Abstract
Background and objectives
Guidelines and indications for exchange transfusion in haemolytic disease of the foetus and newborn (HDFN) have changed drastically in the past decades, causing a decline in exchange transfusion rate. This study aims to evaluate the incidence of exchange transfusions (ETs) in neonates with Rh‐mediated HDFN over the past 20 years at our centre, and report potentially ET‐related complications as well as indicators for bilirubin encephalopathy.
Material and methods
In this observational study, 438 neonates were included with HDFN, born ≥ 35 weeks gestational age at the Leiden University Medical Centre between January 2000 and July 2020. The incidence of ET and procedure‐related complications were assessed in three consecutive time periods determined by changes in guidelines and indications for ET.
Results
The incidence of ET in our centre declined from (104/156) 67% (time period 2000–2005), to (39/181) 22% (2006–2015) and to (10/101) 10% (2015–2020, p < 0·001). The maximum bilirubin levels in neonates after birth increased from 13·6 mg/dL (or 233 μmol/L), to 15·0 mg/dL (257 μmol/L) and to 15·3 mg/dL (263 μmol/L). The incidence of complications associated with the use of ET (including sepsis, haematologic disorders and respiratory failure) remained stable throughout the years, and no neonates died during the study period.
Conclusion
Exchange transfusion incidence declined significantly over the past two decades. Decrease in ET incidence, and concomitant decrease in exposure and expertise, was not associated with an increase in procedure‐related complications.
Keywords: haemolytic disease of the foetus and newborn, exchange transfusion, alloimmunization, hyperbilirubinaemia
Introduction
Haemolytic disease of the foetus and newborn (HDFN) is caused by an incompatibility between maternal and foetal red blood cell antigens. Destruction of the foetal red blood cells (RBC) by maternal alloantibodies results in foetal and neonatal haemolytic anaemia, which can be treated antenatally with intrauterine transfusions (IUTs) and post‐natally with RBC transfusions. Haemolysis may also lead to hyperbilirubinaemia and can result in acute and chronic bilirubin encephalopathy.
Hyperbilirubinaemia is treated with intensive phototherapy and exchange transfusion (ET). ET is recommended for infants whose bilirubin levels continue to rise to exchange levels, despite intensive phototherapy [1]. ET removes excess bilirubin from the neonatal blood circulation as well as maternal antibodies and antibody‐coated erythrocytes [1, 2, 3]. Approximately 85% of the neonatal blood is replaced by irradiated donor blood by double‐volume exchange transfusion [3, 4]. While being effective in the acute treatment of hyperbilirubinaemia, ET is an invasive procedure requiring central lines and has potentially severe side effects. Mortality rates around 0·3% are reported in term neonates, but increase above 10% in preterm neonates [4, 5, 6]. Morbidity rates are reported up to 24% and include cardiorespiratory instability, catheter‐related complications, thrombocytopenia and sepsis [7, 8, 9].
Data on changes in the incidence and complications of ET in severe HDFN are limited. In our centre, we noticed a reduction in the need for ET in the past decades, partly related to changes in our guidelines and indications for ET, which have become more restrictive over the years. Whether a reduction in exposure and expertise in performing an invasive and complex procedure as an ET is also associated with an increased risk of complications, is not well known.
The aim of this study is to give an overview of the incidence of exchange transfusions in neonates with severe Rh‐mediated HDFN over the past 20 years at our centre and to report potentially ET‐related complications, as well as indicators for bilirubin encephalopathy.
Methods
Study design and population
This is an observational cohort study conducted at the Leiden University Medical Centre (LUMC), the Dutch National Referral centre for severe HDFN and foetal therapy. In the Netherlands, all pregnant women with RhD immunization and an ADCC (antibody‐dependent cell‐mediated cytotoxicity) of >50% and/or antibody titre ≥ 16, as well as with Rh immunization other than D and ADCC > 30% and/or antibody titre ≥ 16, are referred to the LUMC [10]. Subsequently, these high‐risk pregnancies are monitored by serial Doppler measurements to assess the velocity of the blood flow in the middle cerebral artery. If this velocity exceeds 1·5 multiples of the median or if signs of hydrops are present, the treatment with IUT is indicated. IUTs can be administered until 34–35 weeks of gestation, after which induced delivery is preferred to IUT treatment. The IUT technique used in the Netherlands has been previously described [11].
All (near‐) term neonates (≥35 weeks of gestation) with HDFN due to maternal red cell alloimmunization against Rh antigens (D, C, c, Cw, E and e) admitted to the LUMC between 1 January 2000 and 31 June 2020 were eligible for this study.
Neonates with blood group alloimmunization caused by non‐Rh antigens were excluded as these antigens show different pathophysiological characteristics and great variation in exchange transfusion risk after birth [12]. Neonates born <35 weeks of gestation were excluded as prematurity itself is a major risk factor for hyperbilirubinaemia and ET treatment and is associated with greater odds of death following ET compared to term infants [13]. In addition, neonates who received intravenous immunoglobulins (IVIg) (n = 41) as part of a randomized controlled trial (RCT) (LIVIN trial, identifier ISRCTN14013064) between 2006 and 2010, were excluded as IVIg is not a standard practice at the LUMC.
The study cohort was divided into three different time periods according to changes in ET guidelines:
Group I: 1 January 2000 to 31 December 2005
In this first group, the bilirubin threshold for ET was a total serum bilirubin level at birth > 3·5 mg/dL (measured in umbilical cord blood, or, more often, in neonatal blood at birth) and/or a rise of bilirubin > 0·5 mg/dL/h despite intensive phototherapy [14]. In all neonates, bilirubin levels were measured every 2–3 hours according to protocol during the first few days after birth. The differences in bilirubin thresholds have been previously described [9].
Group II: 1 January 2006 to 31 March 2015
On 1 January 2006, we implemented new, more restrictive, ET guidelines based on the updated guideline of the American Academy of Pediatrics (AAP) [2]. After the guideline change, the new criteria for ET were: (1) total serum bilirubin above thresholds according to the AAP guideline, and/or (2) rise of bilirubin > 0·5 mL/dL/h despite intensive phototherapy, and/or (3) clinical symptoms of acute bilirubin encephalopathy regardless of bilirubin level [9].
Group III: 1 April 2015 to 31 June 2020
This third group encloses the years after the most recent guideline change at our centre, implemented in April 2015. The AAP guideline advises to classify proven blood group alloimmunization as ‘high risk’ for the rise of bilirubin to levels that might cause bilirubin encephalopathy [2]. After a consensus meeting at our centre, we decided not to categorize proven blood group antagonism as an extra risk factor as the blood‐brain barrier does not decrease in function with the presence thereof. Neonates with proven blood antagonism born after April 2015 were therefore categorized as ‘standard risk’ when choosing which threshold curve (low, standard or high risk) to use for phototherapy and ET.
Throughout the study period, ET was performed with blood exchange volumes of 100–200 mL/kg. The irradiated blood product consists of leukocyte‐reduced erythrocytes and plasma of two donors, less than 5 days old with a haematocrit of 0·50–0·65 g/L. No albumin or calcium infusions were given prior or during ET.
Outcome measures
The primary outcome was the incidence of ETs. Secondary outcomes included the timing of the first ET in hours, the duration of phototherapy in days, post‐natal RBC transfusion dependency, length of stay at the neonatal intensive care unit (NICU) and ET‐related complications.
Data collection
The following obstetric and neonatal data were directly recorded at our centre and obtained from patient’s medical files: foetal haemoglobin at first IUT and number of IUTs, gestational age and weight at birth, gender, mode of delivery, haemoglobin and bilirubin level at birth (conjugated and unconjugated), type of alloimmunization, maximum bilirubin level during admission, number of ETs and timing of ETs, post‐natal RBC transfusions (not including RBCs given as part of ET), respiratory distress (defined as need for mechanical ventilation), umbilical vein catheterization, number of days of phototherapy before discharge home or transfer to another hospital, results from standard cerebral ultrasound (intraventricular haemorrhage graded according to the grading system of Papile [15] and periventricular leukomalacia (PVL) according to de Vries et al. [16]), results from standard hearing screening and total length of hospital stay. The following complications of ET were recorded: proven sepsis (defined as clinical symptoms of infection combined with positive blood culture after the moment of ET), thrombosis (defined as the detection of a vascular thrombosis on ultrasound examination), mechanical ventilation, leukopenia (defined as leukocytes < 5·109/L), thrombocytopenia (defined as platelets < 100·109/L), platelet transfusion rate, hypocalcaemia (defined as calcium < 8·0 mg/dL), hyperkalaemia (defined as potassium > 6·5 mEq/L) and neonatal mortality [17]. Follow‐up data on RBC transfusions after discharge from our centre were collected from referral hospitals with written consent from parents or caregivers. RBC transfusions were administered in term neonates with HDFN when haemoglobin levels fall below 10·5 g/dL for day 0–6, below 8·9 g/dL for day 7–13 and below 7·2 g/dL from day 14 onwards. Before February 2014, haemoglobin thresholds for transfusion were 9·6 g/dL for days 7–13 and 8·0 g/dL from day 14 onwards. A transfusion of 15 mL/kg irradiated packed erythrocytes less than 5 days old was advised throughout the study period, with a haematocrit of 0·50–0·65 g/L.
Statistical analysis
Data was reported as means and standard deviations (SD), or as medians and interquartile range (IQR), when appropriate. The primary outcome was tested by χ2 test. Statistical analysis was performed using IBM SPSS Statistics version 26.
Ethical considerations
Due to the non‐invasive nature of this study, a waiver of consent was granted by the medical ethics committee of our centre.
Results
During the study period of 20 years, 612 neonates with severe HDFN were admitted to the neonatal intensive care unit (NICU) of the LUMC, and 438 neonates met the inclusion criteria and were eligible for this study. We excluded 87 neonates due to HDFN primarily caused by non‐Rh antibodies, 56 neonates with a gestational age < 35 weeks, 10 neonates who fulfilled both exclusion criteria and 41 neonates treated with IVIg as part of the LIVIN trial (Fig. 1).
Fig. 1.
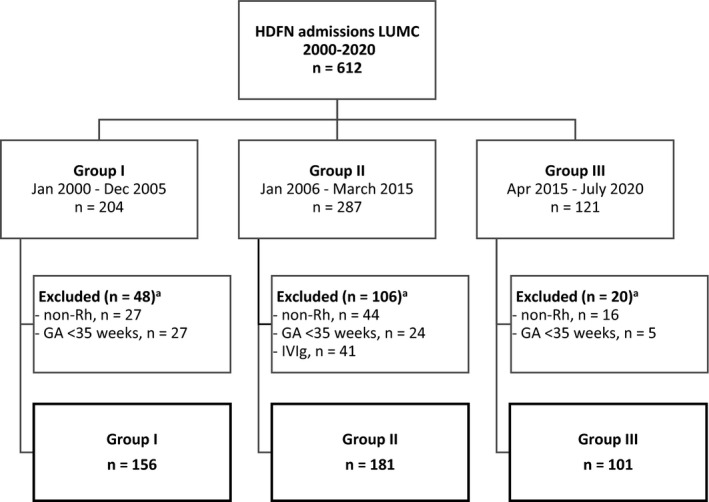
Flowchart of study participants. aSome neonates meet more than one exclusion criteria; hence, combined numbers per criterion can exceed the total number of exclusions. GA, gestational age; IVIg, intravenous immunoglobulin.
Baseline characteristics for each of the three cohorts are shown in Table 1. RhD was the most common causative Rh antigen of HDFN in all three groups (89%, 85% and 92%, respectively), followed by Rhc. The occurrence of multiple Rh‐alloantibodies was 51% in group I, 43% in group II and 39% in group III. The occurrence of a non‐Rh alloantibody, besides a primary Rh‐alloantibody, was, respectively, 7% in group I, 6% in group II and 3% in group III. This most often involved alloantibodies against Kell or Jka antigens.
Table 1.
Baseline characteristics.
|
Group I n = 156 |
Group II n = 181 |
Group III n = 101 |
|
|---|---|---|---|
| Neonates treated with IUT(s) ‐ n (%) | 99 (63) | 102 (56) | 61 (60) |
| Number of IUT(s) per neonate a | 3 (2–4) | 2 (2–4) | 2 (1–3) |
| Gestational age at birth ‐ weeks a | 37 (36–37) | 37 (36–37) | 37 (36–37) |
| Birthweight ‐ grams b | 2972 ± 443 | 2987 ± 470 | 2913 ± 348 |
| Caesarean delivery ‐ n (%) | 48 (31) | 41 (23) | 27 (27) |
| Male gender ‐ n (%) | 87 (56) | 114 (63) | 47 (47) |
| Primary type of alloantibodies | |||
| Rh D ‐ n (%) | 139 (89) | 153 (85) | 93 (92) |
| Rh C ‐ n (%) | 1 (1) | 2 (1) | 0 (0) |
| Rh c ‐ n (%) | 13 (8) | 17 (9) | 8 (8) |
| Rh Cw ‐ n (%) | 0 (0) | 2 (1) | 0 (0) |
| Rh E ‐ n (%) | 3 (2) | 7 (4) | 0 (0) |
| Additional non‐Rh alloantibodies ‐ n (%) | 11 (7) | 10 (6) | 3 (3) |
| Haemoglobin level at birth ‐ g/dL b , c | 11·6 ± 2·6 | 13·4 ± 3 | 13·5 ± 2·6 |
| Unconjugated bilirubin level at birth ‐ mg/dL b , d | 5·5 ± 2·8 | 5·7 ± 2·9 | 5·1 ± 2·3 |
| Unconjugated bilirubin level at birth ‐ μmol/L b , d | 94 ± 48 | 97 ± 49 | 87 ± 39 |
| Conjugated bilirubin level at birth ‐ mg/dL a , e | 0·5 (0·4–0·8) | 0·6 (0·5–0·8) | 0·5 (0·4–0·6) |
| Conjugated bilirubin level at birth ‐ μmol/L a , e | 9 (6–14) | 10 (8–13) | 8 (6–11) |
IQR, interquartile range; IUT, intrauterine transfusion; SD, standard deviation.
Median (IQR).
Mean ± SD.
0 missing values in group I, five missing values in group II, one missing value in group III.
one missing value in group I, five missing values in group II, one missing value in group III.
17 missing values in group I, 35 missing values in group II, five missing values in group III.
Neonatal treatment data and outcome measures are presented in Table 2. The ET incidence decreased significantly from 104/156 (67%) in group I (before the AAP guideline change) to 39/181 (22%) in group II (after the AAP guideline change) and was further reduced since then to 10/101 (10%) in the most recent group III (p < 0·001). Numbers of ET per year are shown in Fig. 2.
Table 2.
Neonatal outcomes.
|
Group I n = 156 |
Group II n = 181 |
Group III n = 101 |
|
|---|---|---|---|
| Neonates treated with ET(s) ‐ n (%) a | 104 (67) | 39 (22) | 10 (10) |
| Maximum unconjugated bilirubin level ‐ mg/dL b | 13·6 ± 4·7 | 15·0 ± 5·2 | 15·3 ± 4·7 |
| Maximum unconjugated bilirubin level ‐ μmol/L b | 233 ± 80 | 257 ± 89 | 262 ± 80 |
| Bilirubin > 25·0 mg/dL ‐ n (%) c | 1 (1) | 6 (3) | 1 (1) |
| Umbilical venous catheter ‐ n (%) | 129 (83) | 68 (38) | 39 (39) |
| Duration of phototherapy ‐ days d , e | 4 (3–5) | 5 (3–6) | 5 (4–6) |
| Neonates receiving RBC transfusion(s) ‐ n (%) f | 106 (69) | 137 (81) | 68 (74) |
| Number of RBC transfusions per neonate d , g | 2 (1–3) | 2 (1–3) | 2 (1–3) |
| Mechanical ventilation ‐ n (%) | 6 (4) | 1 (1) | 1 (1) |
| Proven sepsis ‐ n (%) h | 6 (4) | 6 (4) | 5 (5) |
| Duration of NICU admission ‐ days | 6 ± 3 | 7 ± 3 | 7 ± 2 |
| Mortality ‐ n (%) | 0 (0) | 0 (0) | 0 (0) |
ET, exchange transfusion; IQR, interquartile range; IUT, intrauterine transfusion; NICU, neonatal intensive care unit; RBC, red blood cell; SD, standard deviation.
p‐value < 0·001.
Mean ± SD.
Absolute ‘medical emergency’ value indicating direct need for intensive phototherapy as recommended by the AAP [2], equal to 428 μmol/L.
Median (IQR).
22 missing values group I, one missing value in group II, 0 missing values in group III.
Two missing values in group I, 12 missing values in group II, nine missing values in group III.
Two missing values in group I, three missing values in group II, six missing values in group III.
0 missing values in group I, eight missing values in group II, one missing value in group III.
Fig. 2.
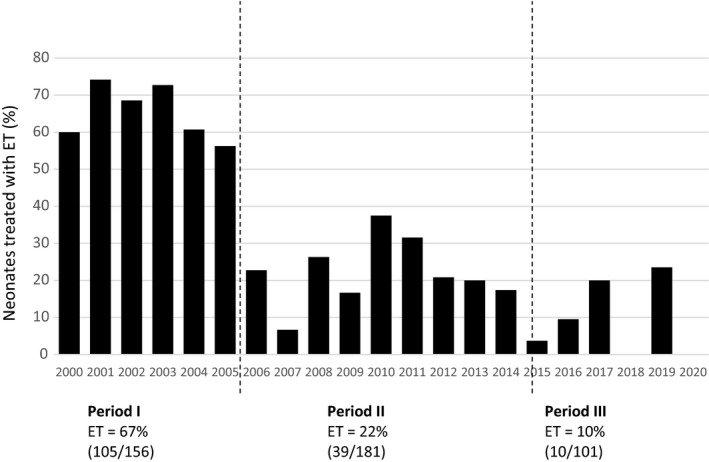
Incidence of exchange transfusion throughout the years. ET, exchange transfusion.
In group I, 106/156 (69%) neonates were treated with post‐natal RBC transfusion(s), 37/156 (81%) in group II and 68/101 (74%) neonates in group III. The maximum bilirubin reached levels > 25·0 mg/dL in eight neonates: one (1%) in group I, six (3%) in group II and one (1%) in group III. In group I, 129/156 (83%) neonates had an umbilical venous catheter, 68/156 (38%) in group II and 39/101 (39%) in group III (Table 2).
Characteristics and complications in subgroup of neonates treated with ET are presented in Table 3. The number of ETs per neonate did not differ between groups (median one ET per neonate); the time after birth before an ET was performed increased from a median of 6 hours in group I to a median of 50 hours in group III.
Table 3.
Characteristics and complications in subgroup of neonates treated with ET.
|
Group I n = 104 |
Group II n = 39 |
Group III n = 10 |
|
|---|---|---|---|
| Number of ET(s) per neonate a | 1 (1–2) | 1 (1–2) | 1 (1–1) |
| Time to first ET ‐ hours after birth a | 6 (5–9) | 31 (15–55) | 50 (18–65) |
| Mechanical ventilation ‐ n (%) | 4 (4) | 0 (0) | 0 (0) |
| Proven sepsis as related to ET ‐ n (%) b | 6 (6) | 4 (11) | 1 (10) |
| Umbilical vein thrombosis ‐ n (%) c | 1 (1) | 0 (0) | 0 (0) |
| Leukocytopenia < 5·109/L ‐ n (%) d | 66 (63) | 27 (71) | 3 (33) |
| Thrombocytopenia < 100·109/L ‐ n (%) e | 101 (98) | 36 (97) | 10 (100) |
| 25‐49·109/L | 41 (40) | 19 (50) | 3 (30) |
| <25·109/L | 21 (20) | 5 (13) | 0 (0) |
| Neonates receiving platelet transfusion(s) ‐ n (%) | 56 (54) | 23 (59) | 3 (30) |
| Hypocalcaemia < 8·0 mg/dL ‐ n (%) | 12 (12) | 6 (15) | 0 (0) |
| Hyperkalaemia > 6·5 mEq/L ‐ n (%) | 1 (1) | 1 (3) | 0 (0) |
| Hearing screening performed ‐ n (%) | 43 (41) | 32 (82) | 8 (80) |
| Hearing screening passed ‐ n (%) f | 43 (100) | 32 (100) | 8 (100) |
| Cerebral ultrasound performed ‐ n (%) | 51 (49) | 35 (90) | 10 (100) |
| Minor IVH, grade I or II ‐ n (%) | 0 (0) | 0 (0) | 0 (0) |
| Major IVH, grade III or IV ‐ n (%) | 0 (0) | 1 (3) | 0 (0) |
| Cystic PVL ‐ n (%) | 0 (0) | 1 (3) | 0 (0) |
| Mortality ‐ n (%) | 0 (0) | 0 (0) | 0 (0) |
ET, exchange transfusion; IQR, interquartile range; IUT, intrauterine transfusion; IVH, intraventricular haemorrhage; PVL, periventricular leukomalacia; SD, standard deviation.
Median (IQR).
Sepsis defined as symptomatic infection with positive blood culture, onset later than first ET.
Only one neonate underwent a catheter ultrasound.
one missing value in group II.
three missing values, one in each group.
61 missing values in group II, seven missing values in group II, two missing values in group III.
In group I, four neonates required mechanical ventilation in relation to ET treatment, none in the other groups. Sepsis after the first or following ETs occurred in 6% of group I, respectively, 11% and 10% in groups II and III. All neonates received the ET through an umbilical vein catheter. Umbilical vein thrombosis was diagnosed in one case, which was detected after an ultrasound examination was performed due to persisting thrombocytopenia. Leukocytopenia occurred in 63% of the cases in group I, 71% in group II and 33% in group III. Thrombocytopenia < 100·109/L occurred in almost all neonates that underwent at least one ET, but severe thrombocytopenia < 25·109/L was rare (21 neonates (20%) in group I, 5 neonates (13%) in group II and none in group III. The occurrence of hypocalcaemia < 8·0 mg/dL after ET was 12% in group I, 15% in group II and did not occur in group III. Hyperkalaemia > 6·5 mEq/L after ET occurred in only two cases, one neonate in group I and one neonate in group II.
Hearing screening was conducted in a great majority of neonates in groups II and III, but only in 43 (41%) of group I. All neonates tested passed the screening. Cerebral ultrasound was conducted in a great majority of group II (90%) and group III (100%), but only in half of the neonates (49%) of group I. Ultrasound showed one case of severe IVH (grade 3) and one case of cystic PVL in group II. No neonates died in our study population.
Discussion
In this study, we showed a significant decline of ET incidence in neonates with severe HDFN due to severe Rh‐mediated HDFN over the past 20 years. The incidence of ET treatment declined from 67% to 10%. The time to first ET after birth was postponed from 6 to 50 hours. This impressive decline in ET can be attributed to the changes and implementation of increasingly restrictive ET guidelines as well as to improved use of intensive phototherapy. Importantly and reassuringly, the strong decline in ET incident and thus decreased exposure and expertise with this complex procedure was not associated with an increase in procedure‐related complications. In the Netherlands, this is presumably due to the centralization and thus specialization of care for neonates with HDFN.
Only few studies have evaluated the changes in the use of ET over the years [8, 13, 18, 19, 20, 21], and although accurate comparison is not possible, these studies also show an overall sharp decline in ETs in HDFN without increase in adverse events related to ET. Comparison with other centres is complicated due to various time cohorts chosen, different study populations as definitions of (severe) HDFN vary greatly, as do local phototherapy and ET protocols and guidelines. A study from the USA compared 71 infants treated with ET between 1986 and 1995 to 36 infants treated with ET between 1996 and 2006 (overall decline of 35/71, 49%). The ET‐related complications’ incidence was 14% vs. 7% in the two cohorts (p = 0·270). Of the studied cases, only 41% was treated with ET due to hyperbilirubinaemia caused by Rh‐mediated HDFN and an additional 28% due to ABO incompatibility [8]. A study from Norway showed a decrease in ET incidence from (39/80) 49% in the period 1993–1998 to (12/96) 12% in the period 1999–2003 [18]. The study group included infants with Rh‐mediated HDFN, as well as ABO incompatibility. This decline was attributed to the introduction of standard IVIg treatment in 1998. A study in India reported a decline of ET incidence from 6·8% of the total neonatal admissions in 2006 to 0·3% in 2016, but did not report on underlying indications for ET [19]. A recently published large multicentre cohort study assessing the prevalence of ET and ET‐associated morbidity and mortality in the USA between 1997 and 2016 reported a decline in ETs of 0·3% to 0·05% among all NICU admissions and found similarly high numbers of thrombocytopenia and leukopenia. However, the study also did not report on underlying causes of hyperbilirubinaemia and included neonates as young as 23 weeks of gestation, complicating accurate comparison [13].
For an increasingly rare disease as HDFN, international research would be highly recommendable to register and address these major differences between countries and treatment centres. It is important to have seemingly simple numbers as the current ET incidence available for counselling of parents and caregivers, organization of care and for further research in which ET incidence can be a potential outcome measure.
In our cohort, no cases of acute transfusion reactions such as transfusion‐related lung injury and transfusion‐associated circulatory overload were reported. The features of these reactions are presumably less outspoken and therefore under recognized in neonates [22].
Several studies that describe the decline of ETs during the last three decades suggest that the decrease in ETs could be explained by the administration of IVIg to neonates [18]. A Cochrane review by Zwiers et al. [23] was inconclusive on this subject since the studies that showed a reduction in ETs after administration of IVIg were of low quality and of high risk of bias. The only two high‐quality placebo‐controlled randomized studies did not provide any evidence that administration of IVIg reduced the need for ETs [24, 25]. In our centre, we do not administer IVIg (except during the study period of the aforementioned LIVIN study [24]), and therefore, the observed decline in ETs in our cohorts cannot be explained by IVIg treatment.
Earlier work by our study group showed a tendency towards an increase in the post‐natal RBC transfusion rate and correlated with a decrease in ET incidence in neonates with severe HDFN. This effect was attributed to the removal of antibodies and IgG‐coated erythrocytes during ET, hence reducing the haemolytic process [10, 18, 23]. Although morbidity of RBC transfusion (reported between 0·014 and 0·04% [26]) is much less than the morbidity of ET treatment (between 7 and 24% [9]), it is still of interest that in the current study, no further increase in neonatal RBC transfusions was observed. A possible explanation could be the implementation of a more restrictive RBC transfusion threshold at our department in February 2014.
This study has several strengths and limitations. One of the major strengths is the setting of one national referral centre for severe HDFN, providing us with a homogenous and near‐complete collection of data and follow‐up records of an increasingly rare disease. However, the results must be carefully interpreted in the context of our study population, as it is a selection of (very) severe HDFN cases as result of the referral guidelines in the Netherlands. Neonates not treated with IUT, particularly the less severe cases that did not require foetal therapy, were probably more likely to have been admitted to other centres.
Another major limitation of this study, and of the research in the field of HDFN in general, is the lack of long‐term outcome results of these neonates. Although cerebral ultrasound and hearing screening are now part of routine care after IUT treatment and ET treatment at our centre and no neonates in this study showed abnormalities in these tests, the occurrence of long‐term (mild) symptoms of bilirubin encephalopathy is unknown, as well as clear, absolute bilirubin cut‐off values which may give rise to such symptoms.
In conclusion, the need for ET in neonates with severe HDFN admitted to our centre has gradually decreased and has now become relatively rare. Reduction in ET incidence and therefore in expertise in performing this complex procedure was not associated with an increase in procedure‐related complications. Nevertheless, if the exposure of physicians to ET treatment will continue to decline, centralization of this procedure in specialized tertiary care centres may be necessary to maintain sufficient experience.
Conflicts of interest
There are no conflicts of interest to report.
References
- 1. Ree IMC, Smits‐Wintjens V, van der Bom JG, et al. Neonatal management and outcome in alloimmune hemolytic disease. Expert Rev Hematol 2017;10:607–16. [DOI] [PubMed] [Google Scholar]
- 2. American Academy of Pediatrics Subcommittee on, H . Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics 2004;114:297–316. [DOI] [PubMed] [Google Scholar]
- 3. Al‐Alaiyan S, Al Omran A. Late hyporegenerative anemia in neonates with rhesus hemolytic disease. J Perinat Med 1999; 27:112–5. [DOI] [PubMed] [Google Scholar]
- 4. Smits‐Wintjens VE, Walther FJ, Lopriore E. Rhesus haemolytic disease of the newborn: Postnatal management, associated morbidity and long‐term outcome. Semin Fetal Neonatal Med 2008;13:265–71. [DOI] [PubMed] [Google Scholar]
- 5. Murki S, Kumar P. Blood exchange transfusion for infants with severe neonatal hyperbilirubinemia. Semin Perinatol 2011;35:175–84. [DOI] [PubMed] [Google Scholar]
- 6. Dennery PA, Seidman DS, Stevenson DK. Neonatal hyperbilirubinemia. N Engl J Med 2001;344:581–90. [DOI] [PubMed] [Google Scholar]
- 7. Chitty HE, Ziegler N, Savoia H, et al. Neonatal exchange transfusions in the 21st century: a single hospital study. J Paediatr Child Health 2013;49:825–32. [DOI] [PubMed] [Google Scholar]
- 8. Steiner LA, Bizzarro MJ, Ehrenkranz RA, et al. A decline in the frequency of neonatal exchange transfusions and its effect on exchange‐related morbidity and mortality. Pediatrics 2007;120:27–32. [DOI] [PubMed] [Google Scholar]
- 9. Rath ME, Smits‐Wintjens VE, Lindenburg I, et al. Top‐up transfusions in neonates with Rh hemolytic disease in relation to exchange transfusions. Vox Sang 2010;99:65–70. [DOI] [PubMed] [Google Scholar]
- 10. Oepkes D, van Kamp IL, Simon MJ, et al. Clinical value of an antibody‐dependent cell‐mediated cytotoxicity assay in the management of Rh D alloimmunization. Am J Obstet Gynecol 2001;184:1015–20. [DOI] [PubMed] [Google Scholar]
- 11. van Kamp IL, Klumper FJ, Meerman RH, et al. Treatment of fetal anemia due to red‐cell alloimmunization with intrauterine transfusions in the Netherlands, 1988–1999. Acta Obstet Gynecol Scand 2004;83:731–7. [DOI] [PubMed] [Google Scholar]
- 12. Rath ME, Smits‐Wintjens VE, Lindenburg IT, et al. Exchange transfusions and top‐up transfusions in neonates with Kell haemolytic disease compared to Rh D haemolytic disease. Vox Sang 2011;100:312–6. [DOI] [PubMed] [Google Scholar]
- 13. Wolf MF, Childers J, Gray KD, et al. Exchange transfusion safety and outcomes in neonatal hyperbilirubinemia. J Perinatol 2020; 40:1506–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. De Boer IP, Zeestraten EC, Lopriore E, et al. Pediatric outcome in Rhesus hemolytic disease treated with and without intrauterine transfusion. Am J Obstet Gynecol 2008;198:54 e1–4. [DOI] [PubMed] [Google Scholar]
- 15. Papile LA, Burstein J, Burstein R, et al. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr 1978;92:529–34. [DOI] [PubMed] [Google Scholar]
- 16. de Vries LS, Eken P, Dubowitz LM. The spectrum of leukomalacia using cranial ultrasound. Behav Brain Res 1992;49:1–6. [DOI] [PubMed] [Google Scholar]
- 17. Smits‐Wintjens VE, Rath ME, van Zwet EW, et al. Neonatal morbidity after exchange transfusion for red cell alloimmune hemolytic disease. Neonatology 2013;103:141–7. [DOI] [PubMed] [Google Scholar]
- 18. Huizing K, Roislien J, Hansen T. Intravenous immune globulin reduces the need for exchange transfusions in Rhesus and AB0 incompatibility. Acta Paediatr 2008;97:1362–5. [DOI] [PubMed] [Google Scholar]
- 19. Chhapola V, Sharma AG, Kanwal SK, et al. Neonatal exchange transfusions at a tertiary care centre in north India: an investigation of historical trends using change‐point analysis and statistical process control. Int Health 2018;10:451–6. [DOI] [PubMed] [Google Scholar]
- 20. Al‐Lawama M, Al‐Rimawi E, Al‐Shibi R, et al. Adoption of the American Academy of Pediatrics' neonatal hyperbilirubinemia guidelines and its effect on blood exchange transfusion rate in a tertiary care center in Amman, Jordan. J Blood Med 2018;9:61–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yu C, Li H, Zhang Q, et al. Report about term infants with severe hyperbilirubinemia undergoing exchange transfusion in Southwestern China during an 11‐year period, from 2001 to 2011. PLoS One 2017;12:e0179550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kelly AM, Williamson LM. Neonatal transfusion. Early Hum Dev 2013;89:855–60. [DOI] [PubMed] [Google Scholar]
- 23. Zwiers C, Scheffer‐Rath ME, Lopriore E, et al. Immunoglobulin for alloimmune hemolytic disease in neonates. Cochrane Database Syst Rev 2018;3:CD003313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Smits‐Wintjens VE, Walther FJ, Rath ME, et al. Intravenous immunoglobulin in neonates with rhesus hemolytic disease: a randomized controlled trial. Pediatrics 2011;127:680–6. [DOI] [PubMed] [Google Scholar]
- 25. Santos MC, Sa C, Gomes SC Jr, et al. The efficacy of the use of intravenous human immunoglobulin in Brazilian newborns with rhesus hemolytic disease: a randomized double‐blind trial. Transfusion 2013;53:777–82. [DOI] [PubMed] [Google Scholar]
- 26. Stainsby D, Jones H, Wells AW, et al. Group SS : Adverse outcomes of blood transfusion in children: analysis of UK reports to the serious hazards of transfusion scheme 1996–2005. Br J Haematol 2008;141:73–9. [DOI] [PubMed] [Google Scholar]


