Abstract
Human alveolar echinococcosis (AE) is a zoonotic infection caused by the fox tapeworm Echinococcus multilocularis. We report the case of a patient who developed an accelerated course of AE with diffuse liver involvement after high-dose steroid treatment for autoimmune encephalitis. Immunosuppressive therapies present us with new challenges regarding the management of AE. With this article, we would like to draw attention to the importance of a screening program for AE before planned immunosuppressive therapy.
Keywords: case report, liver metastases, reactivation, steroids, immunosuppression, echinococcosis
Introduction
Human alveolar echinococcosis (AE) is a zoonotic infection caused by the larval form (metacestode) of the fox tapeworm Echinococcus multilocularis [1]. For decades before 2000, only sporadic AE infections were reported with Switzerland recording about eight to 12 new AE cases per year [2]. In the last 10-20 years, however, there has been a geographical and numerical increase or expansion within the European endemic area [3]. Switzerland has also been affected by this increase, with now approximately 24-30 new AE cases being annually diagnosed, which corresponds to a two- to three-fold increase compared to the past [2].
Several important factors have been identified as reasons for the expansion of the European endemic area as well as for the increase in AE case numbers in humans. The fox population and concurrently the parasite population itself have increased, on the one hand, due to the successful control of fox rabies, and due to the urbanization of foxes on the other hand. Dogs also act as the definitive host; the parasite was introduced into new areas via this animal species ("traveling" dogs and dog translocations). Another reason is the increased administration of immunomodulatory therapies in humans, with the effect that AE is occurring more frequently as an opportunistic infection. Finally, an overall increased application of crossectional imaging (computer tomography (CT), magnetic resonance tomography (MRI)) is resulting in an increased coincidental early detection of, in most cases, yet asymptomatic AE [4,5].
Vuitton and Gottstein have suggested that immunocompromised (ICR) individuals may present with rapid disease progression compared with immunocompetent patients who seem to be able to control the disease development better, and present with a slowly growing tumor clinically presenting only many years after initial infection [5,6].
We report a case of a male patient who developed an accelerated course of a liver AE, 5.5 months after high dose treatment with steroids (90mg/day (corresponding to 1mg/kgbw/day), initially 1000mg/day intravenously for five days) for autoimmune encephalitis.
Case presentation
A 59-year-old male with no previous medical or psychiatric history was brought to the hospital following a change of character with apathy, listlessness and fatigue, as well as a deterioration of his general condition and apraxia, which occurred after initial flu-like symptoms and were accompanied by an additional unclear weight loss and a slightly increased C-reactive protein (CRP) value. In addition, in the week prior to admission, there had been repeated transient tingling paresthesias of the left arm, partly accompanied by dysarthria, unilateral facial nerve paralysis, and left arm as well as leg weakness.
The findings in the clinical examination showed neuropsychological deficits with leading frontal brain performance conspicuous with a Montreal Cognitive Assessment (MoCA) test score of 21 out of 30 points. The detailed neuropsychological examination showed a mild to moderate cognitive brain dysfunction (memory, attention, cognitive processing speed, executive functions). The suspicion of the patient's complaints was an autoimmune encephalitis.
On the one hand, this diagnosis was supported by the clinical picture with progressive, subacute neuropsychological and neurocognitive changes. On the other hand, the recurrent transient focal neurological deficits were compatible with focal epileptic seizures [7].
Furthermore, the inflammatory cerebrospinal fluid (CSF) syndrome with a moderate mononuclear pleocytosis (cell count 120 M/L) and negative smear and culture matched autoimmune encephalitis. The positive antibody test in the blood and the CSF for the Ma-2 antibody finally confirmed the diagnosis of a Ma-2 antibody-associated autoimmune encephalitis.
However, the etiology of Ma-2 limbic encephalitis ultimately had to be left open. Since a paraneoplastic genesis occurs in 90% of cases, a broad tumor search including whole-body positron emission tomography (PET) scan was performed, which showed no evidence of a neoplastic event and especially no enhancement of the liver. This was then continued by CT thorax/abdomen every four months.
Therapeutically, we established a seizure-suppressing therapy with oxcarbazepine. Under this therapy, no new epileptic seizures occurred. In addition, in the case of positive antibodies and no evidence of neoplasia, therapy with steroids (1000mg intravenous) was given for five days. This resulted in an improvement in the clinical status with a new score of 26 points on the MoCA test. To avoid the risk of recurrence, a longer-term, immunomodulatory, steroid-sparing therapy with immunoglobulins was started, and the therapy with peroral steroids (initially 1mg/kgbw/day; 90mg/day) was gradually reduced and stopped after four months.
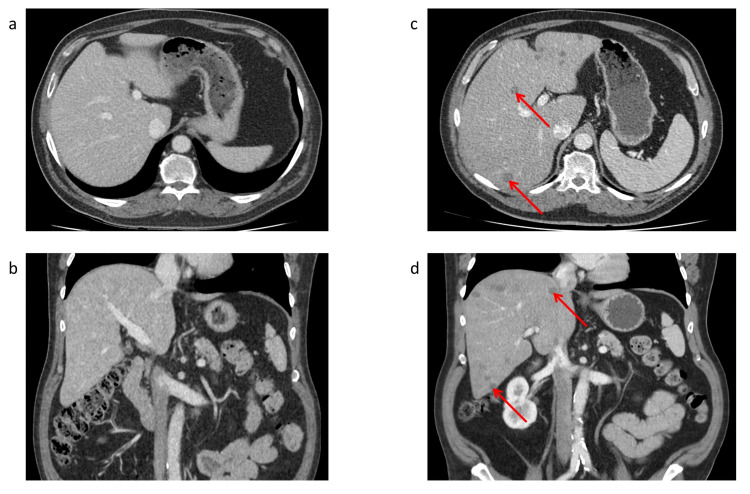
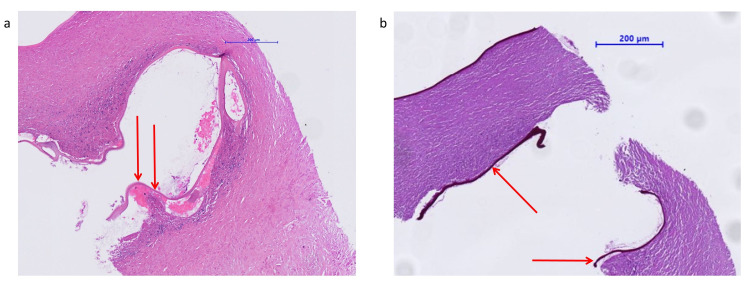
The CT scan of October 2020 showed new onset of multiple hypodense lesions of the liver, primarily suspected as metastases, compared to the CT of June 2020 (Figure 1). Therefore, a liver biopsy was performed, showing granulomatous demarcated necrosis zones and evidence of amorphous cuticular membranes with periodic acid-Schiff (PAS)-positivity compatible with Echinococcus spp. infestation (Figure 2). Malignancy could be excluded. The serology testing for Echinococcus multilocularis was positive (Table 1).
Table 1. Positive serology testing for Echinococcus multilocularis.
Testing for E. multilocularis showed a positive Western blot but a negative result for E. multilocularis antigens (Em2 and Em18). An expression of an active AE is, in particular, a positivity of Em18, which, however, is not present here. The positivity for E. granulosus in the Western blot and EgHF ELISA is due to a cross-reaction with E. multilocularis.
Abbreviations: AE = alveolar echinococcosis, EgHF = E. granulosus hydatid fluid antigen, ELISA = enzyme-linked immunosorbent assay
| Name of investigation | Results | Normal ranges |
| E. granulosus EgHF, ELISA | 22 | < 1 AE |
| E. multilocularis Em2, ELISA | < 1 | < 1 AE |
| E. multilocularis Em18, ELISA | < 1 | < 1 AE |
| E. granulosus, Western Blot | weakly positive | negative |
| E. multilocularis, Western Blot | weakly positive | negative |
Figure 1. Contrast-enhanced abdominal CT scan.
Contrast-enhanced abdominal CT scan, (a, b) at time of the diagnosis of autoimmune encephalitis, which reveals normal liver parenchyma, and (c, d) under therapy with glucocorticoids, which reveals multiple hypodense lesions of the liver (arrows).
Abbreviations: CT = computer tomography
Figure 2. Liver biopsy showing alveolar echinococcosis.
The mass shows a multiloculated cyst with a germinal layer (arrows): (a) HE stain, (b) PAS stain.
Abbreviations: HE = hematoxylin-eosin, PAS = periodic acid-Schiff
Besides, liver enzymes and eosinophils were elevated (Table 2). A polymerase chain reaction (PCR) from the liver specimen confirmed E. multilocularis to be the infecting organism.
Table 2. Evolution of laboratory values.
Stage 1: Diagnosis of autoimmune encephalitis 04/2020. Stage 2: Under therapy with steroids (90mg/day). Stage 3: Diagnosis of AE 11/2020. Therapy with steroids stopped 1.5 months ago. Stage 4: Under therapy with albendazole for one month. Stage 5: Under therapy with albendazole for four months.
Abbreviations: AE = alveolar echinococcosis, ALAT = alanine aminotransferase, AP = alkaline phosphatase, ASAT = aspartate aminotransferase, CRP = C-reactive protein, gamma-GT = gamma-glutamyltransferase
| Name of investigation | Stage 1 | Stage 2 | Stage 3 | Stage 4 | Stage 5 | Normal ranges |
| Leucocytes | 5.88 | 8.07 | 7.28 | 6.0 | 6.66 | 3.0-10.5 G/L |
| Eosinophils | - | 0.06 | 0.92 | 0.64 | - | 0.02-0.40 G/L |
| ASAT | 24 | 38 | 70 | 57 | 40 | < 50 U/L |
| ALAT | 19 | 43 | 110 | 73 | 43 | < 50 U/L |
| Bilirubin total | - | - | 4 | 4 | 4 | < 17 umol/l |
| AP | - | - | 118 | 104 | 90 | 40-129 U/L |
| Gamma-GT | 45 | 51 | 96 | 167 | 94 | < 60 U/L |
| CRP | 8 | <3 | 12 | 6 | 4 | < 5 mg/l |
There was no involvement of other organs except the before-mentioned liver involvement. Continuous treatment with albendazole (400mg twice a day) was immediately initiated. The six-month follow-up showed no deterioration of the liver lesions in the CT scan, but liver enzymes normalized in the meantime.
Discussion
This report describes a case of AE with disseminated liver involvement after initiation of immunosuppressive treatment with steroids and immunoglobulins due to a Ma-2 antibody-associated limbic encephalitis. In the literature, we do not find any correlation between Ma-2 antibody-associated limbic encephalitis and AE. In contrast, the role of immunosuppression in the development of AE is well known [5,6].
AE is a parasitic infection with a tumor-like and potentially invasive and metastasizing growth pattern. The current incidence of AE is three-fold higher than 20 years ago. This is associated with dense fox populations and high prevalence for E. multilocularis, respective urbanization of foxes, translocation of infected dogs, an increased frequency of medicating with immunosuppressive drugs, and modern imaging techniques that contribute to higher detection rates [2,3].
In immunocompetent individuals, infection with E. multilocularis may be controlled by the immune system, leading either to abortion of the parasite lesion or to a very slow-growing potential for the parasite, leading to late clinical manifestation [8]. Conversely, Lachenmayer et al. observed a higher incidence of AE in patients with immunocompromised conditions [5]. These results are comparable with data from a large French registry, which showed that almost 10% of the patients with AE had some immunosuppressive condition, including different malignancies, autoimmune diseases, or the intake of immunosuppressive drugs [4]. However, it is important to consider that type and extent of immune deficiency are difficult to compare between the different series.
In the study of Lachenmayer et al., there is no correlation between immunocompromised conditions and disease course [5]. In contrast, previous studies showed a rapid disease progression of AE in immunocompromised patients compared with immunocompetent patients [4,6].
Gottstein et al. showed three different disease courses of AE in general, depending on immune response elicited by the host: (i) seroconversion and the parasite fails to establish chronic infection, and detection of either no lesions, or only “dying” or “aborted” lesions; (ii) seroconversion and metacestodes grow slowly and establish a chronic infection, and first clinical symptoms occur putatively after five to 15 years post-infection, and (iii) uncontrolled and rapid metacestode proliferation, as it occurs in individuals with impaired immunity such as acquired immune deficiency syndrome (AIDS) patients or patients undergoing transplantation or being treated by immunosuppressive drugs or biological agents [8].
Hübner et al. discussed the T-cell-mediated immune response and showed that Th2-predominated immunity is associated with an increased susceptibility to disease in patients suffering from chronic AE. Conversely, protective immunity is induced by a Th-1-based immune response, even in aborted forms of AE [9]. A mix of a Th1/Th2 pattern in the chronic stages of AE is seen in most AE cases investigated [9].
Based on this knowledge, we can better understand the course of the disease in our patient. In fact, a therapy with glucocorticoids leads to an inhibition of the acute generation of both Th1- and Th2-derived cytokines by activated T cells, although the inhibitory effect on the expression of Th1 cytokines appears to be greater [10]. Thus, treatment with glucocorticoids may be associated with a shift in the expression of Th2-derived cytokines relative to Th1 cytokines [11]. In the case of infection with E. multilocularis, this imbalance favors an uncontrolled and rapid metacestode proliferation and thus explains the accelerated disease course of our patient.
In the case of our patient, steroids not only promoted the disease but also falsified the diagnosis. The negative serologies for Em2 and Em18 are probably due to the effect of steroids on antibody production. Corticosteroids used in higher than physiologic doses can also reduce the immune response to vaccines or other infections [12].
All these data raise the question of whether a screening program for AE before planned immunosuppressive therapy is useful and what such a program should include (e.g., serology, sonography). In case of the appearance of new liver lesions such as cysts or single or multiple tumor-like lesions, AE should always be included as a differential diagnosis, and further workup with an appropriate serology should be initiated [13].
The therapy of choice and the only curative approach available for AE is surgical resection. In unresectable cases, treatment with antihelminthic benzimidazoles (e.g., mebendazole, albendazole) has a markedly prolonged survival. Finally, intensified research on the treatment of this deadly disease is still necessary, especially to find parasiticidal compounds and supportive immunostimulatory medications.
Conclusions
In conclusion, the increasing incidence of AE plays an important role in the management of patients, especially in the context of immunosuppressive therapy. In patients with immunosuppressive therapy, clinical presentation and diagnostics such as serology may be atypical or false-negative, respectively. In case of new findings or complaints after initiation of immunosuppressive therapy, AE should be sought at a low threshold.
Therefore, it is important that adequate screening for AE is considered for patients on immunosuppressive therapy, taking into account any influencing factors of immunosuppression on outcomes, e.g., false-negative results of serology. Furthermore, under established immunosuppressive therapy, close monitoring is indicated in order to recognise new complaints or findings at an early stage and to be able to adapt the further procedure accordingly.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study
References
- 1.Biological, epidemiological, and clinical aspects of echinococcosis, a zoonosis of increasing concern. Eckert J, Deplazes P. Clin Microbiol Rev. 2004;17:107–135. doi: 10.1128/CMR.17.1.107-135.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Human alveolar echinococcosis after fox population increase, Switzerland. Schweiger A, Ammann RW, Candinas D, et al. Emerg Infect Dis. 2007;13:878–882. doi: 10.3201/eid1306.061074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Threat of alveolar echinococcosis to public health--a challenge for Europe. Gottstein B, Stojkovic M, Vuitton DA, Millon L, Marcinkute A, Deplazes P. Trends Parasitol. 2015;31:407–412. doi: 10.1016/j.pt.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Increased incidence and characteristics of alveolar echinococcosis in patients with immunosuppression-associated conditions. Chauchet A, Grenouillet F, Knapp J, et al. Clin Infect Dis. 2014;59:1095–1104. doi: 10.1093/cid/ciu520. [DOI] [PubMed] [Google Scholar]
- 5.Elevated incidence of alveolar echinococcosis in immunocompromised patients. Lachenmayer A, Gebbers D, Gottstein B, Candinas D, Beldi G. Food Waterborne Parasitol. 2019;16:0. doi: 10.1016/j.fawpar.2019.e00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Echinococcus multilocularis and its intermediate host: a model of parasite-host interplay. Vuitton DA, Gottstein B. J Biomed Biotechnol. 2010;2010:923193. doi: 10.1155/2010/923193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.A clinical approach to diagnosis of autoimmune encephalitis. Graus F, Titulaer MJ, Balu R, et al. Lancet Neurol. 2016;15:391–404. doi: 10.1016/S1474-4422(15)00401-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Susceptibility versus resistance in alveolar echinococcosis (larval infection with Echinococcus multilocularis) Gottstein B, Wang J, Boubaker G, Marinova I, Spiliotis M, Müller N, Hemphill A. Vet Parasitol. 2015;213:103–109. doi: 10.1016/j.vetpar.2015.07.029. [DOI] [PubMed] [Google Scholar]
- 9.Echinococcus multilocularis metacestodes modulate cellular cytokine and chemokine release by peripheral blood mononuclear cells in alveolar echinococcosis patients. Hübner MP, Manfras BJ, Margos MC, et al. Clin Exp Immunol. 2006;145:243–251. doi: 10.1111/j.1365-2249.2006.03142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Effects of dexamethasone on the profile of cytokine secretion in human whole blood cell cultures. Franchimont D, Louis E, Dewe W, et al. Regul Pept. 1998;73:59–65. doi: 10.1016/s0167-0115(97)01063-x. [DOI] [PubMed] [Google Scholar]
- 11.Glucocorticoids in T cell development and function*. Ashwell JD, Lu FW, Vacchio MS. Annu Rev Immunol. 2000;18:309–345. doi: 10.1146/annurev.immunol.18.1.309. [DOI] [PubMed] [Google Scholar]
- 12.2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Rubin LG, Levin MJ, Ljungman P, et al. Clin Infect Dis. 2014;58:309–318. doi: 10.1093/cid/cit816. [DOI] [PubMed] [Google Scholar]
- 13.Diagnostic and follow-up performance of serological tests for different forms/courses of alveolar echinococcosis. Gottstein B, Lachenmayer A, Beldi G, et al. Food Waterborne Parasitol. 2019;16:0. doi: 10.1016/j.fawpar.2019.e00055. [DOI] [PMC free article] [PubMed] [Google Scholar]