Abstract
Aim
To determine the diagnostic accuracy of pulmonary artery to aorta ratio in screening for pulmonary hypertension in advanced chronic obstructive pulmonary disease (COPD) patients.
Methods
A prospective, diagnostic study was conducted in University Hospital Center Zagreb between January 2015 and March 2018. The study enrolled 100 patients who consecutively underwent chest computed tomography (CT), echocardiographic exam, and right heart catheterization. Two independent observers measured pulmonary artery and ascending aorta diameters. The correlation between the ratio and mean pulmonary artery pressure, measured invasively, was assessed. Patients with echocardiographic signs of moderate systolic or diastolic left ventricular dysfunction were excluded (n = 44).
Results
Sixty-six patients (55.5% men), with a median age of 61, were identified. Median forced expiratory volume during the first second (FEV1) was 34 ± 12, FEV1/forced vital capacity <0.70. Patients with and without pulmonary hypertension had pulmonary artery diameter of 36 ± 7 mm and 27 ± 4.6 mm, respectively (P < 0.001). Median pulmonary artery/aorta (PA/A) ratios for patients with and without pulmonary hypertension were 1.05 and 0.81, respectively (P < 0.001). PA/A ratio above 0.95 was an independent predictor of pulmonary hypertension with a specificity of 100% and a sensitivity of 74.51% (area under the curve = 0.882; standard error = 0.041; P < 0.001).
Conclusion
PA/A ratio as measured on chest CT images can be used as a screening tool instead of echocardiography.
In patients suffering from late-stage chronic obstructive pulmonary disease (COPD), pulmonary vasculature disease is a predictor of COPD progression and exacerbation. The association may be explained by the resultant pulmonary arterial hypertension (1-3). As pulmonary hypertension develops throughout a patient’s lifetime, varying degrees of subclinical and clinical pulmonary vascular pathology are found. In a subset of patients with advanced COPD, pulmonary hypertension development is associated with a decreased functional status and an increased mortality (4,5). Furthermore, transplant-free patients with pulmonary hypertension have a markedly reduced survival rate. In some patient groups, the timing of lung transplantation is determined by elevated pressures in the pulmonary circulation (6). For these reasons, detection of pulmonary hypertension is vital as it influences the decisions on medical and surgical treatment.
Pulmonary hypertension remains a diagnostic challenge due to its non-specific symptoms: malaise, dyspnea on exertion, and fatigue, along with many other pulmonary disease presentations. Pulmonary hypertension is ultimately treated by surgery: single lung transplant, bilateral lung transplant, or heart and lung transplant (6). However, lung volume reduction surgery can be contraindicated in patients with advanced lung emphysema (7). Hence, adequate treatment requires early and precise detection of pulmonary hypertension (8).
Currently, right heart catheterization (RHC) is the gold standard in pulmonary hypertension verification (9,10), but this invasive method carries the risk of complications (ie, malignant arrhythmias, heart chamber perforation, pericardial effusion).
A non-invasive, widely available method of measurement of the right heart and pulmonary artery pressures is echocardiography; it is validated and well-established in clinical practice for pulmonary hypertension detection. However, in COPD patients it is often burdened with inadequate acoustic windows due to hyperinflation (11).
Late-stage COPD patients require routine follow-up computed tomography (CT) scanning. CT enables pulmonary artery (PA) and aorta (A) diameter measurement and PA/A ratio determination on a single scan. Hence, in these patients the PA/A ratio could be used as a valuable diagnostic tool for pulmonary hypertension assessment, obviating the need for RHC. On the other hand, patients with a high PA/A score could benefit from a timely referral for invasive diagnostic assessment. Recent studies suggested that PA/A ratio could be a relevant marker of PH in COPD patients (3,12,13). However, only a few studies have evaluated PA diameter in end-stage COPD patients (14,15). Thus, the aim of this study was to determine the cut-off value of PA/A ratio measured on CT that could be applied as a screening tool for pulmonary hypertension in end-stage COPD patients.
Patients and methods
Patients
This prospective, diagnostic study enrolled all patients diagnosed with COPD in the group C or D according to the GOLD standards. All patients were evaluated for lung transplantation from January 2015 to March 2018 in the University Hospital Center Zagreb. The patients' data obtained by clinical assessment, RHC, echocardiography (ECHO), and CT were collected within a six-month period. The inclusion criteria included stable disease with no patient in acute exacerbation during CT, ECHO examination, or RHC. We excluded the patients with another pulmonary pathology (asthma, pneumonia, tumor, idiopathic pulmonary fibrosis), those with systolic dysfunction of the left ventricle, those with greater than mild diastolic dysfunction or any valvular pathology, and non-compliant patients.
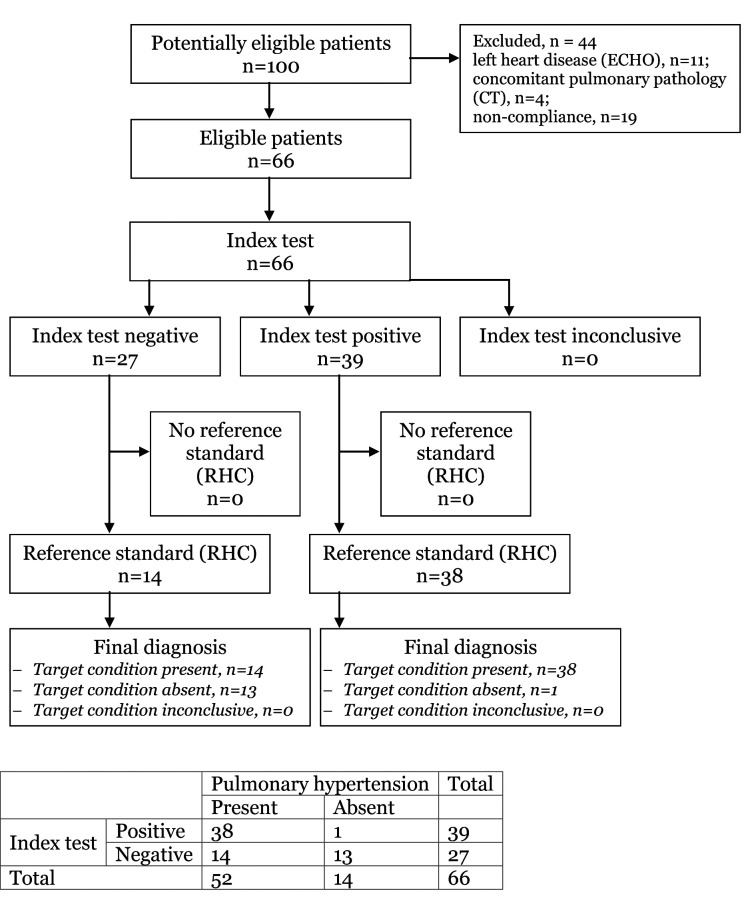
One hundred patients with verified end-stage COPD based on spirometry findings were consecutively enrolled (Figure 1). We presumed the prevalence of pulmonary hypertension within the studied population to be 75%, and the sensitivity for target condition to be 80%, which allows the study power of 90%. After ECHO examination and CT, 34 patients were excluded: 11 because of left heart disease verified by ECHO, 4 because of concomitant pulmonary pathology verified on CT, and 19 were lost to follow-up because of non-compliance. In total, 66 patients continued the study, with data sets of RHC data, ECHO parameters, and CT findings. Based on mPAP assessed by RHC, the study population was divided into two groups: a control group with mPAP<25 mm Hg and a PH group with mPAP≥25 mm Hg. PA and A diameters were measured on CT in both study groups. Indeterminate results would have been considered as negative, but there was none. The study was approved by the Ethics Committee of UHC Zagreb (02/21/JG).
Figure 1.
Study flowchart. ECHO – echocardiography; CT – computed tomography; RHC – right heart catheterization.
Demographic and clinical assessment
Demographic and clinical variables obtained were sex, age, body mass index (BMI), and smoking habits. Functional status was derived from the BODE index including 6-minute walking test (6-MWT). All patients completed the COPD Assessment Test (CAT) questionnaire and modified Medical Research Council (mMRC) dyspnea scale.
Spirometry revealed information regarding forced expiratory volume in the first second of exhalation (FEV1); predicted values of FEV1, predicted values of total lung capacity, and functional vital capacity (FVC). A patient with COPD was defined as one with post-bronchodilator spirometry FEV1/FVC ratio of less than 70%.
Echocardiographic assessment
ECHO examination included the assessment of the systolic and diastolic left ventricular function. Pulmonary hypertension values were obtained by calculating the maximum velocity of the jet of tricuspid insufficiency using the Bernoulli equation and adding the presumed pressure in the right atrium (regarding flow in the inferior vena cava).
Right heart catheterization
RHC was carried out by three different specialists, and the data collected were mean mPAP, systolic arterial pressure, and diastolic pulmonary arterial pressure, cardiac output, cardiac index, and pulmonary capillary wedge pressure. Pulmonary hypertension was defined as mPAP≥25 mm Hg.
Computed tomography scan
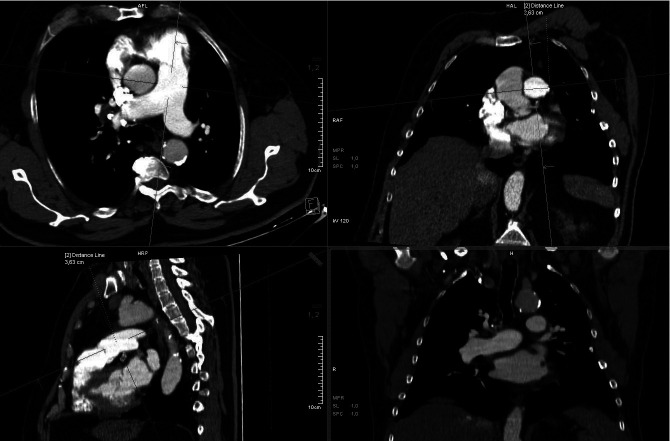
Pulmonary CT angiography was performed using a 40-detector row CT scanner (SOMATOM Sensation 40, Siemens Medical Solutions, Erlangen, Germany) with breath-hold technique. CT scans were analyzed by two radiology specialists blinded to patients’ clinical history. The measurements were performed at the level of the bifurcation of the main PA, and the aorta diameter was measured at the same slice (Figure 2). Both radiologists performed precise and accurate measurements, with no inter-rater bias (kappa test performed, K1 = 0.874, K2 = 0.836, respectively). The patients with abnormal pulmonary CT findings were excluded from the study (interstitial lung disease, bronchiectasis, any mass or fibrosis).
Figure 2.
Measurement of the pulmonary artery and aorta width on the same slice of the computed tomography scan.
Statistical analysis
Demographic and clinical characteristics were summarized as medians and 25th and 75th percentile for continuous variables and as the number and percentage for categorical variables. The Shapiro-Wilk test was used to test the normality of distribution. The Fischer exact and Fischer-Freeman-Halton exact tests were used to assess the differences in categorical variables. The Mann-Whitney U test was implemented to analyze the difference between PA and aorta dimensions in patients with and without pulmonary hypertension. Changes in the specificity and sensitivity of PA/A ratio for the detection of pulmonary hypertension were analyzed by receiver operating characteristic (ROC) curve. Youden index was used to determine the cut/off value of PA/A ratio with the best specificity/sensitivity relationship for pulmonary hypertension prediction. P values lower than 0.05 were considered statistically significant. Data were analyzed with MedCalc Statistical Software, version 18.2.1 (MedCalc Software bvba, Ostend, Belgium).
Results
The baseline clinical characteristics are shown in Table 1. RHC revealed pulmonary hypertension in 52 patients, and excluded pulmonary hypertension in 14 patients (control group). The median age of controls was 58.5 and that of the pulmonary hypertension group was 60 years. There were 30 women included (45.5%).
Table 1.
Differences in sociodemographic and clinical characteristics of patients with pulmonary hypertension and control group*
| Variable | Group | Median | 25-75 percentile | P‡ |
|---|---|---|---|---|
| Age (years)* |
control |
58.50 |
48.00-66.00 |
0.278 |
| PH |
60.00 |
39.00-75.00 |
||
| Smoking years (n) |
control |
20.00 |
0.00-35.75 |
0.651 |
| PH |
30.00 |
20.00-30.00 |
||
| Pack per year (n) |
control |
35.00 |
0.00-52.50 |
0.788 |
| PH |
30.00 |
20.00-40.00 |
||
| BMI (kg/m2) |
control |
27.53 |
26.42-30.10 |
0.068 |
| PH |
26.32 |
23.60-28.25 |
||
| BSA (m2) |
control |
1.94 |
1.90-2.02 |
0.245 |
| PH |
1.90 |
1.77-2.02 |
||
| Exacerbations in one year (n) |
control |
3.00 |
2.00-3.00 |
0.083 |
| PH |
3.00 |
3.00-3.75 |
||
| CAT |
control |
28.00 |
24.50-31.25 |
0.894 |
| PH |
28.50 |
25.00-32.75 |
||
| FVC (%) |
control |
59.00 |
40.05-78.00 |
0.784 |
| PH |
57.20 |
47.43-72.03 |
||
| FEV1 (%) |
control |
32.50 |
20.43-50.00 |
0.551 |
| PH |
33.70 |
22.20-46.08 |
||
| FEV1/FVC |
control |
45.30 |
35.20-65.00 |
0.678 |
| PH |
49.95 |
39.00-61.00 |
||
| DLCO |
control |
37.50 |
32.50-55.00 |
0.090 |
| PH |
50.00 |
40.00-65.00 |
||
| 6-MWT |
control |
240.00 |
180.00-370.00 |
0.052 |
| PH |
182.50 |
125.00-267.50 |
||
| BODE | control |
5.50 |
2.00-6.25 |
0.006 |
| PH | 6.00 | 4.25-7.00 |
*Abbreviations: PH – pulmonary hypertension; BMI – body mass index; BSA – body surface area; CAT – COPD assessment test; FVC – forced volume vital capacity; FEV1 – forced expiratory volume in 1st second; FEV1/FVC – the Tiffeneau-Pinelli index; DLCO – diffusing capacity for carbon monoxide; 6-MWT six-minute walking test; BODE – body mass index, airflow obstruction, dyspnea, and exercise.
‡Mann-Whitney U test.
Median (standard deviation [SD]) FEV1% was 32.5 (20.4-50) in the control group and 33.7 (22.2–46.0) in the pulmonary hypertension group, with no significant difference between the groups. The median mPAP in the control group was 23 (21.7–24) mmHg and that in the pulmonary hypertension group was 40.5 (34.0-47.75) mmHg.
Our study group consisted of 52 patients (78.8%) with pulmonary hypertension; 23 were women (44.2%). The PH group and controls did not significantly differ in age, sex, BMI, FEV1, and FEV1/FVC.
Six-minute walking test result was lower in the pulmonary hypertension group, but the difference did not reach significance (P = 0.052). The groups did not differ in the CAT questionnaire results. Patients with pulmonary hypertension had a significantly higher BODE index (FEV1, 6-MWT, MMRC dyspnea scale, BMI) than controls: 6.0 (4.3-7.0) vs 5.5 (2.0-6.3) (P = 0.006).
Patients with PH had a higher median PA diameter than controls (36.10 [22-63] mm vs 27.50 [17.8-38] mm, P < 0.001) and a significantly higher median PA/A ratio (1.06 vs 0.80, P < 0.001) (Table 2, Figure 3).
Table 2.
Computed tomography and right heart catheterization measurements in the PH and control group
| Variable | Group | Median | 25-75 percentile | P† |
|---|---|---|---|---|
| PA (mm) |
control |
27.50 |
24.75-29.00 |
<0.001 |
| PH |
36.10 |
31.05-40.75 |
||
| Aorta (mm) |
control |
32.50 |
30.75-36.00 |
0.387 |
| PH |
34.00 |
31.40-37.85 |
||
| PA/A |
control |
0.80 |
0.76-0.89 |
<0.001 |
| PH |
1.06 |
0.97-1.17 |
||
| Vena cava superior (mmHg) |
control |
9.50 |
5.00-10.50 |
0.028 |
| PH |
12.00 |
9.00-15.00 |
||
| Right atrium pressure (mmHg) |
control |
9.00 |
5.75-11.75 |
0.024 |
| PH |
12.00 |
10.00-14.75 |
||
| RVsis (mmHg) |
control |
34.00 |
33.00-37.00 |
<0.001 |
| PH |
59.05 |
51.25-76.50 |
||
| RVd (mmHg) |
control |
2.00 |
1.00-5.00 |
0.004 |
| PH |
5.00 |
2.00-7.00 |
||
| RVm (mmHg) |
control |
15.50 |
13.75-16.25 |
<0.001 |
| PH |
26.00 |
21.50-35.75 |
||
| PAs (mmHg) |
control |
35.00 |
33.00-37.00 |
<0.001 |
| PH |
59.50 |
53.25-79.50 |
||
| PAd (mmHg) |
control |
14.00 |
12.00-16.00 |
0.001 |
| PH |
28.00 |
24.25-33.00 |
||
| PAm (mmHg) |
control |
23.00 |
21.75-24.00 |
<0.001 |
| PH |
40.50 |
34.00-47.75 |
||
| PCWP (mmHg) |
control |
10.00 |
8.00-11.25 |
0.037 |
| PH |
11.00 |
9.00-13.00 |
||
| Cardiac output (L/min) |
control |
4.88 |
4.45-5.74 |
0.330 |
| PH |
5.46 |
4.53-5.80 |
||
| Cardiac index (L/min/m2) |
Control |
2.79 |
2.28-2.95 |
0.742 |
| PH |
2.70 |
2.50-3.09 |
||
| PVR (dynes/s/cm5) |
control |
220.70 |
189.75-239.80 |
<0.001 |
| PH |
461.17 |
360.25-568.43 |
||
| SVR (dynes/s/cm5) |
control |
1472.50 |
1309.20-1657.50 |
0.475 |
| PH |
1376.70 |
1210.75-1667.25 |
||
| PVR/SVR |
control |
0.14 |
0.12-0.18 |
0.001 |
| PH |
0.35 |
0.24-0.41 |
||
| EF (%) | control |
60.00 |
60.00-65.00 |
0.907 |
| PH | 60.00 | 60.00-65.00 |
*Abbreviations: PH – pulmonary hypertension; PA – pulmonary artery diameter; A – aortal diameter; RVsis – systolic pressure in the right ventricle; RVd – diastolic pressure in the right ventricle; RVm – mean pressure in the right ventricle; PAs – systolic pressure in the pulmonary artery; PAd – diastolic pressure in the pulmonary artery; PAm – mean pressure in the pulmonary artery; PCWP – pulmonary capillary wedge pressure; PVR – pulmonary vascular resistance; SVR – systemic vascular resistance; EF – ejection fraction of the left ventricle.
†Mann-Whitney U test.
Figure 3.
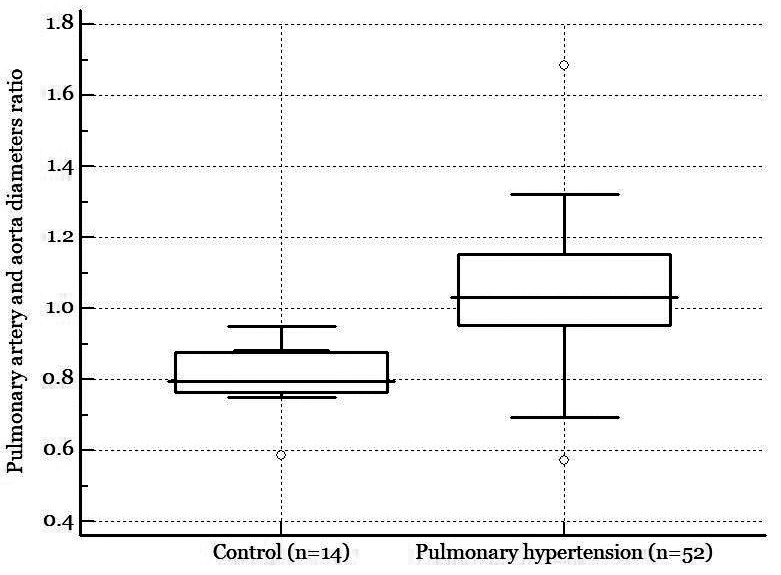
Higher pulmonary artery/aorta diameters ratio in pulmonary hypertension group compared with the control group.
ROC analysis showed an excellent area under the curve when PA/A ratio was explored as a diagnostic tool for the presence of pulmonary hypertension (Figure 4). The highest Youden index (J = 0.750) gave us a cut-off value of ≥0.95 for the presence of PH, with a sensitivity of 74.51% (95% CI 61.1-86.0), specificity of 92.86% (95% CI 66.1-99.8) (AUC = 0.882; SE = 0.041; z = 9.266; P < 0.001), and accuracy of 82.97% (95% CI 71.71-91.10). The odds ratio for having PA/A ratio above 0.95 in late-stage COPD patients was 42.25 (95% CI 5.00-357.10; z = 3.438; P = 0.001).
Figure 4.
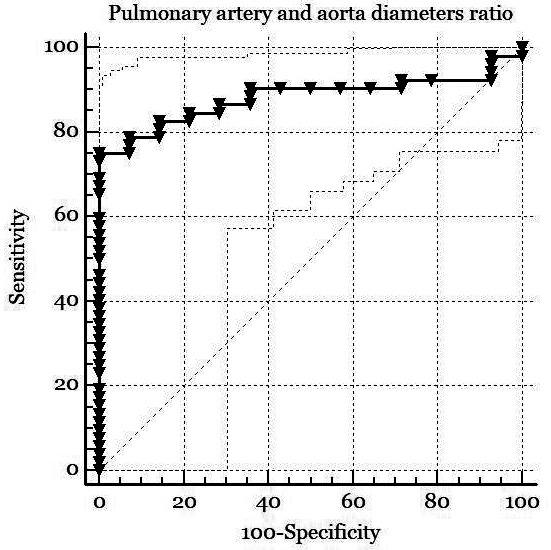
Receiver operating characteristic (ROC) analysis showing pulmonary artery/aorta (PA/A) diameter as an excellent classifier for the presence of pulmonary hypertension. Area under the curve = 0.882; standard error = 0.042; z = 9.266; P < 0.001, sensitivity 74.51% (95% CI 61.1-86.0); specificity 92.86% (95% CI 66.1-99.8) at PA/A diameter ratio of ≥0.95; accuracy of test = 82.97% (95% CI 71.71-91.10).
In the pulmonary hypertension group, PA/A moderately positively correlated with mPAP (rho = 0.381, P = 0.006). In the control group, the correlation coefficient was not significant (rho = -0.402, P = 0.154). The difference between the two correlation coefficients was significant (z = 2.480, P = 0.013), confirming the importance of the PA/A ratio as a marker of pulmonary hypertension. The difference in correlation coefficients of PA/A ratio and mPAP was assessed by z-statistics (Figure 5). No adverse events were reported during CT scanning and RHC.
Figure 5.
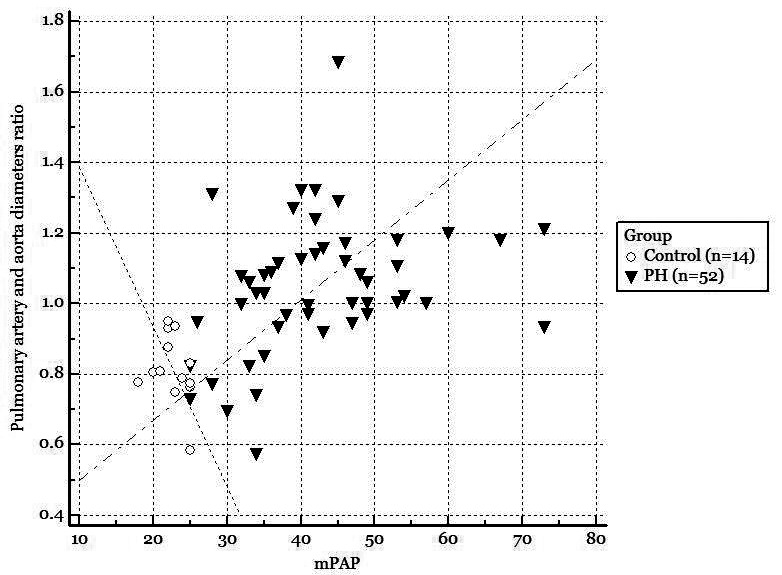
Significantly higher correlation coefficients of pulmonary artery/aorta (PA/A) and mean pulmonary artery pressure (mPAP) in the pulmonary hypertension (PH) croup compared with the control group.
Discussion
In our study, PA/A ratio as measured on CT was associated with pulmonary hypertension in patients suffering from late-stage COPD. PA/A≥0.95 showed a significant diagnostic accuracy for predicting elevated mPAP on RHC, with a sensitivity of 74.5% and specificity of 92.86%. All patients with PA/A≥0.95 had PH confirmed invasively, while 23% of patients with verified PH did not have PA/A≥0.95.
In the PH group, PA/A significantly positively correlated with mPAP, while no such correlation was found in the control group. The significant difference between these two correlation coefficients indicates the potential of PA/A ratio as a new diagnostic parameter.
Pulmonary artery diameter measurement is a relatively new method in clinical practice. This method can help us to identify the patients with pulmonary hypertension who are at risk of increased mortality (16,17) and COPD exacerbation.
Iliaz et al (18) showed, on 156 patients hospitalized for COPD exacerbations, that PA/A ratio positively correlated with the number of hospitalizations due to COPD in one year. In another study, PA/A>1 was significantly related to the number of exacerbations (19). However, this study made no comparison with the level of pulmonary hypertension (19).
Terzikan et al (20), in 2197 participants from the population-based Rotterdam Study, demonstrated no association between 1-SD increase in PA/A and mortality in the general population, but observed this association in moderate-to-severe COPD patients. Previous studies reported an association between PA/A ratio >1 and pulmonary hypertension in COPD, but most of them included patients with various lung pathologies (21-23).
An early pulmonary hypertension diagnosis in COPD patients is important because an adequate approach may improve the patient’s quality of life and survival (24,25). This is in part due to the higher complication risk and increased morbidity and mortality in patients with pulmonary hypertension (6,26).
Ersoy et al (27) observed a correlation between echocardiography-guided measurements of PAP and PA/A>1.0 in patients with acute exacerbation of COPD. However, no significant association between higher PA/A ratio and increased mortality was observed. Furthermore, an increased PA/A ratio or enlarged PA diameter on CT were found to be a useful tool for a timely and proper clinical assessment (28).
Our study involved a well-defined sample of patients with an end-stage COPD. In a similar study, Hoesein et al (15) retrospectively studied 92 patients, 32.6% of whom had pulmonary hypertension, and observed that PA/A>1 had a 50% sensitivity and 85.5% specificity in identifying pulmonary hypertension. Higher sensitivity and specificity obtained in our study may be explained by a proportionally larger number of patients with pulmonary hypertension.
Patients in advanced stages of COPD require periodic CTs, and our method of assessing pulmonary hypertension by PA/A ratio can indicate an invasive diagnostic approach. CT is a non-invasive and accessible tool in routine clinical practice, allowing us to get additional valuable information using this novel approach.
Symptoms of pulmonary hypertension are unspecific and overlap with those of COPD without pulmonary hypertension. However, the PA/A ratio can be used to guide the clinical decision concerning the use of RHC.
Our study showed that an elevated PA/A ratio and mPAP are mainly influenced by PA enlargement rather than by changes in aortic dimensions. However, some other mechanisms could also contribute to PA enlargement, such as pulmonary arterial distensibility (23) and redistribution of blood flow from capillary loss on periphery (29).
In this study, we excluded patients with left ventricular systolic dysfunction and greater than mild diastolic dysfunction. Some studies found a correlation between left heart disease and PA size (30). Echocardiography is widely used as a screening tool for estimating pulmonary hypertension (31-34). However, the presence of a hyper-inflated thorax in COPD patients makes the examination impossible to perform in about 40% of the patients (21). We were able to measure systolic PAP non-invasively in 69% of patients, while in one third of patients echocardiography was insufficient in PH detection.
The limitations of the study include a relatively small sample size. However, the high number of patients with proven PH makes the assessment of pulmonary vasculature reliable. All patients were thoroughly examined by echocardiography, and all patients with a reduced ejection fraction, a greater than mild diastolic dysfunction, and valvular pathology were excluded. There is concern regarding the study applicability since 44/100 patients were not enrolled in the study (left heart disease [ECHO], n = 11; concomitant pulmonary pathology [CT], n = 4; non-compliance, n = 19). Additionally, due to the small number of published studies on this subject there is no reference standard for PA/A ratio. The advantages of the study include no risk of bias due to flow and timing, and no inter-rater bias.
To summarize, according to our data, the PA/A ratio ≥0.95 as measured by CT could be a highly accurate tool to predict the presence of pulmonary hypertension in end-stage COPD patients. The odds ratio, due to small sample size, had a huge 95% confidence interval. Stage C and D COPD patients who had a PA/A ratio ≥0.95 had a 42 times greater probability of having pulmonary hypertension in comparison with patients with PA/A ratio <0.95, or at least a 5 times greater risk, if we take the lower value of 95% confidence interval. However, the presence of pulmonary hypertension cannot be excluded in patients with PA/A<0.95. Meta-analyses including more data are necessary to confirm the reliability and accuracy of PA/A ratio as a measure of the pulmonary hypertension risk in stage C and D COPD patients. Therefore, invasive RHC measurement remains the most important and unavoidable method for detecting pulmonary hypertension.
Acknowledgments
Funding None.
Ethical approval given by the Ethics Committee of UHC Zagreb (02/21/JG).
Declaration of authorship KG and DM conceived and designed the study; GP, MHP, MB, MLB, JŠH, DM, MS, and JJ acquired the data; KG and JJ analyzed and interpreted the data; JJ and MHP drafted the manuscript; KG, GP, MB, MLB, JŠH, DM, MS, and JJ critically revised the manuscript for important intellectual content; all authors gave approval of the version to be submitted; all authors agree to be accountable for all aspects of the work.
Competing interests JJ is a statistical editor in the Croatian Medical Journal. To ensure that any possible conflict of interest relevant to the journal has been addressed, this article was reviewed according to best practice guidelines of international editorial organizations. All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
References
- 1.Seeger W, Adir Y, Barbera JA,, Champion H,, Coghlan JG,, Cottin V,, et al. Pulmonary hypertension in chronic lung diseasesJ AmColl Cardiol 2013. 62(25):109-116 10.1016/j.jacc.2013.10.036 [DOI] [PubMed] [Google Scholar]
- 2. Barbera JA, Blanco I. Pulmonary hypertension in patients with chronic obstructive pulmonary disease: advances in pathophysiology and management. Drugs. 2009;18:1153–71. doi: 10.2165/00003495-200969090-00002. [DOI] [PubMed] [Google Scholar]
- 3. Fayngersh V, Drakopanagiotakis F, Dennis McCool F, Klinger JR. Pulmonary hypertension in a stable community-based COPD population. Lung. 2011;189:377–82. doi: 10.1007/s00408-011-9315-2. [DOI] [PubMed] [Google Scholar]
- 4. Cuttica MJ, Kalhan R, Shlobin OA, Ahmad S, Gladwin M, Machado RF, et al. Categorization and impact of pulmonary hypertension in patients with advanced COPD. Respir Med. 2010;104:1877–82. doi: 10.1016/j.rmed.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 5. Shujaat A, Bajva AA, Cery JD. Pulmonary hypertension secondary to COPD. Pulm Med. 2012;2012:203952. doi: 10.1155/2012/203952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Conte JV, Borja MJ, Patel CB, Yang SC, Jhaveri RM, Orens JB. Lung transplantation for primary and secondary pulmonary hypertension. Ann Thorac Surg. 2001;72:1673–80. doi: 10.1016/S0003-4975(01)03081-8. [DOI] [PubMed] [Google Scholar]
- 7. Watz H, Waschki B, Meyer T, Kretschmar G, Kirsten A, Claussen M, et al. Decreasing cardiac chamber sizes and associated heart dysfunction in COPD: role of hyperinflation. Chest. 2010;138:32–8. doi: 10.1378/chest.09-2810. [DOI] [PubMed] [Google Scholar]
- 8. McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, et al. ACCF/AHA. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc., and the Pulmonary Hypertension Association. Circulation. 2009;119:2250–94. doi: 10.1161/CIRCULATIONAHA.109.192230. [DOI] [PubMed] [Google Scholar]
- 9. Galie N, Humbert M, Vachery JL, Gibbs S, Lang I, Torbicki A, et al. 2015 ESC-ERS GUIDELINES for the diagnosis and treatment of pulmonary hypertension. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016;37:67–119. doi: 10.1093/eurheartj/ehv317. [DOI] [PubMed] [Google Scholar]
- 10. Hoeper MM, Bogaard HJ, Condliffe R, Frantz R, Khanna D, Kurzyna M, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25) Suppl:D42–50. doi: 10.1016/j.jacc.2013.10.032. [DOI] [PubMed] [Google Scholar]
- 11. Janda S, Shahidi N, Gin K, Swiston J. Diagnostic accuracy of echocardiography for pulmonary hypertension: a systematic review and meta- analysis. Heart. 2011;97:612–22. doi: 10.1136/hrt.2010.212084. [DOI] [PubMed] [Google Scholar]
- 12. Cuttica MJ, Bhatt SP, Rosenberg SR, Beussink L, Shah SJ, Smith LJ, et al. Pulmonary artery to aorta ratio is associated with cardiac structure and functional changes in mild-to-moderate COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:1439–46. doi: 10.2147/COPD.S131413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ng CS, Wells AV, Padley SP. A CT sign of chronic pulmonary arterial hypertension: the ratio of main pulmonary artery to aortic diameter. J Thorac Imaging. 1999;14:270–8. doi: 10.1097/00005382-199910000-00007. [DOI] [PubMed] [Google Scholar]
- 14. Iyer AS, Wells M, Viskin S, Bhatt SP, Wille KM, Dransfiels MT. CT scan-measured pulmonary to aorta ratio and echocardiography for detecting pulmonary hypertension in severe COPD. Chest. 2014;145:824–32. doi: 10.1378/chest.13-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mohamed Hoesein FA, Besselink T, Pompe E, Oudijk EJ, de Graaf EA, Kwakkel-van Erp JM, et al. Accuracy of CT pulmonary artery diameter for pulmonary hypertension in end-stage COPD. Lung. 2016;194:813–9. doi: 10.1007/s00408-016-9926-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Andersen KH, Iversen M, Kjaergaard J, Mortensen J, Nielsen-Kudsk JE, Bendstrup E, et al. Prevalence, predictors, and survival in pulmonary hypertension related to end-stage chronic obstructive pulmonary disease. J Heart Lung Transplant. 2012;31:373–80. doi: 10.1016/j.healun.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 17. Benza RL, Miller DP, Gomberg-Maitland M, Frantz RP, Foreman AJ, Coffey CS, et al. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL). Circulation. 2010;122:164–72. doi: 10.1161/CIRCULATIONAHA.109.898122. [DOI] [PubMed] [Google Scholar]
- 18. Iliaz S, Tanriverdio E, Chousein EGU, Ozturk S, Iliaz R, Cetinkaya E, et al. Importance of pulmonary artery to ascending aorta ratio in chronic obstructive pulmonary disease. Clin Respir J. 2018;12:961–5. doi: 10.1111/crj.12612. [DOI] [PubMed] [Google Scholar]
- 19. Wells JM, Washko GR, Han MK, Abbas N, Nath H, Mamary AJ, et al. COPDGene Investigators; ECLIPSE Study Investigators. Pulmonary arterial enlargement and acute exacerbations of COPD. N Engl J Med. 2012;367:913–21. doi: 10.1056/NEJMoa1203830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Terzikhan N, Bos D, Lahousse L, Wolff L, Verhamme KMC, Leening MJG, et al. Pulmonary artery to aorta ratio and risk of all-cause mortality in the general population: the Rotterdam Study. Eur Respir J. 2017;49:1602168. doi: 10.1183/13993003.02168-2016. [DOI] [PubMed] [Google Scholar]
- 21. Alhamad EH, Al-Boukai AA, Al-Kassimi FA, Alfaleh HF, Alshamiri MQ, Alzeer AH, et al. Prediction of pulmonary hypertension in patients with or without interstitial lung disease: reliability of CT findings. Radiology. 2011;260:875–83. doi: 10.1148/radiol.11103532. [DOI] [PubMed] [Google Scholar]
- 22. Chan AL, Juarez MM, Shelton DK, MacDonald T, Li CS, Lin TC, et al. Novel computed tomographic chest metrics to detect pulmonary hypertension. BMC Med Imaging. 2011;11:7. doi: 10.1186/1471-2342-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wells JM, Dransfield MT. Pathophysiology and clinical implications of pulmonary enlargement in COPD. Int Journal of COPD. 2013;8:509–21. doi: 10.2147/COPD.S52204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Terzano C, Conti V, Di Stefano F, Petroianni A, Ceccarelli D, Graziani E, et al. Comorbidity, hospitalization, and mortality in COPD: results from a longitudinal study. Lung. 2010;188:321–9. doi: 10.1007/s00408-009-9222-y. [DOI] [PubMed] [Google Scholar]
- 25. McGhan R, Radcliff T, Fish R, Sutherland ER, Welsh C, Make B. Predictors of rehospitalization and death after a severe exacerbation of COPD. Chest. 2007;132:1748–55. doi: 10.1378/chest.06-3018. [DOI] [PubMed] [Google Scholar]
- 26. Kessler R, Faller M, Weitzenblum E, Chaouat A, Aykut A, Ducoloné A, et al. “Natural history” of pulmonary hypertension in a series of 131 patients with chronic obstructive lung disease. Am J Respir Crit Care Med. 2001;164:219–24. doi: 10.1164/ajrccm.164.2.2006129. [DOI] [PubMed] [Google Scholar]
- 27. Ortac Ersoy E, Durusu Tanriover M, Ocal S, Gulsun Akpinor M, Topeli A. Measurement of pulmonary to aorta ratio in computed tomography is correlated with pulmonary artery pressure in critically ill chronic obstructive pulmonary disease patients. J Crit Care. 2016;33:42–6. doi: 10.1016/j.jcrc.2016.01.020. [DOI] [PubMed] [Google Scholar]
- 28. Mohammedi A, Oshmyansky A, Hassoun PM, Thiemann DR, Siegelman SS. Pulmonary artery measurement in pulmonary hypertension: the role of computed tomography. J Thorac Imaging. 2013;28:96–103. doi: 10.1097/RTI.0b013e318271c2eb. [DOI] [PubMed] [Google Scholar]
- 29. Malhotra R, Dhakal BP, Eisman AS, Pappagianopoulos PP, Dress A, Weiner RB, et al. Pulmonary vascular distensibility predicts pulmonary hypertension severity, exercise capacity, and survival in heart failure. Circ Heart Fail. 2016;9 doi: 10.1161/CIRCHEARTFAILURE.115.003011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dupuis J, Guazzi M. Pathophysiology and clinical relevance of pulmonary hypertension due to left heart diseases. Can J Cardiol. 2015;31:416–29. doi: 10.1016/j.cjca.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 31. Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. doi: 10.1016/j.echo.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 32. Tamborini G, Pepi M, Galli CA, Maltagliati A, Celeste F, Muratori M, et al. Feasibility and accuracy of a routine echocardiographic assessment of right ventricular function. Int J Cardiol. 2007;115:86–9. doi: 10.1016/j.ijcard.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 33. Lai WW, Gauvreau K, Rivera ES, Saleeb S, Powell AJ, Geva T. Accuracy of guideline recommendations for two-dimensional quantification of the right ventricle by echocardiography. Int J Cardiovasc Imaging. 2008;24:691–8. doi: 10.1007/s10554-008-9314-4. [DOI] [PubMed] [Google Scholar]
- 34. Fisher MR, Forfia PR, Chamera E, Housten-Harris T, Champion HC, Girgis RE, et al. Accuracy of Doppler echocardiography in the hemodynamic assessment of pulmonary hypertension. Am J Respir Crit Care Med. 2009;179:615–21. doi: 10.1164/rccm.200811-1691OC. [DOI] [PMC free article] [PubMed] [Google Scholar]