0112–82), CD45 (Cat no. 11 0451–82), CD34 (Cat no. 11–0341–82)] and their isotype controls] were added in cell suspension from eBioscience according to company instruction, and cells were incubated for 1 hr at 4 °C in the dark. Then, Cells were centrifuged at 500 × g, 5 min at 4 °C to remove the unbound antibody. Finally, cell pellets were vortexed and resuspended in a 100 μl staining buffer. About 50,000 cells were analyzed by BD LSR-II using BD FACSDiva 8.0.1 software, and the data was analyzed by FlowJo_v10.6.2 software.
2.2. Immunofluorescence and their differentiation
Extracellular surface marker CD44 expression was studied in the young and aged MSCs by immunofluorescence. Cells (10×104) were seeded on the glass bottom dish and incubated in the CO2 humidified incubator for one day at 37 °C. First, cells were washed with 1 ml 1xPBS, fixed with 2 ml, 4% paraformaldehyde (PFA) for 30 min at RT. Then PFA was aspirated, cells were washed with 2 ml 1xPBS and cells were then incubated in 100 mM glycine/PBS for 20 min at RT. Next, glycine was aspirated, washed with 1xPBS three times, and permeabilized with cold methanol (−70 °C) for 5 min. Then methanol was aspirated, and cells were washed with 1xPBS three times, and blocking was done with 5% donkey serum in 1xPBS/0.5% BSA for 20 min with shaking at RT. Next, donkey serum was aspirated, and cells were washed with 1xPBS and incubated with antibodies conjugated with PE CD44 (Cat no. 12–0441–82) at 1:250 overnight at 4 °C. First, unbound primary antibodies were removed by washing with 2 ml PBST and then Vectasheild with DAPI (Vector Labs H-1200) was applied to the cells and mounted. Images were captured by a Leica fluorescence confocal microscope.
For adipocyte and osteocyte differentiation, MSC-P18 cells were seeded at the density of 1×104 cells/well in 6-well tissue culture plates, and then cells were incubated at 37 °C in a 5% CO2 humidified incubator. Following two days, cultured media was removed. Adipocyte differentiation/induction medium [DMEM-LG (Cat no. D6046, Sigma), 2% FBS, 1x-Anti-anti solution, 5 μg/ml insulin (Cat. no. 19278, Sigma), 1 μM Dexamethasone (Cat. no. G9891, Sigma), 50 μM indomethacin (Cat. no. 17378, Sigma) and 500 nM isobutyl methyl xanthine, IBMX (Cat. no. 17018, Sigma)] holding different calcium concentration was added. Osteocyte differentiation/induction medium [DMEM-LG, 2% FBS, 1x Anti-anti solution, 50 μM ascorbic acid (Cat. no. A8960, Sigma), 10 nM Dexamethasone, 10 mM β-glycerolphosphate (Cat. no. G9891, Sigma)] comprising different calcium concentrations were added to the respective plates. The induction medium was replaced every three days for 14–21 days. The production of oil droplets and adipocytes was estimated by oil-red O staining, and dark brown, blackish mineral deposition and osteocytes generation were assessed by Von Kossa staining. A bright-field microscope was used to captured images.
2.3. Cell viability and cell proliferation
MTT [3-(4, 5-dimethyl thiazolyl-2)-2,5 diphenyltetrazolium bromide] (Cat no. 475989, Calbiochem) assay was used to determine cell viability. Briefly, young, and old MSCs (2×104 cells/cm2) were seeded in 96-well plates and were incubated for one day at 37 °C in a 5% CO2 humidified incubator. After one day, the medium was aspirated from each well, and a new medium containing different calcium conc. (0 mM Ca2+, 2 mM Ca2+, and 5 mM Ca2+) and calcium channel blockers (25 μm SKF 96,365 hydrochloride (Cat no. 1147, Tocris) and 50 μm 2-APB (D9754, Sigma)) were added to the wells, and cells were grown for 1 and 3 days. After day one and day 3, the medium was aspirated from each well, and 100 μl media containing 20 μl MTT reagent (5 mg/ml MTT in 1xPBS) was added to each well and incubated in a CO2 humidified incubator for 4 hr. The medium was removed from each well, and purple bluish formazan crystals were dissolved in 100 μl of acid alcohol (0.04 N HCl in isopropanol) for 15 min at RT. The absorbance was measured in a Synergy™ HTX Multi-Mode Microplate Reader at 570 and 630 nm.
Cell proliferation was studied by Cell Proliferation ELISA BrdU colorimetric kit (Cat no. 11647229001, Roche). Briefly, MSCs (2×104 cells/PBS) were seeded in 96-well plates and treated with different calcium conc. (0 mM Ca2+, 2 mM Ca2+, and 5 mM Ca2+) and calcium channel blockers (25 μm SKF 96,365 and 50 μm 2-APB) for 1 and 3 days. After day one and day 3, the medium was aspirated from each well. According to kit instructions, BrdU labeling, fixing, incubation with anti-BrdU antibody, washing, and substrate reaction steps were performed. The absorbance was measured in a microplate reader (Synergy™ HTX Multi-Mode Microplate Reader) at 370 and 492 nm. Cell proliferation was also determined by Cell Proliferation CFDA-SE assay kit (Cat no. 135–1201, BioRad). MSCs were seeded in a 100 mm dish and treated with different calcium channel blockers (25 μm SKF 96,365 and 50 μm 2-APB) for one day. After day 1, the medium containing CFDA was added to each dish for 30 min, and then media was aspirated from each dish. Cells were washed with 1x-PBS and harvested by trypsinization. Cells were divided into two parts. Part one was used to analyze the cells using a flow cytometer, and the other part seeded into a cell culture plate and let them grow for three days. After the third day, the medium with media was aspirated from each dish. Cells were washed with 1x-PBS and harvested by trypsinization. Cells were analyzed using a flow cytometer as previously described.
2.4. Cell cycle assessment and Colony-forming unit‑fibroblasts (CFU‑F) assay
At 80% confluency, the medium was aspirated from each dish, and cells were treated with different calcium conc. (0 mM Ca2+, 2 mM Ca2+, and 5 mM Ca2+) and calcium channel blockers (25 μm SKF 96,365 and 50 μm 2-APB) for one day. After treatment, the medium was aspirated from each dish. Cells were washed with 1xPBS and were harvested by trypsinization. MSCs (1×106) were fixed in 70% ethanol for one day, washed in 1xPBS, resuspended in 100 μl 1xPBS. Cells were incubated with 100 μg/ml RNase A (Cat no. AM2286, Invitrogen) at 37 °C for 1 hr and then treated with 10 μg/μl PI (Cat no. P3566, Invitrogen) on ice for 15 min in the dark. About 20,000 events were captured by BD LSR-II using BD FACSDiva 8.0.1 software, and data was analyzed by Modfit software. The CFU-F assay was used to analyze the stem cell potential of the MSCs. Briefly, young and old MSCs (2×106 cells/well) were seeded in 6-well plates and incubated for one day at 37 °C in a 5% CO2 humidified incubator. After one day, the medium was aspirated from each well, and cells were treated with different calcium conc. (0 mM Ca2+, 2 mM Ca2+, and 5 mM Ca2+) and calcium channel blockers (25 μm SKF 96,365 and 50 μm 2-APB) for one day. After treatment, the medium was aspirated from each well, and a new medium was added to the cells. On day 6th, the medium was aspirated from each well, and cells were fixed and stained with 1 ml crystal violet (CV) solution (Cat no. V5265, Sigma). The stain was aspirated, and cells were washed with 1xPBS. Then, 500 μl acetic acid (10%) was added to each well and incubated for 30 min on an RT shaker. CV solution was collected from each well, and absorbance was measured in a Synergy™ HTX Multi-Mode Microplate Reader at 550 nm and 590 nm.
2.5. Calcium imaging and gene silencing
Calcium imaging was performed using fluorescence Molecular Probes, Fura-2/AM (Cat no. 344905, Calbiochem), as described earlier 1. In brief, cells were cultured in a glass-bottom dish and then incubated with 2 μM fura-2/AM for 30 min. Cells’ media was drained off, and cells were washed three times with Ca2+ free Standard External Solution, SES (10 mM HEPES, 120 mM NaCl, 5.4 mM KCl, 1 mM MgCl2, 10 mM glucose, pH7.4). The fluorescence measurements were performed using the fluorescence intensity of Fura-2-loaded cells, taken by Olympus XL70 inverted microscope equipped with an Olympus 340 (1.3NA) objective and a CCD camera-based imaging system (Compix, Lake Oswego, USA). Several pictures of cells were collected at excitation 340 nm and excitation at 380 nm wavelength and were processed using Metamorph software. The ratio (F340/F380) of 20–50 cells was collected, and fluorescence traces were made from three individual experiments. Gene silencing was determined by si-RNA. Briefly, MSCs (2×104 cells/cm2) were seeded in 96-well plates and incubated for 24 hr at 37 °C in a 5% CO2 humidified incubator. After one day, the medium was aspirated from each well, and a fresh medium were added to the respective wells, and cells were grown for 1 day. After day one, cell viability was measured by MTT assay.
2.6. Statistical analysis
All experiments were repeated at least 3–4 times, and experimental data values are presented as the mean ± standard error of the mean (SEM). One-way ANOVA or Student t-test were conducted for significant differences among the experimental groups. P-value > 0.05 was not significant (n.s.) and P < 0.05 was statistically significant. P ≤ 0.05, P ≤ 0.01, and P ≤ 0.001 are denoted by *, **, and *** respectively.
3. Results
3.1. Isolation, culture, and characterization of young and aged MSCs
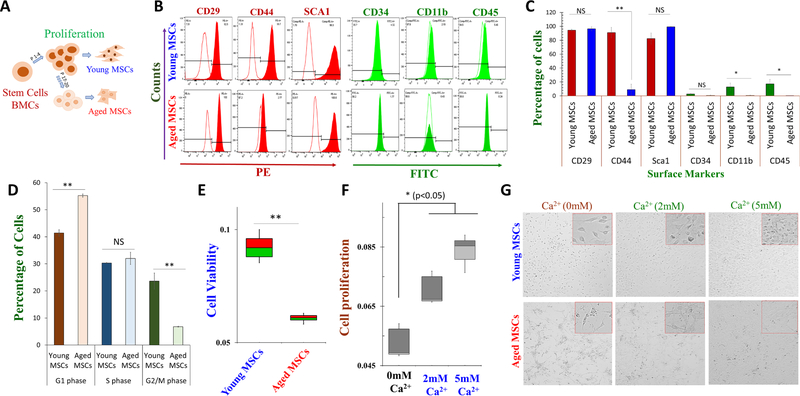
Bone marrow derived stem cells were isolated from the central cavity (bone marrow) of young (8 weeks) C57BL/J6 mice. Long bones, the femur, and the tibia were cleaned and flushed using media, and cells were seeded at the density of 1×106 cells/cm2 in T-75 tissue culture flasks and passaged to grow them up to P20. Mesenchymal stem cells (MSCs) from early passages (P2–4) are called young MSCs, whereas prolonged passages (P18–20) are called aged MSCs (Fig. 1A). Young and aged MSCs were further characterized using positive and negative mesenchymal surface markers, and expression of CD29, Sca-1 (positive markers for stem cells) was observed in young and aged MSC cells (Fig. 1B). In contrast, aged MSCs showed a significant decrease in the expression of CD44 surface markers when compared to young MSCs (Fig. 1B, C). Young MSC cells showed expression for all the positive markers tested: CD29 (94.43%±1.37%), CD44 (91.00% ± 7.60%), Sca-1 (82.36% ± 8.07%), whereas aged MSCs showed expression of CD29 (96.4%±3.25%), CD44 (9.09% ± 7.74%), Sca-1 (99.26% ± 0.58%). Similarly, young MSC cell showed a decrease in all negative stem cell markers (CD34 (2.71% ± 0.81%), CD11b (12.89% ± 5.47%), and CD45 (17.18% ± 6.57%)), which was further decreased in aged MSCs (CD34 (0.76% ± 0.50%), CD11b (0.70% ± 0.50%), and CD45 (0.13% ± 0.06%)) when compared to young MSC cells (Fig. 1C).
Fig. 1.
Characterization of young and aged mesenchymal stem cells (MSCs) from mouse bone marrow. A Schematic showing culture protocols of mouse bone marrow derived MSCs. Young MSCs were cultured till passage 4 and used for our experiments, while aged MSCs were cultured up to 20 passages and used for the study. B Immunophenotypic characterization of young and aged MSCs show the expression of MSC-positive markers (CD29, CD44, and SCA1, conjugated with PE, phycoerythrin) and significantly less expression of MSC-negative markers (CD34, CD45, and CD11b, conjugated with FITC, fluorescein isothiocyanate). C The bar graph shows the percentage of cells expressing the MSC-positive and -negative markers, respectively. D Cell cycle assay was done to study the phases of the cell cycle in young and aged MSCs. E Cell survival assays (MTT) and cell proliferation assays (BrdU shown in F) were performed to investigate aging effect on cell viability and proliferative capability of young and aged MSCs respectively. G MSCs were treated with different calcium conc. for three days and cell proliferation was evaluated. Cultured and enriched adhered young and aged MSCs were visualized using a 40X microscope to show their morphological characteristic (eye-shaped morphology). Bar graphs depict average ± SE in 3–4 independent experiments, NS = non-significant, *p ≤ 0.001 (Student’s t-test).
Next, we evaluated how aging affects cell cycle and again, young MSCs cell showed a decrease in G1 phase (41.40 ± 1.21%), along with an increase in the S phase (30.3 ± 0.11%), and in the G2/M phase (23.65 ± 2.97%)] when compared with aged MSCs that showed G1 arrest stage [increase in G1 phase (55.25 ± 0.60%), along with a decrease in G2/M phase (6.77 ± 0.17%) (Fig. 1D). Importantly, the percentage of G1 phase cells was 1.33-fold higher in aged MSCs, whereas, the percentage of G2/M phase cells was 3.49-fold lower than young MSC cells (Fig. 1D). To further evaluate their proliferation status, young and aged MSC cells were seeded in 96-well plates and cell viability was measured. Importantly, our study showed that increasing the passage number, significantly increase in cell death of aged MSC cells and cell viability was decreased when compared with young MSCs (Fig. 1E). Further, we evaluated the factors that are essential for stem cell function. Importantly, Ca2+ has been shown to play a vital role in modulating cell proliferation; thus, we used BrdU assays to establish the role of Ca2+ in cell proliferation. Aged MSCs were seeded in 96-well plates and treated with different Ca2+ concentrations (0 mM Ca2+, 2 mM Ca2+, and 5 mM Ca2+) for 3 days, and cell proliferation was evaluated. Importantly, increasing external Ca2+ levels showed a significant increase in the proliferation of aged MSCs (Fig. 1F). This result revealed that aged MSCs showed significantly higher cell proliferation rate in media that were supplemented with high Ca2+ levels. To further characterize these cells microscopic study was assessed in these conditions. Again, MSCs were treated with different Ca2+ concentrations (0 mM Ca2+, 2 mM Ca2+, and 5 mM Ca2+), and cell morphology was evaluated. Our study found that the morphology of aged MSC cells was improved in conditions where external Ca2+ was higher (Fig. 1G). Cultured young MSCs showed their characteristic 3D elongated spindle-shaped morphology. In contrast, aged MSCs showed plastic adherence properties to the surface, and showed a 2D type structure, and flatter shaped, which was restored at higher Ca2+ concentrations. Together these results show that aging decreases stem cell function and Ca2+ may be critical in restoring stem cell function.
3.2. Role of calcium in differentiation and expression of cell surface markers in aged MSC cells
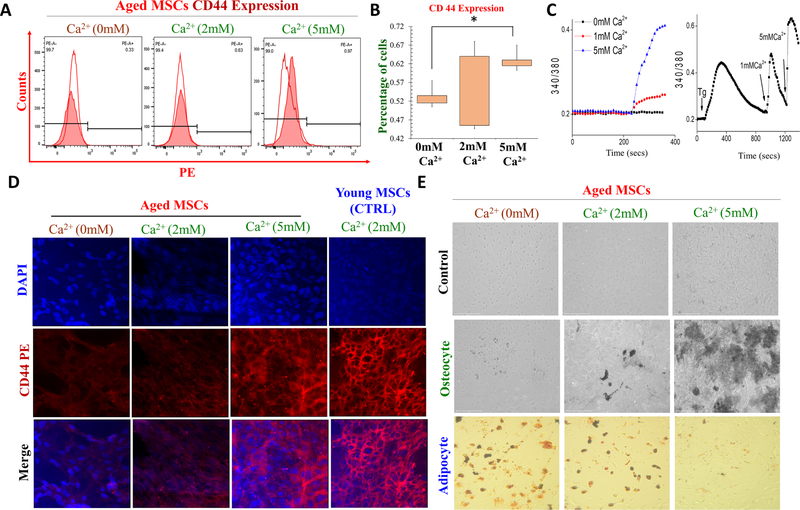
To further identify how Ca2+ treated cells showed improved cell morphology in aged MSCs. Again, aged MSC cells were treated with different Ca2+ concentrations (0 mM Ca2+, 2 mM Ca2+, and 5 mM Ca2+) for 3 days, and expression of cell surface marker (CD44) was judged using Flow cytometry. We found that CD44 expression of aged MSCs was improved in conditions where external Ca2+ was higher, especially at 5 mM Ca2+ (Fig. 2A). Importantly, quantification of the data showed that aged MSCs showed different expression of CD44 at different calcium concentrations: 0.58 %± 0.13% (at 0 mm Ca2+), 0.64 % ± 0.21% (at 2 mm Ca2+), 0.73 % ± 0.22% (at 5 mm Ca2+), respectively (Fig. 2B). Importantly, increasing external Ca2+, significantly increased intracellular Ca2+ levels and higher intracellular Ca2+ was observed in 1 or 5 mm external Ca2+ (Fig. 2C). Similarly, immunohistochemical analysis of CD44 in young and aged MSC cells further showed that exogenous calcium improves CD44 expression in aged MSCs and increase in CD44 expression was observed at 5 mM Ca2+ (Fig. 2D). Ca2+ has been shown to play a vital role in modulating various cellular functions such as cell differentiation into adipocyte, osteocyte, and chondrocyte. Thus, aged cells were seeded in 6-well plates and for 1 days, and post 1 day, induction media with different Ca2+ concentrations (0 mM Ca2+, 2 mM Ca2+, and 5 mM Ca2+) was used for 1–2 weeks and stem cell differentiation was studied. Importantly, increasing external Ca2+ levels showed a significant increase in osteocyte differentiation at 1–2 weeks, while increasing external Ca2+ levels showed a significant decrease in adipocyte differentiation at 1–2 weeks (Fig. 2E). This result revealed that aged MSC cells showed significantly higher cell osteocyte differentiation but, decline adipocyte differentiation after high Ca2+ treatment.
Fig. 2.
Role of calcium in differentiation and surface marker expression on young and old MSCs. A Immunophenotyping assay was done to study the CD44 surface marker expression after various calcium treatment (0 mm Ca2+, 2 and 5 mM Ca2+ for 3 days). B, shows the histogram showing the expression of the CD44 surface marker expression in varying dose of Ca2+ treatment. C Representative traces showing changes in intracellular Ca2+ levels after various Ca2+ treatment. D represent CD44 expression in aged MSCs in various Ca2+ concentrations. Young MSCs grow in in 2 mM Ca2+ was used as control. E Adipocyte and osteocyte differentiation assay was performed to study the stem cell differentiation potential of aged MSCs after treatment with different doses of Ca2+.
3.3. Role of calcium in cell proliferation of aged MSCs
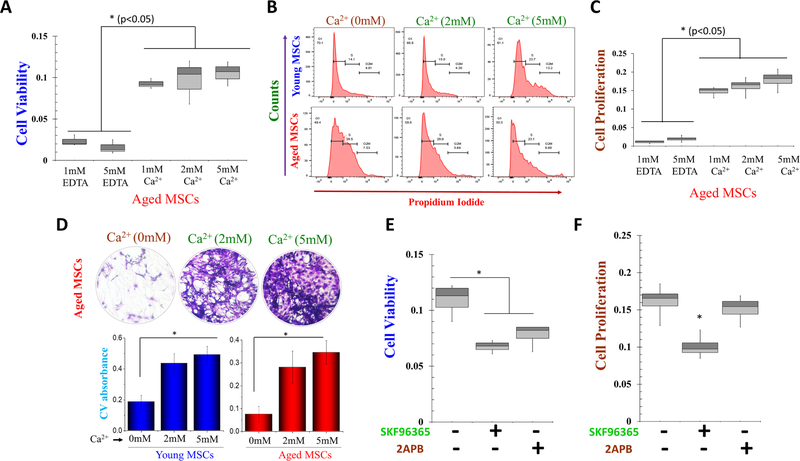
To further establish that Ca2+ levels are essential for stem cell function, we initially used MTT and BrdU assay. Aged MSC cells were seeded in 96-well plates and treated with different Ca2+ concentrations and cell viability were measured. Importantly, increasing external Ca2+ levels showed a dose dependent increase in aged MSCs viability, and aged cells that were devoid of external Ca2+ showed significant cell death (Fig. 3A). To further know how Ca2+ levels affects cell survival and its potency, the cell cycle progression was evaluated in young and aged MSCs. Interestingly, addition of 5 mM Ca2+ showed a reversal in G1 phase in aged MSCs, where similar to young MSCs, no difference in the percentage of G1 or G2/M phase cells was observed (Fig. 3B and Fig. 1D). In contrast, loss of external Ca2+ showed a significant increase in the percentage of cells in G1 phase (G1 arrest) in both aged and young MSCs (data not shown). For cell proliferation analysis, aged MSCs were again treated with higher external Ca2+, which also showed a significant increase in cell survival at 3-day post-treatment; no significant increase in cell viability was observed in 5 mM EDTA treated cells at day3 (Fig. 3C).
Fig. 3.
Role of calcium on aged MSCs cell viability and cell proliferation after increasing calcium concentrations. A MTT assay was performed to investigate calcium’s effect on aged MSCs cell viability. Aged MSCs cells were treated with 5 mM EDTA (mimic 0 mM Ca2+) and increasing Ca2+ concentrations for 1 day. B Cell cycle assay was done to study the phases of the cell cycle after Ca2+ treatment. The bar graph shows the increment of the cell growth phase, i.e., S phase, and diving phase, i.e., G2/M phase with increasing Ca2+ conc. C BrdU assay was performed to study the cell proliferation of MSCs after treatment with 5 mM EDTA and different doses of Ca2+ for and 3 days. D The colony-forming unit (CFU) assay shows the stem cell potential after the treatment of exogenous Ca2+. CFU image and graph show increased stem cell potential with increasing Ca2+ concentration. E MTT assay and F BrdU assay were performed to investigate calcium’s proliferative effect. MSCs cells were treated with SKF 96,365 and 2-APB for 24 h. Bar graphs depicts average ± SE in 3–4 separate experiments, NS = non-significant, *p ≤ 0.001 (Student’s t test).
Colony-forming units (CFU) assay were also performed to analyze calcium’s role in the stem cell potential using both young and aged MSCs. Young and aged MSCs were seeded and treated with different doses of calcium (0 mM Ca2+, 2 mM Ca2+, and 5 mM Ca2+) for 6 day, and CV absorbance was analyzed. Interestingly, again a dose-dependent increase in stem cell potential was observed in the presence of external Ca2+, and absorbance values were gradually increased in Ca2+ treated cells (Fig. 3D). Again 2 mM Ca2+treated young and aged MSCs showed a 2.12 and 1.95 -fold increase in CV absorbance than 0 mM Ca2+ treated cells, respectively (Fig. 3D). Data presented thus far shows that Ca2+ entry is essential for stem cell function; however, the ion channel essential for Ca2+ entry is not yet defined. To identify the molecular identity of the Ca2+ channel, we initially used different Ca2+ channel inhibitors. Interestingly, when cells were treated with non -specific Ca2+ entry channel blockers SKF 96,365 (25 μm), a significant decrease in cell proliferation was observed. In contrast, another Ca2+ channel inhibitor, 2-APB (50 μm), failed to show any loss of cell proliferation (Fig. 3E). Furthermore, our results also showed that SKF 96,365 or 2APB treated cells exhibit a 1.60-fold, and 1.3-fold decrease in cell viability compared to control untreated cells (Fig. 3F); however, the data with 2APB was not significant. These results indicate that Ca2+ entry via the non-selective Ca2+ channels may be responsible for cell proliferation/cell viability in aged MSCs.
3.4. Characterization of calcium channels responsible for cell proliferation in aged MSCs
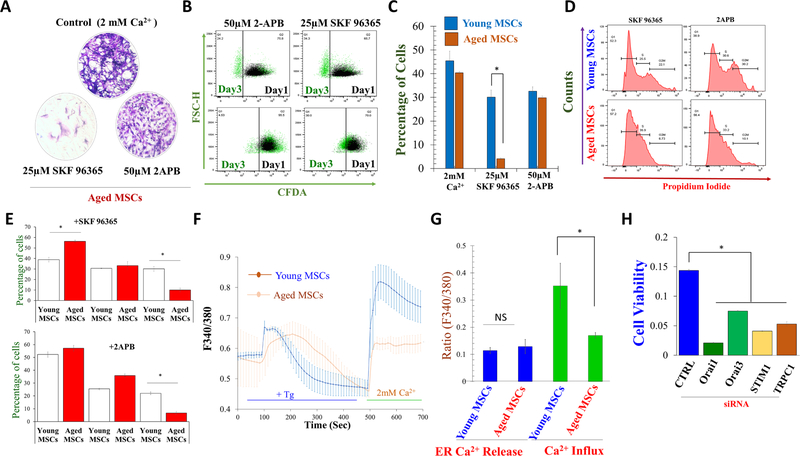
Young and aged MSCs were treated with calcium channel blockers and stem cell potential was evaluated. Interestingly, SKF 96,365 treated cells showed a prominent decrease in CV absorbance. In contrast, 2-APB treated cells showed no significant change in CV absorbance when compared with control, which indicates that 2-APB is perhaps still able to potentiate stem cell potential (Fig. 4A). Further, cell proliferation was also studied by CFDA kit. Again, young and aged MSCs were seeded in 100 mm culture dish and treated with Ca2+ entry channel blockers SKF 96,365 (25 μm), which showed a significant decrease in aged MSCs proliferation (4.07 ± 0.39%) than young MSC cells (30.06 ± 3.05%) respectively, was observed, while 2-APB (50 μm) treated aged MSCs also showed a decline in aged MSC cell proliferation (29.83 ± 0.72 %) than young MSCs (32.56 ± 1.88 %), respectively, but were not significantly different (Fig. 4B, C). Similarly, we found that SKF 96,365 treated, but not 2-APB treated, young and aged MSCs showed significant increase in the G1 phase (G1 arrest) (Fig. 4D. E). In contrast, a significant decrease in G2/M phase was observed in both SKF 96,365 and 2-APB treated cells, without much change observed in the S phase (Fig. 4D. E).
Fig. 4.
Characterization of SOCE activities of young and aged MSCs. A The colony-forming unit (CFU) assay showed the stem cell potential after the treatment of SKF 96,365 and 2-APB for 24 h. CFU image and graph showed changes in stem cell potential upon SKF 96,365 and 2-APB treatment. B CFDA (cell proliferation) assay was performed to investigate Ca2+ Ca2+ Ca2+ proliferative effect on aged MSCs. Young and aged MSCs cells were treated with SKF 96,365 and 2-APB for 24 h. C The bar graph show the decrease the cell proliferation with calcium channel inhibitors. D Cell cycle assay was evaluated after SKF 96,365 and 2-APB treatment. E The histogram show the arrest of cells in G1 phase in SKF-96365 treated cells and dividing phase, i.e., G2/M phase with SKF 96,365 and 2-APB treatment. F Individual traces of fura 2 loaded cells were stimulated with Tg to evaluate changes in cytosolic Ca2+ levels (ER Ca2+ release and Ca2+ entry), and G shows quantification of Ca2+ evoked [Ca2+]i changes in young and aged MSC cells. H Aged MSCs were silenced with siRNAs (STIM1, TRPC1, Orai1, and Orai3), and cell viability were studied. *p ≤ 0.001 (Student’s t test).
Interestingly, when young and aged MSCs were treated with Thapsigargin (Tg), a potent inhibitor of sarco-endoplasmic reticulum Ca-ATPases that causes ER depletion, a rapid increase in cytosolic Ca2+ levels were observed, which was not significantly different in young and aged MSCs (Fig. 4F, G). In contrast, addition of 2 mM Ca2+, which represents Ca2+ entry upon store-depletion was significantly decreased in aged MSCs (Fig. 4F, G). This data further strengthens that Ca2+ entry required for MSCs proliferation was through the SOCE mechanism in both young and aged MSCs. However, young MSC cells showed a significant calcium peak compared to aged MSC cells (Fig. 4F, G). To further confirm which channel(s) is responsible for stem cell function, MSCs were seeded in 96-well plates, and STIM1, TRPC1, Orai1, and Orai3 genes were silenced individually. Interestingly, silencing of either TRPC1 or STIM1, or Orai1, or Orai3 showed a significant decrease in cell proliferation after 24 hr. (Fig. 4H). Thus, these results confirmed that cell proliferation was dependent on Ca2+ entry that is perhaps mediated via the Orai1/3/TRPC1 channels in aged-stem cells.
3.5. Role of calcium in cell proliferation in young and old mice MSCs
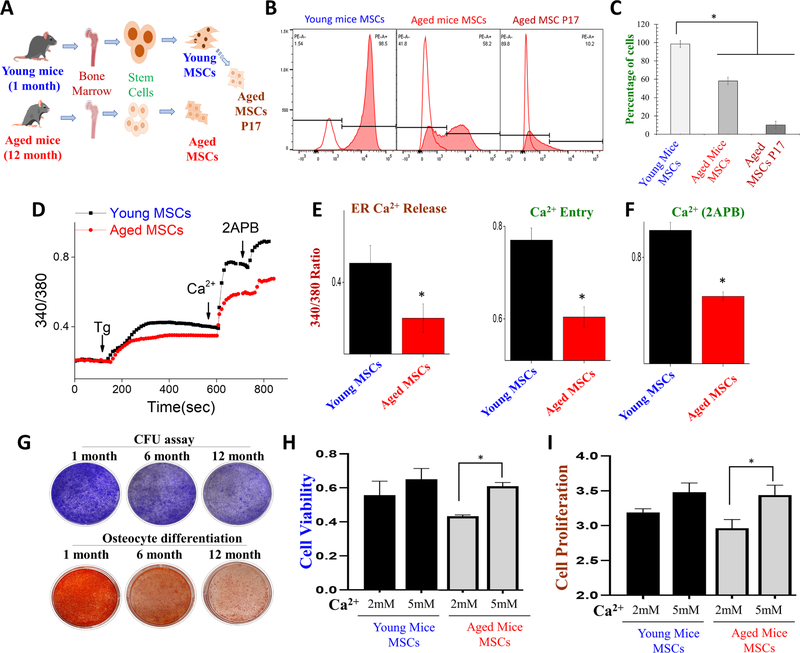
Data presented thus far uses higher passage number to differentiate between young and aged cells; thus to see if similar effects are observed, we used both young and aged mice. Mesenchymal stem cells were isolated from both young (1 month) and aged (12 month) old mice which were further studied (Fig. 5A). Initially, we want to study CD44 expression in aged mice MSCs, and we found that CD44 expression in aged mice MSCs declined when compared to young mice MSCs (Fig. 5B, C). These data are consistent with our aged cells (via passages, Fig. 1B) where aged MSCs showed a differential expression of CD44. To evaluate the calcium retention capabilities in aged and young mice MSCs, we again evaluated cytosolic Ca2+ levels especially upon store depletion. Interestingly, when young mice and aged mice MSC cells were treated with Thapsigargin (Tg), a rapid increase in cytosolic Ca2+ levels were observed in young mice MSC, when compared to aged mice MSCs. Moreover, young mice MSCs significantly showed a higher calcium peak (calcium entry) than aged mice MSCs (Fig. 5D, E). Interestingly addition of 2-APB further increased calcium entry in young MSCs, when compared with aged MSCs (Fig. 5D, F).
Fig. 5.
MSCs surface markers, cell viability, cell proliferation and differentiation potential after treatment of calcium and calcium channel blockers. A Cartoon showing the isolation, culture, and enrichment of mouse bone marrow derived MSCs of young (1 month) and aged mice (12 month). B Immunophenotypic characterization of young, aged mice MSCs, and young MSCs that were passaged 7 times show the CD44 marker expression. C The graph shows the percentage of cells expressing the CD44 markers in various cells. D Representative trace showing changes in cytosolic calcium levels in young and aged MSCs E, F show histograms of Er calcium release, calcium entry, and 2-APB (100 μM) evoked [Ca2 + ]i changes in young and aged mice MSC cells. G The colony-forming unit (CFU) assay was performed to study the stem cell potential in aged MSCs. Image also shows the differentiation capacity of stem cells toward osteocyte differentiation in aged MSCs. H MTT assay was performed to investigate calcium’s effect on cell viability in aged MSCs. Aged mice MSCs cells were treated with a different dose of calcium for 1 day prior to MTT assay evaluation. I BrdU assay was performed to study the cell proliferation of young and aged mice MSCs after treatment with different doses of calcium for and 3 days. *p ≤ 0.001 (Student’s t test).
Next, we evaluated stem cell potential using colony-forming units (CFU) assay and to analyze calcium’s role in modulating their differential capability in aged stem cells. Interestingly, our study found that stem cell potential declines in a age-dependent manner and as the mice aged, the potential for stem cell function gradually decreased (Fig. 5G). Furthermore, we evaluated the differentiation potential of stem cells, which was also decreased as the mice ages and very little differentiation into osteocyte was observed in stem cells isolated from 12 month mice (Fig. 5G). To evaluate the role of calcium in stem cell proliferation and viability, again BrdU and MTT assays were performed. Importantly, MSCs from aged mice showed a decrease in cell viability, when compared with younger MSCs (Fig. 5H). In contrast, aged mice MSCs treated with 5 mM Ca2+ showed significantly higher cell survival (Fig. 5H), again suggesting that calcium influx is able to restore cell viability in aged MSCs. Furthermore, cell proliferation was also increased in aged MSCs that were treated with 5 mM Ca2+ (Fig. 5I). Together these results suggest that effect of aging or age-related loss of stem cell function can be rescued with calcium supplementation.
4. Discussion
Mammalian bone marrow is recognized as the largest reservoir of adult stem cells and a variety of stem cells, including hematopoietic stem cells (HSCs), mesenchymal stem cells (MSCs), multipotent adult progenitor cells (MAPCs), and even small embryonic-like stem cells (VSELs) (Ahamad and Singh, 2021). Though bone marrow is the primary source of MSCs in adults, other tissues such as adipose tissues, placenta, amniotic fluid, umbilical cord blood, periodontal ligament, and dental pulp also harbor MSCs. However, bone marrow still remains the most common and consistently trustworthy source for in vitro studies, including isolation, culture, propagation, and characterization (Ahamad and Rath, 2019; Ahamad and Singh, 2021). In addition, recent research has revealed that stem cells do not remain static, and their properties, including surface marker expression and stem cell potential, are decreased as they are passaged and in aged animals. Similarly, growth factors, physicochemical factors, and transcription factors are also responsible for changing their phenotypes, morphology and decreasing their proliferation and cell viability, but different passage and aging also alter the cell functions (Iwata et al., 2021). Thus, factors that could maintain their proliferation over the passage and during age are demanded to be assessed and identified. Aging is a more complex process. Similarly, Cell proliferation is a complex mechanism orchestrated by several proteins involved in Ca2+ signaling mechanisms. Stem cells, the most proliferative cell, require high external Ca2+ concentrations to trigger cell function such as proliferation, while cancerous cells demand less Ca2+ (Borowiec et al., 2014; Dry et al., 2013). Our data shows that external Ca2+ is fundamental in preserving stem cell propagation in passaged cells and aged mice stem cells. Raising external Ca2+ starts to improve morphology, stem cell surface marker expression, proliferation, and stem cell potential in aged MSCs and aged mice MSCs. On the other hand, excluding external Ca2+ was adequate to repress cell growth and its stem cell potency. Thus, understanding the regulation of Ca2+ signaling during stem cell proliferation is critical to maintaining cell physiology and its function in tissue regeneration during healthy aging.
Our results also indicate Ca2+ entry increases the downstream signaling and increases the CD44 surface marker expression. Cell adhesion protein CD44 is a marker that differentiates between partially and fully reprogrammed cells, and HA-mediated CD44 interaction coordinated intracellular signaling pathways (e.g., Ca2+ mobilization, Rho signaling, PI3 kinase-AKT activation). Importantly, higher Ca2+ entry also promotes osteocyte differentiation while decline adipocyte differentiation was observed. Thus, high extracellular Ca2+ inhibits adipogenesis which is one of the characteristics of aging. Higher Ca2+ levels could also inhibit the aging process, at least at a cellular level. Ca2+ signaling is crucial to sustaining cytosolic Ca2+ levels compelled to stimulate transcription factors that modulate cell proliferation. Several Ca2+ entry channels are present in excitable and non-excitable cells, but the stem’s major Ca2+ entry mechanism is through SOCE (Parekh and Putney, 2005; Srikanth and Gwack, 2012; Uslu et al., 2020). Our data further show that both young and aged MSCs failed to show any increase in Ca2+ entry upon Ca2+ channel blocker treatment that activates either receptor or voltage-gated channels. Interestingly, earlier investigations have revealed that Ca2+ entry is essential for MSC proliferation, but the molecular identity of the Ca2+ entry channel is not yet determined in aged stem cells.
Our earlier research has identified two distinct Ca2+ entry channels that are activated by store depletion, and both Ca2+ entry channels were essential for stem cell function. Interestingly, loss of Orai1 revealed a decrease in cell proliferation, suggesting that it could modulate stem cell function. Interestingly, the silencing of STIM1 (Ca2+ sensor in the ER) also showed a significant decrease in cell proliferation and viability that performed a crucial role in modulating Ca2+ entry (Ambudkar et al., 2017; Sun et al., 2020). Upon store depletion, STIM1 senses a decrease in ER Ca2+, allowing STIM1 translocation in ER-PM junctions where it couples with both Orai1 and TRPC1 and facilitates Ca2+ entry (Ambudkar et al., 2017; Pani et al., 2012). Our data further explain that the cell cycle is also essential in maintaining stem cell function, and loss of Ca2+ entry has been confirmed to induce cell cycle arrest at the G0/G1 phase in aged MSC cells and aged mice MSCs. Interestingly, increasing Ca2+ allowed stem cells to move from G1 to the S phase that could also play a critical role in modulating stem cell functions and increased cell viability and proliferation in aged MSC cells and aged mice MSCs.
5. Conclusions
Our data indicate that higher extracellular Ca2+ concentration protects aged MSCs and MSCs isolated from aged mice against damage due to aging and can partially recover aged MSCs cell function. Importantly, as higher calcium rendered aged MSCs in functionally similar as young MSCs, this mechanism could be utilized against human aging and age-related disorder that are dependent on tissue regeneration therapy.
Acknowledgments
This work was funded by grant support from the National Institutes of Health (R01DE017102; R21DE028265) awarded to B.B.S. The funders had no further role in study design, data analysis, and/or interpretation of the data. Flow Cytometry Facility is supported by UTHSCSA, NIH-NCI P30 CA054174-20 and UL1 TR001120.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Ahamad N, Rath PC, 2019. Expression of interferon regulatory factors (IRF-1 and IRF-2) during radiation-induced damage and regeneration of bone marrow by transplantation in mouse. Mol. Biol. Rep 46 (1), 551–567. [DOI] [PubMed] [Google Scholar]
- Ahamad N, Singh BB, 2021. Calcium channels and their role in regenerative medicine. World J. Stem Cells 13 (4), 260–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed ASI, Sheng MHC, Wasnik S, Baylink DJ, Lau K-H, 2017. Effect of aging on stem cells. World J. Exp. Med 7 (1), 1. 10.5493/wjem.v7.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambudkar IS, de Souza LB, Ong HL, 2017. TRPC1, Orai1, and STIM1 in SOCE: Friends in tight spaces. Cell Calcium 63, 33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop AE, Buttery LDK, Polak JM, 2002. Embryonic stem cells. J. Pathol 197 (4), 424–429. [DOI] [PubMed] [Google Scholar]
- Borowiec A-S, Bidaux G, Pigat N, Goffin V, Bernichtein S, Capiod T, 2014. Calcium channels, external calcium concentration and cell proliferation. Eur. J. Pharmacol 739, 19–25. [DOI] [PubMed] [Google Scholar]
- Brini M, Ottolini D, Cali T, Carafoli E, 2013. Calcium in health and disease. Met Ions Life Sci 13, 81–137. [DOI] [PubMed] [Google Scholar]
- Broxmeyer HE, 2019. Hematopoietic Stem Cell Intracellular Levels of Ca2+ to the Rescue! What Next? Cell Stem Cell 25, 171–173. [DOI] [PubMed] [Google Scholar]
- Dry H, Jorgenson K, Ando W, Hart DA, Frank CB, Sen A, 2013. Effect of calcium on the proliferation kinetics of synovium-derived mesenchymal stromal cells. Cytotherapy 15 (7), 805–819. [DOI] [PubMed] [Google Scholar]
- Ergen AV, Goodell MA, 2010. Mechanisms of hematopoietic stem cell aging. Exp. Gerontol 45 (4), 286–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermakov A, Daks A, Fedorova O, Shuvalov O, Barlev NA, 2018. Ca(2+) -depended signaling pathways regulate self-renewal and pluripotency of stem cells. Cell Biol. Int 42 (9), 1086–1096. [DOI] [PubMed] [Google Scholar]
- Gross L, 2007. Mechanisms of aging in bone marrow stem cells. PLoS Biol. 5 (8), e215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata T, Mizuno N, Ishida S, Kajiya M, Nagahara T, Kaneda-Ikeda E, Yoshioka M, Munenaga S, Ouhara K, Fujita T, 2021. Functional Regulatory Mechanisms Underlying Bone Marrow Mesenchymal Stem Cell Senescence During Cell Passages. Cell Biochem. Biophys 1–16. [DOI] [PubMed] [Google Scholar]
- Bollimuntha S, Selvaraj S, Singh BB, 2011. Emerging roles of canonical TRP channels in neuronal function. Ad. Exp. Med. Biol 704, 573–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovtonyuk LV, Fritsch K, Feng X, Manz MG, Takizawa H, 2016. Inflamm-aging of hematopoiesis, hematopoietic stem cells, and the bone marrow microenvironment. Front. Immunol 7, 502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam BS, Cunningham C, Adams GB, 2011. Pharmacologic modulation of the calcium-sensing receptor enhances hematopoietic stem cell lodgment in the adult bone marrow. Blood, The Journal of the American Society of Hematology 117, 1167–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Couteur DG, Anderson RM, Newman AB, and de Cabo R (2017). Stem cell transplantation for frailty. The Journals of Gerontology: Series A 72, 1503–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerou P, Daley G, 2005. Therapeutic potential of embryonic stem cells. Blood Rev. 19 (6), 321–331. [DOI] [PubMed] [Google Scholar]
- Liang Y, Van Zant G, Szilvassy SJ, 2005. Effects of aging on the homing and engraftment of murine hematopoietic stem and progenitor cells. Blood 106, 1479–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DT, Moynagh MR, Eustace SJ, Kavanagh EC, 2010. Bone marrow. Magn. Reson. Imaging Clin. N. Am 18 (4), 727–735. [DOI] [PubMed] [Google Scholar]
- Pani B, Bollimuntha S, Singh BB, 2012. The TR (i) P to Ca2+ signaling just got STIMy: an update on STIM1 activated TRPC channels. Front. Biosci.: J. Virtual Library 17, 805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichard L, Brondelo J-M, Becker F, Desprat R, De Ceuninck F, Pastoureau P, Noel D, Jorgensen C, Lemaitre J-M, 2020. Generation of human pluripotent stem cell lines (iPSCs) from mesenchymal stem cells (MSCs) from three elderly patients with osteoarthritis. Stem Cell Res. 44, 101721. 10.1016/j.scr.2020.101721. [DOI] [PubMed] [Google Scholar]
- Pleyer L, Valent P, Greil R, 2016. Mesenchymal Stem and Progenitor Cells in Normal and Dysplastic Hematopoiesis-Masters of Survival and Clonality? Int. J. Mol. Sci 17 (7), 1009. 10.3390/ijms17071009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakar M, Ershler WB, Longo DL, 2009. Bone marrow, thymus and blood: changes across the lifespan. Aging health 5 (3), 385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh AB, Putney JW, 2005. Store-operated calcium channels. Physiol. Rev 85 (2), 757–810. [DOI] [PubMed] [Google Scholar]
- Racioppi L, Lento W, Huang W, Arvai S, Doan PL, Harris JR, Marcon F, Nakaya HI, Liu Y, and Chao N (2017). Calcium/calmodulin-dependent kinase kinase 2 regulates hematopoietic stem and progenitor cell regeneration. Cell death & disease 8, e3076–e3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpless NE, DePinho RA, 2007. How stem cells age and why this makes us grow old. Nat. Rev. Mol. Cell Biol 8 (9), 703–713. [DOI] [PubMed] [Google Scholar]
- Srikanth S, Gwack Y, 2012. Orai1, STIM1, and their associating partners. The Journal of physiology 590, 4169–4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Da Conceicao VN, Ahamad N, Madesh M, and Singh BB (2020). Spatial localization of SOCE channels and its modulators regulate neuronal physiology and contributes to pathology. Current Opinion in Physiology. [Google Scholar]
- Sun Y, Sukumaran P, Bandyopadhyay B, Singh B, 2014. Physiological function and characterization of TRPCs in neurons. Cells 3 (2), 455–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah M, Sun Z, 2018. Stem cells and anti-aging genes: double-edged sword—do the same job of life extension. Stem Cell Res. Ther 9, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemoto T, Hashimoto M, Matsumura T, Nakamura-Ishizu A, Suda T, 2018. Ca2+–mitochondria axis drives cell division in hematopoietic stem cells. J. Exp. Med 215, 2097–2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uslu M, Albayrak E, Kocabaş F, 2020. Temporal modulation of calcium sensing in hematopoietic stem cells is crucial for proper stem cell expansion and engraftment. J. Cell. Physiol 235 (12), 9644–9666. [DOI] [PubMed] [Google Scholar]
- Wong C-K, So W-Y, Law S-K, Leung F-P, Yau K-L, Yao X, Huang Y.u., Li X, Tsang S-Y, 2012. Estrogen controls embryonic stem cell proliferation via store-operated calcium entry and the nuclear factor of activated T-cells (NFAT). J. Cell. Physiol 227 (6), 2519–2530. [DOI] [PubMed] [Google Scholar]
- Yajima Y, Kawashima S, 2002. Calpain function in the differentiation of mesenchymal stem cells. Biol. Chem 383, 757–764. [DOI] [PubMed] [Google Scholar]