Abstract
The prezizane‐type sesquiterpene agarozizanol B was synthesized employing a photochemical cascade reaction as the key step. Starting from a readily available 1‐indanone with a tethered olefin, a strained tetracyclic skeleton was assembled which contained all carbon atoms of the sesquiterpene with the correct relative configuration. The conversion into the tricyclic prezizane skeleton was accomplished by a strategic cyclopropane bond cleavage. Prior to the cyclopropane ring opening an adaption of the oxidation state was required, which could be combined with a reductive resolution step. After removal of two functional groups, the natural product was obtained both in racemic form or, if resolved, as the (+)‐enantiomer which was shown to be identical to the natural product.
Keywords: cycloaddition, diastereoselectivity, domino reactions, photochemistry, terpenoids, total synthesis
Starting from a simple indanone derivative, diastereomerically pure intermediate 1 was formed in a photochemical cascade and served as precursor to agarozizanol B (2), either as racemate or as single enantiomer after a resolution step.
The plethora of products originating from farnesyl pyrophosphate impressively demonstrates the enormous structural diversity, that nature achieves from simple precursors. [1] In multiple reaction sequences, molecular skeletons are created with a highly diversified set of C−C bonds and carbocyclic rings. In fact, sesquiterpenes represent the structurally most complex class of all terpenoids and have provided organic chemists with an abundant playground to probe new synthetic methods and strategies. [2] In the present study, the focus is on the synthesis of sesquiterpenes with a prezizane skeleton. Members of this family such as (+)‐prezizaene (Scheme 1, 1), and (+)‐jinkohol II (2) were isolated from vetiver roots (vetiver oil) and from agarwood. [3] The enantiomeric (−)‐form of 1 and (−)‐prezizanol (3) were found in the oil of Eremophila georgei, a flowering plant of the figwort family.[ 4 , 5 ] More recently, di‐ and trioxygenated sesquiterpenes with a prezizane skeleton (agarozizanols, [6] aquilarenes [7] ) were isolated from agarwood and arose some interest due to their activity as α‐glucosidase inhibitors.
Scheme 1.
Structure of the naturally occurring prezizane‐type sesquiterpenes (+)‐prezizaene (1), (+)‐jinkohol II (2), (−)‐prezizanol (3), (+)‐agarozizanol B (4), and retrosynthetic access to the tricyclic prezizane skeleton I by bond cleavage from a strained tetracyclic precursor. The bond set for the precursor (new bonds in gray) requires to bring in carbon atoms C6, C7, and C13‐C15 by a tethered trisubstituted olefin (tether indicated as dashed line).
The key element of the prezizane skeleton is the tricyclo[6.2.1.01,5]undecane core which carries the remaining four carbon atoms of the sesquiterpene unit at carbon atoms C2, C6 (twice), and C7. Apart from the construction of the existing stereogenic centers, the formation of the bonds at the quaternary carbon atom C1 and the introduction of the bridging carbon atoms C9 and C10 pose structural challenges which need to be met by total synthetic efforts. Followed by the first total synthesis of (−)‐prezizaene and (−)‐prezizanol by Vettel and Coates, [8] syntheses of compounds 1–3 were achieved by the groups of Piers, [9] Mori, [10] and Rao. [11] All syntheses had to pay tribute to the complexity of the molecules by a relative large number of steps (>15) in the longest linear sequence. Banwell and co‐workers reported an elegant approach to (−)‐prezizaene based on a cationic rearrangement of khusiol. [12] However, khusiol was prepared in a 17‐step sequence starting from a chiral 1,2‐dihydrocatechol. [13] The more highly oxygenated prezizane skeletons of the agarozizanols and aquilarenes have not yet been approached by total synthesis.
We envisaged a facile and concise access to compounds with a prezizane skeleton I (Scheme 1) from a strained tetracyclic precursor which we hoped to access by a recently discovered photochemical cascade reaction.[ 14 , 15 ] Cleavage of the indicated bond [16] would allow for immediate formation of the skeleton with all 15 carbon atoms already present in the key intermediate. We now report the successful implementation of this strategy which allowed us to access agarozizanol B (4) [6] both in racemic and in enantiopure form. The absolute configuration of its naturally occurring (+)‐enantiomer (+)‐4 could be established.
As indicated in Scheme 1, the reaction cascade required to tether the reactive component that brings in carbon atoms C6, C7, and C13‐C15 to an existing photoactive building block which carries the remaining ten carbon atoms of the sesquiterpene skeleton. Following our retrosynthetic considerations, the latter building block is represented by an indanone with a methyl substituent at the carbon atom which would be C2 in the natural product. Gratifyingly, an access to this starting material in racemic form (rac‐5, Scheme 2) has been reported and relies on a tandem Friedel–Crafts acylation/alkylation of phenol with γ‐butyrolactone. [17] Compound rac‐5 required attachment of an olefin component representing the above mentioned carbon atoms. Initial attempts to employ a carbonate (‐OCOO‐) or a dioxysilyl (‐OSiR2O‐; R=Me, Ph, i Pr) linker remained unsuccessful due to the insufficient reactivity of the compounds in the ensuing photochemical reaction. Instead, alkylation of phenol rac‐5 with the chloromethylether 6 (CAUTION!) [18] delivered in reasonable yield the precursor rac‐7 for the photochemical cascade reaction. It should be noted that the combination of the dioxymethyl (‐OCH2O‐) linker with a trisubstituted alkene and a chiral indanone is without precedence. We were consequently pleased to note that irradiation at λ=350 nm for 24 h led to the desired product rac‐11 with exquisite control of the constitution and relative configuration.
Scheme 2.
Synthesis of photosubstrate rac‐7 and formation of the desired product rac‐11 in a cascade reaction that includes three photochemical steps. Two carbon atoms are marked to facilitate a better understanding of the skeletal rearrangements. The relative configuration of three new stereogenic centers (at C1, C7, C8) present in agarozizanol B is correctly established in this transformation based on the existing stereogenic center at C2. The relative configuration was proven by single crystal X‐ray crystallographic analysis of product rac‐11. [25] .
The course of the reaction can be understood by an initial ortho photocycloaddition[ 19 , 20 , 21 ] which sets up the relative configuration between carbon atoms C2 and C7 (prezizane numbering). The approach of the olefin [22] to the top face of the benzene ring is preferred which accounts for the observed facial diastereoselectivity (d.r.=diastereomeric ratio). Although intermediate rac‐8 could not be isolated, it appears likely that the cyclobutane ring and the 1,3‐dioxane ring are cis‐ but not trans‐connected. Disrotatory ring opening produces cyclooctatriene rac‐9 which undergoes the second photochemical step, a disrotatory [4π] cyclization. The cascade can be interrupted at the stage of cyclobutene rac‐10 and compounds of this type have been shown to be useful for total synthesis. [23] However, continued irradiation induces a di‐π‐methane rearrangement [24] which generates the final product with the correct relative configuration at carbon atoms C1 and C8. The formation of the quaternary carbon atom C1 with correct relative configuration to C2 in a single step is particularly notable. As a result of the photochemical reaction cascade, the desired diastereoisomer rac‐11 was isolated in a remarkable yield of 42 %. Its constitution and relative configuration was proven by single crystal X‐ray crystallography. [25] The minor diastereoisomer isolated in 20 % yield was not further studied but it likely stems from a bottom face attack in the ortho photocycloaddition step. Attempted hydrogenation reactions performed with compound rac‐11 led under a variety of conditions to concomitant cleavage of the cyclopropane ring and the unwanted product rac‐12 was obtained (Scheme 3, see the Supporting Information for details).
Scheme 3.
An undesired cyclopropane ring cleavage occurring upon attempted hydrogenation of compound rac‐11 led to product rac‐12. Reduction of the ketone to alcohol 14 allowed for a selective hydrogenation and the desired ketone 15 could be prepared in enantiopure (15) or racemic (rac‐15, crystal structure shown [25] ) form (for details see the narrative).
The synthesis of the required ketone 15 was accomplished by a sequence of reduction‐hydrogenation‐oxidation. The reduction could be performed with NaBH4 in MeOH to generate a diastereomeric mixture of racemic alcohols in 96 % yield. However, it was found that the reduction step can also be combined with a resolution step employing chiral oxazaborolidine 13 in a Corey–Bakshi–Shibata (CBS) reaction. [26] Under the given conditions the reduction is known to occur from the Re face [27] delivering compound 14 with the depicted absolute and relative configuration. Double bond hydrogenation and oxidation with the Dess–Martin periodinane (DMP) [28] delivered ketone 15. All subsequent steps were performed both with racemic ketone rac‐15 and with the enantiopure compound 15 derived from alcohol 14 (96 % ee). The relative configuration of ketone rac‐15 was secured by single crystal X‐ray crystallography. [25]
The desired selective opening of the cyclopropane ring as outlined in Scheme 1 was eventually achieved by treatment with sodium iodide and chlorotrimethylsilane (TMSCl). [29] Mechanistically, the opening is suggested to occur by TMS activation of the carbonyl group and substitution by the iodide in an SN2 fashion (Scheme 4). The intermediate silyl enol ether is hydrolyzed upon work‐up and iodide 16 was obtained in 80 % yield (7 % recovered starting material). At the stage of iodide 16 it was also possible to corroborate the absolute configuration of the carbon skeleton by anomalous X‐ray diffraction (see the Supporting Information for details).
Scheme 4.
Formation of iodide 16 by regioselective ring opening of cyclopropyl ketone 15. The indicated SN2 type pathway was secured by single crystal X‐ray crystallographic analysis of product rac‐16. [25]
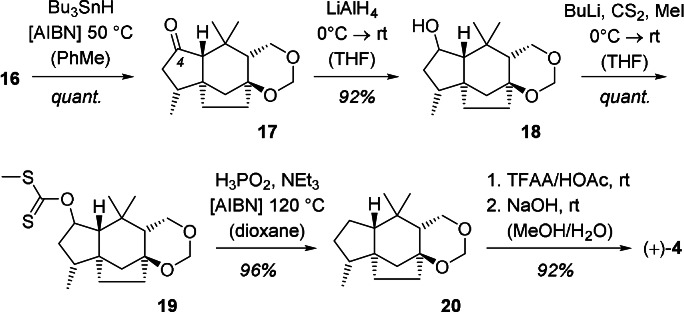
The conclusion of the synthesis required removal of the iodide at carbon atom C11 and the oxygen atom at position C4. The steps were performed in successive order and, given the high yields achieved in the individual steps, it was not attempted to combine the deiodination with the deoxygenation event (Scheme 5). The former reaction was performed with tributyltinhydride as the reductant in a radical chain reaction. [30] Reduction of ketone 17 to secondary alcohol 18 set the stage for a Barton‐McCombie reaction. [31] Xanthate 19 was preferably reduced with phosphinic acid [32] and delivered in high yield the immediate precursor 20 to agarozizanol B. The deprotection of the latent diol was performed with a mixture of trifluoroacetic anhydride and acetic acid and subsequent saponification. [33]
Scheme 5.
Final step of the total synthesis of agarozizanol B (4) starting from iodide 16 [AIBN=azobis(isobutyronitril); TFAA=trifluoroacetic anhydride; HOAc=acetic acid].
The relative configuration and constitution of the final product were secured by single‐crystal X‐ray crystallographic analysis (see the Supporting Information for details). The specific rotation of the final product was determined as [α]D 20=+15 (c=0.3, MeOH) and was identical with the specific rotation of naturally occurring agarozizanol B. [6] Since the absolute configuration of the synthetic material is known, the synthesis establishes the absolute configuration of the natural product.
In summary, a concise synthetic route to oxygenated prezizaene sesquiterpenes has been discovered. Starting from commodity chemicals (phenol, γ‐butyrolactone, prenol), agarozizanol B has been prepared in eleven steps in racemic (4 %) and enantiopure form (2 %). The comparably low overall yields are mainly due to the fact that the initial formation of compound rac‐5 [16] proceeded in our hands in only 27 % yield. The pivotal photochemical reaction cascade holds promise for further applications in the synthesis of natural products. Its compatibility with functional groups needs to be explored and its scope further expanded.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Supporting Information
Acknowledgements
Financial support by the Deutsche Forschungsgemeinschaft (Ba 1372/22‐1) is gratefully acknowledged. L.N. thanks the Carlsberg foundation for a postdoctoral fellowship. O. Ackermann, F. Pecho, and J. Kudermann are acknowledged for their help with HPLC and GLC analyses. Open Access funding enabled and organized by Projekt DEAL.
N. Rauscher, L. Næsborg, C. Jandl, T. Bach, Angew. Chem. Int. Ed. 2021, 60, 24039.
Contributor Information
Niklas Rauscher, https://www.oc1.ch.tum.de/hom/en/.
Prof. Dr. Thorsten Bach, Email: thorsten.bach@ch.tum.de.
References
- 1.
- 1a. Cane D. E., Chem. Rev. 1990, 90, 1089–1103; [Google Scholar]
- 1b. Cane D. E. in Comprehensive Natural Product Chemistry, Vol. 2 (Eds.: Barton D., Nakanishi K., Meth-Cohn O.), Elsevier, Amsterdam, 1999, 155–200; [Google Scholar]
- 1c. Chappell J., Coates R. M. in Comprehensive Natural Product Chemistry, 2nd ed. Vol. 1 (Eds. H.-W. Liu ,, Mander L.), Elsevier, Amsterdam, 2010, pp. 609–641; [Google Scholar]
- 1d. Le Bideau F., Kousara M., Chen L., Wei L., Dumas F., Chem. Rev. 2017, 117, 6110–6159; [DOI] [PubMed] [Google Scholar]
- 1e. Christianson D. W., Chem. Rev. 2017, 117, 11570–11648; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1f. Xu H., Dickschat J. S., Chem. Eur. J. 2020, 26, 17318–17341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Selected reviews:
- 2a. Vandewalle M., De Clercq P., Tetrahedron 1985, 41, 1765–1831; [Google Scholar]
- 2b. Maimone T. J., Baran P., Nat. Chem. Biol. 2007, 3, 396–407; [DOI] [PubMed] [Google Scholar]
- 2c. Urabe D., Inoue M., Tetrahedron 2009, 65, 6271–6289; [Google Scholar]
- 2d. Siengalewicz P., Mulzer J., Rinner U., Eur. J. Org. Chem. 2011, 7041–7055; [Google Scholar]
- 2e. Brill Z. G., Condakes M. L., Ting C. P., Maimone T. J., Chem. Rev. 2017, 117, 11753–11795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.
- 3a. Andersen N. H., Falcone M. S., Chem. Ind. 1971, 62–63; [Google Scholar]
- 3b. Nakanishi T., Yamagata E., Yoneda K., Miura I., Mori H., J. Chem. Soc. Perkin Trans. 1 1983, 601–604. [Google Scholar]
- 4.
- 4a. Carrol P. J., Ghisalberti E. L., Ralph D. E., Phytochemistry 1976, 15, 777–780; [Google Scholar]
- 4b. Ghisalberti E. L., Whilte A. H., Willis A. C., J. Chem. Soc. Perkin Trans. 2 1976, 1300–1303. [Google Scholar]
- 5.For additional references to prezizane-type sesquiterpenes, see:
- 5a. Nakanishi T., Yamagata E., Yoneda K., Miura I., Phytochemistry 1981, 20, 1597–1599; [Google Scholar]
- 5b. Raharivelomanana P., Bianchini J.-P., Faure R., Cambon A., Azzaro M., Phytochemistry 1994, 35, 1059–1060; [Google Scholar]
- 5c. Faraldos J. A., O'Maille P. E., Dellas N., Noel J. P., Coates R. M., J. Am. Chem. Soc. 2010, 132, 4281–4289; [DOI] [PubMed] [Google Scholar]
- 5d. Garcia G. P., Sutour S., Rabehaja D., Tissandié L., Filippi J.-J., Tomi F., Phytochemistry 2019, 162, 29–38. [DOI] [PubMed] [Google Scholar]
- 6. Yang L., Yang Y.-L., Dong W.-H., Li W., Wang P., Cao X., Yuan J.-Z., Cai C.-H., Chen H.-Q., Mei W.-L., Dai H.-F., J. Enzyme Inhib. Med. Chem. 2019, 34, 853–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yang Y.-L., Li W., Wang H., Yang L., Yuan J.-Z., Cai C.-H., Chen H.-Q., Dong W.-H., Ding X.-P., Jiang B., Mándi A., Kurtán T., Mei W.-L., Dai H.-F., Filoterapia 2019, 138, 104301. [DOI] [PubMed] [Google Scholar]
- 8. Vettel P. R., Coates R. M., J. Org. Chem. 1980, 45, 5430–5432. [Google Scholar]
- 9. Piers E., Jean M., Marrs P. S., Tetrahedron Lett. 1987, 28, 5075–5078. [Google Scholar]
- 10. Sakurai K., Kitahara T., Mori K., Tetrahedron 1990, 46, 761–774. [Google Scholar]
- 11. Selvakumar N., Rao G. S. R. S., J. Chem. Soc. Perkin Trans. 1 1994, 3217–3223. [Google Scholar]
- 12. Sharma M. K., Banwell M. G., Willis A. C., Asian J. Org. Chem. 2014, 3, 632–637. [Google Scholar]
- 13. Sharma M. K., Banwell M. G., Willis A. C., Rae A. D., Chem. Asian J. 2012, 7, 676–679. [DOI] [PubMed] [Google Scholar]
- 14. Næsborg L., Jandl C., Zech A., Bach T., Angew. Chem. Int. Ed. 2020, 59, 5656–5659; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2020, 132, 5705–5708. [Google Scholar]
- 15.For general reviews on photochemical key steps in the context of natural product total synthesis, see:
- 15a. Kärkäs M. D., J. A. Porco, Jr. , Stephenson C. R. J., Chem. Rev. 2016, 116, 9683–9747; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15b. Bach T., Hehn J. P., Angew. Chem. Int. Ed. 2011, 50, 1000–1045; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2011, 123, 1032–1077; [Google Scholar]
- 15c. Hoffmann N., Chem. Rev. 2008, 108, 1052–1103. [DOI] [PubMed] [Google Scholar]
- 16.For a recent review, see: Luque A., Paternoga J., Opatz T., Chem. Eur. J. 2021, 27, 4500–4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bruce D. B., Sorrie A. J. S., Thomson R. H., J. Chem. Soc. 1953, 2403–2406. [Google Scholar]
- 18.The compound was prepared from prenol (3-methyl-2-buten-1-ol) according to a known procedure: Bedford C. D., Harris R. N., Howd R. A., Goff D. A., Koolpe G. A., Petesch M., Koplovitz I., Sultan W. E., Musallam H. A., J. Med. Chem. 1989, 32, 504–516. The preparation and handling of the potential carcinogenic should be performed with utmost care and adequate protection. [DOI] [PubMed] [Google Scholar]
- 19.Reviews:
- 19a. Remy R., Bochet C. G., Chem. Rev. 2016, 116, 9816–9849; [DOI] [PubMed] [Google Scholar]
- 19b. Hoffmann N., Photochem. Photobiol. Sci. 2012, 11, 1613–1641; [DOI] [PubMed] [Google Scholar]
- 19c. Streit U., Bochet C. G., Beilstein J. Org. Chem. 2011, 7, 525–542; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19d. Hoffmann N., Synthesis 2004, 481–495; [Google Scholar]
- 19e. Cornelisse J., de Haan R. in Molecular and Supramolecular Photochemistry, Vol. 8 (Eds.: Ramamurthy V., Schanze K.), Dekker, New York, 2001, pp. 1–126. [Google Scholar]
- 20.For the use of arene photocycloaddition reactions in synthesis, see:
- 20a. Zhang Z., Zhou Y.-j., Liang X.-W., Org. Biomol. Chem. 2020, 18, 5558–5566; [DOI] [PubMed] [Google Scholar]
- 20b. Okumura M., Sarlah D., Eur. J. Org. Chem. 2020, 1259–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.For a recent application of an intramolecular ortho photocycloaddition in the synthesis of a complex diterpene, see: Schneider F., Samarin K., Zanella S., Gaich T., Science 2020, 367, 676–681. [DOI] [PubMed] [Google Scholar]
- 22.The observed regioselectivity is in line with previous results on the intramolecular ortho photocycloaddition of related ketones:
- 22a. Wagner P. J., Nahm K., J. Am. Chem. Soc. 1987, 109, 6528–6530; [Google Scholar]
- 22b. Wagner P. J., Acc. Chem. Res. 2001, 34, 1–8. [DOI] [PubMed] [Google Scholar]
- 23.
- 23a. Zech A., Jandl C., Bach T., Angew. Chem. Int. Ed. 2019, 58, 14629–14632; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2019, 131, 14771–14774; [Google Scholar]
- 23b. Proessdorf J., Zech A., Jandl C., Bach T., Synlett 2020, 31, 1598–1602. [Google Scholar]
- 24.Reviews:
- 24a. Banwell M. G., Bon D. J.-Y. D. in Molecular Rearrangements in Organic Synthesis (Ed.: Rojas C. M.), Wiley, Hoboken, 2015, pp. 261–288; [Google Scholar]
- 24b. Riguet E., Hoffmann N. in Comprehensive Organic Synthesis, 2nd ed. (Eds.: Knochel P., Molander G. A.), Elsevier, Amsterdam, 2014, 5, pp. 200–221; [Google Scholar]
- 24c. Tsuno T. in Handbook of Synthetic Photochemistry (Eds.: Albini A., Fagnoni M.), Wiley-VCH, Weinheim, 2010, pp. 95–135; [Google Scholar]
- 24d. Zimmerman H. E., Armesto D., Chem. Rev. 1996, 96, 3065–3112. [DOI] [PubMed] [Google Scholar]
- 25. Deposition Numbers 2095909 (for rac-11), 2095909 (for rac-15), and 2095910 (for rac-16) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service www.ccdc.cam.ac.uk/structures.
- 26.
- 26a. Corey E. J., Bakshi R. K., Shibata S. S., J. Am. Chem. Soc. 1987, 109, 5551–5553; [Google Scholar]
- 26b. Corey E. J., Helal C. J., Angew. Chem. Int. Ed. 1998, 37, 1986–2012; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 1998, 110, 2092–2118. [Google Scholar]
- 27.
- 27a. Lebsack A. D., Overman L. E., Valentekovich R. J., J. Am. Chem. Soc. 2001, 123, 4851–4852; [DOI] [PubMed] [Google Scholar]
- 27b. Watanabe H., Iwamoto M., Nakada M., J. Org. Chem. 2005, 70, 4652–4658; [DOI] [PubMed] [Google Scholar]
- 27c. Larionov O. V., Corey E. J., J. Am. Chem. Soc. 2008, 130, 2954–2955. [DOI] [PubMed] [Google Scholar]
- 28. Dess D. B., Martin J. C., J. Am. Chem. Soc. 1991, 113, 7277–7287. [Google Scholar]
- 29.
- 29a. Olah G. A., Narang S. C., Gupta B. G. B., Malhotra R., J. Org. Chem. 1979, 44, 1247–1251; [Google Scholar]
- 29b. Demuth M. in Modern Synthetic Methods, Vol. 4 (Ed.: Scheffold R.), Springer, Berlin, 1986, pp. 102–104. [Google Scholar]
- 30. Ziegler F. E., Fang J.-M., Tam C. C., J. Am. Chem. Soc. 1982, 104, 7174–7181. [Google Scholar]
- 31. Barton D. H. R., McCombie S. W., J. Chem. Soc. Perkin Trans. 1 1975, 1574–1585. [Google Scholar]
- 32.
- 32a. Barton D. H. R., Jang D. O., Jaszberenyi J. C., J. Org. Chem. 1993, 58, 6838–6842; [Google Scholar]
- 32b. Sathya Shanker P., Rao G. S. R. S., J. Chem. Soc. Perkin Trans. 1 1998, 539–547. [Google Scholar]
- 33. Boto A., Hernández D., Hernández R., Suárez E., J. Org. Chem. 2006, 71, 1938–1948. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Supporting Information