Abstract
Objectives
Histoplasmosis and cryptococcosis are important public health problems in people living with HIV (PLHIV) in Central America. Conventional laboratory assays, based on microscopy and culture, are not optimal for the diagnosis of either disease. However, antigen (Ag) assays are rapid and highly accurate for the diagnosis of these infections.
Methods
Laboratory surveillance of PLHIV was carried out in four hospitals in Panama, Honduras and Nicaragua, between 2015 and 2019. Detection of Histoplasma antigens in urine was performed by enzyme immunoassay (EIA), and Cryptococcus antigen detection in sera and cerebrospinal fluid specimens was performed by lateral flow assay (LFA).
Results
A total of 4,453 PLHIV with clinical suspicion of histoplasmosis (n = 1,343) or cryptococcosis (n = 3,110; 2,721 sera and 389 CSF) were tested. Of 1,343 patients suspected of having histoplasmosis, 269 (20%) were Histoplasma Ag positive. Of 3,110 patients tested using the Cryptococcus Ag assay, 329 (11%) were positive. Honduras reported the highest positivity rates (32% for Histoplasma Ag, and 16% for Cryptococcus Ag); Panama reported the largest number of patients testing positive using the Histoplasma Ag assay (n = 201); and Nicaragua reported the largest number of patients testing positive using the Cryptococcus Ag assay (n = 170).
Conclusion
Here, we show how the implementation of rapid diagnostics assays impacted case detection and was useful for the care of people with advanced HIV. Rapid and accurate diagnosis could reduce mortality associated with histoplasmosis and cryptococcosis in PLHIV.
Keywords: AIDS, cryptococcosis, Cryptococcus, Histoplasma, histoplasmosis, HIV
1. INTRODUCTION
People living with HIV (PLHIV) with advanced diseases are at high risk of developing multiple opportunistic infections including histoplasmosis and cryptococcosis. 1 , 2 , 3 , 4 Clinical signs and symptoms of these infections are often non‐specific and therefore difficult to accurately diagnose and differentiate from other infections caused by non‐fungal pathogens (eg tuberculosis). 5 , 6 , 7 Conventional laboratory methods for diagnosing histoplasmosis and cryptococcosis, such as culture and histopathology, are challenging because they require complex laboratory infrastructure, laboratory technicians with training in mycology, and long turnaround times for results (days to weeks). 8 , 9 , 10
In the Latin America regions, there was an estimation of 1.9 million of PLHIV, and about 132,000 PLHIV were estimated in the Central America Region (CAR), as well as the highest estimated number of new HIV infections each year as reported by UNAIDS gap report. 11 High mortality is seen among persons who develop progressive disseminated histoplasmosis (PDH), and data from a cohort of HIV/AIDS patients with suspected histoplasmosis in Guatemala suggest that mortality from patients with maybe as high as 44% before 2009. 12 Similarly, over 90% of the meningitis cases caused by Cryptococcus neoformans, known as cryptococcal meningitis, occur in PLHIV, a condition that represents the main risk factor for development of this mycosis. Cryptococcal meningitis is the most important clinical presentation in these patients and is associated with high mortality rates, from 9% to 70% depending of the world's region. 13
Rapid diagnostics assays (RDA) directly impact the quality of patient care. 3 , 7 , 10 , 12 , 14 , 15 , 16 , 17 For opportunistic fungal diseases, RDAs have been shown to impact several aspects of patient care such as increasing the number of histoplasmosis cases detected (up to 3 times compared with conventional assays) and reducing mortality of patients with histoplasmosis and cryptococcosis by 50% compared with conventional assays. 3 , 7 , 10 , 12 , 15 , 16 , 17 , 18 , 19 Several RDAs for the diagnosis of histoplasmosis and cryptococcosis are available commercially. They were developed for the detection of circulating fungal antigens (Ag) and are available as enzyme immunoassays (EIA) and in lateral flow assay (LFA) formats. Current World Health Organization (WHO) guidelines for the diagnosis and treatment of cryptococcosis and histoplasmosis in PLHIV recommend Ag detection assays for rapid and accurate diagnosis of both diseases. 20 , 21 Additionally, both assays have been validated in various laboratories in Latin America, showing high analytical performance (sensitivity and specificity higher than 95%). 22 , 23 , 24
This report presents the experience of implementation of RDAs for detection of histoplasmosis and cryptococcosis in PLHIV in three Panama, Honduras and Nicaragua.
2. MATERIAL AND METHODS
2.1. Patient population
In this study, hospitalised adult patients (older than 18 years) with advanced HIV disease were enrolled. A diagnostic workup for histoplasmosis and cryptococcosis was performed in hospitalised patients with advanced HIV disease, defined as a CD4 cell count less than 200 cells/mm3 or a WHO clinical stage 3 or 4 event at presentation for care. 25 Selection of antigen test was done by clinical suspicion for histoplasmosis or cryptococcosis. Clinical suspicion for histoplasmosis was defined as at least three of the following signs or symptoms: fever, pancytopenia, weight loss, lesions of skin or mucosa, pulmonary lesions on radiography or clinical suspicion of tuberculosis (TB). Clinical suspicion of cryptococcosis was defined as one of the following signs or symptoms: headache, loss of vision, stiff neck or neurological deficit.
The study was carried out in four hospitals in three Central American countries: Hospital A was located in Panama City, Panama, and patients were enrolled between August 2016 to June 2019; Hospital B was located in Tegucigalpa, Honduras and enrolment took place from April 2015 to July 2019; Hospital C was located in San Pedro Sula, Honduras, and patients were enrolled from May 2017 to June 2019; and Hospital D was located in Managua, Nicaragua, and patients were enrolled from February 2016 to September 2018. Histoplasma Ag testing was performed between April 2016 to September 2018, 29 in Hospitals A, B and D; and Cryptococcus Ag testing was performed from April 2015 to September 2019 (54 months) in all hospitals.
2.2. Laboratory assays
Diagnosis of histoplasmosis and cryptococcosis was done according to the recommendation of WHO guidelines for diagnosing and managing both infections on PLHIV. 20 , 21 Detection of Histoplasma urinary antigen was performed using a commercial EIA kit (Clarus Histoplasma GM, product reference HGM201. IMMY). Cryptococcus Ag was detected in sera and cerebrospinal fluid (CSF), using a commercial LFA kit (CrAg® LFA, product reference CR2003. IMMY). RDAs used on this report were previously validated in PLHIV from Colombia and Guatemala. 22 , 23 None of the study places were using the antigen detection assays described, reason why before testing samples from patients, all personnel from laboratories participating in this study were trained in assay performance as well as other aspects related to assay implementation. Hospital medical staff was also trained on different aspects of the histoplasmosis and cryptococcosis including clinical, epidemiological, diagnostic and treatment characteristics of both diseases. All laboratories involved in this study were national accredited.
2.3. Statistical analysis
Information was summarised by calculation of absolute and relative frequencies for categorical variables, continuous variables were evaluated for normality, and summary measures were done. Variable associations or differences were identified using chi‐square tests, or tests of the null hypothesis (Student's t test or Mann‐Whitney U test according to data distribution). These analyses were performed at a 95% confidence level using STATA software version 11.
2.4. Ethical considerations
A non‐research determination study protocol for data use analysis was developed and approved by the local and international institutions collaborating in this project.
3. RESULTS
A total of 4,453 hospitalised PLHIV with clinical suspicion of histoplasmosis (n = 1,343) or cryptococcosis (n = 3,110; 2,721 sera and 389 CSF) were tested during the implementation period (Figure 1). For Cryptococcus Ag LFA (CrAg LFA), the largest number of samples was tested in the third quarter of 2017 (July to September; n = 434 samples tested); the median number of samples tested by quarter was 141 samples/quarter (Interquartile range [IQR]: 6‐299 samples/quarter) (Figure 2). For Histoplasma Ag EIA (HisAg EIA), the second quarter of 2018 (April–June) was the period with most samples tested, n = 230, a median of 165 samples were tested by quarter (IQR: 39–05 samples/quarter).
FIGURE 1.
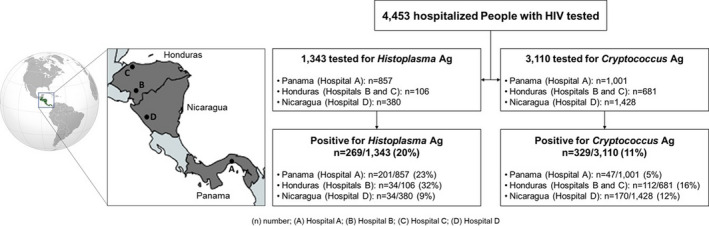
Flowchart of patients tested and detected as positive using Histoplasma and Cryptococcus antigen assays
FIGURE 2.
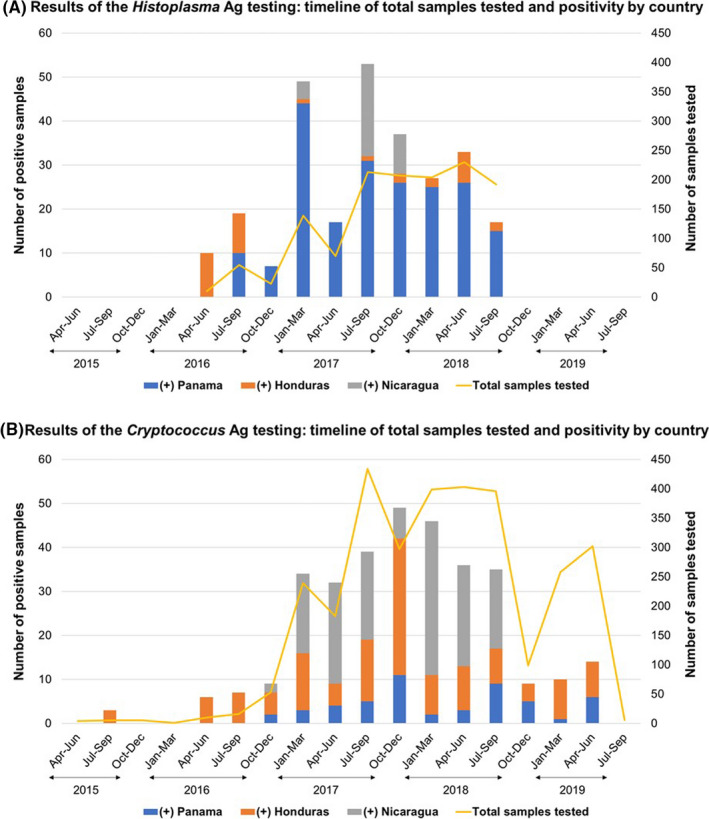
Timeline: description of total samples tested, and positivity according to antigen assay
In Panama (Hospital A), 857 patients were tested using the Histoplasma urinary antigen EIA (HisAg EIA), and 1,001 using the Cryptococcus Ag LFA (CrAg LFA); in Honduras (Hospitals B and C), 106 patients were tested using the HisAg EIA, and 681 patients were tested using the CrAg LFA; and in Nicaragua (Hospital D), 380 patients were tested using the HisAg EIA, and 1,428 patients using the CrAg LFA (Figure 1). Of 4,453 patients, 3,271 (74%) were males. Patient median age was 34 years (IQR: 27–42 years); no statistically significant differences were observed in patient age grouped by sex (p = 0.127). CD4 cell count data were available for 193 of 4,453 (4%) patients tested; median CD4 cell count was 51 cells/µl (IQR: 24–83 cells/µl).
3.1. Positivity of HisAg EIA
Of 1,343 patients tested for urinary Histoplasma Ag 269 (20%) were positive. There were 213 (79%) males and 56 (21%) female patients (sex ratio: 4:1), and the median patient age was 33 years (IQR: 27–38 years old). CD4 cell count data were available in 17 of 269 (6%) histoplasmosis cases with a median CD4 count of 32 cells/µl (IQR: 27–70 cells/µl). By country, in Panama (Hospital A) from 857 patients tested, 201 (23%) were Histoplasma Ag positive; in Honduras, in hospital B, from 106 patients tested, 34 (32%) were Histoplasma Ag positive; and in Nicaragua (Hospital D), from 380 patients HisAg EIA tested, 34 (9%) had a positive result (Figure 1).
3.2. Positivity of CrAg LFA
Of 3,110 (11%) patients tested for CrAg, 329 (11%) were positive (224 [68%] sera and 105 [32%] CSF). Two hundred forty‐one (73%) were males and 88 (27%) were females (sex ratio: 3:1). The median patient age was 36 years old (IQR: 29–43 years old). CD4 cell count data were available in 106 of 329 (32%) cryptococcosis cases, with a median count of 51 cells/µl (IQR: 24–78 cells/µl). In Panama (Hospital A), 47 of 1,001 patients (5%) were Cryptococcus Ag positive; in Honduras, 112 of 681 patients (16%) were Cryptococcus Ag positive (72 of 290 [25%] in hospital B, and 40 of 391 [10%] in hospital C); and in Nicaragua (Hospital D), 170 of 1,428 patients (12%) were Cryptococcus Ag positive (Figure 1).
4. DISCUSSION AND CONCLUSION
This report describes the experience of the implementation of RDAs for the detection of histoplasmosis and cryptococcosis in three Central American countries. In the study, we reported high test positivity, observing that one fifth (20%) patients tested were HisAg positive, and one tenth (10%) were CrAg positive. Honduras presented the highest test positivity, with 32% of those tested positive for HisAg EIA and 16% of those tested positive for CrAg LFA. This study principally entails the description of the implementation of novel laboratory technologies in facilities without laboratory capacity for the diagnosis of histoplasmosis and cryptococcus. It is important to highlight that the transferred assays were previously well validated and personnel on study sited were training and technical support were offered during the study. 22 , 23
The burden of histoplasmosis and cryptococcosis in PLHIV is unclear in Panama, Nicaragua and Honduras; case series are limited and prior seroprevalence studies for histoplasmosis and case series for cryptococcosis. 4 , 26 Historically, histoplasmosis has been described as a global travel‐related disease, but most of infections reported has been linked with travels to countries in the Americas and the Caribbean region, with multiple histoplasmosis outbreaks reported in people travelling to this region, mostly associated with recreational activities (most commonly cave visits). 27 , 28 , 29 , 30 , 31 , 32 , 33 Recently, a modelling study estimated the burden of histoplasmosis in PLHIV based on histoplasmosis seroprevalence studies and regional data about HIV cases in the Latin American region. 4 The authors were able to identify hotspots for histoplasmosis, including the three countries participating in this study, reporting an estimated prevalence of histoplasmosis greater than 30%. 4 A case series from Panama City described 182 cases of histoplasmosis in PLHIV. These cases were diagnosed using conventional diagnostic methods (histopathology and culture) over a period of seven years (from 1997 to 2003). 34 Based on the number of hospitalisations, an 8% prevalence of histoplasmosis was reported; this is much lower than the 23% positivity we found in the Panama hospital in the present study. This discrepancy could be because the use of the HisAg detection assay increases the number of cases detected up to 3 times compared with conventional assays. 7 , 18 , 24
Data on cryptococcosis in PLHIV from Panama, Nicaragua and Honduras are limited. 35 , 36 One report from Panama described 28 cases of cryptococcal meningitis identified over a period of five years between 2007 and 2011(~5 cases/years). However, these patients were diagnosed using India Ink stain and culture. 36 In the present study, in a period of 33 months (2.7 years), we identified 47 patients with positive Cryptococcus Ag in Hospital A (from Panama), approximately 12 cases/years. It is known that the sensitivity of the diagnosis of cryptococcal meningitis by culture and direct examination is near 85%. In comparison, antigen detection has a sensitivity near 100%. 37 Additionally, Ag testing can be performed more easily than conventional assays, Ag testing generate results in a couple of hours in comparison with days or weeks need for culture isolation, these factors related to assay performance may have directly impacted case detection. 10 , 14 , 38
In 2017, a diagnostic laboratory in Guatemala tested a total of 1,953 PLHIV for cryptococcosis and histoplasmosis. This laboratory diagnosed opportunistic infections in 16% of PLHIV tested. 7 Histoplasmosis was diagnosed in 120 of 1,850 (7%) of PLHIV tested, and cryptococcosis was diagnosed in 76 of 1,732 (4%) of PLHIV tested. The prevalence of these infections varies according to the patient's immunological status and are generally observed in PLHIV with advanced disease (CD4 cells <200 cells/mm3). The prevalence increased to 11% for histoplasmosis and 9% on cryptococcosis in patients with CD4 cells <200 cells/mm3, and in patients with CD4 cell counts <100 cells/mm3, prevalence of 15% in histoplasmosis and 11% in cryptococcosis have been reported, frequencies that are like those observed in our study. 7
There were several limitations to this study, including the inability to access patient's clinical and epidemiological information related to risk factors, treatment, outcomes and long‐term impact of RDAs implementation experience. We note that our study does not report prevalence estimates or incidences of these fungal infections, and we limited our description to the report of positive and negative results. All the countries currently follow national and international guidelines on case management for advanced HIV fungal opportunistic infections. 20 , 21 By obtaining early diagnosis, these guidelines can be implemented correctly.
This study shows the importance of implementing rapid and highly accurate diagnostic assays in resource‐limited settings where diagnosis of histoplasmosis and cryptococcosis is limited or lacking, resulting in an impact through early detection for appropriated adequate treatment and case management of both diseases. Since 2017, WHO developed and released guidelines to address advanced HIV disease, including screening, diagnosis and treatment of most common opportunistic infections and co‐infections in PLHIV. In addition, WHO in recent years developed specific guidelines for the diagnosis and treatment of histoplasmosis and cryptococcosis among PLHIV, both guidelines recommend the use of Ag testing on these patients. 20 , 21 , 25 These assays also present other advantages, high analytical performance and shorter turnaround time for results, in comparison with conventional diagnostics, such as commercial availability of the kits, which facilitates the implementation of these assays in resource‐limited settings. Since 2019, the WHO has included these Ag detection assays in the second model list of essential in vitro diagnostics. 39 Further investigation evaluating the impact of these technologies in reduction of morbidity and mortality, economic impact and improvement of patient quality of life are needed.
AUTHOR CONTRIBUTION
Diego H Caceres: Conceptualization (equal); Data curation (equal); Formal analysis (equal); Investigation (equal); Methodology (equal); Project administration (equal); Resources (equal); Software (equal); Supervision (equal); Validation (equal); Visualization (equal); Writing‐original draft (equal); Writing‐review & editing (equal). Ana Belen Arauz: Investigation (equal); Resources (equal); Supervision (equal); Writing‐review & editing (equal). Carlos Flores: Investigation (equal); Writing‐review & editing (equal). Sandra Montoya: Investigation (equal); Writing‐review & editing (equal). Carlos Saenz: Investigation (equal); Writing‐review & editing (equal). Felipe A. Torres‐Meneses: Investigation (equal); Writing‐review & editing (equal). Hortencia Esther Peralta Lara: Investigation (equal); Writing‐review & editing (equal). Julio Cesar Zuniga‐Moya: Investigation (equal); Writing‐review & editing (equal). Isis Zohar Lainez Arteaga: Investigation (equal); Writing‐review & editing (equal). Arturo Garcia: Investigation (equal); Writing‐review & editing (equal). Jose Abdo: Investigation (equal); Writing‐review & editing (equal). Paul E Verweij: Investigation (equal); Writing‐original draft (equal); Writing‐review & editing (equal). Tom Chiller: Conceptualization (equal); Funding acquisition (equal); Investigation (equal); Methodology (equal); Project administration (equal); Resources (equal); Writing‐original draft (equal); Writing‐review & editing (equal). Diana Forno: Conceptualization (equal); Data curation (equal); Formal analysis (equal); Funding acquisition (equal); Investigation (equal); Methodology (equal); Project administration (equal); Supervision (equal); Writing‐original draft (equal); Writing‐review & editing (equal).
DISCLAIMER
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC).
ACKNOWLEDGEMENTS
We want to thank Jovanna Borace, Evelyn Gutierrez de Rodriguez and Roberto Flores‐Reyna for support activities related to the implementation of this study, and Mark Mezzullo for editing the manuscript. Diego H. Caceres would like to acknowledge Oak Ridge Institute for Science and Education (ORISE).
Caceres DH, Arauz AB, Flores C, et al. Implementation of rapid diagnostics assays for detection of histoplasmosis and cryptococcosis in central american people living with HIV. Mycoses. 2021;64:1396–1401. 10.1111/myc.13303
Funding information
All authors report no potential conflicts of interest. This study was funded by the United States President's Emergency Plan for AIDS Relief (PEPFAR). The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the CDC.
Contributor Information
Diego H. Caceres, Email: diegocaceres84@gmail.com.
Ana B. Arauz, Email: anabelenarauz@gmail.com.
Paul E. Verweij, Email: Paul.Verweij@radboudumc.nl.
Tom M. Chiller, Email: tnc3@cdc.gov.
REFERENCES
- 1. Deepe GS Jr, Dolin R, Blaser MJ. 265‐Histoplasma capsulatum (Histoplasmosis) A2. In: Bennett JE, Mandell D, (Eds.). and Bennett's Principles and Practice of Infectious Diseases (Eighth Edition). Philadelphia: Content Repository Only!; 2015:2949‐2962. [Google Scholar]
- 2. Perfect JR, Dolin R, Blaser MJ. 264‐Cryptococcosis (Cryptococcus neoformans and Cryptococcus gattii) A2. In: Bennett JE, Mandell D, (Eds.). and Bennett's Principles and Practice of Infectious Diseases (Eighth Edition). Philadelphia: Content Repository Only!; 2015:2934‐2948. [Google Scholar]
- 3. Rajasingham R, Smith RM, Park BJ, et al. Global burden of disease of HIV‐associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis. 2017;17(8):873‐881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Adenis AA, Valdes A, Cropet C, et al. Burden of HIV‐associated histoplasmosis compared with tuberculosis in Latin America: a modelling study. Lancet Infect Dis. 2018;18(10):1150‐1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Adenis A, Nacher M, Hanf M, et al. Tuberculosis and histoplasmosis among human immunodeficiency virus‐infected patients: a comparative study. Am J Trop Med Hyg. 2014;90(2):216‐223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Caceres DH, Valdes A. Histoplasmosis and Tuberculosis Co‐occurrence in people with advanced HIV. J Fungi. 2019;5):73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Samayoa B, Aguirre L, Bonilla O, et al. The diagnostic laboratory hub: a new health care system reveals the incidence and mortality of tuberculosis, histoplasmosis, and cryptococcosis of PWH in guatemala. Open Forum Infectious Diseases. 2020;7(1):ofz534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Caceres DH, Knuth M, Derado G, Lindsley MD. Diagnosis of progressive disseminated histoplasmosis in advanced HIV: a meta‐analysis of assay analytical performance. J Fungi (Basel). 2019;5(3):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Azar MM, Hage CA. Laboratory diagnostics for histoplasmosis. J Clin Microbiol. 2017;55(6):1612‐1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rajasingham R, Wake RM, Beyene T, Katende A, Letang E, Boulware DR. Cryptococcal meningitis diagnostics and screening in the era of point‐of‐care laboratory testing. J Clin Microbiol. 2019;57(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. UNAIDS . The GAP report. http://www.unaids.org/sites/default/files/media_asset/UNAIDS_Gap_report_en.pdf2013 [Google Scholar]
- 12. Samayoa B, Roy M, Cleveland AA, et al. High mortality and coinfection in a prospective cohort of human immunodeficiency virus/acquired immune deficiency syndrome patients with histoplasmosis in Guatemala. Am J Trop Med Hyg. 2017;97(1):42‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. Aids. 2009;23(4):525‐530. [DOI] [PubMed] [Google Scholar]
- 14. Valencia Y, Cáceres DH, de Bedout C, Cano LE, Restrepo Á. Frequency of invasive fungal disease in adults: experience of a specialized laboratory in medellín, Colombia (2009–2015). J Fungi (Basel). 2020;6(1):2009‐2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Falci DR, Monteiro AA, Braz Caurio CF, et al. Histoplasmosis, an underdiagnosed disease affecting people living with HIV/AIDS in Brazil: results of a multicenter prospective cohort study using both classical mycology tests and histoplasma urine antigen detection. Open Forum Infectious Dis. 2019;6(4). ofz073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nalintya E, Meya DB, Lofgren S, Huppler Hullsiek K, Boulware DR, Rajasingham R. A prospective evaluation of a multisite cryptococcal screening and treatment program in HIV clinics in Uganda. J Acquir Immune Defic Syndr. 2018;78(2):231‐238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Beyene T, Zewde AG, Balcha A, et al. Inadequacy of high‐dose fluconazole monotherapy among cerebrospinal fluid cryptococcal antigen (CrAg)‐positive human immunodeficiency virus‐infected persons in an ethiopian CrAg screening program. Clin Infect Dis. 2017;65(12):2126‐2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Caceres DH, Zuluaga A, Arango‐Bustamante K, et al. Implementation of a training course increased the diagnosis of histoplasmosis in Colombia. Am J Trop Med Hyg. 2015;93(3):662‐667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bansal N, Sethuraman N, Gopalakrishnan R, et al. Can urinary histoplasma antigen test improve the diagnosis of histoplasmosis in a tuberculosis endemic region? Mycoses. 2019;62(6):502‐507. [DOI] [PubMed] [Google Scholar]
- 20. PAHO/WHO . Guidelines for Diagnosing and Managing Disseminated Histoplasmosis among People Living with HIV 2020. https://iris.paho.org/bitstream/handle/10665.2/52304/9789275122495_eng.pdf?sequence=1&isAllowed= [PubMed] [Google Scholar]
- 21. World Health Organization (WHO) . Guidelines for the Diagnosis, Prevention and Management of Cryptococcal Disease in HIV‐Infected Adults, Adolescents and Children. Supplement to the 2016 Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection. 2018. https://www.who.int/hiv/pub/guidelines/cryptococcal‐disease/en/ [PubMed] [Google Scholar]
- 22. Caceres DH, Samayoa BE, Medina NG, et al. Multicenter validation of commercial antigenuria reagents to diagnose progressive disseminated histoplasmosis in people living with HIV/AIDS in two Latin American countries. J Clin Microbiol. 2018;56(6):e01959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Caceres DH, Zuluaga A, Tabares AM, Chiller T, Gonzalez A, Gomez BL. Evaluation of a cryptococcal antigen lateral flow assay in serum and cerebrospinal fluid for rapid diagnosis of cryptococcosis in Colombia. Rev Inst Med Trop Sao Paulo. 2017;59:e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Medina N, Alastruey‐Izquierdo A, Mercado D, et al. Comparative performance of the laboratory assays used by a diagnostic laboratory hub for opportunistic infections in people living with HIV. Aids. 2020;34(11):1625‐1632. [DOI] [PubMed] [Google Scholar]
- 25. World Health Organization (WHO) . Guidelines for managing advanced HIV disease and rapid initiation of antiretroviral therapy. 2017.https://www.who.int/hiv/pub/guidelines/advanced‐HIV‐disease/en/ [PubMed] [Google Scholar]
- 26. Firacative C, Lizarazo J, Illnait‐Zaragozí MT, Castañeda E. The status of cryptococcosis in Latin America. Mem Inst Oswaldo Cruz. 2018;113(7):e170554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lyon GM, Bravo AV, Espino A, et al. Histoplasmosis associated with exploring a bat‐inhabited cave in Costa Rica, 1998–1999. Am J Trop Med Hyg. 2004;70(4):438‐442. [PubMed] [Google Scholar]
- 28. Ariaans M, Valladares MJ, Keuter M, Verweij P, van der Ven AJ, de Mast Q. Fever and arthralgia after 'volcano boarding' in Nicaragua. Travel Med Infect Dis. 2017;16:68‐69. [DOI] [PubMed] [Google Scholar]
- 29. Farina C, Rizzi M, Ricci L, Gabbi E, Caligaris S, Goglio A. Imported and autochthonous histoplasmosis in Italy: new cases and old problems. Rev Iberoam Micol. 2005;22(3):169‐171. [DOI] [PubMed] [Google Scholar]
- 30. Weinberg M, Weeks J, Lance‐Parker S, et al. Severe histoplasmosis in travelers to Nicaragua. Emerg Infect Dis. 2003;9(10):1322‐1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Flor A, Estivills D, Pérez R, et al. Acute pulmonary histoplasmosis in a Spanish traveller to Nicaragua: an imported disease case. Rev Iberoam Micol. 2003;20(1):24‐28. [PubMed] [Google Scholar]
- 32. Nygård K, Brantsaeter A, Feruglio S, et al. Histoplasmosis among travellers to central America. Tidsskr Nor Laegeforen. 2006;126(21):2838‐2842. [PubMed] [Google Scholar]
- 33. Bahr NC, Antinori S, Wheat LJ, Sarosi GA. Histoplasmosis infections worldwide: thinking outside of the Ohio River valley. Curr Trop Med Rep. 2015;2(2):70‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gutierrez ME, Canton A, Sosa N, Puga E, Talavera L. Disseminated histoplasmosis in patients with AIDS in Panama: a review of 104 cases. Clin Infect Dis. 2005;40(8):1199‐1202. [DOI] [PubMed] [Google Scholar]
- 35. Crabtree Ramírez B, Caro Vega Y, Shepherd BE, et al. Outcomes of HIV‐positive patients with cryptococcal meningitis in the Americas. Int J Infect Dis. 2017;63:57‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gonzalez DC, Arauz A, Rodríguez FA. Meningitis por cryptococcus neoformans en pacientes con SIDA. Rev Med Panama. 2013;33(2):3‐7. [Google Scholar]
- 37. Medina N, Alastruey‐Izquierdo A, Bonilla O, et al. A Rapid Screening Program for Histoplasmosis, Tuberculosis, and Cryptococcosis Reduces Mortality in HIV Patients from Guatemala. J Fungi (Basel). 2021;7(4):268. 10.3390/jof7040268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Temfack E, Rim JJB, Spijker R, et al. Cryptococcal antigen in serum and cerebrospinal fluid for detecting cryptococcal meningitis in adults living with HIV: systematic review and meta‐analysis of diagnostic test accuracy studies. Clin Infect Dis. 2020;72(7):1268‐1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. World Health Organization (WHO) . Second WHO Model List of Essential In Vitro Diagnostics. 2019. https://www.who.int/medical_devices/publications/Standalone_document_v8.pdf?ua=1 [Google Scholar]


