Abstract
A1 adenosine receptors (A1Rs) are G protein-coupled heptaspanning receptors that interact at the outer face of the plasma membrane with cell surface ecto-adenosine deaminase (ecto-ADA). By affinity chromatography the heat shock cognate protein hsc73 was identified as a cytosolic component able to interact with the third intracellular loop of the receptor. As demonstrated by surface plasmon resonance, purified A1Rs interact specifically with hsc73 with a dissociation constant in the nanomolar range (0.5 ± 0.1 nM). The interaction between hsc73 and A1R led to a marked reduction in the binding of the ligands and prevented activation of G proteins, as deduced from 35S-labeled guanosine-5′-O-(3-thio)triphosphate binding assays. Interestingly this effect was stronger than that exerted by guanine nucleotide analogs, which uncouple receptors from G proteins, and was completely prevented by ADA. As assessed by immunoprecipitation a high percentage of A1Rs in cell lysates are coupled to hsc73. A relatively high level of colocalization between A1R and hsc73 was detected in DDT1MF-2 cells by means of confocal microscopy, and no similar results were obtained for other G protein-coupled receptors. Colocalization between hsc73 and A1R was detected in specific regions of rat cerebellum and in the body of cortical neurons but not in dendrites or synapses. Remarkably, agonist-induced receptor internalization leads to the endocytosis of A1Rs by two qualitatively different vesicle types, one in which A1R and hsc73 colocalize and another in which hsc73 is absent. These results open the interesting possibility that signaling via G protein-coupled receptors may be regulated by heat shock proteins.
The ubiquitous nucleoside adenosine exerts multiple physiological actions via specific receptors, four of which (A1, A2a, A2b, and A3) have been cloned (see reference 25 for a review). Activation of A1 adenosine receptors (A1Rs) by adenosine or synthetic analogs, in turn regulates the activity of several membrane and intracellular proteins, such as adenylate cyclase, Ca2+ channels, K+ channels and phospholipase C (see reference 25 for a review). Towards the intracellular side of the plasma membrane, A1Rs are functionally coupled to members of the pertussis toxin-sensitive family of G proteins, Gi1, Gi2, Gi3, and Go (13, 20). For this coupling Nanoff et al. (23) have shown that a proteic factor of 30 to 40 kDa is necessary (23). On the other side of the plasma membrane, A1Rs interact with ecto-adenosine deaminase (ecto-ADA) an interaction which is needed for efficient signaling (11, 27). Moreover, in response to agonists, A1Rs and ADA are internalized together via the same endocytic pathway (28).
There is recent evidence indicating that members of the superfamily of G protein-coupled receptors interact with cytosolic proteins. As an example a recent report has described the interaction of the C terminus of metabotropic glutamate receptor type 1α with glyceraldehyde-3-phosphate dehydrogenase, tubulin, and an unknown protein (9). The aim of this study was to identify proteins interacting with the third intracellular loop of A1R. By means of affinity chromatography, coimmunoprecipitation, and biosensor technology we have found that the heat shock cognate protein hsc73 is able to bind to A1R. Interestingly, the interaction between this protein and the A1R results in reduced binding of ligands, thus suggesting a role for hsc73 in the regulation of the receptor function.
MATERIALS AND METHODS
Materials.
ADA (EC 3.5.4.4), electrophoresis reagents, fetal bovine serum and 5′-guanylylimidodiphosphate [Gpp(NH)p] were purchased from Boehringer Mannheim (Barcelona, Spain). Activated thiol Sepharose-4B, activated CH-Sepharose 4B, Sephadex G-25, and protein-A Sepharose CL4B were from Pharmacia Biotech (Barcelona, Spain). NP-40, digitonin, and 3-[(3-cholamidopropyl) dimethylammonio]-1-propanesulfonate (CHAPS) were from Calbiochem Novabiochem Corp. (La Jolla, Calif.). Protease inhibitors, phenylarsine oxide, N6-(R)-phenylisopropyladenosine (R-PIA), guanosine-5′-O-(3-thio)triphosphate (GTPγS) bovine gamma globulins, polyethylene glycol 8000, and 50% polyethylenimine were from Sigma (St. Louis, Mo.). 1,3-Dipropyl-8-cyclopentylxantine (DPCPX), xanthine amine congener (XAC), and 8-cyclopentyl-1,3-dimethylxanthine (CPT) were from Research Biochemicals Inc. (Natick, Mass.). Bovine brain 73-kDa heat shock protein (hsc73) was purchased from StressGen Biotechnologies Corp. (Victoria, Canada). (−)N6-R-[G-3H]phenylisopropyladenosine ([3H]R-PIA) (46.0 Ci/mmol) and guanosine-5′-[γ-35S]-triphosphate ([35S]GTPγS) (1,033 Ci/mmol) were from Amersham Pharmacia Biotech (Barcelona, Spain). Cyclopentyl-1,3-dipropylxanthine,8-[dipropyl-2,3-3H(N)] ([3H]DPCPX) (109.0 Ci/mmol) was from New England Nuclear. BIAcore sensor chip CM5, the amine coupling kit, and certified HBS BIA solution were from Pharmacia Biosensor AB (Uppsala, Sweden). Dulbecco's modified Eagle's medium (DMEM), 2-mercaptoethanol, nonessential amino acids, l-glutamine, horse serum, and sodium pyruvate were from GIBCO Life Technologies (Gaithersburg, Md.). Antibiotics (penicillin, amphotericin B, and streptomycin) were from Biological Industries (Kibbutz Beit Haemek, Israel). All other products were the best grade available and were purchased from Merck (Darmstadt, Germany). Deionized water further purified with a Millipore Milli-Q system was used throughout.
Antibodies.
Antipeptide antisera against the A1R, A2AR, and A2BR were generated in New Zealand White rabbits and characterized as described elsewhere (10, 18). The peptides used for immunization correspond to a 19-amino-acid sequence of the third intracellular loop of A1R (PC11 antibody), a 14-amino-acid sequence of the second extracellular loop of A1R (PC21 antibody), a 13-amino-acid sequence of the second extracellular loop of A2AR (anti-A2AR), and a 12-amino-acid sequence of the second extracellular loop of A2BR (anti-a2R). Antisera generated were purified by affinity chromatography using the specific peptide coupled to activated thiol-Sepharose 4B. Polyclonal affinity-purified antibody anti-mGluR2/3 was genereously provided by J. McIlhinney (Medical Research Council, Oxford, United Kingdom). Anti-hsc73 monoclonal antibody (1B5) was purchased from StressGen Biotechnologies Corp. Anti-G-protein, α subunit internal antibody was from Calbiochem Novabiochem Corp. Horseradish peroxidase-conjugated sheep anti-rat immunoglobulin G (IgG) and Fab fragments were from Boehringer Mannheim. Anti-rat IgG, rhodamine-conjugated anti-rabbit IgG, and horseradish-peroxidase-conjugated goat anti-rabbit IgG were from Sigma Chemical Co. For immunocytochemistry the antibodies were conjugated with fluorescein isothiocyanate (FITC) (1B5) or rhodamine (PC21). Conjugation was carried out with protein (20 μl/mg) and FITC or rhodamine (5 mg/ml) stored in anhydrous dimethyl sulfoxide. Fluorescent reagents were added to the purified antibodies (200 μg) and the conjugation reaction took place in 0.05 M borate buffer, pH 9.0, containing 0.2 M NaCl. The mixture was incubated in the dark for 2 h at room temperature. Finally, labeled antibodies were filtered through a NAP-5 column (Pharmacia Biotech).
Purification of A1R.
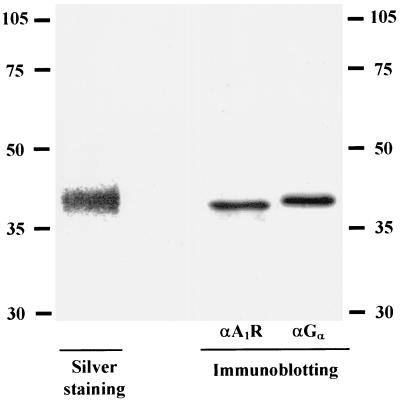
Receptor solubilization from porcine cortical brain membranes was carried out as described elsewhere (6). Receptors solubilized in 0.5% (wt/vol) CHAPS and 0.5% (wt/vol) digitonin were passed through an affinity column prepared, as described by Nakata (22), by coupling 17 mg of XAC in 12 ml of a mixture of DMSO-ethyl alcohol (3:1, vol/vol) to 2 g of activated CH-Sepharose 4B. Before sample application, the affinity column was equilibrated with 50 mM Tris-HCl buffer, pH 7.4, containing 0.1% (wt/vol) CHAPS and 0.1% (wt/vol) digitonin (column buffer). Solubilized extracts of pig brain cortical membranes (60 ml; 2 mg of protein/ml) were applied to a 5-ml XAC affinity column (1.7 by 2.5 cm) at a flow rate of 20 ml/h at 12°C. After application, the column was cooled to 4°C and washed with 25 ml of column buffer at 20 ml/h. Elution was carried out with 40 ml of 100 μM CPT in column buffer at 10 ml/h at room temperature. Fractions (5 ml) were collected over ice and desalted on a Sephadex G-25 column to separate the ligand and the receptor; radioligand binding assays in these fractions or in fractions subjected to a second chromatography through Sephadex G-25 were similar. Routinely, purified A1R was eluted in fractions 2 to 4, which were collected and used for radioligand binding experiments at a receptor concentration of 0.15 to 0.30 nM. Collected fractions were concentrated 5- to 10-fold using Centricon 10 devices (Millipore Ibérica, Barcelona, Spain) for characterization. Electrophoresis of the purified fraction led to a single band by silver staining (Fig. 1). This band of 40,000 Da corresponded to the A1R (26) but also to the αi subunit of G proteins, which have similar molecular masses (19) and copurify with the receptor (Fig. 1). For surface plasmon resonance analysis of protein-protein interactions, the preparation was filtered, equilibrated with 10 mM HEPES (pH 7.4) supplemented with 0.1% CHAPS and 0.1% digitonin, and concentrated using a Centricon 10 device. Taking into account that the stoichiometry of the interaction between a ligand and a receptor is 1:1, the receptor concentration in the purified fractions was calculated as nanomoles of [3H]R-PIA specific binding (at a radioligand concentration of 50 nM) per liter, which is enough to completely saturate the receptor.
FIG. 1.
Identification of purified A1Rs. A1Rs purified as described in Material and Methods were electrophoresed and developed by silver staining and by Western blotting using specific anti-A1R and anti-Gα antibodies. Numbers to the left and right are molecular masses in kilodaltons.
Cell cultures.
DDT1MF-2 smooth muscle cells, originally isolated from a steroid-induced Leiomyosarcoma of Syrian hamster vas deferens (24), were cultured as described elsewhere (11). GH4C1 cells, provided by Francesc Villarroya (Department of Biochemistry and Molecular Biology of the University of Barcelona), were cultured (37°C, 5% CO2) in DMEM supplemented with 15% (vol/vol) horse serum and 2.5% (vol/vol) fetal calf serum, 0.5 μM 2-mercaptoethanol, 1% nonessential amino acids, 2 mM l-glutamine, 1 mM sodium pyruvate, and 1% antibiotics (per milliliter, 10,000 U of penicillin, 10 mg of streptomycin, and 25 μg of amphotericin B). The human leukemia T-cell line Jurkat 32 was grown in RPMI-1640 supplemented with 10% (vol/vol) fetal calf serum, 2 mM l-glutamine, and 1% antibiotics. Human A2AR-transfected Chinese hamster ovary cells were grown in DMEM–Ham's F-12 medium 10% (vol/vol) fetal calf serum, 2 mM l-glutamine, 1% antibiotics, and 400 μg of G418 (Geneticin) per ml.
Chromatography through a peptide-containing Sepharose 4B.
An affinity column was prepared by direct coupling of 2.5 mg of P1 peptide to a 0.5 g of activated thiol-Sepharose 4B via sulfhydryl group chemistry. The P1 peptide, KKVSASSGDPQKYYGKELKC, corresponds to part of the third intracellular loop of rat A1R (between transmembrane-spanning domains V and VI). Cell extracts (4 mg of protein/ml) obtained from 50 million DDT1MF-2 or GH4 cells by lysis (30 min, 4°C) in lysis buffer (20 mM HEPES [pH 7.6], 1% NP-40, 150 mM NaCl, 50 mM NaF, 1 mM phenylmethylsulfonyl fluoride, 20 μM phenylarsine oxide, 10 mM iodoacetamide, 1 mM sodium orthovanadate, 1 mM EGTA, and a cocktail of protease inhibitors [each at 1 μg/ml]: leupeptin, chymostatin, antipain, and pepstatin), were centrifuged at 105,000 × g for 1 h at 4°C. Supernatants were diluted (1:4) using lysis buffer without protease inhibitors. As assessed by immunoblotting this fraction does not contain significant amounts of A1Rs (not shown). After sample application at 4°C, the column was washed at 4°C with 25 ml of a solution containing 20 mM HEPES (pH 7.6), 150 mM NaCl, 50 mM NaF, 1 mM Na3VO4, and 0.25% NP-40. Elution was performed at room temperature using 100 mM glycine buffer, pH 5. Pooled fractions were assayed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and protein staining (see below).
Gel electrophoresis and immunoblotting.
Samples were treated with 10× sample buffer (125 mM Tris-HCl, 10% SDS, 20% glycerol, 5% 2-mercaptoethanol, 0.01% bromophenol blue) for 2 min at 100°C before loading onto the polyacrylamide gels. Electrophoresis was performed according to the method of Laemmli (16), using homogeneous slab gels containing a 15 or 8% acrylamide separating gel and a 6% acrylamide stacking gel, which were running for 1 h at a 30-mA constant current using the Mini-Protean system (Bio-Rad Lab, Hercules, Calif.). Proteins were detected by either silver staining or Coomassie brilliant blue R-250 staining. For silver staining, gels were fixed for 2 min at 50°C with 50% ethanol and 10% acetic acid, sensitized for 6 min with 8.3% glutardialdehyde at 50°C, washed twice (2 min/wash) at 50°C with water, stained for 13 min at 50°C with 0.25% silver nitrate, washed twice (0.5 min/wash) with water at room temperature, and developed with 0.015% formaldehyde–2.5% Na2CO3 at room temperature. Gels were finally washed for 2 min with 5% acetic acid and 3 min with 10% acetic acid and 2.5% glycerol at 50°C. For Coomassie brillant blue R-250 staining, gels were fixed for 1 h with 50% (vol/vol) methanol and 10% (vol/vol) acetic acid in water, stained for 1 h with 0.1% Coomassie brilliant blue diluted in 50% (vol/vol) methanol, and destained with four washes (10 min/wash) with 10% (vol/vol) methanol–7% acetic acid.
For immunoblotting, proteins from acrylamide gels were transferred (1 h at a 125-mA constant current) to Hybond-P polyvinylidene difluoride (PVDF) membranes (Amersham Ibérica SA, Madrid, Spain) using a wet Bio-Rad Trans-Blot, in transfer buffer (25 mM Tris-HCl, 192 mM glycine, 20% methanol, pH 8.3). Nonspecific protein binding sites on the PVDF membranes were blocked by incubation overnight at 4°C using 5% (wt/vol) bovine serum albumin (BSA) in TBS-I (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 0.05% [vol/vol] Tween 20) containing 0.02% (wt/vol) NaN3. After blocking, PVDF membranes were washed three times (10 min/wash) in TBS-II (10 mM Tris-HCl [pH 7.4], 500 mM NaCl, 0.5% Tween 20) and incubated for 2 h with the specific antibody (PC21 [10 μg/ml], PC11 [10 μg/ml], anti-G protein [0.5 μg/ml], or 1:1,000 dilution for 1B5) diluted in TBS-II containing 0.1% (wt/vol) NaN3. The filters were washed three times (10 min/wash) with TBS-II before incubation with the second antibody (horseradish peroxidase-conjugated goat anti-rabbit IgG diluted 1:15,000 for PC11 and PC21 and peroxidase conjugated anti-rat Ig Fab fragments diluted 1:10,000 for 1B5), which was diluted in TBS-I. The filters were washed five times (10 min/wash) with TBS-II and incubated in equal volumes of enhanced chemiluminescence detection solution 1 and solution 2 (Amersham Ibérica). Filters were then placed in contact with Hyperfilms ECL (Amersham Ibérica). Films were developed using a standard photographic procedure.
Protein sequencing.
Eluted fractions containing the proteins interacting with P1 peptide were pooled and concentrated using Ultrafree-15 filter devices (Millipore Ibérica) prior SDS-PAGE. Proteins separated by SDS–8% PAGE were detected with Coomassie brilliant blue. The selected bands were cut with a scalpel and dehydrated by means of two treatments with 50% methanol (10 min each). Samples were sent frozen to the Harvard Microchemistry facility (Cambridge, Mass.), where sequencing was carried out by collisionally activated dissociation on a Finnigan TSQ 7000 triple quadrupole mass spectrometer. Digestion of the protein was performed using Lys-C, and isolation of the peptides was achieved by high-performance liquid chromatography–mass spectroscopy at the Harvard Microchemistry facility.
Immunoprecipitation of hsc73 and A1R.
For immunoprecipitation 60 × 106 DDT1MF-2 cells were suspended in 3.5 ml of 50 mM Tris-HCl, pH 7.4, supplemented with the above-indicated cocktail of protease inhibitors and homogenized using a Polytron (Kinematica) disrupter (PTA 20TS rotor, setting 3; three periods of 5 s separated by an interval of 15 s). Digitonin and CHAPS (final concentrations, 0.5% each) were added to the homogenate, and the mixture was stirred at 4°C for 15 min. The supernatant was obtained by ultracentrifugation at 105,000 × g (1 h, 4°C). Aliquots of the detergent extracts were incubated with the antibodies coupled to protein A-Sepharose CL4B. The antibodies used were PC21 (40 μg/ml) or 1B5 (1:200 dilution). Coupling 1B5 to the protein A-Sepharose required pretreatment of the beads with rabbit anti-rat IgG (1:200 dilution). Fractions were incubated for 2 h at 4°C with constant tumbling. Controls for unspecific binding were performed using the same protocol but omitting the specific antibodies. Immunoprecipitates were washed once with 0.5% CHAPS–0.5% digitonin in tryptic soy agar (TSA) buffer, once with 0.1% CHAPS–0.1% digitonin in TSA buffer, and once with TSA buffer alone. Then, samples were suspended in 100 μl of 10× SDS-PAGE sample buffer and treated 2 min at 100°C prior to the electrophoretic procedure.
Immunocytochemistry.
Double immunofluorescence assays for hsc73 and A1R, A2AR, or A2BR were performed in DDT1MF-2, CHO-A2A, and Jurkat 32 cells, respectively. Cells grown on glass coverslips were permeabilized and fixed using a solution containing 10 mM phosphate-buffered saline (PBS) (pH 7.4), 2% paraformaldehyde, 2% sucrose, and 0.02% saponin; washed for 5 min with 10 mM PBS (pH 7.4); washed for 15 min with this buffer supplemented with 20 mM glycine and 0.02% saponin; and washed for 15 min with incubation buffer (10 mM PBS [pH 7.4], 20 mM glycine, 1% BSA, 0.05% N3Na, and 0.02% saponin). Cells were then incubated for 1 h in the dark at 37°C with fluorescein-conjugated 1B5 (1:200 dilution) and/or rhodamine-conjugated PC21 (25 μg/ml). Alternatively, cells were incubated with fluorescein-conjugated 1B5 (1:200 dilution) and anti-A2AR (40 μg/ml) or anti-A2BR (75 μg/ml) and rhodamine-conjugated anti-rabbit IgG (1:200). After five washes (10 min/wash) with incubation buffer, coverslips were mounted in immunofluorescence medium. Observations were performed using a Leica TCS 4D confocal scanning laser microscope.
Cortical hemispheres from embryonic day 17 rat embryos were dissected, and primary cultures of rat cortical neurons were prepared as previously described (32). Primary cultures of neurons, grown on glass coverslips, were fixed with 4% paraformaldehyde, washed for 10 min with 10 mM PBS–20 mM glycine, permeabilized with 0.5% Triton X-100 for 5 min, and washed for 15 min with PBS–20 mM glycine–1% BSA–0.05% N3Na. Cells were then incubated for 1 h in the dark at 37°C with fluorescein-conjugated 1B5 (1:200 dilution) and rhodamine-conjugated PC21 (25 μg/ml). Rinsed coverslips were mounted, and fluorescence was visualized.
Rat cerebellum was embedded in OCT (Electron Microscopy Sciences) and frozen in liquid nitrogen-cooled isopentane. Eight-micrometer-thick sections of frozen rat cerebella were cut on a cryostat cooled to −18°C. Sections were collected onto SuperFrost Plus (BDH) slides, air dried, and stored at −70°C. For immunofluorescence, the sections were blocked for 30 min in 10% donkey serum in Tris-buffered saline (TBS) (150 mM NaCl–50 mM Tris-HCl, pH 7.5). Slides were incubated with fluorescein-conjugated 1B5 (1:200 dilution) and rhodamine-conjugated anti-A1R (PC21) (5 to 10 μg/ml) for 1 h at room temperature and then washed twice for 5 min in TBS. Sections were mounted with Vectashield immunofluorescence medium (Vector Laboratories, Bretton, United Kingdom).
Radioligand binding experiments.
Membrane suspensions of porcine cortical brain were obtained according to the method of Casadó et al. (6). Radioligand binding assays of membrane suspensions (0.5 mg of protein/ml) or purified receptors (0.15 to 0.30 nM) were carried out at 25°C after preincubating with or without modulators for 30 min in 50 mM Tris-HCl buffer, pH 7.4. Additions of 2 nM [3H]R-PIA, 2 nM [3H]DPCPX, or 0.2 nM [35S]GTPγS were then made. After 90 min of radioligand incubation, free and membrane-bound ligand were separated by rapid filtration of 500-μl aliquots in a Brandel (Gaithersburg, Md.) cell harvester through Whatman GF/C filters embedded in polyethylenimine (except for [35S]GTPγS) as described previously (6). In the case of purified receptors, free and bound ligand were separated by precipitation with polyethylene glycol 8000 and bovine gamma globulins as described previously for solubilized receptors (6). Nonspecific binding was determined in the presence of 1 μM unlabeled ligand.
Saturation binding experiments of purified receptors were carried out under identical conditions by incubating with [3H]R-PIA (0.2 to 28 nM) for the time necessary (0.5 to 6 h) to achieve the equilibrium for each ligand concentration at 25°C.
In all cases, the filters were incubated with 10 ml of Ecoscint H scintillation cocktail (National Diagnostics, Atlanta, Ga.) overnight at room temperature. Radioactivity counts in the vials were determined using a Packard 1600 TRI-CARB scintillation counter with an efficiency of 58%.
Surface plasmon resonance assays.
The surface plasmon resonance assays were performed using a BIAcore equipment (Pharmacia Biosensor AB). The immobilization of hsc73 on the CM5 sensor surface was performed according to method of Johnsson et al. (15). Carboxyl groups on the matrix were transformed into reactive N-hydroxysuccinimide esters by injection of 35 μl of a mixture of N-hydroxysuccinimide (11.5 mg/ml) and N-ethyl-N′-(dimethylaminopropyl) carbodiimide hydrochloride (7.5 mg/ml) in water. Covalent coupling of amine groups in hsc73 was achieved using 35 μl of hsc73 (50 μg/ml) dissolved in 10 mM acetate buffer, pH 4. Then, 80 μl of 1 M ethanolamine hydrochloride (pH 8.5) was added to block excess reactive groups. The coupling process was performed under a continuous flow (5 μl/min) of HBS (10 mM HEPES [pH 7.4], 150 mM NaCl, 3.4 mM EDTA, 0.005% surfactant P20). Noncovalently bound proteins were removed by washing overnight with HBS. In a typical immobilization, the equivalent to 5,000 resonance units was immobilized.
For analysis of protein-protein interactions, samples were filtered, equilibrated with 10 mM HEPES (pH 7.4) supplemented with 0.1% CHAPS and 0.1% digitonin, and concentrated using a Centricon 10 device. To minimize solvent effects, antibodies, all control proteins, and the A1R purified preparation (see above) were placed in the same buffer, containing the indicated detergents, prior to the assays. Assays were performed at 25°C and a constant flow of 5 μl/min using the same solution. The Quickinject command was used to manage all the steps. The volume used for sample injection was 35 μl. For kinetic parameter calculations, five concentrations of purified A1Rs and an injection volume of 40 μl were used.
Data analysis.
Equilibrium binding isotherms and the hsc73 inhibition curve of [3H]R-PIA binding to purified receptors were analyzed by nonlinear regression using the GRAFIT program (Erithacus software Ltd.). The total binding data (specific binding to A1R plus nonspecific binding) were fitted to the previously described equations (6, 7). Three replicates of each point were performed. In surface plasmon resonance assays, association and dissociation data were fitted, using the same nonlinear regression program, to the previously described equations (6). In all cases, goodness of fit was tested according to the reduced χ2 value given by the program. Models were compared using an F test as described by Munson and Rodbard (21).
When means ± standard deviations (SD) are given, differences between groups have been tested for significance (P < 0.05) using Student's t test for unpaired samples.
RESULTS
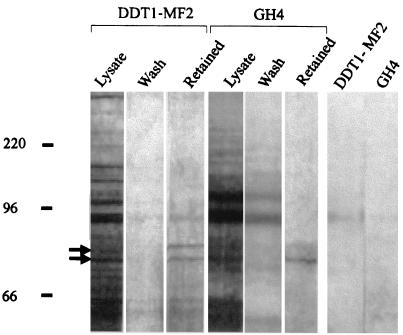
A peptide (P1, KKVSASSGDPQKYYGKELK) corresponding to a sequence in the third intracellular loop of rat A1R (see Materials and Methods) was used in the search for proteins interacting specifically with A1Rs. The peptide was coupled to thiol-activated Sepharose 4B, and lysates from rat pituitary-derived cells (GH4 cells) were subjected to affinity chromatography. After exhaustive washing, elution of retained proteins was performed by using an acidic buffer. Electrophoresis of the eluted fractions led to the identification of two proteins of 73 and 77 kDa (Fig. 2).
FIG. 2.
Identification of proteins interacting with P1 peptide. An affinity column prepared with P1 peptide, which corresponds to the third intracellular loop of the rat sequence of A1R, was used to search for proteins interacting with A1Rs. Lysates from DDT1MF-2 or GH4 cells were chromatographed, and aliquots of the eluates were assayed by electrophoresis. Silver staining led to the detection of two specific bands in the retained fraction, which is that eluted by an acidic wash (see Materials and Methods). The major (73-kDa) band and the minor (77-kDa) band are marked by arrows. The control for nonspecific binding to the column was obtained by analyzing acidic wash eluates corresponding to a column in which P1 peptide was not coupled to the resin (last two lanes on the right).
Unlike the 73-kDa protein, the molecule of 77 kDa could not be sequenced. For analysis the 73-kDa band was digested with Lys-C, and two peptides were sequenced as indicated in Materials and Methods. The sequences of these peptides are SQIHDIVLVGGSTRIPK and VCNPIITK.
These sequences are identical to the predicted sequences of peptides obtained after digestion with Lys-C of rat heat shock cognate protein hsc73, which is the major hsp70-like protein in unstressed rat cells. Although other members of the hsp70 family share homology with hsc73, the sequence of these peptides obtained by hydrolysis of the peptide bond in lysines shows 100% identity only with that of hsc73. Thus, the electrophoretic band isolated after affinity chromatography using P1 corresponds unequivocally to rat hsc73.
The same type of analysis was performed using extracts from Syrian hamster was deferens DDT1MF-2 cells. The pattern of elution of the gel chromatography was similar to that found with the GH4 cell line (Fig. 2), and digestion and sequencing of the 73-kDa band led to the identification of peptides SFYPEEVSSMVLTK and EIAEAYLGK.
Although, to our knowledge, hsc73 from Syrian hamster has not been cloned, the sequence of these peptides is identical to that of rat hsc73. The band from GH4 and DDT1MF-2 cells was also identified by immunoblotting using commercial anti-rat hsc73 antibodies (data not shown). These results indicate that the homology of hsc73 from rat and Syrian hamster is very high and that hsc73 from DDT1MF-2 or GH4 cells extracts binds the P1 peptide.
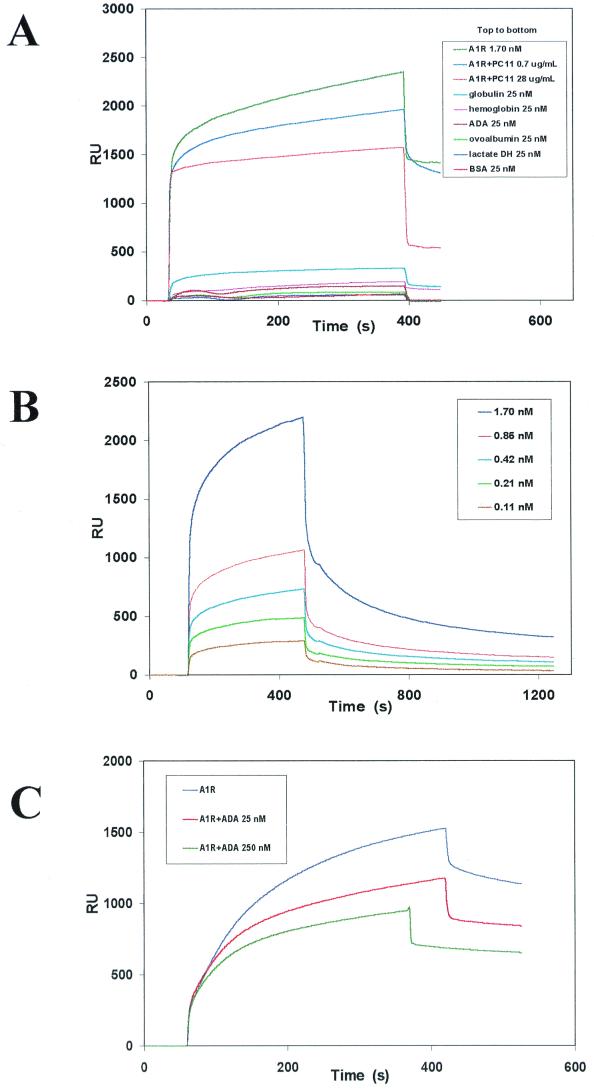
Investigation of a possible interaction between hsc73 and the complete A1R molecule was first attempted by using a purified preparation of the receptor and biosensor technology. BIAcore equipment, which detects specific molecular interactions based on surface plasmon resonance techniques, was used to analyze the interaction between A1R and hsc73. When hsc73 was coupled to the biosensor surface (see Materials and Methods) and a preparation of purified A1Rs from pig cerebral cortex was tested, a specific binding signal in the sensorgram, which is indicated by the curve at the upper-left part of the trace, was observed (Fig. 3). No specific signal was seen using ADA, serum albumin, ovalbumin, or lactate dehydrogenase (up to 25 nM each). With 25 nM bovine hemoglobin or 25 nM bovine gamma globulin a very small signal was observed (Fig. 3A). None of these proteins, except ADA (see below), significantly reduced the binding of A1R to hsc73 (not shown). The specificity of the signal was also assessed by a dose-dependent reduction of the signal in the presence of PC11 antibody, which is directed against the P1 peptide (Fig. 3A). At a concentration of PC11 antibody of 28 μg/ml, the specific signal disappeared (orange trace in Fig. 3A), indicating that the antibody is able to completely block the hsc73-A1R interaction. The signal due to A1R binding to hsc73 was dose dependent (Fig. 3B), and from the real-time association (upper left-hand curves for each trace) and dissociation (upper right-hand curves for each trace) curves the kinetic constants of association and dissociation (kas and kdis, respectively) were calculated (kas = 0.016 ± 0.006 s−1 nM−1; kdis = 0.0078 ± 0.0009 s−1). The affinity of the interaction (Kd = 0.5 ± 0.1 nM) was similar irrespective of the method of calculation (quotient [kdis/kas] or calculation based on saturation data by nonlinear regression). This high-affinity interaction reinforces the view that the A1R-hsc73 recognition is specific.
FIG. 3.
Surface plasmon resonance analysis of proteins interacting with commercial hsc73. (A) A1Rs and different commercial proteins were analyzed for their capacity to bind to commercial hsc73 coupled to the sensor surface. The interaction between A1R and hsc73 was analyzed in the absence or in the presence of the anti-P1 antibody PC11 at two concentrations (0.7 and 28 μg/ml). Real time monitoring of a biospecific interaction was plotted as a function of resonance units (RU) versus time. (B) Dose-response analysis of the interaction of purified A1Rs to commercial hsc73 coupled to the sensor surface. (C) Effect of two different concentrations of ADA upon binding of A1Rs to hsc73. In all panels, the specific signal is indicated by the upper, curved, part of the traces. The vertical part of the traces is irrelevant since it is due to a solvent and/or concentration effect. In panel A, traces corresponding to control proteins have been shifted downward by eliminating the unspecific signal to avoid overlapping with the three upper traces.
In 1996 we described how ADA interacts with A1R on the surface of DDT1MF-2 cells (11). The same applies to GH4 cells (unpublished data). Furthermore, the binding of ADA to A1R is necessary for an efficient signal transduction via agonist-induced activation of A1Rs (11). Therefore, we tested whether addition of ADA affected the surface plasmon resonance signal elicited via the A1R-hsc73 interaction. The binding of purified A1R to hsc73 was significantly reduced in a dose-dependent fashion by ADA (Fig. 3C).
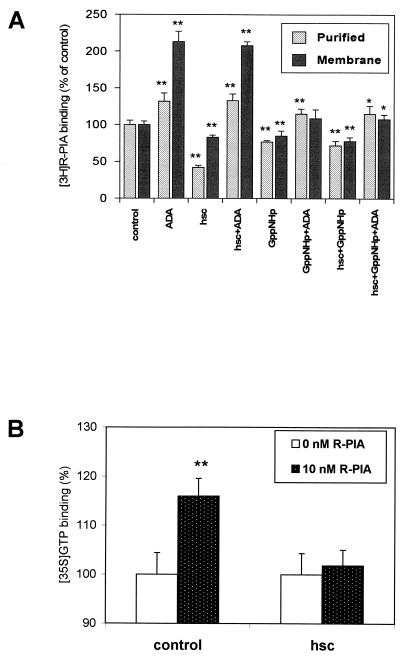
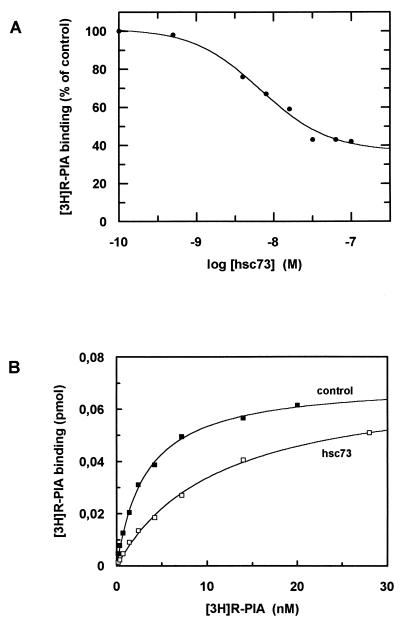
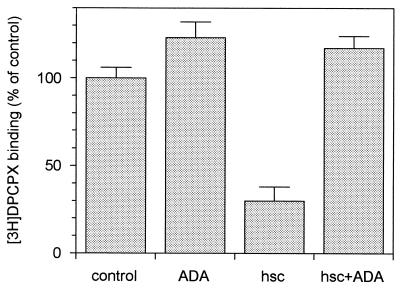
Assays of ligand binding to A1R in brain-derived membranes were performed in the presence of ADA, hsc73, and/or the guanine nucleotide analog Gpp(NH)p (Fig. 4A). Consistent with our previous findings (27), 0.2 U of ADA per ml increased the 2 nM [3H]R-PIA binding to membranes. As expected, 100 μM Gpp(NH)p had a negative effect upon ligand binding, which was more pronounced in the presence of ADA (Fig. 4A). Interestingly, 30 nM hsc73 led to a reduction in ligand binding, which was reverted by ADA (Fig. 4A). In contrast, hsc73 had no effect when Gpp(NH)p was present, irrespective of the presence or absence of ADA. Similar assays were performed with a preparation of purified receptors, in which heterotrimeric G proteins were present as assessed by immunoblotting using antibodies directed against a common epitope of α subunits (Fig. 1). Due to the presence of G proteins, Gpp(NH)p also decreased the binding of [3H]R-PIA to purified receptors as happened in cell membranes. The results concerning the effects of hsc73 in the purified preparation were similar to those obtained in membranes. Remarkably, the effect of hsc73 was stronger than that of Gpp(NH)p (Fig. 4A). The binding of [35S]GTPγS in the presence or absence of hsc73 was investigated in cell membranes. Again the effect of the heat shock protein was the prevention of an R-PIA-induced increase in [35S]GTPγS binding (Fig. 4B). From dose-response data shown in Fig. 5A, the hsc73-mediated maximum reduction in [3H]R-PIA binding was 63 ± 2% with respect to the binding in the absence of hsc73 and the 50% inhibitory concentration was 7 ± 1 nM. hsc73 modified the dissociation constant of the binding of [3H]R-PIA to A1Rs (from 3.2 ± 0.2 to 11 ± 1 nM for an hsc73 concentration of 30 nM) without change in the maximum capacity of specific binding (Fig. 5B). As occurred in membranes, this strong effect was completely prevented by Gpp(NH)p and/or ADA (Fig. 4A). hsc73 also reduced the antagonist binding, an effect which was also blocked by ADA (Fig. 6).
FIG. 4.
Effect of different reagents upon the binding of [3H]R-PIA or [35S]GTPγS. (A) Effect of 25 nM ADA, 30 nM hsc73, and/or 100 μM Gpp(NH)p upon 2 nM [3H]R-PIA binding to membranes or purified A1R preparations. (B) Effect of 30 nM hsc73 on 0.2 nM [35S]GTPγS binding to membranes in the absence of R-PIA (0 nM R-PIA) or presence of 10 nM R-PIA. Values are given as percentages of R-PIA-induced increase of [35S]GTPγS binding with respect to membranes in absence of R-PIA. Data are means ± SD (error bars) of a representative experiment performed in quadruplicate. ∗, P < 0.05; ∗∗, P < 0.01.
FIG. 5.
Characterization of the effect of hsc73 upon 2 nM [3H]R-PIA binding to purified A1R preparations. Data are from a representative experiment performed in triplicate. The parameters were maximum binding (Bmax) = 0.071 ± 0.002 pmol/mg of protein and Kd = 3.2 ± 0.2 nM in the absence of hsc73 and Bmax = 0.072 ± 0.001 pmol/mg of protein and Kd = 11 ± 1 nM in the presence of hsc73. (A) Dose-response curve of the effect of hsc73 upon 2 nM [3H]R-PIA specific binding. (B) Saturation isotherm of [3H]R-PIA specific binding to purified A1Rs in the absence or presence of 30 nM hsc73.
FIG. 6.
Effect of 30 nM hsc73 upon 2 nM [3H]DPCPX binding to purified A1Rs in the absence or presence of 25 nM ADA. Data are means ± SD (error bars) of a representative experiment performed in quadruplicate.
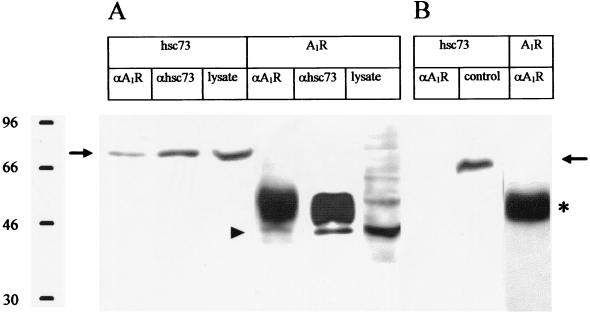
Further analysis of the molecular recognition between A1R and hsc73 in DDT1MF-2 cells was carried out by immunoprecipitation and confocal microscopy. Using the PC21 antibody, which is directed against an extracellular loop of the receptor (see Materials and Methods), hsc73 coprecipitated with A1Rs. Commercial monoclonal antibodies against hsc73 also coprecipitated a band of 43 kDa, corresponding to intact A1R (Fig. 7A). PC21 antibody did not detect any band of 43 kDa when a commercial preparation of hsc73 was used (Fig. 7B). This experiment was performed as a control to rule out the presence of nonspecific bands in the 43-kDa region, which is proximal to the region where the heavy chain of immunoglobulins appears, and also to show that the anti-A1 antibody does not recognize hsc73. As described elsewhere (11) and as deduced from Fig. 7A, PC21 immunoprecipitates A1Rs with low efficiency (about 10% of the total amount of A1Rs in the lysates). In contrast, the efficiency of the antibody against the heat shock cognate protein is high (Fig. 7A). Nevertheless, the hsc73 molecules coprecipitating with A1Rs were detectable by SDS-PAGE (Fig. 7A). These results indicate that a high percentage of 43-kDa A1R molecules are bound to hsc73.
FIG. 7.
Coprecipitation assays. (A) A1Rs and hsc73 were immunoprecipitated from DDT1MF-2 lysates using either a polyclonal antibody (PC21) directed against an extracellular loop of A1Rs (αA1R) (11) or a commercial anti-hsc73 monoclonal antibody (αhsc73). Immunoprecipitates and lysates were immunoblotted and developed using either of the two antibodies (αA1R or αhsc73). (B) Same as in panel A, but using a commercial preparation of hsc73 instead of cell lysates. The control lane corresponds to a preparation of commercial hsc73 immunoblotted using antibodies directed against hsc73 (αhsc73). The hsc73 band is indicated by an arrow. The 43-kDa band corresponding to A1Rs in indicated by an arrowhead. The heavy chain of immunoglobulins is marked by an asterisk.
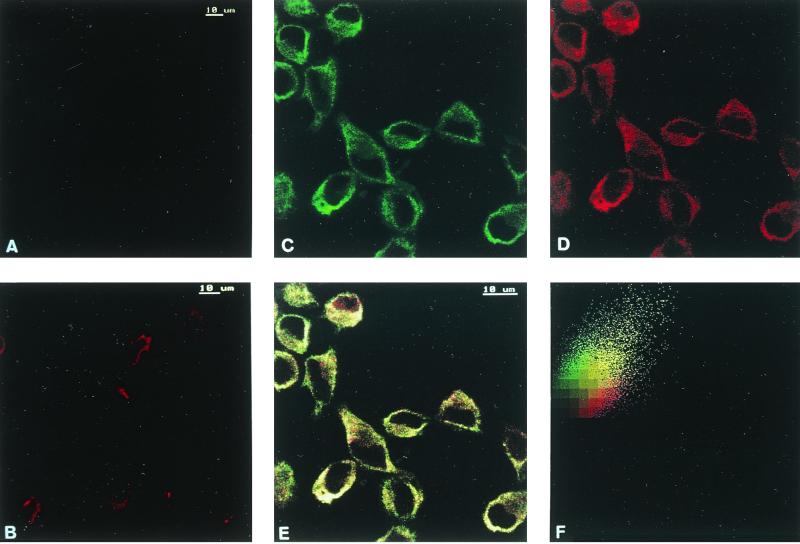
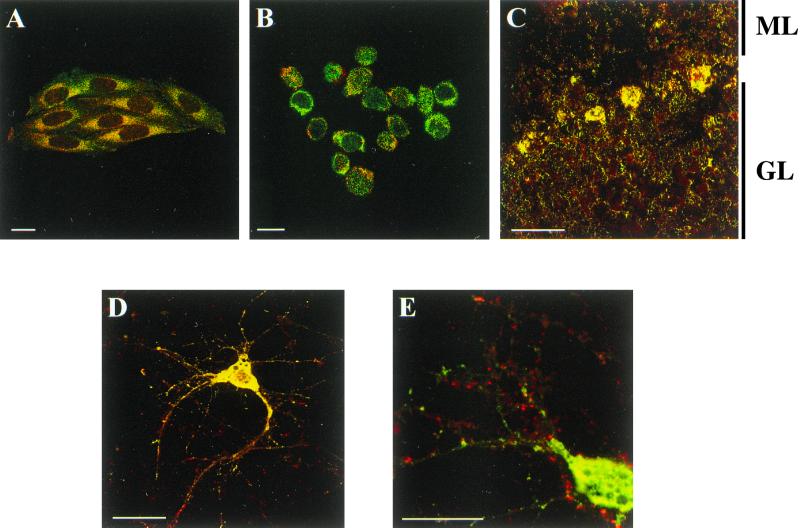
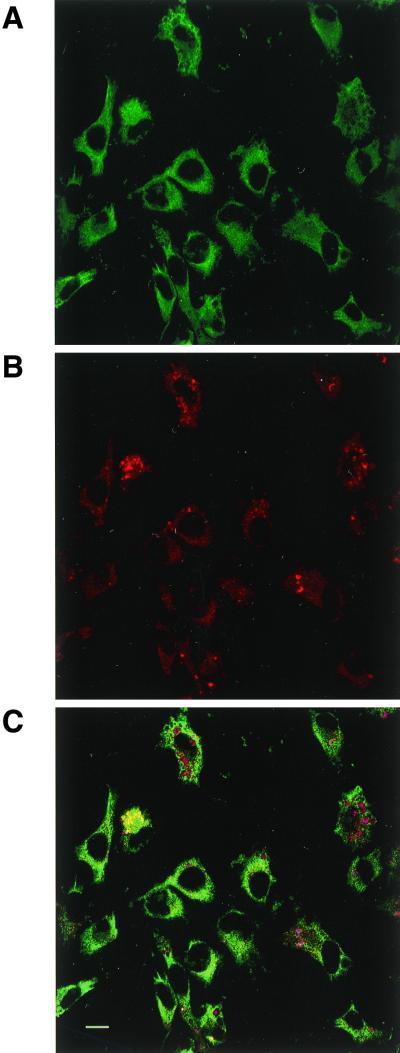
Codistribution assays between A1R and hsc73 were performed in DDT1MF-2 cells by immunocytochemistry and confocal microscopy. Immunofluorescence studies performed in nonpermeabilized cells showed very low levels of hsc73 (Fig. 8A) and confirmed the presence of A1R on the cell surface (Fig. 8B). Consistent with the cytosolic localization of hsc73, the label corresponding to this protein was very low in nonpermeabilized cells (Fig. 8A) and high in permeabilized cells (Fig. 8C). In permeabilized cells double immunofluorescence staining showed that the patterns of distribution of A1R (Fig. 8D) and hsc73 (Fig. 8C) were similar, with a relatively high degree of colocalization (Fig. 8E and F). There were no specific zones of colocalization, thus suggesting that the A1R-hsc73 interaction is not restricted to a specific region within the cell. Similar results were obtained in CHO cells transfected with the cDNA for A1R (not shown). Colocalization between hsc73 and other adenosine receptors, such as A2AR, overexpressed in CHO cells (Fig. 9A), or A2BR, which are naturally expressed in Jurkat T lymphocytes (18) (Fig. 9B) was very low. A1R-hsc73 colocalization was also investigated in slices of rat cerebellum, where A1Rs are distributed among different layers of the tissue in a widespread manner. There, the colocalization between A1R and hsc73 was restricted to some cells in the molecular layer and a few cells in the granulosa layer (Fig. 9C). On the other hand, A1R-hsc73 colocalization was markedly high in the body of primary cultures of cortical neurons from rat embryos (Fig. 9D). In these cortical neurons it was shown that A1R also appear in dendrites and synapses, whereas hsc73 did not. This was also proved by the poor colocalization between hsc73 and metabotropic glutamate 4 receptor, which is a marker of synaptic terminals (Fig. 9D). These results strongly suggest that A1R-hsc73 coupling occurs in vivo and is specific for A1R and not for other members of the G protein-coupled receptor superfamily.
FIG. 8.
Immunostaining of hsc73 (in green) and A1Rs (in red) in DDT1MF-2 cells. Immunostaining of hsc73 was performed in nonpermeabilized (A) and permeabilized (C) cells using fluorescein-conjugated anti-hsc73 1B5 antibody. Immunostaining of A1Rs was performed in nonpermeabilized (B) or permeabilized (D) cells using rhodamine-conjugated anti-A1R PC21 antibody. (E) The hsc73-A1R colocalization is indicated in yellow. See Materials and Methods for details about the immunocytochemical procedure.
FIG. 9.
Colocalization of hsc73 (green) and A1Rs, A2ARs, A2BRs or mGluR2/3 (red). (A) Immunostaining of hsc73 and A2ARs in CHO-A2A cells. (B) Immunostaining of hsc73 and A2BRs in Jurkat 32 cells. (C) Immunolocalization of hsc73 and A1R in horizontal sections from rat cerebellum. ML, molecular layer; GL, granular layer. (D) Immunostaining of hsc73 and A1Rs in primary cultures of rat cortical neurons. (E) Immunostaining of hsc73 and mGluR4 in primary cultures of rat cortical neurons. Scale bar, 10 μm. See Materials and Methods for details about immunocytochemical procedures.
R-PIA-induced internalization of A1Rs results in sequestration of the receptor in intracellular vesicles, an effect that is potentiated in the presence of ADA (3, 28). Internalization of A1R was monitored by confocal microscopy after culturing the cells in the presence of a fluorochrome-conjugated anti-A1R antibody (PC21), ADA, and the ligand R-PIA (see reference 3). Colocalization studies performed in chronically treated cells further permeabilized and immunolabeled using the anti-hsc73 antibody showed that hsc73 is present in some of the A1R-containing vesicles but it is absent from others (Fig. 10). In the vesicles where both proteins are present, the colocalization approaches 100% (Fig. 10C). In contrast, colocalization is negligible in the vesicles where only the rhodamine-conjugated anti-A1R antibody is detected (Fig. 10C).
FIG. 10.
Immunostaining of hsc73 and A1R in internalization vesicles. DDT1MF-2 cells were cultured in the presence of rhodamine-conjugated PC21 anti-A1R antibody, and of 2 U of ADA per ml and 50 nM R-PIA to induce internalization of the receptor (28). After 24 h of culture, cells were washed, permeabilized, and immunolabeled using the FITC-conjugated anti-hsc73 antibody (see Materials and Methods and the legend to Fig. 8). (A) Immunostaining of hsc73 appears in green; (B) immunostaining of A1R appears in red; (C) colocalization is indicated in yellow.
DISCUSSION
Members of the hsp70 family interact with a number of cellular proteins. Due to the molecular chaperone function of hsp70 proteins, they appear capable of recognizing nonnative forms of proteins (3). Therefore, interaction between partially unfolded proteins may occur in members of the molecular chaperone family. The results presented here indicate that hsc73 interacts with functional A1Rs. The first indication of this is the coimmunoprecipitation of hsc73 and 43-kDa A1Rs. Cytosolic hsp70 proteins acting as chaperones can be coimmunoprecipitated with nascent chains but not with completely synthesized and folded proteins (1, 2, 29). On the other hand, adenosine receptors are heptaspanning membrane proteins whose ligand binding capacity is very sensitive to changes in structure. As a matter of fact, a precise combination of detergent and salts is required to achieve a high recovery of binding sites in solubilized preparations of A1Rs. Thus, the strong effect of nanomolar concentrations of hsc73 upon ligand binding to purified A1Rs is evidence for a specific interaction between hsc73 and functional A1Rs. The fact that colocalization between A1R and hsc73 is not restricted to a specific zone of the cell (Fig. 8) is evidence that the interaction occurs with with completely folded, functional receptors.
The substrate binding specificity of hsc73 is still a matter of controversy (31). Despite the fact that some of the interactions established with hsc73 seem to be nonspecific, some requires a precise structure. Thus, Terlecky et al. (31) found that hsc73 binds to a variety of proteins at peptide regions structurally similar to KFERQ. These motifs target proteins interacting with hsc73 for degradation in lysosomes (8, 31). BiP, a member of the hsp70 family localized in the endoplasmic reticulum, recognizes motifs containing heptapeptides in which hydrophobic amino acids alternate with any other amino acid (3). Inoue et al. (14) suggested that a similar motif in the retinoblastoma gene product, pRb, is involved in the interaction with hsc73. The third intracellular loop region in which hsc73 binds to A1R does not contain any of these motifs. Moreover, there is no similarity between P1 and the N-terminal region of p53 with which hsc73 seems to interact (17). The A1R binding domain in hsc73 is thus probably different from the carboxyl-terminal region of the protein, which is involved in the recognition of KFERQ-like sequences (26). On the other hand, due to the poor sequence homology between A1R and calmodulin, the A1R-binding domain might be different from the calmodulin-binding domain, which seems to reside in an amphipathic helical region near the center of hsp70 protein molecules (30). On the basis of this and the high affinity of the binding it is probable that A1R interacts with a specific region in hsc73.
Regulation of ligand binding and G protein activation, measured by the binding of radioligands or [35S]GTPγS, by the coupling between hsc73 and A1R is a relevant finding. Overall, the effect of hsc73 is negative upon ligand binding and upon ligand-induced activation of G proteins. As mentioned above (Results), the effect of hsc73 upon purified A1Rs was even stronger than that exerted by Gpp(NH)p, which uncouples receptors from G proteins. The blocking of this hsc73-induced structural change by ADA is also a very interesting finding since it is not due to competition for the binding to A1R. Although the region of A1R involved in the binding to ADA has not been mapped it is known that the interaction occurs via A1R extracellular domains (11). On the other hand, the ADA-induced modification of equilibrium parameters of ligand binding to A1Rs (27) indicates that the interaction between ADA and A1R leads to conformational changes in the receptor molecule. The surface plasmon resonance technique has been helpful in demonstrating that ADA, which does not bind to hsc73 (Fig. 8A), prevents binding of A1R to hsc73. Taken together these data suggest that conformational changes induced by ADA interacting with the extracellular motifs of A1R affect the structure of the third intracellular loop and inhibit the binding of hsc73 to A1R. This would explain, at least in part, the ADA-induced increase in the affinity of agonist binding (11). The increase in ligand binding affinity due to the presence of ADA may be a consequence of an ADA-induced partial disruption of the binding of hsc73 to A1R.
The high colocalization obtained in some specific areas of rat cerebellum and in the neuronal body but not in dendrites or terminals suggest that A1R-hsc73 interaction occurs in vivo and illustrates its specificity. In fact, we have failed to obtain in neuronal primary cultures evidence for a similar type of interaction with other members of the G protein-coupled receptor superfamily. Thus, hsc73 does not colocalize with either A2AR (Fig. 9A), A2BR (Fig. 9B), or metabotropic glutamate 4 (Fig. 9E) receptors.
The experiments of agonist-induced receptor internalization demonstrate that internalized cell surface receptors, which are found in intracellular vesicles as previously described (3), can be found associated or not with hsc73. In fact, part of the intracellular label due to sequestration of the anti-A1R antibody is found in yellow vesicles, which indicate the presence and colocalization of the receptor and the hsc73 in these vesicles. In contrast, part of the internalized anti-A1R antibody is found in red vesicles where hsc73 is not present (Fig. 10C). This finding would fit with a mechanism of receptor internalization in which hsc73 participates in the formation of early endosomes but is released from early formed vesicles and, therefore, will be absent from vesicles formed at later steps. As discussed above, ADA could be a physiological modulator of the hsc73-cell surface A1R recognition. On the other hand, ecto-ADA can, to some extent, be internalized together with the A1R (28). Taken together, these data open the possibility that cell surface A1R-ADA complexes and A1Rs not bound to ADA follow distinct internalization pathways; A1R-ADA complexes would not likely traffic in association with hsc73, whereas A1R not bound to ADA would be internalized in hsc73-containing vesicles. In fact the number of vesicles lacking hsc73 is smaller when assays are performed in the absence of exogenous ADA (not shown), thus supporting the view that cell surface A1Rs are internalized in two qualitative different types of vesicles, one containing hsc73 and another lacking the heat shock cognate protein.
The novel findings presented here suggest a specific role for hsp70 proteins in regulating the activation and operation of A1Rs. Although a relevant role for chaperones in signaling by steroid hormone receptors has already been demonstrated (4, 5), our results are the first evidence suggesting the control by hsc73 of signaling via plasma membrane G protein-coupled receptors.
ACKNOWLEDGMENTS
S.S. and V.C. contributed equally to this work.
This work was supported by grants from the Fondo de Investigaciones Sanitarias de la Seguridad Social (reference no. 91/0272), from CIRIT-CICYT (QFN93/4423), and from CICYT (PB97/0984, SAF97/0066, and BIO99-0601-C02-02).
We thank Catalina Relaño (Servei de Cultius Cel.lulars) and Susana Castel and Jaume Comas (Serveis Científico-Tècnics) for their excellent technical assistance and Robin Rycroft (Servei d'Assesorament Lingüístic) for assistance in the preparation of the manuscript. We thank Ampar Castell (Biokit company, Lliça d'Amunt, Barcelona, Spain) for the advice which facilitated the production of rabbit antibodies.
REFERENCES
- 1.Beckmann R P, Mizzen L A, Welch W J. Interaction of hsp70 with newly synthesized proteins: implications for protein folding and assembly. Science. 1990;248:850–854. doi: 10.1126/science.2188360. [DOI] [PubMed] [Google Scholar]
- 2.Beckmann R P, Lovett M, Welch W J. Examining the function and regulation of hsp70 in cells subject to metabolic stress. J Cell Biol. 1992;117:1137–1150. doi: 10.1083/jcb.117.6.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blond-Elguindi S, Cwirla S E, Dower W J, Lipscutz R J, Sprang S R, Sambrook J F, Gething M-J H. Affinity panning of a library of peptides displayed on bacteriophages reveals the binding specificity of BiP. Cell. 1993;75:717–728. doi: 10.1016/0092-8674(93)90492-9. [DOI] [PubMed] [Google Scholar]
- 4.Bohen S P, Kralli A, Yamamoto K R. Hold'em and fold'em: chaperones and signal transduction. Science. 1995;268:1303–1304. doi: 10.1126/science.7761850. [DOI] [PubMed] [Google Scholar]
- 5.Caplan A J, Langley E, Wilson E M, Vidal J. Hormone-dependent transactivation by the human androgen receptor is regulated by a dnaJ protein. J Biol Chem. 1995;270:5251–5257. doi: 10.1074/jbc.270.10.5251. [DOI] [PubMed] [Google Scholar]
- 6.Casadó V, Cantí C, Mallol J, Canela E I, Lluís C, Franco R. Solubilization of A1 adenosine receptor from pig brain: characterization and evidence of the role of the cell membrane on the coexistence of high and low affinity states. J Neurosci Res. 1990;26:461–473. doi: 10.1002/jnr.490260409. [DOI] [PubMed] [Google Scholar]
- 7.Casadó V, Casillas T, Mallol J, Canela E I, Lluis C, Franco R. The adenosine receptors present on the plasma membrane of chromaffin cells are of the A2b subtype. J Neurochem. 1992;59:425–431. doi: 10.1111/j.1471-4159.1992.tb09388.x. [DOI] [PubMed] [Google Scholar]
- 8.Chiang H-L, Terlecky S R, Plant C P, Dice J F. A role for a 70-kilodalton heat shock protein in lysosomal degradation of intracellular proteins. Science. 1989;246:382–385. doi: 10.1126/science.2799391. [DOI] [PubMed] [Google Scholar]
- 9.Ciruela F, Robbins M J, Willis A C, McIlhinney R A. Interactions of the C terminus of metabotropic glutamate receptor type 1alpha with rat brain proteins: evidence for a direct interaction with tubulin. J Neurochem. 1999;72:346–354. [PubMed] [Google Scholar]
- 10.Ciruela F, Casado V, Mallol J, Canela E I, Lluis C, Franco R. Immunological identification of A1 adenosine receptors in brain cortex. J Neurosci Res. 1995;42:818–828. doi: 10.1002/jnr.490420610. [DOI] [PubMed] [Google Scholar]
- 11.Ciruela F, Saura C, Canela E I, Mallol J, Lluis C, Franco R. Adenosine deaminase affects ligand-induced signalling by interacting with cell surface adenosine deaminase. FEBS Lett. 1996;380:219–223. doi: 10.1016/0014-5793(96)00023-3. [DOI] [PubMed] [Google Scholar]
- 12.Ciruela F, Soloviev M M, Chan W Y, McIlhinney R A J. Homer 1c/Ves1-1L modulates the cell surface targeting of metabotropic glutamate receptor type 1α: evidence for an anchoring function. Mol Cell Neurosci. 2000;15:36–50. doi: 10.1006/mcne.1999.0808. [DOI] [PubMed] [Google Scholar]
- 13.Freissmuth M, Schutz W, Linder M E. Interactions of the bovine brain A1-adenosine receptor with recombinant G protein α subunits. Selectivity for rGiα-3. J Biol Chem. 1991;266:17778–17783. [PubMed] [Google Scholar]
- 14.Inoue A, Torigoe T, Sogahata K, Kamiguchi K, Takahashi S, Sawada Y, Saijo M, Taya Y, Ishii S, Sato N, Kikuchi K. 70-kDa heat shock cognate protein interacts directly with the N-terminal region of the retinoblastoma gene product pRB. J Biol Chem. 1995;270:22571–22576. doi: 10.1074/jbc.270.38.22571. [DOI] [PubMed] [Google Scholar]
- 15.Johnsson B, Löfas S, Lindquist F. Immobilization of proteins to a carboxymethyldextran-modified gold surface for biospecific interaction analysis in surface plasmon resonance sensors. Anal Biochem. 1991;198:268–277. doi: 10.1016/0003-2697(91)90424-r. [DOI] [PubMed] [Google Scholar]
- 16.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 17.Lam K T, Calderwood S K. hsp70 binds specifically to a peptide derived from the highly conserved domain (I) region of p53. Biochem Biophys Res Commun. 1992;184:167–174. doi: 10.1016/0006-291x(92)91174-o. [DOI] [PubMed] [Google Scholar]
- 18.Mirabet M, Herrera C, Cordero O J, Mallol J, Lluis C, Franco R. Expression of A2B adenosine receptors in human lymphocytes: their role in T cell activation. J Cell Sci. 1999;112:491–502. doi: 10.1242/jcs.112.4.491. [DOI] [PubMed] [Google Scholar]
- 19.Mumby S M, Gilman A G. Synthetic peptide antisera with determined specificity for G protein alpha or beta subunits. Methods Enzymol. 1991;195:215–233. doi: 10.1016/0076-6879(91)95168-j. [DOI] [PubMed] [Google Scholar]
- 20.Munshi R, Pang I-H, Sternweis P C, Linden J. A1 adenosine receptors of bovine brain couple to guanine nucleotide-binding proteins Gi1, Gi2 and Go. J Biol Chem. 1991;266:22285–22289. [PubMed] [Google Scholar]
- 21.Munson P J, Rodbard D. LIGAND: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980;107:220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- 22.Nakata H. Affinity chromatography of A1 adenosine receptors of rat brain membranes. Mol Pharmacol. 1989;35:780–786. [PubMed] [Google Scholar]
- 23.Nanoff C, Waldhoer M, Roka F, Freissmuth M. G protein coupling of the rat A1-adenosine receptor. Partial purification of a protein which stabilizes the receptor-G protein association. Neuropharmacology. 1997;36:1211–1219. doi: 10.1016/s0028-3908(97)00135-4. [DOI] [PubMed] [Google Scholar]
- 24.Norris J S, Gorski J, Kohler P O. Androgen receptors in Syrian hamster ductus deferens tumor cell line. Nature. 1974;248:422–424. doi: 10.1038/248422a0. [DOI] [PubMed] [Google Scholar]
- 25.Palmer T M, Stiles G L. Adenosine receptors. Neuropharmacology. 1995;34:683–694. doi: 10.1016/0028-3908(95)00044-7. [DOI] [PubMed] [Google Scholar]
- 26.Rippmann F, Taylor W R, Rothbard J B, Green N M. A hypothetical model for the peptide binding domain of hsp70 based on the peptide binding domain of HLA. EMBO J. 1991;10:1053–1059. doi: 10.1002/j.1460-2075.1991.tb08044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saura C, Ciruela F, Casado V, Canela E I, Mallol J, Lluis C, Franco R. Adenosine deaminase interacts with A1 adenosine receptors in pig brain cortical membranes. J Neurochem. 1996;66:1675–1682. doi: 10.1046/j.1471-4159.1996.66041675.x. [DOI] [PubMed] [Google Scholar]
- 28.Saura C, Mallol J, Canela E I, Lluis C, Franco R. Adenosine deaminase and A1 adenosine receptors are internalized together following agonist-induced receptor desensitization. J Biol Chem. 1998;273:17610–17617. doi: 10.1074/jbc.273.28.17610. [DOI] [PubMed] [Google Scholar]
- 29.Sheffield W P, Shore G C, Randall S K. Mitochondrial precursor protein. Effects of 70-kilodalton heat shock protein on polypeptide folding, aggregation and import competence. J Biol Chem. 1990;265:11069–11076. [PubMed] [Google Scholar]
- 30.Stevenson M A, Calderwood S K. Members of the 70-kilodalton heat shock protein family contain a highly conserved calmodulin-binding domain. Mol Cell Biol. 1990;10:1234–1238. doi: 10.1128/mcb.10.3.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Terlecky S R, Chiang H-L, Olson T S, Dice J F. Protein and peptide binding and stimulation of in vitro lysosomal proteolysis by the 73-kDa heat shock cognate protein. J Biol Chem. 1992;267:9202–9209. [PubMed] [Google Scholar]