Abstract
Objective
There are only a few treatment algorithms for first‐episode schizophrenia. Moreover, all the algorithms apply to acute treatment, but not maintenance treatment. Therefore, we aimed to develop acute and maintenance treatment algorithms for first‐episode schizophrenia.
Methods
The algorithm committee of the Japanese Society of Clinical Neuropsychopharmacology developed pharmacological treatment algorithms for the acute phase, agitation, and maintenance phase of first‐episode schizophrenia.
Results
The acute treatment algorithm focuses on drug‐naïve patients with first‐episode schizophrenia who are not old or very agitated and recommends first‐line treatment with aripiprazole, second‐ or third‐line treatment with risperidone/paliperidone or olanzapine, and fourth‐line treatment with clozapine. Long‐acting injection of the current antipsychotic agent can be used for poor medication adherence or based on patient preference. The agitation treatment algorithm recommends first‐line treatment with lorazepam and second‐ or third‐line treatment with quetiapine or levomepromazine and clearly instructs that the medication used for agitation should be reduced and then discontinued after remission of agitation. The maintenance treatment algorithm recommends the gradual reduction of antipsychotics to the minimum effective dose after remission of positive symptoms.
Conclusions
We hope that our unique algorithms will be used broadly and will contribute to minimizing patients' burden related to antipsychotic treatment.
Keywords: algorithm, antipsychotics, first episode, schizophrenia, treatment
1. INTRODUCTION
Implementation of treatment guidelines may improve the quality of clinical practice in mental health (Nguyen et al., 2020). Interestingly, a meta‐analysis of six randomized controlled trials (RCTs) examining the effects of guideline implementation strategies on clinical practice in schizophrenia did not demonstrate robust positive effects (Bighelli et al., 2016). However, a recent study showed that adherence to guideline recommendations reduced medical costs and improved quality‐adjusted life‐years in schizophrenia (Jin et al., 2020).
Treatment algorithms significantly differ from treatment guidelines; the former shows a clear flow of treatment procedure, while the latter lists recommendations for treatment in general. A systematic review identified only a few treatment algorithms for schizophrenia in general, although a substantial number of treatment guidelines are available. To our knowledge, there have been only three and one treatment algorithms designed specifically for first‐episode schizophrenia (Agid et al., 2011; Drosos et al., 2020; Yoshimura et al., 2019) and psychosis (Yeisen et al., 2016), respectively. Moreover, all the algorithms were used for acute treatment, but not for maintenance treatment. Therefore, we aimed to develop both acute and maintenance treatment algorithms for patients with first‐episode schizophrenia. We also aimed to enhance the use of clozapine in Japan through an acute treatment algorithm due to the lower rate of clozapine prescription in Japan when compared to other countries (Bachmann et al., 2017).
2. METHODS
Pharmacological treatment algorithms for first‐episode schizophrenia were developed by the algorithm committee of the Japanese Society of Clinical Neuropsychopharmacology (JSCNP). The JSCNP is made up of approximately 1500 members, including more than 250 psychiatrists certified as specialists in clinical psychopharmacology. The committee consisted of seven members of the Society (all listed as authors). The committee drafted the treatment algorithms for the acute phase, agitation, and maintenance phase of first‐episode schizophrenia through several discussions, referring to relevant recent evidence, including meta‐analyses, systematic reviews, and RCTs. The medications were chosen based on the JSCNP expert consensus pharmacological treatment for schizophrenia (Sakurai et al., 2021). Public hearing was performed with all the members of the JSCNP. The committee revised the algorithms according to the 10 comments made during the public hearing. The vast majority of the comments were on the use of aripiprazole long‐acting injection (LAI) as the maintenance treatment. Finally, the algorithms were approved by the JSCNP executive committee and first published on the JSCNP website (http://www.jscnp.org/algorithm/index.html) on January 19, 2021. The JSCNP algorithm committee continues to update the algorithms.
3. RESULTS
3.1. Pharmacological treatment algorithm for the acute phase of first‐episode schizophrenia
The treatment algorithm for the acute phase of first‐episode schizophrenia is shown in Figure 1.
FIGURE 1.
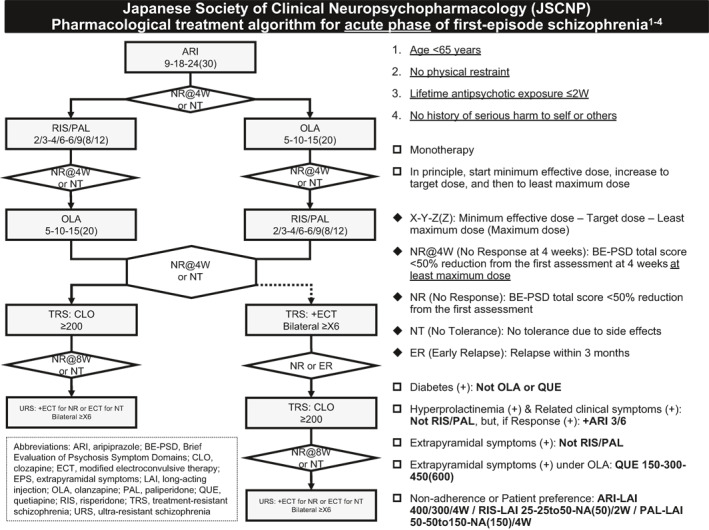
Japanese Society of Clinical Neuropsychopharmacology (JSCNP) pharmacological treatment algorithm for acute phase of first‐episode schizophrenia
The algorithm was designed for outpatients or inpatients with first‐episode schizophrenia who met the following criteria: (1) aged <65 years, (1) those for whom physical restraints are not necessary, (3) those with a ≤2‐week lifetime antipsychotic exposure, and (4) those with no history of serious harm to self or others. Schizophrenia should be diagnosed using standard criteria such as the International Classification of Diseases (ICD) and the Diagnostic and Statistical Manual of Mental Disorders (DSM). Patients with a first episode can be subjected to the algorithm regardless of the duration of untreated psychosis.
The algorithm recommends aripiprazole, risperidone, and olanzapine as antipsychotic monotherapy, which are listed as first‐order drugs for two of four prominent symptoms in the JSCNP expert consensus (Sakurai et al., 2021). The expert consensus was developed by the medical education panel of the JSCNP, which is independent of the algorithm committee. This was achieved through a survey asking the board‐certified experts of the JSCNP about drug choices for clinically relevant issues in the treatment of schizophrenia. Aripiprazole was chosen as a first‐line treatment because (1) the Japanese expert consensus recommended this drug for three of four prominent symptoms in the first order (Sakurai et al., 2021), (2) the drug is associated with the minimum risk of adverse effects (Huhn et al., 2019), and (3) patients with first‐episode schizophrenia respond well to antipsychotic treatment compared to those with multiple‐episode schizophrenia (Takeuchi et al., 2019). The doses of aripiprazole are 9, 18, 24, and 30 mg/day, because tablets of only 1, 3, 6, and 12 mg are available in Japan and the product monograph recommends 6–24 mg/day. In this algorithm, paliperidone, an active metabolite of risperidone, was considered equivalent to risperidone. The dose equivalency of risperidone and paliperidone (1:1.5) was determined following a list made in Japan (Inada & Inagaki, 2015).
The algorithm defined “minimum effective dose” as the lowest dose in the clinically effective dose range, “target dose” as clinically effective dose, and “maximum effective dose” as the highest dose in the clinically effective dose range (Table 1). The minimum effective dose of each antipsychotic agent was determined according to a systematic review that identified the lowest doses that were significantly different from placebo (Leucht et al., 2014). It should be noted that the algorithm adopted the concept of “least maximum dose,” which corresponds to chlorpromazine equivalent 600 mg/day (Inada & Inagaki, 2015), to align with ≥2 antipsychotic trials with chlorpromazine equivalent ≥600 mg/day for ≥4 weeks, required for the use of clozapine in Japan.
TABLE 1.
Minimum effective dose, target dose, least maximum dose, and maximum dose of antipsychotics indicated in the algorithms
| Minimum effective dose | Target dose | Least maximum dose | Maximum dose | |
|---|---|---|---|---|
| Oral formulation | ||||
| Aripiprazole | 9 mg/day | 18 mg/day | 24 mg/day | 30 mg/day |
| Risperidone | 2 mg/day | 4 mg/day | 6 mg/day | 8 mg/day |
| Paliperidone | 3 mg/day | 6 mg/day | 9 mg/day | 12 mg/day |
| Olanzapine | 5 mg/day | 10 mg/day | 15 mg/day | 20 mg/day |
| Clozapine | NA | 200 mg/day | NA | NA |
| Long‐acting injectable formulation | ||||
| Aripiprazole | NA | 400/300 mg/4 weeks | NA | NA |
| Risperidone | 25 mg/2 weeks | 25‐50 mg/2 weeks | NA | 50 mg/2 weeks |
| Paliperidone | 50 mg/4 weeks | 50‐150 mg/4 weeks | NA | 150 mg/4 weeks |
Abbreviation: NA, not applicable.
Treatment non‐response was defined as a <50% reduction from the first assessment in the total score on the Brief Evaluation of Psychosis Symptom Domains (BE‐PSD) (Takeuchi et al., 2016), which can assess five symptom domains of schizophrenia in a short time, referring to a <50% reduction in the total score on the Positive and Negative Syndrome Scale, equivalent to <2 (much improved) on the Clinical Global Impression–Improvement scale (Leucht et al., 2005). Treatment response was evaluated at 4 weeks after treatment with the least maximum dose of an antipsychotic agent, both of which are required for the use of clozapine in Japan, as mentioned above.
If a patient does not respond to aripiprazole, the algorithm recommends switching to risperidone/paliperidone or olanzapine. Clinicians can chose any of the drugs based on their clinical judgement. If a patient does not respond to risperidone/paliperidone or olanzapine, switching to the other is recommended. The algorithm recommends quetiapine for patients with extrapyramidal symptoms under olanzapine treatment. Use of olanzapine or quetiapine is not allowed if a patient has diabetes because these drugs are contraindicated in patients with diabetes in Japan. In addition, the algorithm does not recommend risperidone/paliperidone if a patient has both hyperprolactinemia and related clinical symptoms; however, when the patient responds to risperidone/paliperidone, adjunctive aripiprazole is permitted, which is the sole antipsychotic combination endorsed by the algorithms, as there is sufficient evidence for this strategy (Labad et al., 2020).
The algorithm recommends clozapine for treatment‐resistant schizophrenia (TRS); however, electroconvulsive therapy (ECT) is allowed as an alternative treatment if it is difficult to use clozapine due to the strict regulations in Japan, patient refusal, etc. (Bachmann et al., 2017; Nielsen et al., 2016). Regarding clozapine, ≥200 mg/day is recommended as Asians can be treated with lower doses of clozapine than Americans and Europeans (De Leon et al., 2020) and ≥8 but not 4 weeks is required for the evaluation of the response, as symptom improvement with clozapine is more sustained than with other antipsychotics in TRS (Suzuki et al., 2011). In terms of ECT, ≥6‐time bitemporal ECT is recommended, provided that the current antipsychotic type and dose remain the same. If a patient does not respond to clozapine, the algorithm recommends clozapine together with ECT.
LAI of the current antipsychotic agent can be used for poor medication adherence or based on patient preference. In Japan, only aripiprazole LAI, risperidone LAI, and paliperidone LAI are available.
3.2. Pharmacological treatment algorithm for agitation of first‐episode schizophrenia
The treatment algorithm for the agitation of first‐episode schizophrenia is shown in Figure 2. The acute treatment algorithm excludes patients who need physical restraints and/or have a history of serious harm to themselves or others. However, the remaining patients can manifest agitation to the degree to which they need seclusion.
FIGURE 2.
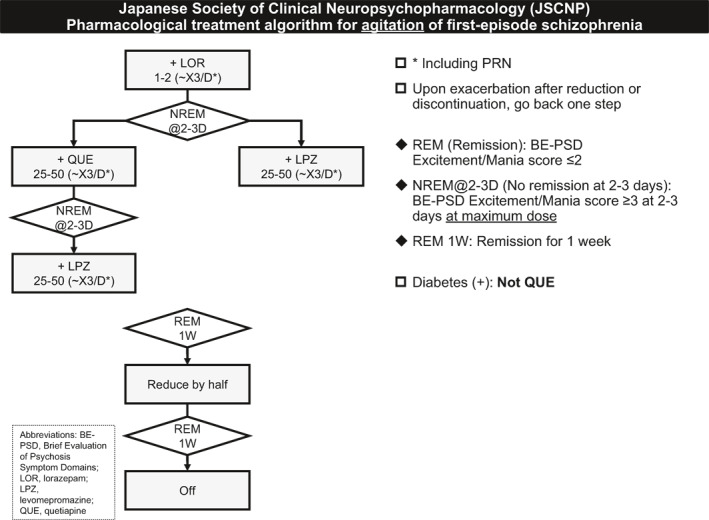
Japanese Society of Clinical Neuropsychopharmacology (JSCNP) pharmacological treatment algorithm for agitation of first‐episode schizophrenia
The algorithm recommends oral lorazepam, quetiapine, and levomepromazine, which are listed as second‐order or higher drugs for two excitement treatment options in the JSCNP expert consensus (Sakurai et al., 2021), as needed or regularly. Lorazepam, the injectable formulation that is indicated only for status epilepticus in Japan, was chosen as a first‐line treatment because it has no affinity for dopamine D2 receptors and its concurrent use does not hinder proper evaluation of the antipsychotic effect of the main agent. Likewise, risperidone and olanzapine were not chosen, regardless of the formulation (e.g., short‐acting injection), because these drugs have moderate‐to‐high affinity for dopamine D2 receptors. The total daily doses of quetiapine and levomepromazine were set to the minimum effective dose or lower. If a patient has diabetes, the use of quetiapine is not allowed because this drug is contraindicated for diabetes in Japan.
The algorithm recommends reducing the medication used for agitation by half 1 week after remission of agitation and then discontinuing it after an additional 1 week if the patient remains in remission to avoid an unnecessary combination of psychotropic medications. Remission of agitation was defined as ≤2 (mild) on excitement/mania of the BE‐PSD (Takeuchi et al, 2016).
3.3. Pharmacological treatment algorithm for the maintenance phase of first‐episode schizophrenia
The treatment algorithm for the maintenance phase of first‐episode schizophrenia is shown in Figure 3.
FIGURE 3.
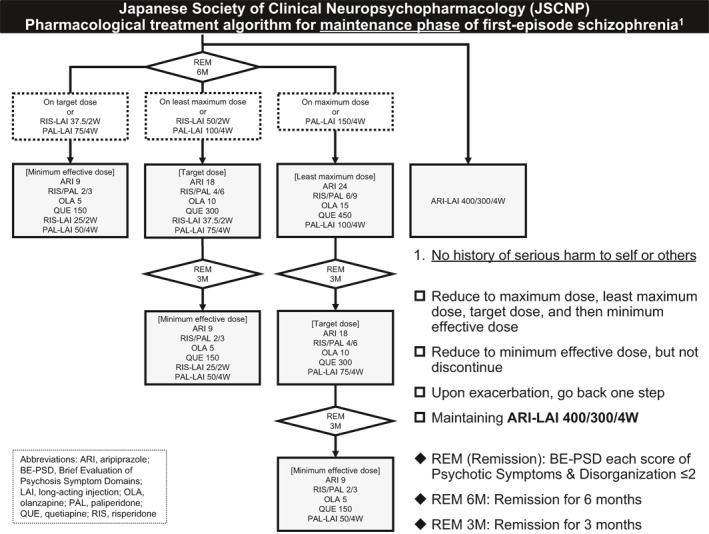
Japanese Society of Clinical Neuropsychopharmacology (JSCNP) pharmacological treatment algorithm for maintenance phase of first‐episode schizophrenia
The algorithm does not recommend discontinuation of antipsychotics for the following reasons. First, it is reported that the discontinuation of antipsychotics is associated with a higher risk of relapse even in first‐episode schizophrenia or psychosis (Kishi et al., 2019; Leucht et al., 2012), several studies are ongoing to address this topic (Begemann et al., 2020; Liu et al., 2021; Moncrieff et al., 2019; Stürup et al., 2017; Weller et al., 2019). Second, there are no established clinical predictors for successful discontinuation of antipsychotics (Bowtell et al., 2018).
On the other hand, the algorithm endorses dose reduction of antipsychotics to the minimum effective dose based on a recent meta‐analysis (Tani et al., 2020). Specifically, the algorithm instructs to begin reducing the dose of the antipsychotic agent used 6 months after remission of positive symptoms and then further reducing the dose every 3 months if the patient remains in remission (e.g., if a patient is treated at a maximum dose, reducing first to the least maximum dose, next to the targeted dose after 3 months, and then to the minimum effective dose after an additional 3 months). If the patient experiences exacerbation, the algorithm recommends a dose increase back to one step, which has been demonstrated to be effective in many trials (Tani et al., 2020). In the case of exacerbation upon dose reduction of LAIs, adding an oral formulation of the same drug is recommended, as dose increase of LAIs does not contribute to a rapid elevation in blood concentration of antipsychotics or a prompt improvement of exacerbation. To minimize the risk of dose reduction, the algorithm targets only patients with no history of serious harm to self or others. In addition, aripiprazole LAI is excluded from antipsychotics targeted at dose reduction, because the product monograph clearly states that this drug is administered at 400 or 300 mg every 4 weeks. Remission of positive symptoms was defined as ≤2 (mild) on both psychotic symptoms and disorganized thought of the BE‐PSD (Takeuchi et al, 2016).
4. DISCUSSION
We developed pharmacological treatment algorithms for the acute phase, agitation, and maintenance phase of first‐episode schizophrenia. Our treatment algorithms are unique, especially in that (1) they mention not only acute treatment but also maintenance treatment, (2) they clearly instruct that the concurrent medication used for agitation is reduced and then discontinued after remission of agitation, and (3) they recommend that antipsychotics be gradually reduced to the minimum effective dose after remission of positive symptoms. It should be noted that we aimed to develop algorithms to show standard treatment strategies for a limited population of drug‐naïve, not elderly, and not very agitated (i.e., those not requiring physical restraint and with no history of serious harm to self or others) patients with first‐episode schizophrenia. Thus, these algorithms cannot be applied to all patients with first‐episode schizophrenia. Moreover, clinicians are allowed to deviate from the algorithms whenever they judge that following the algorithms is difficult. This is because there are several second‐generation antipsychotics (SGAs) available in Japan other than those indicated in the algorithms such as asenapine, blonanserin, brexpiprazole, lurasidone, and perospirone.
The acute treatment algorithm recommends only aripiprazole as a first‐line antipsychotic agent, which is different from other treatment algorithms for first‐episode schizophrenia/psychosis (Agid et al., 2011; Drosos et al., 2020; Yeisen et al., 2016; Yoshimura et al., 2019). We prioritized minimizing adverse effects over maximizing efficacy for the first place, because long‐term treatment with antipsychotics is needed to prevent relapse and exacerbation in patients with schizophrenia (Leucht et al., 2012; Takeuchi et al, 2017). Nonetheless, recent RCTs demonstrated no difference in efficacy between aripiprazole and other SGAs in the acute phase of first‐episode schizophrenia or psychosis (Cheng et al., 2019; Robinson et al., 2015; C. Wang et al., 2017). Moreover, a 12‐week double‐blind RCT showed that aripiprazole was not inferior to risperidone for response of positive symptoms in 198 patients with first‐episode psychosis (Robinson et al., 2015), while a 6‐week open‐label RCT reported that aripiprazole was inferior to risperidone for reduction in total symptoms in 266 patients with first‐episode psychosis (Gómez‐Revuelta et al., 2021). These RCTs showed that aripiprazole was associated with less metabolic disturbance, and hyperprolactinemia and related symptoms than other SGAs (Cheng et al., 2019; Gómez‐Revuelta et al., 2021; Robinson et al., 2015; C. Wang et al., 2017).
As stated in the introduction, one of the purposes of developing these algorithms was to enhance the use of clozapine in Japan. Clozapine is underutilized in Japan compared to other countries (Bachmann et al., 2017) as it was introduced in Japan only 12 years ago, and it is regulated very strictly, including requirements for long‐term hospitalization and frequent blood monitoring (Bachmann et al., 2017; Nielsen et al., 2016). Furthermore, the criteria for the use of clozapine are also strict; the product monograph clearly states that ≥2 antipsychotic trials with chlorpromazine equivalent ≥600 mg/day for ≥4 weeks are required before introducing clozapine. Given the evidence that delay in clozapine initiation is associated with a poor response to clozapine in TRS (Shah et al., 2018), clozapine should be used as early as possible after a patient meets the criteria of TRS. Furthermore, a recent RCT suggested that clozapine could be used as a second‐line treatment in first‐episode schizophrenia (Kahn et al., 2018). Nonetheless, our algorithm positioned clozapine as the fourth‐line drug because we recommended aripiprazole, a partial agonist of dopamine D2 receptors, as a first‐line treatment, which is different from other antipsychotics. It is also thought that it would be difficult for clinicians in Japan to accept such a dramatic change in clinical practice, considering the current situation of clozapine underutilization.
The maintenance treatment algorithm recommends dose reduction of SGAs, which is consistent with the current evidence. Recent clinical guidelines for schizophrenia tend to shift to endorsing dose reduction of antipsychotics in the maintenance treatment (Shimomura et al., 2020), given the fact that SGAs are associated with dose‐dependent adverse effects (Yoshida & Takeuchi, 2021) and accumulating evidence from RCTs examining dose reduction of SGAs (Rouillon, Chartier, & Gasquet, 2008; Takeuchi et al., 2013; Wang et al., 2010; Zhou, Li, Li, Cui, & Ning, 2018). In addition, a recent meta‐analysis of RCTs revealed that dose reduction up to chlorpromazine equivalent 200 mg/day was not associated with a higher relapse risk but with improvement in negative symptoms, extrapyramidal symptoms, and neurocognitive impairment (Tani et al., 2020). On the other hand, several issues remain to be addressed regarding dose reduction of antipsychotics, such as the timing and speed of dose reduction (Shimomura et al., 2020). We recommended starting dose reduction 6 months after remission of positive symptoms and a gradual reduction by every 3 months, according to some recent clinical guidelines (note: all of them only said “gradually” in terms of the speed of dose reduction) (Shimomura et al., 2020).
4.1. Limitations
There are several limitations to the current treatment algorithms. First, some recommendations were not based on evidence, but by committee member consensus. Furthermore, a survey of the board‐certified experts of the JSCNP was not performed, as in the case with the JSCNP expert consensus (Sakurai et al., 2021), although the medications for the acute treatment and agitation were chosen based on the JSCNP expert consensus. Second, the algorithms may not be suitable for clinical settings in other countries because we developed them, considering the current situations of clinical practice in Japan. For instance, in addition to the issues with clozapine such as the delay in the introduction and the strict regulations as described above, amisulpride, ziprasidone, iloperidone, cariprazine, and olanzapine LAI are not available. Besides, olanzapine and quetiapine are contraindicated in patients with diabetes. Third, the algorithms did not cover all areas of treatment, such as strategies after failure of clozapine plus ECT. Finally, it should be noted that the algorithms focused on pharmacological treatments but do not ignore the importance of non‐pharmacological treatments such as cognitive behavioral therapy and cognitive remediation therapy.
5. CONCLUSIONS
We developed treatment algorithms for the acute phase, agitation, and maintenance phase of first‐episode schizophrenia. We intend not to force but to encourage clinicians to follow standard treatment strategies by utilizing these algorithms. Treatment should be optimized according to each patient's background and characteristics. We hope that our algorithms will be used broadly and will contribute to minimizing patients' burden related to psychosis and antipsychotic treatment.
CONFLICT OF INTEREST
Dr. Takeuchi has received speaker's fees from EA Pharma, Kyowa, Janssen, Lundbeck, Meiji Seika Pharma, Mochida, Otsuka, Sumitomo Dainippon Pharma, Takeda, and Yoshitomiyakuhin.
Dr. Takekita has received grant funding from the Japan Society for the Promotion of Science, and speaker's honoraria from Daiichi Sankyo, Eisai, Janssen, Meiji Seika Pharma, MSD, Novartis Pharma, Ono, Otsuka, Pfizer, Sumitomo Dainippon Pharma, and UCB Japan.
Dr. Hori has received speaker's honoraria from Eisai, Eli Lilly, Janssen, Meiji Seika Pharma, Otsuka, Pfizer, Sumitomo Dainippon Pharma, and Takeda.
Dr. Oya has received speaker's honoraria from Eisai, Kissei, Kyowa, Meiji Seika Pharma, Mochida, Otsuka, Sumitomo Dainippon Pharma, Takeda, Tanabe‐Mitsubishi, and Viatris.
Dr. Miura has received speaker's honoraria from Daiichi Sankyo, Janssen, Meiji Seika Pharma, Mochida, MSD, Mylan, Otsuka, Pfizer, Sumitomo Dainippon Pharma, and Takeda.
Dr. Hashimoto has received speaker's honoraria from Janssen, Meiji Seika Pharma, Novartis Pharma, Otsuka, Sumitomo Dainippon Pharma, and Yoshitomiyakuhin.
Dr. Yasui‐Furukori has received speaker's fees from Meiji Seika Pharma, Mochida, Otsuka, and Sumitomo Dainippon Pharma.
Takeuchi, H. , Takekita, Y. , Hori, H. , Oya, K. , Miura, I. , Hashimoto, N ., & Yasui‐Furukori, N . (2021). Pharmacological treatment algorithms for the acute phase, agitation, and maintenance phase of first‐episode schizophrenia: Japanese Society of Clinical Neuropsychopharmacology treatment algorithms. Human Psychopharmacology: Clinical and Experimental, 36(6), e2804. 10.1002/hup.2804
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
REFERENCES
- Agid, O. , Arenovich, T. , Sajeev, G. , Zipursky, R. B. , Kapur, S. , Foussias, G. , & Remington, G. (2011). An algorithm‐based approach to first‐episode schizophrenia: Response rates over 3 prospective antipsychotic trials with a retrospective data analysis. Journal of Clinical Psychiatry, 72(11), 1439–1444. 10.4088/JCP.09m05785yel [DOI] [PubMed] [Google Scholar]
- Bachmann, C. J. , Aagaard, L. , Bernardo, M. , Brandt, L. , Cartabia, M. , Clavenna, A. , Furu, K. , Garuoliené, K. , Hoffmann, F. , Hollingworth, S. , Huybrechts, K. F. , Kalverdijk, L. J. , Kawakami, K. , Kieler, H. , Kinoshita, T. , López, S. C. , Machado‐Alba, J. E. , Machado‐Duque, M. E. , Mahesri, M. , … Taylor, D. (2017). International trends in clozapine use: A study in 17 countries. Acta Psychiatrica Scandinavica, 136(1), 37–51. 10.1111/acps.12742 [DOI] [PubMed] [Google Scholar]
- Begemann, M. J. H. , Thompson, I. A. , Veling, W. , Gangadin, S. S. , Geraets, C. N. W. , Van 't Hag, E. , Müller‐Kuperus, S. J. , Oomen, P. P. , Voppel, A. E. , van der Gaag, M. , Kikkert, M. J. , Van Os, J. , Smit, H. F. E , Knegtering, R. H. , Wiersma, S. , Stouten, L. H. , Gijsman, H. J. , Wunderink, L. , & Staring, A. B. P. , et al. (2020). To continue or not to continue? Antipsychotic medication maintenance versus dose‐reduction/discontinuation in first episode psychosis: HAMLETT, a pragmatic multicenter single‐blind randomized controlled trial. Trials, 21(1). 10.1186/s13063-019-3822-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bighelli, I. , Ostuzzi, G. , Girlanda, F. , Cipriani, A. , Becker, T. , Koesters, M. , & Barbui, C . (2016). Implementation of treatment guidelines for specialist mental health care. Cochrane Database of Systematic Reviews. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowtell, M. , Ratheesh, A. , McGorry, P. , Killackey, E. , & O'Donoghue, B. (2018). Clinical and demographic predictors of continuing remission or relapse following discontinuation of antipsychotic medication after a first episode of psychosis. A systematic review. Schizophrenia Research, 197, 9–18. 10.1016/j.schres.2017.11.010 [DOI] [PubMed] [Google Scholar]
- Cheng, Z. , Yuan, Y. , Han, X. , Yang, L. , Cai, S. , Yang, F. , Wang, C. , Deng, H. , Zhao, J. , Xiang, Y. , Correll, C. U. , & Yu, X. (2019). An open‐label randomised comparison of aripiprazole, olanzapine and risperidone for the acute treatment of first‐episode schizophrenia: Eight‐week outcomes. Journal of Psychopharmacology, 33(10), 1227–1236. 10.1177/0269881119872193 [DOI] [PubMed] [Google Scholar]
- De Leon, J. , Rajkumar, A. , Kaithi, A. , Schoretsanitis, G. , Kane, J. , Wang, C. Y. , Lin, S.‐K. , Hong, K. S. , Farooq, S. , Ng, C. H. , Ruan, C. J. , & Andrade, C. (2020, January 1). Do Asian patients require only half of the clozapine dose prescribed for Caucasians? A critical overview. Indian Journal of Psychological Medicine, 42, 4–10. 10.4103/IJPSYM.IJPSYM_379_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drosos, P. , Brønnick, K. , Joa, I. , Johannessen, J. O. , Johnsen, E. , Kroken, R. A. , Stain, H. J. , Hegelstad, W. T. V ., & Larsen, T. K. (2020). One‐year outcome and adherence to pharmacological guidelines in first‐episode schizophrenia: Results from a consecutive cohort study. Journal of Clinical Psychopharmacology, 40(6), 534–540. 10.1097/JCP.0000000000001303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez‐Revuelta, M. , Pelayo‐Terán, J. M. , Vázquez‐Bourgon, J. , Ortiz‐García de la Foz, V. , Mayoral‐van Son, J. , Ayesa‐Arriola, R. , & Crespo‐Facorro, B. (2021). Aripiprazole vs risperidone for the acute‐phase treatment of first‐episode psychosis: A 6‐week randomized, flexible‐dose, open‐label clinical trial. European Neuropsychopharmacology, 47, 74–85. 10.1016/j.euroneuro.2021.02.009 [DOI] [PubMed] [Google Scholar]
- Huhn, M. , Nikolakopoulou, A. , Schneider‐Thoma, J. , Krause, M. , Samara, M. , Peter, N. , Bäckers, L. , Rothe, P. , Cipriani, A. , Davis, J. , Salanti, G. , & Leucht, S. (2019). Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi‐episode schizophrenia: A systematic review and network meta‐analysis. The Lancet, 394(10202), 939–951. 10.1016/S0140-6736(19)31135-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inada, T. , & Inagaki, A. (2015). Psychotropic dose equivalence in Japan. Psychiatry and Clinical Neurosciences, 69(8), 440–447. 10.1111/pcn.12275 [DOI] [PubMed] [Google Scholar]
- Jin, H. , Tappenden, P. , MacCabe, J. H. , Robinson, S. , McCrone, P. , & Byford, S. (2020). Cost and health impacts of adherence to the National Institute for Health and Care Excellence schizophrenia guideline recommendations. The British Journal of Psychiatry, 1, 224–229. 10.1192/bjp.2020.241 [DOI] [PubMed] [Google Scholar]
- Kahn, R. S. , Winter van Rossum, I. , Leucht, S. , McGuire, P. , Lewis, S. W. , Leboyer, M. , Dazzan, P. , Drake, R. , Heres, S. , Díaz‐Caneja, C. M. , Rujescu, D. , Weiser, M. , Galderisi, S. , Glenthøj, B. , Eijkemans, M. J. C. , Fleischhacker, W. W. , Kapur, S. , Sommer, I. E. , … Eijkemans, M. J. C. (2018). Amisulpride and olanzapine followed by open‐label treatment with clozapine in first‐episode schizophrenia and schizophreniform disorder (OPTiMiSE): A three‐phase switching study. The Lancet Psychiatry, 5(10), 797–807. 10.1016/S2215-0366(18)30252-9 [DOI] [PubMed] [Google Scholar]
- Kishi, T. , Ikuta, T. , Matsui, Y. , Inada, K. , Matsuda, Y. , Mishima, K. , & Iwata, N. (2019). Effect of discontinuation v. maintenance of antipsychotic medication on relapse rates in patients with remitted/stable first‐episode psychosis: A meta‐analysis. Psychological Medicine, 49(5), 772–779. 10.1017/S0033291718001393 [DOI] [PubMed] [Google Scholar]
- Labad, J. , Montalvo, I. , González‐Rodríguez, A. , García‐Rizo, C. , Crespo‐Facorro, B. , Monreal, J. A. , & Palao, D. (2020, August 1). Pharmacological treatment strategies for lowering prolactin in people with a psychotic disorder and hyperprolactinaemia: A systematic review and meta‐analysis. Schizophrenia Research, 222, 88–96. 10.1016/j.schres.2020.04.031 [DOI] [PubMed] [Google Scholar]
- Leucht, S. , Kane, J. M. , Kissling, W. , Hamann, J. , Etschel, E. , & Engel, R. R. (2005). What does the PANSS mean? Schizophrenia Research, 79(2–3), 231–238. 10.1016/j.schres.2005.04.008 [DOI] [PubMed] [Google Scholar]
- Leucht, S. , Samara, M. , Heres, S. , Patel, M. X. , Woods, S. W. , & Davis, J. M. (2014). Dose equivalents for second‐generation antipsychotics: The minimum effective dose method. Schizophrenia Bulletin, 40(2), 314–326. 10.1093/schbul/sbu001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leucht, S. , Tardy, M. , Komossa, K. , Heres, S. , Kissling, W. , Salanti, G. , & Davis, J. M. (2012). Antipsychotic drugs versus placebo for relapse prevention in schizophrenia: A systematic review and meta‐analysis. Lancet, 379(9831), 2063–2071. 10.1016/S0140-6736(12)60239-6 [DOI] [PubMed] [Google Scholar]
- Liu, C.‐C. , Hsieh, M. H. , Chien, Y.‐L. , Liu, C.‐M. , Lin, Y.‐T. , Hwang, T.‐J. , & Hwu, H.‐G. (2021). Protocol of guided antipsychotic reduction to reach minimum effective dose (GARMED) in patients with remitted psychosis based on pragmatic design. Early Intervention in Psychiatry. 10.1111/eip.13144 [DOI] [PubMed] [Google Scholar]
- Moncrieff, J. , Lewis, G. , Freemantle, N. , Johnson, S. , Barnes, T. R. E. , Morant, N. , Hunter, R. , Kent, L. J. , Smith, R. , Darton, K. , Horne, R. , Crellin, N. E. , Cooper, R. E. , & Priebe, S. (2019). Randomised controlled trial of gradual antipsychotic reduction and discontinuation in people with schizophrenia and related disorders: The RADAR trial (Research into Antipsychotic Discontinuation and Reduction). BMJ Open, 9(11), e030912. 10.1136/bmjopen-2019-030912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen, T. , Seiler, N. , Brown, E. , & O'Donoghue, B. (2020, February 1). The effect of clinical practice guidelines on prescribing practice in mental health: A systematic review, Psychiatry Research, 284, 112671. 10.1016/j.psychres.2019.112671 [DOI] [PubMed] [Google Scholar]
- Nielsen, J. , Young, C. , Ifteni, P. , Kishimoto, T. , Xiang, Y. T. , Schulte, P. F. J. , & Taylor, D. (2016, February 1). Worldwide differences in regulations of clozapine use. CNS Drugs, 30, 149–161. 10.1007/s40263-016-0311-1 [DOI] [PubMed] [Google Scholar]
- Robinson, D. G. , Gallego, J. A. , John, M. , Petrides, G. , Hassoun, Y. , Zhang, J. P. , Braga, R. J. , Sevy, S. M. , Addington, J. , Kellner, C. H. , Tohen, M. , Naraine, M. , Bennett, N. , Greenberg, J. , Lencz, T. , Correll, C. U. , Kane, J. M. , & Malhotra, A. K. (2015). A randomized comparison of aripiprazole and risperidone for the acute treatment of first‐episode schizophrenia and related disorders: 3‐Month outcomes. Schizophrenia Bulletin, 41(6), 1227–1236. 10.1093/schbul/sbv125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouillon, F. , Chartier, F. , & Gasquet, I. (2008). Strategies of treatment with olanzapine in schizophrenic patients during stable phase: Results of a pilot study. European Neuropsychopharmacology, 18(9), 646–652. 10.1016/j.euroneuro.2008.04.012 [DOI] [PubMed] [Google Scholar]
- Sakurai, H. , Yasui‐Furukori, N. , Suzuki, T. , Uchida, H. , Baba, H. , Watanabe, K. , Kikuchi, Y. S. , Kikuchi, T. , Katsuki, A. , Kishida, I. , & Kato, M. (2021). Pharmacological treatment of schizophrenia: Japanese expert consensus. Pharmacopsychiatry, 54(2), 60–67. 10.1055/a-1324-3517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah, P. , Iwata, Y. , Plitman, E. , Brown, E. E. , Caravaggio, F. , Kim, J. , Hahn, M. , Remington, G. , Gerretsen, P. , & Graff‐Guerrero, A. (2018). The impact of delay in clozapine initiation on treatment outcomes in patients with treatment‐resistant schizophrenia: A systematic review. Psychiatry Research, 268, 114–122. 10.1016/j.psychres.2018.06.070 [DOI] [PubMed] [Google Scholar]
- Shimomura, Y. , Kikuchi, Y. , Suzuki, T. , Uchida, H. , Mimura, M. , & Takeuchi, H. (2020). Antipsychotic treatment in the maintenance phase of schizophrenia: An updated systematic review of the guidelines and algorithms. Schizophrenia Research, 215, 8–16. 10.1016/j.schres.2019.09.013 [DOI] [PubMed] [Google Scholar]
- Stürup, A. E. , Jensen, H. D. , Dolmer, S. , Birk, M. , Albert, N. , Nielsen, M. , Eplov, L. , Ebdrup, B. H. , Mors, O. , & Nordentoft, M. (2017). TAILOR – Tapered discontinuation versus maintenance therapy of antipsychotic medication in patients with newly diagnosed schizophrenia or persistent delusional disorder in remission of psychotic symptoms: Study protocol for a randomized clinical trial. Trials, 18(1). 10.1186/s13063-017-2172-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki, T. , Remington, G. , Arenovich, T. , Uchida, H. , Agid, O. , Graff‐Guerrero, A. , & Mamo, D. C. (2011). Time course of improvement with antipsychotic medication in treatment‐resistant schizophrenia. British Journal of Psychiatry, 199(4), 275–280. 10.1192/bjp.bp.110.083907 [DOI] [PubMed] [Google Scholar]
- Takeuchi, H. , Suzuki, T. , Remington, G. , Bies, R. R. , Abe, T. , Graff‐Guerrero, A. , Mimura, M. , & Uchida, H. (2013). Effects of risperidone and olanzapine dose reduction on cognitive function in stable patients with schizophrenia: An open‐label, randomized, controlled, pilot study. Schizophrenia Bulletin, 39(5), 993–998. 10.1093/schbul/sbt090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi, H. , Fervaha, G. , Lee, J. , Agid, O. , & Remington, G. (2016). A preliminary examination of the validity and reliability of a new brief rating scale for symptom domains of psychosis: Brief Evaluation of Psychosis Symptom Domains (BE‐PSD). Journal of Psychiatric Research, 80, 87–92. 10.1016/j.jpsychires.2016.06.005 [DOI] [PubMed] [Google Scholar]
- Takeuchi, H. , Kantor, N. , Sanches, M. , Fervaha, G. , Agid, O. , & Remington, G. (2017). One‐year symptom trajectories in patients with stable schizophrenia maintained on antipsychotics versus placebo: Meta‐analysis. The British Journal of Psychiatry, 211(3), 137–143. 10.1192/bjp.bp.116.186007 [DOI] [PubMed] [Google Scholar]
- Takeuchi, H. , Siu, C. , Remington, G. , Fervaha, G. , Zipursky, R. B. , Foussias, G. , & Agid, O. (2019). Does relapse contribute to treatment resistance? Antipsychotic response in first‐ vs. second‐episode schizophrenia. Neuropsychopharmacology, 44(6), 1036–1042. 10.1038/s41386-018-0278-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tani, H. , Takasu, S. , Uchida, H. , Suzuki, T. , Mimura, M. , & Takeuchi, H. (2020). Factors associated with successful antipsychotic dose reduction in schizophrenia: A systematic review of prospective clinical trials and meta‐analysis of randomized controlled trials. Neuropsychopharmacology, 45, 887–901. 10.1038/s41386-019-0573-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, C. , Shi, W. , Huang, C. , Zhu, J. , Huang, W. , & Chen, G. (2017). The efficacy, acceptability, and safety of five atypical antipsychotics in patients with first‐episode drug‐naïve schizophrenia: A randomized comparative trial. Annals of General Psychiatry, 16(1). 10.1186/s12991-017-0170-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, C. Y. , Xiang, Y. T. , Cai, Z. J. , Weng, Y. Z. , Bo, Q. J. , Zhao, J. P. , Wang, G.‐H. , Weng, S.‐M. , Zhang, H.‐Y. , Chen, D.‐F. , Tang, W.‐K. , & Ungvari, G. S. (2010). Risperidone maintenance treatment in schizophrenia: A randomized, controlled trial. American Journal of Psychiatry, 167(6), 676–685. 10.1176/appi.ajp.2009.09030358 [DOI] [PubMed] [Google Scholar]
- Weller, A. , Gleeson, J. , Alvarez‐Jimenez, M. , McGorry, P. , Nelson, B. , Allott, K. , Bartholomeusz, C. , Koval, P. , Harrigan, S. , O'Donoghue, B. , Fornito, A. , Pantelis, C. , Paul Amminger, G. , Ratheesh, A. , Polari, A. , Wood, S. J. , van der El, K. , Ellinghaus, C. , Gates, J. , … Killackey, E. (2019). Can antipsychotic dose reduction lead to better functional recovery in first‐episode psychosis? A randomized controlled‐trial of antipsychotic dose reduction. The reduce trial: Study protocol. Early Intervention in Psychiatry, 13(6), 1345–1356. 10.1111/eip.12769 [DOI] [PubMed] [Google Scholar]
- Yeisen, R. A. H. , Joa, I. , Johannessen, J. O. , & Opjordsmoen, S. (2016). Use of medication algorithms in first episode psychosis: A naturalistic observational study. Early Intervention in Psychiatry, 10(6), 503–510. 10.1111/eip.12203 [DOI] [PubMed] [Google Scholar]
- Yoshida, K. , & Takeuchi, H. (2021). Dose‐dependent effects of antipsychotics on efficacy and adverse effects in schizophrenia. Behavioural Brain Research, 402, 113098. 10.1016/j.bbr.2020.113098 [DOI] [PubMed] [Google Scholar]
- Yoshimura, B. , Sato, K. , Takaki, M. , & Yamada, N. (2019). Algorithm‐based pharmacotherapy for first‐episode schizophrenia involuntarily hospitalized: A retrospective analysis of real‐world practice. Early Intervention in Psychiatry, 13(1), 39–46. 10.1111/eip.12442 [DOI] [PubMed] [Google Scholar]
- Zhou, Y. , Li, G. , Li, D. , Cui, H. , & Ning, Y. (2018). Dose reduction of risperidone and olanzapine can improve cognitive function and negative symptoms in stable schizophrenic patients: A single‐blinded, 52‐week, randomized controlled study. Journal of Psychopharmacology, 32(5), 524–532. 10.1177/0269881118756062 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.


