Abstract
Problem
Repeated implantation failure and recurrent pregnancy loss are associated with chronic endometritis, a persistent endometrial inflammation. Its diagnosis and treatment may increase pregnancy and live birth rates. The aim of this study was to assess the effectiveness of endometrial diagnostic biopsy and subsequent antibiotic treatment in cases of chronic endometritis on reproductive outcomes over a long observation period.
Method of Study
We conducted a historical cohort study (2014–2018) at our University‐based infertility center that included women (n = 108) with repeated implantation failure or recurrent pregnancy loss without known pathologies associated with either condition. Forty‐one women underwent a hysteroscopy only (reference group); the remaining 67 women underwent, in addition to the hysteroscopy, an endometrial diagnostic biopsy with immunohistochemically staining for CD138 to detect plasma cells (biopsy group). If one or more plasma cells were detected, the women were treated with doxycycline 100 mg twice a day orally for 2 weeks. We performed stratified survival analysis (Kaplan‐Meier) and Cox regression.
Results
The biopsy group had higher chances of pregnancy (hazard ratio 2.28; 95% confidence interval 1.23–4.24; p = .009) and of live birth (hazard ratio 2.76; 95% confidence interval 1.30–5.87; p = .008) compared with the reference group. In the sensitivity analysis, repeated implantation failure or recurrent pregnancy loss did not affect the outcome.
Conclusion
Endometrial diagnostic biopsy followed by antibiotic treatment in case of chronic endometritis in women with repeated implantation failure or recurrent pregnancy loss may increase the chances for live birth.
Keywords: chronic endometritis, endometrial diagnostic biopsy, plasma cells, recurrent pregnancy loss, repeated implantation failure, time to live birth, time to pregnancy
1. INTRODUCTION
Repeated implantation failure (RIF) and recurrent pregnancy loss (RPL) impose a heavy burden on women desiring children, especially when etiology is unclear. Implantation failure has been identified by the European Society of Human Reproduction and Embryology as one of the main unresolved issues in reproductive medicine.
Known risk factors for RIF and RPL are parental age, obesity, environmental exposure (including smoking and alcohol), genetic factors, uterine malformations or pathologies, and thyroid autoimmunity. 1 , 2 , 3 In addition, antiphospholipid syndrome is a risk factor for RPL. 3 According to Coughlan et al., 4 RIF is defined as “the failure to achieve a clinical pregnancy after transfer of at least four good‐quality embryos in a minimum of three cycles in a women under the age of 40 years.” The World Health Organization defines RPL as three or more consecutive miscarriages; it affects up to 5% of women. 2 , 5 Endometrial dysfunction and reduced endometrial receptivity caused by inflammatory or immunological processes are found to be associated with both RIF and RPL. 6 , 7
These conditions can be due to chronic endometritis (CE), which is often subtle and asymptomatic or presents with atypical symptoms such as pelvic pain, vaginal discharge, abnormal bleeding, dyspareunia, or leucorrhea. While there is no doubt that CE is of importance clinically, there is still no universally accepted definition and there is a lack of clear criteria, 8 , 9 standardized diagnostic procedure, or treatment confirmed by randomized clinical trials. 8 , 9 , 10 , 11 , 12 , 13 The prevalence of CE in women with RIF reported in published studies ranges from 7.7% to 67.5% 14 , 15 ; the prevalence of CE in women with RPL from 7.0% up to 67.6%. 9 , 16 , 17 It is unclear whether these differences in prevalence result from differences in study populations, prevalence of different pathogens, or diagnostic assessment techniques and thresholds. 14 , 18
Using hysteroscopy (HSC), CE can be suspected in the presence of mucosal edema, focal or diffuse endometrial hyperemia, and/or micro polyps (<1 mm). 15 , 19 , 20 , 21 The diagnosis should be confirmed by an endometrial biopsy stained immunohistochemically with Syndecan‐1 for plasma (CD138) cells. 14 , 18 , 22 , 23 , 24 A meta‐analysis showed higher pregnancy and live birth rates after antibiotic treatment for plasma cell‐positive CE in women with RIF. 25 This was also found in a meta‐analysis of randomized controlled trials on endometrial scratch injury in women who had at least one failed embryo transfer. 26
Many different antibiotics have been used for the treatment of CE. A broad‐spectrum antibiotic such as doxycycline (2 × 100 mg per day for 2 weeks) is mostly used as a first‐line treatment. 16 , 27 , 28 Metronidazole (250 mg 2 × per day or 500 mg per day) in combination with ciprofloxacin 27 (500 mg per day or 200 mg 2 × a day) or ofloxacin (800 mg per day) have been used as second‐line treatments. 16 , 28 In a study published 2019, a mixture of a suitable antibiotic plus dexamethasone was administered directly to the uterus. 29 The choice of the antibiotic should depend on the regional recommendations based on the incidence of pathogens on a population level, as well as on the profile of pathogens found. 30
The aim of this study was to assess the effectiveness of endometrial diagnostic biopsy and subsequent antibiotic treatment in cases of CE in women with RIF and RPL over a long observation period. The focus was on reproductive outcomes, notably chance of live birth and chance of pregnancy over time. Most studies in this area focused on pregnancy and live birth rates achieved after just one in vitro fertilization (IVF) cycle following diagnosis and treatment of CE. We studied the effect of endometrial diagnostic biopsy and antibiotic treatment in women undergoing hysteroscopy for the investigation of RIF or RPL with a focus on time to live birth by using the method of survival analysis (Kaplan‐Meier). This method allowed us to compare reproductive outcomes over a longer period.
2. MATERIALS AND METHODS
2.1. Study population
We screened retrospectively all women treated at our center for RIF or RPL between January 2014 and December 2018. We defined RIF as a failure to achieve a pregnancy after the transfer of six or more good‐quality cleavage‐stage embryos. 4 For RPL, we used the WHO definition of three or more RPLs. 2 , 5
Screening work‐up consisted of thyroid function and antibody testing, exclusion of antiphospholipid syndrome, and assessment of the uterine cavity by ultrasound. Hysterosalpingosonography and transvaginal ultrasound to assess uterine anatomy had already been performed during assessment of infertility. We included all women undergoing HSC or both HSC and biopsy 42 years old or younger at the time of HSC and with a body mass index (BMI) between 18 and 35 kg/m2. We excluded women with conditions known to be associated with RIF and RPL such as parental chromosomal abnormalities, sperm retrieved by testicular sperm extraction, antiphospholipid syndrome, or severe thyroid dysfunction (Figure 1). 31 , 32
FIGURE 1.
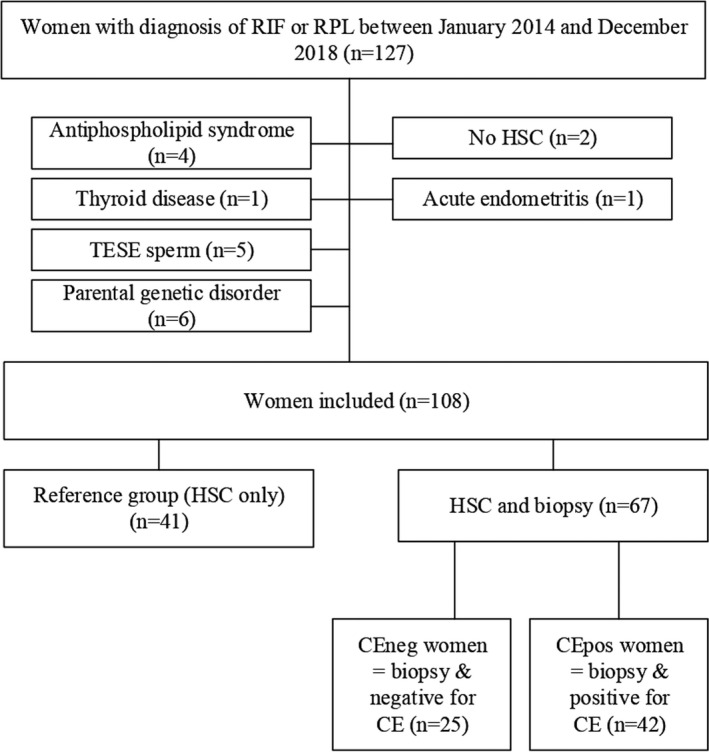
Study population. CE, chronic endometritis; CEneg, women diagnosed negative for chronic endometritis; CEpos, women diagnosed positive for chronic endometritis; HSC, hysteroscopy; RIF, repeated implantation failure; RPL, recurrent pregnancy loss; TESE, testicular sperm extraction
2.2. Diagnosis and histological assessment of CE
Before conducting HSC, we ruled out chlamydia (Chlamydia trachomatis) and gonorrhea (Neisseria gonorrhoeae) using cervical‐vaginal swabs. We performed HSC and biopsy during the late follicular phase, in an office setting without anesthesia. For HSC, we used a rigid 30° view 2.9 mm diameter hysteroscope with an atraumatic tip (TROPHYscope; Karl Storz). We did not provide preoperative analgesia, sedation, or antibiotics; we undertook cervical preparation with dequalinium chloride (Fluomizin®). We photo‐documented the appearance of the uterine cavity and the endometrium (Figure 2A,B). In the biopsy group, we performed the endometrial diagnostic biopsy at the end of the HSC examination of the uterine cavity, using a pipelle (flexible biopsy device). We then conserved the endometrial tissue in a 10% formaldehyde solution. We immunohistochemically stained the sections of the paraformaldehyde‐fixed paraffin‐embedded endometrial biopsy samples with syndecan‐1 according to routine protocols. Syndecan‐1 is a cell surface heparan sulfate proteoglycan used as a specific marker for CD138 plasma cells (Figure 2C‐F). 18 Formalin‐fixed paraffin‐embedded slides with a thickness of 3 µm were pretreated with heat (95°C for 20 min) and then incubated with the CD138 antibody (Serotec) in EDTA buffer at pH 9 for 15 min at a dilution of 1:1600, with diacetylbenzene serving as the chromogenic agent. We counterstained with hematoxylin‐eosin (HE) to compare the two diagnostic methods. For the diagnosis of CE as defined in this study, we relied on immunohistochemistry, considering one or more plasma cell per whole‐slide tissue section (one plasma cell in the hotspot) as sufficient for the diagnosis of CE. 19 Before 2016, HSC without biopsy was the standard diagnostic intervention in women with RPL or RIF; these women served as historical references compared with the women in the biopsy group.
FIGURE 2.

Images of patients with and without CE in hysteroscopy, hemalaun‐eosin staining and immunohistochemically CD138 staining. Comparison of patients with bland endometrium (A,C,E) vs. chronic endometritis (B,D,F) in hysteroscopy (A,B), conventional histology (C,D) and immunohistochemistry for CD138 (E,F). Note, the reddish inflamed mucosal surface in chronic endometritis (B) and the intermingled plasma cells in endometrial stroma in immunohistochemistry (F, central region). These differences in plasma cell densities cannot be distinguished in conventional histology (C,D) of the same patients. Of note, regular endometrial glands serve as a positive internal control as CD138 also known as syndecan‐1 is positive in epithelial cells. Pictures were taken at 200× magnification. CE, chronic endometritis; HE, hemalaun‐eosin staining; HSC, hysteroscopy
2.3. Treatment of CE
Women diagnosed with CE on the basis of immunohistochemistry were treated with 100 mg of doxycycline orally twice a day for 2 weeks. Women with an intolerance to doxycycline received 400 mg of ciprofloxacin a day for 2 weeks. We did not perform a test of cure. In case of RPL after treatment, the women were re‐biopsied and retreated with ciprofloxacin as a second‐line treatment, as described above. Tetracyclines (eg, doxycycline) and quinolones (eg, ciprafloxacine) also act against bacteria lacking cell walls such as chlamydia (Chlamydia trachomatis) and gonorrhea (Neisseria gonorrhea) as well as against parasites. Additionally, doxycycline has an anti‐inflammatory potential, which we considered beneficial for the treatment of an inflammatory condition.
2.4. Fertility treatment
For women with RIF, the same IVF protocol as applied previously was initiated after biopsy, 1 month after termination of antibiotic therapy, or when the couple felt ready. The IVF treatment cycles were conducted either as natural IVF 33 or as conventional gonadotropin‐stimulated IVF, either in an agonist or antagonist protocol. All embryos were transferred at cleavage stage; IVF procedures did not change over the study period. We provided luteal phase support using progesterone for up to 12 weeks of gestation. 34 Women with RPL did not receive IVF treatment. We prescribed 200 mg of vaginal micronized progesterone per day in the subsequent pregnancy. 35 , 36
2.5. Statistical analysis
We compared the women from the biopsy group to the women who had HSC only, the reference group. For subgroup analysis, we grouped the women of the biopsy group as follows, according to the immunohistochemical diagnosis of plasma cells: (a) biopsy without diagnosis of CE (CEneg) and (b) biopsy with diagnosis of CE (CEpos). We compared baseline characteristics and outcomes (pregnancy rates, live birth rates, chance of pregnancy, and of live birth over time) among the biopsy group and the reference group and between women with RIF or RPL. We used the chi‐square test or Fisher’s exact test for categorical variables and univariable linear regression for continuous outcomes. For the time‐to‐event analyses, follow‐up time started at the date of HSC or biopsy (biopsy is performed during HSC). The follow‐up time ended at the event of interest or at last contact date recorded for each woman; the event of interest is either the date of clinical pregnancy confirmed by ultrasound display of an amniotic cavity (pregnancy) or the delivery date (live birth). We used Kaplan‐Meier survival analysis to compare time to pregnancy and live birth between the groups. In further comparisons, we used multivariable Cox regression models that included the following variables: age of the women (continuous) and parity (parous vs. nulliparous). We stratified Cox regression models for RIF and RPL women allowing for different baseline hazards via separate risk‐set definitions in the RIF and RPL groups. In a sensitivity analysis, we conducted separate Cox regressions for women with RIF and women with RPL and among the two subgroups, CEneg, and CEpos, always compared with the reference group. For the survival curves in Figure 3, we used inverse probability weighting to account for different proportions of RIF or RPL women and different age structure of mothers within the treatment groups. 37 For statistical analysis, we used STATA version 16.0 (STATA Corporation LLC). We considered a p‐value <.05 as significant.
FIGURE 3.

Time to pregnancy and time to live birth. Kaplan‐Meier failure estimates. Observation time: from hysteroscopy or biopsy date to clinical pregnancy or live birth; with applied inverse probability weighting to account for the proportion of women with repeated implantation failure and recurrent pregnancy loss, respectively, as well as the maternal age structure within each of the groups compared (according to Cole SR & Hernan MA). p‐values for the two groups compared: (a) p = .009 B) p = .008 C) Reference group compared with biopsy CEpos group: p = .010; biopsy CEpos group compared with biopsy CEneg group: p = .800. (b) D) Reference group compared with biopsy CEpos group: p = .004; biopsy CEpos group compared with biopsy CEneg group: p = .327
2.6. Ethical approval
The local ethical committee approved this study on the December 21, 2017 (BASEC & Kantonale Ethik Kommission Bern: 2017‐01739), approving the use of encoded clinical data after patient information or the further use of biomaterials after informed consent retrieval.
3. RESULTS
3.1. Population, diagnosis, and treatment of CE
The flow chart of the study population is presented in Figure 1. In total, 108 women fulfilled the inclusion criteria, of whom 47 (43.5%) had RIF and 61 (56.5%) had RPL. From 2014 to 2016, we performed only HSC in 41 (38.0%) women (reference group); from 2016–2018, we performed HSC with subsequent biopsy in 67 women. Twenty‐four (35.8%) women showed suspicion of CE at hysteroscopy, with strawberry aspects, endometrial edema, irregular endometrium, and hyperemic areas with prominent leukoplakia (Figure 2). Among the women who underwent a biopsy, a total of 42 women were diagnosed positive for CE (CEpos), which constitutes a prevalence of 62.7%; 25 (37.3%) women did not have any plasma cells according to histology and immunohistochemical CD138 staining (CEneg).
Among the 67 women who underwent a biopsy, with HSC, we could identify 18 women as CEpos out of 42 (sensitivity of 42.8%) and 19 as CEneg out of 25 (specificity of 76.0%) when comparing to immunohistochemically stained biopsy samples (p = .119). HE staining alone was not sufficient to identify plasma cells as compared to IHC as well, with only 11 women correctly diagnosed as CEpos (sensitivity of 26.2%) and 21 as CEneg (specificity of 84.0%) in comparison with immunohistochemical staining (p = .333). False‐positive cells in HE staining were due mainly to pseudodecidual of endometrial stromal cells, which could display a crescent‐like cytoplasmic rim and represent a mimicker of plasma cells (Figure 2). Of the 42 women diagnosed as CEpos, 41 took a first treatment of doxycycline and one woman took ciprofloxacin.
3.2. Population characteristics
Patient characteristics are shown in Table 1. The reasons for infertility are different between RIF and RPL patients (p < .005) but not among the three subgroups studied. Comparison of patient characteristics between CEneg, CEpos, and the reference group are displayed in the Table S1.
TABLE 1.
Patient characteristics and fertility characteristics
| Biopsy group | Reference group | Biopsy vs. reference group (total) | RIF vs. RPL (total) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RIF | RPL | Total | RIF vs. RPL | RIF | RPL | Total | RIF vs. RPL | |||||||||
| m/n | SD/% | m/n | SD/% | m/n | SD/% | p value | m/n | SD/% | m/n | SD/% | m/n | SD/% | p value | p value | p value | |
| n | 28 | 42 | 39 | 58 | 67 | 62 | 19 | 46 | 22 | 54 | 41 | 38 | 67 vs. 41 | 61 vs. 47 | ||
| Mean age (years) a | 37.8 | 3.1 | 36.9 | 5.2 | 37.3 | 4.4 | .422 | 37.6 | 4.0 | 35.3 | 3.5 | 36.4 | 3.8 | .061 | .294 | .094 |
| Median age (years) | 37.9 | na | 37.4 | na | 37.7 | na | na | 37.8 | na | 35.2 | na | 37.0 | na | na | na | na |
| Mean weight (kg) a | 65.6 | 11.1 | 66.4 | 10.3 | 66.1 | 10.5 | .777 | 64.3 | 10.1 | 63.5 | 9.8 | 63.9 | 9.8 | .806 | .301 | .883 |
| Mean height (cm) a | 166.6 | 4.8 | 166.1 | 7.6 | 166.3 | 6.6 | .782 | 166.5 | 5.6 | 165.2 | 6.6 | 165.8 | 6.1 | .529 | .731 | .561 |
| Mean BMI (kg/m2 a | 23.7 | 4.2 | 24.1 | 3.8 | 23.9 | 3.9 | .689 | 23.2 | 3.5 | 23.3 | 3.8 | 23.2 | 3.5 | .924 | .396 | .661 |
| Current smoker (K) b | 3 | 10.7 | 8 | 20.5 | 11 | 16.4 | .286 | 3 | 15.8 | 0.0 | 0 | 3 | 7.3 | .053 | .172 | .957 |
| AMH (pmol/L) a | 34.1 | 38.6 | 26.0 | 31.1 | 29.7 | 34.7 | .375 | 23.1 | 18.5 | 23.4 | 32.3 | 23.2 | 26.5 | .971 | .334 | .459 |
| Time from intention to conceive until HSC (years) a | 5.3 | 3.6 | 4.0 | 2.7 | 4.5 | 3.1 | .080 | 4.6 | 3.3 | 3.2 | 1.9 | 3.9 | 2.7 | .104 | .257 | 5.0 vs. 3.7 (p = .02) |
| Deliveries before treatment (n) b | ||||||||||||||||
| 0 | 23 | 82.1 | 23.0 | 59.0 | 46.0 | 68.6 | .087 | 14.0 | 73.7 | 11.0 | 50.0 | 25.0 | 0.6 | .244 | .522 | .027 |
| 1 | 5 | 17.9 | 13.0 | 33.3 | 18.0 | 26.7 | 5.0 | 26.3 | 10.0 | 45.5 | 15.0 | 36.6 | ||||
| 2 | 0 | 0.0 | 3.0 | 7.7 | 3.0 | 4.5 | 0.0 | 0.0 | 1.0 | 4.5 | 1.0 | 2.4 | ||||
| No. of embryos transferred before treatment (RIF) a | 11 | 7.4 | na | 10.3 | 3.5 | na | .645 | na | ||||||||
| Reason for infertility (n) b | ||||||||||||||||
| Male factor | 10 | 35.7 | na | 7 | 3.7 | na | .492 | na | ||||||||
| Tubal factor | 2 | 7.1 | 0 | 0.0 | ||||||||||||
| Endometriosis | 3 | 10.7 | 4 | 21.0 | ||||||||||||
| Idiopathic | 12 | 42.9 | 6 | 31.6 | ||||||||||||
| Anovulation/PCOS | 1 | 3.6 | 2 | 10.5 | ||||||||||||
AMH, anti‐müllerian hormone; BMI, body mass index; CE, chronic endometritis; cm, centimeter; HSC, hysteroscopy; m, mean; na, not applicable; n, number; PCOS, polycystic ovary syndrome; RIF, repeated implantation failure; RPL, recurrent pregnancy loss; SD, standard deviation.
linear regression.
Pearson’s chi‐squared test.
3.3. Observation time
The mean observation time until live birth or last follow‐up date was the longest for the reference group, with 1.86 years (SD 1.34). For CEneg women, the mean time was 0.88 years (SD 0.61), and for CEpos, it was 0.75 years (SD 0.47).
3.4. Fertility outcomes
The chance of a clinical pregnancy and a live birth was significantly higher for women with biopsy and subsequent management of endometrial pathology compared with the reference group (Table 2). The hazard ratio (HR) stratified for indication is 2.28 (95% CI 1.23 – 4.24, p = .009) for a clinical pregnancy and 2.76 for a live birth (95% CI 1.30–5.86, p =.008).
TABLE 2.
Chances for clinical pregnancy and live birth (Cox models)
| Outcome: Clinical pregnancy | Outcome: Live birth | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted stratified Cox models | Adjusted stratified Cox models a | Unadjusted stratified Cox models | Adjusted stratified Cox models a | |||||||||
| HR | 95% CI | p value | HR | 95% CI | p value | HR | 95% CI | p value | HR | 95% CI | p value | |
| Reference group (baseline) | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||
| Biopsy group | 2.28 | 1.23–4.24 | .009 * | 2.62 | 1.39–4.49 | .003 ** | 2.76 | 1.30–5.86 | .008 * | 2.88 | 1.35–6.16 | .006 * |
| Maternal age (continuous) | 0.94 | 0.88–1.01 | .083 | 0.92 | 0.86–0.99 | .018 * | 0.97 | 0.89–1.06 | .547 | 0.96 | 0.87–1.05 | .364 |
| Duration of subfertility (continuous) | 1.05 | 0.96–1.14 | .301 | 0.95 | 0.81–1.12 | .572 | ||||||
| Parity (y/n) | 0.84 | 0.47–1.51 | .570 | 0.84 | 0.47–1.52 | .568 | 0.98 | 0.47–2.05 | .958 | 0.86 | 0.40–1.85 | .697 |
| Smoking (y/n) | 1.05 | 0.44–2.51 | .904 | 1.04 | 0.40–2.74 | .924 | ||||||
| Subgroup analyses (stratified for RIF/RPL) | ||||||||||||
| Reference group (baseline) | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||
| Diagnosed positive for CE | 2.39 | 1.20–4.36 | .01 * | 2.86 | 1.46–5.63 | .002 ** | 3.53 | 1.48–8.40 | .004 ** | 3.91 | 1.62–9.41 | .002 ** |
| Diagnosed negative for CE | 2.04 | 0.85–4.86 | .110 | 2.11 | 0.88–5.08 | .095 | 1.95 | 0.70–5.42 | .203 | 1.92 | 0.69–5.35 | .213 |
| Subgroup analyses (not stratified for RIF/RPL) | ||||||||||||
| RIF and biopsy | 2.18 | 0.73–6.50 | .162 | 2.12 | 0.71–6.35 | .179 | 2.95 | 0.91–9.62 | .073 | 2.9 | 0.89–9.46 | .077 |
| RIF and CEpos | 3.03 | 0.85–10.90 | .089 | 3.23 | 0.88–11.89 | .078 | 5.88 | 1.42 – 24.28 | .014 * | 6.78 | 1.63–28.10 | .008 * |
| RIF and CEneg | 1.73 | 0.49–6.04 | .390 | 1.61 | 0.46–5.67 | .454 | 2.04 | 0.52–8.02 | .309 | 1.88 | 0.48–7.42 | .367 |
| RPL and biopsy | 2.33 | 1.10–4.96 | .027 * | 2.98 | 1.36–6.52 | .006 * | 2.63 | 0.99–7.00 | .052 | 2.76 | 1.04–7.35 | .042 * |
| RPL and CEpos | 2.28 | 1.06–4.92 | .036* | 3.06 | 1.37–6.85 | .006 * | 2.74 | 0.97–7.70 | .056 | 3.34 | 1.13–9.83 | .029 * |
| RPL and CEneg | 2.73 | 0.81–9.20 | .107 | 2.61 | 0.73–9.35 | .138 | 2.31 | 0.75–11.15 | .298 | 1.62 | 0.30–8.69 | .573 |
95% CI, 95% confidence interval; CE, chronic endometritis; CEneg, women diagnosed negative for chronic endometritis; CEpos, women diagnosed positive for chronic endometritis; HR, hazard ratio; n, no; RIF, repeated implantation failure; RPL, recurrent pregnancy loss; y, yes.
Stratified Cox regression models all stratified for repeated implantation failure or recurrent pregnancy loss.
Cox regression models adjusted for maternal age (continuous) and parity (nullipar vs. par).
p < .05.
p < .005.
Time‐to‐event analysis shows a higher chance for pregnancy and live birth for the biopsied women at any given time point during the follow‐up period. (Figure 3).
A comparative analysis of subgroups shows that the chance of clinical pregnancy is significantly higher for CEpos women (HR 2.39, 95% CI 1.20–4.36, p = .010) and slightly less pronounced for CEneg women (HR 2.04, 95% CI 0.85–4.86, p = .110) compared with the reference group. This trend is also confirmed by analyzing women with RIF and RPL separately. When directly comparing CEpos to CEneg women to each other, both have almost similar chances for a clinical pregnancy (HR 1.12, 95% CI 0.46–2.72, p = .800) but CEpos women have a somewhat elevated chance for a live birth (HR 1.75, 95% CI 0.57–5.38, p = .327). Adjustment for maternal age and parity did not substantially affect the outcomes for clinical pregnancy (HR 1.28 (0.53–3.10; p = .579) nor for live birth (HR 2.17, 95% CI 0.73−6.49, p = .164). All detailed results of the Cox regression models are presented in Table 2. Detailed fertility outcomes are presented in Table S2.
4. DISCUSSION
This is a historical cohort study on the effect of biopsy with subsequent management of CE on chance of live birth over a longer time period in women with RIF and RPL. Our results show that a diagnostic endometrial biopsy with subsequent diagnosis and possibly treatment in case of CE is associated with a higher chance of pregnancy and live birth in both conditions, RIF and RPL. However, it remains unclear, which of the interventions, and the biopsy or the treatment or a combination of both improved the outcome as women who had a biopsy and were diagnosed and treated for CE (CEpos) had a similar chance compared with those without CE (CEneg), but a better chance compared with women with HSC only (reference group).
This leads us to two hypotheses: first, diagnostic endometrial biopsy and CD138 staining and probably in combination with subsequent treatment of CE are important in the high‐risk population of women with RIF and RPL, and second, our study supports the superiority of immunohistochemistry over hysteroscopic assessment; even women not diagnosed with CE may possibly achieve a better reproductive outcome in a shorter time after a diagnostic endometrial biopsy.
Our results are based on the indication that hysteroscopy alone or HE staining is no longer considered sufficient to clearly diagnose CE or to identify plasma cells. 22 Immunohistochemical staining of plasma cells with Syndecan‐1 is considered to be the best practice, but there are various methods regarding their quantification and threshold leading to the confirmation of a diagnosis of CE. 14 , 18 We chose a more conservative approach of diagnosing with the threshold of one plasma cell per high power field in the hot spot, which may have led to an overestimation of CE prevalence.
Our results show a difference in chance of pregnancy and, more important, chance of live birth between the biopsy group and the reference group. Our results suggest that the biopsy may have an impact also on women without CE (CEneg). Our results show that their reproductive outcome also seems to benefit from the biopsy. A biopsy, similar to an endometrial scratch, induces an inflammatory response and may trigger immunological reconstitution. 38 , 39 , 40 This might be particularly relevant in a more vulnerable RIF and RPL population. A large randomized controlled trial has recently shown that endometrial scratching before an IVF or ICSI cycle does not increase the implantation rate in a subsequent transfer. However, in this trial, the effect in women with RIF or RPL or specifically the long‐term effects were not addressed. 41 Endometrial scratch was shown to be beneficial in a meta‐analysis in women who had two or more implantation failures, but not for women with only one failed embryo transfer. The greatest effect was associated with double luteal endometrial scratch with a pipelle. 26 Reactions caused by endometrial scratch and by endometrial diagnostic biopsy and the extent to which they can be considered as comparable have not been well researched. 39
Chronic endometritis is often associated with the presence of diverse bacterial pathogens, which may desire different antibiotics to treat. 42 , 43 This leads to an unbalanced resident microbiota of the uterus and an abnormal pattern of lymphocyte subset in the endometrium, which may influence reproductive immunology. 44 An invasion of plasma cells and other lymphocytes in the endometrial gland can sometimes be observed before menstruation and is possibly associated with higher uterine contractibility. 45 In case of CE, both cell types are present in numbers greater than normal, independent of the menstrual cycle.
A prospective study analyzing immunologic cells within the endometrium of 178 RIF and 155 RPL women revealed a significantly higher prevalence of uterine natural killer cells (53.2 vs. 45.2 and 42.9%, p < .001) in women with RIF compared with women with RPL. However, in all sub‐fertile populations, the percentage of peripheral uterine natural killer cells was higher (p = .001), as well as CD69+ activation (p = .005). Furthermore, the levels of B cells were higher (p < .001), and the ratio of CD4 to CD8 (p < .001) was elevated. In addition, a higher proportion of CD4 positive Th1+ cells (p = .001) was found. 43 Lastly, defective endometrial prostaglandin synthesis was observed in women with RIF. 46 Based on the presence of uterine natural killer cells and defective prostaglandin synthesis, the different immunological responses in the implantation process seem to be even more unfavorable in women with RIF than in women with RPL.
A recent study looked at the immune status of women with reproductive failure diagnosed for CE. The proportions of uterine CD68+ macrophages, CD83+ mature dendritic cells, CD8+ T cells, and regulatory T cells were significantly elevated in patients with CE, independent of whether they had RIF or RPL. The immunological changes associated with CE may, therefore, be associated with poor endometrial receptivity. 47 , 48 The immunological pattern was normalized after antibiotic treatment. 7 This might strengthen the endometrial receptivity and improve reproductive outcome.
4.1. Strengths and limitations
Our study has two important strengths: (1) differentiated diagnostics with HSC, endometrial biopsy, and immunohistochemically staining for CD138 and (2) an outcome that was not yet investigated in combination with CE, namely chance of pregnancy and live birth over time. The sensitivity of our HSC investigation correlates well with a study published recently. 21
Our study also has limitations. First, it is an observational study with a historical cohort as reference group and a limited sample size. Gain of experience, development of treatment protocols, and embryo culture techniques over time might challenge comparability between the groups. Second, we analyzed patients with RIF and RPL as one group, addressing this problem by stratifying the Cox regression models for RIF and RPL as well as by conducting subgroup analyses. We focused on chance of live birth over time as the main outcome. For the survival curves in Figure 3, we used inverse probability weighting to account for different proportions of RIF and RPL and different age structures of mothers. 37 Live birth as outcome is valid for both conditions, and subgroup analysis confirmed that the procedure is beneficial primarily for women diagnosed and treated for CE (CEpos) independent of whether they had RIF and RPL. The small difference between CEpos and CEneg could indicate, that the biopsy is more important than the antibiotic treatment or, that the treatment success is influenced by the choice of the antibiotic treatment, which was not based on antibiogram. We were interested in the long‐term follow‐up of women with RIF and RPL after the intervention, independent of the course of events between intervention and the recorded outcome. Compared with many other studies looking at one subsequent embryo transfer or the next subsequent pregnancy, our study provides information on the longer‐term perspectives. We did not restrict the observation time under the assumptions that immunological processes may take time and the influence of interventions might be beneficial later.
Our results support the increasing evidence that in women with either RIF or RPL, CE needs to be diagnosed and treated. Our results show in particular the higher chance of live birth at any time point during the longer observation period. However, prospective and randomized studies with the assessment of different intervention in larger number of participants are needed as well as further discussion on the definition of CE.
5. CONCLUSIONS
Diagnostic endometrial biopsy with subsequent antibiotic treatment in case of CE increases chances of pregnancy and live birth compared with women undergoing HSC only. The causal associations between the diagnosis of CE with biopsy with or without antibiotic treatment in case of CE and subsequent fertility remain to be resolved. Therefore, randomized controlled trials with longer follow‐up times are needed to establish standards for diagnosis and treatment of CE and to find therapies for implantation failure in the future. Randomized controlled trials should be conducted in distinct RIF or RPL populations diagnosed with CE. As interventions, endometrial scratch, as well as antibiotic treatment, ideally be guided by antibiogram should be controlled for.
CONFLICT OF INTEREST
The authors report no conflicts of interest.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
The authors are thankful for the support of Merck (Switzerland) SA and IBSA Institute Biochimique SA. They further gratefully acknowledge Kathryn Imboden for the editing of the manuscript, Dave Meier for support in data analysis, Julia Wilhelm for data collection, and Heidrun Janka for literature research.
Mitter VR, Meier S, Rau TT, et al. Treatment following hysteroscopy and endometrial diagnostic biopsy increases the chance for live birth in women with chronic endometritis. Am J Reprod Immunol. 2021;86:e13482. 10.1111/aji.13482
Funding Information
The doctoral research of Vera Mitter is supported by an unrestricted grant from IBSA Institute Biochimique SA. Data collection was supported by an independent medical grant form Merck (Switzerland) AG, Zug, Switzerland. The funding bodies did not have any role in designing or conducting the study or in preparing the manuscript
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Maconochie N, Doyle P, Prior S, Simmons R. Risk factors for first trimester miscarriage‐results from a UK‐population‐based case‐control study. BJOG. 2007;114(2):170‐186. 10.1111/j.1471-0528.2006.01193.x [DOI] [PubMed] [Google Scholar]
- 2. Bender Atik R, Christiansen OB, Elson J, et al. ESHRE guideline: recurrent pregnancy loss. Hum Reprod Open. 2018;2018(2):1‐12. 10.1093/hropen/hoy004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bashiri A, Halper KI, Orvieto R. Recurrent implantation failure‐update overview on etiology, diagnosis, treatment and future directions. Reprod Biol Endocrinol. 2018;16(121):1‐18. 10.1186/s12958-018-0414-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Coughlan C, Ledger W, Wang Q, et al. Recurrent implantation failure: definition and management. Reprod Biomed Online. 2014;28(1):14‐38. 10.1016/j.rbmo.2013.08.011 [DOI] [PubMed] [Google Scholar]
- 5. World Health Organization . Recommended definitions, terminology and format for statistical tables related to the perinatal period and use of a new certificate for cause of perinatal deaths. Acta Obstet Gynecol Scand. 1977;56(3):247‐253. 10.3109/00016347709162009 [DOI] [PubMed] [Google Scholar]
- 6. Vitagliano A, Noventa M, Gizzo S. Autoimmunity, systemic inflammation, and their correlation with repeated implantation failure and recurrent miscarriage: is chronic endometritis the missing piece of the jigsaw? Am J Reprod Immunol. 2017;77(1):e12597. 10.1111/aji.12597 [DOI] [PubMed] [Google Scholar]
- 7. Li Y, Yu S, Huang C, et al. Evaluation of peripheral and uterine immune status of chronic endometritis in patients with recurrent reproductive failure. Fertil Steril. 2020;113(1):187‐196.e1. 10.1016/j.fertnstert.2019.09.001 [DOI] [PubMed] [Google Scholar]
- 8. Groth JV. Chronic endometritis and the plasma cell, fact versus fiction. Fertil Steril. 2018;109(5):788. 10.1016/j.fertnstert.2018.02.116 [DOI] [PubMed] [Google Scholar]
- 9. Kimura F, Takebayashi A, Ishida M, et al. Review: Chronic endometritis and its effect on reproduction. J Obstet Gynaecol Res. 2019;45(5):951‐960. 10.1111/jog.13937 [DOI] [PubMed] [Google Scholar]
- 10. Kitaya K, Takeuchi T, Mizuta S, Matsubayashi H, Ishikawa T. Endometritis, new time, new concepts. Fertil Steril. 2018;110(3):344‐350. 10.1016/j.fertnstert.2018.04.012 [DOI] [PubMed] [Google Scholar]
- 11. Bouet PE, El Hachem H, Monceau E, Gariépy G, Kadoch IJ, Sylvestre C. Chronic endometritis in women with recurrent pregnancy loss and recurrent implantation failure: prevalence and role of office hysteroscopy and immunohistochemistry in diagnosis. Fertil Steril. 2016;105(1):106‐110. 10.1016/j.fertnstert.2015.09.025 [DOI] [PubMed] [Google Scholar]
- 12. El Hachem H, Crepaux V, May‐Panloup P, Descamps P, Legendre G, Bouet PE. Recurrent pregnancy loss: current perspectives. Int J Womens Health. 2017;9:331‐345. 10.2147/IJWH.S100817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vitagliano A, Saccardi C, Litta PS, Noventa M. Chronic endometritis: really so relevant in repeated IVF failure? Am J Reprod Immunol. 2017;78(6):e12758. 10.1111/aji.12758 [DOI] [PubMed] [Google Scholar]
- 14. Liu Y, Chen X, Huang J, et al. Comparison of the prevalence of chronic endometritis as determined by means of different diagnostic methods in women with and without reproductive failure. Fertil Steril. 2018;109(5):832‐839. 10.1016/j.fertnstert.2018.01.022 [DOI] [PubMed] [Google Scholar]
- 15. Yang R, Du X, Wang Y, Song X, Yang Y, Qiao J. The hysteroscopy and histological diagnosis and treatment value of chronic endometritis in recurrent implantation failure patients. Arch Gynecol Obstet. 2014;289(6):1363‐1369. 10.1007/s00404-013-3131-2 [DOI] [PubMed] [Google Scholar]
- 16. McQueen DB, Bernardi LA, Stephenson MD. Chronic endometritis in women with recurrent early pregnancy loss and/or fetal demise. Fertil Steril. 2014;101(4):1026‐1030. 10.1016/j.fertnstert.2013.12.031 [DOI] [PubMed] [Google Scholar]
- 17. Zolghadri J, Momtahan M, Aminian K, Ghaffarpasand F, Tavana Z. The value of hysteroscopy in diagnosis of chronic endometritis in patients with unexplained recurrent spontaneous abortion. Eur J Obstet Gynecol Reprod Biol. 2011;155(2):217‐220. 10.1016/j.ejogrb.2010.12.010 [DOI] [PubMed] [Google Scholar]
- 18. Xu Y, Mei J, Diao L, Li Y, Ding L. Chronic endometritis and reproductive failure: role of syndecan‐1. Am J Reprod Immunol. 2020;84(3): 10.1111/aji.13255 [DOI] [PubMed] [Google Scholar]
- 19. Cicinelli E, Resta L, Nicoletti R, Zappimbulso V, Tartagni M, Saliani N. Endometrial micropolyps at fluid hysteroscopy suggest the existence of chronic endometritis. Hum Reprod. 2005;20(5):1386‐1389. 10.1093/humrep/deh779 [DOI] [PubMed] [Google Scholar]
- 20. Cicinelli E, Resta L, Nicoletti R, et al. Detection of chronic endometritis at fluid hysteroscopy. J Minim Invasive Gynecol. 2005;12:514‐518. 10.1016/j.jmig.2005.07.394 [DOI] [PubMed] [Google Scholar]
- 21. Song D, Li T‐C, Zhang Y, et al. Correlation between hysteroscopy findings and chronic endometritis. Fertil Steril. 2019;111(4):772‐779. 10.1016/j.fertnstert.2018.12.007 [DOI] [PubMed] [Google Scholar]
- 22. Bayer‐Garner IB, Korourian S. Plasma cells in chronic endometritis are easily identified when stained with syndecan‐1. Mod Pathol. 2001;14(9):877‐879. 10.1038/modpathol.3880405 [DOI] [PubMed] [Google Scholar]
- 23. Zargar M, Ghafourian M, Nikbakht R, Mir Hosseini V, Moradi CP. Evaluating chronic endometritis in women with recurrent implantation failure and recurrent pregnancy loss by hysteroscopy and immunohistochemistry. J Minim Invasive Gynecol. 2020;27(1):116‐121. 10.1016/j.jmig.2019.02.016 [DOI] [PubMed] [Google Scholar]
- 24. Chen Y, Fang R, Luo Y, Luo C. Analysis of the diagnostic value of CD138 for chronic endometritis, the risk factors for the pathogenesis of chronic endometritis and the effect of chronic endometritis on pregnancy: a cohort study. BMC Womens Health. 2016;16(1):60. 10.1186/s12905-016-0341-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vitagliano A, Saccardi C, Noventa M, et al. Effects of chronic endometritis therapy on in vitro fertilization outcome in women with repeated implantation failure: a systematic review and meta‐analysis. Fertil Steril. 2018;110(1):103‐112. 10.1016/j.fertnstert.2018.03.017 [DOI] [PubMed] [Google Scholar]
- 26. Vitagliano A, Di Spiezio SA, Saccone G, et al. Endometrial scratch injury for women with one or more previous failed embryo transfers: a systematic review and meta‐analysis of randomized controlled trials. Fertil Steril. 2018;110(4):687‐702.e2. 10.1016/j.fertnstert.2018.04.040 [DOI] [PubMed] [Google Scholar]
- 27. Johnston‐MacAnanny EB, Hartnett J, Engmann LL, Nulsen JC, Sanders MM, Benadiva CA. Chronic endometritis is a frequent finding in women with recurrent implantation failure after in vitro fertilization. Fertil Steril. 2010;93(2):437‐441. 10.1016/j.fertnstert.2008.12.131 [DOI] [PubMed] [Google Scholar]
- 28. Kitaya K, Matsubayashi H, Takaya Y, et al. Live birth rate following oral antibiotic treatment for chronic endometritis in infertile women with repeated implantation failure. Am J Reprod Immunol. 2017;78(5):e12719. 10.1111/aji.12719 [DOI] [PubMed] [Google Scholar]
- 29. Zhang Y, Xu H, Liu Y, et al. Confirmation of chronic endometritis in repeated implantation failure and success outcome in IVF‐ET after intrauterine delivery of the combined administration of antibiotic and dexamethasone. Am J Reprod Immunol. 2019;82(5):e13177. 10.1111/aji.13177 [DOI] [PubMed] [Google Scholar]
- 30. Cicinelli E, Matteo M, Tinelli R, et al. Chronic endometritis due to common bacteria is prevalent in women with recurrent miscarriage as confirmed by improved pregnancy outcome after antibiotic treatment. Reprod Sci. 2014;21(5):640‐647. 10.1177/1933719113508817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Demarmels BF. Significance of coagulopathy in recurrent abortions. Pathophysiology, diagnostics, and therapy. Gynäkologische Endokrinol. 2013;2(11):95‐99. [Google Scholar]
- 32. Steinvil A, Raz R, Berliner S, et al. Association of common thrombophilias and antiphospholipid antibodies with success rate of in vitro fertilisation. Thromb Haemost. 2012;108:1192–1197. 10.1160/TH12-06-0381 [DOI] [PubMed] [Google Scholar]
- 33. Von Wolff M. The role of Natural Cycle IVF in assisted reproduction. Best Pract Res Clin Endocrinol Metab. 2019;33:35‐45. 10.1016/J.BEEM.2018.10.005 [DOI] [PubMed] [Google Scholar]
- 34. van der Linden M, Buckingham K, Farquhar C, Kremer JA, Metwally M. Luteal phase support for assisted reproduction cycles. van der Linden M, ed. Cochrane Database Syst Rev. 2011;(10):Art.No.: CD009154. 10.1002/14651858.CD009154.pub2 [DOI] [PubMed] [Google Scholar]
- 35. Stephenson MD, McQueen D, Winter M, Kliman HJ. Luteal start vaginal micronized progesterone improves pregnancy success in women with recurrent pregnancy loss. Fertil Steril. 2017;107(3):684‐690.e2. 10.1016/j.fertnstert.2016.11.029 [DOI] [PubMed] [Google Scholar]
- 36. Haas DM, Hathaway TJ, Ramsey PS. Progestogen for preventing miscarriage in women with recurrent miscarriage of unclear etiology. Cochrane Database Syst Rev. 2018;(10):Art.No:CD003511. 10.1002/14651858.CD003511.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cole SR, Hernán MA. Adjusted survival curves with inverse probability weights. Comput Methods Programs Biomed. 2004;75(1):45‐49. 10.1016/j.cmpb.2003.10.004 [DOI] [PubMed] [Google Scholar]
- 38. Gnainsky Y, Granot I, Aldo PB, et al. Local injury of the endometrium induces an inflammatory response that promotes successful implantation. Fertil Steril. 2010;94(6):2030‐2036. 10.1016/j.fertnstert.2010.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nastri CO, Gibreel A, Raine‐Fenning N, et al. Endometrial injury in women undergoing assisted reproductive techniques. Cochrane Database Syst Rev. 2012;(7):Art.No:CD009517. 10.1002/14651858.CD009517.pub2 [DOI] [PubMed] [Google Scholar]
- 40. Gnainsky Y, Granot I, Aldo P, et al. Biopsy‐induced inflammatory conditions improve endometrial receptivity: the mechanism of action. Reproduction. 2015;149(1):75‐85. doi: 10.1530/REP-14-0395 [DOI] [PubMed] [Google Scholar]
- 41. Lensen S, Osavlyuk D, Armstrong S, et al. A randomized trial of endometrial scratching before in vitro fertilization. N Engl J Med. 2019;380(4):325‐334. 10.1056/NEJMoa1808737 [DOI] [PubMed] [Google Scholar]
- 42. Baker JM, Chase DM, Herbst‐Kralovetz MM. Uterine microbiota: residents, tourists, or invaders? Front Immunol. 2018;9. 10.3389/fimmu.2018.00208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Marron K, Walsh D, Harrity C. Detailed endometrial immune assessment of both normal and adverse reproductive outcome populations. J Assist Reprod Genet. 2019;36(2):199‐210. 10.1007/s10815-018-1300-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Moreno I, Cicinelli E, Garcia‐Grau I, et al. The diagnosis of chronic endometritis in infertile asymptomatic women: a comparative study of histology, microbial cultures, hysteroscopy, and molecular microbiology. Am J Obstet Gynecol. 2018;218(6):602.e1‐602.e16. 10.1016/j.ajog.2018.02.012 [DOI] [PubMed] [Google Scholar]
- 45. Kitaya K, Yasuo T. Immunohistochemistrical and Clinicopathological Characterization of Chronic Endometritis. Am J Reprod Immunol. 2011;66(5):410‐415. 10.1111/j.1600-0897.2011.01051.x [DOI] [PubMed] [Google Scholar]
- 46. Achache H, Tsafrir A, Prus D, Reich R, Revel A. Defective endometrial prostaglandin synthesis identified in patients with repeated implantation failure undergoing in vitro fertilization. Fertil Steril. 2010;94(4):1271‐1278. 10.1016/j.fertnstert.2009.07.1668 [DOI] [PubMed] [Google Scholar]
- 47. Matteo M, Cicinelli E, Greco P, et al. Abnormal pattern of lymphocyte subpopulations in the endometrium of infertile women with chronic endometritis. Am J Reprod Immunol. 2009;61(5):322‐329. 10.1111/j.1600-0897.2009.00698.x [DOI] [PubMed] [Google Scholar]
- 48. Cicinelli E, Matteo M, Tinelli R, et al. Prevalence of chronic endometritis in repeated unexplained implantation failure and the IVF success rate after antibiotic therapy. Hum Reprod. 2015;30(2):323‐330. 10.1093/humrep/deu292 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


