Abstract
The Ni‐catalysed cross‐coupling of aryl ethers is a powerful method to forge new C−C and C−heteroatom bonds. However, the inert C(sp2)−O bond means that a canonical mechanism that relies on the oxidative addition of the aryl ether to a Ni0 centre is thermodynamically and kinetically unfavourable, which suggests that alternative mechanisms may be involved. Here, we provide spectroscopic and structural insights into the anionic pathway, which relies on the formation of electron‐rich hetero‐bimetallic nickelates by adding organometallic nucleophiles to a Ni0 centre. Assessing the rich co‐complexation chemistry between Ni(COD)2 and PhLi has led to the structures and solution‐state chemistry of a diverse family of catalytically competent lithium nickelates being unveiled. In addition, we demonstrate dramatic solvent and donor effects, which suggest that the cooperative activation of the aryl ether substrate by Ni0‐ate complexes plays a key role in the catalytic cycle.
Keywords: catalysis, cross-coupling, hetero-bimetallic compounds, nickel, organolithium
Assessment of the rich co‐complexation chemistry of Ni(COD)2 and PhLi has led to a new family of structurally diverse lithium nickelates being uncovered. Combined stoichiometric, catalytic, and kinetic studies reveal that these hetero‐bimetallic complexes may be the key intermediates that facilitate the smooth C−O bond cleavage and cross‐coupling of aryl ethers under mild conditions.
Introduction
Since the discovery of the Ni‐catalysed cross‐coupling of Grignard reagents by the groups of Kumada [1] and Corriu [2] in 1972, nickel has remained a popular choice for forging a range of C−C and C−heteroatom bonds.[ 3 , 4 ] Although other methods using milder nucleophiles combined with a Pd catalyst are typically favoured due to their exceptional functional group tolerance,[ 5 , 6 ] there are many examples where nickel catalysts still reign supreme. For example, Ni can engage in single‐electron reactivity and manoeuvre across different oxidation states, thereby enabling new bond‐forming strategies. [7] In addition, nickel catalysts can activate strong σ‐bonds outside the scope of Pd catalysis, thus allowing unconventional, but readily available electrophiles, such as phenol derivatives, to be functionalised.[ 8 , 9 , 10 ]
In 1979, Wenkert et al. [11] reported that aryl ethers could serve as electrophiles in Kumada–Corriu type cross‐coupling reactions with Grignard reagents under Ni catalysis. Unlike aryl esters and other activated phenol‐derived electrophiles[ 8 , 10 ] in which the C(sp2)−O bond can be cleaved by Ni0 species, the direct oxidative addition of aryl ethers is thermodynamically and kinetically unfavourable,[ 12 , 13 , 14 , 15 , 16 ] which suggests that non‐conventional mechanisms are in operation, particularly for transformations occurring under mild conditions. Wang, Uchiyama, and co‐workers have provided theoretical support for an alternative anionic pathway (Scheme 1) through DFT calculations, which identified that anionic Ni0‐ate complexes are key intermediates that enable C−O bond cleavage.[ 14 , 16 ] Nickelate intermediates have been proposed in several Ni‐promoted functionalisation reactions,[ 17 , 18 , 19 , 20 ] but catalytically relevant examples have rarely been isolated [21] and direct experimental evidence for their role in the anionic pathway has yet to be established.
Scheme 1.
Proposed anionic pathway in the nickel‐catalysed cross‐coupling of aryl ethers.
Anionic nickelates were widely studied in the 1970s and 1980s to gain insights into the “nickel effect”, [22] a key starting point for the development of Ziegler catalysts. These investigations also succeeded the chemistry of “naked nickel”, [23] from which many ubiquitous Ni0 complexes spawned. [24] Early studies realised that Ni0‐olefin complexes react with organometallic nucleophiles to form a hetero‐bimetallic nickelate.[ 25 , 26 , 27 , 28 , 29 , 30 , 31 ] However, the ability to observe or isolate these sensitive complexes depends on the Lewis acidity and basicity of the two species, which is influenced by the choice of ligand and substituents, and this interaction can be represented as an equilibrium that often lies towards the mono‐metallic components. [19]
Herein, we provide detailed spectroscopic and structural insights into the anionic pathway through the isolation and characterisation of a series of structurally diverse lithium nickelates derived from Ni(COD)2 and PhLi. In addition, we demonstrate dramatic solvent and donor effects that suggest that the hetero‐bimetallic nickelates work cooperatively to enable the smooth activation of the aryl ether substrate.
Results and Discussion
We have probed the addition of PhLi to the ubiquitous Ni0 source Ni(COD)2 (COD=1,5‐cyclooctadiene). Although COD alone is rarely the optimal ligand in Ni‐catalysed reactions, there are examples where it is still effective, particularly for the functionalisation of aryl ethers under mild conditions.[ 18 , 32 , 33 ] We chose an organolithium reagent because of its enhanced nucleophilicity, spectroscopic handle (7Li NMR), and to avoid complications arising from Schlenk‐type equilibria which often plague Grignard reagents. Several examples of Ni‐catalysed cross‐coupling reactions of organolithium compounds and aryl ethers have been reported,[ 32 , 33 , 34 ] and this has also been extended to the synthesis of π‐conjugated polymers. [35]
Adding one equivalent of PhLi to Ni(COD)2 in [D8]THF led to complete consumption of PhLi, as shown by 1H and 7Li NMR spectroscopy, whereas about 50 % of the Ni(COD)2 remained unreacted, which suggests that formation of a 2:1 lithium nickelate was favoured (Figure 1 a). Studies on the effect of the concentration (Figure 1 b) revealed that at high concentrations an additional minor species, assigned as the 1:1 lithium nickelate Li(THF)2PhNiCOD (1 a), formed. This concentration dependence is proposed to originate from an equilibrium between a contacted ion pair (1 a) and a solvent‐separated ion pair (1 a′), with 1 a′ undergoing a rapid dissociation to give a 2:1 lithium nickelate Li2(THF)4Ph2NiCOD (2 a) and Ni(COD)2. 1H DOSY NMR studies support the assignment of each proposed species in solution on the basis of diffusion coefficients and estimated molecular weights (Figure 1 c). The complex solution chemistry of 1 a/1 a′ and its equilibrium with 2 a and Ni(COD)2 contrasts with that of Li(TMEDA) n PhNi(C2H4)2, reported by Cornella and co‐workers, [36] which was obtained from Ni(C2H4)3 and PhLi with excess TMEDA. However, evidence for the interconversion between the contact ion pair and solvent‐separated ion pair was also seen. Equilibria between lower order (1:1 Li/M) and higher order (2:1 Li/M) hetero‐bimetallic species have been observed for lithium zincates[ 37 , 38 ] but not explicitly reported for nickel, where the formation of nickelates is typically governed by steric demand and geometrical preferences.[ 39 , 40 , 41 ]
Figure 1.
a) 1:1 reaction between Ni(COD)2 and PhLi in THF showing the proposed equilibrium between different species. b) Stacked 1H NMR spectra in [D8]THF at various concentrations. c) 1H DOSY NMR spectrum at 0.15 m in [D8]THF.
A series of 2:1 lithium nickelates was prepared by treating Ni(COD)2 with two equivalents of PhLi in the presence of a donor (Scheme 2). The THF solvate Li2(THF)4Ph2NiCOD (2 a) was isolated in 65 % crystalline yield, whereas Li2(TMEDA)2Ph2NiCOD (2 b) precipitated from Et2O as a golden micro‐crystalline solid in 95 % yield. Attempts to isolate Li2(PMDETA)2Ph2NiCOD (2 c) were unsuccessful due to its high solubility in alkane solvents, but single crystals suitable for X‐ray diffraction could nevertheless be grown by slow evaporation at −30 °C. Li2(PMDETA)2Ph2NiCOD (2 c) can, however, be prepared cleanly in situ directly from Ni(COD)2 and PhLi(PMDETA) in C6D6 and characterised by multinuclear NMR spectroscopy.
Scheme 2.
Synthesis of 2:1 lithium nickelates (2 a–c).
Organometallic Ni0‐ates have been reviewed by Jonas and Krüger;[ 28 , 42 ] however, only examples with ethylene as the ligand have been structurally authenticated by XRD studies.[ 19 , 25 , 26 , 31 , 36 ] The lithium nickelates (2 a–c) are extremely sensitive to air and moisture, and must be stored at low temperatures to prevent decomposition.
The identity and structure of each 2:1 lithium nickelate (2 a–c) was confirmed by single‐crystal XRD (Figure 2). The two Ph groups and η2‐coordinated olefin adopt a trigonal‐planar geometry around Ni, and only one olefin in the 1,5‐cyclooctadiene ligand coordinates to the Ni centre, with significant elongation of the double bond (1.446(2)–1.452(2) Å) compared to Ni(COD)2 (1.376(5)–1.388(5) Å) [43] and the uncoordinated double bond (1.321(5)–1.327(2) Å). This is consistent with high electron density at the Ni centre and sizable back‐donation into the empty olefin π* orbital. The solvated Li cations occupy sites in between the organic substituents, and have short contacts to the Ph ipso‐carbon atoms and/or the bound olefin. In 2 c, only two of the three nitrogen atoms of PMDETA coordinate to Li.
Figure 2.
Molecular structures of 2 a–c. Thermal ellipsoids are shown at 50 % probability. Hydrogen atoms are omitted for clarity. a) Selected bond lengths [Å] for 2 a: Ni1‐C1 1.947(3), Ni1‐C8 1.977(3), Ni1‐C9 1.963(3), Ni1‐C15 1.941(2), Li1‐C8 2.272(5), Li1‐C9 2.244(6), Li2‐C9 2.279(6), Li2‐C15 2.243(6), C1‐C8 1.448(3), C4‐C5 1.321(5). b) Only one of the two molecules in the asymmetric unit is shown. Selected bond lengths [Å] for 2 b: Ni1‐C1 1.937(1), Ni1‐C8 1.971(1), Ni1‐C9 1.970(1), Ni1‐C15 1.949(1), Li1‐C8 2.296(3), Li1‐C9 2.256(3), Li2‐C9 2.246(3), Li2‐C15 2.246(3), C1‐C8 1.452(2), C4‐C5 1.326(2). c) Selected bond lengths [Å] for 2 c: Ni1‐C1 1.937(1), Ni1‐C8 1.978(1), Ni1‐C9 1.963(1), Ni1‐C15 1.948(1), Li1‐C8 2.398(2), Li1‐C9 2.256(2), Li2‐C9 2.307(2), Li2‐C15 2.252(3), C1‐C8 1.446(2), C4‐C5 1.327(2).
In the 1H NMR spectrum of 2 a recorded in [D8]THF at room temperature, two broad resonances are seen for COD at δ=3.14 and 1.95, which indicates fluxionality, although they do not de‐coalesce upon cooling to −80 °C and only increased line broadening occurs (Figure S2). The 13C{1H} signal of the Ni‐Ph ipso‐carbon atoms in 2 a is observed at δ=192.0, downfield shifted with respect to [PhLi(THF)2]2 (δ=188.2), [44] which indicates increased charge density at the ipso‐carbon atom, consistent with a formal dianionic Ni0 species.
Interestingly, the 2:1 lithium nickelates slowly lose half an equivalent of COD in solution to give bridged hexanuclear complexes [Li2(donor) n Ph2Ni]2COD (Scheme 3). These compounds can be accessed by repeated or slow recrystallisation of 2 a or 2 b, or by varying the reaction conditions slightly, to give [Li2(THF)4Ph2Ni]2COD (3 a) or [Li2(TMEDA)2Ph2Ni]2COD (3 b), respectively. The dissociation of COD is nevertheless reversible and isolated 3 a can be converted back into 2 a through adding excess COD, although this process is not immediate and Ni(COD)2 is also reformed (Figure S3).
Scheme 3.
Interconversion between trinuclear (2 a,b) and hexanuclear 2:1 lithium nickelates (3 a,b).
The crystal structures of 3 a,b (Figure 3) show no significant changes in bond lengths and angles compared to the non‐bridged trinuclear variants 2 a,b. Conformational differences between 3 a and 3 b are noted, however, and whereas 3 a adopts a cisoid arrangement (i.e. Li1 and Li3 lie on the same side of the COD bridge), 3 b contains an inversion centre and thus adopts a transoid arrangement.
Figure 3.
Molecular structures of 3 a,b. Thermal ellipsoids are shown at 50 % probability. Hydrogen atoms are omitted for clarity. a) Selected bond lengths [Å] for 3 a: Ni1‐C1 1.985(5), Ni1‐C2 1.926(5), Ni1‐C9 1.942(4), Ni1‐C15 1.944(4), Li1‐C1 2.29(1), Li1‐C9 2.26(1), Li2‐C9 2.34(1), Li2‐C15 2.25(1), C1‐C2 1.439(5), Ni2‐C5 1.939(5), Ni2‐C6 1.992(5), Ni2‐C21 1.951(4), Ni2‐C27 1.959(4), Li3‐C6 2.31(1), Li3‐C21 2.28(1), Li4‐C21 2.301(9), Li4‐C27 2.26(1), C5‐C6 1.455(6). b) Selected bond lengths [Å] for 3 b: Ni1‐C1 1.972(1), Ni1‐C2 1.947(1), Ni1‐C9 1.978(1), Ni1‐C15 1.948(1), Li1‐C1 2.236(3), Li1‐C9 2.306(3), Li2‐C9 2.373(3), Li2‐C15 2.277(3), C1‐C2 1.443(2).
When Ni(COD)2 is treated with 5–10 equivalents of PhLi in [D8]THF, to mimic catalytic reaction conditions, trinuclear 2:1 lithium nickelate 2 a remains the major species, as identified by 1H NMR spectroscopy. Although significant decomposition occurs over time due to the sensitivity of these complexes, a crystalline bridged octanuclear 3:1 lithium nickelate [Li3(THF)4Ph3Ni]2COD (4 a) could be isolated from the reaction mixture in low yields (Scheme 4, see Figure S37 for molecular structure).
Scheme 4.
Synthesis of octanuclear bridged 3:1 lithium nickelate 4 a.
Next we studied the role of the lithium nickelates within the Ni‐catalysed cross‐coupling of aryl ethers in a model reaction—specifically, the room‐temperature cross‐coupling of PhLi and 2‐methoxynaphthalene (Scheme 5). Previously reported by Wang, Uchiyama, and co‐workers, [33] this reaction works with 5 mol % Ni(COD)2 as catalyst, although slight improvements in yield and selectivity were found using saturated N‐heterocyclic carbene (NHC) ligands (73 % vs. 86 % yield). [33] A dramatic solvent effect was observed, with only 6 % of the cross‐coupled product (2‐phenylnaphthalene) seen when using THF instead of toluene. This prompted us to investigate the impact of solvent and donor effects, anticipating that this was an overlooked factor that could shed light on the anionic pathway. Furthermore, solvation and aggregation strongly dictate the reactivity and selectivity of organolithium species, [45] especially in directed ortho‐metalation (DoM) reactions.[ 46 , 47 , 48 , 49 ]
Scheme 5.
Nickel‐catalysed cross‐coupling of 2‐methoxynaphthane with PhLi, as reported by Wang, Uchiyama, and co‐workers. [33]
PhLi is polymeric in the solid state, [50] and is poorly soluble in non‐donor solvents. Nevertheless, several well‐defined and hydrocarbon‐soluble PhLi aggregates can be readily prepared by adding donors. Intrigued by the solution chemistry of PhLi, we first probed its reactivity with 2‐methoxynaphthalene (5) in the absence of Ni(COD)2 (Table 1). Surprisingly, we found that when using [D8]THF as the reaction solvent, where PhLi exists as a mixture of dimers and monomers, [44] almost all of the 2‐methoxynaphthalene is consumed to give a mixture of ortho‐lithiation products 6 and 7 in yields of 13 % and 77 %, respectively (entry 1). In contrast to when C6D6 was used as the solvent, no reaction is observed, even after 12 hours (entry 2).
Table 1.
Ortho‐lithiation of 2‐methoxynaphthalene with PhLi using different donor additives.[a]

|
Entry |
Donor |
Solvent |
Consumption of 5 [%] |
Yield of 6 [%] |
Yield of 7 [%] |
|---|---|---|---|---|---|
|
1 |
– |
[D8]THF |
94 |
13 |
77 |
|
2 |
– |
C6D6 |
0 |
0 |
0 |
|
3 |
Et2O |
C6D6 |
14 |
0 |
14 |
|
4 |
THF |
C6D6 |
25 |
5 |
20 |
|
5 |
DME |
C6D6 |
63 |
15 |
46 |
|
6 |
TMEDA |
C6D6 |
81 |
16 |
65 |
|
7 |
PMDETA |
C6D6 |
72 |
16 |
57 |
[a] Standard conditions: 0.20 mmol 2‐methoxynaphthalene (5), 0.22 mmol PhLi, 0.22 mmol donor, and 0.05 mmol (25 mol %) Me4Si (internal standard) in 500 μL deuterated solvent. The consumption and yield were determined against an internal standard (Me4Si).
Extending these studies to a range of soluble and well‐defined PhLi aggregates (see the Supporting Information for DOSY NMR studies) illustrate that they can also promote the ortho‐lithiation of 2‐methoxynaphthalene. When Et2O or THF were used as stoichiometric donor additives, the monosolvated PhLi tetramers [PhLi(Et2O)]4 and [PhLi(THF)]4 are present in solution and moderate consumption of 5 is observed (entries 3 and 4). Multidentate Lewis donors such as DME, TMEDA, and PMDETA, which form smaller aggregates in the solid state[ 51 , 52 ] and in solution [44] (dimers for DME and TMEDA, monomer for PMDETA), drastically increase the formation of ortho‐metalated products (entries 5–7). These results can be rationalised in terms of forming smaller, kinetically more reactive PhLi aggregates that can promote directed ortho‐metalation.
Having assessed the impact of PhLi solvation and aggregation on the competing ortho‐lithiation of 2‐methoxynaphthalene, we next investigated how these factors would influence the nickel‐catalysed cross‐coupling reaction (Table 2). Using donor‐free PhLi in C6D6, the cross‐coupled product, 2‐phenylnaphthalene (8), was obtained in 72 % yield after just 2 hours at room temperature with 5 mol % Ni(COD)2 (entry 1). Although PhLi is poorly soluble in C6D6, a sufficient concentration is solubilised by the aryl ether (see below) or by a direct reaction with Ni(COD)2. In contrast, when using PhLi in [D8]THF, no cross‐coupled product was seen and only a mixture of the two ortho‐lithiation products (6 and 7) formed (entry 2). Et2O‐ and THF‐solvated PhLi tetramers gave a lower yield of 8 compared to donor‐free PhLi, with the more labile Et2O solvate slightly outperforming the THF analogue (entries 3 and 4). The use of [PhLi(DME)]2 led to only traces of the cross‐coupled product being observed (entry 5), whereas in the case of [PhLi(TMEDA)]2 and PhLi(PMDETA), the ortho‐lithiation products were the sole organic species formed (entries 6 and 7). The cross‐coupling of 2‐methoxynaphthalene using Ni(COD)2 was also effective with the Grignard reagent PhMgCl (entries 8 and 9). No tangible solvent effects were apparent, likely due to the poor metalating ability of PhMgCl. Along with the formation of 8, significant quantities (14–21 %) of the homo‐coupled products (biphenyl 9 and 2,2′‐binaphthyl 10) were also formed.
Table 2.
Cross‐coupling of 2‐methoxynaphthalene with various PhM nucleophiles.[a]

|
Entry |
Nucleophile |
Solvent |
t [h] |
Consumption of 5 [%] |
Yield of 8 [%] |
|---|---|---|---|---|---|
|
1 |
[PhLi]∞ |
C6D6 |
2 |
86[b] |
72 |
|
2 |
[PhLi]∞ |
[D8]THF |
12 |
87[c] |
0 |
|
3 |
[PhLi(Et2O)]4 |
C6D6 |
2 |
>95[b] |
62 |
|
4 |
[PhLi(THF)]4 |
C6D6 |
6 |
>95[b] |
58 |
|
5 |
[PhLi(DME)]2 |
C6D6 |
2 |
52[c] |
traces |
|
6 |
[PhLi(TMEDA)]2 |
C6D6 |
12 |
80[c] |
0 |
|
7 |
PhLi(PMDETA) |
C6D6 |
12 |
84[c] |
0 |
|
8 |
[PhMgCl(THF)2] n |
C6D6 |
2 |
80[b] |
54 |
|
9 |
[PhMgCl(THF)2] n |
[D8]THF |
2 |
80[b] |
50 |
[a] Standard conditions: 0.20 mmol 2‐methoxynaphthalene (5), 0.22 mmol PhM(donor), 0.01 mmol (5 mol %) Ni(COD)2, and 0.05 mmol (25 mol %) Me4Si (internal standard) in 500 μL deuterated solvent. The consumption and yield were determined against an internal standard (Me4Si). [b] Significant quantities (14–21 %) of each homo‐coupled side product (biphenyl and 2,2′‐binaphthyl) were observed. [c] Mixture of ortho‐lithiated products (6 and 7) observed.
Having shed light on the influence of the solvent and donor additives on the cross‐coupling process, we next assessed the stoichiometric reactivity between Ni(COD)2, [PhLi(donor)] n , and 2‐methoxynaphthalene in a 1:2:2 ratio. This gave cross‐coupled product (8) regardless of the donor, with no evidence of ortho‐lithiated species (6 and 7). Combining Ni(COD)2 with two equivalents of [PhLi(donor)] n led to the immediate formation of the 2:1 lithium nickelate (2), as evident by 1H and 7Li NMR spectroscopy. The Et2O and THF solvates are poorly soluble in C6D6, whereas the soluble TMEDA and PMDETA solvates are the same as the crystalline 2:1 lithium nickelates 2 b,c. On addition of 2‐methoxynaphthalene, immediate conversion (<1 minute) to the cross‐coupled (and homo‐coupled) products is observed for the Et2O and THF solvates, despite their poor solubility. In contrast, the reaction is significantly slower for the TMEDA solvate (6 days at 25 °C) and PMDETA solvate (12 hours at 25 °C). The yields and selectivity are similar regardless of the donor (and comparable to catalytic trials), but the choice of donor heavily influences the rate of the coupling reaction. In all cases, Ni(COD)2 is cleanly regenerated with no signs of decomposition (Scheme 6 and see Figure S14 for representative spectroscopic examples).
Scheme 6.
Stoichiometric studies supporting the active role of lithium nickelates in the cross‐coupling reaction.
These stoichiometric studies suggest that lithium nickelates may indeed be key intermediates in the cross‐coupling reaction. Further supporting the involvement of lithium nickelates in the anionic pathway, isolated compounds 2 a, 3 a, and 4 a were all competent catalysts for the cross‐coupling reaction. Using 5 mol % 2 a (Li2(THF)4Ph2NiCOD), 2.5 mol % 3 a ([Li2(THF)4Ph2Ni]2COD) or 4 a ([Li3(THF)4Ph3Ni]2COD) together with [PhLi(THF)]4 led to the cross‐coupled product 8 being obtained in almost identical yields (ca. 60 % vs. 58 % in Table 2, entry 4) after similar reaction times (6 hours at 25 °C) compared to Ni(COD)2. Although 3 a and 4 a are catalytically competent, the reaction conditions required to access and observe these species suggest they are not on‐cycle intermediates (see below). Interestingly, the stoichiometric studies also reveal that the choice of donor influences the rate of the cross‐coupling reaction between lithium nickelates and 2‐methoxynaphthalene, which under catalytic conditions can then be outcompeted by the undesirable ortho‐lithiation reaction. This indirectly suggests that the coordination of the aryl ether substrate to the lithium cation(s) in the lithium nickelate may be a crucial step, since this would bring the electrophile into proximity to the nucleophilic Ni centre, as well as polarising the Caryl−O bond to facilitate oxidative addition.[ 14 , 15 , 16 , 53 , 54 ] Although we were unable to directly observe or isolate any further intermediates in the anionic pathway, we could show that aryl ethers can solvate PhLi, despite being weak Lewis donors. Treating a suspension of donor‐free PhLi with a 10‐fold excess of 4‐methylanisole (p‐Tol‐OMe) gave a homogeneous solution, which afforded crystals of the mono‐solvated PhLi tetramer [PhLi(p‐Tol‐OMe)]4 (Figure 4). p‐Tol‐OMe was chosen due to its improved solubility compared to 2‐methoxynaphthalene. No unambiguous spectroscopic evidence of adduct formation was observed on adding p‐Tol‐OMe to isolated Li2(THF)4Ph2NiCOD (2 a) in C6D6, and only slow formation of cross‐coupled (and homo‐coupled) products were noted. Recent studies on the related Ni‐catalysed homo‐coupling of aryl ethers also support that donor exchange and substrate coordination to a Lewis acidic metal is a key reaction step. [54]
Figure 4.
Molecular structure of [PhLi(p‐Tol‐OMe)]4. Thermal ellipsoids are shown at 50 % probability. Hydrogen atoms are omitted for clarity.
Kinetic studies were carried out to further probe the role of hetero‐bimetallic nickelates in the anionic pathway, and to provide experimental insights into this alternative cross‐coupling mechanism (see the Supporting Information for full details). When using the isolated 2:1 lithium nickelate Li2(THF)4Ph2NiCOD (2 a) as a catalyst, a plot of product concentration versus time shows a linear reaction profile (Figure 5) independent of the concentration of the substrates (Figure S30). This behaviour is attributed to a pre‐equilibrium (Figure S28) which relies on donor dissociation to enable coordination of the aryl ether; [54] this is supported by a dramatic rate reduction when changing from Li2(THF)4Ph2NiCOD (2 a) to Li2(TMEDA)2Ph2NiCOD (2 b) as the catalyst (Figure 5). In contrast, using Ni(COD)2 as the catalyst gives a different reaction profile (Figure 5), although a comparable yield of the cross‐coupled product is formed after similar reaction times. Spectroscopic monitoring (Figures S15–20) reveals that lithium nickelate intermediates form rapidly in the early stages of the reaction but quickly decline as the concentration of the substrates decreases, a process consistent with saturation kinetics and a change in the catalyst resting state. The reaction rate is independent of both substrate concentrations, but first order in catalyst concentration (Figures S21 and S29). The rate‐determining step is thus proposed to be oxidative addition and C−O bond cleavage, with the zeroth order dependence in both substrates suggesting that all three reaction components combine prior to this step, thus supporting the role of hetero‐bimetallic nickelates as key reaction intermediates. Since the PhLi(donor) n concentration has no impact on the reaction rate, and due to limitations associated with the solubility of donor‐free PhLi, it is not possible to distinguish between catalytic cycles involving 1:1 or 2:1 lithium nickelates. However, since 1:1 lithium nickelates derived from Ni(COD)2 and PhLi could only be observed under specific reaction conditions, a mechanism involving the 2:1 species is currently favoured. Moreover, the enhanced nucleophilicity of the higher order (2:1 Li/Ni) species is expected to lead to increased reactivity at Ni. This has been demonstrated in other studies with hetero‐bimetallic systems such as lithium manganates. [55]
Figure 5.
A plot of product (2‐phenylnaphthalene, 8) concentration versus time using 5 mol % Ni(COD)2 or isolated 2:1 lithium nickelates (Li2(donor) n Ph2NiCOD) 2 a,b.
Taking our spectroscopic, structural, and kinetic data into account, together with related computational studies,[ 14 , 16 ] a mechanistic proposal for the anionic pathway can be postulated (Scheme 7). Starting from Ni(COD)2 (A), addition of PhLi(donor) n can form either a 1:1 (B) or 2:1 (F) lithium nickelate. Stoichiometric studies show that although 1:1 lithium nickelates (1 a) can be observed under certain conditions, the 2:1 lithium nickelates (2 a–c) are favoured. It has also been proposed that this co‐complexation is an equilibrium that often lies towards the starting components, [19] and only nickelates derived from highly nucleophilic organometallic reagents in combination with a highly Lewis acidic Ni0 source have been isolated to date.[ 19 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 36 ] Nevertheless, this does not discount the possibility that transient nickelate intermediates may still form when milder nucleophiles such as organoboron reagents are used, but the requirement for electron‐rich ligands and harsh reaction conditions does suggest that a different mechanism may be in operation. [15]
Scheme 7.
Proposed catalytic cycles (anionic and dianionic) for the nickel‐catalysed cross‐coupling of aryl ethers.
The next step of the catalytic cycle is the coordination of the aryl ether substrate to the Lewis acidic metal (C or G). [54] Wang, Uchiyama, and co‐workers[ 14 , 16 ] proposed that association of the aryl ether may occur at an earlier stage in the anionic pathway via formation of a Ni0 η2‐arene complex.[ 13 , 56 , 57 , 58 , 59 , 60 ] These species involve a d→π* back‐donation interaction, which is commonly observed for electron‐rich Ni0 species supported by phosphine or NHC ligands, but has not been documented for olefins, again suggesting that a different mechanism may operate for electron‐rich ligands. Regardless of the ligand and nucleophile, however, the coordination of the ethereal oxygen atom of the aryl ether to a Lewis acidic metal is consistently proposed and identified in computational studies.[ 14 , 15 , 16 , 53 , 54 ] Although we did not observe coordination to lithium nickelate intermediates, the dramatic solvent and donor effects in the catalytic and stoichiometric cross‐coupling reaction, as well as the isolation of [PhLi(p‐Tol‐OMe)]4, provides strong support for this hypothesis. Differences in the reaction profile when using Ni(COD)2 or Li2(THF)4Ph2NiCOD (2 a) as the catalyst, are attributed to donor displacement and substrate coordination, which is significantly slower in the presence of donors (even sub‐stoichiometric quantities; see Figure S24), which bind more strongly than the aryl ether to the lithium centre(s). We believe that the lithium nickelate intermediates that can be observed spectroscopically when using catalytic Ni(COD)2 are consistent with C or G (Figures S17 and S18). Pre‐coordination of the aryl ether substrate prior to oxidative addition is consistent with the zeroth order dependence, since it is not directly involved in the rate‐determining step (i.e. it is already part of an intermediate and, therefore, only a first order dependence in the catalyst concentration is seen).
Along with bringing the substrate into proximity to the active Ni centre, coordination of the aryl ether can also polarise the Caryl−O bond to facilitate oxidative addition to Ni0.[ 53 , 59 ] Cleavage of the C−OMe bond may occur via the putative transition state (D) to furnish the oxidative addition NiII compound (E or H), alongside the LiOMe by‐product, consistent with theoretical studies.[ 14 , 16 , 54 ] Although direct experimental insight into this oxidative addition step is limited for catalytically relevant systems, it is likely that the C−O bond cleavage occurs through a hetero‐bimetallic pathway (ligand assisted oxidative addition), a process that has been studied stoichiometrically using well‐defined hetero‐bimetallic complexes. [61] Furthermore, this pathway also avoids the formation of unstable NiII‐OMe species which can undergo competitive β‐hydride elimination.[ 13 , 15 , 61 ]
The proposed transition state (D) could also provide another explanation for the “naphthalene problem”, [9] the observation that the cross‐coupling of aryl ethers works more smoothly for π‐extended aromatic substrates, since these would form more stable de‐aromatised intermediates. Finally, reductive elimination from the NiII compound furnishes the cross‐coupled product and regenerates a Ni0 species (A or B).
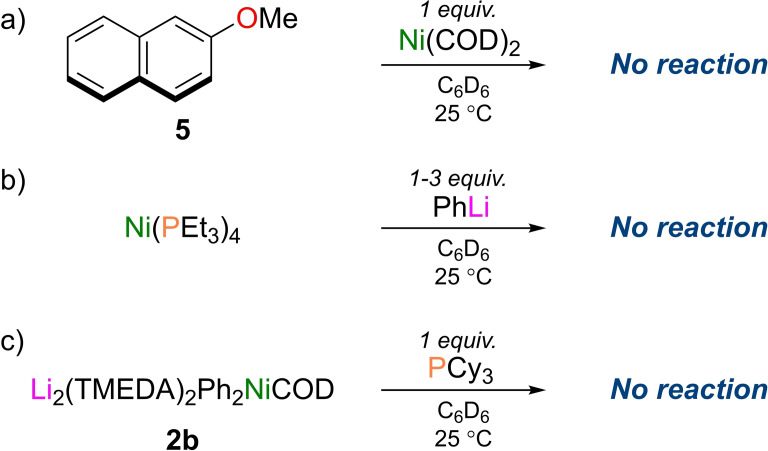
This mechanism bears similarities to one that has been recently reported by Cornella and co‐workers for a low‐temperature Kumada–Corriu cross‐coupling reaction catalysed by 16‐electron Ni0 tris‐olefin complexes. [19] Notably, the authors found that Ni0‐tris‐olefin complexes do not oxidatively add electrophiles such as a vinyl bromides, unlike NHC or phosphine Ni0 complexes which typically operate under the canonical two‐electron Ni0/NiII catalytic cycle. [7] A similar classification can, therefore, be put forward to highlight the differences between Lewis acidic Ni0‐olefin complexes and electron‐rich Ni0 catalysts, and the dramatic ligand‐dependent reactivity. No reaction is observed between 2‐methoxynaphthalene (5) and Ni(COD)2 (Scheme 8 a), which supports that co‐complexation between Ni(COD)2 and PhLi is indeed the preferred pathway in the cross‐coupling reaction under mild conditions.
Scheme 8.
Control reactions showing the contrasting reactivity of different Ni0 complexes and ligands.
For phosphine and NHC Ni0 complexes, the direct oxidative addition and cleavage of C(sp2)−OR bonds often proceeds with very high, and potentially inaccessible, barriers,[ 13 , 14 , 15 , 16 , 53 , 62 ] although this can be lowered partially by careful selection of ligands and/or additives.[ 15 , 62 ] In many cases, the direct oxidative addition also relies on an initial η2‐arene complex formed between the aryl ether and Ni0 centre. [60] As a consequence of the quenched Lewis acidity at the Ni centre in phosphine and NHC species, co‐complexation of the organometallic nucleophile is less likely to occur. Indeed, no nickelate formation or ligand dissociation is observed when Ni(PEt3)4 is treated with PhLi (Scheme 8 b). Finally, we also observed no evidence of ligand dissociation when Li2(TMEDA)2Ph2NiCOD (2 b) was treated with PCy3 (Scheme 8 c). This both illustrates the strong σ‐donation of the carbanionic Ph fragment and highlights that the coordination flexibility, lability, and π‐accepting properties of COD (and other olefins) are essential to stabilise and modulate the electron‐rich Ni0 centre in nickelate complexes.
Conclusion
By studying the rich co‐complexation chemistry of PhLi with Ni(COD)2, we discovered and isolated a series of diverse lithium nickelates which could be characterised by spectroscopic and structural methods. Catalytic, stoichiometry, and kinetic investigations support the involvement of hetero‐bimetallic nickelate intermediates in the catalytic cross‐coupling of aryl ethers, providing strong support for the alternative anionic pathway and illustrating how Ni0 enables the smooth cleavage of inert C(sp2)−O bonds under mild reaction conditions. We also disclosed the drastic solvent and donor influences in the cross‐coupling reaction, which suggest that hetero‐bimetallic nickelate intermediates operate cooperatively through a nucleophilic Ni centre and Lewis acidic lithium cation.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Supporting Information
Acknowledgements
The X‐ray crystal structure determination service unit of the Department of Chemistry and Biochemistry of the University of Bern is acknowledged for measuring, solving, refining, and summarising all new structures. The Synergy diffractometer was partially funded by the Swiss National Science Foundation (SNF) within the R'Equip programme (project number 206021_177033). We also thank Dr. Alberto Hernán‐Gómez (Universidad de Alcalá) and Wowa Stroek for kinetics advice, Dr. Ilche Gjuroski (NMR) and Claudia Bühr (CHN) for analytical services, and Dr. Leonie Bole for assistance in processing DOSY data. E.H. thanks the University of Bern and the SNF (grant 188573) for generous sponsorship of this research. Open access funding provided by Universitat Bern.
A. M. Borys, E. Hevia, Angew. Chem. Int. Ed. 2021, 60, 24659.
Contributor Information
Dr. Andryj M. Borys, Email: andryj.borys-smith@unibe.ch.
Prof. Dr. Eva Hevia, Email: eva.hevia@unibe.ch.
References
- 1. Tamao K., Sumitani K., Kumada M., J. Am. Chem. Soc. 1972, 94, 4374–4376. [Google Scholar]
- 2. Corriu R. J. P., Masse J. P., J. Chem. Soc. Chem. Commun. 1972, 144. [Google Scholar]
- 3. Tasker S. Z., Standley E. A., Jamison T. F., Nature 2014, 509, 299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hazari N., Melvin P. R., Beromi M. M., Nat. Rev. Chem. 2017, 1, 0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Miyaura N., Cross-Coupling Reactions, Springer Berlin Heidelberg, Berlin, 2002. [Google Scholar]
- 6. Biffis A., Centomo P., Del Zotto A., Zecca M., Chem. Rev. 2018, 118, 2249–2295. [DOI] [PubMed] [Google Scholar]
- 7. Diccianni J. B., Diao T., Trends Chem. 2019, 1, 830–844. [Google Scholar]
- 8. Rosen B. M., Quasdorf K. W., Wilson D. A., Zhang N., Resmerita A., Garg N. K., Percec V., Chem. Rev. 2011, 111, 1346–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tobisu M., Chatani N., Acc. Chem. Res. 2015, 48, 1717–1726. [DOI] [PubMed] [Google Scholar]
- 10. Zarate C., van Gemmeren M., Somerville R. J., Martin R., in Adv. Organomet. Chem. Elsevier Inc., 2016, pp. 143–222. [Google Scholar]
- 11. Wenkert E., Michelotti E. L., Swindell C. S., J. Am. Chem. Soc. 1979, 101, 2246–2247. [Google Scholar]
- 12. Quasdorf K. W., Antoft-Finch A., Liu P., Silberstein A. L., Komaromi A., Blackburn T., Ramgren S. D., Houk K. N., Snieckus V., Garg N. K., J. Am. Chem. Soc. 2011, 133, 6352–6363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cornella J., Gómez-Bengoa E., Martin R., J. Am. Chem. Soc. 2013, 135, 1997–2009. [DOI] [PubMed] [Google Scholar]
- 14. Ogawa H., Minami H., Ozaki T., Komagawa S., Wang C., Uchiyama M., Chem. Eur. J. 2015, 21, 13904–13908. [DOI] [PubMed] [Google Scholar]
- 15. Schwarzer M. C., Konno R., Hojo T., Ohtsuki A., Nakamura K., Yasutome A., Takahashi H., Shimasaki T., Tobisu M., Chatani N., Mori S., J. Am. Chem. Soc. 2017, 139, 10347–10358. [DOI] [PubMed] [Google Scholar]
- 16. Kojima K., Yang Z. K., Wang C., Uchiyama M., Chem. Pharm. Bull. 2017, 65, 862–868. [DOI] [PubMed] [Google Scholar]
- 17. Terao J., Kambe N., Acc. Chem. Res. 2008, 41, 1545–1554. [DOI] [PubMed] [Google Scholar]
- 18. Zarate C., Nakajima M., Martin R., J. Am. Chem. Soc. 2017, 139, 1191–1197. [DOI] [PubMed] [Google Scholar]
- 19. Lutz S., Nattmann L., Nöthling N., Cornella J., Organometallics 2021, 40, 2220–2230. [Google Scholar]
- 20. Nohira I., Chatani N., ACS Catal. 2021, 11, 4644–4649. [Google Scholar]
- 21. Iwasaki T., Fukuoka A., Min X., Yokoyama W., Kuniyasu H., Kambe N., Org. Lett. 2016, 18, 4868–4871. [DOI] [PubMed] [Google Scholar]
- 22. Fischer K., Jonas K., Misbach P., Stabba R., Wilke G., Angew. Chem. Int. Ed. Engl. 1973, 12, 943–1026; [Google Scholar]; Angew. Chem. 1973, 85, 1001–1012. [Google Scholar]
- 23. Wilke G., Bogdanović V. B., Borner P., Briel H., Hardt P., Heimbach P., Herrmann G., Kaminsky H. J., Keim W., Kröner M., Müller H., Müller E. W., Oberkirch W., Schneider J., Stedefeder J., Tanaka K., Weyer K., Wilke G., Angew. Chem. Int. Ed. Engl. 1963, 2, 105–115; [Google Scholar]; Angew. Chem. 1963, 75, 10–20. [Google Scholar]
- 24. Bogdanović V. B., Kröner M., Wilke G., Justus Liebigs Ann. Chem. 1966, 699, 1–23. [DOI] [PubMed] [Google Scholar]
- 25. Jonas K., Porschke K. R., Krüger C., Tsay Y.-H., Angew. Chem. Int. Ed. Engl. 1976, 15, 621–922; [Google Scholar]; Angew. Chem. 1976, 88, 682–682. [Google Scholar]
- 26. Brauer B. D. J., Krüger C., Roberts P. J., Tsay Y.-H., Angew. Chem. Int. Ed. Engl. 1976, 15, 48–49; [Google Scholar]; Angew. Chem. 1976, 88, 52–53. [Google Scholar]
- 27. Jonas K., Angew. Chem. Int. Ed. Engl. 1976, 15, 47; [Google Scholar]; Angew. Chem. 1976, 88, 51. [Google Scholar]
- 28. Jonas V. K., Krüger C., Angew. Chem. Int. Ed. Engl. 1980, 19, 520–537; [Google Scholar]; Angew. Chem. 1980, 92, 513–531. [Google Scholar]
- 29. Pörschke K.-R., Jonas K., Wilke G., Benn R., Mynott R., Goddard R., Krüger C., Chem. Ber. 1985, 118, 275–297. [Google Scholar]
- 30. Pörschke K.-R., Jonas K., Wilke G., Chem. Ber. 1988, 121,1913–1919. [Google Scholar]
- 31. Kaschube W., Pörschke K.-R., Angermund K., Kriiger C., Wilke G., Chem. Ber. 1988, 121, 1921–1929. [Google Scholar]
- 32. Leiendecker M., Hsiao C.-C., Guo L., Alandini N., Rueping M., Angew. Chem. Int. Ed. 2014, 53, 12912–12915; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2014, 126, 13126–13129. [Google Scholar]
- 33. Yang Z., Wang D., Minami H., Ogawa H., Ozaki T., Saito T., Miyamoto K., Wang C., Uchiyama M., Chem. Eur. J. 2016, 22, 15693–15699. [DOI] [PubMed] [Google Scholar]
- 34. Heijnen D., Gualtierotti J., Hornillos V., Feringa B. L., Chem. Eur. J. 2016, 22, 3991–3995. [DOI] [PubMed] [Google Scholar]
- 35. Yang Z., Xu N., Takita R., Muranaka A., Wang C., Uchi, Nat. Commun. 2018, 9, 1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nattmann L., Lutz S., Ortsack P., Goddard R., Cornella J., J. Am. Chem. Soc. 2018, 140, 13628–13633. [DOI] [PubMed] [Google Scholar]
- 37. Armstrong D. R., Dougan C., Graham D. V., Hevia E., Kennedy A. R., Organometallics 2008, 27, 6063–6070. [Google Scholar]
- 38. Furuyama T., Yonehara M., Arimoto S., Kobayashi M., Matsumoto Y., Uchiyama M., Chem. Eur. J. 2008, 14, 10348–10356. [DOI] [PubMed] [Google Scholar]
- 39. Borys A. M., Hevia E., Organometallics 2021, 40, 442–447. [Google Scholar]
- 40. Hay-Motherwell R., Wilkinson G., Sweet T. K. N., Hursthouse M. B., Polyhedron 1996, 15, 3163–3166. [Google Scholar]
- 41. Lamm K., Stollenz M., Meier M., Görls H., Walther D., J. Organomet. Chem. 2003, 681, 24–36. [Google Scholar]
- 42.It should be noted that Li2(THF)4Ph2NiCOD and Li2(TMEDA)2Ph2NiCOD, as well as other related alkali-metal nickelates, were first documented and reviewed in a progress report published by Jonas and Krüger,[28] which cites a series of work that was unfortunately never published. Details of their preparation and characterisation were not included in these reports.
- 43.M. Bolte, CSD Commun. 2016, 10.5517/cc1kkh1n. [DOI]
- 44. Reich H. J., Green D. P., Medina M. A., Goldenberg W. S., Gudmundsson B. Ö., Dykstra R. R., Phillips N. H., J. Am. Chem. Soc. 1998, 120, 7201–7210. [Google Scholar]
- 45. Reich H. J., Chem. Rev. 2013, 113, 7130–7178. [DOI] [PubMed] [Google Scholar]
- 46. Snieckus V., Chem. Rev. 1990, 90, 879–933. [Google Scholar]
- 47. Rennels R. A., Maliakal A. J., Collum D. B., J. Am. Chem. Soc. 1998, 120, 421–422. [Google Scholar]
- 48. Betz J., Bauer W., J. Am. Chem. Soc. 2002, 124, 8699–8706. [DOI] [PubMed] [Google Scholar]
- 49. Whisler M. C., Macneil S., Snieckus V., Beak P., Angew. Chem. Int. Ed. 2004, 43, 2206–2225; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2004, 116, 2256–2276. [Google Scholar]
- 50. Dinnebier R. E., Behrens U., Olbrich F., J. Am. Chem. Soc. 1998, 120, 1430–1433. [Google Scholar]
- 51. Thoennes D., Weiss E., Chem. Ber. 1978, 111, 3157–3161. [Google Scholar]
- 52. Uwe S., Kopf J., Weiss E., Angew. Chem. Int. Ed. Engl. 1985, 24, 215–216; [Google Scholar]; Angew. Chem. 1985, 97, 222–222. [Google Scholar]
- 53. Liu X., Hsiao C. C., Kalvet I., Leiendecker M., Guo L., Schoenebeck F., Rueping M., Angew. Chem. Int. Ed. 2016, 55, 6093–6098; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2016, 128, 6198–6203. [Google Scholar]
- 54. Rawat V. K., Higashida K., Sawamura M., Synthesis 2021, 53, 3397–3403. [Google Scholar]
- 55. Uzelac M., Mastropierro P., de Tullio M., Borilovic I., Tarrés M., Kennedy A. R., Aromí G., Hevia E., Angew. Chem. Int. Ed. 2021, 60, 3247–3253; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2021, 133, 3284–3290. [Google Scholar]
- 56. Bach I., Pörschke K.-R., Goddard R., Kopiske C., Krüger C., Rufińska A., Seevogel K., Organometallics 1996, 15, 4959–4966. [Google Scholar]
- 57. Yoshikai N., Matsuda H., Nakamura E., J. Am. Chem. Soc. 2008, 130, 15258–15259. [DOI] [PubMed] [Google Scholar]
- 58. Li Z., Zhang S. L., Fu Y., Guo Q. X., Liu L., J. Am. Chem. Soc. 2009, 131, 8815–8823. [DOI] [PubMed] [Google Scholar]
- 59. Yoshikai N., Matsuda H., Nakamura E., J. Am. Chem. Soc. 2009, 131, 9590–9599. [DOI] [PubMed] [Google Scholar]
- 60. Kelley P., Lin S., Edouard G., Day M. W., Agapie T., J. Am. Chem. Soc. 2012, 134, 5480–5483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tobisu M., Morioka T., Ohtsuki A., Chatani N., Chem. Sci. 2015, 6, 3410–3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Xu L., Chung L. W., Wu Y. D., ACS Catal. 2016, 6, 483–493. [Google Scholar]
- 63.Deposition Numbers 2083511 (for 2a), 2083512 (for 2b), 2083513 (for 2c), 2083514 (for 3a), 2083515 (for 3b), 2083516 (for 4a), 2083510 (for [PhLi(p-Tol-OMe)]4) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service www.ccdc.cam.ac.uk/structures.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Supporting Information