Abstract
A series of cobalt complexes, stabilized by a monoanionic tridentate NCN pincer ligand, was synthetized and characterized. Preparation of the paramagnetic 15 VE complex [Co(NCNCH2−Et)Br] (1) was accomplished by transmetalation of Li[2,6‐(Et2NCH2)2C6H3] with CoBr2 in THF. Treatment of this air‐sensitive compound with NO gas resulted in the formation of the diamagnetic Co(III) species [Co(NCNCH2−Et)(NO)Br] (2) as confirmed by X‐ray diffraction. This complex features a strongly bent NO ligand (Co−N−O∠135.0°). The νNO is observed at 1609 cm−1 which is typical for a bent metal‐N−O arrangement. Coordinatively unsaturated 1 could further be treated with pyridine, isocyanides, phosphines and CO to form five‐coordinate 17 VE complexes. Oxidation of 1 with CuBr2 led to the formation of the Co(III) complex [Co(NCNCH2−Et)Br2]. Treatment of [Co(NCNCH2−Et)Br2] with TlBF4 as halide scavenger in acetonitrile led to the formation of the cationic octahedral complex [Co(NCNCH2−Et)(MeCN)3](BF4)2. A combination of X‐ray crystallography, IR‐, NMR‐ and EPR‐spectroscopy as well as DFT/CAS‐SCF calculations were used to characterize all compounds.
Keywords: Cobalt, DFT studies, EPR studies, Nitrosyl ligand, Pincer complexes
The preparation of several Co(II) and Co(III) NCN pincer complexes is described. A combination of X‐ray diffraction, EPR‐, IR‐ and NMR spectroscopy together with computational methods was used to characterize and study the properties of all products.

Introduction
Despite the wide prominence of pincer complexes [1] with phosphine donors and their diversity amongst d‐block elements, the chemistry of NCN ([2,6‐(R2NCH2)2C6H3]−, R=alkyl) pincer transition metal complexes is rich but largely limited to Ni, Pd, and Pt. Most notably by van Koten and coworkers, numerous Pd and Pt complexes have been prepared for applications in catalysis, sensor systems or even as building blocks for biomolecular and peptide chemistry. [2] Ever since the first PCP systems were reported, pincer ligands evolved to be extremely valuable scaffolds for stabilization of transition metal fragments in various configurations and oxidation states. The major difference affecting coordination chemistry of the NCN ligand, is the N‐atom being significantly smaller than the corresponding P‐atom in PNP or PCP ligands and the aliphatic NR2 group acting exclusively as a σ‐donor. Moreover, NCN ligands are coordinated in typically in planar tridentate mer‐fashion, but in some cases also a fac geometry was observed. [2g] It was also shown that a direct regio‐selective Carene−H activation of the ipso position is unfavorable when strongly coordinating groups (σ and π) are missing, a problem that is well known for simple metal salts and for thermodynamic reasons. It needs to be mentioned that aside transition metal coordination chemistry, NCNCH2−R (R=alkyl) systems were also reported to successfully stabilize main group elements such as Ge, Sn, and Te. [3] As far as cobalt is concerned, only the κ3‐NCN bis(amino)aryl complex [Co(2,6‐(Me2NCH2)2C6H3)X(L)] (X=Cl, Br, L=py, PPh3) was reported by van Koten in 1986 and studied by EPR and UV‐VIS‐NIR spectroscopy. [4] Aside bis(amino)aryl ligands, bis(imino)aryl and bis(oxazolinyl) ligands constitute important representatives of NCN pincer systems and a few cobalt complexes are known thereof (Scheme 1).[ 5 , 6 ]
Scheme 1.

Literature known NCN cobalt pincer complexes.
In this contribution we report on the synthesis and characterization of several new cobalt NCN pincer complexes. X‐Ray structures, EPR‐spectra, and DFT/CAS‐SCF calculations are presented.
Results and Discussion
In an attempt to reappraise van Koten's seminal work, we used a direct lithiation protocol starting from the free ligand N(C−Br)NCH2−Et that itself was prepared by reacting bis(benzylic bromide) with diethyl amine at room temperature. [7] Treatment of the lithium species with stoichiometric amount of CoBr2 suspended in THF at low temperature resulted in a color change to dark violet. After careful workup, the highly air sensitive complex [Co(NCNCH2−Et)Br] (1) was obtained in 67 % isolated yield (Scheme 2). The measurement of the solution magnetic properties (Evans method, benzene) revealed an effective magnetic moment of 2.3(1) μB. This value is in agreement with other reported Co(II) PCP pincer complexes suggesting a d7 low spin system. In order to unequivocally establish the ligand arrangement and geometry, single crystals were grown from a saturated pentane solution kept at −20 °C. A view of the molecular structure is depicted in Figure 1 with selected metrical parameters reported in captions. The complex adopts a square planar conformation with almost C2 molecular symmetry.
Scheme 2.
Synthesis of complex 1 via transmetalation.
Figure 1.

Structural view of [Co(NCNCH2−Et)Br] (1) showing 50 % displacement ellipsoids (H atoms omitted for clarity). Selected bond lengths [Å] and angles [°]: C1−Co1 1.850(3), Co1−Br1 2.438(3), Co1−N1 2.033(3), Co1−N2 2.044(3), N1−Co1−N2 167.76(9), C1−Co1−Br1 179.35(8), C1−Co1−N1 83.9(1).
The NCN ligand is coordinated to the metal center in a tridentate meridional fashion. The C1−Co1−Br1 angle is essentially linearly being 179.35(8)° and the N1−Co1−N2 angle is 167.76(9)°. The Co1−C1 distance of 1.850(3) Å is significantly shorter than in corresponding PCPCH2 and PCPO (cf 1.955 and 1.914 Å) complexes. [8]
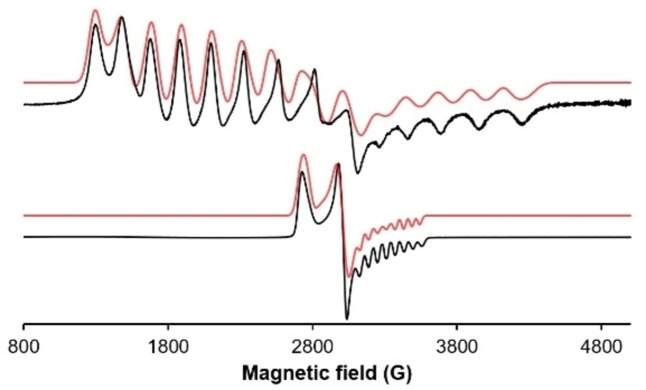
The EPR spectrum of 1 recorded at 100 K shows a strongly broadened rhombic signal with g1=3.387, g2=2.958 and g3=1.953 (giso=2.766) as shown in Figure 2 (top). The hyperfine couplings to 59Co (I=7/2) are clearly resolved with coupling constants of A1=200 G, A2=226 G and A3=230 G whereas couplings to the NCN scaffold could not be observed or simulated. The high anisotropy and large values for A(Co) are likely caused by strong spin‐orbit coupling and a nearly degenerate set of d‐orbitals. [9] The present data are consistent with a Co(II) S= system with the unpaired electron highly localized on the metal center rather than on the ligand.
Figure 2.
EPR spectra of complexes 1 (top) and 3 a (bottom) in toluene glass at 100 K microwave frequency of 9.43 GHz and microwave power 15.9 mW. Associated simulations are depicted in red color.
The electronic structure of [CoII(NCNCH2−Et)Br] (1) was further evaluated by means of computational chemistry. A DFT (BP86/def2‐TZVP) optimized structure of the low spin species on a full model agrees favorably with the metrical parameters of the experimentally determined molecular structure of 1. DFT calculations reveal that a high spin species (S=3/2) is 15.6 kcal/mol less stable than the doublet species and therefore not observed. Figure 3 shows the qualitative d‐splitting obtained from the alpha MO set with the dz2 orbital being the SOMO and the dx2–y2 being the LUMO consistent with a d7 configuration. To confirm these results and better understand the EPR experiment a complete active space self‐consistent field (CAS‐SCF) calculation was performed with additional NEVPT2 correction of the wavefunction (see Supporting Information). [10] The CAS(7,5) calculation supports the DFT results and gives a ground state configuration (96 %) of (dxy)2(dxz)2(dyz)2(dz2)1(dx2–y2)0. Within this methodology the g‐values were computed to gx=1.91, gy=2.81 and gz=3.13 (A>150 G) and thus agree satisfactorily well with the experiment.
Figure 3.
BP86/def2‐TZVP computed frontier orbitals (d‐splitting) for [Co(NCNCH2−Et)Br] (1).
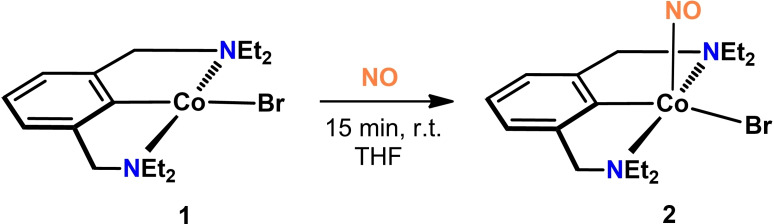
In analogy to our previous studies on {CoNO}8 complexes, [Co(NCNCH2−Et)Br] reacts with NO gas to form the closed‐shell diamagnetic complex [Co(NCNCH2−Et)(NO)Br] (2) (Scheme 3). [8] In the infrared spectrum 2 exhibits one strong band at 1609 cm−1 which is comparable to related cobalt PCP nitrosyl systems and characteristic for a bent coordination mode of the Co−NO moiety. This value indicates the formal presence of an NO− anion and therefore suggests a Co(III) oxidation state. In the 1H NMR spectrum the aliphatic protons are giving rise to two separate signals each for CH 2, CH 3 and CH 2NEt2, respectively as proved by an 1H,13C−HSQC NMR experiment. The signals of the CH 2NEt2 (linker) protons give rise to resonances at 3.26 and 2.76 ppm (c.f. 3.72 in the free ligand, C6D6) and show coupling to each other as evidenced by 1H,1H‐COSY NMR. The inspection of the 13C{1H} NMR suggests the presence of a second minor species that could be an isomer not found in the solid state. Earlier studies on Ni and Pt NCN pincer complexes already demonstrated the complexity of NMR spectra and the possibility of stereo isomerism. [11]
Scheme 3.
Synthesis of the nitrosyl complex [Co(NCNCH2−Et)(NO)Br] (2).
The solid‐state structure of 2 was determined by single crystal X‐ray diffraction. Suitable crystals were grown from a saturated pentane solution kept at −20 °C. A view of the molecular structure is depicted in Figure 4 with selected bond distances and angles reported in captions. The complex adopts a distorted square pyramidal geometry (τ5=0.31) [12] with the NCN ligand coordinated in a tridentate fashion to the metal center and almost CS point group symmetry. The N−O group is occupying the apical position and is strongly bent towards the aromatic scaffold. The N−O bond distance is 1.178(2) Å and the Co−N−O angle is 135.0(2)° both in accordance with earlier reported {CoNO}8 PCP pincer complexes. All attempts to generate the cationic complex [Co(NCNCH2−Et)(NO)]+ using halide scavengers such as AgBF4 or TlBF4 failed and resulted in partial decomposition of the starting material.
Figure 4.

Structural view of [Co(NCNCH2−Et)(NO)Br] (2) showing 50 % displacement ellipsoids (H atoms omitted for clarity). Selected bond lengths [Å] and angles [°]: Co1−C1 1.883(2), Co1−Br1 2.4915(4), Co1−N1 2.0753(18), Co1−N2 2.0641(19), Co1−N3 1.7400(17), N3−O1 1.178(2), C1−Co1−Br1 162.84(6), N1−Co1−N2 143.71(7), C1−Co1−N3 93.72(9), Co1−N3−O1 135.0(2).
Due to the sensitivity of the generated substances, in situ spectroscopic experiments were performed wherein coordinatively unsaturated complex 1 was reacted with L=pyridine, tBuNC, P(OMe)3 and CO to form complexes tentatively assigned as [Co(NCNCH2−Et)(L)Br] (3 a–d) (Scheme 4). These 17 VE complexes were not isolated but directly studied by X‐Band EPR spectroscopy in frozen toluene glass and IR spectroscopy in the case of 3 d. In comparison to complex 1, complex 3 a gives rise to a much more compact signal with g1=2.017, g2=2.228 and g3=2.459 (giso=2.235). The hyperfine couplings to 59Co are well resolved with Azz=58 G and comparable with van Koten's earlier contribution. [4] The spectra for the tBuNC and P(OMe)3 coordinated species show similarly well resolved signals with giso=2.120 and giso=2.144, respectively (see Supporting Information). The infrared spectrum of 3 d gives rise to a distinctive band at 1967 cm−1 which is in accordance with earlier reported PCP cobalt mono carbonyl complexes. [13] An analogous computational protocol (vide supra) was applied to study complex 3 a and to theoretically justify the strongly differing EPR parameters with respect to 1. The structure of the putative pyridine adduct was optimized using DFT on a full model and used for a consecutive CAS(7,5)/NEVPT2 calculation (96 % of configuration [22210]). The EPR parameters were calculated to gx=2.01, gy=2.19 and gz=2.60 agreeing with the observed experimental trend.
Scheme 4.
Synthesis of 17 VE complexes 3 a–d.
The oxidation of complex 1 with Cu(II) bromide in THF leads to a color change from violet to green and formation of the paramagnetic five‐coordinate Co(III) species [Co(NCNCH2−Et)Br2] (4) in 88 % isolated yield (Scheme 5). The solution magnetic moment (Evans method, THF) of 4.8(1) μB is consistent with a d6 high spin system, corresponding to four unpaired electrons, and is within the observed range of other five‐coordinate Co(III) complexes known. Compound 4 was then treated with TlBF4 and in acetonitrile as solvent to generate the diamagnetic tris acetonitrile complex [Co(NCNCH2−iPr)(CH3CN)3]2+ (5). Surprisingly, this complex turned out to be very unstable and all attempts to isolate this compound in pure form failed due to decomposition and formation of intractable paramagnetic compounds. This compound was thus merely characterized by 1H NMR spectroscopy. This behavior is in sharp contrast to the analogous Co(III) complex [Co(PCPNMe−iPr)(CH3CN)3]2+ bearing PCP ligands described previously. [13]
Scheme 5.
Synthesis of the Co(III) species 4 and 5.
Conclusion
The preparation of several Co(II) and Co(III) NCN pincer complexes is described. A simple transmetalation protocol allowed for the synthesis of the highly air‐sensitive 15 VE complex [CoII(NCNCH2−Et)Br] (1) which provided the starting material for subsequent transformations. The reaction with NO gas yields the diamagnetic {CoNO}8 species [Co(NCNCH2−Et)(NO)Br] featuring a strongly bent NO ligand (Co−N−O∠135.0°). The νNO is observed at 1609 cm−1 which is typical for a bent metal‐N−O arrangement. Addition of various co‐ligands L=py, tBuCN, P(OMe)3, and CO to 1 in toluene leads to the formation of very unstable and not isolable five‐coordinate complexes of the type [Co(NCNCH2−Et)(L)Br]. Oxidation of 1 with CuBr2 results in the formation of the high‐spin complex [CoIII(NCNCH2−Et)Br2] that can be transformed into the diamagnetic, but very unstable, tris‐acetonitrile complex [CoIII(NCNCH2−Et)(MeCN)3]2+. A combination of X‐ray diffraction, EPR‐, IR‐ and NMR spectroscopy together with computational methods was used to characterize and study the properties of all products.
Experimental section
General information
All manipulations were performed under an inert atmosphere of Argon by using Schlenk techniques or in an MBraun inert‐gas glovebox. The solvents were purified according to standard procedures. The deuterated solvents were purchased from Aldrich and dried over 4 Å molecular sieves. Nitric oxide (NO 2.5) was purchased from MESSER GmbH (Gumpoldskirchen, Austria). The ligand N(C−Br)NCH2−Et was synthesized according to literature and purified via distillation.14 1H, 13C{1H}, and COSY NMR spectra were recorded on an AVANCE‐400 spectrometer. 1H and 13C{1H} NMR spectra were referenced internally to residual protio‐solvent and solvent resonances, respectively, and are reported relative to tetramethylsilane (δ=0 ppm). Infrared spectra were recorded in attenuated total reflection (ATR) mode on a PerkinElmer Spectrum Two FT‐IR spectrometer. Elemental analysis was performed on an elementar vario MACRO (Elementar Analysensysteme GmbH, Germany) CHNS analyzer. High‐resolution mass spectra were recorded on an Agilent 6545 QTOF equipped with an Agilent Dual AJS ESI ion source (Agilent Technologies, Santa Clara, USA). Measured accurate mass data for confirming elemental compositions were typically within ±3 ppm accuracy. In all experiments a direct infusion technique was used, and samples prepared in a glovebox. Electron Paramagnetic Resonance (EPR) spectra were recorded on an X‐band Bruker Elexsys‐II E500 CW‐EPR spectrometer (Bruker Biospin GmbH, Rheinstetten, Germany) equipped with a high sensitivity cavity (SHQE1119) at 100±1 K. The instrument parameters were set as follows: microwave frequency, 9.43 GHz; modulation frequency, 100 kHz, and microwave power, 15.9 mW. The spectra were analyzed using Xepr software and the Anisotropic SpinFit simulation program (both Bruker Biospin GmbH).
Syntheses
[Co(NCNCH2−Et)Br] (1). To a solution of N(C−Br)NCH2−Et (215 mg, 0.65 mmol) in THF (10 mL) was slowly added nBuLi (0.45 mL, 1.6 M, 0.72 mmol) at −90 °C and then stirred at low temperature for 1 h. After allowing to warm to 0 °C, a suspension of anhydrous CoBr2 (150 mg, 0.68 mmol) in THF (5 mL) was added dropwise. The reaction mixture was stirred for further 30 min and all volatiles were removed under reduced pressure. The remaining dark solid was extracted into n‐pentane and the extract filtered through a syringe filter. The solvent was removed under reduced pressure to give a violet solid. Yield: 169 mg (67 %). μeff=2.3(1) μB. (benzene, Evans method). Elemental analysis: C16H27BrCoN2 (386.23) calc. C 49.75, H 7.05, N 7.25 found C 49.23, H 7.20, N 7.33.
[Co(NCNCH2−Et)(NO)Br] (2). Nitric oxide was injected into the headspace of a solution of [Co(NCNCH2−Et)Br] (50 mg, 0.13 mmol) in THF (2 mL) whereupon the color changed from violet to dark purple. The reaction mixture was stirred for additional 15 min and all volatiles were removed under reduced pressure. Yield: 49 mg (91 %). 1H NMR (600 MHz, δ, C6D6): 7.01 (t, J=7.3 Hz, 1H, ph), 6.56 (d, J=7.6 Hz, 2H, ph), 3.26 (m, 2H, CH 2NEt2), 2.96 (m, 4H, NCH 2CH3), 2.76 (m, 2H, CH 2NEt2), 2.49 (m, 4H, NCH 2CH3), 0.61 (m, 4H, CH 3), 0.54 (m, 8H, CH 3). 13C{1H} NMR (151 MHz, δ, C6D6): 147.1 (s, phCCH2), 124.8 (s, phCH), 118.2 (s, phCH), 60.7 (CH2NEt2), 52.8 (NCH2CH3), 51.9 (NCH2CH3), 10.6 (CH3), 9.0 (CH3). IR (ATR, cm−1): 1609 (νNO). HR‐MS (ESI+, CH3CN) m/z calcd for C16H27CoN3O [M−Br]+ 336.1480 found 336.1483.
EPR experiments. Reaction of 1 with L=pyridine, t BuNC and P(OMe)3. Formation of [Co(NCNCH2−Et)(L)Br] (3 a–c). To a solution of complex 1 (5 mg) in dry toluene (1 mL) was added an excess of ligand (pyridine, 30 μL; tBuNC, 5 mg; P(OMe)3, 30 μL) and the solution was stirred for 5 min. An aliquot was transferred to a heat‐dried EPR tube in a Glovebox and measured at 100 K revealing the formation of [Co(NCNCH2−Et)(L)Br]. EPR parameters: 3 a g1=2.017. g2=2.228, g3=2.459; 3 b g1=2.136, g2=2.110, g3=2.112 and 3 c g1=2.073, g2=2.282, g3=2.076. (see Supporting Information for details)
Reaction of 1 with CO. Formation of [Co(NCNCH2−Et)(CO)Br] (3 d). CO was injected into the headspace of a solution of 1 (10 mg) in toluene whereupon the color changed to green. All volatiles were removed and the solid was analyzed by IR spectroscopy. IR (ATR, cm−1): 1967 (νCO).
[Co(NCNCH2−Et)Br2] (4). To a solution of [Co(NCNCH2−Et)Br] (50 mg, 0.13 mmol) in THF (2 mL) was added solid CuBr2 (30 mg, 0.13 mmol) and the reaction mixture stirred for 5 min. After removal of the solvent under reduced pressure, CH2Cl2 (3 mL) was added, and the solution filtered through a syringe filter. All volatiles were removed and the obtained green solid washed with n‐pentane (5 mL). Yield: 53 mg (88 %). μeff=4.8(1) μB (THF, Evans method). Elemental analysis: C16H27Br2CoN2 (466.14) calc. C 41.23, H 5.84, N 6.01 found C 41.66 H 5.97 N 5.91.
Reaction of 1 with CH3CN and TlBF4 to form [Co(NCNCH2−Et)(MeCN)3](BF4)2 (5). To a solution of complex 4 (35 mg, 0.07 mmol) in dry acetonitrile (2 mL) was added TlBF4 (46 mg, 0.15 mmol). The reaction mixture was stirred for 3 h and all volatiles were removed under reduced pressure. The crude product was redissolved in CH2Cl2 (3 mL) and filtered through a syringe filter. The solvent was evaporated to afford a red‐brown solid. 1H NMR (400 MHz, δ, CD3CN): 7.76 (m, 1H, ph), 7.66 (m, 2H, ph), 4.13 (m, 4H, CH 2NEt2), 3.04 (m, 8H, CH 2CH3), 2.01 (bs, 9H, CH 3CN), 1.42 (t, J=6.1 Hz, 12H, CH 3).
Computational Details
All calculations were performed using the ORCA 4.2.1 software package [15] utilizing the Vienna Scientific Cluster (VSC3) in part. Electronic ground state calculations, including geometry optimizations and frequencies were carried out with density functional theory (DFT) using the GGA functional BP86 [16] and Ahlrichs [17] def2‐TZVP basis set on all atoms. The resolution of identity (RI) approximation was used along with the corresponding auxiliary basis sets to accelerate the calculations. State averaged and state specific CAS‐SCF [18] calculations were carried out with an active space comprised of seven electrons in five d‐orbitals using the obtained DFT‐geometry of the low spin species. To capture the effect of the dynamic correlation, NEVPT2 correction [19] was employed on top of the CAS‐SCF wave function. The def2‐TZVP basis set and a very fine integration grid (Grid6) was used in all calculations. Orbital plots and graphics were generated with ChemCraft. [20]
X‐Ray Structure Determination
X‐ray diffraction data of 1 and 2 (CCDC 2098276, 2098277) were collected at T=100 K in a dry stream of nitrogen on a Bruker Kappa APEX II diffractometer system using graphite‐monochromatized Mo‐Kα radiation (λ=0.71073 Å) and fine sliced ϕ‐ and ω‐scans. Data were reduced to intensity values with SAINT and an absorption correction was applied with the multi‐scan approach implemented in SADABS. [21] The structure was solved by the dual‐space approach implemented in SHELXT [22] and refined against F 2 with SHELXL. [23] Non‐hydrogen atoms were refined with anisotropic displacement parameters. The H atoms were placed in calculated positions and thereafter refined as riding on the parent C atoms. Molecular graphics were generated with the program MERCURY. [24]
Deposition Numbers 2098276 (for 1) and 2098277 (for 2) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service www.ccdc.cam.ac.uk/structures.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Acknowledgements
Financial support by the Austrian Science Fund (FWF) is gratefully acknowledged (Project No. P 32570‐N). The X‐Ray center of the Vienna University of Technology is acknowledged for financial support and for providing access to the single‐crystal diffractometer.
J. Pecak, W. Eder, G. Tomsu, B. Stöger, M. Pignitter, K. Kirchner, Eur. J. Inorg. Chem. 2021, 2021, 4280.
Contributor Information
Jan Pecak, https://www.ias.tuwien.ac.at/research‐units/inorganic‐chemistry/kk/home/.
Prof. Dr. Karl Kirchner, Email: karl.kirchner@tuwien.ac.at.
References
- 1.For reviews on pincer complexes, see:
- 1a. Albrecht M., van Koten G., Angew. Chem. Int. Ed. 2001, 40, 3750–3781; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2001, 113, 3866–3898; [Google Scholar]
- 1b. Valdos H., García-Eleno M. A., Canseco-Gonzalez D., Morales-Morales D., ChemCatChem 2018, 10, 3136–3172; [Google Scholar]
- 1c. Benito-Garagorri D., Kirchner K., Acc. Chem. Res. 2008, 41, 201–213; [DOI] [PubMed] [Google Scholar]
- 1d. Peris E., Crabtree R. H., Chem. Soc. Rev. 2018, 47, 1959–1968; [DOI] [PubMed] [Google Scholar]
- 1e. Murugesan S., Kirchner K., Dalton Trans. 2016, 45, 416–439; [DOI] [PubMed] [Google Scholar]
- 1f. Morales-Morales D., Jensen C. M. (Eds.), The Chemistry of Pincer Compounds, Elsevier: Amsterdam; 2007; [Google Scholar]
- 1g. van Koten G., Milstein D. (Eds.), Organometallic Pincer Chemistry, Springer: Berlin; 2013; [Google Scholar]
- 1h. Vogt M., Langer R., Eur. J. Inorg. Chem. 2020, 3885–3898. [Google Scholar]
- 2.
- 2a. Grove D. M., van Koten G., Ubbels H. J. C., Zoet R., Organometallics 1984, 3, 1003–1009; [Google Scholar]
- 2b. Slagt M. Q., van Zwieten D. A. P., Moerkerk A. J. C. M., Klein Gebbink R. J. M., van Koten G., Coord. Chem. Rev. 2004, 248, 2275–2282; [Google Scholar]
- 2c. Back S., Gossage R. A., Lang H., van Koten G., Eur. J. Inorg. Chem. 2000, 1457–1464; [Google Scholar]
- 2d. Rodriguez G., Lutz M., Spek A. L., van Koten G., Chem. Eur. J. 2002, 8, 45–57; [DOI] [PubMed] [Google Scholar]
- 2e. van der Zeijden A. A. H., van Koten G., Luijk R., Vrieze K., Slob C., Krabbendam H., Spek A. L., Inorg. Chem. 1988, 27, 1014–1019; [Google Scholar]
- 2f. Schimmelpfennig U., Zimmering R., Schleinitz K. D., Stößer R., Wenschuh E., Z. Anorg. Allg. Chem. 1993, 619, 1931–1938; [Google Scholar]
- 2g. Adams J. J., Arulsamy N., Roddick D. M., Organometallics 2012, 31, 1439–1447. [Google Scholar]
- 3.
- 3a. Bibal C., Mazieres S., Gornitzka H., Couret C., Polyhedron 2002, 21, 2827–2834; [Google Scholar]
- 3b. Cui C., Roesky H. W., Noltemeyer M., Schmidt H.-G., Inorg. Chem. 2000, 39, 3678–3681; [DOI] [PubMed] [Google Scholar]
- 3c. Jastrzebski J. T. B. H., van der Schaaf P. A., Boersma J., van Koten G., Zoutberg M. C., Heijdenrijk D., Organometallics 1989, 8, 1373–1375. [Google Scholar]
- 4. van der Zeijden A. A. H., van Koten G., Inorg. Chem. 1986, 25, 4723–4725. [Google Scholar]
- 5. Huang L. C., Zhang J. S., Jia T., Mu Y., Gao W., Dalton Trans. 2020, 49, 5219–5227. [DOI] [PubMed] [Google Scholar]
- 6. Hosokawa S., Ito J., Nishiyama H., Organometallics 2013, 32, 3980–3985. [Google Scholar]
- 7. Jastrzebski J. T. B. H., van Koten G., Konijn M., Stam C. H., J. Am. Chem. Soc. 1982, 104, 5490–5492. [Google Scholar]
- 8. Pecak J., Eder W., Stöger B., Realista S., Martinho P. N., Calhorda M. J., Linert W., Kirchner K., Organometallics 2020, 39, 2594–2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.
- 9a. Hojilla Atienza C. C., Milsmann C., Lobkovsky E., Chirik P. J., Angew. Chem. Int. Ed. 2011, 50, 8143–8147; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2011, 123, 8293–8297; [Google Scholar]
- 9b. Semproni S. P., Milsmann C., Chirik P. J., J. Am. Chem. Soc. 2014, 136, 9211–9224. [DOI] [PubMed] [Google Scholar]
- 10. Singh S. K., Atanasov M., Neese F., J. Chem. Theory Comput. 2018, 14, 4662–4677. [DOI] [PubMed] [Google Scholar]
- 11. van Beek J. A. M., van Koten G., Ramp M. J., Coenjaarts N. C., Grove D. M., Goubitz K., Zoutberg M. C., Stam C. H., Smeets W. J. J., Spek A. L., Inorg. Chem. 1991, 30, 3059–3068. [Google Scholar]
- 12. Addison A. W., Nageswara Rao T., Reedijk J., van Rijn J., Verschoor G. C., J. Chem. Soc. Dalton Trans. 1984, 1349–1356. [Google Scholar]
- 13. Murugesan S., Stöger B., Carvalho M. D., Ferreira L. P., Pittenauer E., Allmaier G., Veiros L. F., Kirchner K., Organometallics 2014, 33, 6132–6140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van Beek J. A. M., van Koten G., Dekker G. P. C. M., Wissing E., Zoutberg M. C., Stam C. H., J. Organomet. Chem. 1990, 394, 659–678. [Google Scholar]
- 15. Neese F., WIREs Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar]
- 16. Becke A. D., Phys. Rev. A 1988, 38, 3098–3100. [DOI] [PubMed] [Google Scholar]
- 17. Weigend F., Ahlrichs R., Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [DOI] [PubMed] [Google Scholar]
- 18. Malmqvist P. A., Roos B. O., Chem. Phys. Lett. 1989, 155, 189–194. [Google Scholar]
- 19. Angeli C., Cimiraglia R., Evangelisti S., Leininger T., Malrieu J. P., J. Chem. Phys. 2001, 114, 10252–10264. [Google Scholar]
- 20.ChemCraft – https://chemcraftprog.com.
- 21.Bruker computer programs: APEX3, SAINT and SADABS (Bruker AXS Inc., Madison, WI, 2020).
- 22. Sheldrick G. M., Acta Crystallogr. 2015, A71, 3–8. [Google Scholar]
- 23. Sheldrick G. M., Acta Crystallogr. 2015, C71, 3–8. [Google Scholar]
- 24. Macrae C. F., Edgington P. R., McCabe P., Pidcock E., Shields G. P., Taylor R., Towler M., van de Streek J., J. Appl. Crystallogr. 2006, 39, 453–457. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information