Abstract
Background
Over the last years, several trials offered new evidence on heart failure (HF) treatment.
Design and results
For HF with reduced left ventricular ejection fraction, type 2 sodium—glucose cotransporter inhibitors, aside from sacubitril—valsartan, demonstrated extraordinary efficacy in ameliorating patients' prognosis. Some new molecules (eg vericiguat, omecamtiv mecarbil and ferric carboxymaltose) correct iron deficiency and have shown to be capable of furthering reducing the burden of HF hospitalisation. Finally, there is new evidence on the possible therapeutic approaches of HF patients with mid‐range or preserved left ventricular ejection fraction.
Conclusions
This review aimed to revise the main novelties in the field of HF therapy and focus on how the daily clinical approach to patient treatment is changing.
Keywords: heart failure, prognosis, therapy
1. INTRODUCTION
It has been 5 years since the last European Society of Cardiology (ESC) guidelines have been published, 1 and some relevant trials have been published (Figure 1) 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 over this period, which will probably modify the current therapeutic approach for acute and chronic heart failure (CHF). After obtaining the results of PARADIGM‐HF, 10 more recent trials have confirmed the relevance of sacubitril‐valsartan in HF with reduced ejection fraction (HFrEF). 2 , 3 A greater and unexpected novelty has been represented by type 2 sodium‐glucose cotransporter inhibitor (SGLT2i), a glycosuric class of drugs that had already demonstrated its extraordinary effects on the prevention of HF hospitalisation among patients with type 2 diabetes mellitus (T2DM). 11 , 12 , 13 , 14 DAPA‐HF, 4 EMPEROR‐reduced 5 and SOLOIST 6 trials have now provided new evidence on the role of two SGLT2i (ie dapagliflozin and empagliflozin) and an SGLT2i/SGLT1i (ie sotagliflozin) in further improving the prognosis of HF in patients with and without diabetes.
FIGURE 1.
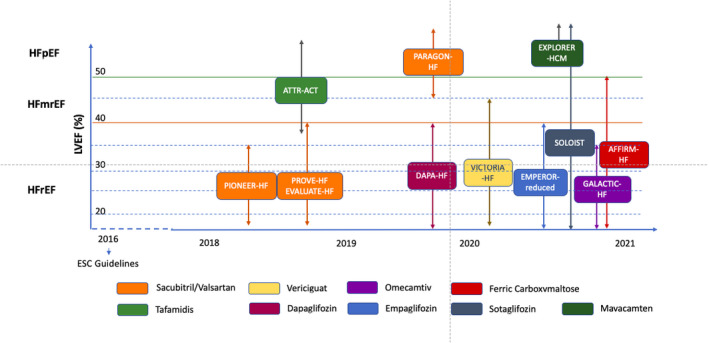
The main trials testing drug therapy after the publication of the last European Society of Cardiology Guidelines. 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 87 , 93 , 94 The distribution of the trials is based on the year of publication and the left ventricular ejection fraction at the time of the enrolment (the mean and range follow the inclusion criteria). The different colours identify the different drugs tested. ESC, European Society of Cardiology; HFmrEF, heart failure with mid‐range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; LVEF, left ventricular ejection fraction
Aside from SGLT2i, there is new evidence on the role of vericiguat (a vasodilator 7 ), omecamtiv (a myotrope 8 ) and iron deficiency (ID) correction by ferric carboxymaltose (FCM) administration. 9 Moreover, new evidence was obtained on the possible therapeutic approaches of patients with HF with mid‐range ejection fraction (HFmrEF) or HF with preserved ejection fraction (HFpEF).
This review aimed to revise the main therapeutic novelties in the field of HF therapy and focus on how the daily clinical approach for the treatment of patients is changing. The reporting of the studies conforms to the broad EQUATOR guidelines. 15
1.1. Neurohormonal modulation and sacubitril‐valsartan in HFrEF
The greatest progress in the HFrEF therapy was indicated by the effects of the drugs antagonising the neurohormonal systems responsible for HF progression since the early 1990s. 16 , 17 The activation of the renin‐angiotensin‐aldosterone system (RAAS) and the increased sympathetic nervous activity (SNA) initially play a compensatory role by increasing inotropic response, enhancing peripheral vascular resistance and promoting fluid and salt retention in the presence of systolic dysfunction. 16 , 17 However, RAAS and SNA overactivity have negative effects in the long term. They are responsible for the alterations in myocyte biology as well as for the induction of cardiomyocyte apoptosis and necrosis as well as myocardial fibrosis. All these effects cause further changes in the left ventricular chamber geometry and the left ventricular remodelling. 16 Based on this pathophysiological background, ACE inhibitors (ACEi), 18 , 19 , 20 beta blockers, 21 , 22 , 23 , 24 , 25 , 26 mineralocorticoid receptor antagonists (MRAs) 27 , 28 and angiotensin (AT) II receptor blockers (ARBs) 1 , 29 have been investigated in HFrEF, leading to an overall prognosis improvement in HFrEF patients.
Recently, this therapeutic approach has been further enhanced by the inhibition of neprilysin, the endothelial endopeptidase involved in the degradation of natriuretic peptides (NPs). The NP system (NPS) counteracts RAAS and SNA 30 by inducing natriuresis and diuresis, exerting an antifibrotic effect at the cardiac level and RAAS vasodilation and inhibition. The serum levels of NPs, that is, atrial (ANP) and brain (BNP), increase with the worsening of HF counterbalancing the negative effects of RAAS and SNA overactivation. However, their effectiveness is progressively reduced due to an altered target organ responsiveness or decreased availability of biologically active NPs. 31 This last condition seems to be related to neprilysin overactivity, 32 , 33 and consequently, its inhibition could lead to an increase in NP activity. 34 However, neprilysin is also responsible for the degradation of other substrates favouring remodelling, such as ATII. 30 Thus, the neprilysin inhibitor, sacubitril, has been associated with an ATII antagonist, valsartan, to privilege its favourable effects.
It has been more than 5 years since the results of PARADIGM‐HF have been published, 10 which demonstrated the effects of sacubitril‐valsartan on the reduction of HF worsening as well as mortality when compared with enalapril. The trial enrolled a number of outpatients in stable clinical conditions, most of whom were classified as NYHA class II, did not have symptomatic hypotension, and have already tolerated a maximum dose of enalapril and sacubitril‐valsartan during the run‐in. Sacubitril‐valsartan was able to reduce HF hospitalisation very early during the follow‐up 35 as well as the 30‐day readmission 36 and the total number of HF hospitalisation. 37 Following PARADIGM‐HF, EVALUATE‐HF 2 and PROVE‐HF 3 provided further data about the effects of sacubitril‐valsartan on cardiac remodelling, thus strengthening its possible usefulness in HFrEF.
EVALUATE‐HF 2 aimed to compare the effects of sacubitril‐valsartan with enalapril on aortic stiffness and ventricular remodelling in 464 patients with left ventricular ejection fraction (LVEF) randomised in a 1:1 ratio. During a short‐term follow‐up of 12 weeks, the sacubitril‐valsartan group demonstrated a significantly greater reduction from the baseline of the left ventricular end‐diastolic volume index (LVEDVI) and left ventricular end‐systolic volume index (LVESVI) as well as of the parameters of diastolic function (mitral E/e′ ratio and left atrial volume index, LAVI). No differences were observed when LVEF and aortic stiffness were evaluated.
PROVE‐HF 3 has tested the efficacy of sacubitril‐valsartan in the left ventricular remodelling with a different design. It was a prospective, single‐group, open‐label study that enrolled 794 patients with HFrEF who were followed up for a longer period of time (up to 12 months). The results of the study indicated a significant and progressive reduction of LVEDVI, LVESVI as well as LAVI and E/e′. Moreover, a significant LVEF improvement and NT‐proBNP reduction were observed. Finally, a significant correlation between changes in NT‐proBNP and reverse remodelling was demonstrated.
The results of EVALUATE‐HF and PROVE‐HF are even more relevant when compared with analogous previous studies that evaluated the effects of ACEis, beta blockers and their combination, 38 as presented in Figure 2.
FIGURE 2.
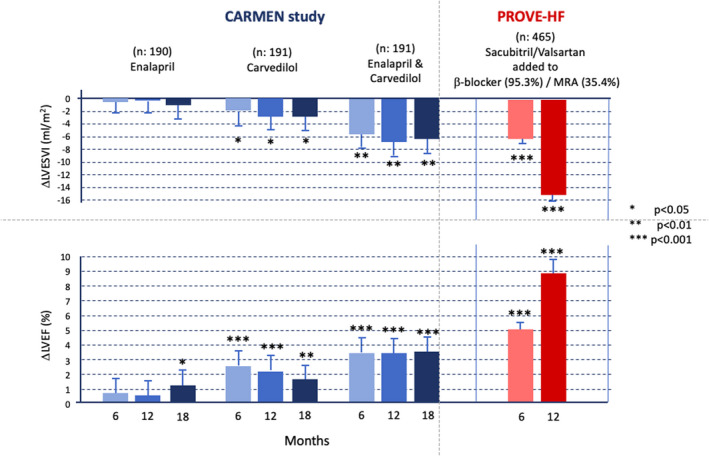
The results of the CARMEN 38 and PROVE‐HF 3 studies. The first study compared the reverse remodelling and improvement of the left ventricular ejection fraction in patients undergoing treatment with enalapril, carvedilol and enalapril plus carvedilol for up to 18 months. The second tested the introduction of sacubitril‐valsartan in patients with heart failure with reduced ejection fraction who have already been treated with beta blockers and, in part, mineralocorticoid receptor antagonists, 75.8% of whom were previously treated with ACEi or angiotensin II receptor blockers. LVEF, left ventricular ejection fraction; LVESVI, left ventricular end‐systolic volume index
The greater anti‐remodelling effect is a relevant clinical aspect that should be considered when starting the drug administration as soon as possible to HFrEF patients. The relevance of the earlier introduction of an effective therapeutic neurohormonal approach has been previously demonstrated in terms of the effect on survival 39 and of the probability of a greater and long‐term persistent reverse remodelling and LVEF improvement. 40 , 41 This hypothesis for sacubitril‐valsartan is strengthened by the results of PROVE‐HF, 2 which have demonstrated a greater effect on reverse remodelling in naïve patients who were not previously taking ACEi/ARBs.
From a clinical point of view, the possible beneficial effects on a more critical disease stage such as acute decompensated HF (ADHF), an adverse event leading to an abrupt change in the HF prognosis, should also be highlighted beyond the relevance of the anti‐remodelling effect derived from the early introduction of sacubitril‐valsartan in HFrEF patients. 42 The neurohormonal modulation proved the beneficial effects on the HFrEF prognosis also in the advanced stage of the syndrome. 18 , 21 , 24 , 28 In this clinical setting, the PIONEER‐HF trial 43 provided new evidence. It was designed to investigate sacubitril‐valsartan in terms of their safety for ADHF subjects during in‐hospital admission by comparing its tolerability with that of enalapril at a short‐term follow‐up. The trial results confirmed that sacubitril‐valsartan was superior to enalapril in decreasing plasma NT‐proBNP concentration as HFrEF therapy surrogate end point. 43 A reduction of HF worsening was observed in the extended follow‐up, although it was only an exploratory end point. 43 , 44 Furthermore, the TRANSITION study provided robust evidence on the safety of sacubitril‐valsartan before and after hospital discharge 45 and further knowledge on the strategy of starting sacubitril‐valsartan in advanced HF syndrome.
The above‐mentioned more recent trial evaluating sacubitril‐valsartan has also confirmed some clinical aspects, which can limit the introduction and up‐titration of the drug in daily clinical practice. In PARADIGM‐HF, hypotension was frequently observed in patients taking sacubitril‐valsartan. With a frequency similar to that of patients taking ACEi, hyperkalaemia and worsening kidney function were the other most frequent adverse effects observed. These adverse effects were even more evident in the trials enrolling patients with ADHF. In EVALUATE‐HF and PROVE‐HF, 82.8% and 65% of the patients, respectively, achieved the target dose, whereas in PIONEER‐HF and TRANSITION‐HF, the percentages were lower, at 55.2% and 45.4%–50.7%, respectively. These data can also partially explain the low dosages of sacubitril‐valsartan observed in actual patients. 46
1.2. Sodium‐glucose cotransporter inhibitors
Recently, a new therapeutic approach, based on the introduction of sodium‐glucose cotransporter inhibitors (SGLTis), has proven to have a positive effect on the natural history of HFrEF. 4 , 5 , 6 , 11 , 12 , 13 , 14 SGLT2is have been investigated at first for their hypoglycaemic effects on patients with T2DM. The new class of drugs unexpectedly demonstrated an extraordinary reduction of HF‐related hospitalisations in the T2DM setting. 11 , 12 , 13 , 14 Recently, as summarised in Table 1, two trials (DAPA‐HF and EMPEROR‐reduced) have demonstrated the beneficial effect of SGLT2i on HFrEF subjects with and without T2DM. 4 , 5 DAPA‐HF demonstrated that dapagliflozin compared with placebo significantly reduced HF hospitalisation, cardiovascular (CV) mortality and all‐cause mortality. 4 Further analyses of DAPA‐HF demonstrated that the beneficial effects of dapagliflozin were independent of the presence of T2DM, 4 , 47 background therapy 48 and LVEF value. 49
TABLE 1.
The design of the study and the main results of the DAPA‐HF, 4 EMPEROR‐reduced 5 and SOLOIST 6 trials are summarised
|
DAPA‐HF 4 (n = 4,744) |
EMPEROR‐reduced 5 (n = 3,730) |
SOLOIST 6 (n = 1,222) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dapagliflozin 10 mg o.d. vs. placebo | Empagliflozin 10 mg o.d. vs. placebo | Sotagliflozin 200 mg o.d. up‐titrated to 400 mg o.d. vs. placebo | ||||||||||
| Clinical setting | ||||||||||||
| Outpatients | Outpatients | Inpatients or outpatients (pre‐ or post‐discharge) | ||||||||||
| Main inclusion criteria | ||||||||||||
| With and without T2DM | With and without T2DM | With T2DM | ||||||||||
| Diagnosis of HFrEF (≥2 months) with NYHA classes II–IV | Diagnosis of HFrEF (≥3 months) with NYHA II–IV | Hospitalisation due to acute decompensated heart failure | ||||||||||
| LVEF ≤40% in the last 12 months | LVEF ≤40% in the last 6 months | No need for oxygen therapy, intravenous inotropic or vasodilator (excluding nitrates) or diuretic therapy. | ||||||||||
|
hHF (last 12 months) Yes No |
If AF |
hHF (last 12 months) Yes No |
||||||||||
| NT‐proBNP | >400 | >600 | >900 |
NT‐proBNP
|
SR AF |
≥600 >1,200 |
≥2,500 ≥5,000 |
BNP |
SR AF |
≥150 >450 |
||
|
SR AF |
≥600 >1,200 |
≥1,000 ≥2,000 |
NT‐proBNP |
SR AF |
≥600 >1,800 |
||||||
|
SR AF |
≥600 >1,200 |
≥600 >1,200 |
|||||||||
|
Optimal treatment, stable in the last ≥4 weeks (ACEI, ARB or ARNI; beta‐bloccanti, MRA) |
Optimal treatment, stable in the last ≥1 weeks (ACEI, ARB or ARNI; beta‐bloccanti, MRA) | |||||||||||
| Main exclusion criteria | ||||||||||||
| History of hypotension or systolic arterial pressure <95 mm Hg | History of hypotension or systolic arterial pressure <100 mm Hg | Systolic arterial pressure <100 mm Hg | ||||||||||
| eGFR <30 mL/min/1.73 m2 | eGFR <20 mL/min/1.73 m2 | eGFR <30 mL/min/1.73 m2 | ||||||||||
| Follow‐up (median) | ||||||||||||
| 18.2 months | 16 months | 9 months | ||||||||||
| Main results | ||||||||||||
|
Primary end point: ‐ CV death and hHF or urgent HF visit: ARR: 4.9 × 100 patients/year HR, 0.74 (95% CI, 0.65‐0.85); P <.001 Main secondary end points: ‐ Total number of hHF: HR, 0.75 (95% CI, 0.65‐0.88); ˆ < 0.001 ‐ CV death: HR, 0.82 (95% CI, 0.69‐0.98); p: NA ‐ All‐cause death: HR, 0.83 (95% CI, 0.71‐0.97); p: NA |
Primary end point: ‐ total number CV death and hHF: ARR, −5.3 × 100 patients/year HR, 0.75 (95% CI, 0.65‐0.86); P <.001 Secondary end points: ‐ Total number hHF: HR, 0.70 (95% CI, 0.58‐0.85); P <.001 ‐ Mean slope of change in eGFR (mL/min/1.73 m2) per year: absolute difference 1.73 (95% CI, 1.10‐2.37); P <.001 Other analysed end points: ‐ CV death: HR, 0.92 (95% CI, 0.75‐1.12); p: NA ‐ All‐cause death: HR, 0.92 (95% CI, 0.77‐1.10); p: NA |
Primary end point: ‐ total number CV death and hHF or urgent HF visit: ARR: −25.3 × 100 patients/year HR, 0.67 (95% CI, 0.52‐0.85); P <.001 Main secondary end points: ‐ Total number HF hospitalisation or urgent visit: HR, 0.64 (95% CI, 0.49‐0.83); HR, P <.001 ‐ CV death: HR 0.84 (95% CI, 0.58‐1.22); P =.36 ‐ All‐cause death: HR, 0.82 (95% CI, 0.59‐1.14); p: NA |
||||||||||
| Non‐HF‐related adverse events | ||||||||||||
|
Dapagliflozin vs. placebo: ‐ symptoms of volume depletion 7.5% vs. 6.8% |
Empagliflozin vs. placebo: ‐ Hypotension: 9.4% vs. 8.7% ‐ Genital infections 1.7% vs. 0.6% |
Sotagliflozin vs. placebo ‐ Hypotension 6.0% vs. 4.6% ‐ Diarrhoea 6.1% vs. 3.4% ‐ Severe hypoglycaemia 1.5% vs. 0.3% |
||||||||||
Abbreviations: ACEi, ACE inhibitor; AF, atrial fibrillation; ARB, angiotensin II receptor blocker; ARNi, angiotensin receptor neprilysin inhibitor; ARR, absolute risk reduction; CI, confidence interval; GFR, estimated glomerular filtration rate; hHF, hospitalisation for heart failure; HR, hazard ratio; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonists; NA, not available; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; SR, sinus rhythm; T2DM, type 2 diabetes mellitus.
More recently, in the EMPEROR‐reduced trial, 5 empagliflozin, when compared with placebo, reduced a composite primary end point based on cardiovascular mortality and first HF hospitalisation. 5 The beneficial effect was mainly driven by the reduction of HF hospitalisations, whereas no significant change was observed in cardiovascular mortality. Empagliflozin was also able to significantly modify the secondary end points represented by the first and recurrent HF hospitalisations, decline in GFR and renal outcomes. 5 In EMPEROR‐reduced trial, the benefits were not related to the presence of diabetes 50 and therapy background. 5 The meta‐analysis of DAPA‐HF and EMPEROR‐reduced trials confirmed the ability of SGLT2is to significantly reduce HF hospitalisation and cardiovascular mortality regardless from background therapy. 51
The SOLOIST trial provided more data on the possible usefulness of sotagliflozin in patients admitted for ADHF. 6 Different from DAPA‐HF and EMPEROR‐reduced, the SOLOIST trial enrolled T2DM patients presenting HFrEF, HFmrEF and HFpEF. In the trial, sotaglifozin was able to significantly reduce the occurrence of primary composite end point, that is, the total number of cardiovascular death and HF hospitalisations and/or urgent visits (first and subsequent events). Moreover, no significant effect was observed in CV reduction and all‐cause mortalities. For the first time, the SOLOIST trial provided data on HFmrEF and HFpEF patients. Although the median LVEF was 35%, with 79.1% of patients having <50% LVEF, a similar reduction of the primary end point was observed in the groups with and without preserved LVEF.
The mechanisms by which the SGLT2i can reduce the risk of HF‐related events have not been fully elucidated, although the effects on prognosis are striking. 4 , 5 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 The administration of SGLT2is can reduce glycosuria threshold and tubular maximal transport of glucose in patients with diabetes, with a consequent reduction of plasma glucose levels, thus improving insulin secretion and sensitivity. 54 Interestingly, the glycosuric effect is also observed in nondiabetic patients, but the SGLT2i benefit goes beyond glycaemic control. 54
One of the possible favourable effects of SGLT2i is its diuretic osmotic effect, 55 , 56 , 57 which could contribute to haemodynamic stability and potentiate the loop diuretics without electrolyte abnormalities and RAAS activation. Metabolic, 58 anti‐inflammatory and antifibrotic effects 59 as well as improvement in myocardial energetics mediated by the increase in haematocrit and oxygen delivery 60 or by the changes in the myocardial energetic substrates 58 are among the other mechanisms hypothesised.
Those at the renal level seem to play a key role in explaining the cardiac and renal protections beyond these effects. 54 , 61 , 62 In particular, SGLT2i leads to the increased delivery of sodium at the level of macula densa by blocking glucose and sodium re‐absorption. Consequently, tubule‐glomerular feedback is inhibited, which induces a re‐balance of the adenosine‐mediated dense macula response and inhibition of the renin‐angiotensin‐aldosterone activation. 54 , 61 This mechanism drives the nephroprotection observed in HF 5 as well as in patients with chronic kidney disease. 62 In both DAPA‐HF 4 and EMPEROR‐reduced 5 trials, an initial small GFR decline was observed, which is the consequence of the reduced glomerular hyperfiltration. However, a slower decline in GFR progression has been observed, following this initial decline, which was significant in the EMPEROR‐reduced trial. Finally, as for the effect on HF hospitalisation, the nephroprotection was independent of the T2DM presence. 5 , 62
DAPA‐HF, EMPEROR‐reduced and SOLOIST trials have also demonstrated high tolerability, indicating the rate of adverse events not related to HF similar to placebo, although a slight increase in genitourinary infection and hypoglycaemic events in the SOLOIST trial has been observed. The safety of SGLT2i as well as its single dosage, not requiring up‐titration, makes this class of drugs extremely easy to be used in clinical practice. However, arterial blood pressure and body‐circulating volume should be monitored after their introduction, particularly in patients with high loop diuretic doses 63 in whom the dosage may be reconsidered.
1.3. New vasodilator and inotropic drugs: Vericiguat and omecamtiv mecarbil
As summarised in Table 2, other recent trials which have tested the effects of other new therapeutic approaches aside from those concerning sacubitril‐valsartan and SGLT2i.
TABLE 2.
The design of the study and main results of the VICTORIA‐HF, 7 GALACTIC‐HF 8 and AFFIRM‐HF 9 trials are summarised
|
VICTORIA‐HF 7 (n = 5,050) |
GALACTIC‐HF 8 (n = 8,256) |
AFFIRM‐HF 9 (n = 1,108) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Vericiguat (2.5‐10 mg once daily) vs. placebo | Omecamtiv mecarbil (25, 37.5 or 50 mg b.i.d.) vs. placebo | Endovenous ferric carboxymaltose vs. placebo | |||||||
| Clinical setting | |||||||||
| Outpatients | Inpatients or outpatients | Inpatients | |||||||
| Main inclusion criteria | |||||||||
| NYHA classes II–IV | NYHA classes II–IV | Hospitalisation due to acute decompensated heart failure | |||||||
| LVEF ≤45% in the last 12 months | LVEF ≤35% |
LVEF <50% Ferritin <100 μg/L, or 100‐299 μg/L with transferrin saturation <20% |
|||||||
| BNP |
SR AF |
≥300 >500 |
BNP |
SR AF |
≥125 >375 |
BNP |
SR AF |
≥400 >600 |
|
|
NT‐proBNP |
SR >1,000 AF >1 600 |
NT‐proBNP |
SR AF |
≥400 >1,200 |
NT‐proBNP |
SR AF |
≥1,200 >2,400 |
||
|
Optimal treatment, ARNI introduction encouraged |
Optimal treatment |
At least 40 mg intravenous furosemide or equivalent |
|||||||
| Main exclusion criteria | |||||||||
|
Arterial pressure <100 mm Hg Use of long‐acting nitrates, soluble guanylate cyclase stimulators or phosphodiesterase type 5 inhibitor |
Mechanical support or intravenous medication for haemodynamic or clinical instability Systolic arterial pressure <85 mm Hg |
Immediate need of transfusion or with Hb <8 g/dL* or with Hb >15 g/dL Renal dialysis |
|||||||
| s | eGFR <20 mL/min/1.73 m2 | ||||||||
| Follow‐up (median) | |||||||||
| 10.8 months | 21.8 months | 12 months | |||||||
| Main results | |||||||||
|
Primary end point: ‐ CV death or first hHF ARR, −3 × 100 patients/year HR, 0.90 (95% CI, 0.82‐0.98); P =.02 Main secondary end points: ‐ Total number HF hospitalisation or urgent visit: HR, 0.91 (95% CI, 0.84‐0.99); P =.02 ‐ hHF: HR, 0.90 (95% CI, 0.81‐1.0); p: NA ‐ CV death: HR, 0.93 (95% CI, 0.81‐1.06); p: NA ‐ All‐cause death: HR, 0.95 (95% CI, 0.84‐1.07); P =.38 |
Primary end point: ‐ CV death and hHF or urgent HF visit: ARR, −2.1 × 100 patients/year HR, 0.92 (95% CI, 0.86‐0.99); P =.03 Main secondary end points: ‐ hHF: HR, 0.95 (95% CI, 0.87‐1.03); p: NA ‐ CV death: HR, 1.02 (95% CI, 0.92‐1.11); P =.86 ‐ All‐cause death: HR, 1.00 (95% CI, 0.92‐1.09); p: NA |
Primary end point: ‐ total number CV death and hHF: ARR, −15.35 × 100 patients/year HR, 0.79 (95% CI, 0.62‐1.01); P =.059 COVID sensitivity analysis ARR, −18.24 × 100 patients/year HR, 0.75 (95% CI, 0.59‐0.96); P =.024 Main secondary end points: ‐ Total number HF hospitalisation: HR, 0.74 (95% CI, 0.58‐0.94); P =.013 COVID sensitivity analysis HR, 0.70 (95% CI, 0.55‐0.90); P =.005 |
|||||||
| Non‐HF‐related adverse events | |||||||||
|
Vericiguat vs. placebo: ‐ Symptomatic hypotension 9.1% vs. 7.9% (P =.12) ‐ Syncope 4.0% vs. 3.5% (P =.30) ‐ Anaemia 7.6% vs. 5.7% |
Omecamtiv vs. placebo: ‐ Similar major cardiac ischaemic events (4.9% vs. 4.6%) and myocardial infarction (3.0% vs. 2.9%) ‐ Similar rate of ventricular arrhythmic events |
Similar rate in ferric carboxymaltose and placebo | |||||||
Abbreviations: ACEi, ACE inhibitor; AF, atrial fibrillation; ARB, angiotensin II receptor blocker; ARNi, angiotensin receptor neprilysin inhibitor; ARR, absolute risk reduction; CI, confidence interval; eGFR, estimated glomerular filtration rate; hHF, hospitalisation for heart failure; HR, hazard ratio; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonists; NA, not available; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; SR sinus rhythm.
VICTORIA‐HF compared vericiguat with placebo in 5,050 patients with CHF. 7 The reduced levels of cyclic guanosine monophosphate can contribute to the progression of HF. Vericiguat is a novel oral soluble drug modulating the nitric oxide (NO)‐guanylyl monophosphate‐phosphodiesterase pathway by stimulating guanylate cyclase or enhancing its sensitivity to endogenous NO 64 . In VICTORIA‐HF, vericiguat was up‐titrated from 2.5 to 10 mg once daily in HF patients classified as NYHA classes II–IV and with <45% LVEF. Vericiguat significantly reduced the composite primary end point (first hospitalisation for HF or cardiovascular mortality) during a median follow‐up of 10.8 months. An insignificant increase in the incidence of hypotension and syncope was observed in the vericiguat group. 7
GALACTIC‐HF 8 tested the effects of a new class of drug with inotropic effect which differs from previous ones, the omecamtiv mecarbil (OM). OM is a myotrope, that is, a drug that is capable of directly activating cardiac myosin in a calcium‐independent manner by increasing the number of myosin heads able to pull on actin filaments during depolarisation. 65 , 66 Consequently, OM increases the duration of ventricular systole, systolic ejection time and, thus, aortic blood flow for each contraction without high oxygen consumption. 66
COSMIC‐HF 67 first evaluated the effect of OM in stable HFrEF patients randomised to receive OM 25 mg b.i.d. (fixed‐dose group), 25 mg twice daily titrated to 50 mg twice daily guided by pharmacokinetics (pharmacokinetic‐titration group) or placebo for 20 weeks. A significant increase in systolic ejection time and stroke volume as well as a reduction of left ventricular end‐systolic and end‐diastolic diameter and NT‐proBNP serum levels was observed in the pharmacokinetic‐titration group versus the placebo group.
OM was added to the standard HFrEF therapy in GALACTIC‐HF 8 , 68 at different daily doses (25, 37.5 or 50 mg twice daily). The OM dose was modified according to its serum plasma levels (Figure S1), on the basis of a scheme that could be not easily adopted in the current clinical practice. During a median follow‐up of 21.8 months, OM significantly reduced the composite primary outcome (hospitalisation or urgent visit for HF or mortality from cardiovascular causes). The significant effect on OM outcome was mainly driven by the reduction of HF hospitalisations. No significant difference was observed between OM and placebo when the Kansas City Cardiomyopathy Questionnaire total symptom score was analysed. In addition, no significant increase in the frequency of cardiac ischaemic and ventricular arrhythmia events was observed in the OM group.
The results of VICTORIA‐HF 7 and GALACTIC‐HF 8 indicate that vericiguat and OM are drugs that are not capable of modifying patients’ survival but could be useful for patients with recent ADHF to reduce admission recurrence. In particular, on the basis of sub‐group analysis, the patients with lower LVEF, worse NYHA class and higher NT‐proBNP serum levels are those who could gain a greater benefit from OM therapy.
1.4. Iron deficiency correction: Ferric carboxymaltose
Reduction of the incidence of ADHF readmission could also be achieved by correcting ID administering FCM 9 (Table 2). ID is a very common comorbidity in CHF with a prevalence that is even greater in ADHF. 69 It is caused by different aetiologic conditions, such as reduced iron absorption, haematic loss, inflammatory status and other abnormalities of the mechanisms regulating iron serum levels and storage. 70 Moreover, it is associated with a worse prognosis independent of anaemia. 71 The pathophysiological background underlying the prognostic impact is complex. ID is not only related to a possible haemopoiesis impairment but is also a key component of mitochondrial respiratory chain proteins involved in ATP production. 72 Consequently, an absolute or functional ID could negatively influence the myocardial 73 as well as skeletal muscle functions, thus worsening functional capacity. 74 Previous studies have demonstrated that the correction of ID by FCM administration, 75 , 76 but not iron sucrose oral supplementation, 77 can improve the patients’ quality of life and 6‐min walking test distance. Moreover, a meta‐analysis of all available studies on intravenous FCM administration revealed the reduction of hospitalisation due to ADHF. 78 Based on this evidence, the correction of ID by FCM administration has been already recommended in the 2016 ESC guidelines. 1 The AFFIRM‐HF trial has confirmed the possible relevance of FCM by evaluating its efficacy in a number of patients admitted due to ADHF with ID (defined as ferritin <100 or 100‐299 μg/L with transferrin saturation <20%) and <50% LVEF. The patients were randomised to placebo or treatment with intravenous FCM. The first dose was administered before discharge and the second dose at week 6. Moreover, 500 or 1,000 mg was administered based on the screening haemoglobin and bodyweight values. During 1‐year follow‐up, FCM administration was not able to significantly reduce the composite primary end point (CV mortality and HF hospitalisation) as well as the secondary end point of CV. However, a significantly lower rate of HF hospitalisation was observed. The study data are relevant by considering the effectiveness of the therapy in reducing the use of hospital resources for HF exacerbations and the chance to adopt FCM administration in HF patients prior to hospital discharge to prevent recurrence.
2. NEW ALGORITHM AND STRATEGY IN HFREF TREATMENT
The availability of new disease‐modifying drugs has led to rethinking of the scheme for therapy optimisation of HFrEF patients. The latest ESC guidelines 1 recommended a step‐by‐step therapeutic approach: first is to introduce beta blockers followed by ACEis in the case of persistence of <35% LVEF before the MRA; then, sacubitril‐valsartan and ivabradine are administered next; finally, ventricular resynchronisation therapy is given. The use of diuretics should be aimed at congestion relief, and the prevention of sudden death with an implantable cardioverter‐defibrillator should be considered. However, this stepwise approach does not consider the possibility that the different drug classes have a synergistic action and that the most effective therapeutic approach should be adopted as soon as possible. 39 Thus, new therapeutic schemes instead of stepwise approach have been proposed over the last months, which focuses on the major four classes of disease‐modifying drugs. 79 , 80 Furthermore, the American College of Cardiology/American Heart Association has already recommended a therapeutic approach based on beta blockers and sacubitril‐valsartan, which are preferable for starting to ACEi or ARB association. Moreover, depending on the specific conditions, the combination of other classes of drugs is recommended. 81 Incoming ESC guidelines will further present a new recommended scheme.
However, some points should be considered about any future therapeutic scheme. First, any scheme entails that it is not generalisable for all patients. The HF physician generally chooses the sequence of the different disease‐modifying drugs and their up‐titration according to the therapeutic target that seems most relevant for every single patient. The second aspect concerns the patient's background therapy. Each scheme assumes that patients are not taking any of the recommended drug classes at the time of HFrEF diagnosis. However, this is unlikely. Due to the prevalence of HFrEF comorbidities (eg essential hypertension), the patient is unlikely to be therapy‐naïve with ACEi, ARBs or beta blockers as all classes of drugs are commonly administered to hypertensive, diabetic or ischaemic patients. Moreover, SGLT2i should be prescribed to diabetic patients as a primary prevention before the onset of HF, as already recommended by the guidelines. 82 Finally, it should be noted that the majority of CHF patients, who could benefit from the new emerging classes of drugs (eg SGLT2i), are already taking previously recommended therapy. In this case, the greatest risk is represented by the therapeutic inertia more frequently involving clinically stable patient management. Probably, in the routine clinical practise, what should mainly guide the optimization of medical therapy in HFrEF patients is the awarness about the terapeutic targets we can intervene and the results we can obtain in terms of reduction of mortality and/or heart failure hospitalization risks with the different available therapeutic strategies (Figure 3).
FIGURE 3.
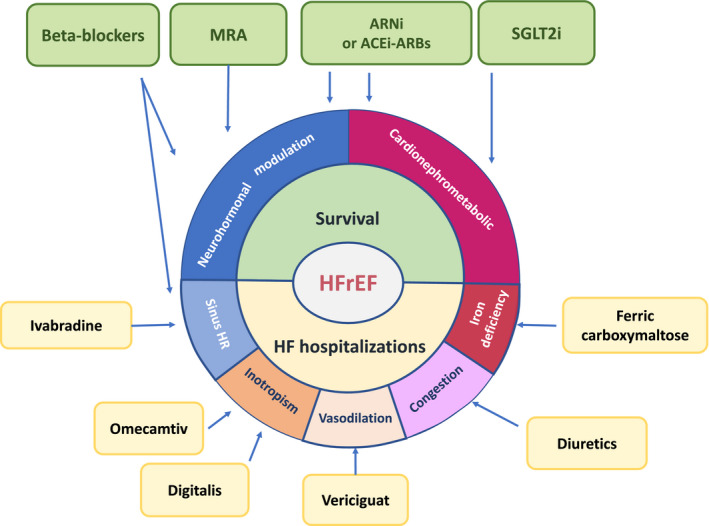
Therapeutic target of pharmacological treatment in patients with heart failure with reduced ejection fraction. ACEi and ARBs should be prescribed when ARNi are not tolerated. ACEi, ACE inhibitor; ARBs, Angiotensin II receptor blockers; ARNi, angiotensin receptor neprilysin inhibitor; HFrEF, heart failure with reduced ejection fraction; HR, heart rate; MRA, mineralocorticoid receptor antagonists; SGLT2i, sodium‐glucose cotransporter‐2 inhibitor
3. HFMREF AND HFPEF: A CHALLENGE STILL TO BE WON
Over the last years, the growing evidence of new classes of drugs for HFrEF has been useful for improving patients’ prognosis. However, the available trials have not generally demonstrated these effects in patients with HFmrEF and HFpEF. 83 , 84 , 85 This is the consequence of a heterogeneous and still not fully understood disease pathophysiology. 86
Many components (eg chronotropic incompetence, volume overload, systolic dysfunction, high body mass index, renal dysfunction and obstructive sleep apnoea) could differently characterise HFpEF phenotypes, thus rendering the efficacy of the therapies successfully adopted in HFrEF uncertain. 86
This pathophysiological background could explain the results of PARAGON‐HF, 87 which was aimed at comparing sacubitril‐valsartan with valsartan alone in 4,822 patients who are ≥55 years, have ≥45% LVEF, have evidence of structural heart disease (left atrial enlargement or left ventricular hypertrophy), classified as NYHA classes II–IV and have elevated NPs. The results of PARAGON‐HF demonstrated that sacubitril‐valsartan was able to reduce the primary end point (total HF hospitalisations and CV mortality) without reaching statistical significance (rate ratio, 0.87; 95% confidence interval [CI], 0.75‐1.01; P =.06). The CV mortality rate (8.5% and 8.9% in the sacubitril‐valsartan and valsartan groups (HR 0.95; 95% CI, 0.79‐1.16), respectively) and the total number of HF hospitalisation (rate ratio, 0.85; 95% CI, 0.72‐1.00) were not statistically different. Among the pre‐specified sub‐groups, evidence of heterogeneity with the possible benefit that can be obtained from sacubitril‐valsartan was noted in the sub‐groups of female patients with lower ejection fraction. In the adjusted rate ratio for the primary end point, patients with LVEF below the median value (57%) demonstrated a significant reduction of relative risk (HR, 0.78; 95% CI, 0.64‐0.95) with a benefit consistent with what was obtained from sacubitril‐valsartan by HFrEF subjects enrolled in the PARADIGM‐HF trial. 87 The possible beneficial effect of sacubitril‐valsartan in patients with mild LVEF reduction and in those with moderate‐to‐severe LVEF reduction has also been suggested by an analysis conducted by combining data from PARADIGM‐HF and PARAGON‐HF. 88
These results are similar to those of the post hoc analyses of TOPCAT 89 and CHARM 90 studies. In TOPCAT, 89 LVEF influenced the effect of spironolactone treatment, particularly on the primary outcome (first of either cardiovascular death, HF hospitalisation or resuscitated sudden death; P =.046) and on HF hospitalisation (P =.039), with higher estimated benefits at the lower end of the LVEF spectrum to the primary end point. The benefit in the relatively impaired LVEF was even superior to the expected benefit in the overall study population.
The positive spironolactone effect detected in the HF sub‐population was observed before than definition of that population and HF middle range EF (HFmrEF) in 2016 ESC guidelines. 1 Following these guidelines, a new post hoc analysis across the LVEF spectrum was conducted on patients enrolled in the CHARM programme. 90 Analogous to PARAGON and TOPCAT, the analysis evaluated the characteristics, treatment effect and outcomes of candesartan according to LVEF as a continuous spline variable. The incidence rates for the primary outcome of candesartan vs. placebo were 7.4 vs. 9.7 and 8.6 vs. 9.1 per 100 patient‐years in HFmrEF (LVEF 40%–49% (HR, 0.76; 95% CI, 0.61‐0.96; P =.02) and HFpEF (HR, 0.95; 95% CI, 0.79‐1.14; P =.57), respectively. The incidence rate ratios for HF hospitalisation were 0.48 and 0.78 in HFmrEF (95% CI, 0.33‐0.70; P <.001) and HFpEF (95% CI, 0.59‐1.03; P =.08), respectively.
Based on this evidence, patients with HFmrEF could benefit from the therapeutic approach based on the neurohormonal modulation analogous to HFrEF. Moreover, HFpEF treatment remains a challenge. The results of the SOLOIST 6 trial suggest the possible usefulness of SGLT2i in this group of patients. However, the ongoing trials designed to test this hypothesis should be awaited. 91 , 92
These trials will test the treatment efficacy in a population similar to that of the PARAGON study. However, it is worth noting that the efficacy of therapy aimed at treating patients with HFpEF due to the same aetiologies, such as transthyretin amyloidosis (ATTR) and hypertrophic cardiomyopathy (HCM), became evident over the last years. The Transthyretin Amyloidosis Cardiomyopathy Clinical Trial 93 recently demonstrated the ability of tafamidis to significantly reduce all‐cause mortality and CV‐related hospitalisations hierarchically analysed in patients with transthyretin. EXPLORER‐HCM demonstrated the possible usefulness of a novel therapeutic approach in the obstructive HCM based on a cardiac myosin ATPase inhibitor, the mavacamten, 94 , 95 to improve functional capacity in patients with an intraventricular gradient ≥50 mm Hg, classified as NYHA classes II–III and who have ≥55% LVEF. The evidence of these two trials proves the efficacy of the HFpEF therapeutic approach personalised according to aetiology and pathophysiological background. 96
4. CONCLUSION
Recent evidence confirmed the relevance of sacubitril‐valsartan and also demonstrated the usefulness of SGLT2i for HFrEF treatment in order to further ameliorate HF outcome. The combination of sacubitril and valsartan or ACEi/ARB, beta blockers, MRA and SGLT2i have synergistic action against HFrEF progression. Aside from the four classes of disease‐modifying drugs (ie ARNi/ACEi/ARBs, beta blockers, MRA and SGLT2i), other therapeutic approaches could act on different pathophysiologic mechanisms, providing further prognosis improvement in HF patients. Despite the impressive progress in understanding the pathophysiology of HFpEF, disease management is still challenging and remains an interesting issue in the cardiovascular arena. New therapies targeting the different aetiologies (eg amyloidosis and HCM) open a new opportunity for future therapeutic progress.
CONFLICT OF INTEREST
M. Iacoviello received honoraria as a consultant in advisory boards from Astra Zeneca, Boehringer Ingelheim, Lilly, Merk Serono, Novartis, Vifor Pharma. A. Palazzuoli received honoraria as a consultant from Menarini, Novartis. E. Gronda has no conflict of interest to declare for the contents of this paper.
Supporting information
Figure S1
ACKNOWLEDGEMENTS
The authors would like to thank Enago (www.enago.com) for the English language review.
Iacoviello M, Palazzuoli A, Gronda E. Recent advances in pharmacological treatment of heart failure. Eur J Clin Invest. 2021;51:e13624. 10.1111/eci.13624
REFERENCES
- 1. Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129‐2200. [DOI] [PubMed] [Google Scholar]
- 2. Desai AS, Solomon SD, Shah AM, et al. Effect of sacubitril‐valsartan vs enalapril on aortic stiffness in patients with heart failure and reduced ejection fraction: a randomized clinical trial. JAMA. 2019;322:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Januzzi JL Jr, Prescott MF, Butler J, et al. Association of change in N‐terminal pro‐B‐type natriuretic peptide following initiation of sacubitril‐valsartan treatment with cardiac structure and function in patients with heart failure with reduced ejection fraction. JAMA. 2019;322:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995‐2008. [DOI] [PubMed] [Google Scholar]
- 5. Packer M, Anker SD, Butler J, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413‐1424. [DOI] [PubMed] [Google Scholar]
- 6. Bhatt DL, Szarek M, Steg PG, et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med. 2020;384:117–128. [DOI] [PubMed] [Google Scholar]
- 7. Armstrong PW, Pieske B, Anstrom KJ, et al. Vericiguat in patients with heart failure and reduced ejection fraction. N Engl J Med. 2020;382:1883‐1893. [DOI] [PubMed] [Google Scholar]
- 8. Teerlink JR, Diaz R, Felker GM, et al. Cardiac myosin activation with omecamtiv mecarbil in systolic heart failure. N Engl J Med. 2021;384:105‐116. [DOI] [PubMed] [Google Scholar]
- 9. Ponikowski P, Kirwan BA, Anker SD, et al. Ferric carboxymaltose for iron deficiency at discharge after acute heart failure: a multicentre, double‐blind, randomised, controlled trial. Lancet. 2020;396:1895‐1904. [DOI] [PubMed] [Google Scholar]
- 10. McMurray JJ, Packer M, Desai AS, et al. Angiotensin‐neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993‐1004. [DOI] [PubMed] [Google Scholar]
- 11. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117‐2128. [DOI] [PubMed] [Google Scholar]
- 12. Perkovic V, de Zeeuw D, Mahaffey KW, et al. Canagliflozin and renal outcomes in type 2 diabetes: results from the CANVAS Program randomised clinical trials. Lancet Diabetes Endocrinol. 2018;6:691‐704. [DOI] [PubMed] [Google Scholar]
- 13. Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295‐2306. [DOI] [PubMed] [Google Scholar]
- 14. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347‐357. [DOI] [PubMed] [Google Scholar]
- 15. Simera I, Moher D, Hoey J, Schulz KF, Altman DG. A catalogue of reporting guidelines for health research. Eur J Clin Invest. 2010;40:35‐53. [DOI] [PubMed] [Google Scholar]
- 16. Mann DL. Mechanisms and models in heart failure: A combinatorial approach. Circulation. 1999;100:999‐1008. [DOI] [PubMed] [Google Scholar]
- 17. Braunwald E. Heart failure. JACC: Heart Fail. 2013;1:1‐20. [DOI] [PubMed] [Google Scholar]
- 18. CONSENSUS Trial Study Group . Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med. 1987;316:1429‐1435. [DOI] [PubMed] [Google Scholar]
- 19. Investigators SOLVD, Yusuf S, Pitt B, Davis CE, Hood WB Jr, Cohn JN. Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. N Engl J Med. 1992;327:685‐691. [DOI] [PubMed] [Google Scholar]
- 20. Investigators SOLVD, Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325:293‐302. [DOI] [PubMed] [Google Scholar]
- 21. Packer M, Coats AJ, Fowler MB, et al. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med. 2001;344:1651‐1658. [DOI] [PubMed] [Google Scholar]
- 22. Packer M, Bristow MR, Cohn JN, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N Engl J Med. 1996;334:1349‐1355. [DOI] [PubMed] [Google Scholar]
- 23. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT‐HF). Lancet. 1999;353:2001‐2007. [PubMed] [Google Scholar]
- 24. Packer M, Fowler MB, Roecker EB, et al. Effect of carvedilol on the morbidity of patients with severe chronic heart failure: results of the carvedilol prospective randomized cumulative survival (COPERNICUS) study. Circulation. 2002;106:2194‐2199. [DOI] [PubMed] [Google Scholar]
- 25. CIBIS‐II Investigators and Committees . The Cardiac Insufficiency Bisoprolol Study II (CIBIS‐II): a randomised trial. Lancet. 1999;353:9‐13. [PubMed] [Google Scholar]
- 26. Flather MD, Shibata MC, Coats AJS, et al. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). Eur Heart J. 2005;26:215‐225. [DOI] [PubMed] [Google Scholar]
- 27. Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709‐717. [DOI] [PubMed] [Google Scholar]
- 28. Zannad F, McMurray JJ, Krum H, et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364:11‐21. [DOI] [PubMed] [Google Scholar]
- 29. Granger CB, McMurray JJ, Yusuf S, et al. Effects of candesartan in patients with chronic heart failure and reduced left‐ventricular systolic function intolerant to angiotensin‐converting‐enzyme inhibitors: the CHARM‐Alternative trial. Lancet. 2003;362:772‐776. [DOI] [PubMed] [Google Scholar]
- 30. Buggey J, Mentz RJ, DeVore AD, Velazquez EJ. Angiotensin receptor neprilysin inhibition in heart failure: mechanistic action and clinical impact. J Card Fail. 2015;21:741‐750. [DOI] [PubMed] [Google Scholar]
- 31. Díez J. Chronic heart failure as a state of reduced effectiveness of the natriuretic peptide system: implications for therapy. Eur J Heart Fail. 2017;19:167‐176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Miller WL, Phelps MA, Wood CM, et al. Comparison of mass spectrometry and clinical assay measurements of circulating fragments of B‐type natriuretic peptide in patients with chronic heart failure. Circ Heart Fail. 2011;4:355‐360. [DOI] [PubMed] [Google Scholar]
- 33. Bayés‐Genís A, Barallat J, Galán A, et al. Soluble neprilysin is predictive of cardiovascular death and heart failure hospitalization in heart failure patients. J Am Coll Cardiol. 2015;65(7):657‐665. [DOI] [PubMed] [Google Scholar]
- 34. Nougué H, Pezel T, Picard F, et al. Effects of sacubitril/valsartan on neprilysin targets and the metabolism of natriuretic peptides in chronic heart failure: a mechanistic clinical study. Eur J Heart Fail. 2019;21:598‐605. [DOI] [PubMed] [Google Scholar]
- 35. Packer M, McMurray JJ, Desa AS, et al. Angiotensin receptor neprilysin inhibition compared with enalapril on the risk of clinical progression in surviving patients with heart failure.. Circulation. 2015;131:54‐61. [DOI] [PubMed] [Google Scholar]
- 36. Desai AS, Claggett BL, Packer M, et al. Influence of Sacubitril/Valsartan (LCZ696) on 30‐day readmission after heart failure hospitalization. J Am Coll Cardiol. 2016;68:241‐248. [DOI] [PubMed] [Google Scholar]
- 37. Packer M, McMurray JJ, Desai AS, et al. Angiotensin receptor neprilysin inhibition compared with enalapril on the risk of clinical progression in surviving patients with heart failure. Circulation. 2015;131:54‐61. [DOI] [PubMed] [Google Scholar]
- 38. Remme WJ; CARMEN Steering Committee and Investigators . The Carvedilol and ACE‐Inhibitor Remodelling Mild Heart Failure EvaluatioN trial (CARMEN)–rationale and design. Cardiovasc Drugs Ther. 2001;15:69‐77. [DOI] [PubMed] [Google Scholar]
- 39. Jong P, Yusuf S, Rousseau MF, et al. Effect of enalapril on 12‐year survival and life expectancy in patients with left ventricular systolic dysfunction: a follow‐up study. Lancet. 2003;31(361):1843‐1848. [DOI] [PubMed] [Google Scholar]
- 40. Aimo A, Gaggin HK, Barison A, et al. Imaging, biomarker, and clinical predictors of cardiac remodeling in heart failure with reduced ejection fraction. JACC Heart Fail. 2019;7:782‐794. [DOI] [PubMed] [Google Scholar]
- 41. Lupón J, Gavidia‐Bovadilla G, Ferrer E, et al. Dynamic trajectories of left ventricular ejection fraction in heart failure. J Am Coll Cardiol. 2018;7(72):591‐601. [DOI] [PubMed] [Google Scholar]
- 42. Tavazzi L, Senni M, Metra M, et al. Multicenter prospective observational study on acute and chronic heart failure: one‐year follow‐up results of IN‐HF (Italian Network on Heart Failure) outcome registry. Circ Heart Fail. 2013;6:473‐481. [DOI] [PubMed] [Google Scholar]
- 43. Velazquez EJ, Morrow DA, DeVore AD, et al. Angiotensin‐neprilysin inhibition in acute decompensated heart failure. N Engl J Med. 2019;380:539‐548. [DOI] [PubMed] [Google Scholar]
- 44. DeVore AD, Braunwald E, Morrow DA, et al. Initiation of angiotensin‐neprilysin inhibition after acute decompensated heart failure: secondary analysis of the open‐label extension of the PIONEER‐HF trial. JAMA Cardiol. 2020;5:202‐207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wachter R, Senni M, Belohlavek J, et al. Initiation of sacubitril/valsartan in haemodynamically stabilised heart failure patients in hospital or early after discharge: primary results of the randomised TRANSITION study. Eur J Heart Fail. 2019;21:998‐1007. [DOI] [PubMed] [Google Scholar]
- 46. Wachter R, Fonseca AF, Balas BK, et al. Real‐world treatment patterns of sacubitril/valsartan: a longitudinal cohort study in Germany. Eur J Heart Fail. 2019;21:588‐597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Petrie MC, Verma S, Docherty KF, et al. Effect of dapagliflozin on worsening heart failure and cardiovascular death in patients with heart failure with and without diabetes. JAMA. 2020;323:1353‐1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Docherty KF, Jhund PS, Inzucchi SE, et al. Effects of dapagliflozin in DAPA‐HF according to background heart failure therapy. Eur Heart J. 2020;41:2379‐2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dewan P, Solomon SD, Jhund PS, et al. Efficacy and safety of sodium‐glucose co‐transporter 2 inhibition according to left ventricular ejection fraction in DAPA‐HF. Eur J Heart Fail. 2020;22:1247‐1258. [DOI] [PubMed] [Google Scholar]
- 50. Anker SD, Butler J, Filippatos G, et al. Effect of empagliflozin on cardiovascular and renal outcomes in patients with heart failure by baseline diabetes status: results from the EMPEROR‐reduced trial. Circulation. 2021;143:337‐349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zannad F, Ferreira JP, Pocock SJ, et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta‐analysis of the EMPEROR‐Reduced and DAPA‐HF trials. Lancet. 2020;396:819‐829. [DOI] [PubMed] [Google Scholar]
- 52. Verma S, McMurray JJV. SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state‐of‐the‐art review. Diabetologia. 2018;61:2108‐2117. [DOI] [PubMed] [Google Scholar]
- 53. Seferović PM, Fragasso G, Petrie M, et al. Sodium‐glucose co‐transporter 2 inhibitors in heart failure: beyond glycaemic control. A position paper of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2020;22:1495‐1503. [DOI] [PubMed] [Google Scholar]
- 54. Gronda E, Jessup M, Iacoviello M, Palazzuoli A, Napoli C. Glucose Metabolism in the Kidney: Neurohormonal Activation and Heart Failure Development. J Am Heart Assoc. 2020;9:e018889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hallow KM, Helmlinger G, Greasley PJ, et al. Why do SGLT2 inhibitors reduce heart failure hospitalization? A differential volume regulation hypothesis. Diabetes Obes Metab. 2018;20(3):479‐487. [DOI] [PubMed] [Google Scholar]
- 56. Griffin M, Rao VS, Ivey‐Miranda J, et al. Empagliflozin in heart failure: diuretic and cardiorenal effects. Circulation. 2020;142:1028‐1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mordi NA, Mordi IR, Singh JS, et al. Renal and cardiovascular effects of SGLT2 inhibition in combination with loop diuretics in patients with type 2 diabetes and chronic heart failure: The RECEDE‐CHF Trial. Circulation. 2020;142:1713‐1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ferrannini E, Baldi S, Frascerra S, et al. Shift to fatty substrate utilization in response to sodium‐glucose cotransporter 2 inhibition in subjects without diabetes and patients with type 2 diabetes. Diabetes. 2016;65:1190‐1195. [DOI] [PubMed] [Google Scholar]
- 59. Heerspink HJL, Perco P, Mulder S, et al. Canagliflozin reduces inflammation and fibrosis biomarkers: a potential mechanism of action for beneficial effects of SGLT2 inhibitors in diabetic kidney disease. Diabetologia. 2019;62:1154‐1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Inzucchi SE, Zinman B, Fitchett D, et al. How does empagliflozin reduce cardiovascular mortality? insights from a mediation analysis of the EMPA‐REG OUTCOME trial. Diabetes Care. 2018;41:356‐363. [DOI] [PubMed] [Google Scholar]
- 61. DeFronzo RA, Norton L, Abdul‐Ghani M. Renal, metabolic and cardiovascular considerations of SGLT2 inhibition. Nat Rev Nephrol. 2017;13:11‐26. [DOI] [PubMed] [Google Scholar]
- 62. Heerspink HJL, Stefánsson BV, Correa‐Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383:1436‐1446. [DOI] [PubMed] [Google Scholar]
- 63. Jackson AM, Dewan P, Anand IS, et al. Dapagliflozin and diuretic use in patients with heart failure and reduced ejection fraction in DAPA‐HF. Circulation. 2020;142:1040‐1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Emdin M, Aimo A, Castiglione V, et al. Targeting cyclic guanosine monophosphate to treat heart failure: JACC Review Topic Of The Week. J Am Coll Cardiol. 2020;76:1795‐1807. [DOI] [PubMed] [Google Scholar]
- 65. Psotka MA, Gottlieb SS, Francis GS, et al. Cardiac calcitropes, myotropes, and mitotropes: JACC review topic of the week. J Am Coll Cardiol. 2019;73:2345‐2353. [DOI] [PubMed] [Google Scholar]
- 66. Malik FI, Hartman JJ, Elias KA, et al. Cardiac myosin activation: a potential therapeutic approach for systolic heart failure. Science. 2011;331:1439‐1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Teerlink JR, Felker GM, McMurray JJ, et al. Chronic Oral Study of Myosin Activation to Increase Contractility in Heart Failure (COSMIC‐HF): a phase 2, pharmacokinetic, randomised, placebo‐controlled trial. Lancet. 2016;388:2895‐2903. [DOI] [PubMed] [Google Scholar]
- 68. Teerlink JR, Diaz R, Felker GM, et al. omecamtiv mecarbil in chronic heart failure with reduced ejection fraction: rationale and design of GALACTIC‐HF. JACC Heart Fail. 2020;8:329‐340. [DOI] [PubMed] [Google Scholar]
- 69. Rocha BML, Cunha GJL, Menezes Falcão LF. The burden of iron deficiency in heart failure: therapeutic approach. J Am Coll Cardiol. 2018;71:782‐793. [DOI] [PubMed] [Google Scholar]
- 70. Ghafourian K, Shapiro JS, Goodman L, Ardehali H. Iron and heart failure: diagnosis, therapies, and future directions. JACC Basic Transl Sci. 2020;5:300‐313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Jankowska EA, Rozentryt P, Witkowska A, et al. Iron deficiency: an ominous sign in patients with systolic chronic heart failure. Eur Heart J. 2010;31:1872‐1880. [DOI] [PubMed] [Google Scholar]
- 72. Melenovsky V, Petrak J, Mracek T, et al. Myocardial iron content and mitochondrial function in human heart failure: a direct tissue analysis. Eur J Heart Fail. 2017;19:522‐530. [DOI] [PubMed] [Google Scholar]
- 73. Hoes MF, Grote Beverborg N, Kijlstra JD, et al. Iron deficiency impairs contractility of human cardiomyocytes through decreased mitochondrial function. Eur J Heart Fail. 2018;20:910‐919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Dziegala M, Josiak K, Kasztura M, et al. Iron deficiency as energetic insult to skeletal muscle in chronic diseases. J Cachexia Sarcopenia Muscle. 2018;9:802‐815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Anker SD, Comin Colet J, Filippatos G, et al. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med. 2009;361:2436‐2448. [DOI] [PubMed] [Google Scholar]
- 76. Ponikowski P, van Veldhuisen DJ, Comin‐Colet J, et al. Beneficial effects of long‐term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency†. Eur Heart J. 2015;36:657‐668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lewis GD, Malhotra R, Hernandez AF, et al. Effect of oral iron repletion on exercise capacity in patients with heart failure with reduced ejection fraction and iron deficiency: The IRONOUT HF randomized clinical trial. JAMA. 2017;317:1958‐1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Anker SD, Kirwan BA, van Veldhuisen DJ, et al. Effects of ferric carboxymaltose on hospitalisations and mortality rates in iron‐deficient heart failure patients: an individual patient data meta‐analysis. Eur J Heart Fail. 2018;20:125‐133. [DOI] [PubMed] [Google Scholar]
- 79. Bauersachs J. Heart failure drug treatment: the fantastic four. Eur Heart J. 2021;11(42):681‐683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. McMurray JJV, Packer M. How should we sequence the treatments for heart failure and a reduced ejection fraction?: a redefinition of evidence‐based medicine. Circulation. 2021;143:875‐877. [DOI] [PubMed] [Google Scholar]
- 81. Writing Committee , Maddox TM, Januzzi JL, Allen LA Jr, et al. Update to the 2017 ACC expert consensus decision pathway for optimization of heart failure treatment: answers to 10 pivotal issues about heart failure with reduced ejection fraction: a report of the american college of cardiology solution set oversight committee. J Am Coll Cardiol. 2021;2021(77):772‐810. [DOI] [PubMed] [Google Scholar]
- 82.Cosentino F, Grant PJ, Aboyans V et al. 2019 ESC Guidelines on diabetes, pre‐diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41 :255‐323. [DOI] [PubMed]
- 83. Yusuf S, Pfeffer MA, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and preserved left‐ventricular ejection fraction: the CHARM Preserved Trial. Lancet. 2003;362:777‐781. [DOI] [PubMed] [Google Scholar]
- 84. Massie BM, Carson PE, McMurray JJ, et al. I‐PRESERVE Investigators. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359:2456‐2467. [DOI] [PubMed] [Google Scholar]
- 85. Pitt B, Pfeffer MA, Assmann SF, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370:1383‐1392. [DOI] [PubMed] [Google Scholar]
- 86. Gronda E, Vanoli E, Iacoviello M. The PARAGON‐HF trial: the sacubitril/valsartan in heart failure with preserved ejection fraction. Eur Heart J Suppl. 2020;22(Suppl L):L77‐L81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Solomon SD, McMurray JJV, Anand IS, et al. Angiotensin‐neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. 2019;381:1609‐1620. [DOI] [PubMed] [Google Scholar]
- 88. Solomon SD, Vaduganathan M, Claggett L, et al. Sacubitril/Valsartan across the spectrum of ejection fraction in heart failure. Circulation. 2020;141:352‐361. [DOI] [PubMed] [Google Scholar]
- 89. Solomon SD, Claggett B, Lewis EF, et al. Influence of ejection fraction on outcomes and efficacy of spironolactone in patients with heart failure with preserved ejection fraction. Eur Heart J. 2016;37:455‐462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Lund LH, Claggett B, Liu J, et al. Heart failure with mid‐range ejection fraction in CHARM: characteristics, outcomes and effect of candesartan across the entire ejection fraction spectrum. Eur J Heart Fail. 2018;20:1230‐1239. [DOI] [PubMed] [Google Scholar]
- 91. Packer M, Butler J, Filippatos G, et al. EMPEROR Trial Committees and Investigators. Design of a prospective patient‐level pooled analysis of two parallel trials of empagliflozin in patients with established heart failure. Eur J Heart Fail. 2020;22:2393‐2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. ClinicalTrials.gov . Dapagliflozin Evaluation to Improve the LIVEs of Patients With PReserved Ejection Fraction Heart Failure. (DELIVER). 2020. https://clinicaltrials.gov/ct2/show/NCT03619213 [Google Scholar]
- 93. Maurer MS, Schwartz JH, Gundapaneni B, et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018;379:1007‐1016. [DOI] [PubMed] [Google Scholar]
- 94. Olivotto I, Oreziak A, Barriales‐Villa R, et al. Mavacamten for treatment of symptomatic obstructive hypertrophic cardiomyopathy (EXPLORER‐HCM): a randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet. 2020;396:759‐769. [DOI] [PubMed] [Google Scholar]
- 95. Argirò A, Zampieri M, Berteotti M, et al. Emerging medical treatment for hypertrophic cardiomyopathy. J Clin Med. 2021;10:951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Senni M, Paulus WJ, Gavazzi A, et al. New strategies for heart failure with preserved ejection fraction: the importance of targeted therapies for heart failure phenotypes. Eur Heart J. 2014;35:2797‐2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1


